This cohort study evaluates maternal and neonatal COVID-19 antibody levels at delivery after receipt of a messenger RNA (mRNA) COVID-19 vaccine during the second trimester of pregnancy.
Key Points
Question
What were the maternal and neonatal SARS-CoV-2 immunoglobulin G antibody levels at birth after messenger RNA (mRNA) COVID-19 vaccination during the second trimester of pregnancy?
Findings
In this cohort study of 130 pregnant women who received the BNT162b2 mRNA vaccine during their second trimester, antibody titers were positive for all women during delivery, and neonatal titers were higher than maternal titers, representing 100% placental antibody transfer.
Meaning
The findings suggest that administration of the mRNA COVID-19 vaccine during the second trimester is associated with a maternal humoral response that is sustained during labor and transfers antibodies to the neonate, supporting early vaccination of pregnant women.
Abstract
Importance
BNT162b2 messenger RNA (mRNA) COVID-19 vaccination in the third trimester was found to be associated with a strong maternal humoral IgG response that crossed the placenta and approached maternal titers in the newborn.
Objective
To evaluate maternal and neonatal SARS-CoV-2 immunoglobulin G (IgG) antibody levels at birth after mRNA COVID-19 vaccination during the second trimester of pregnancy.
Design, Setting, and Participants
This prospective cohort study, conducted at a single medical center in Haifa, Israel, from May to July 2021, included women with a singleton pregnancy over 24 weeks of gestation at least 7 days after receipt of their second COVID-19 vaccine dose who were not known to be previously infected with COVID-19.
Exposures
BNT162b2 (Pfizer/BioNTech) vaccination.
Main Outcomes and Measures
The primary outcomes were SARS-CoV-2 IgG antibody titers measured in the parturient at admission and in the umbilical cord blood within 30 minutes after delivery. Secondary outcomes were the correlation between antibody titers, feto-maternal characteristics, maternal adverse effects after vaccination, and time interval from vaccination to delivery.
Results
Antibody levels were measured for 129 women (mean [SD] age, 31.9 [4.9] years) and 114 neonates, with 100% of the tests having positive results. The mean (SD) gestational age at administration of the second vaccine dose was 24.9 (3.3) weeks. Neonatal IgG titers were 2.6 times higher than maternal titers (median [range], 3315.7 [350.1-17 643.5] AU/mL vs 1185.2 [146.6-32 415.1] AU/mL). A positive correlation was demonstrated between maternal and neonatal antibodies (r = 0.92; 95% CI, 0.89-0.94). Multivariable analysis revealed that for each week that passed since receipt of the second vaccine dose, maternal and neonatal antibody levels changed by −10.9% (95% CI, −17.2% to −4.2%; P = .002) and −11.7% (95% CI, −19.0 to −3.8%; P = .005), respectively. For each 1-year increase in the mother’s age, maternal and neonatal antibody levels changed by −3.1% (95% CI, −5.3% to −0.9%; P = .007) and −2.7% (95% CI, −5.2% to −0.1%; P = .04), respectively.
Conclusions and Relevance
In this cohort study, receipt of the BNT162b2 mRNA COVID-19 vaccine during the second trimester of pregnancy was associated with maternal and neonatal humoral responses, as reflected in maternal and neonatal SARS-CoV-2 IgG antibody levels measured after delivery. These findings support COVID-19 vaccination of pregnant individuals during the second trimester to achieve maternal protection and newborn safety during the pandemic.
