Abstract
Randomized controlled trials (RCTs) have shown an antidepressant effect of glabellar botulinum toxin (BoNT) injections. In the FDA Adverse Event Reporting System (FAERS) database, BoNT injection is associated with reduced incidence rates of depression across various non-psychiatric indications, which confirms the previous findings independently of specific expectations to an antidepressant effect of BoNT. The rationale of using BoNT to treat depression is to interrupt proprioceptive body feedback that may reinforce negative emotions. Negative emotions also occur in other mental disorders, suggesting a transdiagnostic therapeutic potential of BoNT in psychiatry. Here we report an analysis of the FAERS database, in which we found that, compared to alternative treatments, BoNT injections were associated with lower incidence of anxiety symptoms and related disorders. Among seven indications/injection sites, we found this protective effect of BoNT in cosmetic use/facial muscles, migraine/facial and head muscles, spasms and spasticity/upper and lower limbs, torticollis and neck pain/neck muscles, and sialorrhea/parotid and submandibular glands (reporting odds ratios 0.79–0.27). These findings are encouraging for possible future RCTs on the use of BoNT as a treatment for anxiety and related disorders.
Subject terms: Computational biology and bioinformatics, Medical research, Neurology
Introduction
A series of randomized placebo-controlled trials (RCTs) and meta-analyses have shown that glabellar injections of botulinum toxin can reduce the symptoms of depression1–6. However, because the noticeable muscle relaxation induced by the toxin makes it impossible to truly blind the study participants for their group allocation, it is unclear to what extent a bias towards expectations/placebo effects in the treatment groups vs. disappointment/nocebo effects in the control groups may have inflated the large effect sizes observed in these trials.
To overcome this methodical limitation, we have reassessed the antidepressant action of botulinum toxin in the absence of specific expectations to that effect. For that purpose, we have gone into the FDA Adverse Event Reporting System (FAERS) and have compared the incidence rates of depression and related symptoms after treatment with botulinum toxin to a benchmark of alternative treatments for the same indications. Confirming and extending the results of the previous RCTs, we have found a significant preventive antidepressant effect of botulinum toxin across a broad spectrum of indications and injection sites7,8.
The rationale for the assessment of botulinum toxin as an antidepressant is the facial feedback hypothesis. The consequential idea that relaxing facial muscles expressing negative emotions would disrupt the proprioceptive afferences from these muscles and their maintaining and reinforcing effect on the expressed emotions9.
Since an excess of negative emotions is not specific for depression, but occurs in and determines the suffering associated with the majority of mental disorders, botulinum toxin therapy may not be specific for depression either, but may rather represent a transdiagnostic, emotion-focused treatment approach10.
Among the excessively experienced negative emotions, anxiety is one the most common.
Anxiety, panic and fear symptoms occur in many psychiatric conditions including depression, schizophrenia, borderline personality disorder, and anxiety disorders, in which panic, fear, or anxiety are the leading symptoms, are the most prevalent mental disorders of all11.
Proprioceptive and interoceptive signals are involved in the experience of panic, fear, and anxiety and in the pathophysiology of anxiety disorders. Conversely, relaxation and biofeedback techniques play a role in the treatment of these conditions12–16.
There is already one case series suggesting that glabellar injection of botulinum toxin may alleviate the symptoms of social anxiety disorder17. Accordingly, BoNT injections as a treatment of glabellar frown lines were associated with lower anxiety levels than other cosmetic treatments18. Moreover, in several studies on BoNT injections for indications like dystonia, facial spasms, chronic migraine, and hyperhidrosis, the treatment improved comorbid anxiety disorders or related symptoms, supporting the hypothesis that BoNT may have an anxiolytic effect19–29. Interestingly, anxiolytic effects of BoNT injections have also been observed in studies with mice and rats30–32. However, to date there are no RCTs investigating the effect of BoNT as a treatment for anxiety disorders. As in depression, expectations and blinding issues may affect the outcome of such trials in this indication33,34. Thus, before committing to any RCTs, we first analysed the FAERS database to investigate whether botulinum toxin injections may prevent incident anxiety symptomatology in patients who have bona fide no specific anticipation of such an effect.
Methods
FDA adverse event reporting system (FAERS)
FAERS database and its older version AERS store AE reports from healthcare professionals, patients, legal representativessubmitted through MedWatch35. If the reports are submitted to the manufacturer, the latter is mandated to evaluate and forward the reports to the FDA. This study used over fifteen million FAERS/AERS reports which, at the time of the analysis, included reports from January 2004 to March 2021. Reports were used to perform a retrospective inverse frequency analysis.
Combining and normalizing FAERS/AERS data sets
Quarterly FAERS and AERS data sets, available as ASCII files online, were individually downloaded and separated in dollar-separated text (.txt) format. Since the data structure was not uniform in all quarterly files, it was necessary to modify the sets into a consistent table structure where missing fields were replaced with blank columns. Unix language/code was used for both data management restructuring, and analysis. Additionally, it was necessary to standardize all the drug names by generic terms due to the variability of brand names in the internationally submitted reports8,36,37.
