Abstract
This paper has selected dicyclohexanocucurbit[6]uril (CyH2Q[6]) as the host and 2-phenylbenzimidazole (G) as the guest to investigate the host–guest interaction mode between CyH2Q[6] and G. Under acidic conditions, the complex was characterized using nuclear magnetic resonance, ultraviolet and fluorescence spectroscopy. The results show that the molecular ratio of CyH2Q[6] to G is 2 : 1. The crystals were cultured with ZnCl2 as a structural inducer under acidic conditions and single crystal X-ray diffraction showed that the molecular ratio of CyH2Q[6] to G is 1 : 3. The G@CyH2Q[6] was used as a fluorescent probe to identify metal cations. The probe exhibits a good selective recognition effect toward Fe3+ ions, which involves a reduced fluorescence intensity with a limit of detection of 1.321 × 10–6 mol l–1.
Keywords: CyH2Q[6], 2-phenylbenzimidazole, host–guest interaction, ion recognition, limit of detection
1. Introduction
Benzimidazole compounds [1–5] are aromatic heterocyclic compounds containing two nitrogen atoms. Benzimidazole derivatives and metal complexes exhibit good biological activity. Benzimidazole and its derivatives containing an imidazole ring have important medicinal value and have important applications as anti-fungal, anti-rheumatic, deworming, analgesic, anti-inflammatory and anti-cancer agents, among others [6–13]. Fe3+ is widely distributed in nature and is one of the indispensable trace elements found in the human body. It plays an important role in metabolism. Cucurbit[n]uril (Q[n] or CB[n]) [14–28] is a new type of macrocyclic compound discovered after cyclodextrins, crown ethers and calixarenes. However, most ordinary cucurbit[n]uril have poor solubility, the development of cucurbit[n]urils has been significantly limited. Through the efforts of some researchers, several modified cucurbit[n]urils, such as methyl-, hydroxyl-, cyclopentyl- and cyclohexyl-substituted cucurbit[n]urils have been reported [29–35]. Dicyclohexanocucurbit[6]uril (CyH2Q[6]) is a modified cucurbituril, which has made great contributions in host–guest chemistry, coordination chemistry, etc. [36–39].
Herein, CyH2Q[6] was selected as the host and 2-phenylbenzimidazole (G) used as the guest to study the host–guest interaction (figure 1). Nuclear magnetic resonance (NMR), ultraviolet (UV) and fluorescence spectroscopy, and single crystal X-ray diffraction were used to characterize the host–guest complex. Regarding the study of cucurbit[nuril induced to sensing for metal ions many scientific researchers have made great efforts and have reported in related fields40,41]. Specifically, CyH2Q[6] with 2-phenylbenzimidazole was designed as a fluorescent probe and its ability to recognize metal ions was explored using the change in its fluorescence intensity. The results show that the fluorescent probe can selectively recognize Fe3+ cations, which exhibit a reduced fluorescence intensity and can be used to detect the Fe3+ concentration in a sample using the reduced fluorescence effect.
Figure 1.
The structure of (a,b) CyH2Q[6] and (c) 2-phenylbenzimidazole.
2. Experimental section
2.1. Instruments and reagents
Bruker D8 Venture X-ray single crystal diffractometer (Bruker, Germany), JNM-ECZ400S/L1 400M superconducting NMR spectrometer, a UV-2700 dual-beam UV–vis spectrophotometer (Shimadzu Instruments Co. Ltd), VARIANCARYE-CLIPSE fluorescence spectrophotometer (Varian, USA). All materials were reagent grade and used without any further purification. CyH2Q[6] (purity ≥97%) was prepared in the Key Laboratory of Macrocyclic and Supramolecular Chemistry of Guizhou Province, China.
2.2. Interaction between CyH2Q[6] and 2-phenylbenzimidazole: 1H NMR titration
CyH2Q[6] was added to deuterated water containing a certain amount of deuterated hydrochloric acid to prepare a solution with a concentration of 1.0 × 10–3 mol l–1 (pH = 1). Fifty microlitres of this solution and 450 µl of deuterium chloride solution was loaded into an 1H NMR tube and a certain amount of 2-phenylbenzimidazole (n(G)/n(CyH2Q[6]) = 0.2, 0.4, 0.6, …) was added in sequence and the 1HNMR spectrum recorded at 293 K.
