Abstract
Background
India, along with the rest of the world, faced the challenging severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. The second wave in India lagged behind that in the Western world, due to different timing of seasons. There is scarce data about the differences between the two waves, for intensive care unit (ICU) patients. We present the data of 3,498 patients from 9 ICUs of western Maharashtra.
Materials and methods
We collected prospective data of hospitalized, RT-PCR confirmed, coronavirus-2019 (COVID-19) patients, from nine tertiary centers, after institutional ethics committee (IEC) approval. Then, we segregated and analyzed the data of patients admitted to the ICU, for comorbidities, high-resolution computed tomography (HRCT) score, ventilatory support, etc. The primary outcomes were ICU and hospital mortality. We also performed multivariable analysis for predictors of ICU mortality.
Results
Overall, there were 3,498 ICU patients. In the first wave, 1,921 patients needed ICU admission, while in the second wave, 1,577 patients. Patients in the second wave had significantly higher ICU (26.1 vs 13.4%, p <0.001) and hospital mortality (29.9 vs 18.2%, p <0.001) and need for ventilatory support of any type. More patients received steroids during the second wave. On multivariable regression, male gender, ICU admission during the second wave, increasing HRCT score, and need for intubation and mechanical ventilation were significant predictors of ICU mortality.
Conclusion
ICU patients admitted during the two waves were of the similar age, but there were more females, and more patients had comorbidities during the second wave. The ICU and hospital mortality were significantly higher during the second wave.
How to cite this article
Zirpe KG, Dixit S, Kulkarni AP, Pandit RA, Ranganathan P, Prasad S, et al. The Second- vs First-wave COVID-19: More of the Same or a Lot Worse? A Comparison of Mortality between the Two Waves in Patients Admitted to Intensive Care Units in Nine Hospitals in Western Maharashtra. Indian J Crit Care Med 2021; 25(12):1343–1348.
Keywords: Comorbidities, COVID-19, First wave, ICU mortality, Second wave, Ventilatory support
Introduction
The world is not the same anymore, since the dreaded severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has affected the mankind across the globe, starting in Wuhan, China, in December 2019. This is the biggest and worst pandemic to affect the mankind since the Great Influenza Pandemic of 1918, caused by H1N1 influenza A virus, which lasted 2 years, killing over 50 million.1 The first cases of coronavirus disease-2019 (COVID-19) were reported in Kerala, India, in January 2020, when three medical students returning from China were found to be positive.2
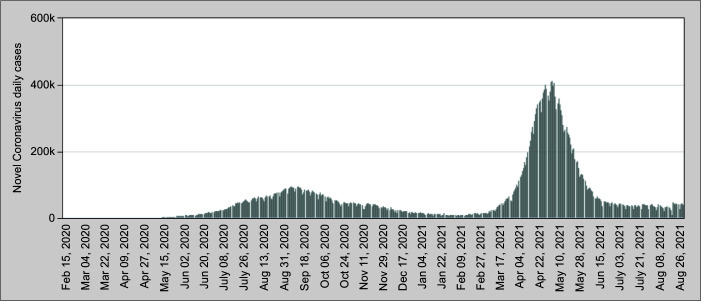
In India, the first wave began in March 2020 and lasted till nearly November 2020, while the second wave began in March 2021 lasting till the end of May 2021 (Fig. 1).3 Thus, we lagged behind the Western countries, probably due to a difference in timing of seasons. The second wave led to widespread devastation, with acute shortages of hospital beds, medications, and oxygen supply.
Fig. 1.
Daily COVID-19 cases in India March 2020–August 2021
We present here data of nearly 3,500 COVID-19 patients admitted to the intensive care unit (ICU) of nine tertiary care centers from Western India. There has been a lot of speculation about the differences in the two COVID-19 waves, about age, gender, and outcomes of patients. We therefore collected and analyzed the data of RT-PCR confirmed COVID-19 patients during both the waves and looked at ICU and hospital outcomes.
