Abstract
Following a request from the European Commission, the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) was asked to deliver an opinion on Eurycoma longifolia (Tongkat Ali) root extract as a novel food (NF) pursuant to Regulation (EU) 2015/2283. The NF is standardised water extract prepared from the dried ground root chips of Tongkat Ali (Eurycoma longifolia Jack) and proposed by the applicant to be used as food supplement in amounts up to 200 mg/day. The target population is the adult population, except pregnant and lactating women. The characteristic components of the NF are glycosaponins (40–65%) and eurycomanone (0.8–1.5%). It can also contain canthin‐6‐one alkaloids and isoscopoletin (coumarin). The NF has been present in various international markets since 2009. The Panel notes positive results from the submitted in vitro chromosome aberration test, which indicates clastogenic properties of the NF. In the requested follow‐up in vivo mammalian alkaline comet assay, the NF induced positive results at the highest dose tested (2,000 mg/kg body weight (bw)) at the tissues of the first site of contact (stomach and duodenum). Histopathological evaluation of the tested tissues indicated that the positive results of the comet assay were rather due to genotoxicity than cytotoxicity. Taken together, the Panel concludes that the NF has the potential to induce DNA damage, which is of concern, particularly locally for tissues that represent first sites of contact. The Panel concludes that the safety of NF has not been established under any condition of use.
Keywords: Novel food, Eurycoma longifolia, water extract, food supplement, genotoxicity
1. Introduction
1.1. Background and Terms of Reference as provided by the European Commission
On 9 August 2016, the company Biotropics Malaysia Berhad submitted a request to the United Kingdom Food Standards Agency (FSA) in accordance with Article 4 of Regulation (EC) No 258/971 to place on the EU market Eurycoma longifolia (Tongkat Ali) root extract as a novel food (NF).
The FSAs’ Advisory Committee on Novel Foods and Processes (ACNFP) raised several concerns which were addressed by the applicant on 4 January 2017, 30 May 2017, 21 June 2017 and 2 October 2017.
The concerns of a scientific nature raised by the ACNFP can be summarised as follows:
The data presented for the compositional analysis was not accurate or robust enough to allow the NF to be fully characterised. Analytical data on the glycosaponin content, which makes up to 40–60% of the total weight of the NF, was considered to be important, given the potential pharmacological nature of the product. Protein content was also relevant for the assessment of allergenic potential;
In view of adverse effects (especially in liver and kidneys) observed in a submitted chronic toxicity study (Unpublished study report, 2015), the ACNFP considered that additional targeted studies were needed to confirm the safety of the NF. Since initially, the proposed uses of the NF included a range of different food categories, the ACNFP considered that additional studies were needed to assess the impact on a wider range of non‐targeted consumers.
Pursuant to Article 35(1) of Regulation (EU) 2015/22832, any request for placing a novel food on the market within the Union submitted to a Member State in accordance with Article 4 of Regulation (EC) No 258/97 of the European Parliament and of the Council concerning novel foods and novel foods ingredients and for which the final decision has not been taken before 1 January 2018, shall be treated as an application submitted under Regulation (EU) 2015/2283.
On 2 May 2018, in accordance with Article 10(3) of Regulation (EU) 2015/2283, the Commission asked EFSA to provide a scientific opinion by carrying out the additional assessment for Eurycoma longifolia (Tongkat Ali) root extract as a NF.
2. Data and methodologies
2.1. Data
The safety assessment of this novel food (NF) is based on data supplied in the application, the concerns and objections of a scientific nature raised by the initial risk assessor ACNFP, information submitted by the applicant following EFSA's requests for supplementary information, information provided by the EFSA Working Group on Compendium of Botanicals, EFSA Scientific Committee Working Group on Genotoxicity and additional data identified by the Panel.
Administrative and scientific requirements for NF applications referred to in Article 10 of Regulation (EU) 2015/2283 are listed in the Commission Implementing Regulation (EU) 2017/2469.3
A common and structured format on the presentation of NF applications is described in the EFSA guidance on the preparation and presentation of an NF application (EFSA NDA Panel, 2016). As indicated in this guidance, it is the duty of the applicant to provide all of the available (proprietary, confidential and published) scientific data, including both data in favour and not in favour to supporting the safety of the proposed NF.
This NF application includes a request for protection of proprietary data in accordance with Article 26 of Regulation (EU) 2015/2283. The data, requested by the applicant to be protected, comprise: compositional data (chemo‐profiling of the NF), toxicological information (studies on genotoxicity, acute, subacute, subchronic, chronic and reproduction/developmental toxicity), human data and allergens analysis report.
2.2. Methodologies
The assessment follows the methodology set out in the EFSA guidance on NF applications and the principles described in the relevant existing guidance documents from the EFSA Scientific Committee. The legal provisions for the assessment are laid down in Article 11 of Regulation (EU) 2015/2283 and in Article 7 of the Commission Implementing Regulation (EU) 2017/2469.
The information provided by the EFSA Working Group on Compendium of Botanicals is based on an extensive literature search on Tongkat Ali (Eurycoma longifolia) using the following scientific databases: ‘Scopus’, ‘Pubmed’, ‘Scifinder’ and ‘Web of Science’. This provided the basis for identifying scientific evidence available in peer‐reviewed scientific papers in relation to substances contained in Tongkat Ali of potential concern, toxicological data and studies reporting adverse health outcomes in humans.
This assessment concerns only risks that might be associated with consumption of the NF under the proposed conditions of use, and is not an assessment of the efficacy of the NF with regard to any claimed benefit.
3. Assessment
3.1. Introduction
The NF, which is the subject of the application, is a standardised water extract prepared from the dried ground root chips of Tongkat Ali (Eurycoma longifolia Jack) that belong to the family of Simaroubaceae DC.4
The NF falls under Regulation (EU) 2015/2283, Article 3(2)(a)(iv): food consisting of, isolated from or produced from plants or their parts.
The NF is proposed by the applicant to be used as food supplement. The target population is the adult population, except pregnant and lactating women.
3.2. Identity of the NF
The NF, named by the applicant ‘Tongkat Ali Root Extract’ is a standardised water extract obtained from the dried ground root chips of the plant Eurycoma longifolia Jack. It is a powder mainly containing polysaccharides (30–55%) and glycosaponins (40–65%). Other commonly used names are Tongkat Ali, Longjack or Malaysian Ginseng (Bhat and Karim, 2010). Ng et al. (2019) performed a phylogenetic analysis of the plant and confirmed its position within the family of Simaroubaceae , based on whole chloroplast genome sequences.
The plant is native to Southeast Asian countries such as Indonesia, Malaysia, Vietnam and also Cambodia, Myanmar, Laos and Thailand (Rehman et al., 2016).
3.3. Production process
Dried root chips of wild E. longifolia serve as the source material. Cultivars are identified using random amplified polymorphic deoxyribonucleic acid (DNA) analysis by polymerase chain reaction (PCR‐RAPD) (Razi et al., 2013). An extraction process with purified hot water is applied on dry chips. The filtered extract is concentrated via evaporation, sterilised and dried. The product is then ground/milled to a fine powder and packaged. No excipients or diluents are used in the formulation of the NF.
