Abstract
Background
Prenatal care is recommended during pregnancy as a method to improve neonatal and maternal outcomes. Improving the use of prenatal care is important, particularly for women at moderate to high risk of adverse outcomes. Incentives are sometimes utilized to encourage women to attend prenatal care visits.
Objectives
To determine whether incentives are an effective tool to increase utilization of timely prenatal care among women.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (31 January 2015) and the reference lists of all retrieved studies.
Selection criteria
Randomized controlled trials (RCTs), quasi‐RCTs, and cluster‐RCTs that utilized direct incentives to pregnant women explicitly linked to initiation and frequency of prenatal care were included. Incentives could include cash, vouchers, coupons or products not generally offered to women as a standard of prenatal care. Comparisons were to no incentives and to incentives not linked directly to utilization of care. We also planned to compare different types of interventions, i.e. monetary versus products or services.
Data collection and analysis
Two review authors independently assessed studies for inclusion and methodological quality. Two review authors independently extracted data. Data were checked for accuracy.
Main results
We identified 11 studies (19 reports), six of which we excluded. Five studies, involving 11,935 pregnancies were included, but only 1893 pregnancies contributed data regarding our specified outcomes. Incentives in the studies included cash, gift card, baby carrier, baby blanket or taxicab voucher and were compared with no incentives. Meta‐analysis was performed for only one outcome 'Return for postpartum care' and this outcome was not pre‐specified in our protocol. Other analyses were restricted to data from single studies.
Trials were at a moderate risk of bias overall. Randomization and allocation were adequate and risk of selection bias was low in three studies and unclear in two studies. None of the studies were blinded to the participants. Blinding of outcome assessors was adequate in one study, but was limited or not described in the remaining four studies. Risk of attrition was deemed to be low in all studies that contributed data to the review. Two of the studies reported or analyzed data in a manner that was not consistent with the predetermined protocol and thus were deemed to be at high risk. The other three studies were low risk for reporting bias. The largest two of the five studies comprising the majority of participants took place in rural, low‐income, homogenously Hispanic communities in Central America. This setting introduces a number of confounding factors that may affect generalizability of these findings to ethnically and economically diverse urban communities in developed countries.
The five included studies of incentive programs did not report any of this review's primary outcomes: preterm birth, small‐for‐gestational age, or perinatal death.
In terms of this review's secondary outcomes, pregnant women receiving incentives were no more likely to initiate prenatal care (risk ratio (RR) 1.04, 95% confidence interval (CI) 0.78 to 1.38, one study, 104 pregnancies). Pregnant women receiving incentives were more likely to attend prenatal visits on a frequent basis (RR 1.18, 95% CI 1.01 to 1.38, one study, 606 pregnancies) and obtain adequate prenatal care defined by number of “procedures” such as testing blood sugar or blood pressure, vaccinations and counseling about breastfeeding and birth control (mean difference (MD) 5.84, 95% CI 1.88 to 9.80, one study, 892 pregnancies). In contrast, women who received incentives were more likely to deliver by cesarean section (RR 1.97, 95% CI 1.18 to 3.30, one study, 979 pregnancies) compared to those women who did not receive incentives.
Women who received incentives were no more likely to return for postpartum care based on results of meta‐analysis (average RR 0.75, 95% CI 0.21 to 2.64, two studies, 833 pregnancies, Tau² = 0.81, I² = 98%). However, there was substantial heterogeneity in this analysis so a subgroup analysis was performed and this identified a clear difference between subgroups based on the type of incentive being offered. In one study, women receiving non‐cash incentives were more likely to return for postpartum care (RR 1.26, 95% CI 1.09 to 1.47, 240 pregnancies) than women who did not receive non‐cash incentives. In another study, women receiving cash incentives were less likely to return for postpartum care (RR 0.43, 95% CI 0.30 to 0.62, 593 pregnancies) than women who did not receive cash incentives.
No data were identified for the following secondary outcomes: frequency of prenatal care; pre‐eclampsia; satisfaction with birth experience; maternal mortality; low birthweight (less than 2500 g); infant macrosomia (birthweight greater than 4000 g); or five‐minute Apgar less than seven.
Authors' conclusions
The included studies did not report on this review's main outcomes: preterm birth, small‐for‐gestational age, or perinatal death. There is limited evidence that incentives may increase utilization and quality of prenatal care, but may also increase cesarean rate. Overall, there is insufficient evidence to fully evaluate the impact of incentives on prenatal care initiation. There are conflicting data as to the impact of incentives on return for postpartum care. Two of the five studies which accounted for the majority of women in this review were conducted in rural, low‐income, overwhelmingly Hispanic communities in Central America, thus limiting the external validity of these results.
There is a need for high‐quality RCTs to determine whether incentive program increase prenatal care use and improve maternal and neonatal outcomes. Incentive programs, in particular cash‐based programs, as suggested in this review and in several observational studies may improve the frequency and ensure adequate quality of prenatal care. No peer‐reviewed data have been made publicly available for one of the largest incentive‐based prenatal programs – the statewide Medicaid‐based programs within the United States. These observational data represent an important starting point for future research with significant implications for policy development and allocation of healthcare resources. The disparate findings related to attending postpartum care should also be further explored as the findings were limited by the number of studies. Future large RCTs are needed to focus on the outcomes of preterm birth, small‐for‐gestational age and perinatal outcomes.
Plain language summary
Impact of offering incentives in exchange for attending prenatal care visits on maternal and neonatal health outcomes
Getting care from a provider during a woman's pregnancy is important to try to ensure the best pregnancy outcomes. Early and regular prenatal care can increase the chances of having a healthy baby. However, many women begin prenatal care late in the pregnancy or do not attend all of their scheduled visits. This can make it difficult for providers to help avert problems in pregnancy. In an effort to encourage pregnant women to begin prenatal care early in the pregnancy and to attend all of their visits, some health systems and providers offer incentives to patients to attend prenatal care. These incentives may be monetary, items such as coupons or car seats, or may be for services.
This review's objective was to find out if offering incentives is an effective way to improve the beginning of prenatal care early in pregnancy and the attendance at all scheduled prenatal visits. We searched for trials on 31 January 2015 and found a total of five trials, involving 11,935 pregnancies, but only 1893 pregnancies contributed data towards this review. Overall, the trials were at a moderate risk of bias. Incentives in these studies included cash, gift card, baby carrier, baby blanket and taxicab voucher.
The studies found did not report on the main outcomes that we wanted to evaluate in this review: preterm delivery, small babies, or deaths of the babies.
One study found that women receiving incentives were more likely to attend frequent prenatal visits during their pregnancy. One study indicated that women who received incentives were more likely to obtain adequate quality prenatal care defined as undergoing a certain number of procedures such as testing blood sugar or blood pressure, vaccinations and counseling about breastfeeding and birth control. One study found that women who received incentives were no more likely to begin prenatal care early in pregnancy. One study found that women receiving incentives were somewhat more likely to be delivered by cesarean section. There were two studies that examined likelihood of returning for postpartum care after delivery and their combined results indicated that women who received incentives were no more likely to return for postpartum care ‐ these two studies had different results. In one of the studies, women who received non‐cash incentives were more likely to return for postpartum care than those who did not receive incentive. Whereas, in other study, women who received cash incentives were less likely to return for postpartum care than those who did not receive incentive.
Overall, the included studies were of moderate risk of bias. Three of the studies adequately described the process of selecting and randomizing women, while two of the studies did not describe this process in detail. All of the studies allowed pregnant women to know whether they were in the treatment group or placebo group. Four of the studies allowed those assessing outcomes to know whether women were in the treatment group or placebo group. All five studies reported results completely and disclosed incomplete data or number of participants who dropped out of the study. Two of the studies reported or analyzed results in a manner different from how they originally planned, while the other three reported results consistent with their plan. No other sources of bias were found. Two of the five studies which accounted for the majority of women in this review were conducted in rural, low‐income, overwhelmingly Hispanic communities in Central America. Therefore, the findings of this review may not accurately predict what would happen if similar studies were performed in developed countries with more ethnic and economic diversity. There is a need for more, high‐quality studies to evaluate the impact of offering incentives to pregnant women for attending prenatal care visits and the effects of this on the health and wellbeing of the mother and her baby.
Background
Description of the condition
Prenatal care refers to the medical and nursing care recommended during pregnancy. This includes both health care and childbirth education and counseling (WHO 2006). The aim of good prenatal care is to detect any potential problems early, to prevent them if possible, and to direct the women to appropriate specialists or hospitals if necessary. Additionally, prenatal care can grant reassurance of wellbeing to a pregnant woman and her family while providing education and information. Community support and engagement for pregnant women is also important to improving outcomes (WHO 2015). Early and regular prenatal care can increase the chances of having a healthy baby (AAP 2012; Alexander 2001). The plan of antenatal care should take into consideration the medical, nutritional, psychosocial, and educational needs of the woman and her family (WHO 2006).
