Abstract
It is well established that a phosphoinositide (PI) cycle which is operationally distinct from the classical plasma membrane PI cycle exists within the nucleus, where it is involved in both cell proliferation and differentiation. However, little is known about the regulation of the nuclear PI cycle. Here, we report that nucleus-localized phospholipase C (PLC) β1, the key enzyme for the initiation of this cycle, is a physiological target of extracellular signal-regulated kinase (ERK). Stimulation of Swiss 3T3 cells with insulin-like growth factor I (IGF-I) caused rapid nuclear translocation of activated ERK and concurrently induced phosphorylation of nuclear PLC β1, which was completely blocked by the MEK inhibitor PD 98059. Coimmunoprecipitation detected a specific association between the activated ERK and PLC β1 within the nucleus. In vitro studies revealed that recombinant PLC β1 could be efficiently phosphorylated by activated mitogen-activated protein kinase but not by PKA. The ERK phosphorylation site was mapped to serine 982, which lies within a PSSP motif located in the characteristic carboxy-terminal tail of PLC β1. In cells overexpressing a PLC β1 mutant in which serine 982 is replaced by glycine (S982G), IGF-I failed to activate the nuclear PI cycle, and its mitogenic effect was also markedly attenuated. Expression of S982G was found to inhibit ERK-mediated phosphorylation of endogenous PLC β1. This result suggests that ERK-evoked phosphorylation of PLC β1 at serine 982 plays a critical role in the activation of the nuclear PI cycle and is also crucial to the mitogenic action of IGF-I.
The mitogen-activated protein kinase signaling cascade, comprising extracellular signal-regulated protein kinase 1 (ERK1) and ERK2, is present in all eukaryotic cells and is the central pathway that is activated by growth factors. It is involved in the regulation of diverse cellular functions, such as cell proliferation, differentiation, and development (8, 29, 43). In response to a wide range of extracellular stimuli, activation of the cascade occurs by coupling receptors to Ras and hence to Raf1 and MEK1. The dual-specificity kinases MEK1 and MEK2 activate ERK1 and ERK2 through direct phosphorylation on threonine and tyrosine residues in their activation loops (42). Activated ERK1 and ERK2 exert their biological functions by phosphorylating a variety of intracellular targets, including protein kinases (52), transcription factors (24), signaling components, and cytoskeletal proteins (16).
The localization of ERK1 and ERK2 is predominantly cytoplasmic in quiescent cells (7, 28). However, upon serum or growth factor stimulation, a large fraction of cytoplasmic ERK rapidly translocates to the nucleus, where it persists for several hours, possibly by binding to a newly synthesized anchoring protein (1, 7, 21, 27, 28). Several recent studies have demonstrated that nuclear translocation of ERK is crucial for its biological action. For instance, nuclear uptake of ERK strongly correlates with proliferation of fibroblasts (40) and neuronal differentiation of PC12 cells (2, 50). Conversely, prevention of ERK nuclear translocation blocks growth factor-induced gene expression and cell proliferation (5). However, a mechanistic explanation of these events is hampered by the relative paucity of identified nuclear targets for ERK.
Phospholipase C (PLC) β1 has been shown to reside within the nucleus in many cell lines (6, 17, 38, 58). Nuclear PLC β1 is the key enzyme responsible for the initiation of the nuclear phosphoinositide (PI) cycle, a nuclear signaling pathway that is activated by insulin-like growth factor I (IGF-I) and involves the hydrolysis of PI lipids in a manner that is analogous to, but quite distinct from, that of plasma membrane PI-mediated signal transduction mechanisms (9–11, 17, 36). Stimulation of the nuclear PI cycle leads to the production of diacyglycerol (15, 46) followed by translocation of protein kinase C (PKC) to the nucleus (15, 39). Activated nuclear PKC has been shown to phosphorylate a number of proteins involved in cell division and appears to be critical for progression through the G1/S (49) and G2/M checkpoints of the cell cycle (19, 20, 22, 48).
PLC β1 exists as two alternatively spliced isoforms, PLC β1a (150 kDa) and PLC β1b (140 kDa), which differ only in a short region of their C termini (3). The nuclear localization of this enzyme is determined by a cluster of lysine residues (between positions 1055 and 1072) which is common to both isoforms (25). Overexpression of PLC β1 and subsequent localization to the nucleus can significantly enhance the mitogenic action of IGF-I in Swiss 3T3 cells (30) and also prevent erythroid differentiation in mouse erythroleukemia cells, indicating a pivotal role of this enzyme in the regulation of cell proliferation and differentiation (37). Indeed, it has recently been demonstrated that even in serum-starved cells, overexpression of PLC β1 alone is sufficient to increase the expression of cyclin D3 and cdk4, enhance hyperphosphorylation of retinoblastoma protein, and consequently activate E2F-1 transcription factor (18). This conclusion is further strengthened by the discovery that in Saccharomyces cerevisiae, nuclear PLC1 (homologous in function to mammalian PLC-β1) and two inositol polyphosphate kinases constitute a nuclear signaling cascade that affects mRNA transport and transcription control (55).
It is currently unclear how the activity of PLC β1 in the nucleus is regulated by extracellular stimuli. A recent study has shown that IGF-I can induce phosphorylation of nuclear PLC β1 in a time-dependent manner (35). However, its physiological relevance remains to be addressed. In the present study, we demonstrate that PLC β1 is the physiological nuclear target of ERK1 and ERK2. In response to IGF-I stimulation, the activated ERK in the nucleus phosphorylates PLC β1 at serine 982, which is located in the characteristic, long carboxyl-terminal domain that has been shown to possess a number of regulatory functions. Expression of a PLC β1 S982G mutant has a dominant-negative affect on IGF-I-induced activation of the nuclear PI cycle and cell proliferation, suggesting that phosphorylation of this site is obligatory for the activation of the enzyme and the mediation of IGF-I's mitogenic action. To our knowledge, this study is the first demonstration of cross talk between the Ras/Raf/MAP kinase cascade and a PLC signaling pathway.
MATERIALS AND METHODS
Materials.
p44/p42 MAP kinase polyclonal antibody, phospho-p44/p42 MAP kinase polyclonal antibody, and the MEK inhibitor PD98059 were purchased from New England Biolabs, Inc. (Beverly, Mass.). Activated rat ERK2 (recombinant, Escherichia coli), U0126, and LY294002 were from Calbiochem (San Diego, Calif..) Protein kinase A (PKA) catalytic subunit, Cy3-conjugated goat anti-rabbit immunoglobulin G, anti-β-tubulin monoclonal antibody (MAb), 5′-bromodeoxyuridine (5′-BrdUrd), aprotinin, and leupeptin were from Sigma (St. Louis, Mo.). Isotopes ([γ-32P]ATP, [32P]orthophosphate, [3H]thymidine, and [3H]phosphatidylinositol biphosphate {[3H]PIP2}) were from ICN (Costa Mesa, Calif.). Lipofectamine Plus, G418, and enhanced chemiluminescence detection kits were from Life Technologies (Paisley, Scotland).
Construction of expression vectors and transfection.
A full-length cDNA encoding wild-type rat PLC β1 (45) was cloned into the multiple-cloning site of the cytomegalovirus promoter-driven eukaryotic expression vector PRc/CMV (Invitrogen Corp., San Diego, Calif.). A PLC β1 clone in which the putative MAP kinase phosphorylation site PSSP (corresponding to amino acids 980 to 983 in the published sequence) was mutated to PSGP was constructed as follows. A PCR was carried out with wild-type PLC β1 template DNA and a forward primer (5′-AAATCTGAACCCAGCGGCCCAGATCATGGC-3′) which contains the serine (AGC)-to-glycine (GGC) mutation (in boldface) and a reverse primer (5′-CATCTGCAGCTTGGGCTTCTCATCCAGGAT-3′) which spans a unique PstI site (in bold) at nucleotides 3424 to 3430 in the published sequence. The resultant 414-bp product was used as a source of reverse primer to perform a second PCR with a forward primer (5′-CAGCATATGAGGAAGGAGGCAAATTTATTG-3′) which spans a unique NdeI site (in boldface) at nucleotides 2236 to 2252 in the published sequence. The 1,191-bp product was digested with NdeI and PstI and inserted into the corresponding sites in the wild-type PLC β1 expression vector. The resulting clone was validated by DNA sequencing and is referred to herein as the S982G PLC β1 mutant. Construction of another PLC β1 mutant, M2b, in which a cluster of lysine residues located within the nuclear localization sequence was replaced by isoleucine, is described elsewhere (18, 37). This mutant lacks the ability to localize to the nucleus (25).
