Abstract
Triclosan (TCS) is widely used and it bioaccumulates in humans. We found that TCS induced DNA damage in TK6 cell in our previous work. Herein, we performed a pilot assay of the TK6 cell/TK gene (TK+/−) mutation assay without metabolic activation for 24 h and found that TCS significantly induced mutation frequency. We further investigated the dose–response toxicity and genotoxicity of TCS. We combined the newly developed Pig-a gene mutation assay with bone marrow micronucleus (MN) test in a 19-day short-term study. ICR mice were administered orally with TCS at six dose levels from 0 to1000 mg/kg/day. We quantitatively assessed the dose–response relationships for the Pig-a assay, MN test, and organ coefficient data for possible points of departure (PoDs) by estimating the benchmark dose using PROAST software. We did not observe elevated Pig-a mutant frequency or MN frequency in TCS-treated mice. But a dose-dependent and statistically significant increase in liver organ coefficient data was observed. The PoD and acceptable daily intake based on organ toxicity were further developed and no greater than 1.82 and 0.00182 mg/kg/day, respectively, indicating that the toxicity of TCS may has been underestimated in previous studies and greater attention should be paid to low-level TCS exposure.
Keywords: triclosan, genotoxicity, Pig-a assay, micronucleus test, points of departure (PoDs)
Introduction
Triclosan (TCS, CAS No. 3380-34-5) is a chlorinated, broad-spectrum antimicrobial chemical which is present in thousands of consumer and industrial products, including toothpastes, antibacterial soaps, deodorants, and cosmetics. As a result, a large amount of TCS has been discharged into the environment, and it has been detected in a variety of matrices worldwide, such as multiple bodies of water and sediment [3, 36, 40, 57, 62]. Humans are exposed to TCS via contact with personal hygiene products, food, drinking water, and many other sources. TCS is persistent and bioaccumulates, and biomonitoring studies report that TCS can be detected in human urine, plasma, breast milk, and other tissues [2, 8, 13, 21]. Historically, TCS was regarded as well tolerated and safe. More recently, however, animal and in vitro studies have identified TCS as an endocrine disruptor which is associated with reproductive and developmental impacts [1, 38, 52]; but these the possible effects of TCS have not been sufficiently evaluated in human studies [22, 58]. Thus, the toxicity of TCS has become a concern for environmental and human health, and several authorities have issued limits on the use of TCS [17, 20, 25].
Since most human carcinogens are genotoxic, TCS has been tested extensively in genetic toxicology assays to evaluate its potential carcinogenicity. TCS has been negative in most studies, including Ames test, in vivo chromosome aberration (CA) test, and in vivo micronucleus (MN) test [14]. But one in vitro CA test was positive [27], and a study conducted in 1978 also observed that TCS induced somatic mutations in vivo in the mouse spot test [18]. However, the Organization for Economic Cooperation and Development deleted Test Guideline 484 “Genetic Toxicology: Mouse Spot Test” in 2014 because of its insensitivity and the large number of animals and high cost necessary to conduct the assay. Another study also indicated that TCS reduced the levels of global DNA methylation in human hepatocellular carcinoma HepG2 cell, which has an association with liver tumor induction [42]. We also performed Ames test, Comet assay, and MN test in TK6 cell in our previous study and only found that TCS induces DNA damage [9]. Moreover, the recent literatures reported that TCS exhibited a genotoxic response in multiple species of aquatic organisms with increasing MN frequency and inducing DNA damage in Comet assay [6, 10, 47, 59].
In addition, TCS was identified as a carcinogen in an early rodent study [41] that reported an increase in mouse liver tumors [4], and long-term TCS exposure enhanced liver fibrogenesis and tumorigenesis [63]. Although these suggestive results produce ambiguity regarding the genotoxicity and carcinogenicity of TCS, so far the International Agency for Research on Cancer has not classified TCS as a human carcinogen, and no further action has been taken on the carcinogenicity assessment of TCS.
Gene mutation is considered to be responsible for the initial and/or critical steps in carcinogenesis [5, 48]; and there is considerable evidence of a positive correlation between the mutagenicity of chemicals in vivo and their carcinogenicity in long-term studies with animals. We performed TK gene (TK+/−) mutation assay in TK6 cell without metabolic activation (S9) for 24 h and found TCS significantly induced mutation frequency (shown in Supplementary Fig. S1). We further speculate that TCS may also cause gene mutation in vivo. However, no in vivo mutagenicity testing has been performed on TCS since the early 1980s, and the existing suggestive observations on the genotoxicity and carcinogenicity of TCS indicate that further work on evaluating its carcinogenic hazard to humans, using more modern and potentially more sensitive methods, is warranted.
The rodent erythrocyte Pig-a assay is a recently developed method to detect gene mutation. The endogenous X-linked phosphatidylinositol glycan class A gene (Pig-a) is present in only one functional copy per cell and codes for an enzyme involved in an early step in the biosynthesis of glycosylphosphatidylinositol (GPI) anchors [34, 35]. Thus, inactivating mutations in the Pig-a gene results in loss of GPI anchors and GPI-anchored proteins on the exterior of the cytoplasmic membrane, and GPI-deficient erythrocytes can be rapidly identified by flow cytometry [44, 45]. Over 100 chemicals have been evaluated in the Pig-a assay, with results displaying remarkable sensitivity and specificity for identifying in vivo mutagens [51]. The assay is minimally invasive (usually performed on a drop of blood from the rodent tail) and can be readily integrated into other rodent studies, including acute, sub-chronic, and chronic general toxicology studies. Both of these characteristics address the 3Rs (replace/reduce/refine) precepts for animal welfare.
