Abstract
High-fat diet (HFD) is the primary cause of metabolic syndrome associated chronic kidney disease. This study aimed to investigate the pathogenesis of HFD-induced kidney injury. ApoE−/− mice were fed with HFD and kidney damage was examined. In addition, HK-2 human renal proximal tubular epithelial cells were treated with fructose and receptor of advanced glycation end products (RAGE) siRNA. The results showed that HFD increased body weight, blood glucose and insulin resistance in ApoE−/− mice. The kidney damage was associated with increased oxidative stress and strong staining of RAGE and NF-κB in kidney tissues, as well as high serum levels of TNF-α, IL-1β and IL-6. Western-blot analysis showed that HFD increased the levels of RAGE, p-IκBα, p-NF-κB, bax, caspase-3 and caspase-9 but decreased the levels of Bcl-2 in kidney tissues. In HK-2 cells, fructose promoted the secretion of TNF-α, IL-1β and IL-6 and increased the levels of RAGE, p-IκBα, p-NF-κB, bax, caspase-3 and caspase-9, but decreased the levels of Bcl-2. Moreover, RAGE siRNA could attenuate increased levels of p-IκBα, p-NF-κB, bax, caspase-3 and caspase-9 while restore decreased levels of Bcl-2 in fructose-treated HK-2 cells. In conclusion, HFD causes kidney injury by promoting oxidative stress, inflammation and apoptosis possibly through the activation of RAGE/NF-κB pathway.
Keywords: high-fat diet, chronic kidney disease, inflammation, apoptosis, oxidative stress, RAGE
Introduction
With the improvement of living conditions, our dietary structure has changed. The irrational dietary structure, especially high-fat diet (HFD), is the primary cause of metabolic syndrome associated chronic kidney disease (CKD) [1]. The onset of dyslipidemia-related renal injury is mainly associated with renal hemodynamic changes, endothelium dysfunction, insulin resistance, chronic inflammation and oxidative stress [2]. However, the pathogenesis of CKD is complicated and not yet fully understood.
The patients who suffer from renal impairment or renal failure for more than 3 months are diagnosed as CKD. CKD is a growing public health concern and the rise in fatality rate of CKD is related to atherosclerosis, heart failure and sudden cardiac death [3]. Recent epidemiological studies have shown that HFD increased the risk of kidney diseases [4–6]. In particular, high fat high sugar dietary pattern was associated with significantly increased incident CKD [4].
Previous studies have suggested that HFD can lead to fat cell growth, adipose tissue inflammation, and the increase of inflammatory cytokines such as interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), which contribute to the development of CKD [7]. Excess reactive oxygen species (ROS) or ineffective antioxidant defense could cause kidney injury by lipid peroxidation, DNA lesion and protein modification. Superoxide dismutase (SOD) is the most important antioxidant enzymes, which removes the free radicals in the organisms. Glutathione exists in both thiol-reduced (GSH) form and disulfide-oxidized (GSSG) form. Glutathione peroxidase (GSH-Px) can catalyze GSH to GSSG in order to decrease its toxicity. Malondialdehyde (MDA) is an important indicator of lipid peroxidation and oxidative damage of cells [8]. It has been shown that the lipid oxidation and apoptotic progression are related to renal lesion in response to HFD stimulation [ 9]. However, the underlying mechanism by which HFD induces kidney injury is not fully understood.
ApoE gene knockout (ApoE−/−) mouse is a hyperlipidemia model widely used in the investigations of atherosclerosis, non-alcoholic cirrhosis and other injury. Therefore, in this study we investigated the pathogenesis of HFD-induced kidney injury using ApoE−/− mouse as in vivo model. In addition, fructose consumption may accelerate kidney injury [10]. Thus, we explored the underlying mechanism of HFD-induced kidney injury using human renal proximal tubular epithelial cell line HK-2 exposed to fructose as in vitro model.
Methods
Reagents
HFD was supplied from Jiakangyuan Technology (Beijing, China). Glucose and insulin detection kits were purchased from Sinopharm Chemical Reagent (Shanghai, China). TNF-α, IL-1β and IL-6 enzyme-linked immunosorbent assay (ELISA) kits were purchased from BioLegend Inc. (San Diego, CA, USA). MDA, SOD and GSH-Px detection kits were provided by Jiancheng Institute of Biotechnology (Nanjing, China). Dulbecco’s modified Eagle’s medium (DMEM) was obtained from Life Technologies (Carlsbad, CA, USA). All antibodies were provided by Cell Signaling Technology (Beverly, MA, USA).
