Abstract
Objective:
Risk factors for mortality in patients with subdural hematoma (SDH) include poor Glasgow Coma Scale (GCS), pupil non-reactivity, and hemodynamic instability on presentation. Little is published regarding prognosticators of SDH in the elderly. This study aims to examine risk factors for hospital mortality and withdrawal of life-sustaining measures in an octogenarian population presenting with SDH.
Methods:
A prospectively collected multi-center database of 3,279 TBI admissions to 45 different U.S. trauma centers between 2017–2019 was queried to identify patients >79 years old presenting with SDH. Factors collected included baseline demographic data, past medical history, antiplatelet/anticoagulant use, and clinical presentation (GCS, pupil reactivity, injury severity scale [ISS]). Primary outcome data included hospital mortality/discharge to hospice care and withdrawal of life-sustaining measures. Multivariate logistic regression analyses were used to identify factors independently associated with primary outcome variables.
Results:
A total of 695 patients were isolated for analysis. Of the total cohort, the rate of hospital mortality or discharge to hospice care was 22% (n=150) and the rate of withdrawal of life-sustaining measures was 10% (n=66). A multivariate logistic regression model identified GCS <13, pupil non-reactivity, increasing ISS, intraventricular hemorrhage, and neurosurgical intervention as factors independently associated with hospital mortality/hospice. Congestive heart failure (CHF), hypotension, GCS<13, and neurosurgical intervention were independently associated with withdrawal of life-sustaining measures.
Conclusions:
Poor GCS, pupil non-reactivity, ISS, and intraventricular hemorrhage are independently associated with hospital mortality or discharge to hospice care in patients >80 years with SDH. Pre-existing CHF may further predict withdrawal of life-sustaining measures.
Keywords: Mortality, Octogenarians, Subdural hematoma, Traumatic Brain Injury
Introduction:
Traumatic brain injury (TBI) represents a growing public health concern, especially among the growing older patient population. According to the CDC (Center for Disease Control), TBI attributed to 10% of all injury-related emergency department visits, hospitalization, and deaths in the United States in 2013, noting a 53% increase from 2006 to 2014.1 There is a substantial body of literature detailing outcomes and prognosticating factors following TBI. For instance, risk factors for mortality include poor Glasgow Coma Scale (GCS),2, 3 especially GCS motor response,4 loss of pupil reactivity,4, 5 hemodynamic instability,6 and higher injury severity score (ISS).5, 6
Of all demographic sub-groups within the TBI population, subdural hematomas (SDHs) in the older patient represent the highest incidence and have the most devastating outcomes.7–11 Normal physiological processes of aging, such as cerebral atrophy that creates a larger subdural space and an increased risk of shearing bridging vessels, are thought to play a role in the poor outcomes seen amongst this population.10, 12, 13 Although numerous risk factors have been established following TBI in general, little has been published regarding prognostication in its highest risk population – SDH in older patients
Our study aims to examine predictors of important key metrics such as hospital mortality, withdrawal of life-sustaining support, and discharge disposition in the octogenarian population following SDH. Detailed study of the risk factors that govern outcomes in this population may help to guide surgical and medical management in an area with little available published guidance.
Material and Methods:
Patient selection
This is a retrospective analysis of prospectively collected data from the Geriatric Traumatic Brain Injury (“Geri-TBI”) study. The observational Geri-TBI study was approved by the American Association for the Surgery of Trauma (AAST) Multicenter Trials Committee. Data were collected from September 2017 through February 2019 across 45 trauma centers. Data were abstracted from medical records and entered into an online data collection portal resource maintained by the AAST. The Geri-TBI study inclusion criteria were CT-verified TBI, age ≥ 40 years, and presentation at a participating hospital within 24 hours of injury. To establish a population with TBI as the primary injury and minimize the confounding influence of polytrauma, we excluded patients with injury to another body region resulting in an abbreviated injury scale (AIS) score >2. Prisoners and pregnant women were also excluded. Data of patients >89 years is considered protected health information under the Health Insurance Portability and Accountability Act, and was permitted for inclusion by some, but not all, sites.
Data Extraction
Patients were included in this study if they were aged >79 years and if they carried a diagnosis of SDH on admission. Factors collected included baseline demographic data, past medical history, antiplatelet/anticoagulant use, and clinical presentation (GCS, pupil reactivity, ISS). Patients were excluded if no discharge disposition was recorded. GCS scores were categorized into one of three categories (GCS 3–8, 9–12, or 13–15) based on previously published benchmarks for TBI severity (i.e. mild, moderate, severe) and was included in the analysis as a categorical variable. For the purposes of the analysis, pupil reactivity was categorized as either 1) both reactive or 2) one or both pupils non-reactive. We included both patients who died in the hospital as well as patients discharged to hospice in the mortality/hospice endpoint. Discharge disposition was categorized into one of four categories – 1) home/assisted living, 2) skilled nursing facility (SNF)/long-term acute care hospital (LTACH), 3) acute rehabilitation facility, and 4) hospital mortality/hospice. The two primary endpoints of this study were mortality/hospice and withdrawal of life-sustaining measures. Discharge disposition to one of the four aforementioned categories was a secondary endpoint.
Statistical Analysis
The data were initially subjected to a bivariate analysis to determine variables significantly associated with withdrawal of life-sustaining measures and hospital mortality/hospice. All factors included in our analysis are listed in Table 1. Chi-squared tests were utilized to evaluate the association of categorical variables (such as gender or CHF) on the outcome variables. Fisher exact tests were used in cases of counts <5 in any of the constructed contingency tables. A 2-tailed student’s t-test was utilized to evaluate the effects of continuous variables on the outcome data. Factors associated with either hospice/mortality or withdrawal of life-sustaining measures were selected for inclusion in multivariate analysis. Two multivariate logistic regression models were created for each of the primary endpoint variables studied. A multinomial logistic regression was utilized to evaluate the odds ratio (OR) for mortality/hospice in relation to a reference category (home/assisted living). This produced ORs for the primary endpoint as well as ORs for discharge to rehabilitation and SNF/LTACH. A binary logistic regression was used for the withdrawal of life-sustaining measures primary endpoint. To evaluate the accuracy of each model for predicting the primary outcome, receiver operating characteristic (ROC) curves were created and the area under the ROC (AUROC) curve was computed. Variables significantly associated with either mortality/hospice or withdrawal of life-sustaining measures were selected for inclusion in both multivariable models.