As stated by the applicant, the manufacturing process is conducted according to Good Manufacturing Practice (GMP) and is compliant with ISO 22000:2005 and Hazard Analysis and Critical Control Points (HACCP) principles.
The Panel considers that the production process is sufficiently described.
3.4. Compositional data
The NF is a standardised powder containing total polysaccharide (30–55%), total protein (22–45%), total glycosaponin (40–65%) and eurycomanone (0.8–1.5%), which is consistent with the specifications established in the Malaysian Standard for this extract (Department of Standards Malaysia, 2011).
The applicant provided chemical and microbiological analyses of the NF which were carried out using three independently produced batches (Table 1).
Table 1.
Batch‐to‐batch analyses of the NF
| Parameter (unit) | Batch no | Method/technique | ||
|---|---|---|---|---|
| #1 | #2 | #3 | ||
| Organoleptic | ||||
| Colour | Light brown | Light brown | Light brown | Visual |
| Odour | Characteristic | Characteristic | Characteristic | Sensory |
| Flavour | Bitter | Bitter | Bitter | Sensory |
| Form/texture | Fine powder | Fine powder | Fine powder | Sensory |
| Extraneous material | Free from foreign matter | Free from foreign matter | Free from foreign matter | Visual |
| Physical characteristics | ||||
| Moisture content (%) | 2.11 | 3.45 | 2.43 | Loss on drying |
| Average mesh size | 90% smaller than 120 mesh | 90% smaller than 120 mesh | 90% smaller than 120 mesh | Sieve analysis |
| Constituents | ||||
| Eurycomanone (%) | 0.91 | 0.87 | 0.81 | In‐house method based on HPLC‐DAD |
| Total protein (%) | 27.6 | 29.9 | 30.8 | Lowry method |
| Total polysaccharide (%) | 33.5 | 34.7 | 36.5 | UV‐spectrophotometry |
| Glycosaponins (%) | 47.2 | 49.3 | 51.5 | Gravimetry |
| Heavy metals | ||||
| Lead (mg/kg) | < 0.10 | < 0.10 | < 0.10 | AAS (Ph. Eu. 2.4.27) |
| Mercury (mg/kg) | < 0.01 | < 0.01 | < 0.01 | AAS (Ph. Eu. 2.4.27) |
| Arsenic (mg/kg) | < 0.01 | < 0.01 | 0.04 | AAS (Ph. Eu. 2.4.27) |
| Cadmium (mg/kg) | < 0.01 | < 0.01 | < 0.01 | AAS (Ph. Eu. 2.4.27) |
| Microbial specifications | ||||
| Total aerobic count (CFU/g) | < 10 | < 10 | < 10 | Ph. Eu. 2.6.12 |
| Total yeast and mould count (CFU/g) | < 10 | < 10 | < 10 | Ph. Eu. 2.6.12 |
| Salmonella (/10 g) | Absent | Absent | Absent | Ph. Eu. 2.6.13 |
| Escherichia coli (/1 g) | Absent | Absent | Absent | Ph. Eu. 2.6.13 |
| Staphylococcus aureus (/1 g) | Absent | Absent | Absent | Ph. Eu. 2.6.13 |
| Bile‐tolerant Gram‐negative bacteria (bacteria/g) | < 10 | < 10 | < 10 | Ph. Eu. 2.6.13 |
HPLC‐DAD: high‐performance liquid chromatography with diode‐array detector; UV: ultraviolet AAS: atomic absorption spectroscopy; Ph. Eu.: European Pharmacopoeia; CFU: Colony Forming Units.
Eurycomanone (a quassinoid, CAS number 84633‐29‐4) was proposed by the applicant as the major marker compound for the specification of the NF (Figure 1). Beside quassinoids (a group of nortriterpenoids), which account for a major portion of the E. longifolia root phytochemicals, the plant is reported to contain also canthin‐6‐one alkaloids, β‐carboline alkaloids, coumarins, squalenes, triterpenes and biphenylneolignans (Bhat and Karim, 2010; Dang et al., 2019; Tam et al., 2014; Rehman et al., 2016; Chaingam et al., 2021). In addition, more than 85 compounds have been reported from aqueous extracts of E. longifolia and characterised by liquid chromatography with tandem mass spectrometry (LC‐MS/MS) by Chua et al. (2011), Rehman et al. (2016) and Ezzat et al. (2019), mostly as aglycones.
Figure 1.
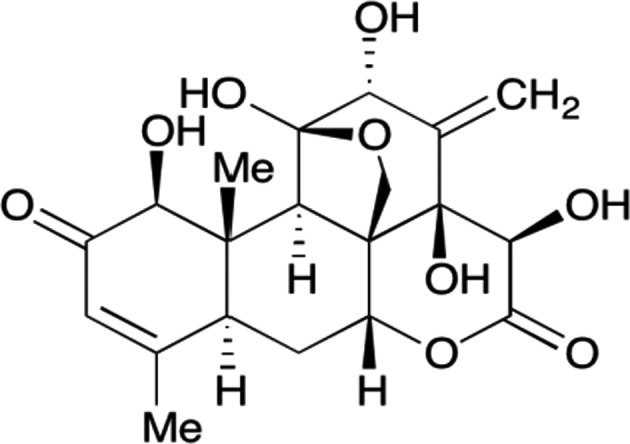
Eurycomanone structure
The ACNFP was concerned that the application contained no substantial analytical data on the glycosaponin component, which makes up 40–65% of the material in the NF.
The applicant provided the LC‐MS/MS analysis of the saponins obtained by precipitation, in which eight peaks have been tentatively identified by molecular weight, peak fragmentation and matching with data from the literature (Chua et al., 2011, 2012). Most signals are not specific for a single compound but are rather compatible with several compounds. They are mainly quassinoid metabolites including eurycomanone and its derivatives, eurycomanol, eurycomalactone, laurycolactone, eurycomalide B and eurycolactone, some of them as glycosides.
In order to better characterise the saponin fraction, the applicant performed liquid–liquid extraction of a sample of the NF dissolved in water using three types of solvents: ethyl acetate, butanol and chloroform. Extraction with ethyl acetate allowed to increase the amounts of extracted saponins, but the identification of these compounds was not possible. The same experiment was conducted by Chua et al. (2019) reaching the same conclusions.