Animals
Male apoE-deficient (ApoE−/−) mice (8-weeks old, weight 18–20 g) with the C57BL/6 J background were purchased from Yangzhou University and housed in specific pathogen-free conditions with 12:12 h light–dark cycles. The animals had free access to water and food. All the experimental procedures were carried out in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and were approved by Animal Use and Care Committee of Capital Medical University. ApoE−/− mice were fed with a HFD for 13 weeks, composed of 21% (wt/wt) fat from lard supplemented with .15% (wt/wt) cholesterol. During the last week of the feeding period, body weight was measured and the blood was collected. The blood was centrifuged at 3000 g for 10 min and then the serum samples were kept at −80°C. Serum glucose and insulin were examined with glucose kit and ELISA kit according to the manufacturer’s protocol. The kidney tissues were harvested for further analysis.
Oral glucose tolerance test
The mice were fasted for 12 h. They were orally treated with glucose (1.5 g/kg). Tail-vein blood samples were collected from 0 to 120 min. After centrifugation at 3000 g for 10 min at 4°C, the serum was collected for glucose assay by using commercial kit.
Histopathological analysis
The kidney tissues were excised and fixed with 10% neutral formalin. The renal specimens were embedded in paraffin blocks. The paraffin-embedded samples were cut into 5-μm sections with a microtome (Leica, Nussloch, Germany) and stained with hematoxylin and eosin (H&E). The sections were deparaffinized in xylene and the histological analysis was performed under a light microscope in a blinded manner. For immunohistochemical analysis, the sections were incubated with primary antibodies overnight and then with biotinylated secondary antibody. Finally, the slides were dehydrated and mounted with neutral gum and observed using a microscope.
Determination of oxidative stress markers
The oxidative stress markers MDA and antioxidants markers SOD, GSH-Px were measured in serum. The levels of SOD, GSH-Px and MDA were evaluated with commercial kits from Jiancheng Biological Engineering Institute.
Cell culture
Human proximal tubular epithelial cell line (HK-2) was purchased from the American Type Culture Collection (Manassas, VA, USA) and cultured in DMEM/F12 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 IU/ml streptomycin and 100 IU/ml penicillin (Amresco, Solon, USA) at 37°C in humidified atmosphere with 5% CO2. For transfection, the cells were seeded in 24-well plates at a density of 1 × 10 [5] cells/well and cultured overnight till the cells reached 70% confluency. Then, 200 nM siRNA for receptor of advanced glycation end products (RAGE) (Santa Cruz Biotechnology, Danvers, MA, USA) were mixed with Lipofectamine 2000 (Invitrogen) and incubated at room temperature for 30 min. Next, the mixtures were added into each well gently, and the cells were incubated at 37°C for 48 h. The cells were stimulated with 5 mM fructose for 48 h, and the dose of fructose was selected based on previous study [11].
Enzyme-linked immunosorbent assay
The contents of TNF-α, IL-1β and IL-6 in serum and supernatants of HK-2 cells were evaluated with enzyme-linked immunosorbent assay (ELISA) kits following the manufacturer’s instructions.
Western-blot analysis
The renal tissues and HK-2 cells were homogenized and lysed with a Radioimmunoprecipitation assay buffer (Beyotime, Nanjing, China). The concentrations of total protein were measured using BCA protein assay kit (Beyotime, Nanjing, China). Equal amount of protein extracts were resolved by polyacrylamide gel electrophoresis and then transferred onto polyvinylidene difluoride membranes (Millipore). The membrane was blocked using 5% non-fat milk in tris buffered saline (TBS) and then incubated with primary antibodies at 4°C overnight. Then, the blots were washed and incubated with horseradish peroxidase conjugated secondary antibodies for 1 h at room temperature. Thereafter, the antibody-reactive bands were visualized using enhance ECL plus reagent (KeyGEN Biotechnology, Nanjing, China).
Statistical analysis
All data were expressed as mean ± standard derivation (SD). Comparison between groups was assessed using the Student’s t-test or one-way ANOVA with Tukey test. Statistical significance was set at P < 0.05.
Results
HFD increased body weight, blood glucose and insulin resistance in ApoE −/− mice
To examine the effects of HFD on ApoE−/− mice, we measured the body weight and blood glucose and insulin levels in mice. HFD caused significant increase in body weight, and the levels of blood glucose and insulin were significantly higher in HFD-treated mice than in control mice (Fig. 1). These results indicated that HFD treatment increased insulin resistance and decreased glucose intolerance in ApoE−/− mice.