Materials and Methods
Data were collected prospectively for patients who need hospitalization during the first (March–November 2020) and second (March–May 2021) waves of COVID-19 pandemic from tertiary care centers located in western Maharashtra. Of the total nine centers, seven centers each contributed data during the first and second waves, i.e., two centers each contributed data only during one of the waves, so we analyzed the data from the total of nine centers. A total of 24,461 patients were admitted to the nine hospitals for COVID-19 (RT-PCR positive) during the two waves (first wave—13,514 and second wave—10,947 patients) of the pandemic. The units initially collected the data on their own; therefore, the manner of data collection and time points of stopping data collection were different. We then combined the data and analyzed it retrospectively. The respective institutional ethics committees (IECs) of the participating hospitals granted a waiver of informed consent. Overall, 3,498 patients required admission to the ICUs of these hospitals. In all, 1,921 patients were admitted during the first wave, while 1,577 patients needed ICU admission during the second wave. The data collected included demographics, comorbidities, duration from symptoms to hospital admission, baseline hemogram and high-resolution computed tomography (HRCT) score, need for ventilatory support, mortality at ICU and hospital discharge, and other management. We then analyzed the data with ICU and hospital mortality as primary outcomes. Different participating hospitals had different follow-up times, so the exact time point for these outcomes is variable between hospitals. We also performed multivariable analysis to find out the predictors of ICU mortality.
Statistics
Data were expressed as proportions for categorical data and means (with standard deviation) or median (with interquartile range) for numerical data. We compared groups using the Chi-squared test for categorical data and the unpaired t-test or the Mann–Whitney U test for numerical data. Time to event analysis by the Kaplan–Meier technique was used to estimate ICU and hospital survival times. We performed univariate followed by multivariable logistic regression analyses, to identify predictors of ICU mortality. The following variables were included in the logistic regression model: age, gender, period (first vs second wave), presence or absence of comorbidities, all comorbidities together (as present or absent), and also individually: hypertension, diabetes, ischemic heart disease (IHD), chronic kidney disease (CKD), respiratory disorders, hypothyroidism, and malignancy. The HRCT score at presentation and change in HRCT score, SpO2 on admission, and need for intubation and mechanical ventilation were recorded. All analyses were interpreted at 5% level of significance.
Results
In this observational study, we found that patients admitted to ICU during the second wave of the COVID-19 pandemic had significantly higher ICU and hospital mortality.
The age of patients needing ICU admission was similar in both the waves; however, the proportion of women who needed ICU admission was higher during the second wave (Table 1). Patients in the second wave had more comorbidities, as compared to those afflicted during the first wave (Table 1). Duration from symptoms to hospital admission was lower during the second wave. Similarly, the total leukocyte count was lower, but the need for any type of ventilatory support and intubation was higher in the patients admitted during the second wave. More patients received steroids for a shorter period of time during the second wave of COVID-19 in our cohort (Table 2).
Table 1.
Demographics and comorbidities
| Parameter | First wave | Second wave | p value |
|---|---|---|---|
| Patients admitted to the ICU (3,498 patients) | 1,921 | 1,577 | |
| Gender * | |||
| Male | 1,412 (73.5%) | 1,079 (68.4%) | |
| Female | 509 (26.5%) | 498 (31.6%) | 0.001 |
| Age distribution | |||
| Age, n (mean ± SD) | 57.6 ± 14.6 | 56.7 ± 15.5 | 0.07 |
| <18 years, 9 pts (0.5%) | 15 ± 3.24 | 15 ± 4.27 | NS |
| 18–64 years, 2,305 pts (65.89%) | 49 ± 10.45 | 50 ± 10.22 | NS |
| ≥65 years, 1,181 pts (33.76%) | 73 ± 6.48 | 73 ± 6 | NS |
| Comorbidity: Yes (No), % with comorbidity* | 1,275/1,907 (67%) | 1,012/1,388 (73%) | <0.001 |
| Comorbidities | |||
| Diabetes mellitus | 765/1,898 (40.3%) | 586/1,298 (45.1%) | 0.007 |
| Hypertension* | 848/1,899 (44.7%) | 645/1,299 (49.7%) | 0.005 |
| Ischemic heart disease* | 265/1,890 (14%) | 199/1,180 (16.9%) | 0.034 |
| Chronic kidney disease* | 110/1,890 (5.3%) | 105/1,135 (9.8%) | 0.001 |
| Malignancy* | 38/1,886 (2.0%) | 67/1,144 (5.9%) | <0.001 |
| Respiratory disorders* | 87/1,884 (4.6%) | 119/1,159 (10.3%) | <0.001 |
| Hypothyroidism* | 96/1,891 (5.1%) | 88/1,146 (7.7%) | 0.005 |
Statistically significant
Table 2.