Cohort selection
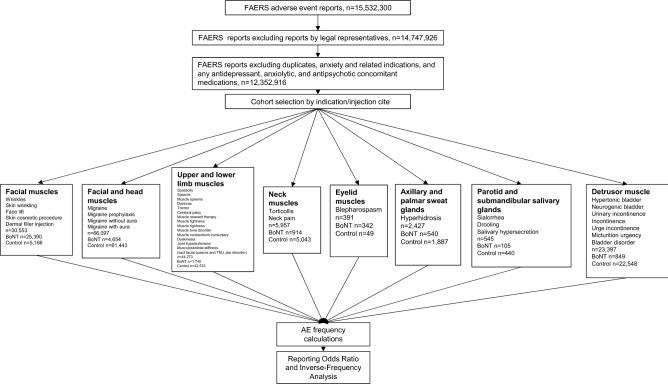
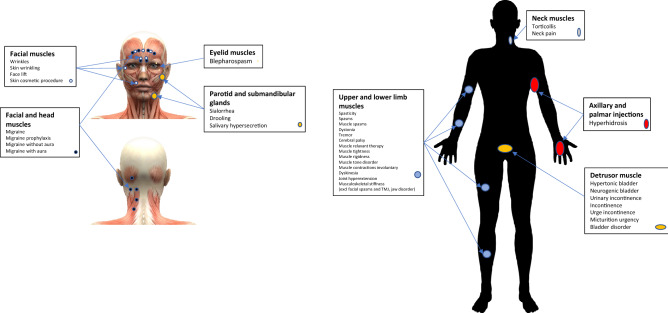
A total of 15,532,300 unique reports until March 2021 were collected prior to the analysis. Reports submitted to the FDA by legal representatives were excluded to avoid potential bias. Additionally, reports related to patients taking both indicated and off-label antidepressants, anxiolytics, and antipsychotics along with reports where patients were comorbid with anxiety and related disorders (see details in S1-S3 Appendices) resulting in 12,352,916 reports. Cases with botulinum toxin (OnabotulinumtoxinA, AbobotulinumtoxinA, IncobotulinumtoxinA, and RimabotulinumtoxinB) were analysed to define eight indication and injection site cohorts (Figs. 1 and 2): (1) Cosmetic use—facial muscles (wrinkles, skin wrinkling, face lift, skin cosmetic procedure, dermal filler injection), n = 30,553; (2) Migraine—facial and head muscles (migraine, migraine prophylaxis, migraine without aura, migraine with aura), n = 66,097; (3) Spasms and Spasticity—upper and lower limbs (spasticity, muscle spasms, dystonia, tremor, cerebral palsy, muscle relaxant therapy, muscle tightness, muscle rigidness, muscle tone disorder, muscle contractions involuntary, dyskinesia, joint hyperextension, musculoskeletal stiffness), n = 44,273, disorders related to facial muscles such as facial spasms, temporomandibular joint disorder and jaw disorder were excluded; (4) Torticollis and neck pain—neck muscles, n = 5,957; (5) Blepharospasm—eyelid muscles, n = 391; (6) Hyperhidrosis—axilla and palm, n = 2427; (7) Sialorrhea—parotid and submandibular glands (drooling, salivary hypersecretion), n = 545; (8) Neurological and urinary bladder disorders—detrusor muscle (hypertonic bladder, neurogenic bladder, urinary incontinence, incontinence, urge incontinence, micturition urgency, bladder disorder), n = 23,397 (Figs. 1 and 2). The cohorts were separated into BoNT (exposed) and non-BoNT (control) sub-cohorts. Anxiety and related AE frequencies were calculated for patients in each sub-cohort and reporting odds ratios (RORs) were calculated to identify any protective effect through Inverse-Frequency Analysis.
Figure 1.
Analysis flow chart, and inclusion/exclusion terms for cohort selection, used in adverse event rate comparison between botulinum toxin and control cohorts.
Figure 2.
Study cohorts by indication and injection site. Christos Georghiou/shutterstock.com, decade3d—anatomy online/shutterstock.com.
Statistical analysis
The statistical analysis of the FAERS and other safety surveillance data is well established, it includes frequencies, reporting odds ratios and 95% confidence intervals38. Below is the summary of the formulae.