Figure 1.
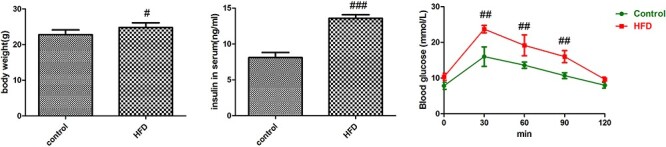
Evaluation of body weight, blood glucose and insulin resistance in HFD-treated ApoE−/− mice. Values were expressed as mean ± SD (n = 5). Compared with control: #P < 0.05, ##P < 0.01, ###P < 0.001.
HFD caused renal damage in ApoE −/− mice
Next, we examined the impairment of glomeruli and podocytes in the kidney of HFD-fed mice. Inflammatory cells were infiltrated into interstitium and various glomeruli cells proliferated in response to HFD stimulation. In control mice, tubular and glomerular structures were clear and tubular endothelial cells were in good arrangement. However, in HFD-treated mice, glomerular structure was unclear, tubular endothelial cells were swollen and had vacuoles (Fig. 2A). These data confirmed that HFD caused kidney damage.
Figure 2.
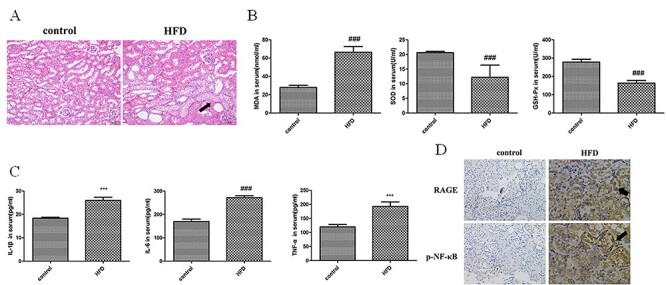
Evaluation of renal injury in HFD-treated ApoE−/− mice. (A) Histological analysis of kidney tissues in control and HFD-treated ApoE−/− mice. In control mice, tubular and glomerular structures were clear and tubular endothelial cells were in good arrangement. However, in HFD-treated mice, glomerular structure was unclear, tubular endothelial cells were swollen and had vacuoles (marked by arrow). (B) Evaluation of oxidative stress markers in serum of control and HFD-treated ApoE−/− mice. Values were expressed as mean ± SD (n = 5). Compared with control: #P < 0.05, ##P < 0.01, ###P < 0.001. (C) Evaluation of inflammatory cytokines in serum of control and HFD-treated ApoE−/− mice. Values were expressed as mean ± SD (n = 5). Compared with control: #P < 0.05, ##P < 0.01, ###P < 0.001. (D) Immunohistochemical staining of RAGE and NF-κB in kidney tissues of control and HFD-treated ApoE−/− mice. The strong staining areas were marked by arrows.
Next, lipid peroxidation was determined by assessing the levels of MDA, SOD and GSH-Px. As shown in Figure 2B, HFD stimulation significantly decreased SOD, GSH-Px activities and increased the MDA content in ApoE−/− mice compared to control group.
To evaluate inflammation in HFD-treated ApoE−/− mice, the levels of inflammatory cytokine including TNF-α, IL-1β and IL-6 were measured. As shown in Figure 2C, the contents of TNF-α, IL-1β and IL-6 in serum significantly increased in HFD-fed ApoE−/− mice. These data suggested that HFD increased the production of inflammatory cytokines in ApoE−/− mice.
RAGE and NF-κB are important mediators of inflammation. Immunohistochemical staining showed that the staining densities of RAGE and NF-κB were significantly stronger in kidney tissues of HFD-treated mice compared with control mice (Fig. 2D). Taken together, these results suggested that HFD induced oxidative stress and inflammation to cause kidney damage.
RAGE/NF-κB signaling mediated apoptosis in kidney tissues of HFD-fed ApoE −/− mice
To confirm the role of RAGE/NF-κB signaling in HFD induced kidney damage, we detected the expression of RAGE, p-IκBα, IκBα, p-NF-κB, NF-κB, bax, bcl-2, caspase-3 and caspase-9 by western-blot analysis. HFD increased the levels of RAGE, p-IκBα, p-NF-κB, bax, caspase-3 and caspase-9, but decreased the levels of Bcl-2 in kidney tissues compared with control group (Fig. 3). These data suggested that HFD may activate RAGE/NF-κB signaling to induce apoptosis in kidney tissues.
