Highlights
-
•
Women living with cystic fibrosis (CF) are increasingly pursuing pregnancy.
-
•
Gestational and pregestational diabetes are highly prevalent in women with CF.
-
•
Diabetes impacts fetal outcomes, and good glycemic control reduces risk of pregnancy complications.
-
•
Diabetes during pregnancy in women with CF requires intensive endocrine and nutrition care, ideally incorporated into the CF multidisciplinary team.
Keywords: Cystic fibrosis, Pregnancy, Diabetes, Gestational, Pregestational, Fetal, Maternal
Abstract
As cystic fibrosis transmembrane regulator (CFTR) modulator therapies offer greater longevity and improved health quality, women living with cystic fibrosis (CF) are increasingly pursuing pregnancy. Maternal risks for pregnant women with CF largely depend on a woman’s baseline pulmonary and pancreatic function, and the majority of CF pregnancies will successfully end in live births.
Diabetes, either gestational or pre-existing cystic fibrosis-related diabetes (CFRD), is highly prevalent in women with CF, affecting 18 to 62% of pregnancies in recent CF center reports. In addition to the rising incidence of CFRD with age, gestational diabetes is also more common in women with CF due to lower insulin secretion, higher insulin resistance, and increased hepatic glucose production as compared to pregnant women without CF. Diabetes occurring during pregnancy has important implications for maternal and fetal health. It is well established in women without CF that glycemic control is directly associated with risks of fetal malformation, neonatal-perinatal mortality, cesarean delivery and need for neonatal intensive care. Small studies in women with CF suggest that pregnancies affected by diabetes have an increased risk of preterm delivery, lower gestational age, and lower fetal birth weight compared to those without diabetes.
Women with CF preparing for pregnancy should be counseled on the risks of diabetes and should undergo routine screening for CFRD with oral glucose tolerance testing (OGTT) if not already completed in the past six months. Glycemic control in those with pre-gestational CFRD should be optimized prior to conception. Insulin is preferred for the management of diabetes in pregnant women with CF via multiple daily injections or insulin pump therapy, and continuous glucose monitors (CGM) can be useful in mitigating hypoglycemia risks. Women with CF face many unique challenges impacting diabetes care during pregnancy and would benefit from support by a multidisciplinary care team, including nutrition and endocrinology, to ensure healthy pregnancies.
Background
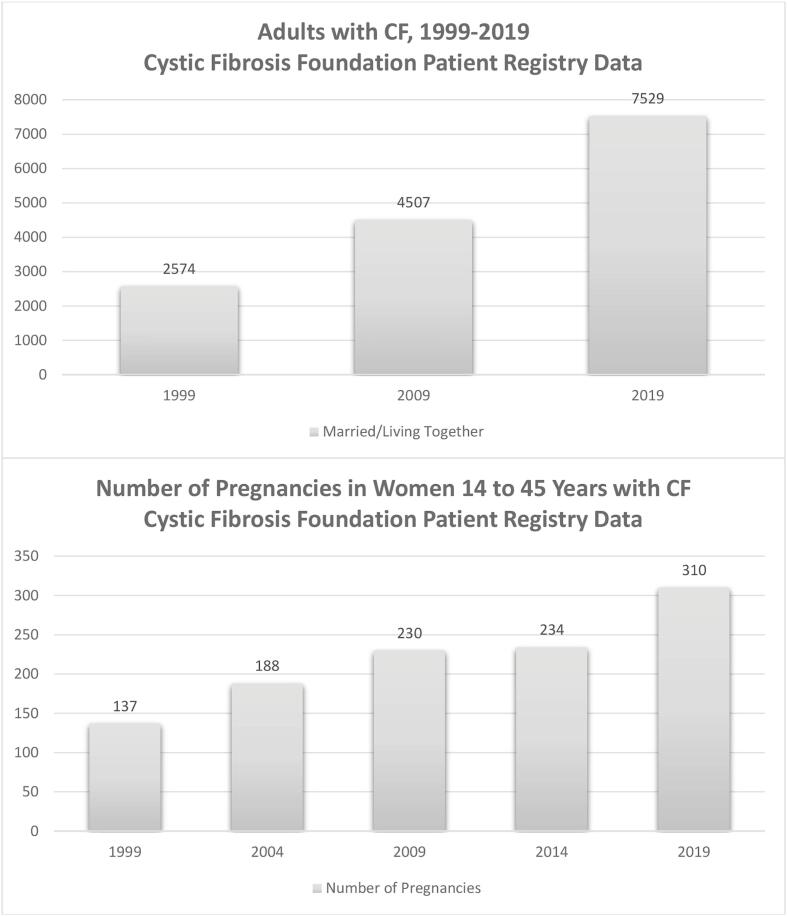
Recent advances in the treatment of cystic fibrosis (CF) have significantly changed survival and health quality for many individuals living with CF. Adults now represent over half of the CF population, and the median predicted survival age for infants born in 2019 is estimated at 48 years [1], [2]. Important demographic changes in the CF community have followed along with these improvements. Among adults with CF, 44% are married or live with a partner, and pregnancies among women with CF have steadily increased with 310 reported in 2019 [1] (Fig. 1). The recent advent of highly-effective cystic fibrosis transmembrane regulator (CFTR) modulator therapy, elexacaftor-tezacaftor-ivacaftor (ETI), for which 90% of the CF population is eligible, has dramatically improved CF morbidity [3], [4]. In addition, highly-effective CFTR modulator therapy is predicted to further increase longevity and possibly fertility [5], effects that will likely continue to impact pregnancy rates.
Fig 1.
