Abstract
Intrafusal fibres are a specialised cell population in skeletal muscle, found within the muscle spindle. These fibres have a mechano-sensory capacity, forming part of the monosynaptic stretch-reflex arc, a key component responsible for proprioceptive function. Impairment of proprioception and associated dysfunction of the muscle spindle is linked with many neuromuscular diseases. Research to-date has largely been undertaken in vivo or using ex vivo preparations. These studies have provided a foundation for our understanding of muscle spindle physiology, however, the cellular and molecular mechanisms which underpin physiological changes are yet to be fully elucidated. Therefrom, the use of in vitro models has been proposed, whereby intrafusal fibres can be generated de novo. Although there has been progress, it is predominantly a developing and evolving area of research. This narrative review presents the current state of art in this area and proposes the direction of future work, with the aim of providing novel pre-clinical and clinical applications.
Keywords: Skeletal muscle, muscle spindle, intrafusal fibre, proprioception, tissue engineering
Introduction
Proprioception is an integrated, multi-system physiological function, which can be described as the ‘sense of position and movement of a part of the body, relative to another part’. Proprioception is imperative to coordinated movements, body posture, balance, postural control and influencing motor learning and relearning. 1 Such a function is achieved through a somatosensory input – feedback loop necessary to determine the change and extent of muscle length, tension (passive and active force), consequential changes in joint angle and associated movement of the skin. 2 Mechanoreceptors situated in the joint, including the joint capsule, ligaments, muscles, tendons and skin all contribute to proprioceptive function.3 –5 However, current knowledge indicates that the muscle spindle (MS) provides the greatest contribution to proprioceptive function. 6
This review will provide a brief overview and summary of the anatomy, physiology and function of the muscle spindle, as well as introducing clinical conditions where muscle spindle dysfunction is observed. The main aim is to summarise how our understanding of muscle spindle morphogenesis, has informed the development of in vitro models of intrafusal fibres. The development and progress of these models will be discussed, with the challenges and future directions related to the development of physiological and biomimetic in vitro models of the muscle spindle.
Anatomy and physiology of the muscle spindle
The MS is described as a mechano-sensory organ, that detects and mediates static and dynamic information about skeletal muscle fibre length and stretch. Sensory information is communicated to the central nervous system, where it mediates the appropriate motor response. 7 Muscle spindles are present within most skeletal muscles and are at a higher density in muscles responsible for fine motor control or postural adjustment, such as the hand, head or neck.8,9 They are embedded within skeletal muscle, running parallel and surrounded by regular fascicles of force producing fibres (extrafusal fibres). They consist of an encapsulated bundle of intrafusal muscle fibres within a distinct extra-cellular matrix (ECM) capsule, innervated by primary (Ia) and secondary (II) afferent sensory neurons, as well as dynamic and static efferent γ-motor neurons, termed fusimotors10,11 (Figure 1).
Figure 1.
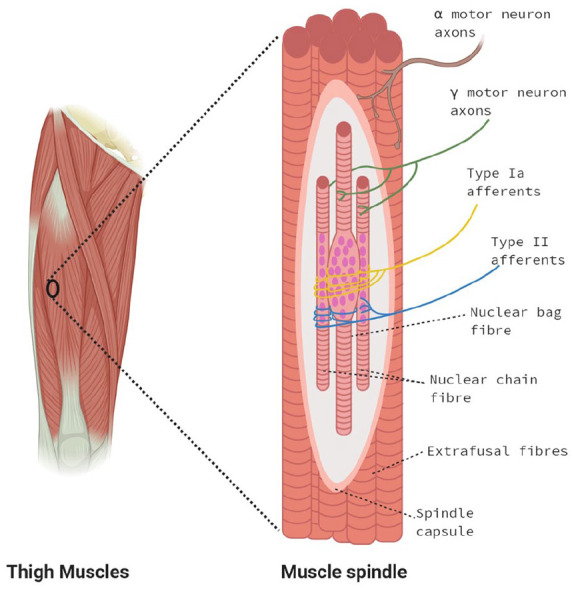
Simplified diagram of the anatomical structure of the muscle spindle. The muscle spindle consists of an encapsulated bundle of intrafusal ‘bag’ and ‘chain’ fibres, which run parallel with normal force producing fibres (extrafusal). The muscle spindle consists of a non-contractile equatorial region (smooth) and contractile (ribbed) polar regions. Type I afferents innervate the equatorial regions of with ASWs. The Type II afferents innervate the juxta equatorial regions with both annulospiral wrappings (ASWs) and flower spray endings (FSEs). In the contractile polar regions, intrafusal fibres are innervated by either static or dynamic gamma motor neurons which modulate the sensitivity of the afferent fibres through contraction of the polar regions. Myonuclei in the bag fibres are clustered at the equatorial regions with a ‘bulging’, appearance. Myonuclei in the chain fibres are aligned linearly across the equatorial regions.
Figure created with BioRender.com.
There are three sub-types of intrafusal fibres; nuclear bag1, bag2 and chain, where there are commonly 2–3 bag fibres and 4–6 chain fibres per MS. 12 To date, these fibres have been categorised based on morphological criteria, myosin ATPase immunohistochemical staining profile, innervation pattern, functional responses and expression of specific myosin heavy chains.6,13 The nuclei of bag1 and bag2 fibres cluster in the equatorial region, creating a bulging ‘bag like’ appearance. The nuclei of chain fibres align linearly, much like extrafusal fibres. 10 The innervation pattern of the sub-types of intrafusal fibres is also specialised according to physiological function. In mammals, type Ia afferent innervation occurs at the equatorial region of all intrafusal fibre types, forming specialised synapses called annulospiral wrappings (ASWs). Type IIa afferents primarily originate at the juxta-equatorial regions and polar regions of nuclear chain fibres, and less frequently bag2 and bag1 bag fibres,14,15 forming either unique flower spray endings (FSEs) or ASWs similar to Ia afferent endings.6,15,16 Investigation of the human MS largely corroborates with mammalian data. 17 Fusimotor activity modulates the sensitivity of muscle spindle afferents through contraction of the polar regions of intrafusal fibres.11,12 It is generally accepted that the dynamic fusimotors only innervate bag1 fibres, while the static fusimotors innervate bag2 and chain fibres. 10 The structural and molecular differences suggest independent functions for each intrafusal fibre type. The number and size of intrafusal fibres, their typing as determined by myosin ATPase staining and MyHC expression is highly heterogeneous between spindles from different human muscles.13,18,19 For example, biceps brachii muscle spindles have a smaller mean intrafusal fibre diameter and proportionally contain significantly less bag2 and chain fibres than spindles from masseter muscle. 18 This highlights the complexity of MS physiology and indicates functional specialisation among different muscles of the same species.
Describing the extensive and complex physiology of the muscle spindle is beyond the scope of this specific review, however to gain further insight into the MS we recommend comprehensive review articles.6,11 –13
Clinical significance
Impairment of proprioception and associated dysfunction of the MS is linked with many neuromuscular diseases including; multiple sclerosis20 –22 Parkinson’s disease,23,24 muscular dystrophy, 25 and peripheral nerve injuries.22,26 –30 Proprioception also deteriorates with age, contributing to overall musculoskeletal frailty and morbidity.31,32 There is a body of literature to support the impairment and decline of proprioception in diabetes,33 –35 thought to be related to degeneration of both the muscle spindle and neurons in the hyperglycaemic/hyperinsulinaemic state. Patients with proprioceptive dysfunction display a variety of difficulties in controlling the speed and magnitude of limb movement, which affects basic motor skills and causes significant physical limitations, such as impaired balance, locomotion and postural stability.34,36 –39
To date, much of the research has been focussed on the cellular mechanisms regulating the effector motor function of skeletal muscle tissue. This work, although highly significant, often neglects the integrated nature of the neuromuscular system by not accounting for the afferent functions of skeletal muscle, mediated via the muscle spindle. A focus in both basic science and clinical studies towards improving complete neuromuscular function following dysfunction, is likely to be more effective in providing the development of novel therapeutic and prognostic strategies to enhance patient care. To this end, the development of robust models, including intrafusal fibres, are needed.
Development of intrafusal fibres and the muscle spindle
The development and maturation of the MS to full physiological function takes considerable time, during gestation and postnatally. In the rat hind limbs, the MS matures by the fourth week post birth. 40 However, it is possible to identify intrafusal fibre specification (based on myosin heavy chain (MyHC) expression) much earlier in the perinatal period. 40 Intrafusal fibres develop from primary myotubes, which are formed from the fusion of committed myoblasts during myogenesis. 41 During foetal developmental stages, as early as day E14.5 in mice, neuronal input is established and correlates with the onset of myogenic differentiation. 42 Sensory neuron Ia-afferent innervation, provides the foremost driving signal for MS morphogenesis. Ia afferent contact with the developing myotubes initiates intrafusal differentiation, through a mechanism involving the transcriptional regulator early growth response protein-3 (Egr3).43 –46 When rats are surgically denervated 47 or treated with a neurotoxin (β bungarotoxin) 48 during prenatal myogenic differentiation, MS do not develop. Surgical manipulation of sensory input at early postnatal stages in the rat, also causes fast-onset MS degeneration,49,50 whereas ablation of motor input fails to cause any noticeable effect in early MS differentiation. 51 As such, this confirms the indispensable requirement of the sensory input for intrafusal fibre development and spindle morphogenesis.