Symptom duration, laboratory parameters, and treatment
| Parameter | First wave (1,921) | Second wave (1,577) | p value |
|---|---|---|---|
| Symptoms and laboratory parameters | |||
| Duration from symptoms to hospital admission (median with IQR) in days* | 5 (4–8) | 5 (4–7) | 0.001 |
| HRCT score (median with IQR) | 14 (9–18) | 14 (10–18) | 0.584 |
| Hb (mean and SD) | 12.8 (2.0) | 12.8 (1.9) | 0.8 |
| TLC (mean and SD)* | 8.9 (4.9) | 8.4 (4.8) | 0.005 |
| Treatment received | |||
| Oxygen required (any modality) | 1,553/1,617 (96%) | 1,458/1,513 (96.8%) | 0.6 |
| Needed some form of ventilation (HFNC/CPAP/NIV)* | 853/1,282 (66.5%) | 853/1,103 (77.3%) | <0.001 |
| Needed intubation* | 404/1,536 (26.3%) | 503/1,275 (39.5%) | <0.001 |
| Received steroid* | 1,796/1,821 (98.6%) | 1,507/1,508 (99.9%) | <0.001 |
| Duration of steroid (median with IQR)* | 12 (9–14) | 9 (5–14) | <0.001 |
| Received LMWH | 1,842/1,852 (99.5%) | 1,543/1,547 (99.7%) | 0.28 |
Statistically significant. The denominator is different for many parameters, due to missing data for that particular parameter in the database
We were able to get complete follow-up data for nearly all our patients and outcome data were missing for only 10 patients in the entire cohort. The ICU and hospital mortality were significantly higher in the second wave compared to the first wave (26.1 vs 13.4%, p <0.001 and 29.9 vs 18.2%, p <0.001, respectively (Table 3). Age, male gender, admission during the second wave, increasing HRCT score, presence of comorbidities, and need for intubation and mechanical ventilation were found to be significant predictors of ICU mortality on univariate analysis (Table 4), while on multivariable regression, male gender, increasing HRCT score, second wave, need for intubation and mechanical ventilation were significant predictors of ICU mortality (Table 5).
Table 3A.
Follow-up data for both waves
| First wave (n = 1,921) | Second wave (n = 1,577) | |||
|---|---|---|---|---|
| Outcome | ICU | Hospital | ICU | Hospital |
| Alive | 1,661 | 1,569 | 1,165 | 1,102 |
| Dead | 258 | 350 | 412 | 469 |
| Missing data | 2 | 2 | 0 | 6 |
Table 4.
Predictors of ICU mortality on univariate analysis
| OR (95% CI) | p value | |
|---|---|---|
| Age | 1.019 (1.013; 1.025) | <0.000 |
| Gender (male compared to female) | 1.285 (1.059; 1.559) | <0.001 |
| Wave (second compared to first) | 2.277 (1.916; 2.706) | <0.001 |
| HRCT score | 1.115 (1.094; 1.136) | <0.001 |
| Comorbidities | 1.449 (1.187; 44.120) | <0.001 |
| Intubation required | 33.972 (26.159; 36.811) | 0.000 |
Only those variables, which were statistically significant, are included here
Table 5.
Predictors of ICU mortality on multivariable analysis
| OR (95% CI) | p value | |
|---|---|---|
| Gender (male compared to female) | 1.491 (1.049; 2.118) | 0.026 |
| Wave (second compared to first) | 2.381 (1.708; 3.321) | 0.000 |
| HRCT score (per unit increase) | 1.088 (1.058; 1.119) | 0.000 |
| Need for intubation and invasive ventilation | 44.099 (30.462; 63.827) | 0.000 |
Only those variables, which were statistically significant, are included here
Table 3B.
ICU and hospital outcomes
| Parameter | First wave (1,921) | Second wave (1,577) | p value |
|---|---|---|---|
| ICU mortality* | 258/1,919 (13.4%) | 412/1,577 (26.1%) | <0.001 |
| Hospital mortality* | 350/1,919 (18.2%) | 469/1,571 (29.9%) | <0.001 |
Statistically significant
Discussion
In our observational cohort study, we found that the ICU and hospital mortality at both 7 and 14 days was significantly higher in patients who developed COVID-19 and were admitted to the ICUs of tertiary care units in western Maharashtra during the second wave of the pandemic.
