Abstract
Glycosylation is one of the most significant and abundant posttranslational modifications in mammalian cells. It mediates a wide range of biofunctions, including cell adhesion, cell communication, immune cell trafficking, and protein stability. Also, aberrant glycosylation has been associated with various diseases such as diabetes, Alzheimer’s disease, inflammation, immune deficiencies, congenital disorders, and cancers. The alterations in the distributions of glycan and glycopeptide isomers are involved in the development and progression of several human diseases. However, the microheterogeneity of glycosylation brings a great challenge to glycomic and glycoproteomic analysis, including the characterization of isomers. Over several decades, different methods and approaches have been developed to facilitate the characterization of glycan and glycopeptide isomers. Mass spectrometry (MS) has been a powerful tool utilized for glycomic and glycoproteomic isomeric analysis due to its high sensitivity and rich structural information using different fragmentation techniques. However, a comprehensive characterization of glycan and glycopeptide isomers remains a challenge when utilizing MS alone. Therefore, various separation methods, including liquid chromatography, capillary electrophoresis, and ion mobility, were developed to resolve glycan and glycopeptide isomers before MS. These separation techniques were coupled to MS for a better identification and quantitation of glycan and glycopeptide isomers. Additionally, bioinformatic tools are essential for the automated processing of glycan and glycopeptide isomeric data to facilitate isomeric studies in biological cohorts. Here in this review, we discuss commonly employed MS-based techniques, separation hyphenated MS methods, and software, facilitating the separation, identification, and quantitation of glycan and glycopeptide isomers.
Keywords: Glycan isomers, Glycopeptide isomers, MS-based isomeric characterization
1. Introduction
As glycosylation is one of the most common posttranslational modifications of proteins, glycosylated proteins -known as glycoproteins- represent more than 50% of total mammalian proteins (Apweiler et al. 1999, Hart and Copeland 2010). Due to their abundance, it is apparent that glycoproteins play a wide range of essential roles in biological functions, including cell adhesion (Phillips et al. 1990, Yoshida-Moriguchi et al. 2010), cell-to-cell recognition (Ohtsubo and Marth 2006), immune cell trafficking (Sperandio et al. 2009), protein solubility and stability (Sola and Griebenow 2009, Ardejani et al. 2021). Glycan moieties on the cell surface regularly interact with other glycans or receptor binding domains specific to the glycan structures (Taylor and Drickamer 2014). Furthermore, the aberrant glycosylation of proteins has been linked to various diseases, including cancers (Mechref et al. 2012, Guo and Abbott 2015, Mehta et al. 2015, Pan et al. 2016, Wooding et al. 2016, Kailemia et al. 2017), immune deficiencies (Back et al. 1994), Alzheimer’s disease (Cho et al. 2019), diabetes (Bermingham et al. 2018, Everest-Dass et al. 2018), inflammation (Chandrasekaran and Lacy 2017), congenital disorders (Freeze et al. 2014, Van Scherpenzeel et al. 2016) and bacterial/viral infections (Blomme et al. 2009, Vigerust 2011, Orlova et al. 2015).
Glycosylation of proteins can be categorized into two types, N- and O- glycosylation. N-Linked glycosylation only occurs on the asparagine within the specific amino acid motif of Asp-X-Ser/Thr, where X is any amino acid except for proline. However, its counterpart, O-linked glycosylation, does not require a specific amino acid motif. O-glycans can be attached to any serine or threonine. These glycans contain vast amounts of complex stereochemical information and glycan structures that reside on proteins are often convoluted for many reasons. Glycans are comprised of various monosaccharides linked by glycosidic bonds with multiple linkage sites, yielding α and β anomeric linkages between the monosaccharides (Mariño et al. 2010). These linkages also generate numerous branching of terminal glycan residues such as sialic acids. Furthermore, functional groups on the monosaccharides can be altered to sulfate or another functional group (Veillon et al. 2017).
This complex nature of glycans is also reflected in isomeric glycans, which have the same monosaccharide composition but differ in monosaccharide linkages and monosaccharide positions. One example of glycan isomers is fucose positional isomers, where one isomer can reside on the core of the glycan. In contrast, other isomers can have fucose on any of the branches associated with a glycan structure. Another example of glycan isomers is terminal sialic acid linkage isomers. Terminal sialic acids can be either α2,3 or α2,6 linked with the galactose. The importance of these glycan isomers in several types of cancers has been previously investigated, highlighting the role of glycan isomers in the development and progression of the disease (Huang et al. 2017, Peng et al. 2019). Peng et al. have examined N-glycan isomer expressions from different breast cancer cell lines and found an increased prevalence of α2,6 sialic acid linked N-glycans in the 231BR cell line being known for brain metastasis (Peng et al. 2019). Furthermore, Chung et al. discovered the hypersensitivity in patients to cancer drug Cetuximab was caused by interaction between the preexisting immunoglobulin E (IgE) antibody and galactose-α1,3-galactose on the Cetuximab’s heavy chain (Chung et al. 2008). On top of this glycan complexity, profiling of glycopeptides is even more complicated due to the microheterogeneity in which many different glycoforms, including isomers, are linked to specific glycosylation sites (Hua et al. 2012, Yu et al. 2018).
Mass spectrometry (MS) has been the analytical tool of choice to study protein glycosylation because of its sensitivity and ability to elucidate structural information of glycans and glycopeptides using tandem MS or MSn, including a variety of fragmentation techniques such as collision, electron, or photo-induced fragmentations (Dong et al. 2018). Although chemical derivatization techniques permit the analysis of glycan and glycopeptide isomers (Reiding et al. 2014), MS alone is often insufficient to characterize glycan and glycopeptide isomers fully. Therefore, MS in conjunction with chromatographic tools is frequently utilized to study glycan and glycopeptide isomers; however, this is only limited to terminal sialic acid isomers. Other glycan isomers such as fucosylated glycan isomers or bisecting glycan isomers are not readily characterized by MS. Although MSn permits the characterization of glycan and glycopeptide isomers, the sensitivity of this approach is not adequate for biological samples in which glycans and glycopeptides concentrations are limited. Therefore, to perform a comprehensive analysis of glycan isomers, the use of chromatographic techniques in conjunction with MS is essential (Veillon et al. 2017). As for intact glycopeptides, not only separation techniques are necessary to resolve glycopeptide isomers, but often it is essential to remove non-glycosylated peptides because they adversely influence the ionization of glycopeptides of interest. Enrichment of the glycopeptides is often performed to address this issue (Yu et al. 2018).
Currently, there are many chromatographic techniques available for protein glycosylation analysis. Although gas-phase separation such as ion mobility (IM) has made strides to resolve glycan isomers in recent years (Chen et al. 2018), liquid-phase chromatography (LC) is most often employed. In this review, a variety of MS methods in conjunction with separation techniques to analyze glycan and glycopeptide isomers are summarized. An array of separation approaches is discussed, and the various MS fragmentation techniques to facilitate the analysis of glycan and glycopeptide isomers. Moreover, glycan and glycopeptide analysis software tools that allow rapid identification and quantitation of such analytes are discussed.
2. MS-based isomeric characterization of glycans and glycopeptides
MS has been widely used as one of the most efficient analytical tools in glycomic and glycoproteomic studies due to its high sensitivity and rich structural information (Mechref and Novotny 2002, Dong et al. 2018). MS fragment techniques enable the identification of glycans and glycopeptides by their fragment patterns or diagnostic fragments. Although isomeric separation is commonly performed before MS analysis, there are several methods that allow the rapid identification and quantitation of glycan and glycoprotein isomers without separation. Matrix-assisted laser desorption/ionization (MALDI)-MS in conjunction with chemical derivatization is one of the most frequently utilized ionization methods to monitor specific glycan isomers such as sialic acid linkage isomers. Besides ionization techniques, multiple dissociation methods have been employed in the identification of glycan and glycopeptide isomeric structures, including ultraviolet photodissociation (UVPD), electron-transfer dissociation (ETD), collision-induced dissociation (CID), higher-energy collisional dissociation (HCD).
Moreover, the MSn technique has been utilized to analyze glycan isomers as it provides sufficient structural information. Different MS/MS modes such as multiple reaction monitoring (MRM) and parallel reaction monitoring (PRM) have been used to improve the isomeric identification of glycans. Together, these MS techniques permit the characterization of glycan and glycopeptide isomers.
2.1. Characterization of glycans and glycopeptide isomers by MALDI-MS.
MALDI is a matrix-based ionization technique that is widely used in glycomic and glycoproteomic analysis due to its simple sample preparation procedures and high throughput. MALDI is commonly coupled to time of fight (TOF) MS, which theoretically has an unlimited m/z range and fast scan speed, thus making it one of the most popular MS tools in clinical glycomic analysis (Everest-Dass et al. 2018). However, the low ionization efficiency of glycans, which is promoted by the hydrophilic nature of the molecules, demands the identification of better matrices. Accordingly, many efficient matrix and co-matrix were evaluated (Stahl et al. 1991, Karas et al. 1993, Metzger et al. 1994, Stahl et al. 1994, Mohr et al. 1995, Mechref and Novotny 1998, Banazadeh et al. 2018), including 2,5-dihydroxybenzoic acid (2,5-DHB), which is the most common matrix used in glycomics and glycoproteomics (Jensen et al. 2013, Chen et al. 2020). However, without separation, the characterization of native glycan and glycopeptide isomers is a great challenge for MALDI-MS due to the lack of isomeric structural information. Therefore, sample preparation strategies that can introduce differences in different glycan isomers have been developed to unravel this issue.
2.1a. Distinguishing isomers using chemical derivatization.
Sialylation of glycans has attracted interest due to its correlation with diseases (Davril et al. 1999, Varki 2008, Mechref et al. 2012). However, distinguishing labile sialylated glycan isomers is analytically challenging for quantifying sialylated glycoforms using MALDI-MS. Different glycan derivatization methods have been reported to add isomeric-specific chemical groups to glycan isomeric structures. This introduces a mass shift between two isomers, allowing the distinction of these glycan isomers in MS. Different derivatization strategies have been utilized for this purpose, including amidation (de Haan et al. 2015) and esterification (Gomes de Oliveira et al. 2015, Powers et al. 2015) of sialylated glycans. The early derivatization method used to distinguish sialic acid linkage isomers employed 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMT-MM) - MeOH approach, which was introduced by Harvey and coworkers (Wheeler et al. 2009). It induced a 32 Da shift between the two sialic acid linkage isomers. The α2,6‐linked sialic acids formed methyl esters, with a mass addition of 14 Da on α2,6‐linked sialic acids (methyl esters) while a mass loss of 18 Da on α2, 3‐linked sialic acids, resulted from the formation of lactones.
In 2004, a better derivatization method was reported by Wuhrer and coworkers (Reiding et al. 2014). This method enables an easy and fast reaction under a mild condition using carboxylic acid activator 1-ethyl-3-(3-dimethylamino)propyl)carbodiimide (EDC) and the catalyst 1-hydroxybenzotriazole (HOBt) in ethanol solution. Then, they reported a dimethylamidation method with the EDC-HOBt to derivatize sialic acid. This method resulted in an 18 Da loss for α2,3-linked sialic acid and a 27 Da gain for α2,6-linked sialic acid (de Haan et al. 2015). The overall 45 Da mass difference between α2,3- and α2,6-linked sialic acids allowed the identification of these isomers in MALDI-TOF. However, the instability of lactones complicated the analysis of α2,3-linked structures.
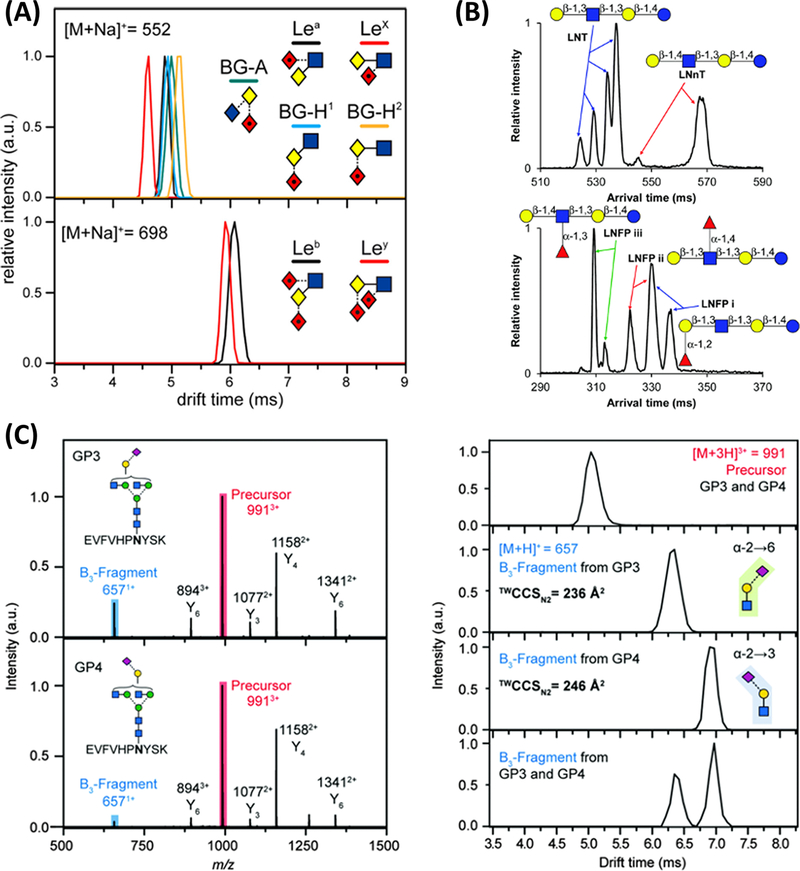
To overcome this disadvantage, Wuhrer and coworkers introduced a two-step derivatization method to further covert lactones to stable amides (Holst et al. 2016). In the first step, α2,3- and α2,6-linked sialic acids were reacted with EDC, HOBt, and dimethylamine as usual to form lactone and dimethylamide, respectively. Then, in the second step, the lactone was hydrolyzed, and the added ammonia reacts with the carboxylic acid to form a stable amide on α2,3-linked sialic acid. In contrast, the dimethlamide on α2,6-linked sialic acid was left unreacted, as shown in Figure 1. This two-step method has been widely employed and modified as the sialic acid linkage specific alkylamidation (SALSA) method (Nishikaze et al. 2017, Hanamatsu et al. 2018, Suzuki et al. 2019) to achieve higher derivatization efficiency. Recently, SALSA was further demonstrated to be able to distinguish α2,3- and α2,8-linked sialic acids as well (Hanamatsu et al. 2018). This methodology was further improved for isomeric α2,3- and α2,8-linked sialic acids derivatization for MALDI-MS as the Lactone-Driven Ester-to-Amide Derivatization for Sialic Acid Linkage-Specific Alkylamidation (LEAD-SALSA) method (Furukawa et al. 2020). More recently, SALSA has been combined with the isotopic labeling technique (iSALSA) for quantitation of sialylated glycan isomers in liver disease to reveal the alteration of α2,3 sialoglycans during liver fibrosis using MALDI-TOF (Hanamatsu et al. 2019).
Figure 1.
Schematic of two-step derivatization of sialic acid linkage isomers. A α2,6-linked sialic acid forms a stable dimethylamide through EDC, HOBt, and dimethylamine reactions in the first step, and keeps the same structure in the second step. B α2,3-linked sialic acid forms an unstable lactone in the first step, and then convert to a stable amide in the second step. C Mass spectra of in situ derivatized (top) and native (bottom) N-glycans derived from leiomyosarcoma FFPE tissue showing the induced mass shift of +28.031 Da between α2,3- and α2,6-linked sialic acids after derivatization, while native N-glycans are detected with additional neutral proton-sodium exchange. Symbols: green circle represents mannose, yellow circle represents galactose (Gal), blue square represents N-acetylglucosamine (GlcNAc), yellow square represents N-acetylgalactosamine (GalNAc), red triangle represents fucose, purple diamond represents N-acetylneuraminic acid (NeuAc), Reproduced with the permission from ref (Holst et al. 2016).
2.1b. Distinguishing isomers using enzymatic digestion.
Exoglycosidase enzymatic digestion has been utilized to elucidate the glycan and glycopeptide isomers (Royle et al. 2006, Cho et al. 2020). However, this approach is commonly combined with LC-MS or CE-MS to separate the digested products from the precursor glycans. Recently, West et al. reported a new enzymatic approach to differentiate fucosylated glycan isomers of N-glycans (West et al. 2020). A recombinant endoglycosidase F3 (Endo F3) was utilized to specifically cleave the glycosidic bond between two N-acetylglucosamines in the core structure of N-glycans, thus introducing 349.137 Da mass shift between Endo F3 digested and PNGase F digested core-fucosylated N-glycans. This method was combined with MALDI imaging to investigate the distribution of fucosylated glycan isomers in liver and prostate tissues. The core-fucosylated glycans were observed to be associated with specific tissue regions, which provided more information for in situ targeted marker screening of diseases.
2.1c. Distinguishing isomers using MALDI imaging mass spectrometry.
Extensive application of glycan structural specific derivatization and specific enzymatic digestion is combined with a MALDI imaging mass spectrometry (MALDI-IMS) to acquire in situ visualization of glycan isomeric distributions on tissues. In early studies, Drake and coworkers introduced a tissue-based glycan imaging workflow that allows for both qualitative and quantitative in situ N-glycan analysis on tissues (Powers et al. 2013). Wuhrer and co-workers employed the EDC-HBOt-EtOH method to investigate the distribution of sialic acid linkage isomers on colon carcinoma formalin-fixed paraffin-embedded (FFPE) tissues using MALDI imaging (Holst et al. 2016). The glycan isomers with different sialic acid linkages were observed to have different distributions across tumor regions. Later, West et al. achieved the visualization of core- and branch-fucosylated glycan isomers on cancer tissues using specific enzymatic digestions coupled with MALD-IMS (West et al. 2020). After treating with Endo F3, N-glycans with core-fucosylation were specifically cleaved to generate diagnostic m/z values that could be utilized to define core-fucosylation. This isomeric MALDI-IMS method was applied to analyze patient breast cancer tumor microarray slides (Herrera et al. 2019) and was further improved for a rapid slide-based IMS N-glycan profiling of serum and plasma which was spotted on an amine-reactive slide (Blaschke et al. 2020). The in situ IMS isomeric studies of tissue samples highlighted the different isomeric distributions of glycans in different disease regions.
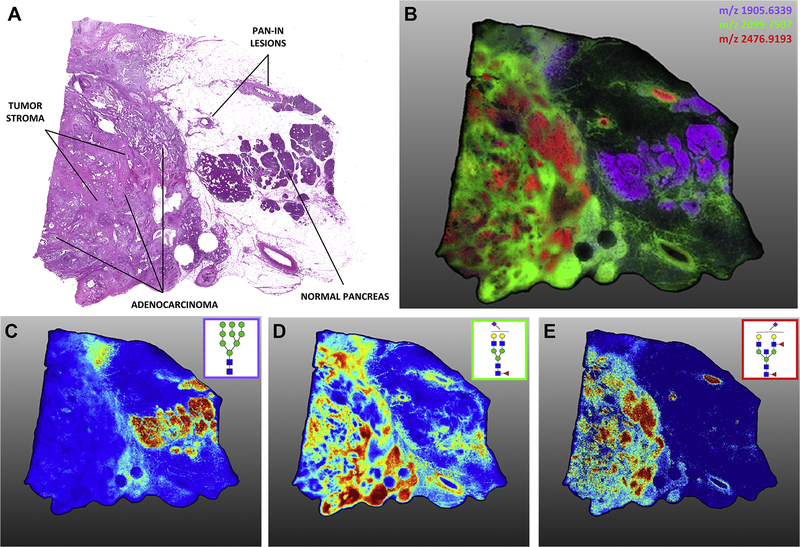
In a recent study, Drake and coworkers combined the linkage specific sialic acid derivatization method and the Endo F3 specific enzymatic digestion method to access the N-glycome of the pancreatic ductal adenocarcinomas (PDAC) patient tissues using MALDI-IMS (McDowell et al. 2020). Figure 2 depicts the high-resolution (40 μm) MALDI-IMS of amidation-amidation derivatized sialylated N-glycans. In different tumor regions (Figure 2A and 2B), the distribution of Man9 (Figure 2C), α2,3 sialylated glycan (Figure 2D), and α2,6 sialylated glycan (Figure 2E) were different and unique. The α2,3 sialylated N-glycans were predominate in tumor stroma regions, while primary adenocarcinoma areas were a mixture of α2,3 and α2,6 sialylation. The core-fucose was confirmed by the treatment of the in-house expressed core-fucose-specific Endo F3 enzyme. This study highlighted the isomeric analysis of MALDI-IMS in biological cohort studies, and the method was further employed to analyze N-glycan isomers on congenital aortic valve stenosis tissues (Angel et al. 2021).
Figure 2.
Sialic acid linkage and expression differences between tissue types in an advanced-stage pancreatic ductal adenocarcinoma (PDAC) tumor tissue. High-resolution (40 μm) MALDI-IMS of AA-stabilized sialylated N-glycans. A, annotated H&E staining of late-stage PDAC tumor. B, the overlay image of three N-glycan structures (depicted individually in panels C–E) localized to adjacent normal tissue (purple, Hex9HexNAc2 m/z 1905.6339), tumor stroma (green, Hex5dHex1HexNAc4NeuAc1(2,3) m/z 2099.7507), and adenocarcinoma (red, Hex5dHex2HexNAc5NeuAc1(2,6) m/z 2476.9193). C, high-mannose glycan structures tended to associate with normal adjacent tissue. N-glycan structures in normal tissue lacked significant sialic acid expression as compared with tumor tissue. D, α2,3-oriented sialic acid–decorated N-glycan structures localized to tumor stroma regions. E, adenocarcinoma-associated N-glycans were predominantly α2,6-sialylated although α2,3 vs. α2,6 sialylation of the same base structures drove different intratumor localization. These same α2,6-sialylated structures were also present within pancreatic intraepithelial neoplasia (Pan-IN) lesions. Symbols as in Figure 1. Reprinted with the permission from ref (McDowell et al. 2020).
Although MALDI-MS or MALDI-IMS distinguishes glycan isomers through structural specific derivatizations or enzymatic digestions, it only resolves specific glycan isomers such as sialic acid linkage isomers or fucosylation isomers. In order to distinguish other types of isomers and provide a more comprehensive characterization of glycan and glycopeptide isomers, these MALDI techniques require additional development. However, there are different MS-based fragmentation techniques that enable the effective characterization of glycan isomers.
2.2. Characterization of glycan and glycopeptide isomers by MSn and different fragmentation techniques.
LC-MS has proven to be extremely useful for the separation of complex isomeric glycan and glycopeptide structures. Unfortunately, the unavailability of standards complicates the characterization of the resolved isomers by using orthogonal derivation or enzymatic strategies (Nishikaze et al. 2017, Zhou et al. 2017, Gutierrez Reyes et al. 2021). The applications of MSn methods have demonstrated to assign structural features independent of standards due to the predictability of fragmentation patterns.