Demographic changes 1999–2019 in the CF community. This figure shows a steady increase in the number of individuals with CF who are married or living with a partner from 1999 to 2019. A similar progressive increase is seen in number of pregnancies among women 14 to 45 years with CF.
Special considerations for pregnancy in women with CF
For women with CF, pregnancy carries added health risks, including higher rates of pneumonia, renal failure, and rarely death, with a mortality rate of 1% [6]. Severe pulmonary disease (FEV1 < 50%), pulmonary hypertension, Burkholderia Cepacia infection, and exocrine pancreatic insufficiency have been shown to be important predictors of poor maternal outcomes [7], [8], [9], [10]. During pregnancy, women with CF experience increased frequency of pulmonary exacerbations, hospitalizations and illness-related visits [11], [12]. In addition to diabetes, which will be the focus this review, other challenges of pregnancy in CF include difficulty meeting the increased nutritional and metabolic demands of pregnancy. Appropriate weight gain can be difficult to achieve, and women may require oral supplements or, in more severe circumstances, enteral feeds [12], [13]. However, despite these challenges, women with CF who tolerate pregnancy do not experience deterioration of CF disease afterwards [10]. When compared to never-pregnant women with CF matched by age and disease status, pregnancy does not impact 10-year survival rates [14] or subsequent progression of pulmonary disease [10], [11], [12], [15], [16]. Fetal outcomes are generally favorable with high live birth rates, [17], [18]. The most common fetal complications are low birth weight and preterm delivery, affecting nearly one-quarter of pregnancies [16], [19], [20].
Prevalence & pathophysiology of diabetes in pregnant women with CF
Diabetes in pregnant women with CF is most often either gestational diabetes mellitus (GDM) or pregestational cystic fibrosis-related diabetes (CFRD). The prevalence of diabetes in pregnancy is high and has risen over time with 18 to 62% of pregnancies affected in more recent retrospective cohort studies performed at CF centers; prevalence rates reported through other sources such as billing codes, national database data and/or surveillance systems vary minorly from this range [7], [8], [12], [18], [19], [21], [22], [23], [23], [24], [25], [26], [27] (Table 1).
Table 1.
Prevalence of diabetes in CF pregnancy among case series, retrospective cohorts & database reviews.
| Reference | Study Period, Location & Source | # Mothers | # Pregnancies | % Diabetes (CFRD & GDM) | % CFRD | % GDM |
|---|---|---|---|---|---|---|
| Gilljam et al. 2000 |
|
49 | 74 | 20%* | 6%* | 14%* |
| Edenborough et al. 2000 |
|
55 | 69 | 18%* | 14%* | 4%* |
| Odegaard et al. 2002 |
|
23 | 33 | 18% ǂ | 6% ǂ | 12% ǂ |
| Barak et al. 2005 |
|
8 | 11 | 45% ǂ | 18% ǂ | 27% ǂ |
| Cheng et al. 2006 |
|
25 ‖ | 43 | 62%* | 31% * | 31%* |
| McMullen et al. 2006 |
|
216 | – | 20.6% ǂ | 9.3% ǂ | |
| Lau et al. 2011 |
|
18 | 20 | 55%* | 22%* | 33%* |
| Thorpe-Beeston et al. 2013 |
|
41 | 48 | 35.4% ǂ | – | – |
| Burden et al. 2012 |
|
12 | 15 | 57% ǂ | 28.5% ǂ | 28.5% ǂ |
| Jelin et al. 2017* |
|
66 | 77 | 15.2%* | 4.6%* | 10.6%* |
| Girault et al. 2016 |
|
29 | 33 | 48.5% ǂ | 30.3% ǂ | 18.2% ǂ |
| Ashcroft et al. 2020 |
|
71 | 71 | 66%* | 32%* | 36%* |
* Percentage of mothers with CF diagnosed with and/or treated for diabetes during pregnancy.
ǂ Percentage of pregnancies in women with CF affected by diabetes.
‖ Diabetes status available for 16 of 25 women.
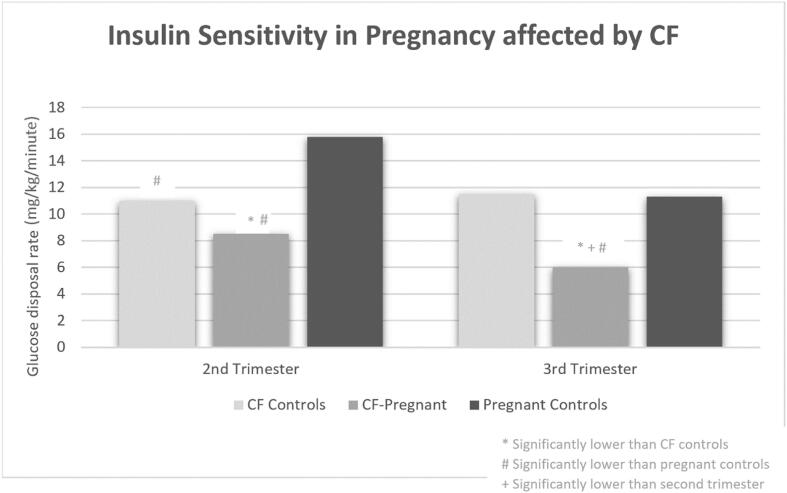
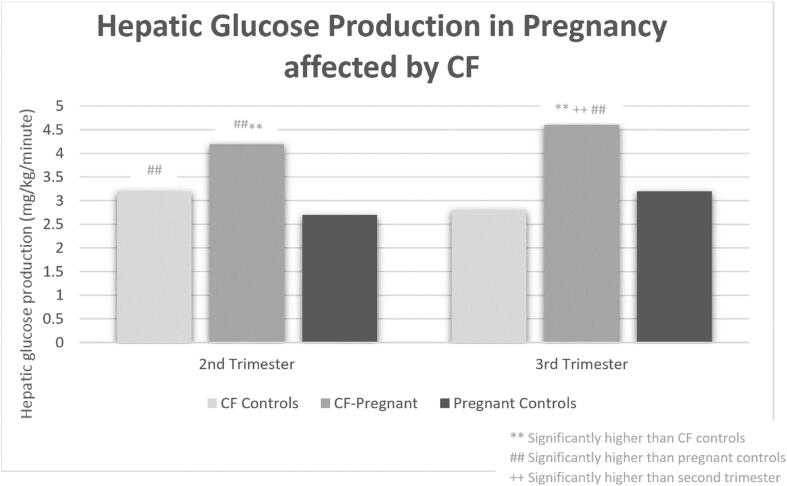
The high and increasing prevalence of diabetes in pregnant women with CF is multifaceted. First, as longevity improves, more women with CF are living to adulthood to consider pregnancy, particularly with the widespread use of highly effective CFTR modulatory therapy. Given that 40–50% of adults with CF have CFRD and there is an increased CFRD prevalence in women aged 30–39 years compared to younger and older age groups, a significant portion of women with CF will develop CFRD during their reproductive years [28], [29]. Secondly, even if a woman with CF does not have pre-gestational CFRD, GDM is more prevalent in women with CF (ranging 10.6–36% in more recent cohorts) than the general U.S. population where GDM prevalence is <10% [18], [22], [23], [27], [30], [31]. Pregnancy is a physiologically demanding time characterized by escalating insulin resistance beginning in the second trimester that necessitates a ∼ 50% increase in insulin production from pre-pregnancy levels to maintain euglycemia [32]. When this increase in