- Descriptive Statistics (Fig. 3a): Frequency for each side effect was calculated by the equation:
- Comparative Statistics (Fig. 3b): Anxiety related report rates were compared via the Reporting Odds Ratio (ROR) using the following equations:
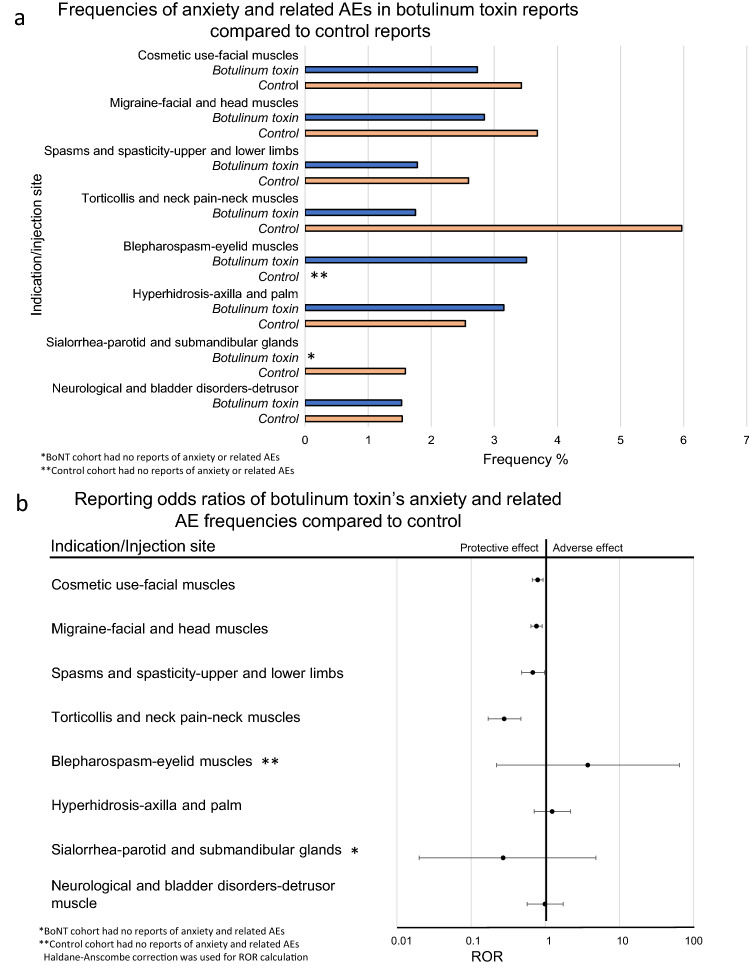
Figure 3.
Frequencies and reporting odds ratios (ROR) of anxiety and related adverse events (AE). (a) Relative frequencies of anxiety events for patients administered botulinum toxin (BoNT) for various indications. (b) Reporting odds ratios with 95% confidence intervals (CI) as calculated by comparing frequencies of anxiety reports in patients administered botulinum toxin for each indication and respective control sub-cohorts.
a = No. of anxiety and related AE reports in exposed group, b = No. in exposed group with no anxiety and related AE reports, c = No. anxiety and related AE reports in control group, d = No. in control group with no anxiety and related AE reports.
Standard Error (SE) of the LnROR value was calculated by the following equation:
Error bars were computed using 95% confidence intervals.
Haldane–Anscombe correction was used in small sample cohorts with zero reports of interest39.
Results
Botulinum toxin: anxiety and anxiety related adverse events
Patients who were administered BoNT had a significantly lower incidence of anxiety and anxiety-related AE reports, compared to the control groups. It was observed not only for cosmetic use in facial muscles (reporting odds ratios (ROR) 0.79, 95% confidence interval (CI) [0.67, 0.93]), but also for other indications and injection sites including:, migraine—facial and head muscles (0.76 [0.64, 0.91]), spasms and spasticity—upper and lower limbs, excluding facial muscles (0.68 [0.48, 0.98]), torticollis and neck pain—neck muscles (0.28 [0.17, 0.47]), There were no reports of anxiety or related AEs in the BoNT sialorrhea—parotid and submandibular glands sub-cohort. The reduced ROR value derived from 0/105 to 7/433 was evaluated as significant at 95% CI level, after the Haldane-Anscombe correction was applied (0.27 [0.020, 4.83]).
Almost no decrease in anxiety and related AE reports where BoNT was injected into the detrusor muscle in the neurological and urinary bladder disorders cohort was observed, but the reduced ROR value did not reach statistical significance (0.99 [0.57, 1.74]) and hyperhidrosis—axilla and palm cohort followed a similar trend (0.85 [0.51, 1.42]) (Fig. 3).
RORs for blepharospasm—eyelid muscles cohort exhibited increased potential risk, however not statistically significant (3.74 [0.22, 64.25]) (Fig. 3).
Discussion
In this survey of the FAERS database we found that treatment with BoNT has a protective effect against incident anxiety disorders or symptoms. This effect was significant for the indications/injection sites cosmetic use/facial muscles, migraine/facial and head muscles, spasms and spasticity/upper and lower limbs, torticollis and neck pain/neck muscles, and sialorrhea/parotid and submandibular glands. There was no effect for hyperhidrosis/axilla and palm neurological and bladder disorders/detrusor muscle. With no reports in the control group, we found a numerically increased incidence of anxiety after BoNT injection in the blepharospasm/eyelid muscles indication. Although a bit less pronounced and consistent, these findings are largely in line with those from an analogous study on depression (ROR ranging from 0.13 to 0.60), supporting the potential of BoNT injections in the management of mental disorders8.
The evaluation of BoNT as a therapeutic for depression and other mental disorders associated with an excess of negative emotions was motivated by the facial feedback hypothesis9. However, the cumulating evidence of the efficacy of BoNT in such indications is not per se evidence of the accuracy of this rationale. Our previous study, which showed an antidepressant effect of BoNT across a broad range of indications and injection sites, opened up a broad spectrum of possible explanations for this effect8,40. Some of theses explanations are compatible with the facial feedback hypothesis while others challenge it. We have discussed these possibilities at length in the corresponding paper. In principle, they may also apply for our findings on anxiety. In the following, we will discuss them shortly in this regard.