Deciphering the diversity of glycan or glycopeptide isomers brings to the forefront multiple challenges. Among them, the stereo and structural isomers of single sugar units are compounded with additional structural isomers found in linkage and branching arrays (Ashline et al. 2014). However, MSn can solve these structural details in the mass analyzers of the MS systems because of the different monomer bonding energies that provide stepwise disassembly to smaller molecules upon collisional activation dissociation (CAD). Thus, CAD induces rupture of the more stable bonds exposing greater structural information (Ashline et al. 2014). Glycan fragmentation is commonly achieved by using collision-induced dissociation (CID), a soft dissociation strategy that produces mostly Y and B ions (Han and Costello 2013). The formation of Y ions depends on charge localization and only can be originated from a single bond rupture to the C1-carbon providing monomer mass intervals; thus, such fragments fail to define linking connectivity. Nevertheless, glycan derivatization strategies such as permethylation enhance the cross-ring fragmentation during CAD, enabling comprehensive characterization of isomeric glycans (Shajahan et al. 2017). The absence of cross-ring fragments is a limitation of CID when applied to molecules with high molecular weight, such as large glycans or glycopeptide structures. Whereas advanced fragmentation techniques such as high energy collision dissociation (HCD), electron transfer/higher-energy collision dissociation (EThcD), electron-detachment dissociation (EDD), infrared multiphoton dissociation (IRMPD), and UV-photon dissociation (UVPD) can provide sufficient fragment ions, and therefore structural information of large molecules as are glycopeptides (Myers et al. 2013, Zhurov et al. 2013, Depraz Depland et al. 2018, Riley and Coon 2018). Besides, targeted-MSn modes such as MRM and PRM provide an increase in specificity and sensitivity that can be extrapolated to accurate quantitation methods of isomeric glycan and glycopeptides (Zhou et al. 2015, Reyes et al. 2021).
2.2a. MSn characterization of glycan isomers.
It is known that permethylation offers advantageous opportunities to achieve a baseline isomeric separation of several N- and O-glycan compositions (Cho et al. 2021, Gutierrez Reyes et al. 2021). Additionally, permethylation enables comprehensive structural characterization of glycans by enhancing cross-ring fragmentation during MSn procedures, the fragmentation that is dependent on the bonding position of adjacent sugars, thus provide evidence of their linkage (Shajahan et al. 2017). Kurz et al. used an LC-MSn approach to describe the sialic acid linkages of isomeric N-glycans derived from HIV gp120 (Kurz et al. 2021). The MS2 fragmentation was generated by CID with 40% of collision energy (CE). Further, MS3 to 5 approaches were used for the identification of the sialic acid linkages. An example is observed in Figure 3A, where a targeted MSn workflow was applied to the neutral loss of the permethylated sialic acid (m/z = 375) to trigger product-dependent MS3−5 acquisitions to reveal the α2,6 linkage of the HexNAc4Hex6Neu5Ac2 N-glycan. Zhou et al. used the MS2 fragmentation patterns acquired with HCD to distinguish between the core and fucosylated isomeric structures of the permethylated glycan HexNAc4Hex4Fuc1. The fragment ions at 432.47 and 638.50 m/z are indicative of branched fucose structures, and the fragment ions at 1481.95 and 1552.94 m/z reflects a structure with branched fucosylation (Zhou et al. 2017). Reinhold and coworkers used an MSn approach to characterize 34 permethylated and 2-amino-N-(2-aminoethyl)-benzamide (AEAB) derivatized isomeric glycans derived from human milk (HMGs). In an example of their work, three isomers of the AEAB derivatized glycan HexNAc3Hex5Fuc1 were identified in the extracted ion chromatogram and characterized using the fucosylated di-LacNAc motifs of the glycan structure observed on the MS3 spectrum with 660 and 646 m/z values, and further MS4 clarified the details of the linkage. CID with a collision energy of 35% were the parameters used to acquire the MSn spectra (Ashline et al. 2014). Later the same research group applied the same MSn strategy to investigate permethylated isomeric N- and O-glycans derived from two breast cancer cell lines, MCF-7 and MDA-MB-231. Among many other isomeric structures, four isoforms were identified for the O-glycan with the composition GalNacGlcNAc2Neu5Ac (1345 m/z) from the MCF-7 sample. The characterization of the isomers was based on the identification of the MS2 fragment parent ions that differ on the localization of the sialic acid in the LacNAc moieties (486 and 847 m/z) or direct to the core Gal-GalNAc (520 and 881 m/z). Further, MS3 and MS4 elucidated the sialic acid linkage (Ashline et al. 2017). Depraz Depland et al.(Depraz Depland et al. 2018) coupled an LC-MS with an InfraRed Multiple Photon Dissociation (IRMPD) approach to study the isomeric forms of N-glycans derived from human milk and N-glycan epitopes. The α2,3 and α2,6 linkages of the glycans 3’-sialyllactose sodium salt, 6’-sialyllactose sodium salt, and 6’-sialyl-N-acetyllactosamine sodium salt were easily identified due the spectral fingerprint observed for both linkages. Figure 3Bi shows the MS/MS spectra of protonated milk oligosaccharides (634 m/z) obtained by CID, and Figure 3Bii shows the IRMPD fingerprint of the milk oligosaccharide oligomers in the 2700–3700 cm−1 spectral range.
Figure 3.
(A) MSn defines sialic acid linkage position for the HexNAc4Hex5Neu5Ac2 N-glycan derived from HIV gp120. (Bi) CID spectra (30% collision energy) of protonated 1 “purple” and 2 “green”, the precursor is labeled P. (Bii) IRMPD spectra of protonated 1“purple” and 2 “green”. Photofragmentation yield (dots), and 5 points FT rolling averaging (line). Symbols as in Figure 1. (A) Reproduced with the permission from ref (Kurz et al. 2021) and (B) reproduced with the permission from ref (Depraz Depland et al. 2018).
2.2b. MSn characterization of glycopeptide isomers.
For glycopeptides, the rupture of glycosidic bonds occurs prior to the peptide backbone during the CAD process leading to the generation of abundant glycan fragments and less information of the peptide backbone. Escobar et al. used an HCD-UVPD approach to address the formation of multiple peptide fragment ions that enable precise localization of O-GlcNAc sites in the O-glycopeptides (Escobar et al. 2020). Using this approach, four positional isoforms of the O-glycopeptide VYPVSSVPSSAQSTSK + GlcNAc (serine glycosylation positions 395, 396, 399, 401) were identified with the information provided by the diagnostic ions. Lee et al. used a combined CID-HCD fragmentation strategy that generated either oxonium ions and B/Y ions (Lee et al. 2016). The method was applied for the characterization of site-specific N-glycopeptide isoforms of α−1-acid glycoprotein (AGP). For each sample, five CID scans were acquired in linear trap quadrupole with a 30 ms activation time and collision energy of 35, and five HCD scans were acquired in the Orbitrap® with 20 ms activation time and collision energy of 35. A total of 165 N-glycopeptide isoforms were identified and used to depict the microheterogeneity of AGP. Ye et al.(Ye et al. 2013) used a hybrid CID-ETD-HCD approach for the qualitative and quantitative characterization of TMT-glycopeptide isoforms tryptic digested from bovine fetuin. With ETD, glycopeptides were cleaved from the peptide backbone without carbohydrate fragmentation and therefore used for site localization. Glycan structure elucidation was accomplished using CID. The glycopeptide quantitation of the TMT-glycopeptides was performed based on the HCD spectra. The isomeric glycopeptides with structure [AA 54–85] + HexNAcNeu5Ac were differentiated by CID MS2. In addition, other approaches that do not show glycopeptide isomeric data but describe important achievements for the characterization of glycopeptides are stepped HCD (Yin et al. 2018), CID/ETD (Mechref 2012), HCD/ETD (Singh et al. 2012), EThcD (Yu et al. 2017, Chen et al. 2018, Glover et al. 2018, Zhu et al. 2019).
Gutierrez Reyes et al. recently reported a comprehensive LC-PRM-MS approach where a targeted parallel reaction monitoring (PRM) strategy was used for the quantitation of isomeric haptoglobin N-glycopeptides derived from cirrhosis and hepatocellular carcinoma (HCC) samples (Reyes et al. 2021). The target glycopeptides were acquired and fragmented by HCD with a collision energy of 25%. This strategy produced abundant and stable Y fragment ions that were used for glycopeptide quantitation. A total of 73 N-glycopeptide structures, including 21 glycopeptide compositions with glycan isomers and 21 compositions without glycan isomers, were identified and quantified under this strategy.
3. LC-MS analysis of native and reducing end-labeled glycan isomers
Although isomeric identification of glycans has been achieved by MS using aforementioned fragmentation strategies such as MSn or photodissociation (Devakumar et al. 2008, Kurz et al. 2021), the relatively large amount of analytes required for MSn hindered the use of such technique for the analysis of biological samples. On the other hand, the structural information provided by MS/MS is insufficient to distinguish all glycan isomers. Therefore, different separation techniques such as liquid chromatography (LC), capillary electrophoresis (CE), and ion-mobility (IM) are coupled with MS to achieve isomeric separation prior to MS, thus improving the characterization of glycan and glycopeptide isomers.
Efficient separation methods are important to characterize glycan isomers, especially for those low abundant structures. Another advantage of separation methods is avoiding ionization competition of multiple glycan structures, which increases the sensitivity of minor glycans. Among separation techniques, LC-MS is one of the most efficient approaches for isomeric glycomic studies. The robustness, reproducibility, sensitivity, and diverse applications of LC-MS make it an ideal tool to analyze glycan isomers in complex systems such as biofluids, cells, and tissues. There are several LC-MS techniques employed in isomeric glycomics, including hydrophilic interaction liquid chromatography (HILIC)-LC-MS, revered-phase (RP)-LC-MS, porous graphitic carbon (PGC)-LC-MS, and mesoporous graphitic carbon (MGC)-LC-MS. These LC techniques can selectively analyze different types of glycans based on what derivatization is utilized.
Although there are many efforts to separate native glycan isomers, the low ionization efficiency of native glycans and glycopeptides is a challenge to LC-MS analysis. Moreover, the equilibrium of the free reducing end of native glycans always forms anomers which sometimes are hard to distinguish from other isomers, complicating the isomeric identification (Cho et al. 2020). Therefore, reducing end derivatization methods are commonly utilized to increase the ionization efficiency, eliminate the anomers, and enhance the quantification of glycans. A variety of reagents can be used to derivatize the reducing end of the released glycans including hydrazide, amines, alkoxyamine and carbamate compounds (Dong et al. 2018). These reagents usually contains nitrogen, aromatic rings, and alkyl chains which have been proved to enhance the ionization efficiency of glycans as well as their separation efficiency (Ruhaak et al. 2010). Chemical reactions that prompt commonly utilized reducing end derivatization techniques include reductive amination (Ruhaak et al. 2010, Jiang et al. 2017, Kovács et al. 2017), hydrazide chemistry (Gil et al. 2008, Unterieser and Mischnick 2011, Walker et al. 2011), and carbamate derivatization (Hosokawa et al. 2008, Wang et al. 2008, Lauber et al. 2015). The most common reducing end labeling reagents used in glycomics are aniline (Jiang et al. 2014), 2-aminobenzamide (2-AB) (Royle et al. 2008), 2-aminobenzoic acid (2-AA) (Ruhaak et al. 2008), 2-aminopyridine (PA) (Deguchi et al. 2008), procainamide (ProA) (Keser et al. 2018), 2-aminonaphthalene trisulfonic acid (ANTS) (Saba et al. 2001), and 1-aminopyrene-3,6,8-trisulfonic acid (APTS) (Bunz et al. 2013) for reductive amination; Girard’s T reagent (GT) (Unterieser and Mischnick 2011), Girard’s P reagent (GP) (Kim et al. 2015), and phenylhydrazine (Lattova and Perreault 2003) for hydrazide labeling; and N-hydroxysuccinimide (NHS)-carbamate such as RapiFluor (Lauber et al. 2015) for carbamate derivatization. Zhou et al. have compared the commonly used reducing end labeling reagents and permethylation methods, revealing the high sensitivity of RapiFluor and the quantitative accuracy of permethylation (Zhou et al. 2017). These derivatization strategies can be utilized in different separation techniques for better characterization of glycan isomers.
3.1. Isomeric separation of native and reducing end-labeled glycans on HILIC columns.
HILIC is a normal phase chromatography technique whose separation is based on the hydrophilic interactions between analytes and the stationary phase. Therefore, glycan isomers that have different hydrophilicity can be separated on HILIC columns. Since glycans are highly hydrophilic, HILIC-MS is considered to be one of the best methods to analyze native and reducing end-labeled glycans and proved to be capable of isomeric separation. Multiple HILIC columns, including zwitterionic (ZIC®)-HILIC (Takegawa et al. 2006), HALO® columns (Tao et al. 2014), and amide/amine columns (Jiang et al. 2014), have been utilized to separate glycan isomers. Recently, the isomeric separation efficiencies of several common HILIC columns have been compared, and HALO® Penta-HILIC was observed to have the best isomeric separation (Molnarova and Kozlík 2020).
Recently, Zhao et al. studied the low abundant isomeric N-glycans in biological therapeutics using HILIC-LC-MS (Zhao et al. 2016). A Waters BEH glycan column was employed to analyze 2-AB labeled glycan isomers derived from immunoglobulin G (IgGs). The separations of positional isomers were observed. However, the separations of linkage isomers were not comprehensively investigated due to the lack of sialylated structures that only differed in sialic acid linkages. Later, Mancera-Arteu et al. reported the separation of aniline labeled sialic acid linkage isomers using a ZIC-HILIC-MS/MS (Mancera-Arteu et al. 2017). The separation of core- and branch-fucosylated isomers were also observed. Most recently, Messina et al. investigated the glycan isomers in congenital disorders of glycosylation (CDG) disease using HILIC-UPLC-MS (Messina et al. 2021). In this study, an amide column was utilized to analyze glycan isomers labeled with RapiFluor. Isomeric separations of positional isomers and sialic acid linkage isomers were achieved. In addition to N-glycan isomers, Zhang et al. developed a targeted MS-based approach to achieve absolute quantification of twelve oligosaccharides, including 9 isomers, in human milk (Zhang et al. 2019). This targeted method can help in determining variations in human milk oligosaccharides (HMOs) among different samples. Although HILIC-MS has been utilized for isomeric glycan studies, the relatively low resolution hindered its application in complex biological samples.
3.2. Isomeric separation of native and reducing end-labeled glycans on PGC columns.
Another widely used technique in isomeric studies of native and reducing end-labeled glycans is PGC-LC-MS (Ruhaak et al. 2009, Stavenhagen et al. 2015, Dong et al. 2018). PGC separation is based on the combination of hydrophobic interaction and distinctive polar retention effect, making it suitable for isomeric glycomic analysis (Pereira 2008). In addition, the retention of analytes is also affected by their shapes and 3D structures due to the planar structure of the PGC material (West et al. 2010). PGC was initially utilized to separate glycan isomers derived from RNase B (Kawasaki et al. 1999) and erythropoietin (Kawasaki et al. 2000) to demonstrate the separation of high-mannose and sialylated structural isomers. Later, Packer and coworkers (Karlsson et al. 2004) reported the use of nanoPGC-LC-MS to increase the sensitivity by ten folds in the low-femtomole range for the minor glycan structures. Lebrilla and co-workers utilized the nanoPGC system to analyze glycans derived from mucin and human milk (Niñonuevo et al. 2005). Since then, analytical PGC columns as well as nano- and microchip-PGC columns have been widely employed in isomeric studies for native and reducing end-labeled glycans.
Lebrilla and coworkers have demonstrated that PGC can provide isomeric-level separation of glycan structures using a nanoPGC-chip-LC-QTOF for efficient characterization of native N-glycan isomers (Hua et al. 2013, Park et al. 2016). Most positional and linkage isomers achieved a baseline separation with the isomeric structures confirmed by multiple exoglycosidase digestions. Recently, the same group also proved the isomeric separation of native O-glycan isomers using the same PGC-LC-MS system (Xu et al. 2020), where 44 O-glycan isomers with the complete elucidation of 25 structures were acquired from cell surfaces.
In a recent study, Wuhrer and coworkers introduced a high throughput 96-well plate sample preparation method for integrated N- and O-glycomics using PGC-LC-MS (Zhang et al. 2020). A 96-well plate (pore size 0.45 μm) with a high protein-binding membrane (hydrophobic Immobilon-P PVDF membrane) was utilized to capture proteins from blood serum or cell lysate, followed by on plate denaturation, and N- and O-glycan release. Released N- and O-glycans were further reduced and separated by a 100 mm × 75 μm Hypercarb nano PGC column. The sialic acid linkage isomers of reduced N-glycans were efficiently resolved in both fetuin and cell line samples, as shown in Figures 4A and 4B. The baseline separations of these isomers were achieved. In addition, two pairs of O-glycan isomers (indicated by purple and orange) from a cell line sample were also baseline separated, as shown in Figure 4C. This promising isomeric separation highlighted the importance of PGC in isomeric glycomic research. Later, they performed an in-depth O-glycomic analysis for 26 different colorectal cancer (CRC) cell lines using the same platform, where more than 100 O-glycan structures were efficiently identified and quantified (Madunić et al. 2021).
Figure 4.
Isomeric separation of glycans derived from different samples following the 96-well plate sample preparation method using nanoPGC-LC-MS/MS. (A) Combined EICs of N-glycans derived from bovine fetuin. Sialic acid linkage isomers were efficiently resolved on PGC column. (B) Combined EICs of N-glycans derived from NMuMG cells. Isomeric separation of sialylated N-glycan isomers was achieved on biological samples. (C) Combined EICs of O-glycans derived from NMuMG cells. Isomeric separation of two pairs of O-glycan isomers were observed, as indicated by purple and orange peaks. Symbols as in Figure 1. Reproduced with the permission from ref (Zhang et al. 2020).
Recently, She et al. achieved a high-resolution isomeric separation of native high-mannose glycan isomers by nanoPGC-LC-MS using an in-house packed 20 cm long PGC column (She et al. 2020). Moreover, four isomers of monosialylated Man4-based hybrid glycan were effectively separated with structure elucidation through unique patterns of MS2 fragments. The specific ratio among several diagnostic ions could indicate the location of branching substituents, distinguish α3 or α6 linkages of the 4th mannose linked to the 6-arm of the core structure. The authors also demonstrated the high-resolution separation of native sulfoglycans derived from the egg-propagated and cell culture-derived influenza vaccines. In addition to N-glycan isomers, the isomeric separation was also achieved for O-glycans using PGC-LC-MS. Lebrilla and coworkers developed annotated libraries of sialylated (Wu et al. 2011) and neutral (Wu et al. 2010) HMOs using HPLC-PGC-Chip/QTOF MS to enable the rapid detection of oligosaccharide structures.
The use of glucose units (GU) for determining the glycan isomers was highlighted by researchers recently. Packer and co-workers standardized the retention times of isomeric native glycans for sample-specific glycotyping using PGC-LC-MS (Ashwood et al. 2019). They utilized hydrolyzed dextran as an internal standard in conjunction with Skyline software to minimize RT and peak area technical variations in isomeric glycan analysis. GUI-based RT normalization can further be used in conjunction with additional experiments like exoglycosidase digestion and sensitive tandem-MS strategies for comprehensive structural determination of isomeric glycans.
4. LC-MS analysis of permethylated glycan isomers
In addition to native and reducing end derivatization, permethylation is another widely employed derivatization method for LC-MS-based glycomics (Costello et al. 2007, Zhou et al. 2017, Zhou et al. 2017, Dong et al. 2019, Gautam et al. 2020). Several permethylation procedures have been utilized over the years; however, solid-phase permethylation is currently deemed as the most efficient method. A solid-phase permethylation approach was successfully demonstrated by Novotny, Mechref, and coworkers in 2005 (Kang et al. 2005). Subsequently, a high-throughput procedure was reported by the same group in 2008 (Kang et al. 2008). Mechref and coworkers (Desantos-Garcia et al. 2011) reported the enhanced sensitivity of LC-MS analysis of permethylated glycans by coupling a miniaturized version of solid-phase permethylation with online purification.
During permethylation, all active hydrogens in the glycan structure are replaced with methyl group from iodomethane. This chemical modification alters the glycans’ properties and augments their stability for LC-MS analysis. Advantages offered by permethylation over the other derivatization strategies are reported by Mechref and coworkers (Zhou et al. 2017). This modification stabilizes glycans by preventing fucose rearrangement, sialic acid loss and enhances glycan ionization efficiency in positive ion mode. Additionally, it forms more informative fragments that facilitate structural elucidation by MSn.
4.1. Isomeric separation of permethylated glycans on C18 columns.
C18 columns are particularly suitable for analyzing permethylated glycans as permethylation enhances their hydrophobicity. This allows the efficient separation of glycans by reversed-phase LC (RPLC) (Zhou et al. 2017). However, the minimal differences in hydrophobicity between complex glycan isomers hinder the ability to achieve baseline separation of isomers, leading to either partial or no isomeric separation (Veillon et al. 2017). Although some smaller glycans like O-glycans and trisaccharides demonstrate isomeric separation on C18, separation of complex isomeric sialylated glycans poses a challenge (Hanisch and Müller 2009).
Isomeric separation of permethylated complex N-glycans on C18 column at 55°C was demonstrated by Zhou et al. According to the researchers, a higher temperature significantly decreases the peak width, thus improving the peak shape (Zhou et al. 2016). The stretching of glycan structures at increased temperatures exposes more methyl groups and increases the nonpolar surface area, which in turn enhances the analyte-stationary phase interactions. These enhanced interactions improve the chromatographic resolution of the isomeric structures. Recently, Kurz et al. reported an optimized workflow including sample preparation, mobile phase optimization, and MSn method development for LC-MS analysis of permethylated glycans (Kurz et al. 2021). They demonstrated baseline separation and MSn of isomeric N- and O-glycan structures using the workflow adapted from standard proteomics-based workflows. This allows the proteomics focused laboratories to acquire glycomic data without excessive deviation from their standard workflows. In addition, C18-LC-MS was recently used in conjunction with Glucose Unite Index (GUI) to eliminate the inter- and intra- laboratory variability in retention times of glycans, arising from various factors including column and instrument performances (Gautam et al. 2020).
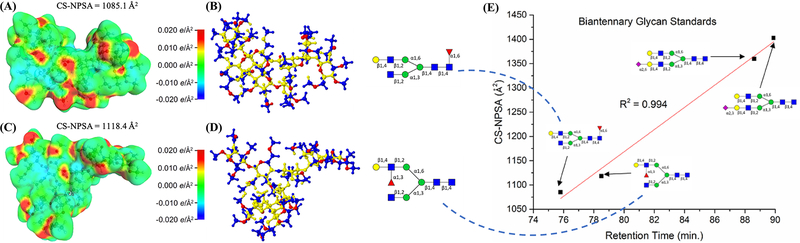
Structural modeling of glycopeptide isomers was also employed to improve the elucidation of glycan isomers. Most recently, Dhakal et al. introduced a new approach for defining and computing nonpolar surface areas of glycan isomers based on continuum solvation models (CS-NPSA) (Dhakal et al. 2021). This approach considered the solvent environment of molecules during LC-MS analysis to acquire a very fine resolution of the surface for NPSA calculation. The CS-NPSA was demonstrated on phenylbutanehydrazide and model oligosaccharides such as dextran, then utilized to model glycan isomers. Figure 5 depicts the modeling of two glycan isomers and the correlations between CS-NPSA and retention times. The two examples of computed glycan structures are core- and branch-fucosylation isomers. Their polarity charges (Figure 5A and C) show a distinct difference of CS-NPSA, which is corresponding to their 3D structures (Figures 5B and 5D). Similar modeling and CS-NPSA were applied to the two sialic acid linkage isomers as well. The promising linear correlation between CS-NPSA and retention time, which was acquired through C18-LC-MS (Figure 5E) indicates the accurate calculation of NPSA through this modeling method and the possibility of using this method to predict the retention time for unknown glycan isomers during LC-MS analysis, thus facilitating the identification of glycan isomers in complex biological samples.
