Abstract
Introduction
Somatic KRAS mutations occur in 25% of patients with NSCLC. Treatment with MEK inhibitor monotherapy has not been successful in clinical trials to date. Compensatory activation of FGFR1 was identified as a mechanism of trametinib resistance in KRAS-mutant NSCLC, and combination therapy with trametinib and ponatinib was synergistic in in vitro and in vivo models. This study sought to evaluate this drug combination in patients with KRAS-mutant NSCLC.
Methods
A phase 1 dose escalation study of trametinib and ponatinib was conducted in patients with advanced NSCLC with KRAS mutations. A standard 3-plus-3 dose escalation was done. Patients were treated with the study therapy until intolerable toxicity or disease progression.
Results
A total of 12 patients with KRAS-mutant NSCLC were treated (seven at trametinib 2 mg and ponatinib 15 mg, five at trametinib 2 mg and ponatinib 30 mg). Common toxicities observed were rash, diarrhea, and fever. Serious adverse events potentially related to therapy were reported in five patients, including one death in the study and four cardiovascular events. Serious events were observed at both dose levels. Of note, 75% (9 of 12) were assessable for radiographic response and no confirmed partial responses were observed. The median time on study was 43 days.
Conclusions
In this phase 1 study, in patients with KRAS-mutant advanced NSCLC, combined treatment with trametinib and ponatinib was associated with cardiovascular and bleeding toxicities. Exploring the combination of MEK and FGFR1 inhibition in future studies is potentially warranted but alternative agents should be considered to improve safety and tolerability.
Keywords: NSCLC, KRAS, targeted therapy, MEK, FGFR1
Introduction
Activating mutations in KRAS occur in 25% of patients with metastatic NSCLC. Although strides have been made in identifying molecular drivers of NSCLC, leading to the development of targeted therapies and resulting in improved survival for patients,1 strategies to combat KRAS-driven tumors have been largely ineffective aside from covalent G12C inhibitors in clinical development.2, 3, 4, 5 For patients with non–G12C mutation subtypes, most efforts have been focused on targeting effector proteins downstream in the MAPK signaling pathway (RAF, MEK, ERK) to halt cancer growth. MEK inhibition in KRAS-mutant NSCLC revealed promise in preclinical studies; however, the efficacy of single-agent MEK inhibitors (e.g., trametinib6 and selumetinib7) in clinical trials has been limited, revealing no benefit compared with docetaxel in the second-line setting.
This lack of efficacy may be, in part, owing to adaptive resistance mechanisms. Compensatory activation of FGFR1 has been identified as a potential critical mechanism for trametinib resistance in KRAS-mutant lung cancer models,8 suggesting a rationale for combinatorial therapy targeting both MEK and FGFR1. Ponatinib9 is a multitargeted tyrosine kinase inhibitor that blocks signaling from FGFR and ABL. The combination of trametinib and ponatinib resulted in synergistic inhibition of the MAPK pathway and tumor shrinkage in in vitro and in vivo KRAS-mutant NSCLC models. On the basis of this preclinical data, we developed and conducted a phase 1 dose escalation study to evaluate the combination of trametinib and ponatinib in patients with metastatic NSCLC harboring a KRAS mutation.
Materials and Methods
This was a single-institution study conducted after approval from the institutional review board at Memorial Sloan Kettering Cancer Center (NCT03704688) and all patients provided informed consent before study screening assessments. Patients with KRAS mutations were identified with routine clinical testing. To be eligible for the study, all patients were required to have documented metastatic disease and have been previously treated with available standard therapies for NSCLC (platinum doublet chemotherapy and immune checkpoint inhibitor). Patients with a history of arterial or venous thromboembolism diagnosed within 6 months before the start of therapy were not eligible for the study. Those with a more distant history of these events were required to be stable on appropriate medical therapy. Patients with symptomatic brain metastases were not eligible.