In order to comprehensively understand the genotoxic potential of TCS, especially to clarify the equivocal in vivo mutagenicity of TCS, we have dosed male ICR mice with TCS over 5 consecutive days, followed by a 10-day treatment-free period, and then a second treatment over 3 consecutive days prior to tissue sampling (Fig. 1). We designed this experimental scheme to combine two endpoints: determining gene mutation with the erythrocyte Pig-a assay and chromosome alteration with the bone marrow MN test. We chose six closely spaced dose levels (separated by a factor of 2). The dose–response relationships for Pig-a mutant frequency (MF), MN frequency, and relative organ weight data were analyzed for possible points of departure (PoDs) using the benchmark-dose (BMD) software PROAST. In addition, we also performed a pilot study on female C57BL/6 mice treated with TCS for 80 days to better understand the mutagenicity of TCS after a long-term exposure. This is the first report on the genotoxicity of TCS performed by integrating two different genetic endpoints and then evaluating the data for identifying PoDs using dose response modeling. This is also is the first study using the new high-throughput Pig-a assay to assess the mutagenicity of TCS.
Figure 1.
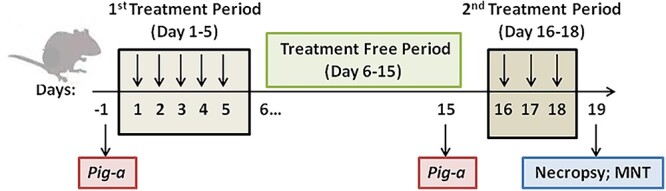
Male ICR mice 19-day study: overview of the experimental design (sampling time points and endpoints).
Material and Methods
Reagents
TCS (CAS NO. 3380-34-5) and N-methyl-N-nitrosourea (MNU; CAS No. 759-73-9) were purchased from Sigma-Aldrich. TCS was prepared in olive oil, which was used as a vehicle, and MNU (positive control) was freshly prepared in phosphate-buffered saline, which was previously adjusted to pH 6.0.
Animal husbandry and welfare
The animal experimental procedures were approved by the Institutional Animal Care and Use Committee of the Shanghai Jiao Tong University School of Medicine, and the animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals. Male ICR mice, aged 6–7 weeks, were obtained from Shanghai Lingchang Biotechnology Co Ltd. The mice were group-housed and maintained in a 12-h light/dark cycle at 20–26°C and 40–70% humidity, with ≥15 air changes/h. The mice were fed with standard laboratory diet purchased from XIETONG ORGANSIM (Jiangsu, China) and were provided water ad libitum.
Study design and treatment
Six male ICR mice per group were assigned randomly to experimental groups. The groups were identified by cage card, and individual animals were identified by marking a number on the tail. The mice were dosed via oral gavage at a volume of 10 ml/kg body weight according to the most recent recorded body weight. As shown in Fig. 1, TCS was administered once per day at dose levels of 0 (vehicle control), 31.25, 62.5, 125, 250, 500, and 1000 mg/kg/day; MNU (positive control) was administered at 40 mg/kg/day over 5 consecutive days (Days 1–5), followed by a treatment-free period of 10 days (Days 6–15). Blood for the Pig-a assay was sampled before (Day −1) and after (Day 15) the dosing period. All groups were dosed an additional 3 consecutive days (Days 16–18) prior to necropsy on Day 19 at which time bone marrow was collected and was used to prepare slides for conducting the MN assay.
Body weights and tissue collection
Body weights were recorded for all animals on Days −1, 1, 2, 3, 4, 5, 10, 15, and 18; in addition, body weights were determined immediately prior to dosing and at necropsy on Day 19. The mice were fasted for 16 h prior to necropsy; at necropsy, heart, liver, spleen, lung, kidneys, testis, and epididymis were removed and weighed. Organ-to-body weight ratios were calculated as organ coefficients.
RBC Pig-a assay
We purchased FITC-anti-mouse CD24, APC anti-mouse TER-119 and PE rat anti-mouse CD71 from BD Pharmingen (catalog numbers # 553261, #557909, and # 553267, respectively). We used a BD Accuri C6 flow cytometer with C6 v1.0.264.21 software in this study.
We performed the RBC Pig-a assay to detect Pig-a MFs, and the percentage of reticulocytes among total erythrocytes (%RET) was used to evaluate the toxicity of TCS to the erythropoietic system. The blood sample labeling and flow cytometer gating strategy were the same as described in our previous study [12]. Briefly, 1 μl of EDTA-anticoagulant-preserved whole blood was labeled with 2 μl FITC-anti-mouse CD24 (or 5 μl rat anti-mouse CD71 for the %RET assay) and 5 μl of APC anti-mouse TER-119. About 2 × 106 TER-119-labeled RBCs were evaluated for CD24 expression to estimate Pig-a MFs; the frequency of RBCCD24- cells was recorded as Pig-a MF−. Approximately, 2 × 105 TER-119-labeled RBCs were evaluated for CD71-PE expression to estimate %RET.
Bone marrow erythrocyte MN assay
Bone marrow cells were obtained from the femurs of mice immediately following euthanasia. The bone marrow from a femur was mixed with a drop of fetal bovine serum, then smeared onto glass slides. The smears were fixed with methanol, air-dried, and stained with freshly prepared 10% Giemsa for 10–15 min. The slides then were flushed with water and were air-dried. The frequency of micronucleated polychromatic erythrocytes (MN-PCE %) was determined in 2000 polychromatic erythrocytes (PCE). The proportion of PCE (%PCE) among total erythrocytes [PCE + normochromatic erythrocytes (NCEs)] was determined for each mouse by counting a total of at least 2000 erythrocytes. %PCE was used as a metric to evaluate bone marrow toxicity.
Pilot study: Pig-a MF in female C57BL/6 mice treated with TCS for 80 days
Female C57BL/6 mice were dosed with TCS via their drinking water starting at 8 weeks of age. About 0.1% DMSO + 0.5% Tween80 was used as the vehicle solvent. Two mice were allocated to a negative control (water) group, five mice for vehicle control group, and six mice per group were administered with TCS at dose levels (a cage average based on water consumption) of 1, 10, and 50 mg/kg/day which. Blood samples collected on Day 80 were used to perform the RBC Pig-a assay.
