Abstract
There is a close relationship between sleep and depression, and certain maladaptive outcomes of sleep problems may only be apparent in individuals with heightened levels of depression. In a sample enriched for preschool depression, we examined how sleep and depression in early childhood interact to predict later trajectories of gray matter volume. Participants (N = 161) were recruited and assessed during preschool (ages 3–6 years) and were later assessed with five waves of structural brain imaging, spanning from late childhood to adolescence. Sleep and depression were assessed using a semi-structured parent interview when the children were preschool-aged, and total gray matter volume was calculated at each scan wave. Although sleep disturbances alone did not predict gray matter volume/trajectories, preschool sleep and depression symptoms interacted to predict later total gray matter volume and the trajectory of decline in total gray matter volume. Sleep disturbances in the form of longer sleep onset latencies, increased irregularity in the child’s sleep schedule, and higher levels of daytime sleepiness in early childhood were all found to interact with early childhood depression severity to predict later trajectories of cortical gray matter volume. Findings provide evidence of the interactive effects of preschool sleep and depression symptoms on later neurodevelopment.
Keywords: Early childhood sleep, Preschool depression, Gray matter volume, Longitudinal
1. Introduction
Healthy sleep, defined as adequate, good quality sleep that has appropriate timing and regularity, is crucial for health and well-being in childhood (Gruber et al., 2014). However, with an estimated 25% of children experiencing some sort of sleep disturbance (Owens, 2007), it is important to understand the developmental consequences of early-occurring sleep disturbances. Sleep disturbances in childhood are associated with a range of maladaptive outcomes, including higher body mass indexes (Butte et al., 2016), elevated blood pressure (Quist et al., 2016), increased later internalizing and externalizing problems (Gregory et al., 2005, Gregory and O’Connor, 2002), poorer cognitive abilities (Bernier et al., 2014), and poorer academic achievement/school performance (Dewald et al., 2010). Although less research has focused on the effects of sleep disturbances in early childhood than in school-age children, growing evidence suggests that similar associations are present for sleep disturbances that emerge in the toddler and preschool period (Hoyniak et al., 2020, Hoyniak et al., 2021, Reynaud et al., 2018).
Despite evidence suggesting far-reaching cognitive, behavioral and health consequences of sleep disturbances in childhood, relatively little research has focused on the relationship of childhood sleep to developing brain structure and function (Spruyt, 2019, Spruyt, 2020). A growing literature suggests that sleep plays a crucial role in neurodevelopment across childhood and adolescence (Dutil et al., 2018, Jan et al., 2010, Maski and Kothare, 2013), and it is likely that sleep plays a similarly crucial role in neurodevelopment in early childhood. Given that early childhood is a time of rapid brain development (Brown and Jernigan, 2012), with the vast majority of recent-large scale studies showing a normative decline in gray matter volume starting by age 5 (Mills et al., 2016, Walhovd et al., 2017), it is plausible that sleep disturbances that occur during this developmental period may have lasting neurodevelopmental consequences. However, to our knowledge, no research has explored how sleep in early childhood relates to trajectories of later brain development. To address this gap in the literature, using a sample enriched for preschool depression, the current study examined how sleep in early childhood is associated with trajectories of neural development, specifically total gray matter volume, across late-childhood into adolescence.
2. Sleep and neurodevelopment in childhood
Experimental research in both humans and animal models suggests that sleep deprivation has a number of neurocognitive effects, including diminished executive functioning, memory performance, and sustained attention (Lowe et al., 2017). Across both experimental and observational studies with children, sleep disturbances have been found to be associated with decrements in various cognitive processes, including poorer concentration, academic difficulties, and intellectual functioning (Astill et al., 2012, Beebe, 2011, Gorgoni et al., 2020, O’Brien, 2011). Research suggests that sleep facilitates the reorganization of brain circuitry (Tononi and Cirelli, 2014) and impacts synaptic density (Kurth et al., 2015), two crucial neurodevelopmental processes. Problematic sleep in childhood, such as inadequate, non-restorative, or irregular sleep, may disrupt these processes, leading to widespread effects on neurodevelopment that may underlie some of the cognitive and behavioral deficits that have been shown to be associated with poor sleep. A recent systematic review examined the association between sleep habits and brain structure/function in childhood (Dutil et al., 2018). Findings suggested an effect of sleep on various brain structures and functions in childhood, although firm conclusions were difficult given the relative dearth of studies and the use of different imaging methodologies (Dutil et al., 2018). It is likely that sleep disturbances have far-reaching neurodevelopmental consequences, impacting the structure and/or function of several brain regions. The current study focuses explicitly on total gray matter volume as a plausible index of these widespread effects. Additionally, as gray matter volume undergoes an extended trajectory of experience-based pruning across childhood into adolescence (Ducharme et al., 2016, Frangou et al., 2021, Gennatas et al., 2017), this index may be particularly susceptible to the effects of sleep disturbances.
Few studies have examined relations between sleep and gray matter volume in children. Kocevska et al. (2016) identified that sleep problems in toddlerhood were associated with smaller gray matter volumes at age 7. In adolescents, weekend shifts in sleep schedules, reduced sleep efficiency, and shorter weekday sleep durations have all been shown to be associated with smaller gray matter volumes (Lapidaire et al., 2021, Teicher et al., 2018, Urrila et al., 2017). Research examining specific regions suggests that sleep disturbances, including shorter weekday sleep durations and obstructive sleep apnea, are associated with reduced regional gray matter volume in the bi-lateral hippocampus (Taki et al., 2012) and across various frontal regions (Musso et al., 2020, Philby et al., 2017) in children and adolescence. These studies indicate a role of sleep and sleep disturbances in gray matter development across childhood and adolescence, but additional research is clearly needed to further clarify this association.
3. Sleep and depression
Research has also identified a close relationship between sleep and depression symptoms, with nearly 90% of adults and 73% of youth diagnosed with Major Depressive Disorder (MDD) reporting some form of sleep disturbance (Liu et al., 2007, Tsuno et al., 2005). There is likely a bi-directional association between depression and sleep disturbances, with sleep disturbances exacerbating depressive symptoms and depressive symptoms, in turn, exacerbating sleep disturbances (Chorney et al., 2007). Additionally, the negative effects of sleep disturbances and depression may be interactive, with certain maladaptive outcomes of sleep loss only apparent in individuals with heightened levels of depression. For example, using ecological momentary assessment in a sample of children and adolescents, more negative moods during the day were associated with less time spent awake the following night and more positive mood during the day was associated with more time spent in bed the following night, but only among youth with depression. Further, reduced sleep latency was associated with more positive and less negative mood the following day in youth with depression (Cousins et al., 2011). These findings highlight how depression might alter the relationship of sleep to various outcomes. Although a similar interaction with depression may be at work in the relationship of sleep disturbances to neurodevelopment, to our knowledge, no research has yet examined how preschool sleep and depression interact to predict later neurodevelopment.
