Abstract
The effect of various media (aqueous, NaCl, urea (U) and thiourea (TU)) on the micellization and adsorption activity of varied mixtures of imipramine hydrochloride (IMP) and benzethonium chloride (BZCl) is investigated via tensiometry. In an aqueous medium, the interactions between IMP and BZCl are also evaluated using UV–visible and FTIR spectroscopy. The interaction between components increases with increased mole fraction (α1) of BZCl in the mixed system (IMP + BZCl). Different parameters, such as micellar and the mixed monolayer component composition, the interaction parameters of the solution and the interface, the activity coefficients of the components in solution and at the interface, and thermodynamic parameters, are computed using different proposed theoretical models (i.e. Clint, Motomura, Rubingh and Rosen). The cmc values obtained for the pure components and mixtures (IMP + BZCl) of all the compositions are found to be less in NaCl than in the aqueous solution while found more in the presence of U or TU. TU is more effective in increasing the cmc of the pure and mixed systems than U. The Gibbs free energy values of the studied pure and mixed systems are negative, showing the spontaneous nature of the reaction.
Keywords: antidepressant drug, micellization, adsorption, micellar/interfacial mole fraction, different spectroscopy
1. Introduction
Most surface-active agents are amphiphilic organic molecules, consisting of hydrophobic groups in a hydrocarbon tail and hydrophilic groups as the head of a single molecule [1–6]. The association within amphiphilic molecules (such as surfactants) called micelles that happens due to the balance between hydrophilic and hydrophobic interactions is the critical micelle concentration (cmc) [7–13]. Because of their exceptional structural characteristics and good solubilization properties [6], micelles have been extensively used in industries such as pharmaceuticals, dyes, make-up and environmental management [4,6]. Recently, the solubilization outcomes of surfactants in micelles have been extensively studied [6]. Micelles are also excellent catalysts [14–18].
In several practical applications, it is acknowledged that two or more types of surfactant mixture are employed in formulations because of their effective solubilization, pharmaceutical and therapeutic formulations, and dispersion along with detergents [19,20]. Compared with the self-association of a single amphiphile, the amphiphile mixtures form mixed associations with excessive efficiency and lower charge. The resulting mixed micelles have much-improved surface activity [19,21,22]. A mixture can have improved air–solution interface properties as well as varied colloidal properties received from both components. Accordingly, in pharmaceutical chemistry, a mixed micelle solution is used to increase the absorption of different medicines in individuals [22,23]. Nevertheless, few studies address the impact of external factors such as electrolytes, urea on the surfactants and drug compounds in micelles. Additional research in this area will significantly ease screening as well as develop micellar systems, realize drug delivery tools and attain drug delivery goals [24,25].
Because it is straightforward to distinguish micelles, cmc is typically used to initially assess the solubilizing capability of surfactants [6]. A lower cmc value means that a lesser amount of the surfactant is required to form micelles [26]. Consequently, the cmc is also important for examining the interactions between drugs and surfactant molecules. Similar to surfactants, several amphiphilic drugs (including tricyclic antidepressants, phenothiazines and tranquillizers) also have the potential to self-associate into micellar forms [27–29]. These amphiphilic drugs are classified based on their different functional groups, for example, hydrophilic and hydrophobic groups, as these groups are possibly the main reason for the therapeutic properties of the drug. Despite their amphiphilic nature, these drugs do not form stable micelles and act as their own carrier because of their low lipophilic nature and low counterion binding [30]. To overcome this situation, a surfactant and amphiphilic drug mixed system is proposed as an alternative to options such as vesicles or soluble polymers. Usually, surfactants consist of a more hydrophobic micellar core that has a superior capability to admit amphiphilic drugs, enhance drug solubility and inhibit drug precipitation [30,31].
In the current study, an amphiphilic drug, imipramine hydrochloride (IMP), which is the hydrochloride salt form of imipramine, is employed as a model drug (scheme 1). This drug is used to treat depression (antidepressants). The IMP molecule comprises a tricyclic ring core with an alkylamine side chain to which a terminal nitrogen atom is attached. Due to the presence of the alkylamine side chain, this drug behaves like a conventional surfactant but forms micelles at a higher concentration than the surfactant [22,32]. The pKa value of IMP has been reported as 9.5; therefore, this drug has a positive charge in the current study condition [20,22].
Scheme 1.
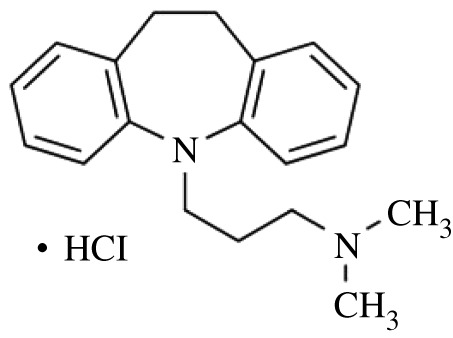
Molecular model of imipramine hydrochloride (IMP).
Apart from the positive outcomes of drugs, they can also have many undesirable side effects. These unwanted effects may be minimized if drugs are properly used with a carrier. The usage of micelles as drug carriers offers many advantages compared with other possible carriers, and nearly all surfactants form micelles [6]. Micelles can solubilize the drugs with low solubility in their hydrophobic interiors and boost bioavailability. Furthermore, micelles are easily prepared on an industrial scale [31,33].
The interaction of benzethonium chloride (BZCl) surfactant (scheme 2) with the drug IMP in aqueous, salt, urea (U) and thiourea (TU) media is studied. BZCl belongs to the category of N-cationic surfactants that have topical anti-infective and antiseptic qualities, which have been acknowledged for an extended period [34]. Because of its ability to form micelles, BZCl can capture hydrophobic compounds in the interior of its hydrophobic core or the Stern layer [35]; thus, it can act as a drug delivery agent by integrating hydrophobic drugs [36]. The IMP drug employed is highly soluble in water but forms micelles at higher concentrations due to low hydrophobicity; therefore, for its safe delivery, a carrier is required. Accordingly, BZCl is employed as a carrier in this study. Solutions containing both components formed mixed micelles, and the cmc value of the drug was too greatly reduced; hence, much less of the drug is required for particular purposes. The effects of using NaCl, U and TU media of fixed concentration on IMP and BZCl mixture interactions were also studied since electrolytes and U are present in the body, which may affect the interaction of IMP and BZCl and influence the drug biological activity. The outcomes of electrolyte and ureas can give more knowledge for drug and surfactant mixtures for developing improved delivery systems than aqueous system, since in the presence of electrolyte or ureas, the value of cmc of singular and mixture of amphiphiles declines or rises as the spontaneity of solution mixture declines or rises. Detail information is forever valuable in attaining drugs' biological activity having the minimum unwanted effects. The surface tension technique was used to assess the mixed association behaviour of IMP + BZCl mixtures in different ratios, and the experimental outcomes were analysed using four theories of mixed micellization (Clint, Rubingh, Rosen and Motomura). FTIR spectroscopic analysis was also carried out to further confirm the interaction between IMP and BZCl. UV–visible measurements were also taken to understand the interaction mechanism between the constituents employed in this study.
Scheme 2.
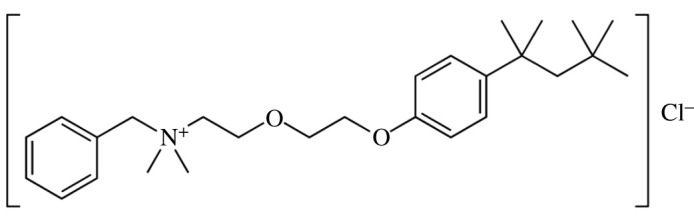
Structure of benzethonium chloride (BZCl) surfactant.
2. Material and methods
2.1. Materials
Every compound used is of analytic grade and was applied as procured from their corresponding seller with no additional purification (table 1). Distilled water (conductivity approx. 1–6 µS cm−1) was used for the solutions preparation in all solvents media.
Table 1.
Source and purity of the compounds used.
| chemical | source | CAS number | purification methods | mass fraction purity |
|---|---|---|---|---|
| IMP | Sigma (USA) | 113-52-0 | vacuum drying | ≥0.98 |
| BZCl | Sigma (USA) | 121-54-0 | vacuum drying | ≥0.98 |
| NaCl | BDH (England) | 7647-14-5 | vacuum drying | 0.98 |
| U | Sigma (Germany) | 57-13-6 | vacuum drying | 0.98 |
| TU | Techno Pharmachem (India) | 62-56-6 | vacuum drying | 0.995 |
2.2. Methods
2.2.1. Surface tension measurement
The surface tension (γ) measurements were conducted using an Attension tensiometer (Sigma 701, Germany), which applies the platinum ring detachment process at 298.15 K. The γ-values of stock solutions of pure and mixed systems (IMP, BZCl and IMP + BZCl mixtures in the presence of various solvents) were determined by adding a fixed quantity of stock solution by micropipette. Similar measurements were repeated until the γ-value became constant with further addition of the solution. The measured γ of pure and mixed systems (IMP + BZCl) in all media versus their respective log concentrations were plotted, and the breakpoint in the plot is the cmc for the system. The temperature of the studied system was retained via circulating water from an ORBIT RS10S thermostat connected with the instrument. The error in temperature obtained during the measurement of γ was attained ±0.2 K. The relative uncertainties limits on cmc were achieved close to 3% and the combined expanded uncertainty of surface tension value was attained close to 1.0 mN m−1. The whole evaluated surface tension data of the employed system are provided in electronic supplementary material, tables S1–S4.
2.2.2. FTIR spectroscopy
The FTIR spectra of pure IMP, BZCl, and a 1 : 1 IMP + BZCl mixture in an aqueous solution were measured at 298.15 K using a Nicolet iS50 FTIR spectrometer (Thermo Scientific, Madison, WI, USA). All spectra were accumulated from 4000 to 400 cm−1; however, only a portion of this range is displayed for clarity. In all samples, the water background was removed from the spectra.
2.2.3. UV–visible spectroscopy
An Evolution 300 UV–visible (Thermo Scientific) spectrometer was used here to record the spectra of IMP solutions with increasing concentration of BZCl in an aqueous solution at room temperature (298.15 K). The UV–visible absorbance spectra were recorded after each increment of BZCl. Distilled water was used for baseline correction.
