Abstract
Parasitism is a divesting problem that is frequently overlooked and may result in severe prominent clinical manifestation. This study aimed to investigate the seasonal and sexual prevalence of the gastrointestinal nematode Ascaridia columbae (A. columbae) infection among domestic pigeons in Giza governorate, Egypt, during the period from 2020 to 2021. One hundred and sixty suspected pigeons were clinically investigated. Blood & tissue samples were collected from infected birds to estimate serum zinc concentration, malondialdehyde (MDA), and nitric oxide levels. As well as tumor necrosis factor-alpha (TNF-α), interleukin 1β (IL1β) activity, and histopathological examination were estimated; also, worms were collected for morphological identification using electron microscope (SEM) and molecularly identified using polymerase chain reaction (PCR), further sequenced, and submitted in GenBank with accession number MZ343369. The average ascarid (length × breadth) were 72.4 ± 3.3 µm (70.5 – 79.9 µm) × 39.9 ± 2.5 µm (37.6 – 42.3 µm). The distinguishing morphological characteristics that have been noticed in ascarid worms were creamy white, cylindrical worm with triradiate lips with wide cephalic alae extending on both the lateral sides and filariform esophagus. In males, spicules were almost equal with the presence of precloacal chitinous-rimmed sucker. The prevalence of A. columbae infection was (63.1%) with a higher incidence in females (79.2%) than males (46.1%). The highest seasonal prevalence was observed in winter (92.5%), followed by summer and spring (87.5% and 55%), respectively while, the lowest prevalence was observed in autumn (17.5%). The intensity of worms in the infected intestine varied from 5 to 120 adult worms. The histopathological examination revealed the presence of chronic diffuse moderate catarrhal enteritis with roundworms in the lumen. Infected birds showed a significant increase in nitric oxide and MDA levels while serum zinc levels were lowered in infected pigeons. Infected pigeons revealed a marked increase in IL1-β and TNFα than apparently healthy ones.
Key words: Ascaridia columbae, Columba livia domestica, electron microscope, nematodes, pigeons, PCR
INTRODUCTION
Domestic pigeons (Columba livia domestica) are common free-living birds that coexist in the environment with humans and animals and avian species (Al-Barwari and Saeed, 2012; Alkharigy et al., 2018). Pigeons serve as reservoir hosts for a variety of pathogens that can be transmitted to other bird species as well as humans (Adang et al., 2008; Sari et al., 2008; Hamzah et al., 2020; Salem et al., 2021). Pigeons travel long distances in quest of food, which exposes them to parasitism and infections (Attia and Salem, 9AD). Parasites adversely affect pigeon's health, resulting in impaired growth, loss of condition, lowered production, and decreased performance and sometimes lead to death, especially in young squabs (Puttalakshmamma et al., 2008; Bizhga et al.,2011). Nematodes are the most significant widespread helminth species that have a direct or indirect life cycle and Pigeons are more sustainable to helminths' varieties, particularly Ascaridia species than other avian species (Belete et al., 2016; Alkharigy et al., 2018). Pigeons can be infected with A. columbae and Ascaridia galli (Abdel Rahman et al., 2019). Ascariasis is a poultry disease due to severe worm infestation, especially in pigeons, chickens and turkeys (Griffiths, 1978). Ascaridia columbae can infect all ages but is often fatal when infecting young birds under 12 wk of age (Jacobs et al., 2003). Infected pigeons with A. columbae show clinical signs of weight loss, emaciation, diarrhea, anemia, and death in case of heavy infestation (Abdel Rahman et al., 2019; Hamzah et al., 2020). Parasitic infestation in pigeons disturbs the body's dynamic equilibrium between reactive free radical generation and elimination (oxidative status) (Samani et al., 2018). Many drug preparations are available for the treatment of nematodes in poultry such as piperazine citrate, albendazole, fenbendazole, etc. (Tarbiata et al., 2016) furthermore, many research have been focused to found natural safe drug alternatives to overcome helminths and other pathogens resistance to chemical drugs and many of the other alternatives showed their efficacy in elimination of worms’ infestation and other infections such as: herbal extracts, essential oils, green synthesized nanoparticles, etc (Abd El-Ghany et al., 2021; Attia et al., 2021; El-Shall et al., 2021; Salem et al., 2021a). Therefore, this study aims to morpho-molecular characterization of A. columbae using scanning electron microscope and molecular analysis; histopathological examination of the infected intestine; determination of the health status of the infected birds by analysis of the immunological genes affected by the ascaridiasis infection.
MATERIALS AND METHODS
Case History and Clinical Investigations
The total numbers of 160 suspected adults (82 males and 78 females) domestic pigeons (Columba domestica) showing signs of (emaciation, ruffled feathers, general weakness, and unable to fly were collected from veterinary clinics and purchased from markets at different localities in Giza Province, Egypt which located in 29.9870°N 31.2118°E with a hot desert climate during the period from January 2020 to January 2021(1 year).
Direct Microscopal Examination
Fresh dropping was collected from each bird and examined (macroscopically and microscopically) for the presence of Ascaridia eggs or adult worms by the method described by William (2001). Positive birds were ethically euthanized and exposed for postmortem examination according to Swayne (2020) with collection of whole blood and its sera using plain tube and tube with EDTA.
All bird handling procedures were followed the generally accepted ethical rules approved by the Faculty of Veterinary Medicine, Cairo University, Egypt.
Postmortem Examination and Samples Collection
Each digestive tract was labeled and removed intact. The esophagus, crop, proventriculus, gizzard, duodenum, jejunum, ileum, ceca, and rectum were separated and placed into Petri dishes. A longitudinal incision was made out in all parts to expose their contents, and then all unattached nematodes were gently collected using forceps in separate Petri-dishes. Recovered nematodes were carefully washed with NaCl (0.9%) physiological saline (NaCl) and kept in a separate jar for further examinations.
Biochemical Analysis
The zinc concentration in sera samples was measured using ionized coupled plasma mass spectrometry as the method recorded by Page et al. (2018), Attia et al. (2019) and Attia et al. (2020).
Measurement of Stress Markers
Oxidative stress due to Ascaris columbae infection was performed by measuring the level of malondialdehyde (MDA) and nitric oxide in the blood, according to Aktas et al. (2017) and Attia et al. (2020).
Evaluation of Tumor Necrosis Factor-Alpha and Interleukin 1β Activity
Infected intestinal mucous and part of intestinal samples with the parasites were aseptically dissected. Samples from 5 uninfected healthy control pigeons were collected in the same manner as negative controls.
RNA Isolation
Isolation of mRNA from 100 mg of intestinal mucous and intestine was performed using total RNA kits (Ambion, Applied Biosystems). Homogenization using a FastPrep-24 homogenizer (MP Biomedicals, 2 cycles of 30 s at 6 m/s) of the sampled tissues were applied in Lysing Matrix D tubes (MP Biomedicals). The purity and quantity of mRNA were measured using Nanodrop (Thermo Scientific, Santa Clara, CA). A 500 nanogram of mRNA was made with DNaseI amplification grade (Invitrogen) following the manufacturer's instructions. The reverse transcription of treated mRNA was performed by High-Capacity cDNA Archive Kit (Applied Biosystems, Waltham, MA) (Attia et al. 2020; Younis et al. 2020).
Quantitative Real-Time PCR Protocol
PCR primer sets specific for tumor necrosis factor-alpha (TNFα) and Interleukin 1β specific for pigeons were designed and based on sequences deposited in the Gene Bank (Table 1). β-actin was used as a reference gene and for sample normalization. The genes expression included in this study was tested on a separate pool of cDNA, generated from 5 noninfected sheep previously examined for the presence of any parasites. Real-time PCR protocol was run according to Attia et al. (2020), with their condition as recorded in Hayashi et al. (2011).
Table 1.
