Key Points
Question
Is there a difference in risk of major ischemic or hemorrhagic events in patients with atrial fibrillation treated with rivaroxaban vs apixaban?
Findings
In this retrospective cohort study that included 581 451 patients 65 years or older enrolled in Medicare with atrial fibrillation, the adjusted incidence of major ischemic or hemorrhagic events was 16.1 per 1000 person-years for rivaroxaban vs 13.4 per 1000 person-years for apixaban, a difference that was statistically significant.
Meaning
Among older adults with atrial fibrillation, treatment with rivaroxaban compared with apixaban was associated with a significantly increased risk of major ischemic or hemorrhagic events.
Abstract
Importance
The comparative effectiveness of rivaroxaban and apixaban, the most frequently prescribed oral anticoagulants for ischemic stroke prevention in patients with atrial fibrillation, is uncertain.
Objective
To compare major ischemic and hemorrhagic outcomes in patients with atrial fibrillation treated with rivaroxaban or apixaban.
Design, Setting, and Participants
Retrospective cohort study using computerized enrollment and claims files for US Medicare beneficiaries 65 years or older. Between January 1, 2013, and November 30, 2018, a total of 581 451 patients with atrial fibrillation began rivaroxaban or apixaban treatment and were followed up for 4 years, through November 30, 2018.
Exposures
Rivaroxaban (n = 227 572) and apixaban (n = 353 879), either standard or reduced dose.
Main Outcomes and Measures
The primary outcome was a composite of major ischemic (stroke/systemic embolism) and hemorrhagic (intracerebral hemorrhage/other intracranial bleeding/fatal extracranial bleeding) events. Secondary outcomes were nonfatal extracranial bleeding and total mortality (fatal ischemic/hemorrhagic event or other death during follow-up). Rates, hazard ratios (HRs), and rate differences (RDs) were adjusted for baseline differences in comorbidity with inverse probability of treatment weighting.
Results
Study patients (mean age, 77.0 years; 291 966 [50.2%] women; 134 393 [23.1%] receiving reduced dose) had 474 605 person-years of follow-up (median [IQR] of 174 [62-397] days). The adjusted primary outcome rate for rivaroxaban was 16.1 per 1000 person-years vs 13.4 per 1000 person-years for apixaban (RD, 2.7 [95% CI, 1.9-3.5]; HR, 1.18 [95% CI, 1.12-1.24]). The rivaroxaban group had increased risk for both major ischemic events (8.6 vs 7.6 per 1000 person-years; RD, 1.1 [95% CI, 0.5-1.7]; HR, 1.12 [95% CI, 1.04-1.20]) and hemorrhagic events (7.5 vs 5.9 per 1000 person-years; RD, 1.6 [95% CI, 1.1-2.1]; HR, 1.26 [95% CI, 1.16-1.36]), including fatal extracranial bleeding (1.4 vs 1.0 per 1000 person-years; RD, 0.4 [95% CI, 0.2-0.7]; HR, 1.41 [95% CI, 1.18-1.70]). Patients receiving rivaroxaban had increased risk of nonfatal extracranial bleeding (39.7 vs 18.5 per 1000 person-years; RD, 21.1 [95% CI, 20.0-22.3]; HR, 2.07 [95% CI, 1.99-2.15]), fatal ischemic/hemorrhagic events (4.5 vs 3.3 per 1000 person-years; RD, 1.2 [95% CI, 0.8-1.6]; HR, 1.34 [95% CI, 1.21-1.48]), and total mortality (44.2 vs 41.0 per 1000 person-years; RD, 3.1 [95% CI, 1.8-4.5]; HR, 1.06 [95% CI, 1.02-1.09]). The risk of the primary outcome was increased for rivaroxaban in both those receiving the reduced dose (27.4 vs 21.0 per 1000 person-years; RD, 6.4 [95% CI, 4.1-8.7]; HR, 1.28 [95% CI, 1.16-1.40]) and the standard dose (13.2 vs 11.4 per 1000 person-years; RD, 1.8 [95% CI, 1.0-2.6]; HR, 1.13 [95% CI, 1.06-1.21]) groups.
Conclusions and Relevance
Among Medicare beneficiaries 65 years or older with atrial fibrillation, treatment with rivaroxaban compared with apixaban was associated with a significantly increased risk of major ischemic or hemorrhagic events.
This cohort study assesses major ischemic and hemorrhagic outcomes in Medicare beneficiaries with atrial fibrillation who were treated with rivaroxaban compared with apixaban.
Introduction
An estimated 3 million to 6 million persons in the US have atrial fibrillation, and these numbers are projected to reach 6 million to 16 million by 2050.1 Atrial fibrillation increases the risk of stroke 5-fold and is thought to cause 15% of all strokes2; thus, anticoagulation to prevent ischemic strokes is a critical component of management for this chronic disease.3 Direct oral anticoagulants—with more predictable pharmacokinetics, greater ease of use, and equally good or better clinical outcomes than vitamin K antagonists—are the preferred anticoagulants for patients with atrial fibrillation.3 Of the 4 drugs in this class licensed in the US for use in atrial fibrillation, rivaroxaban and apixaban now account for nearly all direct oral anticoagulant prescriptions and are prescribed more frequently than warfarin.4
The beneficial and adverse effects of direct oral anticoagulants are closely related to plasma concentrations; plasma concentrations that are too low fail to prevent ischemic strokes or systemic embolisms and those that are too high increase the risk of severe bleeding.5,6,7 Although apixaban and rivaroxaban are both reversible inhibitors of activated factor X (Xa) and have comparable elimination half-lives,5 apixaban is taken twice daily, whereas there is only a single daily dose for rivaroxaban. Thus, there is substantially greater peak-trough variation in rivaroxaban concentrations,5,8 which raises the concern that this medication may have poorer efficacy and safety. Because differences in the clinical outcomes of rivaroxaban and apixaban would have major health implications for the millions of patients with prolonged use for stroke prevention, the objective of this retrospective cohort study was to compare major ischemic and hemorrhagic outcomes in Medicare beneficiaries with atrial fibrillation who initiated anticoagulation treatment with rivaroxaban or apixaban.
Methods
Cohort and Follow-up
Medicare Data
The computerized files of the US Medicare program, which provides health care insurance for US citizens 65 years or older and younger persons with disabilities,9 provided the study data. Files included the Medicare Master Beneficiary Summary File, which includes enrollment status and identifies deaths for beneficiaries,9 as well as claims files for medical care services (pharmacy, hospital, outpatient, and nursing home). The data resided in the Centers for Medicare & Medicaid Services Chronic Condition Warehouse and were accessed through the Virtual Research Data Center, a cloud-based repository of deidentified Medicare files.10 In accordance with the Centers for Medicare & Medicaid Services policy, no table cells with fewer than 11 patients were reported. The study was approved by the Vanderbilt University Medical Center Institutional Review Board, with waiver of informed consent.
