Abstract
Objective: The associations of dietary and circulating vitamin E level with metabolic syndrome (MetS) remains conflicting. This meta-analysis of observational study was therefore employed to investigate the issue above.
Methods: The PubMed, Web of Science and Embase database were searched up to April 2021. The observational studies on the associations of dietary and circulating vitamin E level with MetS were specified. The pooled relative risk (RR) of MetS for the highest vs. lowest dietary and circulating vitamin E level, and the standard mean difference (SMD) of dietary and circulating vitamin E level for MetS vs. control subjects, were calculated.
Results: A total of 25 observational studies with 51,276 participants, were included in this meta-analysis. The overall multi-variable adjusted RR demonstrated that the dietary vitamin E level was inversely associated with MetS (RR = 0.92, 95%CI: 0.85–1.00; P = 0.044). In addition, the dietary vitamin E level in MetS was also lower than that in control subjects according to the overall combined SMD (SMD = −0.08, 95%CI: −0.14 to −0.02; P = 0.024). On the other hand, the overall multi-variable adjusted RR showed no significant relationship between the circulating vitamin E level and MetS (RR = 1.46, 95%CI: 0.85–2.48; P = 0.17). However, the circulating vitamin E level in MetS was lower than that in control subjects according to the overall combined SMD (SMD = −0.58, 95%CI: −1.04 to −0.13; P = 0.013).
Conclusions: The results of this meta-analysis suggest that the dietary vitamin E level is inversely associated with MetS. On the other hand, current evidence is still insufficient to conclude a relationship between the circulating vitamin E level and MetS. More well-designed prospective cohort studies are needed to address the issues further.
Keywords: dietary vitamin E, circulating vitamin E, metabolic syndrome, meta-analysis, observational studies
Introduction
Metabolic syndrome (MetS) is a pathological state characterized by the following clinical features: elevated waist circumference, blood pressure, fasting blood glucose, triglycerides and reduced high-density lipoprotein cholesterol (1). As a common risk factor for cardiovascular disease, diabetes and death, MetS has gradually become a major global public health issue (2). With the deepening of our knowledge, nutritional factors are considered to be involved in MetS (3–7).
As a fat-soluble vitamin with antioxidant properties, vitamin E is considered to be involved in signal transduction, gene expression and immunomodulatory capabilities (8). Higher vitamin E level was reported to be inversely associated with hypertension and diabetes (9–11). Moreover, a meta-analysis of randomized controlled trials demonstrated that vitamin E supplementation was beneficial for subclinical inflammation in adults (12). Experimental evidence also indicated that vitamin E supplementation could ameliorate hypertension and diabetes (13–15), and decrease the total serum cholesterol and lipid peroxides level (16) in animal model.
A number of observational studies have examined the associations of dietary and circulating vitamin E level with MetS. However, their results are still conflicting (17–41). The present meta-analysis of observational studies was therefore employed to address the issues. It was hypothesized that both dietary and circulating vitamin E was inversely associated with MetS.
Materials and Methods
Search Strategy
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (42). The PubMed, Web of Science and Embase electronic database were searched during April 2021 by using a combination of keywords and in-text words related to metabolic syndrome (“metabolic syndrome”) and vitamin E (“vitamin E”, “tocopherol”) (43). No language restrictions were set in the search strategy. The titles and abstracts of all articles were screened firstly, and then the full articles were read to include the eligible studies. To identify the additional studies, the reference lists for the retrieved articles were also reviewed.
Study Selection
The titles, abstracts and full texts of all retrieved studies were reviewed by two researchers (YZ and DZZ) independently. Disagreements were resolved by discussions. The included studies were required to meet the following criteria: (1) observational studies; (2) the associations of dietary and circulating vitamin E level with MetS were reported; (3) relative risk (RR), odds ratio (OR) or standard mean difference (SMD) with 95% confidence interval (CI) were reported. The exclusion criteria were listed as follows: (1) duplicated or irrelevant articles; (2) reviews, letters or case reports; (3) randomized controlled trials; and (4) non-human studies.
Data Extraction
The data were extracted by two researchers (YZ and DZZ) independently, and disagreements were resolved by discussions. The information about first author, year of publication, location, age, gender, sample size, study design, adjustments, exposure, category of exposure, effect estimates and diagnostic criteria of MetS, was collected respectively. The corresponding effect estimates adjusted for the maximum number of confounding variables with 95% CIs for the highest vs. lowest dietary and circulating vitamin E level were extracted. Moreover, the dietary and circulating vitamin E level (mean ± SD) was also extracted to calculate the SMD (MetS vs. control).
Quality Assessment
Quality assessment was conducted according to the Newcastle-Ottawa (NOS) criteria for non-randomized studies, which was based on three broad perspectives: the selection process of study cohorts, the comparability among different cohorts and the identification of exposure or outcome of study cohorts. Disagreements with respect to the methodological quality were resolved by discussion and mutual-consultation. A study awarded seven or more stars was considered as a high-quality study (44).
Statistical Analyses
The RR for MetS and SMD for both dietary and circulating vitamin E level were the outcome measures in our study. The I2 statistic, which measures the percentage of total variation across studies due to heterogeneity, was examined (I2 > 50% was considered heterogeneity). If significant heterogeneity was observed among the studies, the random-effects model was used; otherwise, the fixed effects model was utilized. Begg's test was employed to assess the publication bias (45). A p-value < 0.05 was considered as statistically significant. Moreover, subgroup analysis was employed for both dietary and circulating vitamin E level, respectively.
Results
Study Identification and Selection
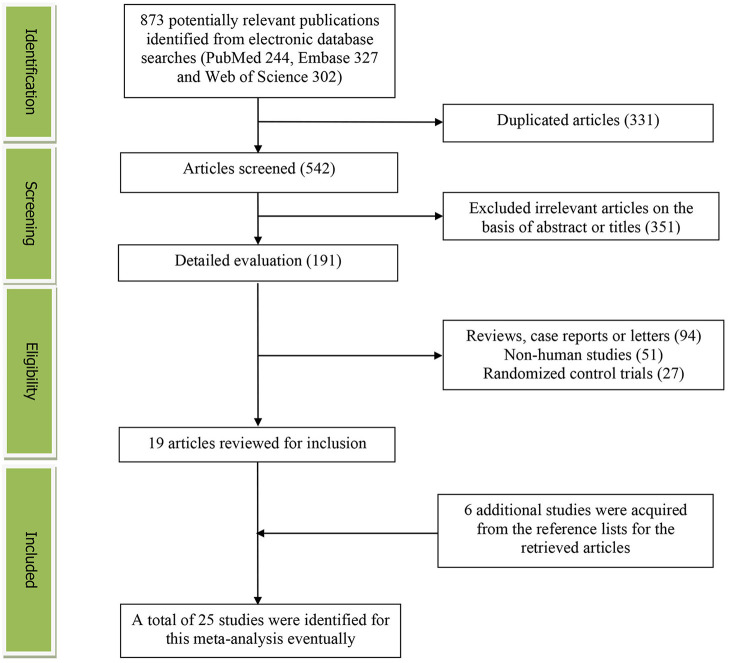
The detailed flow diagram of the study identification and selection was presented in Figure 1. A total of 873 potentially relevant articles (244 for PubMed, 327 for Embase and 302 for Web of Science) were retrieved during the initial literature search. After eliminating 331 duplicated articles, 542 articles were screened. 351 irrelevant studies were excluded according to the titles and abstracts. Then, 94 reviews, case reports or letters, 51 non-human studies, 27 randomized control trials studies were removed. Thereafter, 6 additional studies were acquired from the reference lists for the retrieved articles. Eventually, a total of 25 studies were selected for this meta-analysis (17–41).
Figure 1.
The detailed flow diagram of the study identification and selection in this meta-analysis.