The sequences of the forward and reverse primer used in the quantitative real-time PCR.
| Gene | Sequence [5-3] | Product size (bp) | Reference |
|---|---|---|---|
| IL1β | Forward GAGGAAGCCGACATCAGGAG Reverse GGGACGTGCAGATGAA CCAG |
136 | Hayashi et al. (2011). |
| IL6 |
Forward AGCGTCGATTTGCTGTGCT Reverse; GATTCCTGGGTAGCT GGGTCT |
107 | Hayashi et al. (2011). |
| β-Actin | Forward; AGGCTACAGCTTCACCACCAC Reverse; CCATCTCCTGCTCAA AATCCA |
95 | Hayashi et al. (2011). |
Parasitological Examination
Ultrastructure Identification of the Collected A. columbae Using Scanning Electron Microscopy Study
Adult A. columbae were freshly collected; then washed several times with saline (NaCl 0.9%) to remove the adhesive debris. The adult nematode was fixed in 2.5% Glutaraldehyde according to Hilali et al. (2015); Attia and Salaeh (2020); then dehydrated through passing in ethanol series (50; 70; 90; 100%) and dried in CO2 critical point drier (Autosamdri-815, Germany). The adult was glued over stubs (as an anterior end and posterior end) and then; coated with 20 nm gold (Abd ElKader et al., 2021) in a sputter coater (Spi-Module sputter Coater, UK). All specimens were photographed with a scanning electron microscope (JSM 5200, Electron prob); Microanalyzer Jeol, Japan; at Faculty of Agriculture, Cairo University, as described by Salem and Attia (2021).
Molecular Identification of A. columbae by Analysis of Internal Transcribed Spacer Genes
Genomic DNA was extracted using the QIAamp DNA Mini Kit (Qiagen, Venlo, the Netherlands) from the ethanol-preserved nematode samples as recommended by the manufacturer. The ITS1-5.8s-ITS2 rDNA partial gene region was amplified using primers designed by Bazh (2013), Primer 2 Forward (5′-GTTTCCGTAGGTGAACCTGC-3′), Reverse (5′-ATATGCTTAAGTTCAGCGGGT-3′) using the standard conditions. The PCR reactions are performed in volumes of 50 µL; according to Bazh (2013), Amplicons are sequenced using ABI Prism Dye Terminator Cycle Sequencing Core Kit (Applied Biosystems; Thermo Fisher Scientific, Waltham, MA) with 310 Automated DNA Sequencer (Applied Biosystems). To identify the related sequences, a BLAST search was conducted on the NCBI database. The ITS1-5.8s-ITS2 rDNA sequences were aligned using multiple alignments of CLUSTAL-X (Abdelsalam et al., 2020). The alignment was corrected manually using the alignment editor software BIOEDIT 4.8.9. They were using MEGA ver. 6.0, a phylogenetic tree was constructed using maximum parsimony (neighbor-interchange [CNI] level 3, random addition trees 100). Bootstrap analysis was performed based on 1,000 replicates to assess the robustness of the tree topologies.
Histopathological Examination
Tissue samples from the intestine of pigeon-containing roundworms were processed by paraffin embedding technique after fixation in 10% neutral buffered formalin. A cross-section of the intestine was made 3 to 4-µm thick using a microtome, followed by deparaffinization and staining with hematoxylin and eosin stain examination (Bancroft and Gamble, 2008). Sections were examined by light microscope and photographed by a digital camera (Olympus XC30, Tokyo, Japan).
Statistical Analysis
Oxidative stress and gene expression were analyzed using Predictive Analytics Software (PASW) Statistics, Version 18.0 software (SPSS Inc., Chicago, IL). A P-value was considered significant when <0.05.
RESULTS
Clinical Examination
Clinically examined suspected birds showed general signs as; weakness, inappetence, loss of weight, severe emaciation (Figure 1), ruffled feather, dropping in wings, and unable to fly. Some birds showed nervous manifestation as twisting in the neck (torticollis) as seen in Figure 2, vomiting, diarrhea, laying pigeons showed a history of decrease in both egg quantity and quality (eggs with soft, thin, and misshaped shells). Positive birds revealed the presence of oval grayish color eggs with the thick wall during the direct microscopic fecal examination as seen in Figure 3. The prevalence of A. columbae infection in the examined governorate was 63.1% during the observation period. Females harbored the infection 79.2% more than males 46.1%, as summarized in Table 2. Ascaridia infestation was highly prevalent during winter 92.5% and summer 87.5% seasons while less prevalent in spring 55% and least prevalent in autumn 17.5%; as summarized in Table 3.
Figure 1.
(A) Adult pigeon is showing ruffled feather greenish color diarrhea. (B and C) Adult pigeons showed severe emaciation, protrusion of keel bone and ruffled feathers.
Figure 2.
(A) Adult pigeon shows general weakness, dropping in the wing. (B and C) Adult pigeons showed nervous manifestation as twisting in the neck (Torticollis).
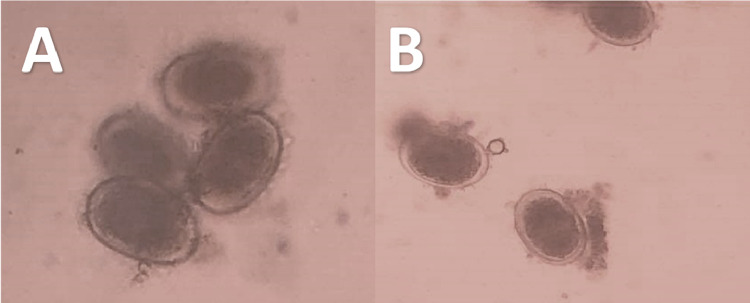
Figure 3.
Microscopic appearance of Ascaridia columbae eggs that appears as oval grayish color embryonated eggs with thick wall.
Table 2.
Prevalence of Ascaridiasis among pigeons in Giza during the period from 2020: 2021.
| Sex | Female | Male | Total |
|---|---|---|---|
| No. of examined | 82 | 78 | 160 |
| No. of positive | 65 | 36 | 101 |
| Prevalence (%) | 79.2 | 46.1 | 63.1 |
Table 3.
The seasonal prevalence of Ascaridia infestation among pigeons in Giza from 2020: 2021.
| Season | Winter | Spring | Summer | Autumn | Total |
|---|---|---|---|---|---|
| No. of examined | 40 | 40 | 40 | 40 | 160 |
| Positive cases | 37 | 22 | 35 | 7 | 101 |
| Prevalence (%) | 92.5 | 55 | 87.5 | 17.5 | 63.1 |
Postmortem Examination
Postmortem examination of positive birds revealed varying degrees of enteritis, impacted intestine with parasitic worms, obstruction, and even ruptured intestine. Mesenteric blood vessels were engorged with blood (Figure 4). The longitudinal section in the intestine revealed adult living worms attached to the mucosa and free worms in the intestinal lumen. Nematode worms were found in all intestinal parts (small intestine and ceca). The intestinal mucosa was congested with the presence of petechial bleeding at the site of worm attachment. The intensity of Ascaridia worms in positive cases varied from 5 to 120 adult worms in serious cases (Figure 5).
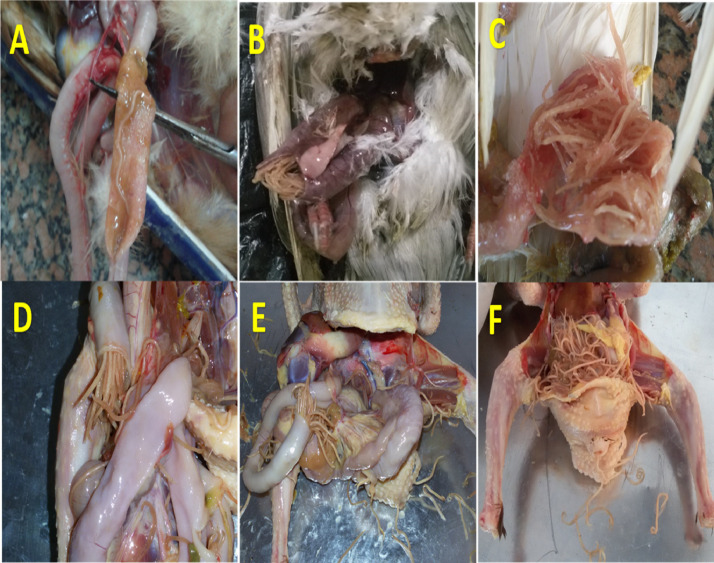
Figure 4.
(A) Opened intestine of pigeon showed attached adult nematode worm with the intestinal mucosa. (B) Opened duodenal loop showed congestion of intestine with impaction with adult nematodes. (C–F) Opened intestine of pigeon showed intestinal impaction with a large number of Ascaridia worms.