Constructs were transfected into Swiss 3T3 cells using Lipofectamine Plus according to the manufacturer's instructions. Stable transfectants were selected in medium containing the neomycin analogue G418 (Life Technologies) at 600 μg/ml. At 10 days after transfection, the clones were harvested using sterilized steel rings and expanded separately in the presence of G418.
In vivo 32P labeling and isolation of nuclei and cytoplasmic fractions.
Confluent Swiss 3T3 cells grown in 10-cm petri dishes were starved for 1 h in Dulbecco modified Eagle medium without sodium phosphate and subsequently labeled with 200 μCi of [32P]orthophosphate/ml for 4 h. Cells were then incubated without or with 40 ng of IGF-I /ml for different times. Nuclei were purified as previously described (36). Briefly, 5 × 106 cells were lysed in 400 μl of nuclear isolation buffer (10 mM Tris-HCl [pH 7.8], 1% NP-40, 10 mM mercaptoethanol, 0.5 mM phenylmethylsulfonyl fluoride, 1 μg of aprotinin and leupeptin/ml, 10 μg of soybean trypsin inhibitor/ml, 15 μg of calpain inhibitor 1 and 2 [Boehringer]/ml, 2.0 mM Na3VO4, and 5 mM NaF) for 3 min on ice. MilliQ water (400 μl) was then added to swell cells for 3 min. The cells were sheared by eight passages through a 23-gauge hypodermic needle. Nuclei were recovered by centrifugation at 400 × g and 4°C for 6 min and washed once in 400 μl of washing buffer (10 mM Tris-HCl [pH 7.4] and 2 mM MgCl2, plus protease and phosphatase inhibitors as described above). The purity of the isolated nuclei was analyzed by transmission electron microscopy, detection of β tubulin, and measurement of glucose-6-phosphatase as described elsewhere (18, 36, 37). Only nuclear preparations that were completely free of cytoplasmic contamination were processed for further experiments.
The cytoplasmic fraction was obtained by homogenizing the cells with 20 strokes in a Dounce homogenizer in 10 mM Tris-HCl (pH 7.8), 10 mM β-mercaptoethanol, and protease inhibitors and then pelleting the nuclei at 400 × g. This procedure allows the recovery of pure cytoplasmic fractions and avoids the risk of contamination by nuclear debris present in the crude supernatant from nuclear preparations (36).
Immunoprecipitation.
Purified nuclei were solubilized in immunoprecipitation (IP) buffer (25 mM HEPES [pH 7.5], 5 mM EDTA and EGTA, 50 mM NaCl, 50 mM NaF, 30 mM sodium pyrophosphate, 10% glycerol, and 1% Triton X-100, plus protease inhibitor cocktail as described above) for 20 min at 4°C with shaking. Cell debris was removed by centrifugation at 12,000 × g and 4°C for 5 min. The supernatants were incubated with 50 μl of a 50% slurry of protein A/G agarose beads for 1 h. The cleared lysates were then incubated with 5 μg of mouse anti-PLC β1 antibody for 16 h. The immunocomplexes were recovered by adding 50 μl of protein A/G agarose beads for another hour and released by boiling in 50 μl of 1× sodium dodecyl sulfate (SDS) buffer for 5 min. Samples were then separated by SDS–8% polyacrylamide gel electrophoresis (PAGE), and the protein phosphorylation was analyzed by autoradiography and quantified by Image software (Pharmacia Biotech). For Western blot analysis, the proteins were transferred from gels to nitrocellulose membranes, blocked with 10% fat-free milk, and incubated with the various primary and secondary antibodies described below. The immunoreactive proteins were detected using enhanced chemiluminescence reagents according to the manufacturer's instructions.
Immunostaining and confocal imaging microscope.
Swiss 3T3 cells grown on coverslips were starved for 24 h in serum-free medium and incubated without or with 40 ng of IGF-I/ml or IGF-I plus the MEK inhibitor PD98059 as described above. Cells were then stained for activated ERK1 and ERK2 as previously reported, using rabbit anti-phospho-p42/p44 MAP kinase antibody (1:100), followed by goat anti-rabbit antibody conjugated with Cy3. To stain the nuclei, DAPI (4′,6′-diamidino-2-phenylindole; Boehringer Mannheim) was added for the last 15 min at a final concentration of 0.2 μg/ml. The specimens were then examined using a Leica TCS 4D confocal laser scanning microscope (Lasertechnik, Heidelberg, Germany) fitted with a mercury vapor lamp and a mixed-gas krypton-argon laser.
Expression and purification of recombinant PLC β1 and in vitro phosphorylation.
The entire open reading frame of rat PLC β1 was amplified by PCR with SmaI and XbaI linkers at the 5′ and 3′ ends, respectively. The PCR product was then digested and ligated into pVL1393 baculovirus transfer vector (Pharmingen), and the fidelity of the new plasmid (pVL1393-PLC β1) was confirmed by sequencing the plasmid on both strands. To generate recombinant baculovirus expressing PLC β1, Spodoptera frugiperda (Sf9) cells were cotransfected with pVL1393-PLC β1, BaculoGold, and modified baculovirus genomic DNA with Lipofectamine transfection reagent. Recombinant virus was subjected to two rounds of purification.
A 1-liter suspension culture of Sf9 cells at 106 cells/ml was infected with recombinant baculovirus expressing PLC β1 at a multiplicity of infection of 10. The cells were harvested at 72 h postinfection, resuspended in ice-cold phosphate-buffered saline (PBS), and repelleted. The cell pellet was resuspended in 10 ml of homogenization buffer (20 mM HEPES-NaOH [pH 7.0], 50 mM KCl, 1 mM EDTA, 1 mM EGTA, 0.1 mM dithiothreitol, and protease inhibitor cocktail as described above) and sonicated with a Branson sonicator. The lysates were centrifuged at 10,000 × g and 4°C for 1 h and the supernatant was collected. The recombinant PLC β1 was purified by sequential chromatography through columns of TSK Phenyl 5 PW, TSKgel DEAE-5PW, and HiTrap heparin. The purity of the protein was confirmed by SDS-PAGE and high-pressure liquid chromatography (HPLC).
Purified recombinant PLC β1 (500 ng) was incubated with 20 U of activated rat ERK2 at 30°C in a reaction mixture containing 10 mM HEPES (pH 8.0), 100 μM ATP, 1 μCi of [γ-32P]ATP, 10 mM MgCl2, 0.5 mM benzamidine, and 1 mM dithiothreitol for the indicated time. For PKA phosphorylation analyses, the recombinant PLC β1 was incubated with 20 U of PKA in the presence of 100 μM ATP and 1 μCi of [γ-32P]ATP in PKA reaction buffer (10 mM Tris [pH 7.0], 5 mM MgCl2). Reactions were terminated by the addition of 2× SDS sample buffer and boiling for 5 min. Proteins were separated by SDS–8% PAGE and visualized by autoradiography or Coomassie blue staining. To calculate the stoichiometry of phosphorylation, the bands corresponding to 32P-labeled PLC β1 were excised from the gel. The gel pieces were solubilized by 27% H2O2 and 0.3 M NH4OH at 55°C overnight, and the radioactivity was determined by liquid scintillation counting.
In-gel trypsin digestion and two-dimensional phosphopeptide mapping analysis.
The bands corresponding to 32P-labeled PLC β1 were excised from the gels, minced, and digested with trypsin as previously described (54). Aliquots of the tryptic mixtures were lyophilized and solubilized in 10 μl of electrophoresis buffer (1% pyridine, 10% acetic acid [pH 3.5]) and applied to the middle of a thin-layer chromatography plate (20 by 20 cm; Sigma) 4 cm from the bottom along with a trace of basic fuchsin dye. Electrophoresis was performed at 350 V, until the basic fuchsin dye had migrated 2.5 cm towards the cathode. The plates were then air dried and subjected to perpendicular chromatography in pyridine-butanol-acetic acid-water (10:15:3:12, vol/vol) until the solvent front reached the top of the plate. Plates were air dried, and phosphopeptides were visualized by autoradiography.
Isolation of the tryptic phosphopeptides by reversed-phase HPLC (RP-HPLC).
Trypsin-digested mixtures of 32P-labeled PLC β1 were separated using a Jupiter 5μ C18 column (25 by 0.2 cm; Phenomenex). The prewarmed column (37°C) was washed with 0.1% trifluoroacetic acid (vol/vol) followed by elution using a linear gradient of 4 to 56% acetonitrile at a flow rate of 300 μl/min. Fractions were collected at 30-s intervals, and aliquots from each fraction were analyzed for 32P by liquid scintillation counting.
Determination of the phosphorylation site of PLC β1 by MALDI-TOF and phosphate-releasing analysis.