Figure 5.
Biantennary standard glycan structures in gg conformation optimized using the PBE-D3/SV method in the COSMO/acetonitrile environment. Representations (A) and (C) show the polarization charges and CS-NPSA values, and (B) and (D) the polar atoms in red, nonpolar atoms contributing to the NPSA in blue, and buried nonpolar atoms that do not contribute to the NPSA in yellow. The core-fucosylated structure is shown in (A) and (B), while the branch-fucolyslated structure is shown in (C) and (D). Linear regression correlating experimentally determined retention times with CS-NPSA values for the core- and branch-fucosylated isomers and the 2,3- and 2,6-sialylated isomers in gg conformation using the PBE-D3/SV approach is shown in (E). Symbols as in Figure 1. Reproduced with the permission from ref (Dhakal et al. 2021).
Micro pillar array columns (μPAC) are recently developed commercial columns that offer advantages over packed bed columns due to their design. The uniformly placed micropillars, coated with C18 material in μPAC, offer an efficient RPLC platform. This uniformity eliminates peak dispersion due to Eddy diffusion. The column lacks any particles or frits, thus preventing column clogging. The ability to operate at a higher flow rate with a bi-directional flow is another advantage offered by these columns. The column has been extensively used in proteomic analysis; however, workflow to analyze reduced and permethylated glycans on μPAC was developed (Cho et al. 2021). The work examined the ability of 50 cm long μPAC to analyze both N- and O-glycans. Additionally, the isomeric separations of oligomannose and other complex glycans were achieved using a 200 cm μPAC-LC-MS/MS.
4.2. Isomeric separation of permethylated glycans on PGC columns.
PGC columns have proved to be precious tools to achieve isomeric separation of both native as well as permethylated glycans (Zhou et al. 2016, Veillon et al. 2017, Ashwood et al. 2019, Peng et al. 2019, Gautam et al. 2020). Unlike C18, PGC columns demonstrate stronger retention for glycans and can also retain small glycans like O-glycans. PGC-based separation relies on both hydrophobic interactions of the analyte with the stationary phase and the polar retention effect of graphite (PREG) which is based on the high polarizing ability of the graphitized carbon material. Additionally, the 3D structure of the analyte also influences the interactions due to the planar nature of the stationary phase (Zhou et al. 2016, Veillon et al. 2017).
Costello et al. first reported the isomeric separation of permethylated glycans on PGC (Costello et al. 2007). They demonstrated the isomeric separation of RNAse B derived Man7 and Man8 and assigned the isomeric structures based on fragment patterns in MS/MS data. However, Man7 only demonstrated partial isomeric separation. Subsequently, Mechref and coworkers demonstrated the isomeric separation of permethylated glycans by using PGC at 75°C, which showed isomeric separation of a variety of glycans, including oligomannose structures as well as positional and linkage isomers derived from fucosylated and sialylated structures (Zhou et al. 2017, Zhou et al. 2017). In addition, 3D structural models of glycan isomers were also simulated to aid the assignment of a pair of galactose positional glycan isomers. Figure 6 illustrates the isomeric separation of galactose positional isomers on the PGC column, along with the 3D molecular modeling of the structures. Also, the isomeric separations of sialylated and fucosylated structures on PGC are depicted. This established high-temperature PGC-LC-MS method was also utilized for isomeric profiling of permethylated N-glycans from serum haptoglobin of hepatocellular carcinoma and cirrhotic patients (Huang et al. 2017). Dong et al. used the same method for serum isomeric profiling of permethylated glycans in patients with primary restless legs syndrome (Dong et al. 2020) and Idiopathic rapid eye movement sleep behavior disorder (Dong et al. 2018). Later, the biological attributes of 144 permethylated N-glycan isomers from 50 N-glycan compositions in breast cancer brain metastasis were investigated using PGC-LC-MS/MS.(Peng et al. 2019) Recently, high-temperature PGC-LC-MS/MS in conjunction with the glucose unit index (GUI) was utilized to normalize the retention time of isomeric permethylated glycans across different instruments and laboratories by using dextrin ladder as an internal reference standard (Gautam et al. 2020).
Figure 6.
(A) EIC of the glycan F1A2G1 with galactose attached to different branches, which were released from human blood serum. (B) CID MS/MS spectrum of the isomeric structure eluted at 32.8 min, with molecular modeling of the structure. (C) CID MS/MS spectrum of the isomeric structure eluted at 34.1 min, with molecular modeling of the structure. (D) EIC of permethylated bi-antennary bi-sialylated glycans released from bovine fetuin. (E) EIC of F1A2G1 with isomers resulting from branch and core fucosylation. Symbols as in Figure 1. Reproduced with the permission from ref (Zhou et al. 2017).
To improve the identification of permethylated glycan isomers, GUI was successfully utilized in both C18-LC-MS/MS and PGC-LC-MS/MS-based separation of permethylated glycans for normalizing RT variations (Gautam et al. 2020). High reproducibility of GUI values across different instruments and laboratories as well as different days was demonstrated. Most recently, Padgett et al.(Padgett et al. 2021) used GUI as a reference frame and proposed a data-driven partial differential equation model to classify unknown N-glycans using diffusivity and absorption.
The efficient separation of permethylated O-glycan and glycosphingolipids (GSL) glycans was also achieved using PGC-LC-MS/MS. Dong et al. examined the reduced and permethylated free oligosaccharide isomers derived from human, bovine, and goat milk samples (Dong et al. 2016). Subsequently, PGC-LC-MS was utilized to demonstrate the isomeric separation of free reducing end and reduced end O-glycans, free oligosaccharides from human milk, and GSL glycans derived from the MDA-MB-231BR cancer cell line (Cho et al. 2020).
4.3. Isomeric separation of permethylated glycans on MGC columns.
Despite the popularity of PGC to achieve isomeric separation of glycans, lack of stability, poor reproducibility, and need for regeneration were constant issues faced by researchers working with the nanoLC columns (Bapiro et al. 2016, Gautam et al. 2021). These problems ultimately led to the discontinuation of the commercial nanoflow HyperCarb PGC columns. PGC columns in capillary flow configuration are still available, but there was a need for a stable and robust column that can provide sensitive and efficient isomeric separation of glycans in nanoflow configuration.
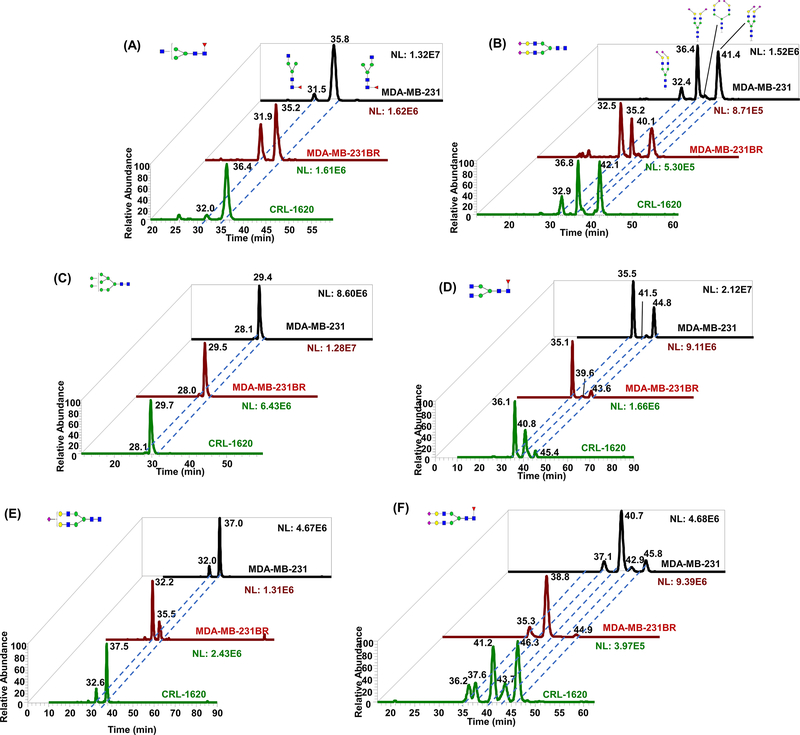
Mechref and co-workers recently introduced an in-house packed 1 cm long mesoporous graphitized carbon (MGC) column that yields efficient isomeric separation of permethylated glycans at 75°C (Gautam et al. 2021). The mechanism of MGC based isomeric separation is believed to be similar to PGC columns; however, the smaller particle size (<500 nm) and pore size of MGC offer a larger surface area for interaction with the analytes providing better retention for permethylated glycans. Comparison of MGC versus PGC reveals the enhanced efficiency and robustness of isomeric glycan separation offered by MGC columns. Figure 7 depicts the isomeric separation of reduced and permethylated glycans derived from breast cancer cell lines MDA-MB-231, MDA-MB-231BR, and a brain cancer cell line CRL-1620 on the MGC column. Decreasing the dead volume was attained by packing the MGC stationary phase in nanospray emitters, thus, improving the resolution. The temperature of these packed nanospray emitters was controlled by an in-house developed Peltier heater (Gautam et al. 2021). The MGC column and packed nanospray emitter are cost-effective and promising candidates for replacing nanoflow PGC columns. Optimization of MGC-LC-MS methods to facilitate the analysis of diverse biomolecules will be critical to providing MGC columns and emitters commercially.
Figure 7.
Separation of isomeric permethylated glycans derived from MDA-MB231, MDA-MB-231BR, and CRL-1620 on the MGC column (A) Separation of HexNAc3Hex3DeoxyHex1. (B) Separation of HexNAc4Hex5NeuAc2. (C) Separation of HexNAc2Hex8. (D) Separation of HexNAc4Hex3DeoxyHex1. (E) Separation of HexNAc4Hex5NeuAc1. (F) Separation of HexNAc4Hex5DeoxyHex1NeuAc2. Symbols as in Figure 1. Reproduced with permission from ref (Gautam et al. 2021).
5. LC-MS analysis of glycopeptide isomers
The analysis of glycopeptides is just as challenging, if not more so than glycans, because of the micro- and macro- heterogeneity of glycoproteins. A combination of LC and MS is one of the most powerful tools for the analysis of protein glycosylation. MS itself, together with different fragmentation modes, provides useful structural information for glycoconjugate analysis (Novotny and Alley 2013, Dong et al. 2018, Yu et al. 2018). However, due to the similar fragmentation patterns and the chemical composition of isomers being the same, it is difficult to distinguish isomeric glycopeptides with structural glycan isomers such as those of sialic acid linkages. Hence, it is necessary to separate isomeric structures prior to MS analysis. LC strategies including RPLC (Zhou et al. 2016, Vreeker and Wuhrer 2017, Zhou et al. 2017, Ji et al. 2019), HILIC (Huang et al. 2016, Mancera-Arteu et al. 2017, Kozlik et al. 2018), and PGC-LC (Zhou et al. 2017, Zhu et al. 2020) have been employed to separate and analyze isomeric glycopeptides. Thus, by using LC strategies to complement MS analysis, it enables accurate characterization of different glycoforms from glycoproteins. In this section, three of the most widely used types of liquid chromatography, including RPLC, HILIC, and PGC coupled with tandem MS are reviewed and compared for their capability in achieving the isomeric separation of glycopeptides.
5.1. Isomeric separation of glycopeptides on C18 columns.
RPLC, one of the most widely used separation methodologies, accomplishes separation by noncovalent interaction between the nonpolar moieties of analytes and the stationary phase of the column (Vreeker and Wuhrer 2017). Unlike normal liquid chromatography, RPLC has nonpolar stationary phases such as octadecyl carbon chain (C18) bonded silica, C8, and C30 as packing materials. Moreover, it normally uses polar solvents as mobile phases, which is one of the advantages due to the safety and low cost of the solvents. In RPLC, both stationary phase materials and mobile phase components play an important role in the elution orders and retention times of analytes. Out of all the packing materials, C18 is the most widely used material in reversed-phase columns. The separation and elution order of glycopeptides in RPLC (C18 columns) are highly dependent on the hydrophobicity of the peptide backbone along with the contribution of the hydrophilic glycan moiety (Kozlik et al. 2017, Ang et al. 2019). However, due to the different positions and linkages of glycan isomers, the contribution of glycan isomers to the retention of glycopeptides differs. Separation of isomeric glycopeptides can be achieved by optimizing the conditions of column properties and mobile phase components.
Several studies have shown that different glycan moieties affect the retention behavior of glycopeptides in reversed-phase columns. Wang et al. have reported that the existence of hexose or N-Acetylhexosamine residues on the glycans attached to glycopeptides decreased in retention while sialylated glycans and phosphorylated glycans increased the retention of glycopeptides in RPLC conditions (Wang et al. 2014). Medzihradszky et al. showed that the presence of N-Glycolylneuraminic acid and N-Acetylneuraminic acid increased the hydrophobicity of glycopeptides, resulting in the increased retention time (Medzihradszky et al. 2015). Kozlik et al. showed that the retention time of a glycopeptide is decreased when the number of neutral monosaccharide units of the glycan increased (Kozlik et al. 2017). However, the addition of the fucose shows a less significant influence on the retention time of glycopeptides with larger glycans such as tri- or tetra-antennary structures than those with smaller ones, such as bi-antennary structures. These features contribute to the separation of different glycopeptides and could prompt the separation of glycopeptide isomers in different monosaccharide linkages and positions using RPLC-MS.
Recently, a C18-RPLC coupled with tandem MS was applied to study the temperature effect on the isomeric separation of N- and O-sialylated glycopeptides by Ji et al.(Ji et al. 2019) In this study, the separation of four O-glycopeptides derived from erythropoietin (EPO) were evaluated using a C18 column at 30°C, 40 °C, 50°C, and 60 °C. The monosialylated O-glycopeptides showed isomeric separation under high temperature, as shown in Figure 8A. The resolution between these two peaks was improved from 1.00 at 40 °C to 1.83 at 60 °C. Using α2,3 neuraminidase, they were able to distinguish the sialic acid linkage isomers on the glycopeptide. By using RPLC at high temperatures, the structural isomers of sialylated O-glycopeptides derived from tryptic digested EPO were separated based on different linkages and positions. A similar strategy was also applied to investigate isomeric separations on tryptic N-glycopeptides from α−1-acid glycoprotein (AGP) under different temperatures. From Figure 8B, C, and D, efficient isomeric separations of sialylated N-glycoforms with mono-, di-, and tri-sialic acids from the same peptide backbone were achieved. Increasing temperature in RPLC enables the enhancement of resolution for both sialylated O- and N- glycopeptides. Adopting this work, Gutierrez Reyes et al.(Reyes et al. 2021) combined a targeted parallel reaction monitoring (PRM) with C18-LC for a comprehensive study on the microheterogeneity of haptoglobin. A total of 73 isomeric structures from 42 N-glycopeptides from sites 184Asn, 207Asn, and 241Asn were identified and quantified.
Figure 8.
Extracted ion chromatograms of (A) O-glycopeptide with EAISPPDAASAAPLR backbone from EPO, and (B, C, D) tri-antennary N-glycopeptides containing LVPVPITNATLDR backbone from AGP, separated using a C18 at 30°C, 40°C, 50°C, and 60 °C. Symbols as in Figure 1. Reproduced with the permission from ref (Ji et al. 2019).
Besides native glycopeptides, structure-specific derivatization could also improve the isomeric study of glycopeptides. Zhong et al. presented derivatization of the sialylated glycopeptides (DOSG) method using site-specific derivatization on glycopeptides derived from AGP and EPO (Zhong et al. 2021). In this study, SALSA was first used to derivatize sialylated glycopeptides, and its effects on the peptide backbone were observed and elucidated. This derivatized method selectively discriminates α2,3, and α2,6 linkage of sialic acids isomers thus enables site-specific isomeric profiling of sialylated N- and O-glycopeptides using C18-LC-MS. With the ability to effectively and reliably characterize sialylated glycopeptide isomers, 11 sialylated glycans, including 28 isoforms, were well profiled on Asn72 of AGP. In addition, the simultaneous derivatization of the peptide backbone carboxylic groups prompted a more than four-times increase in signal, making it a promising technique to study sialylated glycopeptide isomers.
5.2. Isomeric separation of glycopeptides on HILIC columns.
HILIC, an alternative type of chromatography for the separation of glycopeptides, has been described to have substantially different selectivity and diverse surface chemistries compared to RPLC. The interaction between glycopeptides and HILIC materials mainly depends on the hydrophilicity of glycan moieties of the glycopeptide, the number of monosaccharides of a glycan moiety, and the polarity of the peptide backbone (Takegawa et al. 2006, Zauner et al. 2010, Kozlik et al. 2017). The mobile phase of HILIC is similar to RPLC, which includes high organic solvents such as acetonitrile with water or some other polar solvents. It is reported that the addition of ammonium formate in the high organic mobile phase enhances the retention of polar analytes due to the presence of solvated ions, which cause the increased volume of water-rich layer on the stationary phase (Hemström and Irgum 2006, Chirita et al. 2011). An obviously increased retention was observed in glycopeptides with monosialylated glycoforms, while only a slight increase in retention was obtained in the neutral glycoforms. By replacing ammonium formate with 0.1% formic acid, the isomeric separation of hemopexin glycopeptides with glycoforms of core and outer arm linked fucose was achieved using a HALO® Penta-HILIC column (Kozlik et al. 2018).
The different packing materials in the HILIC columns also play an important role in glycopeptide separations. Amide, ZIC-HILIC, and Penta-HILIC columns are most widely used for glycopeptide separations as they stand out for their resolution and versatility (Takegawa et al. 2006, Pedrali et al. 2014, Yin et al. 2016, Badgett et al. 2017). The amide column has ethylene bridged hybrid (BEH) particles with trifunctionally-bonded amide as the stationary phase. The ZIC-HILIC column consists of a stationary phase with a zwitterionic sulfoalkybetaine covalently attached to porous silica. As for the Penta-HILIC column, it contains five hydroxyl groups on the bonded ligand. Recently, Gilar et al. have successfully applied an amide HILIC column to separate glycopeptides and their glycoforms from monoclonal antibodies and multiple glycoproteins (Gilar et al. 2011). Hernandez-Hernandez et al. has used a ZIC-HILIC column to separate isomeric O-glycopeptides with linear or branched sialylated trisaccharide glycoforms from bovine caseinomacropeptide (Hernandez-Hernandez et al. 2016). For the penta-HILIC columns, they have been utilized for isomeric separation of fucosylated and sialylated glycopeptides from hemopexin, bovine fetuin, and IgG (Huang et al. 2016, Kozlik et al. 2018).
To understand the separation potential of these three types of HILIC columns, Molnarova et al.(Molnarova and Kozlík 2020) have compared their abilities to separate glycopeptides from well-studied glycoproteins, including human hemopexin and IgG. As shown in Figure 9, the separation efficiencies of fucosylated glycopeptide isomers derived from hemopexin were compared among three different HILIC stationary phases. Figures 9A and D illustrate two peaks representing the core and outer arm A2G2F1 glycoforms of SWPAVGN187CSSALR and ALPQPQN453VTSLLGCTH separated on the HALO® Penta-HILIC column, respectively. Although isomeric separation of A2G2F1 glycoforms of SWPAVGN187CSSALR was observed with 1.72 resolution in the Glycan BEH amide column, there was no separation in the other glycopeptide as shown in Figure 9B and E. For the ZIC-HILIC column, the core and outer arm fucosylated glycoforms of two peptide backbones were not separated even in the optimized gradient condition (Figure 9C and F). In addition to the fucosylated glycopeptide, their result shows that the best separation of sialylated glycopeptides was observed in the HALO® Penta-HILIC column, followed by the Glycan BEH amide column, whereas the ZIC-HILIC column shows the worse separation efficiency. The findings of all these studies above illustrate that HILIC columns are potentially useful tools for isomeric separation of glycopeptides with different glycoforms. By optimizing the mobile phase compositions and choosing the right HILIC stationary phase, isomeric separation on complex glycopeptides can be achieved.
Figure 9.
Normalized EIC chromatograms of A2G2F1 glycoforms of SWPAVGN187CSSALR (A–C) and ALPQPQN453VTSLLGCTH (D–F) in different HILIC columns. PEP1 refers to SWPAVGN187CSSALR, and PEP2 to ALPQPQN453VTSLLGCTH. Symbols as in Figure 1. Reprinted with the permission from ref (Molnarova and Kozlík 2020).
5.3. Isomeric separation of glycopeptides on PGC columns.
PGC, whose retention mechanism is based on the combination of hydrophobic and electrostatic interaction, is another suitable material for the isomeric separation of glycopeptides. PGC was first used to separate glycans and glycopeptides in early 1994 (Hounsell 1994). It has been widely used as a useful stationary phase for LC-MS studies for many years (West et al. 2010). PGC-LC coupled with MS is an efficient method that provides good resolution for the separation of isomeric glycans and is stable within a wide pH range (Wuhrer et al. 2005). This ability has led researchers to investigate the capability of PGC to resolve and separate glycopeptides and their isomers
Since the retention of the glycopeptides on PGC is mainly driven by the hydrophobicity from the peptide backbones or sialylated glycan moieties, glycopeptides with long peptide backbones or high occupancy of sialic acid residues have difficulties in elution (Alley et al. 2009, Thaysen-Andersen et al. 2011). To overcome this problem, several studies have applied nonspecific proteases to create glycopeptides with small peptide backbones (<4–6 amino acids) (An et al. 2003, Froehlich et al. 2011, Hua et al. 2013, Stavenhagen et al. 2015). Although shorter glycopeptides generated from nonspecific proteases treatment were more compatible with PGC-LC analysis, multiple glycopeptides generated simultaneously from the same glycosylation site limit the accuracy of identification and quantification of glycosylation. To address the issue caused by nonspecific proteases, Hua et al.(Hua et al. 2013) proposed a glyco-analytical multispecific proteolysis (Glyco-AMP) strategy to characterize and quantify glycopeptide isomers. Quantitative accuracy, sensitivity, and digestion kinetics of multispecific proteases subtilisin, pronase, and proteinase K were characterized and compared. Instead of lacking specificity, these enzymes possess multiple substrate specificities, such as hydrolyzing peptide backbones at a finite number of sites on a protein. By optimizing glycopeptide digestion using Glyco-AMP, glycopeptides were separated and characterized using an isomer-sensitive PGC nanoLC/MS. Based on their study, O-glycopeptides isomers that have peptide SPPDAASAAP attached to tri-O-acetylated glycoforms from pronase digestion of darbepoetin alfa were successfully separated by the PGC column.
