Abstract
Background
Elevated cardiac troponin levels in blood are associated with increased risk of cardiovascular diseases (CVDs) and mortality. Cardiac troponin levels are heritable, but their genetic architecture remains elusive.
Methods
We conducted a trans-ethnic genome-wide association analysis on high-sensitivity cardiac troponin T and I levels (hs-cTnT and hs-cTnI) in 24,617 and 14,336 participants free of coronary heart disease and heart failure from six population-based cohorts, followed by a series of bioinformatic analyses to decipher the genetic architecture of hs-cTnT and hs-cTnI.
Results
We identified four genome-wide significant loci for hs-cTnT including a novel locus, rs3737882 in PPFIA4, and three previously reported loci at NCOA2, TRAM1 and BCL2. One known locus at VCL was replicated for hs-cTnI. One copy of C allele for rs3737882 was associated with a 6% increase in hs-cTnT levels (MAF=0.18, p-value=2.80×10−9). We observed pleiotropic loci located at BAG3 and ANO5. The proportions of variances explained by single nucleotide polymorphisms (SNPs) were 10.15% and 7.74% for hs-cTnT and hs-cTnI, respectively. SNPs were co-localized with BCL2 expression in heart tissues and hs-cTnT, and with ANO5 expression in artery, heart tissues and whole blood and both troponins. Mendelian randomization analyses showed that genetically increased hs-cTnT and hs-cTnI levels were associated with higher odds of atrial fibrillation (OR [95% CI] =1.38 [1.25, 1.54] for hs-cTnT and 1.21 [1.06, 1.37] for hs-cTnI).
Conclusions
We identified a novel genetic locus associated with hs-cTnT in a multi-ethnic population, and found that genetically regulated troponin levels were associated with atrial fibrillation.
Keywords: Cardiovascular disease, genome-wide association analysis, cardiac troponin, Mendelian randomization analysis, co-localization analysis
Introduction
Cardiac troponin is a biomarker of cardiomyocyte necrosis,1 consisting of three units, T, I and C, collocated with tropomyosin on the actin filament. The troponin complex is essential for calcium-mediated regulation of cardiac muscle contraction.2 Cardiac troponin T and I (cTnT and cTnI) are established biomarkers for myocardial infarction diagnosis and prognosis1 and have been shown to be associated with increased risk for cardiovascular disease (CVD) and mortality in the general population.3–6
Circulating cardiac troponin levels are heritable; the estimated heritability is 35% for cTnT and 25% for cTnI.6 A genome-wide association study (GWAS) of serum levels of high-sensitivity cTnT (hs-cTnT) identified two loci – an intergenic region at 8q13 and TNNT2 (1q32) - in 11,544 European and African Americans.7 Recently, a GWAS in 19,130 Scottish subjects has identified multiple loci for hs-cTnI (KLKB1 (4q35.2), VCL (10q22.2), ANO5 (11p14.3), CEP95 (17q23.3), and CPLX4 (18q21.32)), and added four novel loci at C1orf112 (1q24.2), TRABD2A (2p11.2), SORBS2 (4q35.1), and PTPRD (9p24.1) for hs-cTnT.6,7 Yet, the impact of genetic variation on the levels of hs-cTnT and I, in ethnically diverse populations has not been described. Using the most-updated high-sensitivity assays,8 we aimed to identify novel genetic variants associated with circulating cTnT and cTnI levels in a large multi-ethnic population consisting of African, Asian, European and Hispanic ancestries, and furthermore, to investigate causal associations with CVDs.
Methods
Availability of data and materials
Full summary GWAS statistics generated in this study are available upon reasonable request made to the corresponding authors. The GTEx version 8 expression quantitative trait loci (eQTL) data used in this study is available from eQTL catalogues (ftp://ftp.ebi.ac.uk/pub/databases/spot/eQTL). The authors declare that all other supporting data are available within the article and its Supplemental Materials.
Ethical declarations and methods
All studies were approved by appropriate institutional review committees, and all subjects provided written informed consent. Full details of data and methods used in this study are presented in Supplemental Data and Methods.
Results
Multi-ethnic GWAS identifies a novel locus associated with hs-cTnT
We conducted multi-ethnic GWAS for hs-cTnT levels in 24,617 participants, including 18,590 from European, 3,806 from African, 775 from Asian, and 1,446 from Hispanic ancestries. The hs-cTnI analyses included 14,336 participants, consisting of 12,730 European and 1,606 African ancestry subjects. The studies had mean ages ranged from 47.13 (SD=16.05) to 76.21 (SD=5.23), with proportions of females ranging from 50.8% to 65.1%. Baseline characteristics were comparable among studies. Detailed demographic information is presented in Supplemental Data V.
We identified 67 variants at four independent loci that were associated with hs-cTnT at genome-wide significance (p-value<5×10−8) (Figure 1 (a) and Table 1). One locus, mapping to the intron of PPFIA4 (liprin-alpha-4), has not been previously reported. One copy of a C allele (minor allele frequency (MAF)=0.18) for the lead SNP rs3737882 in PPFIA4 was associated with 6% increased hs-cTnT level (p-value=2.80×10−19). The MAF of rs3737882 was similar and the direction of effect was consistent across ethnic groups (Supplemental Table I). We also replicated three previously reported loci near NCOA2 (nuclear receptor coactivator 2) and TRAM1 (translocation associated membrane protein 1), and at BCL2 (B-cell lymphoma, apoptosis regulator). We did not observe any genome-wide significant association for hs-cTnI (Figure 1 (b)); however, one previously reported locus at VCL showed suggestive association with hs-cTnI (p-value=5.51×10−8). No genomic inflation was observed for both troponin analyses (Supplemental Figure I).
Figure 1. Manhattan Plots of Genome-wide Associations for hs-cTnT (a) and hs-cTnI (b).

SNPs are positioned along the x-axis according to chromosomal position with −log10(p-value) along the y-axis. Genome-wide significance threshold (p-value=5×10−8) is presented as a dashed black horizontal line and suggestive significance threshold (p-value=1×10−5) is presented as a dotted black horizontal line. Sentinel SNPs (±50kb) with p-value<1×10−6 are labeled with the nearest genes. Novel findings are colored in green, while the previously reported loci are highlighted in yellow.
Table 1.