Benefits of prenatal care
A number of studies have demonstrated a relationship between fewer prenatal visits and poorer pregnancy outcomes such as low birthweight, preterm birth and fetal or infant death. One large retrospective cohort study in Finland found that women who attended no prenatal visits or between one to five prenatal visits had increased risk for low birthweight (odds ratio (OR) 5.46, 95% confidence interval (CI) 3.90 to 7.65 and OR 9.18, 95% CI 6.65 to 12.68, respectively), fetal death (OR 5.19, 95% CI 2.04 to 13.22 and OR 12.05, 95% CI 5.95 to 24.40, respectively), and neonatal death (OR 8.66, 95% CI 3.59 to 20.86 and OR 10.03, 95% CI 3.85 to 26.13, respectively) compared to women who attended six or more prenatal visits (Raatikainen 2007). A large retrospective cohort in the United States demonstrated that women with no prenatal care or inadequate prenatal care (defined as attending fewer than 50% of recommended visits) had an increased risk of preterm birth (OR 4.4, 95% CI 4.0 to 4.8 and OR 2.0, 95% CI 1.9 to 2.0, respectively), low birthweight (OR 4.8, 95% CI 4.4 to 5.3 and OR 1.7, 95% CI 1.6 to 1.7, respectively), and infant mortality (OR 4.7, 95% 3.7 to 6.0 and OR 1.5, 95% 1.3 to 1.7, respectively) compared to women who received adequate prenatal care (Cox 2011). A cross‐sectional study in Brazil found that number of prenatal care appointments was more predictive (beta = 28.21, P = 0.007) of low birthweight than maternal age (beta = ‐10.28, P = 0.024) or pre‐gestational body mass index (BMI) (beta = 13.02, P = 0.037) (Carvahlo Padilha 2009). Ensuring that women obtain adequate quality and frequency of prenatal care appears to be an effective method to improve perinatal outcomes.
In addition, adequate prenatal care has been shown to be cost‐effective. This effect is largely due to the exponential cost associated with care of preterm and low birthweight infants. A retrospective cohort study in the United States found that healthcare costs in the first year of life were $18,900 (US) greater for low birthweight infants compared to normal birthweight infants. The same study demonstrated that 3% of women who participated in a statewide augmented prenatal care model had a low birthweight delivery verses 6% of women who received standard prenatal care. The savings associated with decreased rate of low birthweight constituted a 37% return on investment for additional costs associated with the augmented program (Sackett 2004). A cost‐benefit analysis of Medicaid data in the United States demonstrated that costs for pregnant teenagers who obtained any prenatal care were $2,400 and $3,200 (US) lower than those for pregnant teenagers who obtained no prenatal care (Hueston 2008). An Institute of Medicine (IOM) report from 1994 demonstrated that for every $1 (US) spent on prenatal care for high‐risk women, $3.38 (US) is saved in medical care costs for low birthweight infants (IOM 1994). Economic benefits of improved prenatal care and subsequent birth outcomes are also likely to be seen in low‐ and middle‐income settings.
Prenatal care models
In evaluation of prenatal care models, it is important to consider that there is no single metric by which prenatal care is judged as adequate. However, there are common themes. The majority of organizations that make recommendations for prenatal care agree that important metrics include early initiation of prenatal care, sufficient number of prenatal care visits, monitoring specific physical and laboratory parameters, providing prenatal and intrapartum education, appropriate supervision of labor process, encouragement of vaginal delivery, promoting breastfeeding, postpartum follow‐up and family planning education, and overall patient satisfaction with the birth experience (AAP 2012; Chalmers 2001; WHO 2006).
Initiation of prenatal care
Early initiation of prenatal care is encouraged for optimization of maternal health and infectious disease screening. The World Health Organization (WHO) recommends initiation of prenatal care as early as possible in pregnancy but ideally before 16 weeks gestational age (WHO 2006). The American College of Obstetricians and Gynecologists (ACOG) recommends a preconception visit to assist in optimizing health prior to pregnancy. Once pregnant, initiation of prenatal care is recommended prior to 14 weeks gestational age when possible, but sooner if the woman has vaginal bleeding, is at risk for ectopic pregnancy, has a multiple gestation pregnancy, or has a history of poor pregnancy outcomes in the past (AAP 2012).
Frequency of prenatal care
The recommended number of prenatal care visits depends on when prenatal care was begun in the pregnancy. Several indices have been used to aid in identifying adequacy of prenatal care, with one of the most widely used ones being the Kotelchuck Adequacy of Prenatal Care Utilization (APNCU) Index (Heaman 2008; Kotelchuck 1994). The APNCU compares the number of attended visits to the number of expected visits, as determined by ACOG recommendations (AAP 2012).
The ACOG recommended standard schedule includes a visit before 14 weeks followed by visits every four weeks for the first 28 weeks, every two to three weeks until 36 weeks, and every week until delivery. More frequent visits are recommended if the patient is complicated by medical or obstetric issues such as gestational diabetes, hypertension or multiple gestation. Less frequent visits are acceptable for women at low risk for complications (AAP 2012).
There has been a global trend toward de‐medicalizing prenatal care which emphasizes less frequent visits for low‐risk pregnant women. The WHO recommends only four routine antepartum visits over the course of pregnancy, with a plan for more frequent visits if that patient has hypertension, severe anemia, HIV or malaria. The recommended standard schedule includes visits before 16 weeks, 24 to 28 weeks, 30 to 32 weeks and 36 to 38 weeks (WHO 2006).
Adequacy of prenatal care
The WHO recommends that each visit includes a number of procedures including screening, history‐taking, medical prophylaxis and advising. Screening for pre‐eclampsia, anemia, syphilis and HIV is recommended at each visit. The prenatal care provider should ask about fetal movement, rupture of membranes, fever, burning with urination, vaginal discharge, signs of HIV infection, tobacco or substance abuse, or difficulty breathing or coughing at each visit. Tetanus toxoid immunization should be given once during each pregnancy. The prenatal provider should prescribe iron and folate, mebendazole and antimalarial medications in the second or third trimester. Each visit should include advice regarding nutrition, self‐care, birth plan and family planning (WHO 2006).
ACOG and other international specialty organizations recommend that each visit include assessment of vital signs, weight, uterine size, fetal heart tones, tobacco use and urinalysis. Initial laboratory screening should include blood type, Rh status, hemoglobin, platelets, Hepatitis B, syphilis, chlamydia, gonorrhea, HIV, cervical cancer, urinalysis and culture, and genetic testing. Tuberculosis screening should be performed if a patient is at risk. Second and third trimester screening should include gestational diabetes, blood type, Rh status, hemoglobin, syphilis and Group B streptococcus. Fetal anatomic ultrasound should be offered between 18 to 20 weeks. Each visit should include advice regarding nutrition, birth plan and family planning (AAP 2012).
Labor and delivery
The WHO recommends delivery in a birthing facility or with a skilled birthing attendant if a facility is not available or desired (WHO 2006). ACOG recommends delivery in a hospital or accredited birthing center (AAP 2012). Both organizations recommend frequent monitoring of maternal vital signs, fetal heart rate and uterine contractions throughout the birthing process. Specific recommendations regarding management of the labor process and delivery are available and vary according to clinical scenario as well as maternal and fetal risk factors.
Postpartum care
Postpartum care is a critical opportunity for counseling regarding appropriate interpregnancy intervals, screening for postpartum depression, addressing chronic health conditions and encouraging continuation of breastfeeding. The WHO recommends postpartum follow‐up within six weeks after delivery (WHO 2006). Postpartum visits should include examination of the uterus and perineum. Blood pressure and temperature should be documented. Patients should be counseled regarding breastfeeding, HIV infection, mood changes and family planning (WHO 1999). ACOG recommends postpartum visit within four to six weeks after delivery, or sooner if the pregnancy was complicated by maternal health conditions. Postpartum visits should include examination of breasts, perineum and cesarean incision, if applicable. Maternal weight and blood pressure should be documented and blood sugar should be tested where applicable. Women should be counseled regarding postpartum depression, breastfeeding and family planning (AAP 2012).
Adverse neonatal and maternal outcomes
Adverse neonatal outcomes, such as preterm birth and low birthweight, have serious short‐ and long‐term effects, such as increased neonatal and infant mortality, respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis and neurodevelopmental delays (Lin 2007; Mikkola 2005; Tommiska 2001). Maternal obesity, diabetes, tobacco use, substance abuse and poor utilization of prenatal care are all associated with increased risk for low birthweight. Other well‐documented maternal risk factors are easily identified but more difficult to mitigate, such as primigravida, low income, low educational achievement, young maternal age and marital status (Canning 2009; McDonald 2010; Moore 1994, Silva 2006). Adverse maternal outcomes such as pre‐eclampsia, cesarean delivery, and maternal mortality are also potential complications of lack of prenatal care. This is particularly problematic in lower socioeconomic groups and others with moderate‐ or high‐risk pregnancies.
Description of the intervention
Despite significant advances in medical technology, the modifiable nature of many risk factors and numerous small‐ and large‐scale interventions, little improvement has been demonstrated in the areas of adverse maternal and neonatal birth outcomes in recent decades. Previous studies have demonstrated that early and consistent prenatal care does help to modify some of the risk factors for adverse birth outcomes and reduce the incidence of preterm birth, particularly for teenage mothers and those from low socioeconomic backgrounds (Debiec 2010; Partington 2009; Quinlivan 2004). The effect has not been as significant in decreasing low birthweight (Ickovics 2007). Improving attendance of antenatal care may be a modifiable factor that could improve outcomes.
It is often the women at highest risk for adverse birth outcomes who receive the least adequate prenatal care. There have been several private‐ and government‐based programs that have attempted to address this disparity through increased access to social services, educational initiatives, or financial incentives.
Augmenting prenatal care with educational programs or social services may improve perinatal outcomes. One retrospective cohort study in Illinois (United States) demonstrated that participants in an augmented model of prenatal care had lower rates of low birthweight compared to non‐participants (7.4% versus 8.2%). The augmented program consisted of prenatal care services provided by a local publicly‐supported healthcare center and a targeted case management program which provided education and referral to social services. The program was only available to low‐income women. The program demonstrated a trend toward lower rates of low birthweight deliveries per visit and an hour spent with a case manager, but this effect was no longer statistically significant once adjusted for confounding factors such as smoking and race (Silva 2006).