4. The current study
Using a sample enriched for preschool depression, we examined how sleep in early childhood predicts trajectories of development of total gray matter volume across late childhood to adolescence. In the current study, sleep was assessed across several, parent-reported domains, including bedtime, sleep onset latency, night awakenings, sleep schedule regularity, daytime sleepiness, and total sleep duration. Sleep disturbances were defined as later bedtimes, longer latencies to fall asleep, increased night awakenings, more irregular sleep schedules, increased daytime sleepiness, and shorter total sleep durations, consistent with theoretical conceptualizations of what constitutes healthy sleep (DeSantis et al., 2019). Building on prior findings (Kocevska et al., 2016, Lapidaire et al., 2021, Urrila et al., 2017), we hypothesized that sleep disturbances in early childhood would predict smaller total gray matter volumes later in development, as well as a more rapid rate of decrease in gray matter volume across time. In the current study, we use the term “predict” in its regression context, to describe the association between the predictors of interest and the outcomes of interest. We are not implying causality or a directionality of association between variables with this term. Given a relative lack of literature specifying which domains of sleep disturbances (e.g., later sleep timing, shorter sleep durations, decreased sleep regularity, etc.) show associations with gray matter, we took a broad approach to examining the relationship between sleep and gray matter across sleep domains. As the effect of sleep disturbances and depression symptoms may be interactive in predicting maladaptive outcomes, we also examined how sleep and depression interact to predict gray matter volume development. We hypothesized that children with co-occurring depression symptoms and sleep disturbances in early childhood would show the smallest total gray matter volumes and a more rapid rate of decrease in total gray matter volume across time. Similarly, we took a broad approach to examining which domains of sleep disturbance might interact with depression to predict gray matter development. We focus specifically on preschool depression symptoms as a continuous measure rather than a dichotomous, diagnostic index in order to capture the broad distribution of preschool depression symptoms in this sample. Given that no prior research has explored how sleep in early childhood predicts later gray matter volume trajectories, and that there was little evidence for specific regional effects, the current study focuses specifically on total gray matter volumes as a measure of the plausible widespread effects of sleep on neurodevelopment.
5. Methods
5.1. Participants
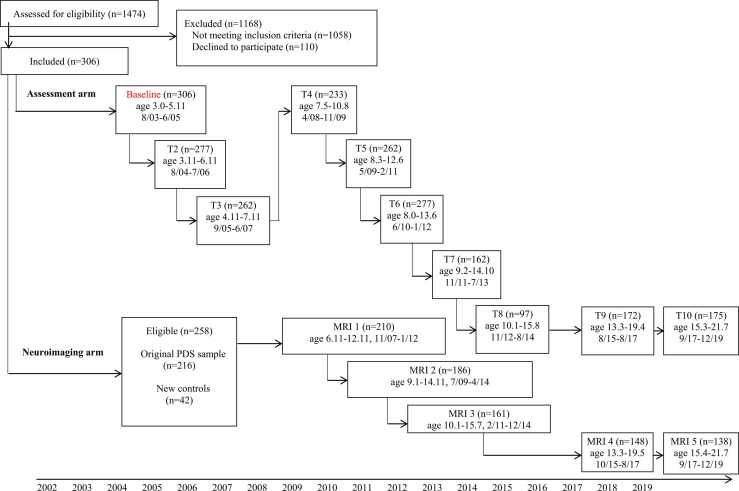
Participants included a subsample of children enrolled in the Preschool Depression Study (PDS; Luby et al., 2009, Luby et al., 2009). The PDS is a prospective, longitudinal study conducted by the Washington University in St. Louis School of Medicine (WUSM) Early Emotional Development Program, designed to investigate the longitudinal course of preschool depression (see Fig. 1 for overview). At baseline (time 1), children (N = 306) between the ages of 3 and 6 years (M = 4.45, SD = 0.80) and their primary caregivers were recruited from daycares, preschools, and primary care sites. Children were recruited based on scores on the Preschool Feelings Checklist (Luby et al., 2004) in order to oversample for children with/at risk for preschool depression. The Preschool Feelings Checklist assesses depression-related symptoms in young children but does not include an assessment of sleep disturbances directly (although, one item does assess whether the child seems “tired/low energy”). Healthy preschoolers and those with other psychiatric disorders (e.g., externalizing problems) were also recruited for the sample and additional healthy subjects were added during the school age period using similar recruitment methods. Participants underwent annual diagnostic and developmental assessments. Once they reached school age (age range 6.11–12.92 years), healthy children and those with a history of depression from the sample were invited to participate in yearly MRI scans (n = 210 completed the first wave of scanning). Exclusion criteria for the MRI portion of the study included (a) head injury with loss of consciousness for at least 5 min, (b) neurological illness, (c) parent-report of a prior diagnosis of Autism and/or Intellectual Disability or parent endorsement of a number of the core symptoms of Autism and/or Intellectual Disability, (d) treatment for lead poisoning, or (e) other contraindications for MRI scanning.
Fig. 1.
Preschool Depression Study (PDS) flowchart.
Children in the PDS study had between one and ten developmental assessment waves and between one and five scan waves. Parental consent and child verbal assent were obtained prior to study participation. The Institutional Review Board at WUSM approved all procedures in accordance with institutional ethical guidelines. The current study focuses only on children whose sleep was assessed at the baseline assessment and who had at least one wave of MRI scan data, yielding a final sample of 158 children. Information about the number of scans gathered from the children in the PDS sample is included in Supplemental Appendix A in Supplemental Fig. S1. The characteristics of this final sample are described in Table 1. For the children in the final sample, an average of 5 years 9 months (SD = 1 year 0 months) elapsed between baseline assessment and the first MRI scan. Additionally, there were no differences in demographics (e.g., race, sex, income-to-needs) or baseline characteristics (e.g., sleep, psychopathology, psychotropic medication usage) between children who were and were not included in the final sample (i.e., had usable imaging data; see Supplemental Table S1 in Supplemental Appendix A).
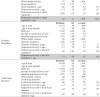
Table 1.
Final sample characteristics (n = 158).
| Total N | Mean | SD | |
|---|---|---|---|
| Age | |||
| Baseline age (years) | 158 | 4.52 | 0.77 |
| Scan 1 age (years) | 144 | 10.31 | 1.26 |
| Scan 2 age (years) | 129 | 11.92 | 1.14 |
| Scan 3 age (years) | 114 | 13.13 | 1.05 |
| Scan 4 age (years) | 115 | 16.52 | 0.99 |
| Scan 5 age(years) | 104 | 18.67 | 1.02 |
| Mean income-to-needs ratio | 158 | 1.77 | 0.85 |
| Baseline depression severity | 158 | 2.39 | 1.70 |
| Percentage | n | ||
| Sex (Male) | 51.9% | 82 | |
| Race | |||
| White | 55.1% | 87 | |
| Black | 33.5% | 53 | |
| Other | 11.4% | 18 | |
| Number of scan waves | |||
| 1 scan | 2.5% | 4 | |
| 2 scans | 9.5% | 15 | |
| 3 scans | 24.7% | 39 | |
| 4 scans | 28.5% | 45 | |
| 5 scans | 34.8% | 55 | |
| Baseline diagnoses based on the PAPA | |||
| MDD | 28.5% | 45 | |
| ADHD | 15.2% | 24 | |
| ODD | 22.8% | 36 | |
| Conduct Disorder | 12.7% | 20 | |
| GAD | 7.6% | 12 | |
| Separation Anxiety | 19.0% | 30 | |
| PTSD | 2.6% | 4 | |
| Baseline psychotropic medication use | 6.3% | 10 | |
Note: PAPA = Preschool Age Psychiatric Assessment; MDD = Major Depressive Disorder; ADHD = Attention Deficit Hyperactivity Disorder; GAD = Generalized Anxiety Disorder; PTSD = Post-Traumatic Stress Disorder.