Cohort
The cohort (eTable 1 in the Supplement) consisted of Medicare beneficiaries 65 years or older with fee-for-service (Parts A and B) and prescription drug (Part D) coverage. Medicare Advantage (Part C) enrollees were excluded because the encounter data were considered less reliable during the study years.9 Participants had to have complete demographic information and fill a prescription for apixaban or rivaroxaban with either the standard (5 mg twice daily for apixaban and 20 mg once daily for rivaroxaban) or reduced (2.5 mg twice daily for apixaban and 15 mg once daily for rivaroxaban) dose for atrial fibrillation between January 1, 2013 (first year of apixaban use in Medicare), and November 30, 2018 (most recent data). The reduced dose is indicated for patients with factors likely to increase plasma concentrations.2,11
During the preceding year, study patients had to have continuous enrollment in Medicare and, to ensure regular contact with medical care, at least 1 outpatient visit and 1 filled prescription (other than the study anticoagulant). They could not have terminal illness, long-term care residence (except <30 days following inpatient stay), mitral valve stenosis, or severe chronic kidney disease (stage 4, 5, or end-stage). The primary analysis included patients with heart valve replacement, because direct oral anticoagulants are an option for those with bioprosthetic heart valves. For those with mechanical heart valves, direct oral anticoagulants are contraindicated and thus those patients would be excluded from the cohort.3 There could be no evidence of outpatient oral anticoagulant use during the past year (eTable 1 in the Supplement), thus restricting the cohort to new users of oral anticoagulants.12
Participants had to have a diagnosis of atrial fibrillation/flutter in the past 90 days. They could not have had conditions that can cause reversible atrial fibrillation (eg, thyrotoxicosis13) or an alternative oral anticoagulant indication in the past 30 days (eTable 1 in the Supplement). Cohort members could not have had a stroke or bleeding-related hospitalization in the past 30 days, because subsequent readmissions might be confused with new events.
Follow-up
Cohort members were followed up for up to 4 years, beginning the day after filling the initial oral anticoagulant prescription. Follow-up ended (eAppendix §1 in the Supplement) with a gap of more than 30 days in anticoagulant days of supply (indicating discontinuation); the filling of a prescription for a different anticoagulant; change in anticoagulant dose; development of stage 4, 5, or end-stage chronic kidney disease; the last study day (November 30, 2018, or 4 years after anticoagulant initiation); loss of full fee-for-service Medicare enrollment; occurrence of any study outcome; or death. Patients who left the cohort could not reenter. The proportion of patients who discontinued or changed the study anticoagulant by the median time of follow-up was estimated with the cumulative incidence function, considering other causes of loss to follow-up as censoring.
Outcomes
Definitions
The primary study outcome was the following major ischemic or hemorrhagic events: ischemic stroke, systemic embolism, hemorrhagic stroke, other intracranial bleeding, and fatal extracranial bleeding (death within 30 days14,15 of bleeding onset). Secondary outcomes were nonfatal extracranial bleeding and total mortality, which included fatal ischemic or hemorrhagic events (death within 30 days of event onset) and other deaths during follow-up.
Identification
Strokes and bleeding events were identified from hospital principal discharge diagnosis codes (eAppendix §2 in the Supplement). The codes for strokes (eAppendix §2.1 and eTable 2 in the Supplement) have positive predictive values greater than 95% and sensitivity of 87% for strokes seen in the hospital.16,17 Intracranial and extracranial bleeding were identified by a previously validated algorithm (eAppendix §2.2, eFigure, eTables 3 and 4 in the Supplement) with positive predictive values of 89% for probable bleeds and 99% for probable/possible bleeds18,19 and a sensitivity of 93% for bleeds with hospitalization.20 The codes for intracranial bleeding also included those for traumatic intracranial hemorrhages (except those indicating open wounds) to capture fall-related events. The outcome occurrence date was generally that of hospital admission, but was set to the prior day when appropriate (eg, emergency department visit on prior day with stroke diagnosis; see eAppendix §2 in the Supplement).
Statistical Analyses
Study comparisons controlled for 208 covariates potentially associated with both outcomes and anticoagulant choice. The covariates (eTable 5 in the Supplement), defined from enrollment history and claims in the year preceding cohort entry, were chosen based on previous studies of anticoagulants,9,15,18,19,21,22 standard measures of comorbidity,23 and indicators of frailty.24 They included demographic characteristics, cardiovascular conditions (including components of the claims-based CHA2DS2-VASc [congestive heart failure, hypertension, age ≥75 years (doubled), diabetes, stroke/transient ischemic attack/thromboembolism (doubled), vascular disease (prior myocardial infarction, peripheral artery disease, or aortic plaque)] score25,26,27,28), risk factors for bleeding, respiratory illness, neurologic conditions, measures of frailty, cancer, and medical care utilization. Race (self-reported to the US Social Security Administration via fixed categories) was included as a marker of social, environmental, and genetic factors that potentially influence anticoagulant outcomes.29
The analysis controlled for covariates with stabilized inverse probability of treatment weights calculated from the propensity score.30 The propensity score, the probability of rivaroxaban use given baseline covariates,30 was estimated with logistic regression that was stratified by anticoagulant dose because factors influencing anticoagulant choice could differ with dose. The propensity score distributions in the rivaroxaban and apixaban groups had good overlap (eTable 6 in the Supplement).
With a properly constituted propensity score, inverse probability of treatment weighting controls for confounding by eliminating imbalances in measured covariates between the study groups. The magnitude of covariate imbalances was assessed with the standardized difference; differences of less than 0.10 are considered to indicate acceptable balance.30
The adjusted relative risk of the outcomes was estimated with hazard ratios (HRs) calculated from an inverse probability of treatment–weighted proportional hazards regression. The regression was stratified by dose, permitting the hazard function to differ according to dose.31 Weighting-induced dependencies were corrected with modified sandwich variance estimation.31 The rate difference (RD), or difference in the absolute adjusted incidence between rivaroxaban and apixaban, was estimated assuming the Poisson distribution, with variances calculated via generalized estimating equations to compensate for weighting-induced dependencies. In a prespecified analysis, HRs and RDs were calculated for patients according to dose.
Sensitivity analyses assessed the effects of alternative study definitions or statistical analyses. Cohort eligibility criteria were modified to exclude patients with heart valve replacement (limited data supporting direct oral anticoagulant use3), unspecified or stage 3 chronic kidney disease (rivaroxaban elimination more dependent on kidney function32), or extreme propensity scores (trimming first percentile contrary to prediction, which can reduce unmeasured confounding33). Follow-up was limited to the year following cohort entry (up to 4 years in the primary analysis), which reduced the effects of changes in baseline covariates and censoring for discontinuation or switching of medication. The maximum allowed gap in anticoagulant days of supply was decreased from 30 days to 7 days, which would reduce exposure misclassification. The stroke definition was broadened to identify strokes with both the principal and secondary hospital discharge diagnoses (eAppendix §2 in the Supplement), which would increase the sensitivity to 93%.17 Other alternative analyses included propensity score–matched treatment groups and estimation of HRs from a proportional hazards regression with all of the study covariates in place of the propensity score weighting. The potential influence of unmeasured confounding on findings was assessed by calculating the E-value, the minimum strength of the association of an unmeasured confounder with both the primary outcome and the initiation of rivaroxaban treatment required to explain the observed study HR.34
All statistical analyses were performed with SAS, version 9.4 (SAS Institute). Statistical significance was defined as a 95% CI that excluded 0 (RD) or 1 (HR). Because CIs were not adjusted for multiple comparisons, analyses of secondary end points should be interpreted as exploratory.
