Abstract
Background:
Frailty is associated with a higher risk for adverse outcomes after aortic valve replacement (AVR) for severe aortic valve stenosis, but whether or not frail patients derive differential benefit from transcatheter (TAVR) vs. surgical (SAVR) AVR is uncertain.
Methods:
We linked adults ≥ 65 years old in the US CoreValve High Risk (HiR) or Surgical or Transcatheter Aortic-Valve Replacement in Intermediate Risk Patients (SURTAVI) trial to Medicare claims, 2/2/2011–9/30/2015. Two frailty measures, a deficit-based (DFI) and phenotype-based (PFI) frailty index, were generated. The treatment effect of TAVR vs. SAVR was evaluated within frailty index (FI) tertiles for the primary endpoint of death and non-death secondary outcomes, using multivariable Cox regression.
Results:
Of 1,442 (linkage rate = 60.0%) individuals included, 741 (51.4%) individuals received TAVR and 701 (48.6%) received SAVR (mean age 81.8 ± 6.1 years, 44.0% female). Though 1-year death rates in the highest FI tertiles (DFI 36.7%, PFI 33.8%) were 2–3-fold higher than the lowest tertiles (DFI 13.4%, HR 3.02, 95% CI 2.26–4.02, p < 0.001; PFI 17.9%; HR 2.05, 95% CI 1.58–2.67, p < 0.001), there were no significant differences in the relative or absolute treatment effect of SAVR vs. TAVR across FI tertiles for all death, non-death, and functional outcomes (all interaction p-values > 0.05). Results remained consistent across individual trials, frailty definitions, and when considering the non-linked trial data.
Conclusion:
Two different frailty indices based on Fried and Rockwood definitions identified individuals at higher risk of death and functional impairment but no differential benefit from TAVR vs. SAVR.
Keywords: frailty, aortic valve disease, TAVR, SAVR, treatment effect
INTRODUCTION
Frailty is an important and often unmeasured risk factor for adverse outcomes in individuals undergoing aortic valve replacement (AVR) for severe aortic stenosis (AS).1, 2 Frailty is present in an estimated 63% of individuals undergoing transcatheter AVR (TAVR), confers a near 4-fold increased risk of death as well as functional impairment at 6 months post-procedure, and is incrementally predictive of risk beyond age and comorbidities alone.3–8
As frail individuals may be at high risk for adverse outcomes with TAVR as well as surgical AVR (SAVR), it remains unclear if frailty identifies individuals with severe AS who derive greater benefit from one procedure versus the other. As a physician’s subjective assessment of a patient’s frailty status may not predict risk in TAVR,9 validated constructs with a known association with excess risk have been advocated for in-person assessment of frailty status prior to TAVR.2, 5, 10 These indices may be generated prior to or at the time of the AVR, or could be ascertained retrospectively using linkage to billing data and construction of claims-based frailty indices (FIs).7–8 However, whether or not measures of frailty identify benefit from TAVR vs. SAVR remains uncertain.
As such, in the current study, we evaluated whether two FIs, based on two different definitions of frailty (e.g. Fried and Rockwood conceptualizations), predicted benefit from TAVR vs. SAVR using data from the US CoreValve High Risk (HiR) and Surgical or Transcatheter Aortic-Valve Replacement in Intermediate Risk Patients (SURTAVI) trials.
METHODS
Data Source
We previously linked Medicare administrative claims to the US CoreValve Pivotal Trials dataset,11, 12 a series of trials comparing TAVR with SAVR, as part of the National Heart, Lung, and Blood Institute-sponsored (1R01HL136708) Extending Trial-Based Evaluations of Medical Therapies Using Novel Sources of Data (EXTEND) Study. Details on this study and linkage have been published previously.13 Patients included in the US CoreValve HiR and SURTAVI trials with procedure dates February 2, 2011 to September 30, 2015 who could be linked successfully to the US Centers for Medicare and Medicaid Services (CMS) Medicare Provider Analysis and Review (MedPAR), were included. The MedPAR dataset used consisted of a 100% sample of inpatient discharge claims for Medicare Fee-for-Service beneficiaries in a given year. The CoreValve HiR trial randomized high-surgical risk individuals with severe AS to undergo TAVR with the self-expanding Medtronic™ CoreValve bioprosthesis vs. SAVR.11 The SURTAVI trial randomized intermediate surgical risk individuals with severe AS to TAVR with the self-expanding Medtronic™ CoreValve bioprosthesis vs. SAVR.12 The choice of these particular studies was based on the high-prevalence of frailty among included individuals and overlap with use of International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) claims prior to October 1, 2015. The study was approved by the Institutional Review Board at Beth Israel Deaconess Medical Center. Per data use agreements, the data supporting this study are not publicly available.
Study Population
As direct patient identifiers were not available in the CoreValve dataset, a deterministic matching strategy was used to link MedPAR and CoreValve datasets, with matches identified by age, sex, date of birth, procedural and admission/discharge dates, and hospital ID.13 Those patients younger than age 65 or those undergoing AVR at a European or Veterans Affairs of hospital were excluded.
A total of 2,410 individuals were initially included in the HiR (N = 750) and SURTAVI (N = 1660) trials (Figure 1). Of these individuals, 1,605 (66.6%) were successfully linked to Medicare claims including 600 (80%) of individuals in the HiR trial and 1005 (60.5%) of individuals in the SURTAVI trial. Among the 750 individuals in the HiR trial, 15 (2.0%) were excluded due to age at procedure < 65 or AVR at a European or Veterans Affairs hospital. Among the 1660 individuals in the SURTAVI trial, 355 (21.4%) were excluded for the same. Additionally, 163 (9.8%) individuals in the SURTAVI trial were excluded due to undergoing AVR after October 1, 2015, the date of transition from ICD-9-CM to International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) in the US. Non-linked individuals likely represent individuals enrolled in Medicare Advantage Health Maintenance Organizations, who represented 13–30% of those enrolled in the Medicare program over the study period.
Figure 1. Study Schematic Displaying Results of Study Linkage.