Aliquots of the 32P-phosphopeptides separated by RP-HPLC were mixed with an equal amount of α-cyano-4-hydroxycinnamic acid. The mixture was applied to mass analysis using a G2025A matrix-assisted laser adsorption ionization time-of-flight (MALDI-TOF) mass spectrometer as previously described (51). Phosphate-releasing assays were performed with a Hewlett-Packard G1000A protein sequencer utilizing routine 3.1 Edman degradation chemistry as recommended by the manufacturer. Aliquots of purified phosphopeptides were covalently linked to a Sequelon AA filter using the Sequelon AA reagent kit (Millipore), and the phosphate was extracted from each cycle with three 0.5-ml aliquots of 90% methanol–0.015% phosphoric acid as the solvent in routine 3.1 of the polyvinylidene difluoride method. Extracts were diverted via valve number RV6 (line 61), and fractions were collected and then counted in a liquid scintillation counter.
PLC activity assay.
The nuclei were purified as described above, and the activity of nuclear PLC was measured as outlined previously (36). Nuclear proteins were incubated with 100 mM MES (morpholineethanesulfonic acid) buffer, pH 6.7, plus 150 mM NaCl, 0.06% sodium deoxycholate, and 3 nmol of [3H]PIP2 (specific activity, 30,000 dpm/nmol) for 30 min at 37°C. Hydrolysis was stopped by adding chloroform-methanol-HCl, and the amount of IP3 in the upper phase was quantified by liquid scintillation counting.
Analysis of cell proliferation by [3H]thymidine incorporation and 5′-BrdUrd fluorescent immunolabeling.
Cells grown in 24-well dishes were starved for 24 h in serum-free medium containing 0.2% bovine serum albumin and subsequently incubated without or with 40 ng of IGF-I/ml for another 15 h. The cells were then pulse-labeled with 0.8 μCi of [3H]thymidine/ml and washed sequentially with cold PBS (2×), 5% cold trichloroacetic acid (2×), and 100% ethanol (2×). After air drying for 30 min, the residues were solubilized in 200 μl of 0.2 N NaOH and neutralized with an equal volume of 0.1 HCl, and radioactivity was counted. A 5′-BrdUrd fluorescent immunolabeling assay was performed according to a previously described protocol (39). Briefly, cells at 15 h after IGF-I incubation were pulse-labeled with 100 μM 5′-BrdUrd for 10 min and then fixed in 4% paraformaldehyde in PBS for 30 min. The cells were treated with 4 N HCl for 30 min at room temperature to denature DNA and subsequently fixed at −20°C in graded ethanol solutions to prevent DNA reannealing. Coverslips were air-dried and reacted with a MAb against 5′-BrdUrd and subsequently with a Cy3-conjugated anti-mouse IgG. After three washings, the samples were mounted on slides for fluorescence analysis.
RESULTS
Nuclear PLC β1 is the direct target of ERK.
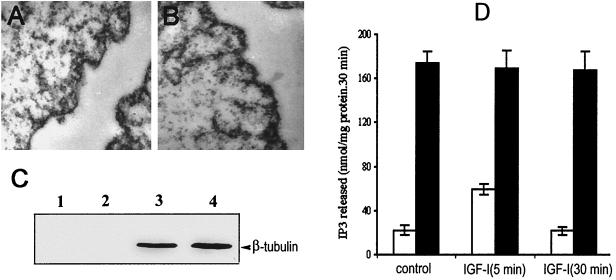
To investigate the effect of IGF-I on nuclear PLC activity, nuclei were isolated from quiescent cells or IGF-I-treated cells as described in Materials and Methods. Electron microscope analysis showed that the nuclei obtained from these cells are completely stripped of their outer envelope and free from cytoplasmic contamination (Fig. 1A and B). The purity of our nuclear preparations was also confirmed by assay for β-tubulin. As shown in Fig. 1C, β-tubulin was completely absent from preparations of membrane-free nuclei, whereas β-tubulin was abundant in the cytoplasmic fraction. Analysis for the activity of glucose-6-phosphatase, a cytoplasmic marker, also revealed that our nuclear preparation is free from cytoplasmic contamination (data not shown). In line with several previous reports, treatment of cells with 40 ng of IGF-I/ml for 5 min increased the activity of nuclear PLC about threefold over the basal level but had no effect on the total PLC activity in the cytoplasmic fraction (Fig. 1D).
FIG. 1.
Analysis of PLC activity in isolated nuclear and cytoplasmic fractions of Swiss 3T3 cells after stimulation with IGF-I. Nucleus and cytoplasm preparation was performed as described in the text. The isolated nuclear pellets from quiescent cells (A) and cells treated with 40 ng of IGF-I/ml for 5 min (B) were analyzed using transmission electron microscopy. (C) Cytoplasmic or nuclear proteins (80 μg) were separated by SDS–7% PAGE and probed with an anti-β tubulin MAb at a dilution of 1:500. Lane 1, nuclei from quiescent Swiss 3T3 cells; lane 2, nuclei from Swiss 3T3 cells stimulated with 40 ng of IGF-1/ml (5 min); lane 3, cytoplasmic fraction from quiescent Swiss 3T3 cells; lane 4, cytoplasmic fraction from Swiss 3T3 cells treated with IGF-1 (5 min). Bands corresponding to β-tubulin with a molecular mass of 55 kDa were detected. (D) Histogram showing the PLC activity of nuclei (white bars) and cytoplasmic fraction (black bars) in quiescent and IGF-I-treated cells. Analysis of enzyme activity was performed as described in Materials and Methods, and the results are expressed as means ± standard deviations (n = 4).
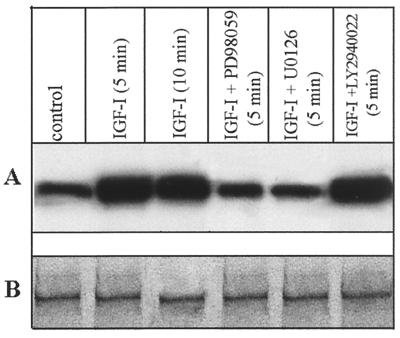
Immunoprecipitation of 32P-labeled nuclear proteins revealed that PLC β1 within the nucleus is phosphorylated in quiescent Swiss 3T3 cells (Fig. 2A). At 5 and 10 min after IGF-I stimulation, the phosphorylation of PLC β1 increased 3.1- and 2.7-fold, respectively, although the protein concentration within the nucleus was unchanged under these conditions (Fig. 2B). This result excludes the possibility that the increased phosphorylation of PLC β1 is due to its enhanced expression or nuclear translocation following IGF-I treatment. IGF-I-induced phosphorylation of nuclear PLC β1 was blocked by the specific MEK inhibitors PD98059 and U0126 but not by the PI-3-kinase inhibitor LY294002, suggesting the involvement of the Raf/MEK/ERK signaling cascade in this process.
FIG. 2.
IGF-I-evoked phosphorylation of nuclear PLC β1 is blocked by the MEK inhibitor PD98059 and U0126. Quiescent Swiss 3T3 cells were radiolabeled with 32P and incubated without or with 40 ng of IGF-1/ml for the times indicated. The kinase inhibitor (20 μM PD98059, 20 μM U0126, or 20 μM LY2940022) was added 30 min before IGF-I. Nuclear proteins purified from each of these treated cultures (500 μg) were immunoprecipitated by anti-PLC β1 MAb. Immunoprecipitated complexes were eluted with 50 μl of 1× SDS-PAGE loading buffer. A 25-μl portion of each sample was separated by SDS–8% PAGE and analyzed by either autoradiography (A) or Western blotting using anti-PLC β1 (B).
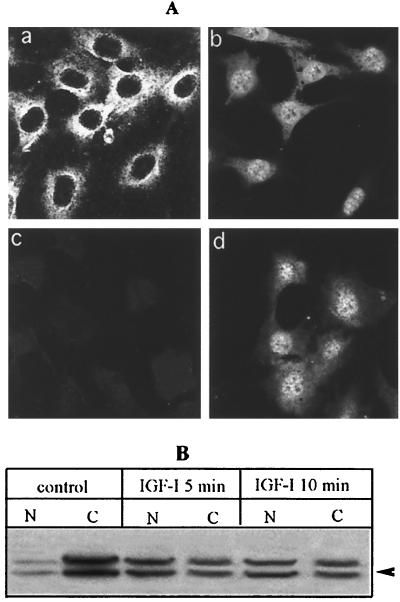
In order to phosphorylate PLC β1 in the nucleus, ERK requires access to this cellular compartment. Indeed, a recent immunocytochemical study has found that a large portion of cytoplasmic ERK1 and ERK2 translocates into the nucleus in IGF-I-treated cells (40). We further confirmed this result by using both anti-p42/p44 MAP kinase and anti-phospho-p42/p44 MAP kinase antibodies. Both immunostaining and immunoblotting with anti-p42/p44 MAP kinase revealed that a majority of ERK is located in the cytoplasm in quiescent cells (Fig. 3). Nuclear accumulation of ERK was observed at 5 min after IGF-I stimulation. Staining the cells with anti-phospho-p42/p44 MAP kinase also revealed that a majority of activated ERK is located in the nucleus of IGF-I-treated cells (Fig. 3A). These results indicate that the increased phosphorylation of nuclear PLC β1 correlates with activation and nuclear translocation of ERK.