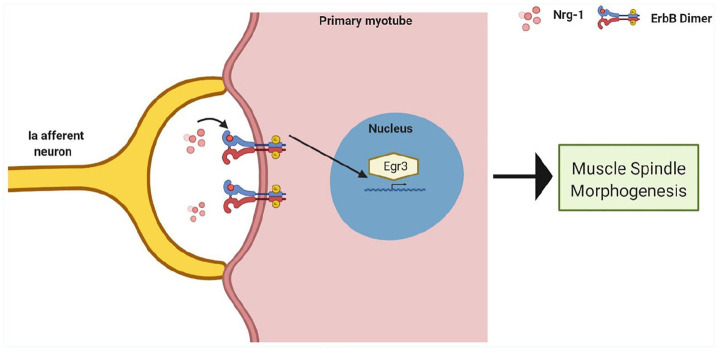
Egr3 is a downstream target of tyrosine kinase receptors ErbB(2–4) (erythroblastic leukemia viral oncogene homologue), which are activated through neuregulin-1 (Nrg-1), a cell adhesion molecule secreted by the afferent neurons. 52 Disruption of these key signalling mechanisms, results in impairments in MS development and function. Indeed, Nrg-1 knockout mice exhibit an early defect in MS differentiation 52 and deletion of ErbB2 in mice prevents the complete formation of the MS, leading to significant neuromuscular defects, such as abnormal gait and posture.53,54 In addition, skeletal muscle specific ablation of Egr3 results in perturbed spindle development, Ia-afferent contacted myotubes developed Egr3 negative spindle remnants with significant physiological abnormalities which persisted into adulthood. 45 As the key developmental mechanisms have been described (Figure 2), this has provided an opportunity to target these proteins when seeking to generate intrafusal fibres in vitro. To this end, the use of Nrg-1 has been largely reported in published work to-date.55–61
Figure 2.
Proposed developmental signalling mechanism which drives intrafusal fibre differentiation and muscle spindle morphogenesis. Nrg-1 is released by an innervating Ia afferent neuron, this activates the ErbB receptors on primary myotubes. Egr3 is activated downstream of activated ErbB receptors and is essential for intrafusal formation and muscle spindle morphogenesis.
Figure created with BioRender.com.
Regenerative capability of the muscle spindle
Post-natal muscle regeneration recapitulates many of the cellular and molecular aspects of muscle embryonic development. 62 Satellite cells are the resident stem cell of skeletal muscle, where they reside beneath the basal lamina surrounding each muscle fibre. When activated, they give rise to committed myoblasts which are essential for muscle fibre regeneration and repair.63,64 There is growing evidence to suggest that intrafusal fibres are in a comparatively immature state, relative to mature extrafusal fibres. In chickens, there is a higher number of satellite cells residing within intrafusal fibres of the MS. These cells also maintain expression of paired-domain transcription factor 3 (PAX3), a satellite cell marker which is repressed in the majority of mouse muscles following embryogenesis.65 –67 Furthermore, myonuclei of intrafusal fibres maintain gene expression of Myf5, 68 the earliest marker of myogenic commitment in satellite cells, and retain expression of embryonic and neonatal isoforms of MyHC into maturity.69,70 Finally, intrafusal fibre growth is arrested shortly after birth, where they remain comparatively small compared to extrafusal fibres. 71 Together, these data suggest a unique cellular and molecular phenotype of intrafusal fibres. The physiological advantage of increased satellite cell number and maintained expression of numerous factors associated with early muscle development in MS, is currently unknown. It has led conjecture that due to the continuous length monitoring of skeletal muscle and adjustment of its own length, intrafusal fibres may require regular regeneration. Further, that by remaining in this comparatively immature state contributes to a greater capacity for intrafusal fibre regeneration, repair and preservation. 66 Recent work supports this hypothesis, whereby adult mice ablated for satellite cells displayed normal adaptability to aerobic exercise but showed decrements in wheel running performance and gross motor coordination. 72 This is a similar phenotype seen in universal or skeletal muscle specific Egr3 knockout mice, which lack functional MS.45,46,73 Upon immunohistochemical analysis, the MS exhibited an increased ECM deposition and presented intrafusal fibre atrophy, while extrafusal fibres displayed no abnormal morphology. In addition, satellite cell depletion lead to functional changes in MS, as determined by decreased firing frequency in response to stretch. Combined, these data indicate satellite cells are essential for MS maintenance and regeneration and that there is a key physiological demand for the relative increased satellite cell density in intrafusal fibres.
Despite this evidence, the specific cellular and molecular mechanisms which regulate regeneration of the MS are yet to be fully elucidated. This highlights a necessity to develop robust and tractable models, to investigate how muscle spindle regeneration is controlled and affected in health and disease.
Generating intrafusal fibres in vitro
Due to the complex integrated physiology of the MS, in vivo experimentation largely conducted in mice, rats and cats has formed the basis of much of our current understanding.6,10,11 In vivo and ex vivo experiments maintain the muscle spindle within the majority of its the physiological niche with functional innervation. Additionally, there are powerful genetic tools, including transgenic animals and optogenetic tools available to induce various physicochemical and cellular alterations, as well as models of injury and disease states. 74
There are however limitations of in vivo and ex vivo models. Invasive experimentation on mammalian models are ethically undesirable. The 3Rs – Replacement, Reduction and Refinement – are embedded into the legislation and guidelines governing the ethics of animal use in experiments, and highlights that, if possible, methods which avoid or replace the use of animals should be developed. 75 Analytical techniques used in animal studies often cannot be safely reproduced in humans, experiments are expensive, time consuming and yield data which may not accurately translate to human physiology.76,77 Furthermore, single factor changes are difficult to study due to the biological complexity of whole-body living systems and organisms.78,79 In vitro modelling is an indispensable tool in biological research and in recent years there has been a shift from flat, two-dimensional (2D) cultures, to three-dimensional (3D) structures which can be manipulated to replicate a more in vivo-like biochemical and biomechanical microenvironment. 80 With this in mind, there is a growing need to develop robust in vitro models of intrafusal fibre development and function, which consider the in vivo anatomy and physiology described. This will help to investigate the basic molecular and cellular mechanisms regulating their phenotype, in a defined, highly controlled, reproducible system. Furthermore, there are potential pre-clinical and clinical applications for intrafusal fibres generated in vitro, which may contribute to the development of novel therapeutic strategies for tissue damage and disease.
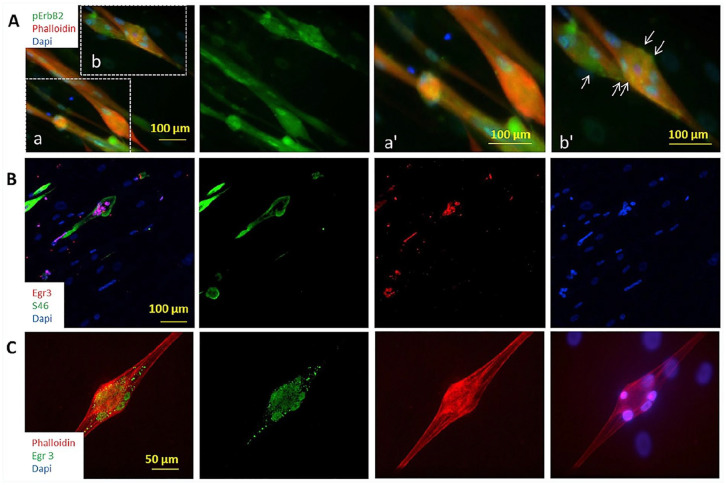
In 2004, Jacobson and colleagues used recombinant Nrg-1(NRG-1) to induce the expression of Egr3 at an mRNA and protein level in a 2D human primary myoblast culture. 56 NRG-1 treated cells showed a significant increase in the expression of muscle spindle-specific slow developmental myosin, replicating the characterised developmental mechanism in vitro. More recent work completed by the Hickman laboratory since 2008, used NRG-1 to influence myotube fate specification towards intrafusal-like myotubes in human and rat myoblasts, or human induced pluripotent stem cells (hiPSCs).57 –61 Immunocytochemistry for intrafusal specific protein MyHC6, Egr3, phosphor-ErbB2 and distinctive morphology allowed identification of intrafusal bag fibres in a heterogenous myotube population (Figure 3). In this regard, NRG-1 induced a five-fold increase in bag-like myotubes, which corroborates with in vivo data highlighting a requirement for Nrg-1 for physiological intrafusal fibre differentiation. In addition, bag-like myotubes (MyHC6 positive) had an increased expression of phospho-ErbB2 and Egr3, indicating the developmental mechanism essential for spindle morphogenesis (Figure 2) has been activated in these cells.59,61 This work provides evidence for the morphological, mechanistic and phenotypic (myosin heavy chain expression) development of intrafusal-like fibres in vitro. The morphological and phenotypic characteristics that can be used to determine the formation of intrafusal fibres in vitro are presented below (Figure 3 and Table 1).
Figure 3.
Activation of Neuregulin signalling pathway demonstrated by immunocytochemistry. (A) Co-immunostaining of Phalloidin and erbB2-℗. To visualise the erbB2-℗ clusters on the cell membrane, two regions of the low magnification image were enlarged. Image a’ and b’ are the higher magnifications of regions a and b respectively. Abundant erbB2-℗ signals (indicated by arrows) were observed only on multi-nuclei bag fibres (b and b’), and rarely observed on the others (a and a′). (B) Immunostaining of Egr3 co-stained with S46. Egr3-positivity was only observed in S46-positive myofibres, confirming its specificity. (C) A bag fibre under higher magnification showing Egr3 staining.