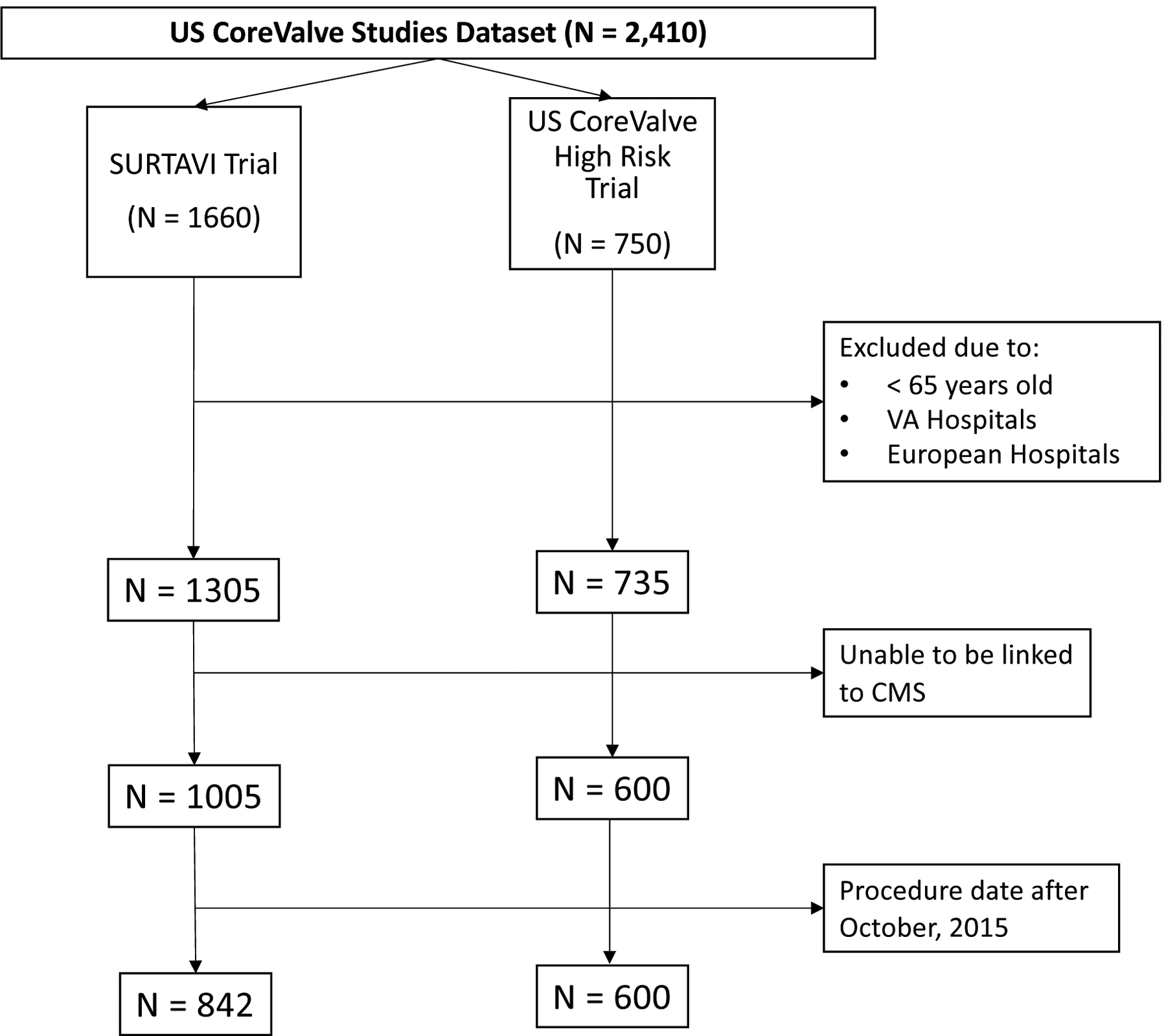
The above schematic details the linkage strategy used in the current study. Of 750 individuals in the US CoreValve High Risk Trial, 15 were excluded due to being under 65 years old or undergoing aortic valve replacement at a Veterans Administration or European hospital. Of the 735 remaining, 135 were unable to linked to Medicare data. Of the 1,660 individuals in the SURTAVI trial, 355 were excluded due to being under 65 years old or undergoing aortic valve replacement at a Veterans Affairs Administration or European hospital. Of the 1,305 remaining, 200 were unable to be linked to Medicare data. Subsequently, 163 were excluded due to procedure dates after October 1, 2015. CMS = Centers for Medicare and Medicaid Services, N = number of individuals, SURTAVI = Surgical or Transcatheter Aortic-Valve Replacement in Intermediate Risk Patients Trial, US = United States, VA = Veterans Administration.
Covariates and Outcomes
Clinical covariates were determined using baseline characteristics as recorded and defined in the individual trials (Supplemental Table I).11, 12
Outcomes included all-cause mortality (primary outcome), acute kidney injury (AKI), bleeding, stroke or transient ischemic attack (TIA), hospitalization, myocardial infarction (MI), and MACCE, defined as a composite of all-cause death, MI, stroke, or aortic valve reintervention. Outcomes were determined as recorded and defined in the individual trials using a 1-year timepoint (Supplemental Table II).11, 12 Additionally, as improvement in quality of life may be more important for certain individuals than overall survival, we examined an additional functional-status endpoint. Functional status was defined using the Kansas City Cardiomyopathy Questionnaire (KCCQ), a 23-item questionnaire, graded from 0–100 with higher scores reflecting better health status.14 In both trials, participants underwent assessment of the KCCQ at baseline and 6 months follow-up. A poor functional outcome at 6 months post-procedure was defined as death, a KCCQ overall summary (OS) score < 45, or a decline in KCCQ-OS ≥ 10 points from baseline.15
Ascertainment of Frailty Status
Frailty can be broadly conceptualized in two main ways: first, as an accumulation of deficits in health related domains (e.g., diseases, disabilities, and symptoms), on the theory that the more deficits accumulated, the more likely that a given person is frail (Rockwood approach),16 and second, as a description of a physical phenotype based on the interrelated concepts of weakness, slowness, weight loss, exhaustion, and low energy (Fried approach).1, 17 While there remains disagreement in the literature regarding the optimal method of measuring frailty, both approaches are supported by a broad-base of supporting literature and represent valid methods for determining frailty.1,16–17 We therefore used FIs derived based on these two approaches to measure frailty status in the current study so as to evaluate the role of frailty as a whole and benefit of TAVR vs. SAVR.
To construct the deficit-based frailty index (DFI), a standard procedure, using the technique by Searle et al., was used to sum the number of in-person frailty-related deficits for each individual in the CoreValve trials (Supplemental Table III).18 This sum was divided by the total number of possible deficits to calculate a frailty index (FI) (range 0–1, higher values = greater frailty). These variables were chosen based on availability in the CoreValve dataset and their ability to satisfy the 5 criteria for creation of a valid DFI,18 namely 1) the variables must be associated with health status, 2) a deficit’s prevalence must generally increase with age, 3) the chosen deficits must not saturate too early, 4) the deficits must cover a range of system (e.g. not unique to cognition for example), and 5) if a single FI is to be used serially in the same individuals, the items that make up this FI need to be the same from one iteration to the next.
To construct the phenotype-based frailty index (PFI), claims for hospitalizations in the 6 months preceding the baseline visit (Supplemental Table IV) were used to construct a PFI (ranging from 0–1, higher values = greater frailty) according to the technique by Segal et al., which uses the Fried frailty phenotype as the reference standard.19 This PFI was derived using ICD-9-CM claims linked to the Cardiovascular Health Study,19 externally validated in the National Health and Aging Trend Study,20 and has been shown to predict outcomes similarly to the frailty phenotype,20,21 We have recently demonstrated that this PFI is associated with worse impairments in in-person assessments of frailty, disability, cognitive dysfunction, and nutrition amongst patients with severe AS in the CoreValve studies.22 Of note, while this PFI was derived based on a dichotomous definition of frailty (i.e. the Fried definition), it nevertheless predicts adverse risk on a continuous basis22 and thus PFI tertiles were used in the analysis to evaluate for a dose-response relationship in the treatment effect across PFI tertiles.
Statistical Analysis
Linked and unlinked cohorts were first compared using t-tests and Fisher’s exact tests for continuous and categorical variables respectively. Subsequently, we divided each FI into tertiles in the overall linked cohort (i.e. both trials combined) as well as in each trial individually. Tertiles were chosen so as to evaluate for a dose-response relationship between FI and effect heterogeneity while maintaining sufficient numbers in each subgroup to detect an effect when present. Baseline characteristics of patients were recorded using means and standard deviations (SDs) for continuous variables and numbers and percentages for categorical variables and compared across FI tertiles using one-way analysis of variance and Chi Squared tests respectively. Q-Q plots were used to verify normality of continuous variables.
For all outcomes except for functional impairment, Kaplan-Meier techniques were used to estimate endpoint event rates at 1-year. Death rates were compared across FI tertiles using Cox regression. The number and proportion with a poor functional outcome at 6 months were determined. Rates of poor functional outcomes were compared across FI tertiles using logistic regression. These estimates were stratified by randomized treatment assignment and FI tertile under an intention-to-treat framework. For non-death outcomes except for functional impairment, Fine-Gray competing risk analysis was used to account for the competing risk of death.23
Subsequently, to evaluate whether relative treatment effect of TAVR vs. SAVR differed across levels of FI, for each outcome and cohort, the adjusted hazard ratio (HR), 95% confidence interval (CI), and log-rank p-value for the TAVR vs. SAVR comparison were estimated using multivariable Cox regression for each FI tertile. For the functional impairment outcome, for each cohort, the adjusted odds ratio (OR), 95% CI, and p-value for the TAVR vs. SAVR comparison were estimated using multivariable logistic regression for each FI tertile. A pre-specified interaction between FI tertile and treatment assignment was evaluated for each outcome and cohort.