3. Results and discussion
3.1. Determination of cmc and cmcid values
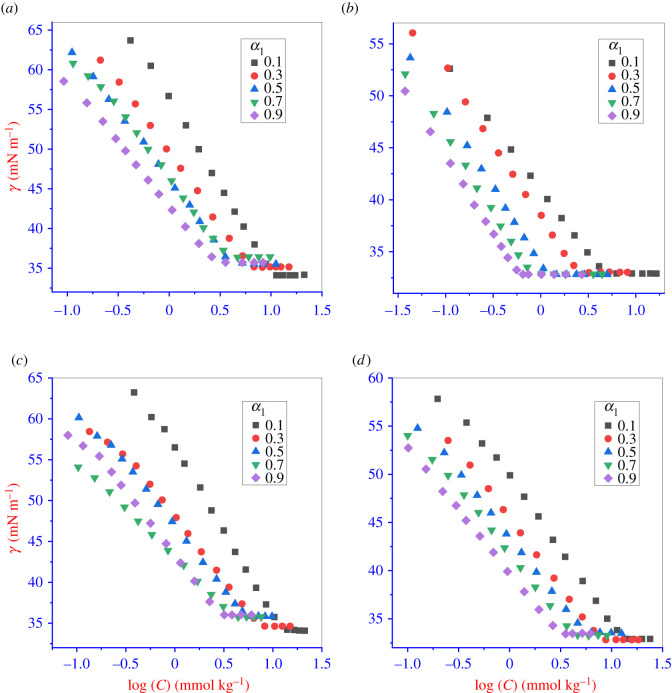
The present research concentrates mainly on the physico-chemical interactions between IMP and the prospective surface-active surfactant BZCl, and several theoretical models are applied to the data collected. The cmc value of individual amphiphiles (IMP and BZCl) and binary amphiphile (IMP + BZCl) mixtures in different ratios determined by a tensiometric method. The amphiphile chain length and hydrophobic interaction are the most important qualities for micellization [6]. Consequently, the cmc of the amphiphile solution can be determined from the surface tension (γ) versus concentration plot. The resulting plots of the IMP + BZCl mixtures at 298.15 K in various media (water, 50 mmol kg−1 NaCl, 300 mmol kg−1 U and 300 mmol kg−1 TU) at different mole fractions of BZCl (α1) are shown in figure 1. With the addition of the amphiphile (pure or mixed) solution into the solvent (aqueous or non-aqueous), the surface tension (γ) value decreased linearly in the pre-micellar region, and after an excess concentration of amphiphile(s) was added to the solvent, the γ value obtained became constant. The breakpoint obtained between γ and concentration (C) is the cmc of the amphiphiles (figure 1) [37]. The obtained cmc values of the current system are given in table 2 and electronic supplementary material, figure S1. The solubility of IMP in aqueous media was tested above a concentration of 400 mmol kg–1, and hence, IMP is found to be freely soluble in an aqueous system.
Figure 1.
Plot of surface tension (γ) against concentration (C) of IMP + BZCl mixture in five different mole fractions (α1) of BZCl: (a) water, (b) 50 mmol kg−1 NaCl, (c) 300 mmol kg−1 urea and (d) 300 mmol kg−1 thiourea system at 298.15 K.
Table 2.
Various physico-chemical parameters for IMP + BZCl mixed systems in various media at 298.15 K.a
| α1 | cmc (mmol kg−1) | cmcid (mmol kg−1) | ln (cmc1/cmc2) | |||||
|---|---|---|---|---|---|---|---|---|
| aqueous solution | ||||||||
| 0 | 41.85 | |||||||
| 0.1 | 10.20 | 18.05 | 0.552 | 0.612 | −2.34 | 0.626 | 0.490 | |
| 0.3 | 6.07 | 8.44 | 0.720 | 0.859 | −1.97 | 0.857 | 0.361 | |
| 0.5 | 3.97 | 5.51 | 0.772 | 0.934 | −2.65 | 0.872 | 0.206 | −2.65 |
| 0.7 | 3.60 | 4.09 | 0.879 | 0.971 | −2.0 | 0.971 | 0.214 | |
| 0.9 | 2.72 | 3.25 | 0.878 | 0.992 | −3.80 | 0.945 | 0.053 | |
| 1 | 2.95 | |||||||
| 50 mmol kg−1 NaCl | ||||||||
| 0 | 37.25 | |||||||
| 0.1 | 4.56 | 15.66 | 0.535 | 0.622 | −5.03 | 0.338 | 0.237 | |
| 0.3 | 2.38 | 7.26 | 0.622 | 0.864 | −5.52 | 0.455 | 0.118 | |
| 0.5 | 1.15 | 4.72 | 0.637 | 0.937 | −7.79 | 0.358 | 0.043 | −2.69 |
| 0.7 | 0.79 | 3.50 | 0.656 | 0.972 | −9.27 | 0.334 | 0.019 | |
| 0.9 | 0.58 | 2.78 | 0.679 | 0.993 | −11.5 | 0.305 | 0.005 | |
| 1 | 2.52 | |||||||
| 300 mmol kg−1 urea | ||||||||
| 0 | 45.20 | |||||||
| 0.1 | 12.05 | 20.13 | 0.549 | 0.599 | −2.10 | 0.653 | 0.532 | |
| 0.3 | 7.20 | 9.54 | 0.728 | 0.852 | −1.69 | 0.883 | 0.408 | |
| 0.5 | 5.15 | 6.26 | 0.815 | 0.931 | −1.79 | 0.940 | 0.304 | −2.60 |
| 0.7 | 4.0 | 4.65 | 0.866 | 0.969 | −2.16 | 0.962 | 0.198 | |
| 0.9 | 2.90 | 3.70 | 0.853 | 0.992 | −4.31 | 0.911 | 0.044 | |
| 1 | 3.36 | |||||||
| 300 mmol kg−1 thiourea | ||||||||
| 0 | 46.0 | |||||||
| 0.1 | 12.5 | 20.70 | 0.547 | 0.595 | −2.06 | 0.656 | 0.540 | |
| 0.3 | 7.52 | 9.86 | 0.730 | 0.850 | −1.63 | 0.888 | 0.421 | |
| 0.5 | 5.35 | 6.47 | 0.816 | 0.929 | −1.74 | 0.943 | 0.315 | −2.58 |
| 0.7 | 4.15 | 4.82 | 0.867 | 0.969 | −2.13 | 0.963 | 0.202 | |
| 0.9 | 3.01 | 3.83 | 0.853 | 0.992 | −4.28 | 0.912 | 0.044 | |
| 1 | 3.48 | |||||||
aStandard uncertainties (u) are u(T) = 0.20 K, u(NaCl) = 1 mmol kg−1, u(urea) = 2 mmol kg−1, u(thiourea) = 2 mmol kg−1 and u(p) = 5 kPa (level of confidence = 0.68). Relative standard uncertainties (ur) are ur(cmc/cmcid) = ±3%, ur(βm) = ±3% and .
The cmc of IMP is 41.85 mmol kg−1 in an aqueous solution at 298.15 K, showing good agreement with earlier published values [20,22,38]. The cmc value of BZCl is 2.95 mmol kg−1, which is also in good agreement with the literature (table 2) [39,40]. As our best knowledge, the cmc value of BZCl by surface tension method was first reported by our group [40]. As shown in table 2, the cmc value of BZCl is much lower than the cmc of IMP because the surfactant used is much more hydrophobic in nature. Consequently, the BZCl surfactant initiates micelle formation at a lower concentration than IMP.
Table 2 also shows that in the case of a mixed system (IMP + BZCl), the cmc of the formed micelles was reduced too much compared with the cmc of pure IMP. With the increased molar fraction (α1) of BZCl in the mixtures, the cmc decreases more, to below the cmc of pure BZCl surfactant, due to the enhanced interactions (table 2 and figure 2). Table 2 also shows that the cmc of the mixed system was closer to the cmc value of pure BZCl, indicating that the constituent with high hydrophobicity initiates micelle formation at an interior concentration than the less hydrophobic component. Among the components used, BZCl is more hydrophobic than IMP. BZCl forms micelles at a lower concentration and persists at much higher compositions in mixed micelles. IMP monomers only join the micelles formed with BZCl. Consequently, the mixed micelles which form contain more BZCl than IMP.
Figure 2.
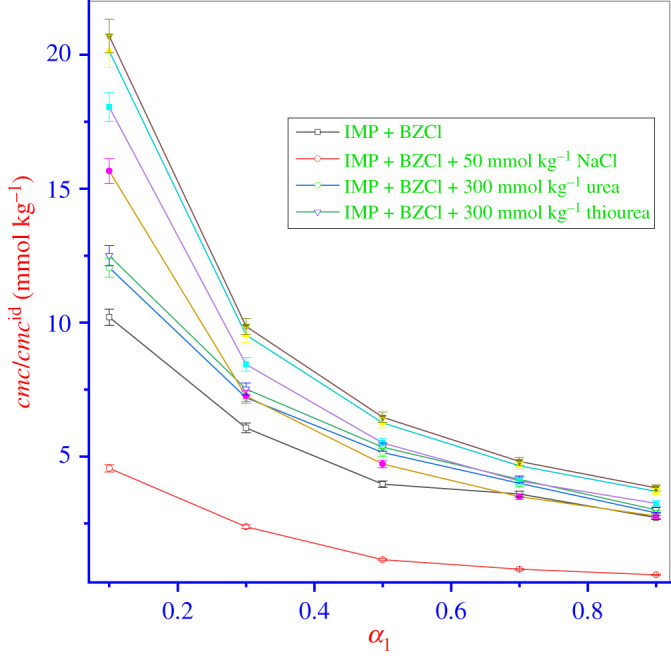
Variation of cmc and cmcid value of IMP + BZCl mixtures with α1 variation of BZCl (experimental cmc (open symbol) and cmcid (filled symbol) (relative standard uncertainties (ur) is ur(cmc/cmcid) = ±3%).
The amphiphile mixing can be either ideal or non-ideal throughout mixed micelle formation. The formation of mixed micelles may be represented through the relation [6]. In the case of a binary mixture, the ideal cmc value (cmcid) of the mixed micelles was assessed via Clint's theory [41], which is valuable for comparing an ideal and a non-ideal mixed system.
| 3.1 |
In equation (3.1), , , cmc1 and cmc2 signify the mole fraction of BZCl, the mole fraction of IMP, the cmc of BZCl and the cmc of IMP, respectively. Table 2 and figure 2 and visibly show that the cmcid (ideal) in each case is greater than the cmc (the experimental values of the binary mixtures), signifying the existence of non-ideal behaviour because of the mutual interaction of the IMP and BZCl in the mixed system (i.e. attractive interaction or synergistic behaviour). In the IMP + BZCl mixture, the BZCl and the IMP have a high tendency to self-association in solution because of the decreasing cmc of mixtures due to lowered repulsion within the head groups owing to the effective screening effect of BZCl/IMP. The cmcid values are higher than the experimental cmc values because the IMP + BZCl mixture strengthens the hydrophobicity of the mixed system, causing the start of micellization at smaller concentrations compared with pure species. Consequently, owing to the attractive interaction between the components, cmcid values are higher than the experimental cmc values. Throughout the interaction, electrostatic interactions occur between the micellar head groups, accompanied by the chain–chain interactions between micelles of different chain lengths [42].
Table 2 also shows that in NaCl media, the cmc values of the pure and mixed systems of various ratios were lower than the corresponding aqueous solution. Salt promotes micelle formation by lowering the repulsion between the head groups and thus lessening the effective area captured with every head group [6]. Adding NaCl causes the reduction of the cmc of the pure and mixed systems since a decline in electrostatic interactions occurs in NaCl media, and consequently, interactions between the molecules are enhanced, which triggers aggregation at lower concentrations [43].
However, in U or TU media, the cmc of the pure and mixed component systems were found to correspond more to those of the aqueous system (table 2), but TU is more efficient than U in increasing the cmc values. In the presence of U or TU, the polarity of the medium decreased [44]. The effects of U and TU on the aggregation behaviour of amphiphiles are still poorly understood, but plausible mechanisms are available in the literature for urea [45]. The first is the indirect mechanism, in which U or TU alters only the solvent, resulting in a change in the water structure [46]. The second is the direct mechanism, in which U or TU is involved in hydrophobic solvation by substituting for water particles in the hydration shells [47]. For U or TU, the indirect mechanism is broadly acknowledged [48]; therefore, U and TU act as water-structure breakers [49]. Through the interruption of the water structure by U or TU, the hydration of the polar head groups increases, raising the solubility of unassociated molecules. The result of breaking the water structure is similar to that of increased temperature [50]. TU is a better water-structure breaker than U [51]; therefore, cmc rises more when TU is added than when U is added. Additionally, in a solution containing U or TU, the repulsive interactions among the head groups of monomers at the micellar interface are enhanced. Therefore, the aggregation of pure IMP, BZCl and their mixtures is more hindered to some extent than in the aqueous system, and the resulting cmc values are higher. In table 2, IMP + BZCl mixtures in NaCl media showed higher non-ideality than in the aqueous, U and TU systems. This non-ideal behaviour measured in the IMP + BZCl mixed system in different media validates the effectiveness of BZCl in strengthening the hydrophobic atmosphere in the mixed state that results in stable mixed micelles.