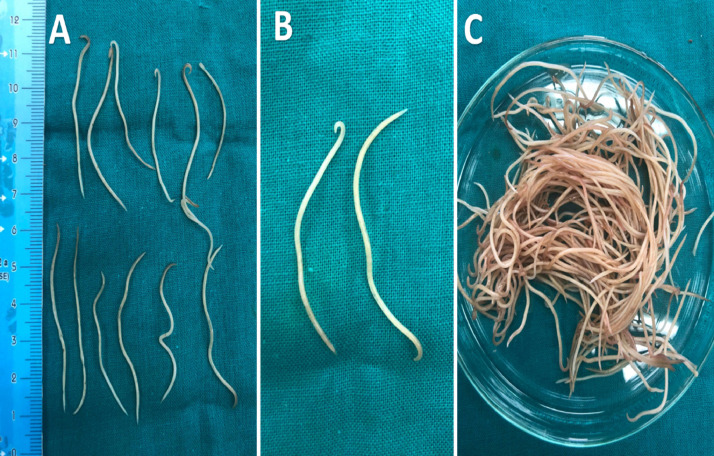
Figure 5.
(A) Adult Ascaridia worms. (B) Left adult male worm, right female Ascaridia worm. (C) A large number of Ascaridia worms collected from intestine of one freshly dead bird.
Morphological Description of the Collected Nematodes From the Intestinal Tract of Pigeons
The collected worms body appeared as semitransparent, creamy-white, and cylindrical shape and its anterior end is characterized by a prominent mouth, which is surrounded by 3 large, trilobed lips. The average ascarid (length × breadth) were 72.4 ± 3.3 µm (70.5 – 79.9 µm) × 39.9 ± 2.5 µm (37.6 – 42.3 µm). The adult ascarid worms were identified based on gross and microscopic morphological examination. Grossly, the length of the ascarid male and female worms (n = 5 each) were measured as 63.2 ± 4.7 mm (56 – 68 mm) and 70.9 ± 4.9 mm (65 – 76 mm), 10.1 ± 0.5 mm (9.4 – 10.8 mm) and 12.6 ± 0.2 mm (10.8 – 15.0 mm) and 11.8 ± 1.9 mm (9 – 14 mm) and 20 ± 2.1 mm (18 – 23 mm), respectively as seen in (Figure 6). The distinctive morphological features observed in ascarid worms were developed triradiate lips (1 dorsal and 2 subventral) with wide cephalic alae extending on both the lateral sides and filariform esophagus (Figure 6). In males, spicules were almost equal and there was the presence of precloacal chitinous-rimmed sucker (Figure 6).
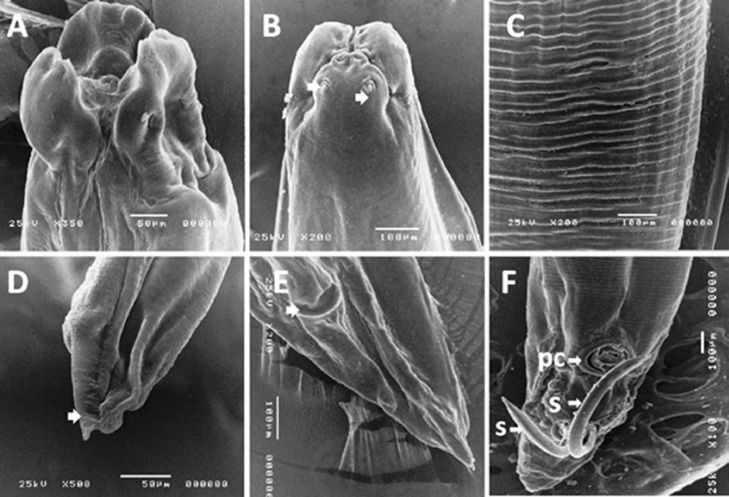
Figure 6.
Scanning electron micrograph of A. columbae showing: (A, B) anterior end with 3 lips and papillae (showing by arrows). (C) Segmentation along the nematode body. (D) Posterior end of female showing pointed posterior end; (E) Female posterior end showing the female genital notch. F: male posterior end with two equal spicule and precloacal sucker.
Molecular Identification
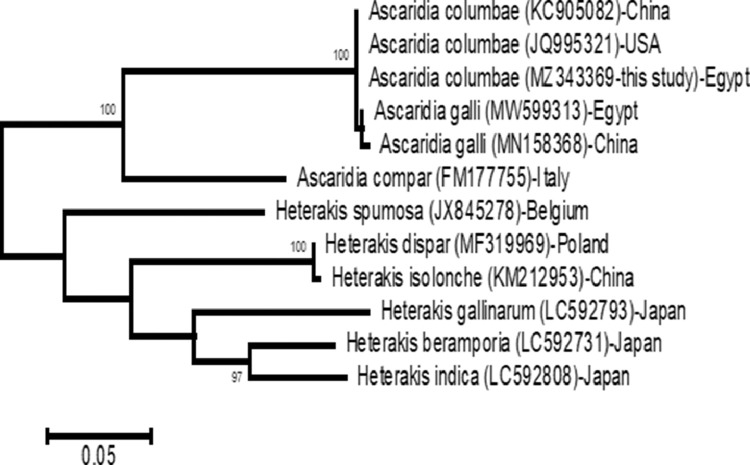
A total of 919 bp of the ITS1-5.8s-ITS2 rDNA sequence was deposited in GenBank under the accession number MZ343369. Comparing this genomic sequence with other ascaridida species showed that this Ascaridia sp. is deeply embedded in the ascaridida with a close relationship with A.columbae. Consequently, this parasite could be identified to the species level as Ascaridia columbae. BLAST analysis of A. columbae (MZ343369) in this study showed 97.95% similarity with A. columbae (KC905082) in China, 97.17% similarity with A. columbae (JQ995321) in the United States, 96.11 and 96.01% similarity of A. galli (MW599313 from Egypt and MN158368 from China, respectively), and 81.10% similarity with A. compar (FM177755) in Italy, 77.41% similarity with Heterakis spumosa (JX845278)-Belgium, 79.29% similarity with Heterakis dispar (MF319969)-Poland, 79.35% similarity with Heterakis isolonche (KM212953)-China, 77.26% similarity with Heterakis gallinarum (LC592793)-Japan, 72.23% similarity with Heterakis beramporia (LC592731)-Japan, 70.12% similarity with Heterakis indica (LC592808)-Japan. The phylogenetic tree based on the ITS1-5.8s-ITS2 rDNA gene sequenced in this work and 11 nucleotide sequences obtained from the database of GenBank showed that the dendrogram comprised of two major clades with a strong bootstrap value. The first clade included A. columbae of this study, was grouped with other A. columbae and A. galli to form a monophyletic branch which was powerfully supported with a 100% bootstrap value. The second clade grouped the Heterakoidea superfamily in one branch (Figure 7).
Figure 7.
Phylogenetic tree based on maximum composite likelihood model for the neighbor-joining method for the presence of A. columbae and another related superfamily retrieved from GenBank.