FIG. 3.
IGF-I induces nuclear translocation of ERK1 and ERK2. (A) Images of cells stained with anti-p42/p44 polyclonal antibody (a and b) and anti-phospho-p42/p44 polyclonal antibody (c and d). Quiescent cells grown on coverslips were incubated without (a and c) or with 40 ng of IGF-I/ml for 5 min (b and d), fixed, and incubated with anti-p42/p44 MAP kinase (1:100) or anti-phospho-p42/p44 MAP kinase (1:100). The samples were then stained with Cy3-conjugated secondary antibody (1:1,000) and analyzed by confocal microscopy (voltage for photo multiplier tube = 450 V; pinhole = 60 nm). Note that the faint staining in panel c can be visualized only by very high laser energy (voltage for photo multiplier tube = 1,000 V; pinhole = 60 nm). (B) Intracellular distribution of ERK in quiescent and IGF-I-treated cells. Quiescent cells grown on 10-cm petri dishes were incubated without or with IGF-I as described above, and nuclei were purified from cells. Nuclear or cytoplasmic proteins (60 μg) were separated by SDS–10% PAGE and probed with anti-p42/p44 MAP kinase antibody. N, nuclear proteins; C, cytoplasmic proteins.
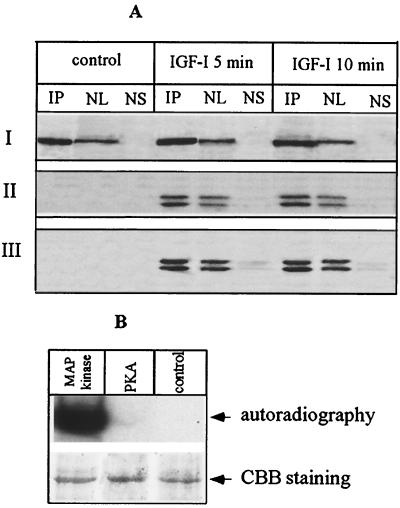
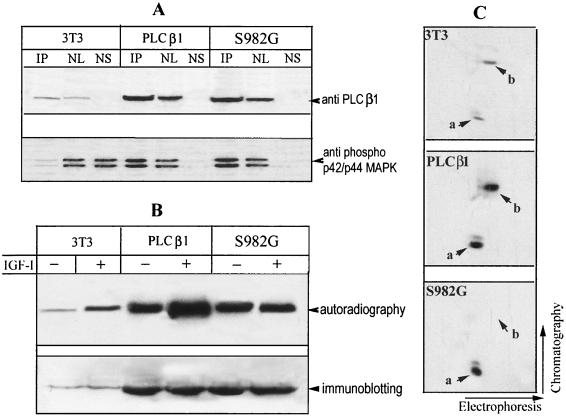
To investigate the potential association of PLC β1 and ERK, PLC β1 was overexpressed in Swiss 3T3 cells, and their nuclei were subjected to IP using a specific anti-PLC β1 MAb (44). The precipitated complex was separated by SDS-PAGE and probed with either anti-PLC β1 or anti-phospho-p42/p44 MAP kinase antibody. This analysis demonstrated an interaction between PLC β1 and activated ERK in the nuclei of cells stimulated with IGF-I (Fig. 4A). Notably, activated ERK was hardly detected in the nuclear supernatant after recovery of IP complexes (Fig. 4A), indicating that a majority of activated ERK was bound to PLC β1 following IGF-I stimulation. A similar scenario was also observed using an antibody against p42/p44 MAP kinase. We were unable to detect the association of ERK and PLC β1 in the nucleus of quiescent cells, perhaps due to the extremely low nuclear concentration of ERK under resting conditions.
FIG. 4.
Nuclear PLC β1 is the direct target of ERK in vivo and in vitro. (A) Cells overexpressing PLC β1 were incubated without or with IGF-I for different periods. The nuclei were purified from these cells and then lysed with IP buffer. Aliquots of nuclear lysate were subjected to IP by anti-PLC β1 MAb. The nuclear supernatant was saved after recovery of IP complexes. IP complexes were released from protein A/G Sepharose beads by incubating with 50 μl of SDS loading buffer at 95°C for 5 min. Nuclear lysate (NL) (20 μg), 25 μl of nuclear supernatant after recovery of IP complexes (NS), or 25 μl of IP complexes was separated by SDS–8% PAGE and blotted with either anti-PLC β1 (panel I), anti-phospho-p42/p44 MAP kinase antibody (panel II), or anti-p42/p44 MAP kinase (panel III). The result is representative of three independent experiments. (B) PLC β1 was expressed and purified using a baculovirus-based system as described in the text. A 500-ng portion of the purified protein was phosphorylated in vitro using the indicated kinases, separated by SDS–8% PAGE, and visualized by either autoradiography or Coomassie brilliant blue (CBB) staining.
To further confirm that PLC β1 is a substrate of ERK1 and ERK2, PLC β1 was expressed and purified from insect cells using the baculovirus system, as described in Materials and Methods. The purity was confirmed by both SDS-PAGE and HPLC (data not shown). The purified protein was then used for an in vitro phosphorylation assay. In the presence of activated ERK, phosphorylation of PLC β1 was observed and increased with time of incubation (data not shown). Maximal phosphate incorporation was achieved within 10 min (Fig. 4B). Under this condition, the stoichiometry of phosphorylation (mean ± standard deviation) is 1.12 ± 0.19 mol of phosphate/mol of PLC β1; n = 4). In contrast, phosphorylation of PLC β1 was not detected if activated ERK was omitted or replaced with PKA (Fig. 4B).
Serine 982, with the surrounding motif PSSP, is the phosphorylation site of ERK.
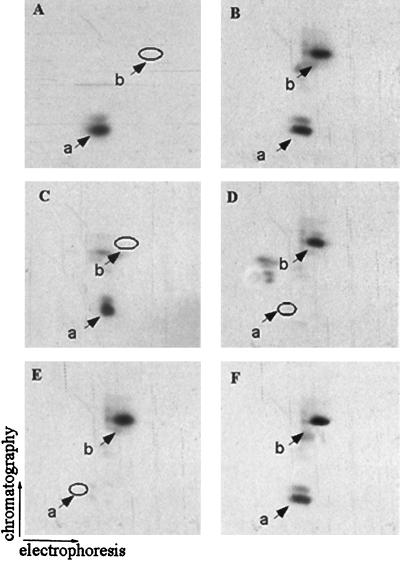
Two-dimensional phosphopeptide mapping analysis of the tryptic mixtures from 32P-labeled PLC β1 revealed one prominent tryptic phosphopeptide in control cells (Fig. 5A). In IGF-I-stimulated cells, one extra phosphopeptide (designated phosphopeptide b) was detected (Fig. 5B). The production of phosphopeptide b was completely inhibited by PD98059 (Fig. 5C) and U0126 (data not shown), suggesting that ERK-evoked phosphorylation possibly occurs on this peptide. Indeed, direct in vitro phosphorylation of PLC β1 by activated ERK also leads to the phosphorylation of the same peptide (Fig. 5D).
FIG. 5.
Characterization and purification of the tryptic peptide of PLC β1 phosphorylated by ERK. The 32P-labeled cells overexpressing PLC β1 were incubated without (A) or with (B) 40 ng of IGF-1/ml or with 50 ng of IGF-1/ml plus 50 μM PD98059 (C). Nuclear 32P-labeled PLC β1 was immunoprecipitated, separated by SDS–8% PAGE, and visualized by autoradiography as described for Fig. 1. The bands corresponding to PLC β1 were excised from the gels and digested in gel with trypsin. The tryptic peptide mixtures from each of these samples were analyzed by two-dimensional phosphopeptide mapping. (D) Tryptic peptides of PLC β1 phosphorylated in vitro by ERK. (E) HPLC fraction 36 as described in the text. (F) Mixture of peptides from panels B and E. The positions of phosphopeptides a and b are indicated.
To purify phosphopeptide b for further analysis, the tryptic peptide mixtures from 32P-labeled PLC β1 were subjected to RP-HPLC as described in Materials and Methods. Fractions were collected at 30-s intervals, and radioactivity was counted. In cells treated with IGF-I, fraction 36 (eluted after 18 min with 43% acetonitrile) contained a 32P-labeled phosphopeptide which does not exist in either control cells or cells treated with IGF-I plus PD98059. A 32P-labeled phosphopeptide with similar characteristics was also detected in the tryptic peptide mixture of 32P-labeled PLC β1 phosphorylated in vitro by ERK. Two-dimensional phosphopeptide mapping revealed that the phosphopeptide in this fraction exactly comigrated with phosphopeptide b (Fig. 5E and F).