Unlike RPLC, which uses hydrophobic interactions for separation, PGC-LC possesses a unique retention mechanism that enables the separation of both hydrophobic and hydrophilic analytes. Hydrophilic glycan moieties of the glycopeptides exhibit a polar retention effect on graphite (PREG), which influences the separation differently from hydrophobic peptide backbones (Pereira 2008). PREG is relevant to the polarizability and the charge states of the glycopeptides. In the glycan moieties, the charge state of the carboxyl group on sialic acid residues varies due to the different pHs of the mobile phase. Therefore, the pH of the mobile phase is an important factor for the elution of glycopeptides. Recently, Mechref and coworkers first investigated the mobile phase composition on the PGC-LC in order to achieve the isomeric separation of glycopeptides (Zhu et al. 2020). This method was applied to multiple standard glycoproteins with different types of glycan moieties, including high mannose, fucosylated, sialylated, and neutral glycans with both linkage and position isomers. Baseline separations were achieved for all glycopeptides with the pH 9.9 solution. The temperature effect on the isomeric separations of glycopeptides on the PGC column was also studied. Elevating column temperature enhanced the interaction between glycopeptides and the stationary phase, thus improving the separation efficiency. Figure 10 depicts isomeric separation of complex glycopeptides from AGP at 75 °C on PGC-LC-MS. Figure 10A and B show elution profiles of glycopeptides with different antennary types and degrees of sialylation from glycosylation sites 56Asn and 103Asn, respectively. A total of 62 glycopeptide isoforms from 5 glycosylation sites of tryptic digested AGP were identified using PGC-LC-MS. This reliable separation technique in basic conditions and at high temperatures shows its potential to study site-specific isomeric glycosylation and glycopeptide identifications.
Figure 10.
Isomeric separation of glycopeptides composed of different peptide backbones, antennary types, and degrees of sialylation from AGP on PGC. Glycopeptide structures were from glycosylation sites 56Asn (A) and 103Asn (B). Symbols as in Figure 1. Reprinted with the permission from ref (Zhu et al. 2020).
The aforementioned methods have demonstrated their ability to separate glycopeptide isomers on C18-, HILIC- or PGC-LC-MS. However, a comprehensive isomeric separation for glycopeptides has yet been achieved. In comparison to glycomic studies, there are few investigations into the isomeric separation of glycopeptides—these methods only capable of separating core- or branch- fucosylated or sialylated glycopeptide isomers. The studies on the isomeric separation of high-mannose glycopeptides are still insufficient. More studies need to be performed in this field to develop better methods for efficient and sensitive isomeric characterization of glycopeptides.
6. CE-MS analysis of glycan and glycopeptides isomers
6.1. CE-MS of glycan isomers.
While liquid chromatography is a highly popular technique, capillary electrophoresis (CE) is another efficient option when discussing techniques for the separation of glycans and their isomers. Unlike LC, whose separation mechanism is related to the equilibrium of analytes between the mobile phase and stationary phase, the CE-based separation mechanism involves different parameters. For example, capillary zone electrophoresis (CZE) is dependent on the charge and hydrodynamic radius of the analytes. Capillary isoelectric focusing (CIEF) is based on the isoelectric point, while the capillary gel electrophoresis (CGE) relies on the molecular weight of the analytes (Stolz et al. 2019). Providing a different separation mechanism via charge-to-mass ratios, CE can be coupled to MS in order to combine its highly efficient separation capabilities with in-depth structural information. Though CE is more commonly associated with laser-induced fluorescence (LIF), LIF lacks the structural information needed for glycomic studies. Thus, CE-MS is widely utilized in isomeric glycomics.
There are multiple CE techniques that have been utilized for glycomic analysis, including capillary electrochromatography (CEC), capillary gel electrophoresis (CGE), capillary zone electrophoresis (CZE), and microfluidics capillary electrophoresis (MCE). Among them, CZE and MCE are more widely used in the analysis of glycan isomers than CEC and CGE due to the simplicity of their preparation and their high throughput. It is common amongst CE techniques for reducing end tags to be used in order to provide charges to glycans and enhance the ionization and separation of the glycans. The most popular derivatization reagent is 9-aminopyrene-1,3,6-trisulfonic acid (APTS) (Adamczyk et al. 2014, Reusch et al. 2014), though other reagents like TMT (Zhong et al. 2015) and 2-AA (Iwatsuka et al. 2014, Varadi et al. 2016) are also common.
6.1a. Isomeric separation of glycans by CZE.
While CE can be used to describe the entire range of techniques, it is commonly used in reference to capillary zone electrophoresis. CZE combines the ultrahigh resolution of up to a million plates with sample injection amounts in the nanoliters, making it capable of separating glycan isomers efficiently.
In a study that combined CE-LIF with MALDI-TOF-MS2, Huang et al. analyzed APTS labeled IgG N-glycans from rheumatoid arthritis patients (Huang et al. 2017), the results showing efficient separation of positional isomers and partial separation of mono-sialylated isomers. Another study combined CE with ESI-MS to analyze the glycans in pooled plasma and was able to identify sialic acid linkage isomers (Lageveen-Kammeijer et al. 2019). In the first on-line coupling of CE with drift tube ion mobility MS (DTIM-MS), Jooß et al. studied both labeled and unlabeled glycans (Jooß et al. 2019). In this study they were able to show differences in both the APTS labeled and native glycans which were able to be attributed to isomeric differences, showing the effectiveness of coupling the two techniques for glycomic analyses.
6.1b. Isomeric separation of glycans by MCE.
As an alternative to CZE, MCE has attracted much interest in separation of glycans recently. MCE has advantages over conventional CE techniques such as CZE due to its faster speed, higher resolution, lower sample volumes, and variety of coating materials within the manufactured network of microchannels on the microchip (Fu et al. 2007). These microchips can integrate several functions such as sample loading, handling, and separation into a single chip.
These microchips have shown to have the capability to separate glycan isomers through recent studies. In an analysis of monosaccharides, oligosaccharides, and glycopeptides, Khatri et al. found their MCE method capable of separating high mannose and sialic acid isomers, including positional isomers as well as linkage isomers (Khatri et al. 2017). Snyder et al. compared the isomeric separation capabilities of CZE and MCE in a recent study (Snyder et al. 2017). In this study MCE shows higher resolution than CZE, which showed broader peaks for the same isomers that MCE separated with greater efficiency. This along with the faster speed and lower injection volumes of MCE showed the method had an overall higher efficiency in isomeric glycan separation. In another comparative study, Song et al. compared and combined MCE, CZE, MALDI-MS, exoglycosidase digestion, and sialic acid linkage specific alkylamidation (Song et al. 2019). Though isomeric separation was achieved in both CE and MCE, MCE displayed a higher resolution and promoted more qualitative and quantitative analysis of the glycan isomers in comparison.
6.1c. Isomeric separation of glycans by CGE.
As a type of CGE, DNA analyzers have proven to be capable of separating glycan isomers efficiently (Reusch et al. 2014). This technique allows for multiple channel analysis to occur simultaneously, thereby greatly increasing throughput. In a recent study, Feng et al. analyzed the N-glycome expression in chronic kidney disease and heart disease using this technique (Feng et al. 2017). They were able to resolve both linkage and positional isomers using the 16-capillary array of the DNA analyzer.
LIF is still the most commonly used detection method for CE; however, its shortage in acquiring structural information hinders its use in protein glycosylation studies. Although exoglycosidase digestions and GU values could aid the glycan identification, CE-MS or CE-LIF-MS have been widely utilized for isomeric glycomics, which are rich in structural information. However, the interface between CE and MS could be a challenge due to the low flow rate of CE. The ultra-sharp peaks might cause identification issues because MS is a pulse-like detection method, thus demanding a higher request for a scan speed of MS. CE-MS provides a secondary separation dimension to the studies of glycan isomers which is an essential complement to LC-MS techniques.
6.2. CE-MS of glycopeptide isomers.
In addition to glycan isomeric separation, CE is also the method of choice for resolving glycopeptide structures due to its mature separation efficiency (Mechref and Novotny 2009, Gomes and Yates 2019). Compared to LC methods, CE provides advantages, including efficient and rapid separation coupled with low sample consumption. Moreover, the consumptions of organic solvent or aqueous is very low in CE, making it an environmentally friendly method (Týčová et al. 2017). With the development of commercially available interfaces such as coaxial sheath-flow interface, porous tip interface, and nano-electrospray ionization interface, CE is effectively coupled with MS and has attracted considerable interest for proteomic and glycoproteomic studies (Bush et al. 2016, Týčová et al. 2017, Stolz et al. 2019). However, the lack of high-sensitivity and highly stable electrospray interfaces hinder the application of CE-MS for glycopeptide separations. To resolve this, Kammeijer et al. combined a dopant enriched nitrogen gas with a sheathless CE-ESI-MS to enhance the sensitivity and repeatability of glycopeptide analysis (Kammeijer et al. 2016). Khatri et al. integrated a microfluidic separation system with a nano-ESI for intact glycopeptide analysis (Khatri et al. 2017). Qu et al. applied an electrokinetically-pumped nanospray interface to CZE-ESI-MS for fast separation of intact glycopeptides within 9 min (Qu et al. 2018).
Glycopeptide separations using CE are mainly driven by their charge and size. The size of glycans attached to peptide backbones shows an important impact on their electrophoretic mobilities. In addition, sialic acid residues of the glycopeptides carry extra charges, making the migration of sialylated glycopeptides different through the separation capillary (Khatri et al. 2017). The separation and characterization of glycopeptides with different sialic acid linkage isomers are important in biomedicine and biotechnology, such as clinical biomarker discovery (Reis et al. 2010, Nagai-Okatani and Minamino 2016). However, due to similar physicochemical properties of the sialic acid isomers, detailed characterization and separation of sialylated linkages of glycopeptides remain to be challenging using MS. Kammeijer et al. developed an approach that applies CE-ESI-MS to distinguish α2,3- and α2,6-sialylated glycopeptides from IgG Fc glycoproteins and prostate specific antigen (PSA) (Kammeijer et al. 2017). Different pKa values of α2,3- and α2,6-sialylated glycopeptides resulting in their differences in electrophoretic mobilities, enable baseline separations of the α2,3- and α2,6-sialylated linkage isomers. This approach shows its ability to discriminate α2,3- and α2,6-sialylated glycopeptides without prior sample treatment. Extending this method for biological application, they developed a high-performance PSA Glycosylation Assay (PGA) to distinguish and quantify α2,3- and α2,6-sialylated glycopeptides from patients’ urine samples (Kammeijer et al. 2018). Through the efficiency separation ability of the CE-ESI-MS, glycopeptides with different linkage isomers were identified and quantified. Based on their result, the increase of α2,6-sialylated glycopeptides may associate with aggressive prostate cancer. The PGA is a promising method for the early detection of prostate cancer and for the diagnosis of aggressive or non-aggressive tumors. Although CE-MS shows its potential to separate glycopeptides, its ability to resolve and separate complex glycopeptides is limited. There is a clear need for the development of the CE-MS method for a comprehensive study of protein glycosylation.
7. Ion Mobility Mass Spectrometry (IM-MS/MS) analysis of glycan and glycopeptide isomers
Recently, as a novel gas-phase separation technique, IM has been utilized for the separation of glycan and glycopeptide isomers. IM separates ionized analytes according to their mobility through a carrier gas (Kanu et al. 2008), the separated ions are subsequently passed through an MS analyzer (Gutierrez Reyes et al. 2021). In IM, species with different collision cross-sections (CCS) travel with different velocities across a drift cell pulled by an electrical field; thus small ions travel faster than big ions (Baker et al. 2007). Therefore, the specific CCS of an ion provides information on its size and shape, differences that are used to achieve separation of important isomeric analytes (Lanucara et al. 2014). The resolution of IM has a large dependence on the separation path length (Nagy et al. 2018). For drift tube ion mobility (DTIMS), the separation is achieved by passing the ions through a drift gas along the axis of an applied electric field (Kalenius et al. 2019). In traveling wave ion mobility (TWIMS), a traveling voltage wave is applied to a series of electrically connected ring electrodes that pushes the ions through the device (Kalenius et al. 2019). For field asymmetry ion mobility (FAIMS), the ions are introduced into an alternating asymmetric electric field that causes them to drift toward two electrodes at different rates (Kalenius et al. 2019). Structures for lossless ion manipulation (SLIM) uses RF waves applied from multiple adjacent electrodes to prevent ions from closely approaching the planar surfaces, thus allowing for serpentine pathways that increase the separation path length compared to the aforementioned IM methods (Deng et al. 2016, Deng et al. 2017). Either for glycomics and glycoproteomics studies, IM-MS has demonstrated to be capable of separating isomeric structures (Manz et al. 2019, Pathak et al. 2020). Moreover, the evaluation of the CCS differences of the fragment ions allows for the easy identification of isoforms, unlike the MS approaches where enzymatic or derivatization reactions are needed (Harvey et al. 2018).
7.1. IM-MS/MS of glycan isomers.
Initial studies of IM-MS in glycoscience incorporated metal ions and ligands to improve the separation of their isoforms (Fenn and McLean 2008, Huang and Dodds 2013, Hofmann et al. 2015, Huang and Dodds 2015). Huang and Dodds (Huang and Dodds 2013) evaluated the influence of metal ions from group I on the CCS of four groups of isomeric oligosaccharides (Lacto-N-difucohexaose I and II, and lacto-N-fucopentaose I and V), and the changes in resolution of their different isomeric species. Overall, they reported an expected increase in the CCS as the radius of the metal ion increase; however, different metal ion sizes have distinct separation effects upon the CCS of different isomers. Later, Xie et al. explored the influence of group II metals in the isomeric separation of similar glyco-compounds (Xie et al. 2020). Manz et al. evaluated the impact of reducing end modifications on the CCS and isomer separation of glycans using an LC-IM-MS approach (Manz et al. 2019). A set of Lewis isomers were derivatized with fluorescent tags (procainamide, 2-aminobenzoic acid, and 2-aminobenzamide). Their results showed that fluorescent labels could significantly influence the gas-phase structure of glycans, improving isomeric separation. Pallister et al. used a similar strategy with RFMS® labeled glycans to investigate the sialic acid and galactose linkages of the glycan structures F(6)A2G1, and F(6)A2G1S1 (Pallister et al. 2020).
To demonstrate the reliability of an analytical technique, the evaluation of complex biological samples is imperative. Hofmann et al. employed an IM-MS approach to study the isoforms of Lewis antigen group, blood, and milk oligosaccharides, as well for released N-glycans from parotid tissues (Hofmann et al. 2017). They effectively identified the isomeric differences of the evaluated analytes by using their diagnostic fragments and CCS fingerprints. Further fragmentation of the diagnostic ions and their evaluation in a new IM cycle allowed them to differentiate α2,3 and α2,6 linked sialic acid isomers, as shown in Figure 11A. Jin et al. compared the separation properties of PGC-LC-MS and IM-MS/MS using O-glycans released from porcine gastric mucin and human saliva samples (Jin et al. 2019). They investigated the separations of acidic structures, Neu5Ac2Hex2HexNAc2 and Neu5AcHexNAc2, and neutral structures, Hex2HexNAc2 and Hex2HexNAc2dHex. The results showed a comparable resolving power between both techniques but clear advantages in time analysis and structural description for IM.
Figure 11.
(A) Arrival time distributions (ATDs) for Lewis and blood group oligosaccharides measured as sodium adducts. (B) SLIM SUPER IM separation of human milk oligosaccharide isomers LNT and LNnT (31.5 M “top” and 45 m “down”). (C) Differentiation of sialic acid linkage for the N-glycopeptide EVFVHPN-[H5N4S1(α2,3 or α2,6)]YSK using CID fragmentation and subsequent IM-MS analysis. Symbols as in Figure 1. (A) Reproduced with the permission from ref (Hofmann et al. 2017), (B) reproduced with the permission from (Nagy et al. 2018), and (C) reproduced with the permission from (Hinneburg et al. 2016).
In an attempt to advance the available strategies, Warnke et al. performed a SLIM-IM-MS-IR approach to study the glycan’s vibrational infrared (IR) spectrum that is highly precise in differentiating isomerism, which permitted the separation and identification of glycan anomers of the reducing end (Warnke et al. 2019). Recently, Ujma et al. utilized a cyclic IM-TOF MS strategy to separate the alpha and beta anomers of cellopentaose, mannopentaose, and maltopentaose (Ujma et al. 2019). Nagy et al. achieved the isomeric separation of glycans using an ultra-long path drift tube (SLIM-SUPER, 31.5 and 45 m) via switching regions that sent the ions through additional cycles (Nagy et al. 2018). This approach was tested on human milk oligosaccharides and lacto-N-fucopentaose samples and exhibited better resolutions between isomeric glycans than previously SLIM-IMS approaches, as shown in Figure 11B.
In addition to N-glycans, the isomeric separation of O-glycans derived from porcine gastric and human salivary mucins using IM in conjunction with LC-MS was recently demonstrated by Karlsson and coworkers (Jin et al. 2019). According to the authors, the conjunction of IM and LC-MS would be a powerful tool for the full glycome determination of unknown samples.
7.2. IM-MS/MS of glycopeptide isomers.
The importance of glycoproteomic studies lies in the elucidation of glycoprotein microheterogeneity. Therefore, IM represents a potential source of structural information to elucidate the abundant glycopeptide isoforms observed in the glycoproteomic analysis. An initial strategy in the field performed by Campbell et al. combined differential mobility spectrometry (DMS) with electron and collision-based fragmentation methods (ECD/CID-MS) to study the structural glycopeptide isomers of mucin 5AC “GTT(3)PSPVPTTSTT(13)SAP” (Campbell et al. 2017). The results demonstrated that for this case, either DMS-ECD-MS or DMS-CID-MS provide enough information to differentiate between both glycopeptide isomers, and DMS-ECD-MS should be the choice for glycopeptides with large glycan moieties because ECD could result in the cleavage of intact glycan structures, which could be further fragmented and analyzed by CID. Later, Pathak et al. used high-definition FAIMS to describe the multilevel isomerism of mucin glycopeptides, where three GalNAc localization area variants, α and β anomers, and GalNAc/GlcNAc isomers were characterized (Pathak et al. 2020). Both et al. utilized an IM-MS-CID approach to investigate the synthesis of epimeric glycopeptides and differentiated between the isomers glycopeptide + [α-GlcNAc and α-GalNAc], and the isomers glycopeptide + [α-GalNAc and β-GlcNAc] (Both et al. 2014). Barroso et al. used a TWIMS-TOF-MS approach to study isomeric glycopeptides with α2,3 and α2,6 sialic acid linkages (Barroso et al. 2018). The tryptic digested N-glycopeptide N-[H5N4S2]STLCDLCIGPLK from mouse transferrin showed two isoforms that were assigned to the α2,6 and α2,3 isomeric structures, respectively. The sialic acid linkage was confirmed by the arrival time distribution (ATD) of the fragment H1N1S1, where their drift time does not change between both isomers; thus the sialic acid linkage causes the CCS differences of the isomeric forms. The theoretical calculation of the CCS of the isomeric N-glycopeptide was used to define the linkage positions. Recently, Pallister et al. demonstrated that isomeric resolution of the evaluated glycopeptides in IM-MS was affected by their charge states, where [M+3H]3+ and [M+Na+H]3+ ionic species could be well resolved while the [M+2H]2+ ionic species could not (Pallister et al. 2020).
In addition to sialic acid linkage glycopeptide isomers, positional isomers that have the same glycans located on the different sites of the same peptide backbone can also be resolved by IM-MS. Hinneburg et al. reported an IM-MS strategy that was able to resolve the positional isoforms of the synthetic N-glycopeptides YGNPN-[H5N4S2 (α2,6)]ETQNNSTSWPVFK and YGNPNETQN-[H5N4S2 (α2,6)]NSTSWPVFK, as well for the sialic acid isomers of the synthetic N-glycopeptides EVFVHPN-[H5N4S1(α2,3 or α2,6)]YSK (Hinneburg et al. 2016), as shown in Figure 11C. This method differentiated α2,3 and α2,6 sialic acid linkages in N-glycosylated peptides based on the CCSs of diagnostic B3-type fragment ions cleaved directly from mass-selected glycopeptide precursor ions.
Although IM-MS has been utilized in isomeric studies of glycans and glycopeptides, the low resolution in complex biological samples and complex structural molecules has limited its application in comprehensive glycomic and glycoproteomic isomeric analysis. However, the different separation mechanisms and ultra-fast analytical speed make it a potential technique that has a large room for improvement in regards to the isomeric separation of glycans and glycopeptides. The combination of IM and LC could provide a secondary dimension of isomeric separation, which might allow a better separation of glycan and glycopeptide isomers.
8. Bioinformatics tools aiding in the automated identification and quantitation of glycan and glycopeptide isomers
Omics analyses are always based on the analysis of a considerable number of samples. Therefore, high-throughput and reliable data processing software are essential. Compared with proteomics software, where data analysis using software tools has become routine, data processing software for glycomics and glycoproteomics is relatively under-developed. Manual identification of glycans and glycopeptides is the most accurate but time-consuming due to the microheterogeneity of glycosylation (Veillon et al. 2017, Yu et al. 2018). It becomes even more impracticable when analyzing glycomics or glycoproteomics from big cohorts of complex biological samples. Moreover, the challenge with glycoproteomic analysis is the accurate identification of both glycans and peptides. Some glycopeptides have the same peptide backbone but different glycans attached. Therefore, they are more likely to coelute if they have similar glycans (Banazadeh et al. 2017). These challenges can be simplified using robust software tools and encompassing different strategies, which automatically and accurately identify and quantify the glycans and glycopeptides. Although there is still a lack of robust glycan and glycopeptide isomer identification and quantification software programs, to keep in line with the rest of this review paper, various software tools, both commercially available and open-source programs that have been recently developed in glycomics and glycoproteomics analysis are briefly discussed in this section.
GlycoMod is an open-source program linked to the SWISS-PROT database, which is designed to find all possible N- and O-linked oligosaccharides on glycopeptides using their experimentally determined mass data (Cooper et al. 2001). The program can be used to predict the composition of any glycoprotein-derived oligosaccharide comprised of either underivatized, methylated, or acetylated monosaccharides or with a derivatized reducing terminus. However, it does not generate quantitative data. On the other hand, SimGlycan™ is commercially available software that allows the identification and quantification of glycans derivatized in various methods, in addition to permethylation, including aminoxyTMT labeling (Apte and Meitei 2010). SimGlycan™ represents one of the most powerful software solutions for quantitative glycan data analysis and structural elucidation based on MS/MS database searching and scoring techniques. GlycoReSoft is another developed software to assist glycan detection from LC-MS data to compare different samples (Maxwell et al. 2012, Wang et al. 2021). MultiGlycan is additional software developed for LC-MS analysis of permethylated glycans (Hu et al. 2015), where an algorithm was used for deconvolution to diminish false negatives. MultiGlycan can also analyze permethylated glycan data to enable the normalization of reagent purity and isotopic distribution differences (Hu et al. 2015).