Lead variants (p-value < 1×10−6) associated with hs-cTnT and hs-cTnI
| rsID | Chr | Position (hg19) | Locus | Nearest gene(s)* | Relation to gene | A1/A2 | AF | Beta (SE) | p-value | CADD |
|---|---|---|---|---|---|---|---|---|---|---|
| hs-cTnT (n=24,617) | ||||||||||
| rs10091864 | 8 | 71359103 | 8q13.3 | NCOA2;TRAM1 | intergenic | c/g | 0.56 | −0.07(0.008) | 2.28E-19 | 0.22 |
| rs9944895 | 18 | 60859974 | 18q21.33 | BCL2 | intronic | c/g | 0.69 | 0.07(0.008) | 1.05E-15 | 2.32 |
| rs3737882 | 1 | 203034955 | 1q32.1 | PPFIA4 | intronic | c/g | 0.82 | 0.06(0.010) | 2.80E-09 | 12.64 |
| rs28581409 | 8 | 71407059 | 8q13.3 | TRAM1 | intergenic | a/g | 0.34 | −0.05(0.008) | 6.63E-09 | 0.62 |
| rs75244633 | 4 | 119879588 | 4q26 | SYNPO2 | intronic | t/c | 0.02 | 0.14(0.027) | 1.44E-07 | 4.14 |
| rs146737477 | 1 | 83763281 | 1p31.1 | TTLL7 | intergenic | a/g | 0.03 | −0.25(0.047) | 1.65E-07 | 1.81 |
| rs12506869 | 4 | 101000987 | 4q23 | DDIT4L | ncRNA_intronic | a/g | 0.26 | −0.05(0.009) | 2.13E-07 | 0.02 |
| rs199460 | 17 | 44764775 | 17q21.31 | NSF | intronic | a/c | 0.74 | −0.05(0.010) | 3.07E-07 | 4.26 |
| rs17618762 | 2 | 19846104 | 2p24.1 | LINC00954;TTC32 | intergenic | a/g | 0.93 | −0.09(0.017) | 3.37E-07 | 0.58 |
| rs13341435 | 17 | 64250605 | 17q24.2 | APOH | intronic | a/g | 0.06 | 0.08(0.016) | 5.61E-07 | 3.86 |
| rs1192168 | 11 | 65730945 | 11q13.1 | SART1 | intronic | t/g | 0.50 | 0.04(0.007) | 7.27E-07 | 0.18 |
| rs9899998 | 17 | 29711014 | 17q11.2 | NF1;RAB11FIP4 | intergenic | a/g | 0.06 | −0.19(0.039) | 8.22E-07 | 1.22 |
| rs4922982 | 11 | 22237365 | 11p14.3 | ANO5 | intronic | t/c | 0.31 | −0.04(0.009) | 9.21E-07 | 0.63 |
| rs116819086 | 3 | 25449004 | 3p24.2 | RARB | intronic | c/g | 0.04 | −0.24(0.048) | 9.23E-07 | 0.10 |
| hs-cTnI (n=14,336) | ||||||||||
| rs7915720 | 10 | 75774139 | 10q22.2 | VCL;AP3M1 | ncRNA_intronic | a/g | 0.32 | 0.07(0.012) | 5.51E-08 | 0.63 |
| rs2915700 | 7 | 38984277 | 7p14.1 | VPS41;POU6F2 | intergenic | a/g | 0.17 | 0.09(0.019) | 7.97E-07 | 1.53 |
| rs26742 | 5 | 16664769 | 5p15.1 | MYO10 | downstream | a/g | 0.57 | −0.06(0.012) | 9.45E-07 | 0.71 |
Abbreviations
Chr: chromosome; AF_A1: allele frequency for allele 1; CADD: combined annotation dependent depletion score. This table presents the top 14 and 3 independent variants associated with hs-cTnT and hs-cTnI, respectively, at the significance level of p-value <1×10−6. Six studies were meta-analyzed using an inverse-variance-based fixed-effect approach. The statistics are based on the allele 1 (A1).
Nearest gene with a functional protein or RNA (e.g. anti-sense RNA) product that either overlaps with the sentinel variant, or for intergenic variants, the nearest genes up- and downstream, respectively.
The European-specific analysis resulted similar findings comparing to the trans-ethnic analysis (Supplemental Table II). The proportions of phenotypic variance explained by common variants were estimated at 10.15% (standard error (SE)=0.025) for hs-cTnT and 7.74 % (SE=0.038) for hs-cTnI in European ancestry. In the African-ancestry-specific analysis, we identified a genome-wide significant locus at LOC105378816;LOC107985037 (rs150095447, p-value=4.63×10−9) for hs-cTnT, and one at CD2BP2 (rs116215614, p-value=1.11×10−8) for hs-cTnI (Supplemental Table III). We did not observe genome-wide significant association in Asian or Hispanic ancestries (Supplemental Table IV and V). We presented ancestry-specific allele frequencies and association statistics for trans-ethnic significant associations in Supplemental Table I.
Variant effects on protein coding sequence
We investigated the predicted deleterious effects of troponin-associated loci using the Combined Annotation Dependent Depletion (CADD) scores. Sentinel SNPs and their proxies with CADD scores greater than 12 are shown in Table 1 and Supplemental Table VI. Among the genome-wide significant loci associated with hs-cTnT, the CADD score was only significantly high (12.64) for the sentinel SNP at PPFIA4 (rs3737882). SNPs associated with hs-cTnI in the VCL and ADK region showed significant CADD score. Additionally, a proxy for the sentinel variant in the pleiotropic BAG3 region, rs2234962, was predicted to be deleterious (CADD score =21.50).
Pleiotropic locus for troponin T and I
We identified three candidate pleiotropic loci, BCL2, ANO5 and BAG3, associated with both hs-cTnT and hs-cTnI at genome-wide significance (Supplemental Figure II and Supplemental Table VII). The sentinel SNP at BCL2, rs12457700, was identified by multi-trait analysis of GWAS (MTAG) with p-values of 3.93×10−12 and 4.84×10−12 for hs-cTnT and hs-cTnI, respectively. Two loci, BAG3 and ANO5 were previously identified with suggestive evidence in both hs-cTnT and hs-cTnI GWAS analyses and had improved significance in MTAG analyses. The sentinel pleiotropic variants at BAG3 and ANO5 were rs7938061 (MTAG p-value for hs-cTnT (PMTAG-hs-cTnT) =1.38×10−9 and PMTAG-hs-cTnI=1.36×10−9, respectively) and rs72842207 (PMTAG-hs-cTnT =1.17×10−8 and PMTAG-hs-cTnI=1.11×10−8, respectively).