insulin requirement cannot be met, GDM develops [32], [33], [34], [35]. It has been demonstrated that pregnant women with CF but without pre-existing CFRD have lower insulin production, reduced insulin sensitivity, increased hepatic gluconeogenesis, and increased protein catabolism over the progression of pregnancy from second to third trimesters compared to pregnant women without CF [36] (Fig. 2). The same study also demonstrated that when compared to non-pregnant women with CF, pregnant women with CF have reduced insulin sensitivity and increased hepatic glucose production but no significant difference in insulin production [36]. These physiologic features highlight why women with CF experience such high rates of GDM due to the combination of both elevated insulin resistance and lower ability to raise insulin production (Fig. 3).
Fig. 2.
Insulin Sensitivity in Pregnancy affected by CF. Adapted from Hardin, D. S., Rice, J., Cohen, R. C., Ellis, K. J. & Nick, J. A. The metabolic effects of pregnancy in cystic fibrosis. Obstet. Gynecol. 106, 367–375 (2005).
Fig. 3.
Hepatic glucose production in pregnancy affected by CF. Adapted from Hardin, D. S., Rice, J., Cohen, R. C., Ellis, K. J. & Nick, J. A. The metabolic effects of pregnancy in cystic fibrosis. Obstet. Gynecol. 106, 367–375 (2005).
Fetal complications of diabetes
Fetal complications of GDM in the general population
Studies of the general population show that there are significant risks for fetal complications when pregnancy is affected by maternal hyperglycemia [37], [38], [39], [40], [41], [42]. Fetal risks differ depending on whether maternal diabetes is gestational or pre-gestational. GDM is classified as hyperglycemia diagnosed in second or third trimester of pregnancy that was not present or suspected prior to pregnancy [43]. GDM places the fetus at elevated risk for macrosomia, birth injury (shoulder dystocia), hypoglycemia, respiratory distress, hyperbilirubinemia, preterm birth, and perinatal mortality (stillbirth) [37], [39], [41], [42], [44]. These risks overall appear to have a direct association with degree of maternal hyperglycemia [37], [38], as demonstrated by the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study, a landmark multicenter multinational prospective observational study of over 25,000 women [37]. This study showed that mild maternal hyperglycemia on 75-gram oral glucose tolerance testing between 24 and 32 weeks gestation conveyed significant risks for multiple fetal complications in a continuous manner with no apparent threshold, highlighting the importance of maintaining maternal glucose levels within normal range as much as possible for optimal pregnancy outcomes [37]. Risk for adverse fetal outcome is also higher for pregnancies with insulin-requiring GDM versus diet-controlled GDM [42].
Fetal complications of pregestational diabetes in the general population
Pregnancies affected by pre-gestational type 1 or type 2 diabetes carry additional fetal risks compared to GDM because of the hyperglycemia present in first trimester pregnancy when organogenesis occurs. Specifically, studies have shown an increased risk for fetal malformation, most commonly occurring as cardiac and neural tube anomalies and less often as gastrointestinal, genitourinary and caudal regression anomalies [45], [46], [47], [48], [49]. The extent of malformation risk is directly related to the degree of maternal hyperglycemia. HbA1c in the first trimester portends risk of fetal malformation and neonatal and perinatal mortality, and risk elevations begin even with minor elevations in HbA1c [47], [50], [51]. One study of 691 pregnancies in women with type 1 diabetes compared to pregnancies without diabetes demonstrated a 3 times increased risk for malformation with first trimester A1c 5.6–6.8%, with risk rising to ∼5 times for A1c 9.4% or higher, which correlated to absolute malformation risk of 4.1% versus 6.6%, respectively; interestingly, the highest relative risk (6.0) was associated with mothers who went without A1c testing in early pregnancy [47]. Another study of 1649 pregnancies affected by pregestational diabetes (type 2 and type 1 diabetes) similarly documented a linear risk for most congenital malformations with higher risk beginning with A1c of 6% or higher, and with fetal malformation incidence progressively reaching 15% in the highest A1c categories [50]. Like GDM, pregestational diabetes carries elevated risk for perinatal mortality, but the risk is even higher [41], [52]. Causes of perinatal mortality are associated with fetal growth abnormalities (intrauterine growth restriction and macrosomia), fetal anomalies, and preterm delivery [40], [46].