5.2. Measures
5.2.1. Preschool sleep
Preschool sleep was assessed using the sleep behaviors module of the Preschool-Age Psychiatric Assessment (PAPA; Egger et al., 2003) administered at the baseline assessment. The PAPA is a valid and reliable parent informant measure that consists of a series of developmentally appropriate questions assessing the DSM-IV criteria for childhood disorders (Egger et al., 2006). The PAPA was administered in-person by trained staff during the developmental assessments. PAPA interviews were audiotaped, reviewed for reliability, and calibrated for accuracy using methods previously described (Luby, Si et al., 2009). Raters were trained to reliability and blind to the child’s previous diagnostic status. All interviews were audiotaped, and methods to maintain reliability and prevent drift, including ongoing calibration of interviews by master raters for 20% of each interviewer’s cases, were implemented in consultation with an experienced clinician (JL) at each study wave.
The Sleep Behaviors module of the PAPA contains a number of items assessing common aspects of child sleep, shows correspondence with other measures of child sleep disturbances (Alfano et al., 2013), and has been used in a number of previous studies (e.g., Alfano et al., 2013, Whalen et al., 2016, Willoughby et al., 2008). In the current study, the sleep measures derived from the PAPA included: 1) current, average bedtime, 2) current, average sleep onset latency – the average amount of time it takes the child to fall asleep after being put to bed, 3) current, average number of nighttime awakenings – the number of signaled awakenings that occur throughout the night, 4) sleep regularity – a composite of three items assessing parent perceptions of regularity of the child’s sleep schedule across the past three months (i.e., “is there a recognizable pattern to child’s sleep and waking schedule?”, and interviewer perception of whether the family has bedtime rituals that they do most nights and whether the child has a regular weekday bedtime, 5) daytime sleepiness – a composite of four items assessing parent perceptions of child sleepiness during the day across the past three months (i.e., “does s/he seem sleepy during the day?”, “does s/he fall asleep almost every time s/he rides in a car when it is not nap time?”, “has s/he been feeling especially tired or weary?” and “has s/he become tired or worn out more easily than usual?”), and 6) current, average total daily sleep duration, including both daytime and nighttime sleep. Descriptive statistics and correlations between these sleep items are included in Table 2. Scores on each sleep measure from the baseline assessment were used in analysis. Each of these sleep measures were examined in separate models, given the conceptual distinctions between these various aspects of sleep.
Table 2.
Descriptive statistics for and Pearson correlations between early childhood sleep measures and control variables.
| Mean (SD) or n (%) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Bedtime (HH:MM in 24-h time) | 20:50 (57 min) | 1.00 | ||||||||
| 2. Sleep onset latency (min) | 24.72 (27.52) | .14 | 1.00 | |||||||
| 3. Nighttime awakenings | 0.79 (0.86) | -0.09 | .04 | 1.00 | ||||||
| 4. Sleep regularity | 5.53 (1.09) | -0.36** | -0.36** | -0.01 | 1.00 | |||||
| 5. Daytime sleepiness | 0.73 (1.51) | .08 | .14 | .15 | -0.12 | 1.00 | ||||
| 6. Total sleep duration (hours) | 11.65 (2.01) | -0.30** | -0.21* | -0.02 | .29** | -0.13 | 1.00 | |||
| 7. Baseline/preschool depression severity | 2.39 (1.70) | .04 | .20* | .11 | -0.08 | .39** | -0.19* | 1.00 | ||
| 8. Mean income-to-needs ratio | 1.77 (0.85) | -0.11 | -0.10 | -0.01 | .01 | -0.22* | .07 | -0.26* | 1.00 | |
| 9. Male sex | 82 (51.9) | -0.06 | .08 | .08 | -0.09 | .13 | -0.08 | .10 | .06 | 1.00 |
Note: *p < .05, **p < .001; min = minutes; baseline depression severity scores do not include the two sleep items (i.e., insomnia/hypersomnia and fatigue) that are included in the core preschool MDD depression symptoms.
5.2.2. Preschool depression severity
Depression symptoms in early childhood were measured using parent-reports on the depression module of the PAPA. To take a continuous, not categorical, approach to quantifying psychopathology, preschool depression was quantified as the number of core depression symptoms endorsed in the depression module. However, as sleep problems are included in the core symptoms of depression, we created a preschool depression severity index from the PAPA that included all of the core depression symptoms except for insomnia/hypersomnia and fatigue. This index included 7 items assessing child depressed and/or irritated mood, anhedonia, cognitive disturbances (e.g., difficulty concentrating), appetite disturbances, psychomotor disturbances (e.g., increased agitation or retardation), feelings of worthlessness and/or excess and inappropriate guilt, and recurrent thoughts of death and/or suicidal ideation and/or suicide attempts. Preschool depression severity, as measured at the baseline assessment, was used in analysis.
5.2.3. Income-to-needs ratio
Mothers reported on the family’s income at each developmental assessment/MRI scan wave. Income-to-needs ratios were computed as the total family income at baseline divided by the federal poverty level, based on family size (McLoyd, 1998). An income-to-needs ratio below 1 is indicative of poverty.
5.3. Imaging acquisition
MRI scans 1–3 were processed using the FreeSurfer Longitudinal pipeline. Scans 4 and 5 switched to using a 3.0 Siemen’s Tesla Prisma whole-body scanner with a 32-channel head coil using Human Connectome Project style acquisitions (Glasser et al., 2016; see Supplemental Appendix A for details). Quality assurance measures included having subjects practice in an MRI simulator, evaluating head motion during structural scans, and recollection of data if necessary. Structural data were obtained using two 3D T1-weighted scans (TR 2300 ms, TE 3.16 ms, TI 1200 ms, flip angle 8°, 160 slices, 256 matrix, field of view 256 mm, 1.0 mm3 voxels, 6:18 min per scan) in the sagittal plane using a magnetization-prepared rapid gradient echo (MPRAGE) sequence. Full details of the imaging acquisition are provided in Supplemental Appendix A.
5.4. Structural imaging processing
Processing of structural data was accomplished using the FreeSurfer Longitudinal pipeline v5.3 (http://surfer.nmr.mgh.harvard.edu; Reuter et al., 2010) with visual inspection of the white and pial surfaces for errors and regeneration with manual intervention to correct for errors when necessary by an experienced rater blinded to diagnostic category. Processing steps included skull stripping, atlas registration, spherical surface registration, and parcellation. Importantly, the longitudinal stream includes initialization from an unbiased within subject template (created across the longitudinal scans), which reduces the bias that would otherwise be present in selecting a single scan as baseline. Using an unbiased longitudinal template significantly increases reliability and statistical power (Reuter et al., 2012). Total gray and white matter volume in the subject’s native space were obtained using FreeSurfer’s “aseg.stats” report. Full details of imaging processing are provided in Supplemental Appendix A.