Table 1.
Baseline patient characteristics (n=695)
| Variable | Count (%) or mean (range) |
|---|---|
| Male | 313 (45%) |
| Age, years | 86 (80–99) |
| Race | |
| White | 578 (83%) |
| Black | 45 (7%) |
| Asian | 22 (3%) |
| Other/Unknown | 50 (7%) |
| PMH | |
| Neuro | 328 (47%) |
| Dementia | 165 (24%) |
| Prior TBI | 32 (5%) |
| CHF | 104 (15%) |
| CAD | 196 (28%) |
| COPD | 71 (10%) |
| ESRD | 17 (2%) |
| Cirrhosis | 3 (0.4%) |
| Diabetes | 196 (28%) |
| Substance abuse | 14 (2%) |
| Anticoagulant use | 163 (24%) |
| Antiplatelet use | 377 (54%) |
| Mechanism of injury | |
| Found down | 41 (6%) |
| Fall >10ft | 12 (2%) |
| Fall <10ft | 592 (85%) |
| MVC | 23 (3%) |
| Other/Unknown | 27 (4%) |
| GCS | |
| 3–8 | 76 (11%) |
| 9–12 | 56 (8%) |
| 13–15 | 563 (81%) |
| ISS | 17 (1–38) |
| Pupillary reactivity | |
| Both reactive | 636 (92%) |
| None reactive | 38 (6%) |
| One reactive | 21 (3%) |
| Subdural hematoma | |
| Acute | 660 (95%) |
| Subacute | 21 (3%) |
| Chronic | 12 (2%) |
| Epidural hematoma | 13 (2%) |
| IVH | 38 (6%) |
| SAH | 215 (31%) |
| Contusion | 84 (12%) |
| DAI | 1 (0.1%) |
| Skull fracture | 48 (7%) |
Abbreviations: PMH - past medical history; TBI - traumatic brain injury; CHF - congestive heart failure; CAD - coronary artery disease; COPD - chronic obstructive pulmonary disease; ESRD - end-stage renal disease; GCS - Glasgow coma scale; ISS - injury severity scale; IVH - intraventricular hemorrhage; SAH - subarachnoid hemorrhage; DAI - diffuse axonal injury
For the purposes of the bivariate analysis, statistical significance was set at p<0.10. On the multivariate logistic regression, variables were deemed statistically significant if the associated p-value was less than 0.05 or if the 95% confidence interval (95% CI) for the odds ratio did not include ‘1.0’. All ORs are displayed with 95% CI. All statistical analyses were computed with SPSS version 27 (IBM, Armonk, NY). Secondary analysis of this de-identified dataset was deemed exempt by our site-specific Institutional Review Board and thus patient consent was not required.
Results:
Baseline patient characteristics
A total of 695 patients (45% male, mean age 86 years) with SDH were included in our analysis (Table 1). Almost all SDHs in the total cohort were acute (95%), with only a small proportion of patients presenting with chronic (2%) or subacute (3%) SDH. The most common comorbidities included coronary artery disease (28%) and dementia (24%). Sixty-nine percent (69%) of patients were using either an antiplatelet or anticoagulant agent prior to admission. The most common mechanism of injury was a low-level fall <10 feet (85%) and the most common associated intracranial injury was subarachnoid hemorrhage (31%). Most patients presented as GCS 13–15 (81%) with both pupils reactive to light (92%). The median ISS was 17 (range, 1–38).
Outcome data
Overall, 150 patients (22%) either died while in the hospital or were discharged to hospice care (Table 2). A total of 101 (15%) patients died within the hospital. The majority of patients (93%) died of their underlying TBI, but some patients died of other reasons including cardiac (14%), respiratory (15%), and sepsis/multi-organ dysfunction (2%). Of note, several patients had multiple causes of death coded, and thus the cause of death frequency data sums to >100%. Sixty-six patients (10%) died secondary to withdrawal of life-sustaining therapies. Regarding discharge disposition, 38% of patients were discharged home or to an assisted living facility, 29% of patients to a SNF or LTACH, and 12% to an acute rehabilitation facility.
Table 2.
Outcomes of all octogenarians following hospitalization for SDH
| Outcome | Count (%) or mean (range) |
|---|---|
| LOS | 5 (0–51) |
| ICU LOS | 4 (1–51) |
| Disposition | |
| Home | 244 (35%) |
| Assisted Living | 22 (3%) |
| SNF/LTACH | 198 (29%) |
| Rehab | 81 (12%) |
| In-house mortality/hospice | 150 (22%) |
| Withdrawal of life-sustaining measures | 66 (10%) |
LOS - length of stay; ICU - intensive care unit; SNF - skilled nursing facility; LTACH - long term acute care hospital; TBI - traumatic brain injury; SDH - subdural hematoma
Mortality/hospice
On bivariate analysis, factors significantly associated with death/hospice included ISS, gender, CHF, COPD, substance abuse, antiplatelets/anticoagulant use, mechanism of injury, GCS, pupil reactivity and associated traumatic cranial injuries (IVH, SAH, contusion, skull fracture), and neurosurgical intervention (Table 3). On multivariate analysis, only six of the original 17 factors included reached statistical significance (Table 3). These included ISS (p<0.001; OR 1.2 [95% CI: 1.1–1.2]), GCS 3–8 (p<0.001; OR 66.6 [8.0–500.0]), GCS 9–12 (p<0.001; OR 15.2 [4.5–50.0]), pupil non-reactivity (p=0.005; OR 6.7 [1.6–27.8]), intraventricular hemorrhage (p=0.019; OR 9.3 [2.0–41.2]), and neurosurgical intervention (p=0.001; OR 4.3 [1.6–11.1]). A binary logistic regression model and an ROC curve using the factors selected from the bivariate analysis were then constructed to predict mortality/hospice (Figure 1A). The AUROC was calculated to be 0.885.