3.2. Mixed micellization study of IMP and BZCl mixed system
From our measurements of cmc, BZCl forms mixed micelles with IMP. The quantitative evaluation of non-ideality and the type of interactions between the constituents are understood using Rubingh's theory [52]. Furthermore, the micellar composition (depending on regular solution theory) have been analysed by solving equation (3.2)
| 3.2 |
where is the composition of the first constituent (BZCl) in the mixed micelles.
The composition of the first constituent (BZCl) in the mixed micelles in the ideal state , is computed using equation (3.3) from Motomura & Aratono [53].
| 3.3 |
The values of of mixed micelles determined at various bulk mole fractions (α1) of BZCl are given in table 2 in each medium. The of BZCl in the mixed micelles is very high compared with (the equivalent value for IMP), signalling the greater extent of transfer of BZCl from the solution into the micellar form compared with IMP in all the media studied. The greater occurrence of BZCl in mixed micelles is attributed to the high hydrophobicity of BZCl, greater than IMP. Furthermore, due to the presence of BZCl in the mixed micelles, the electrostatic repulsions between the head groups of IMP declined. The values were also larger than the α1 of BZCl used except at the highest α1, reflecting the higher amount of surfactant in the mixed micelles. The values in all cases were lower than the value at each α1 in aqueous, salt, U and TU media, demonstrating that the composition of BZCl in mixed micelles is less than predicted from the ideal values (table 2) [54]. The calculated value is higher than the α1 value in all corresponding cases, meaning that the calculated value will certainly be higher than . In addition, increases because of an increase in α1 of the mixtures (table 2).
Rubingh's theory [52] is also employed to assess the nature and strength of the interactions of the constituents via the interaction parameter determined by equation (3.4)
| 3.4 |
The calculated values of in each medium are presented in table 2. As stated in the literature [6], for the mixed system, a positive indicates antagonistic behaviour, a negative indicates attractive interactions or synergistic effects, and a equal to 0 represents ideal behaviour (neither interaction nor repulsion) between both components during mixed micelle formation. In our case, table 2 shows that in all media, the values were negative, and their values do not follow any trend with increased α1. Overall, the βm values increased at higher α1, revealing that attractive interactions were detected between the components in the mixed systems. Here, negative values were obtained because of the decline in electrostatic self-repulsion of the cationic molecules [55]. Table 2 shows that in all media, the negative value varies between −11.50 and −1.63, showing high amounts of interactions or synergistic impacts between the mixture components. From the average value of , it is evident that in the presence of NaCl, the mixture components interact more strongly than in aqueous, U or TU media, hence more negative value of was obtained in NaCl media [56]. The effect of NaCl on is consistent with the effect on the cmc described earlier. In the presence of NaCl, repulsive interactions among the components of the mixed micelles further decreased. Rubingh's theory predicts that the value of any mixed system should be constant despite changes in the component composition, but here the value varies with the change in component compositions, showing the limitation of the above theory [6].
Regardless, a negative value suggests attractive interactions occur between components, but to verify synergistic effects despite the attractive interactions, one more condition should be obeyed by the system: . As shown in table 2, all the values are below zero but is higher than for all α1 of BZCl in NaCl media, but only the higher or highest α1 in aqueous, U and TU media. Consequently, synergistic effects between mixture components were attained only at the higher or highest α1 in aqueous, U, TU media, and all α1 in NaCl media. In the remaining cases, only attractive interactions were detected.
Another parameter, the activity coefficients of both components ( (BZCl) and (IMP)), were calculated using the previously obtained values of and via the following equations:
| 3.5 |
and
| 3.6 |
Table 2 shows the calculated values of and in all media. The values of as well as are less than one in all cases, indicating non-ideal behaviour and interactions between the components [57]. Activity coefficient is a thing used in thermodynamics to describe for deviations from ideal behaviour in a mixed system of chemical ingredients. The activity coefficient is a portion of the excess chemical potential of ingredients from a mixed system. Table 2 shows that with an increase in the α1 value, the value consistently declined, confirming that with increasing in α1, the non-ideal behaviour and interactions between the components increase. This trend explains the difference in the distribution of the components in the mixed micelles.
3.3. Properties of IMP + BZCl mixtures at interfacial surfaces
The fundamental properties of pure and mixed amphiphile systems occur at the interfacial surface, affecting the changes in the surface properties of the aqueous system. The number of amphiphilic molecules adsorbed per unit area of the surface is called the maximum surface excess concentration (Γmax) and is determined using the Gibbs adsorption equation [58]. The Γmax value in dilute aqueous or non-aqueous solutions is calculated using the Gibbs adsorption isotherm [58].
| 3.7 |
where γ, R, T and C are the surface tension (mN m−1), the ideal gas constant, the temperature (K) and the total added concentration of compounds in pure and mixed forms, respectively. n is the entire count number of each species of amphiphile molecule participating in the adsorption process [6], and n is determined for both components (IMP and BZCl). However, in the case of a mixture, the n value is calculated using [59], where is the composition of the first component (i.e. BZCl) at the mixed interface (table 3). For all systems, the value of the slope, was determined at a fixed concentration to evaluate Γmax.
Table 3.
Numerous interfacial parameters for IMP + BZCl mixed systems in different solvents at 298.15 K.a
| α1 | X1σ | βσ | f1σ | f2σ | Γmax 107 (mol m−2) | Amin./Aid (nm2) | γcmc | πcmc (mN m−1) | pC20 | ln (C1/C2) |
|---|---|---|---|---|---|---|---|---|---|---|
| aqueous solution | ||||||||||
| 0 | 12.8 | 1.30 | 42.6 | 28.4 | 1.95 | |||||
| 0.1 | 0.560 | −3.65 | 0.493 | 0.319 | 19.2 | 0.87/1.13 | 34.1 | 36.9 | 2.76 | |
| 0.3 | 0.658 | −4.36 | 0.599 | 0.152 | 16.2 | 1.03/1.09 | 35.1 | 35.9 | 3.08 | |
| 0.5 | 0.692 | −5.39 | 0.599 | 0.076 | 15.6 | 1.07/1.08 | 35.6 | 35.4 | 3.28 | −2.87 |
| 0.7 | 0.773 | −4.58 | 0.790 | 0.065 | 16.1 | 1.03/1.06 | 36.4 | 34.7 | 3.26 | |
| 0.9 | 0.747 | −8.14 | 0.594 | 0.011 | 14.0 | 1.18/1.07 | 35.7 | 35.4 | 3.51 | |
| 1 | 16.8 | 0.99 | 36.8 | 34.2 | 3.20 | |||||
| 50 mmol kg−1 NaCl | ||||||||||
| 0 | 8.86 | 1.87 | 44.7 | 26.3 | 2.07 | |||||
| 0.1 | 0.654 | −7.25 | 0.420 | 0.045 | 10.8 | 1.54/1.83 | 32.9 | 38.1 | 3.83 | |
| 0.3 | 0.808 | −4.52 | 0.847 | 0.052 | 12.1 | 1.37/1.82 | 33.0 | 38.0 | 3.91 | |
| 0.5 | 0.830 | −5.29 | 0.857 | 0.026 | 13.1 | 1.27/1.82 | 32.9 | 38.1 | 4.12 | −5.07 |
| 0.7 | 0.818 | −6.95 | 0.794 | 0.010 | 13.3 | 1.25/1.82 | 32.9 | 38.1 | 4.30 | |
| 0.9 | 0.812 | −9.33 | 0.718 | 0.002 | 13.0 | 1.28/1.82 | 32.8 | 38.2 | 4.46 | |
| 1 | 9.17 | 1.81 | 32.7 | 38.3 | 4.27 | |||||
| 300 mmol kg−1 urea | ||||||||||
| 0 | 16.5 | 1.0 | 45.3 | 25.7 | 1.64 | |||||
| 0.1 | 0.691 | −3.01 | 0.751 | 0.237 | 18.2 | 0.91/1.20 | 34.2 | 36.8 | 2.73 | |
| 0.3 | 0.743 | −4.61 | 0.738 | 0.078 | 13.7 | 1.21/1.22 | 34.6 | 36.4 | 3.19 | |
| 0.5 | 0.840 | −3.69 | 0.909 | 0.074 | 13.7 | 1.21/1.25 | 35.8 | 35.2 | 3.26 | −4.15 |
| 0.7 | 0.741 | −8.18 | 0.578 | 0.011 | 10.6 | 1.57/1.22 | 35.8 | 35.2 | 3.66 | |
| 0.9 | 0.876 | −5.90 | 0.914 | 0.011 | 13.8 | 1.20/1.26 | 36.0 | 35.0 | 3.50 | |
| 1 | 12.8 | 1.29 | 36.8 | 34.2 | 3.45 | |||||
| 300 mmol kg−1 thiourea | ||||||||||
| 0 | 15.4 | 1.08 | 47.4 | 23.6 | 1.54 | |||||
| 0.1 | 0.693 | −5.04 | 0.622 | 0.089 | 13.6 | 1.22/1.51 | 32.9 | 38.1 | 3.06 | |
| 0.3 | 0.782 | −5.04 | 0.787 | 0.046 | 12.6 | 1.32/1.57 | 32.8 | 38.2 | 3.38 | |
| 0.5 | 0.828 | −5.18 | 0.857 | 0.029 | 11.9 | 1.40/1.60 | 33.5 | 37.5 | 3.54 | −4.96 |
| 0.7 | 0.838 | −6.19 | 0.849 | 0.013 | 12.0 | 1.38/1.60 | 33.3 | 37.7 | 3.69 | |
| 0.9 | 0.817 | −8.94 | 0.741 | 0.003 | 11.5 | 1.45/1.59 | 33.5 | 37.5 | 3.87 | |
| 1 | 9.74 | 1.71 | 32.6 | 38.4 | 3.99 | |||||
aStandard uncertainties (u) are u(T) = 0.20 K, u(NaCl) = 1 mmol kg−1, u(urea) = 2 mmol kg−1, u(thiourea) = 2 mmol kg−1 and u(p) = 5 kPa (level of confidence = 0.68). Relative standard uncertainties (ur) are , ur(βσ) = ±3%, , ur(Γmax) = ±5%, ur(Amin/Aid) = ±5%, ur(πcmc) = ±2%, ur(pC20) = ±3% and ur(γcmc) = ±2%.
Once a monolayer is saturated, then an additional layer of the monomer molecules initiates micelle formation. The minimum area occupied by each monomer (Amin) on the saturated monolayer is correlated to the Γmax and can be determined from the following equation [58]:
| 3.8 |
where NA denotes Avogadro's number.
All computed values of Γmax and for all pure compounds and mixtures in all media are presented in table 3. The Γmax value of pure IMP is lower than the Γmax value of pure BZCl, which indicates that Amin is greater for IMP than BZCl as these parameters are inversely proportional, signifying that BZCl is more surface active than IMP. For IMP + BZCl mixtures, the Γmax value in all media except NaCl was either above or close to the Γmax of the pure components. With an increase in the α1 of BZCl in all media except NaCl, the Γmax value decreases with few exceptions (table 3).