As for modulation of facial feedback, as a mechanism of action, behind the observed effects on anxiety, it may explain the findings for cosmetic use and migraine. The corrugator muscles, which represent the key effectors in the facial expression of any emotions with negative valence, are the main site of BoNT injections in the cosmetic indication and are targeted in the migraine injection scheme, too. Raising the eyebrows belongs to the expression of anxiety and is accomplished by the frontalis muscle, which is also covered by the migraine scheme and is frequently injected for cosmetic reasons, too41,42. Hence, interruption of the corresponding proprioceptive feedback may explain the reduced incidence of anxiety. In blepharospasm, the numerically higher incidence of anxiety after BoNT treatment also fits into a similar concept. The main target in this indication is the orbicularis oculi muscle, which is involved in the expression of happiness (Duchenne’s smile) and narrows the palpebral fissure43. Its relaxation widens the eyes and may confer a negative shift in emotional expression and experience which, in turn, may promote anxiety. Of note, we observed a strong antidepressant effect of BoNT in the blepharospasm indication in our previous study with an overlapping population and an identical analytic approach8. Thus, BoNT injections around the eyes may have a differential effect on different psychiatric symptomatology. However, in the present study it is impossible to make a sharp distinction between the glabellar and orbital injections and their possibly opposite emotional effects, because the former is sometimes included in the treatment of blepharospasm and the latter may be injected in the cosmetic treatment of crow’s feet.
The reverberating interrelation between muscle activity and emotions is effective beyond the face44. Increased muscle tone in various body regions is a common phenomenon in anxiety disorders and may be both cause and effect of anxiety. In the treatment of anxiety disorders progressive muscle relaxation (PMR) is used to induce mental relaxation via tension and subsequent relaxation of skeletal muscles12,15. Proprioceptive afferences from the hypertonic musculature may account for the high prevalence of comorbid anxiety disorders or symptoms in patients suffering from dystonia or spasticity14,19,23,25. Accordingly, the anti-anxiety effect of BoNT injections in spasms and spasticity/upper and lower limbs as well as torticollis and neck pain/neck muscles may be explained by the interruption of these afferences45.
The body feedback concept may be extended to vegetative feedback mechanisms: hyperhidrosis is strongly associated with anxiety, and it is conceivable that increased sweating is not only a vegetative manifestation of anxiety but may also have an anxiety-enhancing feedback effect46–48. Botulinum toxin treatment has been successfully used as a treatment of anxiety disorders associated with hyperhidrosis49. However, we did not find a significant effect in our analyses. Bladder hyperactivity is also a vegetative correlate of anxiety, but we did not find association between BoNT treatment for this indication and decreased incidence of anxiety either50. As for saliva production, xerostomia is rather associated with anxiety than sialorrhea51. However, we found an association of BoNT treatment of sialorrhea with absence of anxiety. In summary, these findings do not support a role of interoceptive/vegetative feedback mechanisms in the observed anti-anxiety effect of BoNT.
It is possible, yet improbable that direct pharmacological BoNT effects within the CNS may explain its psychotropic action. BoNT may undergo targeted, transneuronal transport into the CNS where it may theoretically reach structures involved in the regulation of emotions52,53. In theory, BoNT may also reach the CNS and accomplish its anti-anxiety effect via systemic distribution. However, the amount of circulating BoNT may be very low, and the anti-anxiety effect shows no dose-dependence across the investigated indications with large vs. small muscles/muscle groups54. More likely, the peripheral action of BoNT may initiate a chain of neurochemical and neuroplastic changes that may be propagated to remote sites within the CNS55. Such neuronal reorganisation has been observed in patients treated for dystonia and spasticity45,56–58. It may also explain anxiolytic effects of BoNT applied at various injection sites in rats or mice30–32.
In the investigated indications, BoNT may have higher efficacy and better tolerability than the treatment options that were taken as comparators. Unfortunately, the FAERS database does not include efficacy data. As some of these conditions are chronic and burdensome, they may lead to secondary, reactive psychiatric comorbidities including anxiety disorders and related symptoms59. Hence, the more a treatment improves the primary condition for which it is given, the more it may also protect against the sequel of this condition. Thus, differential relief from the burden of disease between the BoNT and the control group may lead to overestimation of a possible specific anti-anxiety effect of BoNT. This may include relief from pain, which is a symptom of some of the investigated indications, especially migraine. However, superior efficacy and tolerability is not a unifying explanation of our findings either, because in the blepharospasm indication, in which it is the most effective treatment, there is no protective effect of BoNT against anxiety, but rather an anxiogenic tendency60. This also applies for the other indications in which BoNT did not show a protective effect against anxiety.
A neuronal structure that may mediate effects of BoNT on emotional experience and anxiety, is the amygdala61–63. Experimental studies have shown that facial injections of BoNT can modify its activity in response to emotional stimuli64,65.