The primary end point for the study was the determination of the maximum tolerated dose (MTD) of trametinib and ponatinib in patients with KRAS-mutant NSCLC. The standard 3-plus-3 dose escalation was done using prespecified dosage levels (Supplementary Table 1). The study planned to enroll a minimum of six patients and a maximum of 30 patients if dose de-escalation levels were used. A starting dose of trametinib 2 mg was planned given its previous exploration in clinical trials for patients with KRAS-mutant NSCLC6 and previous determination as to the maximal tolerated dose both as monotherapy and in combination with dabrafenib for the management of BRAF V600E–mutant NSCLC.10 Escalating doses with ponatinib were planned, starting at the lowest available dose of ponatinib (15 mg in dose level 1 and increasing to 30 mg in dose level 2). Patients were assessed for dose-limiting toxicities (DLTs), defined as grade 3 or 4 toxicity or any grade toxicity that required drug discontinuation, for 28 days.
Secondary objectives of the study included exploration of clinical efficacy and description of the toxicities of the combination in this study population. Toxicities were assessed according to National Cancer Institute Common Terminology Criteria version 4.1 and summarized using descriptive statistics. Disease assessments were performed every two cycles (28-day cycles) and the response was assessed using the Response Evaluation Criteria in Solid Tumors 1.1 criteria. Patients were treated with study therapy until intolerable toxicity or disease progression.
After determination of the MTD of the combination therapy, the study was designed to continue into phase 2 with a Simon optimal two-stage trial design to assess the primary end point of the response rate (Response Evaluation Criteria in Solid Tumors 1.1 complete response + partial response [PR]). A null hypothesis of a 10% response rate against the alternative hypothesis of a 30% response rate for patients with progressive disease after at least two previous lines of therapy was used. The planned phase 2 portion of the study would enroll 15 patients in the first stage. If one or more responses were seen, then the study would expand to 25 patients. To declare ponatinib and trametinib at this dose and schedule as promising, there would need to be at least five confirmed PRs in 25 response-assessable patients.
Results
A total of 12 patients with KRAS-mutant NSCLC were enrolled, treated between October 2018 and October 2019 at the Memorial Sloan Kettering Cancer Center. The baseline characteristics of patients treated are summarized in Table 1. The most common mutation subtype of patients treated was G12C mutation and 42% of patients had concurrent STK11 mutation. Three patients were initially enrolled treated at dose level 1 and after no DLTs were observed, dose level 2 was explored. In total, five patients were treated at dose level 2 (trametinib 2 mg and ponatinib 30 mg). Two patients experienced DLTs, one myocardial infarction, and one patient with intolerable grade 1 fever requiring dose reduction; therefore, further enrollment at this dose level was halted. A total of seven patients were treated at dose level 1 (trametinib 2 mg and ponatinib 15 mg). These seven patients included one patient who was replaced in the cohort as the patient was not DLT-assessable and discontinued study therapy owing to clinical progression of the disease.
Table 1.
Baseline Characteristics of Patients
| Patient Characteristic | No. of Patients (%) |
|---|---|
| Age, median (range) | 61 (38–71) |
| Sex | |
| M | 6 (50) |
| F | 6 (50) |
| History of smoking | |
| Current/former | 9 (75) |
| Never | 3 (25) |
| ECOG performance Status | |
| 0 | 3 (25) |
| 1 | 9 (75) |
| Previous lines of therapy | |
| 1 | 3 (25) |
| 2 | 5 (42) |
| 3 | 4 (33) |
| KRAS mutation | |
| G12C | 5 (42) |
| G12D | 2 (17) |
| G13C | 2 (17) |
| G12V | 2 (16) |
| Q61H | 1 (8) |
| STK11 comutation | 5 (42) |
| KEAP1 comutation | 2 (17) |
ECOG, Eastern Cooperative Oncology Group; F, female; M, male.
Table 2 summarizes the most common toxicities that were observed during treatment. Diarrhea, rash, and fever were the most common toxicities observed. Treatment-related grade 3 to 5 toxicities were observed in 6 of 12 patients, including one death owing to gastrointestinal hemorrhage that was deemed possibly related to study therapy. Multiple serious adverse events were observed. Serious adverse events potentially owing to study therapy were reported in 5 of 12 patients (Table 3). These events included cardiovascular complications of new heart failure, myocardial infarction, atrial fibrillation, and venous thromboembolism, in addition to the gastrointestinal bleeding event previously described. These events occurred in both dose level 1 and dose level 2, and four of five of the events occurred during the first two cycles of treatment.
