Abstract
Background
Cellulitis and erysipelas are now usually considered manifestations of the same condition, a skin infection associated with severe pain and systemic symptoms. A range of antibiotic treatments are suggested in guidelines.
Objectives
To assess the efficacy and safety of interventions for non‐surgically‐acquired cellulitis.
Search methods
In May 2010 we searched for randomised controlled trials in the Cochrane Skin Group Specialised Register, the Cochrane Central Register of Controlled Trials in The Cochrane Library, MEDLINE, EMBASE, and the ongoing trials databases.
Selection criteria
We selected randomised controlled trials comparing two or more different interventions for cellulitis.
Data collection and analysis
Two authors independently assessed trial quality and extracted data.
Main results
We included 25 studies with a total of 2488 participants. Our primary outcome 'symptoms rated by participant or medical practitioner or proportion symptom‐free' was commonly reported. No two trials examined the same drugs, therefore we grouped similar types of drugs together.
Macrolides/streptogramins were found to be more effective than penicillin antibiotics (Risk ratio (RR) 0.84, 95% CI 0.73 to 0.97). In 3 trials involving 419 people, 2 of these studies used oral macrolide against intravenous (iv) penicillin demonstrating that oral therapies can be more effective than iv therapies (RR 0.85, 95% CI 0.73 to 0.98).
Three studies with a total of 88 people comparing a penicillin with a cephalosporin showed no difference in treatment effect (RR 0.99, 95% CI 0.68 to 1.43).
Six trials which included 538 people that compared different generations of cephalosporin, showed no difference in treatment effect (RR 1.00, 95% CI 0.94 to1.06).
We found only small single studies for duration of antibiotic treatment, intramuscular versus intravenous route, the addition of corticosteroid to antibiotic treatment compared with antibiotic alone, and vibration therapy, so there was insufficient evidence to form conclusions. Only two studies investigated treatments for severe cellulitis and these selected different antibiotics for their comparisons, so we cannot make firm conclusions.
Authors' conclusions
We cannot define the best treatment for cellulitis and most recommendations are made on single trials. There is a need for trials to evaluate the efficacy of oral antibiotics against intravenous antibiotics in the community setting as there are service implications for cost and comfort.
Plain language summary
Interventions for cellulitis and erysipelas
This review looks at interventions for the skin infections 'cellulitis' and 'erysipelas'. These two terms are now considered different presentations of the same condition by most experts, so they are considered together for this review. For simplicity we used the one term 'cellulitis' to refer to both conditions.
Cellulitis is a common painful skin infection, usually bacterial, that may require hospitalisation in severe cases. There is variation in the types of treatments prescribed, so this review aims to collate evidence on the best treatments available.
The infection most commonly affects the skin of the lower leg but can infect the skin in any part of the body, usually following an injury to the skin. The symptoms include severe pain, swelling, and inflammation, often accompanied by fever, rigours, nausea, and feeling generally unwell. The infection is usually treated with antibiotics, however corticosteroids and physical treatments have been used to reduce pain, redness, and swelling, and improve the circulation to the skin.
We identified 25 randomised controlled trials. No two trials investigated the same antibiotics, and there was no standard treatment regime used as a comparison. We are not able to define the best treatment for cellulitis and our limited conclusions are mostly based on single trials. No single treatment was clearly superior. Surprisingly, oral antibiotics appeared to be more effective than antibiotics given into a vein for moderate and severe cellulitis. This merits further study. Antibiotics given by injection into a muscle were as effective as when given into a vein, with a lower incidence of adverse events. In one study the addition of corticosteroids to an antibiotic appeared to shorten the length of hospital stay, however further trials are needed. A single small study indicated vibration therapy may increase the rate of recovery but the results of single trials should be viewed with caution. We had insufficient data to give meaningful results for adverse events.
Background
Description of the condition
Cellulitis and definitions
Cellulitis is an acute, subacute, or chronic inflammation of loose connective tissue, but the term has been applied mainly to inflammation of subcutaneous tissue in which an infective, generally bacterial cause is proven or assumed. Erysipelas is a bacterial infection of the dermis and upper subcutaneous tissue; its hallmark is a well‐defined, raised edge reflecting the more superficial (dermal) involvement. However, cellulitis may extend superficially and erysipelas deeply, so that in many cases the two processes coexist and it is impossible to make a meaningful distinction. Current usage tends to regard erysipelas as a form of cellulitis rather than a distinct entity, so that the definition of cellulitis would include inflammation of dermal as well as subcutaneous tissue (Hay 2004). There are no internationally accepted criteria for mild, moderate, and severe cellulitis although this classification is widely used in clinical practice (Morris 2001). In this review we have used the term cellulitis to include erysipelas.
Incidence
Cellulitis is thought to be relatively common, but there are few published data on its incidence. A cohort study conducted in the USA indicated that the incidence of cellulitis and leg abscess (grouped together) ranged from 4 to 25 cases per 10,000 person years in the over 65 age group and that the incidence of cases diagnosed in hospital increased over a 10‐year period (Haan 1997). A further USA study carried out in 1997 to 2002 indicated a higher incidence of 246/10,000 person years (Ellis Simonsen 2006). In England alone, people admitted with a diagnosis of cellulitis took up to 360,000 bed days (UK DOH 2001).
Infective agents
Microbiological studies are positive in only a quarter of people who present to hospital with erysipelas or cellulitis, using classical testing with blood cultures, swabs from skin lesions, or fine needle aspiration from affected skin. The use of latex agglutination techniques and direct immunofluorescence on skin biopsy specimens increases the yield and has clearly shown that beta Haemolytic streptococci (usually group A or group G) represent the most prominent bacteria in studies of cellulitis and erysipelas, accounting for almost 80% of isolated organisms (Bernard 1987; Brook 1995). Staphylococcus aureus probably does not cause a clinical picture of classical erysipelas, but may sometimes cause cellulitis (Eriksson 1996). However with the rise in the number of community‐acquired methicillin‐resistant Staphylococcus aureus (MRSA)‐related infections (Kluytmans‐Vandenbergh 2006; Purcell 2005) this picture may change in the future. Enterococci are occasionally isolated from people with leg ulcers, often mixed with gram‐negative bacteria and/or Staphylococcus aureus (Eriksson 1996). Anaerobic organisms are much less commonly isolated and include Peptostreptococcus species, Bacteroides fragilis, Prevotella species, Porphyromonas species, and Clostridium species. (Brook 1995). In rare cases fungal species may be implicated in the disease process (Baddour 1984). There was a decrease in the frequency of childhood periorbital and orbital cellulitis caused by Haemophilus influenzae B (Hib) coinciding with the introduction of the Hib vaccine (Ambati 2000).
Causes and risk factors
Research data on risk factors for developing cellulitis is scant. Three case‐control studies (Dupuy 1999; Mokni 2006; Roujeau 2004) focused on cellulitis of the leg. Of the variables investigated, a disruption of the cutaneous barrier caused by such factors as leg ulcer, wound, athlete's foot, pressure ulcer, dermatosis, or leg oedema were shown to be risk factors in these studies. Venous insufficiency, lymphoedema, and being overweight were additional risk factors in the Dupuy 1999 study. In contrast to anecdotal and case‐series reports, diabetes, alcohol misuse, intravenous drug misuse, or smoking were not risk factors. Age was not analysed as a risk factor. Cellulitis may be reported as a complication following surgery, with incidence being reported in case‐series studies as 1% to 5% (Critchley 1997; Escalante 1995; Lasley 1997; Thomas 1999) and those with lymphatic abnormalities are over represented in acute and recurrent cellulitis cases (Soo 2008).
Impact and complications of cellulitis
Cellulitis is a localised skin infection, most commonly affecting the lower limbs, although it can involve any part of the skin. It is characterised by an area of redness and inflammation of the skin, with associated pain and swelling. It has an acute onset and it is usually accompanied by generalised symptoms, such as fever and rigours, nausea, and vomiting. A minority of sufferers have severe sepsis, local gangrene, or necrotising fasciitis, but most people are not seriously ill and have a low risk of severe complications (Eriksson 1996). There are no scientific studies investigating risk factors for complications. In most people the condition is treatable with antibiotics (Morris 2001), however, longer‐term problems, such as persistent swelling and venous ulcers, can occur in about one in every ten hospital inpatients (Cox 1998). Between 25% and 46% of people admitted to hospital may have recurrent episodes of cellulitis (Cox 1998; Jorup‐Rönström 1987; Pavlotsky 2004). However, in a population‐based cohort study, in which nearly 80% of cases were treated in the community, only 11% had a recurrence within 1 year (Ellis Simonsen 2006). Factors associated with recurrence have been examined using case‐control (Pavlotsky 2004), cohort (Jorup‐Rönström 1987), and case‐series designs (Cox 2006), and identified factors included venous insufficiency (Jorup‐Rönström 1987; Pavlotsky 2004) and obesity, lymphoedema, (Cox 2006, Pavlotsky 2004) smoking, tinea pedis, and local injury (Pavlotsky 2004).
Description of the intervention
The standard treatment for cellulitis is antibiotics as cellulitis is usually a bacterial infection. However, as symptoms may persist due to inflammation, anti‐inflammatory agents may be effective. Physical treatments to reduce inflammation have also been used. This review does not include prophylaxis for recurrent infections: currently a large multicentre trial on 'Prophylactic Antibiotics for the Treatment of Cellulitis at Home' (PATCH I and II) is investigating penicillin to prevent recurrence
Guidelines for treatment
No single specialty of medicine can claim cellulitis as exclusively part of their remit. The condition is diagnosed and treated by general practitioners, emergency department doctors, dermatologists, paediatricians, surgeons, and physicians from a variety of sub‐specialties. If it occurs following surgery, orthopaedic, vascular and general surgeons, ophthalmologists, ear, nose and throat surgeons, gynaecologists, and paediatricians may treat it. Anecdotal discussions with clinicians within the UK have highlighted that there is variation in practice for treating cellulitis. There are a few published guidelines for cellulitis (British Lymphology Society 2007; CREST 2005; Eron 2003; Societe Francaise de Dermatologie 2001; Stevens 2005). Due to the paucity of relevant research, recommendations from these guidelines are mostly based on evidence extrapolated from studies of other infections or based on expert opinion.
Why it is important to do this review
Cellulitis is a common condition taking up a large number of occupied bed days in hospital. No national guidelines for treating cellulitis have been published, although some are currently in preparation and the Dermatological Society of France have issued a consensus guideline. Trials of treatment options have been published but these are often small and inconclusive. This review of the effects of interventions for cellulitis provides a valuable resource for clinicians in summarising current best evidence and highlighting gaps in the research.
Objectives
To assess the efficacy and possible adverse effects of interventions to treat non‐surgically‐acquired cellulitis.
Methods
Criteria for considering studies for this review
Types of studies
We included studies that allocated participants to groups using randomisation in order to reduce bias.
Types of participants
Adults or children diagnosed with cellulitis. Diagnosis could be based on clinical diagnosis, such as that described by Hay 2004 with or without further microbiological or physiological inclusion criteria. Cellulitis was the primary clinical problem for antibiotic therapy and studies that included participants on concurrent antibiotic treatments or prophylactic therapy were not included unless the data from the populations could be separated.
Types of interventions
This review focused on treatment rather than prophylaxis.
We considered trials if a comparison was made between different treatment regimens including, but not limited to:
Different drug(s);
Different routes of administration of drugs; and
Different duration of therapy.
Therapy could include antibiotics or antibiotics with anti‐inflammatory agents, or physical treatment (such as topical heat, cold, vibration, or elevation).
Types of outcome measures
Primary outcomes
a) Symptoms rated by participant or medical practitioner, e.g. duration and intensity of fever, pain, redness of the affected area, swelling of the skin surface and subcutaneous tissue, blister formation, or proportion symptom‐free ('cure'), at a time specified by the study authors.
b) Proportion with severe complications (such as severe sepsis, multi‐organ failure, death).
c) Quality of life scores (including generic and disease‐specific items and return to normal activity).
Secondary outcomes
a) Changes in laboratory markers of inflammation and infection, such as C‐reactive protein and Interleukin 6, or isolation of the presumed aetiological organism.
b) Therapeutic failure, defined as 'any changes to the initial antibiotic regimen including duration of treatment, type, dose, or route of antibiotic instituted as part of primary disease management'.
c) Adverse events, including diarrhoea, skin rash, or nausea.
Search methods for identification of studies
Electronic searches
We searched the following databases on 4th May 2010:
The Cochrane Skin Group Specialised Register using the terms 'cellulitis' or 'erysipelas';
The Cochrane Central Register of Controlled Trials (Clinical Trials) in The Cochrane Library using the search strategy in Appendix 1;
MEDLINE (OVID) from 2003 to present using the search strategy displayed in Appendix 2; and
EMBASE from 2005 to present using the strategy in Appendix 3.
The UK Cochrane Centre (UKCC) has an ongoing project to systematically search MEDLINE and EMBASE for reports of trials which are then included in the Cochrane Central Register of Controlled Trials. Searching has currently been completed in MEDLINE to 2003 and in EMBASE to 2005. Further searching has been undertaken for this review by the Cochrane Skin Group to cover the years that have not been searched by the UKCC.
In addition we searched PubMed on 11th February 2008 for recent publications using the search strategy: 'Search cellulitis or erysipelas Limits: added to PubMed in the last 180 days, published in the last 180 days, Humans, Clinical Trial, Randomized Controlled Trial'.
Ongoing Trials
We searched for ongoing trials most recently in May 2010 in the following databases using the terms 'cellulitis' and 'erysipelas':
The metaRegister of Controlled Trials www.controlled-trials.com.
The U.S. National Institutes of Health ongoing trials register www.clinicaltrials.gov.
The Australian and New Zealand Clinical Trials Registry www.anzctr.org.au.
The World Health Organization International Clinical Trials Registry platform www.who.int/trialsearch.
The Ongoing Skin Trials register on www.nottingham.ac.uk/ongoingskintrials.
Searching other resources
References from published studies
We searched the reference lists of all reviewed trials and recent review articles for relevant trials.
Language restrictions
We made no restrictions on searching for foreign language articles. One author (PF) translated some French studies which did not meet our inclusion criteria.
Data collection and analysis
Selection of studies
Two authors (PF, SK) independently reviewed potentially relevant articles to determine if they met the specified criteria and extracted the key outcome data from unmasked copies of the studies onto data extraction forms. If both authors agreed that the reported study was not relevant to the objectives of the review the study was not included. If it was unclear from the abstract, the full text of the paper or report was obtained for independent assessment by the two authors (SK, PF). Any disagreement among authors was resolved by consensus, with referral to a third author (RB) if necessary, and the reasons for exclusion recorded.
The data recorded included the description of the population, interventions, treatment duration, number of participants randomised into each treatment group, the number of participants in each group who were cured or failed treatment, the numbers lost to follow up, and the duration of follow‐up.
Data extraction and management
Two authors (SK, PF) independently entered data onto the extraction form. The data recorded included the description of the population, interventions, treatment duration, number of participants randomised into each treatment group, and the number of participants in each group who were cured or failed treatment, the numbers lost to follow up and the duration of follow‐up. A third author (BH) resolved discrepancies. One author (SK) entered the data into RevMan.
Assessment of risk of bias in included studies
For all potential studies, two review authors (SK, PF) independently extracted and analysed the data from the publications or reports and, where necessary, this was done also by a statistician (BH). Review authors were not blinded to the study authors or the sources of the articles. Any discrepancies between the authors were resolved by discussion, with referral to a third author (RB) if necessary.
Four types of bias were assessed since there is evidence that these are associated with biased estimates of treatment effect (the topic was reviewed by Jüni 2001): a) selection bias; b) performance bias; c) detection bias; and d) attrition bias which is dealt with under 'Dealing with missing data'.
For this review we followed the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008) and completed a 'Risk of bias' assessment for each included study. See Characteristics of included studies.
Measures of treatment effect
For studies where similar types of interventions were compared and the same primary outcome measures were used, we performed a meta‐analysis, to calculate a weighted treatment effect across trials. The results are expressed as risk ratio with 95% confidence intervals (CI) for dichotomous outcomes.
Dealing with missing data
We assessed the reporting of withdrawals, dropouts, protocol deviations, and whether participants were analysed in the group to which they were originally randomised (intention‐to‐treat).
Assessment of heterogeneity
We assessed heterogeneity of the included studies with the Chi² test using the statistical package provided by The Cochrane Collaboration (Higgins 2008). As the number of included studies was low, we interpreted I² statistic values of 50% or more as representing substantial heterogeneity, where the P value was less than 0.10.
Subgroup analysis and investigation of heterogeneity
Due to the small number of trials available within each category we were unable to carry out subgroup analysis. The subgroups chosen a priori included a history of recurrent cellulitis and plausible risk factors for disease development or complications such as persistent disruption of the skin (leg ulcer, pressure ulcer, or leg dermatosis), venous insufficiency, phlebitis (superficial vein inflammation), leg oedema, lymphoedema (swelling due to obstruction of lymphatic channels), and being overweight.
Sensitivity analysis
Due to the small number of trials available within each category we were unable to carry out sensitivity analysis.
Results
Description of studies
Results of the search
From the 371 abstracts viewed, 64 papers were selected for retrieval of the full text paper from the electronic search and an additional 47 papers were selected from the reference lists of trials and review articles. In total 111 full text papers were assessed, of which 23 were included, 8 are awaiting assessment, 71 were excluded, and 9 were review papers so were not relevant.
Included studies
Of the 23 papers included, 2 studies consisted of 2 sets of comparisons and so these results were treated as separate studies making a total of 25. One study (Grayson 2002) was a duplicate publication having been previously published as an abstract in 2001.
Participants
The 25 studies included 2488 participants with a typical range of from 16 to 90 years of age. Details are as follows:
Seventeen studies of skin and skin structure infections (SSSIs), such as abscess, impetigo, folliculitis (inflammation of hair follicles), furunculosis (boils), and wound infection, with cellulitis as a subgroup (Bucko 2002 part 1; Bucko 2002 part 2; Chan 1995; Daniel 1991 part 1; Daniel 1991 part 2; DiMattia 1981; Fabian 2005; Giordano 2005; Iannini 1985; Kiani 1991; Leman 2005; Rao 1985; Sachs 1990; Schwartz 1996; Tack 1998; Tarshis 2001; Weigelt 2005).
Eight studies with cellulitis or erysipelas as the main inclusion criteria (Bergkvist 1997; Bernard 1992; Bernard 2002; Grayson 2002; Hepburn 2004; Johnson 2007; Vinen 1996; Zeglaoui 2004).
All studies stated that inclusion criteria consisted of signs of skin inflammation and evidence of bacterial infection. In studies specialising in cellulitis, criteria were expanded in keeping with the definition of cellulitis. For example, 'a well delineated dermal‐hypodermal inflammation' (Bernard 2002) or the less specific 'erythema' (Grayson 2002). The reports of mixed populations did not usually provide a definition for individual conditions. Three studies of SSSIs restricted their studies to more severe cases. Their inclusion criteria were stated as 'complicated' infections (Fabian 2005; Giordano 2005; Weigelt 2005), with 'deep tissue involvement' or 'the presence of co‐morbid conditions'. In addition, Weigelt 2005 included only those where there was suspected methicillin‐resistant Staphylococcus aureus (MRSA). Trial authors did not give definitions of severity of disease or state the range of severity in the baseline characteristics, with the exception of two studies of moderate‐to‐severe cases: Bernard 2002, who applied a scoring system, and Grayson 2002, who used 'the severity of symptoms or failure of previous treatment'. Statements were sometimes used to imply severity within the studies inclusion criteria, for example 'treatable with oral antibiotics' for mild to moderate severity (Bucko 2002 part 1), 'requiring intravenous antibiotics' for more severe cases (Rao 1985), or by the setting for recruitment, which varied (Characteristics of included studies) as follows:
Inpatients ‐ 16 trials (Bergkvist 1997; Bernard 1992; Bernard 2002; Chan 1995; Daniel 1991 part 1; Daniel 1991 part 2; Fabian 2005; Giordano 2005; Iannini 1985; Johnson 2007; Rao 1985; Sachs 1990; Schwartz 1996; Vinen 1996; Weigelt 2005; Zeglaoui 2004);
Outpatients ‐ four trials (Bucko 2002 part 1; Bucko 2002 part 2; DiMattia 1981; Tarshis 2001);
A mixture of hospital inpatients, outpatients, and primary care ‐ two trials (Kiani 1991; Hepburn 2004);
Hospital emergency department ‐ one trial (Leman 2005); and
Community‐based care ‐ two trials (Grayson 2002; Tack 1998).