Introduction
COVID-19 can lead to severe respiratory disease in pregnant women as well as quicker disease progression and higher rates of intensive care unit admissions than in the general population.1 It has been suggested that pregnancies complicated by COVID-19 have a higher risk for preterm birth, cesarean delivery, fetal distress, preeclampsia,2 and perinatal death.3 The chance of children becoming infected with COVID-19 is similar to that for adults; however, disease in children is usually more subtle.4,5 A severe infection in neonates and infants is uncommon5 despite the suboptimal defense of their humoral immune system.6 Several studies have shown that SARS-CoV-2 immunoglobulin G (IgG) antibodies are transferred through breast milk.7 However, IgG antibodies were not detected in the plasma of infants whose mothers were vaccinated during lactation.8
A study9 of parturient women with antibodies as a result of an infection found that SARS-CoV-2 IgG antibody concentrations in umbilical cord blood correlated with maternal antibody concentrations and with duration between onset of infection and delivery. Another study10 reported that SARS-CoV-2 antibody transfer to infants in the third trimester was significantly reduced compared with transfer of influenza- and pertussis-specific antibodies. Furthermore, levels of neutralizing SARS-CoV-2 IgG antibodies created in the beginning of pregnancy remain high at later stages and are transferred to the newborn,11 which may suggest a potential advantage in vaccinating women during early stages of pregnancy.
Gray et al12 found that the messenger RNA (mRNA) COVID-19 vaccines generate a robust humoral immune response in pregnant women similar to that observed in nonpregnant women. Similarly, Goldshtein et al13 demonstrated that mRNA COVID-19 vaccination was associated with a significantly lower risk of COVID-19 in pregnant women compared with unvaccinated women. Moreover, a multicenter study14 found that mRNA COVID-19 vaccination during the third trimester was associated with a strong maternal humoral IgG response that crossed the placental barrier and approached maternal titers in the fetus within 15 days after the first dose. A small cohort study15 found that antibody concentrations in umbilical cord blood were correlated with maternal levels and with time since vaccination.
Ideally, vaccination of pregnant individuals should result in a maximum coverage period during pregnancy while ensuring protection through delivery. In this study, we aimed to evaluate maternal and neonatal SARS-CoV-2 antibody levels at birth after vaccination during the second trimester of pregnancy. Furthermore, we reevaluated the correlation between the period of vaccination during pregnancy and antibody levels in the mother and neonate.
Methods
This prospective cohort study was conducted between May 2021 to July 2021 at the delivery room of Carmel Medical Center, Haifa, Israel. The study was approved by the Carmel Medical Center institutional review board on April 26, 2021. Written informed consent was obtained from all study participants before recruitment. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Pregnant women over 24 weeks of gestation expected to give birth within 3 days who had received their second BNT162b2 mRNA COVID-19 vaccine (Pfizer/BioNTech) within the past 7 days (vaccinated women) and were not known to be previously infected with the virus were enrolled and recruited consecutively at admission to the delivery room. Women who reported previous COVID-19 infection and those who did not receive 2 vaccine doses were excluded.
After recruitment, we obtained a maternal blood sample. An umbilical cord blood sample was obtained within 30 minutes after delivery. Both maternal and umbilical cord blood samples were assessed using SARS-CoV-2 IgG II Quant (Abbott Core Laboratory), a 2-step chemiluminescent microparticle immunoassay used for the quantitative determination of IgG antibodies to SARS-CoV-2 on Architect and Alinity i systems (Abbott Core Laboratory). The SARS-CoV-2 IgG II Quant assay is designed to detect IgG antibodies, including neutralizing antibodies, to the receptor-binding domain of the S1 subunit of the spike protein of SARS-CoV-2 virus in human serum and plasma.
After enrollment, demographic and clinical data were collected, including ethnicity (classified by the investigator), maternal age, body mass index, history of systemic disease, parity, date and gestational age at the first and second vaccine doses, systemic adverse effects including adverse events after the first and second vaccine doses, and gestational age at birth. The time interval between the first and second vaccine doses and delivery was calculated. Post partum, additional data were extracted from patients’ medical records, including newborn sex and weight.