For each outcome and cohort, to evaluate whether absolute treatment effects of TAVR vs. SAVR differed across levels of FI, cumulative incidence functions were used to estimate outcome rates at 1-year and the adjusted risk difference (RD) and 95% CI for the TAVR vs. SAVR comparison within each FI. Log-rank tests were used to compare the fatal outcomes between treatment assignments within each FI, and Fine-Gray tests were used to compare the non-death outcomes, accounting for the competing risk of death.23 A pre-specified additive interaction between FI tertile and treatment assessment was evaluated for each outcome and cohort using variance-weighted least squares regression with RDs included as a response variable and FI tertile included as a continuous predictor variable, with weights calculated as the inverse of the variance of treatment differences within each tertile.24
In both cases, estimates were adjusted for all variables significant on a p < 0.05 basis on univariate analyses. In survival models, individuals without events were censored at 1-year after their procedure. No adjustment for multiple comparisons was made. All analyses were performed using SAS v9.4 or JMP v15.0 (SAS Institute, Cary, NC) using a two-tailed p-value < 0.05 to define significance for all comparisons.
Sensitivity Analysis
As results could differ shortly after the index procedure, we evaluated pre-specified interactions between FI tertile and treatment assignment for death within 30-days of the date of AVR. Additionally, as CMS-linkage could reduce the overall power to detect an interaction between FI tertile and treatment assignment, analyses were additionally repeated in the unlinked combined trial dataset (N = 2410) using a DFI, constructed using the technique by Searle et al. (Supplemental Table III)18
RESULTS
Overall Results
A total of 1,442 (60.0%) individuals from the HiR and SURTAVI trials were successfully linked to Medicare data and were included in the analytic cohort (Figure 1). Individuals whose records could not be linked (Supplemental Table V) were generally similar to linked individuals though age, Society of Thoracic Surgeons (STS) risk score,25 and the proportion with heart failure were higher in the linked group and the Logistic EuroSCORE26 was lower.
Amongst included individuals, the mean age was 81.8 ± 6.1 years and 635 (44.0%) were female. The mean STS score was 5.9 ± 2.7, the mean DFI was 0.25 ± 0.06 (Supplemental Figure I; range 0.04 to 0.59), and the mean PFI was 0.25 ± 0.12 (Supplemental Figure II; range 0.03 to 0.82). Overall, 741 (51.4%) individuals received TAVR, including 314 (52.3%) HiR participants and 427 (50.7%) SURTAVI participants, and 701 (48.6%) individuals received SAVR, including 286 (47.7%) HiR participants and 415 (49.3%) SURTAVI participants.
In the overall cohort (i.e. both trials combined), 479 (33.2%) individuals were in DFI tertile 1 (T1; DFI ≤ 0.22) and 481 (33.3%) individuals were in PFI T1 (T1; PFI ≤ 0.18), 468 (32.5%) individuals were in DFI tertile 2 (T2; DFI 0.23–0.27) and 481 (33.3%) individuals were in PFI tertile 2 (T2; PFI 0.19–0.28), and 463 (32.1%) were in DFI tertile 3 (T3; DFI ≥ 0.28) and 480 (33.3%) individuals were in PFI tertile 3 (T3; PFI ≥ 0.29).
Comparison of Baseline Characteristics
In the overall cohort (Supplemental Table VI), individuals in higher DFI tertiles were younger (T3 vs. T1, 80.9 ± 6.4 vs. 82.7 ± 5.7 years, p < 0.001) and had higher STS risk scores (T3 vs. T1, 7.0 ± 3.2 vs. 4.9 ± 2.0, p < 0.001). They also additionally had higher Logistic EuroSCORE and Charlson comorbidity indices, and higher rates of comorbidities (p < 0.05 for all but immunosuppressive therapy [p = 0.10]). Treatment assignment was not different across tertiles (p = 0.69). Results were overall similar across trials (Supplemental Tables VII–VIII)
In the overall cohort (Supplemental Table IX), individuals in higher PFI tertiles were older (T3 vs. T1, 85.9 ± 4.0 vs. 76.6 ± 5.6 years, p < 0.001), more frequently female (T3 vs. T1, 56.9% vs. 32.4%, p < 0.001), had higher STS risk scores (T3 vs. T1, 7.1 ± 3.0 vs. 4.8 ± 2.1, p < 0.001), and had more congestive heart failure (T3 vs. T1, 86.3% vs. 46.8%, p < 0.001) and severe aortic calcification (T3 vs. T1, 10.0% vs. 8.5%, p = 0.03). Individuals in higher PFI tertiles less frequently had diabetes, peripheral vascular disease, coronary artery disease, and receipt of coronary artery bypass grafting (CABG) (all p < 0.05). Treatment assignment was not different across DFI tertiles (p = 0.051). Results were overall similar across trials (Supplemental Tables X–XI)
Primary Endpoint
At 1-year follow-up, 337 individuals died (23.4%) including 165 (23.5%) of those receiving SAVR and 184 (24.8%) of those receiving TAVR (p = 0.65). Overall, 170 (36.7%) of those in DFI tertile 3 died vs. 64 (13.4%) of those in DFI tertile 1 (HR 3.02, 95% CI 2.26–4.02, p < 0.001) (Supplemental Table XII and Figures 2–3). Amongst those in DFI tertile 1, 10.2% of those receiving SAVR and 16.5% of those receiving TAVR died (SAVR vs. TAVR, adjusted HR 0.63, 95% CI 0.37–1.06, p = 0.08; adjusted RD, −6.3%, 95% CI −12.4% to −0.2%, p = 0.06). In DFI tertile 2, 24.9% of those receiving SAVR and 19.4% of those receiving TAVR died (SAVR vs. TAVR, adjusted HR 1.30, 95% CI 0.88–1.92, p = 0.19; adjusted RD, 5.5%, 95% CI −2.1% to 13.0%, p = 0.13). In DFI tertile 3, 35.9% of those receiving SAVR and 37.5% of those receiving TAVR died (SAVR vs. TAVR, adjusted HR, 1.02, 95% CI 0.75–1.39 p = 0.91; adjusted RD, −1.6%, 95% CI −10.4% to 7.2%, p = 0.73). There was no significant interaction between PFI tertile and treatment assignment with respect to risk of death, on a relative or absolute scale (p > 0.05 for all interactions). Results were consistent across individual trials (Supplemental Table XII and Figures III–VI).
Figure 2. Relative Treatment Effect for TAVR vs. SAVR in the Overall Study Cohort by DFI Tertile and Trial Outcome.