Source: Figure from Guo 59 with permission from Elsevier.
Table 1.
Methods proposed for the characterisation of intrafusal fibres in vitro.
| Parameter | Description | Reference(s) |
|---|---|---|
| Morphological structure | Clustering of nuclei and expanded equatorial region of the myotube | Rumsey et al. 57 |
| Expression of developmental proteins | Expression of phospho-ErbB2 and Egr3 | Rumsey et al., 57 Colón et al.,58,61 and Guo et al. 59 |
| Expression of distinctive phenotypic proteins | Expression of MyHC6 | Rumsey et al., 57 Colón et al., 58 and Guo et al.59,81 |
| Expression of key gene transcripts | Expression of genes highly regulated by Egr3 with restricted expression to developing spindles | Albert et al. 44 |
| Sensory neuron innervation | Sensory neuron ASW and FSE co-localisation with myotubes | Qiao et al., 55 Guo et al., 59 and Rumsey et al. 60 |
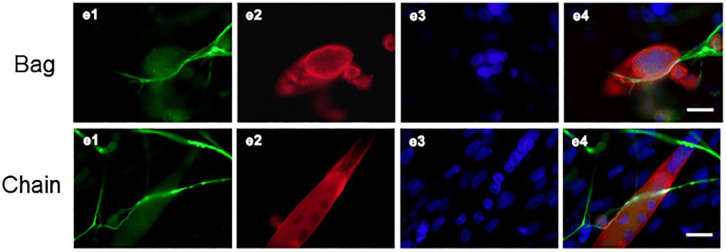
S46 and BA-G5 myosin heavy chain antibodies against MyHC6, are reported to be the most intrafusal-bag fibre specific markers available.69,82,83 In addition, MyHC15 and MyHC7b/14 have displayed specificity to specialised muscle, including intrafusal fibres.84,85 To accurately model the extent of de novo intrafusal fibre formation in vitro, it is necessary to characterise intrafusal nuclear chain fibres separate from morphologically similar extrafusal fibres in a heterogeneous myotube population. The combination of Egr3 and phosphor-ErbB2 positive staining has been used to identify both intrafusal bag and chain fibres, however in some published manuscripts where intrafusal fibres are quantified, nuclear chain fibres have not been considered.57 –59 Intrafusal chain fibres were considered in a co-culture of dorsal root ganglion (DRG) explants and skeletal muscle cells, classification relied on an overlay in fluorescent staining for neurofilament 200, α-actin and DAPI, visualising sensory neurons and myotubes respectively, suggesting innervation 55 (Figure 4). The supplementation of NRG-1 into the co-culture system lead to increased neuronal outgrowth and migration from DRG explants and increased level of intrafusal bag and chain myotubes.
Figure 4.
Bag and chain fibres visualised in a co-culture of DRG explants and dissociated skeletal muscle cells: e1 NF-200 immunoreactive neurons, e2 SKM cells, e3 DAPI, and e4 overlay of e1, e2, and e3. Scale bar = 20 μm. ‘Bag’, a–e Intrafusal nuclear bag fibre (red) with gathered nuclei (blue) inside and sensory nerve terminals wrapping around the bag fibre surface. ‘Chain’, a–e Intrafusal nuclear chain fibre (red) with linear assembled nuclei (blue) inside and sensory nerve terminals wrapping around the chain fibre surface.
Source: Figure taken and edited from Qiao, 55 Published through BioMed central and with permission of the Creative Commons license http://creativecommons.org/licenses/by/4.0/.
Defining bag1 from bag2 fibres presents another challenge in vitro; bag1 fibres, compared to bag2 fibres have a slower phenotype, distinguishable myosin ATPase reactivity and the of lack key M-band protein expression. 13 The in vivo assays have not yet been adapted or utilised in vitro to define bag1 from bag2 fibres. Alongside this, MyHC isoforms are very similar, meaning there is possible limitations caused by cross-reactivity of antibodies. 13 MyHC expression is controlled at the transcriptional level, utilising mRNA transcriptional profiling will also greatly overcome issues relating to the use of antibodies. Table 2 displays the current extent of human myosin heavy chain genes (MYH) and the corresponding phenotype.13,84,86
Table 2.
Myosin heavy chain profiles: Nomenclature and phenotypes (modified from Schiaffino and Reggiani 84 ).
| Functional phenotype | Gene | Protein alias |
|---|---|---|
| Fast isoforms | MYH1 | MyHC-IIx/d |
| MYH2 | MyHC-IIa | |
| MYH4 | MyHC-IIb | |
| Embryonic isoforms | MYH3 | MyHC-embryonic |
| MYH8 | MyHC-neonatal | |
| Slow/cardiac isoforms | MYH6 | MyHC-α |
| MYH7 | MyHC-β | |
| Specialised/other isoforms | MYH13 | MyHC-extraocular |
| MYH7b/14 | MyHC-slow/tonic | |
| MYH15 | MyHC-15 | |
| MYH16 | MyHC-masticatory |
Structure and role of the extracellular matrix
Muscle fibres reside within a three-dimensional scaffold consisting of numerous glycoproteins, collagens, proteoglycans and elastin, known as the extracellular matrix (ECM). The ECM contributes to the regulation many key physiological processes of skeletal muscle development, growth, repair and contractile function.87 –89 To date, in vitro models of intrafusal fibres are in a 2D environment, which alongside many limitations discussed in the future work section below, do not account for cell interactions with the ECM. The MS is enveloped by a capsular sleeve with two cellular portions, an outer capsule, that encloses a peri-axial space and an inner capsule that defines the axial compartments, where the intrafusal fibres and their nerve endings reside.90,91 A basal lamina surrounds individual intrafusal fibres and the perineural epithelium of the outer capsule, 92 and unlike neuromuscular junctions, which lack basal lamina where the axon and muscle fibre meet, myo-sensory junctions have a common basal lamina that surrounds both intrafusal fibres and sensory terminals. The region has a modified structure and ECM composition in comparison to non-sensory regions. 93 There are reports of capsular thickening of the MS capsule in several neuromuscular disorders, aging25,94 and as described previously, in adult mice ablated of satellite cells. 72 The specialised physiology and subsequent degradation in diseases associated with MS dysfunction, further suggests the MS ECM plays a significant role in muscle spindle function. 95
Collagen IV has been proposed as a key ECM component to intrafusal development, as it is a significant component of the basal lamina surrounding muscle spindles.56,92 However, it has been demonstrated that coating coverslips with Collagen IV, resulted in no measurable differences in NRG-1 induced intrafusal specific differentiation of primary rat myocytes in vitro. 57 This lack of effect, may be a result of the 2D environment, where cells on coated coverslips will respond and interact differently with themselves and their environment compared to cells in 3D, leading to distinct variation in mechanical and chemical cues, cell adhesions and migration. 80 Interestingly, MS lamina proteins Laminin and Agrin, can independently induce the expression of Egr3 in vitro through interaction with the a-dystroglycan receptor. The resulting expression is induced through the same ErbB receptors as Nrg-1, indicating an important role of the ECM in intrafusal fibre development, independent of Nrg-1. 96 The challenge ahead is to precisely identify the cell–ECM interactions involved and how the connective tissue ECM orients and regulates the intrafusal fibre phenotype. Candidates such as Collagen VI, 97 chondroitin sulphate proteoglycan, laminin, heparan sulphate proteoglycan and vimentin, show strong immunostaining in avian or mammalian MS extracellular spaces,92,93,97,98 suggesting a strong role for these proteins in the development and maintenance of the MS. In vitro models investigating the functional role of MS ECM in its physiology, regeneration and degeneration are required to provide further understanding of how it could contribute to MS dysfunction in disease and aging. This can be achieved through the use of tissue engineered models, similar to that developed by our group and others (reviews99 –101).
Functional responses
In addition to characterisation outlined in Table 1, functional responses of the MS are key in determining if an in vivo-like physiological response is achieved in vitro. To this end, a short summary of the electrophysiological responses will be highlighted.
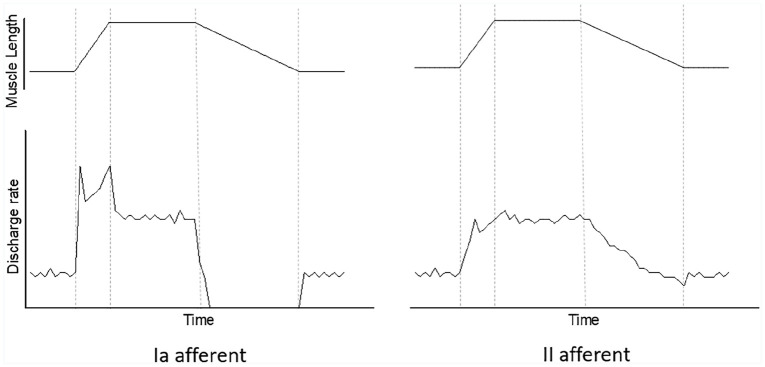
Using electro-physiological techniques, the firing patterns as muscles stretch have been well-characterised experimentally in various mammals102 –109 and in humans in vivo.110 –113 Such analyses have also been replicated ex vivo and in explanted in vitro conditions.74,114 –118 To summarise, type Ia and II exhibit different dynamic responses to stretch, producing a unique patterns of discharge rate during stretch (Figure 5). Type Ia afferents display a burst of high impulse rate at the beginning of the ramp stretch, a transient fall in discharge rate at the beginning of the hold phase and an absence of impulses during the majority of shortening. In comparison, type II afferents keep a steady firing pattern throughout the ramp hold stretch which correlates with the length of the muscle. This firing pattern is not dependent on fusimotor innervation, as this pattern of response is elicited in de-efferented spindles. 119 There are two functionally distinct fusimotor fibres, static and dynamic, categorised by the excitatory effect upon afferent endings. 120 Stimulation of either fusimotor increases type Ia afferent firing rate at a constant length, with a greater increase in firing rate observed with static fusimotor stimulation. During the dynamic phase of stretching, the Ia afferent ending fires at a much higher rate when the dynamic fusimotor is stimulated compared to the static fusimotor, in which the dynamic response was lost, replicating a response similar to a type II afferent ending.106,109,120,121
Figure 5.