Study Characteristics
Table 1 showed the main characteristics of the included studies. These studies were published between 2003 and 2021. Among which, 14 studies were performed in Asian countries [Korea (18, 19, 21, 35–39), China (29, 30, 33, 40), Iran (31, 41) and Saudi Arabia (28)], and 4 ones were conducted in European countries [Poland (34, 38), France (20) and Finland (24)]. The other 7 studies were from US (17, 23, 25, 26, 32), Brazil (22) and Nigeria (27). Both male and female participants were considered in all included studies, except for Bruscate's and Cho's study (21, 22). The sample size ranged from 20 to 10,351 for a total number of 51,276. The dietary vitamin E level was assessed by food-frequency questionnaire (FFQ) in 5 studies (17, 19, 26, 33, 41), and 24 h or 3-day recall method in 18 studies (18, 20, 22, 23, 25, 27–32, 34–40), and 4-day record in 1 study (24). The circulating vitamin E level was assessed by high performance liquid chromatography (HPLC) in 11 studies (17, 18, 20, 21, 23, 25, 27, 30, 32, 38, 39), and spectrophotometric method in 1 study (34). The criteria for MetS were National Cholesterol Education Program-Adult Treatment Panel III (NCEP ATP III) in 16 (17–21, 23, 24, 27, 29, 30, 35–37, 39–41) and International Diabetes Federation (IDF) in 6 studies (22, 25, 28, 31, 34, 38). The others utilized American Heart Association (AHA) (26, 33) or Joint Interim Statement (JIS) (32), respectively.
Table 1.
Characteristics of the individual studies included in this meta-analysis.
| Reference | Location | Age years | Gender | Sample Size | Study design | Adjustments | Exposure assessment | Category of exposure | Effect Estimates (RR or SMD) | Diagnostic criteria of MetS | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ford (17) | US | >20 | Both | 8,808 | Cross-sectional | NA | FFQ and HPLC | Control subjects MetS subjects Control subjects MetS subjects |
Dietary vitamin E 9.9 (9.5, 10.3) 9.4 (8.8, 10.0) Circulating vitamin E 25.9 (25.5, 26.4) 30.1 (29.2, 30.9) |
NCEP ATP III | 8 |
| Kim (18) | Korea | >60 | Both | 404 | Cross-sectional | Age, BMI, energy intake, smoking status, alcohol, physical activity, vitamin, and mineral supplements | 24 h recall and HPLC | Male Control subjects MetS subjects |
Dietary vitamin E 8.6 (7.0, 10.2) 7.0 (5.6, 8.4) |
NCEP ATP III | 7 |
| Female Control subjects MetS subjects |
Dietary vitamin E 8.6 (7.6, 9.6) 6.7 (5.9, 7.5) |
||||||||||
| Dietary vitamin E Male Quartiles 1 Quartiles 2 Quartiles 3 Quartiles 4 |
RR 1.0 0.71 (0.25, 2.1) 0.94 (0.33, 2.67) 0.54 (0.18, 1.62) |
||||||||||
| Female Quartiles 1 Quartiles 2 Quartiles 3 Quartiles 4 |
RR 1.0 1.0 (0.52, 1.93) 0.52 (0.43, 1.58) 0.50 (0.26, 0.98) |
||||||||||
| Control subjects MetS subjects |
Circulating vitamin E 11.7 (10.5, 12.9) 10.8 (10.0, 11.6) |
||||||||||
| Circulating vitamin E Male Tertiles 1 Tertiles 2 Tertiles 3 |
RR 1.0 1.3 (0.2, 7.3) 3.5 (0.7, 17.7) |
||||||||||
| Female Tertiles 1 Tertiles 2 Tertiles 3 |
RR 1.0 1.1 (0.5, 2.9) 0.8 (0.3, 1.9) |
||||||||||
| Kim (19) | Korea | Middle-aged | Both | 688 | Cross-sectional | NA | FFQ | Male Control subjects MetS subjects |
Dietary vitamin E 7.9 (7.5, 8.3) 7.7 (7.3, 8.2) |
NCEP ATP III | 6 |
| Female Control subjects MetS subjects |
Dietary vitamin E 8.7 (8.1, 9.3) 8.1 (7.6, 8.6) |
||||||||||
| Czernichow (20) | France | 49 | Both | 5,520 | Cohort | Age, sex, intervention group, educational level, smoking status, physical activity and alcohol consumption | HPLC | Circulating vitamin E Tertiles 1 Tertiles 2 Tertiles 3 |
RR 1.0 0.94 (0.64, 1.38) 1.02 (0.70, 1.49) |
NCEP ATP III | 9 |
| Cho (21) | Korea | >20 | Male | 163 | Cross-sectional | NA | HPLC | Control subjects MetS subjects |
Circulating vitamin E 1,153.3 (1,100.7, 1,206.0) 1,315.7 (1,148.8, 1,482.5) |
NCEP ATP III | 6 |
| Bruscate (22) | Brazil | 69.3 | Female | 284 | Cross-sectional | Age, smoking, education, physical activity and dietary fiber | 24 h recall | Control subjects MetS subjects |
Dietary vitamin E 17.8 (16.7, 18.9) 17.4 (15.7, 19.1) |
IDF | 7 |
| Dietary vitamin E Quartiles 1 Quartiles 2 Quartiles 3 Quartiles 4 |
RR 1.0 0.52 (0.25, 1.08) 0.65 (0.32, 1.33) 0.70 (0.34, 1.42) |
||||||||||
| Beydoun (23) | US | 20–85 | Both | 3,202 | Cross-sectional | Age, sex, race/ethnicity, marital status, educational level, PIR, smoking status, total energy intake, alcohol, caffeine, b-carotene, vitamin C, vitamin E, and dietary supplement use, serum levels of folate, tHcy, vitamin B12, 25(OH)D, total cholesterol, and TG | 24 h recall and HPLC | Male Control subjects MetS subjects |
Dietary vitamin E 8.2 (7.8, 8.6) 8.0 (7.6, 8.4) |
NCEP ATP III | 8 |
| Female Control subjects MetS subjects |
Dietary vitamin E 6.6 (6.4, 6.8) 6.1 (5.7, 6.5) |
||||||||||
| Male Control subjects MetS subjects |
Circulating vitamin E 28.5 (27.5, 29.5) 33.8 (32.0, 35.6) |
||||||||||
| Female Control subjects MetS subjects |
Circulating vitamin E 28.2 (27.2, 29.2) 35.3 (33.5, 37.1) |
||||||||||
| Circulating vitamin E Quartiles 1 Quartiles 2 Quartiles 3 Quartiles 4 |
RR 1.0 0.79 (0.41, 1.52) 1.54 (0.84, 2.84) 1.09 (0.44, 2.67) |
||||||||||
| Kouki (24) | Finland | 57–78 | Both | 1,334 | Cross-sectional | Age, alcohol consumption, smoking, education and VO2 max | 4-day food record | Male Control subjects MetS subjects |
Dietary vitamin E 11.1 (10.6, 11.6) 9.9 (9.0, 10.8) |
NCEP ATP III | 8 |
| Female Control subjects MetS subjects |
Dietary vitamin E 9.8 (9.2, 10.4) 9.5 (8.6, 10.4) |
||||||||||
| Dietary vitamin E Male <10 mg/d >10 mg/d |
RR 1.0 0.99 (0.96, 1.03) |
||||||||||
| Female <10 mg/d >10 mg/d |
RR 1.0 1.01 (0.98, 1.05) |
||||||||||
| Beydoun (25) | US | 12–19 | Both | 1,339 | Cross-sectional | NA | 24 h recall and HPLC | Control subjects MetS subjects |
Dietary vitamin E 6.4 (6.2, 6.6) 5.9 (4.3, 7.5) |
IDF | 7 |
| Control subjects MetS subjects |
Circulating vitamin E 18.6 (18.2, 19.0) 20.2 (18.0, 22.4) |
||||||||||
| de Oliveira Otto (26) | US | 45–84 | Both | 3,828 | Cohort | Energy intake, age, sex, race-ethnicity, education, study center, alcohol intake, physical activity, BMI, fiber intake, cigarette smoking, dietary supplement use, the ratio of polyunsaturated fat intake and saturated fat intake, Mg, Zn, heme iron, non-heme iron, and antioxidant intake | FFQ | Dietary vitamin E Quintiles 1 Quintiles 2 Quintiles 3 Quintiles 4 Quintiles 5 |
