Abstract
Cardiovascular disease (CVD) is the leading cause of mortality among the human species, however the non-existence of successful therapies to curtail the effect of Myocardial Infarction (MI) is a disquieting reality. Even though successful herbal formulations using Crataegus oxycantha (COC) is available, however, it is not recognized as an alternative medicine due to the lack of explanation on the molecular mechanism of COC extract on CVD conditions. In vivo studies revealed that COC extract significantly prevented caspase activation in conditions like post-MI; however, the role of a specific secondary metabolite that could be involved in this action is under quest. The present study, therefore, aims at predicting the plausible mechanism of action of key secondary metabolite in COC extract on apoptotic executioner caspase - caspase 3 during MI through in silico tools. The protein-protein interaction network, QikProp, and molecular docking studies were performed to identify the lead compound that revealed Epicatechin Gallate (ECG) of COC as an effective inhibitor against candidate MI/apoptosis mediator – caspase 3. The docked complex was further taken for molecular dynamics simulation, which was achieved through Desmond. Molecular dynamics further confirmed the stability of the binding interactions between the docked complex. The overall in silico results proved that ECG could prevent the dissociation of cleaved caspases, which is essential for their activation. Computational observations were strongly supported by experimental evidence obtained from in vivo studies in the MI-model system. From the above observations, it was concluded that computational analysis was in good agreement with the experimental analysis on ECG’s potential to prevent caspase 3 activation during MI.
Keywords: Molecular docking, cardiovascular disease, apoptosis, flavonoids
Graphical Abstract

Introduction
Molecular docking as one of the emerging fields in computational biology, in particular, structure-based drug designing (SBDD) a subcategory of molecular docking, has seen numerous developments and enhancements to the concepts in the last decade. Molecular docking refers to the identification of the preferred orientation and binding affinity of the ligand to a protein when bound to each other in a stable complex (Lengauer & Rarey, 1996). This can be simulated by computational approaches with well-defined mathematical algorithms, kinetics and critical consideration of atomic forces from the end of ligand or protein or both thereby forming the stable complex. These molecular docking algorithms can be implemented on a larger dataset of small molecules against single or multiple protein(s) by virtual screening to rationally assess their binding interactions with the target enzyme/receptor active site residues (Ajay & Murcko, 1995). However, this provides limited information on the potential mechanism of action of the selected lead compound. When a small molecule is chosen as the best interacting compound based on the computational scoring functions, the chances of it leading to false positives can be higher (Abagyan & Totrov, 2001). Nevertheless, supporting experimental data from earlier or existing literature is strongly believed to potentially suggest its role as a potent therapeutic agent against a particular disease target. This forms the basis of our present study to identify key molecular agents that target Myocardial Infarction (MI). This envisages the development of potential therapeutic drug molecules by the pharmaceutical industries, as in most cases a target (receptor or an enzyme) is activated or inhibited leading to disease alleviation or abrogation. This rational approach saves both cost and time and helps in development and improvement of selectivity and specificity of the potential drug-like molecule against the target.
MI is the major killer worldwide in the division of non-communicable diseases. Each year, more than 17.9 million people die because of cardiovascular disease (CVD) in which MI and stroke contribute 85% of the mortality. Furthermore, this doesn’t seem to decrease as time progresses. MI morbidity and mortality is increasing drastically in developing countries like India in comparison to well-developed countries (World Health Organization, 2017). Reports suggest that rapid urbanization, consumption of westernized foods, low physical activity, smoking, and stress contribute to the increase in MI mediated death. Ethnically Indian population is reported to have a higher susceptibility to MI because of their genetic makeup (Prabhakaran et al., 2016). Even though several medicines are considered for MI, most of them are reported for their adverse effects in the chronic usage (Moore et al., 2015; Vostinaru, 2017; mach et al., 2018).
Flavonoid based secondary metabolites (drug/ligands) used for this computational study is from Crataegus oxycantha (COC) plant extract, a well-known cardio tonic agent used for several centuries in the treatment of cardiovascular disease (CVD) associated complications (Dasagrandhi et al., 2018; Elango et al., 2009; Jayachandran et al., 2010; Swaminathan et al., 2010; Thiruchenduran et al., 2011) in the Homeopathic Medicine. However, essential compounds and specific targets of MI are not clearly determined and the underlying mechanism remains poorly defined. Therefore, to reveal and understand the treatment targets and mechanism of MI is a subject which is worthy of study. In extension to the previous work on pharmacological studies in identifying the presence of active secondary metabolites (oligomericproanthocyanidins - OPCs) from COC extract, this is the first attempt to study the interaction between the active compounds present in the COC extract and specific protein targets involved in MI using a comprehensive multifaceted computational and experimental mode of analysis.
Materials and Methods
Computational
Selection of target Proteins
From the earlier experimental works published in various journals and from our group; we have data-mined sixteen proteins expressed under critical conditions during the progression of MI. These proteins were studied for their association and relation with nineteen other proteins involved in CVD development using GeneMANIA (Warde-Farley et al., 2010). Consequently, all thirty-five proteins were considered as a target for virtual screening studies as shown in Table 1.
Table 1:
Protein details and structural information
| S. No | Protein1 | Pathway Involved2 | UNIPROT ID3 | PDB ID4 | Template5 | Structure Validation6 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Modellera | Swiss modelb | Phyre 2c | ||||||||||||||
| Temp ID | %Id | %Qc | Fav | All | Dis | Fav | All | Dis | Fav | All | Dis | |||||
| Information of the proteins collected through literature review | ||||||||||||||||
| 1 | Alpha-1 antichymotripsin | Acute inflammation | P01011 | 1QMN | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 2 | Endothelial protein C receptor | Venous thrombosis | Q9UNN8 | 1L8J | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 3 | Thrombospondin-1 | Inflammation | P07996 | 1UX6 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 4 | Interleukin-6 | Inflammation | P05231 | 1ALU | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 5 | Tumour necrosis factor-α | Inflammation | P01375 | 1A8M | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 6 | Apoptosis regulator BAX | Apoptosis | Q07812 | 1F16 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 7 | Caspase 3 | Apoptosis | P42574 | 1CP3 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 8 | Calmodulin | Arrhythmias | P62158 | 1CLL | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 9 | Caspase 7 | Apoptosis | P55210 | 1F1J | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 10 | Glyceraldehyde-3-phosphate dehydrogenase | Myocardial necrosis | P04406 | 4WNC | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 11 | Angiotensin-converting enzyme | Blood pressure | P12821 | 1O86 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 12 | HMG-CoA reductase | Inflammation and atherosclerosis | P04035 | 2Q1L | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 13 | NF-κB | Inflammation | P19838 | -- | 1SVC_P | 99 | 37 | 69.4 | 27.3 | 3.3 | 85.0 | 14.3 | 0.8 | 85.3 | 13.9 | 0.8 |
| 14 | Caspase 8 | Apoptosis | Q14790 | -- | 4JJ7_A | 100 | 54 | 80.5 | 17.0 | 2.5 | 85.7 | 14.2 | 0.0 | 75.5 | 22.3 | 2.2 |
| 15 | Caspase 9 | Apoptosis | P55211 | -- | 1JXQ_A | 99 | 66 | 83.0 | 16.5 | 0.6 | 91.4 | 8.3 | 0.2 | 86.8 | 13.2 | 0.0 |
| 16 | High mobility group protein B1 | Inflammation | P09429 | -- | 2YRQ_A | 100 | 77 | 94.7 | 4.8 | 0.5 | 88.8 | 11.2 | 0.0 | 86.7 | 13.3 | 0.0 |
| Information of the additional proteins that are obtained from Gene Mania results | ||||||||||||||||
| 17 | Interleukin-6 receptor subunit alpha | Inflammation | P08887 | 1N26 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 18 | Bcl-2-like protein 1 | Apoptosis | Q07817 | 1R2D | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 19 | Bcl-2-like protein 10 | Apoptosis | Q9HD36 | 4B4S | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 20 | Caspase 2 | Apoptosis | P42575 | 1PYO | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 21 | Caspase 1 | Apoptosis | P08887 | 1N26 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 22 | Caspase 6 | Apoptosis | Q07817 | 1R2D | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 23 | Angiotensin-converting enzyme 2 | Inflammation | Q9BYF1 | 1R42 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 24 | Glyceraldehyde-3-phosphate dehydrogenase, testis-specific | Apoptosis | O14556 | 3H9E | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 25 | Apoptotic protease-activating factor 1 | Inflammation | O14727 | 3JBT | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 26 | Apoptosis regulator Bcl-2 | Apoptosis | P10415 | 2XA0 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 27 | Caspase 14 | Apoptosis | P31944 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 28 | Caspase 12 | Inflammation | Q6UXS9 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 29 | Caspase 10 | Inflammation | Q92851 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 30 | Caspase 4 | Inflammation | P49662 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 31 | B2 bradykinin receptor | Apoptosis | P30411 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 32 | Alpha-adducin | Apoptosis | P35611 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 33 | Proto-oncogene c-Rel | Inflammation, Apoptosis | Q04864 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 34 | E3 ubiquitin-protein ligase XIAP | Inflammation, Apoptosis | P98170 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 35 | CASP8 and FADD-like apoptosis regulator | Apoptosis | O15519 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
Important proteins involved in the progression of MI
Stages where the proteins expressed
UNIPROT ID of the target proteins (Gives protein sequence information)
PDB ID of the target proteins (Gives tertiary structure of the proteins)
Template information for the proteins without structures obtained from BLAST (Gives information of the template, % identity and % query coverage between the target and template)
Validation results of the structure predicted through various servers done by SAVES.