Gene-based association test and gene-set enrichment
The Multi-marker Analysis of GenoMic Annotation (MAGMA) gene-based association analysis identified seven and three loci associated with hs-cTnT and hs-cTnI (p-value<2.58×10−6), respectively (Supplemental Table VIII). The significant associations for hs-cTnT included the GWAS loci at BCL2 and PPFIA4, with five other novel genes NSF, MANBA, NPC1, TMEM127, and C18orf8. For hs-cTnI, VCL, ADK, and AP3M1 were identified as significant. Genes mapped to GWAS associations with p-value <1×10−5 were further investigated for gene-set enrichment (Supplemental Table IX). Two genome-wide significant loci for hs-cTnT, BCL2 and PPFIA4, were enriched in the hypoxia hallmark gene set composed of genes up-regulated in response to low oxygen levels (adjusted p-value =9.60×10−3). Genes mapped to hs-cTnI SNPs were enriched among the gene ontologies associated with mitochondrion targeting (adjusted p-value =6.38×10−6) and protein localization to mitochondrion (adjusted p-value =1.19×10−5).
Tissue-specific co-localization and transcriptome-wide association analyses
We performed co-localization analysis for the nineteen loci identified in the GWAS and MTAG analysis with gene expression using Genotype-Tissue Expression (GTEx) v8 expression quantitative trait locus (eQTL) data (Supplemental Table X). We identified SNPs associated with ANO5 expression and either hs-cTnT or hs-cTnI in aortic artery, coronary artery, heart atrial appendage, and whole blood (Figure 2). The eQTL associations for ANO5 were remarkably high in two artery tissues. We also identified SNPs at BCL2 in left ventricular and atrial appendage tissues (Supplemental Figure III), and SNPs at NSF in aorta artery tissue, which co-localized with either hs-cTnT or hs-cTnI levels. Using predicted expression levels, we performed a transcriptome-wide association analysis in aorta artery, coronary artery, atrial appendage, left ventricle, and whole blood (Supplemental Table XI). At the transcriptome-wide significance level (p-value<1.59×10−6), we found that ANO5 in whole blood (p-value=1.51×10−6) and in atrial appendage (p-value=6.94×10−7), BCL2 in left ventricle (p-value=4.41×10−11) and in atrial appendage (p-value=3.49×10−8) were associated with hs-cTnT. For hs-cTnI, we identified a novel locus at PLAU in left ventricle (p-value= 8.05×10−7).
Figure 2. Scatter Plot of GWAS and eQTL Associations at ANO5.
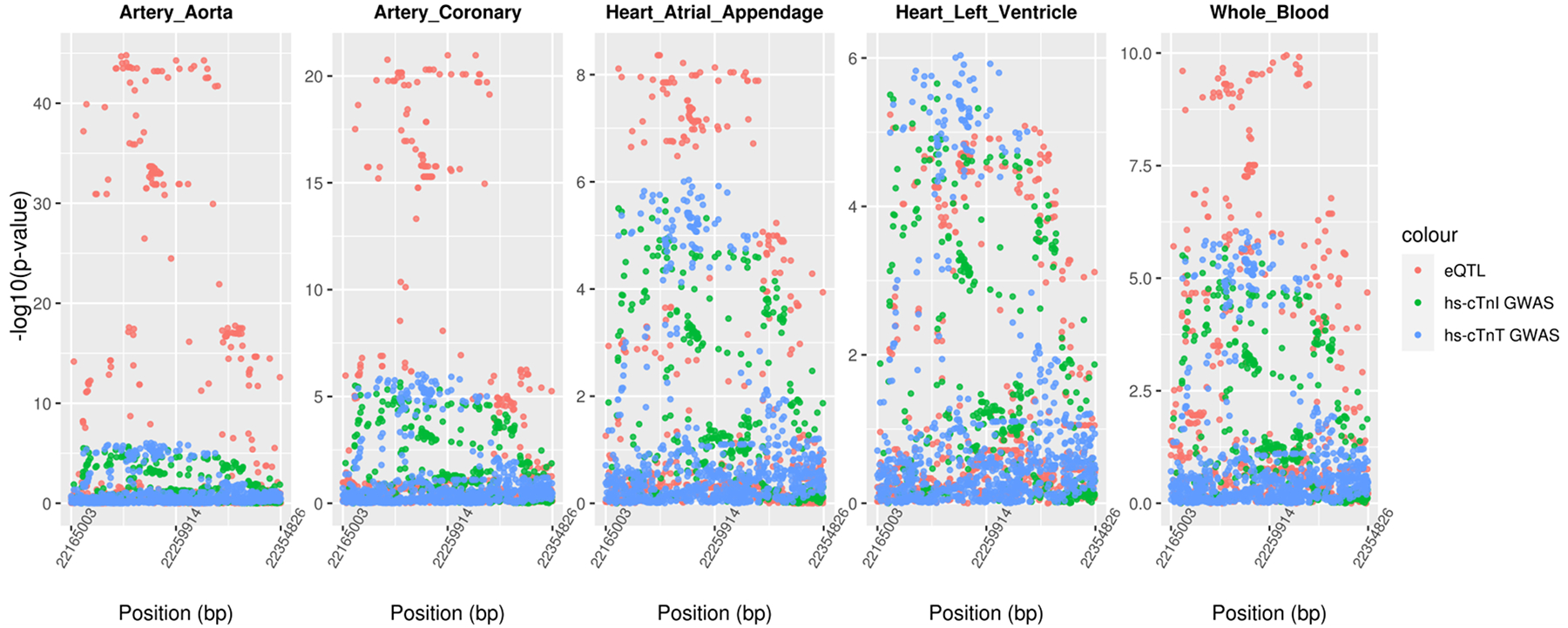
SNPs located ±50kb of ANO5 are plotted with −log10(p-value) along the y-axis against their genomic positions on the x-axis. Associations for gene expression, hs-cTnT and hs-cTnI are shown in red, green, and blue points, respectively.
Phenotypic effects of troponin-associated loci
The genetic correlation between hs-cTnT and hs-cTnI was estimated to be 0.99 (p-value=2.00×10−3). Genetic correlations with CVDs and related-traits are provided in Supplemental Table XII. Atrial Fibrillation (AF) (r=0.27, p-value=1.00×10−4), body mass index (BMI) (r=0.18, p-value=2.00×10−4), and estimated glomerular filtration rate (eGFR) (r=−0.30, p-value=1.17×10−5) were significantly genetically correlated with hs-cTnT, and heart failure (HF) was correlated with hs-cTnI (r=0.53, p-value=2.00×10−3).