Timing of program initiation may affect success. A retrospective cohort study in Canada demonstrated that low‐income pregnant women who participated in a prenatal support program beginning early in pregnancy (before 21 weeks' gestation), had significantly decreased rates of low birthweight infant compared to women who enrolled later in pregnancy (after 30 weeks' gestation) (risk ratio (RR) 0.47, 95% CI 0.22 to 0.98). The study also demonstrated that rate of low birthweight in this high‐risk group decreased to levels comparable to the surrounding population level when participants enrolled early (RR 1.29, 95% CI 0.71 to 2.32), whereas participants who enrolled later in pregnancy had rates of low birthweight significantly higher than the surrounding population (RR 2.76, 95% CI 1.61 to 4.74). The support program provided a small monthly cash supplement, educational materials and access to public health nursing (Canning 2009).
Incentives offered in exchange for participation in prenatal care or educational programs may provide the necessary motivation to prompt action. A retrospective cohort study in Nevada (United States) assessed perinatal outcomes for low‐income patients covered by an insurance company that offered a cash incentive to both the pregnant woman and prenatal care provider if the woman enrolled in prenatal care during the first trimester and maintained adequate frequency of prenatal visits thereafter. This cohort was compared to pregnant women who were covered by the insurance company prior to initiation of the incentive program. The pregnant women who received cash incentives had significantly lower rates of infant neonatal intensive care admission (OR 0.45, 95% CI 0.23 to 0.88). There was also a trend towards decreased rate of low birthweight (OR 0.53, 95% CI 0.23 to 1.18), but this effect was no longer statistically significant once adjusted for confounding factors (Rosenthal 2009). The impact of incentives has also been demonstrated in interventions within low‐ and middle‐income countries. One article reviewed several government‐based interventions in South Asia that offered cash or voucher incentives to pregnant women in exchange for obtaining prenatal care or delivery in a skilled facility. In Nepal, both pregnant women and healthcare providers received cash incentives in exchange for delivery in a skilled facility. This program resulted in a 2% to 3% increase in both deliveries within skilled facility as well as presence of a skilled birth attendant for home deliveries. In India, a program combined cash incentives to pregnant women in exchange for delivery in a skilled facility with implementation of network of "social health activists" who accompany pregnant women to prenatal, delivery and postpartum visits. This program resulted in a 43% increase in delivery within a skilled facility and a modest reduction in the neonatal death rate. In Bangladesh, a program combined vouchers that could be used for prenatal and postpartum care and delivery with skilled birth attendant and cash incentives to both pregnant women and healthcare providers in exchange for utilizing or providing skilled birth attendants. This program resulted in a 16% increase in the number of women attending at least one prenatal visit (P < 0.001), a 36% increase in number of deliveries with a skilled birth attendant present (P < 0.001), an 18% increase in number of deliveries within skilled facility (P < 0.001), and a 15% increase in the number of women who had a postpartum visit (P < 0.001). In Pakistan, a novel program sold booklets containing vouchers to pregnant women for a minimal fee. The vouchers granted free access to three prenatal visits, delivery in a skilled facility and one postpartum visit. The program resulted in a 21% increase in attendance of prenatal care, a 22% increase in delivery in skilled facility, and a 35% increase in attendance of a postpartum visit (Jehan 2012).
How the intervention might work
This review focuses on programs that offer incentives directly in exchange for participation in prenatal care. Incentives may include direct financial incentives, tangible items such as baby supplies or increased access to social services in exchange for initiation or maintenance of adequate prenatal care or participation in small group educational settings. Prenatal care includes any visit for childbirth care, education or counseling.
The hypothesis being testing with this review is that pregnant women will attend prenatal care visits earlier in pregnancy and will attend more visits during pregnancy if there is some tangible incentive for them to do so. This assumes that for these women, the knowledge of a "need" to attend early and frequent prenatal visits is not lacking or insufficient incentive enough to engage in prenatal care.
Why it is important to do this review
A better understanding of the best practices and pitfalls in this area of research can lead to improved maternal and neonatal clinical outcomes, as well as more effective use of resources for healthcare providers and public health initiatives.
Objectives
To determine whether incentives are an effective tool to increase utilization of timely prenatal care among women.
Methods
Criteria for considering studies for this review
Types of studies
Randomized, quasi‐randomized, and cluster‐randomized studies were eligible for inclusion in this review. Cross‐over studies were not eligible for inclusion. Trials identified only as published abstracts or conference proceedings were included only if outcome and trial characteristics data were able to be extracted from the published abstract or after communication with the authors.
Types of participants
All pregnant women were included.
Types of interventions
Interventions included direct incentives to pregnant women explicitly linked to initiation and frequency of prenatal care. Incentives could include cash, vouchers, coupons or products not generally offered to patients as a standard of prenatal care. Comparisons were to no incentives and to incentives not linked directly to utilization of care. We also planned to compare different types of interventions, i.e. monetary versus products or services.
Types of outcome measures
Primary outcomes
Preterm birth < 37 weeks.
Small‐for‐gestational age.
Perinatal deaths (fetal, neonatal, infant deaths).
Secondary outcomes
Adequacy of prenatal care.
Frequency of prenatal care.
Initiation of prenatal care.
Return for postpartum care (this outcome was added after the approved protocol).
Pre‐eclampsia.
Cesarean delivery.
Satisfaction with birth experience.
Maternal mortality.
Low birthweight (less than 2500 g).
Infant macrosomia (birthweight greater than 4000 g).
Five‐minute Apgar less than seven.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (31 January 2015).
For full search methods used to populate the PCG Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth Group in The Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, the Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth Group review topic (or topics), and is then added to the Register. The Trials Search Co‐ordinator searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that review authors then fully account for in the relevant review sections (Included, Excluded, Awaiting Classification or Ongoing).
Searching other resources
We searched the reference lists of all retrieved studies. We did not apply any language or date restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third person. We entered data into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or was supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed the methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We planned to use the standardized mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomized trials
We included cluster‐randomized trials in the analyses along with individually‐randomized trials. We adjusted their standard errors using the methods described in the Handbook where applicable, using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we had used ICCs from other sources, we planned to report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identified both cluster‐randomized trials and individually‐randomized trials, we planned to synthesize the relevant information. We considered it reasonable to combine the results from both if there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomization unit was considered to be unlikely.
We also acknowledged heterogeneity in the randomization unit and performed a sensitivity analysis to investigate the effects of the randomization unit.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomized to each group in the analyses, and all participants were analyzed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was be the number randomized minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We were only able to combine two studies in meta‐analysis for the outcome 'Return for postpartum care'. We assessed statistical heterogeneity in this meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if the I² was greater than 30% and either the T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using Review Manager Software (RevMan 2014). We combined studies in meta‐analysis for the outcome 'Return for postpartum care'. We used random‐effects meta‐analysis, given the substantial heterogeneity between the two trials. Meta‐analysis was not performed for any other outcomes due to insufficient data. In future updates of this review, we will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
Where we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of T2 and I2.
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, used random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses.
High‐income versus low‐income settings.
Women at moderate to high risk of adverse outcomes versus women at low risk.
Cash versus non‐cash incentives.
High‐quality (low risk of bias) study versus low‐quality (high risk of bias) study.
We planned to consider the following primary outcomes in subgroup analysis: preterm birth, low birthweight, perinatal mortality. Return for postpartum care was examined with subgroup analysis given the substantial heterogeneity in meta‐analysis. Subgroup analysis was not completed for any other secondary outcomes.
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the χ2 statistic and P value, and the interaction test I² value.
In future updates of this review, if sufficient data are available, we will carry out subgroup analyses on the primary outcomes.
Sensitivity analysis
We observed substantial heterogeneity in one analysis (where a cluster‐RCT trials was included with an individually‐randomised trial) and carried out sensitivity analysis in order to investigate the effect of the randomisation unit. In future updates, we will carry out planned sensitivity analysis, as appropriate. Sensitivity analyses will be performed to explore the effects of allocation concealment or other aspects of study quality. Heterogeneity may also be explored comparing fixed‐effect or random‐effects analyses for outcomes with statistical heterogeneity. This will be done for primary outcomes only.
Results
Description of studies
Results of the search
(See: Figure 1)
1.
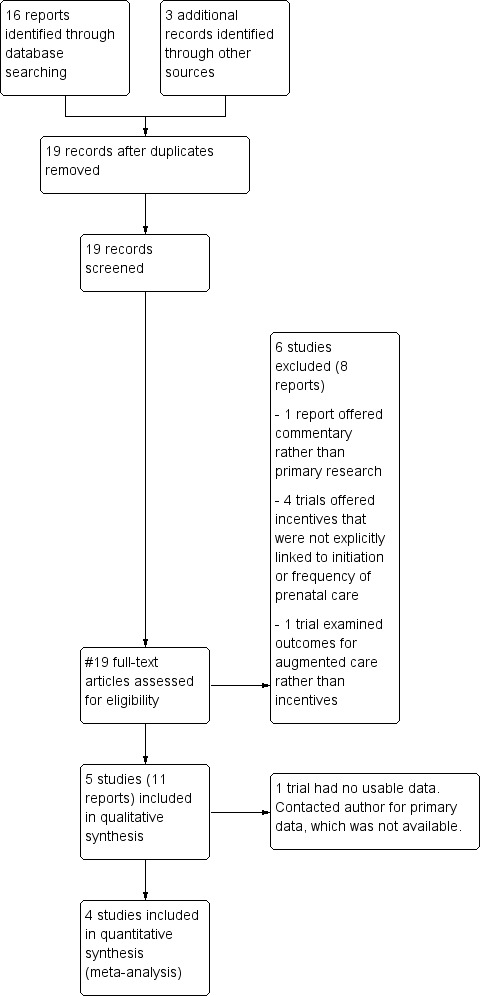
Study flow diagram.