% of residues in the favoured, allowed and disallowed regions for the proteins predicted through modeller
% of residues in the favoured, allowed and disallowed regions for the proteins predicted through swiss model
% of residues in the favoured, allowed and disallowed regions for the proteins predicted through phyre 2
Selection of active components from COC
Crataegus oxycantha (COC) commonly known as hawthorn is a well-known cardio tonic agent. It is used to treat several CVD (Dasagrandhi et al., 2018; Elango et al., 2009; Jayachandran et al., 2010; Swaminathan et al., 2010; Thiruchenduran et al., 2011). Important OPCs, to name a few: Epicatechin, Epicatechin Gallate (ECG), Rutin, Tyramine and Vitexin were used for the current study and structures were collected from previous literature survey (Swaminathan et al., 2010; Kumar et al., 2012) and given in Figure 1.
Figure 1:
Structure of major active flavonoids (Epicatechin, Epicatechin Gallate, Rutin, Vitexin) and amine (Tyramine) present in COC. The structural information’s of these compounds were obtained through Pubchem compound database.
Structure prediction and validation
Of these thirty-five target proteins, twenty-two proteins were found to have the crystallography structure solved and deposited in PDB. For the remaining thirteen proteins, templates were selected through BLAST based on high sequence similarity, high crystallographic resolution, and overall fold. Proteins from 27–35 of Table 1, were eliminated from further investigations since they do not have structural templates. Four proteins from 13–16 of Table 1 were considered for the development of 3D structure by comparative modelling as it contains sufficient information about template, hence the model was generated. The models were generated using three modelling programs: Modeller (http://www.salilab.org/modeller/9v7/), Swiss model (http://swissmodel.expasy.org/) and Phyre2 (http://www.sbg.bio.ic.ac.uk/phyre2). The three-dimensional structures generated through these methods were evaluated for its quality through SAVES (http://https://servicesn.mbi.ucla.edu/SAVES/). The least amount of amino acid residues in the disallowed region gives a better resolution of the protein crystallographic structure. Based on this criterion the predicted structures with a minimal amount of residues in the disallowed region were taken for further studies.
Molecular dynamics simulation was performed to understand the physical stability of the predicted 3D protein structures. The conformational features and a reasonable structure of the modelled proteins (NF-κB, Caspase 8, Caspase 9 and High mobility group protein 1) were analysed through molecular dynamics simulations using GROMACS (Groningen Machine for Chemical Simulations) version 5.1.1. GROMOS96 43a1 force field was used. The protein structures were relaxed to eliminate bad atomic contracts and then solvated by adding SPC 216 water to the system by applying cubic box with 10 Å cutoffs. Counterion, Na+ or Cl- were added to the system, to replace the water molecules by bringing it to the neutrality. Steepest descent algorithms have been used to minimize the system to remove bad Van der Waals contacts before submitting the same to the molecular dynamic simulation. Then the solvated system was subjected to the constant temperature and pressure bath. The constant temperature of 300K and pressure of 1 bar, the Berendsen thermostat was maintained using a coupling time of 0.1 and 1.0 pico second (ps). Under equilibrium period, a free molecular dynamics simulation of 10 ps was simulated with a force constant of 1000 kJ mol-1 nm-2 were applied (Karthikeyan et al., 2014). Finally, the free molecular dynamics simulations of 50 nano second (ns) were performed for the modelled structures separately. Each trajectory was graphically visualized using the Xmgrace tool.
Active site prediction
For all the target proteins, the binding sites were predicted using SiteMap protocol (Schrödinger, LLC, New York, NY, 2015) that gives information about the location of protein binding sites and functional residues that involved in protein-ligand interactions. The tool provides information about one or more regions on the protein surface that suits for binding of a ligand to the receptor. These binding sites were finally evaluated by calculating various properties such as the size of the site, the degree of the enclosure by the protein, the rigidity of the site, the degree of the bound ligand which donate or accept hydrogen bonds at the binding site of the protein.
ADME prediction
Unfavourable absorption, distribution, metabolism, excretion and toxicity prediction plays an important role in the drug discovery process. This standard criterion was identified as the major cause of failure in the drug discovery process. This can be observed through the QikProp program (Schrödinger suite). It also evaluates more than 42 pharmaceutical relevant properties for essential lead generation and lead optimization. The ADME properties of Epicatechin, Epicatechin Gallate (ECG), Rutin, Tyramine and Vitexin were predicted.
Molecular Docking
Evaluation of the binding conformation of COC compounds in the active site of all the 26 target proteins was performed using Glide-XP (Schrödinger suite). Initially, the protein was prepared using the Protein Preparation Wizard (Schrödinger suite) workflow implemented in the Maestro. Using the default parameters it removes all water molecules and generated polar hydrogen atoms to the parent carbon atoms. All atoms were charged with OPLS 2001 force fields. The docking area was defined by a grid box generation using receptor-grid generation protocol (Schrödinger) which selects 15 Å radius for the active site cavity. Five different active compounds of COC were collected from the literature and the 3D structures were retrieved from the PubChem compound database (http://www.ncbi.nlm.nih.gov/pccompound). These structures were imported into the glide window and converted into maestro format and ligands were prepared using the LigPrep module (Schrödinger suite). The ligands were geometry optimized by using the OPLS-2005 force field. The molecular docking experiments were carried out using default parameters (Glide protocol). The results of the molecular docking studies provide information about the drug-like molecules that interact with the binding site of the target proteins. The docking results were interpreted based on docking score and glide energy, with the information on the binding affinity, mode of interaction with the key amino acid residues in the active site.