Causal pathway from troponins to cardiovascular diseases
To establish a causal pathway from troponins to CVDs, we performed an MR analysis for coronary artery disease (CAD), HF, AF, and stroke. As shown in Figure 3, both troponins were causally associated with the risk of AF. A one standard deviation (SD) higher level of inverse-normalized hs-cTnT and hs-cTnI was associated with a 32% (odds ratio (OR) [95% confidence interval (CI)] = 1.32 [1.17, 1.50], p-value= 1.14×10−5) or a 21% (OR [95% CI] = 1.21 [1.06, 1.37], p-value= 4.72×10−3) greater risk of AF, respectively. We did not observe any association with CAD, HF, or stroke. The MR associations of hs-cTnT for AF showed the presence of heterogeneity and horizontal pleiotropy (Supplemental Table XIII), so we conducted sensitivity analyses by excluding outliers identified by MR-Pleiotropy RESidual Sum and Outlier (MR-PRESSO). The adjusted OR [95% CI] for an association between hs-cTnT and AF association was 1.38 [1.25, 1.54] (p-value =1.04×10−9), consistent with the primary analysis. Scatter plots of causal estimates for different MR test methods are presented in Supplemental Figure 4.
Figure 3. Forest Plots for Mendelian randomization associations between troponins and cardiovascular diseases.

cTnT: cardiac troponin T; cTnI: cardiac troponin I; AF: atrial fibrillation; CAD: coronary artery disease; HF: heart failure; AS: All stroke.
*Indicates significant MR association after Bonferroni adjustment for multiple testing burden.
Discussion
We are the first study to examine genetic determinants of hs-cTnT and hs-cTnI in a multi-ethnic population, and we identified novel genetic determinants as well as validated previous findings to improve our understanding of troponin genetic susceptibility. Beyond mapping to nearest genes, we also showed the biological impacts of our findings using in silico functional analyses and increasingly abundant publicly available data. Our results demonstrated that multiple genetic loci were coupled with gene expression information, which imply biologically relevant pathways. Furthermore, we observed a putative causal association between troponins and AF using MR approaches. Our study provides insights into the genetic etiology of circulating troponin levels and its potential impact on CVD.
We identified a novel hs-cTnT locus at 1q32.1 mapped to PPFIA4. PPFIA4 encodes liprin-alpha-1, which may regulate cell interaction with the extracellular environment. Our sentinel variant, rs3737882, is a PPFIA4 intron variant with a deleterious effect (CADD score =12.64). A previous multi-ethnic GWAS7 has reported another gene at 1q32.1, TNNT2, associated with a 99th percentile dichotomized hs-cTnT trait. PPFIA4 is a novel finding, as it lies 1.6 Mb away from TNNT2 and the sentinel SNPs at the two genes are independent (r2<0.01). Our study, for the first time, identified BCL2 with genome-wide significance in hs-cTnT. BCL2 showed only suggestive evidence in Yu et al., 2013 (p-value= 6.57×10−6).7 The sentinel variant mapped to BCL2 was rs9944895, and we functionally confirmed its co-localization with BCL2 expression in heart atrial appendage and left ventricular tissues (Supplemental Figure III).
Troponin associated loci are involved in cardiac cell responses to oxidative stress,9,10 which can potentially influence the devilment of AF. Hs-cTnT-associated loci, PPFIA4 and BCL2, are enriched in the hypoxia-mediated mechanism (Supplemental Table IX). When cells are stressed by hypoxia, PPFIA4 (liprin-alpha-4) is up-regulated by a hypoxia-inducible factor, HIF-1a,9 and dissociates cell contacts.11 PPFIA4 is specifically expressed in the brain, and skeletal and cardiac muscle tissues (The Human Protein Atlas, https://www.proteinatlas.org/)12. According to GTEx version 8 data, PPFIA4 is significantly over-expressed in the cerebellum and cerebellar hemispheres. The cerebellum is believed to have a unique modular structure made, including the control tower of the cardiovascular system, especially blood vessels.13 Over-expression of PPFIA4 in the cerebellum may potentially implicate a role for the brain in controlling the cardiovascular system under hypoxic conditions.
BCL2 encodes an integral outer mitochondrial membrane protein that inhibits the apoptotic death of cells such as lymphocytes. BCL2 proteins have highly redundant structures indicating the evolutionary importance of apoptosis - generally implicated in the pathogenesis of many conditions including cardiac failure.14 Apoptosis of cardiomyocytes is the major pathological change in cardiomyopathy, leading to excessive intercellular space. In vivo, decreased expression of Bcl-2 is associated with production of reactive oxygen species,10 which can cause arrhythmic conditions.15 Cardiomyocytes with overexpressed peroxisome proliferator-activated receptor gamma (PPARgamma) were reportedly resistant to oxidative stress-induced apoptosis, and a knock-down study suggested that Bcl-2 up-regulation mediated the protective effect of PPARgamma by regulating cell’s sensitivity to oxidative stress.16 At the apex and both ventricles of the heart, three transplanted individuals with dilated cardiomyopathy exhibited increased Bcl-2 expression possibly as a compensatory mechanism to the increased level of apoptosis.17 In the co-localization analysis, we observed that the sentinel SNP, rs9944895, was associated with a decreased hs-cTnT levels and an increased BCL2 expression in heart tissues (for an additional G allele, beta= −0.07 in GWAS; beta = 0.25 (left ventricle) and 0.38 (atrial appendage) in GTEx version 8 eQTL data), supporting the anti-apoptotic protective effect of BCL2. BCL2 expression associated with hs-cTnT variants is specific to heart tissues (Supplemental Figure III), highlighting the importance of hs-cTnT as a detectable biomarker in the blood.