Under ideal conditions, the minimum area engaged by each molecule was calculated using equation (3.9).
| 3.9 |
where is the of pure BZCl and is the of pure IMP. The calculated of IMP + BZCl in aqueous, NaCl, U and TU media is greater than the values at all α1 of BZCl with a few exceptions (table 3). This phenomenon shows that the reduced repulsion between the components with similar head groups is probably responsible for the decreased value. Overall, these results indicate the non-ideal behaviour of the IMP + BZCl mixture in various media.
3.4. IMP–BZCl interaction at the interfacial surface
Using Rosen & Hua's theoretical approach [60], the interaction parameters and the compositions of the components at the air–water interface between IMP and BZCl at the formation of the Gibbs monolayer are evaluated. In agreement with the Rubingh model [52], the proportions of the components of the adsorbed mixed monolayer and the interaction parameters at the interface were evaluated by Rosen & Hua's approach as follows [60,61]:
| 3.10 |
and
| 3.11 |
where C1, C2 and C are the concentrations of pure BZCl, pure IMP and the IMP + BZCl mixture at various α1 in all media of fixed selected surface tension in all cases and is the mixed monolayer composition of BZCl. The evaluated and βσ values of the currently employed system are given in table 3. We measured the value between 0.5598 and 0.8761; the interfacial surface contains a large amount of BZCl. Tables 2 and 3 show that the average value feel in a range similar to the average value, which suggests that both the mixed monolayer and the mixed micelles contain a high concentration of BZCl. These values do not signify any regular increase in the α1 of BZCl, but overall they are higher at higher α1. Compared with the aqueous medium, the value is greater for the NaCl medium, as NaCl screens the repulsive interactions between IMP and BZCl so that more BZCl monomers are incorporated in the mixed monolayer (table 3).
Akin to , negative, positive and zero (or close to zero) values of βσ indicate attractive interaction, repulsion interaction, and neither interaction nor repulsion (ideal mixing), respectively, among the components of a mixed monolayer [6]. In this work, all the βσ values are negative, indicating attractive interactions between the component monomers at the interface (table 3). In all media, the average value of βσ in all systems is greater than the average value of , signifying the interactions between components are greater in the mixed monolayer than the mixed micelles. The βσ values in the NaCl are the most negative among all the media, suggesting that, in the presence of NaCl, the interaction between the constituents increased at the monolayer.
The mixtures of IMP and BZCl display higher surface properties and considerably lower cmc values than pure IMP. The synergism in the binary mixed systems also depends on the associated characteristics of each component other than the interaction strength . The states for synergism at the interfacial surface which reduce surface tension efficiency are (a) the total required concentration of the mixture of the components to reduce the surface tension of water or any other media to the 20 mN m−1, as the surface tension should be lower than that of a particular component at the interface, and (b) the mixed system subsequently obeys the following conditions [6]:
-
(i)
βσ < zero
-
(ii)
Table 3 shows that the values were negative in all cases and the value of was greater than the value of for all systems with a few exceptions. Therefore, the IMP + BZCl mixed system showed synergism in surface tension reduction efficiency.
As for the mixed micelles, the activity coefficients of both constituents of the formed monolayer, (BZCl) and (IMP), were also calculated using and through the following equations [6].
| 3.12 |
and
| 3.13 |
Table 3 shows the obtained and values of the mixed systems in all media, and in every case, are below 1, indicating attractive interactions among the components at the interface as well as non-ideal behaviour. Table 3 also shows the involvement of BZCl is greater than the involvement of IMP in all media at the monolayer, as in each system and the involvement of IMP at the monolayer decreased with increases in α1.
Another parameter, which is frequently applied to assess the efficiency of the adsorption of amphiphile solutions, is pC20, which is determined using equation (3.14) [6]:
| 3.14 |
where pC20 is the concentration required to lessen the surface tension of aqueous or non-aqueous systems by 20 mN m−1. The higher the pC20 value, the more effectively the amphiphiles are adsorbed at the interfacial surface, and the more effectively it lessens surface tension. This effect determines the concentrations of amphiphiles essential to achieve saturation adsorption or decrease the surface tension by 20 mN m−1. The calculated pC20 values of pure IMP, BZCl and IMP + BZCl mixtures are given in table 3. The results reveal that the computed value of pC20 of BZCl is greater than that for IMP in each medium, showing that the surfactant has greater surface adsorption efficiency than IMP. In addition, their corresponding cmc values, determined previously, indicate this. In the case of a mixed system (IMP + BZCl), the surface adsorption efficiency was greater than pure IMP, and with an increase in α1, a considerable increase in pC20 value is seen, which means the surface adsorption efficiency of mixed systems increases with an increase in α1. However, pC20 for the IMP + BZCl mixture was less than the pC20 of BZCl at lower α1 and close to the pC20 of BZCl at 0.5 α1. At higher α1, the pC20 value of the mixture surpasses the pC20 value of both components (table 3).
The capability of an amphiphile to lessen surface tension is assessed via the extent of reduction, or the surface pressure (πcmc = (γ0 − γcmc)), at the cmc, because the reduction of the surface tension beyond the cmc is somewhat unimportant. Here, γ0 describes the surface tension of pure aqueous or non-aqueous media, and γcmc indicates the γ at the cmc of a system (pure or mixed). Table 3 presents the γcmc and πcmc of all the systems studied. IMP has the highest γcmc, while pure BZCl and IMP + BZCl mixtures have similar values (table 3). The πcmc value of IMP was less than that of pure BZCl in each medium; but the πcmc of the IMP + BZCl mixtures is greater than the πcmc of IMP and less than the πcmc of BZCl.
3.5. Thermodynamic parameters
It is important to evaluate the micellar solution thermodynamic parameters in the aqueous and non-aqueous systems because they indicate the comparative significance of hydrophobic interactions, water associated with amphiphiles, as well as head group repulsions. The Gibbs free energy of micellization is evaluated using equation (3.15) [62].
| 3.15 |
Here, the degree of ionization is considered one [6] because tensiometry cannot measure the degree of ionization; therefore the ionization of amphiphiles is considered complete. Xcmc is the cmc in mole fraction, and T and R have their usual meanings.
The computed values for pure IMP, BZCl and IMP + BZCl mixtures in various ratios in each medium are given in table 4 and show that their values are negative in every case. The negative values suggest that the association process is spontaneous and thermodynamically stabilized. The larger the magnitude of , showed the greater the spontaneity of the aggregation process. Through an increase in the α1 of BZCl in solution, the magnitude of increases regularly and reaches the maximum negative value at the maximum α1 tested, indicating that the mixed systems become more spontaneous and thermodynamically stabilized with an increase in α1 [63]. The value of pure IMP is in good agreement with earlier reported work [64]. The value for pure BZCl is also in good agreement with previously measured values [40,65]. Table 4 also shows that for the BZCl surfactant, the value was greater than the value of IMP, which means the association of BZCl is more spontaneous because BZCl has more hydrophobicity than IMP. Table 4 also shows that in the NaCl media, the values of the of the mixed systems are more negative than in the aqueous system, indicating that in the presence of NaCl, the hydrophobicity of pure as well as mixed systems is enhanced as the interactions between similar and dissimilar molecules are raised, and the electrostatic repulsions are reduced. As a result, the pure or mixed association process starts at a lower concentration than in the aqueous system. Instead, thevalue of the tested mixture is less negative in U or TU media than in aqueous solution, revealing that the interactions among similar and dissimilar molecules are weaker in the U or TU media. The aggregation behaviour of compounds remains spontaneous in U or TU media; however, the spontaneity decreases to some extent compared with the other systems studied (table 4). As the concentration of U and TU used is the same, but in TU media, the system is less spontaneous than in U media (i.e. the magnitude of is lowest in TU media). It is concluded from the overall results that the magnitude of is inversely proportional to the cmc value of the corresponding system. In TU medium, the cmc of pure compounds and mixtures is greater than in aqueous, NaCl and U media, and the magnitude of is greater in the U medium than in the other media used. The negative value of the studied system in different media are found in the following order:
Table 4.
Numerous thermodynamic parameters and packing parameter (P) for IMP + BZCl mixed systems in various solvents at 298.15 K.a
| α1 | (kJ mol−1) | Gmin (kJ mol−1) | p-value | |||
|---|---|---|---|---|---|---|
| aqueous system | ||||||
| 0 | −17.8 | −40.1 | 33.3 | 0.34 | ||
| 0.1 | −21.3 | −40.5 | 17.8 | −1.43 | −2.23 | 0.50 |
| 0.3 | −22.6 | −44.8 | 21.7 | −0.98 | −2.44 | 0.42 |
| 0.5 | −23.7 | −46.4 | 22.9 | −1.15 | −2.85 | 0.41 |
| 0.7 | −23.9 | −45.4 | 22.6 | −0.52 | −1.99 | 0.42 |
| 0.9 | −24.6 | −49.8 | 25.4 | −1.01 | −3.81 | 0.37 |
| 1 | −24.4 | −44.7 | 21.9 | 0.45 | ||
| 50 mmol kg−1 NaCl | ||||||
| 0 | −18.1 | −47.8 | 50.4 | 0.24 | ||
| 0.1 | −23.3 | −58.6 | 30.4 | −3.10 | −4.06 | 0.28 |
| 0.3 | −24.9 | −56.3 | 27.2 | −3.21 | −1.74 | 0.32 |
| 0.5 | −26.7 | −55.9 | 25.1 | −4.46 | −1.86 | 0.34 |
| 0.7 | −27.7 | −56.3 | 24.6 | −5.18 | −2.57 | 0.35 |
| 0.9 | −28.4 | −57.8 | 25.3 | −6.23 | −3.54 | 0.34 |
| 1 | −24.8 | −66.6 | 35.7 | 0.24 | ||
| 300 mmol kg−1 urea | ||||||
| 0 | −17.6 | −33.2 | 27.4 | 0.44 | ||
| 0.1 | −20.9 | −41.1 | 18.8 | −1.29 | −1.59 | 0.48 |
| 0.3 | −22.2 | −48.7 | 25.2 | −0.83 | −2.18 | 0.36 |
| 0.5 | −23.0 | −48.6 | 26.1 | −0.67 | −1.23 | 0.36 |
| 0.7 | −23.6 | −56.9 | 33.8 | −0.62 | −3.89 | 0.28 |
| 0.9 | −24.4 | −49.8 | 26.2 | −1.34 | −1.59 | 0.36 |
| 1 | −24.1 | −50.7 | 28.7 | 0.34 | ||
| 300 mmol kg−1 thiourea | ||||||
| 0 | −17.58 | −32.9 | 30.8 | 0.41 | ||
| 0.1 | −20.81 | −48.8 | 24.2 | −1.26 | −2.65 | 0.36 |
| 0.3 | −22.07 | −52.3 | 26.0 | −0.79 | −2.13 | 0.33 |
| 0.5 | −22.91 | −54.5 | 28.3 | −0.65 | −1.83 | 0.31 |
| 0.7 | −23.54 | −54.8 | 27.7 | −0.61 | −2.09 | 0.32 |
| 0.9 | −24.34 | −57.1 | 29.2 | −1.33 | −3.31 | 0.30 |
| 1 | −23.98 | −63.4 | 33.5 | 0.26 | ||
aStandard uncertainties (u) are u(T) = 0.20 K, u(NaCl) = 1 mmol kg−1, u(urea) = 2 mmol kg−1, u(thiourea) = 2 mmol kg−1 and u(p) = 5 kPa (level of confidence = 0.68). Relative standard uncertainties (ur) are , , ur(Gmin) = ±4%, .