Determination of PoDs
PoD values were calculated using PROAST software (version 67.0, available at https://www.rivm.nl/proast). For Pig-a MF and MN-PCE % data, critical effect sizes (CES) of 0.1 and 0.5 were used to calculate PoDs; CESs of 0.05 and 0.1 were used for organ coefficient data. The CES represents a minimum effect size that has biological significance and was used to define a lower bound (CEDL) and an upper bound (CEDU). The CEDL was use as a PoD.
Statistical methods
Pig-a MF and MN-PCE % were log (10)-transformed to approximate normal distributions; when data points with zero values occurred in a data set, a constant of 0.01 was added to all values in the data set to enable transformation. The transformed Pig-a MF and MN-PCE % data and the organ coefficient data were analyzed using one-way analysis of variance (ANOVA) followed by Dunnett’s multiple test for pairwise comparisons to evaluate differences between the responses for each TCS treatment groups and the vehicle control. The Wilcoxon matched-pairs signed rank test was used to compare differences between Pig-a MFs on Day −1 and Day 15. In addition, a one-way ANOVA for trend was used to detect dose-dependent responses for each endpoint. All statistical tests were two-tailed and used P < 0.05 to determine the statistical significance. All the statistical analyses were performed and graphs were prepared using GraphPad Prism (Prism Software, version 8.02, Nashville, TN).
Results
In the 19-day study, no mortality or clinical signs of toxicity were observed among the ICR mice dosed with 0–500 mg/kg/day TCS. At the highest TCS dose of 1000 mg/kg/day, however, five of six ICR mice were found dead during the first cycle of TCS treatment, including two mice found dead on Day 3 before the third administration; two mice and one mouse were found dead on Day 5 and Day 6, respectively, while hypothermia was observed in these mice 4 h after the administration on Day 5; and only one mice was survived. The one surviving mouse from the 1000 mg/kg/day group was still dosed in the second treatment cycle and showed no clinical signs of toxicity during the entire study period. We did not use data from this mouse to evaluate differences between groups.
Body weight
Mice treated with 0–500 mg/kg/day TCS showed a slight increase in body weight gain, while the body weight gain of the surviving mice dosed with 1000 mg/kg/day appeared to decrease with time (Fig. 2).
Figure 2.
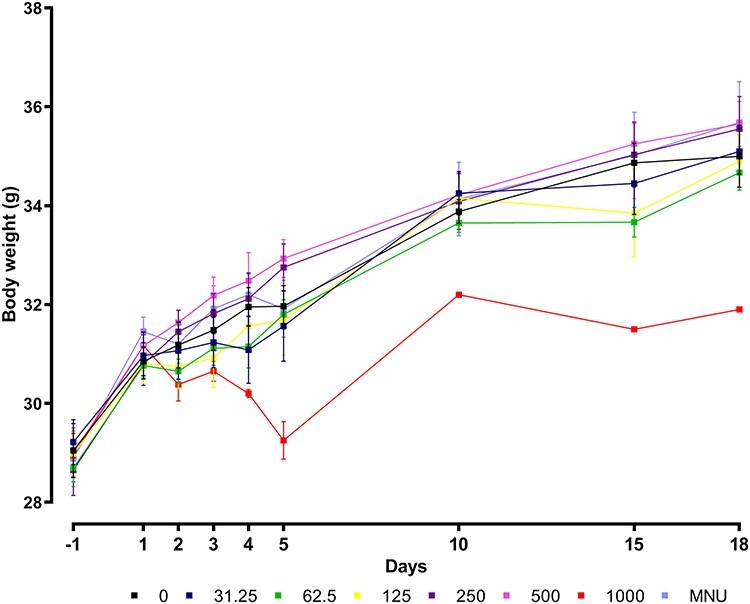
Body weight gain of mice dosed with 0–1000 mg/kg/day Triclosan dose levels and 40 mg/kg N-methyl-N-nitrosourea (MNU). Each data point represents the mean per group (n = 6, except group 1000 mg/kg/day from day 4 onwards: n = 3 and from day 6 onwards: n = 1).
Organ coefficients for liver displayed significant dose-dependent increases
No increase or decrease was observed in organ coefficients (organ weight in comparison with animal weight) for heart, spleen, lung, kidneys, testis, or epididymis. The coefficients for liver, however, were significantly increased for groups dosed with 31.25–500 mg/kg/day TCS in comparison with the 0 mg/kg/day group. In addition, a one-way ANOVA for trend indicated that the increases displayed a significant trend (linear trend significant P < 0.0001, R2 = 0.9370) (Fig. 3).
Figure 3.
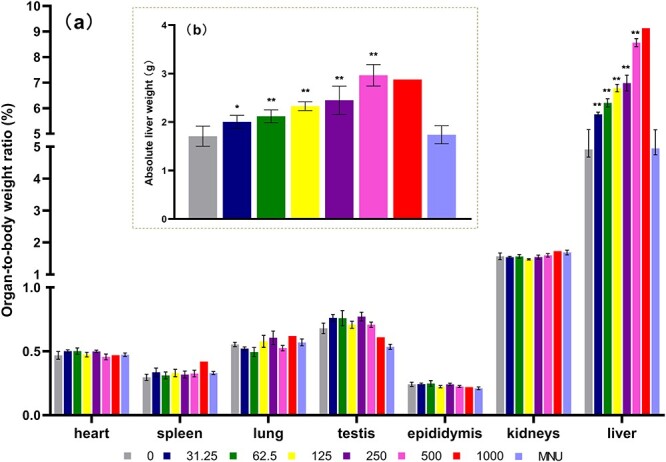
Organ-to-body weight ratio (%) of heart, spleen, lung, testis, epididymis, kidneys and liver, treated with Triclosan dose levels (0–1000 mg/kg/day) and N-methyl-N-nitrosourea (MNU) 40 mg/kg (a) and absolute liver weight of all groups (b). Each data point represents the mean per group (n = 6, except group 1000 mg/kg/day from day 4 onwards: n = 3 and from day 6 onwards: n = 1). Statistically significant difference to control group (*P < 0.05 and **P < 0.01) based on one-way analysis of variance (ANOVA) followed by Dunnett’s multiple test.