Formulated figure illustrating typical firing patterns for muscle spindle type Ia and II afferents, respectively. Discharge rate (imp/sec), muscle length (joint angle position or mechanical stretch) and time are on linear scales. Type Ia afferents display a burst of high impulse rate at the beginning of the ramp stretch, a transient fall in discharge rate at the beginning of the hold phase and an absence of impulses during most of the shortening. In comparison, type II afferents keep a steady firing pattern throughout the ramp hold stretch which correlates with the length of the muscle. The dotted lines from left to right show beginning of ramp stretch, hold phase, muscle shortening and finally the end of muscle shortening with a return to resting discharge rate.
Source: Figure adapted from Crowe and Matthews, 106 Kakuda and Nagaoka 110 and Edin and Vallbo. 122
Both sensory and motor neurons have been individually co-cultured with intrafusal fibres in vitro.60,61 Afferent and fusimotor connections have been morphologically characterised through identification of ASWs and FSEs endings, localised on intrafusal fibres with co-localisation with brain sodium channels 1 and 2 ( BNaC1 and BNaC2) and protein kinase C alpha (PICK1), proteins associated with the mechanosensory endings in non-mammalian studies. 60 Ca2+ flux has been shown to be important for muscle spindle afferent activity in vivo and was therefore used to visualise a functional connection between intrafusal fibre and afferent neuron. 123 Afferent electrical activity was also visualised, following field stimulation to initiate myotube contraction, in afferent-intrafusal fibre co-cultures but absent in afferent only controls. 60
In addition to mechanically stimulated action potential responses, the processes of mechanotransduction could be utilised to assess the intrafusal phenotype in vitro. Stretch-sensitive channels are responsible for transducing mechanical stimuli in spindle afferents. Most mammalian mechanosensory channels await definitive identification, however, ENaCs, 114 ASICs,114,124 TRPs, Piezo 2 125 and Tentonin 3 126 have been associated with mechanosensory function, with strong evidence of sensory terminal localisation within the MS for ENaC and ASIC proteins.124,127 One of the most compelling candidates is Piezo 2, a nonselective cation channel, which when ablated from sensory neurons in mice leads to severe proprioceptive defects and absent stretch evoked afferent responses to stretch in over 85% of muscles. 125 This suggests Piezo 2 is a principal mechanically activated ion channel required for physiological proprioception mediated through the muscle spindle.
Clusters of synaptic-like vesicles (SLVs) are a feature of muscle spindle primary afferent terminals when visualised via electron microscopy. 127 Furthermore, several functionally important pre-synaptic proteins are expressed at the spindle primary afferent terminals. Including vesicle clustering protein synapsin I, synaptophysin, vesicle docking SNARE complex protein, syntaxin 1B and many presynaptic Ca2+ binding proteins; calbindin-D28k, calretinin, neurocalcin, NAP-22 and frequenin.127,128 There is evidence of tonic Ca2+ dependent, glutamate releasing, SLV exo/endocytosis, which significantly increases with mechanical activity 128 These data suggest a Ca2+ dependent synaptic/secretory vesicle turnover system as part of MS mechanotransduction. Although a full review of this topic is beyond the scope of this article (see Bewick and Banks 127 and Bewick 128 for more details), it does highlight novel in vitro targets for displaying functionality in an innervated MS model. But also, how a sophisticated model will also offer a platform to help further define mammalian sensory mechanotransduction.
Fusimotors form cholinergic acetylcholine (ACh)-based neuromuscular interactions that appear in some aspects, similar to the neuromuscular junction formed by α-motoneurons on extrafusal muscle.129,130 Therefore, functional fusimotor synapses with intrafusal fibres has been demonstrated using glutamate, an excitatory neurotransmitter capable of stimulating neurons in vitro. 131 Myotube action potentials (APs) were induced in the majority of the supposed innervated intrafusal fibres upon glutamate addition, which were not replicated in non-innervated intrafusal controls. The addition of curare, a competitive antagonist of ACh receptor, also caused immediate cessation APs. 61
Future electrophysiological assessments should also focus on direct afferent firing in response to stretch and the effects of type specific fusimotor stimulation, trying to replicate the in vivo responses. This topic will be further discussed in the following section.
Future directions and challenges
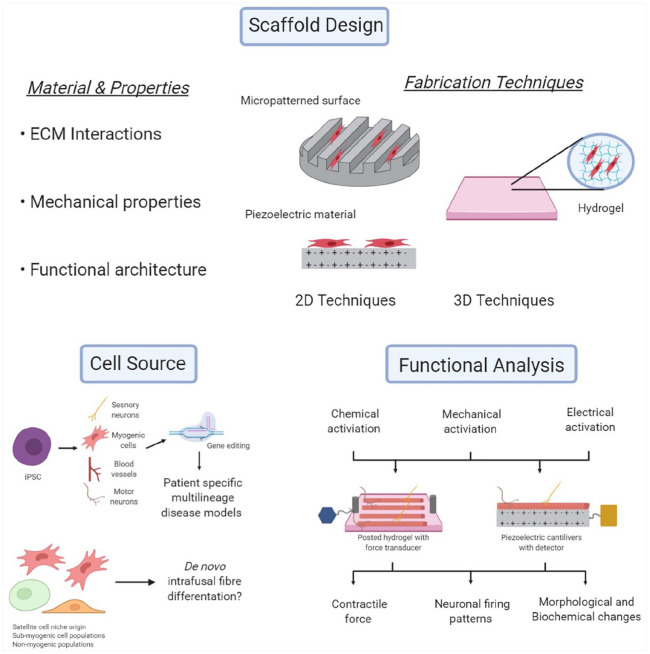
Although there has been impressive recent progress in engineering and characterising intrafusal fibres in vitro, there are multiple avenues for researchers to consider (Figure 6), which have not been fully discussed in the sections above.
Figure 6.
Schematic representation summarising key future research areas that can be explored to improve tissue engineering and characterisation of intrafusal skeletal muscle fibres.
Figure created with BioRender.com.
The greatest area for development, is the translation of current 2D progress towards more advanced 3D tissue engineered models. Cell behaviour is dependent on the niche they inhabit and external cues they receive, a 3D model better replicates the in vivo environment, offering a geometric similarity, allowing cells to interact with other cells and the surrounding matrix in three directions. The user can also control parameters such as stiffness, orientation, and porosity, all of which effect cell proliferation, migration, self-renewal and differentation. 132 3D muscle cultures give rise to more mature molecular, structural and functional differentiation, characteristic of native adult muscle.133 –136 They also permit far longer culture times,134,137,138 support higher expression of adult MyHc isoforms and Ca2+ handling genes, display increased alignment and hypertrophy, form developed sarcomeres and triads101,134 and demonstrate basic contractile physiological properties such as positive force-frequency and length-tension.89,101,134,139,140 From this, we can assume intrafusal fibres engineered in a 3D, aligned microenvironment, should result in a more mature phenotype and hence researchers should seek to develop and utilise these models.
Further structural advances include the morphogenic patterning of differentiating cells,141,142 using physical and chemical patterning. Indeed, it is possible to control the freeform morphology of neurons using polymer brushes, which allows control at the micro-level (20µm). 143 Physical patterning methods (such as the use of electrospinning polymers, 3D printing, focussed ion beam and other micro-patterning methods) can also be used to generate surfaces on materials with micro- and nano-patterned features, which alters myogenic differentiation.144 –151 These methods could be used to chemically and/or physically pattern the necessary ‘morphological template’ for the differentiation of intrafusal fibre subtypes (i.e. bag and chain).
It is essential that future models of intrafusal fibres use functional assays to further validate and characterise the phenotype. It has been described extensively in this manuscript that the function of the muscle spindle is only possible where intrafusal fibres synapse with both sensory and motor neurons, creating an integrated feedback and feedforward system. Neuronal innervation of intrafusal fibres generated in vitro is therefore of paramount importance, when trying to recapitulate the in vivo structure. The previously published methods to assess in vitro intrafusal fibre function, have assessed the electro-chemical and synaptic properties.57,59,61 However, to facilitate physiological analysis of the model system, the platform would also need to support both mechanical stretch and load and permit for direct recording of afferent fibres and stimulation of fusimotor fibres. This could be achieved through the use of biomaterials (either natural or synthetic polymers) in a planar environment or the use of a 3D hydrogel.101,133,135
Biomedical microelectromechanical systems (BioMEMS) devices measure small scale mechanical movement, often relying on microcantilever sensors and can serve as a powerful functional assay for tissue engineered skeletal muscle. 152 A promising avenue for BioMEMS in muscle tissue engineering is piezoelectric materials, which can transduce mechanical stress to generate surface charges (or the inverse). Therefore, they can be used sense and quantify muscle contraction,153,154 or as an actuator to apply mechanical force to cells152,155 in real time with high throughput. Intrafusal models that facilitate direct afferent recording could be further utilised to characterise afferent Ia to type II depending on firing patterns observed during stretch. In addition, fusimotor stimulation permits the categorisation of static and dynamic fusimotors, depending on its excitatory effect upon afferent endings.109,120 –122 This integrated model could be used to investigate the relationship between afferent feedback, fusimotor feedforward and intrafusal fibre type in various diseased or damaged states.