The search retrieved 19 reports. Six studies (eight reports) were excluded (Burr 2007; Cueto 2009; Dykema 2012; Klerman 2001; Liu 2011; Lund 2014). Five trials (11 reports) were included (Barber 2009; Laken 1995; Melnikow 1997; Morris 2004; Stevens‐Simon 1994).
Included studies
Five trials were included in the review (Barber 2009; Laken 1995; Melnikow 1997; Morris 2004; Stevens‐Simon 1994). See Figure 1. One of these (Laken 1995) trials met criteria for design and outcomes but did not present data in a standard format allowing for meta‐analysis. The trial author was contacted and invited to offer primary data, but was unable to locate the appropriate information. Thus, the article did not contribute data to the review.
Participants and design
Five studies involving a total of 11,935 pregnancies were included (Barber 2009; Laken 1995; Melnikow 1997; Morris 2004; Stevens‐Simon 1994). However, only 1893 pregnancies contributed data to the review. Several of the reports included more than one pregnancy event for each woman. Outcomes from each pregnancy event were analyzed separately, thus the number of pregnancy events was reported rather than number of participating women.
Seven articles presented data on different outcomes from the same trial, and thus were analyzed as a single trial (Barber 2009). One of these articles examined an included outcome but did not present data in a standard format allowing for meta‐analysis. The author was contacted and invited to offer primary data in order to allow for inclusion, but was unable to locate the appropriate data. Thus, this article did not contribute data to the review. Four of these articles met the inclusion criteria but did not address any of the included study outcomes. Therefore, only two of the articles contributed data to our analysis. Each of the seven articles separately surveyed participants within the study population for their reports. It was not clear whether there was participant overlap within these surveys. However, the two reports that contributed data to our analysis were written by the same author. Thus, the larger of the participant numbers (n = 979) was used in an effort to avoid over‐reporting the number of participants.
Three trials were randomized controlled trials (RCTs) (Laken 1995; Melnikow 1997; Stevens‐Simon 1994) and two were cluster‐RCTs (Barber 2009; Morris 2004). The two cluster‐RCT trials randomized entire communities or villages to a particular intervention and compared them to similar communities who had not received the intervention. Participants in all five studies were pregnant women living in selected low‐income communities. Trials were conducted over periods of 24 months to six years. Both cluster‐RCTs adequately accounted for the cluster unit of randomization in their analysis and reported adjusted figures according to their calculated intra‐cluster correlation. Morris 2004 included their calculated intra‐cluster correlation of 0.016, whereas Barber 2009 discussed that analysis accounted for intra‐cluster correlation but did not list the calculation. Only adjusted data were used for this analysis.
Setting
One trial was conducted in Mexico (Barber 2009), one in Honduras (Morris 2004) and three in the United States (Laken 1995; Melnikow 1997; Stevens‐Simon 1994). All trials were conducted in low‐income communities. Two studies (Barber 2009; Morris 2004) were government‐based public health interventions designed to improve maternal and child health in impoverished communities. The other three (Laken 1995; Melnikow 1997; Stevens‐Simon 1994) were small university‐based research studies that enrolled patients who were already participating in existing state‐based programs designed to improve perinatal outcomes among impoverished or high‐risk populations.
Intervention
Eligibility criteria for this review mandated that all incentives were explicitly offered in exchange for attendance of prenatal care visits. Three trials examined cash incentives (Barber 2009; Laken 1995; Morris 2004). In the "Oportunidades" trial (Barber 2009), selected communities were randomized by the Mexican government to "early implementation" versus "late implementation" two years later. Intervention households received a conditional cash transfer of ˜$15/month dependent on obtaining regular preventive health, attending a minimum of five prenatal visits and participating in monthly health education talks. Participants were eligible to receive education bonuses for ensuring regular school attendance for school‐aged children. Households could receive benefits for up to three years. Non‐intervention households received standard prenatal care and primary school opportunities. The study compared pregnancies that were exposed to the intervention to those that were not.
The Morris 2004 trial examined results of the "Programa de Asginaction Familiar" implemented by the Honduran government, which identified 70 communities with the highest rates of malnutrition in rural Honduras and randomized these communities into four groups, 20 to control, 20 to household‐level package, 10 to service‐level package and 20 to dual‐package. Within household‐level package communities, eligible households could receive vouchers equal to cash for each pregnant woman, a child under age three or a child between six to 12 years who was enrolled in school, dependent on regular prenatal and well‐child preventive care, as well as regular school attendance. Service‐level package communities received quality improvement teams aimed at strengthening health centers and community‐based nutrition programs. Dual‐package communities received both household‐level and service‐level interventions. Control groups received standard prenatal care and primary school opportunities. Of note, the service‐level package was only fully implemented in 17% of selected communities due to difficulty in transferring specified resources from the government to the community‐based teams responsible for implementation. Teams were able to implement community‐based nutrition programs, but most were not able to implement the individual‐based nutrition counseling as intended. Thus, this report only compared pregnancies that occurred within household‐level package and control groups and did not address pregnancies within the service‐level package or dual‐package communities.
The Laken 1995 trial randomized patients at a single prenatal care site to three groups. The first intervention group received $5 store gift card for each appointment attended. The second intervention group received $5 store gift card for each appointment attended plus a chance at a $100 raffle. The control group received standard prenatal care. This trial did not contribute data to the review as the data were not presented in a standard format allowing for meta‐analysis and the author was unable to locate the appropriate data.
Two trials offered non‐cash incentives in the form of a baby carrier (Stevens‐Simon 1994), taxicab voucher or baby blanket voucher (Melnikow 1997). The Stevens‐Simon 1994 trial randomized patients at a single prenatal care site to intervention and control groups. Both groups received standard prenatal care throughout pregnancy with randomization at 34 weeks. The intervention group received a Gerry Cuddler if they returned for postpartum visit within 12 weeks of delivery. The control group received standard prenatal care and was instructed to return for postpartum visit. The Melnikow 1997 trial randomized newly diagnosed pregnant patients at a single prenatal care site to three groups. The first intervention group received a taxicab voucher to/from first prenatal visit. The second intervention group received a baby blanket voucher to be redeemed at first prenatal visit. The control group received standard prenatal care. The primary outcome was return for initiation of prenatal care within the following six weeks. The blanket intervention group had poor compliance with the primary outcome and was combined with the control group for stratified analysis in the original study, although data for the primary outcome were reported accurately. Given that the two interventions were similar in terms of potential value to patients, we felt it was more accurate to combine data from the two intervention groups for the purposes of this review.
Excluded studies
We excluded six studies (Burr 2007; Cueto 2009; Dykema 2012; Klerman 2001; Liu 2011; Lund 2014).
One trial was excluded as it provided commentary rather than primary research (Cueto 2009). Three trials were excluded as they did not provide incentives explicitly linked to initiation or frequency of prenatal care (Burr 2007; Dykema 2012; Liu 2011). The remaining two trials were excluded as they examined outcomes for augmented prenatal care rather than incentives linked to initiation and frequency of prenatal care (Klerman 2001; Lund 2014).
For further details please see Characteristics of excluded studies.
Risk of bias in included studies
The quality of available studies was mixed. See 'Risk of bias' tables in Characteristics of included studies and Figure 2.
2.
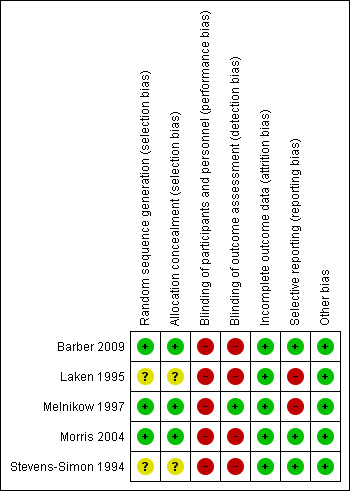
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The two largest studies (Barber 2009; Morris 2004) were cluster‐RCTs implemented by government entities. Reports obtained data from retrospective field surveys. In both studies, randomization occurred at the community level. One study (Barber 2009) randomized low‐income communities to "early implementation" and "late implementation". Assignment was performed at the community level using STATA randomization commands. Low‐income households within that community were then identified using census data and offered enrollment. Within each selected community, eligible households were randomly selected to participate in a retrospective field survey. In another study (Morris 2004), communities with high prevalence of malnourishment were identified and stratified according to severity of malnourishment. Within each stratum, communities were randomly assigned to control, household‐level intervention, service‐level intervention or dual intervention. Within each community, low‐income households were identified and offered enrollment. Eligible households were randomly selected to participate in pre‐ and post‐intervention surveys. In both of these studies, randomization was deemed adequate and risk of selection bias was deemed to be low.
The three remaining studies (Laken 1995; Melnikow 1997; Stevens‐Simon 1994) were RCTs. Randomization sequencing and allocation concealment were clearly delineated and deemed adequate in one report (Melnikow 1997). The other two (Laken 1995; Stevens‐Simon 1994) did not describe these processes in detail and risk for selection bias was deemed unclear.