Molecular dynamic simulation for complex structure
The conformational stability of the protein-ligand complex in motion was obtained through molecular dynamic simulation using Desmond module (Schrödinger suite). The protein-ligand complex was bound by a predefined TIP4P water model, by an orthorhombic box with 10Å distance and the box volume was minimized. The complete charge of the system was neutralized by adding Na+ and Cl- ions. OPLS 2005 force field was used for energy calculation. Temperature and pressure were kept default at 300 K and 1.01325 bar using the Nose-Hoover thermostat algorithm and Martyna-Tobias-Klein Barostat algorithm. The complex of structural dynamic simulation for the best complex was carried out with NPT ensemble for 50ns and the trajectory was set at an interval of 1.2ps
In vivo Experiments
In vivo experiments such as the grouping of animals, surgical preparation, left anterior descending artery ligation, TTC staining and western blotting caspase 3 (Cell Signaling Technology, Catalogue number: #9915) were performed as described by Swaminathan et al., 2010 without any modification.
Results
Computational
Structure prediction and validation
Based on the literature survey and GeneMANIA analysis (Figure 2), thirty-five proteins were data mined and reported for their involvement in the development and progression of MI.
Figure 2:
Interaction network image obtained from GeneMANIA, with sixteen proteins (which are involved in the progression of MI) as target input. GeneMANIA assessed the interactions between these targets and also retrieved nineteen other proteins associated with these targets in interactions with the help of its available genomics and proteomics data from linked databases. Physical interaction information’s are in pink colour and interactions through co-expression studies are indicated in violet colour.
Among thirty-five proteins, only twenty-two have their 3D structure and the remaining thirteen proteins structures were predicted using modeller, swiss model, and phyre2 (Table 1). The predicted structures were further validated through the Ramachandran plot (Figure 3I A–D) for their reliability. The structures that have their maximum number of residues in the favoured region on the Ramachandran map were selected as the best fit and were considered for further studies. Of these thirteen proteins only NF-κB, Caspase 8, Caspase-9 and High mobility group protein B1satisfied Ramachandran plot, hence were included for further experiments. The structure prediction for the rest of proteins viz., caspases 14, 10, 12, 4, B2 bradykinin receptor, Alpha-adducin, Proto-oncogene c-Rel, E3 ubiquitin-protein ligase XIAP, CASP8 and FADD-like apoptosis regulator was not positive. Hence these nine proteins were excluded from further study. The complete structural information of all the thirty-five inputs is provided in Table 1.
Figure 3I-A:
Final 3D structure of NF-κB predicted through Swiss model with 1SVC as template. The Ramachandran plot assessment of the predicted structure showed 226 (85.0%) residues in the favoured region, 36 (13.5%) residues in the additionally allowed region, 2 (0.8%) residues in the generously allowed region and 2 (0.8%) residues in the disallowed region.
Figure 3I-D:
Final 3D structure of HMGB1 predicted through Modeller with 2YRQ as template. The Ramachandran plot assessment of the predicted structure showed 179 (94.7%) residues in the favoured region, 9 (4.8%) residues in the additionally allowed region, 0 (0.0%) residues in the generously allowed region and 1 (0.5%) residues in the disallowed region.
Further validation of the predicted structure on its physical stability for 50 ns molecular dynamics simulation was performed to investigate the stability pattern of the modelled proteins. The root mean square deviations were calculated for the trajectories of the protein from starting structure as a function of time. The RMSD plots for all the structures were given in the following Figure 3II A–D.
Figure 3II:
Stability analysis of modelled proteins using GROMACS showing RMSD plot for A) NF-κB, B) Caspase 8, C) Caspase 9 and D) High mobility group protein 1.
ADME prediction
The prediction of ADME provides information about the suitability of any organic compound to become a drug-like molecule. QikProp module in the Schrödinger suite will analyse more than 42 pharmacokinetics properties as descriptors and the salient ones are represented in Table 2. Of the 5 compounds taken from the COC extract, all four compounds except Rutin satisfied the criteria for drug likeliness. Therefore Rutin was eliminated from further experiments.
Table 2:
ADME Prediction
| Comp Namea | Mol MW b | Rule of 5c | Rule of 3 d | Acceptor HB e | Donor HB f | Human oral absorption g | QPP MDCKh | QPlog HERGi | QPP Cacoj | Stars k |
|---|---|---|---|---|---|---|---|---|---|---|
| Epicatechin | 290.272 | 0 | 1 | 5.45 | 5 | 2 | 21.591 | −4.675 | 55.168 | 0 |
| Vitexin | 432.383 | 1 | 2 | 12.25 | 6 | 2 | 3.053 | −5.161 | 9.032 | 1 |
| Tyramine | 137.181 | 0 | 0 | 1.75 | 3 | 2 | 87.121 | −4.452 | 182.649 | 1 |
| Rutin | 610.524 | 3 | 2 | 20.55 | 9 | 1 | 1.151 | −5.202 | 3.664 | 9 |
| Epicatechin Gallate | 442.378 | 1 | 2 | 8 | 7 | 1 | 0.714 | −5.897 | 2.354 | 4 |
Compound Name
Molecular weight (acceptable range 130–725 is good)
Number of violations of Lipinski rule of five (maximum is 4)
Number of violations of Jorgensen’s rule of three (maximum is 4)
Donor hydrogen bond (acceptable range 0.0 – 6.0)
Acceptor hydrogen bond (acceptable range 2.0 – 20.0)
Predicted human oral absorption (1, 2, or 3 for low, medium, or high)
Predicted apparent MDCK cell permeability (acceptable range <25 is poor, > 500 is high)
Predicted IC50 value for blockage of HERG K+ channels (concern below −7)
Predicted Caco-2 cell permeability (acceptable range <25 is poor, >500 is high)
Stars (acceptable range 0–5)
Molecular Docking
Docking is the procedure to identify the preferred orientation of any small organic compound to that of a given macromolecule such as protein. Several parameters are used in docking to understand the preferred orientation. In docking, the number of hydrogen bonds formed between the organic compound and the chargeable amino acids present in the active site of the protein decides the efficiency of a drug-like molecule. The docking glide score denotes the stability of the bound organic compound with docked proteins. The docking glide score is always indirectly proportional to binding affinity. Lower the docking glide score indicates the higher binding affinity of the compound with docked protein. Of the four compounds taken for the analysis, ECG proved as a promising molecule with effective drug-like properties and all docking results were given in Table 3.