Fetal complications of diabetes in pregnant women with CF
The available studies investigating outcomes of pregnancy in CF by diabetes status (GDM or pregestational CFRD) are limited in number, have small sample sizes, and assess fewer fetal outcomes compared to studies of diabetes in pregnancy in the general population [25], [53], [54]. Additionally, results must be interpreted carefully given that pregnant women with CF and diabetes commonly have lower FEV1 and/or older age compared to pregnant women with CF without diabetes. With this context in mind, multiple studies suggest an elevated risk of preterm delivery in CF pregnancies affected by diabetes compared to those that are not [25], [53], [54]. Over 20 years ago, Edenborough et al studied a cohort of 72 CF pregnancies with a 17% prevalence of diabetes and found that diabetic pregnancies disproportionately accounted for 25% of the preterm deliveries and only 9% of term deliveries [25]. Raynaud et al found similar results in a French cohort of 189 CF pregnancies comparing outcomes in those with pre-gestational diabetes to those without. In this study, 38.5% of pre-gestational diabetic pregnancies ended with preterm labor compared to 29.5% of non-diabetic pregnancies; however, this difference did not reach significance likely in part because women diagnosed with diabetes during pregnancy were included in the pre-gestational non-diabetic group [53]. In comparison, a study by Giacobbe et al identified 23 pregnancies in women with CF affected by either gestational or pregestational diabetes compared to 31 pregnancies without diabetes, finding a significant increase in preterm delivery (52.4% versus 20%, p = 0.02), lower gestational age at delivery (36.9 weeks versus 38 weeks, p = 0.04), and lower birthweights (2,825 gm versus 3,185 gm, p = 0.03) in those with diabetes [54].
Aside from prematurity and low birth weight, there are few data regarding other fetal risks associated with diabetes in pregnancy in CF. In the Edenborough et al cohort, it was noted that premature infants were at higher risk of significant complications including artificial ventilation and nasogastric feeding when compared to term infants though no specific association could be identified based on maternal diabetes status [25]. Three fetal anomalies were also documented including diaphragmatic hernia and polysplenia, dextrocardia and unilateral renal cystic dysplasia, and multiple muscular ventricular septal defects, but maternal diabetes status was not reported for these pregnancies [25]. A recent review of California discharge and vital statistics data also found higher prevalence of fetal anomalies (14.3% vs 6.4%) in pregnancies affected by CF including a prominent increase in cardiac anomalies (3.9% vs 0.5%) when compared to the general population [27]. The same study found a higher prevalence of diabetes in pregnancies affected by CF compared to those that were not but it did not evaluate if there was an association between diabetes status and congenital anomalies [27].
Maternal complications of diabetes
Maternal complications of GDM in the general population
Studies of the general population have shown that there are significant maternal risks when pregnancy is affected by GDM. The landmark HAPO trial and Australian Carbohydrate Intolerance in Pregnancy Study both demonstrated increased risks for c-section based on glucose results during 75-gram oral glucose tolerance testing during pregnancy [37], [38]. The HAPO study also demonstrated increased risk for preeclampsia based on fasting, 1-hour and 2-hour glucose results during 75-gram oral glucose tolerance testing between 24 and 32 weeks gestation [37]. Additional studies have found GDM to be an independent risk factor for pregnancy hypertension (HTN) and preeclampsia even when controlling for risk factors such as body mass index (BMI), ethnicity, and age [55], [56], [57], [58].
GDM also portends long-term maternal health risks. Women with a history of GDM have a ∼ 7 times greater risk for developing type 2 diabetes later in life [59]. This risk is higher for those women with elevated BMI, greater post-partum weight gain, higher fasting glucose on pregnancy screening (OGTT) and if insulin was required for GDM treatment [60], [61], [62]. Additionally, women with GDM are at higher risk for developing cardiovascular disease, which may be in part related to BMI and subsequent development of type 2 diabetes [63], [64], [65], [66].
Maternal complications of pregestational diabetes in the general population
Similar to women with GDM, women with pregestational diabetes experience higher rates of c-section and preeclampsia during pregnancy compared to the general population [56], [58], [67], [68], [69], [70]. Unlike women with GDM, women with pregestational diabetes are also at risk for exacerbation of microvascular diabetic complications. Pregnancy is an independent risk factor for progression of diabetic retinopathy and is seen in both type 1 or type 2 diabetes, though risk for type 1 diabetes appears to be higher [71], [72], [73], [74]. Women are at greater risk for diabetic retinopathy progression during pregnancy if they have had longer duration of diabetes ≥ 10 years, history of poorly controlled diabetes, uncontrolled hypertension, and preeclampsia [73]. Women with diabetic nephropathy also experience greater risks, including increased frequency of preeclampsia, when compared to pregnant women with diabetes but normal kidney function [75]. Furthermore, women with more significant diabetic nephropathy (baseline macroalbuminuria, eGFR < 60 ml/min) at the start of pregnancy and poorly controlled HTN during pregnancy can have permanent kidney decline and in the worst cases progression to kidney failure [75]. Finally, diabetes has long been recognized as a risk factor for increased maternal mortality in both type 1 and type 2 diabetes [68], [76], [77]. Treatment of diabetes in pregnancy can also be complicated by hypoglycemia, which occurs more often with the stringent glucose targets in pregnancy and fluctuating insulin resistance including period of insulin sensitization at the end of first trimester [78], [79]. Severe hypoglycemia is common during pregnancy in women with type 1 diabetes, affecting nearly a quarter of women with the highest prevalence in the first trimester and among those women with a history of hypoglycemic unawareness [78], [79].