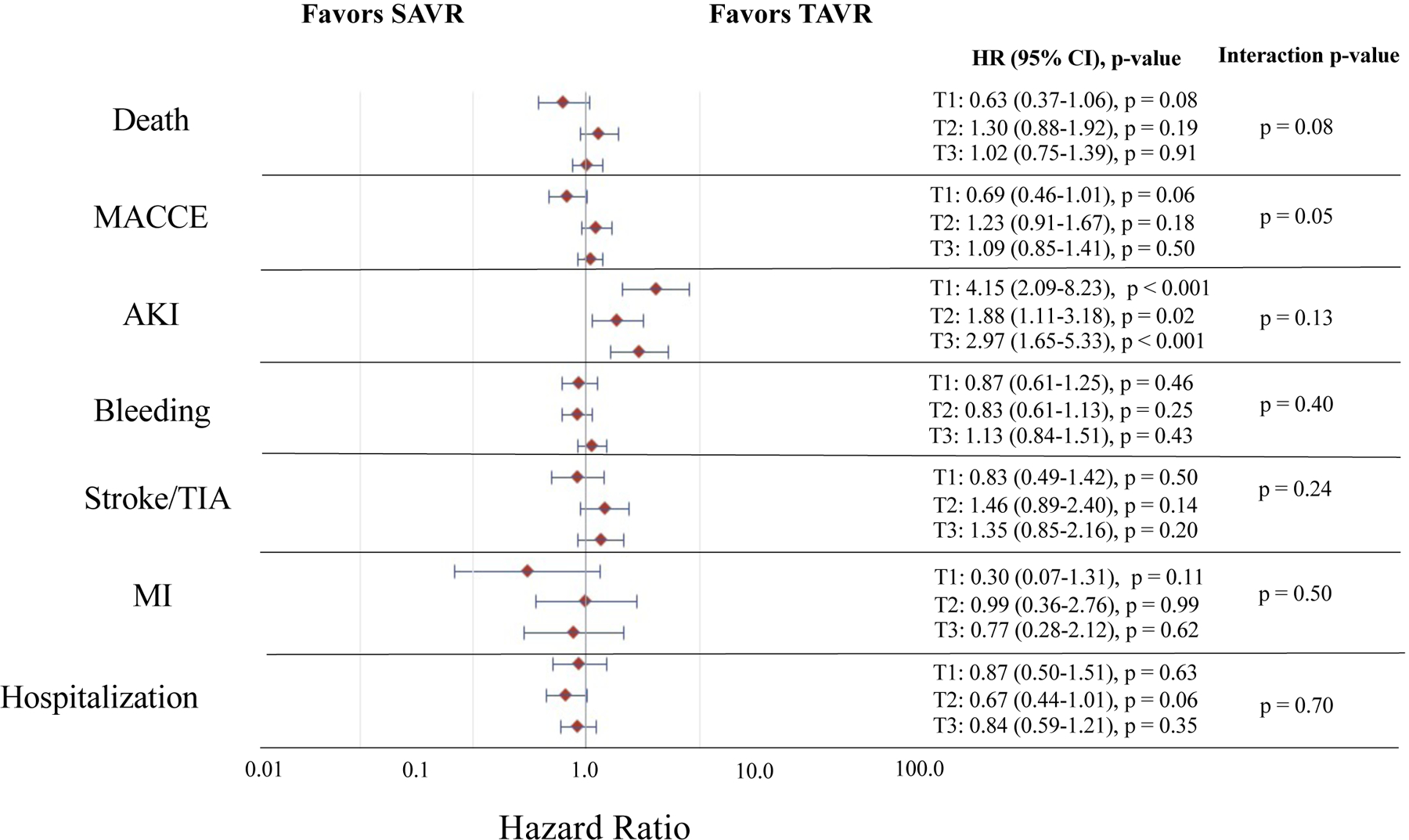
Represents the relative treatment effect for TAVR vs. SAVR in the combined Medicare linked CoreValve SURTAVI and High Risk trials by DFI tertile and trial outcome. The red diamonds indicate the point estimate for the adjusted hazard ratio and the horizontal blue lines indicate the 95% confidence interval for the adjusted hazard ratio for each outcome. The estimates, 95% confidence intervals, and p-values for TAVR vs. SAVR (TAVR as reference) within each DFI tertile are provided to the right of the forest plot. The p-value for the interaction of DFI tertile and treatment group (i.e. TAVR vs. SAVR) for each given outcome is provided. Estimates are adjusted for age, sex, Society of Thoracic Surgeons risk score, Logistic EuroSCORE, Charlson comorbidity index, history of diabetes mellitus, hypertension, peripheral vascular disease, prior stroke and TIA, immunosuppressive therapy, coronary artery disease, coronary artery bypass grafting, receipt of percutaneous coronary intervention, pacemaker or implantable defibrillator, congestive heart failure, atrial fibrillation and flutter, and aortic calcification. Individuals in tertile 1 (T1) had a DFI ≤ 0.22, those in tertile 2 (T2) had a DFI 0.23–0.37, and those in tertile 3 (T3) had a DFI ≥ 0.28. AKI = acute kidney injury, DFI = deficit-based frailty index, HR = hazard ratio, MACCE = major adverse cardiovascular and cerebrovascular event, MI = myocardial infarction, SAVR = surgical aortic valve replacement, TAVR = transcatheter aortic valve replacement, TIA = transient ischemic attack.
Figure 3. Absolute Treatment Effect for TAVR vs. SAVR in the Overall Study Cohort by DFI Tertile and Trial Outcome.
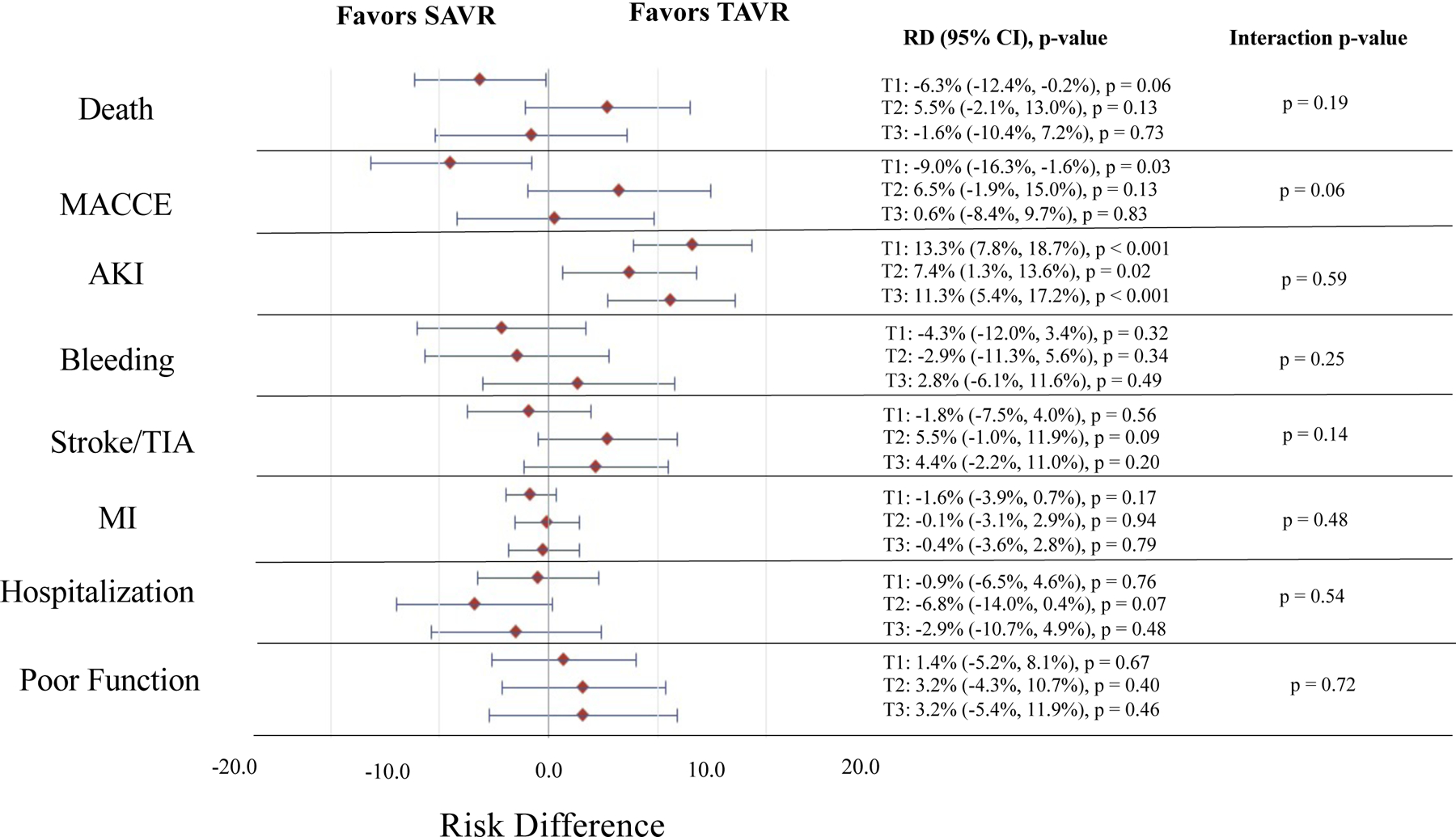
Represents the absolute treatment effect for TAVR vs. SAVR in the combined Medicare linked CoreValve SURTAVI and High Risk trials by DFI tertile and trial outcome. The red diamonds indicate the point estimate for the adjusted risk difference and the horizontal blue lines indicate the 95% confidence interval for the adjusted risk difference for each outcome. The estimates, 95% confidence intervals, and p-values for TAVR vs. SAVR (TAVR as reference) within each DFI tertile are provided to the right of the forest plot. The p-value for the interaction of DFI tertile and treatment group (i.e. TAVR vs. SAVR) for each given outcome is provided. Estimates are adjusted for age, sex, Society of Thoracic Surgeons risk score, Logistic EuroSCORE, Charlson comorbidity index, history of diabetes mellitus, hypertension, peripheral vascular disease, prior stroke and TIA, immunosuppressive therapy, coronary artery disease, coronary artery bypass grafting, receipt of percutaneous coronary intervention, pacemaker or implantable defibrillator, congestive heart failure, atrial fibrillation and flutter, and aortic calcification. Individuals in tertile 1 (T1) had a DFI ≤ 0.22, those in tertile 2 (T2) had a DFI 0.23–0.27, and those in tertile 3 (T3) had a DFI ≥ 0.28. AKI = acute kidney injury, DFI = deficit-based frailty index, Poor Function = functional impairment or death at 6 months, MACCE = major adverse cardiovascular and cerebrovascular event, MI = myocardial infarction, RD = risk difference, SAVR = surgical aortic valve replacement, TAVR = transcatheter aortic valve replacement, TIA = transient ischemic attack.