Statistical Analysis
After data collection, the correlation between antibody titers, feto-maternal characteristics, and the time interval from vaccination to delivery were analyzed. Continuous variables are presented as means and SDs or as medians and IQRs. Categorical variables are presented as percentages. To estimate correlations with antibody levels, we used a univariable linear model (maternal and neonatal separately) with a logarithmic transformation for the antibody level because it is not normally distributed. Correlation coefficients (r) with 95% CIs for the correlation between the antibody level and duration from receipt of the second vaccine dose are presented. Variables that were found to be statistically significant (2-sided P < .05) were entered into a multivariable regression model to estimate adjusted associations with antibody levels. Correlation between the maternal and neonatal antibody levels was analyzed using the Spearman correlation. The 95% CI for the correlation coefficient was performed using SAS, version 9.4 (SAS Institute Inc). All other analyses were performed using IBM statistics, version 24 (SPSS).
Results
From May 2021 to July 2021, 130 women were recruited. Antibody titers were measured for 129 women (mean [SD] age, 31.9 [4.9] years) and 114 neonates born at a mean (SD) gestational age of 39.3 (1.3) weeks. One maternal sample was inadequately obtained (wrong test tube sent). Of 16 neonates whose antibody titers were not measured, 5 neonates did not have samples obtained; 11 additional samples were not analyzed owing to invalid blood sample obtainment (ie, wrong test tube sent or insufficient amount of blood sample sent). The mean (SD) gestational ages at administration of the first and second vaccine doses were 21.9 (3.3) weeks and 24.9 (3.3) weeks, respectively. The mean (SD) duration from the second vaccine dose to birth was 14.4 (3.0) weeks. All maternal and newborn SARS-CoV-2 IgG tests had positive results. The median level of IgG antibodies at birth was 1185.2 AU/mL (range, 146.6-32 415.1 AU/mL) for parturient women and 3315.7 AU/mL (range, 350.1-17 643.5 AU/mL) for neonates, with neonatal titers measuring approximately 2.6 times higher than maternal titers. A positive correlation was demonstrated between maternal and neonatal antibodies (r = 0.92; 95% CI, 0.89 to 0.94; P < .001). Demographic and clinical characteristics are presented in Table 1.
Table 1. Demographic and Clinical Characteristics of Pregnant Women Vaccinated With the BNT162b2 mRNA COVID-19 Vaccine.
| Characteristic | Women (N = 130)a |
|---|---|
| Maternal age, mean (SD), y | 31.9 (4.9) |
| Body mass index, median (IQR)b | 27.5 (24.9-30.0) |
| Background systemic diseasec | 30 (23.1) |
| Jewish religion | 119 (91.5) |
| Arab ethnicity | 11 (8.5) |
| Primipara | 61 (46.9) |
| Multipara (≥2 births) | 30 (23.1) |
| Grand multipara (≥5 births) | 2 (1.5) |
| Gestational age at first vaccine dose, mean (SD), wk | 21.9 (3.2) |
| Systemic adverse effects after first vaccine dosed | 13 (10) |
| Gestational age at second vaccine dose, mean (SD), wk | 24.9 (3.3) |
| Systemic adverse effectsd | |
| After second vaccine dose | 41 (31.5) |
| After first and/or second vaccine dose | 47 (36.2) |
| Gestational age at birth, mean (SD), wk | 39.3 (1.3) |
| Duration from second vaccine dose to birth, mean (SD), wk | 14.4 (3.0) |
| SARS-CoV-2 IgG antibody level, median (range), AU/mL | |
| Intrapartum maternal | 1185.2 (146.6-32 415.1) |
| Newborn | 3315.7 (350.1-17 643.5) |
| Newborn sex | |
| Male | 70 (53.8) |
| Female | 60 (46.2) |
| Newborn weight, mean (SD), g | 3265.2 (429.1) |
Data are presented as number (percentage) of women unless otherwise indicated.
Calculated as weight in kilograms divided by height in meters squared.
Hypertension (5 women), diabetes (7 women), asthma (4 women), thyroid disease (7 women), celiac (2 women), Crohn disease (1 woman), familial Mediterranean fever (1 woman), multiple sclerosis (2 women), and epidermolysis bullosa (1 woman).
General weakness, dizziness, fever, headache, general muscle aches, fatigue, and general rash.