Maternal complications of diabetes in pregnant women with CF
CFRD has been associated with increased mortality of individuals with CF, though increased screening and treatment of CFRD may improve survival [28], [80]. In contrast, when specifically considering diabetes in pregnant women with CF, studies have not shown a significant difference in maternal survival based on diabetes status [14], [25]. It is also reassuring that while pregnant women with CF and pre-gestational diabetes may have baseline lower FEV1 and BMI compared to their nondiabetic pregnant peers, there was no significant difference in rate of decline for either parameter over 2-year follow up following delivery [53].
It is presently unclear if pregnant women with CF and diabetes have greater risk for c-section. Raynaud et al found significantly increased c-section risk for those with diabetes during pregnancy (48% versus 21.4%, p = 0.005) while Giacobbe et al did not (19.1% versus 10%, p = 0.36) [53], [54]. There are also no data regarding risk of other maternal complications in women with CF and diabetes, including pre-eclampsia and gestational hypertension. Although prevalence of microvascular complications is lower in those with CFRD than other diabetes populations, it is unknown how pregnancy in women with pregestational CFRD may affect progression of diabetic retinopathy or nephropathy.
Effect of diabetes treatment on pregnancy outcomes
The effect of diabetes treatment in pregnancy has not been studied in the CF population. However, there is high quality evidence in the general population that diabetes treatment in pregnancy can significantly reduce risk of maternal and fetal complications in both gestational and pregestational diabetes [81], [82], [83], [84]. Two large randomized trials have documented that providing women with GDM dietary counseling, glucose monitoring, and initiation of insulin therapy when indicated can significantly reduce risks of major birth complications including fetal death, shoulder dystocia, bone fracture, and nerve palsy [82] as well as minor birth complications including large for gestational age (LGA), cesarean delivery, and preclampsia [81]. Similarly, optimization of pregestational diabetes through programs to improve preconception and pregnancy care have also effectively reduced complications such as fetal malformations, still births/late miscarriage, neonatal death, LGA, and preeclampsia [83], [84].
Screening for pregestational CFRD & GDM in women with CF
Given that women with CF of childbearing age have high risk for both CFRD and GDM and given the beneficial effects of diabetes treatment on pregnancy outcomes, screening both before and during pregnancy is recommended [85], [86], [87]. According to guidelines established by the CF Foundation and American Diabetes Association (ADA), women with CF without pre-existing diabetes who are considering pregnancy or are confirmed pregnant should have 75-gram OGTT if not performed within the prior 6 months [85]. This early OGTT testing is intended to identify pre-existing CFRD and to allow for prompt optimization of glycemic status given the associated malformation risks in first trimester. Women with a normal OGTT prior to or at the start of pregnancy should have repeat 75-gram OGTT testing at the end of first (12–16 weeks) and second (24–28 weeks) trimesters to screen for interval development of GDM [85], [87]. Clinicians may also consider initiating glucose monitoring during periods of medical stress such as infection, distal intestinal obstruction syndrome (DIOS) flares, or steroid exposures when women may experience transient hyperglycemia [86].
Pre-conceptional care
Given the established risks of pregnancy, women with CF should be counselled on reproductive potential so that pregnancies are planned and they have the opportunity for comprehensive pre-conceptional care, including diabetes screening and management [86], [88]. An essential component to optimizing diabetes care is to undergo recommended OGTT screenings to ensure timely diagnoses of CFRD. In those with pre-gestational CFRD, ensuring tight glycemic control prior to conception is important in reducing maternal and fetal risks. In addition, all women with diabetes should receive adequate folic acid supplementation to reduce risks of neural tube malformations [8], [16], [35], [86]. Finally, regardless of diabetes status, any woman with CF planning for pregnancy is recommended to achieve a BMI of 22 kg/m2 or higher prior to conception given the possibility of maternal CF-related disease complications during pregnancy including insufficient weight gain [10], [13], [85]. Obtaining a target BMI and the related concerns of sufficient nutrition in pregnancy is especially important for women with CFRD since diabetes in CF has a known association with nutritional decline [89]. Consultation with a nutritionist may be helpful to guide women to weight targets with high quality, calorie dense food choices, and initiation of enteral feeds may be considered if this is not successful [86], [89], [90].
Glycemic targets in pregnancy
HbA1c targets in pregnancy
For women with pre-gestational type 1 and type 2 diabetes, general guidelines recommend an HbA1c at time of conception of < 6.5% to minimize risks of fetal malformations [35]. However, in the CF population, HbA1c may not adequately capture the prandial hyperglycemia occurring early in the course of CFRD and may therefore be a less reliable measure of glycemia [85], [91]. Therefore, if HbA1c is ≥ 6.5%, it can be assumed that further optimization of CFRD is needed prior to conception; however, if HbA1c is < 6.5%, clinicians should confirm that available glucose data (continuous glucose monitor and/or fingerstick glucose levels) also correlate with adequate diabetes control for conception. Once pregnant, HbA1c is ideally maintained < 6.0% and tested on monthly basis accounting for physiologic changes to red blood cell turnover which occur in pregnancy [35], [92].