Twelve studies were performed in North America, six in Europe, one in North Africa, three in Australia, and three were international trials (Included studies). Only a minority of studies included information on the ethnic group of recruits (Chan 1995; Giordano 2005; Hepburn 2004; Schwartz 1996; Tack 1998).
Just over half of the studies of participants with cellulitis included bacteriological examination with the baseline data, however most of the studies of SSSIs did not show separated data for cellulitis. We have summarised the information on isolated organisms in the Characteristics of included studies tables. In many cases the organism was not isolated, in those that did they reported Streptococcus pyogenes and Staphylococcus aureus present. Only one study selected participants on the suspected type of organism Weigelt 2005. A number of studies excluded people because the causal organism was not sensitive to the study drug, and this led to high rates of post‐random exclusions and withdrawals. Fabian 2005 and Kiani 1991 used the postrandomisation exclusion criteria of 'resistance/tolerance to either study drugs', whereas Iannini 1985 and Schwartz 1996 excluded those with 'resistance/tolerance' to the assigned drug only. In the absence of sensitivity data, some trial authors (Hepburn 2004; Schwartz 1996) excluded those with 'deterioration or no improvement after several days treatment' while Tarshis 2001 excluded all culture‐negative participants.
Interventions
Different types of drug
The majority of studies compared different antibiotic treatments or durations. No studies compared antibiotic against placebo. There is no agreed 'gold standard' treatment for cellulitis, although the French Consensus Statement (Societe Francaise de Dermatologie 2001) recommended penicillin as the initial standard treatment for community‐acquired erysipelas and cellulitis, because 'the majority is caused by penicillin‐sensitive streptococci'. The choice of a comparison antibiotic varied from study to study.
No two trials have made the same comparison. However, different antibiotics can be grouped by the nature of their site of action and chemical structure. See Table 1.
1. Grouping of antibiotics included in this review.
| Site of Action | Group | Generation | Generic Name |
| Act against cell wall synthesis and contain a beta‐lactam ring in their structure that binds to penicillin binding protein and inhibits bacterial cell wall synthesis. | Penicillins sometimes supplemented with beta‐lactamase inhibitors that prevent the bacteria from inactivating the antibiotic. | penicillin ampicillin with sulbactam cloxacillin flucloxacillin ticarcillin | |
| Cephalosporins | Generation 1 | cefadroxil cefazolin cephalexin tack cefazolin cefonicid cephalexin | |
| Generation 2 | cefuroxime axetil | ||
| Generation 3 | cefditoren pivoxil ceftriaxone ceftazidime cefdinir | ||
| Generation 4 | cefipime | ||
| carbapenem | meropenen | ||
| Act against cell wall synthesis | oxacephem | moxolactan rao | |
| Act against cell wall synthesis | glycopeptide | vancomycin | |
| Inhibit protein synthesis by binding to 50S subunit of the ribosome | macrolides, lincosamines and streptogramin (MLS) | erythromycin roxithromycin azithromycin pristinamycin | |
| Inhibit protein synthesis | oxazolidonones | linezolid | |
| Inhibitors of nucleic acid synthesis | quinolones (including fluoroquinolones *) | Generation 1 | |
| Generation 2* | |||
| Generation 3* | levofloxacin moxifloxacin | ||
| Generation 4* | gatifloxacin |
The drug groups compared were as follows.
Three trials compared a penicillin with a macrolide or streptogramin (Bernard 1992; Bernard 2002; Daniel 1991 part 2).
Three trials compared a cephalosporin with penicillin (Chan 1995; Sachs 1990; Vinen 1996).
One trial compared the addition of benzylpenicillin or placebo to a standard treatment of flucloxacillin Leman 2005.
Six trials compared different cephalosporins (Bucko 2002 part 1; Bucko 2002 part 2; Grayson 2002; Iannini 1985; Schwartz 1996; Tack 1998).
One trial compared two macrolides Daniel 1991 part 1.
One trial compared two carbapenems Fabian 2005.
One trial compared quinolone against a beta‐lactam plus beta‐lactamase inhibitor Giordano 2005.
One trial compared linezolid (an oxazolidinone) against vancomycin Weigelt 2005.
One trial compared two quinolones (Tarshis 2001).
One trial compared a penicillin against an oxacephem (Rao 1985).
One trial compared steroid against placebo in a population who were receiving antibiotic treatment Bergkvist 1997.
One trial compared a macrolide against a first generation cephalosporin Kiani 1991.
Different routes of administration
Studies involving oral, intramuscular, and intravenous treatments were included. One trial (Zeglaoui 2004) compared intravenous with intramuscular penicillin, one excluded study compared an oral versus intravenous penicillin (Jorup‐Rönström 1984). There were no other comparisons of different routes of administration for the same drug. Two trials compared an oral macrolide (Bernard 1992) or oral streptogramin (Bernard 2002) against intravenous penicillin.
Different duration and frequency of administration
For most studies the duration of treatment was allowed to vary, depending on clinical need. Only one trial compared treatment duration, a five‐day course of a quinolone with a ten‐day course (Hepburn 2004). One trial compared four times‐ against two times‐a‐day treatment of a cephalosporin (DiMattia 1981).
Non‐pharmacological
One study looked at the effectiveness of 'cycloidal vibration' (Vibrio‐Pulse, Vibrant Medical) (Johnson 2007) a form of small amplitude low‐frequency vibration therapy. This therapy aims to increase the microcirculation in the tissues.
Outcomes
One of our primary outcome measures was duration and intensity of symptoms. Only one study reported duration of symptoms (Bernard 1992). However, as trial authors often defined cure as 'resolution of symptoms and signs', we have accepted 'proportion cured' as analogous to 'the proportion with improved/reduced symptoms'. In a number of studies the 'cure' group also contains those with 'clinical improvement' (Bucko 2002 part 1; Bucko 2002 part 2; Chan 1995; DiMattia 1981; Grayson 2002; Hepburn 2004; Iannini 1985; Rao 1985; Tack 1998). The 'time to follow‐up' for assessment of cure or improvement varied widely. The quality of follow‐up ranged from all participants being assessed to an assumption that cure had occurred unless the participant returned to the emergency department (Leman 2005).
A few studies reported the proportion with severe complications, but the numbers were too low in the cellulitis subpopulations to yield reliable data.
None of the trials described quality of life scores. In one study the primary outcome measure was the length of stay as a hospital inpatient (Bergkvist 1997).
Of our secondary outcomes only two studies included 'changes in laboratory markers of infection and inflammation' in the Methods section (Bernard 1992; Bernard 2002). However, the results were not reported. Trials generally described adverse events, but none of the SSSI studies separated the data for those with cellulitis.
Therapeutic failure, as defined within this review, was not reported in the studies as a primary outcome measure. A few trials provided outcome data for cure, improvement, or failure. For example, Chan 1995 defined failure as 'no significant effect on signs and symptoms', Sachs 1990 'no apparent response to therapy', and Vinen 1996 as 'persistence of all symptoms and signs at 48 to 72 hours'. Within many studies the response to treatment was reported as a dichotomous outcome; failure as no response or requiring additional antibiotic treatment; cure as 'reduced signs and symptoms or none', e.g. Bernard 2002. For this reason these two outcomes are reported together in the results section.
Some studies of SSSIs showed data for microbiological eradication, however the results for the cellulitis subset were not shown (Bucko 2002 part 1; Bucko 2002 part 2; Daniel 1991 part 1; Daniel 1991 part 2; DiMattia 1981; Fabian 2005; Giordano 2005; Kiani 1991; Rao 1985; Sachs 1990; Schwartz 1996; Tack 1998; Vinen 1996; Weigelt 2005). Two studies of cellulitis participants intended to show microbiological eradication, however one did not pursue this because of lack of data (Grayson 2002), and the other used the organism, not the person, as the unit of analysis (Vinen 1996).
Excluded studies
Seventy one studies were excluded: 70 because the results for the population with cellulitis were not presented (see Characteristics of excluded studies), and 1 because it was a quasi‐randomised controlled trial Jorup‐Rönström 1984.
Studies awaiting classification
Eight studies are awaiting classification, four are reports of studies investigating vancomycin, one compared outpatient intravenous antibiotic therapy against an oral drug, one compared cefditoren pivoxil vs cefdinir and two studies are very old controlled trials (Characteristics of studies awaiting classification).
Risk of bias in included studies
Allocation
All included studies were described as randomised as this was a selection criterion. However, in some studies the method of sequence generation or concealment of allocation was not stated (Characteristics of included studies).
Blinding
In trials described as double‐blind, it was not always clear whether the participants, healthcare staff, and outcome assessor(s) were all blinded (see Characteristics of included studies).
Incomplete outcome data
Dropouts and exclusions were determined slightly differently in the trials, which made it difficult to standardise our assessment of quality in this regard. For participants who did not return for follow‐up, one trial assumed that they had been cured (Leman 2005), while others called them dropouts. In one study of military personnel and their families, follow up of participants appears to have been 100% (Hepburn 2004). There was also 100% follow‐up of the study by Zeglaoui 2004.
Only six studies included an intention‐to‐treat analysis for the outcomes of interest for this review (Bergkvist 1997; Bernard 2002; Grayson 2002; Hepburn 2004; Weigelt 2005; Zeglaoui 2004). Other studies involving participants with a mixture of infections provided intention‐to ‐treat analysis for the whole mixed population but not for the cellulitis subgroup. In a similar way details of loss to follow up for the cellulitis subgroup was not shown.
A number of drug‐company‐sponsored studies excluded participants where the bacteria isolated were not sensitive to study antibiotics. These may have represented postrandomisation exclusions, although this was not stated. Participants with cellulitis will only yield a positive culture in a minority of cases unless sophisticated immunofluorescence techniques are employed (Bernard 1987). So, if being 'culture negative' at baseline counted as an exclusion factor, a large proportion of participants with cellulitis were excluded from the trial, which may have skewed the results.
There was inconsistency in trials dealing with the situation where bacteria were found to be resistant to the study drugs at the baseline assessment.
In some trials the investigators isolated the organism, tested sensitivities, and then excluded those participants postrandomisation where the bacteria isolated were not sensitive to study antibiotics. In other studies the organisms' sensitivity to trial antibiotics was not assessed and this group of participants was be included in the final analysis.
Other potential sources of bias
A number of other difficulties were encountered in reviewing the trials. Presentation of results was sometimes poor, especially in older papers. A narrative description of outcomes, especially of adverse events, made it difficult to ascertain percentages and participants lost to follow up. There was no accepted definition of cure of cellulitis, so many publications included phrases such as, 'the investigators agreed that the condition was cured'.
Effects of interventions
Where possible, we have grouped studies that used related treatments in the following manner and dealt with the review outcomes under these four headings:
Different types of drug
a) Penicillin versus a member of the macrolides, lincosamines and streptogramin group (MLS) b) Penicillin versus a cephalosporin c) Penicillin co‐treatment versus placebo d) Cephalosporin versus cephalosporin e) Miscellaneous antibiotics f) Antibiotic plus oral corticosteroid versus antibiotic alone
Different routes of administration
g) Intravenous versus oral antibiotics h) Intravenous versus intramuscular antibiotics
Non‐pharmacological.
The study reports did not provide data for all of the three primary outcomes listed in our protocol. Of our primary outcomes, only symptoms rated by the participant or a medical practitioner were reported. These were presented as 'absence of symptoms', 'reduced symptoms at end of treatment', or 'cure at end of treatment'. None of the studies reported our second outcome, severe complications of cellulitis, such as necrotizing fasciitis, severe sepsis, or multi‐organ failure. One study, Rao 1985, reported a death, however this was due to gastrointestinal bleeding and was unrelated to cellulitis. No studies reported our third primary outcome, quality of life.
Of our three secondary outcomes there was no data for changes in laboratory markers of inflammation. There was little data on adverse events ‐ these are shown in Figure 1. In the SSSI studies adverse events were reported for the whole population, but this data was not separated for the cellulitis subgroup. Some studies reported therapeutic failure, and these results are shown below.
1.
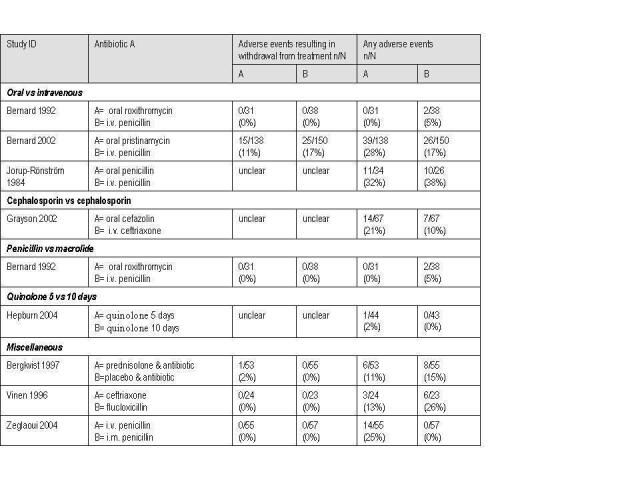
Adverse events table.
Studies of mixed populations of skin and skin structure infections did not supply adverse event results for the subgroup with cellulitis
Different types of drug
a) Penicillin versus a member of the macrolides, lincosamines and streptogramin group (MLS)
Primary outcome 1 (PO1): Symptoms rated by participant or medical practitioner (proportion symptom‐free ('cure'))
Two studies (Bernard 1992; Bernard 2002) examined intravenous benzyl penicillin against an oral macrolide or streptogramin, roxithromycin, and pristinamycin respectively. Participants in both studies had uncomplicated erysipelas, presumed by the author to be caused by Streptococcal infection and therefore likely to be penicillin‐sensitive. Another study compared oral cloxacillin, a semisynthetic penicillin, against the macrolide azithromycin (Daniel 1991 part 2). Data from 419 people were included in the analysis comparing a penicillin against an MLS. The combined RR was 0.84 (95% CI 0.73 to 0.97) (Analysis 1.1).
1.1. Analysis.

Comparison 1: Penicillin vs macrolides, lincosamines and streptogramin (MLS), Outcome 1: Symptom‐free/reduced at the end of treatment
Secondary outcome 2 (SO2): Therapeutic failure
One paper Bernard 2002 reported the number of participants who withdrew prematurely, these withdrawals were due to adverse events, 17% for the penicillin and 11% MLS groups respectively, this difference was not significant.
Secondary outcome 3 (SO3): Adverse events
Two studies reported adverse events. There were no significant differences for Bernard 1992, however in Bernard 2002 there were more mild or moderate gastrointestinal adverse events in the oral macrolide group (P = 0.034 ), but no difference in the number where the drug had to be withdrawn, 17% versus 11% (Figure 1).
b) Penicillin versus a cephalosporin
PO1: Symptoms rated by participant or medical practitioner (Cure at the end of treatment)
Three studies (Chan 1995; Sachs 1990; Vinen 1996) involving 88 participants compared a cephalosporin administered with a penicillin against a cephalosporin. In two studies (Chan 1995; Sachs 1990) intravenous (iv) ampicillin/sulbactam was compared with iv cefazolin for the treatment of cellulitis. In the Vinen study (Vinen 1996), iv ceftriaxone was compared with iv flucloxacillin. The combined RR was 0.99 (95% CI 0.68 to1.43) and there was significant heterogeneity: Chi² = 6.52, df = 2 (P = 0.04), I² = 69% (Analysis 2.1). Subgroup analysis for the generation of the cephalosporin removed the heterogeneity: two studies used a 1st generation cephalosporin (Chan 1995; Sachs 1990) and there was no strong evidence of an effect (RR 1.17, 0.91 to 1.50) (Analysis 2.1) and the evidence from the one study using a third generation cephalosporin showed no strong effect (Vinen 1996) (RR 0.7, 95% CI 0.48 to 1.00) (Analysis 2.1).
2.1. Analysis.

Comparison 2: Penicillin vs cephalosporin, Outcome 1: Symptom‐free/reduced at end of treatment
SO2: Therapeutic failure
Three studies (Chan 1995; Sachs 1990; Vinen 1996) compared a cephalosporin with a penicillin with 88 participants. In both studies iv ampicillin/sulbactam was compared with iv cefazolin for the treatment of cellulitis. In Chan (Chan 1995) there were no therapeutic failures reported. In Sachs (Sachs 1990) there was one therapeutic failure in the penicillin group, and none in the cephalosporin group. In the third study (Vinen 1996) there was 1 (out of the 23) with treatment failure in the iv ceftriaxone group and 6 out of 23 in the iv flucloxacillin group. As there were so few outcomes reported in two of the papers, meta‐analysis was not performed.
SO3: Adverse Events
No studies reported this outcome for the cellulitis subgroup.
c) Penicillin co‐treatment versus placebo
PO1: Symptoms rated by participant or medical practitioner (Cure at the end of treatment, primary outcome 1)
In the 1 study of 81 participants, comparing the addition of benzyl penicillin in those receiving a standard therapy of flucloxicillin (Leman 2005), there was no significant effect on symptoms, temperature, pain, or diameter of infected area assessed on day 1 and 2 of treatment.
SO2: Therapeutic failure
In this sole study of 81 participants, where this outcome was reported, there was no difference in the failure rates between the 2 groups: 3/41 benzyl penicillin and 2/40 placebo (Leman 2005).
SO3: Adverse events
There were no side‐effects of treatment in either arm of the study.
d) Cephalosporin versus cephalosporin
PO1: Symptoms rated by participant or medical practitioner (Cure at the end of treatment)
There were 6 trials (Bucko 2002 part 1; Bucko 2002 part 2; Grayson 2002; Iannini 1985; Schwartz 1996; Tack 1998) including a total of 538 people, that compared 1 cephalosporin with another. There is no single cephalosporin accepted as a 'standard' for comparison. We chose to define the 'new' cephalosporin as cephalosporin A and the 'older' drug as cephalosporin B for our analysis, but this was arbitrary, which made the meta‐analysis statistic hard to interpret. There were no significant differences between the cephalosporins (RR 1.00, 95% CI 0.94 to 1.06) (Analysis 3.1).
3.1. Analysis.

Comparison 3: Newer vs older generation cephalosporin, Outcome 1: Symptom‐free/reduced at the end of treatment
SO2: Therapeutic failure
Not reported as a separate outcome.
SO3: Adverse Events
None of the studies reported separated data for severe adverse events. Only one (Grayson 2002) reported data for any adverse event for the cellulitis subgroup (Figure 1), and in this study the cefazolin‐probenecid group suffered more adverse events than the ceftriaxone arm: 21% compared to 10%, and the study authors reported this was a significant increase. Adverse events reported were mainly nausea and vomiting.
e) Miscellaneous antibiotics
PO1: Symptoms rated by participant or medical practitioner (Cure at the end of treatment)
Three studies investigated antibiotics normally set aside for antibiotic resistant organisms or severe infections, (Fabian 2005; Tarshis 2001; Weigelt 2005). Other studies not dealt with in the previous sections are Daniel 1991 part 1, Kiani 1991, and Rao 1985. The outcome data from these studies are shown in Table 2.