The majority of publications in this area to-date, have utilised a tissue digestion methodology for the isolation primary muscle derived cells (MDCs).55 –57,59 –61 As such, there is no control over the origin of the satellite cell progeny; these cells could be derived from the extrafusal tissue, or indeed from within the muscle spindle niche. It is currently unknown whether there are differences in the propensity for intrafusal differentiation of myoblasts derived from these two anatomically distinct regions. It also important to consider, that MDCs in vivo are a heterogeneous population of cells containing myogenic and non-myogenic (fibroblasts, pericytes, vascular endothelial cells, mesenchymal stromal cells, fibro-adipogenic progenitors, immune cells etc) populations.156 –158 Evidence suggests non-myogenic cells play an important supportive role in myogenesis, 157 therefore, future work should seek to identify the extent to which the non-myogenic populations contribute to intrafusal differentiation.158 –160 Additionally, there are skeletal muscle cell populations with myogenic potential, other than satellite cells158,161 (mesoangioblasts, endothelial progenitor cells, adipose-derived stem cells, CD133+ muscle derived stem cells etc.). Many are capable of in vitro myogenic differentiation, 161 but fail to rescue myogenesis following injury in the absence of satellite cells, suggesting a supporting role. 158 They have attracted interest as a candidate for myogenic cell transplantation therapies, proving a possible alternative to the progeny of satellite cells, which have historically been largely inefficient.158,161,162 These sub-myogenic cell populations should be investigated for their ability for de novo intrafusal fibre differentiation.
Developments in hiPSCs and human amniotic mesenchymal cells (hAMCs) offer an exciting alternative to primary MDCs, they have the ability to self-renew and differentiate in a controlled manner into myogenic cells. iPSCs and hAMCs largely overcome issues relating to primary MDCs (large variability, limited passage capacity, ethical issues etc.) and offer an extremely powerful tool in personalised regenerative medicine, disease modelling and developmental biology.163 –165 Work with hAMCs in skeletal muscle tissue engineering is still limited, initial work has shown them as a suitable stem cell source for in vitro skeletal muscle tissue engineering and a viable method to treat volumetric tissue loss within the rat, causing increased angiogenesis and improved local tissue repair 4 weeks post implantation. 165 hiPSCs have been under the spotlight since there discovery in 2006, 166 they are generated by reprogramming donor somatic cells into a pluripotent state, which can then be reprogrammed to the desired cell type 164 and easily edited using genome editing tools such as CRISPR/Cas9 system. 167 hiPSCs represent an ideal source to produce patient and disease-specific adult cells for cell therapy and disease modelling. Indeed 3D hiPSC derived, multilineage muscle models containing myotubes, vascular endothelial cells, pericytes, and motor neurons, has been recently achieved. 168 Integration of hiPSC developed sensory neurons169,170 and intrafusal fibres 58 into such a model, would provide the foundation of producing a complex human derived biomimetic tissue engineered model of skeletal muscle, that unlike any to date, will account for the afferent function of the muscle spindle.
Conclusion
There has been significant progress towards the generation of intrafusal fibres in vitro. This work has often been limited to the basic science of development and fundamental cell engineering. With the rapid and continued progress in tissue-engineered skeletal muscle models, there is an opportunity to develop anatomically and physiologically relevant models of the MS. These models could be used for developmental or disease state studies, pre-clinical screening of therapeutics, or clinical applications such as tissue replacement and regeneration for diseased and dysfunctional tissue.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: PB is in receipt of the John Scales studentship, UCL.
ORCID iD: Philip Barrett  https://orcid.org/0000-0003-3963-4601
https://orcid.org/0000-0003-3963-4601
References
- 1. Ribeiro F, Oliveir J. Factors influencing proprioception: what do they reveal? In: Klika V. (ed.) Biomechanics in applications. Croatia, Balkans: InTech, 2011, pp.323–346. [Google Scholar]
- 2. Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev 2012; 92: 1651–1697. [DOI] [PubMed] [Google Scholar]
- 3. Macefield VG. Physiological characteristics of low-threshold mechanoreceptors in joints, muscle and skin in human subjects. Clin Exp Pharmacol Physiol 2005; 32: 135–144. [DOI] [PubMed] [Google Scholar]
- 4. Blecher R, Heinemann-Yerushalmi L, Assaraf E, et al. New functions for the proprioceptive system in skeletal biology. Philos Trans R Soc B Biol Sci 2018; 373(1759): 20170327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jami L. Golgi tendon organs in mammalian skeletal muscle: functional properties and central actions. Physiol Rev 1992; 72: 627–657. [DOI] [PubMed] [Google Scholar]
- 6. Macefield VG, Knellwolf TP. Functional properties of human muscle spindles. J Neurophysiol 2018; 120: 452–467. [DOI] [PubMed] [Google Scholar]
- 7. Stifani N. Motor neurons and the generation of spinal motor neuron diversity. Front Cell Neurosci 2014; 8: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Banks RW. An allometric analysis of the number of muscle spindles in mammalian skeletal muscles. J Anat 2006; 208: 753–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saverino D, De Santanna A, Simone R, et al. Observational study on the occurrence of muscle spindles in human digastric and mylohyoideus muscles. Biomed Res Int 2014; 2014: 294263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Banks RW. The innervation of the muscle spindle: a personal history. J Anat 2015; 227: 115–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Banks RW. The motor innervation of mammalian muscle spindles. Prog Neurobiol 1994; 43: 323–362. [DOI] [PubMed] [Google Scholar]
- 12. Matthews PBC. Muscle spindles: their messages and their fusimotor supply. In: Brookhart JM, Mountcastle WB, Brooks VB. (eds) Handbook of physiology: the nervous system. Bethesda, MD: American Physiological Society, 1981, pp.189–228. [Google Scholar]
- 13. Thornell L-E, Carlsson L, Eriksson P-O, et al. Fibre typing of intrafusal fibres. J Anat 2015; 227: 136–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schröder JM, Bodden H, Hamacher A, et al. Scanning electron microscopy of teased intrafusal muscle fibers from rat muscle spindles. Muscle Nerve 1989; 12: 221–232. [DOI] [PubMed] [Google Scholar]
- 15. Fox KP, Koeze TH, Swash M. Sensory innervation of baboon muscle spindles. J Anat 1975; 119: 557–568. [PMC free article] [PubMed] [Google Scholar]
- 16. Banks RW, Barker D, Stacey MJ. Form and distribution of sensory terminals in cat hindlimb muscle spindles. Phil Trans R Soc Lond B 1982; 299: 329–364. [DOI] [PubMed] [Google Scholar]
- 17. Swash M, Fox KP. Muscle spindle innervation in man. J Anat 1972; 112: 61–80. [PMC free article] [PubMed] [Google Scholar]
- 18. Österlund C, Liu J-X, Thornell L-E, et al. Muscle spindle composition and distribution in human young masseter and biceps brachii muscles reveal early growth and maturation. Anat Rec 2011; 294: 683–693. [DOI] [PubMed] [Google Scholar]
- 19. Österlund C, Liu J-X, Thornell L-E, et al. Intrafusal myosin heavy chain expression of human masseter and biceps muscles at young age shows fundamental similarities but also marked differences. Histochem Cell Biol 2013; 139: 895–907. [DOI] [PubMed] [Google Scholar]
- 20. Feys P, Helsen W, Ilsbroukx S, et al. Is MS intention tremor amplitude related to changed peripheral reflexes? ISRN Neurol 2011; 2011: 192414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fling BW, Dutta GG, Schlueter H, et al. Associations between proprioceptive neural pathway structural connectivity and balance in people with multiple sclerosis. Front Hum Neurosci 2014; 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prather JF, Nardelli P, Nakanishi ST, et al. Recovery of proprioceptive feedback from nerve crush. J Physiol 2011; 589: 4935–4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Conte A, Khan N, Defazio G, et al. Pathophysiology of somatosensory abnormalities in Parkinson disease. Nat Rev Neurol 2013; 9: 687–697. [DOI] [PubMed] [Google Scholar]
- 24. Teasdale H, Preston E, Waddington G. Proprioception of the ankle is impaired in people with Parkinson’s disease. Mov Disord Clin Pract 2017; 4: 524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ovalle WK, Dow PR. Alterations in muscle spindle morphology in advanced stages of murine muscular dystrophy. Anat Rec 1986; 216: 111–126. [DOI] [PubMed] [Google Scholar]
- 26. Haftel VK. Central suppression of regenerated proprioceptive afferents. J Neurosci 2005; 25: 4733–4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cope TC, Bonasera SJ, Nichols TR. Reinnervated muscles fail to produce stretch reflexes. J Neurophysiol 1994; 71: 817–820. [DOI] [PubMed] [Google Scholar]