Table 3:
Docking score, Glide energy, interacting residues and number of hydrogen bonds of the docked molecules.
| S. No | Proteins | Compounds | Docking score | Glide energy | H-Bond | H-Bond interaction |
|---|---|---|---|---|---|---|
| 1 | Alpha-1 antichymotripsin 1QMN |
Epicatechin | −7.594 | −48.041 | 3 | SER 95, ASN 247, HIS 98 |
| Epicatechin Gallate | −8.634 | −58.413 | 6 | SER 95, ASN 385, HIS 98, ASN 247(2) | ||
| Tyramine | −3.235 | −26.784 | 1 | THR 102 | ||
| Vitexin | −6.753 | −53.680 | 2 | THR 102, ASN 385 | ||
| 2 | Angiotensin -converting enzyme 1O86 |
Epicatechin | −5.987 | −55.254 | 4 | ALA 354, GLU 403 (2), GLU 411 |
| Epicatechin Gallate | −7.067 | −80.611 | 8 | CYS 370, GLU 376 (2), ASP 377 (2), ALA 354, LYS 511, GLY 2000 | ||
| Tyramine | −3.437 | −28.046 | 3 | ASP 377, LYS 511, GLN 281 | ||
| Vitexin | −7.229 | −78.894 | 6 | ARG 522(2), GLU 411, ALA 356, GLU 143, HIS 353 | ||
| 3 | Apoptosis regulator BAX 1F16 |
Epicatechin | −5.551 | −38.839 | 3 | ASP 86, LYS 123, THR 127 |
| Epicatechin Gallate | −6.233 | −52.732 | 2 | ILE 80, THR 127 | ||
| Tyramine | −5.709 | −32.155 | 3 | LEU 185, LYS 189, ASP 84 | ||
| Vitexin | −5.672 | −50.591 | 2 | LYS 123, LYS 119 | ||
| 4 | Calmodulin 1CLL |
Epicatechin | −6.904 | −38.252 | 2 | MET 124, GLU 127 |
| Epicatechin Gallate | −5.410 | −43.608 | 3 | GLU 127(2), MET 144 | ||
| Tyramine | −6.271 | −29.783 | 0 | −- | ||
| Vitexin | −6.559 | −45.260 | 2 | GLU 127, MET 124 | ||
| 5 | Caspase 3 1CP3 |
Epicatechin | −7.434 | −54.028 | 6 | SER 205, GLN 161, ARG 64(2), THR 62, HIS 121 |
| Epicatechin Gallate | −9.931 | −79.319 | 7 | GLN 161, ARG 64(2), GLY 122(2), SER 209, HIS 121 | ||
| Tyramine | −3.441 | −30.392 | 2 | SER 120, ARG 64 | ||
| Vitexin | −6.669 | −60.466 | 3 | SER 205, ARG 207, PHE 250 | ||
| 6 | Caspase 7 1F1J |
Epicatechin | −6.224 | −42.725 | 4 | ARG 233 (2), HIS 144 (2) |
| Epicatechin Gallate | −9.240 | −77.358 | 7 | ARG 237, GLN 276 (2), SER 231 (2), ASP 278 (2) | ||
| Tyramine | −3.595 | −23.036 | 1 | ARG 233 | ||
| Vitexin | −6.953 | −53.204 | 4 | ARG 233 (2), GLN 184, SER 143 | ||
| 7 | Endothelial protein C receptor 1L8J |
Epicatechin | −10.619 | −48.761 | 1 | LEU 14 |
| Epicatechin Gallate | −6.868 | −70.101 | 4 | THR 65, ARG 156, GLU 160(2) | ||
| Tyramine | −5.450 | −27.946 | 1 | GLN 75 | ||
| Vitexin | −7.547 | −57.292 | 6 | GLU 160(3), GLN 75(2), THR 65 | ||
| 8 | Glyceraldehyde-3-phosphate dehydrogenase 4WNC |
Epicatechin | −6.473 | −52.557 | 5 | ARG 13, GLY 15, ASN 316, SER 98, SER 122 |
| Epicatechin Gallate | −9.694 | −73.633 | 7 | SER 98(2), ILE 14, ARG 13, ASN 316(3) | ||
| Tyramine | −6.170 | −28.618 | 3 | ARG 80, ASP 35(2) | ||
| Vitexin | −6.982 | −54.343 | 4 | ILE 14, SER 98, SER 122, CYS 152 | ||
| 9 | HMG-CoA reductase 2Q1L |
Epicatechin | −4.439 | −33.381 | 2 | GLU 559, ALA 856 |
| Epicatechin Gallate | −6.102 | −56.481 | 3 | GLU 559, ALA 856(2) | ||
| Tyramine | −2.669 | −23.079 | 1 | GLU 559 | ||
| Vitexin | −4.528 | −44.497 | 3 | GLU 559, HIS 861, ALA 751 | ||
| 10 | Interleukin-6 1ALU |
Epicatechin | −4.848 | −40.467 | 4 | ASP 160(2), GLU 42, LYS 46 |
| Epicatechin Gallate | −7.985 | −67.762 | 7 | THR 43(2), GLU 42, ARG 104, GLU 106(2), SER 107 | ||
| Tyramine | −4.786 | −37.603 | 4 | ASP 160, THR 43, GLU 106, SER 107 | ||
| Vitexin | −5.768 | −50.690 | 3 | ARG 104, GLU 106, SER 107 | ||
| 11 | Thrombospondin-1 1UX6 |
Epicatechin | −4.725 | −50.048 | 3 | ASP 983, GLU 821, TYR 982 |
| Epicatechin Gallate | −7.554 | −75.101 | 4 | GLU 984, GLU 821(2), ARG 833 | ||
| Tyramine | −5.891 | −38.252 | 3 | ASP 933, ARG 833, TYR 982 | ||
| Vitexin | −7.091 | −66.164 | 4 | ASN 863, GLN 864, TYR 982, GLU 821 | ||
| 12 | Tumour necrosis factor-α 1A8M |
Epicatechin | −5.922 | −49.030 | 6 | LEU 26, ILE 136, ALA 134, ASN 46(2), TRP 28 |
| Epicatechin Gallate | −7.272 | −68.147 | 7 | ASP 45(2), GLN 47, ASN 46, ALA 134(2), TRP 28 | ||
| Tyramine | −5.461 | −27.065 | 4 | GLU 135, TRP 28, ASN 46, ALA 134 | ||
| Vitexin | −8.644 | −58.615 | 8 | ASP 45(2), GLN 47(2), GLU 135, ASN 46, ALA 134, TRP 28 | ||
| 13 | NF-κB | Epicatechin | −3.907 | −41.935 | 4 | GLY 63, VAL 60, CYS 118(2) |
| Epicatechin Gallate | −5.752 | −54.606 | 7 | ASN 138(2), LYS 79, ARG 56(2), GLY 54, LYS 51 | ||
| Tyramine | −0.594 | −15.102 | 2 | VAL 60, ILE 141 | ||
| Vitexin | −4.648 | −38.428 | 4 | LYS 116, ASN 138, GLY 67, SER 65 | ||
| 14 | Caspase 8 | Epicatechin | −5.667 | −43.948 | 2 | PHE 468, GLU 396 |
| Epicatechin Gallate | −7.337 | −59.747 | 3 | SER 386, PHE 468, THR 337 | ||
| Tyramine | −3.733 | −21.598 | 2 | THR 467, GLU 396 | ||
| Vitexin | −5.960 | −46.899 | 3 | THR 337, GLU 396, SER 386 | ||
| 15 | Caspase 9 | Epicatechin | −4.631 | −37.191 | 6 | LYS 292 (2), GLU 290 (2), HIS 237, GLY 238 |
| Epicatechin Gallate | −4.973 | −60.871 | 6 | GLU 290 (4), GLY 238, ARG 178 | ||
| Tyramine | −3.450 | −20.486 | 2 | GLU 290, HIS237 | ||
| Vitexin | −5.056 | −41.965 | 5 | SER 175, GLY 238, GLY 289, GLU 290 (2) | ||
| 16 | High mobility group protein B1 | Epicatechin | −5.224 | −50.457 | 5 | PHE 89, PRO 95, TYR 155, LYS 88, ARG 97 |
| Epicatechin Gallate | −6.645 | −77.457 | 5 | ASP 91, LYS 90, ARG 97(2), PRO 98 | ||