Table 2.
Summary of Most Common Toxicities Observed
| Toxicity Observed | Grade 1 (%) | Grade 2 (%) | Grade 3 (%) | Total, Any Grade (%) |
|---|---|---|---|---|
| Rash | 6 (50) | 2 (17) | 8 (67) | |
| Diarrhea | 4 (33) | 1 (8) | 5 (42) | |
| Dry skin | 3 (25) | 3 (25) | ||
| Constipation | 2 (17) | 2 (17) | ||
| Fever | 2 (17) | 1 (8) | 3 (25) | |
| Dry mouth | 2 (17) | 2 (17) | ||
| Headache | 2 (17) | 2 (17) | ||
| Nausea | 2 (17) | 2 (17) | ||
| Vomiting | 2 (17) | 2 (17) | ||
| Anorexia | 2 (17) | 2 (17) | ||
| Fatigue | 1 (8) | 1 (8) | 2 (17) | |
| Skin infection | 2 (17) | 2 (17) | ||
| Thromboembolic event | 1 (8) | 1 (8) | 2 (17) | |
| AST increased | 1 (8) | 1 (8) | 2 (17) |
AST, aspartate transaminase.
Table 3.
Treatment-Related Serious Adverse Events Observed
| Toxicity Observed | Timing of Event | Dose Level | Comments |
|---|---|---|---|
| Left ventricular ejection fraction decrease | C2D1 | 1 | Asymptomatic, LV thrombus identified |
| Atrial fibrillation | C2D8 | 1 | Clinical heart failure |
| Pulmonary embolus | C1D12 | 1 | with hemoptysis, ICU stay |
| Myocardial infarction | C1D19 | 2 | ST elevation myocardial infarction |
| Gastrointestinal bleed | C4D8 | 2 | Grade 5 (required before dose reduction) |
ICU, intensive care unit; LV, left ventricle.
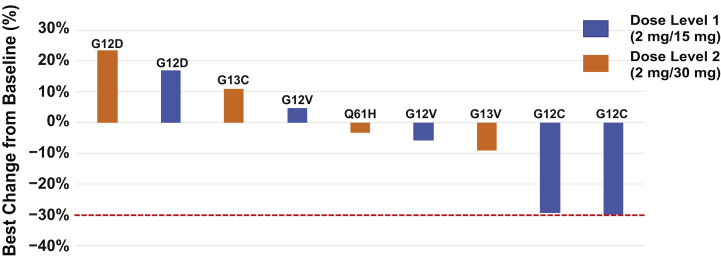
The key efficacy end point of this study was the radiographic response rate. The response rate was 0% (0 of 12, 95% confidence interval: 0%–25%). Five of nine patients were observed to have disease control on initial response assessments, including two patients treated at dose level 1 (trametinib 2 mg and ponatinib 15 mg) both with a 30% reduction in target lesions on first follow-up imaging. Disease control was not sustained, however, and both patients developed progressive disease at subsequent follow-up assessment and, therefore, no confirmed partial or complete responses occurred. Three patients discontinued study therapy before the first disease assessment and were categorized as not assessable. Figure 1 shows tumor response assessments of target lesions. The median duration on study was 43 days (range: 12–112) with the median time on therapy being 37 days.
Figure 1.
Change from baseline in tumor burden in patients assessable for response (9 of 12). Three patients discontinued study therapy before the initial disease assessment.
Discussion
Here, we report the results of a phase 1 dose escalation clinical trial of the combination of trametinib and ponatinib in KRAS-mutant lung cancer conducted to evaluate the hypothesis that combined treatment with a MEK inhibitor and FGFR inhibitor would lead to synergistic inhibition of the MAPK pathway and tumor shrinkage in patients, as was reported in KRAS-mutant NSCLC models. Although modest disease shrinkage was observed in some patients, disease control was not durable and no confirmed PRs were observed. The combination of trametinib and ponatinib led to substantial toxicities.