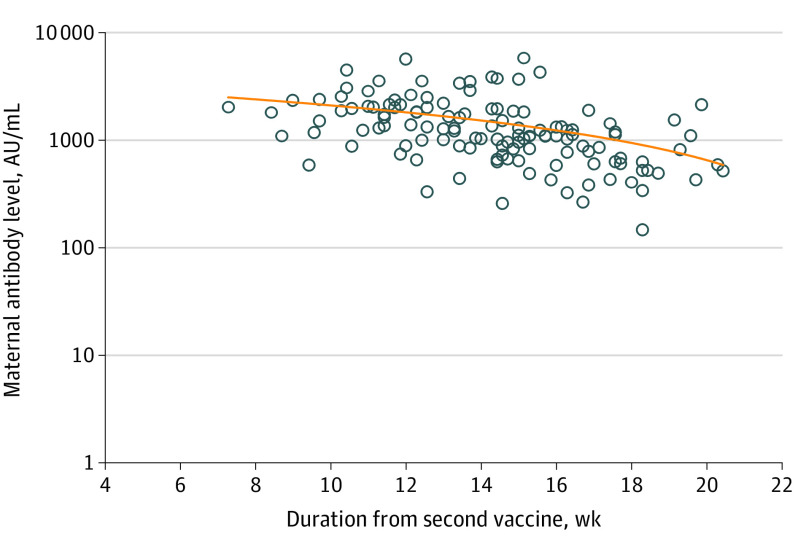
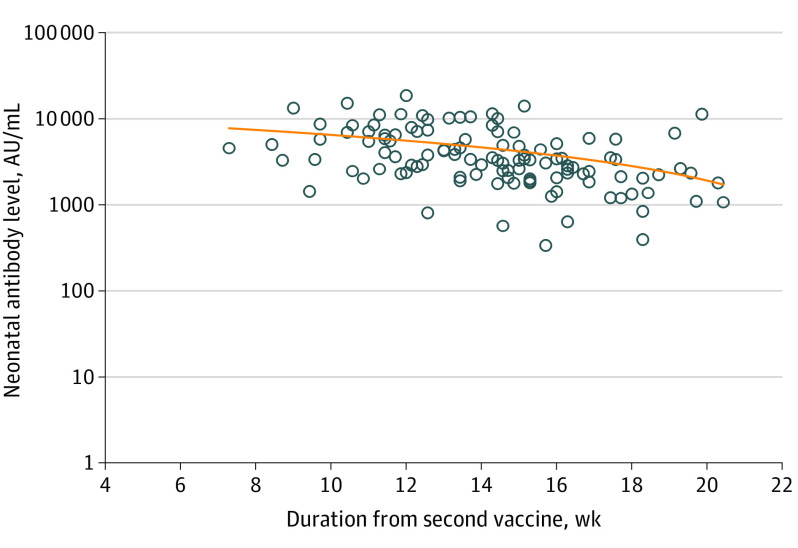
Univariable analysis demonstrated correlation of maternal and newborn SARS-CoV-2 IgG antibody titers at delivery with maternal age, gestational age at the second vaccine dose, and duration from the second vaccine dose to birth. For each 1-year increase in the mother’s age, the maternal and neonatal antibody levels changed by −3.9% (95% CI, −6.8% to −2.0%; P = .01) and −3.9% (95% CI, −5.8% to −1.0%; P = .01), respectively. In addition, for each 1-week increase in gestational age at the second vaccine dose, the maternal and neonatal antibody levels increased by 10.5% (95% CI, 7.2%-13.9%; P < .001) and 9.4% (95% CI, 5.1%-13.9%; P < .001), respectively. Furthermore, for each week that passed since the second vaccine dose, maternal and neonatal antibody levels changed by −12.1% (95% CI, −15.6% to −8.6%; P < .001) and −11.3% (95% CI, −15.0 to −6.9%; P < .001), respectively. No correlation was found between maternal SARS-CoV-2 IgG antibody levels and maternal body mass index, any maternal background systemic disease, parity, systemic adverse effects after the first and/or second vaccine dose, and gestational age at birth. No correlation was found between newborn SARS-CoV-2 IgG antibody levels and maternal body mass index, any maternal background systemic disease, parity, systemic adverse effects after the first and/or second vaccine dose, gestational age at birth, newborn sex, newborn weight, and neonatal intensive care unit admission. The correlations between the time interval from the second vaccine dose to birth and maternal or neonatal antibody levels are presented in Figure 1 and Figure 2.