One additional thermodynamic parameter is the standard free energy of adsorption of the pure and mixed amphiphiles system at the air–water interface as calculated from equation (3.16) [66,67].
| 3.16 |
Table 4 shows that the values for the pure and mixed systems in each medium were negative, illustrating that the adsorption of constituents at the interface occurs spontaneously. Additionally, the value of is much more negative than the value of , signifying that the association is a secondary process with respect to interfacial adsorption; therefore, work must be required to convert monomers from the monomeric form to the micellar form. This difference also indicates that the adsorption process is more favourable [68]. Additionally, the higher magnitude of shows that the adsorption process is preferred over the micellization process in bulk systems because the hydrophobic portion of molecules always prefers the interface (table 4). In all media, of pure BZCl is greater than for pure IMP, showing that adsorption in BZCl is easier and comparatively more spontaneous than IMP. However, for IMP + BZCl mixtures, the value was greater than the value of IMP but less than the value of pure BZCl. Overall, the value shows that the adsorption process in the case of IMP + BZCl mixtures is easier than pure IMP adsorption. In NaCl media, the value of of all systems is more negative than in the aqueous system. This rise in the magnitude of in NaCl media is due to the diminished electrostatic repulsions between the molecules. Consequently, more molecules can reside at the surface, which enriches the density of the monomers at the monolayer, and hence additional work is needed to transport more monomers from the surface to the micellar form than in the aqueous system.
Sugihara et al. [69] have computed the minimum free energy of the interface at the maximum adsorption (Gmin) achieved at cmc, another thermodynamic parameter, using equation (3.17).
| 3.17 |
The values calculated for all of the systems studied are given in table 4. The smaller value of Gmin displays the more stable interfacial surface formation; this parameter is directly related to the attractive interactions among monomers. In this work, smaller values of Gmin are achieved in the case of pure compounds and their mixtures in each medium, showing that a thermodynamically stable surface is fashioned. This requires that interactions among components are valued. The Gmin of the mixed system at each α1 of their respective media is less than the pure compound in the same medium, verifying that a mixed monolayer is more thermodynamically stable than a single component monolayer (table 4).
An additional important thermodynamic parameter called excess free energy is computed by applying equations (3.18) and (3.19) [70–73].
| 3.18 |
and
| 3.19 |
In equations (3.18) and (3.19), and denote the excess free energy of mixed micelles and mixed monolayers, respectively, and their values in each medium are given in table 4. All the are negative, demonstrating that the stability of the mixed micelles, as well as the mixed monolayers, is greater for mixtures than pure systems because of the interactions between the different components. Mixed micelles and mixed interfaces have distinct physico-chemical characteristics from micelles/interfaces formed by single species. From the perspective of applications, mixed micelles and mixed interfaces show immense significance in different fields (such as pharmaceutical, enhanced oil recovery activity, biological, solubilization of hydrophobic molecules and dispersion). The cmc values of binary mixtures were much lower than those of either component alone, and the interfacial properties of the binary mixed system were greatly enhanced. These qualities are of significant value, as the use of mixed components systems for any purpose leads to lower costs as well as lower environmental impact. Consequently, mixed micelles and mixed monolayers formed by binary components mixtures are more stable than micelles and monolayers formed from either of the pure components. Overall, and do not follow any specific trend with increased α1, even in different media. Through comparing the and values, the average value is greater than the average value, implying that the mixed monolayer shows additional stability (table 4). Herein, it is found that the value of interaction parameters was not constant through the change in α1 of ingredients which means that the involved will not be symmetrical, or it can say that molecular interactions diverge from symmetrical solutions displaying the limitations of Rubingh's model [52,74].
3.6. Packing parameters of IMP + BZCl
The micellar shape generated in an aqueous or non-aqueous system is significant in evaluating various properties of the amphiphile solutions, such as viscosity, capability to solubilize water-insoluble hydrophobic compounds, and phase separation [6]. The packing parameter (P) is used to evaluate the shape of the micelles formed in an aqueous or non-aqueous system using Israelachvili et al.'s equation [75].
| 3.20 |
where V0 is the volume engaged through the hydrophobic groups in the core of the micelles, lc is the hydrophobic chain length in the micellar core and is the minimum area of the hydrophilic group at the interfacial surface. ; and . Vo and lc are evaluated using the formula given by Tanford [76], where nc is the total number of C atoms in the hydrocarbon chain. nc is considered less than the actual count of C atoms as the C atom which was connected directly with the head group, which is highly solvated, so this is also considered as part of the head group [77].
The calculated p-values of the pure (IMP and BZCl) and mixed systems (IMP + BZCl) in all the media studied are presented in table 4. Moreover, from the literature, a p-value between 0 and 1/3 will be obtained for spherical micelles, between 1/3 and 1/2 signifies cylindrical micelles, and between 0.5 and 1 shows vesicular micelles [6]. Results in table 4 indicate that the p-value for pure IMP in NaCl medium is 0.24, indicating spherical micelles, while in aqueous, U and TU media, the p-value is between 0.33 and 0.5, indicating the formation of cylindrical micelles. In the case of BZCl in NaCl and TU, the p-values are less than 0.24 and 0.26, respectively, due to the formation of spherical micelles, while in aqueous and U media, the p-values are 0.45 and 0.34, respectively, indicating cylindrical micelles have formed. In the IMP + BZCl mixture, the p-value is between 0.33 and 0.50 in most cases and is attributable to vesicular micelles. However, in some other cases, the value is less than 0.33, indicating the formation of spherical micelles.
3.7. FTIR spectroscopy
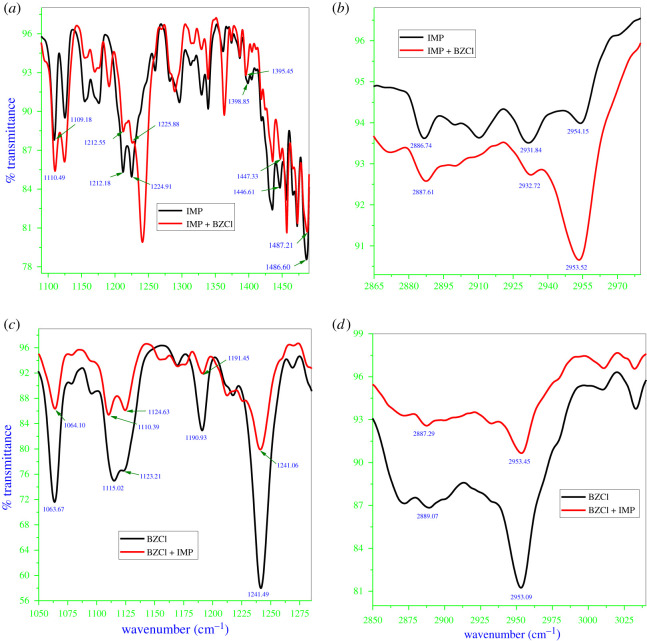
FTIR spectroscopy is used to explore the interactions between components of the micellar solutions [78,79]. In particular, the frequencies of the hydrophobic and head group parts of the amphiphiles deliver information about structural changes in the micellar monomers [80]. Background-subtracted FTIR spectra of pure IMP in aqueous solution and the 1 : 1 IMP + BZCl mixed system are shown in figure 3a,b. The interaction of IMP with BZCl is possibly observed in the shifting of C–N stretching, C–H bending and stretching of the head of IMP. Figure 3a shows FTIR spectra of IMP and IMP + BZCl mixtures from 1090 to 1490 cm−1 to assess the influence of BZCl on C–N stretching (aliphatic) along with C–H bending in IMP monomers. IMP is cationic, contains three alkyl groups, and shows C–N stretching (aliphatic) at three different frequencies. These vibrational bands occur at 1109.18, 1212.18 and 1224.91 cm−1 in the spectrum of the pure compound, but in the presence of BZCl, that is, for the IMP + BZCl mixtures, these obtained frequencies (C–N stretching) shift to higher frequencies of 1110.49, 1212.55 and 1225.88 cm−1. In IMP, C–H bending is detected at three distinct frequencies: 1398.85, 1446.61 and 1486.60 cm−1; in IMP + BZCl mixtures, the frequencies of C–H bending in IMP shift to 1395.45, 1447.33 and 1487.21 cm−1.
Figure 3.
FTIR spectra of pure IMP and in existence of BZCl ((a) and (b)), and BZCl spectra in pure form and presence of IMP ((c) and (d)).
For further analysis of the interaction of IMP with BZCl, figure 3b shows the frequency range from 2865 to 2980 cm–1 to view the effect of BZCl on the frequency of the C–H stretching of IMP. IMP showed C–H stretching at three frequencies: 2886.74, 2931.84 and 2954.15 cm−1. But in the case of the IMP + BZCl mixed system, the C–H stretching bands in IMP move to 2887.61, 2932.72 and 2953.52 cm−1. Therefore, in the presence of BZCl, the observed shifts in C–N stretching, C–H stretching, as well as the bending frequency in the head group of IMP indicate the interaction between IMP and BZCl [81].
The FTIR spectra of pure BZCl and 1 : 1 BZCl + IMP were recorded between 1050 and 1285 cm−1, and their spectra are given in figure 3c. Pure BZCl shows the C–O stretching (aliphatic ether and alkyl aryl ether) bands at different frequencies. The C–O stretching (aliphatic ether) bands in BZCl are detected at 1063.67, 1115.02 and 1123.21 cm−1, and in the presence of IMP (BZCl + IMP), the position of the C–O stretching (aliphatic ether) bands in BZCl is shifted to different wavelengths: 1064.10, 1110.39 and 1124.63 cm−1, respectively. The C–O stretching (alkyl aryl ether) bands in BZCl appear at 1190.93 and 1241.49 cm−1. However, in the case of the BZCl + IMP mixed system, the C–O stretching (alkyl aryl ether) bands shift from their original positions to 1191.45 and 1241.06 cm−1 (figure 3c). The shifting in the frequency of the C–O stretching (aliphatic ether and alkyl aryl ether) bands in BZCl in the presence of IMP shows the clear-cut interaction between the components [82].
The frequency range of 2865 to 2980 cm−1 also shows the effect of IMP on the C–H stretching bands (methyl and/or methylene) in BZCl. Their spectra are shown in figure 3d. The C–H stretching bands in BZCl are present at three different wavelengths (2886.74, 2931.84 and 2954.15 cm−1). However, in the BZCl + IMP mixed system, the C–H stretching band of BZCl is changed to different frequencies: the first two frequencies increase, and the third decreases (that is, 2886.74 to 2887.61 cm−1, 2931.84 to 2932.72 cm−1, 2954.15 to 2953.52 cm−1). These frequency shifts show the interactions between the components of the mixed micelles (BZCl + IMP). Because of these interactions, the shifts in frequency in the mixed system compared with the pure system were small but reproducible [83]. Overall, the shifting in the C–N stretching (aliphatic), C–H bending and stretching, and C–O stretching (aliphatic ether and alkyl aryl ether) frequencies in the mixed system compared with the pure systems signifies the interaction between the components.