There are some general limitations of this study. FAERS/AERS reporting is voluntary and often incomplete. Thus, the investigated data sets represent only a fraction of actual cases and the frequencies do not represent population incidences. Moreover, legal and scientific variables as well as newsworthiness may influence reporting to FAERS/AERS66,67. To address these limitations and to assess the significance of the difference between the sub-cohorts, we used disproportionality analysis with reporting odds ratios and 95% CI. Other limitations to consider include occasionally missing demographic variables, treatment doses and durations, and comprehensive medical records as well as bias associated with the comparator (differential efficacy, undetected differences between patients treated with the substance of interest and the comparator). Moreover, unreported life events and situations may have an imponderable impact on the incidence of anxiety. We excluded all the reports with comorbid anxiety disorders or anxiolytic medications (both labelled and off-label use); however, both may be underreported, which may affect the results. Exclusion of these reports may lead to underestimation of the efficacy of BoNT against anxiety, because we capture only preventive effects on incident anxiety. Therapeutic effects on prevalent anxiety may be more pronounced, but are not accessible to our analytic approach. Across all indications, there are differences in the concurrent medications between the BoNT and the reference groups, which may have confounding effects.
In conclusion, our findings show that BoNT administered for various indications and injection sites may have a protective effect against incident anxiety. The anti-anxiety effect represents an advantage over the alternative treatment options, because anxiety disorders and related symptoms are a frequent comorbidity in the respective indications. Even though afflicted with several limitations, our findings are encouraging to pursue the anxiolytic potential of BoNT in RCTs with patients suffering from anxiety disorders. Though there are effective pharmacological and psychotherapeutic treatments for these disorders, there is a need for further therapeutic options and BoNT may be one of them.
Supplementary Information
Acknowledgements
We thank Dr. Da Shi for his contributions to data preparation. We also thank members of the Abagyan lab for the support during the project. We also thank clinical faculty at Skaggs School of Pharmacy and Pharmaceutical Sciences for useful discussions.
Author contributions
R.A., M.A.W, and T.M. designed the study, R.A. processed the data set, T.M. performed the analyses, M.A.W., T.M., and T.H.C.K. drafted the manuscript, and all authors reviewed its final version.
Data availability
The data sets are de-identified and made available to the public online by the United States Food and Drug Administration. Institutional Review Board requirements do not apply under 45 CFR 46.102. https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-latest-quarterly-data-files. Both FAERS and AERS datasets are de-identified and are made available online at: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm082193.htm. Institutional Review Board Requirements do not apply under 45 CFR 46.102. There was no direct human participation in the study. Thus, all experiments were performed in accordance with relevant guidelines and regulations.
Competing interests
M.A.W. and T.H.C.K. have consulted for and received honoraria from Allergan/Abbvie pharmaceuticals. Other authors declare no conflict of financial or non-financial interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: M. Axel Wollmer and Tigran Makunts.
Contributor Information
M. Axel Wollmer, Email: m.wollmer@asklepios.com.
Ruben Abagyan, Email: rabagyan@health.ucsd.edu.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-03713-x.
References
- 1.Wollmer MA, et al. Facing depression with botulinum toxin: A randomized controlled trial. J. Psychiatr. Res. 2012;46:574–581. doi: 10.1016/j.jpsychires.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 2.Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA: A randomized, double-blind, placebo controlled trial. J. Psychiatr. Res. 2014;52:1–6. doi: 10.1016/j.jpsychires.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Magid M, et al. Treatment of major depressive disorder using botulinum toxin A: A 24-week randomized, double-blind, placebo-controlled study. J. Clin. Psychiatry. 2014;75:837–844. doi: 10.4088/JCP.13m08845. [DOI] [PubMed] [Google Scholar]
- 4.Magid M, et al. Treating depression with botulinum toxin: A pooled analysis of randomized controlled trials. Pharmacopsychiatry. 2015;48:205–210. doi: 10.1055/s-0035-1559621. [DOI] [PubMed] [Google Scholar]
- 5.Brin MF, et al. OnabotulinumtoxinA for the treatment of major depressive disorder: A phase 2 randomized, double-blind, placebo-controlled trial in adult females. Int. Clin. Psychopharmacol. 2020;35:19–28. doi: 10.1097/YIC.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulze J, et al. Botulinum toxin for the management of depression: An updated review of the evidence and meta-analysis. J. Psychiatr. Res. 2021;135:332–340. doi: 10.1016/j.jpsychires.2021.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Cohen IV, Makunts T, Atayee R, Abagyan R. Population scale data reveals the antidepressant effects of ketamine and other therapeutics approved for non-psychiatric indications. Sci. Rep. 2017;7:1450. doi: 10.1038/s41598-017-01590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makunts T, Wollmer MA, Abagyan R. Postmarketing safety surveillance data reveals antidepressant effects of botulinum toxin across various indications and injection sites. Sci Rep. 2020;10:12851. doi: 10.1038/s41598-020-69773-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finzi E, Rosenthal NE. Emotional proprioception: Treatment of depression with afferent facial feedback. J. Psychiatr. Res. 2016;80:93–96. doi: 10.1016/j.jpsychires.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Thoits PA. Self-labeling processes in mental illness: The role of emotional deviance. Am. J. Sociol. 1985;92:221–249. [Google Scholar]
- 11.Ritchie H. & Roser M. Mental Health. OurWorldInData.org. https://ourworldindata.org/mental-health (2018).