Blinding
All five of the studies were, by design, unblinded to the pregnant women. Clinician blinding was only described in one study (Laken 1995). Double‐blinding is rarely a suitable design strategy for interventions in which participants receive incentives in exchange for action. All five studies were therefore deemed high risk for performance bias. Blinding of outcome assessors was poorly described or inadequate in four trials (Barber 2009; Laken 1995; Morris 2004; Stevens‐Simon 1994), and thus deemed high risk for detection bias. Blinding of outcome assessors was adequate in the other trial (Melnikow 1997).
Incomplete outcome data
All five studies carried out intent‐to‐treat analyses. Attrition did not differ significantly among treatment groups in any study, except where it was the primary outcome. All studies were deemed low risk for attrition bias.
Selective reporting
Three studies adhered to their stated reporting protocols (Barber 2009; Morris 2004; Stevens‐Simon 1994) and were deemed low risk for reporting bias. The Melnikow 1997 study was designed to compare two intervention groups versus a control group. One of the interventions had essentially no effect on the primary outcome and that intervention group was combined with the control group for stratified analysis, although data for the primary outcome were reported accurately. Within this review, we examined data from the control and both intervention groups separately. Given that the two interventions were similar in terms of potential value to patients, we felt it was more accurate to analyze combined data from the two intervention groups. In one study (Laken 1995), the two incentive groups were combined for analysis after comparison revealed no significant differences in outcomes. However, this study did not contribute data to the review as primary data were not available. Both Melnikow 1997 and Laken 1995 were deemed high risk for reporting bias.
Other potential sources of bias
Other potential sources of bias include the entities commissioning these trials. Two of the five trials were commissioned by either the Mexican or Honduran governments with the purpose of evaluating these government‐administered programs (Barber 2009; Morris 2004). In these cases, the evaluations were performed by independent entities with non‐governmental funding. There was no indication in any of these articles that the respective governments attempted to influence findings or reporting of results. Thus, these were determined to be low‐risk indicators for bias. No other sources of bias were identified and all five studies were considered low risk for bias.
Effects of interventions
This review did not identify data regarding the impact of incentives on neonatal outcomes. The impact of incentives on various indicators of prenatal care utilization was mixed.
Outcomes
None of the trials examined the primary outcomes identified in our protocol. Initiation of prenatal care was analyzed in one trial (Melnikow 1997). Adequacy of prenatal care was analyzed in three trials, although reported using different metrics. Two trials reported on the frequency of prenatal care visits (Barber 2009; Laken 1995), and one reported on quality of prenatal care as defined by number of “procedures” received throughout perinatal period (Morris 2004). Three trials analyzed compliance with return for postpartum care (Laken 1995; Morris 2004; Stevens‐Simon 1994). We added the outcome Return for postpartum care after the publication of the approved protocol. The review authors felt that it was consistent with the spirit of the Frequency of prenatal care and Adequacy of prenatal care outcome measures and should be included as a separate secondary outcome. One trial reported on cesarean rate among pregnant women (Barber 2009). Again, Laken 1995 did not contribute data to the review.
Pregnant women who received incentives versus pregnant women who did not receive incentives
Primary outcomes
Preterm birth < 37 weeks.
No data regarding the impact of incentives on preterm birth were available for this review.
Small‐for‐gestational age
No data regarding the impact of incentives on small‐for‐gestational age infants were available for this review.
Perinatal deaths (fetal, neonatal, later deaths)
No data regarding the impact of incentives on perinatal death were available for this review.
Secondary outcomes
Adequacy of antenatal care
Pregnant women receiving incentives were more likely to obtain adequate quality prenatal care defined by number of “procedures” (mean difference (MD) 5.84, 95% confidence interval (CI) 1.88 to 9.80, one study, 892 pregnancies. See Analysis 1.1). Procedures in this trial included history‐taking, diagnostic tests, physical examination, immunizations, iron supplementation, lactation counseling and family planning counseling.
1.1. Analysis.
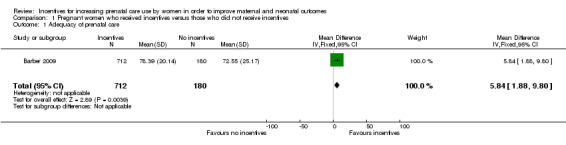
Comparison 1 Pregnant women who received incentives versus those who did not receive incentives, Outcome 1 Adequacy of prenatal care.
Frequency of prenatal care
Pregnant women receiving incentives were more likely to obtain frequent prenatal care (risk ratio (RR) 1.18, 95% CI 1.01 to 1.38, one study, 606 pregnancies. See Analysis 1.2). Frequent prenatal care was defined as five or more prenatal visits in this trial.
1.2. Analysis.
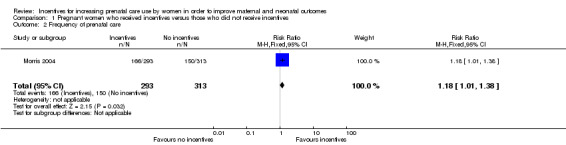
Comparison 1 Pregnant women who received incentives versus those who did not receive incentives, Outcome 2 Frequency of prenatal care.
Initiation of prenatal care
Pregnant women receiving incentives were no more likely to initiate early prenatal care (RR 1.04, 95% CI 0.78 to 1.38, one study, 104 pregnancies. See Analysis 1.3).
1.3. Analysis.
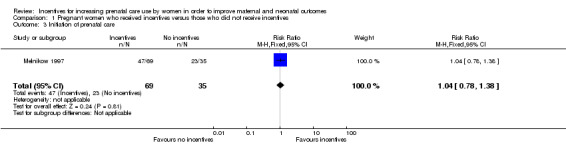
Comparison 1 Pregnant women who received incentives versus those who did not receive incentives, Outcome 3 Initiation of prenatal care.
Return for postpartum care (outcome added after the approved protocol)
Data regarding return for postpartum care were combined in meta‐analysis, which demonstrated that women receiving incentives were no more likely to return for postpartum care (average RR 0.75, 95% CI 0.21 to 2.64, two studies, 833 pregnancies, Tau² = 0.81, I² = 98%. See Analysis 1.4). However, there was substantial heterogeneity between the two studies and a clear difference between subgroups based on the type of incentives being offered (test for subgroup differences: Chi² = 28.85, df = 1, P < 0.00001, I² = 96.5%). In one study, women receiving non‐cash incentives were more likely to return for postpartum care (RR 1.26, 95% CI 1.09 to 1.47, 240 pregnancies) than women who did not receive non‐cash incentives. In contrast, in the other study, women receiving cash incentives were less likely to return for postpartum care (RR 0.43, 95% CI 0.30 to 0.62, 593 pregnancies) than women who did not receive cash incentives.
1.4. Analysis.
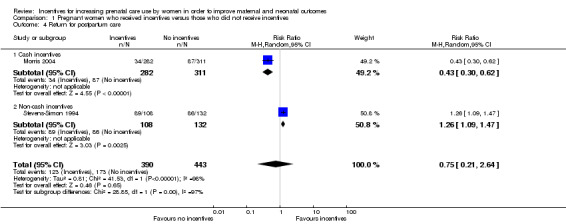
Comparison 1 Pregnant women who received incentives versus those who did not receive incentives, Outcome 4 Return for postpartum care.
Pre‐eclampsia
No data regarding impact of incentives on pre‐eclampsia were available for this review.
Cesarean delivery
Recipients of incentives were more likely to deliver by cesarean (RR 1.97, 95% CI 1.18 to 3.30, one study, 979 pregnancies. See Analysis 1.5). This study examined delivery location (birthing center verses home birth) to evaluate for possible confounding and found that there were no significant differences in delivery location among participants and non‐participants.
1.5. Analysis.
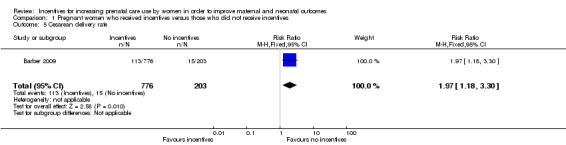
Comparison 1 Pregnant women who received incentives versus those who did not receive incentives, Outcome 5 Cesarean delivery rate.
Satisfaction with birth experience
No data regarding impact of incentives on satisfaction with birth experience were available for this review.
Maternal mortality
No data regarding impact of incentives on maternal mortality were available for this review.
Low birthweight (less than 2500 g)
No data regarding impact of incentives on low birthweight were available for this review.
Infant macrosomia (birthweight greater than 4000 g)
No data regarding impact of incentives on infant macrosomia were available for this review.
Five‐minute Apgar less than seven
No data regarding impact of incentives on five‐minute Apgar score of less than seven were available for this review.
Discussion
Five studies, involving 11,935 pregnancies were included, but only 1893 pregnancies contributed data regarding our specified outcomes. Incentives in the studies included cash, gift card, baby carrier, baby blanket or taxicab voucher and were compared with no incentives. Meta‐analysis was performed for only one outcome 'Return for postpartum care' and this outcome was not pre‐specified in our protocol. Other analyses were restricted to data from single studies.
The use of incentives may produce modest improvements in frequency or quality of prenatal care, but there is not adequate evidence to determine the impact on maternal or neonatal outcomes. There were no data regarding the impact of incentives on our primary outcomes of preterm birth, small‐for‐gestational age or perinatal mortality. There are also sparse data on most of our secondary outcomes. Another important consideration is the fact that the largest two of the five trials took place in rural, low‐income, homogenously Hispanic communities in Latin America. This setting introduces a number of confounding factors that may affect generalizability of these findings to ethnically and economically diverse urban communities.