2.3. UV and fluorescence titration
CyH2Q[6] and 2-phenylbenzimidazole were formulated into an acidic aqueous solution (pH = 1) with a concentration of 1.00 × 10–2 and 1.00 × 10–3 mol l–1, respectively, to keep the concentration of the guest unchanged and an appropriate amount of CyH2Q[6] (n(G)/n(CyH2Q[6]) = 0.2, 0.4, 0.6, …) was then added and analysed using UV absorption and fluorescence spectroscopy.
2.4. Crystal culture and testing
CyH2Q[6] (10 mg, 9.05 µmol) and 2-phenylbenzimidazole (5 mg, 25.76 µmol) were dissolved in a 10 ml round bottom flask containing 5 ml of HCl (3 mol l−1), and ZnCl2 (0.67 g) was added. The resulting mixture was shaken for 3 min at room temperature and heated to reflux for 5 min with stirring. The mixture was cooled and left to stand for about one week to obtain colourless crystals. A regular and transparent crystal of the complex was selected and fixed on a glass filament using petroleum jelly for X-ray single crystal diffraction analysis on a Bruker D8 Venture X-ray single crystal diffractometer in ω-scan mode using graphite to monochromatize the Mo-Kα rays (λ = 0.71073 Å, μ = 0.828 mm–1). The crystal data were collected and Lorentz polarization, and absorption correction carried out. SHELXT-14 and SHELXL-14 program packages were used for structural analysis and full matrix least-squares refinement. All non-hydrogen atoms were anisotropically refined using the analytical expression of the neutral atom scattering factor and combined with anomalous dispersion correction. The SQUEEZE program in the PLATON package was used to delete some of the disordered solvent molecules. The X-ray crystallographic data for structures reported in this study have been deposited in the Cambridge Crystallographic Data Center under accession no. CCDC: 2100651. These data can be obtained free of charge via https://www.ccdc.cam.ac.uk/data_request/cif. The crystal data and structure modification parameters are shown in table 1.
Table 1.
The crystallographic parameters of the complex.
| empirical formula | C83H82Cl8N30O14Zn2 | Dc (g cm −3) | 1.349 |
| Mr | 2138.12 | F(000) | 4392 |
| crystal system | monoclinic | μ (mm−1) | 0.729 |
| space group | P21/c | data/params | 17 898/1187 |
| a (Å) | 15.6869(8) | Rint | 0.1002 |
| b (Å) | 48.224(2) | R[I > 2σ(I)]a | 0.1320 |
| c (Å) | 14.3174(6) | wR[I > 2σ(I)]b | 0.3543 |
| α (deg) | 90 | R(all data) | 0.1779 |
| β (deg) | 103.561(2) | wR(all data) | 0.3808 |
| γ (deg) | 90 | GOF (F2) | 1.350 |
| V [Å3] | 10529.0(9) | T (K) | 300.0 |
| Z | 4 | CCDC | 2 100 651 |
aConventional R on Fhkl: ∑||Fo| − |Fc||/∑|Fo|.
bWeighted R on |Fhkl|2: .
2.5. Selective fluorescence measurement of metal ions
First, all the metal ions studied were accurately prepared into 0.2 mol l–1 solutions using deionized water and then, under acidic conditions (pH = 1), a solution of G at a concentration of 5 × 10−5 mol l–1 prepared with deionized water was used to form a G@CyH2Q[6] solution. Three millilitres of the 5 × 10–5 mol l–1 G@CyH2Q[6] solution was added to a quartz fluorescent cuvette and the different metal cation solutions added. The amount of metal cation added to the G@CyH2Q[6] probe was 10 times the equimolar concentration. A fluorophotometer was used to detect the fluorescence intensity change at the maximum excitation wavelength (λex = 298 nm); the slit width was 5 nm/5 nm, voltage was 400 V, and scanning range was 250–600 nm.
2.6. Fluorescent quenching experiment of metal ions
Under acidic conditions (pH = 1), a solution of G@CyH2Q[6] and Fe3+ at a concentration of 5 × 10–5 mol l–1 was prepared with deionized water and 3 ml of the resulting solution was added to a quartz fluorescent cuvette. Different metal cation solutions were added to the solution; the amount of metal cation added was 10 times the equimolar concentration of the G@CyH2Q[6] and Fe3+ solution. After mixing uniformly, a fluorophotometer was used to detect the fluorescence signal intensity within 2 min at the maximum excitation wavelength (λex = 298 nm); the slit width was 5 nm/5 nm, voltage was 400 V, and scanning range was 250–600 nm.