| Tyramine | −3.259 | −27.574 | 3 | LYS 88, PHE 89, ASP 91 | ||
| Vitexin | −5.678 | −57.434 | 8 | LYS 86, PHE 89, ASP 91(2), LYS 88(2), ALA 94, ARG 97 | ||
| 17 | Angiotensin-converting enzyme 2 1R42 |
Epicatechin | −5.287 | −47.755 | 2 | ASN 290, GLU 406 |
| Epicatechin Gallate | −8.109 | −60.427 | 5 | ALA 413, ASN 290(2), THR 445, LYS 441 | ||
| Tyramine | −6.612 | −28.031 | 2 | GLU 435, ILE 291 | ||
| Vitexin | −5.898 | −52.820 | 3 | GLN 442, GLU 406, ASP 367 | ||
| 18 | Apoptosis Regulator BCL2 2XA0 |
Epicatechin | −4.938 | −44.109 | 2 | LYS 441- O, ASP 367- H |
| Epicatechin Gallate | −7.912 | −58.684 | 3 | ALA 149, GLU 136, ARG 139 | ||
| Tyramine | −4.462 | −25.523 | 1 | ALA 149 | ||
| Vitexin | −6.190 | −52.611 | 3 | GLN 118, TYR 108, GLU 136 | ||
| 19 | Apoptotic protease-activating factor 1 3JBT |
Epicatechin | −7.005 | −57.616 | 3 | SER 161, VAL 125, VAL 127 |
| Epicatechin Gallate | −10.282 | −82.899 | 3 | GLY 159, VAL 127(2) | ||
| Tyramine | −3.833 | −28.842 | 3 | ASP 244, ASP 360, LYS 160 | ||
| Vitexin | −7.684 | −68.641 | 3 | GLY 159(2), VAL 125 | ||
| 20 | Bcl-2-like protein 1 1R2D |
Epicatechin | −4.685 | −38.786 | 3 | ASP 156(2), SER 145 |
| Epicatechin Gallate | −5.983 | −54.973 | 7 | ARG 102, ALA 104, TYR 101, SER 145, SER 106, GLU 153(2) | ||
| Tyramine | −2.719 | −32.462 | 2 | GLU 153, ASP 156 | ||
| Vitexin | −6.079 | −46.170 | 3 | GLU 98, TYR 101, LYS 20 | ||
| 21 | Bcl-2-like protein 10 4B4S |
Epicatechin | −5.392 | −47.508 | 5 | ARG 99, ARG 21(2), GLY 24, TYR 18 |
| Epicatechin Gallate | −5.472 | −64.725 | 7 | ARG 46, GLU 98(2), ARG 21(2), GLU 27(2) | ||
| Tyramine | −4.406 | −31.696 | 3 | ARG 99, SER 19, GLU 98 | ||
| Vitexin | −5.713 | −51.034 | 5 | LEU 102, ARG 99, GLU 98, TYR 18, ALA 43 | ||
| 22 | Caspase 1 3E4C |
Epicatechin | −5.524 | −46.567 | 3 | HIS 342, GLY 346, ASP 381 |
| Epicatechin Gallate | −10.130 | −74.127 | 6 | ASP 381(2), VAL 348, GLY 346, THR 180(2) | ||
| Tyramine | −6.557 | −31.561 | 0 | −- | ||
| Vitexin | −5.730 | −46.275 | 2 | ALA 285, ASP 336 | ||
| 23 | Caspase 2 1PYO |
Epicatechin | −6.028 | −41.938 | 3 | ALA 229, ARG 231, TYR 273 |
| Epicatechin Gallate | −5.667 | −53.648 | 3 | ALA 229(2), THR 233 | ||
| Tyramine | −3.137 | −20.541 | 2 | ASN 232, ARG 231 | ||
| Vitexin | −5.438 | −47.447 | 4 | THR 233(2), ASN 232(2) | ||
| 24 | Caspase 6 2WDP |
Epicatechin | −6.083 | −34.033 | 3 | CYS 163, VAL 212, ALA 213 |
| Epicatechin Gallate | −6.123 | −52.203 | 3 | GLU 214, ALA 129, LYS 133 | ||
| Tyramine | −4.826 | −27.056 | 2 | CYS 163, ALA 213 | ||
| Vitexin | −7.010 | −51.889 | 4 | GLU 214(2), CYS 163, LYS 133 | ||
| 25 | Glyceraldehyde-3-phosphate dehydrogenase, testis-specific 3H9E |
Epicatechin | −7.162 | −55.401 | 5 | SER 169, GLY 87, ARG 85, ASN 388, SER 193 |
| Epicatechin Gallate | −9.626 | −74.353 | 6 | GLY 284, SER 169, ARG 85, ASN 388(3) | ||
| Tyramine | −4.062 | −21.903 | 3 | GLU 389, PRO 310, THR 254 | ||
| Vitexin | −6.456 | −51.840 | 3 | SER 193, ASN 388(2) | ||
| 26 | Interleukin-6 receptor subunit alpha 1N26 |
Epicatechin | −5.102 | −44.978 | 3 | CYS 146, ALA 127, PRO 121 |
| Epicatechin Gallate | −6.267 | −61.546 | 3 | GLU 10, ALA 127, CYS 146 | ||
| Tyramine | −3.130 | −25.246 | 3 | THR 125, PRO 121, LYS 126 | ||
| Vitexin | −6.227 | −61.559 | 5 | CYS 146, ALA 127, PRO 121, TYR 148(2) |
Molecular dynamics simulation
The molecular dynamic simulation was done for caspase 3-ECG complex. The protein-ligand RMSD (root mean square deviation) plot is provided in Figure 4A and the results showed that caspase 3-ECG complex achieved stable kinetics from 38 to 50 ns. The histogram chart and 2D interaction poses are also included here as Figure 4B&C.
Figure 4:
4A sows the stability of protein under the given environment and interaction of ECG with protein. 4B Histogram and percentage of interaction in molecular dynamics simulation of Caspase-3- ECG. 4C A schematic of detailed ligand atom interactions with the protein residues. Interactions that occur more than 30.0% of the simulation time in the selected trajectory.
In vivo experiments
Evaluation of Infarction Size
TTC staining revealed the quantity of MI created in the control and experimental groups. Figure 5 shows the amount of left ventricular area at risk (AAR) in control and experimental groups. The amount of infarction is substantially increased in induced (Ischemia/reperfusion (I/R)) group in comparison to control. However, treatment with COC reduced AAR in the treatment group significantly (mean±SD; n = 3; *P<0.05).
Figure 5:
Effect of COC extract administration on infarct size. Irreversible infarction was determined by staining the heart ventricular sections with 1% TTC. Representative photomicrographs of the infarct area of (a) control, (b) I/R and (c) COC extract-treated hearts, showing infarct (white zones) and non-infarct (red zones) after TTC staining. (d) Percentage of infarct area from the left-ventricular area of the sections determined using MetaMorph software. Values are expressed as mean±S.D. (n=6). The COC extract-treated hearts show a significant reduction in infarction size ($p<0.05) compared to I/R group. I/R heart shows an increase in the infarction compared to control.