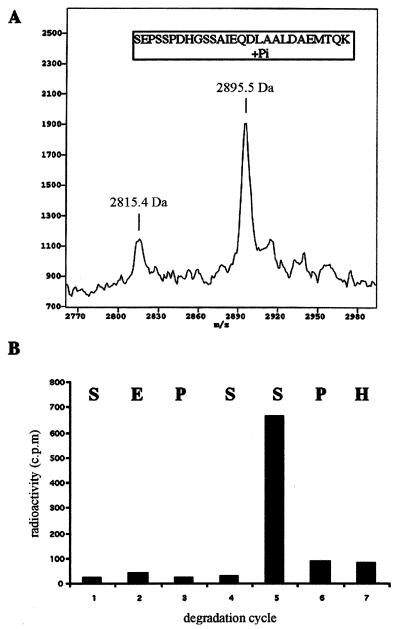
MALDI-TOF analysis for HPLC fraction 36 revealed two peptides, with molecular masses of 2815.4 and 2895.5 Da (Fig. 6A). The difference of 80 Da between these two peptides is equivalent to one phosphate residue. By reference to the theoretical masses for the tryptic peptides of PLC β1 (http://www.expasy.ch), the two peptides can be assigned to the unphosphorylated and monophosphorylated tryptic fragments, respectively, corresponding to the residues between 978 and 1004 of PLC β1 (SEPSSPDHGSSAIEQDLAALDAEMTQK). The identity of this peptide fragment was further confirmed by amino acid sequencing. Phosphoamino acid analysis revealed that phosphorylation occurred exclusively on a serine residue (data not shown). There are five serine residues within this fragment. To determine the precise phosphorylation site, a ∼1,000-cpm aliquot from fraction 36 was subjected to a phosphate-releasing assay. This analysis showed that the preponderance of 32P is released at cycle 5, which corresponds to serine 982 (Fig. 6B). Thus, we conclude that the ERK phosphorylation site is located at serine 982, within the surrounding motif PSSP. This motif exactly conforms to the consensus sequence for ERK.
FIG. 6.
Determination of the precise ERK phosphorylation site of PLC β1. (A) Mass spectra of the peptides for HPLC fraction 36. The peptide samples were mixed with α-cyano-4-hydroxycinnamic acid. The mixture was applied to mass analysis using a G2025A MALDI-TOF mass spectrometer as previously described (51). (B) Phosphate release analysis of the 32P-labeled peptide. An aliquot of HPLC fraction 36 (∼1,000 cpm) was subjected to N-terminal sequencing, and 32P released from each cycle was analyzed for radioactivity. The corresponding amino acid residues released from each cycle are shown at the top of the chart.
Overexpression of the PLC β1 S982G variant regulates the biological action of IGF-I in a dominant-negative manner.
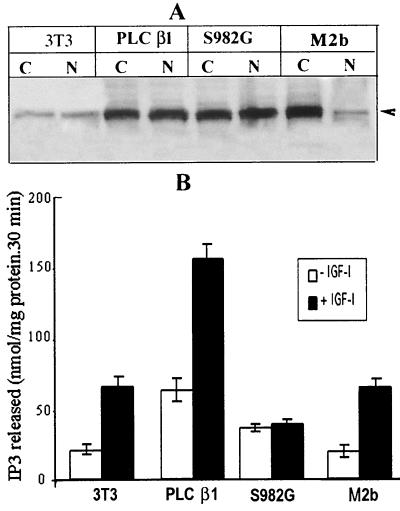
To investigate the effect of ERK-mediated phosphorylation on the enzymatic activity of PLC β1, we generated stably transfected cell lines that overexpress either wild-type PLC β1 or its S982G variant. Another PLC β1 variant, M2b, which lacks a cluster of lysine residues responsible for its nuclear localization (18, 25), was also expressed to discriminate the biological effects of the cytoplasmic and nuclear enzymes. Western blot analysis of nuclear proteins from the transfected cell lines indicated that the concentration of wild-type PLC β1 and its S981G mutant are similar, suggesting that the nuclear localization of the PLC β1 S982G mutant was not affected (Fig. 7A). In the cells overexpressing PLC β1 variant M2b, an increased level of immunoreactive PLC β1 was detected in cytoplasm but not in the nucleus.
FIG. 7.
The responsiveness of wild-type PLC β1 and PLC S982G mutant or M2b to IGF-I stimulation in Swiss 3T3 cells. (A) Western blot analysis of nuclear PLC β1 from wild-type 3T3 cells and 3T3 cells overexpressing wild-type PLC β1, the PLC β1 S982G mutant, or the mutant M2b. The stable transfectants were selected as described in the text. Sixty micrograms of cytoplasmic proteins or 20 μg of nuclear proteins from these cells was separated by SDS–8% PAGE and probed with anti-PLC β1 MAb. (B) Nuclei from cells were analyzed for PLC activity as described in the text. The results are presented as the means ± standard deviations (n = 4). Note that the figure shows the result of typical experiments, and similar results were obtained from at least another two independent transfectants that express wild-type PLC β1, the S982G mutant, or M2b.
Consistent with previous findings (30), IGF-I stimulation increased the activity of nuclear PLC β1 about threefold over basal levels in control 3T3 cells (Fig. 7B). In cells overexpressing wild-type PLC β1, the PLC enzyme activity in both basal and IGF-I-stimulated states is drastically increased compared to that in control 3T3 cells (Fig. 7B). In contrast, the nuclear PLC from cells overexpressing the S982G mutant failed to respond to IGF-I stimulation and was significantly lower than that of the IGF-I-stimulated control cells, indicating that the PLC S982G mutant regulates the IGF-I-dependent nuclear PLC activity in a dominant-negative fashion. The direct involvement of ERK in the regulation of the nuclear PI cycle was also confirmed by the finding that the MEK inhibitor PD98059 blocked the IGF-I-induced increase in enzyme activity of PLC β1 (data not shown). In cells overexpressing the PLC β1 variant M2b, the basal level of nuclear PLC activity and the activity following IGF-I treatment are similar to those in control cells (Fig. 7B).
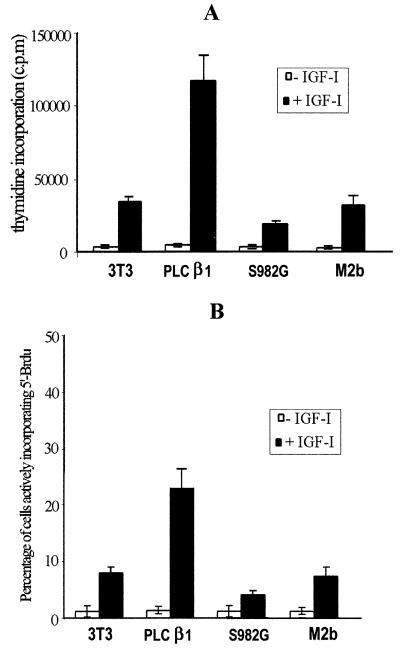
It has previously been demonstrated that nuclear PLC β1 plays an essential role in the mitogenic action of IGF-I (30, 39). Therefore, we further evaluated the mitogenic response for the stable transfectants using a [3H]thymidine incorporation assay (Fig. 8A). Compared to control 3T3 cells, overexpression of wild-type PLC β1 increased by 3.5-fold the number of cells in S phase actively incorporating thymidine after IGF-1 stimulation. In contrast, in cells overexpressing the PLC S982G mutant, the IGF-I-stimulated DNA synthesis was decreased to about 15% of the response obtained with overexpressed wild-type PLC β1. Noticeably, IGF-I-induced [3H]thymidine incorporation in this cell line was 43% lower than that in control (untransfected) cells, suggesting that abolition of ERK-mediated phosphorylation at serine 982 of PLC β1 substantially blocked IGF-I-induced mitogenesis in a dominant-negative manner. This result was further reinforced by a 5′-BrdUrd fluorescent immunolabeling assay, which showed that the percentage of IGF-I-induced cell entry into S phase in S982G mutant-overexpressing cells was drastically decreased, relative to both the cells overexpressing wild-type PLC β1 and control cells (Fig. 8B). The fact that the S982G mutant does not completely block IGF-I-induced DNA synthesis may be due either to the increased basal level of nuclear PLC activity in this cell line (Fig. 7B) or to IGF-dependent mechanisms which do not involve activation of nuclear PLC β1. Western blot analysis using anti-phospho-p42/p44 antibody revealed that overall activation of ERK by IGF-I was not affected by overexpression of either wild-type PLC β1 or its S982G mutant (data not shown).