2.7. Titration experiment of Fe3+
Three millilitres of a 5 × 10–5 mol l–1 solution of the G@CyH2Q[6] probe was added to a quartz cuvette, followed by Fe3+ and G@CyH2Q[6] = 0, 0.5, 1.0, 1.5, 2.0, 2.5. After adding 3.0, 3.5, 4.0, … , 28.0 equivalent of the Fe3+ solution to the quartz cuvette in turn and mixing uniformly, a fluorophotometer was used to detect the fluorescence signal intensity within 2 min at the maximum excitation wavelength (λex = 298 nm); the slit width was 5 nm/5 nm, voltage was 400 V, and scanning range was 250–600 nm.
3. Results and discussion
3.1. 1HNMR Titration of the host–guest interaction
Figure 2 shows the 1HNMR titration spectra obtained when adding different equivalents of 2-phenylbenzimidazole (G) to CyH2Q[6]. It can be seen from the figure that, upon the addition of G, each proton peak of G splits into two groups of peaks, moving to the high field and low field, respectively. A preliminary judgement can be made on this. There are two modes of action for G and CyH2Q[6] (shown by the black and red curves in figure 2), namely mode a and mode b. In mode a, the benzimidazole part enters the cavity of CyH2Q[6] and the benzene ring is outside the port of CyH2Q[6]; the benzimidazole part of G is shielded and the benzene ring is unshielded. In mode b, the benzene ring enters the cavity of CyH2Q[6] and the benzimidazole part is outside the port of CyH2Q[6]; the benzene ring part of G is shielded and the benzimidazole is unshielded. When 0.4 equivalents of G were added, the chemical shift values of H2a and H1a in G correspond to their free state moving 0.74 and 0.64 ppm to the high field, respectively; H3a and H4a move 0.37 and 0.09 ppm to the low field, respectively. The chemical shift values of H3b and H4b correspond to their free state moving 1.27 and 0.52 ppm to the high field, respectively; H2b and H1b move 0.24 and 0.07 ppm to the low field, respectively. When 0.6 equivalents of G were added, the chemical shift of the proton signal peaks of G are the same as the chemical shifts observed after adding 1.0 equivalent, and a free guest peak appears at the same time. This shows that the amount of the guest was excessive, indicating that the ratio of CyH2Q[6] to G was 2 : 1.
Figure 2.
1HNMR spectra (25°C, 400 MHz) of the interaction between CyH2Q[6] and G recorded in D2O upon adding (a) 0, (b) 0.4, (c) 0.6 and (d) 1.0 equivalents of G, and (e) pure G.
3.2. UV absorption spectroscopy of the host–guest interaction
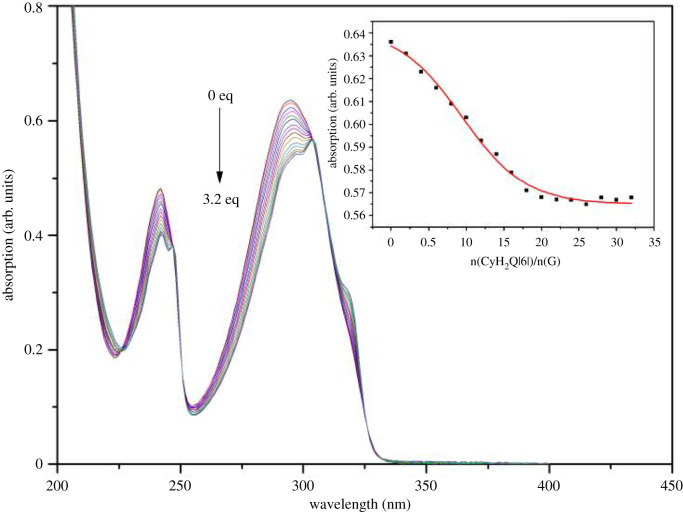
Figure 3 shows the UV absorption spectra obtained upon the interaction of CyH2Q[6] with G. n(CyH2Q[6])/n(G) = 0.2, 0.4, 0.6, … , 3.2, was added to the guest solution and the UV spectra recorded. As the amount of CyH2Q[6] added to the guest solution increases, the UV absorption of the guest shows a regular change and gradually decreases. When the host–guest ratio was 2 : 1, the fitting curve exhibits an inflection point and as the concentration of CyH2Q[6] further increases, the absorption intensity basically remains unchanged, indicating that the ratio of CyH2Q[6] to G was 2 : 1.
Figure 3.