Figure 3.
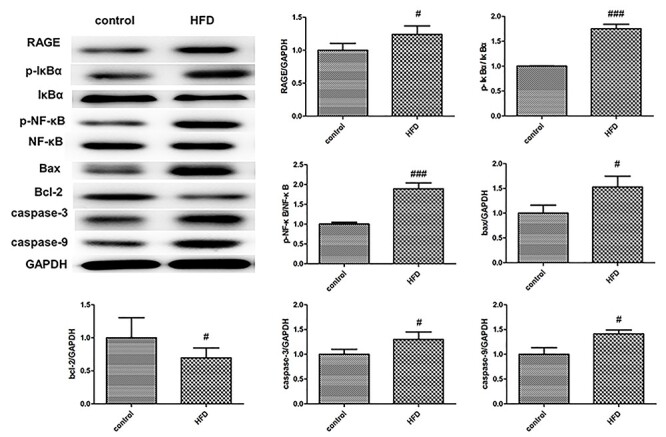
Western-blot analysis of RAGE/NF-κB signaling components and apoptosis-related proteins in kidney tissues of HFD-treated ApoE−/− mice. GAPDH was loading control. Values were expressed as mean ± SD (n = 5). Compared with control: #P < 0.05, ##P < 0.01, ###P < 0.001.
RAGE-activated inflammation in HK-2 cells
To reveal the mechanism of RAGE-mediated inflammation, we used HK-2 kidney cells as in vitro model. We found that the activation of RAGE by fructose led to increased secretion of inflammatory factors TNF-α, IL-1β and IL-6 by HK-2 cells, but RAGE siRNA could attenuate the secretion of inflammatory factors (Fig. 4).
Figure 4.
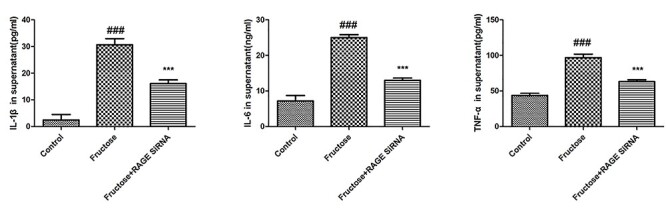
Evaluation of inflammatory cytokines in supernatant of fructose-treated HK-2 cells. Values were expressed as mean ± SD (n = 5). Compared with control: #P < 0.05, ##P < 0.01, ###P < 0.001.
RAGE-activated NF-κB signaling in HK-2 cells
Furthermore, we evaluated the expression of components of RAGE/NF-κB signaling and apoptosis-related proteins in HK-2 cells. Fructose increased the levels of RAGE, p-IκBα, p-NF-κB, bax, caspase-3 and caspase-9, but decreased the levels of Bcl-2 in HK-2 cells. However, RAGE siRNA could attenuate increased levels of p-IκBα, p-NF-κB, bax, caspase-3 and caspase-9, but restore decreased levels of Bcl-2 (Fig. 5). Collectively, these results indicated that the activation of RAGE/NF-κB signaling may promote the apoptosis in HK-2 cells.
Figure 5.
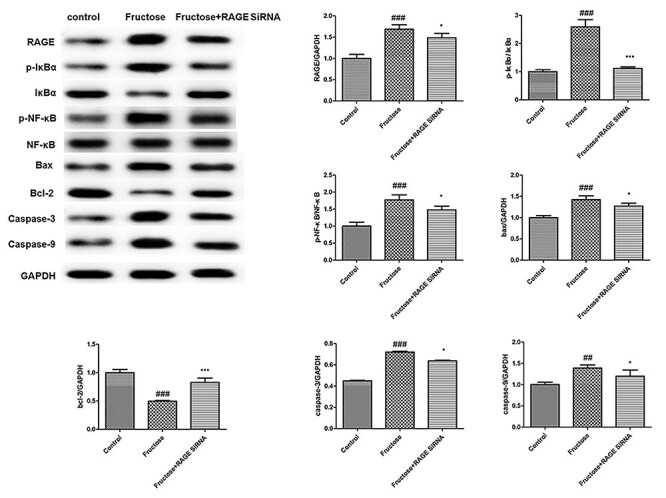
Western-blot analysis of RAGE/NF-κB signaling components and apoptosis-related proteins in HK-2 cells. GAPDH was loading control. Values were expressed as mean ± SD (n = 5). Compared with control: #P < 0.05, ##P < 0.01, ###P < 0.001.