Western blotting analysis of caspase 3
Pro caspase 3, 32kDa inactive form was down-regulated significantly (P<0.05) in I/R induced group in comparison to control. Simultaneously, the 17 kDa cleaved form was up-regulated significantly (P<0.05) in I/R induced group in comparison to control. Upon treatment with COC extract, the up-regulated 17 kDa cleaved form in I/R group was significantly (P<0.05) down-regulated in COC treated animals without any change in the overall expression and is shown in Figure 6.
Figure 6: Western blot analysis of caspase 3.
Level pro caspase 3 ((a) 32 kDa) was significantly downregulated (p<0.05) in I/R group (Group II) compared to control (Group I), and simultaneously, the levels of cleaved caspase-3 ((a) 17 kDa) was upregulated (p<0.05) in I/R group (Group II). Upon treatment with COC extract, these levels were brought back to near-normal levels. (b) Values are expressed as mean±S.D. (n=3). (c)) shows levels of β-actin, served as control. Values are expressed as mean±S.D. (n=3). Values are significant at $ - p < 0.05; a - when compared to Group I; b - when compared to Group II. (d) Values are expressed as mean±S.D. (n=3).
Discussion
Earlier studies from our laboratory have reported the presence of several secondary metabolites and remedial significance of COC extract (Dasagrandhi et al., 2018; Elango et al., 2009; Jayachandran et al., 2010; Swaminathan et al., 2010; Thiruchenduran et al., 2011). COC extract was reported for its potent antioxidant property which is beneficial against CVD and Ischemia/reperfusion (I/R) injury associated with MI (Afsheen et al., 2018; Ranjbar et al, 2018). Since I/R injury mediated MI involves multiple molecular signalling pathways (Cadenas, 2018), the investigation was started with selecting the most important signalling pathways involved in the CVD-I/R-MI axis and was listed in Table 1. Figure 2 shows the output of the product by GeneMANIA, an online tool to investigate the network hub link between the above-mentioned axis and the signalling pathways that were integrated from several databases (https://genemania.org). The details of the secondary metabolites present in the COC extract were directly selected from the literature (Svedström et al., 2006; Swaminathan et al., 2010), and its structures were obtained from the PubChem database (Figure 1). ADME predictions provided the details of the suitability and biological likeliness of any given compound to act as a drug. Out of the 5 compounds analysed, Rutin was eliminated, as it violated the Lipinski’s rule of five and other salient descriptors as mentioned in Table 2. Furthermore, as per the respective active site details of 26 proteins, the COC extract active compounds were docked (Table 3). Based on all this information, the following mechanism is proposed and is supported with additional results.
ECG - a potential secondary metabolite that prevents caspase 3 activation.
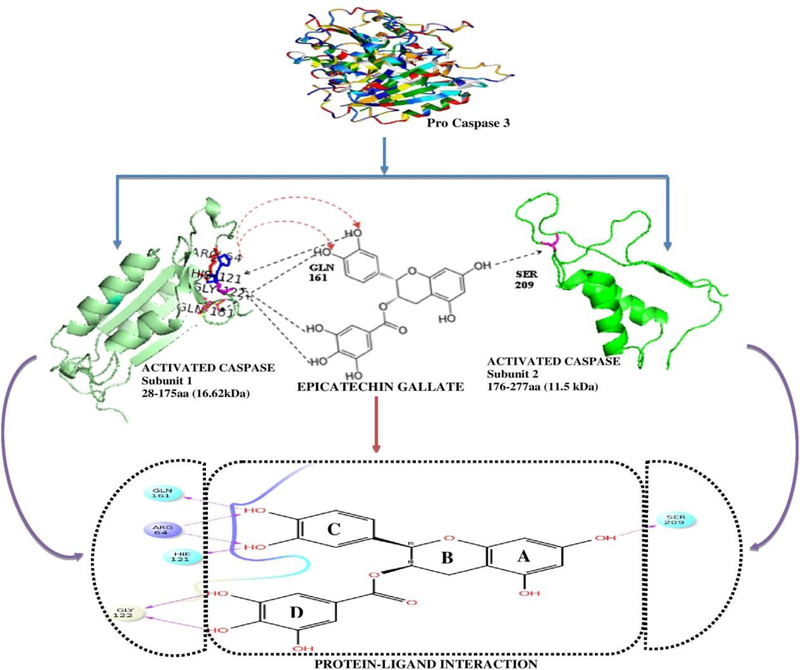
The key focus of the current study is to explain the mechanism of action of active compound in COC extract with the help of integrated computational and experimental approaches. Pro caspase 3 is a 32 kDa inactive form of protein (Brentnall et al., 2013; Cepero et al., 2005). The primary structure revealed that it has 277 amino acids, where the first 28 amino acids of the Pro caspase 3 will be removed during activation. Amino acid position 29 to 175 forms the 17 kDa unit and from 176 to 277 amino acids forms 12 kDa unit (Brentnall et al., 2013). The molecular weight calculator (https://www.bioinformatics.org/sms/prot_mw.html) also shows the molecular weight of cleaved forms which is 28–175aa with 16.62 kDa and 176–277 amino acids are 11.5 kDa. Also, pro caspase 3 after activation gets converted to 17 kDa and 12 kDa fragments inside the cells and then it executes its biological function. But it should be noted that only free 17 kDa and 12 kDa forms can execute its function, not a bound integrated form. From the docking analysis, it was found that caspase 3 may gain hydrogen bond interactions with ECG at GLN 161, ARG 64, GLY 122, HIS 121 and SER 209 as a donor or an acceptor (Figure 7). The 32 kDa form of Pro caspase 3 must be cleaved into 17kDa and 12kDa forms to exhibit its biological function while ECG acts like a chelator by connecting two cleaved forms through hydrogen bond interactions.
Figure 7:
2D Ligplot image of docked Caspase 3- Epicatechin Gallate complex.
To provide a plausible explanation, we modelled 17 kDa and 12 kDa fragments to its 3D structure and the interacting bonds were visualized with ECG based on the docking results. After cleavage, SER 209 of 12 kDa unit binds with ECG on the A ring and all six hydrogen bonds were attached with 17 kDa form on the C and D rings respectively, which is represented in Figure 8. Ring C of ECG increased the number of bonded interactions with 17 kDa unit using GLN 161, ARG 64(2), HIS 121. Ring D of ECG forms two hydrogen bonds with 17kDa using GLY 122. This observation was similar to the findings of docking studies, where ECG was reported to hold six bonded interactions in the 17 kDa region (Rings C and D) and one interaction with 12 kDa region (Ring A) that may not allow caspase 3 to become free to execute its biological function. To confirm the above, we further performed molecular dynamics to check the stability of the above mentioned ligand-protein complex.
Figure 8:
Pictorial representation of efficiency of Epicatechin Gallate in anchoring the two subunits of pro caspase 3, which becomes freed upon activation. The upper part showed the schematic representation with the modelled structure of two active subunits of proteins with ECG and the lower part shows the results as in docking analysis. This binding may prevent the cleavage of caspase 3.
Molecular dynamics are in silico based studies that are used to check the stability of a protein-ligand binding efficiency in a controlled simulated kinetic environment (Cui et al., 2015). Results from molecular dynamics revealed that the complex is not bound with protein at the initial stages, it starts achieving the stability of the projectile from 38th ns till 50 ns as shown in Figure 4. This shows that the ECG is capable of preventing the activation of caspase 3 by making a strong interaction/attachment with cleaved forms. Previously, studies from our lab presented the protective role of ethanolic extract of COC against apoptosis in I/R mediated MI using animal experiments (Jayachandran et al., 2010). But the mechanism of action of the active compound - ECG at the individual component level of the extract was not ascertained. Considering the strong in silico results of ECG and its involvement in the COC extract against caspase 3, this work was further extended to confirm pro caspase 3 expressions and its activation by western blotting in an invivo experimental model.