RR 1.0 1.00 (0.79, 1.26) 1.03 (0.81, 1.31) 0.84 (0.65, 1.09) 0.76 (0.56, 1.03) |
AHA | 8 |
| Odum (27) | Nigeria | 50 | Both | 192 | Case-control | NA | HPLC | Control subjects MetS subjects |
Circulating vitamin E 30.8 (29.6, 32.0) 16.9 (15.9, 17.9) |
NCEP ATP III | 7 |
| Al-Daghri (28) | Saudi Arabia | 19–60 | Both | 185 | Cross-sectional | Age, BMI and physical activity | 24 h recall | Control subjects MetS subjects |
Dietary vitamin E 2.3 (2.1, 2.5) 2.0 (1.8, 2.2) |
IDF | 7 |
| Dietary vitamin E Quartiles 1 Quartiles 2 Quartiles 3 Quartiles 4 |
RR 1.0 0.20 (0.07, 0.60) 0.14 (0.05, 0.40) 0.17 (0.06, 0.51) |
||||||||||
| Motamed (31) | Iran | 35–65 | Both | 3,800 | Cross-sectional | Sex, age, physical activity level, smoking, past medical history, energy intake, and BMI | 24 h recall | Male Control subjects MetS subjects |
Dietary vitamin E 14.8 (14.2, 15.4) 16.6 (15.9, 17.3) |
IDF | 7 |
| Female Control subjects MetS subjects |
Dietary vitamin E 14.7 (14.3, 15.1) 13.8 (13.4, 14.2) |
||||||||||
| Dietary vitamin E Quintiles 1 Quintiles 2 Quintiles 3 Quintiles 4 Quintiles 5 |
RR 1.0 0.96 (0.70, 1.10) 0.86 (0.60, 1.00) 1.06 (0.80, 1.30) 0.90 (0.70, 1.10) |
||||||||||
| Bian (29) | China | 30–70 | Both | 258 | Cross-sectional | NA | 24 h recall | Control subjects MetS subjects |
Dietary vitamin E 29.9 (28.4, 31.4) 27.5 (25.9, 29.1) |
NCEP ATP III | 8 |
| Li (30) | China | 18–65 | Both | 550 | Cross-sectional | Age and sex | 3-day food record and HPLC | Control subjects MetS subjects |
Dietary vitamin E 45.7 (43.2, 48.2) 45.1 (42.1, 48.1) |
NCEP ATP III | 8 |
| Dietary vitamin E Quartiles 1 Quartiles 2 Quartiles 3 Quartiles 4 Control subjects |
RR 1.0 0.65 (0.37,1.04) 0.85 (0.51–1.41) 0.62 (0.37–1.04) Circulating vitamin |
||||||||||
| MetS subjects | E 12.1 (11.7, 12.5) 12.9 (12.2, 13.6) |
||||||||||
| Circulating vitamin E Quartiles 1 Quartiles 2 Quartiles 3 Quartiles 4 |
RR 1.0 0.44 (0.15,1.25) 0.88 (0.32–2.44) 1.58 (0.55–4.54) |
||||||||||
| Mah (32) | US | 24–40 | Both | 20 | Case-control | NA | HPLC | Control subjects MetS subjects |
Circulating vitamin E 22.2 (19.5, 24.9) 23.9 (21.9, 25.9) |
JIS | 5 |
| Wei (33) | China | 18–84 | Both | 2,069 | Cross-sectional | Age, sex, cigarette smoking, alcohol, drinking, nutritional supplementary, activity level, dietary energy intake, fiber intake and protein intake | FFQ | Control subjects MetS subjects |
Dietary vitamin E 29.8 (29.1, 30.6) 30.7 (28.8, 32.6) |
AHA | 7 |
| Dietary vitamin E Quartiles 1 Quartiles 2 Quartiles 3 Quartiles 4 |
RR 1.0 1.07 (0.77, 1.50) 0.98 (0.67, 1.41) 1.20 (0.77, 1.87) |
||||||||||
| Godala (34) | Poland | 30–65 | Both | 273 | Case-control | NA | 3-day food record and spectrophotometric method | Control subjects MetS subjects |
Dietary vitamin E 9.33 (8.27, 10.39) 8.85 (8.03, 9.67) |
IDF | 7 |
| Control subjects MetS subjects |
Circulating vitamin E 25.49 (24.86, 26.12) 12.47 (12.08, 12.86) |
||||||||||
| Lim (36) | Korea | Middle-aged | Both | 143 | Cross-sectional | Not mentioned | 3-day food record | Control subjects MetS subjects |
Dietary vitamin E 12.6 (11.5, 13.7) 12.7 (11.3, 14.1) |
NCEP ATP III | 6 |
| Ahn (35) | Korea | 30–60 | Both | 614 | Cross-sectional | Age, smoking, alcohol consumption and physical activity | 3-day food record | Male Control subjects MetS subjects |
Dietary vitamin E 3.5 (3.3, 3.7) 3.3 (3.2, 3.4) |
NCEP ATP III | 7 |
| Female Control subjects MetS subjects |
Dietary vitamin E 3.5 (3.3, 3.7) 3.8 (3.5, 4.1) |
||||||||||
| ‘Dietary vitamin E Male Tertile 1 Tertile 2 Tertile 3 |
RR 1.0 1.00 (0.56, 1.76) 0.52 (0.30, 0.92) |
||||||||||
| Female Tertile 1 Tertile 2 Tertile 3 |
RR 1.0 1.16 (0.65, 2.09) 1.70 (0.94, 3.08) |
||||||||||
| Ahn (37) | Korea | 19–64 | Both | 10,351 | Cross-sectional | Age, BMI, alcohol consumption, smoking, physical activity, household income, education level and energy intake | 24 h recall | Dietary vitamin E Male Tertile 1 Tertile 2 Tertile 3 |
RR 1.0 0.88 (0.71, 1.11) 0.76 (0.60, 0.96) |
NCEP ATP III | 8 |
| Female Tertile 1 Tertile 2 Tertile 3 |
RR 1.0 0.87 (0.67, 1.14) 1.02 (0.79, 1.31) |
||||||||||
| Godala (38) | Poland | 57 | Both | 332 | Cross-sectional | NA | 24 h recall and HPLC | Control subjects MetS subjects |
Dietary vitamin E 9.31 (8.26, 10.35) 9.01 (8.37, 9.66) |
IDF | 7 |
| Control subjects MetS subjects |
Circulating vitamin E 23.67 (22.88, 24.46) 14.22 (13.93, 14.51) |
||||||||||
| Kim (39) | Korea | 47.1 | Both | 5,885 | Cross-sectional | Age, sex, residence, household income, education, alcohol consumption, smoking status, physical activity, hs-CRP and BMI | HPLC | Circulating vitamin E Quartiles 1 Quartiles 2 Quartiles 3 Quartiles 4 |
RR 1.0 1.30 (0.97, 1.74) 1.71 (1.30, 2.25) 2.56 (1.95, 3.35) |
NCEP ATP III | 9 |
| Peng (40) | China | >99 | Both | 992 | Cohort | Aex, marital status, physical activity, smoking status, alcohol intake, family history of chronic diseases and daily total energy intake | 24 h recall | Control subjects MetS subjects |
Dietary vitamin E 17.74 (10.45, 25.03) 12.18 (9.00, 15.36) |
NCEP ATP III | 7 |
| Zaeemzadeh (41) | Iran | 30 | Both | 42 | Case-control | NA | FFQ | Control subjects MetS subjects |
Dietary vitamin E 17.21 (14.49, 19.93) 6.17 (2.01, 10.33) |
NCEP ATP III | 6 |
RR of MetS for the Highest vs. Lowest Dietary Vitamin E Level
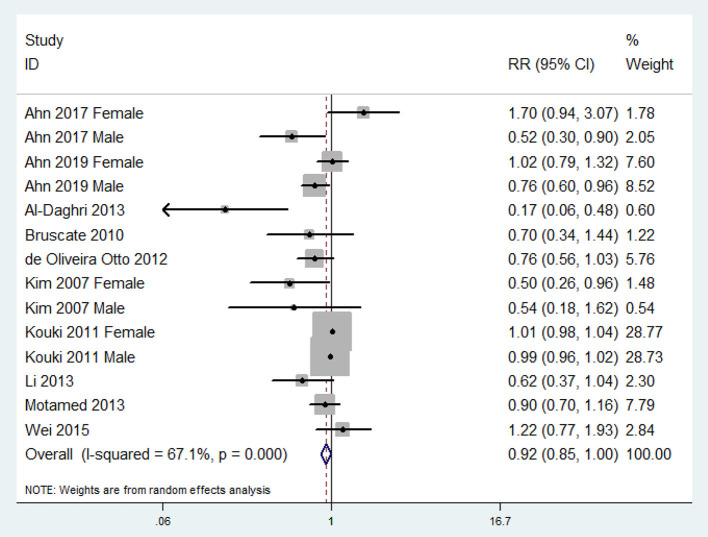
The overall multi-variable adjusted RR demonstrated that the dietary vitamin E level was negatively associated with MetS (RR = 0.92, 95%CI: 0.85–1.00; P = 0.044) (Figure 2). A substantial level of heterogeneity among various studies was obtained (P < 0.001, I2 = 67.1%). The Begg's rank-correlation test showed no evidence of publication bias (P = 0.189). The results of subgroup analysis were presented in Table 2. The aforementioned results only existed in studies with the adjustment of BMI (RR = 0.75, 95%CI: 0.59 to 0.94; P = 0.01), physical activity (RR = 0.76, 95%CI: 0.61–0.95; P = 0.02), and energy intake (RR = 0.86, 95%CI: 0.76–0.97; P = 0.01), respectively.