3.8. UV–visible spectroscopy
UV–visible spectroscopy is also employed to investigate the interactions between the constituent compounds of the mixture. A fixed concentration (0.11 mmol kg−1) of the amphiphilic drug IMP is used for UV–Vis measurements. The titration of IMP (1.5 ml in quartz cuvette) was performed by increasing the concentration of BZCl. To prevent dilution, the stock solution of surfactant (BZCl) (5 mol kg−1) was prepared in the 0.11 mmol kg−1 IMP solution. First, the absorbance spectrum of the 0.11 mmol kg−1 IMP solution was recorded, which shows an absorbance maximum at 249 nm (figure 4). This obtained maximum absorbance wavelength is a π–π* transition. Figure 4 also shows that when BZCl is added to the IMP solution, the absorbance intensity increases. With further increases in the BZCl concentration (from 0.03 to 0.37 mmol kg−1) in the IMP solution, the absorbance intensity again increases, confirming that the hyperchromic effect arises because of the attractive interaction between IMP and BZCl [84,85]. However, it shows that at a lower added concentration of BZCl, the maximum absorbance peak of IMP does not change, but at a higher concentration, the peak shifts to a somewhat higher concentration. This indicates that the redshift of a mixed system occurs due to greater interaction between mixture components at higher concentrations (figure 4). At the higher concentration of BZCl in the solution, mixtures also showed maxima at around 272 nm. Overall, the titration results show the interaction between IMP and BZCl, since the absorbance peak of IMP disappears to some extent because of the formation of the complex [86]. An insignificant redshift (2–3 nm) in the maxima of IMP in the presence of BZCl does not describe the complex formation between IMP and BZCl. Therefore, for quantitative evaluation of the binding of BZCl with IMP monomers, absorbance statistics are applied using the Benesi–Hildebrand equation [87,88]
| 3.21 |
where K is the binding constant and A0, A and Amax are the absorbance values of IMP in the absence, presence and infinite concentration of BZCl, respectively.
Figure 4.
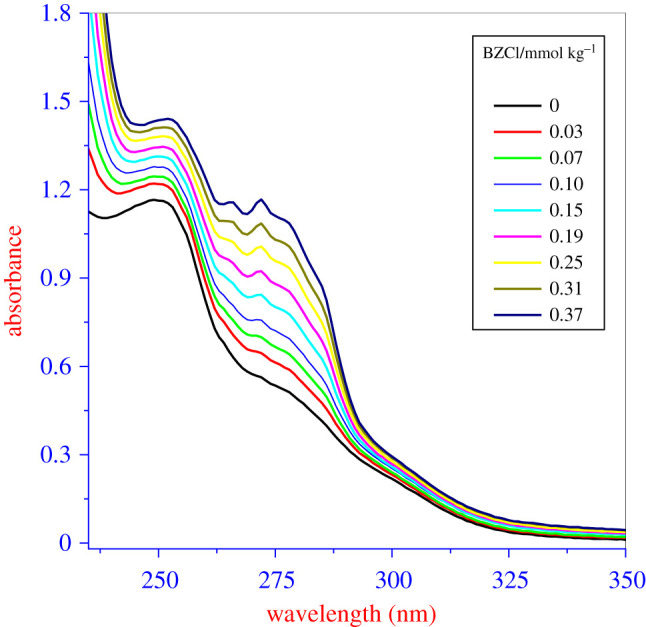
UV–visible spectra of pure IMP and in occurrence of different concentration of BZCl in different media ((a) water, (b) 50 mmol kg−1 NaCl, (c) 300 mmol kg−1 urea and (d) 300 mmol kg−1 thiourea).
The plot of 1/(A − A0) versus 1/[BZCl] shows straight lines (figure 5), which is more evidence in favour of the formation of a 1 : 1 IMP : BZCl complex. The K value for the IMP + BZCl complexes is determined as 2510 mol kg−3 by evaluating the ratio of the intercept and the slope of the Benesi– Hildebrand plot. The correlation coefficient (r) is 0.998, which supports the good linear fit. The obtained value of K is further used to determine the free energy change using equation (3.22) [89].
| 3.22 |
Figure 5.
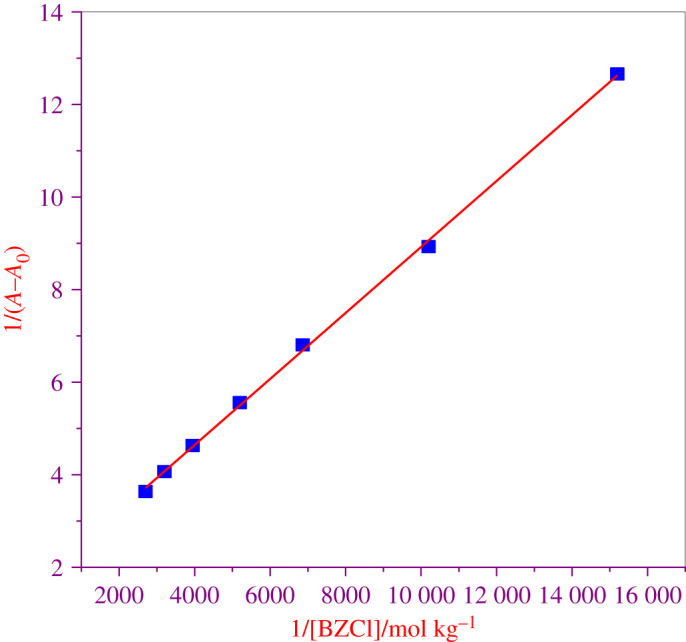
The plot of 1/(A–A0) against 1/[BZCl] for the interaction of IMP with BZCl.
The ΔG value obtained for the IMP + BZCl mixed system is negative (−19.4 kJ mol−1), which confirms the spontaneity of the complexation process. This may be caused by a reduction in self-electrostatic repulsion among the cationic head group molecules, which shows preferential binding between components. At 0.1 α1 of BZCl, the value of was found to be −21.3 kJ mol−1 showing the micellization process was more spontaneous as compared with the complexation process.
4. Conclusion
Before, establishing a surfactant as a suitable drug vehicle, a broad investigation must be completed to inspect and understand the aggregation behaviours of the surfactant with the projected drug. Herein, the mixed micellization behaviour of the IMP drug and BZCl surfactant mixed system in aqueous and other media is investigated via the tensiometry technique. The relationship between cmc and cmcid shows non-ideal behaviour, indicating the presence of attractive interactions and synergism between the components. In NaCl media, the cmc of pure components and mixtures decreases compared with the aqueous system, and in U or TU media, the cmc values increase compared with the aqueous solution. Between U and TU, TU is more effective in increasing the cmc of the system. The values were found larger than the employed α1 of BZCl except α1 = 0.9, revealing the higher BZCl contribution in the mixed micelles. The negative values of βm and βσ showed the attractive interactions among components in mixed micelles and the mixed monolayers, respectively. The activity coefficients of both components in solution and at the surface were found to be less than 1, showing the non-ideal behaviour and interaction between components. The Γmax value for IMP is found lower than BZCl, implying that BZCl has an additional surface active than IMP. In all media, the pC20 value for BZCl is higher than IMP, revealing that the BZCl has more surface adsorption efficiency as compared with IMP. The value is negative in all media, which is indicative of the spontaneity in the pure and mixed systems, and their spontaneity rises with increased α1; however, is greater than the corresponding in all media. and are larger in NaCl media than the other media tested in this study. Excess free energies showed that the mixed interfacial surface of mixed micelles is more stable than singular component micelles and monolayers. The FTIR spectra of aqueous IMP in the presence of BZCl or vice versa showed frequencies shifting from their original position owing to the interaction among constituent compounds. UV–Vis spectra display the interaction between IMP and BZCl as shifts in the absorbance maxima of IMP. The results of this study show a straightforward approach to the design of BZCl, which possibly will be an effective drug delivery agent for antidepressant drugs.
Supplementary Material
Data accessibility
Data that support this study have been uploaded as electronic supplementary material [90].
Authors' contributions
D.K. and M.A.R. did the experiments and wrote the manuscript. Y.G.A. and A.M.A. analysed and interpreted data. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
The authors declare no competing interest.
Funding
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. (G:102-130-1441). The authors, therefore, acknowledge with thanks DSR for technical and financial support.