Glucose targets in pregnancy
In addition to HbA1c testing, pregnant women with diabetes should perform regular fingerstick glucose testing before and after meals [35], [92], [93]. Recommended glucose targets for women with CF are the same as those for all women with diabetes during pregnancy and are as follows: fasting glucose < 95 mg/dL, 1 h post-prandial reading < 140 mg/dL, and 2 h post-prandial reading < 120 mg/dL [35], [93]. Continuous glucose monitoring (CGM) can be a helpful adjunct to fingerstick testing. CGM devices consist of a sensor with a small filament inserted under the skin that measures interstitial glucose levels every 5–15 min, providing comprehensive glycemic data to users in real-time. In 2021, American Diabetes Association (ADA) guidelines newly incorporated glycemic targets for interpretation of CGM data in pregnancies affected by type 1 diabetes given documented improvement in fetal outcomes including reduced LGA, macrosomia, NICU care and fetal hypoglycemia [35], [94], [95], [96], [97] (Table 2). These recommendations were not generalized to other types of diabetes given the lack of data. However, CGM has been validated in individuals with CF [98], and CGM use during pregnancy in women with type 1 diabetes has been shown to reduce maternal hypoglycemia, which is a common occurrence for individuals with cystic fibrosis [99], suggesting that this may be a useful tool for the management of diabetes in women with CF during pregnancy.
Table 2.
Continuous Glucose Monitor Glucose Targets during Pregnancy. Adapted from American Diabetes Association, 14. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care44, S200–S210 (2021).
|
Continuous Glucose Monitor Glucose Targets during Pregnancy | |||
|---|---|---|---|
| Time Below< 54 mg/dL | Time Below< 63 mg/dL | Time in Target 63–140 mg/dL | Time Above >140 mg/dL |
| <1% | <4% | >70% | <25% |
Treatment
Dietary modification
Although diet modification is commonly used to address GDM in pregnant women without CF, diet modification is often not a viable option in pregnant women with CF. Pregnant women with CF frequently struggle to gain sufficient weight during pregnancy because their high baseline caloric needs from CF are further increased by pregnancy [86], [89]. For this reason, pregnant women with CF and diabetes should not restrict carbohydrates because this can precipitate or exacerbate insufficient weight gain [89]. Similar to non-pregnant patients with CFRD, high-sugar foods and beverages should ideally be consumed in moderation during pregnancy in those with diabetes given the difficulty in controlling the hyperglycemia induced by this type of intake [89]. There are no studies investigating the safety and efficacy of dietary modifications in the management of GDM in women with CF.
Insulin treatment
Insulin is the recommended therapy for CFRD as well as for GDM in women with CF [85], [86], [87], [100]. It is also first-line therapy for all other diabetes in pregnancy in the general population [35], [101]. Glyburide and metformin, which are second line therapies for GDM without CF [35], are not recommended for women with CF.
Not all insulins have been equally assessed for safety in pregnancy. In general, insulins that have undergone human studies to confirm safety in pregnancy are preferred, including NPH, detemir, aspart, lispro, and regular insulin [102]. Several newer insulins like glargine, degludec and glulisine have not been sufficiently studied though may be considered with caution if there is strong clinical indication that they are needed to maintain adequate control or to reduce risks associated with treatment [102]. The choice of insulin therapy should be personalized based on the glycemic pattern on glucose testing and any pertinent comorbid conditions that may affect response.
Insulin therapy may be administered through multiple daily injections (MDI) or insulin pumps. In non-pregnant individuals with CF, insulin pumps were found to be effective and have good metabolic benefits in one small study [103]. However, acceptability and continuation of insulin pump therapy are generally lower among individuals with CF than in other diabetes populations [104], [105]. Insulin pump therapy has not been studied in pregnant women with CF. Retrospective observational studies in type 1 diabetes show mixed results regarding whether insulin pump therapy can achieve better glycemic control than MDI [106], [107], [108], [109], and maternal and fetal outcomes were found to be overall similar [108], [110], [111], [112], [113]. Insulin pumps are often utilized for pre-gestational insulin requiring diabetes, and it is recommended that pump therapy is initiated prior to conception to allow for an appropriate adjustment period [101]. Commercially available hybrid closed-loop pumps, which automatically adjust insulin delivery based on CGM input, are not FDA-approved for pregnancy. These devices have set glucose targets that cannot be adjusted to accommodate the lower targets recommended during pregnancy, and individuals may need to change to manual pump settings to achieve pregnancy targets. Nonetheless, insulin pumps can offer greater flexibility in insulin dosing through a variety of other features including precise dosing amounts, bolus features (i.e. square wave, dual wave or extended), variable basal rates, among others [100], [114]. Ultimately, the choice of insulin delivery method should be tailored to individual clinical situations [111].
Unique challenges of diabetes in CF pregnancy
Women with CF face multiple challenges in managing diabetes during pregnancy. First, CFRD is unique in many ways compared to other forms of diabetes. Dysglycemia in CF typically begins with reduced first phase insulin secretion leading to post prandial hyperglycemia, which precedes changes to fasting glucose levels [29], [115]. Patients with CF, even those not treated with exogenous insulin, are at risk for spontaneous fasting and reactive hypoglycemia secondary to abnormal insulin and glucagon secretion [99]. The combination of these factors may present clinically with an individual with CF experiencing alternating episodes of hyperglycemia and hypoglycemia, which can be challenging to manage with insulin therapy. Hormonal changes of pregnancy cause insulin requirements to progressively increase beginning in the second trimester, and women with CF may also experience fluctuations in insulin requirements with health changes and/or medications like glucocorticoids, as routinely seen in non-pregnant individuals with CF [29], [100]. In pregnancy, these challenges are especially difficult given the strict glycemic targets recommended to optimize fetal outcomes.
Additionally, individuals with CF often have comorbid gastrointestinal complications that affect digestion. Dysmotility related to CF may affect every level of the digestive system resulting in DIOS, gastroparesis and/or gastroesophageal reflux disease (GERD) [116], [117]. These alterations can make it difficult to achieve optimal insulin and carbohydrate matching at mealtimes. Gastrointestinal dysmotility may also impact food choices. While not recommended, an individual with CF may favor foods lower in fat and fiber if they are experiencing frequent exacerbations of GERD or gastroparesis [117], which could impact both sufficient nutrition and post-prandial glucose response in pregnancy.