Table 3.
Predictors of discharge disposition for all patients with SDH**
| Factor | p-value (bivariate) | p-value (multivariate) | Odds ratio (95% CI)* | ||
|---|---|---|---|---|---|
| Rehab | SNF/LTACH | Death/Hospice | |||
| ISS | <0.001 | <0.001 | 1.1 (1.0–1.1) | 1.1 (1.0–1.1) | 1.2 (1.1–1.2) |
| Gender | 0.063 | 0.184 | |||
| CHF | 0.051 | 0.228 | |||
| COPD | 0.019 | 0.180 | |||
| Substance abuse | 0.005 | 0.578 | |||
| Antiplatelet agents | <0.001 | 0.609 | |||
| Anticoagulants | <0.001 | 0.888 | |||
| Low level fall | 0.005 | 0.499 | |||
| SBP <90 | <0.001 | 0.161 | |||
| GCS 3–8 | <0.001 | <0.001 | 15.2 (1.7–142.9) | 66.6 (8.0–500.0) | |
| GCS 9–12 | <0.001 | <0.001 | 5.6 (1.5–20.4) | 5.7 (1.8–17.9) | 15.2 (4.5–50.0) |
| Pupil non-reactivity | <0.001 | 0.005 | 6.7 (1.6–27.8) | ||
| Intraventricular hemorrhage | <0.001 | 0.019 | 9.3 (2.0–41.7) | ||
| Subarachnoid hemorrhage | <0.001 | 0.056 | |||
| Intraparenchymal contusion | 0.001 | 0.674 | |||
| Skull fracture | <0.001 | 0.252 | |||
| Neurosurgical intervention | <0.001 | 0.001 | 5.3 (2.0–13.7) | 4.3 (1.6–11.1) | |
Relative to Home/assisted living (reference category)
blank spaces had non-significant odds ratios
Figure 1.
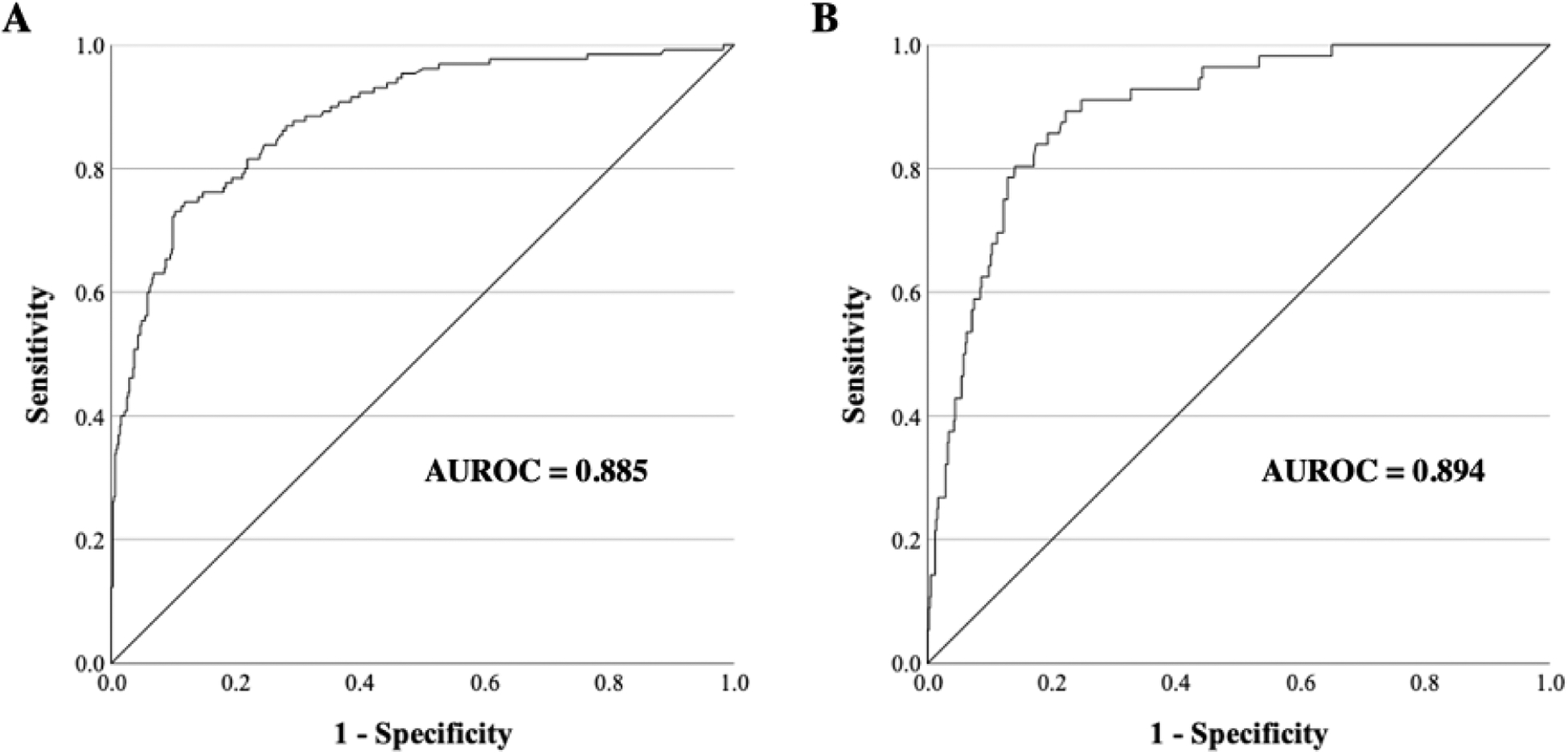
ROC (receiver operating characteristic) curves with AUROC (area under ROC) for binary logistic regression models predicting (A) mortality/hospice and (B) withdrawal of life-sustaining measures
Withdrawal of Life-Sustaining Measures
On bivariate analysis, factors associated with withdrawal of life-sustaining measures included ISS, CHF, antiplatelet/anticoagulant use, hypotension, GCS, pupillary non-reactivity, associated traumatic cranial injuries (IVH, SAH, contusion, skull fracture), and neurosurgical intervention (Table 4). Several factors (such as COPD and substance abuse), which reached statistical significance in relation to mortality/hospice did not reach significance with respect to withdrawal of life-sustaining measures. On multivariate regression, the only factors independently associated with the outcome were CHF (p=0.011; OR 3.0 [1.3–6.9]), SBP <90 (p=0.024; OR 11.1 [1.4–89.6]), GCS 3–8 (p<0.001; OR 12.1 [5.0–29.4]), GCS 9–12 (p=0.050; OR 2.8 [1.0–7.6]), and neurosurgical intervention (p=0.003; OR 3.3 [1.5–7.4]). The AUROC for the multivariate logistic regression model predicting withdrawal of life-sustaining measures was 0.894 (Figure 1B).
Table 4.
Predictors of withdrawal of life-sustaining measures for all patients with SDH
| Factor | p-value (bivariate) | p-value (multivariate) | Odds ratio (95% CI)* |
|---|---|---|---|
| ISS | <0.001 | 0.057 | |
| Gender | 0.375 | 0.107 | |
| CHF | 0.010 | 0.011 | 3.0 (1.3–6.9) |
| COPD | 0.164 | 0.291 | |
| Substance abuse | 0.215 | 0.881 | |
| Antiplatelet agents | 0.009 | 0.849 | |
| Anticoagulants | <0.001 | 0.333 | |
| Low level fall | 0.126 | 0.33 | |
| SBP <90 | 0.001 | 0.024 | 11.1 (1.4–89.6) |
| GCS 3–8 | <0.001 | <0.001 | 12.1 (5.0–29.4) |
| GCS 9–12 | 0.202 | 0.050 | 2.8 (1.0–7.6) |
| Pupil non-reactivity | <0.001 | 0.976 | |
| Intraventricular | |||
| hemorrhage | <0.001 | 0.418 | |
| Subarachnoid hemorrhage | 0.007 | 0.103 | |
| Intraparenchymal | |||
| contusion | 0.046 | 0.766 | |
| Skull fracture | 0.023 | 0.783 | |
| Neurosurgical intervention | <0.001 | 0.003 | 3.3 (1.5–7.4) |
Blank spaces had non-significant odds ratios
Discussion:
Our study describes predictive factors of mortality, withdrawal of life-sustaining measures, and discharge disposition in a large population of octogenarians presenting with SDH across 45 trauma centers. Despite a diverse patient cohort with various comorbidities and a wide range of injury severity, most patients passed away as a result of their underlying TBI, rather than other medical causes. In addition to known predictors of mortality such as poor GCS and pupil non-reactivity, our study suggests that other factors such as CHF may further enhance prognostication of patients with SDH. Furthermore, several factors, such as poor GCS, although predictive of poor outcome were also associated with favorable outcomes, such as discharge to an inpatient rehabilitation facility.
Many prior studies have examined and validated various clinical and radiographic predictors of poor outcome after TBI and SDH such as GCS, loss of pupil reactivity, age, hypotension, ISS, Rotterdam CT score, and Marshall classification.2–6 The TBI population is diverse and ranges in baseline patient characteristics, mechanism of injury, and injury severity. Thus, although many prognostic factors are known and likely generalizable to allcomers with TBI, our study seeks to investigate if there are unique predictors of outcome in an octogenarian population which may have its own unique set of governing baseline patient characteristics. For instance, a recent study by Deeb et al. demonstrated that elderly patients are less likely to present with motor deficits compared to younger patients, which could lead to under-triaging of older patients following the initial trauma.14 Other studies showed that the elderly population has its own unique set of independent risk factors for mortality such as the clinical frailty and the Geriatric Nutritional Risk Index.15, 16 We further chose to isolate the SDH population in specific, as this sub-type of TBI is very common and lends itself readily to surgical evacuation. Furthermore, the decision to offer surgery in the octogenarian population is often complicated by other factors such as baseline patient functional status, underlying comorbidity load, antiplatelet/anticoagulant agent use, and prior goals of care discussions between family members. Thus, a more detailed understanding of the relevant factors that influence outcome in this population may further inform medical and surgical decision-making throughout hospital admission.
Our study redemonstrates several known predictors of mortality following TBI in the octogenarian population presenting with SDH, establishing internal validity of our study. Here, we show that GCS 3–8, loss of pupil reactivity, and ISS are independently predictive of mortality or discharge to hospice care. Associated intracranial injury (specifically intraventricular hemorrhage) may further increase the risk of death as this may indicate a higher injury severity, which is more difficult to surmount. Neurosurgical intervention was also associated with hospice care and mortality, however the effect of neurosurgical intervention is difficult to interpret in this context. Although there is some expected morbidity following neurosurgical intervention that may increase the risk of hospital mortality, the overall effect on mortality is confounded by the fact that patients offered surgery generally have more severe injuries than those that are not.