References
- 1.Wu X, Zhong C, Lian X, Yang Y. 2018. Solution properties and aggregating structures for a fluorine-containing polymeric surfactant with a poly(ethylene oxide) macro-monomer. R. Soc. Open Sci. 5, 180610. ( 10.1098/rsos.180610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hazrina HZ, Noorashikin MS, Beh SY, Loh SH, Zain NNM. 2018. Formulation of chelating agent with surfactant in cloud point extraction of methylphenol in water. R. Soc. Open Sci. 5, 180070. ( 10.1098/rsos.180070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohd NI, Zain NNM, Raoov M, Mohamad S. 2018. Determination of carcinogenic herbicides in milk samples using green non-ionic silicone surfactant of cloud point extraction and spectrophotometry. R. Soc. Open Sci. 5, 171500. ( 10.1098/rsos.171500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheng R, et al. 2021. Molecular interaction between sodium dodecylbenzene sulfonate and octylphenol polyoxyethylene ether and effect of hydrophilic chain. Colloids Surfaces A 626, 127048. ( 10.1016/j.colsurfa.2021.127048) [DOI] [Google Scholar]
- 5.Azum N, Rub MA, Asiri AM. 2018. Interaction of antipsychotic drug with novel surfactants: micellization and binding studies. Chinese J. Chem. Eng. 26, 566-573. ( 10.1016/j.cjche.2017.09.009) [DOI] [Google Scholar]
- 6.Rosen MJ. 2004. Surfactants and interfacial phenomena, 3rd edn. New York, NY: John Wiley & Sons. [Google Scholar]
- 7.Kumar D, Rub MA. 2018. Interaction of ninhydrin with chromium-glycylglycine complex in the presence of dimeric gemini surfactants. J. Mol. Liquids 250, 329-334. ( 10.1016/j.molliq.2017.11.172) [DOI] [Google Scholar]
- 8.Kumar D, Rub MA. 2019. Role of cetyltrimethylammonium bromide (CTAB) surfactant micelles on kinetics of [Zn(II)-Gly-Leu]+ and ninhydrin. J. Mol. Liquids 274, 639-645. ( 10.1016/j.molliq.2018.11.035) [DOI] [Google Scholar]
- 9.Huang J, Ren ZH. 2020. Mechanism on micellization of amino sulfonate amphoteric surfactant inaqueous solutions containing different alcohols and its interfacial adsorption. J. Mol. Liquids 316, 113793. ( 10.1016/j.molliq.2020.113793) [DOI] [Google Scholar]
- 10.Zhou X, Hu S, Wang Y, Ullah S, Hu J, Liu H, Xu B. 2019. The surface adsorption, aggregate structure and antibacterial activity of Gemini quaternary ammonium surfactants with carboxylic counterions. R. Soc. Open Sci. 6, 190378. ( 10.1098/rsos.190378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sachin KM, Karpe SA, Singh M, Bhattarai A. 2019. Self-assembly of sodium dodecylsulfate and dodecyltrimethylammonium bromide mixed surfactants with dyes in aqueous mixtures. R. Soc. Open Sci. 6, 181979. ( 10.1098/rsos.181979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar D, Rub MA. 2020. Catalytic influence of 16-s-16 gemini surfactants on the rate constant of histidine and ninhydrin. R. Soc. Open Sci. 7, 191648. ( 10.1098/rsos.191648) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang J, Ren ZH. 2019. Micellization and interactions for ternary mixtures of amino sulfonate surfactant and nonionic octylphenol polyoxyethylene ethers in aqueous solution: 1 Blending with nonionic surfactants with smaller numbers of hydrophilic unit. J. Mol. Liquids 278, 53-60. ( 10.1016/j.molliq.2019.01.035) [DOI] [Google Scholar]
- 14.Ren ZH, Huang J, Zheng YC, Lai L, Mei P, Yu XR, Chang YL. 2018. Micellization and interaction for ternary mixtures of amino sulfonate surfactant and nonionic octylphenol polyoxyethylene ethers in aqueous solution: 2 Blending with nonionic surfactant with a longer or shorter hydrophilic chain. J. Mol. Liquids 272, 380-386. ( 10.1016/j.molliq.2018.09.088) [DOI] [Google Scholar]
- 15.Ren ZH, Huang J, Zheng YC, Lai L, Li Hu LL. 2018. Interaction and micellar behavior of binary mixture of amino sulfonate amphoteric surfactant with octadecyltrimethylammonium bromide in aqueous solutions of NaCl. J. Chem. Eng. Data 62, 1782-1787. ( 10.1021/acs.jced.6b00968) [DOI] [Google Scholar]
- 16.Ren ZH, Huang J, Luo Y, Zheng YC, Mei P, Lai L, Chang YL. 2016. Micellization behavior of binary mixtures of amino sulfonate amphoteric surfactant with different octylphenol polyoxyethylene ethers in aqueous salt solution: both cationic and hydrophilic effects. J. Ind. Eng. Chem. 36, 263-270. ( 10.1016/j.jiec.2016.02.009) [DOI] [Google Scholar]
- 17.Kumar D, Rub MA, Asiri AM. 2020. Synthesis and characterization of geminis and implications of their micellar solution on ninhydrin and metal amino acid complex. R. Soc. Open Sci. 7, 200775. ( 10.1098/rsos.200775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar D, Rub MA. 2020. Influence of dicationic quaternary ammonium gemini surfactant system on metal-amino acid complex-ninhydrin reaction. Mater. Chem. Phys. 248, 122926. ( 10.1016/j.matchemphys.2020.122926) [DOI] [Google Scholar]
- 19.Jafari-Chashmi, P, Bagheri A. 2018. The strong synergistic interaction between surface active ionic liquid and anionic surfactant in the mixed micelle using the spectrophotometric method. J. Mol. Liquids 269, 816-823. ( 10.1016/j.molliq.2018.08.094) [DOI] [Google Scholar]
- 20.Ren ZH. 2015. Mechanism of the salt effect on micellization of an aminosulfonate amphoteric surfactant. Ind. Eng. Chem. Res. 54, 9683-9688. ( 10.1021/acs.iecr.5b02169) [DOI] [Google Scholar]
- 21.Rub MA, Kumar D. 2020. Micellization behavior of antidepressant imipramine hydrochloride drug and hydrotrope (sodium tosylate) mixtures at different compositions and temperatures in different media. J. Chem. Eng. Data 65, 2659-2672. ( 10.1021/acs.jced.0c00038) [DOI] [Google Scholar]
- 22.Attwood D, Florence AT. 1983. Surfactant systems, their chemistry, pharmacy and biology. New York, NY: Chapman and Hall. [Google Scholar]
- 23.Kumar D, Khan F, Rub MA, Azum N, Asiri AM. 2021. Interaction of promethazine hydrochloride drug and sodium benzoate hydrotrope mixtures in different solvent media at different temperatures. J. Mol. Liquids 325, 115188. ( 10.1016/j.molliq.2020.115188) [DOI] [Google Scholar]
- 24.Kocabay OG, Ismail O. 2021. Preparation and optimization of biodegradable self-assembled PCL-PEG-PCL nano sized micelles for drug delivery systems. Int. J. Polym. Mater. Polymeric Biomaterials 70, 328-337. ( 10.1080/00914037.2020.1713784) [DOI] [Google Scholar]
- 25.Lu Q, Yi M, Zhang M, Shi Z, Zhang S. 2019. Folate-conjugated cell membrane mimetic polymer micelles for tumor-cell-targeted delivery of doxorubicin. Langmuir 35, 504-512. ( 10.1021/acs.langmuir.8b03693) [DOI] [PubMed] [Google Scholar]
- 26.Rub MA, Azum N, Asiri AM. 2016. Interaction of cationic amphiphilic drug nortriptyline hydrochloride with TX-100 in aqueous and urea solutions and the studies of physicochemical parameters of the mixed micelles. J. Mol. Liquids 218, 595-603. ( 10.1016/j.molliq.2016.02.049) [DOI] [Google Scholar]
- 27.Mahbub S, Akter S, Luthfunnessa AP, Hoque MA, Rub MA, Kumar D, Alghamdi YG, Asiri AM, Caňcar HD. 2020. Effect of temperature and polyols on the drug (ciprofloxacin hydrochloride) mediated micellization of sodium dodecyl sulfate. RSC Adv. 10, 14 531-14 541. ( 10.1039/D0RA00213E) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azum N, Ahmed A, Rub MA, Asiri AM, Alamery SF. 2019. Investigation of aggregation behavior of ibuprofen sodium drug under the influence of gelatin protein and salt. J. Mol. Liquids 290, 111187. ( 10.1016/j.molliq.2019.111187) [DOI] [Google Scholar]
- 29.Alfaifi SYM, Kumar D, Rub MA, Khan F, Azum N, Khan A, Asiri AM, Čančar HD. 2021. Effect of low levels of hydrotropes on micellization of phenothiazine drug. Korean J. Chem. Eng. 38, 386-399. ( 10.1007/s11814-020-0710-3) [DOI] [Google Scholar]
- 30.Rub MA, Azum N, Khan SB, Marwani HM, Asiri AM. 2015. Micellization behavior of amphiphilic drug promazine hydrochloride and sodium dodecyl sulfate mixtures at various temperatures: effect of electrolyte and urea. J. Mol. Liquids 212, 532-543. ( 10.1016/j.molliq.2015.09.049) [DOI] [Google Scholar]
- 31.Torchilin VP. 2001. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Rel. 73, 137-172. ( 10.1016/s0168-3659(01)00299-1) [DOI] [PubMed] [Google Scholar]
- 32.Taboada P, Attwood D, Ruso JM, Suarez MJ, Sarmiento F, Mosquera V. 1999. Concentration dependence of the osmotic and activity coefficients of imipramine and clomipramine hydrochlorides in aqueous solution. J. Chem. Eng. Data 44, 820-822. ( 10.1021/je990060o) [DOI] [Google Scholar]
- 33.Jones M, Leroux J. 1999. Polymeric micelles – a new generation of colloidal drug carriers. Eur. J. Pharm. Biopharm. 48, 101-111. ( 10.1016/s0939-6411(99)00039-9) [DOI] [PubMed] [Google Scholar]
- 34.Budavari S (ed.). 1989 The Merck index: an encyclopedia of chemicals, drugs, and biologicals, 11th edn, p. 1084. Rahway, NJ: Merck. [Google Scholar]
- 35.Menger FM. 1979. The structure of micelles. Acc. Chem. Res. 12, 111-117. ( 10.1021/ar50136a001) [DOI] [Google Scholar]
- 36.Paradies HH. 1989. US-Patent. 4074850.
- 37.Alam MS, Kabir-ud-Din, Mandal AB. 2010. Thermodynamics of some amphiphilic drugs in presence of additives. J. Chem. Eng. Data 55, 2630-2635. ( 10.1021/je900749a) [DOI] [Google Scholar]
- 38.Khan F, Rub MA, Azum N, Asiri AM. 2018. Mixtures of antidepressant amphiphilic drug imipramine hydrochloride and anionic surfactant: micellar and thermodynamic investigation. J. Phys. Org. Chem. 31, e3812. ( 10.1002/poc.3812) [DOI] [Google Scholar]
- 39.Rodríguez JL, Minardi RM, Schulz EP, Pieroni O, Schulz PC. 2012. The composition of mixed micelles formed by dodecyl trimethyl ammonium bromide and benzethonium chloride in water. J. Surfact. Deterg. 15, 147-155. ( 10.1007/s11743-011-1302-3) [DOI] [Google Scholar]
- 40.Rub MA, Alabbasi A, Azum N, Asiri AM. 2020. Aggregation and surface phenomena of amitriptyline hydrochloride and cationic benzethonium chloride surfactant mixture in different media. J. Mol. Liquids 300, 112346. ( 10.1016/j.molliq.2019.112346) [DOI] [Google Scholar]
- 41.Clint JH. 1975. Micellization of mixed nonionic surface-active agents. J. Chem. Soc. Faraday Trans. 71, 1327-1334. ( 10.1039/F19757101327) [DOI] [Google Scholar]
- 42.Bagheri A, Ahmadi SMA. 2017. Mixed micellization between amphiphilic drug propranolol hydrochloride and cetyltrimethylammonium bromide surfactant in aqueous medium. J. Mol. Liquids 230, 254-260. ( 10.1016/j.molliq.2017.01.024) [DOI] [Google Scholar]
- 43.Jakubowska A. 2010. Interactions of different counterions with cationic and anionic surfactants. J. Colloid Interface Sci. 346, 398-404. ( 10.1016/j.jcis.2010.03.043) [DOI] [PubMed] [Google Scholar]
- 44.Ruiz CC, Sanchez FG. 1994. Effect of urea on aggregation behavior of triton X-100 micellar solutions: a photophysical study. J. Colloid Interface Sci. 165, 110-115. ( 10.1006/jcis.1994.1211) [DOI] [Google Scholar]
- 45.Enea O, Jolicoeur CJ. 1982. Heat capacities and volumes of several oligopeptides in urea-water mixtures at 25.degree.C. Some implications for protein unfolding. J. Phys. Chem. 86, 3870-3881. ( 10.1021/j100216a033) [DOI] [Google Scholar]