Discussion
In this study, we demonstrated that HFD-fed ApoE−/− mice exhibited increased lipid accumulation, body weight gain, serum blood glucose and kidney injury, which may be related to HFD-induced oxidative stress and inflammatory response. In addition, we found increased secretion of inflammatory cytokines and the activation of RAGE/NF-κB signaling in HK-2 cells exposed to fructose.
The imbalance between antioxidant system and free radical production contributes to lipid peroxidation and renal injury. It is well established that HFD would alter the antioxidant/oxidative imbalance in kidney tissues and induce lipid peroxidation [12]. The activities of antioxidant enzymes decrease in response to HFD, and MDA is the end product from lipid breakdown and serves as a reliable index sensitive to oxidative stress [13]. Our results confirmed that HFD-induced oxidative stress in the kidney tissues of ApoE−/− mice, which may contribute to kidney injury.
RAGE is a pattern recognition receptor that participates in the innate immune response, but upon binding to legends such as AGE, RAGE could activate downstream component NF-κB transcription factor to induce inflammatory response and kidney diseases [14]. It has been widely acknowledged that inflammatory cytokines are important modulators of renal lesion [7]. The inflammatory cytokines, such as IL-1β, IL-6 and TNF-α, are responsible for initiating and amplifying inflammatory reactions and are overexpressed during HFD-induced kidney injury [15, 16]. The phosphorylation and degradation of inhibitory protein IκBα is required for the activation of NF-κB. Our results demonstrated that HFD treatment remarkably upregulated the expression of RAGE, p-IκBα and NF-κB, while the silencing of RAGE in HK-2 cells effectively attenuated the activation of NF-κB and the secretion of inflammatory cytokines.
Furthermore, HFD activates apoptotic process in kidney tissues [17]. Our data showed that HFD administration upregulated caspase-3, caspase-9, bax and downregulated Bcl-2. Nevertheless, RAGE siRNA evidently increased Bcl-2 level and decreased caspase-3, caspase-9, bax levels. Increased mitochondrial oxidative stress has been proposed as an important mechanism in the pathogenesis of kidney disease [18]. Interestingly, a recent study reported that HFD promoted renal injury by inducing oxidative stress and mitochondrial dysfunction [ 19]. These results suggest that HFD induces oxidative stress to activate RAGE/NF-κB pathway and promote the apoptosis in kidney tissues, ultimately leading to renal injury. It should be pointed out that although we observed significant differences in inflammation and apoptosis markers between RAGE knockdown cells and fructose-treated cells, there were differences in inflammation and apoptosis markers between RAGE knockdown cells and control cells. Therefore, it is possible that other factors in addition to RAGE contribute to inflammation mediated by high fat.
This study has some limitations. ApoE−/− mouse is an animal model of dyslipidemia, which may have kidney injury. To better assess the effects of HFD on kidney injury, it would be important to use normal C57BL/6 J mice as control in future investigations.
In summary, we demonstrated that HFD caused kidney injury by promoting oxidative stress and inflammatory reactions possibly through the activation of RAGE/NF-κB pathway. Our findings suggest that RAGE/NF-κB pathway is a potential target for the prevention and treatment of HFD-induced kidney disease.
Authors’ Contributions
J.X. designed the study and wrote the manuscript. Y. Hong, Y. Hu, Y.S. and J.S. performed the experiments and analyzed the data. All authors read and approved the final manuscript.
Contributor Information
Yin Hong, Department of Health Management, Beijing Tian Tan Hospital, Capital Medical University, Beijing 100050, China.
Yue Hu, Department of Neurology, Affiliated Hospital of Yangzhou University, Yangzhou, Jiangsu 225001, China.
Yong-an Sun, Department of Neurology, Peking University First Hospital, Beijing 10068, China.
Jian-quan Shi, Department of Neurology, Cognitive Center, Beijing Tian Tan Hospital, Capital Medical University, Beijing 100070, China.
Jun Xu, Department of Neurology, Cognitive Center, Beijing Tian Tan Hospital, Capital Medical University, Beijing 100070, China.
Availability of Data and Material
All data and material are available upon request.
Competing Interests
The authors declare no competing interests.
Funding
We received no funding for this study.