Upon a request by EFSA for a better characterisation of glycosaponins, the applicant has provided information on additional work. Upon fractionation of a crude extract of the NF by column chromatography, two fractions were collected, which were analysed by Liquid Chromatography‐Diode Array Detector (LC‐DAD‐MS/MS) and Quadrupole Time of Flight Mass Spectrometer (QTOF‐MS). The first fraction was further analysed by Ultra Performance Liquid Chromatography‐tandem Mass Spectrometry (UPLC‐MS/MS) and showed the presence of three compounds: eurycomanone glycoside, eurycomanol glycoside and eurycomanol trimer. The presence of eurycomanol was confirmed by proton nuclear magnetic resonance (1H‐NMR) by data matching to the predicted data using ACD (Advanced Chemistry Development)/Labs and published data (Ebrahimi et al., 2016). The second fraction, analysed by LC‐DAD‐MS/MS and NMR, allowed to identify a diglycoside with the aglycone very similar to glyyunnanprosapogenin D, previously isolated from the roots of Glycyrrhiza yunnanensis, first reported by Zeng et al. (1990). The only difference is the presence of an additional hydroxyl group at carbon‐2. The above compound is identified as saponin, but no quantitative data have been provided.
As previously mentioned, available literature (Bhat and Karim, 2010; Chua et al., 2011; Dang et al., 2019; Tam et al., 2014; Rehman et al., 2016; Chaingam et al., 2021) suggests the possible presence of compounds in E. longifolia extracts, which could raise safety concerns: canthin‐6‐one alkaloids and isoscopoletin (coumarin). The applicant confirmed the presence of these compounds in small amounts, based on the chromatogram of the crude extract and of the ethyl acetate fraction.
Finally, the applicant provided results of an analysis of contaminants in one sample of the NF, which has been analysed for the presence of organochlorine and organophosphorus pesticides (using in‐house method based on AOAC 200.03) and aflatoxins (using in‐house method based on AOAC 991.31 & 990.33). All results were below the limit of detection5 for the particular parameter.
The Panel considers that the information provided on the composition is sufficient for characterising the NF.
3.5. Stability
In order to demonstrate the stability of the NF, the applicant conducted two studies of a shelf‐life using two independently produced batches of the NF. The first study was conducted under normal storage conditions (30 ± 2°C, relative humidity of 75 ± 5%), with a duration of up to 36 months for one batch and 24 months for another batch, respectively. The second study was conducted under accelerated conditions (40 ± 2°C, relative humidity of 75 ± 5%) up to 6 months.
The results of both studies indicate that the NF conforms to the established physical, chemical and microbial specifications for at least its recommended shelf‐life of 24 months (data not shown).
The NF is recommended to be stored at room temperature below 30°C and relative humidity of 60%.
The Panel considers that the data provided sufficient information with respect to the stability of the NF for 24 months.
3.6. Specifications
Product specifications proposed by the applicant are reported in Table 2. The applicant stated that specifications were based on the standardisation parameters outlined in The Malaysian Standard's phytopharmaceutical aspect of dried water extract from Tongkat Ali roots (Department of Standards Malaysia, 2011).
Table 2.
Product specifications for the NF
| Parameter | Specification (unit) |
|---|---|
| Organoleptic | |
| Colour | Light brown to brown |
| Odour | Characteristic |
| Flavour | Bitter |
| Form/texture | Fine powder |
| Extraneous material | Free from foreign matter |
| Physical characteristics | |
| Moisture content (%) | < 8.0 |
| Average mesh size | 90% smaller than 100 mesh |
| Constituents | |
| Eurycomanone (%) | 0.8–1.5 |
| Total protein (%) | 22–45 |
| Total polysaccharide (%) | 30–55 |
| Glycosaponins (%) | 40–65 |
| Heavy metals | |
| Lead (mg/kg) | < 2.0 |
| Mercury (mg/kg) | < 0.05 |
| Arsenic (mg/kg) | < 1.0 |
| Cadmium (mg/kg) | < 0.3 |
| Microbial parameters | |
| Total aerobic count (CFU/g) | < 10,000 |
| Yeasts and moulds (CFU/g) | < 100 |
| Salmonella (/10 g) | Absent |
| Escherichia coli (/1 g) | Absent |
| Staphylococcus aureus (/1 g) | Absent |
| Bile‐tolerant Gram‐negative bacteria (bacteria/g) | < 100 |
CFU: Colony Forming Units.
3.7. History of use of the NF and/or of its source
3.7.1. History of use of the source
Eurycoma longifolia is a slow‐growing tree with pinnate, spiral leaves and panicle flowers (Elliott and Brimacombe, 1987; Bhat and Karim, 2010). The applicant provided specimen vouchers from the ‘Universiti Putra Malaysia’ for the verification and authentication of a plant.
In addition, the applicant listed several publications describing the history of the consumption of E. longifolia as a herbal remedy (Oxley, 1850; Burkill, 1935; Gimlette, 1939; Elliott and Brimacombe, 1987; Bhat and Karim, 2010; Ong et al., 2012) prevalent in South‐East Asian countries such as Malaysia, Indonesia and Vietnam. Most of the parts of the plant have a history of consumption and particularly the root extract has been used for different, putative health benefits.
3.7.2. History of use of the NF
The applicant provided information about the distribution of the NF in various international markets. It has been on the Malaysian market since 2009 as a food supplement, followed by Hong Kong, as an herbal‐based product (traditional) and other major markets, including Singapore, Russia, Japan, United States and Canada where it is mainly marketed as an herbal food product and dietary supplement. The global circulated volume for the NF in 2014 was ~ 3,000 kg (equivalent to 15 million servings of 200 mg or approx. 60 million servings of 50 mg).
■■■■■
3.8. Proposed uses and use levels and anticipated intake
3.8.1. Target population
The applicant specified that the target population for the consumption of the NF would be adults, excluding pregnant and lactating women.
3.8.2. Proposed uses and use levels
The applicants’ intention is to use the NF as a food supplement in an amount up to 200 mg/day, equivalent to approximately 2.86 mg/kg bw per day for 70 kg adult.
3.9. Absorption, distribution, metabolism and excretion (ADME)
The applicant presented a study by Low et al. (2005) in which the bioavailability and pharmacokinetics of eurycomanone have been evaluated (by HPLC) in rat plasma following oral (PO) and intravenous (IV) administration. The study authors concluded that eurycomanone exhibited low bioavailability (PO), a short biological half‐life and appeared to be well distributed in the extravascular fluids.
In another study (Ahmad et al., 2018) presented by the applicant, the bioavailability of eurycomanone in its pure form or as a compound of the NF was characterised by a series of in vitro and in vivo studies in rats and mice. This study confirmed the similar bioavailability of eurycomanone in rats following oral administration in comparison to the study by Low et al. (2005). However, a higher bioavailability for mice suggested interspecies differences and that the pharmacokinetic values may not be directly extrapolated from rodents to humans, as concluded by the study authors.
3.10. Nutritional information
Taking into account the proposed conditions of use (i.e. 200 mg/day), the Panel considers that the consumption of the NF is not nutritionally disadvantageous.
3.11. Toxicological information
The applicant provided comprehensive toxicological information with several study reports on the NF, overviewed in Table 3. Some of the studies on genotoxicity were performed upon the request from the Panel (see the following section).
Table 3.