More advanced software for glycomics analysis uses partial de novo sequencing, including GlycoDeNovo (Hong et al. 2017) and Glycoforest 1.0 (Horlacher et al. 2017). Unlike the catalog-library approaches (Tseng et al. 1999, Cooper et al. 2001, Joshi et al. 2004, Lohmann and von der Lieth 2004), GlycoDeNovo does not rely on any known glycans database and can be used to determine new structures. It first makes an interpretation-graph specifying how to interpret each peak using preceding interpreted peaks. It subsequently reconstructs the topologies of peaks that contribute to interpreting the precursor ion. These precursor ions are then inspected by IonClassifier, which has initially learned the fragmentation patterns previously from mass spectra of known glycans. Afterward, IonClassifier scores predicted glycan structures. Glycoforest 1.0 is another partial de novo algorithm that utilizes fragmentation information to generate glycan identification (Horlacher et al. 2017).
Additionally, it is good to mention that GlycoWorkbench (Ceroni et al. 2008, Damerell et al. 2012, Damerell et al. 2015) acquires predicting fragment ions for glycans; Cartoonist (Goldberg et al. 2005) provides automated annotation of MALDI-MS analyzed glycans; and software like Glyquest (Gao 2009), Glyco-Peakfinder (Maass et al. 2007), and SysBioWare (Vakhrushev et al. 2009) are also employed in the glycomic data processing. Glyquest, uses a database in conjunction with an integrated search engine to define the composition of peptide-attached N-glycans from CID MS/MS data (Gao 2009). Glyco-Peakfinder is suitable for de novo calculation and annotation of glycan fragment ions within tandem mass spectra (Maass et al. 2007). SysBioWare assesses glycan compositions by searching the spectral peak list of user-input MS data for matches between calculated theoretical glycan masses and corresponding m/z values (Vakhrushev et al. 2009).
Skyline is another freely available and open-source software for targeted proteomics (MacLean et al. 2010), lipidomics (Peng and Ahrends 2016), and metabolomics (Adams et al. 2020), previously used for glycomics study (Loziuk et al. 2017), representing glycome profiling study using its MS1 filtering feature. It was also utilized to analyze glycan data using MS/MS (Ashwood et al. 2018) and later used in conjugation with glucose unit to normalize the retention time and assign each glycan structure with its glucose unit value (Ashwood et al. 2019). Skyline is a versatile software that can simplify data analysis and allow quantitation in omics’ diverse fields.
Many of the available tools to analyze glycopeptides are software developments of tools that have been established previously to analyze released glycans (Woodin et al. 2013). One drawback of expanding glycan analysis tools to glycopeptides is that these tools are generally insufficient for analyzing and scoring the peptide backbone of glycopeptides. SimGlycan™ is one such specimen, which has been updated to perform fragmentation analysis for glycopeptides, in addition to glycans (Blow 2009, Apte and Meitei 2010). Many other open-source tools to illuminate glycosylation profiles of glycopeptides have also emerged out of glycan analysis software. GlycoWorkbench and Glyco-Peakfinder can interpret glycan fragmentation in glycopeptide data, while the peptide backbone of the glycopeptide must be determined by other tools than the use of these software (Maass et al. 2007, Ceroni et al. 2008). Glyco-Peakfinder can de novo glycopeptide analysis through glycan fragmentation profiling for spectra after the peptide sequence is input by the user (Maass et al. 2007). GlycoMod is another open-source software that can determine potential glycopeptide compositions based on mass from MS data (Cooper et al. 2001).
A completely different approach is used in GlycoPep ID (Irungu et al. 2007). GlycoPep ID is a web-based tool to analyze glycopeptides from MS/MS spectra of complex mixtures by identifying the peptide portion based on expected product ions. GlycoMiner is another publicly accessible software, which was specifically designed to interpret the fragmentation of glycopeptides (Ozohanics et al. 2008). The program can identify glycopeptides from qTOF MS/MS data and is able to perform compositional analysis when the spectra are of good quality. Although this software is an excellent progression towards the automated interpretation of glycopeptides MS/MS data, it often generates multiple probable compositions and fails to rank the correct glycopeptides as the top candidate (Ozohanics et al. 2008). Like GlycoMiner, GlycoPeptide Search (GPS) is developed to determine glycopeptide composition from CID data (Pompach et al. 2012). GPS applies a glycan database to generate glycopeptide compositions after searching LC-MS/MS data of purified proteins and matching fragmentation patterns. GlypID is another open-source software, which was designed to identify glycopeptides from LC-MS/MS experiments using a combination of MS1 and MS2 data (Wu et al. 2010). The newer software, GlypID 2.0, utilized high-resolution MS1 data along with CID and HCD scan information to identify glycan type, monosaccharide composition, and attachment site for N-linked glycopeptide MS/MS data (Mayampurath et al. 2011).
GlycoPep Grader (GPG) was also developed as a freely available web-based tool to assign a glycopeptides composition to MS/MS data in an automated fashion (Woodin et al. 2012). This software is particularly designed for data collected in an ion trap mass spectrometer. It features an algorithm that helps users identify the correct glycopeptide composition from a large number of candidate compositions of the same nominal mass (Woodin et al. 2012). MSFragger-Glyco is also recently developed as a glycoproteomics mode of the MSFragger search engine for fast and sensitive identification of N- and O-linked glycopeptides (Polasky et al. 2020). With significantly improved spectral annotation, coupled with the speed of index-based scoring, this open-source software makes it possible to expansively interrogate glycoproteomics data and elucidate the many roles of glycosylation (Polasky et al. 2020).
Besides the abovementioned software, several computational tools have been established for the automated identification of glycopeptides. An enormous number of academically developed computational tools showed potential on automated glycopeptide identification studies, for instance, GlyDB (Ren et al. 2007), GlyFragWork (Mayampurath et al. 2014), GlycoMaster DB (He et al. 2014), GlycoPeptideSearch (Chandler et al. 2013), GlycoPep Detector (Zhu et al. 2013), GlycoPep Evaluator (Zhu et al. 2014), MAGIC (Lynn et al. 2015), Integrated Glyco-Proteome Analyzer (I-GPA) (Park et al. 2016), pGlyco (Zeng et al. 2016), Glyco-DIA (Ye et al. 2019), Protein Prospector (Darula et al. 2010, Baker et al. 2011), SweetNET (Nasir et al. 2016), Sweet-Heart (Wu et al. 2013), and others (Hu et al. 2016, Hu et al. 2017).
Most academic tools are usually open-source and are mainly designed for specific needs, and do not follow up frequently. Moreover, very often, they are lacking an appropriate graphic design making them less user-friendly. On the other hand, commercial tools are designed to be user-friendly, continuously developed further, and upgraded based on the research demands. Although SimGlycan™ (Premier Biosoft) (Apte and Meitei 2010), GlycoQuest, and Byonic™ (Bern et al. 2012) are among the commercially available glycopeptide identification tools, Byonic™ has developed as the most utilized in glycoproteomics analysis due to its flexibility to examine MS/MS data and incorporation with Proteome Discoverer (Thermo Scientific) (Bollineni et al. 2018).
Potent software tools are enormously advancing the depth and precision of glycans and glycopeptides analysis. Such analysis tools will significantly facilitate an inclusive and accurate understanding of the role of glycosylation in organisms. Without them, data interpretation is a challenging and tedious task. However, several difficulties must be resolved in this progression. The first is that most of the available tools are established with N-glycopeptides, excluding O-glycopeptides. Although O-glycans’ strategies and interpretation tools are developing, they are immature and not generic and defined software tools as N-glycopeptides. The second concern is addressing the precise site-specific localization of the glycan, especially for O-glycopeptides. Several glycosylation sites occur contiguously in one digested O-glycopeptides, which presents a substantial challenge for both MS identification and software interpretation. The third is that universal software tools supporting both MS and tandem MS quantification of intact glycopeptides are urgently needed, which is vital for investigations of the glycosylation roles in different biological phases. Last but not least, because the current glycopeptide analysis methods can generate only the composition and a small degree of topology, the analyses of glycan structures and glycan isoforms on glycopeptides are challenges due to the inherent intricacy of glycans. So, widespread improvements in techniques and software tools are desired to attain more delicate and outstanding tools for intact glycopeptide characterization.
9. Concluding remarks and future directions
We have discussed the advances in different techniques commonly utilized for isomeric studies in glycomics and glycoproteomics. MS-based methods have been widely utilized as powerful tools in glycan and glycopeptide isomeric analysis. MALDI-MS is preferred in some clinical research due to the fast analytical time and simple sample preparations. Different structural-specific derivatization methods enable the identification of glycans and glycopeptides in MALDI-MS and significantly benefit from MALDI imaging to visualize the glyco-isomer distributions on a tissue section. Different fragmentation methods in MSn provide structural information of glycan and glycopeptides.
However, the lack of comprehensive isomeric identification of these MS methods without separation prior to MS limited their applications in isomeric glycomics and glycoproteomics. Therefore, LC-MS has become the most common technique to analyze glycan and glycopeptide isomers. According to different derivatizations of glycans and glycopeptides, different columns are employed. HILIC-LC-MS provides separation of several glycan and glycopeptide isomers for native and reducing end derivatized or sialic acid linkage specific derivatized glycans and glycopeptides. C18-LC-MS provides partial separation for permethylated glycans. PGC/MGC, to date, packed with graphitized carbon, permits the most promising separation for both native and permethylated glycan and glycopeptide isomers. Although the isomeric separation of glycans and glycopeptides has been demonstrated, CE-MS was limited by its sensitivity and glycoprotein ID numbers due to its low flow rate and ultra-high resolution, IM-MS provides a novel gas-phase separation of glycan isomers but is hindered by its low resolution. However, CE and IM could be a satisfactory complementary technique to LC-MS because they introduced different separation dimensions than LC, which could contribute to the separation of glycan and glycopeptide isomers. In addition to analysis methods, the development of bioinformatics tools such as glycan and glycopeptide automatic processing software facilitates the characterization of glycans and glycopeptides from biological cohorts. However, to date, none of the software can achieve automated isomeric identification and quantitation of glycans and glycopeptides. The fact that most software is able to assign different retention times to the same structure indicates that this software has potential capabilities to achieve automatically isomeric processing, which demands more communication and collaboration between software developers and glycomic and glycoproteomic scientists.
Although other techniques such as ion exchange chromatography (Veillon et al. 2017) and gas chromatography (Karlsson et al. 1989) can analyze glycan isomers, they are not the major approaches due to the limited resolution and sensitivity. However, none of the existing techniques can achieve a complete elucidation of all glycan and glycopeptide isomer structures from complex biological samples. The issues are usually insufficient isomeric separation and lack of information for the accurate assignment of glycan and glycopeptide isomers. Therefore, more effort should be put in this field to improve the isomeric separation as well as the structural identification of glycan and glycopeptides. The combination of different techniques such as LC, CE, and IM may enhance the isomeric studies. In addition, compared to glycomics, there are fewer studies focused on glycopeptide isomeric analysis due to the higher complexity of glycopeptide isomers, which also needs to be addressed.
Acknowledgment
This work is supported by grants from the National Institutes of Health (R01GM112490, R01GM130091 and U01CA225753).
Biography

Wenjing Peng is a Research Assistant Professor in Dr. Mechref’s group, Chemistry and Biochemistry Department, Texas Tech University. Before working in Texas Tech University, Dr. Peng completed his undergraduate program and master’s degree in Sichuan University, and Ph.D.’s degree in Chengdu Biological Institute, Chinese Academy of Sciences, China. His recent research involves LC-MS based glycomic, proteomic and glycoproteomic studies for methods development and biomarker discovery of cancer diagnosis and progression.

Cristian D. Gutierrez Reyes is currently a Ph.D. student in Analytical Chemistry at Texas Tech University (Dr. Mechref’s Research Laboratory), He received his MS degree in Organic Chemistry from Prairie View A&M University, and BS degree in Biochemical Engineering from Universidad Autonoma de Aguascalientes, Mexico. He also has twelve years of experience in the manufacture of pharmaceutical products.

Sakshi Gautam is a PhD candidate in the Department of Chemistry & Biochemistry at Texas Tech University. She earned her bachelor’s in Biotechnology and master’s in Molecular Biology and Biochemistry from Guru Nanak Dev University in India. She is currently working in the research group of Dr. Yehia Mechref where her research focuses on the development of LC-MS based analytical methods for efficient separation and identification of isomeric glycans and glycopeptides from biological samples.

Aiying Yu is working as a research assistant in Dr. Mechref’s research group. Her research focus on using mass spectrometry for proteomics, glycomics and glycoproteomics studies on biological samples. She gains experience in development and application of LC-MS/MS methods for characterization and identification of protein glycosylation, microfluidic chip application for glycomics and proteomics study using MALDI-TOF and LC-MS/MS analytical techniques. Her current work includes optimization conditions for glycan and glycopeptide separations, LC-MS based glycomic and proteomic studies for potential biomarker discovery of Chronic kidney disease diagnosis.

Byeong Gwan Cho is a doctoral candidate in chemistry at Texas Tech University under supervision of Dr. Yehia Mechref. He earned his Bachelor of Science Degree in Chemistry in 2014 from the University of Texas at Austin. His research is focused on developing bioanalytical methods to characterize glycans derived from complex biological samples using liquid chromatography and mass spectrometry.

Mona Goli received her B.S. degree in Pure Chemistry from Shahid Beheshti University and her M.S. degree in Analytical Chemistry from Mazandaran University, Iran. She had been a college lecturer before pursuing her Ph.D. at Texas Tech University. She is currently a Ph.D. candidate in Analytical Chemistry under the supervision of Prof. Yehia Mechref. Her main research focus is the biomedical application of liquid chromatography-mass spectrometry, especially proteomics, glycomics, and glycoproteomics.

Kaitlyn Donohoo is a second year graduate student at Texas Tech University, where her research focuses on mass spectrometry based glycomics and proteomics. She received a Bachelors of Science in Chemistry in 2018 from Texas Tech University.

Stefania Mondello is a physician-scientist (MD, MPH, PhD) with a sub-specialty in critical care medicine and extensive experience in clinical neurotrauma, biomarker research, and statistical analysis methods. She has done advanced academic work at Banyan Biomarkers, Inc. as Director of Clinical Research on the clinical validation of novel biochemical markers of traumatic brain injury (TBI). Currently, Dr. Mondello has an appointment at the University of Messina as Associate Professor, where she coordinates multidisciplinary laboratories and transitional neuroscience research projects as principal investigator and co-investigator. The pioneering work she has been leading has contributed to developing new biomarkers of brain injury being used in clinical studies worldwide and recently cleared by the FDA. The ultimate goal of her research is to address the clinical utility of brain injury biomarkers and support their adoption in clinical practice as tools to improve patient characterization, assist in clinical decision-making, and inform a rational approach to personalized therapeutic interventions.

Firas Kobeissy is an Associate Professor at the American University of Beirut at the Department of Biochemistry and Molecular Genetics. Dr. Kobeissy obtained his PhD from the University of Florida in the area of Neuroscience with focus on brain injury biomarkers. He is a trained neuroscientist with extensive expertise in the areas of neurotrauma biomarker research and drug abuse research utilizing neuroproteomics approaches. His experience spans different proteomics applications related to brain injury models including closed head injuries, blast injuries as well as clinical studies. Dr. Kobeissy has authored more than 200 articles, reviews, and book chapters along with two patents. He is the associate director of the Center of Neuroproteomics and Biomarker Research (CNBR) at the McKnight Brain Institute at the University of Florida, Department of Emergency Medicine. He is the editor of seven books (Amazon: Springer, Elsevier, CRC Press and Taylor & Francis); these books involves brain injury, biomarker and proteomics research. Currently, Dr. Kobeissy has served on several national and international grant review panels including the NIH, DoD and the Department of the Veteran Affairs; he is the current president for the International Brain Research Organization-MENA chapter (2020–2023).

Yehia Mechref is a Paul W. Horn Distinguished Professor in the department of Chemistry and Biochemistry at Texas Tech University. He is also the Associate Vice President of Research, Chairman of the Department of Chemistry and Biochemistry and the Director of the Center for Biotechnology and Genomics. He received a B.Sc. in chemistry from the American University of Beirut (Beirut, Lebanon) and a Ph.D. with an honorable mention from Oklahoma State University with Professor Ziad El Rassi (Stillwater, OK, USA). He was a postdoctoral fellow at Indiana University where he worked with Dr. Milos V. Novotny before moving to Texas Tech University. Dr. Mechref’s research efforts are focused on the development of sensitive biomolecular mass spectrometry methods that enable qualitative and quantitative assessments of the roles of proteins, glycoproteins, and glycans in biological systems. Dr. Mechref is a member of the editorial boards of several journals. He is currently a standing member of the NIH Enabling Bioanalytical and Imaging Technologies (EBIT) study section panel and has chaired, co-chaired, and participated as an ad-hoc reviewer for over 35 NIH study section panels. Dr. Mechref is currently the co-chair of the steering committee of the NCI Alliance of Glycobiologists for Cancer Research. Dr. Mechref is the recipient of numerous awards, including the 2019 TTU President’s Academic Achievement Award, the 2019 TTU Nancy J. Bell Faculty Excellence in Mentoring Award, the 2016 Barnie E. Rushing Jr. Faculty Distinguished Research Award, and the 2015 Barnie E. Rushing Jr. Faculty Outstanding Research Award.
Footnotes
Conflict of Interest
The authors declare that there is no conflict of interest.