- 28. Maas H, Prilutsky BI, Nichols TR, et al. The effects of self-reinnervation of cat medial and lateral gastrocnemius muscles on hindlimb kinematics in slope walking. Exp Brain Res 2007; 181: 377–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bullinger KL, Nardelli P, Pinter MJ, et al. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. II. Loss of functional connectivity with motoneurons. J Neurophysiol 2011; 106: 2471–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ruijs ACJ, Jaquet JB, Kalmijn S, et al. Median and ulnar nerve injuries: a meta-analysis of predictors of motor and sensory recovery after modern microsurgical nerve repair. Plast Reconstr Surg 2005; 116(2): 484–494. [DOI] [PubMed] [Google Scholar]
- 31. Shaffer SW, Harrison AL. Aging of the somatosensory system: a translational perspective. Phys Ther 2007; 87: 193–207. [DOI] [PubMed] [Google Scholar]
- 32. Miwa T, Miwa Y, Kanda K. Dynamic and static sensitivities of muscle spindle primary endings in aged rats to ramp stretch. Neurosci Lett 1995; 201: 179–182. [DOI] [PubMed] [Google Scholar]
- 33. Muller KA, Ryals JM, Feldman EL, et al. Abnormal muscle spindle innervation and large-fiber neuropathy in diabetic mice. Diabetes 2008; 57: 1693–1701. [DOI] [PubMed] [Google Scholar]
- 34. van Deursen RWM, Sanchez MM, Ulbrecht JS, et al. The role of muscle spindles in ankle movement perception in human subjects with diabetic neuropathy. Exp Brain Res 1998; 120(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 35. Muramatsu K, Niwa M, Tamaki T, et al. Effect of streptozotocin-induced diabetes on motoneurons and muscle spindles in rats. Neurosci Res 2017; 115: 21–28. [DOI] [PubMed] [Google Scholar]
- 36. Ettinger LR, Boucher A, Simonovich E. Patients with type 2 diabetes demonstrate proprioceptive deficit in the knee. World J Diabetes 2018; 9: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. D’Silva LJ, Lin J, Staecker H, et al. Impact of diabetic complications on balance and falls: contribution of the vestibular system. Phys Ther 2016; 96: 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ferlinc A, Fabiani E, Velnar T, et al. The importance and role of proprioception in the elderly: a short review. Mater Socio Medica 2019; 31: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Van Deursen M, Simoneau GG, Van Deursen WM. Foot and ankle sensory neuropathy, proprioception, and postural stability. J Orthop Sport Phys Ther 1999; 29: 718–726. [DOI] [PubMed] [Google Scholar]
- 40. Soukup T, Pedrosa-Domellöf F, Thornell L-E. Expression of myosin heavy chain isoforms and myogenesis of intrafusal fibres in rat muscle spindles. Microsc Res Tech 1995; 30: 390–407. [DOI] [PubMed] [Google Scholar]
- 41. Chal J, Pourquié O. Making muscle: skeletal myogenesis in vivo and in vitro. Development 2017; 144: 2104–2122. [DOI] [PubMed] [Google Scholar]
- 42. Hurren B, Collins JJP, Duxson MJ, et al. First neuromuscular contact correlates with onset of primary myogenesis in rat and mouse limb muscles. PLoS One 2015; 10: e0133811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tourtellotte WG, Keller-Peck C, Milbrandt J, et al. The transcription factor Egr3 modulates sensory axon-myotube interactions during muscle spindle morphogenesis. Dev Biol 2001; 232: 388–399. [DOI] [PubMed] [Google Scholar]
- 44. Albert Y, Whitehead J, Eldredge L, et al. Transcriptional regulation of myotube fate specification and intrafusal muscle fiber morphogenesis. J Cell Biol 2005; 169: 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oliveira Fernandes M, Tourtellotte WG. Egr3-dependent muscle spindle stretch receptor intrafusal muscle fiber differentiation and fusimotor innervation homeostasis. J Neurosci 2015; 35: 5566–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tourtellotte WG, Milbrandt J. Sensory ataxia and muscle spindle agenesis in mice lacking the transcription factor Egr3. Nat Genet 1998; 20: 87–91. [DOI] [PubMed] [Google Scholar]
- 47. Zelena J. The morphogenetic influence of innervation on the ontogenetic development of muscle-spindles. Development 1957; 5(3): 283–292. [Google Scholar]
- 48. Kucera J, Waldro JM. Treatment with beta bungarotoxin blocks muscle spindle formation in fetal rats. Development 1990; 110(2): 483–489. [DOI] [PubMed] [Google Scholar]
- 49. Kucera J, Walro JM, Reichler J. Differential effects of neonatal denervation on intrafusal muscle fibers in the rat. Anat Embryol (Berl) 1993; 187: 397–408. [DOI] [PubMed] [Google Scholar]
- 50. Kucera J, Walro JM. Postnatal maturation of spindles in deafferented rat soleus muscles. Anat Embryol (Berl) 1987; 176: 449–461. [DOI] [PubMed] [Google Scholar]
- 51. Kucera J, Walro JM. Formation of muscle spindles in the absence of motor innervation. Neurosci Lett 1992; 145: 47–50. [DOI] [PubMed] [Google Scholar]
- 52. Hippenmeyer S, Shneider NA, Birchmeier C, et al. A role for neuregulin1 signaling in muscle spindle differentiation. Neuron 2002; 36: 1035–1049. [DOI] [PubMed] [Google Scholar]
- 53. Andrechek ER, Hardy WR, Girgis-Gabardo AA, et al. ErbB2 is required for muscle spindle and myoblast cell survival. Mol Cell Biol 2002; 22: 4714–4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leu M, Bellmunt E, Schwander M, et al. Erbb2 regulates neuromuscular synapse formation and is essential for muscle spindle development. Development 2003; 124: 2603–2613. [DOI] [PubMed] [Google Scholar]
- 55. Qiao Y, Cong M, Li J, et al. The effects of neuregulin-1β on intrafusal muscle fiber formation in neuromuscular coculture of dorsal root ganglion explants and skeletal muscle cells. Skelet Muscle 2018; 8: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jacobson C, Duggan D, Fischbach G. Neuregulin induces the expression of transcription factors and myosin heavy chains typical of muscle spindles in cultured human muscle. Proc Natl Acad Sci 2004; 101: 12218–12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rumsey JW, Das M, Kang J-F, et al. Tissue engineering intrafusal fibers: dose- and time-dependent differentiation of nuclear bag fibers in a defined in vitro system using neuregulin 1-beta-1. Biomaterials 2008; 29: 994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Colón A, Badu-Mensah A, Guo X, et al. Differentiation of intrafusal fibers from human induced pluripotent stem cells. ACS Chem Neurosci 2020; 11: 1085–1092. [DOI] [PubMed] [Google Scholar]
- 59. Guo X, Colon A, Akanda N, et al. Tissue engineering the mechanosensory circuit of the stretch reflex arc with human stem cells: sensory neuron innervation of intrafusal muscle fibers. Biomaterials 2017; 122: 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rumsey JW, Das M, Bhalkikar A, et al. Tissue engineering the mechanosensory circuit of the stretch reflex arc: sensory neuron innervation of intrafusal muscle fibers. Biomaterials 2010; 31: 8218–8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Colón A, Guo X, Akanda N, et al. Functional analysis of human intrafusal fiber innervation by human γ-motoneurons. Sci Rep 2017; 7: 17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Musarò A. The basis of muscle regeneration. Adv Biol 2014; 2014: 1–16. [Google Scholar]
- 63. Prüller J, Mannhardt I, Eschenhagen T, et al. Satellite cells delivered in their niche efficiently generate functional myotubes in three-dimensional cell culture. PLoS One 2018; 13: e0202574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Relaix F, Zammit PS. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development 2012; 139: 2845–2856. [DOI] [PubMed] [Google Scholar]
- 65. Kirkpatrick LJ, Allouh MZ, Nightingale CN, et al. Pax7 shows higher satellite cell frequencies and concentrations within intrafusal fibers of muscle spindles. J Histochem Cytochem 2008; 56: 831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kirkpatrick LJ, Yablonka-Reuveni Z, Rosser BWC. Retention of Pax3 expression in satellite cells of muscle spindles. J Histochem Cytochem 2010; 58: 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Horst D, Ustanina S, Sergi C, et al. Comparative expression analysis of Pax3 and Pax7 during mouse myogenesis. Int J Dev Biol 2006; 50: 47–54. [DOI] [PubMed] [Google Scholar]
- 68. Zammit PS, Carvajal JJ, Golding JP, et al. Myf5 expression in satellite cells and spindles in adult muscle is controlled by separate genetic elements. Dev Biol 2004; 273: 454–465. [DOI] [PubMed] [Google Scholar]
- 69. Walro JM, Kucera J. Why adult mammalian intrafusal and extrafusal fibers contain different myosin heavy-chain isoforms. Trends Neurosci 1999; 22: 180–184. [DOI] [PubMed] [Google Scholar]
- 70. Liu J-X, Eriksson P-O, Thornell L-E, et al. Myosin heavy chain composition of muscle spindles in human biceps brachii. J Histochem Cytochem 2002; 50: 171–183. [DOI] [PubMed] [Google Scholar]
- 71. Kozeka K, Ontell M. The three-dimensional cytoarchitecture of developing murine muscle spindles. Dev Biol 1981; 87: 133–147. [DOI] [PubMed] [Google Scholar]
- 72. Jackson JR, Kirby TJ, Fry CS, et al. Reduced voluntary running performance is associated with impaired coordination as a result of muscle satellite cell depletion in adult mice. Skelet Muscle 2015; 5: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Akay T, Tourtellotte WG, Arber S, et al. Degradation of mouse locomotor pattern in the absence of proprioceptive sensory feedback. Proc Natl Acad Sci U S A 2014; 111: 16877–16882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Franco JA, Kloefkorn HE, Hochman S, et al. An in vitro adult mouse muscle-nerve preparation for studying the firing properties of muscle afferents. J Vis Exp 2014; 91: e51948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fenwick N, Griffin G, Gauthier C. The welfare of animals used in science: how the ‘Three Rs’ ethic guides improvements. Can Vet J 2009; 50: 523–530. [PMC free article] [PubMed] [Google Scholar]