Biochemical and Immunological Evaluation of Infected Pigeon
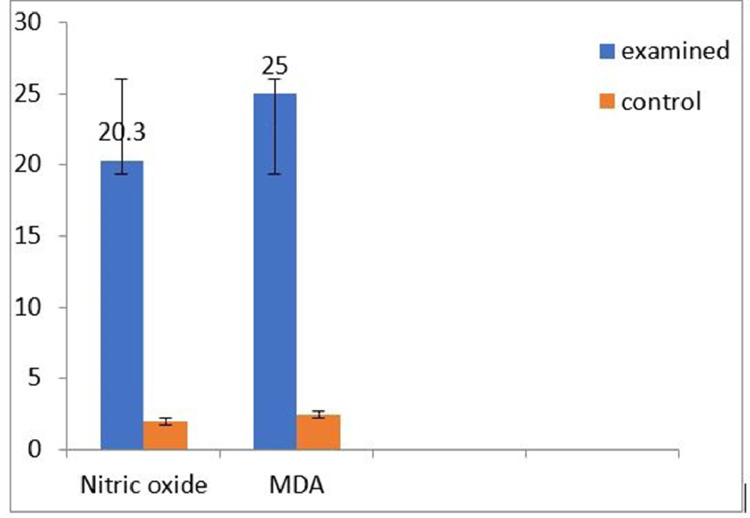
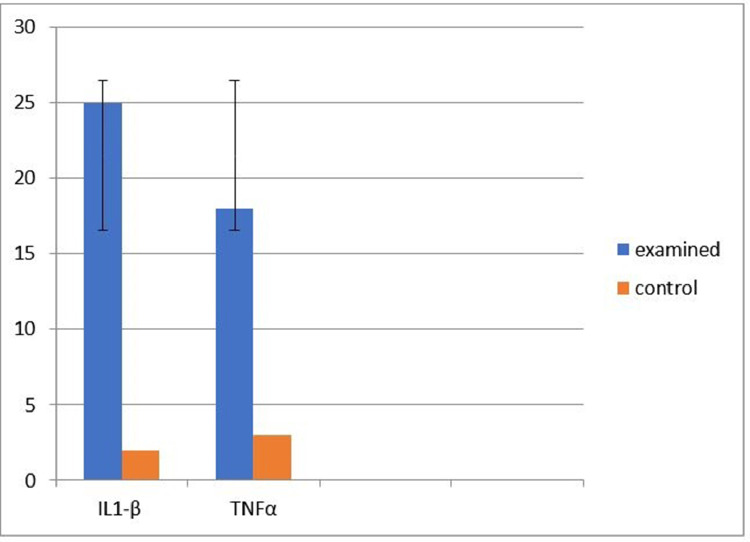
Infested pigeon had a significantly higher Nitric oxide and MDA (20.30 [±5.98, ±95% C.I.]; P = 0.010); (25.00 (±5.89, ±95% C.I.]; P = 0.001), respectively than noninfected pigeon (Figure 8). Concerning the zinc levels in infected pigeons were lower than that of non-infected pigeons by 50.35 (±6.79, ±95% C.I.); (Figure 8). Infested pigeons showed significantly higher IL1-β, upregulated by 25-fold, than that of control one, and TNFα was upregulated by 18-fold than that of control one (Figure 9).
Figure 8.
Biochemical evaluation of infected pigeon with nitric oxide and MDA analysis is examined and control pigeon. Abbreviation: MDA, malondialdehyde.
Figure 9.
Immunological evaluation of the infected pigeon with analysis of IL1-β and TNFα in examined and control one.
Histopathological Examination
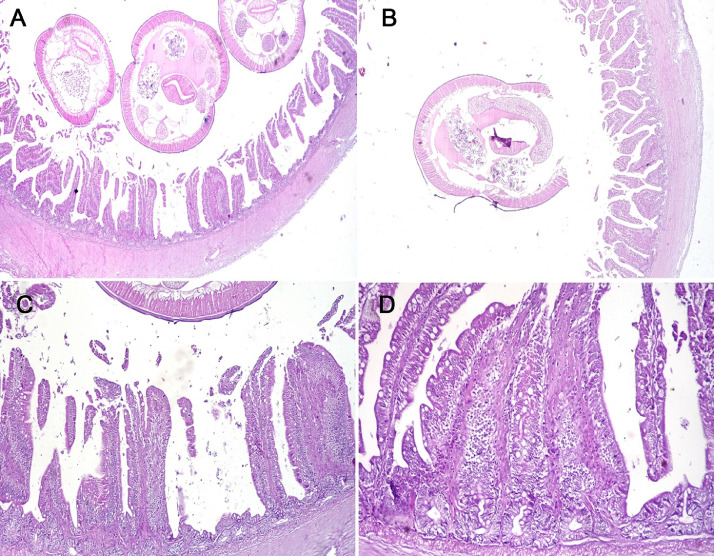
Microscopy of the intestine infected with Ascaridia species revealed multiple worms in the lumen oval in shape with dorsal and ventral indentation. The adult worm had outer prominent muscle cells covered by cuticle, multiple gravid uteri, intestine, and coiled ovary. The intestinal villi were short and wide. The mucosa showed epithelial and goblet cell hyperplasia and the lamina propria had fibrosis and leukocyte infiltration. The tunica musculosa was also segmentally hypertrophied with the absence of Meissner's plexuses (Figure 10).
Figure 10.
Intestine of pigeon showing (A) multiple roundworms in the lumen, shortening of intestinal villi, and segmental muscular hypertrophy of the tunica musculosa, (B) single roundworm in the lumen of less infested chicken, (C, D) epithelial and goblet cell hyperplasia in the lamina epithelialis, and fibrosis of lamina propria and submucosa with leukocytes infiltration.
DISCUSSION
Endoparasites are responsible for severe health problems in domestic pigeons (Adang et al., 2008). Helminth infestation has particularly deleterious and debilitating effects on infested birds, especially squabs. It lowers weight, interferes with healthy growth, makes older birds more susceptible to secondary infections, and causes mortalities between domesticated pigeons (Galloway, 1972; Cheng, 1973; Soulsby, 1982). Gastrointestinal nematodes cause subclinical infestation and result in severe economic losses. Our study focused on monitoring the prevalence of A. columbi in domesticated adult pigeons in the Giza governorate from 2020 to 2021. Clinical signs observed in the present study were like those recorded by Abdel Rahman et al. (2019). The poor health condition represented by emaciation, ruffled feathers, and inability to fly is mainly due to worms competitively feeding on semidigested food in the intestine, making it unavailable to the bird (Adang et al., 2008; Frantova, 2000). Furthermore, worms cause mechanical interference to food absorption and satiety feeling resulting in bird inappetence. Postmortem examination of examined birds revealed adult worms in all parts of the small intestine and ceca with worms’ intensity from 5 to 120 adult Ascaridia worms. This result agreed with Adang et al. (2008) and Frantova (2000) who attributed this finding to the presence of an optimal concentration of glucose, saline, debris, and semidigested food in these parts which are essential for worms’ growth.
Ascaridia columbae is the most common problematic parasite in pigeons and doves in various parts of the world, causing ascariasis (Boado et al., 1992; Senlik et al., 2005; Msoffe et al., 2010). In our study, the prevalence of Ascaridia columbae infestation in the Giza governorate was (63.1%) during the observation period, unlike other studies, which recorded a lower prevalence of Ascaridia spp. (1.20, 4.04, 5.10, 15.21, 15.50, 15.62 and 16.66%) (Sari et al., 2008; Natala et al., 2009; Bahrami et al., 2013; Radfar et al., 2011; Radfar et al., 2012; Faraj and Al- Amery, 2020; Msoffe et al., 2010). In Albania, a higher prevalence (40–90%) was also reported. The differences in Ascaridiasis prevalence may be attributed to the variation in climatic conditions, different localities, the presence of intermediate hosts (beetles, ants, pillbugs, earthworms, and snails), reduced host immunity, ineffective treatment, and bad management.
In our study, females harbored the infection (79.2%) more than males (46.1%). A similar observation was recorded by Bachaya et al. (2015), who attributed the high prevalence rate in females to the hormonal changes, stress during the laying of eggs, and the voracious feeding habits of female birds, especially during egg production than the males which remain largely selective. On the other hand, Adang et al. (2008) showed no significant association between infection and sex and a similarity in the mean intensity of infection in male and female birds, indicating that both sexes are equally exposed to the same risk of infection.
Our results revealed that the highest prevalence of A. columbae infection was observed during winter (92.5%) and summer (87.5%) seasons, while the prevalence was decreased in spring (55%) and the lowest in autumn (17.5%). Similarly, a parallel study demonstrated the high prevalence rate of Ascaridia spp. in the winter season and contributed that to farming activities in Zaria during the early months of the wet season, which may help to expose the eggs of nematodes that are hidden below the surface of the soil for the birds to ingest particularly as the birds visit newly cultivated farms in large flock to feed on spilled grains during planting (Adang et al., 2008). In our study, the high prevalence in summer may have contributed to the spread of beetles, pill bugs, ants, earthworms, and snails, which act as an intermediate host to nematodes. In contrast, a study found that the harsh and unfavorable climatic conditions in the summer season may be responsible for the low population of invertebrate hosts, and they negatively affected the viability of the hatched nematode eggs, resulting in low infection rates and worm loads in the summer season (Dede and Richards, 1998). The discrepancies in overall prevalence rate are attributed to climatic conditions, breeding methods, geographical location, presence of infectious stage, and variability in poultry immunity (Zada et al., 2015).