Figure 1. Correlation Between the Time Interval From the Second COVID-19 Vaccine Dose and Maternal SARS-CoV-2 Immunoglobulin G Antibody Level.
Figure 2. Correlation Between the Time Interval From the Second COVID-19 Vaccine Dose and Neonatal SARS-CoV-2 Immunoglobulin G Antibody Level.
Multivariable analysis revealed an inverse correlation of maternal and neonatal antibody titers at delivery with the time interval from the second vaccine dose and with maternal age (Table 2 and Table 3). For each week that passed since the second vaccine dose, maternal and neonatal antibody levels changed by −10.9% (95% CI, −17.2% to −4.2%; P = .002) and −11.7% (95% CI, −19.0% to −3.8%; P = .005), respectively. Furthermore, for each 1-year increase in the mother’s age, the maternal and neonatal antibody levels changed by −3.1% (95% CI, −5.3% to −0.9%; P = .007) and −2.7% (95% CI, −5.2% to −0.1%; P = .04), respectively.
Table 2. Multivariable Linear Regression Analysis of Maternal SARS-CoV-2 IgG Antibody Levels at Birth After BNT162b2 mRNA COVID-19 Vaccination During the Second Trimester of Pregnancy.
| Variable | Change in maternal antibody level, % (95% CI) | P value |
|---|---|---|
| Per 1-y increase in maternal age | −3.1 (−5.3 to −0.9) | .007 |
| Per 1-wk increase from second vaccine dose to birth | −10.9 (−17.2 to −4.2) | .002 |
| Per 1-wk increase in gestational age at second vaccine dose | 0.9 (−5.3 to 7.4) | .79 |
Table 3. Multivariable Linear Regression Analysis of Newborn SARS-CoV-2 IgG Antibody Levels at Birth After BNT162b2 mRNA COVID-19 Vaccination During Pregnancy.
| Variable | Change in newborn antibody level, % (95% CI) | P value |
|---|---|---|
| Per 1-y increase in maternal age | −2.7 (−5.2 to −0.1) | .04 |
| Per 1-wk increase from second vaccine dose to birth | −11.7 (−19.0 to −3.8) | .005 |
| Per 1-wk increase in gestational age at second vaccine dose | −1.4 (−8.6 to 6.3) | .71 |
Discussion
In this prospective cohort study, receipt of the mRNA COVID-19 vaccine during the second trimester of pregnancy was associated with maternal and neonatal humoral responses at the time of delivery. Antibody titers were positive for all women during delivery, and furthermore, neonatal titers were approximately 2.6 times higher than maternal titers, representing 100% placental antibody transfer. Neonatal antibody titers were positively correlated with maternal antibody titers, and as such, higher maternal antibody titers were associated with higher neonatal antibody titers. Maternal and neonatal antibody titers were found to be inversely correlated with maternal age, meaning that in younger pregnant women, maternal and neonatal antibody levels were higher. In addition, we showed an inverse correlation of antibody titers with the time interval from the second vaccination; thus, if the second vaccination was performed earlier in pregnancy, both maternal and neonatal titers were found to be lower at delivery. No correlation was found between maternal and neonatal antibody titers and the presence of systemic adverse effects after vaccination, gestational age at birth, or newborn weight or sex.