- 76. Esch MB, King TL, Shuler ML. The role of body-on-a-chip devices in drug and toxicity studies. Annu Rev Biomed Eng 2011; 13: 55–72. [DOI] [PubMed] [Google Scholar]
- 77. Astashkina A, Mann B, Grainger DW. A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol Ther 2012; 134: 82–106. [DOI] [PubMed] [Google Scholar]
- 78. Sakolish CM, Esch MB, Hickman JJ, et al. Modeling barrier tissues in vitro: methods, achievements, and challenges. EBioMedicine 2016; 5: 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Smith LR, Meyer GA. Skeletal muscle explants: ex-vivo models to study cellular behavior in a complex tissue environment. Connect Tissue Res 2020; 61(3–4): 248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Duval K, Grover H, Han L-H, et al. Modeling physiological events in 2D vs. 3D cell culture. Physiology 2017; 32: 266–277. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 81. Guo X, Das M, Rumsey J, et al. Neuromuscular junction formation between human stem-cell-derived motoneurons and rat skeletal muscle in a defined system. Tissue Eng Part C Methods 2010; 16(6): 1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pedrosa F, Soukup T, Thornell L-E. Expression of an alpha cardiac-like myosin heavy chain in muscle spindle fibres. Histochemistry 1990; 95: 105–113. [DOI] [PubMed] [Google Scholar]
- 83. Kucera J, Walro JM, Gorza L. Expression of type-specific MHC isoforms in rat intrafusal muscle fibers. Jourd Histochem Cytochem 1992; 40: 293–307. [DOI] [PubMed] [Google Scholar]
- 84. Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev 2011; 91: 1447–1531. [DOI] [PubMed] [Google Scholar]
- 85. Rossi AC, Mammucari C, Argentini C, et al. Two novel/ancient myosins in mammalian skeletal muscles: MYH14/7b and MYH15 are expressed in extraocular muscles and muscle spindles. J Physiol 2010; 588: 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lee LA, Karabina A, Broadwell LJ, et al. The ancient sarcomeric myosins found in specialized muscles. Skelet Muscle 2019; 9: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Csapo R, Gumpenberger M, Wessner B. Skeletal muscle extracellular matrix – what do we know about its composition, regulation, and physiological roles? A narrative review. Front Physiol 2020; 11: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Thorsteinsdottir S, Deries M, Cachaço AS, et al. The extracellular matrix dimension of skeletal muscle development. Dev Biol 2011; 354: 191–207. [DOI] [PubMed] [Google Scholar]
- 89. Hinds S, Bian W, Dennis RG, et al. The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle. Biomaterials 2011; 32: 3575–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ovalle WK, Dow PR. The capsular sleeve of muscle spindles in mouse and man with special reference to the cytoskeleton. In: Hník P, Soukup T, Vejsada R, et al. (eds) Mechanoreceptors. Boston, MA: Springer, 1988, pp.255–261. [Google Scholar]
- 91. Ovalle WK, Dow PR. Morphological aspects of the muscle spindle capsule and its functional significance. In: Boyd IA, Gladden MH.(eds) The muscle spindle. London: Palgrave Macmillan, pp.23–28. [Google Scholar]
- 92. Maier A, Mayne R. Basal lamina development in chicken muscle spindles. Dev Dyn 1995; 202: 284–293. [DOI] [PubMed] [Google Scholar]
- 93. Maier A, Mayne R. Regional differences in organization of the extracellular matrix and cytoskeleton at the equator of chicken intrafusal muscle fibres. Cell Motil 1993; 14: 35–46. [DOI] [PubMed] [Google Scholar]
- 94. Swash M, Fox KP. The effect of age on human skeletal muscle studies of the morphology and innervation of muscle spindles 1972; 16(4): 417–432. [DOI] [PubMed] [Google Scholar]
- 95. Pedrosa-Domellöf F, Virtanen I, Thornell L-E. Diverse expression of extracellular matrix proteins in human muscle spindles. In: Taylor A, Gladden MH, Durbaba R. (eds) Alpha and gamma motor systems. Boston, MA: Springer, 1995, pp.243–245. [Google Scholar]
- 96. Williams S, Jacobson C. α-Dystroglycan is essential for the induction of Egr3, a transcription factor important in muscle spindle formation. Dev Neurobiol 2010; 70(7): 498–507. [DOI] [PubMed] [Google Scholar]
- 97. Maier A. Extracellular matrix and transmembrane linkages at the termination of intrafusal fibers and the outer capsule in chicken muscle spindles. J Morphol 1996; 228: 335–346. [DOI] [PubMed] [Google Scholar]
- 98. Čížková D, Soukup T, Mokrý J. Expression of nestin, desmin and vimentin in intact and regenerating muscle spindles of rat hind limb skeletal muscles. Histochem Cell Biol 2009; 131: 197–206. [DOI] [PubMed] [Google Scholar]
- 99. Benam KH, Dauth S, Hassell B, et al. Engineered in vitro disease models. Annu Rev Pathol Mech Dis 2015; 10: 245. [DOI] [PubMed] [Google Scholar]
- 100. Morgan J, Partridge T. Skeletal muscle in health and disease. Dis Model Mech 2020; 13(2): dmm042192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Khodabukus A, Prabhu N, Wang J, et al. In vitro tissue-engineered skeletal muscle models for studying muscle physiology and disease. Adv Healthc Mater 2018; 7(15): e1701498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cheney PD, Preston JB. Classification and response characteristics of muscle spindle afferents in the primate. J Neurophysiol 1976; 39: 1–8. [DOI] [PubMed] [Google Scholar]
- 103. Jansen JKS, Matthews PBC. The effects of fusimotor activity on the static responsiveness of primary and secondary endings of muscle spindles in the decerebrate cat. Acta Physiol Scand 1962; 55: 376–386. [DOI] [PubMed] [Google Scholar]
- 104. Hunt CC, Kuffler SW. Stretch receptor discharges during muscle contraction. J Physiol 1951; 113: 298–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hunt CC. Mammalian muscle spindle: peripheral mechanisms. Physiol Rev 1990; 70: 643–663. [DOI] [PubMed] [Google Scholar]
- 106. Crowe A, Matthews PBC. The effects of stimulation of static and dynamic fusimotor fibres on the response to stretching of the primary endings of muscle spindles. J Physiol 1964; 174: 109–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Matthews PBC. Muscle spindles and their motor control. Physiol Rev 1964; 44: 219–288. [DOI] [PubMed] [Google Scholar]
- 108. Gerwin L, Haupt C, Wilkinson KA, et al. Acetylcholine receptors in the equatorial region of intrafusal muscle fibres modulate mouse muscle spindle sensitivity. J Physiol 2019; 597: 1993–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Cheney PD, Preston JB. Classification of fusimotor fibers in the primate. J Neurophysiol 1976; 39: 9–19. [DOI] [PubMed] [Google Scholar]
- 110. Kakuda N, Nagaoka M. Dynamic response of human muscle spindle afferents to stretch during voluntary contraction. J Physiol 1998; 513: 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hagbarth KE, Vallbo ÅB. Discharge characteristics of human muscle afferents during muscle stretch and contraction. Exp Neurol 1968; 22: 674–694. [DOI] [PubMed] [Google Scholar]
- 112. Ribot-Ciscar E, Rossi-Durand C, Roll J-P. Increased muscle spindle sensitivity to movement during reinforcement manoeuvres in relaxed human subjects. J Physiol 2000; 523: 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dimitriou M. Human muscle spindle sensitivity reflects the balance of activity between antagonistic muscles. J Neurosci 2014; 34: 13644–13655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Simon A, Shenton F, Hunter I, et al. Amiloride-sensitive channels are a major contributor to mechanotransduction in mammalian muscle spindles. J Physiol 2010; 588: 171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wilkinson KA, Kloefkorn HE, Hochman S. Characterization of muscle spindle afferents in the adult mouse using an in vitro muscle-nerve preparation. PLoS One 2012; 7: e39140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Fischer M, Schäfer SS. Temperature effects on the discharge frequency of primary and secondary endings of isolated cat muscle spindles recorded under a ramp-and-hold stretch. Brain Res 1999; 840: 1–15. [DOI] [PubMed] [Google Scholar]
- 117. Hunt CC, Ottoson D. Impulse activity and receptor potential of primary and secondary endings of isolated mammalian muscle spindles. J Physiol 1975; 252: 259–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hunt CC, Ottoson D. Responses of primary and secondary endings of isolated mammalian muscle spindles to sinusoidal length changes. J Neurophysiol 1977; 40: 1113–1120. [DOI] [PubMed] [Google Scholar]
- 119. Matthews PBC. The response of de-efferented muscle spindle receptors to stretching at different velocities. J Physiol 1963; 168: 660–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Cheney PD, Preston JB. Effects of fusimotor stimulation on dynamic and position sensitivities of spindle afferents in the primate. J Neurophysiol 1976; 39: 20–30. [DOI] [PubMed] [Google Scholar]
- 121. Bessou P, Laporte Y, Pagès B. Frequencygrams of spindle primary endings elicited by stimulation of static and dynamic fusimotor fibres. J Physiol 1968; 196: 47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Edin BB, Vallbo AB. Dynamic response of human muscle spindle afferents to stretch. J Neurophysiol 1990; 63(6): 1297–1306. [DOI] [PubMed] [Google Scholar]