Results
Cohort
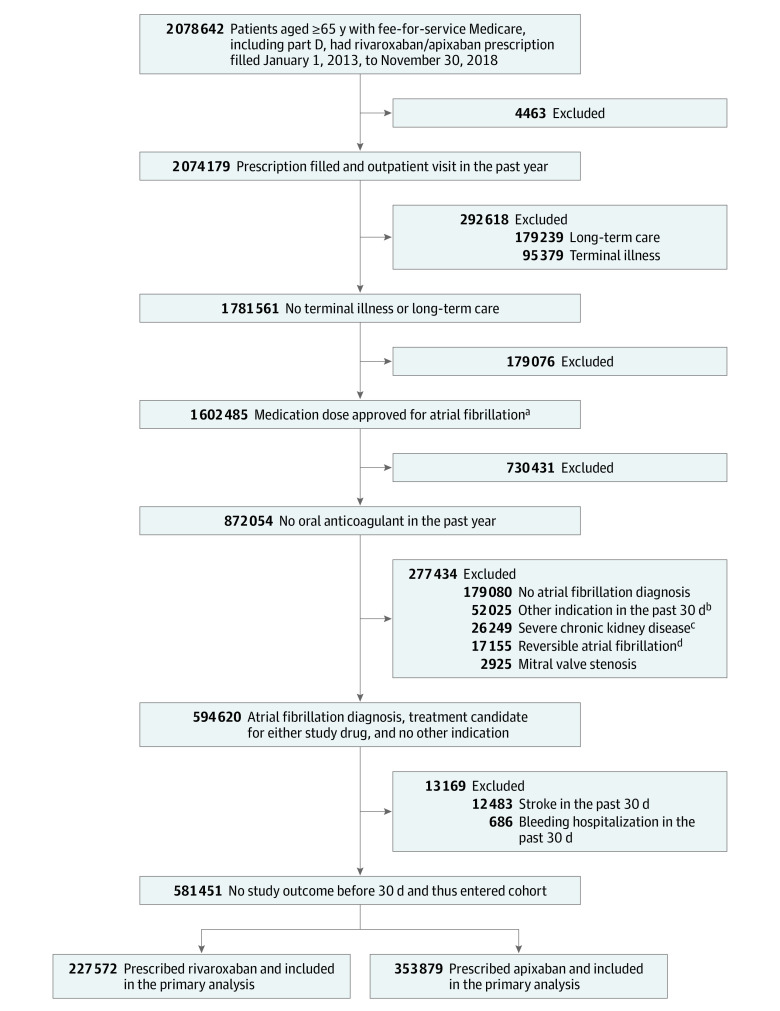
The cohort included 581 451 patients with atrial fibrillation initiating oral anticoagulant treatment, with 227 572 patients in the rivaroxaban group and 353 879 in the apixaban group (Figure 1). Participants had a mean age of 77.0 years, 291 966 (50.2%) were women, and 134 393 (23.1%) received the reduced anticoagulant dose. Prior to inverse probability of treatment weighting, patients in the rivaroxaban group (Table 1; eTable 5 in the Supplement) were younger (76.3 vs 77.4 years), were less likely to be women (48.3% vs 51.4%), and had a lower mean CHA2DS2-VASc score (4.2 vs 4.4) than patients in the apixaban group. Patients in the rivaroxaban group also had lower prevalence of several risk factors for study outcomes, including history of stroke or intracranial bleeding (8.5% vs 9.8%), myocardial infarction (5.6% vs 6.8%), heart failure (27.9% vs 31.2%), acute kidney failure (7.6% vs 10.5%), stage 3 or unspecified chronic kidney disease (13.8% vs 17.9%), history of bleeding (14.4% vs 15.6%), anemia (24.6% vs 27.1%), unintentional fall (9.5% vs 11.5%), and hospitalization in the 30 days before anticoagulant initiation (26.7% vs 29.0%). After weighting (Table 1; eTable 5 in the Supplement), differences in covariate prevalence were minimal, with all standardized differences no more than 0.01.
Figure 1. Selection and Inclusion of Participants in a Study of the Association of Rivaroxaban vs Apixaban With Major Ischemic or Hemorrhagic Events in Atrial Fibrillation.
aApixaban (2.5 mg or 5 mg twice/d) or rivaroxaban (15 mg or 20 mg once/d).
bDeep vein thrombosis/pulmonary embolism, hip/knee replacement, femur/tibia/patella fracture, thrombectomy, or chronic hypercoagulable state.
cStage 4 or 5 or end-stage chronic kidney disease.
dDiagnosis of hyperthyroidism or open coronary artery bypass graft/open cardiac valve surgery.
Table 1. Participant Demographics, Medical History, and Medications at Baseline in a Study of the Association of Rivaroxaban vs Apixaban With Major Ischemic or Hemorrhagic Events in Atrial Fibrillationa,b.
| Characteristic | Unweighted | Weighted | ||||
|---|---|---|---|---|---|---|
| Rivaroxaban, % | Apixaban, % | Standardized difference | Rivaroxaban, % | Apixaban, % | Standardized difference | |
| No. of participants | 227 572 | 353 879 | 227 572 | 353 879 | ||
| Anticoagulant dose reduced | 23.0 | 23.2 | 0.0039 | 23.2 | 23.1 | 0.0012 |
| Demographics | ||||||
| Age, mean (SD), y | 76.3 (6.8) | 77.4 (7.2) | 0.1586 | 77.0 (7.1) | 77.0 (7.0) | 0.0023 |
| Year anticoagulant started, mean | 2015.5 | 2016.3 | 0.5409 | 2016.0 | 2016.0 | 0.0018 |
| Women | 48.3 | 51.4 | 0.0627 | 50.0 | 50.0 | 0.0007 |
| Men | 51.7 | 48.6 | 0.0627 | 50.0 | 50.0 | 0.0007 |
| Race and ethnicityc | ||||||
| Asian | 1.6 | 1.3 | 0.0205 | 1.5 | 1.4 | 0.0014 |
| Black | 3.6 | 3.7 | 0.0017 | 3.6 | 3.7 | 0.0008 |
| Hispanic | 1.2 | 1.0 | 0.0182 | 1.1 | 1.0 | 0.0087 |
| North American Native | 0.3 | 0.2 | 0.0162 | 0.3 | 0.2 | 0.0092 |
| White | 92.0 | 92.6 | 0.0224 | 92.4 | 92.4 | 0.0020 |
| Other | 1.2 | 1.1 | 0.0100 | 1.1 | 1.2 | 0.0081 |
| Region of residence | ||||||
| West | 16.1 | 14.1 | 0.0567 | 15.0 | 15.0 | 0.0002 |
| Southwest | 10.6 | 10.6 | 0.0007 | 10.6 | 10.6 | 0.0006 |
| Midwest | 22.2 | 21.3 | 0.0226 | 21.5 | 21.5 | 0.0010 |
| Southeast | 33.1 | 36.7 | 0.0747 | 35.4 | 35.3 | 0.0020 |
| Northeast | 17.9 | 17.3 | 0.0153 | 17.5 | 17.6 | 0.0018 |
| Dual Medicare-Medicaid enrollment | 13.9 | 12.8 | 0.0332 | 13.1 | 13.2 | 0.0032 |
| Cardiologist-prescribed anticoagulant | 49.1 | 49.0 | 0.0030 | 49.8 | 49.6 | 0.0037 |
| Medical history (past year unless otherwise noted) | ||||||
| CHA2DS2-VASc score, meand | 4.2 | 4.4 | 0.1276 | 4.3 | 4.3 | 0.0038 |
| Hypertension | 89.7 | 90.7 | 0.0309 | 90.3 | 90.3 | 0.0008 |
| Diabetes | 35.0 | 34.8 | 0.0031 | 34.8 | 34.9 | 0.0009 |
| Heart failure | 27.9 | 31.2 | 0.0723 | 29.8 | 29.8 | 0.0005 |
| Anemia | 24.6 | 27.1 | 0.0572 | 26.1 | 26.1 | 0.0018 |
| Chronic obstructive pulmonary disease | 21.9 | 22.9 | 0.0249 | 22.3 | 22.3 | 0.0003 |
| Cancer other than nonmelanoma skin cancer | 17.8 | 18.4 | 0.0174 | 18.1 | 18.2 | 0.0008 |
| Bleeding at gastrointestinal or other sites | 14.4 | 15.6 | 0.0314 | 15.1 | 15.1 | 0.0006 |
| Kidney disease, stage 3 or unspecified chronic | 13.8 | 17.9 | 0.1113 | 16.3 | 16.3 | 0.0002 |
| Percutaneous coronary intervention | 11.4 | 12.9 | 0.0447 | 12.3 | 12.3 | 0.0004 |
| Dysphagia/malnutrition | 11.1 | 12.9 | 0.0559 | 12.2 | 12.2 | 0.0004 |
| Coronary artery bypass graft | 9.8 | 10.9 | 0.0339 | 10.4 | 10.4 | 0.0001 |
| Fall | 9.5 | 11.5 | 0.0637 | 10.7 | 10.7 | 0.0003 |