UV spectra and trend chart obtained for the solution of G (5 × 10–5 mol l–1) upon gradually adding CyH2Q[6] (0, 0.2, 0.4, 1.6, … , 3.2 equivalents).
3.3. Crystal structure
CyH2Q[6] and 2-phenylbenzimidazole (G) were added with ZnCl2 as a structural inducer and the crystal structure of the resulting complex was determined using X-single crystal diffraction. The asymmetric structural unit of the complex includes one CyH2Q[6] molecule, three molecules of G, two [ZnCl4]2– ions and two free H2O molecules (figure 4a). Figure 4b clearly shows that G enters the cavity of the cucurbit[n]uril, and the two nitrogen atoms on the imidazole ring interact with the carbonyl oxygen atoms at the port of the cucurbit[n]uril via hydrogen bonding (as shown in figure 4b). Figure 4c shows the stacking diagram of the crystal structure of the complex viewed along the c-axis. The other two molecules of 2-phenylbenzimidazole act as a bridge connecting the two layers of cucurbit[n]uril, so that the complexes form a regular arrangement.
Figure 4.
Crystal structure of CyH2Q[6] and G: (a) Asymmetric structural unit, (b) host–guest interaction, and (c) stacked diagram of the structure along the c-axis.
3.4. Fluorescence spectroscopy of the host–guest interaction
Figure 5 shows the fluorescence spectra obtained for the interaction between CyH2Q[6] and G at the maximum fluorescence emission wavelength of 363.07 nm and maximum absorption wavelength of 295 nm. Three millilitres of a 5 × 10–5 mol l–1 solution of G was added to a quartz cuvette and CyH2Q[6] slowly added to the guest solution at the following ratios: (CyH2Q[6])/n(G) = 0.2, 0.4, 0.6, … , 4.0. The UV spectra indicate that the fluorescence intensity gradually increases, but upon reaching n(CyH2Q[6])/n(G) = 2 : 1, the fluorescence intensity only gradually changes.
Figure 5.
Fluorescence titration spectra of the host–guest interaction between CyH2Q[6] and G.
3.5. Determination of the metal cations selectivity using fluorescence spectroscopy
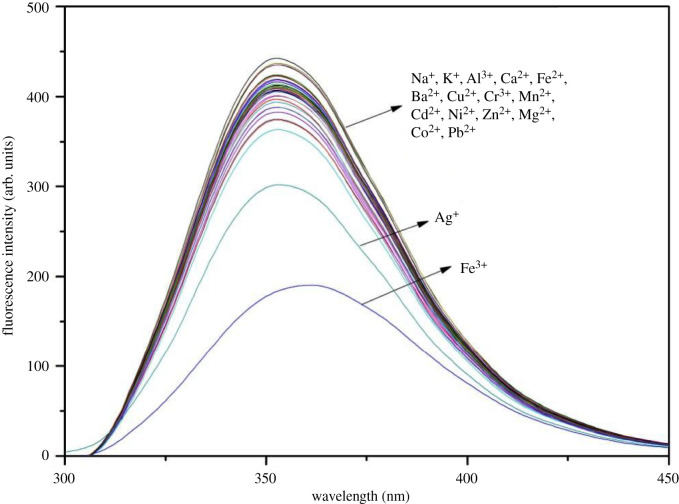
The selectivity of G@CyH2Q[6] toward common metal cations was monitored using fluorescence spectroscopy (figure 6). In the fluorescence range of 600 nm, the G@CyH2Q[6] probe exhibits a strong fluorescence intensity. Upon adding ten equivalents of different metal cations (Hg2+, Ca2+, Na+, Mg2+, Al3+, Cd2+, Cu2+, Pb2+, Ni2+, Co2+, Cr3+, …), most of the metal cations do not affect the fluorescence intensity of the host and guest complex. However, Fe3+ ions cause a significant decrease in the fluorescence intensity, which indicates that the probe was highly selective toward Fe3+ ions.
Figure 6.
The fluorescence spectra of the G@CyH2Q[6] (5 × 10–5 mol l–1) probe in the presence of various metal cations (10 eq) recorded in an acidic aqueous solution (pH = 1).