Summary of main results
The use of incentives may produce modest improvements in the frequency or quality of prenatal care, but there is not adequate evidence to determine the impact on maternal or neonatal outcomes. This review combined data regarding 'Return for postpartum care' in meta‐analysis. However, there were no other areas of adequately overlapping data allowing for meta‐analysis in any other primary or secondary outcomes.
In terms of subgroup analyses, there was substantial homogeneity among trials in terms of participant demographic characteristics and study quality. Therefore, formal subgroup analyses relative to these characteristics were not undertaken. Subgroup analysis was performed for cash and non‐cash incentives, but only for the outcome of returning for postpartum care (Analysis 1.4). For this outcome, non‐cash incentives led to higher postpartum visit rates, while cash incentives actually led to lower postpartum visit rates. It is unclear why this would be the case. As there was no overlap of other outcomes with more than one study, subgroup comparisons were not possible. Interestingly, three of the five trials offered monetary incentives rather than goods. The two trials that did not involve monetary incentives offered a baby carrier, taxicab voucher or a baby blanket. Results of one of these trials were significant, while the results of the other were not. Thus, there were not adequate data to make conclusions regarding efficacy of monetary incentives versus goods.
There was a large range of the size of financial incentive relative to income. The incentives provided in the Barber 2009 and Morris 2004 trials represented considerable augmentation of a participating family’s income, whereas the Laken 1995 trial provided a very small stipend. The non‐cash incentive in the Stevens‐Simon 1994 trial was considered more valuable than that offered in Melnikow 1997. Overall, it is difficult to argue that either financial or non‐cash incentives provided equivalent motivation among all of these participant groups.
Overall completeness and applicability of evidence
The impact of the intervention was limited in all studies, even those that demonstrated results which were statistically significant. There were no data regarding the impact of incentives on our primary outcomes of preterm birth, small‐for‐gestational age or perinatal mortality. There were also no data on many of our secondary outcomes.
An important consideration is the fact that the largest two of the five studies comprising the majority of participants took place in rural, low‐income, homogenously Hispanic communities in Central America. This setting introduces a number of confounding factors that may affect generalizability of these findings to ethnically and economically diverse urban communities in developed countries.
Quality of the evidence
Overall, the studies examined in this systematic review were of moderate risk of bias. Performance and detection bias was assessed as high risk in nearly all of the studies. However, double‐blinding is rarely a suitable design strategy for interventions in which participants receive incentives in exchange for action.
Potential biases in the review process
Two publications met the inclusion criteria but did not offer data in a standard format allowing for meta‐analysis (Barber 2009; Laken 1995). We contacted both authors and invited them to provide additional data, but both were unable to locate the necessary information. Therefore, these two publications did not contribute data to the review. Data from other publications of the Opportunidades trial (Barber 2009) were presented in an appropriate format. It is conceivable that results from either of these publications could have influenced results of the analysis. No other potential sources of bias were identified related to the review process.
The findings are limited by the location of the studies in that they may not be generalizable to all healthcare settings. In addition, the type of incentive may not be as attractive in all settings.
Agreements and disagreements with other studies or reviews
No comparable observational or randomized studies examined the outcomes of interest. No other systematic reviews on this topic were available for comparison.
Authors' conclusions
Implications for practice.
There was no evidence to determine whether incentive programs can decrease the incidence of preterm birth, small‐for‐gestational‐age babies, or perinatal mortality. We found limited evidence to suggest that incentives may improve the frequency and ensure adequate quality of prenatal care, but at the cost of increased cesarean rates. However, these findings should be interpreted with caution due to the small number of studies reporting these outcomes. In addition, these findings are of limited generalizability as the majority of participants were drawn from impoverished communities in Central America.
Implications for research.
The absence of reporting of all of our primary outcomes in the currently published literature represents an opportunity for future research. Incentive programs, in particular cash‐based programs, have been demonstrated in this review and in several observational studies to improve the frequency and ensure adequate quality of prenatal care. No peer‐reviewed data have been made publicly available for one of the largest incentive‐based prenatal programs – the statewide Medicaid‐based programs within the United States. These observational data represent an important starting point for future research with significant implications for policy development and allocation of healthcare resources. The disparate findings related to attending postpartum care should also be further explored as the findings were limited by the number of studies. Future large randomized controlled trials are needed to focus on the outcomes of preterm birth, low birthweight and perinatal outcomes.
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Pregnant women who received incentives versus those who did not receive incentives.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adequacy of prenatal care | 1 | 892 | Mean Difference (IV, Fixed, 95% CI) | 5.84 [1.88, 9.80] |
| 2 Frequency of prenatal care | 1 | 606 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.01, 1.38] |
| 3 Initiation of prenatal care | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.78, 1.38] |
| 4 Return for postpartum care | 2 | 833 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.21, 2.64] |
| 4.1 Cash incentives | 1 | 593 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.30, 0.62] |
| 4.2 Non‐cash incentives | 1 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.09, 1.47] |
| 5 Cesarean delivery rate | 1 | 979 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [1.18, 3.30] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barber 2009.
| Methods | Cluster‐randomized controlled trial. The duration of this study was six years. The intervention "Opportunidades" included a total of 506 low‐income communities in rural Mexico, 320 randomized to "early implementation" in 1998 and 186 randomized to "late implementation" in 2000. Reports randomly selected communities within both "early" and "late" intervention groups. Within each selected community, eligible households were randomly selected to participate in a retrospective field survey. Comparison was to pregnancies that occurred within "early" and "late" implementation periods. |
|
| Participants | Low‐income women age 15‐49 living in an intervention or control community who had a live singleton birth from 1997‐2003. Each report surveyed a different number of pregnant women. It was not clear whether there was overlap within these surveys among different reports. Summary for each of the reports is as follows. ‐ Barber 2008: 892 pregnancies. 712 beneficiary and 180 non‐beneficiary. ‐ Barber 2009: 979 pregnancies. 776 beneficiary and 203 non‐beneficiary. ‐ Barber, Gertler 2009: 840 pregnancies. 666 beneficiary and 174 non‐beneficiary. ‐ Fernald 2008: 3780 pregnancies. 2273 early beneficiary and 1507 late beneficiary. ‐ Fernald 2009: 1793 pregnancies. 1093 early beneficiary and 700 late beneficiary. ‐ Leroy 2008: 432 pregnancies. 344 beneficiary and 88 non‐beneficiary. ‐ Rivera 2004: 650 pregnancies. 373 early beneficiary and 277 late beneficiary. |
|
| Interventions | Intervention households received conditional cash transfer of ˜$15/month dependent on obtaining regular preventive health, attending a minimum of 5 prenatal visits and participating in monthly health education talks. Participants were eligible to receive education bonuses for ensuring regular school attendance for school‐aged children. Households could receive benefits for up to 3 years. | |
| Outcomes | Cesarean rate, delivery location. Quality of prenatal care, measured by number of "procedures", including defined measures within history‐taking and diagnostics, physical examination and prevention and case management. Birthweight, child growth, hemoglobin, cognitive development, language and behavioral problems. | |
| Notes | This study (Opportunidades) was evaluated in 7 publications included within this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster‐randomization. Assignment was performed at the community level using STATA randomization commands. Low‐income households within that community were then identified using census data and offered enrollment; 97% of eligible households enrolled in the program. Less than 1% of enrolled households failed to receive benefits due to non‐compliance. Retrospective field surveys identified participants via a 2‐stage stratified sampling design using computer‐generated randomization sequences. |
| Allocation concealment (selection bias) | Low risk | Randomization was performed centrally and via computer‐generated sequence. Communities were not aware that they would be participating in the study and timing of program roll‐out was not made public. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded as they knew whether they received incentives. It was unclear whether clinicians knew about participation status. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | Reporting consistent with protocol. |
| Other bias | Low risk | Mexican government implemented this intervention and commissioned an independent evaluation of program impact. This study examining that data was funded by an NIH grant. |
Laken 1995.
| Methods | Randomized controlled trial, antenatal clinic in Ohio (United States), all Medicaid patients, 205 participants. | |
| Participants | Low‐income women, prenatal care < 32 weeks and delivered at a tertiary care hospital. | |
| Interventions | 2 intervention groups. 1 received $5 store gift card for each appointment kept (n = 51). The second received $5 store gift card for each appointment kept plus a chance at a $100 raffle (n = 53). Control group received routine prenatal care without incentive, and was interviewed after the delivery (n = 101). | |
| Outcomes | Attendance of prenatal and postpartum visits, gestational age, birthweight. | |
| Notes | This study did not contribute data to the review because primary data were not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Random numbers were used." Not otherwise described. |
| Allocation concealment (selection bias) | Unclear risk | "Random assignment was used to eliminate bias." Not otherwise described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were aware of allocation status. Clinicians were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | High risk | The 2 incentive groups were combined for analysis after comparison revealed no significant differences in outcomes. Difficult to assess without primary data. |
| Other bias | Low risk | No other sources of bias detected. |
Melnikow 1997.