The identification of A. columbae depends on ultramicroscopic identification of the nematode and analysis of the ITS gene; this study reveals that the morphological identification confirmed the infection of examined pigeon with A. columbae nematod. In this study; the morphological identification was similar to a previous study recorded by Al Quraishy et al. 2020; which studied the A. columbae by SEM, which based upon different characteristic features that confirm the recovered nematod is A. columbae; these features are (well-developed lips; a cuticular organization with the presence of transverse annuli; pre-cloacal sucker the number and distribution of the cloacal papillae; a vulva near the middle region of the body) this study similar as Khalil et al. 2014; Al Quraishy et al. 2020.
Our study in molecular characterization used the PCR sequencing of the (ITS1-5.8sITS2) of the rDNA gene region, which confirmed that the collected Ascidida belongs to A. columbae nematod. A similar study was recorded by Al Quraishy et al. 2020 (A. columbae) and Gomes et al. 2015 (Ancylostoma buckleyi, Pterigodermatites pluripectinata, and Ascaridia galli). The moleculer approach in A. columbae revealing that the genus Ascaridia and is closely related and within the A. columbae species among Ascaridiidae (MZ343369) as recorded by Al Quraishy et al. (2020).
From our results, the sequenced ITS1-5.8s-ITS2 rDNA gene of A. columbae nematode was collected from domestic pigeon with the accession numbers MZ343369, which were displayed 100% identity with each other. All submitted sequences showed 97.95% identity with A. columbae collected from China, the United States, Egypt, Italy, and Belgium.
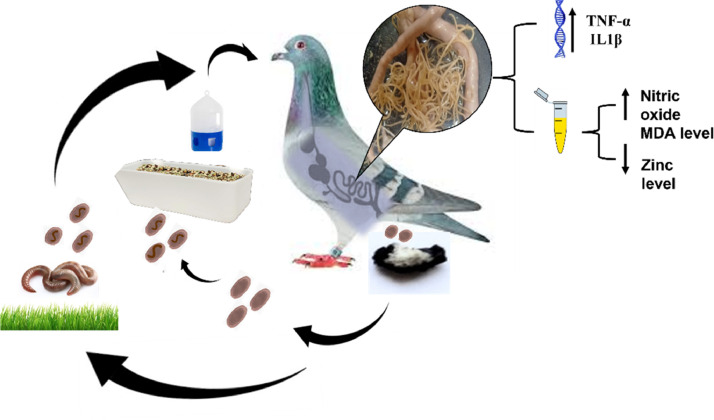
Ascaridia columbae has a direct or in-direct life cycle, as described in Figure 11. From our observations, infected pigeons with ascridiasis showed a significant decrease in serum zinc concentration. At the same time, MDA, Nitric oxide levels, and gene expression for IL1-β and TNF α were significantly increased. Also, Kim et al. (2001) confirmed that TNF-α and IL-1β could be used as markers for noticing the immune function. In a parallel study conducted by Olias et al. (2013), they found that IFN-γ and tumor necrosis factor (TNF α)-related cytokines were significantly up-modulated during the late central-nervous phase of Apicomplexa protozoon parasite infection in domestic pigeons as well as Basit et al. (2006) found that pigeons infected with gastrointestinal nematodes showed changes in blood hemoglobin level and total leucocytic count. On the other hand, Adang et al. (2012) found that experimentally infected pigeons with Ascaridia galli didn't show statistically significant differences in blood parameters (packed cell volume, hemoglobin level and total plasma protein) when compared with noninfected ones. We contributed this disagreement to the differences in the infected Ascaridia species or the infective dose.
Figure 11.
Life cycle of A. columbae showing that infected pigeon containing adult worms in its intestine which passes embryonated eggs in droppings, then eggs pass in the environment and 1st larva appears in the eggs within 2 wk then larva molts to 2nd stage larvae (infective stage) that may contaminate feed & water as a direct life cycle or may engulfed by earthworm then picked up by pigeon as indirect life cycle after that eggs liberare 2nd larvae in pigeons proventriculus or duodenum then molt to form 3rd stage larvae which molt to form adult in pigeon intestine. Our findings showed that during ascardiasis, genes expression for IL1-β and TNFα increased, and serum Nitric oxide and MDA levels decreased while serum zinc levels decreased. Abbreviation: MDA, malondialdehyde.
Histopathology of the intestine revealed chronic diffuse enteritis due to the presence of roundworm, which was partially consistent with previous studies (Abdel Rahman et al., 2019). In the current study, lesions of intestinal villi were limited to shortening of villi, but in previous studies, necrosis was also observed (Adang et al., 2010; Abdel Rahman et al., 2019). The segmental hypertrophy of tunica musculosa was similarly recorded with other ascaridia infestations (Lagundoye, 1972). The intestinal smooth muscle hyperplasia was greater in ringdove pigeon than with ornamental chicken, which could be due to an increase in intraluminal pressure caused by large amounts of intestinal parasites (Pavone et al., 2019). The absence of Meissner's plexuses which sense the lumen environment might be related to smooth muscle hypertrophy (Pavone et al., 2019). Through this study, we recommend paying attention to pigeon breeding and public awareness to reduce the risk of contracting diseases and transmitting these diseases to other avian and animal species as well as humans, through the application of biosecurity & quarantine measures, strengthening the general health of pigeons by using of balanced feed and supplying the bird with safe environmentally friend preparations as herbal extracts (Abou-Kassem et al., 2021a; Abd El-Hack et al., 2021a), essential oils (Alagawany et al., 2021a; Dosoky et al., 2021; El-Tarabily et al., 2021; Mohamed et al., 2021), essential amino acids (Abou-Kassem et al., 2021b), bioactive peptides (El-Saadony et al., 2021a,b), probiotics (Alagawany et al., 2021b, El-Saadony et al., 2021c), prebiotics (Abd El-Hack et al., 2020, 2021b; Yaqoob et al., 2021), plant-derived natural bioactive compounds (El-Saadony et al., 2021d; Raza et al., 2021; Seidavi et al., 2021a,b), biological synthesized nanoparticles (Abd El‐Hack et al., 2021c,d; El-Saadony et al., 2021e), and phytogenic compounds (Abo Ghanima et al., 2021, Ashour et al., 2021a; Abdel-Moneim et al.; Ashour et al., 2020b, Reda et al., 2021).
CONCLUSIONS
In Egypt, Ascaridia columbae is a widespread and it has a negative impact on domestic pigeons. Ascaridiasis in pigeons caused oxidative stress alterations in the form of a significant increase in nitric oxide and MDA levels, as well as a drop in serum zinc levels with a considerable increase in gene expression for IL1-β and TNFα in infected pigeons. Ascariasis revealed chronic diffuse catarrhal enteritis that consequent affect nutrients absorbability and affect negatively on the bird productivity. Further investigations should be adopted to found effective safe anthelmintic to overcome such parasitic infestation.
ACKNOWLEDGMENTS
The authors thank the Researchers Supporting Project for their funding this work number (RSP-2021/232) at King Saud University, Riyadh, Saudi Arabia.
Author contributions: Conceptualization, H.M.S., M.S.K., N.Y. and M.M.A.; methodology, H.M.S., M.S.K., N.Y. and M.M.A.; formal analysis, M.E.A.E-H.; investigation, H.M.S., M.S.K., N.Y. and M.M.A.; data curation, M.E.A.E-H. and M.T.E.-S.; writing—original draft preparation, M.E.A.E-H. and M.T.E.-S.; writing—review and editing, M.E.A.E-H.; visualization, M.E.A.E-H. and M.T.E.-S. All authors have read and agreed to the published version of the manuscript.
DSICLOSURES
Authors declare no conflict of interests.