5.5. Statistical analysis
Longitudinal multi-level modeling (MLM) in SAS version 9.4 was used to investigate whether each of the sleep variables in early childhood were significantly associated with later gray matter volume trajectories. Gray matter volume, in cubic centimeters at scans 1–5, was examined as the dependent variable, and separate models were fitted with each of the 6 sleep measures as the independent variable. Time was defined as age at scan and centered at the median age of 13 years, and age squared was included in the models to allow for quadratic trajectories. Modeling a quadratic trajectory allows for the possibility that change in the outcome variable across time is non-linear (Grimm et al., 2011). As prior research indicates that gray matter volume shows a non-linear, decreasing trajectory across childhood into adolescence, we opted to examine both linear and quadratic trajectories in all models. Child sex and preschool depression severity were included as time invariant covariates in all models, and income-to-needs ratio was included as a time-varying covariate. In order to examine the specificity of the effect of preschool sleep on total gray matter volume, white matter volume was included as a time-varying covariate in all models. White matter volumes at each age were not associated with any of the preschool sleep variables after correcting for multiple comparisons, and the inclusion of this covariate allowed us to explicitly test the specificity of the relationship to gray matter. All models included the three-way interaction between age, the sleep measure, and preschool depression severity and all embedded two-way interactions. The longitudinal MLM’s included both random intercept and slope components and assumed an unstructured covariance structure. In all MLMs, missing data were handled using maximum likelihood estimation (Allison, 2012). False discovery rate (FDR) correction was used to account for multiple comparisons. Significance of the three-way interaction between the sleep variable, preschool depression severity, and age was determined by applying FDR correction to those p-values from the 6 MLM’s. Analogously, FDR correction was applied across the 6 MLMs for each of the lower order effects.
6. Results
Descriptive statistics and correlations between all of the predictors included in the longitudinal MLMs are presented in Table 2. Results from the correlation analysis suggest that preschool sleep disturbances were associated with preschool depression severity (as quantified without the sleep items). Specifically, longer sleep onset latencies, increased daytime sleepiness, and lower total sleep durations were associated with more severe preschool depression symptoms. Next, we compared across children who did (n = 44) and did not (n = 114) meet diagnostic criteria for preschool MDD at the baseline assessment. Of note, the diagnostic criteria for preschool MDD requires that the child displays at least 4 of the 9 core depression symptoms. Given that two sleep items are included in the core depression symptoms, we adjusted the diagnostic criteria for the purpose of this analysis so that children needed to display at least 4 “non-sleep core” depression symptoms (i.e., not insomnia/hypersomnia or fatigue). Using these altered criteria, children with preschool MDD were reported by their parents as being significantly more tired during the day and were reported to get less sleep overall (11.88 h/day in non-depressed children vs 11.05 h/day in depressed children; see Table 3).
Table 3.
Differences in baseline sleep variables between children who did and did not meet diagnostic criteria for baseline MDD.
| No Baseline MDD (n = 114) |
Baseline MDD (n = 44) |
No Baseline MDD vs. Baseline MDD |
|||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | df | p | |
| Bedtime (HH:MM in 24-h time) | 20:50 | 59 min | 20:52 | 49 min | -0.28 | 149 | .78 |
| Sleep onset latency (minutes) | 22.59 | 23.31 | 30.33 | 36.09 | -1.31 | 56 | .20 |
| Nighttime awakenings | 0.72 | 0.72 | 0.95 | 1.14 | -1.27 | 57 | .21 |
| Sleep regularity | 5.58 | 1.05 | 5.41 | 1.19 | .88 | 156 | .38 |
| Daytime sleepiness | 0.40 | 0.93 | 1.57 | 2.25 | -3.33 | 49 | .002 |
| Total sleep duration (hours) | 11.88 | 1.76 | 11.05 | 2.47 | 2.05 | 61 | .04 |
Note: MDD = Major Depressive Disorder; Baseline MDD defined as ≥ 4 non-sleep core MDD symptoms at the baseline assessment.
Results of the longitudinal MLM’s examining the relationship of preschool sleep and gray matter volume trajectories are detailed in Table 4. In all models, there was a significant effect of the quadratic age term, indicating a non-linear, decreasing trajectory across late childhood to adolescence. This is consistent with other research examining gray matter volume development across this age range (Giedd and Rapoport, 2010). After correction for multiple comparisons, significant associations between three of the six sleep measures, preschool depression, and gray matter volume trajectories were identified.
Table 4.
Multi-level models of preschool sleep and total cortical gray matter volume at scans 1–5 including the interaction between the sleep variable, depression severity, and age.
 |
 |
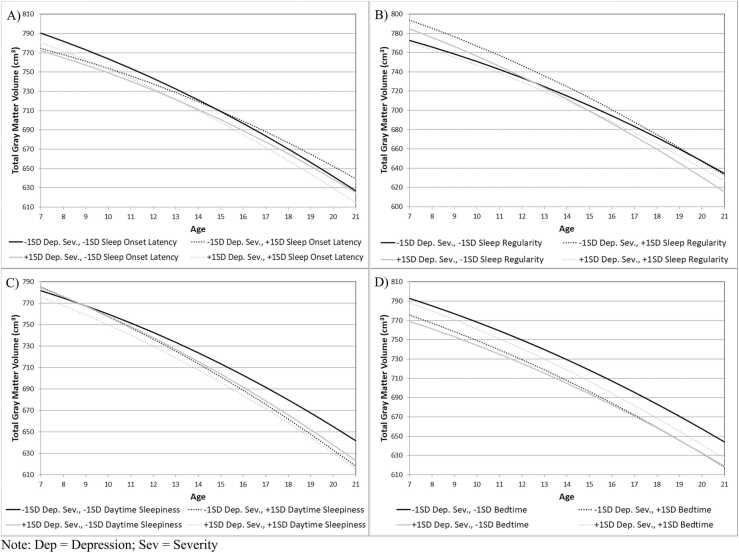
Note: Gray boxes indicate significant interactions that are plotted in Fig. 2; x = interaction term.
In the model examining sleep onset latency as a predictor of gray matter volume trajectories, a significant three-way interaction between sleep onset latency, preschool depression, and linear age emerged. Consistent with prior literature, gray matter declined across age for all children (Mills et al., 2016, Walhovd et al., 2017), but children with longer sleep latencies and higher levels of preschool depression and children with shorter sleep latencies and lower levels of preschool depression both showed the fastest decline in cortical gray matter volume across late childhood to adolescence (Fig. 2A). Although the trajectories were similar, children with shorter sleep latencies and lower levels of preschool depression had greater cortical gray matter volume across the age range than children with longer sleep latencies and higher levels of preschool depression. This significant interaction held when controlling for child sex, income-to-needs ratio, and cortical white matter volume.
Fig. 2.
Significant interactions between baseline sleep, baseline depression severity, and age in predicting gray matter volumes.
In the model examining sleep regularity as a predictor of gray matter volume trajectories, a significant three-way interaction between sleep regularity, preschool depression, and linear age emerged; children with lower levels of sleep regularity and higher levels of preschool depression showed the fastest decline in cortical gray matter volume across late childhood to adolescence (Fig. 2B). This significant interaction held when controlling for child sex, income-to-needs ratio, and cortical white matter volume.