- 12.Conrad A, Roth WT. Muscle relaxation therapy for anxiety disorders: It works but how? J. Anxiety Disord. 2007;21:243–264. doi: 10.1016/j.janxdis.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Domschke K, Stevens S, Pfleiderer B, Gerlach AL. Interoceptive sensitivity in anxiety and anxiety disorders: An overview and integration of neurobiological findings. Clin. Psychol. Rev. 2010;30:1–11. doi: 10.1016/j.cpr.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Montero-Marin J, Garcia-Campayo J, López-Montoyo A, Zabaleta-Del-Olmo E, Cuijpers P. Is cognitive-behavioural therapy more effective than relaxation therapy in the treatment of anxiety disorders? A meta-analysis. Psychol. Med. 2018;48:1427–1436. doi: 10.1017/S0033291717003099. [DOI] [PubMed] [Google Scholar]
- 15.Tarsha MS, Park S, Tortora S. Body-centered interventions for psychopathological conditions: A review. Front. Psychol. 2020;10:2907. doi: 10.3389/fpsyg.2019.02907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weineck F, Schultchen D, Hauke G, Messner M, Pollatos O. Using bodily postures to reduce anxiety and improve interoception: A comparison between powerful and neutral poses. PLoS ONE. 2020;15:e0242578. doi: 10.1371/journal.pone.0242578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finzi E, Rosenthal NE. Botulinum toxin therapy of social anxiety disorder: A case series. J. Clin. Psychopharmacol. 2019;39:410–412. doi: 10.1097/JCP.0000000000001066. [DOI] [PubMed] [Google Scholar]
- 18.Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J. Cosmet. Dermatol. 2009;8:24–26. doi: 10.1111/j.1473-2165.2009.00419.x. [DOI] [PubMed] [Google Scholar]
- 19.Moraru E, et al. Relation between depression and anxiety in dystonic patients: Implications for clinical management. Depress. Anxiety. 2002;16:100–103. doi: 10.1002/da.10039. [DOI] [PubMed] [Google Scholar]
- 20.Weber A, et al. Psychosocial aspects of patients with focal hyperhidrosis: Marked reduction of social phobia, anxiety and depression and increased quality of life after treatment with botulinum toxin A. Br. J. Dermatol. 2005;152:342–345. doi: 10.1111/j.1365-2133.2004.06334.x. [DOI] [PubMed] [Google Scholar]
- 21.Demiryurek BE, et al. Effects of onabotulinumtoxinA treatment on efficacy, depression, anxiety, and disability in Turkish patients with chronic migraine. Neurol. Sci. 2016;37:1779–1784. doi: 10.1007/s10072-016-2665-z. [DOI] [PubMed] [Google Scholar]
- 22.Shayesteh A, Boman J, Janlert U, Brulin C, Nylander E. Primary hyperhidrosis: Implications on symptoms, daily life, health and alcohol consumption when treated with botulinum toxin. J. Dermatol. 2016;43:928–933. doi: 10.1111/1346-8138.13291. [DOI] [PubMed] [Google Scholar]
- 23.Tomic S, Petkovic I, Pucic T, Resan B, Juric S, Rotim T. Cervical dystonia and quality of life. Acta Neurol. Belg. 2016;116:589–592. doi: 10.1007/s13760-016-0634-1. [DOI] [PubMed] [Google Scholar]
- 24.Dong H, Fan S, Luo Y, Peng B. Botulinum toxin relieves anxiety and depression in patients with hemifacial spasm and blepharospasm. Neuropsychiatr. Dis. Treat. 2018;15:33–36. doi: 10.2147/NDT.S181820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu A, Hillel A, Zhao W, Meyer T. Anxiety and depression in spasmodic dysphonia patients. World J. Otorhinolaryngol. Head Neck Surg. 2018;4:110–116. doi: 10.1016/j.wjorl.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blumenfeld AM, et al. Effects of onabotulinumtoxinA treatment for chronic migraine on common comorbidities including depression and anxiety. J. Neurol. Neurosurg. Psychiatry. 2019;90:353–360. doi: 10.1136/jnnp-2018-319290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ceylan D, Erer S, Zarifoğlu M, Türkeş N, Özkaya G. Evaluation of anxiety and depression scales and quality of LIFE in cervical dystonia patients on botulinum toxin therapy and their relatives. Neurol. Sci. 2019;40:725–731. doi: 10.1007/s10072-019-3719-9. [DOI] [PubMed] [Google Scholar]
- 28.d'Onofrio F, et al. Impulse control disorders in chronic migraine with medication overuse after onabotulinumtoxinA: A single-center prospective cohort study. J. Clin. Neurosci. 2020;80:152–155. doi: 10.1016/j.jocn.2020.07.075. [DOI] [PubMed] [Google Scholar]
- 29.Wang C, et al. Botulinum toxin A improves psychological distress in patients with hemifacial spasm. Acta Neurol. Belg. 2021 doi: 10.1007/s13760-021-01601-9. [DOI] [PubMed] [Google Scholar]