Future directions
There are many unanswered questions regarding the optimal diagnosis, management, and clinical impact of diabetes in pregnant women with CF. Further studies are needed to understand the unique fetal and maternal risks of diabetes in this patient population as well as to investigate the most effective approaches to glucose monitoring, insulin treatment, glycemic targets, and nutrition for optimizing pregnancy outcomes. Furthermore, highly effective modulator therapy has dramatically changed health for many individuals living with CF but has unknown effects on pregnancy and diabetes. We eagerly anticipate answers to some of these questions from the Prospective Study Evaluating Maternal and Fetal Outcomes in the Era of Modulators (MAYFLOWERS), a large multicenter prospective observational study in women with CF characterizing the change in pulmonary function over the course of pregnancy and evaluating the effect of CFTR modulators and other factors on fetal and maternal outcomes. This study will also include a sub-study collecting comprehensive CGM data in participants that will provide novel and important information about glycemia throughout pregnancy in this patient population.
Conclusions
Women living with CF are increasingly pursuing pregnancy as CFTR modulator therapies offer greater health and longevity. Diabetes, either GDM or pre-gestational CFRD, is highly prevalent in pregnancy in CF and has important implications for maternal care and fetal outcomes. Aggressive screening, timely diagnosis, and prompt treatment of diabetes are critical to reduce risks of maternal and fetal complications. For those with pre-gestational CFRD, diabetes management should be optimized prior to conception to ensure best pregnancy outcomes. Insulin is the only recommended therapy for diabetes in pregnant women with CF and may be administered through multiple daily injections or insulin pump therapy. Management of diabetes in pregnant women with CF can be uniquely challenging given high risk for hypoglycemia, altered digestion, fluctuating insulin resistance levels and difficulties with nutrition in CF diabetes and pregnancy. Ultimately, diabetes in pregnant women with CF requires intensive endocrine and nutrition care, ideally incorporated into the CF multidisciplinary team.
Funding sources
This work was supported by the Cystic Fibrosis Foundation EnVision II CF: Emerging Leaders in CF Endocrinology Program and the grant numbers are Oxman19 GEO, Roe19GEO and Ullal19GEO. The sponsor provided financial support and national faculty mentorship to Dr. Oxman, Dr. Roe and Dr. Jagdeesh to develop expertise in CF Endocrinology but was not involved in the development of this manuscript.
CRediT authorship contribution statement
Rachael Oxman: Conceptualization, Writing – original draft, Writing – review & editing, Visualization. Andrea H. Roe: Writing – original draft, Writing – review & editing. Jagdeesh Ullal: Writing – original draft, Writing – review & editing. Melissa S. Putman: Writing – original draft, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Cystic Fibrosis Foundation Patient Registry Annual Data Report. 2019;2019:92. [Google Scholar]
- 2.Elborn JS. Cystic fibrosis. Lancet Lond Engl 2016;388:2519–31. https://doi.org/10.1016/S0140-6736(16)00576-6. [DOI] [PubMed]
- 3.Middleton P.G., Mall M.A., Dřevínek P., Lands L.C., McKone E.F., Polineni D., et al. Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N Engl J Med. 2019;381(19):1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heijerman H.G.M., McKone E.F., Downey D.G., Van Braeckel E., Rowe S.M., Tullis E., et al. Efficacy and safety of the elexacaftor/tezacaftor/ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet Lond Engl. 2019;394:1940–1948. doi: 10.1016/S0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones G.H., Walshaw M.J. Potential impact on fertility of new systemic therapies for cystic fibrosis. Paediatr Respir Rev. 2015;16(Suppl 1):25–27. doi: 10.1016/j.prrv.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Patel E.M., Swamy G.K., Heine R.P., Kuller J.A., James A.H., Grotegut C.A. Medical and obstetric complications among pregnant women with cystic fibrosis. Am J Obstet Gynecol. 2015;212(98):e1–e9. doi: 10.1016/j.ajog.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Gilljam M., Antoniou M., Shin J., Dupuis A., Corey M., Tullis D.E. Pregnancy in cystic fibrosis. Fetal and maternal outcome. Chest. 2000;118(1):85–91. doi: 10.1378/chest.118.1.85. [DOI] [PubMed] [Google Scholar]
- 8.Thorpe-Beeston J.G., Madge S., Gyi K., Hodson M., Bilton D. The outcome of pregnancies in women with cystic fibrosis–single centre experience 1998–2011. BJOG Int J Obstet Gynaecol. 2013;120(3):354–361. doi: 10.1111/1471-0528.12040. [DOI] [PubMed] [Google Scholar]
- 9.Gillet D., Braekeleer M., Bellis G., Durieu I. French Cystic Fibrosis Registry. Cystic fibrosis and pregnancy. Report from French data (1980–1999) BJOG Int J Obstet Gynaecol. 2002;109(8):912–918. doi: 10.1111/j.1471-0528.2002.01511.x. [DOI] [PubMed] [Google Scholar]