Our results are in accordance with previous studies presenting a positive association between maternal and neonatal antibody titers.14,15,16,17,18 During the COVID-19 pandemic, research regarding vaccination of pregnant women, who may develop severe COVID-19, is of great importance. The aim of vaccinating pregnant women should be a maximal period of protection with sufficient antibody titers during labor and thus passive vaccination of neonates via placental transmission. A previous study16 found that 96% of 27 vaccinated parturient health care workers had a positive SARS-CoV-2 IgG test result at the time of delivery and that all infants of women who received their first vaccine dose more than 3 weeks before delivery had a positive SARS-CoV-2 IgG test result. However, in contrast to our findings, the authors16 found that infant antibody levels were approximately equal to maternal levels and that the transfer ratio increased with latency from vaccination. In contrast to the present study, which included mainly women vaccinated during their second trimester, Mithal et al16 included mainly women who received the COVID-19 vaccine during their third trimester. This difference suggests a natural humoral response in pregnant women, showing that earlier vaccination during pregnancy is associated with higher maternal and neonatal antibody titers at delivery, allowing sufficient time for a complete immune response. On the basis of what is known regarding different vaccines, titers crossing the placenta are suggested to differ by trimester of vaccination.12 This interpretation is in line with a previous study14 that demonstrated that the IgG transfer ratio at birth was significantly lower after infection during the third trimester than after infection during the second trimester. Passively acquired maternal antibodies with different antigen specificity have been reported to have distinct half-lives in infants.19 For example, although in pregnancy, pertussis-specific IgG levels in umbilical cord blood reach over 200% of maternal levels, maternal pertussis-specific IgG has a half-life of 6 weeks in infants and wanes to undetectable levels as early as 4 months of life.19 In contrast, maternal passively acquired measles-specific IgG remain near above protective levels in infants aged 6 months and are still detectable by 1 year of life.19
In the present study, neonatal samples had higher antibody titers than maternal samples, suggesting possible active and passive placental antibody transmission. Another explanation for this finding may be that antibody levels wane faster among mothers owing to their older age.20 Understanding the kinetics that antibodies create after COVID-19 vaccination is important when considering timing of optimal vaccination during pregnancy, which should result in a maximum coverage period during pregnancy while ensuring protection through delivery. In the present study, vaccination during the second trimester was shown to be associated with a maternal humoral response up to delivery. We found that earlier vaccination in pregnancy was inversely correlated with antibody titers; thus, there may be a point at which the antibody levels begin to stabilize and possibly decrease. The long-term antibody response to COVID-19 vaccination has not been well studied. Experience with seasonal coronaviruses and the present experience with COVID-19 suggest that immunity to natural infection might wane over time, and reinfection has been reported.21 As in natural infection, vaccine immunity has been suggested to decrease over time, and planning additional booster doses might be necessary to extend the duration of protection, particularly in pregnant women.21
The immune response to COVID-10 vaccination was shown in the present study to be negatively correlated with maternal age. Advanced maternal age was associated with lower maternal and neonatal antibody titers. This observation is in line with previous studies22,23 in the general population that showed that age was one of the important factors associated with lower antibody titers. In general, age and sex have been shown to be factors influencing the effectiveness of vaccines and antigen-specific antibodies (ie, influenza vaccine), but the level of seroprotection may not always translate to inferior clinical protection.24 Thus, physicians of pregnant women with advanced maternal age should be aware of the lower antibody levels.
Immunity derived from COVID-19 vaccination was shown to correlate with antibody titers. A recent study25 showed that in patients infected with SARS-CoV-2 despite being vaccinated, the antibody titers were lower during the peri-infection period compared with those in uninfected control individuals.
Unvaccinated pregnant women are at increased risk for severe COVID-19, with quicker disease progression and higher rates of intensive care unit admissions.1 Dagan et al18 found an estimated mRNA COVID-19 vaccine effectiveness of 96% in pregnant women but demonstrated a low prevalence of severe COVID-19 in unvaccinated pregnant women. To date, the evidence supports the safety of COVID-19 vaccination during pregnancy, but future surveillance and data are needed regarding long-term maternal and neonatal COVID-19 vaccine safety.26,27 Further research is needed to establish the duration for which maternal passively acquired SARS-CoV-2 IgG levels remain above protective levels in infants. In addition, the protective level of antibodies needed to prevent COVID-19 is still unknown, and future studies are needed to interpret the meaning of different SARS-CoV-2 antibody levels.