Figure 2.
Forest plot of meta-analysis: Overall multi-variable adjusted RR of MetS for the highest vs. lowest category of dietary vitamin E level.
Table 2.
Subgroup analysis of MetS for the highest vs. lowest dietary vitamin E level category.
| Stratification | Number of studies | Pooled RR | 95% CI | P value | Heterogeneity |
|---|---|---|---|---|---|
| All studies | 10 | 0.92 | 0.85, 1.00 | P = 0.04 | P < 0.001; I2 = 67% |
| Adjustment of BMI | |||||
| Adjusted | 5 | 0.75 | 0.59, 0.94 | P = 0.01 | P = 0.02; I2 = 61% |
| Unadjusted | 5 | 0.99 | 0.93, 1.06 | P = 0.79 | P = 0.03; I2 = 58% |
| Adjustment of physical activity | |||||
| Adjusted | 7 | 0.76 | 0.61, 0.95 | P = 0.02 | P = 0.004; I2 = 63% |
| Unadjusted | 3 | 1 | 0.98, 1.02 | P = 0.98 | P = 0.18; I2 = 38% |
| Adjustment of energy intake | |||||
| Adjusted | 5 | 0.86 | 0.76, 0.97 | P = 0.01 | P = 0.17; I2 = 34% |
| Unadjusted | 5 | 0.96 | 0.88, 1.05 | P = 0.42 | P < 0.001; I2 = 76% |
| Adjustment for vitamin E supplement | |||||
| Adjusted | 3 | 0.8 | 0.64, 1.01 | P = 0.06 | P = 0.12; I2 = 48% |
| Unadjusted | 7 | 0.94 | 0.87, 1.02 | P = 0.14 | P < 0.001; I2 = 70% |
| Study design | |||||
| Cross-sectional | 9 | 0.93 | 0.86, 1.01 | P = 0.10 | P < 0.001; I2 = 67% |
| Cohort | 1 | 0.76 | 0.56, 1.03 | / | / |
SMD of Dietary Vitamin E Level for MetS vs. Control Subjects
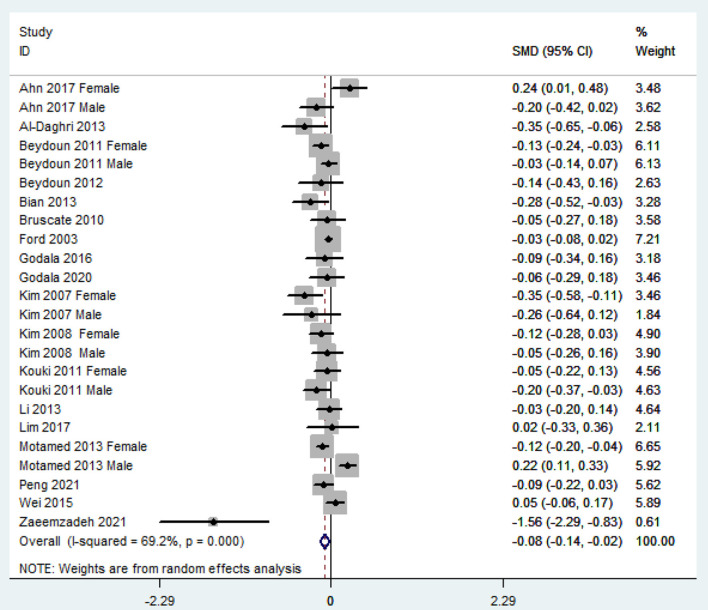
The overall combined SMD showed that the dietary vitamin E level in MetS was lower than that in control subjects (SMD = −0.08, 95%CI: −0.14 to −0.02; P = 0.006) (Figure 3). A substantial level of heterogeneity was obtained among the various studies (P < 0.001, I2 = 69.2%). The Begg's rank-correlation test showed no evidence of publication bias (P = 0.07). The results of subgroup analysis were presented in Table 3. The aforementioned results only existed in female (SMD = −0.10, 95%CI: −0.20 to 0.00; P = 0.05), NCEP ATP III (SMD = −0.11, 95%CI: −0.07 to −0.04; P = 0.002), 24 h or 3 days recall (SMD = −0.09, 95%CI: −0.16 to −0.02; P = 0.01) and high-quality (SMD = −0.07, 95%CI: −0.13 to −0.01; P = 0.02) studies, respectively.
Figure 3.
Forest plot of meta-analysis: SMD of dietary vitamin E level for MetS vs. control subjects.
Table 3.