In the model examining daytime sleepiness, there was again a significant three-way interaction of daytimes sleepiness, preschool depression severity, and age (Fig. 2C). Children with lower levels of daytime sleepiness and lower levels of depression showed a slower decline in gray matter volume across late childhood to adolescence. This significant interaction held when controlling for child sex, income-to-needs ratio, and cortical white matter volume.
Although it did not survive FDR correction, we also identified a significant interaction between bedtime and preschool depression severity, indicating that children with the earliest bedtimes and the lowest levels of depression showed the largest gray matter volumes (Fig. 2D). Although this finding corresponds with our other findings, given that the significance of this effect did not survive multiple comparisons correction, we will not discuss this further.
The MLMs for the two other sleep measures (i.e., nighttime awakenings and total sleep duration) were not found to predict either values at the median age of 13 (i.e., intercept) or change in gray matter volume over development, either alone or when interacting with preschool depression severity. Additionally, given the possibility that child usage of psychotropic may have affected our results, all longitudinal MLMs were re-run controlling for psychotropic medication usage as a time-varying covariate. The pattern of results did not change when including this as a covariate.
7. Discussion
The current study examined how preschool sleep and depression interact to predict gray matter volume trajectories across late childhood to adolescence in a sample enriched for preschool depression. In this sample, there was an association between preschool sleep disturbances and preschool depression, such that preschoolers who were reported to have longer sleep onset latencies, increased daytime sleepiness, and lower total sleep durations also had more severe preschool depression symptoms. Our hypotheses were only partially supported. Although sleep disturbances alone did not predict gray matter volume/trajectories, preschool sleep and depression symptoms interacted to predict later total gray matter volume and the trajectory of decline in total gray matter volume. These findings extend previous research demonstrating the effect of experiences in early childhood on later neurodevelopmental trajectories and provide critical evidence of the interactive effects of both early childhood sleep and early childhood depression on neurodevelopment.
Relatively few studies have examined predictors of within-individual, longitudinal trajectories of gray matter development across childhood into adolescence. The current study adds to this literature, examining how sleep and depression interact to predict changes in total gray matter across this developmental window in a sample enriched for preschool depression. In this analysis, three, three-way interactions emerged indicating that sleep and depression interact to predict the trajectory of gray matter decline across childhood. First, children with longer sleep latencies and higher levels of preschool depression and children with shorter sleep latencies and lower levels of preschool depression both showed the fastest decline in cortical gray matter volume across late childhood to adolescence. Although these trajectories were similar, children with shorter sleep latencies and lower levels of preschool depression had greater cortical gray matter volume across the age range of examination. Research indicates that reduced global gray matter volume in childhood is a characteristic of children with and at risk for various forms of psychopathology, including the children with a parent with schizophrenia (Sugranyes et al., 2015), infants whose mothers experienced perinatal depression (Zou et al., 2019), and children/adolescents who experienced preschool depression (Luby et al., 2016). Recent work with a large sample of 9–10-year-olds suggests that reduced gray matter volume, globally, is associated with increased general risk for psychopathology (Durham et al., 2021). Our findings suggest that the combination of longer sleep latencies and higher depression symptoms in early childhood put children at risk for reduced cortical gray matter volumes in later childhood.
Next, children with more irregular sleep patterns and more depression symptoms during preschool showed the fastest rate of decline in gray matter volume. Similarly, children with lower levels of daytime sleepiness and lower levels of depression showed a slower decline in gray matter volume across late childhood to adolescence. Although gray matter volume has been shown to normatively decline across childhood into adolescence, individual differences in the rates at which this decline occurs may be an informative phenotype underlying psychopathology (Giedd et al., 2008). Decreases in gray matter volume across development are thought to be attributable to normative neurodevelopmental processes, including synaptic pruning of excess connections and increased white matter myelination/expansion that causes gray matter to contract (Stiles and Jernigan, 2010). However, overly rapid decreases in gray matter volume, resulting in lower total gray matter volumes at any given age, may be maladaptive, and associated with increased risk for psychopathology (Durham et al., 2021). Although our index of sleep regularity relied on parent reports of the consistency of the child’s sleep schedule, it is possible that this index served as a proxy measure of chronodisruption. Chronodisruption occurs when an individual’s circadian system becomes misaligned either to the local light cycle (e.g., shift work disorder) or within an individual (e.g., daily rhythms in sleep offset do not coincide with the daily surge in cortisol; Erren and Reiter, 2009, Vetter, 2020). One way this might manifest is through variability in an individual’s sleep schedule (Dijk and Lockley, 2002). Chronodisruption has been linked to both psychopathology and adverse health outcomes (Vetter, 2020, Baron and Reid, 2014), and future research incorporating psychophysiological data, such as accelerometry, should examine whether more precise measures of chronodisruption are associated with neurodevelopment across childhood.
Our findings suggest that the processes by which sleep and depression relate to neurodevelopment are broad, possibly contributing to total gray matter volumetric alterations across development. This observed whole-brain effect likely arises from the aggregation of differences across several cortical regions. Of course, the reverse association is also plausible, in which total gray matter volumetric alterations lead to sleep disturbances and depression in early childhood, an association that is propagated across development. Without preschool-age measures of gray matter volume, we are unable to confirm the directionality of the association between sleep, depression, and gray matter volume, so future longitudinal research should explore this possibility. Additionally, these findings provide evidence that the effect of early sleep disturbances on neurodevelopment are not solely “secondary” to early depression. Early sleep disturbances showed relationships with neurodevelopment trajectories when considered in the context of early depression symptoms. This provides additional evidence that early sleep disturbances are an important, co-occurring condition that should be considered in conjunction with early depression when understanding early, modifiable factors that contribute to later neurodevelopment. Our prior work has demonstrated the central role of preschool depression in relating to total gray matter volume trajectories (Luby et al., 2016) and volume in specific brain regions (i.e., the hippocampus; Barch et al., 2019). The current findings expand upon these finding, highlighting the critical role of additionally exploring sleep as a predictor of neurodevelopment (Dutil et al., 2018). Given the overlap of depression and sleep disturbances (Tsuno et al., 2005), the repercussions of sleep disturbances in early childhood may be best understood when considered in the context of depression. Future research should examine whether the effects found in the current study replicate when examining specific brain regions.