Since activation of pro caspase 3 is central to both the intrinsic and extrinsic mediated apoptotic pathways of the I/R mediated MI or CVD axis (Porter & Jänicke, 1999), it was decided to ascertain our hypothesis with caspase 3 as the target protein. Caspases are the family of proteins mainly involved in programmed cell death, inflammation and fetal development (Busso et al., 2010; McIlwain et al., 2013). Caspases, also known as Interleukin converting enzymes that have the specificity of cleaving in-between cysteine and aspartic acid residue (Julien & Wells, 2017). Activation of apoptosis requires the formation of the apoptosome complex, which contains Cytochrome C and Apaf-1 that bonds with ATP (Zhou et al., 2015). Cytochrome C is a classical marker for the initiation of apoptosis, and its release from the mitochondria causes severe damage to the cell (Tait & Green, 2013). As soon as the apoptosome complex is formed, it will recruit the initiator caspase, pro caspase 9 and activates it. The activated caspase 9 then will activate the execution caspases, most importantly caspase 3 (Brentnall et al., 2013). Furthermore, among all the caspases, caspase 3 is highly vulnerable and inhibition of caspase 3 is proved to protect the cell against apoptosis (McComb et al., 2019; Nicholson et al., 1995). Additionally, the present computational study revealed a very good docking glide score for caspase 3 against ECG and therefore decided to study its mechanism of action of inhibition against caspase 3 in a detailed way.
In order to investigate the above hypothesis, experimental MI was created in the rat model and treated for 28 days post-operative with COC extract. The beneficial effects of COC extract were published previously (Jayachandran et al., 2010; Swaminathan et al., 2010) and in the current study, TTC staining was done to confirm the presence of infarction in control and experimental animals. Triphenyl tetrazolium chloride (TTC) can enter only in the living mitochondria. Hence it will stain the live-cell cells in red colour and leave the dead cell in white colour and it is called as AAR. Increased AAR was observed in MI induced group compared to control and the AAR was reduced in the treatment group compared to the MI induced group, which shows the effect of COC extract in the reduction of infarction (Figure 5).
Western blot results from in vivo experiments confirm that there is no significant alteration in the expression profile of pro caspase 3 in the control and experimental group (Figure 6), whereas the activation in conditions like Post-MI/during MI is inhibited by the COC extract. Down regulation of pro caspase 3 (32 kDa) in the I/R group represents the activation of caspase in the untreated MI group, while COC administration displayed significant down regulation of cleaved caspase 3 (17 kDa) that strongly suggest the prevention of apoptosis by COC extract. Accumulated evidence suggests that the ECG is capable of protecting the cells from different types of apoptosis. Recently it was reported that ECG protects human brain micro vascular endothelial cells from ischemia/ reperfusion-induced injury (Malik et al., 2016) and attenuation of cisplatin-induced kidney injury by ECG (Fu et al., 2019). Mostly the mechanism of action of the drug via signalling pathways was analysed in these reports and there is no explanation of how the drug interacts at the individual protein level. However, in our study altered levels of the cleaved form of caspases are evident in apoptosis and found only in I/R mediated MI-induced group. Nevertheless, their activation is prevented only in the treatment group. These experimental results strongly support the computational evidences as hypothesized on preventing the activation of caspase 3 via ECG.
Conclusion
Secondary metabolites from many plant materials are now attracting medical attention by many research groups. But understanding the mechanism of action is a challenging task. In the present study, we hypothesized and proposed the possible mechanism of action of one of the powerful secondary metabolite - Epicatechin Gallate, present in COC extract with significant medicinal properties through computational and experimental studies against I/R mediated MI axis. Further, the information obtained from this study is believed to aid as motivation for future rational designing of new caspase 3 inhibitors with improved selectivity against MI.
Figure 3I-B:
Final 3D structure of CASP 8 predicted through Swiss model with 4JJ7 as template. The Ramachandran plot assessment of the predicted structure showed 192 (85.7%) residues in the favoured region, 31 (13.8%) residues in the additionally allowed region, 1 (0.4%) residues in the generously allowed region and 0 (0.0%) residues in the disallowed region.
Figure 3I-C:
Final 3D structure of CASP 9 predicted through Swiss model with 1JXQ as template. The Ramachandran plot assessment of the predicted structure showed 425 (91.4%) residues in the favoured region, 36 (7.7%) residues in the additionally allowed region, 3 (0.6%) residues in the generously allowed region and 1 (0.2%) residues in the disallowed region.
Acknowledgement
We acknowledge the financial support from [NIH] under grant [number EB006153]; [DST-SERB] under grant [number SB/EMEQ-175/2014] and [DST-PURSE] under grant [number SR/FT/LS-113/2009]
Abbreviations:
- AAR
Area At Risk
- ADME
Absorption, Distribution, Metabolism, Excretion
- BLAST
Basic Local Alignment Search Tool
- COC
Crataegus oxycantha
- CVD
Cardiovascular Disease
- ECG
Epicatechin Gallate
- I/R
Ischemia / Reperfusion
- MI
Myocardial Infarction
- NPT
amount of substance (N), pressure (P) and temperature (T)
- OPC’s
Oligomericproanthocyanidins
- OPLS
Optimized Potentials for Liquid Simulations
- PDB
Protein Data Bank
- PPIN
Protein-protein interaction network
- RMSD
Root Mean Square Deviation
- SAVES
Structure Analysis and VErification Server
- SBDD
structure-based drug designing
- TTC
TriphenylTetrazolium Chloride
- WHO
World Health Organization
Footnotes
Author Declarations:
We hereby declare that there is no conflict of interest among the authors.
References
- Abagyan R, & Totrov M (2001). High-throughput docking for lead generation. Current Opinion in Chemical Biology, 5(4), 375–382. doi: 10.1016/S1367-5931(00)00217-9 [DOI] [PubMed] [Google Scholar]
- Afsheen N, Khalil-ur-Rehman, Jahan N, Ijaz M, Manzoor A, Khan KM (2018). Cardioprotective and Metabolomic Profiling of Selected Medicinal Plants against Oxidative Stress. Oxidative Medicine and Cellular Longevity, 2018, 17. doi: 10.1155/2018/9819360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajay, & Murcko MA (1995). Computational Methods to Predict Binding Free Energy in Ligand-Receptor Complexes. Journal of Medicinal Chemistry, 38(26), 4953–4967. doi: 10.1021/jm00026a001 [DOI] [PubMed] [Google Scholar]
- Brentnall M, Rodriguez-Menocal L, De Guevara RL, Cepero E, & Boise LH (2013). Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC cell biology, 14, 32–32. doi: 10.1186/1471-2121-14-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busso D, Domínguez C, Pérez-Acle T, & Moreno RD (2010). Life-giving caspases: revealing new roles during mouse embryo preimplantation development. The International journal of developmental biology, 54 5, 857–865 [DOI] [PubMed] [Google Scholar]
- Cadenas S (2018). ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radical Biology and Medicine, 117, 76–89. doi: 10.1016/j.freeradbiomed.2018.01.024 [DOI] [PubMed] [Google Scholar]
- Cepero E, King AM, Coffey LM, Perez RG, & Boise LH (2005). Caspase-9 and effector caspases have sequential and distinct effects on mitochondria. Oncogene, 24(42), 6354–6366. doi: 10.1038/sj.onc.1208793 [DOI] [PubMed] [Google Scholar]
- Cui F, Yang K, & Li Y (2015). Investigate the binding of catechins to trypsin using docking and molecular dynamics simulation. PloS one, 10(5), e0125848–e0125848. doi: 10.1371/journal.pone.0125848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasagrandhi D, R ASK, Muthuswamy A, Lennox AM, Jayavelu T, Devanathan V (2018). Ischemia/reperfusion injury in male guinea pigs: An efficient model to investigate myocardial damage in cardiovascular complications. Biomedicine & Pharmacotherapy, 99, 469–479. doi: 10.1016/j.biopha.2018.01.087 [DOI] [PubMed] [Google Scholar]
- Elango C, Jayachandaran KS, & Niranjali Devaraj S (2009). Hawthorn extract reduces infarct volume and improves neurological score by reducing oxidative stress in rat brain following middle cerebral artery occlusion. International Journal of Developmental Neuroscience, 27(8), 799–803. doi: 10.1016/j.ijdevneu.2009.08.008 [DOI] [PubMed] [Google Scholar]
- Fu B, Zeng Q, Zhang Z, Qian M, Chen J, Dong W (2019). Epicatechin Gallate Protects HBMVECs from Ischemia/Reperfusion Injury through Ameliorating Apoptosis and Autophagy and Promoting Neovascularization. Oxidative Medicine and Cellular Longevity, 2019, 7824684–7824684. doi: 10.1155/2019/7824684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://genemania.org.). Retrieved from https://genemania.org
- https://www.bioinformatics.org/sms/prot_mw.html.