2. Miscellaneous antibiotics.
| Study ID | Drug A | cure rate n/N (%) |
Drug B | cure rate n/N (%) |
| Daniel 1991 part 1 | Azithromycin (macrolide) | 51/72 (72) | Erythromycin (macrolide) | 37/50 (74) |
| Fabian 2005 | Meropene (carbapenem) | 27/39 (68) | Imipenem‐silastatin (carbapenem) |
33/42 (79%) |
| Giordano 2005 | Moxifloxacin (3rd generation quinolone) | 36/43 (84) |
Piperacillin‐tazobactam (beta‐lactamase inhibitor) | 38/43 (88) |
| Kiani 1991 | Azithromycin (macrolide) | 23/24 (96) | Cephalexin (cephalosporin) | 22/23 (96) |
| Rao 1985 | Ticarcillin and clavulanic Acid (a penicillin) | 9/9 (100) | Moxolactan (oxacephem) | 9/10 (90) |
| Tarshis 2001 | Gatifloxaci (4th generation quinolone) | 39/40 (98) | Levofloxacin (4th generation quinolone) | 35/42 (83) |
| Weigelt 2005 | Linezolid (oxazolidinone) | 205/224(92) | Vancomycin (glycopeptide) | 184/201 (92) |
SO2: Therapeutic failure
Only one paper showed separated data for the cellulitis subgroup. Kiani 1991 showed failure rates of 1/24 azithromycin and 1/23 cephalexin.
SO3: Adverse Events
In the SSSI studies this data was not separated for cellulitis.
f) Antibiotic plus oral corticosteroid versus antibiotic alone
Symptoms rated by participant or medical practitioner (Cure at the end of treatment)
One study (Bergkvist 1997), with108 participants, compared the addition of a corticosteroid (oral prednisolone) to antibiotic treatment with an antibiotic alone. There was no difference in the rate of cure at the end of treatment, although length of hospital stay was found by the authors to be one day less for the active treatment group (P < 0.01). Days until healing which was defined as lack of redness and normal temperature, was one day shorter for the active treatment group (P < 0.01). Both these results are presented in the paper as medians so we have not been able to calculate in Review Manager 5.
SO2: Therapeutic failure
Not reported as a separate outcome.
SO3: Adverse Events
There were no significant differences (Figure 1).
Different routes of administration
g) Intravenous versus oral antibiotics
PO1: Symptoms rated by participant or medical practitioner (Cure at the end of treatment)
Only 2 studies looked at an intravenous versus an oral antibiotic (Bernard 1992; Bernard 2002), both investigating oral MLS against iv penicillin (benzyl penicillin) with a total of 357 participants. The oral MLS was shown to be more effective than the intravenous benzyl penicillin (RR 0.85, 95% CI 0.73 to 0.98) (Analysis 1.1).
SO2: Therapeutic failure
Not reported as a separate outcome.
SO3: Adverse Events
Both included studies reported adverse events. Bernard 2002 showed a significant difference, with higher adverse events for the oral drug. There were only five adverse events in total for Bernard 1992 (Figure 1).
h) Intravenous versus intramuscular antibiotics
PO1: Symptoms rated by participant or medical practitioner (Cure at the end of treatment)
There were no studies reporting symptom rate.
SO2: Therapeutic failure
One study compared intravenous with intramuscular penicillin for cellulitis (Zeglaoui 2004) in a hospital‐based dermatology trial. There was no significant difference in therapeutic failure at the end of treatment ‐ 20% of 55 (intramuscular) vs 14% of 57 (intravenous), where the authors defined failure as 'complications or no clinical improvement' after 10 days of treatment.
SO3: Adverse Events
In the one included study there were no severe adverse events, but for any adverse events the study authors found significantly that there were more adverse events with the intravenous route: 25% compared to the intramuscular route. These were mostly venitis at the site of insertion of the needle (Zeglaoui 2004) (Figure 1).
Different duration and frequency of administration
PO1: Symptoms rated by participant or medical practitioner (Cure at the end of treatment)
The study by Hepburn 2004 compared a five‐day treatment regimen with the quinolone, oral levofloxacin to a ten‐day regimen. There was no difference in cure rates at the end of treatment, both were at 98% (RR 1.00, 95% CI 0.94 to 1.07) (Analysis 5.1).
5.1. Analysis.

Comparison 5: Quinolone 5 days vs 10 days, Outcome 1: Symptom‐free/reduced at end of treatment
One study with only 19 participants with cellulitis, looked at administration 4 vs 2 times a day and found that all the participants' symptoms resolved in both groups (DiMattia 1981), and so meta‐analysis was not possible.
SO2: Therapeutic failure
Not reported as a separate outcome.
SO3: Adverse Events
Only one participant suffered an adverse event.
Non‐pharmacological
There was only one study in this group (Johnson 2007) and this looked at a physical therapy based on vibration as a method of reducing inflammation, in addition to antibiotics.
PO1: Symptoms rated by participant or medical practitioner (Cure at the end of treatment)
This was assessed at day seven. In the treatment group 12/18 recovered fully, compared to 2/18 in the control group. The authors used the log rank test to compare recovery times (data was censored for those who had not recovered by day 7) and despite the small sample size the difference was significant (P = 0.0004).
SO2: Therapeutic Failure
Although the area of erythema had not resolved in the remaining cases by day seven, no therapeutic failures were reported and all were showing signs of recovery, for example all were pain free by day four.
SO3: Adverse Events
No adverse events were reported.
Discussion
Summary of main results
We cannot define the best treatment for cellulitis and most recommendations are made on single trials.
Some groups suggest using combinations of intravenous benzyl penicillin and intravenous flucloxacillin to ensure that the treatment is suitable for the majority with Streptococcus as the causal bacterial agent, and the largest minority with Staphylococcus aureus (British Lymphology Society 2007; CREST 2005; ;Eron 2003; Royal Pharmaceutical Society of Great Britain 2007). However, one included trial (Leman 2005) demonstrated that the addition of benzyl penicillin to flucloxacillin did not increase efficacy, perhaps indicating that flucloxacillin could be given alone. For penicillin‐allergic participants the usual recommended alternatives are erythromycin or clindamycin. There are no studies on the latter and only one study on the former (Daniel 1991 part 1), which showed no difference between oral azithromycin and oral erythromycin.
In three studies of complicated skin infections, researchers used long courses of newer broad‐spectrum antibiotics, employing high intravenous doses. Clinicians would be unlikely to use these in simple, community‐acquired cellulitis. This again makes it difficult to assess the 'best treatment' for cellulitis. Only one study focused on participants suspected of having MRSA (Weigelt 2005).
There is little evidence supporting any particular duration of treatment. Opinion suggests that residual symptoms may persist due to inflammation, rather than active infection (Eron 2003) and one small study (Hepburn 2004) supported this view by showing that five days of treatment may be as effective as ten days. Also Bergkvist's study (Bergkvist 1997) showed that symptoms resolved more quickly in response to the anti‐inflammatory agent, prednisolone, however this finding needs corroboration by further studies.
Few studies investigated the route of treatment. One study showed similar efficacy but greater safety for intramuscular compared to intravenous antibiotics (Zeglaoui 2004). In this review when oral antibiotics were compared with intravenous treatments, the oral treatments appeared more effective. These results are inconclusive due to the small number of studies, but merit further investigation. Only two studies compared oral macrolides against intravenous benzyl penicillin (Bernard 1992; Bernard 2002) and demonstrated an overall benefit for the oral therapy. This observation has been shown in other conditions, including community‐acquired pneumonia in the hospital setting (Chan R 1995) and severe pneumonia (Rojas 2006). For severe pneumonia in children, no difference was found between the two routes and no difference was found for neutropenic sepsis after chemotherapy (Kern 1999), nor for urinary tract infections in children (Hoberman 1999).
There is an increasing trend towards home treatment for cellulitis, with claims that it leads to greater satisfaction with fewer adverse events (Corwin 2005). Route and frequency of drug administration has a great impact on such a service. For parenteral drugs, once‐a‐day treatment is preferable to multiple doses and clinicians commonly use intravenous ceftriaxone as an alternative to benzyl penicillin for participants with cellulitis. However, without firm evidence in favour of intravenous therapy the need for Outpatient Parenteral Antibiotic Therapy (OPAT) has not been established.
One study investigated the addition of anti‐inflammatory treatments. Bergkvist 1997 showed that the addition of high‐dose oral steroids shortened the length of hospital stay by one day, without an increase in side‐effects. More efficacy and safety evidence would be required to adopt this as standard therapy.
Overall completeness and applicability of evidence
Thirty three studies investigating treatment for 'skin and skin structure infections' were excluded as they gave no separated data for the cellulitis subgroup.
There is no internationally agreed standard treatment for comparison. Several European guidelines for erysipelas/cellulitis or skin and skin structure infections recommend penicillin as the standard treatment, with the pathogenic organism assumed to be a Streptococcal infection. However, there were limited studies with penicillin as a comparator to confirm or refute this approach and there is tentative evidence that a macrolide/streptogramin antibiotic may be more effective, although probably more expensive.
The majority of studies were hospital‐based, as a consequence these studies are likely to include people who failed to respond to treatment in the community. Only Giordano 2005 and Zeglaoui 2004 specifically excluded those who had previously taken antibiotics for this episode of cellulitis.
Interviews demonstrate that the outcomes of interest to participants are time‐to‐resolution of unpleasant symptoms, such as pain (Carter 2007), yet only three studies gave the rate of symptom reduction. A more common outcome was proportion cured or improved. This assessment was often timed 'at the end of treatment' or 'up to two weeks after treatment', and defined as 'reduction or absence of the original signs and symptoms'. This does not allow for discrimination between treatments that may influence duration of symptoms or length of hospital stay.
In many cases the organism was not isolated, in those that did, the presence of Streptococcus pyogenes and Staphylococcus aureus reported. This review did not investigate the effectiveness of isolation and sensitivity tests in the management of this condition. This review did not investigate the effectiveness of prophylaxis to prevent recurrent disease.
Quality of the evidence
A low rate of included studies explained the process for concealment of allocation (Characteristics of included studies) and no studies provided sample size calculations. A number of studies were 'open' (9/25) and this lack of blinding, in combination with a lack of objective outcome measures, could increase the risk of bias (Jüni 2001).
Many reports of studies with mixed populations showed subgroup data for causal organisms, but not the type of tissue involvement, for example abscess or cellulitis. Isolation rates for causal organisms were low for cellulitis infections (Fabian 2005, Iannini 1985), rarely higher than 25%, meaning that up to 75% of cellulitis participants would be excluded from some studies.
Potential biases in the review process
There are a number of clinical trials that include a mixed population of people with a range of skin and skin structure infections. Unless these studies presented subgroup data for those with cellulitis we were unable to include the studies. There may be bias in the decision to show this data in reports of clinical trials. For example researchers may be more likely to show data for specific disease groups if these groups varied in their response to the treatments. It may be difficult to assess the external validity of the studies as the prevalence of antibiotic resistant strains in the community was not presented by the authors of the clinical trials.
Agreements and disagreements with other studies or reviews
There are no other systematic reviews on this topic.
Authors' conclusions
Implications for practice.
There is limited evidence that macrolide and streptogramin are slightly better than penicillin for eliminating or reducing symptoms at the end of treatment for cellulitis.
The studies comparing a penicillin with different generations of cephalosporin did not agree and there was heterogeneity, only one study investigated a third generation cephalosporin, consequently no recommendations can be made.
There is limited evidence to indicate that oral antibiotics can be more effective than intravenous antibiotics, although this evidence is limited to two studies.
There was insufficient data on varying duration of therapy, addition of corticosteroids, or vibration therapy as only single trials were found.
Implications for research.
In future trials of populations with a variety of distinguishable skin and soft tissue infections, baseline and outcome data on each of the disease group subpopulations e.g. abscess or cellulitis needs to be recorded.
In the absence of an effective method for isolating and identifying the infective agent the exclusion of culture negative participants leads to a very high postrandomisation exclusion rate.
The evidence from this review indicates that some oral antibiotics may be as effective as intravenous therapies, however, the number of trials found was small. Randomised controlled trials should be carried out comparing intravenous versus oral antibiotics such as penicillins and carbopenems for participants within a community setting, as this would have implications for delivering a more cost‐effective home therapy that does not involve a home intravenous service or frequent outpatient hospital visits.
Feedback
Non‐Cochrane update of review published, 16 June 2020
Summary
A comment was received from co‐author Richard Brindle that he has published an update to this review in JAMA dermatology. The update includes only antibiotic therapy and doubles the number of clinical trials reviewed in 2010.
Brindle R, Williams OM, Barton E, Featherstone P. Assessment of Antibiotic Treatment of Cellulitis and Erysipelas: A Systematic Review and Meta‐analysis. JAMA Dermatol. 2019;155(9):1033–1040. doi:10.1001/jamadermatol.2019.0884.
Reply
Cochrane Skin wish to clarify that the publication is not a full update of the 2010 Cochrane review 'Interventions for cellulitis and erysipelas'.
Contributors
Cochrane Skin feedback editor Urbà González and Cochrane Managing Editor Support.
What's new
| Date | Event | Description |
|---|---|---|
| 7 December 2020 | Amended | Feedback was received 16 June 2020 |
History
Protocol first published: Issue 3, 2003 Review first published: Issue 6, 2010
| Date | Event | Description |
|---|---|---|
| 9 February 2009 | Amended | responded to feedback, details attached in word file |
| 23 June 2008 | Amended | uploaded to revman 5 |
| 23 October 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Many thanks to Professor Martin Severs who reviewed the protocol and to the School of Health Sciences and Social Work, University of Portsmouth and Portsmouth Hospitals NHS Trust who hosted the review and the medical librarians at Portmouth Hospitals NHS Trust who helped retrieve the research papers.
The Cochrane Skin Group editorial base would like to thank Robert Dellavalle who acted as key editor, Matthew Grainge as statistics editor, and Philippa Middleton as methods editor on this review. We would also like to thank John English and the late Neil Cox who were the content referees, and Peter Smart who was the consumer referee.
Appendices
Appendix 1. Cochrane Library search strategy
#1(cellulitis):ti,ab,kw or (erysipelas):ti,ab,kw #2MeSH descriptor Cellulitis explode all trees #3MeSH descriptor Erysipelas explode all trees #4(#1 OR #2 OR #3) #5SR‐SKIN #6(#4 AND NOT #5)
Appendix 2. MEDLINE search strategy
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. clinical trials as topic.sh. 6. randomly.ab. 7. trial.ti. 8. 1 or 2 or 3 or 4 or 5 or 6 or 7 9. (animals not (human and animals)).sh. 10. 8 not 9 11. exp Cellulitis/ or cellulitis.mp. 12. exp Erysipelas/ or erysipelas.mp. 13. 11 or 12 14. 10 and 13
Appendix 3. EMBASE search strategy
1. random$.mp. 2. factorial$.mp. 3. (crossover$ or cross‐over$).mp. 4. placebo$.mp. or PLACEBO/ 5. (doubl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 6. (singl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 7. (assign$ or allocat$).mp. 8. volunteer$.mp. or VOLUNTEER/ 9. Crossover Procedure/ 10. Double Blind Procedure/ 11. Randomized Controlled Trial/ 12. Single Blind Procedure/ 13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 14. cellulitis.mp. or exp CELLULITIS/ 15. erysipelas.mp. or exp ERYSIPELAS/ 16. 14 or 15 17. 13 and 16
Data and analyses
Comparison 1. Penicillin vs macrolides, lincosamines and streptogramin (MLS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Symptom‐free/reduced at the end of treatment | 3 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.73, 0.97] |
| 1.1.1 iv penicillin | 2 | 357 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.73, 0.98] |
| 1.1.2 oral penicillin | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.50, 1.26] |
Comparison 2. Penicillin vs cephalosporin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Symptom‐free/reduced at end of treatment | 3 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.68, 1.43] |
| 2.1.1 1st generation cephalosporin | 2 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.91, 1.50] |
| 2.1.2 3rd generation cephalosporin | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.48, 1.00] |
Comparison 3. Newer vs older generation cephalosporin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Symptom‐free/reduced at the end of treatment | 6 | 538 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 3.1.1 2nd vs 1st generation | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.78, 1.25] |
| 3.1.2 3rd vs 1st generation | 3 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.93, 1.13] |
| 3.1.3 3rd vs 2nd generation | 1 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.81, 1.08] |
| 3.1.4 4th vs 3rd generation | 1 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.91, 1.11] |
Comparison 4. Prednisolone+ antibiotic vs antibiotic alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Symptom‐free/reduced at end of treatment | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.88, 1.17] |
4.1. Analysis.

Comparison 4: Prednisolone+ antibiotic vs antibiotic alone, Outcome 1: Symptom‐free/reduced at end of treatment
Comparison 5. Quinolone 5 days vs 10 days.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Symptom‐free/reduced at end of treatment | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.07] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bergkvist 1997.
| Study characteristics | ||
| Methods | 2‐centre randomised controlled trial | |
| Participants | Erysipelas; severity unclear Source: hospital Included: pre‐treatment with antibiotics was allowed Excluded: previous steroids, history of diabetes, peptic ulcer, psychosis, allergy to study drug, active tuberculosis, DVT, osteoporosis, swallowing difficulties Randomised(evaluable): prednisolone: 55 (52) placebo: 57 (55) Age: mean 62 vs 61 Bacteria isolated: mainly Streptococcus pyogenes and Staphylococcus aureus |
|
| Interventions |
Setting: initially hospital A: prednisolone, reducing doses starting at 30 mg/day for 8 days and 'standard antibiotic therapy' (iv benzyl penicillin 3 g 3 times a day or isoxazoyl penicillin 2 g 3 times a day, followed by oral penicillin for 7 days after 'day of cure'). B: placebo plus 'standard antibiotic therapy' as above. |
|
| Outcomes | 1. Time‐to‐cure (cure = no fever > 37.8 C and no skin flush) 2. Side‐effects 3. Recurrence within 3 weeks 4. Duration of iv hospital therapy |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...computer generated random numbers table..sequence block size was four... prospective stratification...for with or without a history of cellulitis." Comment: Adequate |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Before the code envelope was broken all classification of side effects and decisions on evaluation of efficacy had been completed." Comment: There is not enough description to make a valid decision. The paper states that placebo was used, and the quote above implies that once all study data was collected the sealed envelopes were opened to reveal the allocation, however, it is not clear that the researcher recruiting participants was unaware of the allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "...double‐ blind, placebo." "Before the code envelope was broken all classification of side effects and decisions on evaluation of efficacy had been completed." Comment: No further description; the blinding of the participants and the clinician making the assessment is implied. |
| Incomplete outcome data (attrition bias) Cure at end of treatment | Low risk | Comment: Within the text the authors describe the withdrawals. Intention‐to‐treat analysis given in the results section. |
Bernard 1992.
| Study characteristics | ||
| Methods | 6‐centre randomised controlled trial | |
| Participants | Erysipelas; severity unclear Source: hospital inpatients Included: fever, elevated white blood cells Excluded: previous antibiotics, erysipelas of head Randomised(evaluable): roxithromycin 34: 3 of which were excluded postrandomisation (31) penicillin: 38 (38) Age: range 19 to 92 Bacteria isolated: mainly Streptococcus pyogenes and Staphylococcus aureus |
|
| Interventions |
Setting: hospital initially A: oral roxithromycin (a macrolide) ‐ 150 mg 2 times day until apyrexial for 10 days. B: iv penicillin ‐ 2.5 MU 3‐hourly until apyrexial, then 2 MU 3 times a day until apyrexial for 10 days. |
|
| Outcomes | 1. Proportion with symptoms day 1 to 7 2. Proportion cured, time not stated 3. Adverse events |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...random." Comment: No details were supplied for sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Unclear; simply described as "random", but no details for concealing allocation from those recruiting the participants. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: Not stated as blind. |
| Incomplete outcome data (attrition bias) Cure at end of treatment | Low risk | Comment: Withdrawals were described. Intention‐to‐treat analysis for their primary outcome. |
Bernard 2002.