FIG. 8.
Effect of overexpressing wild-type PLC β1, the PLC β1 S982G mutant, or M2b on the mitogenic action of IGF-I. Control 3T3 cells and cells overexpressing wild-type PLC β1, the S982G mutant, or M2b were starved for 24 h and incubated without or with 40 ng of IGF-I/ml for another 15 h. These cells were then incubated either with [3H]thymidine or with 5′-BrdUrd as described in the text. (A) Histogram of data obtained from analysis of [3H]thymidine. (B) Percentage of cells positive for 5′-BrdUrd immunostaining following 15 h of stimulation with IGF-I. The results are from three independent experiments and are means ± standard deviations. Note that the data reported here are representative of at least two identical clones for each type of transfectant.
Both [3H]thymidine incorporation (Fig. 8A) and a 5′BrdUrd fluorescent immunolabeling assay (Fig. 8B) showed that the IGF-I-induced DNA synthesis and cell proliferation were similar between untransfected control cells and cells overexpressing the PLC β1 variant M2b, which lacks a nuclear localization signal. This result further confirms that only nuclear PLC β1 contributes to the mitogenic response of IGF-I.
Effect of the PLC β1 S982G mutant on ERK-mediated phosphorylation of endogenous PLC β1.
One of the potential mechanism underlying the dominant-negative effect of S982G mutant could be due to its inhibition on ERK-mediated phosphorylation of endogenous PLC β1, by competing for the binding sites on ERK. To explore this possibility, nuclei were purified from IGF-I-treated Swiss 3T3 cells and cells overexpressing either wild-type PLC β1 or the S982 mutant. Immunoprecipitation of nuclear proteins using an antibody against PLC β1 revealed that ERK recovered from immunocomplexes of untransfected control cells is hardly detectable, perhaps due to the low expression level of endogenous PLC β1 (Fig. 9A). In contrast, a large portion of ERK was recovered from immunocomplexes of both cells overexpressing wild-type PLC β1 and the PLC β1 S982G variant, suggesting that this variant still retains its ability to interact with ERK in the nucleus. In both untransfected control cells and cells overexpressing wild-type PLC β1, IGF-I stimulation increased phosphorylation of PLC β1 about threefold over basal levels (Fig. 9B). However, phosphorylation of nuclear PLC β1 in cells expressing the S982G mutant was refractory to IGF-I stimulation. Two-dimensional phosphopeptide mapping revealed that phosphopeptide b, which is produced by ERK-mediated phosphorylation at serine 982, was present in both IGF-I-treated control 3T3 cells and cells overexpressing PLC β1 (Fig. 9C; also see Fig. 5). Although the cells expressing the S982G mutant also contain the endogenous PLC β1, phosphopeptide b was not detected in these cells, even after exposure of the film for 2 weeks. These results indicate that expression of the S982G mutant can prevent ERK-mediated phosphorylation of endogenous PLC β1.
FIG. 9.
The PLC β1 S982G mutant associates with ERK and blocks ERK-mediated phosphorylation of endogenous PLC β1 in the nucleus. (A) Quiescent Swiss 3T3 cells and stable transfectants expressing wild-type PLC β1and S982G mutant cells (for details, see the legend to Fig. 7) were treated with 40 ng of IGF-I/ml for 5 min. The nuclei from these cells were purified and then lysed in IP buffer. Aliquots of nuclear lysate were subjected to IP by anti-PLC β1 MAb. IP complexes, nuclear lysate (NL), and nuclear supernatant after IP recovery (NS) were separated by SDS–8% PAGE and blotted with either anti-PLC β1 or anti-phospho-p42/p44 MAP kinase antibody, as described for Fig. 4. (B) Quiescent cells described for panel A were radiolabeled in vivo with [32P]orthophosphate for 4 h as in Fig. 2. Cells were then incubated without or with 40 ng of IGF-I/ml for another 5 min, and the nuclei from these cells were immunoprecipitated with anti-PLC β1 MAb. The immunocomplexes were separated by SDS-PAGE and then either analyzed by autoradiography or probed with anti-PLC β1 MAb, as for Fig. 2. (C) The 32P-labeled PLC β1 from IGF-I-treated 3T3 cells or cells overexpressing the S982G mutant was excised from the gels in panel B. The phosphoproteins were digested in gel by trypsin and analyzed by two-dimensional phosphopeptide mapping as described for Fig. 5. Note that phosphopeptide b was absent from cells expressing S982G. The figure is representative of three identical clones for each type of the transfectant.
DISCUSSION
Several previous studies have showed that the PI cycles within the nucleus and at the plasma membrane are under separate controls (31, 36). Activation of PLC β isoforms at the plasma membrane is controlled by the Gαq class of heterotrimeric G proteins (23, 26, 53) as well as by βγ subunits (41). However, there is no evidence that the Gαq class of heterotrimeric G proteins reside in the nucleus or that they are translocated there. A previous study failed to demonstrate activation of PLC β1 by GTP-γ-S in isolated nuclei, although evidence of stimulation of nuclear PI kinase activity was seen (33). However, evidence does exist for the growth factor-mediated translocation of Gi and for its role in mitosis (12–14). There are also reports suggesting that a novel class of G proteins may exist in the nucleus (4, 47), but none of these data has been linked with regulation of PLC β1 activity.
In the present study, we provide several lines of evidence supporting the notion that nuclear PLC β1 is the physiological target of ERK. Firstly, IGF-I-induced phosphorylation of nuclear PLC β1 is completely blocked by the MEK inhibitor PD98059 or U0126 (Fig. 2). Secondly, following IGF-I stimulation, PLC β1 is found to interact specifically with ERK within the nucleus (Fig. 4A). Thirdly, activated ERK can phosphorylate recombinant PLC β1 in vitro (Fig. 4B). Fourthly, the motif surrounding the phosphorylation site Ser 982 (PSSP) exactly conforms to the consensus sequence recognized by ERK (29). Sequence alignment analysis revealed that Ser 982 is highly conserved among different species of PLC β1 but does not exist in other isoforms of the β family, suggesting that ERK-mediated activation is specific for β1 isoforms. The physiological relevance of this ERK-mediated phosphorylation is supported by the finding that the enzyme activity of the mutant is not increased by IGF-I (Fig. 7).
The details of how ERK-mediated phosphorylation can regulate the enzyme activity of nuclear PLC β1 remain to be defined. Phosphorylation at serine 982 may not be a prerequisite for the maintenance of basal enzyme activity, since this site is not phosphorylated in quiescent cells (Fig. 5). Our preliminary result suggests that ERK-mediated phosphorylation does not directly increase the enzyme activity of PLC β1 in vitro (A. Xu and S. Gilmour, unpublished observation). Phosphorylation of PLC β isoforms by other kinases have been shown to affect the lipid binding ability or the association with its regulatory molecules (56). It is notable that the ERK-mediated phosphorylation site is within the regulatory domain of the characteristic carboxyl terminus, which has been shown to be essential for Gαq-mediated activation of this enzyme at the plasma membrane (25). Thus, it is conceivable that ERK-mediated phosphorylation at Ser 982 might affect the binding to this region of other, as-yet-unidentified nuclear proteins which consequently enhance PLC activity.
Nuclear PLC β1 has been shown to be critical for the mitogenic action of IGF-I (18, 30). This conclusion was also supported by our finding that overexpression of PLC β1 can potentiate the mitogenic response of cells to this growth factor (Fig. 7). It is important to note that IGF-I activates PLC β1 only in the nucleus and does not affect the enzyme activity at the plasma membrane (34). Overexpression of a PLC β1 variant lacking the nuclear localization sequence has no effect on cell proliferation (18). In agreement with these findings, our results also demonstrated that overexpression of a cytoplasm-confined PLC β1 mutant, M2b, has no effect on the mitogenic action of IGF-I (Fig. 8). Taken together, these observations exclude the possibility that overexpression of PLC β1 in nonnuclear compartments may also play a role in the mitogenic action of IGF-I.
While the role of ERK and its nuclear translocation is a well-documented feature of the mitogenic response, little is known about how this kinase exerts its actions, especially within the nucleus. Here, we found that overexpression of the PLC β1 S982G variant, in which the ERK phosphorylation site is mutated, negatively affects the IGF-I-dependent activation of nuclear PLC activity, as well as significantly blocking the proliferative response of the cells to this growth factor. This result strongly suggests that nuclear PLC β1 is one of the critical downstream targets of ERK and that activation of the nuclear PI cycle by ERK is indispensable for the mitogenic actions of IGF-I and possibly other growth factors.