No increase in Pig-a MF in TCS-treated male ICR mice
Pig-a MFs and %RET were evaluated before (Day −1) and after (Day 15) the administration of TCS or MNU (Fig. 4). No increase in Pig-a MF was found for the TCS-treated groups, while the MNU group exhibited a significant increase in Pig-a RBC MF (MNU Day 15 vs. MNU Day −1, P = 0.0313; MNU Day 15 vs. vehicle control Day 15, P = 0.0022). Compared with the Day 1 data, %RET increased at Day 15 for all TCS-treated groups, the vehicle control, and the MNU positive control group; however, only the MNU group exhibited a significant increase (MNU Day 15 vs. MNU Day −1, P < 0.0001; MNU Day 15 vs. vehicle control Day 15, P = 0.0021).
Figure 4.
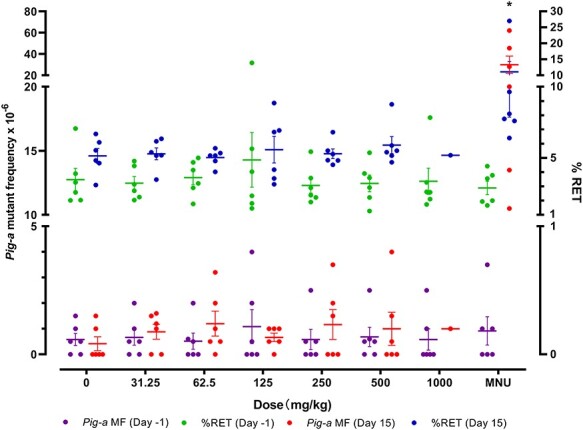
Pig-a mutant frequencies and % reticulocytes in red blood cells from male ICR mice treated with 0–1000 mg/kg/day Triclosan and 40 mg/kg N-methyl-N-nitrosourea (MNU). The Wilcoxon matched-pairs signed rank test was used to compare differences between Pig-a MFs on Day -1 and Day 15. Statistically significant difference from Day -1 data: *P < 0.05.
No increase in MN frequency in TCS-treated mice
As shown in Fig. 5,there was a slight dose-related decrease of %PCE in TCS-treated mice; however, these differences were not significant. No increase in MN-PCE % was found in the TCS-dosed groups, while the MNU group exhibited a significant increase in MN-PCE % (MNU vs. vehicle control, P = 0.0022); the %PCE for MNU group slightly decreased, but the decrease was not significant.
Figure 5.
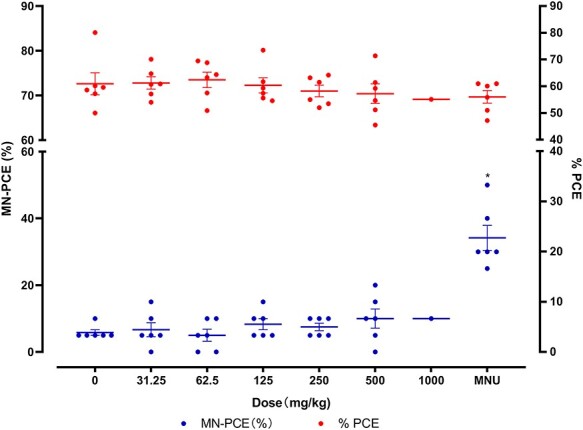
The frequency of micronucleated polychromatic erythrocytes (MN-PCE %) and the proportion of PCE (%PCE) in male ICR mice treated with 0–1000 mg/kg/day Triclosan and 40 mg/kg N-methyl-N-nitrosourea (MNU). Statistically significant difference to control group (*P < 0.05) based on one-way analysis of variance (ANOVA) followed by Dunnett’s multiple test.
Pilot study: no increase in Pig-a MF in TCS-treated female C57BL/6 mice
Pig-a MF and %RET were evaluated on Day 80 of a pilot study evaluating the toxicity of TCS administered in the drinking water. There was a small but significant decrease in %RET for mice dosed with TCS (P = 0.0249), while no increase in Pig-a MF was observed (shown in Supplementary Fig. S2).
Determination of PoDs
PoDs were not calculated using Pig-a MF or MN-PCE % data or organ coefficients for heart, spleen, lung, kidneys, testis, or epididymis, since these responses were not significantly different from the vehicle control group.
For the liver organ coefficient data, setting CES at 0.05, the CED, the lowest CEDL, and highest CEDU were 1.2, 0.213, and 4.78 mg/kg/day, respectively; while setting CES at 0.1, the CED, the lowest CEDL, and highest CEDU were 4.8, 1.82, and 18.4 mg/kg/day, respectively.
Thus, PoD values for TCS, as estimated by the CEDL, were 0.213 mg/kg/day (CES: 0.05) or 1.82 mg/kg/day (CES: 0.1) based on hepatotoxicity in male ICR mice.