Reference
- Adamczyk B, Tharmalingam-Jaikaran T, Schomberg M, Szekrenyes A, Kelly RM, Karlsson NG, Guttman A and Rudd PM Comparison of separation techniques for the elucidation of IgG N-glycans pooled from healthy mammalian species. Carbohydr Res 2014; 389: 174–185. [DOI] [PubMed] [Google Scholar]
- Adams KJ, Pratt B, Bose N, Dubois LG, St John-Williams L, Perrott KM, Ky K, Kapahi P, Sharma V, MacCoss MJ, Moseley MA, Colton CA, MacLean BX, Schilling B and Thompson JW Skyline for Small Molecules: A Unifying Software Package for Quantitative Metabolomics. J Proteome Res 2020; 19(4): 1447–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alley WR Jr., Mechref Y and Novotny MV Use of activated graphitized carbon chips for liquid chromatography/mass spectrometric and tandem mass spectrometric analysis of tryptic glycopeptides. Rapid Commun Mass Spectrom 2009; 23(4): 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An HJ, Peavy TR, Hedrick JL and Lebrilla CB Determination of N-glycosylation sites and site heterogeneity in glycoproteins. Anal Chem 2003; 75(20): 5628–5637. [DOI] [PubMed] [Google Scholar]
- Ang E, Neustaeter H, Spicer V, Perreault H and Krokhin O Retention Time Prediction for Glycopeptides in Reversed-Phase Chromatography for Glycoproteomic Applications. Anal Chem 2019; 91(21): 13360–13366. [DOI] [PubMed] [Google Scholar]
- Angel PM, Drake RR, Park Y, Clift CL, West C, Berkhiser S, Hardiman G, Mehta AS, Bichell DP and Su YR Spatial N-glycomics of the human aortic valve in development and pediatric endstage congenital aortic valve stenosis. J Mol Cell Cardiol 2021; 154: 6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte A and Meitei NS Bioinformatics in glycomics: glycan characterization with mass spectrometric data using SimGlycan. Methods Mol Biol 2010; 600: 269–281. [DOI] [PubMed] [Google Scholar]
- Apweiler R, Hermjakob H and Sharon N On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database11Dedicated to Prof. Akira Kobata and Prof. Harry Schachter on the occasion of their 65th birthdays. Biochim Biophys Acta 1999; 1473(1): 4–8. [DOI] [PubMed] [Google Scholar]
- Ardejani MS, Noodleman L, Powers ET and Kelly JW Stereoelectronic effects in stabilizing protein-N-glycan interactions revealed by experiment and machine learning. Nat Chem 2021: doi: 10.1038/s41557-41021-00646-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashline DJ, Yu Y, Lasanajak Y, Song X, Hu L, Ramani S, Prasad V, Estes MK, Cummings RD, Smith DF and Reinhold VN Structural characterization by multistage mass spectrometry (MSn) of human milk glycans recognized by human rotaviruses. Mol Cell Proteomics 2014; 13(11): 2961–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashline DJ, Zhang H and Reinhold VN Isomeric complexity of glycosylation documented by MSn. Anal Bioanal Chem 2017; 409(2): 439–451. [DOI] [PubMed] [Google Scholar]
- Ashwood C, Lin CH, Thaysen-Andersen M and Packer NH Discrimination of Isomers of Released N- and O-Glycans Using Diagnostic Product Ions in Negative Ion PGC-LC-ESI-MS/MS. J Am Soc Mass Spectrom 2018; 29(6): 1194–1209. [DOI] [PubMed] [Google Scholar]
- Ashwood C, Pratt B, MacLean BX, Gundry RL and Packer NH Standardization of PGC-LC-MS-based glycomics for sample specific glycotyping. Analyst 2019; 144(11): 3601–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back NK, Smit L, De Jong JJ, Keulen W, Schutten M, Goudsmit J and Tersmette M An N-glycan within the human immunodeficiency virus type 1 gp120 V3 loop affects virus neutralization. Virology 1994; 199(2): 431–438. [DOI] [PubMed] [Google Scholar]
- Badgett MJ, Boyes B and Orlando R Predicting the Retention Behavior of Specific O-Linked Glycopeptides. J Biomol Tech 2017; 28(3): 122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker ES, Clowers BH, Li F, Tang K, Tolmachev AV, Prior DC, Belov ME and Smith RD Ion mobility spectrometry-mass spectrometry performance using electrodynamic ion funnels and elevated drift gas pressures. J Am Soc Mass Spectrom 2007; 18(7): 1176–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PR, Trinidad JC and Chalkley RJ Modification site localization scoring integrated into a search engine. Mol Cell Proteomics 2011; 10(7): M111.008078. doi: 008010.001074/mcp.M008111.008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banazadeh A, Peng W, Veillon L and Mechref Y Carbon Nanoparticles and Graphene Nanosheets as MALDI Matrices in Glycomics: a New Approach to Improve Glycan Profiling in Biological Samples. J Am Soc Mass Spectrom 2018; 29(9): 1892–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banazadeh A, Veillon L, Wooding KM, Zabet-Moghaddam M and Mechref Y Recent advances in mass spectrometric analysis of glycoproteins. Electrophoresis 2017; 38(1): 162–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bapiro TE, Richards FM and Jodrell DI Understanding the Complexity of Porous Graphitic Carbon (PGC) Chromatography: Modulation of Mobile-Stationary Phase Interactions Overcomes Loss of Retention and Reduces Variability. Anal Chem 2016; 88(12): 6190–6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso A, Giménez E, Konijnenberg A, Sancho J, Sanz-Nebot V and Sobott F Evaluation of ion mobility for the separation of glycoconjugate isomers due to different types of sialic acid linkage, at the intact glycoprotein, glycopeptide and glycan level. J Proteom 2018; 173: 22–31. [DOI] [PubMed] [Google Scholar]
- Bermingham ML, Colombo M, McGurnaghan SJ, Blackbourn LAK, Vučković F, Pučić Baković M, Trbojević-Akmačić I, Lauc G, Agakov F, Agakova AS, Hayward C, Klarić L, Palmer CNA, Petrie JR, Chalmers J, Collier A, Green F, Lindsay RS, Macrury S, McKnight JA, Patrick AW, Thekkepat S, Gornik O, McKeigue PM and Colhoun HM N-Glycan Profile and Kidney Disease in Type 1 Diabetes. Diabetes Care 2018; 41(1): 79–87. [DOI] [PubMed] [Google Scholar]
- Bern M, Kil YJ and Becker C Byonic: advanced peptide and protein identification software. Curr Protoc Bioinformatics 2012; Chapter 13: Unit13.20. doi: 10.1002/0471250953.bi0471251320s0471250940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschke CRK, Black AP, Mehta AS, Angel PM and Drake RR Rapid N-Glycan Profiling of Serum and Plasma by a Novel Slide-Based Imaging Mass Spectrometry Workflow. J Am Soc Mass Spectrom 2020; 31(12): 2511–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomme B, Van Steenkiste C, Callewaert N and Van Vlierberghe H Alteration of protein glycosylation in liver diseases. J Hepatol 2009; 50(3): 592–603. [DOI] [PubMed] [Google Scholar]
- Blow N Glycobiology: A spoonful of sugar. Nature 2009; 457(7229): 617–620. [DOI] [PubMed] [Google Scholar]
- Bollineni RC, Koehler CJ, Gislefoss RE, Anonsen JH and Thiede B Large-scale intact glycopeptide identification by Mascot database search. Sci Rep 2018; 8(1): 2117. doi: 2110.1038/s41598–41018-20331–41592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both P, Green AP, Gray CJ, Šardzík R, Voglmeir J, Fontana C, Austeri M, Rejzek M, Richardson D, Field RA, Widmalm G, Flitsch SL and Eyers CE Discrimination of epimeric glycans and glycopeptides using IM-MS and its potential for carbohydrate sequencing. Nat Chem 2014; 6(1): 65–74. [DOI] [PubMed] [Google Scholar]
- Bunz SC, Cutillo F and Neusüß C Analysis of native and APTS-labeled N-glycans by capillary electrophoresis/time-of-flight mass spectrometry. Anal Bioanal Chem 2013; 405(25): 8277–8284. [DOI] [PubMed] [Google Scholar]
- Bush DR, Zang L, Belov AM, Ivanov AR and Karger BL High Resolution CZE-MS Quantitative Characterization of Intact Biopharmaceutical Proteins: Proteoforms of Interferon-β1. Anal Chem 2016; 88(2): 1138–1146. [DOI] [PubMed] [Google Scholar]
- Campbell JL, Baba T, Liu C, Lane CS, Le Blanc JCY and Hager JW Analyzing Glycopeptide Isomers by Combining Differential Mobility Spectrometry with Electron- and Collision-Based Tandem Mass Spectrometry. J Am Soc Mass Spectrom 2017; 28(7): 1374–1381. [DOI] [PubMed] [Google Scholar]
- Ceroni A, Maass K, Geyer H, Geyer R, Dell A and Haslam SM GlycoWorkbench: a tool for the computer-assisted annotation of mass spectra of glycans. J Proteome Res 2008; 7(4): 1650–1659. [DOI] [PubMed] [Google Scholar]
- Chandler KB, Pompach P, Goldman R and Edwards N Exploring site-specific N-glycosylation microheterogeneity of haptoglobin using glycopeptide CID tandem mass spectra and glycan database search. J Proteome Res 2013; 12(8): 3652–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran R and Lacy DB The role of toxins in Clostridium difficile infection. FEMS Microbiol Rev 2017; 41(6): 723–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SY, Dong M, Yang G, Zhou Y, Clark DJ, Lih TM, Schnaubelt M, Liu Z and Zhang H Glycans, Glycosite, and Intact Glycopeptide Analysis of N-Linked Glycoproteins Using Liquid Handling Systems. Anal Chem 2020; 92(2): 1680–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Glover MS and Li L Recent advances in ion mobility-mass spectrometry for improved structural characterization of glycans and glycoconjugates. Curr Opin Chem Biol 2018; 42: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Yu Q, Hao L, Liu F, Johnson J, Tian Z, Kao WJ, Xu W and Li L Site-specific characterization and quantitation of N-glycopeptides in PKM2 knockout breast cancer cells using DiLeu isobaric tags enabled by electron-transfer/higher-energy collision dissociation (EThcD). Analyst 2018; 143(11): 2508–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirita RI, West C, Zubrzycki S, Finaru AL and Elfakir C Investigations on the chromatographic behaviour of zwitterionic stationary phases used in hydrophilic interaction chromatography. J Chromatogr A 2011; 1218(35): 5939–5963. [DOI] [PubMed] [Google Scholar]
- Cho BG, Gutierrez Reyes CD and Mechref Y N-Glycomics of Cerebrospinal Fluid: Method Comparison. Molecules 2021; 26(6): 1712. doi: 1710.3390/molecules26061712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho BG, Jiang P, Goli M, Gautam S and Mechref Y Using Micro Pillar Array Columns (μPAC) for the Analysis of Permethylated Glycans. Analyst 2021: Submitted. [DOI] [PubMed] [Google Scholar]
- Cho BG, Peng W and Mechref Y Separation of Permethylated O-Glycans, Free Oligosaccharides, and Glycosphingolipid-Glycans Using Porous Graphitized Carbon (PGC) Column. Metabolites 2020; 10(11): 433. doi: 410.3390/metabo10110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho BG, Veillon L and Mechref Y N-Glycan Profile of Cerebrospinal Fluids from Alzheimer’s Disease Patients Using Liquid Chromatography with Mass Spectrometry. J Proteome Res 2019; 18(10): 3770–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, Murphy BA, Satinover SM, Hosen J, Mauro D, Slebos RJ, Zhou Q, Gold D, Hatley T, Hicklin DJ and Platts-Mills TA Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med 2008; 358(11): 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CA, Gasteiger E and Packer NH GlycoMod--a software tool for determining glycosylation compositions from mass spectrometric data. Proteomics 2001; 1(2): 340–349. [DOI] [PubMed] [Google Scholar]
- Costello CE, Contado-Miller JM and Cipollo JF A glycomics platform for the analysis of permethylated oligosaccharide alditols. J Am Soc Mass Spectrom 2007; 18(10): 1799–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damerell D, Ceroni A, Maass K, Ranzinger R, Dell A and Haslam SM Annotation of glycomics MS and MS/MS spectra using the GlycoWorkbench software tool. Methods Mol Biol 2015; 1273: 3–15. [DOI] [PubMed] [Google Scholar]
- Damerell D, Ceroni A, Maass K, Ranzinger R, Dell A and Haslam SM The GlycanBuilder and GlycoWorkbench glycoinformatics tools: updates and new developments. Biol Chem 2012; 393(11): 1357–1362. [DOI] [PubMed] [Google Scholar]
- Darula Z, Chalkley RJ, Baker P, Burlingame AL and Medzihradszky KF Mass spectrometric analysis, automated identification and complete annotation of O-linked glycopeptides. Eur J Mass Spectrom (Chichester) 2010; 16(3): 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davril M, Degroote S, Humbert P, Galabert C, Dumur V, Lafitte J-J, Lamblin G and Roussel P The sialylation of bronchial mucins secreted by patients suffering from cystic fibrosis or from chronic bronchitis is related to the severity of airway infection. Glycobiology 1999; 9(3): 311–321. [DOI] [PubMed] [Google Scholar]
- de Haan N, Reiding KR, Haberger M, Reusch D, Falck D and Wuhrer M Linkage-specific sialic acid derivatization for MALDI-TOF-MS profiling of IgG glycopeptides. Anal Chem 2015; 87(16): 8284–8291. [DOI] [PubMed] [Google Scholar]
- Deguchi K, Keira T, Yamada K, Ito H, Takegawa Y, Nakagawa H and Nishimura S Two-dimensional hydrophilic interaction chromatography coupling anion-exchange and hydrophilic interaction columns for separation of 2-pyridylamino derivatives of neutral and sialylated N-glycans. J Chromatogr A 2008; 1189(1–2): 169–174. [DOI] [PubMed] [Google Scholar]
- Deng L, Ibrahim YM, Hamid AM, Garimella SVB, Webb IK, Zheng X, Prost SA, Sandoval JA, Norheim RV, Anderson GA, Tolmachev AV, Baker ES and Smith RD Ultra-High Resolution Ion Mobility Separations Utilizing Traveling Waves in a 13 m Serpentine Path Length Structures for Lossless Ion Manipulations Module. Anal Chem 2016; 88(18): 8957–8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Webb IK, Garimella SVB, Hamid AM, Zheng X, Norheim RV, Prost SA, Anderson GA, Sandoval JA, Baker ES, Ibrahim YM and Smith RD Serpentine Ultralong Path with Extended Routing (SUPER) High Resolution Traveling Wave Ion Mobility-MS using Structures for Lossless Ion Manipulations. Anal Chem 2017; 89(8): 4628–4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depraz Depland A, Renois-Predelus G, Schindler B and Compagnon I Identification of sialic acid linkage isomers in glycans using coupled InfraRed Multiple Photon Dissociation (IRMPD) spectroscopy and mass spectrometry. Int J Mass Spectrom 2018; 434: 65–69. [Google Scholar]
- Desantos-Garcia JL, Khalil SI, Hussein A, Hu Y and Mechref Y Enhanced sensitivity of LC-MS analysis of permethylated N-glycans through online purification. Electrophoresis 2011; 32(24): 3516–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devakumar A, Mechref Y, Kang P, Novotny MV and Reilly JP Identification of isomeric N-glycan structures by mass spectrometry with 157 nm laser-induced photofragmentation. J Am Soc Mass Spectrom 2008; 19(7): 1027–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhakal R, Nieman R, Valente DCA, Cardozo TM, Jayee B, Aqdas A, Peng W, Aquino AJA, Mechref Y, Lischka H and Moussa H A General New Method for Calculating the Molecular Nonpolar Surface for Analysis of LC-MS Data. Int J Mass Spectrom 2021; 461: 116495. doi: 116410.111016/j.ijms.112020.116495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Huang Y, Cho BG, Zhong J, Gautam S, Peng W, Williamson SD, Banazadeh A, Torres-Ulloa KY and Mechref Y Advances in mass spectrometry-based glycomics. Electrophoresis 2018; 39(24): 3063–3081. [DOI] [PubMed] [Google Scholar]
- Dong X, Mondello S, Kobeissy F, Ferri R and Mechref Y Serum Glycomics Profiling of Patients with Primary Restless Legs Syndrome Using LC-MS/MS. J Proteome Res 2020; 19(8): 2933–2941. [DOI] [PubMed] [Google Scholar]
- Dong X, Mondello S, Kobeissy F, Talih F, Ferri R and Mechref Y LC-MS/MS glycomics of idiopathic rapid eye movement sleep behavior disorder. Electrophoresis 2018; 39(24): 3096–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Peng W, Yu CY, Zhou S, Donohoo KB, Tang H and Mechref Y 8-plex LC-MS/MS Analysis of Permethylated N-Glycans Achieved by Using Stable Isotopic Iodomethane. Anal Chem 2019; 91(18): 11794–11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Zhou S and Mechref Y LC-MS/MS analysis of permethylated free oligosaccharides and N-glycans derived from human, bovine, and goat milk samples. Electrophoresis 2016; 37(11): 1532–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar EE, King DT, Serrano-Negrón JE, Alteen MG, Vocadlo DJ and Brodbelt JS Precision Mapping of O-Linked N-Acetylglucosamine Sites in Proteins Using Ultraviolet Photodissociation Mass Spectrometry. J Am Chem Soc 2020; 142(26): 11569–11577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everest-Dass AV, Moh ESX, Ashwood C, Shathili AMM and Packer NH Human disease glycomics: technology advances enabling protein glycosylation analysis - part 1. Expert Rev Proteomics 2018; 15(2): 165–182. [DOI] [PubMed] [Google Scholar]
- Everest-Dass AV, Moh ESX, Ashwood C, Shathili AMM and Packer NH Human disease glycomics: technology advances enabling protein glycosylation analysis - part 2. Expert Rev Proteomics 2018; 15(4): 341–352. [DOI] [PubMed] [Google Scholar]
- Feng HT, Lim S, Laserna AKC, Li P, Yin X, Simsek E, Khan SH, Chen SM and Li SFY High throughput human plasma N-glycan analysis using DNA analyzer and multivariate analysis for biomarker discovery. Anal Chim Acta 2017; 995: 106–113. [DOI] [PubMed] [Google Scholar]
- Fenn LS and McLean JA Enhanced carbohydrate structural selectivity in ion mobility-mass spectrometry analyses by boronic acid derivatization. Chem Commun (Camb) 2008;(43): 5505–5507. [DOI] [PubMed] [Google Scholar]
- Freeze HH, Chong JX, Bamshad MJ and Ng BG Solving glycosylation disorders: fundamental approaches reveal complicated pathways. Am J Hum Genet 2014; 94(2): 161–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JW, Barboza M, Chu C, Lerno LA Jr., Clowers BH, Zivkovic AM, German JB and Lebrilla CB Nano-LC-MS/MS of glycopeptides produced by nonspecific proteolysis enables rapid and extensive site-specific glycosylation determination. Anal Chem 2011; 83(14): 5541–5547. [DOI] [PubMed] [Google Scholar]
- Fu LM, Leong JC, Lin CF, Tai CH and Tsai CH High performance microfluidic capillary electrophoresis devices. Biomed Microdevices 2007; 9(3): 405–412. [DOI] [PubMed] [Google Scholar]
- Furukawa JI, Hanamatsu H, Nishikaze T, Manya H, Miura N, Yagi H, Yokota I, Akasaka-Manya K, Endo T, Kanagawa M, Iwasaki N and Tanaka K Lactone-Driven Ester-to-Amide Derivatization for Sialic Acid Linkage-Specific Alkylamidation. Anal Chem 2020; 92(21): 14383–14392. [DOI] [PubMed] [Google Scholar]
- Gao HY Generation of asparagine-linked glycan structure databases and their use. J Am Soc Mass Spectrom 2009; 20(9): 1739–1742. [DOI] [PubMed] [Google Scholar]
- Gautam S, Banazadeh A, Cho BG, Goli M, Zhong J and Mechref Y Mesoporous Graphitized Carbon Column for Efficient Isomeric Separation of Permethylated Glycans. Anal Chem 2021; 93(12): 5061–5070. [DOI] [PubMed] [Google Scholar]
- Gautam S, Peng W, Cho BG, Huang Y, Banazadeh A, Yu A, Dong X and Mechref Y Glucose unit index (GUI) of permethylated glycans for effective identification of glycans and glycan isomers. Analyst 2020; 145(20): 6656–6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil GC, Kim YG and Kim BG A relative and absolute quantification of neutral N-linked oligosaccharides using modification with carboxymethyl trimethylammonium hydrazide and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal Biochem 2008; 379(1): 45–59. [DOI] [PubMed] [Google Scholar]
- Gilar M, Yu YQ, Ahn J, Xie H, Han H, Ying W and Qian X Characterization of glycoprotein digests with hydrophilic interaction chromatography and mass spectrometry. Anal Biochem 2011; 417(1): 80–88. [DOI] [PubMed] [Google Scholar]
- Glover MS, Yu Q, Chen Z, Shi X, Kent KC and Li L Characterization of intact sialylated glycopeptides and phosphorylated glycopeptides from IMAC enriched samples by EThcD fragmentation: Toward combining phosphoproteomics and glycoproteomics. Int J Mass Spectrom 2018; 427: 35–42. [Google Scholar]
- Goldberg D, Sutton-Smith M, Paulson J and Dell A Automatic annotation of matrix-assisted laser desorption/ionization N-glycan spectra. Proteomics 2005; 5(4): 865–875. [DOI] [PubMed] [Google Scholar]
- Gomes de Oliveira AG, Roy R, Raymond C, Bodnar ED, Tayi VS, Butler M, Durocher Y and Perreault H A systematic study of glycopeptide esterification for the semi‐quantitative determination of sialylation in antibodies. Rapid Commun Mass Spectrom 2015; 29(19): 1817–1826. [DOI] [PubMed] [Google Scholar]
- Gomes FP and Yates JR 3rd Recent trends of capillary electrophoresis-mass spectrometry in proteomics research. Mass Spectrom Rev 2019; 38(6): 445–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H and Abbott KL Functional impact of tumor-specific N-linked glycan changes in breast and ovarian cancers. Adv Cancer Res 2015; 126: 281–303. [DOI] [PubMed] [Google Scholar]
- Gutierrez Reyes CD, Jiang P, Donohoo K, Atashi M and Mechref YS Glycomics and glycoproteomics: Approaches to address isomeric separation of glycans and glycopeptides. J Sep Sci 2021; 44(1): 403–425. [DOI] [PubMed] [Google Scholar]
- Han L and Costello CE Mass spectrometry of glycans. Biochemistry (Mosc) 2013; 78(7): 710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamatsu H, Nishikaze T, Miura N, Piao J, Okada K, Sekiya S, Iwamoto S, Sakamoto N, Tanaka K and Furukawa JI Sialic Acid Linkage Specific Derivatization of Glycosphingolipid Glycans by Ring-Opening Aminolysis of Lactones. Anal Chem 2018; 90(22): 13193–13199. [DOI] [PubMed] [Google Scholar]
- Hanamatsu H, Nishikaze T, Tsumoto H, Ogawa K, Kobayashi T, Yokota I, Morikawa K, Suda G, Sho T, Nakai M, Miura N, Higashino K, Sekiya S, Iwamoto S, Miura Y, Furukawa JI, Tanaka K and Sakamoto N Comparative Glycomic Analysis of Sialyl Linkage Isomers by Sialic Acid Linkage-Specific Alkylamidation in Combination with Stable Isotope Labeling of α2,3-Linked Sialic Acid Residues. Anal Chem 2019; 91(21): 13343–13348. [DOI] [PubMed] [Google Scholar]
- Hanisch FG and Müller S Analysis of methylated O-glycan alditols by reversed-phase NanoLC coupled CAD-ESI mass spectrometry. Methods Mol Biol 2009; 534: 107–115. [DOI] [PubMed] [Google Scholar]
- Hart GW and Copeland RJ Glycomics hits the big time. Cell 2010; 143(5): 672–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey DJ, Seabright GE, Vasiljevic S, Crispin M and Struwe WB Isomer Information from Ion Mobility Separation of High-Mannose Glycan Fragments. J Am Soc Mass Spectrom 2018; 29(5): 972–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Xin L, Shan B, Lajoie GA and Ma B GlycoMaster DB: software to assist the automated identification of N-linked glycopeptides by tandem mass spectrometry. J Proteome Res 2014; 13(9): 3881–3895. [DOI] [PubMed] [Google Scholar]
- Hemström P and Irgum K Hydrophilic interaction chromatography. J Sep Sci 2006; 29(12): 1784–1821. [DOI] [PubMed] [Google Scholar]
- Hernandez-Hernandez O, Quintanilla-Lopez JE, Lebron-Aguilar R, Sanz ML and Moreno FJ Characterization of post-translationally modified peptides by hydrophilic interaction and reverse phase liquid chromatography coupled to quadrupole-time-of-flight mass spectrometry. J Chromatogr A 2016; 1428: 202–211. [DOI] [PubMed] [Google Scholar]