3.6. Investigation of the interference of metal cations on the probe using florescence spectroscopy
Figure 7 shows the interference of other metal cations on the probe's recognition of Fe3+. The specific operation of this experiment was as follows: 3 ml of a 5 × 10–5 mol l–1 solution of G@CyH2Q[6] and Fe3+ was added to a quartz cuvette and its fluorescence intensity measured. Different metal cation solutions (the concentration of the metal cations was 10 times that of the probe) were then added to compare whether the fluorescence intensity changes upon adding the different metal cations. The black bars in the figure shows the fluorescence intensity of the G@CyH2Q[6] and Fe3+ solution at an excitation wavelength of 298 nm and the red bars indicate the fluorescence intensity of the G@CyH2Q[6] and Fe3+ solution upon the addition of other metal cations. It can be seen from the figure that after adding other metal cations to the G@CyH2Q[6] and Fe3+ solution, the fluorescence intensity was changed, but the degree of change was not large. This fully shows that the Ag+, Tb3+, Er3+ and Hg2+ ions have weak interference to the recognition of Fe3+, and other metal cations cannot interfere with the recognition of Fe3+ by the complex.
Figure 7.
The change in the fluorescence intensity after adding different metal ions to the G@CyH2Q[6] and Fe3+ system.
3.7. Limit of detection for the Fe3+ titration detection probe
As shown in figure 8, the concentration of the fixed probe G@CyH2Q[6] is 5 × 10–5 mol l–1, and the Fe3+ solution of 0.2 molar ratio is added dropwise in turn. With the increase of the concentration of Fe3+ added, the fluorescence intensity gradually decreases. According to the detection limit formula (3σ/K), the detection limit of G@CyH2Q[6] for Fe3+ is calculated, and the detection limit is 1.321 × 10–6 mol l−1, and the linear correlation is R2 = 0.99055. It is lower than the maximum Fe3+ content of 0.3 mg l−1 specified in the national drinking water sanitation standard (GB5749-2006), so the probe G@CyH2Q[6] can effectively identify Fe3+ in drinking water.
Figure 8.
Fluorescence titration of Fe3+ and G@CyH2Q[6] (a) and fluorescence intensity calibration curve (b).
4. Conclusion
In this paper, 2-phenylbenzimidazole was selected as the guest molecule and CyH2Q[6] was used as the host to form an inclusion complex, which was used as a fluorescent probe to identify metal cations. Firstly, the interaction between the CyH2Q[6] host and 2-phenylbenzimidazole guest in the liquid phase was studied using NMR, UV and fluorescence spectroscopy. The experimental results showed that CyH2Q[6] and 2-phenylbenzimidazole formed a 2 : 1 inclusion compound. There are two modes of action under acidic conditions (pH = 1). One mode is the entry of the benzimidazole into the cavity of the cucurbit[n]uril and the portal interacts with the benzene ring. The other mode is that the benzene ring enters into the cavity of the cucurbit[n]uril and the benzimidazole interacts with the portal. The interaction between CyH2Q[6] and 2-phenylbenzimidazole in the solid phase was studied using single crystal X-ray diffraction. The crystal structure results showed that CyH2Q[6] forms a 1 : 3 inclusion compound with 2-phenylbenzimidazole. The recognition of metal cations between CyH2Q[6] and 2-phenylbenzimidazole was studied and the fluorescent probe (G@CyH2Q[6]) was constructed under acidic conditions (pH = 1). The recognition ability of the probe toward metal cations has been studied. The G@CyH2Q[6] probe can selectively identify Fe3+ ions with a limit of detection of 1.321 × 10–6 mol l–1 and has a strong ability to resist cation interference.
Supplementary Material
Acknowledgements
We thank the experts of the Key Laboratory of Macrocyclic Chemistry and Supramolecular Chemistry of Guizhou University for their technical guidance. We also thank several anonymous reviewers and editors who provided comments that greatly improved the manuscript.
Data accessibility
Data available from: https://www.ccdc.cam.ac.uk/data_request/cif.
Authors' contributions
J.Z. and L.A.: conceptualization, methodology, software, data curation, investigation, writing—original draft; J.G. and L.Z.: validation, formal analysis, visualization; X.Y., W.Z. and S.C.: resources, writing—review, editing, supervision, data curation; P.M.: writing—review and editing. All authors gave final approval for publication. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 21762011) and Guizhou Science and Technology Planning Project (Guizhou Science and Technology Cooperation Platform Talent [2017]5788).