| Cardioversion | 8.9 | 9.4 | 0.0147 | 9.2 | 9.2 | 0.0012 |
| Ischemic stroke, systemic embolism, intracranial bleeding | 8.5 | 9.8 | 0.0466 | 9.3 | 9.2 | 0.0019 |
| Acute kidney failure | 7.6 | 10.5 | 0.0999 | 9.3 | 9.3 | 0.0006 |
| Alzheimer disease and other dementia | 6.9 | 8.2 | 0.0496 | 7.7 | 7.7 | 0.0005 |
| Transient ischemic attack | 6.2 | 6.8 | 0.0263 | 6.6 | 6.5 | 0.0009 |
| Myocardial infarction | 5.6 | 6.8 | 0.0498 | 6.3 | 6.3 | 0.0005 |
| Inpatient discharge past 30 d | 26.7 | 29.0 | 0.0512 | 27.2 | 27.3 | 0.0032 |
| Medication history (past year) | ||||||
| Angiotensin-converting enzyme inhibitor/receptor blocker | 61.5 | 61.7 | 0.0053 | 61.6 | 61.7 | 0.0018 |
| Proton pump inhibitors | 30.2 | 31.5 | 0.0281 | 31.0 | 31.0 | 0.0003 |
| Loop diuretics | 26.1 | 28.9 | 0.0634 | 27.7 | 27.8 | 0.0005 |
| Oral corticosteroids | 24.2 | 25.7 | 0.0334 | 25.0 | 25.0 | 0.0006 |
| Diltiazem or verapamil | 21.5 | 21.1 | 0.0091 | 21.2 | 21.2 | 0.0003 |
| Nonselective nonsteroidal anti-inflammatory drugs | 17.3 | 16.3 | 0.0261 | 16.7 | 16.7 | 0.0002 |
| Platelet ADP P2Y12 receptor inhibitors and other antiplatelet drugs | 15.1 | 16.6 | 0.0404 | 16.0 | 16.0 | 0.0004 |
| Amiodarone | 10.9 | 11.8 | 0.0275 | 11.4 | 11.4 | 0.0005 |
| Home oxygen | 9.8 | 10.6 | 0.0266 | 10.2 | 10.2 | 0.0000 |
| Insulin | 6.1 | 6.6 | 0.0188 | 6.4 | 6.4 | 0.0015 |
| Dronedarone | 2.7 | 2.6 | 0.0099 | 2.7 | 2.6 | 0.0006 |
| No. of medications, mean | 11.8 | 12.1 | 0.0577 | 12.0 | 12.0 | 0.0043 |
Some important covariates selected by the authors as examples of the study covariates; see eTable 5 in the Supplement for the complete set of covariates.
The standardized difference (absolute value of the difference between variable means divided by the SD of the difference) is a measure of balance of covariates between treatment groups, with values <0.1 considered to indicate good balance.
Self-reported race and ethnicity excludes 2369 patients in the rivaroxaban group and 3703 in the apixaban group for whom information was unknown. The categories are as defined in the Medicare data, including the "other" category. The low proportion of Black participants may be related to the substantially lower incidence of atrial fibrillation in this population.29
The CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years [doubled], diabetes, stroke/transient ischemic attack/thromboembolism [doubled], vascular disease [prior myocardial infarction, peripheral artery disease, or aortic plaque]) score for stroke risk stratification25 gives 1 point for age 65-74 y, female sex, heart failure, hypertension, diabetes, vascular disease (myocardial infarction, peripheral artery disease, aortic plaque) and 2 points for age ≥75 y, stroke/transient ischemic attack/thromboembolism. The claims-based version of the score26,27,28 in the table was calculated from Medicare encounters.
Follow-up
Between January 1, 2013, and November 30, 2018, participants had 474 605 person-years of follow-up, with median (IQR) follow-up of 174 (62-397) days (171 [59-407] d for rivaroxaban and 176 [64-392] d for apixaban). By day 174 of follow-up, patients treated with rivaroxaban were more likely to discontinue the study drug or switch to a different oral anticoagulant than patients treated with apixaban (discontinue: 33.4% [95% CI, 33.1%-33.6%] vs 30.4% [95% CI, 30.3%-30.6%]; switch: 7.0% [95% CI, 6.9%-7.1%] vs 4.5% [95% CI, 4.5%-4.6%]; eTable 7 in the Supplement).
Primary Outcome
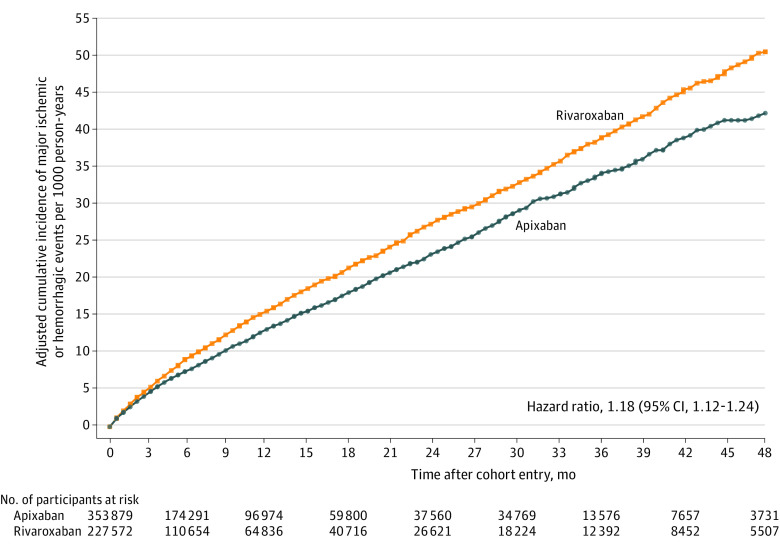
There were 6946 (14.6 per 1000 person-years) major ischemic or hemorrhagic events during follow-up: 3807 (8.0/1000 person-years) ischemic events and 3139 (6.6/1000 person-years) hemorrhagic events. The risk of the primary outcome (Figure 2; Table 2) was greater for rivaroxaban, with adjusted rates of 16.1 vs 13.4 per 1000 person-years (RD, 2.7 [95% CI, 1.9-3.5] per 1000 person-years; HR, 1.18 [95% CI, 1.12-1.24]). The rivaroxaban group had increased risk for both major ischemic events (adjusted rate, 8.6 vs 7.6 per 1000 person-years; RD, 1.1 [95% CI, 0.5-1.7]; HR, 1.12 [95% CI, 1.04-1.20]) and major hemorrhagic events (adjusted rate, 7.5 vs 5.9 per 1000 person-years; RD, 1.6 [95% CI, 1.1-2.1]; HR, 1.26 [95% CI, 1.16-1.36]). Patients treated with rivaroxaban had increased risk for ischemic stroke (adjusted rate, 8.3 vs 7.2 per 1000 person-years; RD, 1.1 [95% CI, 0.5-1.6]; HR, 1.12 [95% CI, 1.05-1.21]), hemorrhagic stroke (adjusted rate, 2.5 vs 1.7 per 1000 person-years; RD, 0.8 [95% CI, 0.5-1.1]; HR, 1.48 [95% CI, 1.30-1.70]), and fatal extracranial bleeding (adjusted rate, 1.4 vs 1.0 per 1000 person-years; RD, 0.4 [95% CI, 0.2-0.7]; HR, 1.41 [95% CI, 1.18-1.70]).