| Study characteristics | ||
| Methods | Randomised controlled trial within 22 centres | |
| Participants | Erysipelas; severity score of 3 or more Source: hospital inpatients Included: fever or chills Excluded: necrotising cellulitis, abscess, HIV infection Randomised (evaluable): pristinamycin: 139 (138) penicillin: 150 (150) Age: 18 to 96 Bacteriaisolated: mainly Streptococcus pyogenes and Staphylococcus aureus Number of participants randomised: 289 |
|
| Interventions |
Setting: hospital A: oral pristinamycin (a streptogramin) ‐ 1 g 3 times a day for 14 days. B: iv penicillin ‐ 18 MU 6 times a day until apyrexial, then 2 MU 3 times a day until day 14. |
|
| Outcomes | 1. Proportion cured at 25 to 45 days 2. Proportion cured at 14 days (end of treatment) 3. Adverse events |
|
| Notes | Severity score oedema, erythema, and pain rated as: absent = 0, moderate = 1, or severe = 2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...centralised computer generated sequence, stratified by centre... numbered containers." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomisation was centralised and balanced by centre. The allocation sequence was generated with a computer list of random set numbers, stratified by centre and blocked. Containers numbered in increasing values were used to implement the random allocation sequence. All study medications were supplied by Laboratoire Aventis." Comment: The central allocation and sequentially numbered containers indicate that concealment may have been achievable but the paper fails to state that the containers were identical for both arms of the study, and that the packages were opened after recruitment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "...open label." |
| Incomplete outcome data (attrition bias) Cure at end of treatment | Low risk | Comment: Withdrawals were described. Intention‐to‐treat analysis. |
Bucko 2002 part 1.
| Study characteristics | ||
| Methods | Randomised controlled trial Multicentre (63 sites) |
|
| Participants | Uncomplicated skin and skin structure infections, cellulitis not defined Source: unclear Included: mild to moderate uncomplicated skin or skin structure bacterial infections, treatable with oral antibiotics Excluded: diabetes mellitus Randomised (evaluable): comparison of cephalosporin vs cephalosporin cefditoren pivoxil: 400 mg, 74 (66) cefuroxime axetil: 87 (79) cefditoren pivoxil: 200 mg, 80 (75) Age: 12 to 95 yrs Bacteria isolated: no separated data for cellulitis |
|
| Interventions |
Setting: outpatients Cephalosporin vs cephalosporin A: oral cefditoren pivoxil (a 3rd generation cephalosporin) ‐ 400 mg 2 times a day. B: oral cefuroxime axetil (a 2nd generation cephalosporin) ‐ 250 mg 2 times a day both for 10 days. C: this study also included a group with cefditoren pivoxil ‐ 200 mg 2 times a day for 10 days. |
|
| Outcomes | 1. Proportion cured at end of treatment (10 days) 2. Treatment failure 3. Adverse events not separated for cellulitis 4. Microbiological eradication, but unit of analysis isolate rather than person |
|
| Notes | Higher dose cefditoren, 400 mg, (A) is shown in the 'results' figures for this review rather than the lower dose of 200 mg (C). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...sealed containers and randomisation schedule." Comment: Although it does not state the method of sequence generation they use the word 'randomised schedule' and the authors state that they followed the US Food and Drugs Administration Guidance for clinical trials, so the review authors assumed a valid method was used. |
| Allocation concealment (selection bias) | Low risk | Quote: "Study drug containers were dispensed in numeric sequence at each investigation site, sealed containers were used." "Double dummy." Comment: The term 'double dummy' indicates that the packages were similar. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "... double dummy." "Patient evaluability and outcomes were assessed under blinded conditions." |
| Incomplete outcome data (attrition bias) Cure at end of treatment | Low risk | Comment: Numbers of withdrawals shown for the participants with cellulitis, but reasons were only supplied for whole group. Intention‐to‐treat analysis was not stated for cellulitis subgroup. |
Bucko 2002 part 2.
| Study characteristics | ||
| Methods | Randomised controlled trial Multicentre (69 sites) |
|
| Participants | Mild to moderate uncomplicated skin or skin structure bacterial infections, cellulitis was not defined Source: unclear Included: treatable with oral antibiotics Excluded: diabetes mellitus Randomised (evaluable): cephalosporin vs cephalosporin cefditoren pivoxil: 400 mg 74 (56) cefadroxil: 500 mg 73 (66) cefditoren pivoxil: 200 mg 70 (67) Age: 12 to 95 yrs Bacteria isolated: no separated data for cellulitis |
|
| Interventions |
Setting: outpatients A: cefditoren pivoxil (a 3rd generation cephalosporin) ‐ 400 mg 2 times a day. B: cefadroxil (a 1st generation cephalosporin) ‐ 500 mg 2 times a day. Both for 10 days. C: the paper also included a group with oral cefditoren pivoxil at the lower dose of 200 mg 2 times a day for 10 days. |
|
| Outcomes | 1. Proportion cured at end of treatment (10 days) 2. Treatment failure 3. Adverse events 4. Microbiological eradication but unit of analysis isolate rather than person |
|
| Notes | The higher dose of cefditoren, 400 mg (A), is shown in the 'results' figures for this review, rather than the lower dose of 200 mg (C). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...sealed containers and randomisation schedule." Comment: Although it does not state the method of sequence generation, they use the words 'randomised schedule' and the authors state that they followed the US Food and Drugs Administration Guidance for clinical trials, so the review authors assumed a valid method was used. |
| Allocation concealment (selection bias) | Low risk | Quote: "Study drug containers were dispensed in numeric sequence at each investigation site, sealed containers were used." "Double dummy". Comment: The term 'double dummy' indicates that the packages were similar. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "...double dummy." "Patient evaluability and outcomes were assessed under blinded conditions." |
| Incomplete outcome data (attrition bias) Cure at end of treatment | Low risk | Comment: Numbers of withdrawals shown for the participants with cellulitis, but reasons were only supplied for whole group. Intention‐to‐treat analysis was not stated for cellulitis subgroup. |
Chan 1995.
| Study characteristics | ||
| Methods | 1‐centre randomised controlled trial | |
| Participants | Skin and skin structure infections, 'clinically compatible with streptococcal or staphylococcal infection, without any open wound' Source: hospital Included: see footnotes Excluded: 'likelihood of death within 48 hours', 'desire to donate blood' Randomised (evaluable): amplicillin/salbactam: 8 (8) cefazolin: 13 (12) Age: mean 50, 19 to 70 years Bacteria isolated: not stated Number of participants randomised: 21 |
|
| Interventions |
Setting: hospital A: iv ampicillin 1g sulbactam ‐ 0.5g 4 times a day B: iv cefazolin (a 1st generation cephalosporin) ‐ 0.5g 4 times a day, min. 4 days, max. depended on clinical response. |
|
| Outcomes | 1. Proportion cured at end of therapy 2. Proportion with eradication of bacteria (no subgroup analysis for cellulitis) 3. Duration of hospitalisation 4. Adverse events (no separated data for cellulitis) 5. Proportion with eradication of bacteria |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: '...randomised.' Comment: No details provided, no mention of a schedule or how the sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No mention of method of sequence generation or method of concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "...double‐blind." |
| Incomplete outcome data (attrition bias) Cure at end of treatment | Low risk | Comment: Numbers of withdrawals supplied. |
Daniel 1991 part 1.
| Study characteristics | ||
| Methods | Randomised controlled trial Multicentre (16 sites) |
|
| Participants | Mixed population of skin and skin structure infections. 'Cellulitis' could include other conditions such as pyoderma and infected eczema Source: hospital Included: secondary infections of ulcers, pyoderma, folliculitis, pustulosis also included Excluded: drug or alcohol abuse, post‐surgical Randomised (evaluable): azithromycin: data not available (72) erythromycin: data not available (50) Age: 16 to 82 Bacteria isolated: no separated data for cellulitis |
|
| Interventions | Setting: hospital A: oral azithromycin (a macrolide) ‐ day 1 = 250 mg 2 times a day, then from day 2 to day 5 = 250 mg 2 times a day. B: oral erythromycin (a macrolide) ‐ 500 mg 4 times a day for 7 days. |
|
| Outcomes | 1. Proportion cured (48 hours after last treatment and azithromycin 8 to 16 days; erythromycin 11 to 16 days) 2. Proportion with eradication of bacteria (unit of analysis isolate, not the person) 3. Proportion with adverse events (no separated data for cellulitis) 4. Proportion with severe side‐effects (no separated data for cellulitis) |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...1:1 ratio, randomisation list." Comment: Review authors assumed that there was a suitable method of sequence generation on the basis of the quote above. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Method of concealment of allocation until after recruitment was not explained. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: Stated as an open study. |
| Incomplete outcome data (attrition bias) Cure at end of treatment | High risk | Comment: For the subpopulation with cellulitis the numbers completing were given, but not the numbers enrolled. |
Daniel 1991 part 2.
| Study characteristics | ||
| Methods | Randomised controlled trial Multicentre |
|
| Participants | Mixed population of skin and skin structure infections, cellulitis may include other conditions, such as pyoderma and infected eczema Source: hospital Included: secondary infections of ulcers, pyoderma, folliculitis, pustulosis also included Excluded: drug or alcohol abuse, post‐surgical Randomised (evaluable): azithromycin: data not available (41) cloxacillin: data not available (21) Age: 16 to 82 Bacteria isolated: no separated data for cellulitis |
|
| Interventions | Setting: Hospital A: azithromycin oral (a macrolide) ‐ 500 mg x 2 day 1, 250 mg x 1 on days 2 to 5. B: cloxacillin oral (a penicillin) ‐ 500 mg x 4/day for 7 days. |
|
| Outcomes | 1. Proportion cured (48 hours and 7 to 10 days post‐treatment) 2. Proportion with eradication of bacteria (isolate, not participant, was the unit of analysis) 3. Proportion adverse events ‐ no separated data for cellulitis 4. Proportion severe side‐effects |
|
| Notes | States that 41 (azithromycin) and 21 (cloxacillin) people were treated in each group but does not state explicitly the number randomised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...2:1 ratio...pre supplied randomisation list." Comment: Review authors assumed that there was a suitable method of sequence generation on the basis of the quote above. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Method of concealment of allocation before recruitment was not explained. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: Presumed open as in part 1 of the study. |
| Incomplete outcome data (attrition bias) Cure at end of treatment | High risk | Comment: Withdrawals were not described. No intention‐to‐treat analysis. |
DiMattia 1981.
| Study characteristics | ||
| Methods | Randomised controlled trial Multicentre (4 sites) |
|
| Participants | Mixture of dermatological infections. Cellulitis was not defined Source: outpatients Included: judged to be due to Staphylococcus or Streptococcus Excluded: not clear Randomised (evaluable): data not available (7) data not available (12) Age: 1 month to 80 years Bacteria isolated: mainly Streptococcus pyogenes and Staphylococcus aureus |
|
| Interventions | Setting: outpatients A: oral cephalexin adults ‐ 500 mg 2 times a day, children 20 to 30 mg/kg/day. B: oral cephalexin adults ‐ 250 mg 4 times a day, children 20 to 30 mg/kg/day. Duration for both 4 to 10 days. |
|
| Outcomes | 1. Proportion cured at end of treatment (time unclear) 2. Proportion eradicated of bacteria 3. Adverse events ‐ no separated data for cellulitis |
|
| Notes | Does not state how many were randomised or when the follow‐up was carried out. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...computer derived randomisation schedule." Comment: Review authors assumed adequate. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No description was given. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: Review authors assumed that this was an open study. |
| Incomplete outcome data (attrition bias) Cure at end of treatment | High risk | Comment: Withdrawals were not described. |
Fabian 2005.
| Study characteristics | ||
| Methods | Randomised controlled trial Multicentre (92 sites) |
|
| Participants | Complicated skin and skin structure infections. Complicated cellulitis i.e. with diabetes, perineum cellulitis, with deep tissue involvement (necrosis, fluctuation needing surgery, or bacteraemia) Source: hospital secondary care Included: 13 years and over. If isolated pre‐therapy pathogen was susceptible to both study drugs, those with sterile cultures were included unless the participant had received prior antibiotic for > 24 hrs in the previous 14 days Excluded: postrandomisation exclusions for isolated organism not susceptible to the study drug. Anti‐epileptic treatment, white blood count < 1000 cells/ml, osteomyelitis, severe peripheral vascular disease, cystic fibrosis, AIDS, necrotising fasciitis, pressure ulcer, infected prostheses. In the main study population over 30% were excluded after randomisation Randomised (evaluable): 88 (39) 89 (42) However this loss to follow up includes postrandomisation exclusions Age: 13 to 95 Bacteria isolated: no separated data |
|
| Interventions |
Setting: inpatients A: iv meropenen (a carbapenem) ‐ 500 mg 3 times a day. B: iv imipenem‐silastatin (a carbapenem) ‐ 500 mg 3 times a day. Duration for at least 3 days and up to 14 days. |
|
| Outcomes | 1. Proportion cured at end of follow‐up (7 to 14 days) 2. Adverse events ‐ no separated data for cellulitis |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomised...blocks of four." Comment: Although the block size is small, the study is blinded so the researchers would be unaware of the allocation. |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomised...blocks of four." "The study was performed in accordance with the International Conference on Harmonization/Good Clinical Practice." (ICH) Comment: The method was not explicitly stated but was assumed to be appropriate as the protocol was compliant with ICH. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Study authors state that the study was double‐blind, but do not describe in any detail. |
| Incomplete outcome data (attrition bias) Cure at end of treatment | Low risk | Comment: Detailed description of withdrawals were given for the whole population, but only the numbers in each arm were provided for the cellulitis subgroup. |
Giordano 2005.
| Study characteristics | ||
| Methods | Randomised controlled trial International multicentre (59 sites) |
|
| Participants | Complicated skin and skin structure infections with cellulitis subset. Complicated defined as involving deep soft tissue or requiring surgical interventions or the presence of complicating factors, such as skin lesions, or factors affecting drug delivery, immune response, or healing Source: hospital secondary care Included: 18 years and over with complicated skin or skin structure infection with at least 3 of; drainage or discharge, erythema, fluctuance, heat or localised warmth, pain or tenderness, swelling or induration, fever, or leukocytosis Excluded: associated with prosthetic materials or untreated osteomyelitis, necrotizing fasciitis, previous antibiotic treatment unless classified as treatment failure Randomised (evaluable): data not available (43) data not available (43) Age: 18 to 90 years. Bacteria isolated: Staphylococcus aureus (54% Moxifloxacin, 50% control) followed by non‐group A beta‐haemolytic streptococci and enterococcus faecalis |
|
| Interventions |
Setting: hospital secondary care A: iv moxifloxacin (3rd generation quinolone) 400 mg once a day for 3 days followed by oral moxifloxacin 7 to 14 days. Total duration 7 to 14 days. B: iv piperacillin‐tazobactam (beta‐lactam plus beta‐lactamase inhibitor) 3.0/0.375 g, 4 times a day for 3 days followed by oral amoxicillin‐clavulanate suspension 800 mg, 2 times a day. Total duration of treatment: 7 to 14 days. |
|
| Outcomes | 1. Clinical cure 2. Adverse events (not separated for cellulitis) |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomised in a 1:1 ratio." Comment: Review authors assessed this as unclear because there was no mention of the generation of a sequence, list, or schedule. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No description provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "...double‐blind double dummy." Comment: It was stated that the investigator making clinical decisions was blind to the allocated treatment. |
| Incomplete outcome data (attrition bias) Cure at end of treatment | High risk | Comment: A detailed description was given for both arms of the whole population, but no information was provided for the cellulitis subset. |
Grayson 2002.
| Study characteristics | ||
| Methods | Randomised controlled trial Multicentre (4 sites) |
|
| Participants | Moderate to severe cellulitis. Erythema associated with signs of infection Source: healthcare centres Included: required admission to hospital and iv antibiotics Excluded: hospital‐acquired or social circumstances not suitable for hospital in the home Randomised (evaluable): ceftriaxone‐placebo: 67, 6 postrandom excluded (57) cefazolin‐probenecid: 67, 6 postrandom excluded (59) Age: 17 to 89 Bacteria isolated: mainly Staphylococcus aureus Number of participants randomised: 134 |
|
| Interventions |
Setting: hospital at home A: iv ceftriaxone (a 3rd generation cephalosporin) ‐ 1 g plus oral placebo once a day. B: iv cefazolin (a 1st generation cephalosporin) ‐ 2 g plus oral probenecid (1 g) once a day. Median duration about 7 days iv, followed by 7 to 10 days of oral cephalexin or clindamycin. |
|
| Outcomes | 1. Proportion cured at 14 days 2. Propotion with recurrence at 1 month 3. Proportion where bacteria were eradicated at 14 days and 1 month, but few isolates made, so the data are not shown 4. Adverse events |
|
| Notes | The postrandom exclusions were for the exclusion criteria of 'concomitant antibiotic therpapy'. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...1:1 ratio, blocks of 4... centrally allocated." Comment: Method not stated but assumed to be adequate as carried out centrally by the Pharmacy Department of the Monash Medical Centre. |
| Allocation concealment (selection bias) | Low risk | Quote: "...all details regarding randomisation, the treatment regimens allocated, and the packaging of probenecid and placebo tablets were managed by the Pharmacy Department at Monash Medical Centre." "..each enrolled participant was automatically allocated the next study number..." Comment: Achieved through central organisation and identical sequentially numbered packages. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "...double‐blind." "Probenecid and placebo tablets were packaged in identical non distinctive containers...labelled with study number...placed with premixed cefazolin or ceftriaxone..." Comment: Central and local pharmacy department prepared the drugs, and they were placed in identical packages labelled with the study number, however in emergency departments if the first treatment was given at night a staff nurse not involved with the study mixed the iv drugs. |
| Incomplete outcome data (attrition bias) Cure at end of treatment | Low risk | Comment: Withdrawals were described and an intention‐to‐treat analysis was provided. |
Hepburn 2004.
| Study characteristics | ||
| Methods | Single‐centre randomised controlled trial | |
| Participants | Uncomplicated cellulitis of the face, trunk, or limbs Source: primary care or emergency department Included: within 24 hours of starting antibiotic therapy Excluded: deep tissue infection, not complying with treatment, or no improvement after 5 days of oral therapy Randomised (evaluable): 5 days: 44 (44) 10 days: 43 (43) Age: mean 56 and 49 respectively Bacteria isolated: not tested Number of participants randomised: 87 |
|
| Interventions | Setting: outpatients and inpatients A: oral levofloxacin (3rd generation quinolone) ‐ 500 mg once a day for 5 days. B: oral levofloxacin (3rd generation quinolone) ‐ 500 mg once a day for 10 days. |
|
| Outcomes | 1. Proportion cured on day 14 2. Recurrence at day 28 3. Adverse effects 4 to 6. Mean swelling, pain and physician scores on day 10 presented in graphs (no number tables). Scales were 0 to 10, 0 to 10, and 0 to 21 respectively. |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...random schedule...blocks of ten." Comment: Assumed that study authors used an adequate method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Comment: As the sequence was not predictable and allocation not revealed until the study complete, concealment at consent was implied. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "...double‐blind, identical capsules, investigators and participant blind to allocation until study complete." |
| Incomplete outcome data (attrition bias) Cure at end of treatment | Low risk | Comment: There were no withdrawals. |
Iannini 1985.
| Study characteristics | ||
| Methods | Randomised controlled trial Multicentre (5 sites) |
|
| Participants | Skin and soft tissue infections. Cellulitis was not defined Source: hospital Included: treatment had to last for 3 days Excluded: pre‐treatment pathogen not sensitive to assigned drug Randomised (evaluable): cefonicid: data not available (35) cefazolin: data not available (10) Age: 18 to 86 Bacteria isolated: mainly Staphylococcus aureus and streptococci Number of participants randomised: 45 |
|
| Interventions | Setting: hospital A: iv or IM cefonicid ‐ 1 or 2 g 1 times a day for at least 3 days, mean 7.7 days. B: iv or IM cefazolin ‐ 0.5 to 1.5 g 3 times a day for at least 3 days, mean 10.6 days. |
|
| Outcomes | 1. Proportion with a satisfactory response, follow‐up duration not stated 2. Adverse events (not separated for cellulitis) |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...were randomised ...according to a 2:1 assignment ratio." Comment: There was no statement about a randomised schedule or list being produced in an appropriate manner. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no mention of how the allocation was concealed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: Review authors assumed that this was an open study. |
| Incomplete outcome data (attrition bias) Cure at end of treatment | High risk | Comment: Withdrawals were not described for the cellulitis subset. In the whole population of people with skin and skin structure infections there were 33% and 51% withdrawals and exclusions respectively. |
Johnson 2007.