References
- 1.Román DO, Peralta AS, Pistolis G, Petrou AL, Kaloudi-Chantzea A, Esturau-Escofet N, Durán-Hernández J, Sosa-Torres ME, Castillo-Blum SE. 2018. Lanthanide coordination compounds with benzimidazole-based ligands. luminescence and EPR. J. Mol. Struct. 1163, 252-261. ( 10.1016/j.molstruc.2018.02.062) [DOI] [Google Scholar]
- 2.Sekerci M, Atalay Y, Yakuphanoglu F, Avcı D, Başoğlu A. 2007. A theoretical study on 1-(thiophen-2-yl-methyl)-2-(thiophen-2-yl)-1H-benzimidazole. Spectrochim. Acta, Part A 67, 503-508. ( 10.1016/j.saa.2006.08.007) [DOI] [PubMed] [Google Scholar]
- 3.Zhao FH, Guo WY, Li SY, Li Z-L, Yan X-Q, Jia X-M, Huang L-W, You J-M. 2019. Two entangled photoluminescent MOFs of naphthalenedisulfonate and bis(benzimidazole) ligands for selective sensing of Fe3+. J. Solid State Chem. 278, 120926. ( 10.1016/j.jssc.2019.120926) [DOI] [Google Scholar]
- 4.Bamberger E, Lorenzen J. 1892. Zur Kenntniss der Benzimidazole. Ber. Dtsch. Chem. Ges. 25, 269-273. ( 10.1002/cber.18920250141) [DOI] [Google Scholar]
- 5.Romburgh P, Huyser HW. 1930. The formation of derivatives of dihydro-benzimidazole and tetrahydroquinoxaline by the action of acetic anhydride and zinc chloride on nitro-derivatives of alkylanilines. Recueil des Travaux Chimiques des Pays-Bas 49, 165-176. ( 10.1002/recl.19300490211) [DOI] [Google Scholar]
- 6.Aanandhi MV, Sachin JA. 2021. Molecular docking Q-SAR studies of benzimidazole as antifungal nucleus. Res. J. Pharm. Technol. 14, 2191-2194. ( 10.52711/0974-360X.2021.00388) [DOI] [Google Scholar]
- 7.Ahuja R, Sidhu A, Bala A, Arora D, Sharma P. 2020. Structure based approach for twin-enzyme targeted benzimidazolyl-1,2,4-triazole molecular hybrids as antifungal agents. Arab. J. Chem. 13, 5832-5848. ( 10.1016/j.arabjc.2020.04.020) [DOI] [Google Scholar]
- 8.Küçükbav H, Durmaz R, Güven M, Günal S. 2001. Synthesis of some benzimidazole derivatives and their antibacterial and antifungal activities. Arzneimittelforschung 51, 420-424. ( 10.1055/s-0031-1300057) [DOI] [PubMed] [Google Scholar]
- 9.Montresor A, Gabrielli AF, Diarra A, Engels D. 2010. Estimation of the cost of large-scale school deworming programmes with benzimidazoles. Trans. R. Soc. Trop. Med. Hyg. 104, 129-132. ( 10.1016/j.trstmh.2009.10.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chikkula KV, Sundararajan R. 2017. Analgesic, anti-inflammatory, and antimicrobial activities of novel isoxazole/pyrimidine/pyrazole substituted benzimidazole analogs. Med. Chem. Res. 26, 3026-3037. ( 10.1007/s00044-017-2000-0) [DOI] [Google Scholar]
- 11.Gaba M, Singh S, Mohan C. 2014. Benzimidazole: an emerging scaffold for analgesic and anti-inflammatory agents. Eur. J. Med. Chem. 76, 494-505. ( 10.1016/j.ejmech.2014.01.030) [DOI] [PubMed] [Google Scholar]
- 12.Fernando CT, Rubiño MEG, López CL, Daniel FK. 2015. Imidazoles and benzimidazoles as tubulin-modulators for anti-cancer therapy. Curr. Med. Chem. 22, 1312-1323. ( 10.2174/0929867322666150114164032) [DOI] [PubMed] [Google Scholar]
- 13.Sireesha R, Sreenivasulu R, Chandrasekhar C, Jadav SS, Pavani Y, Rao MVB, Subbarao M. 2021. Design, synthesis, anti-cancer evaluation and binding mode studies of benzimidazole/benzoxazole linked β-carboline derivatives. J. Mol. Struct. 1226, 129351. ( 10.1016/j.molstruc.2020.129351) [DOI] [Google Scholar]