Figure 2. Primary Outcome in a Study of the Association of Rivaroxaban vs Apixaban With Major Ischemic or Hemorrhagic Events in Atrial Fibrillation.
Adjusted cumulative incidence of major ischemic (ischemic stroke or systemic embolism) or hemorrhagic (hemorrhagic stroke, other intracranial bleeding, fatal extracranial bleeding) events. The median (IQR) follow-up time was 5.8 (2.1-13.2) months. Adjusted with inverse probability of treatment weighting; the variables used in the adjustment are shown in eTable 5 in the Supplement.
Table 2. Outcomes in a Study of the Association of Rivaroxaban vs Apixaban With Major Ischemic or Hemorrhagic Events in Atrial Fibrillationa.
| Outcome | Rivaroxaban (191 153 person-years) |
Apixaban (283 452 person-years) |
Adjusted | |||||
|---|---|---|---|---|---|---|---|---|
| Rate per 1000 person-years | Rate difference (95% CI) |
Hazard ratio (95% CI) | ||||||
| Patients with event, No. | Rate/1000 person-years | Patients with event, No. | Rate/1000 person-years | Rivaroxaban | Apixaban | |||
| Primary outcome and its components | ||||||||
| Major ischemic or hemorrhagic event | 2838 | 14.8 | 4108 | 14.5 | 16.1 | 13.4 | 2.7 (1.9 to 3.5) | 1.18 (1.12 to 1.24) |
| Ischemic event | 1514 | 7.9 | 2293 | 8.1 | 8.6 | 7.6 | 1.1 (0.5 to 1.7) | 1.12 (1.04 to 1.20) |
| Ischemic stroke | 1447 | 7.6 | 2196 | 7.7 | 8.3 | 7.2 | 1.1 (0.5 to 1.6) | 1.12 (1.05 to 1.21) |
| Systemic embolism | 67 | 0.4 | 97 | 0.3 | 0.4 | 0.3 | 0.0 (−0.1 to 0.1) | 1.05 (0.75 to 1.46) |
| Hemorrhagic event | 1324 | 6.9 | 1815 | 6.4 | 7.5 | 5.9 | 1.6 (1.1 to 2.1) | 1.26 (1.16 to 1.36) |
| Hemorrhagic stroke | 459 | 2.4 | 515 | 1.8 | 2.5 | 1.7 | 0.8 (0.5 to 1.1) | 1.48 (1.30 to 1.70) |
| Other intracranial hemorrhage | 624 | 3.3 | 994 | 3.5 | 3.5 | 3.2 | 0.3 (−0.1 to 0.7) | 1.09 (0.98 to 1.22) |
| Fatal extracranial bleeding | 241 | 1.3 | 306 | 1.1 | 1.4 | 1.0 | 0.4 (0.2 to 0.7) | 1.41 (1.18 to 1.70) |
| Secondary outcomes | ||||||||
| Nonfatal extracranial bleeding | 6919 | 36.2 | 5672 | 20.0 | 39.7 | 18.5 | 21.1 (20.0 to 22.3) | 2.07 (1.99 to 2.15) |
| Gastrointestinal | 6132 | 32.1 | 4974 | 17.5 | 35.2 | 16.3 | 19.0 (17.9 to 20.1) | 2.09 (2.01 to 2.18) |
| Other or unspecified | 787 | 4.1 | 698 | 2.5 | 4.4 | 2.3 | 2.2 (1.8 to 2.5) | 1.89 (1.69 to 2.11) |
| Total mortality | 7497 | 39.2 | 12 839 | 45.3 | 44.2 | 41.0 | 3.1 (1.8 to 4.5) | 1.06 (1.02 to 1.09) |
| Fatal ischemic or hemorrhagic event | 767 | 4.0 | 1039 | 3.7 | 4.5 | 3.3 | 1.2 (0.8 to 1.6) | 1.34 (1.21 to 1.48) |
| Other death during follow-up | 6730 | 35.2 | 11 800 | 41.6 | 39.7 | 37.7 | 1.9 (0.6 to 3.2) | 1.03 (0.995 to 1.07) |
Adjusted rates, rate differences, and hazard ratios are adjusted with inverse probability of treatment weighting. The variables used in the adjustment are shown in eTable 5 in the Supplement.
Secondary Outcomes
Patients receiving rivaroxaban had increased risk of the secondary outcomes (Table 2). The risk for nonfatal extracranial bleeding in the rivaroxaban group was increased relative to that in the apixaban group (39.7 vs 18.5 per 1000 person-years; RD, 21.1 [95% CI, 20.0-22.3]; HR, 2.07 [95% CI, 1.99-2.15]), including the risk for bleeding at gastrointestinal sites (35.2 vs 16.3 per 1000 person-years; RD, 19.0 [95% CI, 17.9-20.1]; HR, 2.09 [95% CI, 2.01-2.18]), the most common location for extracranial bleeding. Although the unadjusted rate for total mortality for rivaroxaban was less than for apixaban (39.2 vs 45.3 per 1000 person-years), the rivaroxaban group had increased total mortality after adjustment (44.2 vs 41.0 per 1000 person-years; RD, 3.1 [95% CI, 1.8-4.5]; HR, 1.06 [95% CI, 1.02-1.09]), including increased risk for fatal ischemic or hemorrhagic events (4.5 vs 3.3 per 1000 person-years; RD, 1.2 [95% CI, 0.8-1.6]; HR, 1.34 [95% CI, 1.21-1.48]).
Dose
There were 134 393 patients beginning treatment with reduced doses (23% of patients in each group). Patients receiving reduced doses, compared with patients receiving standard doses (eTable 8 in the Supplement), were older (82.8 vs 75.2 years), were more likely to be women (62.8% vs 46.4%), and had greater prevalence of other risk factors for stroke (mean CHA2DS2-VASc score, 5.0 vs 4.1) and bleeding.