REFERENCES
- Abd El-Ghany W.A, Shaalan M., Salem H.M. Nanoparticles applications in poultry production: an updated review. Worlds Poult. Sci. J. 2021 doi: 10.1080/00439339.2021.1960235. [DOI] [Google Scholar]
- Abd El-Hack M.E., Abdelnour S.A., Taha A.E., Khafaga A.F., Arif M., Ayasan T., Swelum A.A., Abukhalil M.H., Alkahtani S., Aleya L. Herbs as thermoregulatory agents in poultry: an overview. Sci. Total Environ. 2020;703 doi: 10.1016/j.scitotenv.2019.134399. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., Alaidaroos B.A., Farsi R.M., Abou-Kassem D.E., El-Saadony M.T., Saad A.M., Shafi M.E., Albaqami N.M., Taha A.E., Ashour E.A. Impacts of supplementing broiler diets with biological curcumin, zinc nanoparticles and Bacillus licheniformis on growth, carcass traits, blood indices, meat quality and cecal microbial load. Animals. 2021;11:1878. doi: 10.3390/ani11071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Alshahrani O.A., Saghir S.A., Al-Wajeeh A.S., Al-Shargi O.Y., Taha A.E., Mesalam N.M., Abdel-Moneim A.-M.E. Prebiotics can restrict Salmonella populations in poultry: a review. Anim. Biotechnol. 2021;19:1–10. doi: 10.1080/10495398.2021.1883637. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shehata A.M., Arif M., Paswan V.K., Batiha G.E.-S., Khafaga A.F., Elbestawy A.R. Approaches to prevent and control Campylobacter spp. colonization in broiler chickens: a review. Environ. Sci. Pollut. Res. 2021;28:4989–5004. doi: 10.1007/s11356-020-11747-3. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Swelum A.A., Arif M., Abo Ghanima M.M., Shukry M., Noreldin A., Taha A.E., El-Tarabily K.A. Curcumin, the active substance of turmeric: its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021;101:5747–5762. doi: 10.1002/jsfa.11372. [DOI] [PubMed] [Google Scholar]
- Abd ElKader N.A., Sheta E., AbuBakr H.O., El-Shamy O.A., Oryan A., Attia M.M. Effects of chitosan nanoparticles, ivermectin and their combination in the treatment of Gasterophilus intestinalis (Diptera: Gasterophilidae) larvae in donkeys (Equus asinus) Int. J. Trop. Insect Sci. 2021;41:43–54. [Google Scholar]
- Abdel Rahman M.M.I.A., Tolba H.M.N., Abdel-Ghany H.M. Ultrastructure, morphological differentiation, and pathological changes of Ascaridia species in pigeons. Adv. Anim.Vet. Sci. 2019;7:66–72. [Google Scholar]
- Abdel-Moneim A.M.E., El-Saadony M.T., Shehata A.M., Saad A.M., Aldhumri S.A., Ouda S.M., Mesalam N.M. Antioxidant and antimicrobial activities of Spirulina platensis extracts and biogenic selenium nanoparticles against selected pathogenic bacteria and fungi. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.09.046. . Accessed September 17, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelsalam M., Attia M.M., Mahmoud M.A. Comparative morpho molecular identification and pathological changes associated with Anisakis simplex larvae (Nematoda: Anisakidae) infecting native and imported chub mackerel (Scomber japonicus) in Egypt. Reg. Stud. Mar. Sci. 2020;39 [Google Scholar]
- Abo Ghanima M.M., Swelum A.A., Shukry M., Ibrahim S.A., Abd El-Hack M.E., Khafaga A.F., Alhimaidi A.R., Ammari A.A., El-Tarabily K.A., Younis M.E.M. Impacts of tea tree or lemongrass essential oils supplementation on growth, immunity, carcass traits, and blood biochemical parameters of broilers reared under different stocking densities. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Kassem D.E., El-Abasy M.M., Al-Harbi M.S., Abol-Ela S., Salem H.M., El-Tahan A.M., El-Saadony M.T., Abd El-Hack M.E., Ashour E.A. Influences of total sulfur amino acids and photoperiod on growth, carcass traits, blood parameters, meat quality and cecal microbial load of broilers. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.10.063. Accessed 30 October 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Kassem D.E., Mahrose K.M., El-Samahy R.A., Shafi M.E., El-Saadony M.T., Abd El-Hack M.E., Emam M., El-Sharnouby M., Taha A.E., Ashour E.A. Influences of dietary herbal blend and feed restriction on growth, carcass characteristics and gut microbiota of growing rabbits. Ital. J. Anim. Sci. 2021;20:896–910. [Google Scholar]
- Adang K.L., Abdu P.A., Ajanusi J.O., Oniye S.J., Ezealor A.U. Histopathology of Ascaridiagalli infection on liver, lung, intestine, heart and kidney of experimentally infected domestic pigeons (C.I.domestica) in Zaria, Nigeria. J. Sci. Technol. 2010;11:511–515. [Google Scholar]
- Adang L.K., Abdu P.A., Ajanusi J.O., Oniye S.J., Ezealor A.U. Effects of Ascaridia galli infection on body weight and blood parameters of experimentally infected domestic pigeons (Columba livia domestica) in Zaria, Nigeria. Short Communication, Revista Científica UDO 960. Agrícola. 2012;12:960–964. [Google Scholar]
- Adang K.L., Oniye S.J., Ezealor A.U., Abdu P.A., Ajanusi O.J. Ectoparasites of domestic pigeon (Columba livia domestica, Linnaeus) in Zaria. Nigeria Parasitol. Res. 2008;3:79–84. [Google Scholar]
- Aktas M.S., Kandemir F.M., Kirbas A., Hanedan B., Aydin M.A. Evaluation of oxidative stress in pigeon infected with Psoroptes ovis using total antioxidant capacity, total oxidant status, and malondialdehyde level. J. Vet. Res. 2017;61:197–201. doi: 10.1515/jvetres-2017-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Quraishy S., AbdelGaber R., Dkhil M.A., Alzuabi K. Morphological and molecular characteristics of the gastrointestinal nematode parasite Ascaridia columbae infecting the domestic pigeon Columba livia domestica in Saudi Arabia. Acta Parasitol. 2020;65:208–224. doi: 10.2478/s11686-019-00151-8. [DOI] [PubMed] [Google Scholar]
- Alagawany M., El-Saadony M.T., Elnesr S.S., Farahat M., Attia G., Madkour M., Reda F. Use of lemongrass essential oil as a feed additive in quail's nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., Madkour M., El-Saadony M.T., Reda F.M. Paenibacillus polymyxa (LM31) as a new feed additive: antioxidant and antimicrobial activity and its effects on growth, blood biochemistry, and intestinal bacterial populations of growing Japanese quail. Anim. Feed Sci. Technol. 2021;276 [Google Scholar]
- Al-Barwari S., Saeed I. The parasitic communities of the rock pigeon Columba livia from Iraq: component and importance. Türkiye Parazitol. Dergisi. 2012;36:232. doi: 10.5152/tpd.2012.56. [DOI] [PubMed] [Google Scholar]
- Alkharigy F.A., El Naas A.S., Maghrbi A.A.E. Survey of parasites in domestic pigeons (Columba livia) in Tripoli. Libya. Open Vet. J. 2018;8:360–366. doi: 10.4314/ovj.v8i4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour E.A., Abd El-Hack M.E., Swelum A.A., Osman A.O., Taha A.E., Alhimaidi A.R., Ismail I.E. Does the dietary graded levels of herbal mixture powder impacts growth, carcass traits, blood indices and meat quality of the broilers? Ital. J. Anim. Sci. 2020;19:1228–1237. [Google Scholar]
- Ashour E.A., Farsi R.M., Alaidaroos B.A., Abdel-Moneim A.M.E., El-Saadony M.T., Osman A.O., Abd El-Hack M.E. Impacts of dietary supplementation of pyocyanin powder on growth performance, carcase traits, blood chemistry, meat quality and gut microbial activity of broilers. Ital. J. Anim. Sci. 2021;20:1357–1372. [Google Scholar]