Only a few previously published studies have specifically examined outcomes following SDH in the older patient. In one study, Kuhn et al. retrospectively reviewed 671 patients with SDH >65 years and found that age>80, GCS 3–4, associated contusions >10 cm3, and increasing SDH volume were independently associated with 30, 60, and 100-day mortality.9 In another study, Lee et al. evaluated 101 patients >90 years presenting with chronic SDH and demonstrated that surgical evacuation was associated with significantly improved 30-day and 6-month survival rates compared to conservative management.17 Chen et al. demonstrated that liver cirrhosis increased hospital mortality, length of stay, and hospital costs in allcomers who underwent burr hole evacuation for chronic SDH.18 Little has been published specifically analyzing the effect of baseline health and functional status on outcomes following SDH in octogenarians specifically, and no studies have utilized prospectively collected data as we have in our current study.
Although we examined several comorbidities, we were unable to establish an independent relationship between mortality and any pre-existing condition in particular. The effect of pre-existing conditions on mortality following SDH is likely summative in nature rather than related to any one condition in particular. Our data were not collected to compile such measures; however the relationship between indices of general health or baseline functional status such as Charleston Comorbidity Index or the Clinical Frailty Scale on outcomes following TBI warrant further study. We did show, however, that CHF was independently associated with withdrawal of life-sustaining measures. This may be indicative that patients with serious or potentially terminal underlying comorbidities such as CHF have already established goals of care prior to presenting with a severe TBI, and thus decide to withdraw care more often than previously healthy patients. Alternatively, surgeons may be less inclined to offer surgery for SDH evacuation in the face of serious underlying medical comorbidities, thus influencing a decision to withdraw care rather than pursue aggressive medical care.
A unique aspect of our study is the inclusion of discharge disposition as a secondary outcome, which drew several important insights into the data. For instance, several factors predictive of hospice/mortality were also predictive of discharge to a rehabilitation facility. For instance, pupil non-reactivity and intraventricular hemorrhage were strictly poor prognostic factors that were strongly predictive of hospital mortality or discharge to a hospice facility. However, other factors such as GCS 3–8, GCS 9–12, ISS, and neurosurgical intervention were predictors of both hospice/mortality and discharge to a rehabilitation facility. This could suggest that even patients with poor GCS and high ISS may still have a favorable outcome in the absence of pupil non-reactivity and other associated intracranial injuries. This is an important feature of the data as healthcare providers may be prone to treatment bias and prematurely deem elderly patients as non-surgical candidates based on a poor initial neurological exam. For instance Skaansar et al. postulates that the knowledge that aging leads to worse outcomes following TBI could lead to a self-fulfilling prophecy, in which healthcare providers are less likely to devote as many resources towards the care of TBI in the elderly compared to their younger counterparts.19 In that study, they showed that elderly patients were less likely to undergo neurosurgical interventions or ventilatory support, which was independently associated with worsening 30-day mortality.19 Another study showed that the super-geriatric population following TBI was associated with lower rates of medical resource utilization, higher rates of interhospital transfers, and non-routine discharges.20
The strengths of our study include the large patient cohort, its multi-center design, the unique population selected for investigation, and the unique outcome variables designated for analysis. The AUROC statistic for our regression models was 0.89 indicating excellent fit of the predictor variables to the outcomes of withdrawal of care and mortality in patients with traumatic SDH. However, there are several limitations of our study. First, there were no radiographic data collected as part of the dataset. Several important factors such as SDH size and degree of midline shift and other derivative information such as the Rotterdam CT scale have been shown to predict outcomes following SDH and other forms of TBI and thus would be important to include in a more complete prognostication model. Second, given the non-randomized observational design of this study, it is impossible to derive causal relations between the underlying input data and our primary endpoints. Furthermore, our dataset combines both patients managed conservatively and those managed with surgical evacuation of their SDH; however, it is impossible to delineate the underlying rationale for the individual management of each patient, which may further confound our results. Future studies should examine the effects of compiled comorbidity and frailty indices in prognostication models for SDH in the older patient. To that effect, new statistical methods as well as machine learning algorithms show promise in further optimizing prognostic prediction tools for clinical outcomes following TBI.21, 22 Moreover, further research is needed to investigate sub-groups of older patients that may benefit from either surgical or conservative management of SDH. Additional research studying prognostication models may help to guide surgical decision-making and inform family counseling discussions regarding end-of-life care in older patients following TBI.
Conclusions:
Poor GCS, pupil non-reactivity, ISS, and intraventricular hemorrhage are independently associated with hospital mortality or discharge to hospice care in octogenarians with SDH. Pre-existing CHF may predict withdrawal of life-sustaining measures.
Acknowledgments:
This publication was made possible by the Clinical and Translational Science Collaborative of Cleveland, KL2TR002547 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Disclosures:
Dr. Ho is supported by the Clinical and Translational Science Collaborative of Cleveland (KL2TR002547) from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research.
No other authors received funding from any funding agency in the public, commercial or not-for-profit sectors.