For patients treated with reduced doses, the rivaroxaban group had increased risk for the primary outcome (adjusted rate, 27.4 vs 21.0 per 1000 person-years; RD, 6.4 [95% CI, 4.1-8.7]; HR, 1.28 [95% CI, 1.16-1.40]) and its ischemic and hemorrhagic components (Figure 3; eTable 9 in the Supplement). Patients receiving reduced-dose rivaroxaban had greater risk for nonfatal extracranial bleeding, whereas total mortality did not differ significantly from those receiving apixaban.
Figure 3. Outcomes by Medication Dose in a Study of the Association of Rivaroxaban vs Apixaban With Major Ischemic or Hemorrhagic Events in Atrial Fibrillation .
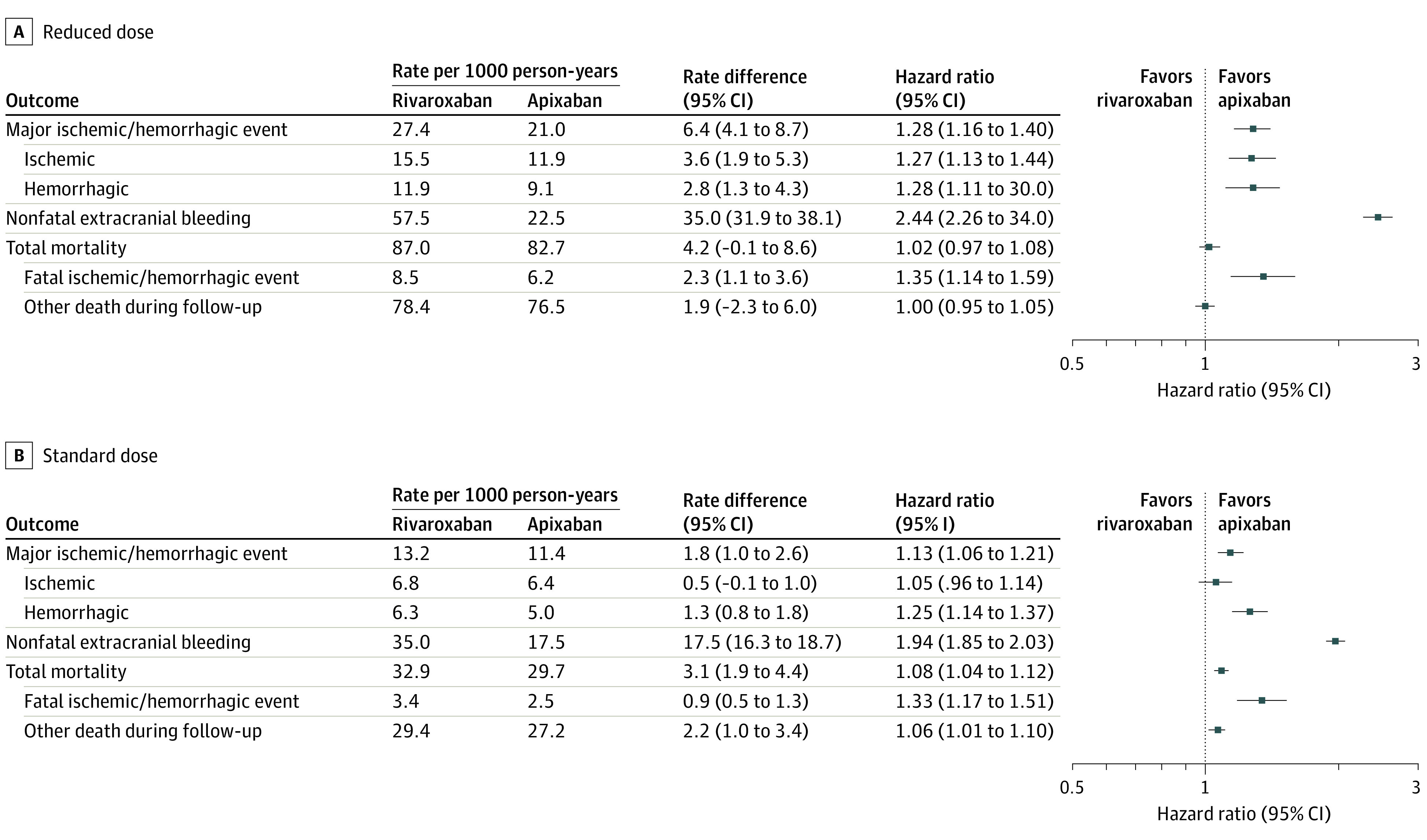
Adjusted incidence of study outcomes according to anticoagulant dose. Rates, rate differences, and hazard ratios were adjusted with inverse probability of treatment weighting; the variables used in the adjustment are shown in eTable 5 in the Supplement. See eTable 9 in the Supplement for numbers of events.
For patients treated with the standard dose (Figure 3; eTable 9 in the Supplement), those receiving rivaroxaban had increased risk for the primary outcome (adjusted rate, 13.2 vs 11.4 per 1000 person-years; RD, 1.8 [95% CI, 1.0-2.6]; HR, 1.13 [95% CI, 1.06-1.21]) and major hemorrhagic events; however, the risk for major ischemic events was not significantly increased. Patients receiving standard-dose rivaroxaban had increased risk for both nonfatal extracranial bleeding and total mortality.
Sensitivity Analyses
The adjusted estimates of incidence, HRs, and RDs changed little with the exclusion of patients with cardiac valve replacement, stage 3 or unspecified chronic kidney disease, or extreme propensity scores (eTable 10 in the Supplement). The estimates did not change materially with restriction of follow-up to 1 year after initiation of the anticoagulant, reduction of the allowable gap in drug supply from 30 days to 7 days, use of a stroke definition with more complete ascertainment, propensity-score matched treatment groups, or estimation of HRs without use of propensity scores. The respective E-values (HRs) for the point estimate and lower confidence bound for major ischemic or hemorrhagic events were 1.64 and 1.48.
Discussion
In this retrospective cohort study of Medicare beneficiaries 65 years or older with atrial fibrillation, initiation of anticoagulation treatment with rivaroxaban compared with apixaban was associated with a significantly increased risk of major ischemic or hemorrhagic events. Although previous retrospective cohort studies have examined the comparative effectiveness of rivaroxaban and apixaban in atrial fibrillation,15,21,22 the present study makes 3 distinct contributions to the evidence needed to guide clinical practice. First, the cohort, with 581 451 new users of rivaroxaban or apixaban, is larger than those of previous studies and consisted entirely of patients 65 years or older, who have both the highest incidence of atrial fibrillation and greatest risk of major ischemic or hemorrhagic events.35,36 The greater sample size permitted more precise quantification of the occurrence of infrequent, but clinically important events, such as fatal extracranial bleeding.
Second, in contrast to other studies,15,21 the cohort included patients treated with reduced doses (23% of participants), whose baseline comorbidity indicated greater susceptibility to differences in anticoagulant efficacy and safety. Although the incidence of major ischemic or hemorrhagic events was increased for patients receiving rivaroxaban in either dose, both the relative and absolute increase in risk were most pronounced for those with reduced doses, which underscores the importance of anticoagulant choice in this population.