Subgroup analysis for SMD of dietary vitamin E level in MetS vs. control subjects.
| Stratification | Number of studies | Pooled SMD | 95% CI | P-value | Heterogeneity |
|---|---|---|---|---|---|
| All studies | 18 | −0.08 | −0.14, −0.02 | P = 0.006 | P < 0.001; I2 = 69% |
| Gender | |||||
| Male | 6 | −0.06 | −0.22, 0.09 | P = 0.44 | P < 0.001; I2 = 80% |
| Female | 6 | −0.10 | −0.20, 0.00 | P = 0.05 | P = 0.02; I2 = 63% |
| Diagnostic criteria of MetS | |||||
| NCEP ATP III | 11 | −0.11 | −0.07, −0.04 | P = 0.002 | P < 0.001; I2 = 63% |
| Other | 7 | −0.04 | −0.16, 0.08 | P = 0.51 | P < 0.001; I2 = 77% |
| Geographical region | |||||
| Asia | 11 | −0.10 | −0.21, 0.00 | P = 0.05 | P < 0.001; I2 = 79% |
| Non-Asia | 7 | −0.06 | −0.09, −0.02 | P = 0.002 | P = 0.62; I2 = 0% |
| Exposure assessment | |||||
| FFQ | 4 | −0.09 | −0.23, 0.06 | P = 0.24 | P = 0.001; I2 = 80% |
| 24 h or 3 days recall | 14 | −0.09 | −0.16, −0.02 | P = 0.01 | P < 0.001; I2 = 66% |
| Study quality | |||||
| High-quality | 15 | −0.07 | −0.13, −0.01 | P = 0.02 | P < 0.001; I2 = 67% |
| Low-quality | 3 | −0.24 | −0.56, 0.08 | P = 0.14 | P = 0.001; I2 = 81% |
RR of MetS for the Highest vs. Lowest Circulating Vitamin E Level
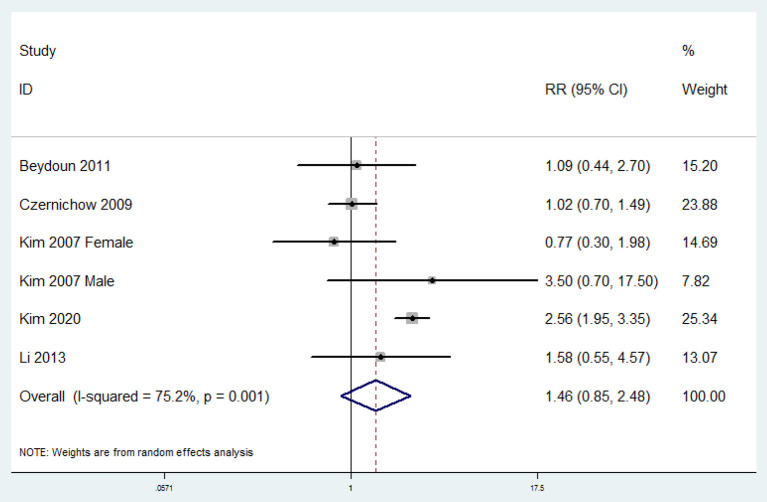
The overall multi-variable adjusted RR showed no significant relationship between circulating vitamin E level and MetS (RR = 1.46, 95%CI: 0.85–2.48; P = 0.168) (Figure 4). A substantial level of heterogeneity was obtained among various studies (P = 0.001, I2 = 75.2%). The Begg's rank-correlation test showed no evidence of publication bias (P = 0.707). The results of subgroup analysis were presented in Table 4.
Figure 4.
Forest plot of meta-analysis: Overall multi-variable adjusted RR of MetS for the highest vs. lowest category of circulating vitamin E level.
Table 4.
Subgroup analysis of MetS for the highest vs. lowest circulating vitamin E level category.
| Stratification | Number of studies | Pooled RR | 95% CI | P value | Heterogeneity |
|---|---|---|---|---|---|
| All studies | 5 | 1.46 | 0.85, 2.48 | P = 0.17 | P = 0.001; I2 = 75% |
| Adjustment of BMI | |||||
| Adjusted | 2 | 1.84 | 0.78, 4.38 | P = 0.16 | P = 0.05; I2 = 67% |
| Unadjusted | 3 | 1.07 | 0.77, 1.49 | P = 0.67 | P = 0.75; I2 = 0% |
| Adjustment of physical activity | |||||
| Adjusted | 2 | 1.84 | 0.78, 4.38 | P = 0.16 | P = 0.05; I2 = 67% |
| Unadjusted | 3 | 1.07 | 0.77, 1.49 | P = 0.67 | P = 0.75; I2 = 0% |
| Adjustment of energy intake | |||||
| Adjusted | 2 | 1.11 | 0.61, 2.04 | P = 0.73 | P = 0.28; I2 = 21% |
| Unadjusted | 3 | 1.62 | 0.79, 3.35 | P = 0.19 | P < 0.001; I2 = 87% |
| Adjustment for vitamin E supplement | |||||
| Adjusted | 2 | 1.11 | 0.61, 2.04 | P = 0.73 | P = 0.28; I2 = 21% |
| Unadjusted | 3 | 1.62 | 0.79, 3.35 | P = 0.19 | P < 0.001; I2 = 87% |
| Study design | |||||
| Cross-sectional | 4 | 1.65 | 0.94, 2.89 | P = 0.08 | P = 0.06; I2 = 55% |
| Cohort | 1 | 1.02 | 0.70, 1.49 | / | / |
SMD of Circulating Vitamin E Level for MetS vs. Control Subjects
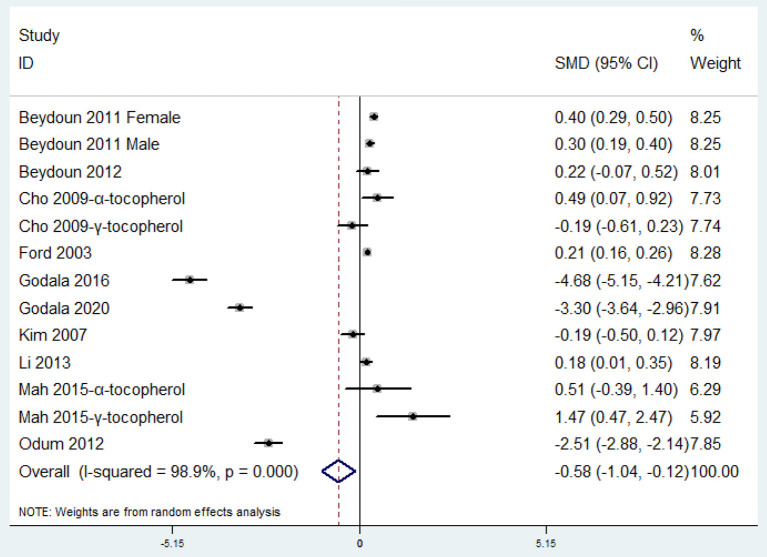
The overall combined SMD showed that the circulating vitamin E level in MetS was lower than that in control subjects (SMD = −0.58, 95%CI: −1.04 to −0.13; P = 0.013) (Figure 5). A substantial level of heterogeneity was obtained among the various studies (P < 0.001, I2 = 98.9%). The Begg's rank-correlation test showed no evidence of publication bias (P = 0.246). The results of subgroup analysis were presented in Table 5. The aforementioned results only existed in spectrophotometric method (SMD = −4.67, 95%CI: −5.14 to −4.20) and high-quality study (SMD = −1.00, 95%CI: −1.54 to −0.45; P < 0.001), but lost in α-tocopherol (SMD = 0.08, 95%CI: −0.16 to 0.33; P = 0.50) and γ-tocopherol (SMD = 0.54, 95%CI: −1.03 to 2.11; P = 0.50), respectively.
Figure 5.
Forest plot of meta-analysis: SMD of circulating vitamin E level for MetS vs. control subjects.
Table 5.
Subgroup analysis for SMD of circulating vitamin E level in MetS vs. control subjects.
| Stratification | Number of studies | Pooled SMD | 95% CI | P-value | Heterogeneity |
|---|---|---|---|---|---|
| All studies | 10 | −0.58 | −1.04, −0.13 | P = 0.01 | P < 0.001; I2 = 99% |
| Assessment of outcome | |||||
| HPLC | 9 | −0.24 | −0.64, 0.12 | P = 0.17 | P < 0.001; I2 = 99% |
| Spectrophotometric method | 1 | −4.67 | −5.14, −4.20 | / | / |
| Study quality | |||||
| High quality | 8 | −1.00 | −1.54, −0.45 | P < 0.001 | P < 0.001; I2 = 99% |
| Low quality | 2 | 0.44 | −0.15, 1.02 | P = 0.14 | P = 0.01; I2 = 73% |
| Type of tocopherol | |||||
| α-tocopherol | 3 | 0.08 | −0.16, 0.33 | P = 0.50 | P = 0.03; I2 = 72% |
| γ-tocopherol | 2 | 0.54 | −1.03, 2.11 | P = 0.50 | P = 0.004; I2 = 88% |
Discussion
In the present meta-analysis, a total of 25 observational studies were identified for examination. The pooled analysis showed that the dietary vitamin E level was inversely associated with MetS.