- 46.Lissi E, Abuin E. 1994. Hydrophobic effects in water and water/urea solutions: a comparison. South. Braz. J. Chem. 2, 71-82. ( 10.48141/SBJCHEM.v2.n2.1994.72_1994.pdf) [DOI] [Google Scholar]
- 47.Tanaka H, Nakanishi K, Touhara H. 1985. Computer experiments on aqueous solutions. VII. Potential energy function for urea dimer and molecular dynamics calculation of 8 mol % aqueous solution of urea. J. Chem. Phys. 82, 5184-5191. ( 10.1063/1.448643) [DOI] [Google Scholar]
- 48.Kumar S, Parveen N, Kabir-ud-Din. 2004. Effect of urea addition on micellization and the related phenomena. J. Phys. Chem. B 108, 9588-9592. ( 10.1021/jp036552w) [DOI] [Google Scholar]
- 49.Manabe M, Koda M, Shirahama K. 1980. The effect of 1-alkanols on ionization of sodium dodecyl sulfate micelles. J. Colloid Interface Sci. 77, 189-194. ( 10.1016/0021-9797(80)90430-0) [DOI] [Google Scholar]
- 50.Bhanumathi R, Vijayalakshamma SK. 1986. Proton NMR chemical shifts of solvent water in aqueous solutions of monosubstituted ammonium compounds. J. Phys. Chem. 90, 4666-4669. ( 10.1021/j100410a040) [DOI] [Google Scholar]
- 51.Burke SE, Rodgers MP, Palepu R. 2001. A thermodynamic and photophysical study of the effects of thiourea on the micellar properties of aqueous sodium dodecyl sulphate solution. Mol. Phys. 99, 517-524. ( 10.1080/00268970010017298) [DOI] [Google Scholar]
- 52.Rubingh DN. 1979. Mixed micelle solutions. In Solution chemistry of surfactants, vol. 1 (ed. Mittal KL), pp. 337-354. New York, NY: Plenum. [Google Scholar]
- 53.Motomura K, Aratono M. 1998. Mixed surfactant systems (eds Ogino K, Abe M). New York, NY: Dekker. [Google Scholar]
- 54.Kumar D, Azum N, Rub MA, Asiri AM. 2018. Aggregation behavior of sodium salt of ibuprofen with conventional and gemini surfactant. J. Mol. Liquids 262, 86-96. ( 10.1016/j.molliq.2018.04.053) [DOI] [Google Scholar]
- 55.Ghosh S. 2001. Surface chemical and micellar properties of binary and ternary surfactant mixtures (cetyl pyridinium chloride, tween-40, and brij-56) in an aqueous medium. J. Colloid Interface Sci. 244, 128-138. ( 10.1006/jcis.2001.7855) [DOI] [Google Scholar]
- 56.Ren ZH. 2015. Effect of sodium chloride on interaction between amino sulfonateamphoteric surfactant and octylphenol polyoxyethylene ether (10) inaqueous solution. J. Ind. Eng. Chem. 30, 44-49. ( 10.1016/j.jiec.2015.04.027) [DOI] [Google Scholar]
- 57.Bagheri A, Jafari-Chashmi P. 2019. Study of aggregation behavior between N-lauryl sarcosine sodium and dodecyltrimethylammonium bromide in aqueous solution, using conductometric and spectrophotometric techniques. J. Mol. Liquids 282, 466-473. ( 10.1016/j.molliq.2019.03.015) [DOI] [Google Scholar]
- 58.Chattoraj DK, Birdi KS. 1984. Adsorption and the Gibbs surface excess. New York, NY: Plenum. [Google Scholar]
- 59.Zhou Q, Rosen MJ. 2003. Molecular interactions of surfactants in mixed monolayers at the air/aqueous solution interface and in mixed micelles in aqueous media: the regular solution approach. Langmuir 19, 4555-4562. ( 10.1021/la020789m) [DOI] [Google Scholar]
- 60.Rosen MJ, Hua XY. 1982. Surface concentrations and molecular interactions in binary mixtures of surfactants. J. Colloid Interface Sci. 86, 164-172. ( 10.1016/0021-9797(82)90052-2) [DOI] [Google Scholar]
- 61.Rub MA, Azum N, Asiri AM. 2017. Binary mixtures of sodium salt of ibuprofen and selected bile salts: interface, micellar, thermodynamic, and spectroscopic study. J. Chem. Eng. Data 62, 3216-3228. ( 10.1021/acs.jced.7b00298) [DOI] [Google Scholar]
- 62.Zana R. 1996. Critical micellization concentration of surfactants in aqueous solution and free energy of micellization. Langmuir 12, 1208-1211. ( 10.1021/la950691q) [DOI] [Google Scholar]
- 63.Bagheri A. 2021. Comparison of the interaction between propranolol hydrochloride (PPL) with anionic surfactant and cationic surface-active ionic liquid in micellar phase. Colloids Surfaces A 615, 126183. ( 10.1016/j.colsurfa.2021.126183) [DOI] [Google Scholar]
- 64.Naqvi AZ, Noori S, Kabir-ud-Din. 2016. Mixed micellization of dimeric surfactant–amphiphilic drug systems: effect of surfactant structure. RSC Adv. 6, 20 324-20 336. ( 10.1039/C5RA24058A) [DOI] [Google Scholar]
- 65.Karumbamkandathil A, Ghosh S, Anand U, Saha P, Mukherjee M, Mukherjee S. 2014. Micelles of benzethonium chloride undergoes spherical to cylindrical shape transformation: an intrinsic fluorescence and calorimetric approach. Chem. Phys. Lett. 593, 115-121. ( 10.1016/j.cplett.2014.01.005) [DOI] [Google Scholar]
- 66.Rub MA, Azum N, Khan F, Asiri AM. 2018. Aggregation of sodium salt of ibuprofen and sodium taurocholate mixture in different media: a tensiometry and fluorometry study. J. Chem. Thermodyn. 121, 199-210. ( 10.1016/j.jct.2018.02.019) [DOI] [Google Scholar]
- 67.Mukerjee P. 1967. The nature of the association equilibria and hydrophobic bonding in aqueous solutions of association colloids. Adv. Colloid Interface Sci. 1, 242-275. ( 10.1016/0001-8686(67)80005-8) [DOI] [Google Scholar]
- 68.Sulthana SB, Rao PVC, Bhat SGT, Rakshit AK. 1998. Interfacial and thermodynamic properties of SDBS-C12E10 mixed micelles in aqueous media: effect of additives. J. Phys. Chem. B 102, 9653-9660. ( 10.1021/jp982060l) [DOI] [Google Scholar]
- 69.Sugihara G, Miyazono A, Nagadome S, Oida T, Hayashi Y, Ko JS. 2001. Adsorption and micelle formation of mixed surfactant systems in water II. A combination of cationic gemini-type surfactant with MEGA-10. J. Oleo Sci. 52, 449-461. ( 10.5650/jos.52.449) [DOI] [Google Scholar]
- 70.Rodenas E, Valiente M, Villafruela MS. 1999. Different theoretical approaches for the study of the mixed tetraethylene glycol mono-n-dodecyl ether/hexadecyltrimethylammonium bromide micelles. J. Phys. Chem. B 103, 4549-4554. ( 10.1021/jp981871m) [DOI] [Google Scholar]
- 71.Kumar D, Rub MA, Azum N, Asiri AM. 2018. Mixed micellization study of ibuprofen (sodium salt) and cationic surfactant (conventional as well as gemini). J. Phys. Org. Chem. 31, e3730. ( 10.1002/poc.3730) [DOI] [Google Scholar]
- 72.Azum N, Rub MA, Asiri AM. 2019. Bile salt–bile salt interaction in mixed monolayer and mixed micelle formation. J. Chem. Thermodyn. 128, 406-414. ( 10.1016/j.jct.2018.08.030) [DOI] [Google Scholar]
- 73.Kumar D, Hidayathulla S, Rub MA. 2018. Association behavior of a mixed system of the antidepressant drug imipramine hydrochloride and dioctyl sulfosuccinate sodium salt: effect of temperature and salt. J. Mol. Liquids 271, 254-264. ( 10.1016/j.molliq.2018.08.147) [DOI] [Google Scholar]
- 74.Reif I, Somasundaran P. 1999. Asymmetric excess free energies and variable interaction parameters in mixed micellization. Langmuir 15, 3411-3417. ( 10.1021/la980103j) [DOI] [Google Scholar]
- 75.Israelachvili JN, Mitchell DJ, Ninham BW. 1976. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. 2, 1525-1568. ( 10.1039/F29767201525) [DOI] [Google Scholar]
- 76.Tanford C. 1980. The hydrophobic effect: formation of micelles and biological membranes. New York, NY: Wiley. [Google Scholar]
- 77.Myers D. 2006. Surfactant science and technology, 3rd edn. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- 78.Mantsch HH, Kartha VB, Cameron DG. 1984. FT-IR studies of aqueous surfactants: the temperature induced micelle formation. In Surfactants in Solutions, vol. 7 (eds Lindman B, Mittal K), pp. 673-690. New York, NY: Plenum Press. [Google Scholar]
- 79.Kawai T, Umemura J, Takenaka T, Gotou M, Sunamoto J. 1988. Fourier transform infrared study on the phase transitions of a 1,2-bis(myristoylamido)-1,2-deoxyphosphatidylcholine-water system. Langmuir 4, 449-452. ( 10.1021/la00080a034) [DOI] [Google Scholar]
- 80.Gaikar VG, Padalkar KV, Aswal VK. 2008. Characterization of mixed micelles of structural isomers of sodium butyl benzene sulfonate and sodium dodecyl sulfate by SANS, FTIR spectroscopy and NMR spectroscopy. J. Mol. Liquids 138, 155-167. ( 10.1016/j.molliq.2007.10.001) [DOI] [Google Scholar]
- 81.Rub MA. 2020. Effect of additives on the aggregation phenomena of amphiphilic drug and hydrotrope mixtures. J. Mol. Liquids 298, 112049. ( 10.1016/j.molliq.2019.112049) [DOI] [Google Scholar]
- 82.Senthilkumar M, Sheelarani B, Joshi RG, Dash S. 2019. Solubilization and interaction of ciprofloxacin with pluronics and their mixed micelles. New J. Chem. 43, 16 530-16 537. ( 10.1039/C9NJ03383A) [DOI] [Google Scholar]
- 83.Padalkar KV, Gaikar VG, Aswal VK. 2009. Characterization of mixed micelles of sodium cumene sulfonate with sodium dodecyl sulfate and cetyl trimethylammonium bromide by SANS, FTIR spectroscopy and NMR spectroscopy. J. Mol. Liquids 144, 40-49. ( 10.1016/j.molliq.2008.09.004) [DOI] [Google Scholar]
- 84.Mahajan S, Mahajan RK. 2012. Interactions of phenothiazine drugs with bile salts: micellization and binding studies. J. Colloid Interface Sci. 387, 194-204. ( 10.1016/j.jcis.2012.07.085) [DOI] [PubMed] [Google Scholar]
- 85.Hoque MA, Mahbub S, Hossain MD, Khan MA, Khan JM, Malik A, Ahmed A, Ahmed MZ. 2021. Influence of NaCl and temperature on the interaction between cephradine monohydrate and surfactants: conductivity and UV–visible measurements. J. Mol. Liquids 328, 115418. ( 10.1016/j.molliq.2021.115418) [DOI] [Google Scholar]
- 86.Sharma R, Mahajan RK. 2012. An investigation of binding ability of ionic surfactants with trifluoperazine dihydrochloride: insights from surface tension, electronic absorption and fluorescence measurements. RSC Adv. 2, 9571-9583. ( 10.1039/C2RA21020G) [DOI] [Google Scholar]
- 87.Benesi HA, Hildebrand JH. 1949. A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. Soc. 71, 2703-2707. ( 10.1021/ja01176a030) [DOI] [Google Scholar]
- 88.Paul BK, Samanta A, Guchhait N. 2010. Exploring hydrophobic subdomain IIA of the protein bovine serum albumin in the native, intermediate, unfolded, and refolded states by a small fluorescence molecular reporter. J. Phys. Chem. B 114, 6183-6196. ( 10.1021/jp100004t) [DOI] [PubMed] [Google Scholar]
- 89.Almgren M, Grieser F, Thomas JK. 1979. Dynamic and static aspects of solubilization of neutral arenes in ionic micellar solutions. J. Am. Chem. Soc. 101, 279-291. ( 10.1021/ja00496a001) [DOI] [Google Scholar]
- 90.Alghamdi YG, Rub MA, Kumar D, Asiri AM. 2021. Effects of various media on micellization, adsorption and thermodynamic behaviour of imipramine hydrochloride and antimicrobial surfactant mixtures. FigShare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Alghamdi YG, Rub MA, Kumar D, Asiri AM. 2021. Effects of various media on micellization, adsorption and thermodynamic behaviour of imipramine hydrochloride and antimicrobial surfactant mixtures. FigShare. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data that support this study have been uploaded as electronic supplementary material [90].