Abbreviations List:
- AAST
American association for the surgery of trauma
- AIS
Abbreviated injury scale
- CAD
Coronary Artery Disease
- CDC
Center for disease control
- CHF
Congestive heart failure
- CI
Confidence interval
- COPD
Chronic obstructive pulmonary disease
- DAI
diffuse axonal injury
- ESRD
End-stage renal disease
- GCS
Glasgow coma scale
- Geri-TBI
Geriatric traumatic brain injury
- ISS
Injury severity scale
- IVH
Intraventricular hemorrhage
- LTACH
Long-term acute care hospital
- OR
Odds ratio
- PMH
Past medical history
- ROC
Receiver operating characteristic
- SAH
Subarachnoid hemorrhage
- SDH
Subdural hematoma
- SNF
Skilled nursing facility
- TBI
Traumatic brain injury
American Association for the Surgery of Trauma GERI-TBI Study Group Authors names and affiliations:
Mira Ghneim MD MS1 (mira.ghneim@som.umaryland.edu);
Jennifer S. Albrecht PhD2 (jalbrecht@som.umaryland.edu);
Karen Brasel26 MD, MPH (brasel@ohsu.edu);
Anna Livaris MD1 (aliveris@gmail.com);
Jill B. Watras MD3 (jill.watras@inova.org);
Christopher P. Michetti MD3 (christopher.michetti@inova.org);
James M. Haan MD4 (James.Haan@ascension.org);
Kelly Lightwine MPH4 (Kelly.Lightwine@ascension.org);
Robert D. Winfield MD5 (rwinfield@kumc.edu);
Sasha D. Adams MD6 (sasha.d.adams@uth.tmc.edu);
Jeanette M. Podbielski RN, BSN, CCRP6 (Jeanette.m.podbielski@uth.tmc.edu);
Scott B. Armen MD7 (sarmen@pennstatehealth.psu.edu);
J. Christopher Zacko MD, MSc7 (jzacko@pennstatehealth.psu.edu);
Fady S. Nasrallah MD8 (Nasrallah.Fady@Scrippshealth.org);
Kathryn B. Schaffer MPH8 (Schaffer.Kathryn@Scrippshealth.org);
Julie Dunn MD, MS9 (Julie.Dunn@uchealth.org);
Lars Widdel MD9 (Lars.Widdel@uchealth.org);
Thomas J. Schroeppel MD, MS10 (Thomas.Schroeppel@uchealth.org);
Zachery Stillman BA10 (Zachery.stillman@uchealth.org);
Zara Cooper MD, MSc11 (zcooper@bwh.harvard.edu);
Deborah Stein MD, MPH12 (deborah.stein@ucsf.edu)
Additional Study Group Members of The American Association for the Surgery of Trauma Geri-TBI Study
Charles Adams MD13 (CAdams1@lifespan.org);
Stephanie Lueckel MD, ScM13 (slueckel@lifespan.org);
Jason Murry MD14 (jason.murry@gmail.com);
Nikita Patel MD14 (NPATEL46@mgh.harvard.edu);
Cindy Hsu MD, PhD15 (hcindy@med.umich.edu);
Umer F. Bhatti MD15 (ufb@med.umich.edu);
Matthew E Lissauer MD16 (ML1141@RWJMS.Rutgers.edu);
Marc LaFonte MD16 (ML1371@RWJMS.Rutgers.edu);
Kaveh Najafi DO17 (Kaveh.najafi@honorhealth.com);
Karen Lewandowski RN, BSN17 (Karen.lewandowski@honorhealth.com);
Kaushik Mukherjee, MD MS18 (kmukherjee@llu.edu);
Kristelle J. Imperio-Lagabon, BS18 (klagabon@llu.edu);
Niels D. Martin MD19 (niels.martin@uphs.upenn.edu);
Kathleen Hirsch CRNP19 (Kathleen.Hirsch@uphs.upenn.edu);
Cherisse Berry MD20 (Cherisse.Berry@nyulangone.org);
Derek Freitas MD20 (Derek.Freitas@nyulangone.org);
Daniel Cullinane MD21 (cullinane.daniel@marshfieldclinic.org);
Roshini Ramawi21 DO (Ramwani.roshini@marshfieldclinic.org);
Michael Truitt MD22 (mike_truitt@hotmail.com);
Chris Pearcy MD22 (ChrisPearcy@mhd.com);
Habiba Hashimi. MD23 (hhashimi@fresno.ucsf.edu);
Krista Kaups MD, MSc23 (kkaupsmd@communitymedical.org);
Jeffrey Claridge MD24 (jclaridge@metrohealth.org);
Jennifer L. Hartwell MD25,40 (jhartwell@iuhealth.org);
Jessica Ballou MD, MPH26 (ballouj@ohsu.edu);
Martin Croce MD27 (mcroce@uthsc.edu);
Stephanie Markle DO, MPH28 (Stephanie.markle@ascension.org);
Sally Osserwaarde MSN, RN, CNS, CNML28 (Sally.ossewaarde@ascension.org);
Joseph Posluszny MD29 (joseph.posluszny@nm.org);
Benjamin Stocker BS29 (benjamin.stocker@northwestern.edu);
Tjasa Hranjec MD30 (thranjec@mhs.net);
Rachele Solomon MPH30 (Rasolomon@mhs.net);
Lucy Martinek MD31 (lcmartin@bidmc.harvard.edu);
Alok Gupta MD31 (agupta4@bidmc.harvard.edu);
Daniel J. Grabo MD32 (daniel.grabo@hsc.wvu.edu);
Uzer Khan MD32 (ukhan@hsc.wvu.edu);
Danielle Tatum PhD33 (Danielle.Tatum@fmolhs.org);
Tomas Jacome MD, MPH33 (Tomas.Jacome@fmolhs.org);
Jonathan Gates MD, MBA34 (Jonathan.Gates@hhchealth.org);
Alisha Jawani MD34 (alisha.z.jawani.gmail.com);
Allison E. Berndtson MD35 (aberndtson@ucsd.edu);
Terry G. Curry RN, BSN35 (tcurry@ucsd.edu);
Miklosh Bala MD36 (rbalam@hadassah.org.il);
Linda A. Dultz MD, MPH37 (linda.dultz@utsouthwestern.edu);
Natasha N. Houshmand BS37 (natasha.houshmand@utsouthwestern.edu);
Paola Pieri MD38 (paolapieri@hotmail.com);
Martin D Zielinski MD39 (Zielinski.martin@mayo.edu);
Joy D. Hughes MD39 (Hughes.joy@mayo.edu);
Jennifer Hartwell MD40 (hartwell.jennifer@gmail.com);
Ajai K. Malhotra MD41 (Ajai.Malhotra@uvmhealth.org);
Tim Lee MD41 (Tim.Lee@uvmhealth.org);
Patrizio Petrone MD, PhD, MPH, MHSA42 (ppetrone@NYUWinthrop.org);
D’andrea Joseph MD42 (djoseph1@nyuwinthrop.org);