Two loci, anoctamin 5 (ANO5) and BCL2-associated athanogene 3 (BAG3), were identified as valid pleiotropic loci for both hs-TnT and hs-cTnI, and included as genetic instruments in the MR analysis. BAG3 encodes an anti-apoptotic co-chaperone protein and variants in BAG3 have been established as causes of dilated cardiomyopathy and myofibrillar myopathy.18 BAG3 interacts with the best characterized inhibitor of apoptosis, BCL2, in preventing cell death.19 In hypoxia-injured cardiomyocytes, BAG3 over-expression activated autophagy and NF-kB promoting cell proliferation and inhibiting apoptosis.20
ANO5 encodes a member of the anoctamin family, a transmembrane protein and a putative calcium activated chloride channel.21 In our study, both troponin associations are significantly co-localized with ANO5 expression in all tissues of interest, but more significant expression is found in artery tissues taken from the left and right coronary arteries and the ascending aorta (rising from the left ventricle of the heart) (Figure 2 and Supplemental Table X). Few is known about function of ANO5 in artery, but anoctaminopathies may apply to arteries since the tunica media or a middle layer of arterial wall contains muscular tissue.22 The recent GWAS in a Scottish family identified ANO5 for hs-cTnI only and stated its relevance to adult-onset cardiomyopathy.6 An increased risk of ventricular arrhythmia has been observed in ANO5 mutation carriers.23
The association between hs-cTnT, hs-cTnI and the risk of AF has been observed repeatedly, where increased troponin levels were observed in AF patients.24,25 Evidence has also shown that troponin is associated with the risk of CVDs and mortality in AF patients.26 The underlying mechanism between troponin and AF is not clear; however, we observed that genetically regulated high hs-cTnT and I levels related to increased risk of AF. The genetic instruments of troponin we used to test the potential causality with AF included PPFIA4, BCL2, BAG3, and ANO5. The novel hs-cTnT locus, PPFIA4, has shown genome-wide significance in the association with AF in large genome-wide studies,27,28 suggesting shared genetic architecture between troponin and AF. The link between PPFIA4 and AF is understudied; we suspect that hypoxia-induced cell disassociation can lead to structural remodeling of the atria, which increases the risk of AF.29 In a canine model of congestive heart failure, the increased apoptosis (i.e. the increased ratio of pro-apoptotic (Bax) to anti-apoptotic BCL2 expression) developed within 24 hours after the onset of tachypacing; which leads to increased cell death and leukocyte infiltration; and progressively increased AF till 5 weeks after the onset.30 Th association between BAG3, ANO5 and AF is largely unknown. Mutations in BAG3 and ANO5 could induce dilated cardiomyopathy,18,23,31 and myocardial interstitial fibrosis was reported in ANO5 knockout rabbits.32 Cardiac troponin is a sensitive biomarker of cardiomyopathy, and elevated hs-cTnT and I levels are associated with myocardial fibrosis.33,34 AF has shared pathology with cardiomyopathy35 and myocardial fibrosis36, suggesting that troponin may mediate the effect of those candidate genes to AF. The potential causal relation between troponin and AF deserves further investigation.
Our study is the largest multi-ethnic GWAS analysis of cardiac troponins; however, it also has limitations. Due to modest sample sizes of African-, Asian- and Hispanic-subjects, the statistical power to detect ancestry-specific associations in these ancestries was limited. In addition, we lacked replication studies to reproduce our novel findings. Of note, we reproduced three previously reported loci: an intergenic region near NCOA2 for hs-cTnT, and VCL and ADK for hs-cTnI.6,7 For our novel finding in PPFIA4, we were not able to get an independent study to replicate the locus. Nevertheless, for the sentinel SNP in PPFIA4, rs3737882, we observed homogeneous positive effect across all ancestries and statistical significance in African, Asian, and European ancestries. We anticipate our novel findings can be generalized to other populations. Lastly, AF polygenic risk score (PRS) has provided prognostic information into clinical factors in risk stratification algorithms.37 Our work can be extended by constructing a troponin PRS and integrating AF loci. Future studies are warranted to exam the added value of troponin-AF PRS in the clinical risk management.
Conclusions
In summary, we identified a novel genome-wide significant locus for hs-cTnT, rs3737882 in PPFIA4 in a large multi-ethnic population. Previously reported loci were also confirmed for hs-cTnT, BCL2 at 8q13.3 and an intergenic region near NCOA2 at 18q21.33, and for hs-cTnI, VCL at 10q22.2. Pleiotropic loci for both hs-cTnT and hs-cTnI were identified at ANO5 and BAG3, supported by co-localization evidence of gene expression in heart and artery tissues. MR analysis showed that hs-cTnT and hs-cTnI were causally associated with 38% and 21% higher risk of AF, respectively. Our findings provide new sights into CVD etiology and demonstrate potential clinical utility of troponin as a preventive target of AF.
Supplementary Material
Acknowledgements
The authors thank the staff and participants of Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study, Atherosclerosis Risk in Communities (ARIC) study, Cardiovascular Health Study (CHS), Multi-Ethnic Study of Atherosclerosis (MESA), Prospective Study of Pravastatin in the Elderly at Risk (PROSPER), and Study of Health in Pomerania (SHIP) for their important contributions.
Source of Funding
The AGES-Reykjavik study is funded by National Institutes of Health contract N01-AG12100, the U.S. National Institute on Aging (NIA) Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). The ARIC study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), Department of Health and Human Services (contract numbers HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I and HHSN268201700005I), R01HL087641, R01HL059367 and R01HL086694; National Human Genome Research Institute (NHGRI) contract U01HG004402; and NIH contract HHSN268200625226C. Infrastructure was partly supported by Grant Number UL1RR025005, a component of the NIH and NIH Roadmap for Medical Research. The CHS research was supported by NHLBI contracts HHSN268201200036C, HHSN268200800007C, HHSN268200960009C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, 75N92021D00006; and NHLBI grants U01HL080295, R01HL085251, R01HL087652, R01HL105756, R01HL103612, R01HL120393, and U01HL130114 with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through R01AG023629 from the NIA. The provision of genotyping data was supported in part by the National Center for Advancing Translational Sciences, CTSI grant UL1TR001881, and the National Institute of Diabetes and Digestive and Kidney Disease Diabetes Research Center (DRC) grant DK063491 to the Southern California Diabetes Endocrinology Research Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The measurement of Troponin T was funded by an investigator-initiated grant to the University of Maryland from Roche Diagnostics. MESA and the MESA SHARe projects are conducted and supported by the NHLBI in collaboration with MESA investigators. Support for MESA is provided by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, UL1-TR-000040, UL1-TR-001079, UL1-TR-001420. Genotyping was performed at Affymetrix (Santa Clara, California, USA) and the Broad Institute of Harvard and MIT (Boston, Massachusetts, USA) using the Affymetrix Genome-Wide Human SNP Array 6.0. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The PROSPER study was supported by an investigator-initiated grant obtained from Bristol-Myers Squibb. Prof. Dr. J. W. Jukema is an Established Clinical Investigator of the Netherlands Heart Foundation (grant 2001 D 032). Support for genotyping was provided by the seventh framework program of the European commission (grant 223004) and by the Netherlands Genomics Initiative (Netherlands Consortium for Healthy Aging grant 050-060-810). The Study of Health in Pomerania (SHIP & SHIP-TREND) is part of the Community Medicine Research net (CMR) at the University of Greifswald, Germany. The CMR encompasses several research projects that share data from the population-based SHIP project (http://ship.community-medicine.de). Funding was provided by grants from the German Federal Ministry of Education and Research, the Ministry for Education, Research and Cultural Affairs (grants no. 01ZZ9603, 01ZZ0103, 01ZZ0403, and 03ZIK012) and the Ministry for Social Affairs of the Federal State of Mecklenburg–West Pomerania (grant 03IS2061A). The work was in part supported by NIH HL105756. Dr. Yu was in part supported by NIH HL105756, HL141824 and HL148218. Dr. Jun was in part supported by NIH DK118631 and HD098552.