- 30.Holzmann C, et al. Effects of intrastriatal botulinum neurotoxin A on the behavior of Wistar rats. Behav. Brain Res. 2012;234:107–116. doi: 10.1016/j.bbr.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Chen WJ, et al. Unilateral facial injection of Botulinum neurotoxin A attenuates bilateral trigeminal neuropathic pain and anxiety-like behaviors through inhibition of TLR2-mediated neuroinflammation in mice. J. Headache Pain. 2021;22:38. doi: 10.1186/s10194-021-01254-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yesudhas A, et al. BOTOX counteracts the innate anxiety-related behaviours in correlation with increased activities of key antioxidant enzymes in the hippocampus of ageing experimental mice. Biochem. Biophys. Res. Commun. 2021;569:54–60. doi: 10.1016/j.bbrc.2021.06.071. [DOI] [PubMed] [Google Scholar]
- 33.Sinyor M, et al. Does inclusion of a placebo arm influence response to active antidepressant treatment in randomized controlled trials? Results from pooled and meta-analyses. J. Clin. Psychiatry. 2010;71:270–279. doi: 10.4088/JCP.08r04516blu. [DOI] [PubMed] [Google Scholar]
- 34.Rutherford BR, et al. Influence of study design on treatment response in anxiety disorder clinical trials. Depress. Anxiety. 2015;32:944–957. doi: 10.1002/da.22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Craigle V. MedWatch: The FDA safety information and adverse event reporting program. J. Med. Libr. Assoc. 2007;95:224–225. [Google Scholar]
- 36.Cohen IV, Makunts T, Moumedjian T, Issa MA, Abagyan R. Cardiac adverse events associated with chloroquine and hydroxychloroquine exposure in 20 years of drug safety surveillance reports. Sci. Rep. 2020;10:19199. doi: 10.1038/s41598-020-76258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen IV, Makunts T, Abagyan R, Thomas K. Concomitant drugs associated with increased mortality for MDMA users reported in a drug safety surveillance database. Sci. Rep. 2021;11:5997. doi: 10.1038/s41598-021-85389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montastruc JL, Sommet A, Bagheri H, Lapeyre-Mestre M. Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. Br. J. Clin. Pharmacol. 2011;72:905–908. doi: 10.1111/j.1365-2125.2011.04037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agresti A. On logit confidence intervals for the odds ratio with small samples. Biometrics. 1999;55:597–602. doi: 10.1111/j.0006-341x.1999.00597.x. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Liu T, Luo W. Botulinum neurotoxin therapy for depression: Therapeutic mechanisms and future perspective. Front. Psychiatry. 2021;12:584416. doi: 10.3389/fpsyt.2021.584416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blumenfeld AM, et al. Insights into the functional anatomy behind the PREEMPT injection paradigm: Guidance on achieving optimal outcomes. Headache. 2017;57:766–777. doi: 10.1111/head.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ekman P, Friesen WV. Facial Action Coding System: A Technique for the Measurement of Facial Movement. Consulting Psychologists Press; 1978. [Google Scholar]
- 43.Sung Y, Nam SM, Lew H. Clinical outcomes of individualized botulinum neurotoxin type A injection techniques in patients with essential blepharospasm. Korean J. Ophthalmol. 2015;29:115–120. doi: 10.3341/kjo.2015.29.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scheer C, Kubowitsch S, Dendorfer S, Jansen P. Happy enough to relax? How positive and negative emotions activate different muscular regions in the back - an explorative study. Front. Psychol. 2021;12:511746. doi: 10.3389/fpsyg.2021.511746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khosravani S, Buchanan J, Johnson MD, Konczak J. Effect of neck botulinum neurotoxin injection on proprioception and somatosensory-motor cortical processing in cervical dystonia. Neurorehabil. Neural Repair. 2020;34:309–320. doi: 10.1177/1545968320905799. [DOI] [PubMed] [Google Scholar]
- 46.Klein SZ, Hull M, Gillard KK, Peterson-Brandt J. Treatment patterns, depression, and anxiety among US patients diagnosed with hyperhidrosis: a retrospective cohort study. Dermatol. Ther. 2020;10:1299–1314. doi: 10.1007/s13555-020-00439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kristensen JK, et al. Anxiety and depression in primary hyperhidrosis: an observational study of 95 consecutive Swedish outpatients. Acta Derm. Venereol. 2020;100:00240. doi: 10.2340/00015555-3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kristensen JK, Vestergaard DG, Swartling C, Bygum A. Association of primary hyperhidrosis with depression and anxiety: A systematic review. Acta Derm. Venereol. 2020;100:00044. doi: 10.2340/00015555-3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Connor KM, Cook JL, Davidson JR. Botulinum toxin treatment of social anxiety disorder with hyperhidrosis: a placebo-controlled double-blind trial. J. Clin. Psychiatry. 2006;67:30–36. doi: 10.4088/jcp.v67n0105. [DOI] [PubMed] [Google Scholar]
- 50.Chess-Williams R, McDermott C, Sellers DJ, West EG, Mills KA. Chronic psychological stress and lower urinary tract symptoms. Low Urin. Tract Symptoms. 2021;13:414–424. doi: 10.1111/luts.12395. [DOI] [PubMed] [Google Scholar]