List of toxicological studies with the NF provided by the applicant
| Reference | Type of the study | Tested population | Doses |
|---|---|---|---|
| Genotoxicity | |||
| Ming et al. (2015a), Unpublished study report (2010a) | Bacterial reverse mutation test (GLP, OECD TG 471) | S. Typhimurium TA98, TA100, TA102, TA1535 and TA1537 | 0, 0.005, 0.01, 0.03, 0.05, 0.3, 1.0, 3.0 or 5.0 mg/plate (absence and presence of S9 mix) |
| Unpublished study report (2013) | In vitro mammalian cell gene mutation test (GLP, OECD TG 476) | L5178Y mouse lymphoma cells | 0, 800, 1,000 or 3,000 μg/mL (absence and presence of S9 mix) |
| Unpublished study report (2011a) | In vitro mammalian chromosome aberration test (GLP, OECD TG 473) | Human lymphocytes | 24 h preparation interval: 0, 62.5, 125, 500 and 1,000 μg/mL (absence and presence of S9 mix) 48 h preparation interval: 0, 500, 1,000 and 2,500 μg/mL (absence and presence of S9 mix) |
| Ming et al. (2015a), Unpublished study report (2011b) | In vivo mammalian erythrocyte micronucleus test (GLP, OECD TG 474) | NMRI mice (n = 5/sex per group) | 0, 100, 250 and 500 mg/kg bw |
| Unpublished study report (2019) | In vitro mammalian cell micronucleus (MN) test (GLP, OECD TG 487) | Human TK6 lymphoblastoid cells | 0, 148.6–424.1 μg/mL for 3 h (presence of S9 mix) 0, 78.04–395.1 μg/mL for 3 h (absence of S9 mix) 0, 23.12–117.1 μg/mL for 24 h (absence of S9 mix) |
| Unpublished study report (2021) | In vivo mammalian alkaline comet assay (GLP, OECD TG 489) | Crl:Wistar Han rats (stomach, duodenum and liver) | 0, 500, 1,000 and 2,000 mg/kg bw |
| Acute, subacute, subchronic and chronic toxicity | |||
| Choudhary et al. (2012), Unpublished study report 2008a) | Acute oral toxicity (OECD TG 420) | Wistar rats (n = 4 females) | 2,000 mg/kg bw |
| Choudhary et al. (2012), Unpublished study report (2008b) | 28‐day repeated dose oral toxicity study (OECD TG 407) | Wistar rats (5/sex per group) | 0, 250, 500 and 1,000 mg/kg bw per day |
| Choudhary et al. (2012), Unpublished study report (2010b) | 90‐day repeated dose oral toxicity study (GLP, OECD TG 408) | Wistar rats (10/sex per group) | 0, 250, 500 and 1,000 mg/kg bw per day |
| Unpublished study report (2015) | 12‐month repeated dose oral toxicity study (GLP, OECD TG 452) | Wistar rats (25/sex per group) | 0, 250, 500 and 1,000 mg/kg bw per day |
| Reproductive and developmental toxicity | |||
| Ming et al. (2015b), Unpublished study report (2016a) | Reproduction/developmental toxicity screening test (OECD TG 421) | Wistar rats (10/sex per group) | 0, 250, 500 and 1,000 mg/kg bw per day |
| Unpublished study report (2016b) | Two‐generation reproduction toxicity study (OECD TG 416) | Wistar rats (30/sex per group) | 0, 250, 500 and 1,000 mg/kg bw per day |
| Solomon et al. (2014) | In vivo effects of E. longifolia Jack (Tongkat Ali) extract on reproductive functions in the rat | Male Sprague‐Dawley rats (14/group) | 0, 200 and 800 mg/kg bw per day |
bw: body weight; GLP: Good Laboratory Practice; MN: micronucleus; OECD TG: Organisation for Economic Co‐operation and Development Test Guideline.
3.11.1. Genotoxicity
A bacterial reverse mutation test (Unpublished study report, 2010a; Ming et al., 2015a) was performed according to OECD Test Guideline No. 471 (OECD, 1997) and in compliance with the principles of Good Laboratory Practice (GLP). Using S. Typhimurium tester strains TA98, TA100, TA102, TA1535 and TA1537 and the plate incorporation method, the NF was tested at dose levels of 0 (vehicle control: deionised water), 0.005, 0.01 and 0.03 (TA100 only), as well as 0.05, 0.3, 1.0, 3.0 or 5.0 mg/plate (all strains), both in the absence and presence of a metabolic activation system (S9 mix). The Panel notes that the test material was partially soluble in water and the remaining sediment was removed before use. No biologically relevant increases in the numbers of revertant colonies were observed at any dose level compared to the negative (vehicle) control.
An in vitro mammalian cell gene mutation test using L5178Y mouse lymphoma cells (Unpublished study report, 2013) was conducted in accordance with OECD Test Guideline 476 (OECD, 1997) and in compliance with GLP. The culture medium was used as the negative control and DMSO (0.8% and 1% or 2.4%) as the vehicle control. In the first experiment, cells were exposed to the NF for 3 h at concentrations up to 3,000 μg/mL, both in the presence and absence of metabolic activation (4% S9 mix). In the absence of metabolic activation, the NF induced an increase in mutant frequency (approximately 4x) at a concentration of 3,000 μg/mL when compared with the negative control. At this concentration, precipitation of the test material, as well as marked cytotoxicity (relative total growth [RTG] 22%), was observed. In the second experiment, cells were exposed for 3 h in the presence of metabolic activation (8% S9 mix) at concentrations up to 3,000 μg/mL, and for 24 h in the absence of S9 mix at concentrations up to 1,250 μg/mL. There was an increase in mutant frequency in the absence of metabolic activation at 800 μg/mL (approximately 2.6x). At this concentration, marked cytotoxicity was observed (RTG 12%) and the higher concentrations were not evaluated. Precipitation of the test material in the absence of S9 mix occurred at 1,250 μg/mL. In the third experiment, cells were treated for 4 h without S9 mix at concentrations up to 3,000 μg/mL. An increase in mutant frequency (4.5x) was observed at 3,000 μg/mL. At this concentration, marked cytotoxicity occurred (RTG 19%), and precipitation was observed above 1,666 μg/mL. Since the increases in mutant frequency occurred only at strongly cytotoxic concentrations, the Panel considers that they are not biologically relevant.
An in vitro mammalian chromosome aberration test using cultured human lymphocytes (Unpublished study report, 2011a) was conducted according to OECD Test Guideline 473 (OECD, 1997) and in compliance with the principles of GLP. In the main experiment, cells were treated with the NF for 4 h with and without S9 mix up to a concentration of 5,000 μg/mL, and prepared for evaluation 24 h after the start of treatment. Based on a pre‐experiment to determine cytotoxicity (using the % Mitotic Index), three concentrations up to 1,000 μg/mL were selected for microscopic evaluation. Using this 24‐h preparation interval, a delay in the cell cycle was observed. Thus, a second experiment was performed with a longer preparation interval. Applying a 48‐h preparation interval, an increase in the rate of structural chromosome aberrations compared with the negative control was observed after treatment with the test material with and without metabolic activation at all concentrations evaluated (500, 1,000 and 2,500 μg/mL). In addition, a dose‐response relationship was observed. Only at the highest concentration, the % Mitotic Index was lower than 50%. Thus, the Panel concludes that the NF was clastogenic in vitro and induced cell cycle delay in this test.