Discussion
In the present study, we found no indication that TCS-dosed mice exhibited elevated Pig-a MFs or MN frequencies relative to vehicle controls. Thus, the results with these two genetic endpoints indicate that TCS neither has mutagenicity nor causes chromosome damage in mice. The MN test results reported herein are consistent with the earlier reports [11, 28]. Our study is the first to use the Pig-a assay to detect the mutagenicity of TCS. Two early studies of in vivo gene mutation using the mouse spot test were inconsistent: one reported that TCS induced mutations [18], and the other did not report so [50]. Hence, the results of our two Pig-a assays (19-day study and 80-day pilot study) provide evidence that TCS does not induce somatic mutation in mice. Note that a weakness of the Pig-a assay, especially when evaluating negative responses, is that the test substance or its metabolites must reach the bone marrow of the test animal for a valid test. The reduction in %RET noted in the 80-day pilot study indicates that biologically significant levels of TCS and/or its metabolites reached the bone marrow, which supports the finding that TCS is negative for in vivo gene mutation.
TCS has also produced inconsistent results in two in vitro chromosomal aberration (CA) tests (unpublished work [7]) [27], but it has been consistently negative in in vivo CA and MN tests [28, 29, 37]. A proof-of-concept study that monitored gene expression responses in human hepatoma-derived HepaRG cells using a microarray platform classified TCS as a non-DNA-reactive compound that only exhibited genotoxicity in vitro. The authors explained, however, that this conclusion required further confirmatory research [15]. Herein, we performed a pilot assay of TK gene (TK+/−) mutation assay at TK6 cell without metabolic activation for 24 h. We found that 35 and 50 μM TCS resulted in 2.9-fold and 12.1-fold increase in total growing MF, respectively (detail shown in Supplementary Fig. S1). Our results in mice study suggest that the rodent Pig-a assay is an appropriate in vivo follow-up method to clarify equivocal or inconsistent in vitro outcomes. International consensus bodies also have recommended that the Pig-a assay could be used as a follow-up for evaluating test articles that are positive in in vitro gene mutation tests [24, 31, 46]. Furthermore, the ready acquisition of quantitative data from the Pig-a assay make it ideal for use in quantitative assessments of genotoxicity, an approach that has shown the potential for estimating human risk [32, 33].
We designed the protocol for our experiments in order to integrate multiple toxicity endpoints in a short-term study that minimizes the need for large numbers of mice. The United States Environmental Protection Agency (US EPA) reported the toxicity of TCS in a 90-day mouse oral study that used seven doses, 15 mice per group, with the highest dose being 900 mg/kg/day. Systemic toxicity was observed at all dose levels and the LOAEL was 25 mg/kg/day; a NOAEL could not be determined [54]. In addition, a 28-day oral study (6.48–135.59 mg/kg/day in males, 8.25–168.78 mg/kg/day in females) used a total of 40 mice and determined a systemic LOAEL of 135.59 mg/kg/day for males and 168.78 mg/kg/day for females; the NOAEL was 6.48 mg/kg/day for males and 8.25 mg/kg/day for females [53]. Considering these findings, we set 31.25 and 1000 mg/kg/day as the lowest and highest doses in our study and dosed over 5 consecutive days in the first treatment period and over 3 consecutive days in the second treatment period (total: 8 days). We expected that the total amount of TCS exposure was sufficient to detect any genotoxicity associated with TCS, and that the lowest dose of TCS should not produce any toxicity. The scheme we used to evaluate the in vivo genotoxicity of TCS integrated the Pig-a assay (which needs at least 14 days after the first dose to detect mutation) with the bone marrow MN test (which needs at least two repeat doses before necropsy for analysis to be conducted in bone marrow samples), but this 19-day short-term repeat dose study also provided information on the general toxicity of TCS. Thus, we used six doses of TCS (six mice per group) to analyze PoDs to obtain data for further risk assessment. Rather than conducting standard in vivo regulatory assays in separate groups of mice, our design makes better use of the mice by combining endpoints in a single study and minimizing animal use, thus conforming with the 3Rs principles.
Recommendations for the selection of CES values, the values for which greatly influence the estimation of BMDs, have been made by several regulatory organizations. The European Food Safety Authority (EFSA) recommends a CES of 5% (or 0.05) for continuous toxicological endpoints such as organ weight (e.g. testis weight), body weight, and hematologic parameters (e.g. white blood cell counts and serum alkaline phosphatase activity) for risk assessment, and 10% (or 0.10) for quantal endpoints such as cancer (vascular tumors) and nephrosis, [16, 26]. Moreover, genotoxicity-specific CES values have been reported for chromosome damage, mutation, and DNA strand breaks that are in the range of 34–76% [64]. Additional studies have established CESs for transgenic rodent (TGR) mutagenicity data in the range of 18–66% [61] and in the range of ~60% for TGR and in the range of 56% for Pig-a mutagenicity [60].
In this study, we conservatively chose two CES values: 10 and 50%, for analyzing Pig-a MFs and MN-PCE data, and chose CES values of 5 and 10% for analyzing organ coefficient data. However, we could not calculate PoDs based on Pig-a assay or MN test results since no dose–responses were detected for these endpoints. By contrast, we found a significant increase in the liver–body weight ratio, which is a result consistent with other studies (unpublished work [53]) [23, 54]. Using organ coefficient liver data, we calculated PoD values of .213 mg/kg/day (CES: 0.05) and 1.82 mg/kg/day (CES: 0.1), and both were much lower than the BMD of 47 mg/kg/ day reported in a review article [49] and the NOAEL values summarized in another report [39]. Thus, our results indicate that the hepatotoxicity of TCS in mice might be much more serious than that reported in previous studies.