- 14.Lagona J, Mukhopadhyay P, Chakrabarti S, Isaacs L. 2005. The Cucurbit[n]uril Family. Angw. Chem. Int. Ed. 44, 4844-4870. ( 10.1002/anie.200460675) [DOI] [PubMed] [Google Scholar]
- 15.Lee JW, Samal S, Selvapalam N, Kim H-J, Kim K. 2003. Cucurbituril homologues and derivatives: new opportunities in supramolecular chemistry. Accounts Chem. Res. 36, 621-630. ( 10.1021/ar020254k) [DOI] [PubMed] [Google Scholar]
- 16.Gerasko OA, Samsonenko DG, Fedin VP. 2002. Supramolecular chemistry of cucurbiturils. Russian Chem. Rev. 71, 741-760. ( 10.1070/RC2002v071n09ABEH000748) [DOI] [Google Scholar]
- 17.Elemans JAAW, Rowan AE, Nolte RJM. et al. 2000. Self-assembled architectures from glycoluril. Ind. Eng. Chem. Res. 39, 3419-3428. ( 10.1021/ie000079g) [DOI] [Google Scholar]
- 18.Hubin TJ, Kolchinski AG, Vance AL, Busch DH. 1999. Advances in supramolecular chemistry. Adv. Supramol. Chem. 5, 237-357. ( 10.1016/S1068-7459(99)80017-4) [DOI] [Google Scholar]
- 19.Mock WL. 1995. Cucurbituril. Top. Curr. Chem. 175, 1-24. ( 10.1007/3-540-58800-0_16) [DOI] [Google Scholar]
- 20.Cintas PJ. 1994. Cucurbituril: supramolecular perspectives for an old ligand. Incl. Phenom. Molec. Reco. Chem. 17, 205-220. ( 10.1007/BF00708781) [DOI] [Google Scholar]
- 21.Yang RY, Deng XY, Huang Y, Zhang Y-Q, Tao Z. 2020. Recognition of lanthanide metal cations by t-DSMI@Alkyl-substituted cucurbit[6]uril probes. ChemistrySelect. 5, 8649-8655. ( 10.1002/slct.202000021) [DOI] [Google Scholar]
- 22.Lin RL, Li R, Shi H, Zhang K, Meng D, Sun W-Q, Chen K, Liu J-X. 2020. Symmetrical-tetramethyl-cucurbit[6]uril-driven movement of cucurbit[7]uril gives rise to heterowheel [4]pseudorotaxanes. J. Org. Chem. 85, 3568-3575. ( 10.1021/acs.joc.9b03283) [DOI] [PubMed] [Google Scholar]
- 23.Day A, Arnold AP, Blanch RJ, Snushall B. 2001. Controlling factors in the synthesis of cucurbituril and its homologues. J. Org. Chem. 66, 8094-8100. ( 10.1021/jo015897c) [DOI] [PubMed] [Google Scholar]
- 24.Jansen K, Buschmann HJ, Wego A, Döpp D, Mayer C, Drexler H-J, Holdt H-J, Schollmeyer E. 2001. Cucurbit [5] uril, decamethylcucurbit [5] uril and cucurbit [6] uril: synthesis, solubility and amine complex formation. J. Incl. Phenom. Macrocycl. Chem. 39, 357-363. ( 10.1023/A:1011184725796) [DOI] [Google Scholar]
- 25.Kim J, Jung IS, Kim SY, Lee E, Kang J-K, Sakamoto S, Yamaguchi K, Kim K. et al. 2000. New cucurbituril homologues: syntheses, isolation, characterization, and X-ray crystal structures of cucurbit[n]uril (n = 5, 7, and 8). J. Am. Chem. Soc. 122, 540-541. ( 10.1021/ja993376p) [DOI] [Google Scholar]
- 26.Freeman WA, Mock WL, Shih NY. 1981. Cucurbituril. J. Am. Chem. Soc. 103, 7367-7368. ( 10.1021/ja00414a070) [DOI] [Google Scholar]
- 27.Xu HP, Dong L, Bin Z, Yansong H, Shaofeng L, Chang L, Chen C, Changli W. 2020. Supramolecular self-assembly of a hybrid ‘hyalurosome’ for targeted photothermal therapy in non-small cell lung cancer. Drug Deliv. 27, 378-386. ( 10.1080/10717544.2020.1730521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Day AI, Blanch RJ, Arnold AP, Lorenzo S, Lewis GR, Dance I. et al. 2002. A cucurbituril-based gyroscane: a new supramolecular form. Angew. Chem. Int. Ed. 41, 275-277. ( 10.1002/1521-3773(20020118)41:2) [DOI] [PubMed] [Google Scholar]