- 123. Fischer M, Schäfer S. Effects of the calcium antagonist nifedipine on the afferent impulse activity of isolated cat muscle spindles. Brain Res 2002; 954: 256–276. [DOI] [PubMed] [Google Scholar]
- 124. Lin SH, Cheng YR, Banks RW, et al. Evidence for the involvement of ASIC3 in sensory mechanotransduction in proprioceptors. Nat Commun 2016; 7: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Woo SH, Lukacs V, De Nooij JC, et al. Piezo2 is the principal mechanotransduction channel for proprioception. Nat Neurosci 2015; 18: 1756–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hong GS, Lee B, Wee J, et al. Tentonin 3/TMEM150c confers distinct mechanosensitive currents in dorsal-root ganglion neurons with proprioceptive function. Neuron 2016; 91: 107–118. [DOI] [PubMed] [Google Scholar]
- 127. Bewick GS, Banks RW. Mechanotransduction in the muscle spindle. Eur J Physiol 2015; 467: 175–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bewick GS. Synaptic-like vesicles and candidate transduction channels in mechanosensory terminals. J Anat 2015; 227: 194–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhang Y, Wesolowski M, Karakatsani A, et al. Formation of cholinergic synapse-like specializations at developing murine muscle spindles. Dev Biol 2014; 393: 227–235. [DOI] [PubMed] [Google Scholar]
- 130. Carr R, Proske U. Action of cholinesters on sensory nerve endings in skin and muscle. Clin Exp Pharmacol Physiol 1996; 23: 355–362. [DOI] [PubMed] [Google Scholar]
- 131. Smith AST, Long CJ, Pirozzi K, et al. A functional system for high-content screening of neuromuscular junctions in vitro. Technology 2013; 1: 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Torii R, Velliou RI, Hodgson D, et al. Modelling multi-scale cell–tissue interaction of tissue-engineered muscle constructs. J Tissue Eng 9. Epub ahead of print 13 August 2018. DOI: 10.1177/2041731418787141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wang J, Khodabukus A, Rao L, et al. Engineered skeletal muscles for disease modeling and drug discovery. Biomaterials 2019; 221: 119416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Rao L, Qian Y, Khodabukus A, et al. Engineering human pluripotent stem cells into a functional skeletal muscle tissue. Nat Commun 2018; 9: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Capel AJ, Rimington RP, Fleming JW, et al. Scalable 3D printed molds for human tissue engineered skeletal muscle. Front Bioeng Biotechnol 2019; 7: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Zhuang P, An J, Chua CK, et al. Bioprinting of 3D in vitro skeletal muscle models: a review. Materials and Design 2020; 193: 108794. [Google Scholar]
- 137. Juhas M, Engelmayr GC, Fontanella AN, et al. Biomimetic engineered muscle with capacity for vascular integration and functional maturation in vivo. Proc Natl Acad Sci 2014; 111: 5508–5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Vandenburgh HH, Karlisch P, Farr L. Maintenance of highly contractile tissue-cultured avian skeletal myotubes in collagen gel. Vitr Cell Dev Biol 1988; 24: 166–174. [DOI] [PubMed] [Google Scholar]
- 139. Madden L, Juhas M, Kraus WE, et al. Bioengineered human myobundles mimic clinical responses of skeletal muscle to drugs. Elife 2015; 4: e04885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Huang YC, Dennis RG, Larkin L, et al. Rapid formation of functional muscle in vitro using fibrin gels. J Appl Physiol 2005; 98: 706–713. [DOI] [PubMed] [Google Scholar]
- 141. Mcguigan AP, Javaherian S. Tissue patterning: translating design principles from in vivo to in vitro. Annu Rev Biomed Eng 2016; 18: 1–24. [DOI] [PubMed] [Google Scholar]
- 142. Martinez-Rivas A, González-Quijano G, Proa-Coronado S, et al. Methods of micropatterning and manipulation of cells for biomedical applications. Micromachines 2017; 8: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Pardo-Figuerez M, Martin NRW, Player DJ, et al. Controlled arrangement of neuronal cells on surfaces functionalized with micropatterned polymer brushes. ACS Omega 2018; 3: 12383–12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Palamà IE, D’Amone S, Coluccia AML, et al. Micropatterned polyelectrolyte nanofilms promote alignment and myogenic differentiation of C2C12 cells in standard growth media. Biotechnol Bioeng 2013; 110: 586–596. [DOI] [PubMed] [Google Scholar]
- 145. Hwang Y, Seo T, Hariri S, et al. Matrix topographical cue-mediated myogenic differentiation of human embryonic stem cell derivatives. Polymers (Basel) 2017; 9: 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Huang NF, Li S. Regulation of the matrix microenvironment for stem cell engineering and regenerative medicine. Ann Biomed Eng 2011; 39: 1201–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Burdick JA, Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng Part A 2009; 15(2): 205–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Ayala R, Zhang C, Yang D, et al. Engineering the cell-material interface for controlling stem cell adhesion, migration, and differentiation. Biomaterials 2011; 32: 3700–3711. [DOI] [PubMed] [Google Scholar]
- 149. Gao C, Tang L, Hong J, et al. Effect of laser induced topography with moderate stiffness on human mesenchymal stem cell behavior. J Phys Mater 2019; 2(3): 34006. [Google Scholar]
- 150. Newman P, Galenano-Ninõ JL, Graney P, et al. Relationship between nanotopographical alignment and stem cell fate with live imaging and shape analysis. Sci Rep 2016; 6: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Metavarayuth K, Sitasuwan P, Zhao X, et al. Influence of surface topographical cues on the differentiation of mesenchymal stem cells in vitro. ACS Biomater Sci Eng 2016; 2: 142–151. [DOI] [PubMed] [Google Scholar]
- 152. Coln EA, Long CJ, Sriram NN, et al. Piezoelectric bioMEMS cantilever for measurement of muscle contraction and for actuation of mechanosensitive cells. MRS Commun 2019; 9(4): 1186–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Wilson K, Das M, Wahl KJ, et al. Measurement of contractile stress generated by cultured rat muscle on silicon cantilevers for toxin detection and muscle performance enhancement. PLoS One 5(6): e11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Mannhardt I, Warncke C, Trieu HK, et al. Piezo-bending actuators for isometric or auxotonic contraction analysis of engineered heart tissue. J Tissue Eng Regen Med 2019; 13: 3–11. [DOI] [PubMed] [Google Scholar]
- 155. Kapat K, Shubhra QTH, Zhou M, et al. Piezoelectric nano-biomaterials for biomedicine and tissue regeneration. Adv Funct Mater 2020; 30(44): 1909045. [Google Scholar]
- 156. Joe AWB, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 2010; 12: 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Paylor B, Natarajan A, Zhang R-H, et al. Nonmyogenic cells in skeletal muscle regeneration. Curr Top Dev Biol 2011; 96: 139–165. [DOI] [PubMed] [Google Scholar]
- 158. Wosczyna MN, Rando TA. A muscle stem cell support group: coordinated cellular responses in muscle regeneration. Dev Cell 2018; 46: 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Birbrair A, Zhang T, Wang Z-M, et al. Pericytes: multitasking cells in the regeneration of injured, diseased, and aged skeletal muscle. Front Aging Neurosci 2014; 6: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Brady MA, Lewis MP, Mudera V. Synergy between myogenic and non-myogenic cells in a 3D tissue-engineered craniofacial skeletal muscle construct. J Tissue Eng Regen Med 2008; 2: 408–417. [DOI] [PubMed] [Google Scholar]
- 161. Tedesco FS, Dellavalle A, Diaz-Manera J, et al. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest 2010; 120: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Negroni E, Riederer I, Chaouch S, et al. In vivo myogenic potential of human CD133+ muscle-derived stem cells: a quantitative study. Mol Ther 2009; 17: 1771–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. del Carmen Ortuño-Costela M, García-López M, Cerrada V, et al. iPSCs: a powerful tool for skeletal muscle tissue engineering. J Cell Mol Med 2019; 23(6): 3784–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Huang CY, Liu CL, Ting CY, et al. Human iPSC banking: barriers and opportunities. J Biomed Sci 2019; 26: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Zhang D, Yan K, Zhou J, et al. Myogenic differentiation of human amniotic mesenchymal cells and its tissue repair capacity on volumetric muscle loss. J Tissue Eng 10. Epub ahead of print 11 November 2019. DOI: 10.1177/2041731419887100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126: 663–676. [DOI] [PubMed] [Google Scholar]
- 167. Geng Bc, Choi KH, Wang Sz, et al. A simple, quick, and efficient CRISPR/Cas9 genome editing method for human induced pluripotent stem cells. Acta Pharmacol Sin. 41(11): 1427–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Maffioletti SM, Sarcar S, Henderson ABH, et al. Three-dimensional human iPSC-derived artificial skeletal muscles model muscular dystrophies and enable multilineage tissue engineering. Cell Rep 2018; 23: 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Guimarães MZP, De Vecchi R, Vitória G, et al. Generation of iPSC-derived human peripheral sensory neurons releasing substance P elicited by TRPV1 agonists. Front Mol Neurosci 2018; 11: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Schwartzentruber J, Foskolou S, Kilpinen H, et al. Molecular and functional variation in iPSC-derived sensory neurons. Nat Genet 2018; 50: 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]