Overall, 162 (33.8%) of those in PFI tertile 3 died vs. 86 (17.9%) of those in PFI tertile 1 (HR 2.05, 95% CI 1.58–2.67, p < 0.001) (Supplemental Table XIII and Figures 4–5). Amongst those in PFI tertile 1, 16.8% of those receiving SAVR and 19.0% receiving TAVR died (SAVR vs. TAVR, adjusted HR, 0.94, 95% CI 0.61–1.46, p = 0.79; adjusted RD, −2.2%, 95% CI −9.1% to 4.7%, p = 0.58). In PFI tertile 2, 23.1% of those receiving SAVR and 19.3% of those receiving TAVR died (SAVR vs. TAVR, adjusted HR, 1.23, 95% CI 0.83–1.84, p = 0.30; adjusted RD, 3.8%, 95% CI −3.6% to 11.2%, p = 0.31). In PFI tertile 3, 30.6% of those receiving SAVR and 37.0% of those receiving TAVR died (SAVR vs TAVR, adjusted HR, 0.77, 95% CI 0.56–1.07, p = 0.17; adjusted RD, −6.4%, 95% CI −14.9% to 2.1%, p = 0.19). There were no significant interactions between PFI tertile and treatment assignment with respect to risk of death, on a relative or absolute scale (p > 0.05 for all interactions). Results were consistent across individual trials (Supplemental Table XIII and Figures VII–X).
Figure 4. Relative Treatment Effect for TAVR vs. SAVR in the Overall Study Cohort by PFI Tertile and Trial Outcome.
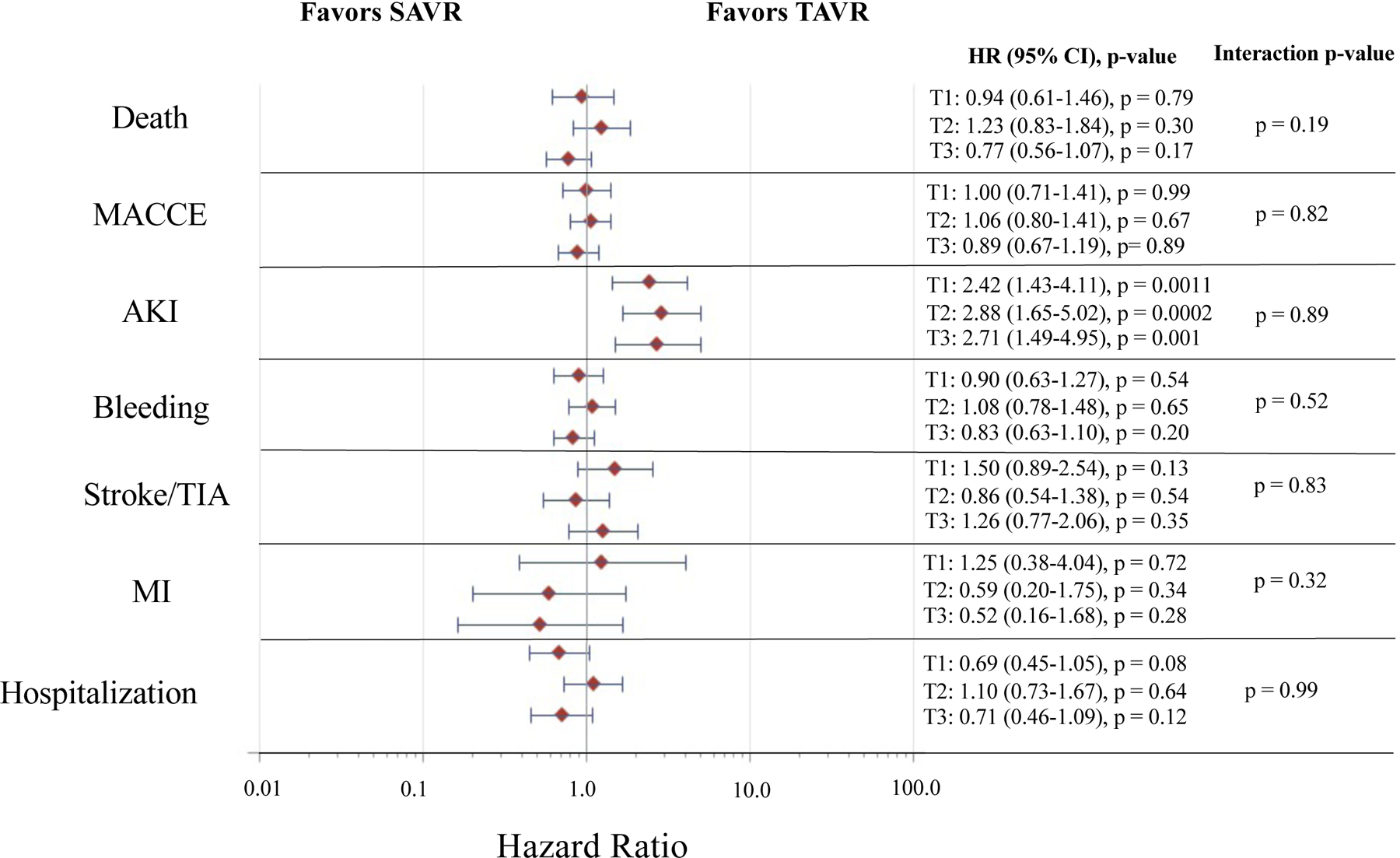
Represents the relative treatment effect for TAVR vs. SAVR in the combined Medicare linked CoreValve SURTAVI and High Risk trials by PFI tertile and trial outcome. The red diamonds indicate the point estimate for the adjusted hazard ratio and the horizontal blue lines indicate the 95% confidence interval for the adjusted hazard ratio for each outcome. The estimates, 95% confidence intervals, and p-values for TAVR vs. SAVR (TAVR as reference) within each PFI tertile are provided to the right of the forest plot. The p-value for the interaction of PFI tertile and treatment group (i.e. TAVR vs. SAVR) for each given outcome is provided. Estimates are adjusted for age, sex, Society of Thoracic Surgeons risk score, Logistic EuroSCORE, history of diabetes mellitus, coronary artery bypass grafting, congestive heart failure, and presence of aortic calcification. Individuals in tertile 1 (T1) had a PFI ≤ 0.18, those in tertile 2 (T2) had a PFI 0.19–0.28, and those in tertile 3 (T3) had a PFI ≥ 0.29. AKI = acute kidney injury, HR = hazard ratio, MACCE = major adverse cardiovascular and cerebrovascular event, MI = myocardial infarction, PFI = phenotype-based frailty index, SAVR = surgical aortic valve replacement, TAVR = transcatheter aortic valve replacement, TIA = transient ischemic attack.
Figure 5. Absolute Treatment Effect for TAVR vs. SAVR in the Overall Study Cohort by PFI Tertile and Trial Outcome.