Disclosures
Dr. Ballantyne has institutional grant supports from Abbott Diagnostics and Roche Diagnostics, and serves as a consultant for Abbott Diagnostics and Roche Diagnostics at modest level, and Denka Seiken at significant level. Dr. Psaty serves on the steering committee of the Yale Open Data Access Project funded by Johnson & Johnson. Dr. deFilippi has received research grants from Roche Diagnostics; has received consulting fees from Abbott Diagnostics, FujiRebio, Metabolomics, Ortho Diagnostics, Roche Diagnostics, and Siemens Healthcare; has received honoraria from WebMD; and has received royalties from UpToDate. The remaining authors have nothing to disclose.
Non-standard Abbreviations and Acronyms
- cTnT and cTnI
Cardiac troponin T and I
- hs-cTnT and hs-cTnI
high-sensitivity cTnT and cTnI
- CVD
cardiovascular disease
- AF
atrial fibrillation
- CAD
coronary artery disease
- CHD
coronary heart disease
- HF
heart failure
- eGFR
estimated glomerular filtration rate
- ARIC
Atherosclerosis Risk in Communities study
- AGES-Reykjavik study
Age, Gene/Environment Susceptibility-Reykjavik Study
- CHS
Cardiovascular Health Study
- MESA
Multi-Ethnic Study of Atherosclerosis
- PROSPER
Prospective Study of Pravastatin in the Elderly at Risk
- SHIP
Study of Health in Pomerania
- SE
standard error
- SD
standard deviation
- OR
odds ratio
- CI
confidence interval
- CADD
combined annotation dependent depletion score
- MTAG
multi-trait analysis of GWAS
- MAGMA
Multi-marker Analysis of GenoMic Annotation
- GWAS
genome-wide association study
- SNP
single nucleotide polymorphism
- MAF
minor allele frequency
- GTEx
Genotype-Tissue Expression
- eQTL
expression quantitative trait locus
- MR
Mendelian Randomization
- MR-PRESSO
MR-Pleiotropy RESidual Sum and Outlier
- PPARgamma
peroxisome proliferator-activated receptor gamma
- PRS
polygenic risk score
Footnotes
Supplemental Materials:
References
- 1.Babuin L, Jaffe AS. Troponin: the biomarker of choice for the detection of cardiac injury. Can Med Assoc J. 2005;173:1191 LP–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takeda S, Yamashita A, Maeda K, Maeda Y. Structure of the core domain of human cardiac troponin in the Ca(2+)-saturated form. Nature. 2003;424:35–41. [DOI] [PubMed] [Google Scholar]
- 3.Blankenberg S, Salomaa V, Makarova N, Ojeda F, Wild P, Lackner KJ, Jorgensen T, Thorand B, Peters A, Nauck M, et al. Troponin I and cardiovascular risk prediction in the general population: the BiomarCaRE consortium. Eur Heart J. 2016;37:2428–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of Serial Measures of Cardiac Troponin T Using a Sensitive Assay With Incident Heart Failure and Cardiovascular Mortality in Older Adults. JAMA. 2010;304:2494–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welsh P, Preiss D, Hayward C, Shah AS v, McAllister D, Briggs A, Boachie C, McConnachie A, Padmanabhan S, Welsh C, et al. Cardiac Troponin T and Troponin I in the General Population. Circulation. 2019;139:2754–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu B, Barbalic M, Brautbar A, Nambi V, Hoogeveen RC, Tang W, Mosley TH, Rotter JI, DeFilippi CR, O’Donnell CJ, et al. Association of genome-wide variation with highly sensitive cardiac troponin-T levels in European Americans and Blacks: a meta-analysis from atherosclerosis risk in communities and cardiovascular health studies. Circ-Cardiovasc Gene. 2013;6:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fathil MFM, Md Arshad MK, Gopinath SCB, Hashim U, Adzhri R, Ayub RM, Ruslinda AR, Nuzaihan M, Azman AH, Zaki M, et al. Diagnostics on acute myocardial infarction: Cardiac troponin biomarkers. Biosens. Bioelectron 2015;70:209–220. [DOI] [PubMed] [Google Scholar]
- 9.Wang V, Davis DA, Haque M, Huang LE, Yarchoan R. Differential gene up-regulation by hypoxia-inducible factor-1alpha and hypoxia-inducible factor-2alpha in HEK293T cells. Cancer Res. 2005;65:3299–3306. [DOI] [PubMed] [Google Scholar]
- 10.Hildeman DA, Mitchell T, Aronow B, Wojciechowski S, Kappler J, Marrack P. Control of Bcl-2 expression by reactive oxygen species. PNAS. 2003;100:15035–15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattauch S, Sachs M, Behrens J. Liprin-α4 is a new hypoxia-inducible target gene required for maintenance of cell–cell contacts. Exp Cell Res. 2010;316:2883–2892. [DOI] [PubMed] [Google Scholar]
- 12.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, et al. Tissue-based map of the human proteome. Science. 2015;347:1260419. [DOI] [PubMed] [Google Scholar]
- 13.Nisimaru N. Cardiovascular Modules in the Cerebellum. JPN J Physiol. 2004;54:431–448. [DOI] [PubMed] [Google Scholar]
- 14.Biala AK, Kirshenbaum LA. The interplay between cell death signaling pathways in the heart. Trends Cardiovas Med. 2014;24:325–331. [DOI] [PubMed] [Google Scholar]
- 15.Jeong E-M, Liu M, Sturdy M, Gao G, Varghese ST, Sovari AA, Dudley SC. Metabolic stress, reactive oxygen species, and arrhythmia. J Mol Cell Cardiol. 2012;52:454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren Y, Sun C, Sun Y, Tan H, Wu Y, Cui B, Wu Z. PPAR gamma protects cardiomyocytes against oxidative stress and apoptosis via Bcl-2 upregulation. Vasc Pharmacol. 2009;51:169–174. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Li M, Xu L, Liu J, Wang D, Li Q, Wang L, Li P, Chen S, Liu T. Expression of Bcl-2 and microRNAs in cardiac tissues of patients with dilated cardiomyopathy. Mol Med Rep. 2017;15:359–365. [DOI] [PubMed] [Google Scholar]