The current study has a number of important strengths, including the use of a semi-structured interview assessment of both sleep and depression symptoms in childhood, along with up to five waves of longitudinal neuroimaging. The longitudinal design of this study spanned from preschool through adolescence, assessing key developmental periods important to sleep and brain maturation. There are several limitations worth noting. First, our assessment of preschool sleep was based solely on parent report. More objective measures of preschool sleep such as actigraphy and polysomnography would enhance the validity of the findings described here and offer exciting opportunities for future research endeavors in this area. Similarly, the current study did not include a thorough assessment of other sleep disorders (e.g., obstructive sleep apnea, parasomnias, etc.) or aspects related to sleep hygiene (e.g., bedtime routines), and as such, we cannot control for or consider these other relevant variables in analysis. Future research exploring the association between sleep and neurodevelopment should include comprehensive assessment of a range of sleep-related disorders and behaviors. This sample was enriched for preschool depression and therefore, findings may not generalize to community-based populations. However, given our interest in the interaction between sleep and depression, it was crucial to have adequate variation in depression symptomology in the sample, necessitating our decision to oversample for preschool depression. Additionally, not every child in the study participated in the MRI scans, so the sample of the current study may not be representative of the entire original sample. Next, given that many of the children in this sample had a baseline diagnosis of depression, we are unable to tease apart causal relationships between sleep and the onset of depression predicting gray matter volume. It will be important for future research using non-clinically-enriched samples to replicate and confirm these findings. Additionally, given the design of the current study, we were unable to examine the directionality of the association between the three main variables of interest, sleep, depression, and gray matter volume or control for prior levels of the outcome variables, and future research should consider implementing study designs that enable an examination of the directionality of the association between these constructs. Similarly, the current study does not address the developmental changes in sleep that are known to occur across early childhood (Acebo et al., 2005, Byars et al., 2012, Galland et al., 2012), focusing on one time point during the preschool period. Future research should consider whether trajectories of change in sleep across the preschool period are associated with later changes in gray matter volumes.
Sleep disturbances are relatively common in early childhood, and in conjunction with early depression symptoms, these disturbances may have widespread effects on neurodevelopmental trajectories. Sleep problems in early childhood have been found to be responsive to brief, cost-effective interventions (Mindell et al., 2006, Meltzer and Mindell, 2014), and, given disruptive effects on the whole family’s sleep, are often readily identified and reported by parents. The findings of the current study underscore the importance of identifying and addressing these sleep disturbances in early childhood in order to prevent possible neurodevelopmental effects. Given research in adults suggesting that treating sleep problems may also reduce depression (Jindal and Thase, 2004, Manber et al., 2008), but that the reverse is not always true (e.g., McGlinchey et al., 2016), future research examining treatment for preschool depression should consider including treatment modules that focus on improving sleep outcomes. The inclusion of sleep as a treatment focus may be a way of both curbing depression and improving neurodevelopmental outcomes.
Data Statement
Due to the sensitive nature of the questions asked in this study, data from this study are not publicly available.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
All phases of this study were supported by a National Institutes of Health (NIH) grant, R01 MH064769-06A1. Dr. Hoyniak’s work was supported by NIH grant: T32 MH100019 (PI’s: Barch and Luby) and K23 MH127305-01 (PI: Hoyniak). Dr. Whalen’s work was supported by NIH grants: L30 MH108015 (PI: Whalen) and K23 MH118426 (PI: Whalen).
Disclosures
None of the authors have any biomedical financial interests or potential conflicts of interest to report.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2021.101053.
Appendix A. Supplementary material
Supplementary material
Supplementary material
References
- Acebo C., Sadeh A., Seifer R., Tzischinsky O., Hafer A., Carskadon M.A. Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1- to 5-year-old children. Sleep. 2005;28:1568. doi: 10.1093/sleep/28.12.1568. [DOI] [PubMed] [Google Scholar]
- Alfano C.A., Smith V.C., Reynolds K.C., Reddy R., Dougherty L.R. The parent-child sleep interactions scale (PSIS) for preschoolers: factor structure and initial psychometric properties. J. Clin. Sleep Med. 2013;09:1153–1160. doi: 10.5664/jcsm.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison, P.D. , 2012. Handling missing data by maximum likelihood. In: SAS Global Forum.
- Astill R.G., Van der Heijden K.B., Van IJzendoorn M.H., Van Someren E.J. Sleep, cognition, and behavioral problems in school-age children: a century of research meta-analyzed. Psychol. Bull. 2012;138:1109–1138. doi: 10.1037/a0028204. [DOI] [PubMed] [Google Scholar]
- Barch D.M., Tillman R., Kelly D., Whalen D., Gilbert K., Luby J.L. Hippocampal volume and depression among young children. Psychiatry Res. Neuroimaging. 2019;288:21–28. doi: 10.1016/j.pscychresns.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron K.G., Reid K.J. Circadian misalignment and health. Int. Rev. Psychiatry. 2014;26:139–154. doi: 10.3109/09540261.2014.911149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe D.W. Cognitive, behavioral, and functional consequences of inadequate sleep in children and adolescents. Pediatr. Clin. N. Am. 2011;58:649–665. doi: 10.1016/j.pcl.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier A., Matte-Gagné C., Bouvette-Turcot A.A. Examining the interface of children’s sleep, executive functioning, and caregiving relationships: a plea against silos in the study of biology, cognition, and relationships. Curr. Dir. Psychol. Sci. 2014;23:284–289. [Google Scholar]
- Brown T.T., Jernigan T.L. Brain development during the preschool years. Neuropsychol. Rev. 2012;22:313–333. doi: 10.1007/s11065-012-9214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butte N.F., Puyau M.R., Wilson T.A., Liu Y., Wong W.W., Adolph A.L., et al. Role of physical activity and sleep duration in growth and body composition of preschool-aged children. Obesity. 2016;24:1328–1335. doi: 10.1002/oby.21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byars K.C., Yolton K., Rausch J., Lanphear B., Beebe D.W. Prevalence, patterns, and persistence of sleep problems in the first 3 years of life. Pediatrics. 2012;129:e276–e284. doi: 10.1542/peds.2011-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorney D.B., Detweiler M.F., Morris T.L., Kuhn B.R. The interplay of sleep disturbance, anxiety, and depression in children. J. Pediatr. Psychol. 2007;33:339–348. doi: 10.1093/jpepsy/jsm105. [DOI] [PubMed] [Google Scholar]
- Cousins J.C., Whalen D.J., Dahl R.E., Forbes E.E., Olino T.M., Ryan N.D., Silk J.S. The bidirectional association between daytime affect and nighttime sleep in youth with anxiety and depression. J. Pediatr. Psychol. 2011;36:969–979. doi: 10.1093/jpepsy/jsr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis A.S., Dubowitz T., Ghosh-Dastidar B., Hunter G.P., Buman M., Buysse D.J., et al. A preliminary study of a composite sleep health score: associations with psychological distress, body mass index, and physical functioning in a low-income African American community. Sleep Health. 2019;5:514–520. doi: 10.1016/j.sleh.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald J.F., Meijer A.M., Oort F.J., Kerkhof G.A., Bögels S.M. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: a meta-analytic review. Sleep Med. Rev. 2010;14:179–189. doi: 10.1016/j.smrv.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Dijk D.J., Lockley S.W. Invited review: Integration of human sleep-wake regulation and circadian rhythmicity. J. Appl. Physiol. 2002;92:852–862. doi: 10.1152/japplphysiol.00924.2001. [DOI] [PubMed] [Google Scholar]
- Ducharme S., Albaugh M.D., Nguyen T.V., Hudziak J.J., Mateos-Pérez J.M., Labbe A., et al. Trajectories of cortical thickness maturation in normal brain development – the importance of quality control procedures. NeuroImage. 2016;125:267–279. doi: 10.1016/j.neuroimage.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham E.L., Jeong H.J., Moore T.M., Dupont R.M., Cardenas-Iniguez C., Cui Z., et al. Association of gray matter volumes with general and specific dimensions of psychopathology in children. Neuropsychopharmacology. 2021:1–7. doi: 10.1038/s41386-020-00952-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutil C., Walsh J.J., Featherstone R.B., Gunnell K.E., Tremblay M.S., Gruber R., et al. Influence of sleep on developing brain functions and structures in children and adolescents: a systematic review. Sleep Med. Rev. 2018;42:184–201. doi: 10.1016/j.smrv.2018.08.003. [DOI] [PubMed] [Google Scholar]
- Egger, H.L., Ascher, B., Angold, A., 2003. The Preschool Age Psychiatric Assessment: Version 1.4. Center for Developmental Epidemiology, Department of Psychiatry and Behavioral Sciences. Duke University Medical Center, Durham, NC.