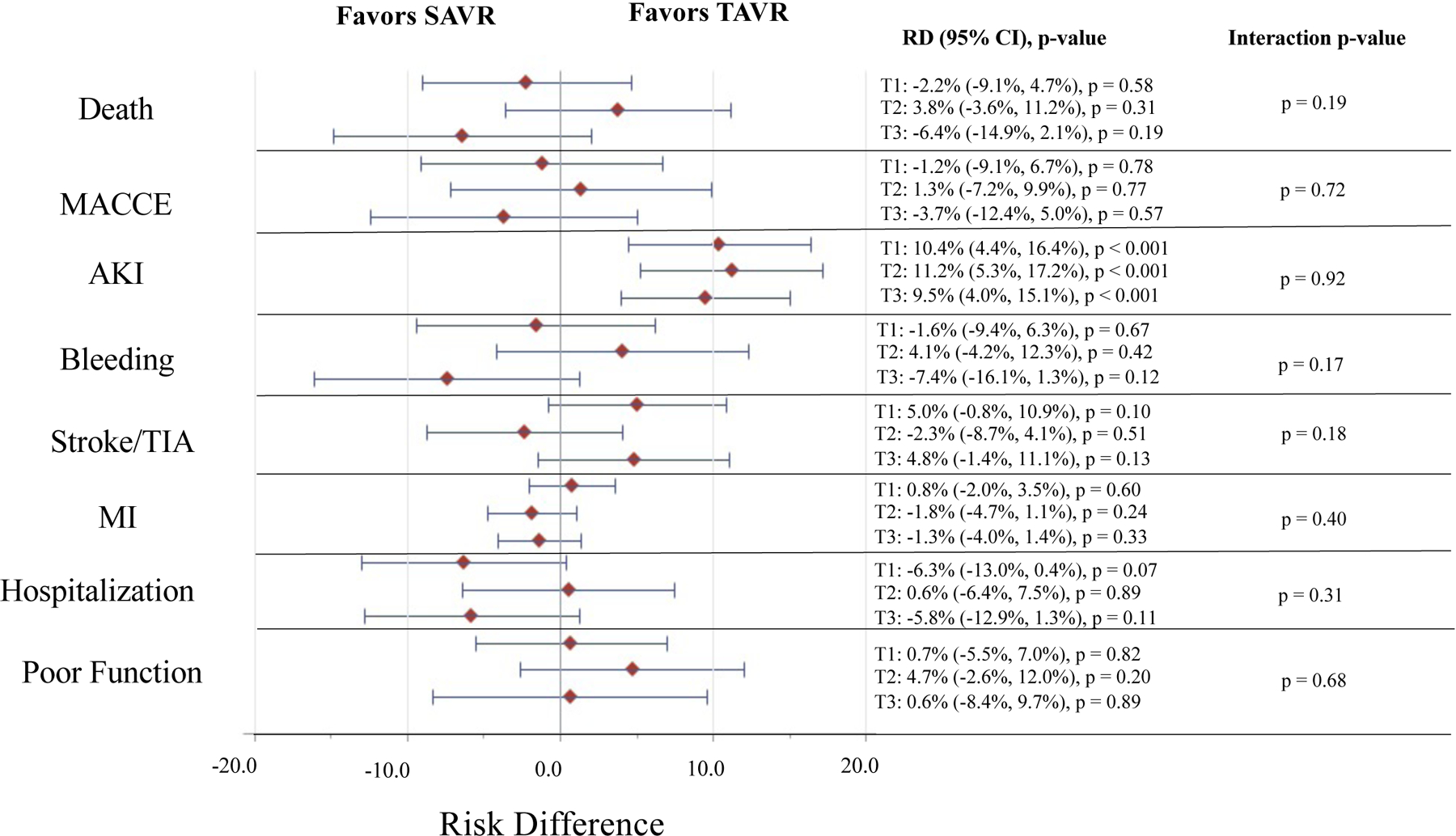
Represents the absolute treatment effect for TAVR vs. SAVR in the combined Medicare linked CoreValve SURTAVI and High Risk trials by PFI tertile and trial outcome. The red diamonds indicate the point estimate for the adjusted risk difference and the horizontal blue lines indicate the 95% confidence interval for the adjusted risk difference for each outcome. The estimates, 95% confidence intervals, and p-values for TAVR vs. SAVR (TAVR as reference) within each PFI tertile are provided to the right of the forest plot. The p-value for the interaction of PFI tertile and treatment group (i.e. TAVR vs. SAVR) for each given outcome is provided. Estimates are adjusted for age, sex, Society of Thoracic Surgeons risk score, Logistic EuroSCORE, history of diabetes mellitus, coronary artery bypass grafting, congestive heart failure, and presence of aortic calcification. Individuals in tertile 1 (T1) had a PFI ≤ 0.18, those in tertile 2 (T2) had a PFI 0.19–0.28, and those in tertile 3 (T3) had a PFI ≥ 0.29. AKI = acute kidney injury, Poor Function = functional impairment or death at 6 months, MACCE = major adverse cardiovascular and cerebrovascular event, MI = myocardial infarction, PFI = phenotype-based frailty index, RD = risk difference, SAVR = surgical aortic valve replacement, TAVR = transcatheter aortic valve replacement, TIA = transient ischemic attack.
Secondary Endpoints
At 1-year in the overall dataset, there were no significant interactions between DFI tertile and treatment assignment with respect to risk of MACCE, AKI, bleeding, stroke/TIA, MI, and hospitalization on both a relative and absolute scale (p > 0.05 for all interactions). (Supplemental Table XII and Figures 2–3) Results were overall consistent across individual trials (Supplemental Table XII and Figures III–VI).
At 1-year in the overall dataset, there were no significant interactions between PFI tertile and treatment assignment with respect to risk of MACCE, AKI, bleeding, stroke/TIA, MI, and hospitalization on both a relative and absolute scale (p > 0.05 for all interactions) (Supplemental Table XIII and Figures 4–5). Though risk of AKI was higher with TAVR across PFI tertiles (all p-values for comparisons of SAVR vs. TAVR within tertiles < 0.05), there was no significant interaction between PFI tertile and treatment assignment with respect to AKI (relative treatment effect, interaction p-value = 0.89; absolute treatment effect, interaction p-value = 0.92). Results were overall consistent across individual trials (Supplemental Table XIII and Figures VII–X).
Functional Outcomes
In the overall cohort, 85 (5.9%) individuals died within 6 months (39 [5.3%] after TAVR and 46 [6.6%] after SAVR, p = 0.32). Of the remaining patients (N = 1,357), 1,118 (82.4%) had nonmissing baseline and 6-month follow-up KCCQ surveys of which 134 (12.0%) had a KCCQ-OS < 45 or a decline from baseline ≥10 points at 6-month follow-up (71 [11.8%] after TAVR and 63 [12.2%] after SAVR, p = 0.85). Thus, the rate of poor functional outcome at 6 months was 14.8% after TAVR and 15.6% after SAVR (p = 0.71). Individuals with missing and non-missing functional data were similar across a range of characteristics, though those with missing functional data were more frequently female, had a slightly lower body mass index, a slightly higher STS score, higher rates of prior defibrillator/pacemaker use, and lower rates of coronary artery disease and PCI (Supplemental Table XIV).
A total of 79 (21.5%) of those in DFI T3 vs. 59 (12.1%) of those in DFI T1 had a poor functional outcome at 6 months (OR 1.98, 95% CI 1.37–2.86, p < 0.001). There was no significant interaction between treatment group and DFI tertile on functional impairment at 6 months on an absolute (p-value for interaction = 0.72) or relative scale (p-value for interaction = 0.50) (Figure 3 and Supplemental Table XV). Results were overall consistent across individual trials (Supplemental Table XV and Figures III–VI).
A total of 107 (28.0%) of those in PFI T3 vs. 52 (12.3%) of those in PFI T1 had a poor functional outcome at 6 months (OR 2.78, 1.93–4.01, p < 0.001). There was no significant interaction between treatment group and PFI tertile on functional impairment at 6 months on an absolute (p-value for interaction = 0.68) or relative scale (p-value for interaction = 0.75) (Figure 5 and Supplemental Table XVI). Results were overall consistent across individual trials (Supplemental Table XVI and Figures VII–X).
Sensitivity Analysis
There were no significant interactions between FI tertile and treatment assignment on risk of death by 30-days post-AVR, on a relative or absolute scale (p > 0.05 for all interactions) (Supplemental Tables XVII–XVIII). Additionally, in the unlinked overall combined trial dataset (N = 2,410), there were no significant interactions between DFI tertile and treatment assignment on risk of death, non-death outcomes, and functional impairment, on a relative or absolute scale (p > 0.05 for all interactions (Supplemental Table XIX and Figures XI–XII).