According to a US EPA report, TCS induced liver toxicity in rodents and dogs, with mice being the most sensitive species [43]. Based on higher levels of TCS in liver than in the plasma of mice, TCS appears to bioaccumulate in mouse liver [55], while bioaccumulation has not been observed in rats [56]. TCS has been classified as a peroxisome proliferator in mice which may cause subsequent tumor formation, but peroxisome proliferators are not likely to cause liver cancer in humans [30]. Contrary to this report, another report found that TCS activated the nuclear receptor constitutive androstane receptor but showed no significant effect on mouse peroxisome proliferation activating receptor. The authors also speculated that the manifestation of TCS-induced hepatic tumorigenesis would occur in humans as it occurs in mice [63]. Furthermore, a higher TCS concentration was found in human liver than in other organs [21]. It also should be noted that the large demand for TCS has consequently led to its high intake by humans, which is currently estimated at ~0.047, 0.065, and 0.073 mg/kg/day for men, women, and children, respectively [49]. According to food additive regulatory procedures (21 CFR 170.22) [19], a safety factor of 100 is used as a general rule in applying animal test data to man. Therefore, the data from our study translate to an acceptable daily intake (ADI) for TCS of no greater than 0.00182 mg/kg/day. Since we are constantly exposed to a variety of genotoxicant every day, no matter TCS is a genotoxicant itself or a tumor promoter, this ADI value here is much lower than the estimates for TCS daily intake, which suggests that more attention should be paid to the potential toxicity of low level TCS exposures.
Conclusion
The data provided herein indicate that the hepatotoxicity of TCS may be underestimated by studies reported in the published literature. Since in vivo human studies of TCS are still rare, whether TCS poses a carcinogenic hazard to humans is unknown. Dose and time dependence are important factors affecting the toxicity of TCS. In order to fully evaluate the health risks of TCS, it will be necessary to conduct further investigations on humans, especially on humans experiencing low-dose and long-term exposure to TCS. Since we have previously employed a human version of the erythrocyte PIG-A assay, performing the human PIG-A assay on a large population could be an efficient way to develop robust mutagenicity data on TCS and to provide further evidence on whether or not TCS is a human carcinogen.
Supplementary Material
Acknowledgements
We would like to thank Dr Robert H. Heflich (Division of Genetic and Molecular Toxicology, National Center for Toxicological Research, US FDA) for much helpful in discussion and for reviewing the manuscript. We would like thank Prof Yan. Feng from our institution for providing the blood of mice in the pilot study.
Contributor Information
Yiyi Cao, School of Public Health, Shanghai Jiaotong University School of Medicine, Shanghai 200025, People’s Republic of China.
Jing Xi, School of Public Health, Shanghai Jiaotong University School of Medicine, Shanghai 200025, People’s Republic of China.
Xinyue You, School of Public Health, Shanghai Jiaotong University School of Medicine, Shanghai 200025, People’s Republic of China.
Weiying Liu, School of Public Health, Shanghai Jiaotong University School of Medicine, Shanghai 200025, People’s Republic of China.
Yang Luan, School of Public Health, Shanghai Jiaotong University School of Medicine, Shanghai 200025, People’s Republic of China.
Authors’ contributions
Y.C. designed the study, analyzed the data and wrote a draft of this manuscript. Y.C., J.X., X.Y., and W.L. executed the benchtop work. Y.L. reviewed a draft of this manuscript and made comments. All authors approved the final manuscript.
Data availability statement
All the readers can request the data from the corresponding author.
Conflict of interest statement
None declared.
Funding
This work was sponsored by Foundation of Shanghai Municipal Heath Commission (grant number 202040009), Natural Science Foundation of Shanghai (grant number 19ZR1428200), and Foundation of Science and Technology Commission of Shanghai Municipality (grant number 20142202700).
References
- 1. Ahn KC, Zhao B, Chen J et al. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: receptor-based bioassay screens. Environ Health Perspect 2008;116:1203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allmyr M, Harden F, Toms LM et al. The influence of age and gender on triclosan concentrations in Australian human blood serum. Sci Total Environ 2008;393:162–7. [DOI] [PubMed] [Google Scholar]
- 3. Anger CT, Sueper C, Blumentritt DJ et al. Quantification of triclosan, chlorinated triclosan derivatives, and their dioxin photoproducts in lacustrine sediment cores. Environ Sci Technol 2013;47:1833–43. [DOI] [PubMed] [Google Scholar]
- 4. Auletta C. An 18-month oral oncogenicity study of tricolsan in the mouse via dietary administration. Pharmaco LSR Study 1995;93–2260. [Google Scholar]
- 5. Basu AK. DNA damage, mutagenesis and cancer. Int J Mol Sci 2018;19:970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Binelli A, Cogni D, Parolini M et al. Cytotoxic and genotoxic effects of in vitro exposure to triclosan and trimethoprim on zebra mussel (Dreissena polymorpha) hemocytes. Comp Biochem Physiol C Toxicol Pharmacol 2009;150:50–6. [DOI] [PubMed] [Google Scholar]
- 7. Broker PC, Gray VM, Howell A. Analysis of Metaphase Chromosomes Obtained from CHO Cells Cultured in vitro and Treated with Triclosan. Huntingdon Research Center, Ltd., 1988. ULR 214/88731; Unilever Test #: KC880171. Unpublished. [Google Scholar]
- 8. Calafat AM, Ye X, Wong LY et al. Urinary concentrations of triclosan in the U.S. population: 2003-2004. Environ Health Perspect 2008;116:303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cao Y, Xi J, Tang W et al. Genotoxicity evaluation of triclosan in vitro. Carcinogenesis Teratogenesis Mutagenesis 2018;30:71–5 (in Chinese). [Google Scholar]
- 10. Capkin E, Ozcelep T, Kayis S et al. Antimicrobial agents, triclosan, chloroxylenol, methylisothiazolinone and borax, used in cleaning had genotoxic and histopathologic effects on rainbow trout. Chemosphere 2017;182:720–9. [DOI] [PubMed] [Google Scholar]
- 11. CCR . Chromosome Aberration Assay in Bone Marrow Cells of the Rat wit FAT 80′023/Q (CCR Project No. 218305). Roßdorf: Cytotest Cell Research GMBH & Co., 1991. [Google Scholar]