- Egger H.L., Erkanli A., Keeler G., Potts E., Waltr B.K., Angold A. Test-retest reliability of the preschool age psychiatric assessment (PAPA) J. Am. Acad. Child Adolesc. Psychiatry. 2006;45:538–549. doi: 10.1097/01.chi.0000205705.71194.b8. [DOI] [PubMed] [Google Scholar]
- Erren T.C., Reiter R.J. Defining chronodisruption. J. Pineal Res. 2009;46:245–247. doi: 10.1111/j.1600-079X.2009.00665.x. [DOI] [PubMed] [Google Scholar]
- Frangou, S., Modabbernia, A., Williams, S.C.R., Papachristou, E., Doucet, G.E., Agartz, I., et al., 2021. Cortical thickness across the lifespan: data from 17,075 healthy individuals aged 3–90 years. Hum. Brain Mapp. [DOI] [PMC free article] [PubMed]
- Galland B.C., Taylor B.J., Elder D.E., Herbison P. Normal sleep patterns in infants and children: a systematic review of observational studies. Sleep Med. Rev. 2012;16:213–222. doi: 10.1016/j.smrv.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Gennatas E.D., Avants B.B., Wolf D.H., Satterthwaite T.D., Ruparel K., Ciric R., et al. Age-related effects and sex differences in gray matter density, volume, mass, and cortical thickness from childhood to young adulthood. J. Neurosci. 2017;37:5065–5073. doi: 10.1523/JNEUROSCI.3550-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd, J.N., Lenroot, R.K., Shaw, P., Lalonde, F., Celano, M., White, S., et al., 2008. Growth factors and psychiatric disorders: Novartis Foundation Symposium 289. Novartis Foundation Symposia, 289, pp. 101–18.
- Giedd J.N., Rapoport J.L. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Smith S.M., Marcus D.S., Andersson J.L.R., Auerbach E.J., Behrens T.E.J., et al. The human connectome project’s neuroimaging approach. Nat. Neurosci. 2016;19:1175–1187. doi: 10.1038/nn.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgoni M., D’Atri A., Scarpelli S., Reda F., Gennaro L.D. Sleep electroencephalography and brain maturation: developmental trajectories and the relation with cognitive functioning. Sleep Med. 2020;66:33–50. doi: 10.1016/j.sleep.2019.06.025. [DOI] [PubMed] [Google Scholar]
- Gregory A.M., Caspi A., Eley T.C., Moffitt T.E., O’Connor T.G., Poulton R. Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. J. Abnorm. Child Psychol. 2005;33:157–163. doi: 10.1007/s10802-005-1824-0. [DOI] [PubMed] [Google Scholar]
- Gregory A.M., O’Connor T.G. Sleep problems in childhood: a longitudinal study of developmental change and association with behavioral problems. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41:964–971. doi: 10.1097/00004583-200208000-00015. [DOI] [PubMed] [Google Scholar]
- Grimm K.J., Ram N., Hamagami F. Nonlinear growth curves in developmental research. Child Dev. 2011;82:1357–1371. doi: 10.1111/j.1467-8624.2011.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber R., Carrey N., Weiss S.K., Frappier J.Y., Rourke L., Brouillette R.T., Wise M.S. Position statement on pediatric sleep for psychiatrists. J. Can. Acad. Child Adolesc. Psychiatry. 2014;23:174–195. [PMC free article] [PubMed] [Google Scholar]
- Hoyniak C.P., Bates J.E., McQuillan M.E., Staples A.D., Petersen I.T., Rudasill K.M., Molfese V.J. Sleep across early childhood: implications for internalizing and externalizing problems, socioemotional skills, and cognitive and academic abilities in preschool. J. Child Psychol. Psychiatry. 2020;61:1080–1091. doi: 10.1111/jcpp.13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyniak C.P., Whalen D.J., Barch D., Luby J.L. Sleep problems in preschool-onset major depressive disorder: the effect of treatment with parent–child interaction therapy-emotion development. Eur. Child Adolesc. Psychiatry. 2021:1–12. doi: 10.1007/s00787-020-01641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan J.E., Reiter R.J., Bax M.C.O., Ribary U., Freeman R.D., Wasdell M.B. Long-term sleep disturbances in children: a cause of neuronal loss. Eur. J. Paediatr. Neurol. 2010;14:380–390. doi: 10.1016/j.ejpn.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Jindal R., Thase M.E. Treatment of insomnia associated with clinical depression. Sleep Med. Rev. 2004;8:19–30. doi: 10.1016/S1087-0792(03)00025-X. [DOI] [PubMed] [Google Scholar]
- Kocevska D., Muetzel R.L., Luik A.I., Luijk M.P.C.M., Jaddoe V.W., Verhulst F.C., et al. The developmental course of sleep disturbances across childhood relates to brain morphology at age 7: the generation R study. Sleep. 2016;40:1–9. doi: 10.1093/sleep/zsw022. [DOI] [PubMed] [Google Scholar]
- Kurth S., Olini N., Huber R., LeBourgeois M. Sleep and early cortical development. Curr. Sleep Med. Rep. 2015;1:64–73. doi: 10.1007/s40675-014-0002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidaire W., Urrila A.S., Artiges E., Miranda R., Vulser H., Bézivin-Frere P., et al. Irregular sleep habits, regional grey matter volumes, and psychological functioning in adolescents. PLoS One. 2021;16 doi: 10.1371/journal.pone.0243720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Buysse D.J., Gentzler A.L., Kiss E., Mayer L., Kapornai K., et al. Insomnia and hypersomnia associated with depressive phenomenology and comorbidity in childhood depression. Sleep. 2007;30:83–90. doi: 10.1093/sleep/30.1.83. [DOI] [PubMed] [Google Scholar]
- Lowe C.J., Safati A., Hall P.A. The neurocognitive consequences of sleep restriction: a meta-analytic review. Neurosci. Biobehav. Rev. 2017;80:586–604. doi: 10.1016/j.neubiorev.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Luby J.L., Belden A.C., Jackson J.J., Lessov-Schlaggar C.N., Harms M.P., Tillman R., et al. Early childhood depression and alterations in the trajectory of gray matter maturation in middle childhood and early adolescence. JAMA Psychiatry. 2016;73:1–8. doi: 10.1001/jamapsychiatry.2015.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J.L., Belden A.C., Pautsch J., Si X., Spitznagel E. The clinical significance of preschool depression: impairment in functioning and clinical markers of the disorder. J. Affect. Disord. 2009;112:111–119. doi: 10.1016/j.jad.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J.L., Heffelfinger A., Koenig-McNaught A.L., Brown K., Spitznagel E. The preschool feelings checklist: a brief and sensitive screening measure for depression in young children. J. Am. Acad. Child Adolesc. Psychiatry. 2004;43:708–717. doi: 10.1097/01.chi.0000121066.29744.08. [DOI] [PubMed] [Google Scholar]