- 51.Gholami N, Sabzvari BH, Razzaghi A, Salah S. Effect of stress, anxiety and depression on unstimulated salivary flow rate and xerostomia. J. Dent. Res. Dent. Clin. Dent. Prospects. 2017;11:247–252. doi: 10.15171/joddd.2017.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caleo M, Schiavo G. Central effects of tetanus and botulinum neurotoxins. Toxicon. 2009;54:593–599. doi: 10.1016/j.toxicon.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 53.Restani L, Antonucci F, Gianfranceschi L, Rossi C, Rossetto O, Caleo M. Evidence for anterograde transport and transcytosis of botulinum neurotoxin A (BoNT/A) J. Neurosci. 2011;31:15650–15659. doi: 10.1523/JNEUROSCI.2618-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hallett M. Explanation of timing of botulinum neurotoxin effects, onset and duration, and clinical ways of influencing them. Toxicon. 2015;107:64–67. doi: 10.1016/j.toxicon.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weise D, Weise CM, Naumann M. Central effects of botulinum neurotoxin-evidence from human studies. Toxins. 2019;11:21. doi: 10.3390/toxins11010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hok P, Veverka T, Hluštík P, Nevrlý M, Kaňovský P. The central effects of botulinum toxin in dystonia and spasticity. Toxins. 2021;13:155. doi: 10.3390/toxins13020155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Opavský R, Hluštík P, Otruba P, Kaňovský P. Somatosensory cortical activation in cervical dystonia and its modulation with botulinum toxin: an fMRI study. Int. J. Neurosci. 2012;122:45–52. doi: 10.3109/00207454.2011.623807. [DOI] [PubMed] [Google Scholar]
- 58.Kikuchi A, et al. Brain metabolic changes of cervical dystonia with spinocerebellar ataxia type 1 after botulinum toxin therapy. Intern. Med. 2016;55:1919–1922. doi: 10.2169/internalmedicine.55.5843. [DOI] [PubMed] [Google Scholar]
- 59.Buse DC, et al. Comorbid and co-occurring conditions in migraine and associated risk of increasing headache pain intensity and headache frequency: Results of the migraine in America symptoms and treatment (MAST) study. J. Headache Pain. 2020;21:23. doi: 10.1186/s10194-020-1084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Green KE, Rastall D, Eggenberger E. Treatment of blepharospasm/hemifacial spasm. Curr. Treat. Options Neurol. 2017;19:41. doi: 10.1007/s11940-017-0475-0. [DOI] [PubMed] [Google Scholar]
- 61.Bandelow B, et al. Biological markers for anxiety disorders, OCD and PTSD—A consensus statement. Part I: Neuroimaging and genetics. World J. Biol. Psychiatry. 2016;17:321–365. doi: 10.1080/15622975.2016.1181783. [DOI] [PubMed] [Google Scholar]
- 62.Bandelow B, et al. Biological markers for anxiety disorders, OCD and PTSD: A consensus statement. Part II: Neurochemistry, neurophysiology and neurocognition. World J. Biol. Psychiatry. 2017;18:162–214. doi: 10.1080/15622975.2016.1190867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Šimić G, et al. Understanding emotions: Origins and roles of the amygdala. Biomolecules. 2021;11:823. doi: 10.3390/biom11060823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hennenlotter A, et al. The link between facial feedback and neural activity within central circuitries of emotion: New insights from botulinum toxin-induced denervation of frown muscles. Cereb. Cortex. 2009;19:537–542. doi: 10.1093/cercor/bhn104. [DOI] [PubMed] [Google Scholar]
- 65.Kim MJ, et al. Botulinum toxin-induced facial muscle paralysis affects amygdala responses to the perception of emotional expressions: Preliminary findings from an A-B-A design. Biol. Mood Anxiety Disord. 2014;4:11. doi: 10.1186/2045-5380-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alatawi YM, Hansen RA. Empirical estimation of under-reporting in the U.S. food and drug administration adverse event reporting system (FAERS) Expert. Opin. Drug Saf. 2017;16:761–767. doi: 10.1080/14740338.2017.1323867. [DOI] [PubMed] [Google Scholar]
- 67.Maciejewski M, et al. Reverse translation of adverse event reports paves the way for de-risking preclinical off-targets. Elife. 2017;6:e25818. doi: 10.7554/eLife.25818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets are de-identified and made available to the public online by the United States Food and Drug Administration. Institutional Review Board requirements do not apply under 45 CFR 46.102. https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-latest-quarterly-data-files. Both FAERS and AERS datasets are de-identified and are made available online at: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm082193.htm. Institutional Review Board Requirements do not apply under 45 CFR 46.102. There was no direct human participation in the study. Thus, all experiments were performed in accordance with relevant guidelines and regulations.