Third, the primary study outcome was an integrated measure of the benefits and harms of anticoagulation for patients with atrial fibrillation. Previous studies reported both an efficacy outcome—ischemic stroke or systemic embolism—and a separate safety outcome that consisted of major bleeding, predominantly gastrointestinal events. However, the relative clinical importance of nonfatal gastrointestinal or other extracranial bleeding vs strokes or other intracranial bleeding has been controversial.37 In contrast, the hemorrhagic component of the primary outcome for the present study included only the most severe extracranial bleeding, that which was fatal, thus permitting a single measure of the clinical impact of anticoagulant choice.
Limitations
This study has several limitations. First, residual confounding by unmeasured factors, including geographic variation in the preferences of patients and physicians, is possible. The effects of such variables could have been reduced by the propensity score analysis, which controlled for 208 covariates derived from medical care encounters that reflected patient health across multiple domains. One investigation that linked such encounter data for direct oral anticoagulants with electronic health records reported that after propensity score adjustment, minimal differences remained between glomerular filtration rate, weight, low-dose aspirin use, and other factors.38 In the study cohort, covariate differences between the 2 groups prior to propensity score adjustment were small (most standardized differences were less than 0.1), which suggests limited channeling of patients to a specific anticoagulant. The differences that were present, as summarized by the CHA2DS2-VASc score, indicated that the rivaroxaban group had lower risk of study outcomes, which is consistent with an unadjusted total mortality rate that was less than that for apixaban. Thus, residual confounding may have caused underestimation of the additional risk associated with rivaroxaban use.
Second, there was potential misclassification of exposure and outcomes. There was no information about adherence, which could bias findings if differential. However, when the cohort was restricted to patients with no more than 7 days between refills—a marker for adherence—findings were unchanged. Study outcomes were identified from the principal inpatient discharge diagnoses. Although these had positive predictive values of greater than 90%, there was the potential for underascertainment of strokes. However, findings did not change materially with use of a more sensitive stroke definition based on all discharge diagnoses. Study outcomes did not include ischemic or hemorrhagic events without hospitalization, unless they resulted in death.
Third, as in other anticoagulant cohort studies9,15 and long-term trials of medications for prevention of cardiovascular disease,39 a substantial proportion of patients discontinued treatment. However, a sensitivity analysis that reduced censoring effects by limiting follow-up to 1 year had results entirely consistent with those of the primary analysis.
Fourth, although it has been hypothesized that greater fluctuation in rivaroxaban plasma concentrations5,8,40 would lead to poorer clinical outcomes, the study data do not permit investigation of mechanisms. Other differences, such as the greater dependence of rivaroxaban bioavailability on food,41 could also be important.
Fifth, the composition of the population studied limits the generalizability of findings. The study was restricted to Medicare beneficiaries in the US 65 years or older. Although more than 90% of persons in this age group have Medicare coverage,42 the study cohort was limited to those beneficiaries with both fee-for-service coverage and enrollment in the Part D program for prescription medications.
Conclusions
Among Medicare beneficiaries aged 65 or older with atrial fibrillation, treatment with rivaroxaban compared with apixaban was associated with a significantly increased risk of major ischemic or hemorrhagic events.
eTable 1. Cohort eligibility criteria
eTable 2. Diagnosis codes for strokes and systemic embolism
eFigure 1. Algorithm for identifying bleeding-related hospitalizations
eTable 3. Diagnosis codes indicating bleeding, according to site
eTable 4. Diagnosis codes that may indicating bleeding
eTable 5. Distribution of covariates according to study anticoagulant, with the standardized difference (SD)
eTable 6. Propensity score distribution according to study anticoagulant
eTable 7. Discontinuation/switching probabilities by the median (174) day of follow-up
eTable 8. Cohort characteristics according to dose
eTable 9. Outcomes according to dose
eTable 10. Sensitivity analyses
References
- 1.Kornej J, Börschel CS, Benjamin EJ, Schnabel RB. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. 2020;127(1):4-20. doi: 10.1161/CIRCRESAHA.120.316340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimetbaum P. Atrial fibrillation. Ann Intern Med. 2017;166(5):ITC33-ITC48. doi: 10.7326/AITC201703070 [DOI] [PubMed] [Google Scholar]
- 3.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125-e151. doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 4.Zhu J, Alexander GC, Nazarian S, Segal JB, Wu AW. Trends and variation in oral anticoagulant choice in patients with atrial fibrillation, 2010-2017. Pharmacotherapy. 2018;38(9):907-920. doi: 10.1002/phar.2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong IY, Kim RB. Importance of pharmacokinetic profile and variability as determinants of dose and response to dabigatran, rivaroxaban, and apixaban. Can J Cardiol. 2013;29(7)(suppl):S24-S33. doi: 10.1016/j.cjca.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 6.Reilly PA, Lehr T, Haertter S, et al. ; RE-LY Investigators . The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY trial (randomized evaluation of long-term anticoagulation therapy). J Am Coll Cardiol. 2014;63(4):321-328. doi: 10.1016/j.jacc.2013.07.104 [DOI] [PubMed] [Google Scholar]
- 7.Ruff CT, Giugliano RP, Braunwald E, et al. Association between edoxaban dose, concentration, anti-Factor Xa activity, and outcomes: an analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet. 2015;385(9984):2288-2295. doi: 10.1016/S0140-6736(14)61943-7 [DOI] [PubMed] [Google Scholar]
- 8.Frost C, Song Y, Barrett YC, et al. A randomized direct comparison of the pharmacokinetics and pharmacodynamics of apixaban and rivaroxaban. Clin Pharmacol. 2014;6:179-187. doi: 10.2147/CPAA.S61131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham DJ, Reichman ME, Wernecke M, et al. Stroke, bleeding, and mortality risks in elderly Medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med. 2016;176(11):1662-1671. doi: 10.1001/jamainternmed.2016.5954 [DOI] [PubMed] [Google Scholar]
- 10.Welcome to the Chronic Conditions Data Warehouse. Centers for Medicare & Medicaid Services . Accessed May 13, 2021. https://www2.ccwdata.org/web/guest/home/
- 11.Which oral anticoagulant for atrial fibrillation. Med Lett Drugs Ther. 2016;58(1492):45-46. [PubMed] [Google Scholar]
- 12.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915-920. doi: 10.1093/aje/kwg231 [DOI] [PubMed] [Google Scholar]
- 13.Ezekowitz MD, Aikens TH, Nagarakanti R, Shapiro T. Atrial fibrillation: outpatient presentation and management. Circulation. 2011;124(1):95-99. doi: 10.1161/CIRCULATIONAHA.110.967455 [DOI] [PubMed] [Google Scholar]
- 14.Sherwood MW, Nessel CC, Hellkamp AS, et al. Gastrointestinal bleeding in patients with atrial fibrillation treated with rivaroxaban or warfarin: ROCKET AF trial. J Am Coll Cardiol. 2015;66(21):2271-2281. doi: 10.1016/j.jacc.2015.09.024 [DOI] [PubMed] [Google Scholar]