A follow‐up in vivo assessment of genotoxicity was undertaken, using the mouse erythrocyte micronucleus assay (Unpublished study report, 2011b; Ming et al., 2015a). This test was conducted in accordance with OECD Test Guideline 474 (OECD, 1997) and in compliance with GLP. Based on a pre‐experiment, an intraperitoneally administered dose of 500 mg/kg bw was chosen as the maximum tolerated dose (MTD). In the main experiment, the NF was administered intraperitoneally as a single dose of 0 (negative control: physiological saline), 100, 250 or 500 mg/kg bw to male and female NMRI mice (n = 5 per dose and sex). The positive control group received cyclophosphamide (40 mg/kg bw). Peripheral blood was collected 44 and 68 h after treatment and analysed using flow cytometry. The proportion of polychromatic (immature) erythrocytes among total erythrocytes (relative PCE) was determined. Statistically significant decreases in the relative PCE, observed in females 44 and 68 h after administration of the NF at a dose of 500 mg/kg bw, when compared with the negative control, indicated that the test material or single constituents contained therein affected the target cells. No statistically significant increases in the number of cells with micronuclei were observed compared to the negative control (slight non‐significant increases were noted for cells obtained from males and females 68 h after treatment with 200 and 500 mg/kg bw, but the mean values were well below the historical negative control means of the testing facility). The positive control cyclophosphamide produced the expected response. The Panel concludes that the NF was not clastogenic or aneugenic in this assay. However, the Panel notes that this experiment was not conducted using the intended route of exposure in humans and the metabolism following the two routes of administration could be different. Additionally, since the NF is a complex mixture, despite the observed bone marrow toxicity, it is not known whether the constituents of the NF, which have induced the effects observed in vitro (clastogenicity and cell cycle delay), have reached the target cells. Thus, the Panel considers that further in vivo investigations are required, and therefore requested the applicant to conduct an in vivo mammalian alkaline comet assay (via oral administration) with the scoring of the liver and of the tissue of the first site of contact (stomach or duodenum) (according to OECD TG 489, 2016), combined with an in vivo mammalian erythrocyte micronucleus assay (OECD TG 474, 2014).
In reply to this request, the applicant submitted only an in vitro mammalian cell micronucleus (MN) test (Unpublished study report, 2019). This test, using human TK6 lymphoblastoid cells was conducted in accordance with OECD TG 487 and in compliance with GLP principles. Two experiments were conducted with the NF up to 2,000 μg/mL and by exposing the cells for 3 h (with S9‐mix). However, both experiments were disregarded by the study authors since in the first one, the control samples did not reach the required population doublings (1.5–2.0) and in the second experiment, the cytotoxicity did not show dose‐dependency. In the third experiment (3‐h exposure, with S9‐mix), the highest concentration tested for the cytotoxicity was 1,000 μg/mL. The MN analysis was conducted with doses of 148.6, 179.9, 318.6, 350.5, 385.5 and 424.1 μg/mL (the highest dose reached cytotoxicity of 58.43%). The Panel notes that also in this experiment, there was no linear, dose‐dependent increase of cytotoxicity (e.g. at the dose of 683 μg/mL, the cytotoxicity was 49.01%). The first experiment included also the treatment conditions of 3‐ and 24‐h exposure (without S9‐mix) and the cytotoxicity showed a linear increase. Thus, the concentrations of 395.1 μg/mL (with cytotoxicity of 52.70%) and 117.1 μg/mL (with cytotoxicity of 53.86%) were selected as the highest doses for MN analysis. Precipitation was not observed in any of the treatment schedules. No statistically significant increase in micronucleus frequency in the exposed cells compared to the control (DMSO) was observed, neither after 3‐h exposure (with or without S9‐mix) nor after 24‐h exposure (without S9‐mix). The positive control substances induced statistically significant increases in MN frequency compared to the negative control. The Panel concludes that the NF did not induce micronucleus formation under the conditions of the study. However, the Panel also concluded that the positive results observed in the chromosomal aberration test (Unpublished study report, 2011a) cannot be ruled out with another standalone in vitro assay and reiterated that, for that purpose, the previously requested in vivo mammalian alkaline comet assay (OECD TG 489) is still needed.
The applicant followed up the request by submitting an in vivo mammalian alkaline comet assay (Unpublished study report, 2021). The study was conducted in accordance with OECD TG 489 and in compliance with GLP principles to assess the potential of the NF, administered by oral gavage, to induce DNA strand breaks in the glandular stomach, duodenum and liver of Crl:Wistar Han rats. Based on the preliminary toxicity test (performed on animals of both sexes), which did not show the signs of toxicity, only male rats were used in the main study. Animals were divided in four dose groups receiving 0 (purified water), 500, 1,000 and 2,000 mg NF/kg bw per day (6 animals per group) and a positive control group treated with a single dose (200 mg/bw) of ethyl methanesulfonate (EMS). The NF was administered to rats on two separate occasions (24 h apart) and animals were sacrificed 3 h after receiving the second dose. No statistically significant increases in the mean % tail intensity (TI) were observed in the liver of rats administered NF at any dose level, compared to vehicle control values. However, a marked and statistically significant increase in the mean %TI was observed in the stomach and even higher in the duodenum of the animals of the high‐dose group (2,000 mg/kg bw per day), accompanied by an increase in the mean % of hedgehog cells (which, according to OECD guidance, were evaluated separately and not scored for TI). The presence of hedgehog cells was argued by the study authors and the applicant to be an indication of cytotoxicity/apoptosis and not genotoxicity. To support this conclusion, tissues (stomach and duodenum) of the animals from the control and high dose group were evaluated for histopathology. No degenerative changes were seen neither in the stomach nor in the duodenum with haematoxylin & eosin (H&E) staining. A follow‐up experiment was conducted to assess the histology of stomach and duodenum with H&E, caspase‐3 (to detect apoptosis) and TUNEL staining (to detect DNA fragmentation as an indicator for apoptosis), without performing slides evaluation for the comet. This follow‐up study was conducted using the same conditions of the main comet assay at the highest dose (2,000 mg/kg bw per day) and animals were sacrificed 3 and 8 h after the last administration of the NF. Minimal to moderate degeneration/necrosis and positive staining for caspace‐3 and TUNEL were seen at an increased incidence in the glandular stomach of the animals sacrificed after 3 h and in both glandular and non‐glandular stomach of the animals sacrificed after 8 h. No degenerative changes were seen in the duodenum of any animal. The Panel notes that the aetiology of the hedgehog cells is still unclear and that scientific literature indicates that their formation can be due to cytotoxicity, but also to genotoxicity or apoptosis (OECD TG 489). Since the NF administered at the dose of 2,000 mg/kg bw per day led to the marked increase in %TI without being associated with signs of cytotoxicity or cell death in the stomach and duodenal tissues in the main experiment and no histological damage in the duodenal tissue was observed in the follow‐up experiment (for which association with comet positive cells was not performed), the Panel concludes that the DNA damage was due to a genotoxic effect of the NF rather than primarily to controlled DNA fragmentation (apoptosis) or unspecific general cell toxicity.