- 12. Chen R, Zhou C, Cao Y et al. Assessment of Pig-a, micronucleus, and comet assay endpoints in Tg.RasH2 mice carcinogenicity study of aristolochic acid I. Environ Mol Mutagen 2020;61:266–75. [DOI] [PubMed] [Google Scholar]
- 13. Dayan AD. Risk assessment of triclosan [Irgasan] in human breast milk. Food Chem Toxicol 2007;45:125–9. [DOI] [PubMed] [Google Scholar]
- 14. DeSalva SJ, Kong BM, Lin YJ. Triclosan: a safety profile. Am J Dent 1989;2 Spec No:185–96. [PubMed] [Google Scholar]
- 15. Doktorova TY, Ates G, Vinken M et al. Way forward in case of a false positive in vitro genotoxicity result for a cosmetic substance? Toxicol in Vitro 2014;28:54–9. [DOI] [PubMed] [Google Scholar]
- 16. EFSA . European Food Safety Authority. Opinion of the Scientific Committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic. EFSA J 2005;3:282. [Google Scholar]
- 17. EU . EU (European Commission). Commission implementing decision (EU) 2016/110 of 27 January 2016 not approving triclosan as an existing active substance for use in biocidal products for product-type 1. Official J European Union L 21/87 2016. http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016D0110&from=EN. [Google Scholar]
- 18. Fahrig R. The effect of Irgasan DP 300 in the “Mammalian Spot Test” an in vivo method for the detection of genetic alterations in somatic cells of mice. Zentrallaboratorium für Mutagenitäsprüfung der Deutschen Forschungsgemeinschaft. 1978. [DOI] [PubMed]
- 19. FDA . US Food Drug Administration. CFR-Code of Federal Regulations Title 21. US Food and Drug Administration, 2019. [Google Scholar]
- 20. Federal Register . Safety and effectiveness of consumer antiseptics; topical antimicrobial drug products for over-the-counter human use. Final rule Fed Regist 2016;81:61106–30. [PubMed] [Google Scholar]
- 21. Geens T, Neels H, Covaci A. Distribution of bisphenol-A, triclosan and n-nonylphenol in human adipose tissue, liver and brain. Chemosphere 2012;87:796–802. [DOI] [PubMed] [Google Scholar]
- 22. Geer LA, Pycke BFG, Waxenbaum J et al. Association of birth outcomes with fetal exposure to parabens, triclosan and triclocarban in an immigrant population in Brooklyn, New York. J Hazard Mater 2017;323:177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goldsmith L, Craig D. 90-Day Oral Toxicity Study in Rats With FAT 80 023/H. Ciba-Geigy Limited, 1983. [Google Scholar]
- 24. Gollapudi BB, Lynch AM, Heflich RH et al. The in vivo Pig-a assay: a report of the International Workshop on Genotoxicity Testing (IWGT) Workgroup. Mutat Res Genet Toxicol Environ Mutagen 2015;783:23–35. [DOI] [PubMed] [Google Scholar]
- 25. Halden RU, Lindeman AE, Aiello AE et al. The Florence statement on triclosan and triclocarban. Environ Health Perspect 2017;125:064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hardy A, Benford D, Halldorsson T et al. Update: use of the benchmark dose approach in risk assessment. EFSA J 2017;15:e04658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heidemann A. Chromosome aberration assay in Chinese Hamster V79 cells in vitro with FAT 80′ 023/Q. Cytotest Cell Research. CCR project (179100). 1990.
- 28. Henderson LM, Proudlock RJ, Haynes P et al. Triclosan Mouse Micronucleus Test (HRC Study No. ULR 213/88492, Unilever Study No. KC 880168). Huntingdon: Huntingdon Research Centre Ltd., 1988. [Google Scholar]
- 29. Hool GSF, Muller D. Chromosome Studies in Male Germinal Epithelium, FAT 80 023/A Mouse (Test for Mutagenic Effects on Spermatogonia). Ciba-Geigy Limited. 1979. Experiment no. 78–2904/1979. [Google Scholar]
- 30. IARC . International Agency for Research on Cancer. Technical Publication No. 24: peroxisome proliferation and its role in carcinogenesis. 1995.
- 31. ICH . ICH Harmonised Guideline M7(R1). Assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic. In: International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Geneva, 2017. [Google Scholar]
- 32. Johnson GE, Slob W, Doak SH et al. New approaches to advance the use of genetic toxicology analyses for human health risk assessment. Toxicology Res 2014;4:667–76. [Google Scholar]
- 33. Johnson GE, Soeteman-Hernández LG, Gollapudi BB et al. Derivation of point of departure (PoD) estimates in genetic toxicology studies and their potential applications in risk assessment. Environ Mol Mutagen 2014;55:609–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kawagoe K, Takeda J, Endo Y et al. Molecular cloning of murine Pig-a, a gene for GPI-anchor biosynthesis, and demonstration of interspecies conservation of its structure, function, and genetic locus. Genomics 1994;23:566–74. [DOI] [PubMed] [Google Scholar]
- 35. Kinoshita T, Fujita M, Maeda Y. Biosynthesis, remodelling and functions of mammalian GPI-anchored proteins: recent progress. J Biochem 2008;144:287–94. [DOI] [PubMed] [Google Scholar]
- 36. Kolpin DW, Furlong ET, Meyer MT et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999-2000: a national reconnaissance. Environ Sci Technol 2002;36:1202–11. [DOI] [PubMed] [Google Scholar]
- 37. Langauer M, Müller D. Nucleus Anomaly Test on Somatic Interphase Nuclei, GP 41 343 (Triclosan), Chinese Hamster. Basel: Ciba-Geigy Ltd, 1974. [Google Scholar]
- 38. Lange A, Sebire M, Rostkowski P et al. Environmental chemicals active as human antiandrogens do not activate a stickleback androgen receptor but enhance a feminising effect of oestrogen in roach. Aquat Toxicol 2015;168:48–59. [DOI] [PubMed] [Google Scholar]
- 39. Lee JD, Lee JY, Kwack SJ et al. Risk assessment of triclosan, a cosmetic preservative. Toxicol Res 2019;35:137–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Loraine GA, Pettigrove ME. Seasonal variations in concentrations of pharmaceuticals and personal care products in drinking water and reclaimed wastewater in southern California. Environ Sci Technol 2006;40:687–95. [DOI] [PubMed] [Google Scholar]
- 41. Lyman FL, Furia T. Toxicology of 2, 4, 4′-trichloro-2′-hydroxy-diphenyl ether. IMS Ind Med Surg 1969;38:64–71. [PubMed] [Google Scholar]
- 42. Ma H, Zheng L, Li Y et al. Triclosan reduces the levels of global DNA methylation in HepG2 cells. Chemosphere 2013;90:1023–9. [DOI] [PubMed] [Google Scholar]
- 43. McMahon T. Revised 5-chloro-2-(2, 4-dichlorophenoxy) phenol (triclosan): toxicology chapter for the Reregistration Eligibility Decision (RED) Document. Case. 2008.