- Herrera H, Dilday T, Uber A, Scott D, Zambrano JN, Wang M, Angel PM, Mehta AS, Drake RR, Hill EG and Yeh ES Core-Fucosylated Tetra-Antennary N-Glycan Containing A Single N-Acetyllactosamine Branch Is Associated with Poor Survival Outcome in Breast Cancer. Int J Mol Sci 2019; 20(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinneburg H, Hofmann J, Struwe WB, Thader A, Altmann F, Varón Silva D, Seeberger PH, Pagel K and Kolarich D Distinguishing N-acetylneuraminic acid linkage isomers on glycopeptides by ion mobility-mass spectrometry. Chem Commun 2016; 52(23): 4381–4384. [DOI] [PubMed] [Google Scholar]
- Hofmann J, Hahm HS, Seeberger PH and Pagel K Identification of carbohydrate anomers using ion mobility–mass spectrometry. Nature 2015; 526(7572): 241–244. [DOI] [PubMed] [Google Scholar]
- Hofmann J, Stuckmann A, Crispin M, Harvey DJ, Pagel K and Struwe WB Identification of Lewis and Blood Group Carbohydrate Epitopes by Ion Mobility-Tandem-Mass Spectrometry Fingerprinting. Anal Chem 2017; 89(4): 2318–2325. [DOI] [PubMed] [Google Scholar]
- Holst S, Heijs B, de Haan N, van Zeijl RJ, Briaire-de Bruijn IH, van Pelt GW, Mehta AS, Angel PM, Mesker WE, Tollenaar RA, Drake RR, Bovée JV, McDonnell LA and Wuhrer M Linkage-Specific in Situ Sialic Acid Derivatization for N-Glycan Mass Spectrometry Imaging of Formalin-Fixed Paraffin-Embedded Tissues. Anal Chem 2016; 88(11): 5904–5913. [DOI] [PubMed] [Google Scholar]
- Hong P, Sun H, Sha L, Pu Y, Khatri K, Yu X, Tang Y and Lin C GlycoDeNovo - an Efficient Algorithm for Accurate de novo Glycan Topology Reconstruction from Tandem Mass Spectra. J Am Soc Mass Spectrom 2017; 28(11): 2288–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horlacher O, Jin C, Alocci D, Mariethoz J, Müller M, Karlsson NG and Lisacek F Glycoforest 1.0. Anal Chem 2017; 89(20): 10932–10940. [DOI] [PubMed] [Google Scholar]
- Hosokawa S, Nakamura K, Fujita Y, Horiuchi R and Yamamoto K Determination of isepamicin in human plasma by HPLC with fluorescence detection after derivatization using 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate. Biol Pharm Bull 2008; 31(10): 1866–1869. [DOI] [PubMed] [Google Scholar]
- Hounsell EF Characterization of the glycosylation status of proteins. Mol Biotechnol 1994; 2(1): 45–60. [DOI] [PubMed] [Google Scholar]
- Hu H, Khatri K, Klein J, Leymarie N and Zaia J A review of methods for interpretation of glycopeptide tandem mass spectral data. Glycoconj J 2016; 33(3): 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Khatri K and Zaia J Algorithms and design strategies towards automated glycoproteomics analysis. Mass Spectrom Rev 2017; 36(4): 475–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Zhou S, Yu CY, Tang H and Mechref Y Automated annotation and quantitation of glycans by liquid chromatography/electrospray ionization mass spectrometric analysis using the MultiGlycan-ESI computational tool. Rapid Commun Mass Spectrom 2015; 29(1): 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua S, Hu CY, Kim BJ, Totten SM, Oh MJ, Yun N, Nwosu CC, Yoo JS, Lebrilla CB and An HJ Glyco-analytical multispecific proteolysis (Glyco-AMP): a simple method for detailed and quantitative Glycoproteomic characterization. J Proteome Res 2013; 12(10): 4414–4423. [DOI] [PubMed] [Google Scholar]
- Hua S, Nwosu CC, Strum JS, Seipert RR, An HJ, Zivkovic AM, German JB and Lebrilla CB Site-specific protein glycosylation analysis with glycan isomer differentiation. Anal Bioanal Chem 2012; 403(5): 1291–1302. [DOI] [PubMed] [Google Scholar]
- Hua S, Williams CC, Dimapasoc LM, Ro GS, Ozcan S, Miyamoto S, Lebrilla CB, An HJ and Leiserowitz GS Isomer-specific chromatographic profiling yields highly sensitive and specific potential N-glycan biomarkers for epithelial ovarian cancer. J Chromatogr A 2013; 1279: 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Liu Y, Wu H, Sun D and Li Y Characterization of IgG glycosylation in rheumatoid arthritis patients by MALDI-TOF-MS(n) and capillary electrophoresis. Anal Bioanal Chem 2017; 409(15): 3731–3739. [DOI] [PubMed] [Google Scholar]
- Huang Y and Dodds ED Discrimination of Isomeric Carbohydrates as the Electron Transfer Products of Group II Cation Adducts by Ion Mobility Spectrometry and Tandem Mass Spectrometry. Anal Chem 2015; 87(11): 5664–5668. [DOI] [PubMed] [Google Scholar]
- Huang Y and Dodds ED Ion mobility studies of carbohydrates as group I adducts: isomer specific collisional cross section dependence on metal ion radius. Anal Chem 2013; 85(20): 9728–9735. [DOI] [PubMed] [Google Scholar]
- Huang Y, Nie Y, Boyes B and Orlando R Resolving Isomeric Glycopeptide Glycoforms with Hydrophilic Interaction Chromatography (HILIC). J Biomol Tech 2016; 27(3): 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Zhou S, Zhu J, Lubman DM and Mechref Y LC-MS/MS isomeric profiling of permethylated N-glycans derived from serum haptoglobin of hepatocellular carcinoma (HCC) and cirrhotic patients. Electrophoresis 2017; 38(17): 2160–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irungu J, Go EP, Dalpathado DS and Desaire H Simplification of mass spectral analysis of acidic glycopeptides using GlycoPep ID. Anal Chem 2007; 79(8): 3065–3074. [DOI] [PubMed] [Google Scholar]
- Iwatsuka K, Iwamoto H, Kinoshita M, Inada K, Yasueda S and Kakehi K Comparative studies of N-glycans and glycosaminoglycans present in SIRC (Statens Seruminstitut rabbit cornea) cells and corneal epithelial cells from rabbit eyes. Curr Eye Res 2014; 39(7): 686–694. [DOI] [PubMed] [Google Scholar]
- Jensen PH, Mysling S, Højrup P and Jensen ON Glycopeptide enrichment for MALDI-TOF mass spectrometry analysis by hydrophilic interaction liquid chromatography solid phase extraction (HILIC SPE). Methods Mol Biol 2013; 951: 131–144. [DOI] [PubMed] [Google Scholar]
- Ji ES, Lee HK, Park GW, Kim KH, Kim JY and Yoo JS Isomer separation of sialylated O- and N-linked glycopeptides using reversed-phase LC-MS/MS at high temperature. J Chromatogr B Analyt Technol Biomed Life Sci 2019; 1110–1111: 101–107. [DOI] [PubMed] [Google Scholar]
- Jiang K, Wang C, Sun Y, Liu Y, Zhang Y, Huang L and Wang Z Comparison of chicken and pheasant ovotransferrin N-glycoforms via electrospray ionization mass spectrometry and liquid chromatography coupled with mass spectrometry. J Agric Food Chem 2014; 62(29): 7245–7254. [DOI] [PubMed] [Google Scholar]
- Jiang K, Zhu H, Xiao C, Liu D, Edmunds G, Wen L, Ma C, Li J and Wang PG Solid-phase reductive amination for glycomic analysis. Anal Chim Acta 2017; 962: 32–40. [DOI] [PubMed] [Google Scholar]
- Jin C, Harvey DJ, Struwe WB and Karlsson NG Separation of Isomeric O-Glycans by Ion Mobility and Liquid Chromatography–Mass Spectrometry. Anal Chem 2019; 91(16): 10604–10613. [DOI] [PubMed] [Google Scholar]
- Jooß K, Meckelmann SW, Klein J, Schmitz OJ and Neususs C Capillary zone electrophoresis coupled to drift tube ion mobility-mass spectrometry for the analysis of native and APTS-labeled N-glycans. Anal Bioanal Chem 2019; 411(24): 6255–6264. [DOI] [PubMed] [Google Scholar]
- Joshi HJ, Harrison MJ, Schulz BL, Cooper CA, Packer NH and Karlsson NG Development of a mass fingerprinting tool for automated interpretation of oligosaccharide fragmentation data. Proteomics 2004; 4(6): 1650–1664. [DOI] [PubMed] [Google Scholar]
- Kailemia MJ, Park D and Lebrilla CB Glycans and glycoproteins as specific biomarkers for cancer. Anal Bioanal Chem 2017; 409(2): 395–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalenius E, Groessl M and Rissanen K Ion mobility–mass spectrometry of supramolecular complexes and assemblies. Nat Rev Chem 2019; 3(1): 4–14. [Google Scholar]
- Kammeijer GS, Kohler I, Jansen BC, Hensbergen PJ, Mayboroda OA, Falck D and Wuhrer M Dopant Enriched Nitrogen Gas Combined with Sheathless Capillary Electrophoresis-Electrospray Ionization-Mass Spectrometry for Improved Sensitivity and Repeatability in Glycopeptide Analysis. Anal Chem 2016; 88(11): 5849–5856. [DOI] [PubMed] [Google Scholar]
- Kammeijer GSM, Jansen BC, Kohler I, Heemskerk AAM, Mayboroda OA, Hensbergen PJ, Schappler J and Wuhrer M Sialic acid linkage differentiation of glycopeptides using capillary electrophoresis - electrospray ionization - mass spectrometry. Sci Rep 2017; 7(1): 3733. doi: 3710.1038/s41598–41017-03838-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammeijer GSM, Nouta J, de la Rosette J, de Reijke TM and Wuhrer M An In-Depth Glycosylation Assay for Urinary Prostate-Specific Antigen. Anal Chem 2018; 90(7): 4414–4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P, Mechref Y, Klouckova I and Novotny MV Solid-phase permethylation of glycans for mass spectrometric analysis. Rapid Commun Mass Spectrom 2005; 19(23): 3421–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P, Mechref Y and Novotny MV High-throughput solid-phase permethylation of glycans prior to mass spectrometry. Rapid Commun Mass Spectrom 2008; 22(5): 721–734. [DOI] [PubMed] [Google Scholar]
- Kanu AB, Dwivedi P, Tam M, Matz L and Hill HH Jr. Ion mobility-mass spectrometry. J Mass Spectrom 2008; 43(1): 1–22. [DOI] [PubMed] [Google Scholar]
- Karas M, Ehring H, Nordhoff E, Stahl B, Strupat K, Hillenkamp F, Grehl M and Krebs B Matrix-Assisted Laser-Desorption Ionization Mass-Spectrometry with Additives to 2,5-Dihydroxybenzoic Acid. Org Mass Spectrom 1993; 28(12): 1476–1481. [Google Scholar]
- Karlsson NG, Wilson NL, Wirth HJ, Dawes P, Joshi H and Packer NH Negative ion graphitised carbon nano-liquid chromatography/mass spectrometry increases sensitivity for glycoprotein oligosaccharide analysis. Rapid Commun Mass Spectrom 2004; 18(19): 2282–2292. [DOI] [PubMed] [Google Scholar]
- Kawasaki N, Ohta M, Hyuga S, Hashimoto O and Hayakawa T Analysis of carbohydrate heterogeneity in a glycoprotein using liquid chromatography/mass spectrometry and liquid chromatography with tandem mass spectrometry. Anal Biochem 1999; 269(2): 297–303. [DOI] [PubMed] [Google Scholar]
- Kawasaki N, Ohta M, Hyuga S, Hyuga M and Hayakawa T Application of liquid chromatography/mass spectrometry and liquid chromatography with tandem mass spectrometry to the analysis of the site-specific carbohydrate heterogeneity in erythropoietin. Anal Biochem 2000; 285(1): 82–91. [DOI] [PubMed] [Google Scholar]
- Keser T, Pavić T, Lauc G and Gornik O Comparison of 2-Aminobenzamide, Procainamide and RapiFluor-MS as Derivatizing Agents for High-Throughput HILIC-UPLC-FLR-MS N-glycan Analysis. Front Chem 2018; 6: 324. doi: 310.3389/fchem.2018.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri K, Klein JA, Haserick JR, Leon DR, Costello CE, McComb ME and Zaia J Microfluidic Capillary Electrophoresis-Mass Spectrometry for Analysis of Monosaccharides, Oligosaccharides, and Glycopeptides. Anal Chem 2017; 89(12): 6645–6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K-J, Kim Y-W, Hwang C-H, Park H-G, Yang Y-H, Koo M and Kim Y-G A MALDI-MS-based quantitative targeted glycomics (MALDI-QTaG) for total N-glycan analysis. Biotechnol Lett 2015; 37(10): 2019–2025. [DOI] [PubMed] [Google Scholar]
- Kovács Z, Papp G, Horváth H, Joó F and Guttman A A novel carbohydrate labeling method utilizing transfer hydrogenation-mediated reductive amination. J Pharm Biomed Anal 2017; 142: 324–327. [DOI] [PubMed] [Google Scholar]
- Kozlik P, Goldman R and Sanda M Hydrophilic interaction liquid chromatography in the separation of glycopeptides and their isomers. Anal Bioanal Chem 2018; 410(20): 5001–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlik P, Goldman R and Sanda M Study of structure-dependent chromatographic behavior of glycopeptides using reversed phase nanoLC. Electrophoresis 2017; 38(17): 2193–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlik P, Sanda M and Goldman R Nano reversed phase versus nano hydrophilic interaction liquid chromatography on a chip in the analysis of hemopexin glycopeptides. J Chromatogr A 2017; 1519: 152–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz S, Sheikh MO, Lu S, Wells L and Tiemeyer M Separation and Identification of Permethylated Glycan Isomers by Reversed Phase NanoLC-NSI-MS(n). Mol Cell Proteomics 2021; 20: 100045. doi: 100010.101074/mcp.RA100120.002266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lageveen-Kammeijer GSM, de Haan N, Mohaupt P, Wagt S, Filius M, Nouta J, Falck D and Wuhrer M Highly sensitive CE-ESI-MS analysis of N-glycans from complex biological samples. Nat Commun 2019; 10(1): 2137. doi: 2110.1038/s41467–41019-09910–41467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanucara F, Holman SW, Gray CJ and Eyers CE The power of ion mobility-mass spectrometry for structural characterization and the study of conformational dynamics. Nat Chem 2014; 6(4): 281–294. [DOI] [PubMed] [Google Scholar]
- Lattova E and Perreault H Labelling saccharides with phenylhydrazine for electrospray and matrix-assisted laser desorption–ionization mass spectrometry. J Chromatogr B 2003; 793(1): 167–179. [DOI] [PubMed] [Google Scholar]
- Lauber MA, Yu YQ, Brousmiche DW, Hua Z, Koza SM, Magnelli P, Guthrie E, Taron CH and Fountain KJ Rapid Preparation of Released N-Glycans for HILIC Analysis Using a Labeling Reagent that Facilitates Sensitive Fluorescence and ESI-MS Detection. Anal Chem 2015; 87(10): 5401–5409. [DOI] [PubMed] [Google Scholar]
- Lee JY, Lee HK, Park GW, Hwang H, Jeong HK, Yun KN, Ji ES, Kim KH, Kim JS, Kim JW, Yun SH, Choi C-W, Kim SI, Lim J-S, Jeong S-K, Paik Y-K, Lee S-Y, Park J, Kim SY, Choi Y-J, Kim Y-I, Seo J, Cho J-Y, Oh MJ, Seo N, An HJ, Kim JY and Yoo JS Characterization of Site-Specific N-Glycopeptide Isoforms of α−1-Acid Glycoprotein from an Interlaboratory Study Using LC–MS/MS. J Proteome Res 2016; 15(12): 4146–4164. [DOI] [PubMed] [Google Scholar]
- Lohmann KK and von der Lieth CW GlycoFragment and GlycoSearchMS: web tools to support the interpretation of mass spectra of complex carbohydrates. Nucleic Acids Res 2004; 32(Web Server issue): W261–266. doi: 210.1093/nar/gkh1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loziuk PL, Hecht ES and Muddiman DC N-linked glycosite profiling and use of Skyline as a platform for characterization and relative quantification of glycans in differentiating xylem of Populus trichocarpa. Anal Bioanal Chem 2017; 409(2): 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn KS, Chen CC, Lih TM, Cheng CW, Su WC, Chang CH, Cheng CY, Hsu WL, Chen YJ and Sung TY MAGIC: an automated N-linked glycoprotein identification tool using a Y1-ion pattern matching algorithm and in silico MS2 approach. Anal Chem 2015; 87(4): 2466–2473. [DOI] [PubMed] [Google Scholar]
- Maass K, Ranzinger R, Geyer H, von der Lieth CW and Geyer R “Glyco-peakfinder”--de novo composition analysis of glycoconjugates. Proteomics 2007; 7(24): 4435–4444. [DOI] [PubMed] [Google Scholar]
- MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, Kern R, Tabb DL, Liebler DC and MacCoss MJ Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010; 26(7): 966–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madunić K, Zhang T, Mayboroda OA, Holst S, Stavenhagen K, Jin C, Karlsson NG, Lageveen-Kammeijer GSM and Wuhrer M Colorectal cancer cell lines show striking diversity of their O-glycome reflecting the cellular differentiation phenotype. Cell Mol Life Sci 2021; 78(1): 337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancera-Arteu M, Giménez E, Barbosa J, Peracaula R and Sanz-Nebot V Zwitterionic-hydrophilic interaction capillary liquid chromatography coupled to tandem mass spectrometry for the characterization of human alpha-acid-glycoprotein N-glycan isomers. Anal Chim Acta 2017; 991: 76–88. [DOI] [PubMed] [Google Scholar]
- Manz C, Grabarics M, Hoberg F, Pugini M, Stuckmann A, Struwe WB and Pagel K Separation of isomeric glycans by ion mobility spectrometry – the impact of fluorescent labelling. Analyst 2019; 144(17): 5292–5298. [DOI] [PubMed] [Google Scholar]
- Mariño K, Bones J, Kattla JJ and Rudd PM A systematic approach to protein glycosylation analysis: a path through the maze. Nat Chem Biol 2010; 6(10): 713–723. [DOI] [PubMed] [Google Scholar]
- Maxwell E, Tan Y, Tan Y, Hu H, Benson G, Aizikov K, Conley S, Staples GO, Slysz GW, Smith RD and Zaia J GlycReSoft: a software package for automated recognition of glycans from LC/MS data. PLoS One 2012; 7(9): e45474. doi: 45410.41371/journal.pone.0045474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayampurath A, Yu CY, Song E, Balan J, Mechref Y and Tang H Computational framework for identification of intact glycopeptides in complex samples. Anal Chem 2014; 86(1): 453–463. [DOI] [PubMed] [Google Scholar]
- Mayampurath AM, Wu Y, Segu ZM, Mechref Y and Tang H Improving confidence in detection and characterization of protein N-glycosylation sites and microheterogeneity. Rapid Commun Mass Spectrom 2011; 25(14): 2007–2019. [DOI] [PubMed] [Google Scholar]
- McDowell CT, Klamer Z, Hall J, West CA, Wisniewski L, Powers TW, Angel PM, Mehta AS, Lewin DN, Haab BB and Drake RR Imaging Mass Spectrometry and Lectin Analysis of N-Linked Glycans in Carbohydrate Antigen-Defined Pancreatic Cancer Tissues. Mol Cell Proteomics 2020; 20: 100012. doi: 100010.101074/mcp.RA100120.002256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechref Y Use of CID/ETD mass spectrometry to analyze glycopeptides. Curr Protoc Protein Sci 2012; Chapter 12: Unit-12.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechref Y, Hu Y, Garcia A and Hussein A Identifying cancer biomarkers by mass spectrometry‐based glycomics. Electrophoresis 2012; 33(12): 1755–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechref Y and Novotny MV Glycomic analysis by capillary electrophoresis-mass spectrometry. Mass Spectrom Rev 2009; 28(2): 207–222. [DOI] [PubMed] [Google Scholar]
- Mechref Y and Novotny MV Matrix-assisted laser desorption ionization mass spectrometry of acidic glycoconjugates facilitated by the use of spermine as a co-matrix. J Am Soc Mass Spectrom 1998; 9(12): 1293–1302. [DOI] [PubMed] [Google Scholar]
- Mechref Y and Novotny MV Structural investigations of glycoconjugates at high sensitivity. Chem Rev 2002; 102(2): 321–370. [DOI] [PubMed] [Google Scholar]
- Medzihradszky KF, Kaasik K and Chalkley RJ Characterizing sialic acid variants at the glycopeptide level. Anal Chem 2015; 87(5): 3064–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A, Herrera H and Block T Glycosylation and liver cancer. Adv Cancer Res 2015; 126: 257–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina A, Palmigiano A, Esposito F, Fiumara A, Bordugo A, Barone R, Sturiale L, Jaeken J and Garozzo D HILIC-UPLC-MS for high throughput and isomeric N-glycan separation and characterization in Congenital Disorders Glycosylation and human diseases. Glycoconj J 2021; 38(2): 201–211. [DOI] [PubMed] [Google Scholar]
- Metzger JO, Woisch R, Tuszynski W and Angermann R New-Type of Matrix for Matrix-Assisted Laser-Desorption Mass-Spectrometry of Polysaccharides and Proteins. Fresenius J Anal Chem 1994; 349(6): 473–474. [Google Scholar]
- Mohr MD, Bornsen KO and Widmer HM Matrix-Assisted Laser-Desorption Ionization Mass-Spectrometry - Improved Matrix for Oligosaccharides. Rapid Commun Mass Spectrom 1995; 9(9): 809–814. [DOI] [PubMed] [Google Scholar]
- Molnarova K and Kozlík P Comparison of Different HILIC Stationary Phases in the Separation of Hemopexin and Immunoglobulin G Glycopeptides and Their Isomers. Molecules 2020; 25(20): 4655. doi: 4610.3390/molecules25204655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers SA, Daou S, Affar el B and Burlingame A Electron transfer dissociation (ETD): the mass spectrometric breakthrough essential for O-GlcNAc protein site assignments-a study of the O-GlcNAcylated protein host cell factor C1. Proteomics 2013; 13(6): 982–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai-Okatani C and Minamino N Aberrant Glycosylation in the Left Ventricle and Plasma of Rats with Cardiac Hypertrophy and Heart Failure. PLoS One 2016; 11(6): e0150210. doi: 0150210.0151371/journal.pone.0150210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy G, Attah IK, Garimella SVB, Tang K, Ibrahim YM, Baker ES and Smith RD Unraveling the isomeric heterogeneity of glycans: ion mobility separations in structures for lossless ion manipulations. Chem Commun 2018; 54(83): 11701–11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasir W, Toledo AG, Noborn F, Nilsson J, Wang M, Bandeira N and Larson G SweetNET: A Bioinformatics Workflow for Glycopeptide MS/MS Spectral Analysis. J Proteome Res 2016; 15(8): 2826–2840. [DOI] [PubMed] [Google Scholar]
- Niñonuevo M, An H, Yin H, Killeen K, Grimm R, Ward R, German B and Lebrilla C Nanoliquid chromatography-mass spectrometry of oligosaccharides employing graphitized carbon chromatography on microchip with a high-accuracy mass analyzer. Electrophoresis 2005; 26(19): 3641–3649. [DOI] [PubMed] [Google Scholar]
- Nishikaze T, Tsumoto H, Sekiya S, Iwamoto S, Miura Y and Tanaka K Differentiation of Sialyl Linkage Isomers by One-Pot Sialic Acid Derivatization for Mass Spectrometry-Based Glycan Profiling. Anal Chem 2017; 89(4): 2353–2360. [DOI] [PubMed] [Google Scholar]
- Novotny MV and Alley WR Jr. Recent trends in analytical and structural glycobiology. Curr Opin Chem Biol 2013; 17(5): 832–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo K and Marth JD Glycosylation in cellular mechanisms of health and disease. Cell 2006; 126(5): 855–867. [DOI] [PubMed] [Google Scholar]
- Orlova OV, Drutsa VL, Spirin PV, Prasolov VS, Rubtsov PM, Kochetkov SN and Beljelarskaya SN The role of HCV e2 protein glycosylation in functioning of virus envelope proteins in insect and Mammalian cells. Acta Naturae 2015; 7(1): 87–97. [PMC free article] [PubMed] [Google Scholar]
- Ozohanics O, Krenyacz J, Ludányi K, Pollreisz F, Vékey K and Drahos L GlycoMiner: a new software tool to elucidate glycopeptide composition. Rapid Commun Mass Spectrom 2008; 22(20): 3245–3254. [DOI] [PubMed] [Google Scholar]
- Padgett JL, Geldiyev Y, Gautam S, Peng W, Mechref Y and Ibraguimov A Object classification in analytical chemistry via data‐driven discovery of partial differential equations. Comput Math Methods 2021: doi: 10.1002/cmm1004.1164. [DOI] [Google Scholar]
- Pallister EG, Choo MSF, Walsh I, Tai JN, Tay SJ, Yang YS, Ng SK, Rudd PM, Flitsch SL and Nguyen-Khuong T Utility of Ion-Mobility Spectrometry for Deducing Branching of Multiply Charged Glycans and Glycopeptides in a High-Throughput Positive ion LC-FLR-IMS-MS Workflow. Anal Chem 2020; 92(23): 15323–15335. [DOI] [PubMed] [Google Scholar]
- Pan S, Brentnall TA and Chen R Glycoproteins and glycoproteomics in pancreatic cancer. World J Gastroenterol 2016; 22(42): 9288–9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Arabyan N, Williams CC, Song T, Mitra A, Weimer BC, Maverakis E and Lebrilla CB Salmonella Typhimurium Enzymatically Landscapes the Host Intestinal Epithelial Cell (IEC) Surface Glycome to Increase Invasion. Mol Cell Proteomics 2016; 15(12): 3653–3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park GW, Kim JY, Hwang H, Lee JY, Ahn YH, Lee HK, Ji ES, Kim KH, Jeong HK, Yun KN, Kim YS, Ko JH, An HJ, Kim JH, Paik YK and Yoo JS Integrated GlycoProteome Analyzer (I-GPA) for Automated Identification and Quantitation of Site-Specific N-Glycosylation. Sci Rep 2016; 6: 21175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak P, Baird MA and Shvartsburg AA High-Resolution Ion Mobility Separations of Isomeric Glycoforms with Variations on the Peptide and Glycan Levels. J Am Soc Mass Spectrom 2020; 31(7): 1603–1609. [DOI] [PubMed] [Google Scholar]