| Study characteristics | ||
| Methods | Randomised controlled trial (single‐centre) | |
| Participants | Cellulitis of lower limb. No definition of cellulitis was provided Source: secondary care Included: prescribed oral or iv antibiotics and bed rest Excluded: non‐concordance with medical treatment Age: mean (range) treatment group 67 (38 to 88), control group 66 (43 to 86) Bacterial isolation not carried out Number randomised: 18 in the treatment group 18 in the control group |
|
| Interventions | Setting: hospital A: cycloidal vibration 30 minutes 3 times a day, plus antibiotics and bed rest. B: antibiotics and bed rest. |
|
| Outcomes | 1. Time to recovery 2. Percentage reduction in erythema at day 7 3. Reduction in pain score at day 7 4. Cost analysis |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No mention of a schedule or a number generation method. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Although researchers state that a 'sealed envelope' was used, they did not state that these envelopes were numbered sequentially. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: Open study as it was not possible to achieve blinding with this type of physical intervention. |
| Incomplete outcome data (attrition bias) Cure at end of treatment | Low risk | Comments: There were no withdrawals or dropouts from the study. |
Kiani 1991.
| Study characteristics | ||
| Methods | Randomised controlled trial Multicentre (22 sites) |
|
| Participants | Acute skin and skin structure infections with cellulitis and erysipelas subpopulations Source: outpatients and inpatients Included: bacteria susceptible to both drugs (postrandom excluded) Excluded: infected ulcer, drug abuse, steroids Randomised (evaluable): azithromycin: 48 (24) cephalexin: 47 (23) Age: 16 to 92 Bacteria isolated: mainly Streptococcus pyogenes,Staphylococcus aureus, and Streptococcusagalactiae Number of participants randomised: 95 |
|
| Interventions |
Setting: unclear A: oral azithromycin ‐ 1 x 500 mg on day 1, 250 mg once a day, day 2 to 5. B: oral cephalexin ‐ 500 mg 2 times a day for 10 days. |
|
| Outcomes | 1. Proportion cured day 11 2. Bacterial eradication (no subgoup for cellulitis) 3. Adverse events (no subgroup for cellulitis) |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomly assigned." Comment: No mention of list or method of generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No description provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "...double blind, double dummy." |
| Incomplete outcome data (attrition bias) Cure at end of treatment | High risk | Comment: Withdrawals were described for the whole population, but not for the cellulitis subset. |
Leman 2005.
| Study characteristics | ||
| Methods | Randomised controlled trial (single‐centre) | |
| Participants | Acute skin and skin structure infections. Cellulitis and erysipelas were not defined Source: outpatients and inpatients Included: lower limb cellulitis with diameter of > 100 mm Excluded: co‐existing illness, diabetes, DVT, abscess Randomised (evaluable): benzyl penicillin: data not available (41) placebo: data not available (40) Age: mean 44.9 year and 46.4 respectively Bacteria isolated: Only 16 with a wound suitable for swabs. Mainly Staphylococcus aureus, Streptococcus, one with Pseudomonas sp Number of participants randomised: 81 |
|
| Interventions | Setting: emergency department A: flucloxacillin 1 g iv and benzyl penicillin 1.2 g iv 4 times a day, and after discharge oral penicillin V 500 mg 4 times a day for 5 days. B: flucloxacillin 1g iv and placebo (10 ml of clear saline) iv 4 times a day. Both groups had oral flucloxacillin 500 mg for 4 times a day after discharge. Iv duration variable, determined using pre‐set criteria. |
|
| Outcomes | 1. Dose of iv drug 2. Change in temperature 3. Size of inflamed area 4. Subjective assessment of outcome (VAS) and an overall subjective assessment of improvement carried out by the participant All outcomes were assessed on day 1 and day 2. There was no follow‐up after they left hospital (discharged after 1 to 2 days) No data on adverse events |
|
| Notes | 99 were randomised, but 81 assessable (41 and 40 per arm respectively). However, further incomplete data collection for each time point for certain outcomes: VAS for Pain/discomfort and subjective feelings of improvement. Failure of iv treatment assessed at day 2, with no further follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...computer generated sequence." Randomisation: 'Computer generated sequence'. |
| Allocation concealment (selection bias) | Low risk | Comment: The allocation was concealed in 'sealed in opaque envelopes, opened only after the participant was considered eligible for the study', although not a gold standard method if carried out correctly this can be considered an acceptable method (Altman 2001). |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "..double‐blind placebo controlled..." Comment: The placebo was 10 ml of normal saline. |
| Incomplete outcome data (attrition bias) Cure at end of treatment | High risk | The total number of withdrawals and postrandom exclusions were described, however the authors did not state from which study arm. |
Rao 1985.
| Study characteristics | ||
| Methods | 1‐centre randomised controlled trial | |
| Participants | Skin and skin structure infections, cellulitis not defined Source: hospital Included: hospitalised Excluded: not requiring iv antibiotic Randomised (evaluable): ticarcillin and clavulanic acid: 9 (9) moxalactam: 10 (9) Age: 18 to 77 Bacteria isolated: not split for cellulitis but most isolates were Streptococci and Staphylococcus aureus Number of participants randomised: 19 |
|
| Interventions |
Setting: hospital A: iv ticarcillin 3 g and clavulanic acid 0.1g, 4 times a day for 5 to 25 days. B: moxalactam iv ‐ 2 g 3 times a day for 3 to 20 days. Cessation reasons not given. |
|
| Outcomes | 1. Clinical cure at end of follow‐up (duration not stated) 2. Eradication of post‐treatment cultures 2. Adverse events: no separated data for cellulitis |
|
| Notes | The number of participants with cellulitis was very small. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "'Randomisation list' supplied by drug company" Comment: Assumed poor reporting and that the sequence was not predictable. |
| Allocation concealment (selection bias) | Low risk | Quote: "'Randomisation list' supplied by drug company... allocated post consent" Comment: The allocation was revealed after the participants agreed to participate in the study. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: Study authors indicate that the study was open, not blind. |
| Incomplete outcome data (attrition bias) Cure at end of treatment | Low risk | Yes, only 1 participant withdrew from the study. |
Sachs 1990.
| Study characteristics | ||
| Methods | 2‐centre randomised controlled trial | |
| Participants | Skin and skin structure infections. Cellulitis defined as acute inflammation of superficial skin with pain, erythema and induration, with absence of purulent exudate Source: hospital Included: see footnotes Excluded: see footnotes Randomised (evaluable): data not available (9) 13 (12) Age: not shown for cellulitis subgroup Bacteria isolated: not shown for cellulitis subset |
|
| Interventions | Setting: Hospital A: ampicillin iv 1.0 g/ sulbactam 500 mg, 4 times a day. B: cefazolin (a 1st generation cephalosporin) ‐ 500 mg iv 4 times a day, variable duration (decision unclear). |
|
| Outcomes | 1. Proportion cured at end of treatment 2. Proportion with eradication of bacteria 3. Proportion with severe side‐effects (not separated for cellulitis subset) |
|
| Notes | Bacteriological eradication included those without pre‐ and post‐therapy culture having 16 doses of study drug. Only 2 participants with cellulitis fulfilled these criteria. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "..drug assignments were made using a table of random numbers in balanced blocks of 8." |
| Allocation concealment (selection bias) | Unclear risk | Comment: The study authors did not explicitly state that the sequence was not known by the researchers before consent. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "An open study." |
| Incomplete outcome data (attrition bias) Cure at end of treatment | High risk | Comment: Withdrawals were described for the whole population, but not for the cellulitis subset. |
Schwartz 1996.
| Study characteristics | ||
| Methods | Randomised controlled trial Multicentre Randomisation: 'randomised' no details, but appears to be 2:1 ratio Blinding: open Withdrawals: not described clearly and no details were given for cellulitis subgroups |
|
| Participants | Skin and skin structure infections. Cellulitis was not defined Source: hospital Included: pathogen thought to be susceptible to study drug Excluded: if there were no susceptible bacteria isolated or if participant did not respond to treatment Randomised (evaluable): data not available (51) data not available (23) Age: no separated data Bacteria isolated: no separated data |
|
| Interventions |
Setting: hospital A: iv cefepime (a 4th generation cephalosporin) ‐ 1 g 2 times a day, 3 to 18 days. B: iv ceftazidime (a 3rd generation cephalosporin) ‐ 1 g 3 times a day, 4 to 16 days. Both treatments were delayed for 48 hours, awaiting culture results. |
|
| Outcomes | 1. Proportion cured: combination of 'at end of treatment ' (3 to 18 days) and 'at follow‐up' (time unspecified) 2. Microbiological eradication (unit of analysis was pathogen, not participants) There were no severe adverse events related to therapy |
|
| Notes | Strict postrandomisation exclusion, including non‐response to treatment, lead to a 60% withdrawal rate for the whole population. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: 'Randomised 2:1' Comments: No details given. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "Open label" |
| Incomplete outcome data (attrition bias) Cure at end of treatment | High risk | Comment: Not described clearly for the whole population and no details were given for the cellulitis subgroups. |
Tack 1998.
| Study characteristics | ||
| Methods | Randomised controlled trial Multicentre (34 centres) |
|
| Participants | Skin and skin structure infections. Cellulitis was not defined Source: primary care Included: 13 years or older Excluded: see note Randomised (evaluable): 35 (17) 43 (17) Age: no details given for the cellulitis subset Bacteria isolated: not shown for the cellulitis subset Number of participants randomised: 78 |
|
| Interventions |
Setting: Primary care A: oral cefdinir (a 3rd generation cephalosporin) ‐ 300 mg capsules 2 times a day B: oral cephalexin (a 1st generation cephalosporin) ‐ 500 mg 4 times a day. Mean duration 10 days for both groups. |
|
| Outcomes | 1. Proportion cured (at follow‐up 7 to 16 days post‐therapy) 2. Microbiological eradication (unit of analysis was the pathogen not the participant) 3. Adverse events (no separated data for cellulitis) |
|
| Notes | There were a large number with postrandom exclusions if susceptible pathogen was not isolated from admission specimen, however, these are not separated from withdrawals for non compliance or no follow‐up visit. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomised ratio 1:1" Comment: No details were given. |
| Allocation concealment (selection bias) | Unclear risk | Comment: It does not state how concealment of allocation was achieved. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: 'Double‐blind' ('Matched placebo capsules were dispensed appropriately to maintain study masking'). |
| Incomplete outcome data (attrition bias) Cure at end of treatment | High risk | Comment: Withdrawals were described for the whole population, but not for the cellulitis subset. |
Tarshis 2001.
| Study characteristics | ||
| Methods | Randomised controlled trial Multicentre |
|
| Participants | Uncomplicated skin and soft tissue infections. Cellulitis was not defined Source: unclear Included: adults Excluded: immediate need for surgical intervention, immune disease, postrandom exclusion if pre‐treatment culture was not carried out. Randomised (evaluable): 53 (40) 50 (42) Age: mean 39 range (18 to 85 )and 40 (18 to 90) Bacteria isolated: not shown for cellulitis subset Number of participants randomised for all infection types: 407 |
|
| Interventions |
Setting: unclear A: oral gatifloxacin (4th generation quinolone) ‐ 400 mg 1 x day 7 to 10 days. B: oral levofloxacin (3rd generation quinolone) ‐ 500 mg 1 x day 7 to 10 days. |
|
| Outcomes | 1. Proportion cured or improved (at follow‐up 7 to 14 days after the end of treatment) | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised by using a dynamic balancing algorithm designed to minimize the imbalance between inequalities within the treatment arms for each study site, for each diagnosis, and for the overall study." |
| Allocation concealment (selection bias) | Low risk | Comment: Not explicitly stated but as the study is double‐blind it is assumed researchers and participants did not know the allocation at recruitment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Double‐blind, double dummy". |
| Incomplete outcome data (attrition bias) Cure at end of treatment | High risk | The reasons participants were defined as unevaluable were described for the whole population, but not for the cellulitis subset. |
Vinen 1996.
| Study characteristics | ||
| Methods | Randomised controlled trial (Single‐centre) | |
| Participants | Cellulitis was defined as fever, erythema, tenderness, and lymphadenitis. Source: emergency department Included: requiring iv antibiotic therapy. Could have received prior oral antibiotics Excluded: see footnotes Randomised(evaluable): in total 58 randomised, 19% drop‐out; data not available (24) data not available (23) Age: mean 61 years Bacteria isolated: mainly Staphylococcus aureus, beta‐haemolytic Streptococcus, and Streptococcus pyogenes. |
|
| Interventions |
Setting: hospital or home A: Setting: hospital or home; iv ceftriaxone (a 3rd generation cephalosporin) ‐ 1 g daily. Mean duration 6.83 days. B: Setting: hospital; iv flucloxacillin (a penicillin) ‐ 1 g 4 times a day. Duration not stated. |
|
| Outcomes | 1. Proportion cured (48 to 72 hours post‐therapy) 2. Microbiological eradication (unit of analysis was the pathogen, not the person) 3. Adverse events |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomised". Comment: No details. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: Not stated and assumed open. |
| Incomplete outcome data (attrition bias) Cure at end of treatment | High risk | It was stated that '19% were not followed up', but the reasons, or which arm of the study they were in, were not given. |
Weigelt 2005.
| Study characteristics | ||
| Methods | Randomised controlled trial Multicentre |
|
| Participants | Complicated Skin and skin structure infections, deeper soft tissue involvement (or large surface area), surgical intervention required or comorbid condition. Cellulitis not defined Source: secondary care Included: suspected or proven MRSA, erythema or induration, fluctuation, heat, pain and drainage, plus one of fever or hypothermia, hypotension, raised white blood count Excluded: gram‐negative infections, underlying infections of bone, heart, brain or joints, implanted devices, necrotising fasciitis or gas gangrene, on another trial, uncomplicated or superficial skin infections Randomised (evaluable): linezolid: 282 (224) vancomycin: 266 (201) Age: mean 52 years for both groups Bacteria isolated: mainly Staphylococcus aureus, almost 45% being MRSA. |
|
| Interventions |
Setting: hospital A: iv or oral linezolid (an oxazolidinone) ‐ 600 mg 2 times a day. Duration: intended 7 to 14 days with a limit of 21 days. Mean for the whole population 11.8 +/‐ 4.9 days. B: iv vancomycin (a glycopeptide) ‐ 1 g 2 times a day. Duration: intended 7 to 14 days with a limit of 21 days. Mean duration for whole population 10.9 +/‐ 5.3 days. |
|
| Outcomes | 1. Proportion cured or improved at end of treatment and 7‐day follow‐up 2. Microbiological cure 3. Adverse events (not separated for the cellulitis population) |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomised." Comment: No details. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "Open label." |
| Incomplete outcome data (attrition bias) Cure at end of treatment | Low risk | Withdrawals were described for whole population, but numbers only given for the cellulitis subgroup. |
Zeglaoui 2004.