- 44. Miyata T, Takeda J, Iida Y et al. The cloning of PIG-A, a component in the early step of GPI-anchor biosynthesis. Science 1993;259:1318–20. [DOI] [PubMed] [Google Scholar]
- 45. Nishimura J, Murakami Y, Kinoshita T. Paroxysmal nocturnal hemoglobinuria: an acquired genetic disease. Am J Hematol 1999;62:175–82. [DOI] [PubMed] [Google Scholar]
- 46. OECD . (Organisation for Economic Cooperation and Development) the in vivo erythrocyte Pig-a gene mutation assay. Part 1: detailed review paper and performance assessment. 2019.
- 47. Parenti CC, Ghilardi A, Della Torre C et al. Environmental concentrations of triclosan activate cellular defence mechanism and generate cytotoxicity on zebrafish (Danio rerio) embryos. Sci Total Environ 2019;650:1752–8. [DOI] [PubMed] [Google Scholar]
- 48. Parsons BL. Modern conception of carcinogenesis creates opportunities to advance cancer risk assessment. Current Opinion Toxicology 2018;11-12:1–9. [Google Scholar]
- 49. Rodricks JV, Swenberg JA, Borzelleca JF et al. Triclosan: a critical review of the experimental data and development of margins of safety for consumer products. Crit Rev Toxicol 2010;40:422–84. [DOI] [PubMed] [Google Scholar]
- 50. Russell LB, Montgomery CS. Use of the mouse spot test to investigate the mutagenic potential of triclosan (Irgasan DP300). Mutat Res 1980;79:7–12. [DOI] [PubMed] [Google Scholar]
- 51. Shemansky JM, McDaniel LP, Klimas C et al. Pig-a gene mutation database. Environ Mol Mutagen 2019. [DOI] [PubMed] [Google Scholar]
- 52. Stoker TE, Gibson EK, Zorrilla LM. Triclosan exposure modulates estrogen-dependent responses in the female wistar rat. Toxicol Sci 2010;117:45–53. [DOI] [PubMed] [Google Scholar]
- 53. Thevenaz DP. Final Report: FAT 80023: 28-Day Toxicity Study in Mice (Administration in Feed) with Special Reference to Histopathology. Basle, Switzerland: Ciba-Geigy Ltd., 1987, Unpublished. [Google Scholar]
- 54. Trutter J. 13-week subchronic oral toxicity study of triclosan in CD-1 mice. Hazleton Washington Inc Report no HWA. 1993, 483–287.
- 55. Van Dijk A. 14C-Triclosan: Absorption, Distribution, Metabolism and Elimination after Single/Repeated Oral and Intravenous Administration to Mice (RCC Project No. 337781) RCC Umweltchemie AG. Itingen/BL, Switzerland, USA 1995. [Google Scholar]
- 56. Van Dijk A. 14C-Triclosan: Absorption, Distribution and Excretion (ADE) after Single Oral and Repeated Oral Administration to Male Rats (RCC Project 341998) RCC Umweltchemie AG. Itingen/BL, Switzerland, 1996. [Google Scholar]
- 57. Venkatesan AK, Pycke BF, Barber LB et al. Occurrence of triclosan, triclocarban, and its lesser chlorinated congeners in Minnesota freshwater sediments collected near wastewater treatment plants. J Hazard Mater 2012;229-230:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang CF, Tian Y. Reproductive endocrine-disrupting effects of triclosan: population exposure, present evidence and potential mechanisms. Environ Pollut 2015;206:195–201. [DOI] [PubMed] [Google Scholar]
- 59. Wang F, Xu R, Zheng F et al. Effects of triclosan on acute toxicity, genetic toxicity and oxidative stress in goldfish (Carassius auratus). Exp Anim 2018;67:j219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. White PA, Long AS, Johnson GE. Quantitative interpretation of genetic toxicity dose-response data for risk assessment and regulatory decision-making: current status and emerging priorities. Environ Mol Mutagen 2020;61:66–83. [DOI] [PubMed] [Google Scholar]
- 61. Wills JW, Johnson GE, Battaion HL et al. Comparing BMD-derived genotoxic potency estimations across variants of the transgenic rodent gene mutation assay. Environ Mol Mutagen 2017;58:632–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xie Z, Ebinghaus R, Flöser G et al. Occurrence and distribution of triclosan in the German Bight (North Sea). Environ Pollut 2008;156:1190–5. [DOI] [PubMed] [Google Scholar]
- 63. Yueh MF, Taniguchi K, Chen S et al. The commonly used antimicrobial additive triclosan is a liver tumor promoter. Proc Natl Acad Sci U S A 2014;111:17200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zeller A, Duran-Pacheco G, Guérard M. An appraisal of critical effect sizes for the benchmark dose approach to assess dose-response relationships in genetic toxicology. Arch Toxicol 2017;91:3799–807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the readers can request the data from the corresponding author.