KRAS-mutant NSCLC is a heterogeneous disease but continues to represent an unmet clinical need. Although KRAS-G12C direct inhibitors are in clinical development, including the recent Food and Drug Administration approval of sotorasib,5 not all patients will respond to therapy and most patients with KRAS-mutant lung cancer have a non-G12C genotype.11 Therefore, there remains a continued need to develop effective targeted therapies for patients with KRAS-mutant NSCLC. Given the lack of significant efficacy of single-agent MEK inhibition,6,7 there has been significant interest in understanding potential mechanisms of resistance to such downstream inhibition. Reactivation of FGFR1 has been observed as a potential mechanism of resistance8 to MEK inhibition, with the combination of trametinib and ponatinib resulting in the greatest synergistic effects, supporting the rationale for this clinical trial.
Ponatinib is a multitargeted tyrosine kinase inhibitor that blocks signaling from FGFR and ABL and is currently approved by the Food and Drug Administration for the treatment of chronic myeloid leukemia (CML).9 Increased risk of arterial and venous thromboembolism has been documented with ponatinib treatment in patients with CML.12 KRAS mutations are more frequently identified in patients with NSCLC who have a history of smoking,13 and therefore, we anticipated that patients with lung cancer may be at a higher risk for these events. In an attempt to decrease this risk, patients with a history of arterial thromboembolism (stroke, myocardial infarction) or venous thromboembolism (pulmonary embolism, deep vein thrombosis) were diagnosed within 6 months of enrollment were not eligible for participation in the clinical trial. Patients with a more distant history of such events were eligible, but only if they were receiving appropriate medical management of these conditions. Despite these restrictions, cardiac events were still observed even in patients with no previous history of such events. Cardiovascular toxicities were observed even in never-smokers, with one patient experiencing an acute myocardial infarction who had no known risk factors for cardiovascular disease. Potential cardiac toxicities, specifically heart failure, have also been reported with trametinib monotherapy,14,15 and, therefore, it is possible that this further contributed to events observed. In addition to serious cardiovascular events, potential overlapping toxicities of both trametinib and ponatinib, most notably diarrhea and rash, lead to difficulty tolerating the combination in several patients.
Although dose de-escalation levels were considered and prespecified for this clinical trial, these dose combinations explored de-escalating doses of trametinib in combination with the lowest available dose of ponatinib (Supplementary Table 1). Given that significant cardiovascular events were observed even at the 15-mg dose of ponatinib, there was significant concern that these events could continue even at dose de-escalation levels. Had the significant toxicities observed been felt to be primarily owing to trametinib (e.g., diarrhea), the use of dose de-escalation levels would have been used. However, given the severity of toxicities observed and the early timing of such events, the determination was made not to proceed with the exploration of other dose levels to determine MTD.
The clinical trial we report here is limited by its small number of patients treated. Although five patients were noted to have disease shrinkage in target lesions, this disease control was not durable with the longest duration on the study of under 4 months. The main limitation of this combination is toxicity with significant risks of arterial and venous thromboembolism. This represents the known risks of ponatinib therapy in patients with CML, though this risk may be higher in patients with NSCLC who are at higher risk for venous thromboembolism16 in the setting of active malignancy, and may have a higher risk for other cardiovascular complications in the setting of previous tobacco use. More recent efforts have identified thrombotic microangiopathy as a cause of cardiovascular toxicity in the setting of ponatinib treatment.17 Other combinations of MEK inhibition and FGFR inhibitors (e.g., erdafitinib or infigratinib) should be explored in KRAS-mutant NSCLC preclinical models and, whether similar synergy is identified, would warrant further evaluation in a clinical trial. This approach may reduce potential toxicity attributable to the multitargeted kinase inhibitor ponatinib and decrease overlapping toxicities with MEK inhibitors, specifically in patients with non–G12C mutation subtypes, in which treatment options remain limited.