- 18.Esslinger U, Garnier S, Korniat A, Proust C, Kararigas G, Müller-Nurasyid M, Empana J-P, Morley MP, Perret C, Stark K, et al. Exome-wide association study reveals novel susceptibility genes to sporadic dilated cardiomyopathy. PloS one. 2017;12:e0172995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J-H, Takahashi T, Yasuhara N, Inazawa J, Kamada S, Tsujimoto Y. Bis, a Bcl-2-binding protein that synergizes with Bcl-2 in preventing cell death. Oncogene. 1999;18:6183–6190. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, He Z, Xiao W, Na Q, Wu T, Su K, Cui X. Overexpression of BAG3 Attenuates Hypoxia-Induced Cardiomyocyte Apoptosis by Inducing Autophagy. Cell Physiol Biochem. 2016;39:491–500. [DOI] [PubMed] [Google Scholar]
- 21.Gerke V, Rescher U. ANO5 in membrane repair - Status: “It’s complicated.” Cell Calcium. 2021;97:102415. [DOI] [PubMed] [Google Scholar]
- 22.Tucker WD, Arora Y, Mahajan K. Anatomy, Blood Vessels. [Updated 2021 Feb 12]. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021. January-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470401/ [PubMed] [Google Scholar]
- 23.Wahbi K, Béhin A, Bécane HM, Leturcq F, Cossée M, Laforêt P, Stojkovic T, Carlier P, Toussaint M, Gaxotte V, et al. Dilated cardiomyopathy in patients with mutations in anoctamin 5. Int J Cardiol. 2013;168:76–79. [DOI] [PubMed] [Google Scholar]
- 24.Parwani AS, Boldt L-H, Huemer M, Wutzler A, Blaschke D, Rolf S, Möckel M, Haverkamp W. Atrial fibrillation-induced cardiac troponin I release. Int J Cardiol. 2013;168:2734–2737. [DOI] [PubMed] [Google Scholar]
- 25.Costabel JP, Urdapilleta M, Lambardi F, Campos R, Vergara JM, Arizavarreta P, Trivi M. High-Sensitivity Cardiac Troponin Levels in Supraventricular Tachyarrhythmias. PACE. 2016;39:588–591. [DOI] [PubMed] [Google Scholar]
- 26.Fan Y, Zhao X, Li X, Li N, Hu X. Cardiac troponin and adverse outcomes in atrial fibrillation: A meta-analysis. Clin Chim Acta. 2018;477:48–52. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen JB, Thorolfsdottir RB, Fritsche LG, Zhou W, Skov MW, Graham SE, Herron TJ, McCarthy S, Schmidt EM, Sveinbjornsson G, et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet. 2018;50:1234–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roselli C, Chaffin MD, Weng L-C, Aeschbacher S, Ahlberg G, Albert CM, Almgren P, Alonso A, Anderson CD, Aragam KG, et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet. 2018;50:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pellman J, Sheikh F. Atrial fibrillation: mechanisms, therapeutics, and future directions. Compr Physiol. 2015;5:649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cardin S, Li D, Thorin-Trescases N, Leung T-K, Thorin E, Nattel S. Evolution of the atrial fibrillation substrate in experimental congestive heart failure: angiotensin-dependent and -independent pathways. Cardiovasc Res. 2003;60:315–325. [DOI] [PubMed] [Google Scholar]
- 31.Garnier S, Harakalova M, Weiss S, Mokry M, Regitz-Zagrosek V, Hengstenberg C, Cappola TP, Isnard R, Arbustini E, Cook SA, et al. Genome-wide association analysis in dilated cardiomyopathy reveals two new players in systolic heart failure on chromosomes 3p25.1 and 22q11.23. Eur Heart J. 2021;42:2000–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sui T, Yao H, Zhang T, Li J, Lai L, Li Z. The Genetic Mutation of ANO5 in Rabbits Recapitulates Human Cardiomyopathy. Appl Sci. 2020;10:4976. [Google Scholar]
- 33.Takashio S, Yamamuro M, Uemura T, Utsunomiya D, Morita K, Izumiya Y, Sugiyama S, Kojima S, Yamamoto E, Tsujita K, et al. Correlation between extent of myocardial fibrosis assessed by cardiac magnetic resonance and cardiac troponin t release in patients with nonischemic heart failure. Am J Cardiol. 2014;113:1697–1704. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Liu J, Cao Y, Han X, Shao G, Zhou X, Gu J, Liu T, Cui Y, Shi H. Predictive values of multiple non-invasive markers for myocardial fibrosis in hypertrophic cardiomyopathy patients with preserved ejection fraction. Sci Rep. 2021;11:4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin D, Mansour MC, Ruskin JN, Heist EK. Atrial Fibrillation–Mediated Cardiomyopathy. Circ Arrhythm. 2019;12:e007809. [DOI] [PubMed] [Google Scholar]
- 36.He X, Gao X, Peng L, Wang S, Zhu Y, Ma H, Lin J, Duan DD. Atrial fibrillation induces myocardial fibrosis through angiotensin II type 1 receptor-specific Arkadia-mediated downregulation of Smad7. Circ Res. 2011;108:164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gladding PA, Legget M, Fatkin D, Larsen P, Doughty R. Polygenic risk scores in coronary artery disease and atrial fibrillation. Heart Lung Circ. 2020;29:634–640. [DOI] [PubMed] [Google Scholar]
- 38.Psaty BM, O’Donnell CJ, Gudnason V, Lunetta KL, Folsom AR, Rotter JI, Uitterlinden AG, Harris TB, Witteman JCM, Boerwinkle E. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: Design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circ-Cardiovasc Gene. 2009;2:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson P v, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF, et al. Age, gene/environment susceptibility–Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 41.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. [DOI] [PubMed] [Google Scholar]
- 42.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux A v, Folsom AR, Greenland P, Jacob DR, Kronmal R, Liu K, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 43.Shepherd J, Blauw GJ, Murphy MB, Cobbe SM, Bollen EL, Buckley BM, Ford I, Jukema JW, Hyland M, Gaw A, et al. The design of a prospective study of Pravastatin in the Elderly at Risk (PROSPER). PROSPER Study Group. PROspective Study of Pravastatin in the Elderly at Risk. Am J Cardiol. 1999;84:1192–1197. [DOI] [PubMed] [Google Scholar]
- 44.Shepherd J, Blauw GJ, Murphy MB, Bollen ELEM, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. [DOI] [PubMed] [Google Scholar]
- 45.Volzke H, Alte D, Schmidt CO, Radke D, Lorbeer R, Friedrich N, Aumann N, Lau K, Piontek M, Born G, et al. Cohort profile: the study of health in Pomerania. Int J Epidemiol. 2011;40:294–307. [DOI] [PubMed] [Google Scholar]