The mechanisms underlying the dominant-negative action of S982G could be due to its inhibitory effect on ERK-mediated phosphorylation of endogenous PLC β1, by competing with the limited number of binding sites for ERK within the nucleus. This conclusion is supported by the demonstration that the S982G mutant retains its ability to interact with ERK (Fig. 9A) and that the phosphorylation of endogenous nuclear PLC β1 in cells expressing the S982G mutant is refractory to IGF-I stimulation (Fig. 9C). Another possible explanation for the dominant-negative effect of S982G may lie in the nature of PLC association within the nucleus. Several previous studies have implied that PLC β1 is associated with the inner nuclear matrix, where phospholipid and PKC can be found (22, 32, 57). The overexpressed variant PLC β1 may therefore compete with endogenous enzyme for intranuclear sites, binding to which may be essential for promoting the proliferative response. The detailed mechanisms underlying the dominant-negative action of the variant S982G are currently under investigation in our laboratory.
ACKNOWLEDGMENTS
We thank Markus Winter for his critical appraisal of the manuscript. Wild-type PLC β1 and its mutant M2b clones were generously provided by Sue Goo Rhee.
This work was funded by Health Research Council of New Zealand and the Marsden Fund of the Royal Society of New Zealand. L.C. is supported by AIRC and Italian CNR PF biotechnology.
REFERENCES
- 1.Adachi M, Fukuda M, Nishida E. Two coexisting mechanisms for nuclear import of MAP kinase: passive diffusion of a monomer and active transport of a dimer. EMBO J. 1999;18:5347–5358. doi: 10.1093/emboj/18.19.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 3.Bahk Y Y, Lee Y H, Lee T G, Seo J, Ryu S H, Suh P G. Two forms of phospholipase C-β1 generated by alternative splicing. J Biol Chem. 1994;269:8240–8245. [PubMed] [Google Scholar]
- 4.Bhullar R P, McCartney D G, Kanfer J N. Heterotrimeric and small molecular mass GTP-binding proteins of rat brain neuronal and glial nuclei. J Neurosci Res. 1999;55:80–86. doi: 10.1002/(SICI)1097-4547(19990101)55:1<80::AID-JNR9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.Brunet A, Roux D, Lenormand P, Dowd S, Keyse S, Pouyssegur J. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 1999;18:664–674. doi: 10.1093/emboj/18.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cataldi A, Caracino A, Di Baldassarre A, Robuffo I, Miscia S. Interferon beta mediated intracellular signalling traffic in human lymphocytes. Cell Signal. 1995;7:627–633. doi: 10.1016/0898-6568(95)00030-s. [DOI] [PubMed] [Google Scholar]
- 7.Chen R H, Sarnecki C, Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cobb M H. MAP kinase pathways. Prog Biophys Mol Biol. 1999;71:479–500. doi: 10.1016/s0079-6107(98)00056-x. [DOI] [PubMed] [Google Scholar]
- 9.Cocco L, Capitani S, Barnabei O, Gilmour R S, Rhee S G, Manzoli F A. Inositides in the nucleus: further developments on phospholipase C beta 1 signalling during erythroid differentiation and IGF-I induced mitogenesis. Adv Enzyme Regul. 1999;39:287–297. doi: 10.1016/s0065-2571(98)00025-9. [DOI] [PubMed] [Google Scholar]
- 10.Cocco L, Martelli A M, Gilmour R S, Ognibene A, Manzoli F A, Irvine R F. Changes in nuclear inositol phospholipids induced in intact cells by insulin-like growth factor I. Biochem Biophys Res Commun. 1989;159:720–725. doi: 10.1016/0006-291x(89)90054-5. [DOI] [PubMed] [Google Scholar]
- 11.Cocco L, Martelli A M, Gilmour R S, Ognibene A, Manzoli F A, Irvine R F. Rapid changes in phospholipid metabolism in the nuclei of Swiss 3T3 cells induced by treatment of the cells with insulin-like growth factor I. Biochem Biophys Res Commun. 1988;154:1266–1272. doi: 10.1016/0006-291x(88)90276-8. [DOI] [PubMed] [Google Scholar]
- 12.Crouch M F. Growth factor-induced cell division is paralleled by translocation of Gi alpha to the nucleus. FASEB J. 1991;5:200–206. doi: 10.1096/fasebj.5.2.1900794. [DOI] [PubMed] [Google Scholar]
- 13.Crouch M F, Osborne G W, Willard F S. The GTP-binding protein Giα translocates to kinetochores and regulates the M-G(1) cell cycle transition of Swiss 3T3 cells. Cell Signal. 2000;12:153–163. doi: 10.1016/s0898-6568(99)00080-7. [DOI] [PubMed] [Google Scholar]
- 14.Crouch M F, Simson L. The G-protein G(i) regulates mitosis but not DNA synthesis in growth factor-activated fibroblasts: a role for the nuclear translocation of G(i) FASEB J. 1997;11:189–198. doi: 10.1096/fasebj.11.2.9039962. [DOI] [PubMed] [Google Scholar]
- 15.Divecha N, Banfic H, Irvine R F. The polyphosphoinositide cycle exists in the nuclei of Swiss 3T3 cells under the control of a receptor (for IGF-I) in the plasma membrane, and stimulation of the cycle increases nuclear diacylglycerol and apparently induces translocation of protein kinase C to the nucleus. EMBO J. 1991;10:3207–3214. doi: 10.1002/j.1460-2075.1991.tb04883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drewes G, Lichtenberg-Kraag B, Doring F, Mandelkow E M, Biernat J, Goris J, Doree M, Mandelkow E. Mitogen activated protein (MAP) kinase transforms tau protein into an Alzheimer-like state. EMBO J. 1992;11:2131–2138. doi: 10.1002/j.1460-2075.1992.tb05272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Santos C S, Clarke J H, Divecha N. Phospholipid signalling in the nucleus. Een DAG uit het leven van de inositide signalering in de nucleus. Biochim Biophys Acta. 1998;1436:201–232. doi: 10.1016/s0005-2760(98)00146-5. [DOI] [PubMed] [Google Scholar]
- 18.Faenza I, Matteucci A, Manzoli F A, Billi A M, Aluigi M, Peruzzi D, Vitale M, Castorina S, Suh P G, Cocco L. A role for nuclear PLCβ1 in cell cycle control. J Biol Chem. 2000;275:30520–30526. doi: 10.1074/jbc.M004630200. [DOI] [PubMed] [Google Scholar]
- 19.Fields A P, Tyler G, Kraft A S, May W S. Role of nuclear protein kinase C in the mitogenic response to platelet-derived growth factor. J Cell Sci. 1990;96:107–114. doi: 10.1242/jcs.96.1.107. [DOI] [PubMed] [Google Scholar]
- 20.Frey R M, Saxon M L, Zhao X, Rollins A, Evans S S, Black J D. Protein kinase C isozyme-mediated cell cycle arrest involves induction of p21(waf1/cip1) and p27(kip1) and hypophosphorylation of the retinoblastoma protein in intestinal epithelial cells. J Biol Chem. 1997;272:9424–9435. doi: 10.1074/jbc.272.14.9424. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez F A, Seth A, Raden D L, Bowman D S, Fay F S, Davis R J. Serum-induced translocation of mitogen-activated protein kinase to the cell surface ruffling membrane and the nucleus. J Cell Biol. 1993;122:1089–1101. doi: 10.1083/jcb.122.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goss V L, Hocevar B A, Thompson L J, Stratton C A, Burns D J, Fields A P. Identification of nuclear beta II protein kinase C as a mitotic lamin kinase. J Biol Chem. 1994;269:19074–19080. [PubMed] [Google Scholar]
- 23.Hepler J R, Kozasa T, Smrcka A V, Simon M I, Rhee S G, Sternweis P C, Gilman A G. Purification from Sf9 cells and characterization of recombinant Gq alpha and G11 alpha. Activation of purified phospholipase C isozymes by G alpha subunits. J Biol Chem. 1993;268:14367–14375. [PubMed] [Google Scholar]
- 24.Hu E, Kim J B, Sarraf P, Spiegelman B M. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARγ. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 25.Kim C G, Park D, Rhee S G. The role of carboxyl-terminal basic amino acids in Gqα-dependent activation, particulate association, and nuclear localization of phospholipase C-β1. J Biol Chem. 1996;271:21187–21192. doi: 10.1074/jbc.271.35.21187. [DOI] [PubMed] [Google Scholar]
- 26.Lee S B, Shin S H, Hepler J R, Gilman A G, Rhee S G. Activation of phospholipase C-β2 mutants by G protein alpha q and beta gamma subunits. J Biol Chem. 1993;268:25952–25957. [PubMed] [Google Scholar]