Since both the oxidative stress and inflammation plays important role in the pathophysiology of MetS (46), the negative relationship between dietary vitamin E level and MetS may be mainly attributed to the antioxidant and anti-inflammatory property of vitamin E (12, 47, 48). Consistently, the clinical trial evidence demonstrates that the vitamin E supplementation may ameliorate MetS via anti-inflammation and anti-oxidative stress (49, 50). Moreover, some experimental animal studies have also indicated the beneficial impact of vitamin E intake on the MetS-related context (13–16). Nevertheless, very limited prospective cohort studies are identified for meta-analysis. The dietary vitamin E level is measured after MetS event occurs in cross-sectional/case-control studies. The factors that matter the dietary vitamin E level may change after MetS, which may reverse the causality. In addition, the significant heterogeneity has also been specified (I2 = 67.1%). Therefore, these above issues should be addressed by further well-designed prospective cohort studies.
In contrast to dietary vitamin E (a general/overall estimation that calculated from the FFQ or recall method), the issue of circulating vitamin E level is rather complicated. On one hand, vitamin E is composed of 8 similar structure compounds: 4 tocopherol and 4 tocotrienol derivatives including α-, β-, γ-, δ-tocopherol and tocotrienol, respectively. All vitamin E isomers from dietary or supplementary sources are absorbed and delivered to the liver. Only α-tocopherol is preferentially recognized by the α-tocopherol transfer protein for incorporation into circulating plasma, whereas the other tocopherol and tocotrienol isomers are mostly excreted (51, 52). Indeed, the concentration of tocotrienol is significantly lower than that of α-tocopherol (52). This is the main reason why α-tocopherol is currently severed as the standard to estimate human vitamin E requirements (53). However, it is increasingly acknowledged that tocopherol and tocotrienol serve different biological functions (tocotrienol may result in superior therapeutic properties than tocopherol), which has greatly challenged the accuracy of α-tocopherol estimation alone (54–56). Unfortunately, only several studies reported the exposure as “tocopherol” (21, 32, 39) (most studies reported the exposure as “circulating vitamin E” directly). The combination of tocopherol/tocotrienol may probably preclude a clear clarification for this issue. On the other hand, chronic inflammation and oxidative stress caused by MetS may lower the circulating antioxidant and anti-inflammatory agent level (57). In turn, the circulating antioxidant and anti-inflammatory agent level may also increase to cope with chronic inflammation and oxidative stress caused by MetS (58). As a consequence, the level of circulating vitamin E might be dynamic in MetS condition. Taken together, the specification of tocopherol/tocotrienol and potential feedback system should be considered for circulating vitamin E in further studies.
The epidemiological data has demonstrated that BMI, physical activity and energy intake is associated with both dietary vitamin E and MetS (59–63). Indeed, our finding is lost in studies without adjustment of these factors (Table 2). The adjustment of confounding factors can exclude the effect on both exposure and outcome (leave the direct relationship between vitamin E and MetS). Therefore, BMI, physical activity and energy intake may be adjusted when further study is employed. In addition, the lower dietary vitamin E level in MetS is only obtained in females, NCEP ATP III, 24 h or 3 days recall and high-quality studies (Table 3). Although females are more precise and reliable in completing the exposure assessment, some genetic gender differences with the diet-related pathology of MetS cannot be fully excluded (64). Moreover, the diagnostic criteria of MetS and exposure assessment may also influence the results. Since a substantial improvement may exist in laboratorial techniques to assess the circulating vitamin E level, a subgroup analysis for assessment method has also been employed. Interestingly, the results only exist in spectrophotometric method, but not HPLC. Notably, the spectrophotometric method is utilized in only one study (34). Taken together, more study with appropriate confounding factors adjustment and different circulating vitamin E assessment is needed.
The strengths of the present meta-analysis are mainly reflected in the following aspects: First, this is the first meta-analysis of observational studies on the associations of dietary and circulating vitamin E level with MetS. Second, our findings for dietary vitamin E level and MetS are consistence with current corresponding experimental and clinical studies. The limitations should also be acknowledged. First, the substantial level of heterogeneity might have distorted the reliability of our results (especially for circulating vitamin E level). Second, due to the limitation in the relevant literature, very few prospective cohort studies are identified totally (precluded causal relationships). Third, the classification of exposure vary greatly among individuals. For example, “tertiles,” “quartiles” and “quintiles” are employed to classify the dietary/circulating vitamin E level. Fourth, the selection of adjusted factors and definition of MetS are not uniform. Last but not the least, very few studies have considered the isomers of tocopherol/tocotrienol. These limitations may weaken the significance of this study.
Conclusions
The results of this meta-analysis suggest that the dietary vitamin E level may be inversely associated with MetS. On the other hand, current evidence is still insufficient to conclude a relationship between the circulating vitamin E level and MetS. More well-designed prospective cohort studies with the specification of circulating tocopherol/tocotrienol are needed to address the issues further.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
YZ, DZ, and JL conceived the idea and drafted this meta-analysis. ZL and QL performed the statistical analysis. YL and JD selected and retrieved relevant papers. HG assessed each study. YZ, DZ, and JL was the guarantor of the overall content. All authors revised and approved the final manuscript.
Funding
This study was supported by National Natural Science Foundation of China (82102581), National Postdoctoral Science Foundation of China (2021M693562), Provincial Outstanding Postdoctoral Innovative Talents Program of Hunan (2021RC2020), Provincial Natural Science Foundation of Hunan (2019JJ40517), Young Investigator Grant of Xiangya Hospital, Central South University (2020Q14), and FuQing Postdoc Program of Xiangya Hospital, Central South University (176).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Zhang Y, Zhang DZ. Relationship between serum zinc level and metabolic syndrome: a meta-analysis of observational studies. J Am Coll Nutr. (2018) 37:708–15. 10.1080/07315724.2018.1463876 [DOI] [PubMed] [Google Scholar]