- Luby J.L., Si X., Belden A.C., Tandon M., Spitznagel E. Preschool depression: homotypic continuity and course over 24 months. Arch. Gen. Psychiatry. 2009;66:897–905. doi: 10.1001/archgenpsychiatry.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manber R., Edinger J.D., Gress J.L., San Pedro-Salcedo M.G., Kuo T.F., Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31:489–495. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maski K.P., Kothare S.V. Sleep deprivation and neurobehavioral functioning in children. Int. J. Psychophysiol. 2013;89:259–264. doi: 10.1016/j.ijpsycho.2013.06.019. [DOI] [PubMed] [Google Scholar]
- McGlinchey E.L., Reyes-Portillo J.A., Turner J.B., Mufson L. Innovations in practice: the relationship between sleep disturbances, depression, and interpersonal functioning in treatment for adolescent depression. Child Adolesc. Ment. Health. 2016;22:96–99. doi: 10.1111/camh.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoyd V.C. Socioeconomic disadvantage and child development. Am. Psychol. 1998;53:185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- Meltzer L.J., Mindell J.A. Systematic review and meta-analysis of behavioral interventions for pediatric insomnia. J. Pediatr. Psychol. 2014;39:932–948. doi: 10.1093/jpepsy/jsu041. [DOI] [PubMed] [Google Scholar]
- Mills K.L., Goddings A.L., Herting M.M., et al. Structural brain development between childhood and adulthood: convergence across four longitudinal samples. NeuroImage. 2016;141:273–281. doi: 10.1016/j.neuroimage.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindell J.A., Kuhn B., Lewin D.S., Meltzer L.J., Sadeh A. Behavioral treatment of bedtime problems and night wakings in infants and young children. Sleep. 2006;29:1263–1276. [PubMed] [Google Scholar]
- Musso M.F., Lindsey H.M., Wilde E.A., Hunter J.V., Glaze D.G., Goodrich-Hunsaker N.J., et al. Volumetric brain magnetic resonance imaging analysis in children with obstructive sleep apnea. Int. J. Pediatr. Otorhinolaryngol. 2020;138 doi: 10.1016/j.ijporl.2020.110369. [DOI] [PubMed] [Google Scholar]
- Owens J. Classification and epidemiology of childhood sleep disorders. Sleep Med. Clin. 2007;2:353–361. [Google Scholar]
- O’Brien L.M. The neurocognitive effects of sleep disruption in children and adolescents. Sleep Med. Clin. 2011;18:813–823. doi: 10.1016/j.chc.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Philby M.F., Macey P.M., Ma R.A., Kumar R., Gozal D., Kheirandish-Gozal L. Reduced regional grey matter volumes in pediatric obstructive sleep apnea. Sci. Rep. 2017;7:44566. doi: 10.1038/srep44566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quist J.S., Sjödin A., Chaput J.P., Hjorth M.F. Sleep and cardiometabolic risk in children and adolescents. Sleep Med. Rev. 2016;29:76–100. doi: 10.1016/j.smrv.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Reuter M., Rosas H.D., Fischl B. Highly accurate inverse consistent registration: a robust approach. Neuroimage. 2010;53:1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Schmansky N.J., Rosas H.D., Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud E., Vecchierini M., Heude B., Charles M., Plancoulaine S. Sleep and its relation to cognition and behaviour in preschool‐aged children of the general population: a systematic review. J. Sleep Res. 2018;27 doi: 10.1111/jsr.12636. [DOI] [PubMed] [Google Scholar]
- Spruyt K. A review of developmental consequences of poor sleep in childhood. Sleep Med. 2019;60:3–12. doi: 10.1016/j.sleep.2018.11.021. [DOI] [PubMed] [Google Scholar]
- Spruyt K. Neurocognitive effects of sleep disruption in children and adolescents. Child Adolesc. Psychiatry Clin. 2020;30:27–45. doi: 10.1016/j.chc.2020.08.003. [DOI] [PubMed] [Google Scholar]
- Stiles J., Jernigan T.L. The basics of brain development. Neuropsychol. Rev. 2010;20:327–348. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugranyes G., Serna E., de la, Romero S., Sanchez-Gistau V., Calvo A., Moreno D., et al. Gray matter volume decrease distinguishes schizophrenia from bipolar offspring during childhood and adolescence. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54:677–684. doi: 10.1016/j.jaac.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Taki Y., Hashizume H., Thyreau B., Sassa Y., Takeuchi H., Wu K., et al. Sleep duration during weekdays affects hippocampal gray matter volume in healthy children. NeuroImage. 2012;60:471–475. doi: 10.1016/j.neuroimage.2011.11.072. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Ohashi K., Khan A., Garcia L.C.H., Klengel T., Anderson C.M., Silveri M.M. Does sleep disruption mediate the effects of childhood maltreatment on brain structure? Eur. J. Psychotraumatol. 2018;8:1450594. doi: 10.1080/20008198.2018.1450594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G., Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81:12–34. doi: 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuno N., Besset A., Ritchie K. Sleep and depression. J. Clin. Psychiatry. 2005;66:1254–1269. doi: 10.4088/jcp.v66n1008. [DOI] [PubMed] [Google Scholar]
- Urrila A.S., Artiges E., Massicotte J., Miranda R., Vulser H., Bézivin-Frere P., et al. Sleep habits, academic performance, and the adolescent brain structure. Sci. Rep. 2017;7:41678. doi: 10.1038/srep41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter C. Circadian disruption: what do we actually mean? Eur. J. Neurosci. 2020;51:531–550. doi: 10.1111/ejn.14255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd K.B., Fjell A.M., Giedd J., Dale A.M., Brown T.T. Through thick and thin: a need to reconcile contradictory results on trajectories in human cortical development. Cereb. Cortex. 2017;27:1472–1481. doi: 10.1093/cercor/bhv301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen D.J., Gilbert K.E., Barch D.M., Luby J.L., Belden A.C. Variation in common preschool sleep problems as an early predictor for depression and anxiety symptom severity across time. J. Child Psychol. Psychiatry. 2016;58:151–159. doi: 10.1111/jcpp.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby M.T., Angold A., Egger H.L. Parent-reported attention-deficit/hyperactivity disorder symptomatology and sleep problems in a preschool-age pediatric clinic sample. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47:1086–1094. doi: 10.1097/CHI.0b013e31817eed1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou R., Tiemeier H., van der Ende J., Verhulst F.C., Muetzel R.L., White T., et al. Exposure to maternal depressive symptoms in fetal life or childhood and offspring brain development: a population-based imaging study. Am. J. Psychiatry. 2019;176:702–710. doi: 10.1176/appi.ajp.2019.18080970. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material