The Panel noted that in the chronic (12 months) study in rats (Unpublished study report, 2015) submitted by the applicant, some histological changes in the stomach were observed in the highest dose group (1,000 mg/kg bw per day). Although this dose is only half of the highest dose applied in the comet assay (Unpublished study report, 2021), the Panel considered the data of possible relevance due to the longer exposure duration. However, the underlying mechanisms of these histological changes in the chronic study remain unexplained and are thus not considered to be a clear indication of cytotoxicity. The Panel also noted that no histopathological changes were observed in the duodenum in this chronic study, with the exception of adenocarcinoma of a single animal in the high‐dose group that died prior to terminal sacrifice. In addition, no histopathological changes in the stomach and duodenum were observed in the studies with shorter durations (Choudhary et al., 2012; Unpublished study report, 2008b; Choudhary et al., 2012; Unpublished study report, 2010b).
Overall, the Panel considers that positive results of the in vitro chromosomal aberration test (Unpublished study report, 2011a) and the outcome of the in vivo comet assay (Unpublished study report, 2021) provide evidence that the NF has the potential to induce DNA damage, which is of concern, particularly locally for tissues that represent first sites of contact.
3.11.2. Human data
Although there are several human studies available and conducted with E. longifolia extracts, the Panel notes that these studies were primarily designed to investigate putative beneficial effects of the NF and addressed only a limited number of safety endpoints that were not relevant in terms of the toxicity profile of the NF.
3.12. Allergenicity
The Panel noted that up to 45% of the NF are proteins and therefore the potential of the NF of causing allergic reactions must be considered. Upon a request from the ACNFP about the better characterisation of the protein content in the NF, the applicant performed allergen screening by ELISA and/or PCR analysis to the known allergens associated with the EU allergy labelling regime. The analysis did not detect any of the allergens tested or cross‐allergenicity. Also, no allergic reactions to Tongkat Ali have been reported in Malaysia.
Upon request from EFSA, the applicant performed a literature review on the botanical relatedness of the source of the NF with other plant species also in regard to cross‐reactivity and reported that within the same family Simaroubaceae, the eventual allergenic potential is seen in two out of 19 plant genera only (Ailanthus and Alvaradoa), whereas no data are available on the genus Eurycoma and on the species E. longifolia.
The Panel considers that the likelihood of allergenic reactions to the NF is low.
4. Discussion
The Panel notes that the NF induced a dose‐dependent increase in aberrant cells in an in vitro mammalian chromosome aberration test using cultured human lymphocytes (Unpublished study report, 2011a), thus showing clastogenic properties. While the positive results observed at the highest dose of the NF tested (2,500 μg/mL) may have been caused by cytotoxicity, no signs of overt cytotoxicity were seen at the low‐ and mid‐dose group (500 and 1,000 μg/mL).
As a follow‐up test to address the relevance of the positive results from the in vitro chromosome aberration test, the in vivo mammalian alkaline comet assay with the scoring of the liver and the tissues of the first site of contact (stomach and duodenum) (Unpublished study report, 2021) was conducted. The Panel notes that this test shows a positive local response within the stomach and duodenum. Considering that the NF is a mixture containing several potentially relevant constituents, the highest dose of the NF tested (2,000 mg/kg bw) would contain less than 2,000 mg/kg bw of individual constituents. Histopathological analyses of stomach tissue revealed evidence of cell necrosis and apoptosis (increased caspase 3 and positive for TUNEL staining) in animals having received the highest dose of NF. However, the fact that the NF dose of 2,000 mg/kg bw led to the marked increase in % TI, without being associated with signs of cytotoxicity or cell death in duodenal tissue, indicates that the DNA damage observed as an increase in % TI was due to a genotoxic effect of the NF rather than primarily to controlled enzymatic DNA fragmentation (apoptosis) or unspecific general cell toxicity.
Taken together, the Panel considers that positive results of the in vitro chromosomal aberration test and the outcome of the in vivo comet assay support the evidence that the NF has the potential to induce DNA damage, which is of concern, particularly locally for tissues that represent first sites of contact.
5. Conclusions
The Panel concludes that the safety of the NF has not been established under any condition of use.
6. Steps taken by EFSA
On 02/05/2018 EFSA received a letter from the European Commission with the request for a scientific opinion on the safety of Eurycoma longifolia (Tongkat Ali) root extract as a novel food. Ref. Ares(2018)2327447 – 02/05/2018.
On 02/05/2018, a valid application on Tongkat Ali root extract as a novel food, which was submitted by Biotropics Malaysia Berhad, was made available to EFSA by the European Commission through the Commission e‐submission portal (NF 2018/0169) and the scientific evaluation procedure was initiated.
On 25/05/2018, 14/12/2018, 20/08/2019 and 27/09/2019 EFSA requested the applicant to provide additional information to accompany the application and the scientific evaluation was suspended.
During its meeting on 27/10/2021, the NDA Panel, having evaluated the data, adopted a scientific opinion on the safety of Eurycoma longifolia (Tongkat Ali) root extract as a NF pursuant to Regulation (EU) 2015/2283.
Abbreviations
- 1H‐NMR
Proton nuclear magnetic resonance
- ACD
Advanced Chemistry Development
- ACNFP
Advisory Committee on Novel Foods and Processes
- ADME
Absorption, distribution, metabolism and excretion
- AOAC
Association of Official Analytical Chemists
- bw
Body weight
- CAS
Chemical Abstracts Service
- CFU
Colony Forming Units
- DMSO
Dimethyl sulfoxide
- ELISA
Enzyme‐linked immunosorbent assay
- EMS
Ethyl methanesulfonate
- FSA
Food Standards Agency
- GLP
Good Laboratory Practice
- GMP
Good Manufacturing Practice
- HACCP
Hazard Analysis and Critical Control Points
- HPLC‐DAD
High‐performance liquid chromatography with diode‐array detector
- H&E
Haematoxylin and eosin
- ISO
International Organization for Standardization
- IV
Intravenous
- LC‐DAD-MS/MS
Liquid chromatography‐diode array detector
- LC‐MS/MS
Liquid chromatography with tandem mass spectrometry
- MN
Micronucleus
- MTD
Maximum tolerated dose
- NDA Panel
Panel on Nutrition, Novel Foods and Food Allergens
- NF
Novel food
- OECD TG
Organisation for Economic Co‐operation and Development Test Guideline
- PCE
Polychromatic erythrocyte
- PCR‐RAPD
Random amplified polymorphic deoxyribonucleic acid (DNA) analysis by polymerase chain reaction
- Ph. Eu.