- 2.Athyros V, Ganotakis E, Elisaf M, Mikhailidis D. The prevalence of the metabolic syndrome using the National Cholesterol Educational Program and International Diabetes Federation definitions. Curr Med Res Opin. (2005) 21:1157–9. 10.1185/030079905X53333 [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Zhang DZ. Relationship between nut consumption and metabolic syndrome: a meta-analysis of observational studies. J Am Coll Nutr. (2019) 38:499–505. 10.1080/07315724.2018.1561341 [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Zhang DZ. Associations of vegetable and fruit consumption with metabolic syndrome. A meta-analysis of observational studies. Public Health Nutr. (2018) 21:1693–703. 10.1017/S1368980018000381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo HB, Ding J, Liang JY, Zhang Y. Associations of whole grain and refined grain consumption with metabolic syndrome. A meta-analysis of observational studies. Front Nutr. (2021) 8:695620. 10.3389/fnut.2021.695620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo HB, Ding J, Liang JY, Zhang Y. Association of red meat and poultry consumption with the risk of metabolic syndrome: a meta-analysis of prospective cohort studies. Front Nutr. (2021) 8:691848. 10.3389/fnut.2021.691848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo HB, Ding J, Liu Q, Li YS, Liang JY, Zhang Y. Vitamin C and metabolic syndrome: a meta-analysis of observational studies. Front Nutr. (2021) 8:728880. 10.3389/fnut.2021.728880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherf-Dagan S, Buch A, Ben-Porat T, Sakran N, Sinai T. Vitamin E status among bariatric surgery patients: a systematic review. Surg Obes Relat Dis. (2021) 17:816–30. 10.1016/j.soard.2020.10.029 [DOI] [PubMed] [Google Scholar]
- 9.Kuwabara A, Nakade M, Tamai H, Tsuboyama-Kasaoka N, Tanaka K. The association between vitamin E intake and hypertension: results from the re-analysis of the National Health and Nutrition Survey. J Nutr Sci Vitaminol. (2014) 60:239–45. 10.3177/jnsv.60.239 [DOI] [PubMed] [Google Scholar]
- 10.Knekt P, Reunanen A, Marniemi J, Leino A, Aromaa A. Low vitamin E status is a potential risk factor for insulin-dependent diabetes mellitus. J Intern Med. (1999) 245:99–102. 10.1046/j.1365-2796.1999.00416.x [DOI] [PubMed] [Google Scholar]
- 11.Salonen JT, Nyyssönen K, Tuomainen TP, Mäenpää PH, Korpela H, Kaplan GA, et al. Increased risk of non-insulin dependent diabetes mellitus at low plasma vitamin E concentrations: a four year follow up study in men. BMJ. (1995) 311:1124–7. 10.1136/bmj.311.7013.1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asbaghi O, Sadeghian M, Nazarian B, Sarreshtedari M, Mozaffari-Khosravi H, Maleki V, et al. The effect of vitamin E supplementation on selected inflammatory biomarkers in adults: a systematic review and meta-analysis of randomized clinical trials. Sci Rep. (2020) 10:17234. 10.1038/s41598-020-73741-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasdev S, Gill V, Parai S, Gadag V. Dietary vitamin e supplementation attenuates hypertension in Dahl salt-sensitive rats. J Cardiovasc Pharmacol Ther. (2005) 10:103–11. 10.1177/107424840501000204 [DOI] [PubMed] [Google Scholar]
- 14.Noguchi T, Ikeda K, Sasaki Y, Yamamoto J, Yamori Y. Effects of vitamin E and sesamin on hypertension and cerebral thrombogenesis in stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. (2004) 31:S24–6. 10.1111/j.1440-1681.2004.04103.x [DOI] [PubMed] [Google Scholar]
- 15.Kamimura W, Doi W, Takemoto K, Ishihara K, Wang D, Sugiyama H, et al. Effect of vitamin E on alloxan-induced mouse diabetes. Clin Biochem. (2013) 46:795–8. 10.1016/j.clinbiochem.2013.02.016 [DOI] [PubMed] [Google Scholar]
- 16.Mardones P, Strobel P, Miranda S, Leighton F, Quiñones V, Amigo L, et al. α-Tocopherol metabolism is abnormal in scavenger receptor clas B type1 (SR-B1)-deficient mice. J Nutr. (2002) 132:443–9. 10.1093/jn/132.3.443 [DOI] [PubMed] [Google Scholar]
- 17.Ford ES, Mokdad AH, Giles WH, Brown DW. The metabolic syndrome and antioxidant concentrations: findings from the Third National Health and Nutrition Examination Survey. Diabetes. (2003) 52:2346–52. 10.2337/diabetes.52.9.2346 [DOI] [PubMed] [Google Scholar]
- 18.Kim MH, Lee HS, Park HJ, Kim WY. Risk factors associated with metabolic syndrome in Korean elderly. Ann Nutr Metab. (2007) 51:533–40. 10.1159/000112977 [DOI] [PubMed] [Google Scholar]
- 19.Kim WY, Kim JE, Choi YJ, Huh KB. Nutritional risk and metabolic syndrome in Korean type 2 diabetes mellitus. Asia Pac J Clin Nutr. (2008) 17 Suppl 1:47–51. [PubMed] [Google Scholar]
- 20.Czernichow S, Vergnaud AC, Galan P, Arnaud J, Favier A, Faure H, et al. Effects of long-term antioxidant supplementation and association of serum antioxidant concentrations with risk of metabolic syndrome in adults. Am J Clin Nutr. (2009) 90:329–5l. 10.3945/ajcn.2009.27635 [DOI] [PubMed] [Google Scholar]
- 21.Cho SW, Paek YM, Kang JY. Park Yk, Choi TI. The relationship between plasma antioxidant levels and metabolic syndrome risk factors in male workers. Korean J Food Nutr. (2009) 22:357–66. [Google Scholar]
- 22.Bruscato NM, Vieira J, Nascimento N, Canto M, Stobbe J, Gottlieb M G, et al. Dietary intake is not associated to the metabolic syndrome in elderly women. N Am J Med Sci. (2010) 2:182–8. 10.4297/najms.2010.2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beydoun MA, Shroff MR, Chen X, Beydoun HA, Wang YF. Zonderman AB. Serum antioxidant status is associated with metabolic syndrome among US adults in recent national surveys. J Nutr. (2011) 141:903–13. 10.3945/jn.110.136580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kouki R, Schwab U, Hassinen M, Komulainen P, Heikkilä H, Lakka TA, et al. Food consumption, nutrient intake and the risk of having metabolic syndrome: the DR's EXTRA Study. Eur J Clin Nutr. (2011) 65:368–77. 10.1038/ejcn.2010.262 [DOI] [PubMed] [Google Scholar]
- 25.Beydoun MA, Canas JA, Beydoun HA, Chen XL, Shroff MR. Zonderman AB. Serum antioxidant concentrations and metabolic syndrome are associated among US adolescents in recent national surveys. J Nutr. (2012) 142:1693–704. 10.3945/jn.112.160416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Oliveira Otto MC, Alonso A, Lee DH, Delclos GL, Bertoni AG, Jiang R, et al. Dietary intakes of zinc and heme iron from red meat, but not from other sources, are associated with greater risk of metabolic syndrome and cardiovascular disease. J Nutr. (2012) 142:526–33. 10.3945/jn.111.149781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odum EP, Orluwene CG, Ejilemele AA, Wakwe VC. Antioxidant status of subjects with Metabolic Syndrome in Port Harcourt, Nigeria. Niger Postgrad Med J. (2012) 19:199–203. [PubMed] [Google Scholar]
- 28.Al-Daghri NM, Khan N, Alkharfy KM, Al-Attas OS, Alokail MS, Alfawaz HA, et al. Selected dietary nutrients and the prevalence of metabolic syndrome in adult males and females in Saudi Arabia: a pilot study. Nutrients. (2013) 5:4587–604. 10.3390/nu5114587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bian S, Gao Y, Zhang M, Wang X, Liu W, Zhang D, et al. Dietary nutrient intake and metabolic syndrome risk in Chinese adults: a case-control study. Nutr J. (2013) 12:106. 10.1186/1475-2891-12-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Guo H, Wu M, Liu M. Serum and dietary antioxidant status is associated with lower prevalence of the metabolic syndrome in a study in Shanghai, China. Asia Pac J Clin Nutr. (2013) 22:60–8. 10.6133/apjcn.2013.22.1.06 [DOI] [PubMed] [Google Scholar]
- 31.Motamed S, Ebrahimi M, Safarian M, Ghayour-Mobarhan M, Mouhebati M, Azarpazhouh M, et al. Micronutrient intake and the presence of the metabolic syndrome. N Am J Med Sci. (2013) 5:377–85. 10.4103/1947-2714.114171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mah E, Sapper TN, Chitchumroonchokchai C, Failla ML, Schill KE, Clinton SK, et al. α-Tocopherol bioavailability is lower in adults with metabolic syndrome regardless of dairy fat co-ingestion: a randomized, double-blind, crossover trial. Am J Clin Nutr. (2015) 102:1070–80. 10.3945/ajcn.115.118570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei J, Zeng C, Gong Q, Li X, Lei G, Yang T. Associations between dietary antioxidant intake and metabolic syndrome. PLoS ONE. (2015) 10:e0130876. 10.1371/journal.pone.0130876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Godala MM, Materek-Kuśmierkiewicz I, Moczulski D, Rutkowski M, Szatko F, Gaszyńska E, et al. Lower plasma levels of antioxidant vitamins in patients with metabolic syndrome: a case control study. Adv Clin Exp Med. (2016) 25:689–700. 10.17219/acem/41049 [DOI] [PubMed] [Google Scholar]