| Study characteristics | ||
| Methods | 1‐centre randomised controlled trial | |
| Participants | Cellulitis was defined as a cutaneous lesion with erythema, warmth, swelling, and temperature > 37oC Source: dermatology department Included: erysipelas of the leg, 15 years or older, symptoms for 5 days or less Excluded: taken antibiotics in previous 7 days, signs of necrotising fasciitis or abscess at recruitment Randomised (evaluable): 112 randomised, with none dropping out; 55 (55) 57 (57) Age: mean 41 and 44 years respectively Bacteria isolated: no data presented |
|
| Interventions |
Setting: hospital A: iv benzyl penicillin ‐ 4 MU 6 time a day B: IM penicillin (benzyl plus procaine) ‐ 2 MU 2 times a day Both arms treated for 10 days. |
|
| Outcomes | 1. Treatment failure 2. Recovery delay 3. Local complications 4. Complications related to treatment |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: 'Random number tables.' |
| Allocation concealment (selection bias) | Low risk | Comment: Allocation was revealed. '..after baseline data collection', however the authors do not state how this was achieved. |
| Blinding (performance bias and detection bias) All outcomes | High risk | ‐ |
| Incomplete outcome data (attrition bias) Cure at end of treatment | Low risk | Comment: The study authors recruited consecutive people admitted with a diagnosis of cellulitis and none withdrew from the study, with no missing data or withdrawals. |
Participants: The majority of studies included people with any skin or skin structure infection likely to involve bacteria. In these cases the numbers randomised and evaluable refers to the cellulitis and erysipelas subpopulation. Exclusion Criteria usually included: Pregnant women, current antibiotic treatment for another reason, allergy to study drugs, severe renal or liver disease; low white Blood Cell Count. Outcomes: Days refer to 'days since initiation of treatment' unless otherwise stated. VA = Visual Analogue Scale MRSA = Methicillin Resistant Staphylococcus Aureus MU = Million unit
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agache 1987 a | RCT of mixed skin and soft tissue infection with no separated outcome data for those with cellulitis. A total of 6 participants had cellulitis. |
| Agache 1987 b | RCT of mixed skin and soft tissue infection with no separated outcome data for those with cellulitis. 3 participants with cellulitis per group. |
| Amaya‐Tapia 1992 | RCT of mixed skin and soft tissue infection with no separated outcome data for those with cellulitis. Unclear how many participants had cellulitis. |
| Amaya‐Tapia 1993 | RCT of mixed skin and soft tissue infection, with no separated outcome data for those with cellulitis. A total of 12 participants with cellulitis. |
| Arata 1989 | RCT of mixed skin and soft tissue infection with no separated outcome data for those with cellulitis. Unclear how many participants had cellulitis. |
| Arata 1994 a | RCT of mixed skin and skin structure infection with no separated outcome data for those with cellulitis. |
| Arata 1994 b | RCT of mixed skin and skin structure infection with no separated outcome data for those with cellulitis. |
| Arata 1995 | RCT of mixed skin and skin structure infections, with no separated outcome data for those with cellulitis. A total of 27 participants with cellulitis and erysipelas. |
| Azimi 1999 | RCT of mixed skin and skin structure infection with no separated outcome data for those with cellulitis. A total of 55 (37 vs 18) with cellulitis. |
| Ballantyne 1980 | RCT of mixed soft tissue infection with no separated outcome data for those with cellulitis. Non with cellulitis. |
| Ballantyne 1985 | RCT of mixed skin and soft tissue infection with no separated outcome data for those with cellulitis. Unclear how many participants had cellulitis. |
| Bechard 1984 | RCT of mixed bacterial infections, with only 11 people having skin or skin structure infections.No separated outcome data for those with cellulitis. Unclear how many participants had cellulitis. |
| Bergkvist 1998 | RCT of erysipelas, follow‐up of included study, with relapse as the only outcome variable (so excluded). |
| Blaszczyk‐Kostanecka | RCT of mixed skin and soft tissue infection, with no separated outcome data for those with cellulitis. |
| Bradsher 1984 | RCT of mixed skin and soft tissue infection with no separated outcome data for those with cellulitis. |
| Breedt 2005 | RCT of mixed skin and soft tissue infection with no separated outcome data for those with cellulitis. |
| Brown 1996 | RCT of mixed skin and soft tissue infection, with no separated outcome data for those with cellulitis. |
| Browning 1981 | RCT of mixed skin and soft tissue infection, with no separated outcome data for those with cellulitis. Unclear how many participants had cellulitis. |
| Carbon 1992 | RCT of mixed bacterial infections, with 60 participants with skin and soft tissue infection, but no separated outcome data for those with cellulitis. Unclear how many participants had cellulitis. |
| Carbon 1995 | RCT of mixed bacterial infections with 203 participants having skin and soft tissue infection, but no separated outcome data for those with cellulitis. Unclear how many participants had cellulitis. |
| Carr 1994 | RCT of mixed skin and soft tissue infections, with no separated outcome data for those with cellulitis. Unclear how many participants had cellulitis. |
| Chiodini 1985 | RCT of treatments for skin and soft tissue infection on ability to mobilise glycogen. No relevant outcomes. |
| Chirurgi 1994 | RCT of mixed skin and soft tissue infection caused by gram‐positive bacteria, but with no separated outcome data for those with cellulitis. 32 participants (14 vs 18) with cellulitis. |
| Colardyn 1996 | RCT of mixed bacterial infections. No separated outcome data for those with cellulitis. Only 1 participant listed with cellulitis. |
| Colin 1988 | RCT of skin and soft tissue infections. No separated data for those with cellulitis. |
| Daly 1990 | RCT of skin and skin structure infections. No separated outcome data for those with cellulitis. |
| Derriennic 1993 | RCT of skin and soft tissue infections. No participants with cellulitis recruited. |
| Echols 1994 | RCT of soft tissue infections. 11 cellulitis participants per arm, but no separated data for those with cellulitis. |
| Edelstein 1991 | RCT with unclear randomisation. Over 24 cellulitis participants per arm, but no separated outcome data. |
| Eron 1983 | RCT of bone and soft tissue infections. Only 4 with cellulitis and no separated outcome data. |
| Fass 1989 | RCT of bacterial infections. 14 people with cellulitis, but no separated outcome data. |
| File 1991 | RCT of skin and skin structure infection. No separated outcome data for those with cellulitis. |
| Fleisher 1983 | RCT of soft tissue infections. No separated outcome data for those with cellulitis. |
| Frank 1989 | RCT of skin and soft tissue infections. No separated outcome data for those with cellulitis. |
| Fujita 1985 | RCT of skin and soft tissue infections. Only 4 participants with cellulitis and no separated outcome data for those with cellulitis. |
| Geckler 1988 | RCT of skin and soft tissue infections. No separated outcome data for those with cellulitis. |
| Gentry 1989 a | RCT of skin and soft tissue infections. No separated outcome data for those with cellulitis. |
| Gentry 1989 b | RCT of mixed skin and soft tissue infection with no separated outcome data for those with cellulitis. |
| Gentry 1996 | RCT of skin and skin structure infections. No separated outcome data for those with cellulitis. |
| Gesser 2004 a | RCT of skin and skin structure infections. No separated outcome data for those with cellulitis. |
| Gesser 2004 b | RCT of skin and skin structure infections. 5 and 3 cellulitis participants per group and no separated outcome data for those with cellulitis. |
| Gordin 1985 | RCT of mixed skin and soft tissue infection with no separated outcome data for those with cellulitis. |
| Gould 1988 | RCT of serious soft tissue infections, with no separated outcome data for those with cellulitis. |
| Graham 2002 | RCT of skin and skin structure infections. No separated outcome data for those with cellulitis. |
| Hanfling 1992 | RCT of skin and skin structure infections. Only 1 and 5 cellulitis participants per group and no separated outcome data for those with cellulitis. |
| Henry 1985 | RCT of participants with gram‐negative infections. No separated outcome data for those with cellulitis. |
| Hubsher 1976 | RCT of skin and soft tissue infections. No separated outcome data for those with cellulitis. |
| Jorup‐Rönström 1984 | Quasi‐experimental hospital based study of cellulitis in adults comparing oral phenoxymethyl penicillin 1.6 g 3 time a day, for 10 days against iv benzyl penicillin 3 g, 3 times a day or more, depending on weight. 'Randomisation' was by "...every other participant admitted stratified by gender and ward." |
| Kulhanjian 1989 | RCT of soft tissue and skeletal infections in children. No separated outcome data for children with cellulitis. |
| Lobo 1995 | RCT of skin infections. Only 2 and 1 cellulitis participants per group. No separated outcome data for those with cellulitis. |
| Miller 1989 | RCT of skin and skin structure infections. Only 12 participants with cellulitis and no separated outcome data for those with cellulitis. |
| Nolen 1992 | RCT of skin and skin structure infections. No separated outcome data for those with cellulitis. |
| Parish 1984 | RCT of skin and skin structure infections. Less than 10 participants with cellulitis per arm. No separated outcome data for those with cellulitis. |
| Parish 1991 | RCT of skin and skin structure infections. No separated outcome data for those with cellulitis. |
| Parish 1993 | RCT of skin and skin structure infections. No separated outcome data for those with cellulitis. |
| Parish 1993a | RCT of mixed skin and skin structure infections with no separated outcome data for those with cellulitis. |
| Pereira 1996 | RCT of folliculitis, furunculosis and cellulitis. No separated outcome data for those with cellulitis. |
| Perez‐Ruvalcaba 1987 | RCT of skin and skin structure infections caused by bacteria. No separated outcome data for those with cellulitis. |
| Pien 1983 | RCT of skin structure infections. No separated outcome data for those with cellulitis. |
| Ramirez‐Ronda 1982 | RCT of skin infections, but no separated outcome data for those with cellulitis. |
| Ramirez‐Ronda 1987 | RCT of skin and skin structure infections. No separated outcome data for those with cellulitis. |
| Sacchidanand 2005 | RCT of skin and skin structure infections. No separated outcome data for those with cellulitis. |
| Schupbach 1992 | RCT of skin and skin structure infections. No separated outcome data for those with cellulitis. |
| Segev 1990 | RCT of soft tissue infections. Excluded as immunocompromised participants and no separated outcome data for those with cellulitis. |
| Siami 2001 | RCT of skin and soft tissue infections. There were 138 participants with cellulitis, but no separated outcome data. |
| Solomkin 1986 | RCT of skin and soft tissue infections. No separated outcome data for those with cellulitis. |
| Stevens 2000 | RCT of skin and skin structure infections. No separated outcome data for those with cellulitis. |
| Tan 1993 | RCT of mixed skin and skin structure infections with no separated outcome data for those with cellulitis. |
| Tassler 1993 | RCT of skin and soft tissue infections. No separated outcome data for those with cellulitis |
| Wasilewski 2000 | RCT of skin and soft tissue infections. There were 28 and 26 cellulitis participants per group but no separated outcome data for those with cellulitis. |
| Wible 2003 | RCT of skin and skin structure infections. No separated outcome data for those with cellulitis. |
Characteristics of studies awaiting classification [ordered by study ID]
Chaudhary 2008.
| Methods | Randomised controlled trial |
| Participants | People with a range of bacterial infections |
| Interventions | Different fixed‐dose combination of ceftriaxone‐vancomycin injection |
| Outcomes | Resolution of symptoms within 7 days |
| Notes | ‐ |
Manaktala 2009.
| Methods | Randomised controlled trial |
| Participants | Uncomplicated skin and skin structure infections |
| Interventions | Cefditoren pivoxil vs cefdinir |
| Outcomes | Clinical cure or improvement |
| Notes | ‐ |
Nichols 1999.
| Methods | Randomised controlled trial |
| Participants | Those with complicated gram‐positive skin and skin structure infections |
| Interventions | Quinupristin/dalfopristin versus cefazolin, oxacillin, or vancomycin |
| Outcomes | Cure plus improvement, bacterial eradication |
| Notes | ‐ |
Noel 2008.
| Methods | Randomised controlled trial |
| Participants | Those with complicated skin and skin‐structure infections |
| Interventions | Ceftobiprole medocaril with vancomycin plus ceftazidime |
| Outcomes | Clinical cure at day 7 to 14. |
| Notes | ‐ |
Pertel 2009.
| Methods | Randomised controlled trial |
| Participants | Those with cellulitis and erysipelas |
| Interventions | Daptomycin vs vancomycin |
| Outcomes | Time to resolution or improvement |
| Notes | ‐ |
Snodgrass 1937.
| Methods | Controlled trial |
| Participants | Erysipelas |
| Interventions | Prontosil |
| Outcomes | Cure |
| Notes | ‐ |
Snodgrass WR 1937.
| Methods | Controlled trial |
| Participants | Erysipelas |
| Interventions | Sulphanilmide |
| Outcomes | Cure |
| Notes | ‐ |
Stein 2008.
| Methods | Randomised controlled trial |
| Participants | Moderately severe skin and soft tissue infections |
| Interventions | Outpatient intravenous antibiotic therapy compared with oral linezolid |
| Outcomes | Visits to clinic, emergency department, wound care, and infusion center as well as hospitalisations |
| Notes | Small pilot study |
Characteristics of ongoing studies [ordered by study ID]
ISRCTN76443564.
| Study name | The efficacy and safety evaluation of ceftriaxone and sulbactam combination (1.5 gram) in participants with skin and soft tissue infections: An open label, parallel, randomised, prospective comparative trial |
| Methods | Randomised controlled trial |
| Participants | Diagnosis of skin and skin structure infections requiring injectable antibiotics. These include deep and extensive cellulitis; abscesses, necrotizing fasciitis, surgical site infections interventions; burns [ > 10% of total body surface area] or requiring surgical interventions or associated with significant underlying disease/s such as diabetes mellitus, peripheral vascular disease, peripheral neuropathy or venous insufficiency |
| Interventions | Ceftriaxone (1 gram) and sulbactam (0.5 gram) every 12 hours for maximum of 7 days. Control group: Ceftriaxone (1 gram) every 12 hours for maximum 7 days |
| Outcomes | Clinical cure at day 7 |
| Starting date | 15/5/2007 |
| Contact information | Dr Pawanindra Lal Maulana Azad Medical College and Hospital New Dehli India 100012 |
| Notes | ClinicalTrials.gov identifier: ISRCTN76443564 Orgnisation contacted |
NCT00295178.
| Study name | A Multicenter Randomized study comparing CUBICIN® (daptomycin for injection) with vancomycin in the treatment of cellulitis or erysipelas |
| Methods | Randomised controlled trial |
| Participants | Primary diagnosis of cellulitis/ erysipelas. Adults with cellulitis/erysipelas requiring hospitalisation and severe enough to warrant iv antibiotics, temperature > 37.5 ºC oral or > 38 ºC rectal, anticipated treatment to be limited to medical (NOT surgical) interventions |
| Interventions | Daptomycin vs vancomycin |
| Outcomes | Time to erythema margin cessation to progress; time to defervescence; time to hospital discharge: degree of improvement of pain and swelling |
| Starting date | March 2006 |
| Contact information | Bruce Friedman MD, Joseph M Still Research Foundation Inc. 4a George C. Wilson Court Augusta GA 30909 |
| Notes | ClinicalTrials.gov identifier: NCT00295178 Organisation contacted |
NCT00424190.
| Study name | Comparative Study of Ceftaroline vs. Vancomycin Plus Aztreonam in Adult Subjects With Complicated Skin Infections |
| Methods | Randomised controlled trial |
| Participants | Skin and skin structure infection (SSSI) that involves deeper soft tissue or requires significant surgical intervention, or cellulitis or abscess on lower extremity which occurs in subjects with diabetes mellitus or well‐documented peripheral vascular disease. |
| Interventions | Ceftaroline vs vancomycin plus aztreonam |
| Outcomes | Cure rate of ceftaroline treatment compared with that of vancomycin plus aztreonam |
| Starting date | 2007 |
| Contact information | Ralph Corey, MD |
| Notes | ClinicalTrials.gov identifier: NCT00424190 |
NCT00948142.
| Study name | Safety and Efficacy of CEM‐102 Compared to Linezolid in Acute Bacterial Skin Infections |
| Methods | Randomised controlled trial |
| Participants | Diagnosis of acute bacterial skin‐structure infection |
| Interventions | Linezolid vs CEM‐102 |
| Outcomes | Clinical Success rates in the clinically evaluable (CE) and Intention‐to‐treat (ITT) patient populations at the test of cure (TOC) visi |
| Starting date | 2009 |
| Contact information | Kay Clark, mailto:kclark%40cempra.com?subject=NCT00948142, CE06‐300, Safety and Efficacy of CEM‐102 Compared to Linezolid in Acute Bacterial Skin Infections |
| Notes | ClinicalTrials.gov identifier: NCT00948142 |
Differences between protocol and review
Background
In the section 'Impact and complications of cellulitis' the following paragraph has been added as a response to recent research on the topic: 'However, in a population‐based cohort study in which nearly 80% of cases were treated in the community, only 11% had a recurrence within 1 year (Ellis Simonsen 2006). Factors associated with recurrence have been examined using case‐control (Pavlotsky 2004) and cohort (Jorup‐Rönström 1987) designs and identified factors included venous insufficiency (Jorup‐Rönström 1987; Pavlotsky 2004;) and obesity, lymphoedema, smoking, tinea pedis, and local injury (Pavlotsky 2004)'.
To reflect the guidelines published since we originally wrote the protocol, in the section 'Guidelines' we deleted the text: 'As far as these authors are aware there is only one published guideline for cellulitis or erysipelas that uses a systematic approach. This guideline was produced by the Société Française de Dermatologie (Soc. Fr. Derm. 2001)'. We then added: 'There are a few published guidelines for cellulitis (British Lymphology Society 2007; CREST 2005; Eron 2003; Societe Francaise de Dermatologie 2001; Stevens 2005). Due to the paucity of relevant research, recommendations from these guidelines are mostly based on evidence extrapolated from studies of other infections or based on expert opinion.'
Methods
We amended the 'Types of outcome measures section'. We had initially stated:
'Symptoms rated by participant or medical practitioner, e.g. duration and intensity of fever, pain, redness of the affected area, swelling of the skin surface and subcutaneous tissue, blister formation.'
Studies seldom listed duration of symptoms, a more widely used outcome was proportion symptom‐free, or with reduced symptoms. We therefore added the following text to examples 'or proportion symptom‐free ('cure'), at a time specified by the study authors'.
In the search section we have deleted:
'(3) UNPUBLISHED LITERATURE Authors of primary studies and companies involved with the development of drugs used for treating cellulitis will be contacted in an attempt to locate data of unpublished trials data and the relevant data extracted.
(4) CONFERENCE PROCEEDINGS Major dermatological conference proceedings and poster abstracts over the last 5 years will be handsearched for further RCTs.'
The number of organisations involved in antibiotic production is very wide, and any of which may be conducting trials on cellulitis. We therefore amended our search strategy to include a more in depth look at registers of ongoing trials.
We originally stated that we would display the results as:
'odds ratio (OR) with 95% confidence intervals (CI) for dichotomous outcomes, and weighted mean difference (WMD) with 95% CI with for continuous outcomes.
However, as the outcome was relatively common the results are expressed as risk ratio in accordance with guidance given by the Cochrane Skin Group editorial base and the Cochrane Handbook for Systematic Reviews of Interventions (See Handbook section 9.2.2.3).
We amended the text from our original protocol with regard to risk of bias to follow current guidelines in the Cochrane Handbook for Systematic Reviews of Interventions.