- 15.Graham DJ, Baro E, Zhang R, et al. Comparative stroke, bleeding, and mortality risks in older Medicare patients treated with oral anticoagulants for nonvalvular atrial fibrillation. Am J Med. 2019;132(5):596-604.e11. doi: 10.1016/j.amjmed.2018.12.023 [DOI] [PubMed] [Google Scholar]
- 16.Chang TE, Tong X, George MG, et al. ; Paul Coverdell National Acute Stroke Program team . Trends and factors associated with concordance between International Classification of Diseases, Ninth and Tenth Revision, clinical modification codes and stroke clinical diagnoses. Stroke. 2019;50(8):1959-1967. doi: 10.1161/STROKEAHA.118.024092 [DOI] [PubMed] [Google Scholar]
- 17.Hsieh MT, Hsieh CY, Tsai TT, Wang YC, Sung SF. Performance of ICD-10-CM diagnosis codes for identifying acute ischemic stroke in a national health insurance claims database. Clin Epidemiol. 2020;12:1007-1013. doi: 10.2147/CLEP.S273853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf. 2011;20(6):560-566. doi: 10.1002/pds.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ray WA, Chung CP, Murray KT, et al. Association of oral anticoagulants and proton pump inhibitor cotherapy with hospitalization for upper gastrointestinal tract bleeding. JAMA. 2018;320(21):2221-2230. doi: 10.1001/jama.2018.17242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnason T, Wells PS, van Walraven C, Forster AJ. Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb Res. 2006;118(2):253-262. doi: 10.1016/j.thromres.2005.06.015 [DOI] [PubMed] [Google Scholar]
- 21.Fralick M, Colacci M, Schneeweiss S, Huybrechts KF, Lin KJ, Gagne JJ. Effectiveness and safety of apixaban compared with rivaroxaban for patients with atrial fibrillation in routine practice: a cohort study. Ann Intern Med. 2020;172(7):463-473. doi: 10.7326/M19-2522 [DOI] [PubMed] [Google Scholar]
- 22.Lip GYH, Keshishian A, Li X, et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. Stroke. 2018;49(12):2933-2944. doi: 10.1161/STROKEAHA.118.020232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simard M, Sirois C, Candas B. Validation of the combined comorbidity index of Charlson and Elixhauser to predict 30-day mortality across ICD-9 and ICD-10. Med Care. 2018;56(5):441-447. doi: 10.1097/MLR.0000000000000905 [DOI] [PubMed] [Google Scholar]
- 24.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in Medicare data: development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci. 2018;73(7):980-987. doi: 10.1093/gerona/glx229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dzeshka MS, Lane DA, Lip GY. Stroke and bleeding risk in atrial fibrillation: navigating the alphabet soup of risk-score acronyms (CHADS2, CHA2 DS2 -VASc, R2 CHADS2, HAS-BLED, ATRIA, and more). Clin Cardiol. 2014;37(10):634-644. doi: 10.1002/clc.22294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen PC, Lip GY, Yeh G, Lin HJ, Chien KL. Risk of bleeding and stroke with oral anticoagulation and antiplatelet therapy in patients with atrial fibrillation in Taiwan: a nationwide cohort study. PLoS One. 2015;10(4):e0125257. doi: 10.1371/journal.pone.0125257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim TH, Yang PS, Uhm JS, et al. CHA2DS2-VASc score (congestive heart failure, hypertension, age ≥75 [doubled], diabetes mellitus, prior stroke or transient ischemic attack [doubled], vascular disease, age 65-74, female) for stroke in Asian patients with atrial fibrillation: a Korean nationwide sample cohort study. Stroke. 2017;48(6):1524-1530. doi: 10.1161/STROKEAHA.117.016926 [DOI] [PubMed] [Google Scholar]
- 28.Webster-Clark M, Huang TY, Hou L, Toh S. Translating claims-based CHA2 DS2 -VaSc and HAS-BLED to ICD-10-CM: impacts of mapping strategies. Pharmacoepidemiol Drug Saf. 2020;29(4):409-418. doi: 10.1002/pds.4973 [DOI] [PubMed] [Google Scholar]
- 29.Alonso A, Agarwal SK, Soliman EZ, et al. Incidence of atrial fibrillation in whites and African-Americans: the atherosclerosis risk in communities (ARIC) study. Am Heart J. 2009;158(1):111-117. doi: 10.1016/j.ahj.2009.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haukoos JS, Lewis RJ. The propensity score. JAMA. 2015;314(15):1637-1638. doi: 10.1001/jama.2015.13480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allison PD. Survival Analysis Using SAS: A Practical Guide. 2nd ed. SAS Institute; 2010. [Google Scholar]
- 32.Kumar S, Lim E, Covic A, et al. Anticoagulation in concomitant chronic kidney disease and atrial fibrillation: JACC review topic of the week. J Am Coll Cardiol. 2019;74(17):2204-2215. doi: 10.1016/j.jacc.2019.08.1031 [DOI] [PubMed] [Google Scholar]
- 33.Stürmer T, Wyss R, Glynn RJ, Brookhart MA. Propensity scores for confounder adjustment when assessing the effects of medical interventions using nonexperimental study designs. J Intern Med. 2014;275(6):570-580. doi: 10.1111/joim.12197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321(6):602-603. doi: 10.1001/jama.2018.21554 [DOI] [PubMed] [Google Scholar]
- 35.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001;285(18):2370-2375. doi: 10.1001/jama.285.18.2370 [DOI] [PubMed] [Google Scholar]
- 36.Lowenstern A, Al-Khatib SM, Sharan L, et al. Interventions for preventing thromboembolic events in patients with atrial fibrillation: a systematic review. Ann Intern Med. 2018;169(11):774-787. doi: 10.7326/M18-1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eikelboom JW, Connolly SJ, Hart RG, et al. Balancing the benefits and risks of 2 doses of dabigatran compared with warfarin in atrial fibrillation. J Am Coll Cardiol. 2013;62(10):900-908. doi: 10.1016/j.jacc.2013.05.042 [DOI] [PubMed] [Google Scholar]
- 38.Huybrechts KF, Gopalakrishnan C, Franklin JM, et al. Claims data studies of direct oral anticoagulants can achieve balance in important clinical parameters only observable in electronic health records. Clin Pharmacol Ther. 2019;105(4):979-993. doi: 10.1002/cpt.1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Navar AM, Roe MT, White JA, et al. Medication discontinuation in the IMPROVE-IT trial. Circ Cardiovasc Qual Outcomes. 2019;12(1):e005041. doi: 10.1161/CIRCOUTCOMES.118.005041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kreutz R, Persson PB, Kubitza D, et al. Dissociation between the pharmacokinetics and pharmacodynamics of once-daily rivaroxaban and twice-daily apixaban: a randomized crossover study. J Thromb Haemost. 2017;15(10):2017-2028. doi: 10.1111/jth.13801 [DOI] [PubMed] [Google Scholar]
- 41.Grześk G, Rogowicz D, Wołowiec Ł, et al. The clinical significance of drug-food interactions of direct oral anticoagulants. Int J Mol Sci. 2021;22(16):8531. doi: 10.3390/ijms22168531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhaskar R, Noon J, O’Hara BJ. The errors in reporting Medicare coverage: a comparison of survey data and administrative records. J Aging Health. 2019;31(10):1806-1829. doi: 10.1177/0898264318797548 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Cohort eligibility criteria
eTable 2. Diagnosis codes for strokes and systemic embolism
eFigure 1. Algorithm for identifying bleeding-related hospitalizations
eTable 3. Diagnosis codes indicating bleeding, according to site
eTable 4. Diagnosis codes that may indicating bleeding
eTable 5. Distribution of covariates according to study anticoagulant, with the standardized difference (SD)
eTable 6. Propensity score distribution according to study anticoagulant
eTable 7. Discontinuation/switching probabilities by the median (174) day of follow-up
eTable 8. Cohort characteristics according to dose
eTable 9. Outcomes according to dose
eTable 10. Sensitivity analyses