- 46.The 1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol. 2010;34:816–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fuchsberger C, Abecasis GR, Hinds DA. minimac2: faster genotype imputation. Bioinformatics. 2015;31:782–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finucane HK, Bulik-Sullivan B, Gusev A, Trynka G, Reshef Y, Loh P-R, Anttila V, Xu H, Zang C, Farh K, et al. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat Genet. 2015;47:1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun. 2017;8:1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46:310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Leeuw CA, Mooij JM, Heskes T, Posthuma D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comp Biol. 2015;11:e1004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.The GTEx Consortium. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348:648–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liberzon A, Subramanian A, Pinchback R, Thorvaldsdóttir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27:1739–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slenter DN, Kutmon M, Hanspers K, Riutta A, Windsor J, Nunes N, Mélius J, Cirillo E, Coort SL, Digles D, et al. WikiPathways: a multifaceted pathway database bridging metabolomics to other omics research. Nucleic Acids Res. 2017;46:D661–D667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, McMahon A, Morales J, Mountjoy E, Sollis E, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019;47:D1005–D1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bulik-Sullivan BK, Loh P-R, Finucane HK, Ripke S, Yang J, Schizophrenia Working Group of the Psychiatric Genomics Consortium, Patterson N, Daly MJ, Price AL, Neale BM. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van der Harst P, Verweij N. Identification of 64 Novel Genetic Loci Provides an Expanded View on the Genetic Architecture of Coronary Artery Disease. Circ Res. 2018;122:433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shah S, Henry A, Roselli C, Lin H, Sveinbjörnsson G, Fatemifar G, Hedman AK, Wilk JB, Morley MP, Chaffin MD, et al. Genome-wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat Commun. 2020;11:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scott RA, Scott LJ, Mägi R, Marullo L, Gaulton KJ, Kaakinen M, Pervjakova N, Pers TH, Johnson AD, Eicher JD, et al. An Expanded Genome-Wide Association Study of Type 2 Diabetes in Europeans. Diabetes. 2017;66:2888–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu Z, Wang X, Li X, Lin Y, Shen S, Liu C-L, Hobbs BD, Hasegawa K, Liang L, Boezen HM, et al. Genetic overlap of chronic obstructive pulmonary disease and cardiovascular disease-related traits: a large-scale genome-wide cross-trait analysis. Respir Res. 2019;20:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pirruccello JP, Bick A, Wang M, Chaffin M, Friedman S, Yao J, Guo X, Venkatesh BA, Taylor KD, Post WS, et al. Analysis of cardiac magnetic resonance imaging in 36,000 individuals yields genetic insights into dilated cardiomyopathy. Nat Commun. 2020;11:2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Evangelou E, Warren HR, Mosen-Ansorena D, Mifsud B, Pazoki R, Gao H, Ntritsos G, Dimou N, Cabrera CP, Karaman I, et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet. 2018;50:1412–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wuttke M, Li Y, Li M, Sieber KB, Feitosa MF, Gorski M, Ganna A, Chen J, Buchkovich ML, Mora S, et al. A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat Genet. 2019;51:957–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Turley P, Walters RK, Maghzian O, Okbay A, Lee JJ, Fontana MA, Nguyen-Viet TA, Wedow R, Zacher M, Furlotte NA, et al. Multi-trait analysis of genome-wide association summary statistics using MTAG. Nat Genet. 2018;50:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giambartolomei C, Vukcevic D, Schadt EE, Franke L, Hingorani AD, Wallace C, Plagnol V. Bayesian Test for Colocalisation between Pairs of Genetic Association Studies Using Summary Statistics. PLoS Genet. 2014;10:e1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kerimov N, Hayhurst JD, Peikova K, Manning JR, Walter P, Kolberg L, Samoviča M, Sakthivel MP, Kuzmin I, Trevanion SJ, et al. A compendium of uniformly processed human gene expression and splicing quantitative trait loci. Nat Genet. 2021;53(9):1290–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barbeira AN, Dickinson SP, Bonazzola R, Zheng J, Wheeler HE, Torres JM, Torstenson ES, Shah KP, Garcia T, Edwards TL, et al. Exploring the phenotypic consequences of tissue specific gene expression variation inferred from GWAS summary statistics. Nat Commun. 2018;9:1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nikpay M, Goel A, Won H-H, Hall LM, Willenborg C, Kanoni S, Saleheen D, Kyriakou T, Nelson CP, Hopewell JC, et al. A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, Rutten-Jacobs L, Giese A-K, van der Laan SW, Gretarsdottir S, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. 2018;50:524–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davies NM, Hill WD, Anderson EL, Sanderson E, Deary IJ, Davey Smith G. Multivariable two-sample Mendelian randomization estimates of the effects of intelligence and education on health. eLife. 2019;8:e43990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife. 2018;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Full summary GWAS statistics generated in this study are available upon reasonable request made to the corresponding authors. The GTEx version 8 expression quantitative trait loci (eQTL) data used in this study is available from eQTL catalogues (ftp://ftp.ebi.ac.uk/pub/databases/spot/eQTL). The authors declare that all other supporting data are available within the article and its Supplemental Materials.