- Pedrali A, Tengattini S, Marrubini G, Bavaro T, Hemström P, Massolini G, Terreni M and Temporini C Characterization of intact neo-glycoproteins by hydrophilic interaction liquid chromatography. Molecules 2014; 19(7): 9070–9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng B and Ahrends R Adaptation of Skyline for Targeted Lipidomics. J Proteome Res 2016; 15(1): 291–301. [DOI] [PubMed] [Google Scholar]
- Peng W, Goli M, Mirzaei P and Mechref Y Revealing the Biological Attributes of N-Glycan Isomers in Breast Cancer Brain Metastasis Using Porous Graphitic Carbon (PGC) Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). J Proteome Res 2019; 18(10): 3731–3740. [DOI] [PubMed] [Google Scholar]
- Pereira L Porous Graphitic Carbon as a Stationary Phase in HPLC: Theory and Applications. J Liq Chromatogr R T 2008; 31(11–12): 1687–1731. [Google Scholar]
- Phillips ML, Nudelman E, Gaeta FC, Perez M, Singhal AK, Hakomori S and Paulson JC ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science 1990; 250(4984): 1130–1132. [DOI] [PubMed] [Google Scholar]
- Polasky DA, Yu F, Teo GC and Nesvizhskii AI Fast and comprehensive N- and O-glycoproteomics analysis with MSFragger-Glyco. Nat Methods 2020; 17(11): 1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompach P, Chandler KB, Lan R, Edwards N and Goldman R Semi-automated identification of N-Glycopeptides by hydrophilic interaction chromatography, nano-reverse-phase LC-MS/MS, and glycan database search. J Proteome Res 2012; 11(3): 1728–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers TW, Holst S, Wuhrer M, Mehta AS and Drake RR Two-Dimensional N-Glycan Distribution Mapping of Hepatocellular Carcinoma Tissues by MALDI-Imaging Mass Spectrometry. Biomolecules 2015; 5(4): 2554–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers TW, Jones EE, Betesh LR, Romano PR, Gao P, Copland JA, Mehta AS and Drake RR Matrix assisted laser desorption ionization imaging mass spectrometry workflow for spatial profiling analysis of N-linked glycan expression in tissues. Anal Chem 2013; 85(20): 9799–9806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Sun L, Zhu G, Zhang Z, Peuchen EH and Dovichi NJ Sensitive and fast characterization of site-specific protein glycosylation with capillary electrophoresis coupled to mass spectrometry. Talanta 2018; 179: 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiding KR, Blank D, Kuijper DM, Deelder AM and Wuhrer M High-throughput profiling of protein N-glycosylation by MALDI-TOF-MS employing linkage-specific sialic acid esterification. Anal Chem 2014; 86(12): 5784–5793. [DOI] [PubMed] [Google Scholar]
- Reis CA, Osorio H, Silva L, Gomes C and David L Alterations in glycosylation as biomarkers for cancer detection. J Clin Pathol 2010; 63(4): 322–329. [DOI] [PubMed] [Google Scholar]
- Ren JM, Rejtar T, Li L and Karger BL N-Glycan structure annotation of glycopeptides using a linearized glycan structure database (GlyDB). J Proteome Res 2007; 6(8): 3162–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch D, Haberger M, Kailich T, Heidenreich AK, Kampe M, Bulau P and Wuhrer M High-throughput glycosylation analysis of therapeutic immunoglobulin G by capillary gel electrophoresis using a DNA analyzer. MAbs 2014; 6(1): 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes CDG, Huang Y, Atashi M, Zhang J, Zhu J, Liu S, Parikh ND, Singal AG, Dai J and Lubman DM PRM-MS Quantitative Analysis of Isomeric N-Glycopeptides Derived From Human Serum Haptoglobin of Patients With Cirrhosis and Hepatocellular Carcinoma. Metabolites 2021: Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley NM and Coon JJ The Role of Electron Transfer Dissociation in Modern Proteomics. Anal Chem 2018; 90(1): 40–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle L, Campbell MP, Radcliffe CM, White DM, Harvey DJ, Abrahams JL, Kim YG, Henry GW, Shadick NA, Weinblatt ME, Lee DM, Rudd PM and Dwek RA HPLC-based analysis of serum N-glycans on a 96-well plate platform with dedicated database software. Anal Biochem 2008; 376(1): 1–12. [DOI] [PubMed] [Google Scholar]
- Royle L, Radcliffe CM, Dwek RA and Rudd PM Detailed structural analysis of N-glycans released from glycoproteins in SDS-PAGE gel bands using HPLC combined with exoglycosidase array digestions. Methods Mol Biol 2006; 347: 125–143. [DOI] [PubMed] [Google Scholar]
- Ruhaak LR, Deelder AM and Wuhrer M Oligosaccharide analysis by graphitized carbon liquid chromatography-mass spectrometry. Anal Bioanal Chem 2009; 394(1): 163–174. [DOI] [PubMed] [Google Scholar]
- Ruhaak LR, Huhn C, Waterreus WJ, de Boer AR, Neusüss C, Hokke CH, Deelder AM and Wuhrer M Hydrophilic interaction chromatography-based high-throughput sample preparation method for N-glycan analysis from total human plasma glycoproteins. Anal Chem 2008; 80(15): 6119–6126. [DOI] [PubMed] [Google Scholar]
- Ruhaak LR, Steenvoorden E, Koeleman CA, Deelder AM and Wuhrer M 2-picoline-borane: a non-toxic reducing agent for oligosaccharide labeling by reductive amination. Proteomics 2010; 10(12): 2330–2336. [DOI] [PubMed] [Google Scholar]
- Ruhaak LR, Zauner G, Huhn C, Bruggink C, Deelder AM and Wuhrer M Glycan labeling strategies and their use in identification and quantification. Anal Bioanal Chem 2010; 397(8): 3457–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba JA, Shen X, Jamieson JC and Perreault H Investigation of different combinations of derivatization, separation methods and electrospray ionization mass spectrometry for standard oligosaccharides and glycans from ovalbumin. J Mass Spectrom 2001; 36(5): 563–574. [DOI] [PubMed] [Google Scholar]
- Shajahan A, Heiss C, Ishihara M and Azadi P Glycomic and glycoproteomic analysis of glycoproteins-a tutorial. Anal Bioanal Chem 2017; 409(19): 4483–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She YM, Tam RY, Li X, Rosu-Myles M and Sauvé S Resolving Isomeric Structures of Native Glycans by Nanoflow Porous Graphitized Carbon Chromatography-Mass Spectrometry. Anal Chem 2020; 92(20): 14038–14046. [DOI] [PubMed] [Google Scholar]
- Singh C, Zampronio CG, Creese AJ and Cooper HJ Higher energy collision dissociation (HCD) product ion-triggered electron transfer dissociation (ETD) mass spectrometry for the analysis of N-linked glycoproteins. J Proteome Res 2012; 11(9): 4517–4525. [DOI] [PubMed] [Google Scholar]
- Snyder CM, Zhou X, Karty JA, Fonslow BR, Novotny MV and Jacobson SC Capillary electrophoresis-mass spectrometry for direct structural identification of serum N-glycans. J Chromatogr A 2017; 1523: 127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola RJ and Griebenow K Effects of glycosylation on the stability of protein pharmaceuticals. J Pharm Sci 2009; 98(4): 1223–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Zhou X, Benktander JD, Gaunitz S, Zou G, Wang Z, Novotny MV and Jacobson SC In-Depth Compositional and Structural Characterization of N-Glycans Derived from Human Urinary Exosomes. Anal Chem 2019; 91(21): 13528–13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio M, Gleissner CA and Ley K Glycosylation in immune cell trafficking. Immunol Rev 2009; 230(1): 97–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl B, Steup M, Karas M and Hillenkamp F Analysis of Neutral Oligosaccharides by Matrix-Assisted Laser Desorption - Ionization Mass-Spectrometry. Anal Chem 1991; 63(14): 1463–1466. [Google Scholar]
- Stahl B, Thurl S, Zeng JR, Karas M, Hillenkamp F, Steup M and Sawatzki G Oligosaccharides from Human-Milk as Revealed by Matrix-Assisted Laser-Desorption Ionization Mass-Spectrometry. Anal Biochem 1994; 223(2): 218–226. [DOI] [PubMed] [Google Scholar]
- Stavenhagen K, Kolarich D and Wuhrer M Clinical Glycomics Employing Graphitized Carbon Liquid Chromatography-Mass Spectrometry. Chromatographia 2015; 78(5–6): 307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavenhagen K, Plomp R and Wuhrer M Site-Specific Protein N- and O-Glycosylation Analysis by a C18-Porous Graphitized Carbon-Liquid Chromatography-Electrospray Ionization Mass Spectrometry Approach Using Pronase Treated Glycopeptides. Anal Chem 2015; 87(23): 11691–11699. [DOI] [PubMed] [Google Scholar]
- Stolz A, Jooß K, Höcker O, Römer J, Schlecht J and Neusüß C Recent advances in capillary electrophoresis-mass spectrometry: Instrumentation, methodology and applications. Electrophoresis 2019; 40(1): 79–112. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Abe T and Natsuka S Quantitative LC-MS and MS/MS analysis of sialylated glycans modified by linkage-specific alkylamidation. Anal Biochem 2019; 567: 117–127. [DOI] [PubMed] [Google Scholar]
- Takegawa Y, Deguchi K, Ito H, Keira T, Nakagawa H and Nishimura S Simple separation of isomeric sialylated N-glycopeptides by a zwitterionic type of hydrophilic interaction chromatography. J Sep Sci 2006; 29(16): 2533–2540. [DOI] [PubMed] [Google Scholar]
- Takegawa Y, Deguchi K, Keira T, Ito H, Nakagawa H and Nishimura S-I Separation of isomeric 2-aminopyridine derivatized N-glycans and N-glycopeptides of human serum immunoglobulin G by using a zwitterionic type of hydrophilic-interaction chromatography. J chromatogr A 2006; 1113(1–2): 177–181. [DOI] [PubMed] [Google Scholar]
- Tao S, Huang Y, Boyes BE and Orlando R Liquid chromatography-selected reaction monitoring (LC-SRM) approach for the separation and quantitation of sialylated N-glycans linkage isomers. Anal chem 2014; 86(21): 10584–10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor ME and Drickamer K Convergent and divergent mechanisms of sugar recognition across kingdoms. Curr Opin Struct Biol 2014; 28: 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaysen-Andersen M, Wilkinson BL, Payne RJ and Packer NH Site-specific characterisation of densely O-glycosylated mucin-type peptides using electron transfer dissociation ESI-MS/MS. Electrophoresis 2011; 32(24): 3536–3545. [DOI] [PubMed] [Google Scholar]
- Tseng K, Hedrick JL and Lebrilla CB Catalog-library approach for the rapid and sensitive structural elucidation of oligosaccharides. Anal Chem 1999; 71(17): 3747–3754. [DOI] [PubMed] [Google Scholar]
- Týčová A, Ledvina V and Klepárník K Recent advances in CE-MS coupling: Instrumentation, methodology, and applications. Electrophoresis 2017; 38(1): 115–134. [DOI] [PubMed] [Google Scholar]
- Ujma J, Ropartz D, Giles K, Richardson K, Langridge D, Wildgoose J, Green M and Pringle S Cyclic Ion Mobility Mass Spectrometry Distinguishes Anomers and Open-Ring Forms of Pentasaccharides. J Am Soc Mass Spectrom 2019; 30(6): 1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterieser I and Mischnick P Labeling of oligosaccharides for quantitative mass spectrometry. Carbohydr Res 2011; 346(1): 68–75. [DOI] [PubMed] [Google Scholar]
- Vakhrushev SY, Dadimov D and Peter-Katalinić J Software platform for high-throughput glycomics. Anal Chem 2009; 81(9): 3252–3260. [DOI] [PubMed] [Google Scholar]
- Van Scherpenzeel M, Willems E and Lefeber DJ Clinical diagnostics and therapy monitoring in the congenital disorders of glycosylation. Glycoconj J 2016; 33(3): 345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadi C, Mittermayr S, Millan-Martin S and Bones J Quantitative twoplex glycan analysis using (12)C6 and (13)C6 stable isotope 2-aminobenzoic acid labelling and capillary electrophoresis mass spectrometry. Anal Bioanal Chem 2016; 408(30): 8691–8700. [DOI] [PubMed] [Google Scholar]
- Varki A Sialic acids in human health and disease. Trends Mol Med 2008; 14(8): 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillon L, Huang Y, Peng W, Dong X, Cho BG and Mechref Y Characterization of isomeric glycan structures by LC-MS/MS. Electrophoresis 2017; 38(17): 2100–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigerust DJ Protein glycosylation in infectious disease pathobiology and treatment. Cent Eur J Biol 2011; 6(5): 802. doi: 810.2478/s11535–11011-10050–11538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeker GC and Wuhrer M Reversed-phase separation methods for glycan analysis. Anal Bioanal Chem 2017; 409(2): 359–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SH, Lilley LM, Enamorado MF, Comins DL and Muddiman DC Hydrophobic derivatization of N-linked glycans for increased ion abundance in electrospray ionization mass spectrometry. J Am Soc Mass Spectrom 2011; 22(8): 1309–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Tsybovsky Y, Palczewski K and Chance MR Reliable determination of site-specific in vivo protein N-glycosylation based on collision-induced MS/MS and chromatographic retention time. J Am Soc Mass Spectrom 2014; 25(5): 729–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Shajahan A, Pepi LE, Azadi P and Zaia J Glycoproteomic Sample Processing, LC-MS, and Data Analysis Using GlycReSoft. Curr Protoc 2021; 1(3): e84. doi: 10.1002/cpz1001.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen X, Chen L, Wang B, Peng C, He C, Tang M, Zhang F, Hu J, Li R, Zhao X and Wei Y Optimizing high-performance liquid chromatography method for quantification of glucosamine using 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate derivatization in rat plasma: application to a pharmacokinetic study. Biomed Chromatogr 2008; 22(11): 1265–1271. [DOI] [PubMed] [Google Scholar]
- Warnke S, Ben Faleh A, Scutelnic V and Rizzo TR Separation and Identification of Glycan Anomers Using Ultrahigh-Resolution Ion-Mobility Spectrometry and Cryogenic Ion Spectroscopy. J Am Soc Mass Spectrom 2019; 30(11): 2204–2211. [DOI] [PubMed] [Google Scholar]
- West C, Elfakir C and Lafosse M Porous graphitic carbon: a versatile stationary phase for liquid chromatography. J Chromatogr A 2010; 1217(19): 3201–3216. [DOI] [PubMed] [Google Scholar]
- West CA, Liang H, Drake RR and Mehta AS New Enzymatic Approach to Distinguish Fucosylation Isomers of N-Linked Glycans in Tissues Using MALDI Imaging Mass Spectrometry. J Proteome Res 2020; 19(8): 2989–2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler SF, Domann P and Harvey DJ Derivatization of sialic acids for stabilization in matrix-assisted laser desorption/ionization mass spectrometry and concomitant differentiation of alpha(2 --> 3)- and alpha(2 --> 6)-isomers. Rapid Commun Mass Spectrom 2009; 23(2): 303–312. [DOI] [PubMed] [Google Scholar]
- Woodin CL, Hua D, Maxon M, Rebecchi KR, Go EP and Desaire H GlycoPep grader: a web-based utility for assigning the composition of N-linked glycopeptides. Anal Chem 2012; 84(11): 4821–4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodin CL, Maxon M and Desaire H Software for automated interpretation of mass spectrometry data from glycans and glycopeptides. Analyst 2013; 138(10): 2793–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooding KM, Peng W and Mechref Y Characterization of Pharmaceutical IgG and Biosimilars Using Miniaturized Platforms and LC-MS-MS. Curr Pharm Biotechnol. 2016; 17(9): 788–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Grimm R, German JB and Lebrilla CB Annotation and structural analysis of sialylated human milk oligosaccharides. J Proteome Res 2011; 10(2): 856–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Tao N, German JB, Grimm R and Lebrilla CB Development of an annotated library of neutral human milk oligosaccharides. J Proteome Res 2010; 9(8): 4138–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SW, Liang SY, Pu TH, Chang FY and Khoo KH Sweet-Heart - an integrated suite of enabling computational tools for automated MS2/MS3 sequencing and identification of glycopeptides. J Proteomics 2013; 84: 1–16. [DOI] [PubMed] [Google Scholar]
- Wu Y, Mechref Y, Klouckova I, Mayampurath A, Novotny MV and Tang H Mapping site-specific protein N-glycosylations through liquid chromatography/mass spectrometry and targeted tandem mass spectrometry. Rapid Commun Mass Spectrom 2010; 24(7): 965–972. [DOI] [PubMed] [Google Scholar]
- Wuhrer M, Deelder AM and Hokke CH Protein glycosylation analysis by liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2005; 825(2): 124–133. [DOI] [PubMed] [Google Scholar]
- Xie C, Wu Q, Zhang S, Wang C, Gao W, Yu J and Tang K Improving glycan isomeric separation via metal ion incorporation for drift tube ion mobility-mass spectrometry. Talanta 2020; 211: 120719. doi: 120710.121016/j.talanta.122020.120719. [DOI] [PubMed] [Google Scholar]
- Xu G, Goonatilleke E, Wongkham S and Lebrilla CB Deep Structural Analysis and Quantitation of O-Linked Glycans on Cell Membrane Reveal High Abundances and Distinct Glycomic Profiles Associated with Cell Type and Stages of Differentiation. Anal Chem 2020; 92(5): 3758–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Boyne MT, Buhse LF and Hill J Direct Approach for Qualitative and Quantitative Characterization of Glycoproteins Using Tandem Mass Tags and an LTQ Orbitrap XL Electron Transfer Dissociation Hybrid Mass Spectrometer. Anal Chem 2013; 85(3): 1531–1539. [DOI] [PubMed] [Google Scholar]
- Ye Z, Mao Y, Clausen H and Vakhrushev SY Glyco-DIA: a method for quantitative O-glycoproteomics with in silico-boosted glycopeptide libraries. Nat Methods 2019; 16(9): 902–910. [DOI] [PubMed] [Google Scholar]
- Yin H, An M, So PK, Wong MY, Lubman DM and Yao Z The analysis of alpha-1-antitrypsin glycosylation with direct LC-MS/MS. Electrophoresis 2018; 39(18): 2351–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Zhu J, Wu J, Tan Z, An M, Zhou S, Mechref Y and Lubman DM A procedure for the analysis of site-specific and structure-specific fucosylation in alpha-1-antitrypsin. Electrophoresis 2016; 37(20): 2624–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida-Moriguchi T, Yu L, Stalnaker SH, Davis S, Kunz S, Madson M, Oldstone MB, Schachter H, Wells L and Campbell KP O-mannosyl phosphorylation of alpha-dystroglycan is required for laminin binding. Science 2010; 327(5961): 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A, Zhao J, Peng W, Banazadeh A, Williamson SD, Goli M, Huang Y and Mechref Y Advances in mass spectrometry-based glycoproteomics. Electrophoresis 2018; 39(24): 3104–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Wang B, Chen Z, Urabe G, Glover MS, Shi X, Guo LW, Kent KC and Li L Electron-Transfer/Higher-Energy Collision Dissociation (EThcD)-Enabled Intact Glycopeptide/Glycoproteome Characterization. J Am Soc Mass Spectrom 2017; 28(9): 1751–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauner G, Koeleman CA, Deelder AM and Wuhrer M Protein glycosylation analysis by HILIC-LC-MS of Proteinase K-generated N- and O-glycopeptides. J Sep Sci 2010; 33(6–7): 903–910. [DOI] [PubMed] [Google Scholar]
- Zeng WF, Liu MQ, Zhang Y, Wu JQ, Fang P, Peng C, Nie A, Yan G, Cao W, Liu C, Chi H, Sun RX, Wong CC, He SM and Yang P pGlyco: a pipeline for the identification of intact N-glycopeptides by using HCD- and CID-MS/MS and MS3. Sci Rep 2016; 6: 25102. doi: 25110.21038/srep25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Madunić K, Holst S, Zhang J, Jin C, Ten Dijke P, Karlsson NG, Stavenhagen K and Wuhrer M Development of a 96-well plate sample preparation method for integrated N- and O-glycomics using porous graphitized carbon liquid chromatography-mass spectrometry. Mol Omics 2020; 16(4): 355–363. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang T, Chen X, Pang X, Zhang S, Obaroakpo JU, Shilong J, Lu J and Lv J Absolute quantification of twelve oligosaccharides in human milk using a targeted mass spectrometry-based approach. Carbohydr Polym 2019; 219: 328–333. [DOI] [PubMed] [Google Scholar]
- Zhao J, Li S, Li C, Wu SL, Xu W, Chen Y, Shameem M, Richardson D and Li H Identification of Low Abundant Isomeric N-Glycan Structures in Biological Therapeutics by LC/MS. Anal Chem 2016; 88(14): 7049–7059. [DOI] [PubMed] [Google Scholar]
- Zhong J, Huang Y and Mechref Y Derivatization of Sialylated Glycopeptides (DOSG) Enabling Site-Specific Isomeric Profiling Using LC-MS/MS. Anal Chem 2021; 93(14): 5763–5772. [DOI] [PubMed] [Google Scholar]
- Zhong X, Chen Z, Snovida S, Liu Y, Rogers JC and Li L Capillary Electrophoresis-Electrospray Ionization-Mass Spectrometry for Quantitative Analysis of Glycans Labeled with Multiplex Carbonyl-Reactive Tandem Mass Tags. Anal Chem 2015; 87(13): 6527–6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Dong X, Veillon L, Huang Y and Mechref Y LC-MS/MS analysis of permethylated N-glycans facilitating isomeric characterization. Anal Bioanal Chem 2017; 409(2): 453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Hu Y, DeSantos-Garcia JL and Mechref Y Quantitation of permethylated N-glycans through multiple-reaction monitoring (MRM) LC-MS/MS. J Am Soc Mass Spectrom 2015; 26(4): 596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Hu Y and Mechref Y High-temperature LC-MS/MS of permethylated glycans derived from glycoproteins. Electrophoresis 2016; 37(11): 1506–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Huang Y, Dong X, Peng W, Veillon L, Kitagawa DAS, Aquino AJA and Mechref Y Isomeric Separation of Permethylated Glycans by Porous Graphitic Carbon (PGC)-LC-MS/MS at High Temperatures. Anal Chem 2017; 89(12): 6590–6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Veillon L, Dong X, Huang Y and Mechref Y Direct comparison of derivatization strategies for LC-MS/MS analysis of N-glycans. Analyst 2017; 142(23): 4446–4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Chen Z, Zhang J, An M, Wu J, Yu Q, Skilton SJ, Bern M, Ilker Sen K, Li L and Lubman DM Differential Quantitative Determination of Site-Specific Intact N-Glycopeptides in Serum Haptoglobin between Hepatocellular Carcinoma and Cirrhosis Using LC-EThcD-MS/MS. J Proteome Res 2019; 18(1): 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu R, Huang Y, Zhao J, Zhong J and Mechref Y Isomeric Separation of N-Glycopeptides Derived from Glycoproteins by Porous Graphitic Carbon (PGC) LC-MS/MS. Anal Chem 2020; 92(14): 9556–9565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Hua D, Clark DF, Go EP and Desaire H GlycoPep Detector: a tool for assigning mass spectrometry data of N-linked glycopeptides on the basis of their electron transfer dissociation spectra. Anal Chem 2013; 85(10): 5023–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Su X, Go EP and Desaire H New glycoproteomics software, GlycoPep Evaluator, generates decoy glycopeptides de novo and enables accurate false discovery rate analysis for small data sets. Anal Chem 2014; 86(18): 9212–9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhurov KO, Fornelli L, Wodrich MD, Laskay Ü A and Tsybin YO Principles of electron capture and transfer dissociation mass spectrometry applied to peptide and protein structure analysis. Chem Soc Rev 2013; 42(12): 5014–5030. [DOI] [PubMed] [Google Scholar]