- Jayachandran KS, Khan M, Selvendiran K, Devaraj SN, & Kuppusamy P (2010). Crataegus oxycantha Extract Attenuates Apoptotic Incidence in Myocardial Ischemia-Reperfusion Injury by Regulating Akt and Hif-1 Signaling Pathways. Journal of Cardiovascular Pharmacology, 56(5), 526–531. doi: 10.1097/FJC.0b013e3181f64c51 [DOI] [PubMed] [Google Scholar]
- Julien O, & Wells JA (2017). Caspases and their substrates. Cell death and differentiation, 24(8), 1380–1389. doi: 10.1038/cdd.2017.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikeyan M, Kirubakaran P, Singh KD, Sampath B, & Krishnasamy G (2014). Understanding the evolutionary relationship of hemagglutinin protein from influenza viruses using phylogenetic and molecular modeling studies. Journal of Biomolecular Structure and Dynamics, 32(5), 816–830. doi: 10.1080/07391102.2013.793211 [DOI] [PubMed] [Google Scholar]
- Kumar D, Arya V, Bhat ZA, Khan NA, & Prasad DN (2012). The genus Crataegus: chemical and pharmacological perspectives. Revista Brasileira de Farmacognosia, 22, 1187–1200 [Google Scholar]
- Lengauer T, & Rarey M (1996). Computational methods for biomolecular docking. Current Opinion in Structural Biology, 6(3), 402–406. doi: 10.1016/S0959-440X(96)80061-3 [DOI] [PubMed] [Google Scholar]
- Mach F, Ray KK, Wiklund O, Corsini A, Catapano AL, Bruckert E (2018). Adverse effects of statin therapy: perception vs. the evidence - focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract. European Heart Journal, 39(27), 2526–2539. doi: 10.1093/eurheartj/ehy182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S, Suchal K, Bhatia J, Gamad N, Dinda AK, Gupta YK (2016). Molecular mechanisms underlying attenuation of cisplatin-induced acute kidney injury by epicatechin gallate. Laboratory Investigation, 96(8), 853–861. doi: 10.1038/labinvest.2016.60 [DOI] [PubMed] [Google Scholar]
- McComb S, Chan PK, Guinot A, Hartmannsdottir H, Jenni S, Dobay MP (2019). Efficient apoptosis requires feedback amplification of upstream apoptotic signals by effector caspase-3 or -7. Science advances, 5(7), eaau9433–eaau9433. doi: 10.1126/sciadv.aau9433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain DR, Berger T, & Mak TW (2013). Caspase functions in cell death and disease. Cold Spring Harbor perspectives in biology, 5(4), a008656–a008656. doi: 10.1101/cshperspect.a008656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore N, Pollack C, & Butkerait P (2015). Adverse drug reactions and drug-drug interactions with over-the-counter NSAIDs. Therapeutics and clinical risk management, 11, 1061–1075. doi: 10.2147/tcrm.s79135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M (1995). Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature, 376(6535), 37–43. doi: 10.1038/376037a0 [DOI] [PubMed] [Google Scholar]
- Porter AG, & Jänicke RU (1999). Emerging roles of caspase-3 in apoptosis. Cell Death & Differentiation, 6(2), 99–104. doi: 10.1038/sj.cdd.4400476 [DOI] [PubMed] [Google Scholar]
- Prabhakaran D, Jeemon P, & Roy A (2016). Cardiovascular Diseases in India. Circulation, 133(16), 1605–1620. doi: 10.1161/circulationaha.114.008729 [DOI] [PubMed] [Google Scholar]
- Ranjbar K, Zarrinkalam E, Salehi I, Komaki A, & Fayazi B (2018). Cardioprotective effect of resistance training and Crataegus oxyacantha extract on ischemia reperfusion–induced oxidative stress in diabetic rats. Biomedicine & Pharmacotherapy, 100, 455–460. doi: 10.1016/j.biopha.2018.02.021 [DOI] [PubMed] [Google Scholar]
- Svedström U, Vuorela H, Kostiainen R, Laakso I, & Hiltunen R (2006). Fractionation of polyphenols in hawthorn into polymeric procyanidins, phenolic acids and flavonoids prior to high-performance liquid chromatographic analysis. Journal of Chromatography A, 1112(1), 103–111. doi: 10.1016/j.chroma.2005.12.080 [DOI] [PubMed] [Google Scholar]
- Swaminathan JK, Khan M, Mohan IK, Selvendiran K, Niranjali Devaraj S, Rivera BK (2010). Cardioprotective properties of Crataegus oxycantha extract against ischemia-reperfusion injury. Phytomedicine, 17(10), 744–752. doi: 10.1016/j.phymed.2010.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait SWG, & Green DR (2013). Mitochondrial regulation of cell death. Cold Spring Harbor perspectives in biology, 5(9), a008706. doi: 10.1101/cshperspect.a008706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruchenduran M, Vijayan NA, Sawaminathan JK, & Devaraj SN (2011). Protective effect of grape seed proanthocyanidins against cholesterol cholic acid diet-induced hypercholesterolemia in rats. Cardiovascular Pathology, 20(6), 361–368. doi: 10.1016/j.carpath.2010.09.002 [DOI] [PubMed] [Google Scholar]
- Vostinaru O (2017). Adverse Effects and Drug Interactions of the Non-Steroidal Anti-Inflammatory Drugs.
- Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P (2010). The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Research, 38(suppl_2), W214–W220. doi: 10.1093/nar/gkq537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. (2017). Cardiovascular diseases (CVDs). Retrieved from https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- Zhou M, Li Y, Hu Q, Bai X-C, Huang W, Yan C (2015). Atomic structure of the apoptosome: mechanism of cytochrome c- and dATP-mediated activation of Apaf-1. Genes & development, 29(22), 2349–2361. doi: 10.1101/gad.272278.115 [DOI] [PMC free article] [PubMed] [Google Scholar]