- Attia M.M., El-Gameel S.M., Ismael E. Evaluation of tumor necrosis factor-alpha (TNF-α); gamma interferon (IFN-γ) genes and oxidative stress in sheep: immunological responses induced by Oestrus ovis (Diptera: Oestridae) infestation. J. Parasit. Dis. 2020;44:332–337. doi: 10.1007/s12639-020-01220-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia M.M., Ismael E., Saleh N.M.K. A sensitive serodiagnostic tool for the detection of active infection of zoonotic visceral and nasopharyngeal linguatulosis. Vet. World. 2019;12:883–889. doi: 10.14202/vetworld.2019.883-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia M.M., Salaeh N.M.K. Ultrastructure of adult Gasterophilus intestinalis (Diptera: Gasterophilidae) and its puparium. Int. J. Trop. Insect Sci. 2020;40:327–335. [Google Scholar]
- Attia M.M. and Salem H.M., Morphological and molecular characterization of Pseudolynchia canariensis (Diptera: Hippoboscidae) infesting domestic pigeons, Int. J. Trop. Insect Sci, Accessed 9 July 2021. 10.1007/s42690-021-00597-2 [DOI]
- Attia M.M., Yehia N., Soliman M.M., Shukry M., El-Saadony M.T., Salem H.M. Evaluation of the antiparasitic activity of the chitosan-silver nanocomposites in the treatment of experimentally infested pigeons with Pseudolynchia canariensis. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.10.067. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachaya H.A., Raza M.A., Anjum M.A., Khan I.A., Aziz Manzoor A., Munawar S.H. Prevalence of Ascaridia galli in white leghorn layers and Fayoumi-Rhode Island red crossbred flock at government poultry farm Dina, Punjab, Pakistan. J. Trop. Biomed. 2015;32:11–16. [PubMed] [Google Scholar]
- Bahrami A.M., HosseinI E., Razmjo R. Important parasite in pigeon, its hematological parameter and pathology of intestine. World Appl. Sci. J. 2013;21:1361–1365. [Google Scholar]
- Bancroft J.D., Gamble M. Elsevier health sciences; China: 2008. Theory and Practice of Histological Techniques. [Google Scholar]
- Basit M.T., Pervez K., Avais M., Rabbani I. Prevalence and chemotherapy of nematodes infestation in wild and domestic pigeons and its effects on various blood components. J. Anim. Pl. Sci. 2006;16:24–27. [Google Scholar]
- Bazh E.A. Molecular characterization of Ascaridia galli infecting native chickens in Egypt. Parasitol Res. 2013;112:3223–3227. doi: 10.1007/s00436-013-3498-9. [DOI] [PubMed] [Google Scholar]
- Belete A., Addis M., Ayele M. Review on major gastrointestinal parasites that affect chickens. J. Biol. Agric. Healthc. 2016;6:11. [Google Scholar]
- Bizhga B., Sotiri E., Boçari A., Kolleshi D. Ascaridia columbae in Columbia livia domestica. Albanian J. Agric. Sci. 2011;2:7–12. [Google Scholar]
- Boado E., Zaldivar L., Gonzales A. Diagnosis, report and incidence of diseases of the pigeon (Columba livia) in Cuba. Rev Cubana de-Ciencia Avicola. 1992;19:74–78. [Google Scholar]
- Cheng T. Academic press; New York, San-Francisco and London: 1973. General Parasitology. [Google Scholar]
- Dede P.M., Richards W.S. Prevalence of helminthiasis in wild and domestic pigeons from North-east zone of Nigeria. Bull. Anim. Health Prod. Afr. 1998;46:193–195. [Google Scholar]
- Dosoky W.M., Zeweil H.S., Ahmed M.H., Zahran S.M., Shaalan M.M., Abdelsalam N.R., Abdel-Moneim A.E., Taha A.E., El-Tarabily K.A., Abd El-Hack M.E. Impacts of onion and cinnamon supplementation as natural additives on the performance, egg quality and immunity in laying Japanese quail. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Khalil O.S., Osman A., Alshilawi M.S., Taha A.E., Aboelenin S.M., Shukry M., Saad A.M. Bioactive peptides supplemented raw buffalo milk: biological activity, shelf life and quality properties during cold preservation. Saudi J. Biol. Sci. 2021;28:4581–4591. doi: 10.1016/j.sjbs.2021.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Abd El-Hack M.E., Swelum A.A., Al-Sultan S.I., El-Ghareeb W.R., Hussein E.O., Ba-Awadh H.A., Akl B.A., Nader M.M. Enhancing quality and safety of raw buffalo meat using the bioactive peptides of pea and red kidney bean under refrigeration conditions. Ital. J. Anim. Sci. 2021;20:762–776. [Google Scholar]
- El-Saadony M. T., M. Alagawany, A. K. Patra, I. Kar, R. Tiwari, M. A. Dawood, K. Dhama, and H. M. Abdel-Latif. 2021c. The functionality of probiotics in aquaculture: an overview, Fish Shellfish Immunol. 117:36–52, doi: 10.1016/j.fsi.2021.07.007. [DOI] [PubMed]
- El-Saadony M.T., Zabermawi N.M., Burollus M.A., Shafi M.E., Alagawany M., Yehia N., Askar A.M., Alsafy S.A., Noreldin A.E., Khafaga A.F., Dhama K., Elnesr S.S., Elwan H.A.M., Di Cerbo A., El-Tarabily K.A., Abd El-Hack M.E. Nutritional aspects and health benefits of bioactive plant compounds against infectious diseases: a review. Food Rev. Int. 2021:1–23. doi: 10.1080/87559129.2021.1944183. Accessed 20 Jul 2021. doi: [DOI] [Google Scholar]
- El- Saadony M. T. , A. M. Saad, T. F. Taha, A. A. Najjar, N. M. Zabermawi, M. M. Nader, S. F. AbuQamar, K. A. El-Tarabily, and A. Salama. 2021e. Selenium nanoparticles, from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi, as a new source from human breast milk, Saudi J. Biol. Sci. 28:6782–6794. [DOI] [PMC free article] [PubMed]
- El-Shall N.A., Abd El-Hack M.E., Albaqami N.M., Khafaga A.F., Taha A.E., Swelum A.A., El-Saadony M.T., Salem H.M., El-Tahan A.M., AbuQamar S.F., El-Tarabily K.A., Elbestawy A.R. Phytochemical control of poultry coccidiosis: a review. Poult. Sci. 2021 doi: 10.1016/j.psj.2021.101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Tarabily K.A., El-Saadony M.T., Alagawany M., Arif M., Batiha G.E., Khafaga A.F., Elwan H.A., Elnesr S.S., Abd El-Hack M.E. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021;28:5145–5156. doi: 10.1016/j.sjbs.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraj A.A., Al- Amery A.M. Microscopic and molecular diagnosis of Ascaridia spp. in domestic pigeons (Columba livia domestica) in Baghdad city. Iraq. Iraqi J. Agric. Sci. 2020;51:1220–1225. [Google Scholar]
- Frantovo D. Some parasitic nematodes (Nematoda) of Birds (Aves) in the Czech Republic. Acta Soc. Zool. Bohem. 2000;66:13–28. [Google Scholar]
- Galloway H.J. Longman; London: 1972. Farm Animal Health and Disease Control. [Google Scholar]
- Gomes A.P., Olifers N., Santos M.M., Simões Rde O., Maldonado Júnior A. New records of three species of nematodes in Cerdocyon thous from the Brazilian Pantanal wetlands. Braz J. Vet. Parasitol. Jaboticabal. 2015;24:324–330. doi: 10.1590/S1984-29612015061. [DOI] [PubMed] [Google Scholar]
- Griffiths H.J. University of Minnesota Press; Minneapolis, MN: 1978. Pages 46-47 in A Handbook of Veterinary Parasitology: Domestic Animals of North America. [Google Scholar]
- Hamzah D.J., Al kardhi I.K.A., Al saegh H.A.H., Muhammed H.A., ALAli F. Molecular identification of Ascaridia columbae in the Local Healthy Pigeon (Columba livia domestica, Gmelin, 1780) in Karbala Province. Indian J. Forensic Med. Toxicol. 2020;14:1008–1012. [Google Scholar]
- Hayashi T., Hiromoto Y., Chaichoune K., Patchimasiri T., Chakritbudsabong W., Prayoonwong N., Chaisilp N., Wiriyarat W., Parchariyanon S., Ratanakorn P., Uchida Y., Saito T. Host cytokine responses of pigeons infected with highly pathogenic thai avian influenza viruses of subtype H5N1 isolated from wild birds. PLoS One. 2011;6:e23103. doi: 10.1371/journal.pone.0023103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilali M., Mahdy O.A., Attia M.M. Monthly variations of Rhinoestrus spp. (Diptera: Oestridae) larvae infesting donkeys in Egypt: morphological and molecular identification of third stage larvae. J. Adv. Res. 2015;6:1015–1021. doi: 10.1016/j.jare.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs R.D., Hogsette J.A., Butcher J.D. Pages 1-3 in The Institute of Food and Agricultural Sciences (IFAS) Series PS18. University of Florida; Gainesville, Florida: 2003. Nematode parasites of poultry (and where to find them) [Google Scholar]