We did not find any difference in the overall age (and subgroups) of patients with SARS-CoV-2 infections between the two waves in our cohort, and also age was not an independent predictor of mortality. This contradicts the widely held belief that the patients infected during the second wave were younger than those infected in the first wave. It also contradicts the supposition that the reason for younger patients getting infected was due to postvaccination status of the elderly patients. However, Jain et al. suggested that the patients affected by the second wave were either younger in age or children, as compared to those infected during the first wave.4 However, they do not provide any data from India to support this. They have quoted data from Hippich et al., which is public health antibody screening study from Bavaria, Germany.5 This screening study suggested that the exposure of children to SARS-CoV-2 increased due to the introduction of more infectious mutations of the virus, higher number of schools being reopened, increased exposure to virus due to environmental factors (i.e., fall and winter). Another multicenter Indian study of patients hospitalized (but not admitted to ICU) for COVID-19, found that there was no difference in the age of patients hospitalized for COVID-19 during the two waves.6 A Spanish study of patients with confirmed diagnosis of COVID-19 found that patients in the second wave were significantly younger [median age: 59.0 (35) vs 51.5 (40) years, p <0.001].7 There was a fivefold increase in patients infected in the second wave who were younger than 18 years [24 (0.9%) vs 46 (5.4%), p <0.001], and also a significant increase in those who were between 18 and 65 years of age [1,433 (57.8%) 531 (62.3%) p <0.0230]. This was accompanied by a decrease in the number of patients infected who were older than 65 years [1,022 (41.2%) vs 275 (32.3%), p <0.001]. However, when they looked at patients who needed hospitalization, this difference disappeared [median age (IQR) 68.0 (27.0) vs 67.0 (24.0) p <0.071]. The odds of mortality increased, with increasing age (OR: 1.079, 95% CI: 1.063; 1.094, p <0.001). Soriano et al. reported no difference in age and gender between the two waves; however, they only looked at confirmed cases of COVID-19 and not those who required ICU admission.8 Iftimie et al. found that the patients hospitalized during the second wave were considerably younger (67 ± 18 vs 58 ± 26 years, p <0.001) and both genders were infected equally in both waves.9 A large multinational study from 14 countries, most of them were high-income countries, found that there was no difference in age in patients infected in the first and second wave; however, mortality was higher during both waves, as the age of the patients increased.10
In our cohort, a higher proportion of female patients were admitted to the ICU during the second wave (31.6 vs 26.5%, p <0.001); however, males were more likely to die than females (OR: 1.969, 95% CI: 1.292; 3.000, p <0.002); this is similar to the report by Domingo et al. (aOR: 1.476, 95% CI: 1.079; 2.018, p = 0.015).7 A single-center study from a French ICU also reported a lower proportion of infections among male patients during the second wave.11 The multicenter study from India also found significantly higher mortality in male patients (4.7 vs 1.4%, p <0.001) younger than 45 years.6 There was no gender difference in mortality in other age groups. The authors did not suggest any possible explanation for this.
The number of patients with various comorbidities was higher during the second wave in our cohort (Table 1). This finding is contrary to that of Domingo et al., where the proportion of patients with comorbidities was significantly lower in the second wave.7 Budhiraja et al. reported higher incidence of comorbidities overall (59.7 vs 54.8%, p <0.001) during the second wave. The number of patients with DM (44.9 vs 43.2%, p = 0.031), hypertension (43.7 vs 41.0%, p = 0.001), and CKD (15.2 vs 13.6%, p = 0.004) were also higher during the second wave.6 In the French study as well as another Spanish study, there was no difference between the two waves, in the proportion of patients with comorbidities, except that there was a small increase in number of postpartum and pregnant patients in the second wave in the Spanish study.8,11 This could also be because during the first wave, clinicians had a lower threshold for admitting, whereas by the second wave, fitter patients and those with no or minimal comorbidities were managed at home. Also, the scarcity of ICU and hospital beds during the second wave resulted in triaging and only those who absolutely needed hospital care were admitted.