DISCUSSION
In this study of CoreValve trial participants linked to Medicare claims, two frailty indices using different conceptualizations of frailty did not identify a clear differential benefit from TAVR vs. SAVR across a range of adverse outcomes. Rates of death and functional impairment were 2–3-fold higher in the highest vs. lowest FI tertile but this increased risk was similar across treatment arms. Overall, these results suggest that frailty is an important risk marker in patients undergoing AVR but may not identify benefit from TAVR vs. SAVR among patients eligible for both treatments.
Frailty is a potent risk factor for adverse outcomes after AVR, conferring a near 4-fold increased risk of death at 1-year and an increased risk of functional impairment at 6-months.2–6 At the same time, a physician’s subjective assessment of a patient’s frailty status has not been associated with risk after AVR,9 leading many to develop and evaluate objective scales to measure frailty in those with severe AS.5 While multiple scales exist to measure frailty, they may be broadly categorized into two main types: (1) a deficit-based frailty index (Rockwood approach) which conceptualizes frailty as an accumulation of deficits over time;16 and (2) a phenotype-based frailty index (Fried approach) which conceptualizes frailty as a syndrome.1 This latter construct conceptualizes frailty as a biologic phenotype consisting of impairments across 5 domains: shrinking (i.e. weight loss), exhaustion, weakness, slowness, and low physical activity.1
Regardless of the approach utilized, frail individuals with severe AS have been found to be at higher risk for adverse outcomes than nonfrail individuals.5 In the FRAILTY-AVR study, Afilalo et al. studied 7 different scales in a cohort of individuals undergoing TAVR or SAVR at 14 centers, including both the Rockwood and Fried scale.5 Though the magnitude of risk differed by scale, all scales evaluated were significantly predictive of 30-day and 1-year mortality or worsening disability after AVR.5
Moreover, the role of frailty as a risk marker is not restricted to in-person assessments.27 As acquisition of in-person measures of frailty may be limited by time, expense, and availability, claims-based FIs have been proposed as a valid alternative to ascertainment of one’s frailty status when in-person measures are not available.20 In a prior study evaluating individuals included in the CoreValve trials,22 we have demonstrated that the claims-based PFI used in this study was associated with greater impairments in nutrition, disability, cognition, and self-reported health and was associated with a higher risk of all-cause death, bleeding, MACCE, and hospitalization at 1-year post-procedure.
Nevertheless, our study is the first, to our knowledge, to examine whether frailty identifies specific benefit from TAVR vs. SAVR. As frailty may be associated with risk of adverse outcomes in both treatment groups, it may not identify benefit from one procedure versus another. In the current study, though the average rate of death at 1-year in the highest FI tertile (DFI 36.7%, PFI 33.8%) was 2–3-fold higher than the average rate of death in the lowest FI tertile (DFI 13.4%, PFI 17.9%), this risk of mortality was similar amongst those assigned to TAVR and SAVR, using two different frailty definitions, such that frailty did not identify a patient subset that derived enhanced benefit from one procedure over the other. This was true regardless of the approach (i.e. Rockwood vs. Fried) used to measure frailty. Moreover, similar results were observed across all secondary endpoints, regardless of the type of treatment interaction sought (i.e. additive vs. relative), or individual trial evaluated. Even considering functional status endpoints, FI tertile was not associated with a differential relative or absolute benefit from TAVR vs. SAVR. Compared with TAVR, SAVR was associated with an increased risk of AKI across all FI tertiles without any significant treatment interaction observed by frailty status. Though it is possible that a benefit from one procedure vs. the other may exist in patients with extreme frailty excluded from these trials due to surgical ineligibility, these findings nevertheless indicate that amongst operable patients, frailty predicts an increased risk of adverse outcomes but no specific advantage to one treatment over another. This finding has particular relevance to interdisciplinary Heart Valve Teams deciding on the optimal type of AVR for patients with severe aortic valve disease, and suggest that frailty should not necessarily be used to assign differential benefits from TAVR or SAVR, recognizing the previously mentioned limitations.
In several other circumstances, frailty has been demonstrated to be an effect modifier of treatment effectiveness in older adults.28 Amongst individuals receiving sleep medications, ambulatory, non-frail patients were more likely to have falls and hip fractures than sedentary, frail adults.29 Similarly, in a post-hoc analysis of the Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation – Thrombolysis in Myocardial Infarction 48 (ENGAGE AF-TIMI-48) trial, edoxaban was associated with lower risk of bleeding than warfarin in all but those with severe frailty.30 As the prevalence difference in frailty in observational studies is larger for high-risk than low-risk interventions, particularly amongst older adults, of whom up to 50% are frail, frailty clearly influences treatment choice in real-world practice.28 However, where treatment choice is randomly assigned, our study suggests that frailty may be an effect modifier of some, but not all treatment-outcome relationships. This observation underlines the need to test the role of frailty as an effect modifier of relevant cardiovascular treatments. Moreover, it emphasizes the need to capture both frailty status and functional outcomes in real-world registries, where it is often unmeasured.22
Despite multiple strengths, including use of large trials datasets with comprehensive data collection and robust outcome ascertainment, our study has several limitations to acknowledge. First, as there are no FIs developed for the Fried frailty phenotype using the ICD-10-CM classification system, we limited our analysis to an ICD-9-CM based PFI and restricted the cohort to those undergoing an AVR prior to the transition to ICD-10-CM on October 1, 2015. As such, these findings may not reflect developments in AVR treatment since this time. Additionally, as other published claims-based FIs require outpatient and durable medical equipment files that are not commonly used or available,31 we chose to focus on the PFI by Segal et al.19 Nevertheless, it is possible that these findings may not generalize to other FIs. While components of FIs may independently be associated with treatment effect heterogeneity, due to the lower power in these subgroups to detect an effect and the possibility of identifying a spurious relationship, we chose to focus this analysis on frailty as a whole. Second, as our analysis was restricted to individuals from a specific group of trials, it is possible that results may not generalize to other trials and treatment relationships. Third, though linked and non-linked study participants were similar across a broad range of characteristics, it is possible that they are different across unmeasured characteristics that could influence study generalizability. In particular, given that individuals with extreme frailty may not have been included in the trials, the results may not generalize to this population. Moreover, the nonsignificant mortality difference observed between treatment arms in the High Risk trial and higher risk of 1-year mortality observed in this study compared to the original High Risk trial may reflect differences between the linked and unlinked cohorts, particularly as linked participants were older and had greater rates of heart failure and higher STS scores than unlinked participants. The absence of a mortality benefit to TAVR in this setting may reduce the ability to identify a treatment benefit by frailty subgroup. Moreover, the greater missingness of functional metrics may limit the ability to detect an effect of frailty on this outcome. CoreValve low-risk trial data was not available for linkage and thus results should not be extrapolated to this population. Fourth, it is possible that use of other FI quantiles may identify an effect at the extremes of frailty or within a particular quantile. However, the absence of a dose-response relationship between frailty and effect heterogeneity may suggest that any effect observed in a particular quantile may be related to chance.
In this study of CoreValve trial participants linked to Medicare claims, two frailty indices based on different theories of frailty, did not identify differential benefit from TAVR vs. SAVR across a range of adverse outcomes. Individuals in the highest FI tertiles had a 2–3-fold higher risk of death or functional impairment, but this increased risk was consistent across treatment arms. Overall, these results suggest that this frailty identifies a significantly increased risk of adverse outcomes but may not identify benefit from TAVR vs. SAVR among patients eligible for both procedures.
Supplementary Material
ACKNOWLEDGEMENTS:
The authors would like to acknowledge Issa Dahabreh, MD, ScD for his assistance with biostatistics.
FUNDING:
This work was supported by grants from the National, Heart, Lung, and Blood Institute [grant numbers 1R01HL136708-01 to R.W.Y., 1K23HL144907 to J.B.S.].
Disclosures:
Dr. Strom additionally reports grant funding from Edwards Lifesciences, consulting for Bracco Diagnostics, and speaker fees from Northwest Imaging Forums, unrelated to the submitted work. Dr. Yeh reports additional grant support from Abbott Vascular, Astra Zeneca, BD Bard, Boston Scientific Cook, Philips Medical Medtronic and Zoll, and consulting fees from Abbott, AstraZeneca, Boston Scientific, Edwards Lifesciences, Medtronic, Shockwave Medical and Zoll outside the submitted work. Changyu Shen is an employee of Biogen. All other authors report no relevant disclosures.