CRediT Authorship Contribution Statement
Kathryn C. Arbour: Conceptualization, Investigation, Writing – original draft, Funding acquisition, Supervision, Project administration.
Eusebio Manchado, Matthew J. Bott, Scott Lowe, Neal Rosen: Conceptualization, Writing – review & editing.
Linda Ahn: Investigation.
Yosef Tobi: Alyssa Shannon, Victoria Perron, Amanda Johnson: Data curation, Writing – review & editing.
Andy Ai Ni: Conceptualization, Writing – review & editing, Formal analysis.
Helena A. Yu, Michelle S. Ginsberg, Andrei Holodny, Charles M. Rudin: Investigation, Writing – review & editing.
Marc Ladanyi, Piro Lito: Writing – review & editing.
Mark G. Kris: Conceptualization, Investigation, Writing – review & editing.
Gregory J. Riely: Conceptualization, Investigation, Writing – original draft, Funding acquisition.
Acknowledgments
This work was supported by the Druckenmiller Center for Lung Cancer Research at Memorial Sloan Kettering Cancer Center, the Ramapo Fund, The John and Georgia Dallepezze Foundation, and the Conquer Cancer Foundation (American Society of Clinical Oncology Young Investigator Award for Arbour). Additional support was provided by the National Cancer Institute (P01CA129243-13). Dr. Lito is supported, in part, by the National Institutes of Health/National Cancer Institute (1R01CA23074501, 1R01CA23026701A1), The Pew Charitable Trusts, the and Damon Runyon Cancer Research Foundation.
Footnotes
Disclosure: Dr. Arbour has received institutional research support from Novartis (drug supply of trametinib for this study), Takeda (drug supply of ponatinib for this study), Revolution Medicines, and Mirati. Dr. Manchado has ownership interest (including stocks and patents) in Novartis. Dr. Bott has received speaking honoraria from Intuitive Surgical and AstraZeneca. Dr. Yu has received institutional research support from AstraZeneca, Daiichi Sankyo, Eli Lilly, Novartis, Pfizer, Cullinan, and Janssen; and has served as a paid consultant for AstraZeneca, Daiichi, Blueprint Medicine, Janssen, C4 Therapeutics, and Cullinan. Dr. Ladanyi has received consulting fees from Takeda. Dr. Kris has received consulting fees from AstraZeneca, Pfizer, Daiichi Sankyo, Sanofi-Genzyme, Novartis, and Janssen; and received institutional research support from Genentech. Dr. Rudin has received consulting fees from AbbVie, Amgen, AstraZeneca, Epizyme, Genentech/Roche, Ipsen, Jazz, Eli Lilly, and Syros; and has served on the scientific advisory board of Bridge Medicine, Harpoon, and Earli. Dr. Lito reports receiving grants to his institution from Amgen, Mirati, Revolution Medicines, and Boehringer Ingelheim; consulting fees from Black Diamond Therapeutics, Repare Therapeutics, and AmMax Bio; compensation as a scientific advisory board member in Revolution Medicines and Boehringer Ingelheim; and is listed as an inventor on patents filed by Memorial Sloan Kettering Cancer Center on the treatment of BRAF or KRAS-mutant cancers. Dr. Rosen served as a paid consultant for Array, AstraZeneca, and Revolution Medicines. Dr. Lowe has received consulting fees from ORIC Pharmaceuticals and Blueprint Medicines. Dr. Riely has been an uncompensated consultant to Daiichi, Pfizer, and Mirati; and received institutional research support from Mirati, Takeda, Merck, Roche, Pfizer, and Novartis. The remaining authors declare no conflict of interest.
Cite this article as: Arbour KC, Manchado E, Bott MJ, et al. Phase 1 clinical trial of trametinib and ponatinib in patients with NSCLC harboring KRAS mutations. JTO Clin Res Rep 2022;3:100256.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2021.100256.