References
- 1. Du J, Li JM. BAS/BSCR23 apocynin treatment reduces high-fat diet-induced obesity and hypertension but has no significant effect on hyperglycaemia. Heart 2010;96(17):e19. [Google Scholar]
- 2. Giraud-Billoud M, Fader CM, Agüero R, Ezquer F, Ezquer M. Diabetic nephropathy, autophagy and proximal tubule protein endocytic transport: a potentially harmful relationship. Biocell 2018;42(2):35–40. [Google Scholar]
- 3. Sánchez E, Betriu À, Arroyo D. et al. Skin autofluorescence and subclinical atherosclerosis in mild to moderate chronic kidney disease: a case-control study. PLoS One 2017;12(1):e0170778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Asghari G, Momenan M, Yuzbashian E. et al. Dietary pattern and incidence of chronic kidney disease among adults: a population-based study. Nutr Metab (Lond) 2018;15:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Westing AC, Küpers LK, Geleijnse JM. Diet and kidney function: a literature review. Curr Hypertens Rep 2020;22(2):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Quintela BCSF, Carioca AAF, de Oliveira JGR, Fraser SDS, da Silva Junior GB. Dietary patterns and chronic kidney disease outcomes: a systematic review. Nephrology (Carlton) 2021;26(7):603–612. [DOI] [PubMed] [Google Scholar]
- 7. Cantero-Navarro E, Rayego-Mateos S, Orejudo M. et al. Role of macrophages and related cytokines in kidney disease. Front Med (Lausanne) 2021;8:688060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gai Z, Wang T, Visentin M, Kullak-Ublick GA, Fu X, Wang Z. Lipid accumulation and chronic kidney disease. Nutrients 2019;11(4):722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo H, Li H, Wang B. et al. Protective effects of glucagon-like peptide-1 analog on renal tubular injury in mice on high-fat diet. Cell Physiol Biochem 2017;41(3):1113–1124. [DOI] [PubMed] [Google Scholar]
- 10. Wyatt CM, Reeves WB. The sweetest thing: blocking fructose metabolism to prevent acute kidney injury? Kidney Int 2017;91(5):998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gersch MS, Mu W, Cirillo P, Reungjui S, Zhang L, Roncal C, Sautin YY, Johnson RJ, Nakagawa T. Fructose, but not dextrose, accelerates the progression of chronic kidney disease. Am J Physiol Renal Physiol 2007;293(4):F1256–61. [DOI] [PubMed] [Google Scholar]
- 12. Pan QR, Ren YL, Zhu JJ. et al. Resveratrol increases nephrin and podocin expression and alleviates renal damage in rats fed a high-fat diet. Nutrients 2014;6(7):2619–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen T, Wang R, Jiang W. et al. Protective effect of astragaloside IV against paraquat-induced lung injury in mice by suppressing rho signaling. Inflammation 2016;39(1):483–492. [DOI] [PubMed] [Google Scholar]
- 14. Wu XQ, Zhang DD, Wang YN. et al. AGE/RAGE in diabetic kidney disease and ageing kidney. Free Radic Biol Med 2021;171:260–71. [DOI] [PubMed] [Google Scholar]
- 15. Na E, Choi M, Park I, Lim S. Effect of Black Sea bream extracts on cytokine production in lipopolysaccharide-induced inflammation. Biocell 2020;44(2): 193–199. [Google Scholar]
- 16. Jang WY, Jeong J, Kim S. et al. Serum amyloid A1 levels and amyloid deposition following a high-fat diet challenge in transgenic mice overexpressing hepatic serum amyloid A1. Appl Physiol Nutr Metab 2016;41(6):640–648. [DOI] [PubMed] [Google Scholar]
- 17. Lian H, Cheng Y, Wu X. TMEM16A exacerbates renal injury by activating P38/JNK signaling pathway to promote podocyte apoptosis in diabetic nephropathy mice. Biochem Biophys Res Commun 2017;487(2):201–208. [DOI] [PubMed] [Google Scholar]
- 18. Ricciardi CA, Gnudi L. Kidney disease in diabetes: from mechanisms to clinical presentation and treatment strategies. Metabolism 2021; 21:154890. [DOI] [PubMed] [Google Scholar]
- 19. Sun Y, Ge X, Li X, He J, Wei X, Du J, Sun J, Li X, Xun Z, Liu W, Zhang H, Wang ZY, Li YC. High-fat diet promotes renal injury by inducing oxidative stress and mitochondrial dysfunction. Cell Death Dis 2020;11(10):914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and material are available upon request.