Gary T. Marshall MD43 (gary.marshall@emcare.com);
Matthew M. Carrick MD43 (matt.carrick@acutesurgical.com);
Abhijit Pathak MD44 (Abhijit.Pathak@tuhs.temple.edu);
Andrea Van Zandt44(Andrea.vanzandt@tuhs.temple.edu);
Nina Glass MD45 (Nina.Glass@rutgers.edu);
David Livingston MD45 (livingst@njms.rutgers.edu);
Shea Gregg MD46 (striamed1@gmail.com);
Travis Webb MD, MHPE47 (trwebb@mcw.edu);
Byron Drumheller MD1(Byron.drumheller@gmail.com);
Rosemary Kozar MD, PhD1(rkozar@som.umaryland.edu);
Robert Barraco MD, MPH48 (robert_d.barraco@lvhn.org);
Bellal Joseph MD49(bjoseph@surgery.arizona.edu);
1R Adams Cowley Shock Trauma, The University of Maryland Medical Center, 22 S Greene St, Baltimore, MD 21201
2 Department of Epidemiology and Public Health, The University of Maryland School of Medicine, 655 W Baltimore Street, Baltimore MD 21201
3Inova Fairfax Medical Campus, 3300 Gallows Rd, Falls Church, VA 22042
4Ascension Via Christi Hospital, 929 North Saint Francis Wichita, KS 67214
5University of Kansas Medical Center, 3901 Rainbow Blvd, Kansas City, KS 66160
6University of Texas Health Science Center at Houston, 7000 Fannin St #1200, Houston, TX 77030
7Penn State Health System, 500 University Dr, Hershey, PA 17033
8Scripps Memorial Hospital La Jolla, 9888 Genesee Ave, La Jolla, CA 92037
9Medical Center of the Rockies, 2500 Rocky Mountain Ave, Loveland, CO 80538
10UC Health Memorial Hospital, 1400 E Boulder St, Colorado Springs, CO 80909
11Brigham and Women’s Hospital, 75 Francis St, Boston, MA 2115
12Univeristy of California San Francisco, 1001 Potrero Ave, San Francisco, CA 94110
13Rhode Island Hospital, 80 Dudley St, Providence, RI 02905
14University of Texas Health Science Center at Tyler, 11937 US-271, Tyler, TX 75708
15University of Michigan, 500 S State St, Ann Arbor, MI 48109
16Rutgers-Robert Wood Johnson Medical School, Clinical Academic Building (CAB, 125 Paterson St, New Brunswick, NJ 08901
17Honor Health Scottdale Osborn Medical Center, 7400 E Osborn Rd, Scottsdale, AZ 85251
18Loma Linda University Medical Center, 11290 Campus St., Suite 200, Loma Linda, CA 92354
19University of Pennsylvania, 3400 Civic Center Boulevard West Pavilion, 2nd Floor & 4th Floor, Philadelphia, PA 19104
20New York University/Bellevue Hospital Center, 462 1st Avenue, New York, NY 10016
21Marshfield Clinic, 1000 N Oak Ave, Marshfield, WI 54449
22Methodist Dallas Medical Center, 1441 N Beckley Ave, Dallas, TX 75203
23University of California San Francisco Fresno, 155 N Fresno St, Fresno, CA 93701
24MetroHealth Medical Center, 2500 Metrohealth Dr, Cleveland, OH 44109
25Indiana University Health Methodist, 1701 N Senate Ave, Indianapolis, IN 46202
26Oregon Health and Science University, 3181 SW Sam Jackson Park Rd, Portland, OR 97239
27University of Tennessee Health Science Center, 920 Madison Ave, Memphis, TN 38163
28Ascension Borgess, 1521 Gull Rd, Kalamazoo, MI 49048
29Northwestern University Feinberg School of Medicine, 420 E Superior St, Chicago, IL 60611
30Memorial Regional Hospital, 1150 N 35th Ave Suite 600, Hollywood, FL 33021
31Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215
32West Virginia University, 1 Medical Center Dr, Morgantown, WV 26506
33Our Lady of the Lake Regional Medical Center, 5000 Hennessy Blvd, Baton Rouge, LA 70808
34Hartford Hospital, 80 Seymour St, Hartford, CT 06106
35University of California San Diego Medical Center, 9500 Gilman Drive La Jolla, CA 92093
36Hadassah Hebrew University Medical Center, Sderot Churchill 8, Jerusalem
37The University of Texas Southwestern Medical Center, Parkland Hospital, 5200 Harry Hines Blvd, Dallas, TX 75235
38Maricopa Intergrated Health Systems, 2601 E Roosevelt St, Phoenix, AZ 85008
39Mayo Clinic Rochester, 201 W Center St, Rochester, MN 55902
40Indiana University at Eskenazi Health, 720 Eskenazi Avenue, Indianapolis, IN 46202
41University of Vermont, Burlington, 111 Colchester Avenue, Burlington, Vermont 05401
42New York University Winthrop Hospital, 259 1st St, Mineola, NY 11501
43Medical Center of Plano, 3901 W 15th St, Plano, TX 75075
44Temple University Hospital, 3401 N Broad St, Philadelphia, PA 19140
45Rutgers Medical School University Hospital, 150 Bergen St, Newark, NJ 07103
46Bridgeport hospital, 267 Grant St, Bridgeport, CT 06610
47Medical College of Wisconsin, 8701 W Watertown Plank Rd, Wauwatosa, WI 53226
49University of San Francisco – Lehigh Valley, 1247 S Cedar Crest Blvd. Suite 202. Allentown PA 18103–6298
48The University of Arizona, 3838 N Campbell Ave #2, Tucson, AZ 85719
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peterson AB, Xu L, Daugherty J, Breiding MJ. Surveillance report of traumatic brain injury-related emergency department visits, hospitalizations, and deaths, United States, 2014. Atlanta, GA Center for Disease Control;2019. [Google Scholar]