We believe that vaccination in this unique group of patients should be recommended especially because worse adverse outcomes were associated with pregnancy. The current study showed that COVID-19 vaccination during the second trimester was associated with high antibody titers in the mother and with even higher levels in neonates, suggesting that earlier timing of vaccination is important for mother and child. In addition, the current study found a maternal and newborn immune response at birth associated with vaccination during the second trimester, suggesting an advantage of early vaccination during pregnancy rather than during the third trimester. Later vaccination may be associated with increased risk of a pregnancy complicated by COVID-19, which has been shown to be associated with a higher risk for hazardous pregnancy outcomes.2 Additional studies are needed to assess maternal and neonatal safety as well as their immune response after COVID-19 vaccination during the first trimester.
Strengths and Limitations
The present study has several strengths. This was a prospective study, and as such, data were accurate and bias was reduced. The study included a relatively large cohort, and thus, correlations were found. Moreover, the conclusions relied on single, academic, objective laboratory results.
This study also has limitation. Selection bias cannot be ruled out because patients from only 1 medical center were included in the study. Moreover, most women in the study were vaccinated during the second trimester; therefore, we were unable to assess and compare maternal and neonatal antibody levels after vaccination during the first and third trimester. In addition, a comparison of vaccinated pregnant women with past naturally infected pregnant women was beyond the reach of the current study. Another possible limitation of our results is the assumption that women did not have a natural infection based on history alone; therefore, some may have been asymptomatic, had a mild illness, or did not have a confirmed diagnosis. Because the antibody test used in our study also recognizes IgG to nucleocapsid protein, it is possible that, in some women, priming through natural exposure exaggerated the immune response.
Conclusions
In this cohort study, receipt of the BNT162b2 mRNA COVID-19 vaccine during the second trimester of pregnancy was associated with maternal and neonatal humoral responses, as reflected by maternal and neonatal SARS-s IgG antibody levels measured after delivery. Additional studies are needed to assess the long-term effects of the COVID-19 vaccine during pregnancy as well as maternal and fetal safety after COVID-19 vaccination.
References
- 1.Allotey J, Stallings E, Bonet M, et al. ; for PregCOV-19 Living Systematic Review Consortium . Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narang K, Enninga EAL, Gunaratne MDSK, et al. SARS-CoV-2 infection and COVID-19 during pregnancy: a multidisciplinary review. Mayo Clin Proc. 2020;95(8):1750-1765. doi: 10.1016/j.mayocp.2020.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Mascio D, Khalil A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2(2):100107. doi: 10.1016/j.ajogmf.2020.100107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmermann P, Curtis N. COVID-19 in children, pregnancy and neonates: a review of epidemiologic and clinical features. Pediatr Infect Dis J. 2020;39(6):469-477. doi: 10.1097/INF.0000000000002700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang P, Wang X, Liu P, et al. Clinical characteristics and risk assessment of newborns born to mothers with COVID-19. J Clin Virol. 2020;127:104356. doi: 10.1016/j.jcv.2020.104356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323(23):2427-2429. doi: 10.1001/jama.2020.8707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pace RM, Williams JE, Järvinen KM, et al. Characterization of SARS-CoV-2 RNA, antibodies, and neutralizing capacity in milk produced by women with COVID-19. mBio. 2021;12(1):e03192-20. doi: 10.1128/mBio.03192-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golan Y, Prahl M, Cassidy AG, et al. COVID-19 mRNA vaccination in lactation: assessment of adverse effects and transfer of anti-SARS-CoV2 antibodies from mother to child. medRxiv. Preprint posted online August 3, 2021. doi: 10.1101/2021.03.09.21253241 [DOI]