In our cohort, the duration of symptoms before ICU admission and the total leukocyte counts were lower in the second wave. A significantly higher proportion of patients required some sort of ventilatory support including invasive mechanical ventilation and corticosteroids; however, the duration of therapy was lower in the second wave (Table 2). Contou et al. reported no differences in laboratory parameters except a higher platelet count in the second wave; however, this was a single-center, very small study, with only 50 patients in the second wave.11 During the second wave in India, the number of patients infected was much higher, and due to paucity of well-equipped beds, only the sickest of patients were admitted to the ICUs. This probably explains why higher proportion of patients in the second wave needed some form of ventilatory support [HFNC, CPAP, or noninvasive ventilation (NIV)] and/or intubation and invasive mechanical ventilation. Domingo et al. also reported an increased requirement of ICU admission (17.5 vs 10.9%, p <0.001), need for invasive mechanical ventilation (11.6 vs 7.9%, p = 0.018), and higher use of steroids (5.2 vs 0%) during the second wave, in comparison with the first wave.7 Iftimie et al. reported contrary findings. In their study, noninvasive modalities of ventilation were used more often in the second than the first wave, but they reported higher use of steroids and anticoagulation during the second wave.9 We also found that steroid use was significantly higher during the second wave, probably because by then the findings of the RECOVERY Trial were widely disseminated and also the clinicians had seen the benefits of steroids themselves first-hand.12
In all studies comparing the two waves, the mortality in the second wave was lower; however, our experience was diametrically opposite.7–9,11 Budhiraja et al. in their multicenter study also reported significantly higher mortality during the second wave (10.5 vs 7.2%, p <0.001).6 There were many reasons for the devastation visited upon India due to the second wave starting with optimistic reopening of state borders, lack of COVID appropriate behavior by general public, large religious, and political gatherings. Another important reason was also restarting the businesses and reopening the marketplaces due to the urgency felt by people to resume normal activities, who were frustrated with economic downturn during the lockdown. The often-touted reason about new variants causing the problems is hardly substantiated by the current evidence.13 However, the most important reasons for the devastation caused by the second wave, though, was probably the failure to anticipate the second wave and not utilizing the time of respite from the first wave to prepare. Vaccination against COVID-19 was begun on January 21, 2021, in the period between the first and the second waves. There was some speculation that the demographics may have changed due to vaccination of the elderly at the beginning of the vaccination; however as described above, there was hardly been any change in the demographics of the population affected by the second wave.
The other factors in our cohort found to be independent predictors of hospital mortality on multivariate analysis, i.e., increasing HRCT score and need for intubation and mechanical ventilation (Table 5), probably reflect the increasing severity of illness of the patients who eventually died, though we did not calculate severity of illness formally, using a scoring system. Domingo et al. found SARS-CoV-2 infection during the first wave, comorbidity, and mechanical ventilation to be the predictors of mortality.7 Iftimie et al. reported the predictors for mortality in two waves separately. The predictors during the first wave were older age, fever, dyspnea, acute respiratory distress syndrome, type 2 diabetes mellitus, and cancer. In the second wave, apart from age, the predictors were male gender, smoking habit, acute respiratory distress syndrome, and chronic neurological diseases.9
This is the first Indian study, with a large number of ICU patients comparing ICU and hospital outcomes, from multiple centers from various large districts of western Maharashtra, with near 100% follow-up. There are no other studies looking at difference between ICU and hospital outcomes between the two COVID-19 waves, from India. All studies from abroad, which had small numbers, were either from a single unit or from a single city. There are some limitations of our study. The data collection was initiated on their own by most of the centers so the time points of follow-up, i.e., data of ICU or hospital discharge, are different for different units, as mentioned earlier. Data for several parameters either were in different formats or was missing, and we could not find a way of combining these data, therefore, several data points were not available for all patients, for full analysis. The other limitation is lack of comparative data about other medications (apart from steroids), and we found it difficult to obtain these data due to retrospective nature of our study.
Nonetheless, as previously stated this is the largest study from India till date and should help in clearing several misconceptions about the outcomes of patients infected in the two waves.
Conclusion
We found that the patients infected during the two waves were of the similar age. The proportion of patients with comorbidities was higher during the second wave. The ICU (26.1 vs 13.4%, p <0.001) and hospital mortality (29.9 vs 18.2%, p <0.001) was significantly higher during the second wave. On multivariable regression, male gender, increasing HRCT score, ICU admission during the second wave, need for intubation and mechanical ventilation were significant predictors of ICU mortality were significant predictors of ICU mortality.