European Pharmacopoeia
- PO
per os
- QTOF‐MS
Quadrupole time of flight mass spectrometer
- RTG
Relative total growth
- TI
Tail intensity
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labelling
- UPLC-MS/MS
Ultra‐performance liquid chromatography‐tandem mass spectrometry
Suggested citation: EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , Turck D, Bohn T, Castenmiller J, De Henauw S, Hirsch‐Ernst KI, Maciuk A, Mangelsdorf I, McArdle HJ, Naska A, Pelaez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Cubadda F, Frenzel T, Heinonen M, Prieto Maradona M, Marchelli R, Neuhäeuser‐Berthold M, Poulsen M, Schlatter JR, van Loveren H, Matijević L and Knutsen HK, 2021. Scientific Opinion on the safety of Eurycoma longifolia (Tongkat Ali) root extract as a novel food pursuant to Regulation (EU) 2015/2283. EFSA Journal 2021;19(12):6937, 16 pp. 10.2903/j.efsa.2021.6937
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00106
Panel members: Dominique Turck, Torsten Bohn, Jacqueline Castenmiller, Stefaan De Henauw, Karen Ildico Hirsch‐Ernst, Helle Katrine Knutsen, Alexandre Maciuk, Inge Mangelsdorf, Harry J McArdle, Androniki Naska, Carmen Pelaez, Kristina Pentieva, Alfonso Siani, Frank Thies, Sophia Tsabouri and Marco Vinceti.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: The Panel wishes to thank the former member of the EFSA Working group on novel foods Annette Pöting for the contribution provided to the development of the opinion until 30 October 2019.
Adopted: 27 October 2021
Notes
Regulation (EU) No 258/97 of the European Parliament and of the Council of 27 January 1997 concerning novel foods and novel food ingredients. OJ L 43, 14.2.1997, p. 1–6.
Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001 (2013/0435 (COD). OJ L 327, 11.12.2015, p. 1–22.
Commission Implementing Regulation (EU) 2017/2469 of 20 December 2017 laying down administrative and scientific requirements for applications referred to in Article 10 of Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. OJ L 351, 30.12.2017, pp. 64–71.
Limit of detection (LOD) for organochlorine and organophosphorus pesticides (based on the analytical method used) was ranging between 0.05 and 0.1 mg/kg. LOD for aflatoxins was 1 µg/kg.
References
- Ahmad N, Samiulla DS, Teh BP, Zainol M, Zolkifli NA, Muhammad A, Matom E, Zulkapli A, Abdullah NR, Ismail Z and Syed Mohamed AF, 2018. Bioavailability of Eurycomanone in its pure form and in a standardised Eurycoma longifolia water extract. Pharmaceutics, 10, 90. 10.3390/pharmaceutics10030090. PMID: 29997335; PMCID: PMC6161288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R and Karim AA, 2010. Tongkat Ali (Eurycoma longifolia Jack): a review on its ethnobotany and pharmacological importance. Fitoterapia, 81, 669–679. 10.1016/j.fitote.2010.04.006 [DOI] [PubMed] [Google Scholar]
- Burkill IH, 1935. A dictionary of the economic products of the Malay Peninsula. Crown Agents for the Colonies, London, UK. Vol. 2, pp. 1219–2402. [Google Scholar]
- Chaingam J, Juengwatanatrakul T, Yusakul G, Kanchanapoom T and Putalun W, 2021. 2021/HPLC‐UV‐based simultaneous determination of Canthin‐6‐one alkaloids, quassinoids, and scopoletin: the active ingredients in Eurycoma Longifolia jack and Eurycoma Harmandiana pierre, and their anti‐inflammatory activities. Journal of AOAC International, 104, 802–810. 10.1093/jaoacint/qsaa141. PMID: 33064798. [DOI] [PubMed] [Google Scholar]
- Choudhary YK, Bommu P, Ming YK and Zulkawi NB, 2012. Acute, sub acute and subchronic 90‐days toxicity of Eurycoma longifolia aqueous extract (Physta) in Wistar rats. Int J Pharm Pharm Sci, 4, 232–238. [Google Scholar]
- Chua LS, Amin NA, Neo JC, Lee CT, Sarmidi MR and Aziz RA, 2011. LC–MS/MS‐based metabolites of Eurycoma longifolia (Tongkat Ali) in Malaysia (Perak and Pahang). Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 879, 3909–3919. 10.1016/j.jchromb.2011.11.002 [DOI] [PubMed] [Google Scholar]
- Chua LS, Abdul‐Rahman N, Rosidi B and Lee CT, 2012. Plant proteins, minerals and trace elements of Eurycoma longifolia (Tongkat ali). Natural Product Research, 1–5. 10.1080/14786419.2012.676552 [DOI] [PubMed] [Google Scholar]
- Chua LS, Lau CH, Chew CY and Dawood D, 2019. Solvent fractionation and acetone precipitation for crude saponins from Eurycoma longifolia extract. Molecules (Basel, Switzerland), 24, 1416. 10.3390/molecules24071416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang NH, Lan DTN, Thu Minh NT, Khanh ND, Trang DT, Cuong PV, Hiep NT, Nam VC, Trung NQ and Dat NT, 2019. Quassinoids and Alkaloids From the Roots of Eurycoma longifolia. Natural Product Communications. May 2019. 10.1177/1934578X19850695 [DOI]
- Department of Standards Malaysia , 2011. Phytopharmaceutical Aspect of Freeze Dried Water Extract from Tongkat Ali Roots ‐ Specification. (Malaysian Standard MS 2409:2011/ICS: 67.040). Department of Standards Malaysia, Ministry of Science, Technology and Innovation, Cyberjaya, Malaysia/Standards & Industrial Research Institute of Malaysia (SIRIM), Shah Alam, Malaysia.