Supplementary Data
References
- 1.Howlader N., Forjaz G., Mooradian M.J., et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383:640–649. doi: 10.1056/NEJMoa1916623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallin J., Engstrom L.D., Hargis L., et al. The KRASG12C inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov. 2020;10:54–71. doi: 10.1158/2159-8290.CD-19-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lito P., Solomon M., Li L.S., Hansen R., Rosen N. Allele-specific inhibitors inactivate mutant KRAS G12C by a trapping mechanism. Science. 2016;351:604–608. doi: 10.1126/science.aad6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostrem J.M., Peters U., Sos M.L., Wells J.A., Shokat K.M. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503:548–551. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skoulidis F., Li B.T., Dy G.K., et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384:2371–2381. doi: 10.1056/NEJMoa2103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumenschein G.R., Smit E.F., Planchard D., et al. A randomized phase II study of the MEK1/MEK2 inhibitor trametinib (GSK1120212) compared with docetaxel in KRAS-mutant advanced non-small-cell lung cancer (NSCLC) Ann Oncol. 2015;26:894–901. doi: 10.1093/annonc/mdv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jänne P.A., Heuvel MM van den, Barlesi F., et al. Selumetinib plus docetaxel compared with docetaxel alone and progression-free survival in patients with KRAS-mutant advanced non-small cell lung cancer: the SELECT-1 randomized clinical trial. JAMA. 2017;317:1844–1853. doi: 10.1001/jama.2017.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manchado E., Weissmueller S., Morris J.P., et al. A combinatorial strategy for treating KRAS-mutant lung cancer. Nature. 2016;534:647–651. doi: 10.1038/nature18600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gozgit J.M., Wong M.J., Moran L., et al. Ponatinib (AP24534), a multitargeted pan-FGFR inhibitor with activity in multiple FGFR-amplified or mutated cancer models. Mol Cancer Ther. 2012;11:690–699. doi: 10.1158/1535-7163.MCT-11-0450. [DOI] [PubMed] [Google Scholar]
- 10.Planchard D., Besse B., Groen H.J.M., et al. Dabrafenib plus trametinib in patients with previously treated BRAFV600E-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. 2016;17:984–993. doi: 10.1016/S1470-2045(16)30146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arbour K.C., Jordan E., Kim H.R., et al. Effects of co-occurring genomic alterations on outcomes in patients with KRAS-mutant non-small cell lung cancer. Clin Cancer Res. 2018;24:334–340. doi: 10.1158/1078-0432.CCR-17-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipton J.H., Chuah C., Guerci-Bresler A., et al. Epic: a phase 3 trial of ponatinib compared with imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CP-CML) Blood. 2014;124 519–519. [Google Scholar]
- 13.Johnson M.L., Sima C.S., Chaft J., et al. Association of KRAS and EGFR mutations with survival in patients with advanced lung adenocarcinomas. Cancer. 2013;119:356–362. doi: 10.1002/cncr.27730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Modak S., Asante-Korang A., Steinherz L.J., Grana N. Trametinib-induced left ventricular dysfunction in a child with relapsed neuroblastoma. J Pediatr Hematol Oncol. 2015;37:e381. doi: 10.1097/MPH.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Rahman O., ElHalawani H., Ahmed H. Risk of selected cardiovascular toxicities in patients with cancer treated with MEK inhibitors: a comparative systematic review and meta-analysis. J Glob Oncol. 2015;1:73–82. doi: 10.1200/JGO.2015.000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tagalakis V., Levi D., Agulnik J.S., Cohen V., Kasymjanova G., Small D. High risk of deep vein thrombosis in patients with non-small cell lung cancer: a cohort study of 493 patients. J Thorac Oncol. 2007;2:729–734. doi: 10.1097/JTO.0b013e31811ea275. [DOI] [PubMed] [Google Scholar]
- 17.Latifi Y., Moccetti F., Wu M., et al. Thrombotic microangiopathy as a cause of cardiovascular toxicity from the BCR-ABL1 tyrosine kinase inhibitor ponatinib. Blood. 2019;133:1597–1606. doi: 10.1182/blood-2018-10-881557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.