Orcid
Kapil G Zirpe https://orcid.org/0000-0002-8140-727X
Subhal Dixit https://orcid.org/0000-0002-1441-0807
Atul P Kulkarni https://orcid.org/0000-0002-5172-7619
Rahul A Pandit https://orcid.org/0000-0002-5846-3708
Priya Ranganathan https://orcid.org/0000-0003-1004-5264
Sayi Prasad https://orcid.org/0000-0003-0077-0513
Zafer Khan Amanulla https://orcid.org/0000-0002-8551-8313
Vatsal Kothari https://orcid.org/0000-0002-3309-3229
Sourabh Ambapkar https://orcid.org/0000-0002-0636-3879
Sushma K Gurav https://orcid.org/0000-0001-68752-071
Shrikant Shastrabuddhe https://orcid.org/0000-0002-60748569
Vinod Gosavi https://orcid.org/0000-0002-3684-6302
Mukund Joshi https://orcid.org/0000-0002-8602-1632
Bindu Mulakavalupil https://orcid.org/0000-0001-9444-9404
Charlotte Saldhanah https://orcid.org/0000-003-2487-5621
Saanvi Ambapkar https://orcid.org/0000-0003-2758-9073
Madhura Bapte https://orcid.org/0000-0001-7877-2527
Sweta Singh https://orcid.org/0000-0003-0661-1470
Abhijit Deshmukh https://orcid.org/0000-0001-5602-291x
Khalid Khatib https://orcid.org/0000-0002-8993-4429
Anmol Zirpe https://orcid.org/0000-0002-8229-1811
Gowri Sayiprasad https://orcid.org/0000-0002-7418-8187
Ameya Joshi https://orcid.org/0000-0001-5383-9668
Acknowledgment
We gratefully acknowledge the help given by Dr Afroz Khan, Junior, Consultant Neuro Trauma Unit, Ruby Hall Clinic, Pune, Maharashtra, India; Dr Shrirang Patwardhan, Medical Superintendent, MJM Hospital, Pune; and Mrs. Nikita Kalan-Kulaye, Data Entry Operator, Department of Anesthesia, Critical Care and Pain, Tata Memorial Hospital, Mumbai, in data entry, assimilation, and corrections.
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Honigsbaum M. Spanish influenza redux: revisiting the mother of all pandemics. Lancet. 2018;391(10139):2492–2495. doi: 10.1016/S0140-6736(18)31360-6. [DOI] [PubMed] [Google Scholar]
- 2.Andrews MA, Areekal B, Rajesh KR, Krishnan J, Suryakala R, Krishnan B, et al. First confirmed case of COVID-19 infection in India: a case report. Indian J Med Res. 2020;151(5):490–492. doi: 10.4103/ijmr.IJMR_2131_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.https://www.worldometers.info/coronavirus/country/india/ [[last accessed on September 17, 2021]]. https://www.worldometers.info/coronavirus/country/india/ Available from:
- 4.Jain VK, Iyengar KP, Vaishya R. Differences between first wave and second wave of COVID-19 in India. Diabetes Metab Syndr. 2021;15(3):1047–1048. doi: 10.1016/j.dsx.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hippich M, Sifft P, Zapardiel-Gonzalo J, Böhmer MM, Lampasona V, Bonifacio E, et al. A public health antibody screening indicates a marked increase of SARS-CoV-2 exposure rate in children during the second wave. Med (NY) 2021;2(5):571–572. doi: 10.1016/j.medj.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budhiraja S, Indrayan A, Aggarwal M, Jha V, Jain D, Tarai B, et al. Differentials in the characteristics of COVID-19 cases in Wave-1 and Wave-2 admitted to a network of hospitals in North India. Available from: https://www.medrxiv.org/content/10.1101/2021.06.24.21259438v1 . [DOI]
- 7.Domingo P, Pomar V, Mur I, Castellví I, Corominas H, de Benito N. Not all COVID-19 pandemic waves are alike. Clin Microbiol Infect. 2021;27(7):1040.e7–1040.e10. doi: 10.1016/j.cmi.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soriano V, Ganado-Pinilla P, Sanchez-Santos M, Gómez-Gallego F, Barreiro P, de Mendoza C, et al. Main differences between the first and second waves of COVID-19 in Madrid, Spain. Int J Infect Dis. 2021;105:374–376. doi: 10.1016/j.ijid.2021.02.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iftimie S, López-Azcona AF, Vallverdú I, Hernández-Flix S, de Febrer G, Parra S, et al. First and second waves of coronavirus disease-19: a comparative study in hospitalized patients in Reus, Spain. PLoS One. 2021;16(3):e0248029. doi: 10.1371/journal.pone.0248029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ioannidis JPA, Axfors C, Contopoulos-Ioannidis DG. Second versus first wave of COVID-19 deaths: shifts in age distribution and in nursing home fatalities. Environ Res. 2021;195:110856. doi: 10.1016/j.envres.2021.110856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Contou D, Fraissé M, Pajot O, Tirolien JA, Mentec H, Plantefève G. Comparison between first and second wave among critically ill COVID-19 patients admitted to a French ICU: no prognostic improvement during the second wave? Crit Care. 2021;25(1):3. doi: 10.1186/s13054-020-03449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joshi RK, Mehendale SM. Prevention and control of COVID-19 in India: strategies and options. Med J Armed Forces India. 2021;77(Suppl. 2):S237–S241. doi: 10.1016/j.mjafi.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]