| Methods | Randomized controlled trial. 24 months. 5 family planning and women's health clinics in northern California (United States), 104 participants. | |
| Participants | Pregnant women who stated intent to obtain prenatal care at participating clinics. | |
| Interventions | 2 intervention groups: 1 received taxicab voucher to/from first prenatal visit; the second received baby blanket voucher to be redeemed at first prenatal visit. Control group received standard prenatal care. | |
| Outcomes | Compliance with attending first prenatal visit. | |
| Notes | The blanket intervention group had poor compliance with primary outcome and was combined with the control group for stratified analysis in the original study, although data for the primary outcome were reported accurately. Given that the 2 interventions were similar in terms of potential value to patients, we felt it was more accurate to combine data from the 2 intervention groups for the purposes of this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked by clinic. Within each clinic, assignment was by computer random number table. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were aware of their voucher offer, but it was not clear whether they were aware of other assignment groups voucher offer. Clinician blinding was not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. Loss to follow‐up was a study outcome. |
| Selective reporting (reporting bias) | High risk | Reporting differed from protocol in that 1 of the intervention groups had poor compliance with primary outcome and was combined with the control group for stratified analysis. The original data for all 3 groups were reported accurately. |
| Other bias | Low risk | No other sources of bias detected. |
Morris 2004.
| Methods | Cluster‐randomized controlled trial. 24 months. The intervention "Programa de Asginaction Familiar" identified 70 communities with highest rates of malnutrition in rural Honduras, which were randomized into 4 groups: 20 to control, 20 to household‐level package, 10 to service‐level package, and 20 to dual‐package. A randomly selected number of households within each group were administered both a pre‐ and post‐intervention survey. Comparison was to pregnancies that occurred within household‐level package and control groups. | |
| Participants | Within household‐level and dual‐level groups, the eligible households were those which had a pregnant woman, child under age 3 or child between age 6‐12 at time of 2000 census. 5545 households participated in the pre‐intervention survey, including 1605 in the control group, 1574 in the household‐level package, 786 in the service‐level package, and 1580 in the dual‐package. 5289 of these households participated in the post‐intervention survey, including 1524 in the control group, 1512 in the household‐level package, 744 in the service‐level package, and 1509 in the dual‐package. | |
| Interventions | Within household‐level package communities, eligible households could receive vouchers equal to cash for each pregnant woman, child under age 3 or child between age 6‐12 who was enrolled in school, dependent on regular prenatal and well‐child preventive care as well as regular school attendance. Service‐level package communities received quality improvement teams aimed at strengthening health centers and community‐based nutrition programs. Dual‐package communities received both household‐level and service‐level interventions. | |
| Outcomes | Primary outcomes included adequate use of prenatal care (defined as at least 5 visits), postpartum checkup within 10 days of delivery and children < 3 years taken to health center within past 30 days. Secondary outcomes included immunization rates and growth monitoring. | |
| Notes | Service‐level package was only fully implemented in 17% of selected communities due to difficulty in transferring specified resources from the government to the community‐based teams responsible for implementation. Teams were able to implement community‐based nutrition programs, but most were not able to implement the individual‐based nutrition counseling as intended. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster‐randomization by community. Communities were stratified by degree of malnutrition. Communities within each stratum were randomized to intervention group by a child blindly drawing colored balls from a box without replacement. |
| Allocation concealment (selection bias) | Low risk | Community was aware of intervention. However, households could not become eligible for vouchers by moving into household‐level community after time of randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No attempt to conceal intervention after time of randomization. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Baseline and post‐intervention surveys administered by independent data collection company which was aware of community intervention grouping. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
| Selective reporting (reporting bias) | Low risk | Reporting consistent with protocol. |
| Other bias | Low risk | Honduran government commissioned an independent evaluation of program impact, which was funded with the assistance of a loan from the Inter‐American Development Bank. |
Stevens‐Simon 1994.
| Methods | Randomized controlled trial, 1 prenatal clinic Colorado (United States), 240 participants. | |
| Participants | 12‐19 years, "poor", receiving prenatal care through the Colorado Adolescent Maternity Program in Denver. | |
| Interventions | Both groups received standard prenatal care throughout pregnancy with randomization at 34 weeks. Intervention group would receive a Gerry Cuddler if they returned for postpartum visit within 12 weeks of delivery. Control group was instructed to return for postpartum visit. | |
| Outcomes | Attendance of postpartum visit. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Consecutive patients randomized by receptionist blind distribution of group assignment on a sheet of paper. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were aware of status. Clinician blinding not described. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intent‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | Reporting consistent with protocol. |
| Other bias | Low risk | No other sources of bias detected. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Burr 2007 | Incentive (fruit juice voucher) was not explicitly linked to initiation or frequency of prenatal care, but rather to fruit juice consumption. |
| Cueto 2009 | Commentary. No data analysis. |
| Dykema 2012 | Incentive (cash or voucher) was not explicitly linked to initiation or frequency of prenatal care, but rather to response rate for a postpartum risk assessment survey. |
| Klerman 2001 | Intervention studied was augmented care, not incentives explicitly linked to initiation or frequency of prenatal care. |
| Liu 2011 | Incentive (voucher) was not explicitly linked to initiation or frequency of prenatal care, but rather to response rate for a postpartum risk assessment survey. |
| Lund 2014 | Intervention studied was augmented care, not incentives explicitly linked to initiation or frequency of prenatal care. |
Differences between protocol and review
'Return for postpartum care' was added as a secondary outcome after publication of our protocol (Haas 2012). Given that postpartum care is widely regarded to be an essential component of perinatal care, the authors felt that return for postpartum care was consistent with the spirit of the frequency and adequacy of prenatal care outcome measures and should be included as a separate secondary outcome.
Some outcomes have been rephrased. Primary outcome
'Perinatal deaths (fetal, neonatal, later deaths)' has been edited to 'Perinatal deaths (fetal, neonatal, infant deaths)'.
Secondary outcomes 'Adequacy of prenatal care (APNCU index or as reported by trialists)' has been edited to 'Adequacy of prenatal care.
'Frequencey of prenatal care (number of episodes per woman)' has been edited to 'Frequency of prenatal care'.
'Initiation of prenatal care (gestational age)' has been edited to 'Initiation of prenatal care'.
We have updated our methods in line with the current standard methods for Cochrane Pregnancy and Childbirth.
Subgroup analysis ‐ 'return for postpartum' care was examined with subgroup analysis given the substantial heterogeneity in meta‐analysis ‐ this was not prespecified in our published protocol.
We have rephrased one of our planned subgroup analyses ‐ 'monetary incentives versus goods/services incentives' was changed to 'cash versus non‐cash incentives',
Contributions of authors
Sara R Till assisted in conceiving the review, designing the review, writing the protocol. She provided a clinical and policy perspective. She reviewed studies, extracted data, and wrote the review.
David Everetts provided general advice on the protocol, its preparation, and contributed to the writing of the review.
David M Haas assisted in designing the review, reviewed studies and extracted data, contributed to the writing of the review, and is guarantor for the review. He provided a clinical and methodological perspective, as well as providing general advice on the protocol.
Declarations of interest
Sara R Till: none known.
David Everetts: none known.
David M Haas: none known.