- 29.Flinn A, Hough GC, Stoddart JF, Williams DJ. 1992. Decamethylcucurbit[5]uril. Angew. Chem. Int. Edit. 31, 1475-1477. ( 10.1002/anie.199214751) [DOI] [Google Scholar]
- 30.Zhao JH, Kim HJ, Oh JH, Kim SY, Lee JW, Sakamoto S, Yamaguchi K, Kim K. 2001. Cucurbit[n]uril derivatives soluble in water and organic solvents. Angew. Chem. 113, 4363-4365. () [DOI] [PubMed] [Google Scholar]
- 31.Wu F, Wu LH, Xiao X, Zhang YQ, Xue SF, Tao Z, Day AI. 2012. Locating the cyclopentano cousins of the cucurbit[n]uril family. J. Org. Chem. 77, 606-611. ( 10.1021/jo2021778) [DOI] [PubMed] [Google Scholar]
- 32.Wu LH, Ni XL, Wu F, Zhang YQ, Zhu QJ, Xue SF, Tao Z. 2009. Crystal structures of three partially cyclopentano-substituted cucurbit[6]urils. J. Mol. Struct. 920, 183-188. ( 10.1016/j.molstruc.2008.10.057) [DOI] [Google Scholar]
- 33.Jon SY, Selvapalam N, Oh DH, Kang JK, Kim SY, Jeon YJ, Lee JW, Kim K. 2003. Facile synthesis of cucurbit[n]uril derivatives via direct functionalization: expanding utilization of cucurbit[n]uril. J. Am. Chem. Soc. 125, 10 186-10 187. ( 10.1021/ja036536c) [DOI] [PubMed] [Google Scholar]
- 34.Isobe H, Sato S, Nakamura E. 2002. Synthesis of disubstituted cucurbit[6]uril and its rotaxane derivative. Org. Lett. 4, 1287-1289. ( 10.1021/ol025749o) [DOI] [PubMed] [Google Scholar]
- 35.Jin YM, Huang TH, Zhao WW, Yang XN, Meng Y, Ma PH. 2020. A study on the self-assembly mode and supramolecular framework of complexes of cucurbit[6]urils and 1-(4-methoxyphenyl)piperazine. RSC Adv. 10, 37 369-37 373. ( 10.1039/D0RA07988J) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang GS, Sun WQ, Zhao WX, Lin RL, Tao Z, Liu JX. 2016. Host–guest complexation of di-cyclohexanocucurbit[6]uril and hexa-cyclohexanocucurbit[6]uril with alkyldiammonium ions: a comparative study. Org. Biomol. Chem. 14, 674-679. ( 10.1039/C5OB01982F) [DOI] [PubMed] [Google Scholar]
- 37.Zheng LM, Zhang K, Lin RL, Chu XF, Liu JX. 2020. Binding interactions of bisbenzimidazolyl derivatives with cyclohexanocucurbit[6]uril. J. Inclusion Phenom. Macrocyclic Chem. 96, 125-135. ( 10.1007/s10847-019-00957-z) [DOI] [Google Scholar]
- 38.Jin YM, et al. 2021. Crystal structure of self-assembled inclusion complex of symmetric dicyclohexanocucurbit[6]uril with 1H-benzotriazole. J. Inclusion Phenom. Macrocyclic Chem. 100, 209-215. ( 10.1007/s10847-021-01076-4) [DOI] [Google Scholar]
- 39.Zhang C, Zhang YQ, Xue SF, Zhu QJ, Tao Z. 2016. Coordination of lanthanide cations to a symmetrical dicyclohexanocucurbit[6]uril in the presence of tetrachlorozincate facilitates isolation of lighter lanthanides. Inorg. Chim. Acta 450, 258-262. ( 10.1016/j.ica.2016.06.016) [DOI] [Google Scholar]
- 40.Xu YQ, Panzner MJ, Li XP, Youngs WJ, Pang Y. 2010. Host–guest assembly of squaraine dye in cucurbit[8]uril: its implication in fluorescent probe for mercury ions. Chem. Commun. (Cambridge, England) 46, 4073-4075. ( 10.1039/c002219p) [DOI] [PubMed] [Google Scholar]
- 41.Geng QX, Cong H, Tao Z, Lindoy LF, Wei G. 2016. Cucurbit[7]uril-improved recognition by a fluorescent sensor for cadmium and zinc cations. Supramol. Chem. 28, 784-791. ( 10.1080/10610278.2015.1117614) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from: https://www.ccdc.cam.ac.uk/data_request/cif.