- Khalil A.I., Lashein G.H., Morsy G.H., Abd El-Mottaleb D.I. Oxyurids of wild and laboratory rodents from Egypt. Life Sci. J. 2014;11:94–107. [Google Scholar]
- Kim G.M, Xu J., Song S.K., Yan P., Ku G., Xu X.M., Hsu C.Y. Tumor necrosis factor receptor deletion reduces nuclear factor-kappaB activation, cellular inhibitor of apoptosis protein 2 expression, and functional recovery after traumatic spinal cord injury. J. Neurosci. 2001;21:6617–6625. doi: 10.1523/JNEUROSCI.21-17-06617.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagundoye S.B. Disordered small bowel pattern in ascariasis. Trop. Geogr. Med. 1972;24:226–231. [PubMed] [Google Scholar]
- Mohamed S.H., Attia A.I., Reda F.M., Abd El-Hack M.E., Ismail I.E. Impacts of dietary supplementation of Boswellia serrata on growth, nutrients digestibility, immunity, antioxidant status, carcass traits and caecum microbiota of broilers. Ital. J. Anim. Sci. 2021;20:205–214. [Google Scholar]
- Msoffe P.L.M., Muhairwa A.P., Chiwanga G.H., Kassuku A.A. A study of ecto- and endo-parasites of domestic pigeons in Morogoro unicipality, Tanzania, African. J. Agric. Res. 2010;5:264–2672. [Google Scholar]
- Natala A.J., Asemadahun N.D., Okubanjo O.O., Ulayi B.M., Owolabi Y.H., Jato I.D., Yusuf K.H. A survey of parasites of domesticated pigeon (Columba livia domestic) in Zaria, Nigeria. Int. J. Soft Comput. 2009;4:148–150. [Google Scholar]
- Olias P.H., Meyer A., Klopfleisch R., Lierz M., Kaspers B., Gruber A.D. Modulation of the host Th1 immune response in pigeon protozoal encephalitis caused by Sarcocystis calchasi. Vet. Res. 2013;44:10. doi: 10.1186/1297-9716-44-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page C.M., Murphy T.W., Van Emon M.L., Bowman J.G.P., Wyffels S.A., Stewart W.C. Blood serum mineral element concentrations of weaned Montana ram lambs and their relationship with water quality characteristics. Prof. Anim. Sci. 2018;34:410–420. [Google Scholar]
- Pavone S., Stazi M., Cambiotti V., Castro V., Gobbi M., Zema J., Filippini G. Cases of intestinal smooth muscle hypertrophy/hyperplasia in pigeon and chickens. J. Vet. Med. Sci. 2019;81:1351–1354. doi: 10.1292/jvms.19-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttalakshmamma G.C., Ananda K.J., Prathiush P.R., Mamatha G.S., Suguna R. Prevalence of gastrointestinal parasites of poultry in and around Banglore. Vet. World. 2008;1:201. [Google Scholar]
- Radfar M.H., Asl E.N., Seghinsara H.R., Dehaghi M.M., Fathi S. Biodiversity and prevalence of parasites of domestic pigeons (Columba livia domestica) in a selected semiarid zone of South Khorasan. Iran. Trop. Anim. Health Prod. 2012;44:225–229. doi: 10.1007/s11250-011-0002-3. [DOI] [PubMed] [Google Scholar]
- Radfar M.H., Fathi S., Asl E.N., Dehaghi M.M., Seghinsara H.R. A survey of parasite of domestic pigeons (Columba livia domestica) in South Khorasan, Iran. Vet. Rese. 2011;4:18–23. doi: 10.1007/s11250-011-0002-3. [DOI] [PubMed] [Google Scholar]
- Raza S.H.A., Naqvi S.R.Z., Abdelnour S.A., Schreurs N., Mohammedsaleh Z.M., Khan I., Shater A.F., Abd El-Hack M.E., Khafaga A.F., Quan G., Khan R., Wang S., Cheng G., Zan L. Beneficial effects and health benefits of Astaxanthin molecules on animal production: a review. Res. Vet. Sci. 2021;138:69–78. doi: 10.1016/j.rvsc.2021.05.023. [DOI] [PubMed] [Google Scholar]
- Reda F., El-Saadony M.T., El-Rayes T., Farahat M., Attia G., Alagawany M. Dietary effect of licorice (Glycyrrhiza glabra) on quail performance, carcass, blood metabolites and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Attia M.M. Accidental intestinal myiasis caused by Musca domestica L. (Diptera: Muscidae) larvae in broiler chickens: a field study. Int. J. Trop. Insect Sci. 2021;41:2549–2554. [Google Scholar]
- Salem H.M., Ismael E., Shaalan M. Evaluation of the effects of silver nanoparticles against experimentally induced necrotic enteritis in broiler chickens. Int. J. Nanomed. 2021;16:6783–6796. doi: 10.2147/IJN.S319708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Yehia N., Al-Otaibi S., El-Shehawi A.M., Elrys A.A.M.E., ElSaadony M.T., Attia M.M. The prevalence and intensity of external parasites in domestic pigeons (Columba livia domestica) in Egypt with special reference to the role of deltamethrin as insecticidal agent. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.10.042. Assesed 22 October, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samani A.D., Kheirabadi K.P., Mohebbi A., Ghahfarokhi S.M., Samani A.D. Effect of blood parasites on biomarkers of oxidative status in pigeons. J. Dairy Vet. Anim. 2018;7:00186. [Google Scholar]
- Sari B., Karatepe B., Karatepe M., Kara M. Parasites of domestic (Columba livia domestica) and wild (Columba livia livia) pigeons in Niğde. Turkey Bull. Vet. Inst. Pulawy. 2008;52:551–554. [Google Scholar]
- Seidavi A., Azizi M., Swelum A.A., Abd El-Hack M.E., Naiel M.A.E. Practical application of some common agro-processing wastes in poultry diets. Worlds Poult. Sci. 2021:1–15. doi: 10.1080/00439339.2021.1960461. Accessed 24 Aug 2021. [DOI] [Google Scholar]
- Seidavi A., Tavakoli M., Slozhenkina M., Gorlov I., Hashem N.M., Asroush F., Taha A.E., Abd El-Hack M.E., Swelum A.A. The use of some plant-derived products as effective alternatives to antibiotic growth promoters in organic poultry production: a review. Environ. Sci. Poll. Res. 2021;28:47856–47868. doi: 10.1007/s11356-021-15460-7. [DOI] [PubMed] [Google Scholar]
- Senlik B., Gulegen E., Akyol V. Effect of age, sex and season on the prevalence and intensity of helminth infections in domestic pigeons (Columba Livia) from Bursa province. Turkey Acta Vet. Hungarica. 2005;53:449–456. doi: 10.1556/AVet.53.2005.4.5. [DOI] [PubMed] [Google Scholar]
- Soulsby E.J.L. Helminths, Arthropods and Protozoa of Domesticated Animals. 7th ed. OXOFORD ACADEMIC; London, UK: 1982. [Google Scholar]
- Swayne D.E. 14th ed. Wiley-Blackwell; Hoboken, NJ: 2020. Diseases of Poultry.https://lccn.loc.gov/2019015575 [Google Scholar]
- Tarbiata B., Janssonb D.S., Tydéna E., Höglunda J. Comparison between anthelmintic treatment strategies against Ascaridia galli in commercial laying hens. Vet. Parasitol. 2016;226:109–115. doi: 10.1016/j.vetpar.2016.07.006. [DOI] [PubMed] [Google Scholar]
- William J.F. 5th ed. Blackwell publisher; UK: 2001. Veterinary Parasitology Reference Manual. [Google Scholar]
- Yaqoob M., Abd El-Hack M.E., Hassan F., El-Saadony M.T., Khafaga A., Batiha G., Yehia N., Elnesr S., Alagawany M., El-Tarabily K., Wang M. The potential mechanistic insights and future implications for the effect of prebiotics on poultry performance, gut microbiome, and intestinal morphology. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis N.A., Laban S.E., Al-Mokaddem A.K., Attia M.M. Immunological status and histopathological appraisal of farmed Oreochromis niloticus exposed to parasitic infections and heavy metal toxicity. Aquac. Int. 2020;28:2247–2262. [Google Scholar]
- Zada L., Rehman T., Niaz S., Zeb M.A., Ruqia B., Salma M.A.Khan, Khan A. Prevalence of Ascaridia galli in some poultry farms of District Mardan. J. Adv. Parasitol. 2015;2:75–79. [Google Scholar]