- 2.Czosnyka M, Balestreri M, Steiner L, et al. Age, intracranial pressure, autoregulation, and outcome after brain trauma. J Neurosurg. March 2005;102(3):450–454. [DOI] [PubMed] [Google Scholar]
- 3.Hsiao KY, Hsiao CT, Weng HH, Chen KH, Lin LJ, Huang YM. Factors predicting mortality in victims of blunt trauma brain injury in emergency department settings. Emerg Med J. October 2008;25(10):670–673. [DOI] [PubMed] [Google Scholar]
- 4.Kilaru S, Garb J, Emhoff T, et al. Long-term functional status and mortality of elderly patients with severe closed head injuries. J Trauma. December 1996;41(6):957–963. [DOI] [PubMed] [Google Scholar]
- 5.Ostermann RC, Joestl J, Tiefenboeck TM, Lang N, Platzer P, Hofbauer M. Risk factors predicting prognosis and outcome of elderly patients with isolated traumatic brain injury. J Orthop Surg Res. November 3 2018;13(1):277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibahashi K, Sugiyama K, Okura Y, Hoda H, Hamabe Y. Multicenter Retrospective Cohort Study of “Talk and Die” After Traumatic Brain Injury. World Neurosurg. November 2017;107:82–86. [DOI] [PubMed] [Google Scholar]
- 7.Mak CH, Wong SK, Wong GK, et al. Traumatic Brain Injury in the Elderly: Is it as Bad as we Think? Curr Transl Geriatr Exp Gerontol Rep. 2012;1:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawley C, Sakr M, Scapinello S, Salvo J, Wrenn P. Traumatic brain injuries in older adults-6 years of data for one UK trauma centre: retrospective analysis of prospectively collected data. Emerg Med J. August 2017;34(8):509–516. [DOI] [PubMed] [Google Scholar]
- 9.Kuhn EN, Erwood MS, Oster RA, et al. Outcomes of Subdural Hematoma in the Elderly with a History of Minor or No Previous Trauma. World Neurosurg. November 2018;119:e374–e382. [DOI] [PubMed] [Google Scholar]
- 10.Ramanathan DM, McWilliams N, Schatz P, Hillary FG. Epidemiological shifts in elderly traumatic brain injury: 18-year trends in Pennsylvania. J Neurotrauma. May 1 2012;29(7):1371–1378. [DOI] [PubMed] [Google Scholar]
- 11.Lee JJ, Segar DJ, Morrison JF, Mangham WM, Lee S, Asaad WF. Subdural hematoma as a major determinant of short-term outcomes in traumatic brain injury. J Neurosurg. January 2018;128(1):236–249. [DOI] [PubMed] [Google Scholar]
- 12.Rakier A, Guilburd JN, Soustiel JF, Zaaroor M, Feinsod M. Head injuries in the elderly. Brain Inj. Feb-Mar 1995;9(2):187–193. [DOI] [PubMed] [Google Scholar]
- 13.Timiras PS. Physiological basis of aging and geriatrics: CRC Press; 2007. [Google Scholar]
- 14.Deeb AP, Phelos HM, Peitzman AB, Billiar TR, Sperry JL, Brown JB. The Whole is Greater Than the Sum of its Parts: GCS Versus GCS-Motor for Triage in Geriatric Trauma. J Surg Res. May 2021;261:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdulle AE, de Koning ME, van der Horn HJ, et al. Early Predictors for Long-Term Functional Outcome After Mild Traumatic Brain Injury in Frail Elderly Patients. J Head Trauma Rehabil. Nov-Dec 2018;33(6):E59–E67. [DOI] [PubMed] [Google Scholar]
- 16.Su WT, Tsai CH, Huang CY, et al. Geriatric Nutritional Risk Index as a Prognostic Factor for Mortality in Elderly Patients with Moderate to Severe Traumatic Brain Injuries. Risk Manag Healthc Policy. 2021;14:2465–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee L, Ker J, Ng HY, et al. Outcomes of chronic subdural hematoma drainage in nonagenarians and centenarians: a multicenter study. J Neurosurg. February 2016;124(2):546–551. [DOI] [PubMed] [Google Scholar]
- 18.Chen CC, Chen SW, Tu PH, et al. Outcomes of chronic subdural hematoma in patients with liver cirrhosis. J Neurosurg. February 2 2018;130(1):302–311. [DOI] [PubMed] [Google Scholar]
- 19.Skaansar O, Tverdal C, Ronning PA, et al. Traumatic brain injury-the effects of patient age on treatment intensity and mortality. BMC Neurol. October 17 2020;20(1):376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bae S, Song SW, Kim WJ, et al. Traumatic brain injury in patients aged >/=65 years versus patients aged >/=80 years: a multicenter prospective study of mortality and medical resource utilization. Clin Exp Emerg Med. June 2021;8(2):94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amorim RL, Oliveira LM, Malbouisson LM, et al. Prediction of Early TBI Mortality Using a Machine Learning Approach in a LMIC Population. Front Neurol. 2019;10:1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raj R, Luostarinen T, Pursiainen E, et al. Machine learning-based dynamic mortality prediction after traumatic brain injury. Sci Rep. November 27 2019;9(1):17672. [DOI] [PMC free article] [PubMed] [Google Scholar]