New
References
References to studies included in this review
Barber 2009 {published data only}
- Barber SL. Mexico's conditional cash transfer programme increases cesarean section rates among the rural poor. European Journal of Public Health 2010;20(4):383‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber SL, Gertler PJ. Empowering women to obtain high quality care: evidence from an evaluation of Mexico's conditional cash transfer programme. Health Policy and Planning 2009;24(1):18‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber SL, Gertler PJ. The impact of Mexico's conditional cash transfer programme, Oportunidades, on birthweight. Tropical Medicine and International Health 2008;13(11):1405‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald LC, Gertler PJ, Neufeld LM. 10‐year effect of Oportunidades, Mexico's conditional cash transfer programme, on child growth, cognition, language, and behaviour: a longitudinal follow‐up study. Lancet 2009;374(9706):1997‐2005. [DOI] [PubMed] [Google Scholar]
- Fernald LCH, Gertler PJ, Neufield LM. The importance of cash in conditional cash transfer programs for child health, growth and development. Lancet 2008;371:828‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy JL, Garcia‐Guerra A, Garcia R, Dominguez C, Rivera J, Neufeld LM. The Oportunidades program increases the linear growth of children enrolled at young ages in urban Mexico. Journal of Nutrition 2008;138(4):793‐8. [DOI] [PubMed] [Google Scholar]
- Rivera JA, Sotres‐Alvarez D, Habicht JP, Shamah T, Villalpando S. Impact of the Mexican program for education, health, and nutrition (Progresa) on rates of growth and anemia in infants and young children: a randomized effectiveness study. JAMA 2004;291(21):2563‐70. [DOI] [PubMed] [Google Scholar]
Laken 1995 {published data only (unpublished sought but not used)}
- Laken MP, Ager J. Using incentives to increase participation in prenatal care. Obstetrics & Gynecology 1995;85:326‐9. [DOI] [PubMed] [Google Scholar]
Melnikow 1997 {published data only}
- Melnikow J, Paliescheskey M, Stewart GK. Effect of a transportation incentive on compliance with the first prenatal appointment: a randomized trial. Obstetrics & Gynecology 1997;89:1023‐7. [DOI] [PubMed] [Google Scholar]
Morris 2004 {published data only}
- Morris SS, Flores R, Olinto P, Medina JM. Monetary incentives in primary health care and effects on use and coverage of preventive health care interventions in rural Honduras: cluster randomised trial. Lancet 2004;364(9450):2030‐7. [DOI] [PubMed] [Google Scholar]
Stevens‐Simon 1994 {published data only}
- Stevens‐Simon C, O'Connor P, Bassford K. Incentives enhance postpartum compliance among adolescent prenatal patients. Journal of Adolescent Health 1994;15(5):396‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Burr 2007 {published data only}
- Burr ML, Trembeth J, Jones KB, Geen J, Lynch LA, Roberts ZE. The effects of dietary advice and vouchers on the intake of fruit and fruit juice by pregnant women in a deprived area: a controlled trial. Public Health Nutrition 2007;10(6):559‐65. [DOI] [PubMed] [Google Scholar]
Cueto 2009 {published data only}
- Cueto S. Conditional cash‐transfer programmes in developing countries. Lancet 2009;374:1952‐3. [DOI] [PubMed] [Google Scholar]
Dykema 2012 {published data only}
- Dykema J, Stevenson J, Kniss C, Kvale K, Gonzalez K, Cautley E. Use of monetary and nonmonetary incentives to increase response rates among African Americans in the Wisconsin Pregnancy Risk Assessment Monitoring System. Maternal & Child Health Journal 2012;16(4):785‐91. [DOI] [PubMed] [Google Scholar]
Klerman 2001 {published data only}
- Klerman LV, Ramey SL, Goldenberg RL, Marbury S, Hou J, Cliver SP. A randomized trial of augmented prenatal care for multiple‐risk, medicaid‐eligible african american women. American Journal of Public Health 2001;91(1):105‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2011 {published data only}
- Liu S, Geidenberger C. Comparing incentives to increase response rates among African Americans in the Ohio Pregnancy Risk Assessment Monitoring System. Maternal & Child Health Journal 2011;15(4):527‐33. [DOI] [PubMed] [Google Scholar]
Lund 2014 {published data only}
- Lund S, Nielsen BB, Hemed M, Boas IM, Said A, Said K, et al. Mobile phones improve antenatal care attendance in Zanzibar: a cluster randomized controlled trial. BMC Pregnancy and Childbirth 2014;14(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund S, Nielsen BB, Hemed M, Said A, Said K, Makungu MH, et al. Mobile phones as a health communication tool to improve maternal and perinatal health in Zanzibar: A cluster randomised controlled trial. Tropical Medicine & International Health 2013;18(Suppl 1):22. [DOI] [PubMed] [Google Scholar]
- Lund S, Rasch V, Hemed M, Boas IM, Said A, Said K, et al. Mobile phone intervention reduces perinatal mortality in zanzibar: secondary outcomes of a cluster randomized controlled trial. Jmir Mhealth and Uhealth 2014;2(1):e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
AAP 2012
- American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. Preconception and antepartum care, intrapartum and postpartum care of the the mother. Guidelines for Perinatal Care. 7th Edition. American Academy of Pediatrics and American College of Obstetricians and Gynecologists, 2012:95‐210. [Google Scholar]
Alexander 2001
- Alexander GR, Kotelchuck M. Assessing the role and effectiveness of prenatal care: history, challenges and directions for future research. Public Health Report 2001;116(4):306‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Canning 2009
- Canning PM, Frizzell LM, Courage ML. Birth outcomes associated with prenatal participation in a government support programme for mothers with low incomes. Child: Care, Health and Development 2009;36:225‐31. [DOI] [PubMed] [Google Scholar]
Carvahlo Padilha 2009
- Carvahlo Padilha CD, Accioly E, Chagas C, Portela E, DaSilva CL, Saunders C. Birth weight variation according to maternal characteristics and gestational weight gain in Brazilian women. Nutricion Hospitalaria 2009;24:207‐12. [PubMed] [Google Scholar]
Chalmers 2001
- Chalmers B, Mangiaterra V, Porter R. WHO principles of perinatal care: the essential antenatal, perinatal, and postpartum care course. Birth 2001;28(3):202‐7. [DOI] [PubMed] [Google Scholar]
Cox 2011
- Cox RG, Zhang L, Zotti ME, Graham J. Prenatal care utilization in Mississippi: racial disparities and implications for unfavorable birth outcomes. Maternal and Child Health Journal 2011;15:931‐42. [DOI] [PubMed] [Google Scholar]
Debiec 2010
- Debiec KE, Paul KJ, Mitchell CM, Hitti JE. Inadequate prenatal care and risk of preterm delivery among adolescents: a retrospective study over 10 years. American Journal of Obstetrics and Gynecology 2010;203:122.e1‐122.e6. [DOI] [PubMed] [Google Scholar]
Heaman 2008
- Heaman MI, Newburn‐Cook CV, Green CG, Elliott LJ, Helewa ME. Inadequate prenatal care and its association with adverse pregnancy outcomes: a comparison of indices. BMC Pregnancy and Childbirth 2008;8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated September 2011]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Hueston 2008
- Hueston WJ, Quattlebaum RG, Benich JJ. How much money can early prenatal care for teen pregnancies save? A cost‐benefit analysis. Journal of the American Board of Family Medicine 2008;21:184‐90. [DOI] [PubMed] [Google Scholar]
Ickovics 2007
- Ickovics JR, Kershaw TS, Westdahl C, Magriples U, Massey Z, Reynolds H, et al. Group prenatal care and perinatal outcomes: a randomized controlled trial. Obstetrics & Gynecology 2007;110:330‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
IOM 1994
- Institute of Medicine. Prenatal care and low birth weight: effects on healthcare expenditures. Preventing Low Birthweight. Washington, DC: National Academy Press, 1994:1450‐7. [Google Scholar]
Jehan 2012
- Jehan K, Sidney K, Smith H, Costa A. Improving access to maternity services: an overview of cash transfer and voucher schemes in South Asia. Reproductive Health Matters 2012;20:142‐54. [DOI] [PubMed] [Google Scholar]
Kotelchuck 1994
- Kotelchuck M. An evaluation of the Kessner Adequacy of Prenatal Care Index and a proposed Adequacy of Prenatal Care Utilization Index. American Journal of Public Health 1994;84(9):1414‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2007
- Lin CM, Chen CW, Chen PT, Lu TH, Li CY. Risks and causes of mortality among low‐birthweight infants in childhood and adolescence. Pediatric and Perinatal Epidemiology 2007;21:465‐72. [DOI] [PubMed] [Google Scholar]
McDonald 2010
- McDonald SD, Han Z, Mulla S, Beyene J. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta‐analyses. BMJ 2010;341:c3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mikkola 2005
- Mikkola K, Ritari N, Tommiska V, Salokorpi T, Lehtonen L, Tammela O, et al. Neurodevelopmental outcome at 5 years of age of a national cohort of extremely low birth weight infants who were born in 1996‐1997. Pediatrics 2005;116:1391‐400. [DOI] [PubMed] [Google Scholar]
Moore 1994
- Moore ML, Michielutte R, Meis PJ, Ernest JM, Wells HB, Buescher PA. Etiology of low‐birthweight birth: a population‐based study. Preventive Medicine 1994;23:793‐9. [DOI] [PubMed] [Google Scholar]
Partington 2009
- Partington SN, Steber DL, Blair KA, Cisler RA. Second births to teenage mothers: risk factors for low birth weight and preterm birth. Perspectives on Sexual and Reproductive Health 2009;41:101‐9. [DOI] [PubMed] [Google Scholar]
Quinlivan 2004
- Quinlivan JA, Evans SF. Teenage antenatal clinics may reduce the rate of preterm birth: a prospective study. BJOG: an international journal of obstetrics and gynaecology 2004;111:571‐8. [DOI] [PubMed] [Google Scholar]
Raatikainen 2007
- Raatikainen K, Heiskanen N, Heinonen S. Under‐attending free antenatal care is associated with adverse pregnancy outcomes. BMC Public Health 2007;7:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenthal 2009
- Rosenthal MB, Li Z, Robertson AD, Milstein A. Impact of financial incentives for prenatal care on birth outcomes and spending. Health Services Research 2009;44:1465‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sackett 2004
- Sackett K, Pope RK, Erdley WS. Demonstrating a positive return on investment for a prenatal program at a managed care organization. Journal of Perinatal and Neonatal Nursing 2004;18:117‐27. [DOI] [PubMed] [Google Scholar]
Silva 2006
- Silva R, Thomas M, Caetano R. Preventing low birth weight in Illinois: outcomes of the family case management program. Maternal and Child Health Journal 2006;10:481‐8. [DOI] [PubMed] [Google Scholar]
Tommiska 2001
- Tommiska V, Heinonen K, Ikonen S, Kero P, Pokela MJ, Renlund M, et al. A national short‐term follow‐up study of extremely low birth weight infants born in Finland in 1996‐1997. Pediatrics 2001;107:e2. [DOI] [PubMed] [Google Scholar]
WHO 1999
- World Health Organization, Technical Working Group. Postpartum care of the mother and newborn: a practical guide. Birth 1999;4:255‐9. [PubMed] [Google Scholar]
WHO 2006
- World Health Organization, United Nations Population Fund, UNICEF, The World Bank. Pregnancy, Childbirth, Postpartum and Newborn Care: A Guide for Essential Practice. 2nd Edition. Geneva: World Health Organization, 2006. [PubMed] [Google Scholar]
WHO 2015
- World Health Organization. WHO recommendations on health promotion interventions for maternal and newborn health 2015. WHO Recommendations on Health Promotion Interventions for Maternal and Newborn Health 2015. Geneva: World Health Organization, 2015. [Google Scholar]
References to other published versions of this review
Haas 2012
- Haas DM, Till SR, Everetts D. Incentives for increasing prenatal care use by women in order to improve maternal and neonatal outcomes. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD009916] [DOI] [PMC free article] [PubMed] [Google Scholar]