- 27.Lenormand P, Brondello J M, Brunet A, Pouyssegur J. Growth factor-induced p42/p44 MAPK nuclear translocation and retention requires both MAPK activation and neosynthesis of nuclear anchoring proteins. J Biol Chem. 1998;142:625–633. doi: 10.1083/jcb.142.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lenormand P, Sardet C, Pages G, L'Allemain G, Brunet A, Pouyssegur J. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J Cell Biol. 1993;122:1079–1088. doi: 10.1083/jcb.122.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis T S, Shapiro P S, Ahn N G. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- 30.Manzoli L, Billi A M, Rubbini S, Bavelloni A, Faenza I, Gilmour R S, Rhee S G, Cocco L. Essential role for nuclear phospholipase C β1 in insulin-like growth factor I-induced mitogenesis. Cancer Res. 1997;57:2137–2139. [PubMed] [Google Scholar]
- 31.Manzoli L, Gilmour R S, Martelli A M, Billi A M, Cocco L. Nuclear inositol lipid cycle: a new central intermediary in signal transduction. Anticancer Res. 1996;16:3283–3286. [PubMed] [Google Scholar]
- 32.Maraldi N M, Cocco L, Capitani S, Mazzotti G, Barnabei O, Manzoli F A. Lipid-dependent nuclear signalling: morphological and functional features. Adv Enzyme Regul. 1994;34:129–143. doi: 10.1016/0065-2571(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 33.Martelli A M, Bareggi R, Cocco L, Manzoli F A. Stimulation of nuclear polyphosphoinositide synthesis by GTP-gamma-S: a potential regulatory role for nuclear GTP-binding proteins. Biochem Biophys Res Commun. 1996;218:182–186. doi: 10.1006/bbrc.1996.0032. [DOI] [PubMed] [Google Scholar]
- 34.Martelli A M, Billi A M, Gilmour R S, Neri L M, Manzoli L, Ognibene A, Cocco L. Phosphoinositide signaling in nuclei of Friend cells: phospholipase C β down-regulation is related to cell differentiation. Cancer Res. 1994;54:2536–2540. [PubMed] [Google Scholar]
- 35.Martelli A M, Cocco L, Bareggi R, Tabellini G, Rizzoli R, Ghibellini M D, Narducci P. Insulin-like growth factor-I-dependent stimulation of nuclear phospholipase C-β1 activity in Swiss 3T3 cells requires an intact cytoskeleton and is paralleled by increased phosphorylation of the phospholipase. J Cell Biochem. 1999;72:339–348. doi: 10.1002/(sici)1097-4644(19990301)72:3<339::aid-jcb3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 36.Martelli A M, Gilmour R S, Bertagnolo V, Neri L M, Manzoli L, Cocco L. Nuclear localization and signalling activity of phosphoinositidase C β1 in Swiss 3T3 cells. Nature. 1992;358:242–245. doi: 10.1038/358242a0. [DOI] [PubMed] [Google Scholar]
- 37.Matteucci A, Faenza I, Gilmour R S, Manzoli L, Billi A M, Peruzzi D, Bavelloni A, Rhee S G, Cocco L. Nuclear but not cytoplasmic phospholipase C β1 inhibits differentiation of erythroleukemia cells. Cancer Res. 1998;58:5057–5060. [PubMed] [Google Scholar]
- 38.Mazzoni M, Bertagnolo V, Neri L M, Carini C, Marchisio M, Milani D, Manzoli F A, Capitani S. Discrete subcellular localization of phosphoinositidase C beta, gamma and delta in PC12 rat pheochromocytoma cells. Biochem Biophys Res Commun. 1992;187:114–120. doi: 10.1016/s0006-291x(05)81466-4. [DOI] [PubMed] [Google Scholar]
- 39.Neri L M, Borgatti P, Capitani S, Martelli A M. Nuclear diacylglycerol produced by phosphoinositide-specific phospholipase C is responsible for nuclear translocation of protein kinase C-alpha. J Biol Chem. 1998;273:29738–29744. doi: 10.1074/jbc.273.45.29738. [DOI] [PubMed] [Google Scholar]
- 40.Pages G, Lenormand P, L'Allemain G, Chambard J C, Meloche S, Pouyssegur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci USA. 1993;90:8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park D, Jhon D Y, Lee C W, Lee K H, Rhee S G. Activation of phospholipase C isozymes by G protein beta gamma subunits. J Biol Chem. 1993;268:4573–4576. [PubMed] [Google Scholar]
- 42.Payne D M, Rossomando A J, Martino P, Erickson A K, Her J H, Shabanowitz J, Hunt D F, Weber M J, Sturgill T W. Identification of the regulatory phosphorylation sites in pp42/mitogen- activated protein kinase (MAP kinase) EMBO J. 1991;10:885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaeffer H J, Weber M J. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suh P G, Ryu S H, Choi W C, Lee K Y, Rhee S G. Monoclonal antibodies to three phospholipase C isozymes from bovine brain. J Biol Chem. 1988;263:14497–14504. [PubMed] [Google Scholar]
- 45.Suh P G, Ryu S H, Moon K H, Suh H W, Rhee S G. Inositol phospholipid-specific phospholipase (C) complete cDNA and protein sequences and sequence homology to tyrosine kinase-related oncogene products. Proc Natl Acad Sci USA. 1988;85:5419–5423. doi: 10.1073/pnas.85.15.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun B, Murray N R, Fields A P. A role for nuclear phosphatidylinositol-specific phospholipase C in the G2/M phase transition. J Biol Chem. 1997;272:26313–26317. doi: 10.1074/jbc.272.42.26313. [DOI] [PubMed] [Google Scholar]
- 47.Takei Y, Kurosu H, Takahashi K, Katada T. A GTP-binding protein in rat liver nuclei serving as the specific substrate of pertussis toxin-catalyzed ADP-ribosylation. J Biol Chem. 1992;267:5085–5089. [PubMed] [Google Scholar]
- 48.Thompson L J, Fields A P. βII protein kinase C is required for the G2/M phase transition of cell cycle. J Biol Chem. 1996;271:15045–15053. doi: 10.1074/jbc.271.25.15045. [DOI] [PubMed] [Google Scholar]
- 49.Topham K M, Bunting M, Zimmerman G A, McIntyre T M, Blackshear P J, Prescott S M. Protein kinase C regulates the nuclear localization of diacylglycerol kinase-zeta. Nature. 1998;394:697–700. doi: 10.1038/29337. [DOI] [PubMed] [Google Scholar]
- 50.Traverse S, Gomez N, Paterson H, Marshall C, Cohen P. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem J. 1992;288:351–355. doi: 10.1042/bj2880351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Xu A, Pearson R B, Cooper G J. Insulin and insulin antagonists evoke phosphorylation of P20 at serine 157 and serine 16 respectively in rat skeletal muscle. FEBS Lett. 1999;462:25–30. doi: 10.1016/s0014-5793(99)01496-9. [DOI] [PubMed] [Google Scholar]
- 52.Waskiewicz A J, Flynn A, Proud C G, Cooper J A. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997;16:1909–1920. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu D Q, Lee C H, Rhee S G, Simon M I. Activation of phospholipase C by the alpha subunits of the Gq and G11 proteins in transfected Cos-7 cells. J Biol Chem. 1992;267:1811–1817. [PubMed] [Google Scholar]
- 54.Xu A, Bellamy A R, Taylor J A. Expression of translationally controlled tumour protein is regulated by calcium at both the transcriptional and post-transcriptional level. Biochem J. 1999;342:683–689. [PMC free article] [PubMed] [Google Scholar]
- 55.York J D, Odom A R, Malviya R, Ives E B, Wente S R. A phospholipase C-dependent inositol polyphosphate kinase pathway required for efficient messenger RNA export. Science. 2000;285:96–100. doi: 10.1126/science.285.5424.96. [DOI] [PubMed] [Google Scholar]
- 56.Yue C, Ku C-Y, Liu M, Simon M I, Sanborn B M. Molecular mechanism of the inhibition of phospholipase C beta3 by protein kinase C. J Biol Chem. 2000;275:30220–30225. doi: 10.1074/jbc.M004276200. [DOI] [PubMed] [Google Scholar]
- 57.Zini N, Martelli A M, Cocco L, Manzoli F A, Maraldi N M. Phosphoinositidase C isoforms are specifically localized in the nuclear matrix and cytoskeleton of Swiss 3T3 cells. Exp Cell Res. 1993;208:257–269. doi: 10.1006/excr.1993.1245. [DOI] [PubMed] [Google Scholar]
- 58.Zini N, Neri L M, Ognibene A, Scotlandi K, Baldini N, Maraldi N M. Increase of nuclear phosphatidylinositol 4,5-bisphosphate and phospholipase C beta 1 is not associated to variations of protein kinase C in multidrug-resistant Saos-2 cells. Microsc Res Tech. 1997;36:172–178. doi: 10.1002/(SICI)1097-0029(19970201)36:3<172::AID-JEMT5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]