ABBREVIATIONS
- AKI
acute kidney injury
- AS
aortic stenosis
- AVR
aortic valve replacement
- CMS
Centers for Medicare and Medicaid Services
- CI
confidence interval
- DFI
deficit-based frailty index
- ENGAGE-AF TIMI-48
Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation – Thrombolysis in Myocardial Infarction 48
- EXTEND
Extending Trial-Based Evaluations of Medical Therapies Using Novel Sources of Data Study
- FI
frailty index
- HiR
US CoreValve High Risk trial
- HR
hazard ratio
- ICD-9-CM
International Classification of Diseases, 9th Revision, Clinical Modification
- ICD-10-CM
International Classification of Diseases, 10th Revision, Clinical Modification
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- MACCE
major adverse cardiovascular and cerebrovascular events
- MedPAR
Medicare Provider Analysis and Review
- OR
odds ratio
- PFI
phenotype-based frailty index
- RD
risk difference
- SAVR
surgical aortic valve replacement
- SD
standard deviation
- STS
Society of Thoracic Surgeons
- SURTAVI
Surgical or Transcatheter Aortic-Valve Replacement in Intermediate Risk Patients trial
- T1
tertile 1
- T2
tertile 2
- T3
tertile 3
- TAVR
transcatheter aortic valve replacement
- TIA
transient ischemic attack
Footnotes
REFERENCES
- 1.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- 2.Afilalo J, Alexander KP, Mack MJ, Maurer MS, Green P, Allen LA, Popma JJ, Ferrucci L and Forman DE. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63:747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chikwe J and Adams DH. Frailty: The Missing Element in Predicting Operative Mortality. Semin Thorac Cardiovasc Surg. 2010;22:109–110. [DOI] [PubMed] [Google Scholar]
- 4.Green P, Woglom AE, Genereux P, Daneault B, Paradis JM, Schnell S, Hawkey M, Maurer MS, Kirtane AJ, Kodali S, et al. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovasc Interv. 2012;5:974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afilalo J, Lauck S, Kim DH, Lefèvre T, Piazza N, Lachapelle K, Martucci G, Lamy A, Labinaz M, Peterson MD, A, et al. Frailty in Older Adults Undergoing Aortic Valve Replacement: The FRAILTY-AVR Study. J Am Coll Cardiol. 2017;70:689–700. [DOI] [PubMed] [Google Scholar]
- 6.Schoenenberger AW, Stortecky S, Neumann S, Moser A, Jüni P, Carrel T, Huber C, Gandon M, Bischoff S, Schoenenberger CM, et al. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI). Eur Heart J. 2013;34:684–692. [DOI] [PubMed] [Google Scholar]
- 7.Farooqi MAM, Gerstein H, Yusuf S and Leong DP. Accumulation of Deficits as a Key Risk Factor for Cardiovascular Morbidity and Mortality: A Pooled Analysis of 154 000 Individuals. J Am Heart Assoc. 2020;9:e014686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orkaby AR, Nussbaum L, Ho YL, Gagnon D, Quach L, Ward R, Quaden R, Yaksic E, Harrington K, Paik JM, et al. The Burden of Frailty Among U.S. Veterans and Its Association With Mortality, 2002–2012. J Gerontol A Biol Sci Med Sci. 2019;74:1257–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodés-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, Osten M, Natarajan MK, Velianou JL, Martucci G, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55:1080–1090. [DOI] [PubMed] [Google Scholar]
- 10.Otto CM, Kumbhani DJ, Alexander KP, Calhoon JH, Desai MY, Kaul S, Lee JC, Ruiz CE and Vassileva CM. 2017 ACC Expert Consensus Decision Pathway for Transcatheter Aortic Valve Replacement in the Management of Adults With Aortic Stenosis. J Am Coll Cardiol. 2017;69:1313–1346. [DOI] [PubMed] [Google Scholar]
- 11.Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J Jr., Kleiman NS, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–1798. [DOI] [PubMed] [Google Scholar]
- 12.Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2017;376:1321–1331. [DOI] [PubMed] [Google Scholar]
- 13.Strom JB, Tamez-Aguilar H, Yuansong Zhao MA, Valsdottir L, Curtis J, Brennan JM, Shen C, Popma JJ, Mauri L and Yeh RW. Validating the Use of Registries and Claims Data to Support Randomized Trials: Rationale and Design of the Extending Trial-Based Evaluations of Medical Therapies Using Novel Sources of Data (EXTEND) Study. Am Heart J. 2019;212:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green CP, Porter CB, Bresnahan DR and Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. [DOI] [PubMed] [Google Scholar]
- 15.Osnabrugge RL, Arnold SV, Reynolds MR, Magnuson EA, Wang K, Gaudiani VA, Stoler RC, Burdon TA, Kleiman N, Reardon MJ, et al. Health status after transcatheter aortic valve replacement in patients at extreme surgical risk: results from the CoreValve U.S. trial. JACC Cardiovasc Interv. 2015;8:315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitnitski AB, Mogilner AJ and Rockwood K. Accumulation of Deficits as a Proxy Measure of Aging. ScientificWorldJournal. 2001;1:321027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogan DB, MacKnight C and Bergman H. Models, definitions, and criteria of frailty. Aging Clin Exp Res. 2003;15:1–29. [PubMed] [Google Scholar]
- 18.Searle SD, Mitnitski A, Gahbauer EA, Gill TM and Rockwood K. A standard procedure for creating a frailty index. BMC Geriatrics. 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segal JB, Chang HY, Du Y, Walston JD, Carlson MC and Varadhan R. Development of a Claims-based Frailty Indicator Anchored to a Well-established Frailty Phenotype. Med Care. 2017;55:716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Segal JB, Huang J, Roth DL and Varadhan R. External Validation of the Claims-based Frailty Index in the National Health and Aging Trends Study Cohort. Am J Epidemiol. 2017;186:745–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kundi H, Valsdottir LR, Popma JJ, Cohen DJ, Strom JB, Pinto DS, Shen C and Yeh RW. Impact of a Claims-Based Frailty Indicator on the Prediction of Long-Term Mortality After Transcatheter Aortic Valve Replacement in Medicare Beneficiaries. Circ Cardiovasc Qual Outcomes. 2018;11:e005048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strom JB XJ, Orkaby AR, Shen C, Charest BR, Kim DH, Cohen DJ, Kramer JB, Spertus JA, Gerszten RE, Yeh RW. Identification of Frailty Using a Claims-Based Frailty Index in the CoreValve Studies: Findings from the EXTEND-FRAILTY Study. J Am Heart Assoc. 2021. [E-pub ahead of print]. doi: 10.1161/JAHA.121.022150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fine JP and Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 24.Harbord RM and Higgins JPT. Meta-Regression in Stata. The Stata Journal. 2008;8:493–519. [Google Scholar]
- 25.O’Brien SM, Feng L, He X, Xian Y, Jacobs JP, Badhwar V, Kurlansky PA, Furnary AP, Cleveland JC Jr., Lobdell KW, et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 2-Statistical Methods and Results. Ann Thorac Surg. 2018;105:1419–1428. [DOI] [PubMed] [Google Scholar]
- 26.Nashef SAM, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R and the Euro Ssg. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg. 1999;16:9–13. [DOI] [PubMed] [Google Scholar]
- 27.Rockwood K, Andrew M and Mitnitski A. A Comparison of Two Approaches to Measuring Frailty in Elderly People. J Gerontol A Biol Sci Med Si. 2007;62:738–743. [DOI] [PubMed] [Google Scholar]
- 28.Kim DH and Schneeweiss S. Measuring frailty using claims data for pharmacoepidemiologic studies of mortality in older adults: evidence and recommendations. Pharmacoepidemiol Drug Saf. 2014;23:891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berry SD, Lee Y, Cai S and Dore DD. Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents. JAMA Int Med. 2013;173:754–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkinson C, Wu J, Searle SD, Todd O, Hall M, Kunadian V, Clegg A, Rockwood K and Gale CP. Clinical outcomes in patients with atrial fibrillation and frailty: insights from the ENGAGE AF-TIMI 48 trial. BMC Medicine. 2020;18:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG and Nijhuis-van der Sanden MW. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10:104–114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