- 9.Flannery DD, Gouma S, Dhudasia MB, et al. Assessment of maternal and neonatal cord blood SARS-CoV-2 antibodies and placental transfer ratios. JAMA Pediatr. 2021;175(6):594-600. doi: 10.1001/jamapediatrics.2021.0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atyeo C, Pullen KM, Bordt EA, et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell. 2021;184(3):628-642.e10. doi: 10.1016/j.cell.2020.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosma S, Carosso AR, Corcione S, et al. Longitudinal analysis of antibody response following SARS-CoV-2 infection in pregnancy: From the first trimester to delivery. J Reprod Immunol. 2021;144:103285. doi: 10.1016/j.jri.2021.103285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray KJ, Bordt EA, Atyeo C, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;225(3):303.e1-303.e17. doi: 10.1016/j.ajog.2021.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldshtein I, Nevo D, Steinberg DM, et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021;326(8):728-735. doi: 10.1001/jama.2021.11035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beharier O, Plitman Mayo R, Raz T, et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J Clin Invest. 2021;131(13):150319. doi: 10.1172/JCI150319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rottenstreich A, Zarbiv G, Oiknine-Djian E, Zigron R, Wolf DG, Porat S. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin Infect Dis. 2021;ciab266. doi: 10.1093/cid/ciab266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mithal LB, Otero S, Shanes ED, Goldstein JA, Miller ES. Cord blood antibodies following maternal coronavirus disease 2019 vaccination during pregnancy. Am J Obstet Gynecol. 2021;225(2):192-194. doi: 10.1016/j.ajog.2021.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zdanowski W, Waśniewski T. Evaluation of SARS-CoV-2 spike protein antibody titers in cord blood after COVID-19 vaccination during pregnancy in Polish healthcare workers: preliminary results. Vaccines (Basel). 2021;9(6):675. doi: 10.3390/vaccines9060675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dagan N, Barda N, Biron-Shental T, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med. 2021;27(10):1693-1695. doi: 10.1038/s41591-021-01490-8 [DOI] [PubMed] [Google Scholar]
- 19.Fouda GG, Martinez DR, Swamy GK, Permar SR. The Impact of IgG transplacental transfer on early life immunity. Immunohorizons. 2018;2(1):14-25. doi: 10.4049/immunohorizons.1700057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stiasny K, Aberle JH, Keller M, Grubeck-Loebenstein B, Heinz FX. Age affects quantity but not quality of antibody responses after vaccination with an inactivated flavivirus vaccine against tick-borne encephalitis. PLoS One. 2012;7(3):e34145. doi: 10.1371/journal.pone.0034145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JH, Marks F, Clemens JD. Looking beyond COVID-19 vaccine phase 3 trials. Nat Med. 2021;27(2):205-211. doi: 10.1038/s41591-021-01230-y [DOI] [PubMed] [Google Scholar]
- 22.Nomura Y, Sawahata M, Nakamura Y, et al. Age and smoking predict antibody titres at 3 months after the second dose of the BNT162b2 COVID-19 vaccine. Vaccines (Basel). 2021;9(9):1042. doi: 10.3390/vaccines9091042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collier DA, Ferreira IATM, Kotagiri P, et al. ; CITIID-NIHR BioResource COVID-19 Collaboration . Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596(7872):417-422. doi: 10.1038/s41586-021-03739-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saeed Z, Greer O, Shah NM. Is the host viral response and the immunogenicity of vaccines altered in pregnancy? Antibodies (Basel). 2020;9(3):E38. doi: 10.3390/antib9030038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergwerk M, Gonen T, Lustig Y, et al. COVID-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474-1484. doi: 10.1056/NEJMoa2109072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia S, Duan K, Zhang Y, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951-960. doi: 10.1001/jama.2020.15543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciapponi A, Bardach A, Mazzoni A, et al. Safety of COVID-19 vaccines, their components or their platforms for pregnant women: a rapid review. medRxiv. Preprint posted online June 6, 2021. doi: 10.1101/2021.06.03.21258283 [DOI]