- 35.Ahn S, Jun S, Kang M, Shin S, Wie G, Baik HW, et al. Association between intake of antioxidant vitamins and metabolic syndrome risk among Korean adults. J Nutr Health. (2017) 50:313–24. 10.4163/jnh.2017.50.4.313 [DOI] [Google Scholar]
- 36.Lim HS, Shin EJ, Yeom JW, Park YH, Kim SK. Association between Nutrient Intake and Metabolic Syndrome in Patients with Colorectal Cancer. Clin Nutr Res. (2017) 6:38–46. 10.7762/cnr.2017.6.1.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahn S, Jun S, Shin J, Ham D, Choi E, Joung H. Association between intake of antioxidant vitamins and metabolic syndrome prevalence among Korean adults. Curr Develop Nutr. (2019) 3:nzz044. 10.1093/cdn/nzz044.P24-001-19 [DOI] [Google Scholar]
- 38.Godala M, Gaszyńska E, Moczulski D, Materek-Kuśmierkiewicz I, Krzyzak M. An assessment of the antioxidant vitamins concentration in people with metabolic syndrome working in agriculture. Med Pr. (2020) 23:128769. 10.13075/mp.5893.01046 [DOI] [PubMed] [Google Scholar]
- 39.Kim T, Kang J. Association between serum retinol and α-tocopherol levels and metabolic syndrome in Korean general population: analysis of population-based nationally representative data. Nutrients. (2020) 12:1689. 10.3390/nu12061689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng Z, Wang Y, Huang X, Zhu Q, Zhao Y, Xie H, et al. Dietary vitamin intake and risk of metabolic syndrome among centenarians in China. Exp Ther Med. (2021) 21:105. 10.3892/etm.2020.9537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaeemzadeh N, Sadatmahalleh SJ, Ziaei S, Kazemnejad A, Movahedinejad M, Mottaghi A, et al. Comparison of dietary micronutrient intake in PCOS patients with and without metabolic syndrome. J Ovarian Res. (2021) 14:10. 10.1186/s13048-020-00746-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339: b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergin P, Leggett A, Cardwell CR, Woodside JV, Thakkinstian A, Maxwell AP, et al. The effects of vitamin E supplementation on malondialdehyde as a biomarker of oxidative stress in haemodialysis patients: a systematic review and meta-analysis. BMC Nephrol. (2021) 22:126. 10.1186/s12882-021-02328-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuhara H, Steinmaus C, Cohen S, Corley D, Tei Y, Buffler P. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am J Gastroenterol. (2011) 106:1911–22. 10.1038/ajg.2011.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 46.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. (2012) 5:9–19. 10.1097/WOX.0b013e3182439613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spiteller G. Peroxyl radicals: inductors of neurodegenerative and other inflammatory diseases. Their origin and how they transform cholesterol, phospholipids, plasmalogens, polyunsaturated fatty acids, sugars, and proteins into deleterious products. Free Radic Biol Med. (2006) 41:362–87. 10.1016/j.freeradbiomed.2006.03.013 [DOI] [PubMed] [Google Scholar]
- 48.Saboori S, Shab-Bidar S, Speakman JR. Rad EYousefi, Djafarian K. Effect of vitamin E supplementation on serum C-reactive protein level: a meta-analysis of randomized controlled trials. Eur J Clin Nutr. (2015) 69:867–73. 10.1038/ejcn.2014.296 [DOI] [PubMed] [Google Scholar]
- 49.Pastor RF, Repetto MG, Lairion F, Lazarowski A, Merelli A, Carabetti ZM, et al. Supplementation with resveratrol, piperine and alpha-tocopherol decreases chronic inflammation in a cluster of older adults with metabolic syndrome. Nutrients. (2020) 12:3149. 10.3390/nu12103149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Devaraj S, Leonard S, Traber MG, Jialal I. Gamma-tocopherol supplementation alone and in combination with alpha-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome. Free Radic Biol Med. (2008) 44:1203–8. 10.1016/j.freeradbiomed.2007.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Traber MG, Vitamin E. Regulatory mechanisms. Annu Rev Nutr. (2007) 27:347–62. 10.1146/annurev.nutr.27.061406.093819 [DOI] [PubMed] [Google Scholar]
- 52.Fairus S, Nor RM, Cheng HM, Sundram K. Alpha-tocotrienol is the most abundant tocotrienol isomer circulated in plasma and lipoproteins after postprandial tocotrienol-rich vitamin E supplementation. Nutr J. (2012) 11:5. 10.1186/1475-2891-11-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Food and Nutrition Board, Institute Institute of Medicine . Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium and Carotenoids. Washington DC: National Academy Press; (2000). [Google Scholar]
- 54.Theriault A, Jun-Tzu C, Wang Q, Gapor A, Adeli K. Tocotrienol: a review of its therapeutic potential. Clin Biochem. (1999) 32:309–19. 10.1016/S0009-9120(99)00027-2 [DOI] [PubMed] [Google Scholar]
- 55.Sylvester PW, Theriault A. Role of tocotrienols in the preventive of cardiovascular disease and breast cancer. Curr Top Nutraceutical Res. (2003) 1:121–36. [Google Scholar]
- 56.Sen CK, Khanna S, Roy S. Tocotrienols: vitamin E beyond tocopherols. Life Sci. (2006) 78:2088–98. 10.1016/j.lfs.2005.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seo JA, Song SW, Han K, Lee KJ, Kim HN. The associations between serum zinc levels and metabolic syndrome in the Korean population: Findings from the 2010 Korean national health and nutrition examination survey. PLoS ONE. (2014) 9:e105990. 10.1371/journal.pone.0105990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim HN, Song SW, Choi WS. Association between serum zinc level and body composition: The Korean National Health and Nutrition Examination Survey. Nutrition. (2016) 32:332–7. 10.1016/j.nut.2015.09.006 [DOI] [PubMed] [Google Scholar]
- 59.Zillikens MC, van Meurs J, Rivadeneira F, Hofman A, Oostra BA, Sijbrands E, et al. Interactions between dietary vitamin E intake and SIRT1 genetic variation influence body mass index. Am J Clin Nutr. (2010) 91:1387–93. 10.3945/ajcn.2009.28627 [DOI] [PubMed] [Google Scholar]
- 60.Ejtahed H, Mahmoodi Z, Qorbani M, Angoorani P, Motlagh ME, Hasani-Ranjbar S, et al. A comparison between body mass index and waist circumference for identifying continuous metabolic syndrome risk score components in Iranian school-aged children using a structural equation modeling approach: the CASPIAN-V study. Eat Weight Disord. (2021) 26:1609–16. 10.1007/s40519-020-00971-y [DOI] [PubMed] [Google Scholar]
- 61.Kang M, Joo M, Hong H, Kang H. Eating speed, physical activity, and cardiorespiratory fitness are independent predictors of metabolic syndrome in Korean University Students. Nutrients. (2021) 13:2420. 10.3390/nu13072420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alghadir AH, Gabr SA, Anwer S, Li H. Associations between vitamin E, oxidative stress markers, total homocysteine levels, and physical activity or cognitive capacity in older adults. Sci Rep. (2021) 11:12867. 10.1038/s41598-021-92076-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hermenegildo-López Y, Donat-Vargas C, Sandoval-Insausti H, Moreno-Franco B, Rodríguez-Ayala M, Rey-García J, et al. A Higher intake of energy at dinner is associated with incident metabolic syndrome: a prospective cohort study in older adults. Nutrients. (2021) 13:3035. 10.3390/nu13093035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shin S, Lee HW, Kim CE, Lim J, Lee J, Lee S, et al. Egg consumption and risk of metabolic syndrome in Korean adults: results from the health examinees study. Nutrients. (2017) 9:687. 10.3390/nu9070687 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.