Contributions of authors
Draft the protocol ‐ PF, SK Search for trials ‐ PF, SK Obtain copies of trials ‐ PF Select which trials to include ‐ PF, SK, and RB (arbiter) Assess the quality of the trials ‐ SK, PF, and BH (arbiter) Extract data from trials ‐ PF, SK Enter data into RevMan ‐ BH, SK Carry out the analysis ‐ BH, SK Interpret the analysis ‐ PF, SK, and BH Draft the final review ‐ PF, SK, BH, and RB Update the review ‐ PF, SK
Sources of support
Internal sources
University of Portsmouth, UK
Portsmouth Hospitals NHS Trust, UK
External sources
The Portsmouth NHS R & D Consortium, UK
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Bergkvist 1997 {published data only}
- Bergkvist PI, Sjobeck K. Antibiotic and prednisolone therapy of erysipelas: A randomized, double blind, placebo-controlled study. Scandinavian Journal of Infectious Diseases 1997;29(4):377-82. [DOI] [PubMed] [Google Scholar]
Bernard 1992 {published data only}
- Bernard P, Plantin P, Roger H, Sassolas B, Villaret E, Legrain V, et al. Roxithromycin versus penicillin in the treatment of erysipelas in adults: A comparative study. British Journal of Dermatology 1992;127(2):155-9. [DOI] [PubMed] [Google Scholar]
Bernard 2002 {published data only}
- Bernard P, Chosidow O, Vaillant L. Oral pristinamycin versus standard penicillin regimen to treat erysipelas in adults: Randomised, non-inferiority, open trial. BMJ 2002;325(7369):864-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bucko 2002 part 1 {published data only}
- Bucko AD, Hunt BJ, Kidd SL, Hom R. Randomized, double-blind, multicenter comparison of oral cefditoren 200 or 400 mg BID with either cefuroxime 250 mg BID or cefadroxil 500 mg BID for the treatment of uncomplicated skin and skin-structure infections. Clinical Therapeutics 2002;24(7):1134-47. [DOI] [PubMed] [Google Scholar]
Bucko 2002 part 2 {published data only}
- Bucko AD, Hunt BJ, Kidd SL, Hom R. Randomized, double-blind, multicenter comparison of oral cefditoren 200 or 400 mg BID with either cefuroxime 250 mg BID or cefadroxil 500 mg BID for the treatment of uncomplicated skin and skin-structure infections. Clinical Therapeutics 2002;24(7):1134-47. [DOI] [PubMed] [Google Scholar]
Chan 1995 {published data only}
- Chan JC. Ampicillin/sulbactam versus cefazolin or cefoxitin in the treatment of skin and skin-structure infections of bacterial etiology. Advances in Therapy 1995;12(2):139-46. [PubMed] [Google Scholar]
Daniel 1991 part 1 {published data only}
- Daniel R. Azithromycin, erythromycin and cloxacillin in the treatment of infections of skin and associated soft tissues. European Azithromycin Study Group. Journal of International Medical Research 1991;19(6):433-45. [DOI] [PubMed] [Google Scholar]
Daniel 1991 part 2 {published data only}
- Daniel R. Azithromycin, erythromycin and cloxacillin in the treatment of infections of skin and associated soft tissues. European Azithromycin Study Group. Journal of International Medical Research 1991;19(6):433-45. [DOI] [PubMed] [Google Scholar]
DiMattia 1981 {published data only}
- DiMattia AF, Sexton MJ, Smialowicz CR, Knapp WH Jr. Efficacy of two dosage schedules of cephalexin in dermatologic infections. Journal of Family Practice 1981;12(4):649-52. [PubMed] [Google Scholar]
Fabian 2005 {published data only}
- Fabian TC, File TM Jr, Embil JM, Krige JEJ, Klein S, Rose A, et al. Meropenem versus imipenem-cilastatin for the treatment of hospitalized patients with complicated skin and skin structure infections: Results of a multicenter, randomized, double blind comparative study. Surgical Infections 2005;6(3):269-82. [DOI] [PubMed] [Google Scholar]
Giordano 2005 {published data only}
- Giordano P, Song J, Pertel P, Herrington J, Kowalsky S. Sequential intravenous/oral moxifloxacin versus intravenous piperacillin-tazobactam followed by oral amoxicillin-clavulanate for the treatment of complicated skin and skin structure infection. International Journal of Antimicrobial Agents 2005;26(5):357-65. [DOI] [PubMed] [Google Scholar]
Grayson 2002 {published data only}
- *.Grayson ML, McDonald M, Gibson K, Athan E, Munckhof WJ, Paull P, et al. Once-daily intravenous cefazolin plus oral probenecid is equivalent to once-daily intravenous ceftriaxone plus oral placebo for the treatment of moderate-to-severe cellulitis in adults. Clinical Infectious Diseases 2002;34(11):1440-8. [DOI] [PubMed] [Google Scholar]
- Grayson ML, McDonald M, Gibson K, Athan E, Munckhof WJ, Paull P, et al. Once-daily IV cefazolin 2g plus oral probenecid 1g is equivalent to once-daily IV ceftriaxone 1g plus oral placebo for the hospital-in-the-home treatment of cellulitis. Internal Medicine Journal 2001;31:A13. [Google Scholar]
Hepburn 2004 {published data only}
- Hepburn MJ, Dooley DP, Skidmore PJ, Ellis MW, Starnes WF, Hasewinkle WC. Comparison of short-course (5 days) and standard (10 days) treatment for uncomplicated cellulitis. Archives of Internal Medicine 2004;164(15):1669-74. [DOI] [PubMed] [Google Scholar]
Iannini 1985 {published data only}
- Iannini PB, Kunkel MJ, Link ASJ, Simons WJ, Lee TJ, Tight RR. Multicenter comparison of cefonicid and cefazolin in hospitalized patients with skin and soft tissue infections. Advances in Therapy 1985;2(5):214-24. [Google Scholar]
Johnson 2007 {published data only}
- Johnson S, Leak K, Singh S, Tan P, Pillay W, Cuschieri R. Can cycloidal vibration plus standard treatment reduce lower limb cellulitis treatment times? Journal of Wound Care 2007;16(4):166-9. [DOI] [PubMed] [Google Scholar]
Kiani 1991 {published data only}
- Kiani R. Double-blind, double-dummy comparison of azithromycin and cephalexin in the treatment of skin and skin structure infections. European Journal of Clinical Microbiology & Infectious Diseases 1991;10(10):880-4. [DOI] [PubMed] [Google Scholar]
Leman 2005 {published data only}
- Leman P, Mukherjee D. Flucloxacillin alone or combined with benzylpenicillin to treat lower limb cellulitis: A randomised controlled trial. Emergency Medicine Journal 2005;22(5):342-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rao 1985 {published data only}
- Rao B, See RC, Chuah Sk, Bansal MB, Lou MA, Thadepalli H. Ticarcillin plus calvulanic acid versus moxalactam in the treatment of skin and soft tissue infections. The American Journal of Medicine 1985;79 Suppl 2(5):126-9. [DOI] [PubMed] [Google Scholar]
Sachs 1990 {published data only}
- Sachs MK, Pilgrim C. Ampicillin/sulbactam compared with cefazolin or cefoxitin for the treatment of skin and skin structure infections. Drug Investigation 1990;2(3):173-83. [Google Scholar]
Schwartz 1996 {published data only}
- Schwartz R, Das-Young LR, Ramirez-Ronda C, Frank E. Current and future management of serious skin and skin-structure infections. American Journal of Medicine 1996;100(6A):90S-5S. [DOI] [PubMed] [Google Scholar]
Tack 1998 {published data only}
- Tack KJ, Littlejohn TW, Mailloux G, Wolf MM, Keyserling CH. Cefdinir versus cephalexin for the treatment of skin and skin-structure infections. The Cefdinir Adult Skin Infection Study Group. Clinical Therapeutics 1998;20(2):244-56. [DOI] [PubMed] [Google Scholar]
Tarshis 2001 {published data only}
- Tarshis GA, Miskin BM, Jones TM, Champlin J, Wingert KJ, Breen JD, et al. Once-daily oral gatifloxacin versus oral levofloxacin in treatment of uncomplicated skin and soft tissue infections: Double blind, multicenter, randomized study. Antimicrobial Agents and Chemotherapy 2001;45(8):2358-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vinen 1996 {published data only}
- Vinen J, Hudson B, Chan B, Fernandes C. A randomised comparative study of once-daily ceftriaxone and 6-hourly flucloxacillin in the treatment of moderate to severe cellulitis. Clinical efficacy, safety and pharmacoeconomic implications. Clinical Drug Investigation 1996;12(5):221-5. [Google Scholar]
Weigelt 2005 {published data only}
- Weigelt J, Itani K, Stevens D, Lau W, Dryden M, Knirsch, Linezolid study group. Linezolid versus vancomycin in treatment of complicated skin and soft tissue infections. Antimicrobial Agents and Chemotherapy 2005;49(6):2260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeglaoui 2004 {published data only}
- Zeglaoui F, Dziri C, Mokhtar I, Ezzine N, Kharfi M, Zghal M, et al. Intramuscular bipenicillin vs. intravenous penicillin in the treatment of erysipelas in adults: Randomized controlled study. Journal of the European Academy of Dermatology and Venereology (JEADV) 2004;18(4):426-8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agache 1987 a {published data only}
- Agache P, Amblard P, Moulin G, Barrière H, Texier L, Beylot C, et al. Roxithromycin in skin and soft tissue infections. Journal of Antimicrobial Chemotherapy 1987;20 Suppl B:153-6. [DOI] [PubMed] [Google Scholar]
Agache 1987 b {published data only}
- Agache P, Amblard P, Moulin G, Barrière H, Texier L, Beylot C, et al. Roxithromycin in skin and soft tissue infections. Journal of Antimicrobial Chemotherapy 1987;20 Suppl B:153-6. [DOI] [PubMed] [Google Scholar]
Amaya‐Tapia 1992 {published data only}
- Amaya-Tapia G, Andrade-Villanueva J, Flores-Glaxiola A, Aguirre-Avalos G, Morfin-Otero R, Esparza-Ahumada S. Safety and efficacy of lomefloxacin and cefaclor in the treatment of skin and skin structure infections. International Journal of Antimicrobial Agents 1992;2(1):55-60. [DOI] [PubMed] [Google Scholar]
Amaya‐Tapia 1993 {published data only}
- Amaya-Tapia G, Aguirre-Avalos G, Andrade-Villanueva J, Peredo-González G, Morfín-Otero R, Esparza-Ahumada S, et al. Once-daily azithromycin in the treatment of adult skin and skin-structure infections. Journal of Antimicrobial Chemotherapy 1993;31 Suppl E:129-35. [DOI] [PubMed] [Google Scholar]
Arata 1989 {published data only}
- Arata J, Yamamoto Y, Tamaki H, Ookawara A, Fukaya T, Ishibashi Y, et al. Double-blind study of lomefloxacin vs. norfloxacin in the treatment of skin and soft tissue infections. Chemotherapy 1989;37(4):482-503. [Google Scholar]
Arata 1994 a {published data only}
- Arata J, Akiyama H, Abe Y, Ishibashi Y, Takehara K, Tsuchida T, et al. A double-blind comparative study of S-1108 and cefaclor in skin and skin structure infections. Chemotherapy 1994;42(3):326-45. [Google Scholar]
Arata 1994 b {published data only}
- Arata J, Kanzaki H, Abe Y, Torigoe R, Ohkawara A, Yamanaka K, et al. A multicenter, double-blind, double-placebo comparative study of SY 5555 versus cefaclor in the treatment of skin and skin structure infections. Chemotherapy 1994;42(6):740-60. [Google Scholar]
Arata 1995 {published data only}
- Arata J, Torigoe R, Ohkawara A, et al. A multicenter, double-blind, double placebo clinical trial of azithromycin versus cefaclor in the treatment of skin and skin structure infections. Japanese Journal of Chemotherapy 1995;43(11):1069-87. [Google Scholar]
Azimi 1999 {published data only}
- Azimi PH, Barson WJ, Janner D, Swanson R. Efficacy and safety of ampicillin/sulbactam and cefuroxime in the treatment of serious skin and skin structure infections in pediatric patients. UNASYN Pediatric Study Group. The Pediatric Infectious Disease Journal 1999;18(7):609-13. [DOI] [PubMed] [Google Scholar]
Ballantyne 1980 {published data only}
- Ballantyne F. A comprehensive study of cefadroxil and cephalexin in the treatment of soft tissue infections. Journal of International Medical Research 1980;8 Suppl 1:70-4. [PubMed] [Google Scholar]
Ballantyne 1985 {published data only}
- Ballantyne FN. Comparative efficacy of cefadroxil and cefaclor in the treatment of skin and soft-tissue infections. Clinical Therapeutics 1985;7(4):487-91. [PubMed] [Google Scholar]
Bechard 1984 {published data only}
- Bechard DL, Hawkins SH, Sutherland SC. Comparative evaluation of cefmenoxime versus cefoxitin in serious infections. American Journal of Medicine 1984;77(6A):13-6. [DOI] [PubMed] [Google Scholar]
Bergkvist 1998 {published data only}
- Bergkvist PI, Sjobeck K. Relapse of erysipelas following treatment with prednisolone or placebo in addition to antibiotics: A 1-year follow-up. Scandinavian Journal of Infectious Diseases 1998;30(2):206-7. [DOI] [PubMed] [Google Scholar]
Blaszczyk‐Kostanecka {published data only}
- Blaszczyk-Kostanecka M, Dobozy A, Dominguez-Soto L, Guerrero R, Hunyadi J, Lopera J, et al. Comparison of two regimens of oral clindamycin versus dicloxacillin in the treatment of mild-to-moderate skin and soft-tissue infections. Current Therapeutic Research - Clinical and Experimental 1998;59(6):341-53. [Google Scholar]
Bradsher 1984 {published data only}
- Bradsher RW Jr, Snow RM. Ceftriaxone treatment of skin and soft tissue infections in a once daily regimen. American Journal of Medicine 1984;77(4C):63-7. [PubMed] [Google Scholar]
Breedt 2005 {published data only}
- Breedt J, Teras J, Gardovskis J, Maritz FJ, Vaasna T, Ross DP, et al. Safety and efficacy of tigecycline in treatment of skin and skin structure infections: Results of a double-blind phase 3 comparison study with vancomycin-aztreonam. Antimicrobial Agents and Chemotherapy 2005;49(11):4658-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 1996 {published data only}
- Brown G, Chamberlain R, Goulding J, Clarke A. Ceftriaxone versus cefazolin with probenecid for severe skin and soft tissue infections. Journal of Emergency Medicine 1996;14(5):547-51. [DOI] [PubMed] [Google Scholar]
Browning 1981 {published data only}
- Browning AK. The efficacy of twice daily cephalexin. Pharmatherapeutica 1981;2(9):559-64. [PubMed] [Google Scholar]
Carbon 1992 {published data only}
- Carbon C. Prospective randomized phase II study of intravenous cefpirome 1g or 2g bd in the treatment of hospitalized patients with different infections. Cefpirome Study Group. Journal of Antimicrobial Chemotherapy 1992;29 Suppl A:87-94. [DOI] [PubMed] [Google Scholar]
Carbon 1995 {published data only}
- Carbon C. Overview of the efficacy of isepamicin in the adult core clinical trial programme. Journal of Chemotherapy 1995;7 Suppl 2:79-85. [PubMed] [Google Scholar]
Carr 1994 {published data only}
- Carr WD, Wall AR, Georgala-Zervogiani S, Stratigos J, Gouriotou K. Fusidic acid tablets in patients with skin and soft-tissue infection: A dose-finding study. European Journal of Clinical Research 1994;5:87-95. [Google Scholar]
Chiodini 1985 {published data only}
- Chiodini PL, Toop MJ, Odugbesan O, Gilbert J, Farrell ID, Barnett AH, et al. Sulbactam/ampicillin: Effects on glucose metabolism in diabetics with soft tissue infection. Journal of Antimicrobial Chemotherapy 1985;16(5):643-7. [DOI] [PubMed] [Google Scholar]
Chirurgi 1994 {published data only}
- Chirurgi VA, Edelstein H, Oster SE, Karp R, Cassano KB, Aiken S, et al. Randomized comparison trial of teicoplanin i.v., teicoplanin i.m., and cefazolin therapy for skin and soft tissue infections caused by gram-positive bacteria. Southern Medical Journal 1994;87(9):875-80. [DOI] [PubMed] [Google Scholar]
Colardyn 1996 {published data only}
- Colardyn F, Faulkner KL. Intravenous meropenem versus imipenem/cilastatin in the treatment of serious bacterial infections in hospitalized patients. Meropenem Serious Infection Study Group. Journal of Antimicrobial Chemotherapy 1996;38(3):523-37. [DOI] [PubMed] [Google Scholar]
Colin 1988 {published data only}
- Colin M, Avon P. Comparative double-blind evaluation of a new topical antibacterial agent, mupirocin, compared with placebo in the treatment of skin and soft tissue infections [Evaluation comparative double aveugle du nouvel agent antibacterien topique, la mupirocine, par rapport a un placebo dans le traitement des infections de la peau et des tissus mous]. Pharmatherapeutica 1988;5(3):198-203. [PubMed] [Google Scholar]
Daly 1990 {published data only}
- Daly JS, Worthington MG, Andrews RJ, Brown RB, Schwartz R, Sexton DJ. Randomized, double-blind trial of cefonicid and nafcillin in the treatment of skin and skin structure infections. Antimicrobial Agents & Chemotherapy 1990;34(4):654-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Derriennic 1993 {published data only}
- Derriennic M, Escande JP. Dirithromycin in the treatment of skin and skin structure infections. Journal of Antimicrobial Chemotherapy 1993;31 Suppl C:159-68. [DOI] [PubMed] [Google Scholar]
Echols 1994 {published data only}
- Echols R, Coulter H. Efficacy and safety of IV ciprofloxacin versus standard antimicrobial therapy in the treatment of selected tissue infections. Infections in Medicine 1994;11(6):461-9. [Google Scholar]
Edelstein 1991 {published data only}
- Edelstein H, Oster SE, Chirurgi VA, Karp RA, Cassano KB, McCabe RE. Intravenous or intramuscular teicoplanin once daily for skin and soft-tissue infections. DICP : the annals of pharmacotherapy 1991;25(9):914-8. [DOI] [PubMed] [Google Scholar]
Eron 1983 {published data only}
- Eron LJ, Park CH, Hixon DL, Goldenberg RI, Poretz DM. Ceftriaxone therapy of bone and soft tissue infections in hospital and outpatient settings. Antimicrobial Agents & Chemotherapy 1983;23(5):731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fass 1989 {published data only}
- Fass RJ, Plouffe JF, Russell JA. Intravenous/oral ciprofloxacin versus ceftazidime in the treatment of serious infections. American Journal of Medicine 1989;87(5A):164S-8S. [DOI] [PubMed] [Google Scholar]
File 1991 {published data only}
- File TM Jr, Tan JS. Ticarcillin-clavulanate therapy for bacterial skin and soft tissue infections. Reviews of Infectious Diseases 1991;13 Suppl 9:S733-6. [DOI] [PubMed] [Google Scholar]
Fleisher 1983 {published data only}
- Fleisher GR, Wilmott CM, Campos JM. Amoxicillin combined with clavulanic acid for the treatment of soft tissue infections in children. Antimicrobial Agents & Chemotherapy 1983;24(5):679-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frank 1989 {published data only}
- Frank E, Phillips SL, Gupta T, Bavishi N, Casey KK. Treatment of skin and soft tissue infections: A comparative study of cefmetazole and cefoxitin. Journal of Antimicrobial Chemotherapy 1989;23 Suppl D:55-60. [DOI] [PubMed] [Google Scholar]
Fujita 1985 {published data only}
- Fujita K, Takahashi H, Mizoguchi A, Takeshima M, Sasaki J, Nonami E, et al. Clinical evaluation of lenampicillin in the treatment of superficial suppurative skin and soft tissue infection. A double-blind study compared to amoxicillin. Japanese Journal of Antibiotics 1985;38(7):1794-818. [PubMed] [Google Scholar]
Geckler 1988 {published data only}
- Geckler RW, Eng RH, Fabian TC, Echols RM, Jemsek JG, LeFrock JL. A multicenter comparative study of cefotetan once daily and cefoxitin thrice daily for the treatment of infections of the skin and superficial soft tissue. American Journal of Surgery 1988;155(5A):91-5. [DOI] [PubMed] [Google Scholar]
Gentry 1989 a {published data only}
- Gentry LO, Rodriguez-Gomez G, Zeluff BJ, Khoshdel A, Price M. A comparative evaluation of oral ofloxacin versus intravenous cefotaxime therapy for serious skin and skin structure infections. American Journal of Medicine 1989;87(6C):57S-60S. [PubMed] [Google Scholar]
Gentry 1989 b {published data only}
- Gentry LO, Ramirez-Ronda CH, Rodriguez-Noriega E, Thadepalli H, Del Rosal PL, Ramirez C. Oral ciprofloxacin vs parenteral cefotaxime in the treatment of difficult skin and skin structure infections. A multicenter trial. Archives of Internal Medicine 1989;149(11):2579-83. [PubMed] [Google Scholar]
Gentry 1996 {published data only}
- Gentry LO, Rodriguez-Gomez G, Pien F, Cubeddu L, Holley HP Jr, Buaron K, et al. Comparative effects of ceftazidime two or three times daily in patients with skin and skin structure infections. American Journal of Therapeutics 1996;3(3):212-8. [PubMed] [Google Scholar]
Gesser 2004 a {published data only}
- Gesser RM, McCarroll KA, Woods GL. Efficacy of ertapenem against methicillin-susceptible Staphylococcus aureus in complicated skin/skin structure infections: Results of a double-blind clinical trial versus piperacillin-tazobactam. International Journal of Antimicrobial Agents 2004;23(3):235-9. [DOI] [PubMed] [Google Scholar]
Gesser 2004 b {published data only}
- Gesser RM, McCarroll KA, Woods GL. Evaluation of outpatient treatment with ertapenem in a double blind controlled clinical trial of complicated skin/skin structure infections. The Journal of Infection 2004;48(1):32-8. [DOI] [PubMed] [Google Scholar]
Gordin 1985 {published data only}
- Gordin FM, Wofsy CB, Mills J. Once-daily ceftriaxone for skin and soft tissue infections. Antimicrobial Agents & Chemotherapy 1985;27(4):648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gould 1988 {published data only}