- Ebrahimi F, Ibrahim B, The CH, Murugaiyah V and Lam CK, 2016. 1HNMR‐based discriminatory analysis of Eurycoma longifolia from different locations and establishing a profile for primary metabolites identification and quassinoids quantification. Planta Medica, 83, 172–182. 10.1055/s-0042-110857 [DOI] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2016. Guidance on the preparation and presentation of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA Journal 2016;14(11):4594, 24 pp. 10.2903/j.efsa.2016.4594 [DOI] [Google Scholar]
- Elliott S and Brimacombe J, 1987. The medicinal plants of Gunung Leuser National Park, Indonesia. Journal of Ethnopharmacology, 19, 285–317. 10.1016/0378-8741(87)90006-7. PMID: 3669690. [DOI] [PubMed] [Google Scholar]
- Ezzat SM, Ezzat MI, Okba MM, Hassan SM, Alkorashy AI, Karar MM, Ahmed SH and Mohamed SO, 2019. Brain cortical and hippocampal dopamine: a new mechanistic approach for Eurycoma longifolia well‐known aphrodisiac activity and its chemical characterization. Evidence‐Based Complementary and Alternative Medicine: eCAM, 2019, 7543460. 10.1155/2019/7543460. PMID: 31275418; PMCID: PMC6582863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimlette JD, 1939. Tongkat Ali. In: Thomson HW (ed.). A dictionary of Malayan medicine. Oxford University Press, London, UK/New York, p. 242. [Google Scholar]
- Low BS, Ng BH, Choy WP, Yuen KH and Chan KL, 2005. Bioavailability and pharmacokinetic studies of eurycomanone from Eurycoma longifolia . Planta Medica, 71, 803–807. 10.1055/s-2005-871259. PMID: 16206032. [DOI] [PubMed] [Google Scholar]
- Ming YK, Zulkawi NB, Choudhary VK and Choudhary YK, 2015a. Evaluation of the genotoxicity of Eurycoma longifolia aqueous extract (Physta®) using in vitro Ames test and in vivo mammalian micronucleus test. International Journal of Pharmacy and Pharmaceutical Sciences, 8, 367–371. Available online: https://innovareacademics.in/journals/index.php/ijpps/article/view/8189 [Google Scholar]
- Ming YK, Zulkawi NB, Choudhary VK and Choudhary YK, 2015b. Reproductive and developmental toxicity of Eurycoma longifolia aqueous extract (Physta®) in Wistar rats. International Journal of Pharmacy and Pharmaceutical Sciences, 8, 372–376. Available online: https://innovareacademics.in/journals/index.php/ijpps/article/view/8190 [Google Scholar]
- Ng WL, Lee SY and Yeap SK, 2019. Characterization of the complete chloroplast genome of an important Southeast Asian medicinal plant, Eurycoma longifolia (Simaroubaceae). Mitochondrial DNA Part B, 4, 128–129. 10.1080/23802359.2018.1540263 [DOI] [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 1997. Test No. 471: Bacterial reverse mutation test. In: OECD guidelines for the testing of chemicals, Section 4, 11 pp. Available at: https://www.oecd.org/chemicalsafety/risk-assessment/1948418.pdf
- OECD (Organisation for Economic Co‐operation and Development), 2014. Test No. 474: Mammalian Erythrocyte Micronucleus Test. OECD Publishing, Paris, 10.1787/9789264224292-en. [DOI]
- OECD (Organisation for Economic Co‐operation and Development), 2016. Test No. 489: In Vivo Mammalian Alkaline Comet Assay, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, 10.1787/9789264264885-en [DOI]
- Ong HC, Faezah AW and Milow P, 2012. Medicinal Plants Used By the Jah Hut Orang Asli at Kampung Pos Penderas, Pahang, Malaysia. Ethno Med, 6, 11–15. 10.1080/09735070.2012.11886414 [DOI] [Google Scholar]
- Oxley T, 1850. The botany of Singapore. In: Logan JR (ed.). The Journal of the Indian Archipelago and Eastern Asia1850, 4, 436–440. [Google Scholar]
- Razi ARM, Abdul‐Aziz A, Alwee SSBS and Aziz R, 2013. Relationship between Malaysians Cultivars of Tongkat Ali (Eurycoma longifolia Jack) Obtained through RAPD Analysis. International Journal of Biotechnology for Wellness Industries, 2, 45–50. 10.6000/1927-3037.2013.02.01.7 [DOI] [Google Scholar]
- Rehman SU, Choe K and Yoo HH, 2016. Review on a traditional herbal medicine, Eurycoma longifolia Jack (Tongkat Ali): its traditional uses, chemistry, evidence‐based pharmacology and toxicology. Molecules, 21, 331. 10.3390/molecules21030331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon MC, Erasmus N and Henkel RR, 2014. In vivo effects of Eurycoma longifolia Jack (Tongkat Ali) extract on reproductive functions in the rat. Andrologia, 46, 339–348. 10.1111/and.12082. Epub 2013 Mar 6. PMID: 23464350. [DOI] [PubMed] [Google Scholar]
- Tam NTT, Thao TTP, Loc TV, Thuy NT, Nhu ND and Sung TV, 2014. Về thành phần hóa học của rễ cây mật nhân (Eurycoma longifolia jack.). Vietnam. Journal of Chemistry, 52, 2014. 10.15625/4984 [DOI] [Google Scholar]
- Unpublished study report , 2008a. Study title: Acute Oral Toxicity Study of BIOEL‐101 in Wistar rats. Study number: 080407/BT/BioEL‐101.
- Unpublished study report , 2008b. (unpublished, claimed as proprietary by the applicant). Prepared by Vedic Lifesciences PVT, LTD, India. Study title: Sub‐Acute Oral Toxicity Study of BIOEL‐101 in Wistar rats. Study number: 080407/BT/BioEL‐101.
- Unpublished study report , 2010a. Study title: Salmonella Typhimurium Reverse Mutation Assay with BioEL‐101. Study Report Number: RPT_BTM‐007-FR. [Google Scholar]
- Unpublished study report , 2010b. Study title: Repeated Dose 90‐Day Oral Toxicity Study of BioEL‐101 (LJ100) in Wistar Rat. Report number: R/10217/SOR-90/10.
- Unpublished study report , 2011a. Study title: In vitro Mammalian Chromosome Aberration Test in Human Lymphocytes with BioEL‐101 (LJ 100). Study number: 103435.
- Unpublished study report , 2011b. Study title: Mammalian Micronucleus Test of Murine Peripheral Blood Cells with BioEL‐101. Study number: 104920.
- Unpublished study report 2013. Study title: evaluation of the mutagenic activity of Physta® in an in vitro mammalian cell gene mutation test with L5178Y mouse lymphoma cells (with independent repeat. Laboratory Project Identification: Project, 502645. [Google Scholar]
- Unpublished study report , 2015. Study title: Repeated Dose 12 Month Toxicity Study of Physta® (Eurycoma Longifolia Freeze Dried Standardized Extract) in Wistar Rats through Oral Gavage. Laboratory Report Number: 429‐1-05-7178.
- Unpublished study report , 2016a. Study title: Reproduction/Developmental Toxicity Screening Test in Rats with BioEL‐101 (LJ 100). Study number: 103437.
- Unpublished study report , 2016b. Study title: Two‐Generation Reproductive Toxicity Study in Wistar Rats with PHYSTA®. Study number: 134271.
- Unpublished study report , 2019. Prepared by Gentronix Limited, UK. Study title: Physta® Standardized Tongkat Ali Extract: In Vitro Mammalian Cell Micronucleus Test. Study number: MNT00644.
- Unpublished study report , 2021. Study title: Physta® Eurycomanone longifolia Standardized Extract: In Vivo Mammalian Alkaline Comet Assay in the Rat. Study number: BR32BJ.
- Zeng L, Zhang RY, Wang D, Pang JH, Zhang ZL, Gao CY and Lou ZC, 1990. Glyyunnanprosapogenin C and glyyunnansapogenin E from the roots of Glycyrrhiza yunnanensis . Yao Xue Xue Bao, 25, 750–757. Chinese. PMID: 2099589. [PubMed] [Google Scholar]