- Gould IM, Hudson M, Morris J, Page G, Douglas JG, Smith CC, et al. Imipenem versus standard therapy in the treatment of serious soft tissue infection. Drugs Under Experimental & Clinical Research 1988;14(8):555-8. [PubMed] [Google Scholar]
Graham 2002 {published data only}
- Graham DR, Talan DA, Nichols RL, Lucasti C, Corrado M, Morgan N, et al. Once-daily, high-dose levofloxacin versus ticarcillin-clavulanate alone or followed by amoxicillin-clavulanate for complicated skin and skin-structure infections: A randomized, open-label trial. Clinical Infectious Diseases 2002;35(4):381-9. [DOI] [PubMed] [Google Scholar]
Hanfling 1992 {published data only}
- Hanfling MJ, Hausinger SA, Squires J. Loracarbef vs. cefaclor in pediatric skin and skin structure infections. The Pediatric Infectious Disease Journal 1992;11(8 Suppl):S27-30. [DOI] [PubMed] [Google Scholar]
Henry 1985 {published data only}
- Henry SA, Bendush CB. Aztreonam: Worldwide overview of the treatment of patients with gram-negative infections. American Journal of Medicine 1985;78(2a):57-64. [DOI] [PubMed] [Google Scholar]
Hubsher 1976 {published data only}
- Hubsher JA, Gadebusch HH, Itkin AG. Double-blind comparison of cephradine and cephalexin in the treatment of skin and soft-tissue infections due to Staphylococcus aureus. Current Therapeutic Research, Clinical & Experimental 1976;19(6):579-88. [PubMed] [Google Scholar]
Jorup‐Rönström 1984 {published data only}
- Jorup-Rönström C, Britton S, Gavlevik A, Gunnarsson K, Redman AC. The course, costs and complications of oral versus intravenous penicillin therapy of erysipelas. Infection 1984;12(6):390-4. [DOI] [PubMed] [Google Scholar]
Kulhanjian 1989 {published data only}
- Kulhanjian J, Dunphy MG, Hamstra S, Levernier K, Rankin M, Petru A, et al. Randomized comparative study of ampicillin/sulbactam vs. ceftriaxone for treatment of soft tissue and skeletal infections in children. The Pediatric Infectious Disease Journal 1989;8(9):605-10. [DOI] [PubMed] [Google Scholar]
Lobo 1995 {published data only}
- Lobo JM, Guedes MM. Treatment of bacterial skin infections. An open, randomized and comparative study among roxithromycin and cephalexin. Revista Brasileira de Medicina 1995;52(9):1047-51. [Google Scholar]
Miller 1989 {published data only}
- Miller LM, Mooney CJ, Hansbrough JF. Comparative evaluation of cefaclor versus cefadroxil in the treatment of skin and skin structure infections. Current Therapeutic Research, Clinical and Experimental 1989;46(3):405-10. [Google Scholar]
Nolen 1992 {published data only}
- Nolen T, Conetta BJ, Durham SJ, Wilber RB. Safety and efficacy of cefprozil vs cefaclor in the treatment of mild to moderate skin and skin structure infections. Infections in Medicine 1992;9 Suppl C:56-68. [Google Scholar]
Parish 1984 {published data only}
- Parish LC, Parks DB, Layne PP, Uri JV. Ceftizoxime treatment of cutaneous and subcutaneous tissue infections. Clinical Therapeutics 1984;6(5):613-9. [PubMed] [Google Scholar]
Parish 1991 {published data only}
- Parish LC, Jungkind DL. Systemic antimicrobial therapy in skin and skin structure infections: Comparison of temafloxacin and ciprofloxacin. American Journal of Medicine 1991;91(6A):115S-9S. [DOI] [PubMed] [Google Scholar]
Parish 1993 {published data only}
- Parish LC. Clarithromycin in the treatment of skin and skin structure infections: Two multicenter clinical studies. Clarithromycin Study Group. International Journal of Dermatology 1993;32(7):528-32. [DOI] [PubMed] [Google Scholar]
Parish 1993a {published data only}
- Parish LC, Jungkind DL. Systemic antimicrobial therapy for skin and skin structure infections: Comparison of fleroxacin and ceftazidime. American Journal of Medicine 1993;94(3A):166S-73S. [PubMed] [Google Scholar]
Pereira 1996 {published data only}
- Pereira LC. Comparative clinical study between roxithromycin and cephalexin in the treatment of folliculitis, furunculosis and Erysipelas /Cellulitis. Revista Brasileira De Medicina 1996;53(1-2):81-6. [Google Scholar]
Perez‐Ruvalcaba 1987 {published data only}
- Pérez-Ruvalcaba JA, Quintero-Pérez NP, Morales-Reyes JJ, Huitrón-Ramirez JA, Rodriguez-Chagollán JJ, Rodríguez-Noriega E. Double-blind comparison of ciprofloxacin with cefotaxime in the treatment of skin and skin structure infections. American Journal of Medicine 1987;82(4A):242-6. [PubMed] [Google Scholar]
Pien 1983 {published data only}
- Pien FD. Double-blind comparative study of two dosage regimens of cefaclor and amoxicillin-clavulanic acid in the outpatient treatment of soft tissue infections. Antimicrobial Agents & Chemotherapy 1983;24(6):856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramirez‐Ronda 1982 {published data only}
- Ramírez-Ronda CH, Gutíerrez J, Bermúdez RH. Comparative effectiveness, safety and tolerance of mezlocillin and ticarcillin: A prospective randomized trial. Journal of Antimicrobial Chemotherapy 1982;9 Suppl A:125-9. [DOI] [PubMed] [Google Scholar]
Ramirez‐Ronda 1987 {published data only}
- Ramirez-Ronda CH, Saavedra S, Rivera-Vázquez CR. Comparative, double-blind study of oral ciprofloxacin and intravenous cefotaxime in skin and skin structure infections. American Journal of Medicine 1987;82(4A):220-3. [PubMed] [Google Scholar]
Sacchidanand 2005 {published data only}
- Sacchidanand S, Penn RL, Embil JM, Campos ME, Curcio D, Ellis-Grosse E, et al. Efficacy and safety of tigecycline monotherapy compared with vancomycin plus aztreonam in patients with complicated skin and skin structure infections: Results from a phase 3, randomized, double-blind trial. International Journal of Infectious Diseases 2005;9(5):251-61. [DOI] [PubMed] [Google Scholar]
Schupbach 1992 {published data only}
- Schupbach CW, Olovich KG, Dere WH. Efficacy of cefaclor AF in the treatment of skin and skin-structure infections. Clinical Therapeutics 1992;14(3):470-9. [PubMed] [Google Scholar]
Segev 1990 {published data only}
- Segev S, Rosen N, Pitlik SD, Block C, Rubinstein E. Pefloxacin versus ceftazidime in therapy of soft tissue infections in compromised patients. Journal of Antimicrobial Chemotherapy 1990;26 Suppl B:193-8. [DOI] [PubMed] [Google Scholar]
Siami 2001 {published data only}
- Siami G, Christou N, Eiseman I, Tack KJ, Severe Skin and Soft Tissue Infections Study Group. Clinafloxacin versus piperacillin-tazobactam in treatment of patients with severe skin and soft tissue infections. Antimicrobial Agents & Chemotherapy 2001;45(2):525-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solomkin 1986 {published data only}
- Solomkin JS, Cocchetto DM. Ceftazidime versus tobramycin plus ticarcillin in the treatment of soft-tissue infections. Clinical Therapeutics 1986;9(1):123-34. [PubMed] [Google Scholar]
Stevens 2000 {published data only}
- Stevens DL, Smith LG, Bruss JB, McConnell-Martin MA, Duvall SE, Todd WM, et al. Randomized comparison of linezolid (PNU-100766) versus oxacillin-dicloxacillin for treatment of complicated skin and soft tissue infections. Antimicrobial Agents & Chemotherapy 2000;44(12):3408-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 1993 {published data only}
- Tan JS, Wishnow RM, Talan DA, Duncanson FP, Norden CW. Treatment of hospitalized patients with complicated skin and skin structure infections: Double-blind, randomized, multicenter study of piperacillin-tazobactam versus ticarcillin-clavulanate. The Piperacillin/Tazobactam Skin and Skin Structure Study Group. Antimicrobial Agents and Chemotherapy 1993;37(8):1580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tassler 1993 {published data only}
- Tassler H. Comparative efficacy and safety of oral fleroxacin and amoxicillin/clavulanate potassium in skin and soft tissue infections. American Journal of Medicine 1993;94(3A):159S-65S. [PubMed] [Google Scholar]
Wasilewski 2000 {published data only}
- Wasilewski MM, Wilson MG, Sides GD, Stotka JL. Comparative efficacy of 5 days of dirithromycin and 7 days of erythromycin in skin and soft tissue infections. Journal of Antimicrobial Chemotherapy 2000;46(2):255-62. [DOI] [PubMed] [Google Scholar]
Wible 2003 {published data only}
- Wible K, Tregnaghi M, Bruss J, Fleishaker D, Naberhuis-Stehouwer S, Hilty M. Linezolid versus cefadroxil in the treatment of skin and skin structure infections in children. The Pediatric Infectious Disease Journal 2003;22(4):315-23. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Chaudhary 2008 {published data only}
- Chaudhary M, Shrivastava SM, Sehgal R. Efficacy and safety study of fixed-dose combination of ceftriaxone-vancomycin injection in patients with various infections. Current Drug Safety 2008;3(1):82-85. [DOI] [PubMed] [Google Scholar]
Manaktala 2009 {published data only}
- Manaktala C, Singh AK, Verma M, Sachdeva A, Sharma H, Roy A, et al. Efficacy and tolerability of cefditoren pivoxil in uncomplicated skin and skin structure infections in Indian patients. Indian Journal of Dermatology 2009;54(4):350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nichols 1999 {published data only}
- Nichols RL, Graham DR, Barriere SL, Rodgers A, Wilson SE, Zervos M, et al. Treatment of hospitalized patients with complicated gram-positive skin and skin structure infections: Two randomized, multicentre studies of quinupristin/dalfopristin versus cefazolin, oxacillin or vancomycin. Journal of Antimicrobial Chemotherapy 1999;44(2):263-273. [DOI] [PubMed] [Google Scholar]
Noel 2008 {published data only}
- Noel GJ, Bush K, Bagchi P, Lanus J, Strauss RS. A randomized, double-blind trial comparing ceftobiprole medocaril with vancomycin plus ceftazidime for the treatment of patients with complicated skin and skin-structure infections. Clinical Infectious Diseases 2008;46(5):647-655. [DOI] [PubMed] [Google Scholar]
Pertel 2009 {published data only}
- Pertel PE, Eisenstein BI, Link AS, Donfrid B, Biermann EJ, Bernardo P, et al. The efficacy and safety of daptomycin vs. vancomycin for the treatment of cellulitis and erysipelas. International Journal of Clinical Practice 2009;63(3):368-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Snodgrass 1937 {published data only}
- *.Snodgrass WR, Anderson T. Prontosil in the treatment of erysipelas, a controlled series of 312 cases. British Medical Journal 1937;2(3993):101-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Snodgrass WR 1937 {published data only}
- Snodgrass WR, Anderson T. Sulphanilamide in the treatment of erysipelas, a controlled series of 312 case. British Medical Journal 1937;2:1156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stein 2008 {published data only}
- Stein GE, Schooley SL, Havlichek DH, Nix DE. Outpatient intravenous antibiotic therapy compared with oral linezolid in patients with skin and soft tissue infections: A pharmacoeconomic analysis. Infectious Diseases in Clinical Practice 2008;16(4):235-239. [Google Scholar]
References to ongoing studies
ISRCTN76443564 {unpublished data only}
- ISRCTN76443564. The efficacy and safety evaluation of ceftriaxone and sulbactam combination (1.5 gram) in patients with skin and soft tissue infections: An open label, parallel, randomized, prospective comparative trial. ISRCTN Register.
NCT00295178 {unpublished data only}
- NCT00295178. A Multicenter, Randomized Study Comparing CUBICIN® (Daptomycin for injection) with Vancomycin in the treatment of cellulitis or erysipelas. World Health Organisation International Clinical Trials Registry.
NCT00424190 {unpublished data only}
- NCT00424190. Comparative study of ceftaroline vs. vancomycin plus aztreonam in adult subjects with complicated skin infections. ClinicalTrials.gov (accessed May 2010).
NCT00948142 {unpublished data only}
- NCT00948142. Safety and efficacy of CEM-102 compared to linezolid in acute bacterial skin infections. ClinicalTrials.gov (accessed May 2010).
Additional references
Altman 2001
- Altman DG, Schulz KF. Concealing treatment allocation in randomised trials. British Medical Journal 2001;323(7310):446-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ambati 2000
- Ambati BK, Ambati J, Azar N, Stratton L, Schmidt EV. Periorbital and orbital cellulitis before and after the advent of Haemophilus influenzae type B vaccination. Ophthalmology 2000;107(8):1450-1453. [DOI] [PubMed] [Google Scholar]
Baddour 1984
- Baddour LM, Bisno AL. Recurrent cellulitis after coronary bypass surgery. Association with superficial fungal infection in saphenous venectomy limbs. JAMA 1984;251(8):1049-52. [PubMed] [Google Scholar]
Bernard 1987
- Bernard P, Toty L, Mounier M, Denis F, Bonnetblanc JM. Early detection of streptococcal group antigens in skin samples by latex particle agglutination. Archives of Dermatology 1987;123(4):468-70. [PubMed] [Google Scholar]
British Lymphology Society 2007
- British Lymphology Society. Consensus Document on the Management of Cellulitis in Lymphoedema. www.lymphoedema.org/lsn/ (accessed July 2007).
Brook 1995
- Brook I, Frazier EH. Clinical features and aerobic and anaerobic microbiological characteristics of cellulitis. Archives of Surgery (Chicago, Ill. : 1960) 1995;130(7):786-92. [DOI] [PubMed] [Google Scholar]
Carter 2007
- Carter K, Kilburn S, Featherstone P. Cellulitis and treatment: A qualitative study of experiences. British Journal of Nursing 2007;16(6):S22-4, S26-8. [DOI] [PubMed] [Google Scholar]
Chan R 1995
- Chan R, Hemeryck L, O'Regan M, Clancy L, Feely J. Oral versus intravenous antibiotics for community acquired lower respiratory tract infection in a general hospital: Open, randomised controlled trial. BMJ 1995;310(6991):1360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corwin 2005
- Corwin P, Toop L, McGeoch G, Than M, Wynn-Thomas S, Wells JE, et al. Randomised controlled trial of intravenous antibiotic treatment for cellulitis at home compared with hospital. BMJ 2005;330(7483):129-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cox 1998
- Cox NH, Colver GB, Paterson WD. Management and morbidity of cellulitis of the leg. Journal of the Royal Society of Medicine 1998;91(12):634-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cox 2006
- Cox MH. Oedema as a risk factor for multiple episodes of cellulitis/erysipelas of the lower leg: A series with community follow-up. British Journal of Dermatology 2006;155(5):947-50. [DOI] [PubMed] [Google Scholar]
CREST 2005
- Clinical Resource Efficiency Support Team (CREST). Guidelines on the management of cellulitis in adults. http//www.crestni.org.uk 2005. [ISBN 1-903982-12-X]
Critchley 1997
- Critchley G, Handa A, Maw A, Harvey A, Harvey MR, Corbett CR. Complications of varicose vein surgery. Annals of the Royal College of Surgeons of England 1997;79(2):105-10. [PMC free article] [PubMed] [Google Scholar]
Dupuy 1999
- Dupuy A, Benchikhi H, Roujeau JC, Bernard P, Vaillant L, Chosidow O, et al. Risk factors for erysipelas of the leg (cellulitis): Case-control study. BMJ 1999;318(7198):1591-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ellis Simonsen 2006
- Ellis Simonsen SM, Van Orman ER, Hatch BE, Jones SS, Gren LH, Hegann KT, et al. Cellulitis incidence in a defined population. Epidemiology and Infection 2006;134(2):293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eriksson 1996
- Eriksson B, Jorup-Rönström C, Karkkonen K, Sjöblom AC, Holm SE. Erysipelas: Clinical and bacteriologic spectrum and serological aspects. Clinical Infectious Diseases 1996;23(5):1091-8. [DOI] [PubMed] [Google Scholar]
Eron 2003
- Eron LJ, Lipsky BA, Low DE, Nathwani D, Tice AD, Volturo GA, et al. Managing skin and soft tissue infections: Expert panel recommendations on key decision points. Journal of Antimicrobial Chemotherapy 2003;52 Suppl 1:i3-17. [DOI] [PubMed] [Google Scholar]
Escalante 1995
- Escalante A, Beardmore TD. Risk factors for early wound complications after orthopedic surgery for rheumatoid arthritis. The Journal of Rheumatology 1995;22(10):1844-51. [PubMed] [Google Scholar]
Haan 1997
- Haan MN, Selby JV, Quesenberry CP Jr, Schmittdiel JA, Fireman BH, Rice DP. The impact of ageing and chronic disease on use of hospital and outpatient services in a large HMO: 1971-1991. Journal of the American Geriatrics Society 1997;45(6):667-74. [DOI] [PubMed] [Google Scholar]
Hay 2004
- Hay RJ, Adriaans BM. Bacterial Infections. In: Burns DA, Breathnach SM, Cox NH, Griffiths CEM, editors(s). Rook's Textbook of Dermatology. 7th edition. Vol. 1. Oxford: Blackwell Publishing, 2004:27.16-27.20. [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008. [Google Scholar]
Hoberman 1999
- Hoberman A, Wald ER, Hicky RW, Baskin M, Charron M, Masoud M, et al. Oral versus initial intravenous therapy for urinary tract infections in young febrile children. Pediatrics 1999;104:79-86. [DOI] [PubMed] [Google Scholar]
Jorup‐Rönström 1987
- Jorup-Rönström C, Britton S. Recurrent erysipelas: Predisposing factors and costs of prophylaxis. Infection 1987;15(2):105-6. [DOI] [PubMed] [Google Scholar]
Jüni 2001
- Jüni P, Altman DG, Egger M. Assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kern 1999
- Kern WV, Cometta A, De Bock R, Langenaeken J, Paesmans M, Gaya H. Oral versus intravenous empirical antimicrobial therapy for fever in patients with granulocytopenia who are receiving cancer chemotherapy. New England Journal of Medicine 1999;341(5):312-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kluytmans‐Vandenbergh 2006
- Kluytmans-Vandenbergh MF, Kluytmans JA. Community-acquired methicillin-resistant Staphylococcus aureus: Current perspectives. Clinical Microbiology and Infection 2006;12 Suppl 1:9-15. [DOI] [PubMed] [Google Scholar]
Lasley 1997
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. American Journal of Obstetrics & Gynecology 1997;176(6):1250-4. [DOI] [PubMed] [Google Scholar]
Mokni 2006
- Mokni M, Dupuy A, Denguezli M, Dhaoui R, Bouassida S, Amri M, et al. Risk factors for erysipelas of the leg in Tunisia: A multicenter case-control study. Dermatology 2006;212(2):108-12. [DOI] [PubMed] [Google Scholar]
Morris 2001
- Morris A. Cellulitis and Erysipelas. In: BMJ editorial board, editors(s). Clinical Evidence. London: BMJ Books, 2001:1146-9. [Google Scholar]
PATCH I and II
- ISRCTN34716921 (PATCH I), ISRCTN03813200 (PATCH II), UKDCTN0501. Randomised controlled trials to investigate whether prophylactic antibiotics can prevent further episodes of cellulitis (erysipelas) of the leg. clinicaltrials.gov.
Pavlotsky 2004
- Pavlotsky F, Amrani S, Trau H. Recurrent erysipelas: Risk factors. Journal der Deutschen Dermatologischen Gesellschaft 2004;2(2):89-95. [DOI] [PubMed] [Google Scholar]
Purcell 2005
- Purcell K, Fergie J. Epidemic of community-acquired methicillin-resistant Staphylococcus aureus infections: A 14-year study at Driscoll Children's Hospital. Archives of Pediatrics & Adolescent Medicine 2005;159(10):980-85. [DOI] [PubMed] [Google Scholar]
Rojas 2006
- Rojas MX, Granados C. Oral antibiotics versus parenteral antibiotics for severe pneumonia in children. Cochrane Database of Systematic Reviews 2006, Issue 2. Art. No: CD004979. [DOI: 10.1002/14651858.CD004979.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roujeau 2004
- Roujeau JC, Sigurgeirsson B, Korting HC, Kerl H, Paul C. Chronic dermatomycoses of the foot as risk factors for acute bacterial cellulitis of the leg: A case-control study. Dermatology 2004;209(4):301-7. [DOI] [PubMed] [Google Scholar]
Royal Pharmaceutical Society of Great Britain 2007
- Royal Pharmaceutical Society of Great Britain. British National Formulary. London: BMJ Publishing Group Ltd and RPS Publishing, 2007. [Google Scholar]
Societe Francaise de Dermatologie 2001
- Societe Francaise de Dermatologie. Erysipelas and necrotizing fasciitis [Erysipele et fasiite necrosante: prise en charge]. Annales de dermatologie et de vénéréologie 2001;128:463-82. [PubMed] [Google Scholar]
Soo 2008
- Soo J K, Bicanic T A, Heenan S, Mortimer P S. Lymphatic abnormalities demonstrated by lymphoscintigraphy after lower limb cellulitis. British Journal of Dermatology 2008;158(6):1350-53. [DOI] [PubMed] [Google Scholar]
Stevens 2005
- Stevens DL, Bisno AL, Chambers HF, Everett ED, Dellinger P, Goldstein EJ, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clinical Infectious Diseases 2005;41(10):1373-406. [DOI] [PubMed] [Google Scholar]
Thomas 1999
- Thomas TA, Taylor SM, Crane MM, Cornett WR, Langan EM 3rd, Snyder BA, et al. An analysis of limb-threatening lower extremity wound complications after 1090 consecutive coronary artery bypass procedures. Vascular medicine (London, England) 1999;4(2):83-8. [DOI] [PubMed] [Google Scholar]
UK DOH 2001
- United Kingdom Department of Health. Hospital Episode Statistics 2001. www.statistics.gov.uk (accessed June 2003).