- 10.Cohen-Cymberknoh M., Gindi Reiss B., Reiter J., Lechtzin N., Melo J., Pérez G., et al. Baseline Cystic fibrosis disease severity has an adverse impact on pregnancy and infant outcomes, but does not impact disease progression. J Cyst Fibros. 2021;20(3):388–394. doi: 10.1016/j.jcf.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Schechter M.S., Quittner A.L., Konstan M.W., Millar S.J., Pasta D.J., McMullen A., et al. Long-term effects of pregnancy and motherhood on disease outcomes of women with cystic fibrosis. Ann Am Thorac Soc. 2013;10:213–219. doi: 10.1513/AnnalsATS.201211-108OC. [DOI] [PubMed] [Google Scholar]
- 12.McMullen A.H., Pasta D.J., Frederick P.D., Konstan M.W., Morgan W.J., Schechter M.S., et al. Impact of pregnancy on women with cystic fibrosis. Chest. 2006;129(3):706–711. doi: 10.1378/chest.129.3.706. [DOI] [PubMed] [Google Scholar]
- 13.Geake J., Tay G., Callaway L., Bell S.C. Pregnancy and cystic fibrosis: Approach to contemporary management. Obstet Med. 2014;7(4):147–155. doi: 10.1177/1753495X14554022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goss C.H., Rubenfeld G.D., Otto K., Aitken M.L. The effect of pregnancy on survival in women with cystic fibrosis. Chest. 2003;124(4):1460–1468. doi: 10.1378/chest.124.4.1460. [DOI] [PubMed] [Google Scholar]
- 15.Ahluwalia M., Hoag J.B., Hadeh A., Ferrin M., Hadjiliadis D. Cystic fibrosis and pregnancy in the modern era: a case control study. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2014;13(1):69–73. doi: 10.1016/j.jcf.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Thorpe-Beeston J.g. Contraception and pregnancy in cystic fibrosis. J R Soc Med. 2009;102(1_suppl):3–10. doi: 10.1258/jrsm.2009.s19002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciavattini A., Ciattaglia F., Cecchi S., Gagliardini R., Tranquilli A.L. Two successful pregnancies in a woman affected by cystic fibrosis: case report and review of the literature. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2012;25(2):113–115. doi: 10.3109/14767058.2011.565839. [DOI] [PubMed] [Google Scholar]
- 18.Ashcroft A., Chapman S.J., Mackillop L. The outcome of pregnancy in women with cystic fibrosis: a UK population-based descriptive study. BJOG Int J Obstet Gynaecol. 2020;127(13):1696–1703. doi: 10.1111/1471-0528.16423. [DOI] [PubMed] [Google Scholar]
- 19.Ødegaard I., Stray-Pedersen B., Hallberg K., Haanaes O.C., Storrøsten O.T., Johannesson M. Maternal and fetal morbidity in pregnancies of Norwegian and Swedish women with cystic fibrosis. Acta Obstet Gynecol Scand. 2002;81:698–705. [PubMed] [Google Scholar]
- 20.Schlüter D.K., Griffiths R., Adam A., Akbari A., Heaven M.L., Paranjothy S., et al. Impact of cystic fibrosis on birthweight: a population based study of children in Denmark and Wales. Thorax. 2019;74(5):447–454. doi: 10.1136/thoraxjnl-2018-211706.supp1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau EMT, Barnes DJ, Moriarty C, Ogle R, Dentice R, Civitico J, et al. Pregnancy outcomes in the current era of cystic fibrosis care: a 15-year experience. Aust N Z J Obstet Gynaecol 2011;51:220–4. https://doi.org/10.1111/j.1479-828X.2010.01287.x. [DOI] [PubMed]
- 22.Burden C., Ion R., Chung Y., Henry A., Downey D.G., Trinder J. Current pregnancy outcomes in women with cystic fibrosis. Eur J Obstet Gynecol Reprod Biol. 2012;164(2):142–145. doi: 10.1016/j.ejogrb.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Girault A., Blanc J., Gayet V., Goffinet F., Hubert D. Maternal and perinatal outcomes of pregnancies in women with cystic fibrosis–A single centre case-control study. Respir Med. 2016;113:22–27. doi: 10.1016/j.rmed.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Barak A., Dulitzki M., Efrati O., Augarten A., Szeinberg A., Reichert N., et al. Pregnancies and outcome in women with cystic fibrosis. Isr Med Assoc J IMAJ. 2005;7:95–98. [PubMed] [Google Scholar]
- 25.Edenborough F.P., Mackenzie W.E., Stableforth D.E. The outcome of 72 pregnancies in 55 women with cystic fibrosis in the United Kingdom 1977–1996. BJOG Int J Obstet Gynaecol. 2000;107(2):254–261. doi: 10.1111/j.1471-0528.2000.tb11697.x. [DOI] [PubMed] [Google Scholar]
- 26.Cheng E.Y., Goss C.H., McKone E.F., Galic V., Debley C.K., Tonelli M.R., et al. Aggressive prenatal care results in successful fetal outcomes in CF women. J Cyst Fibros. 2006;5(2):85–91. doi: 10.1016/j.jcf.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Jelin A.C., Sharshiner R., Caughey A.B. Maternal co-morbidities and neonatal outcomes associated with cystic fibrosis. J Matern Fetal Neonatal Med. 2017;30(1):4–7. doi: 10.3109/14767058.2016.1161747. [DOI] [PubMed] [Google Scholar]
- 28.Moran A., Dunitz J., Nathan B., Saeed A., Holme B., Thomas W. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care. 2009;32(9):1626–1631. doi: 10.2337/dc09-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Granados A., Chan C.L., Ode K.L., Moheet A., Moran A., Holl R. Cystic fibrosis related diabetes: Pathophysiology, screening and diagnosis. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2019;18(Suppl 2):S3–S9. doi: 10.1016/j.jcf.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Zhou T, Sun D, Li X, Heianza Y, Nisa H, Hu G, et al. Prevalence and Trends in Gestational Diabetes Mellitus among Women in the United States, 2006–2016. Diabetes 2018;67. https://doi.org/10.2337/db18-121-OR.
- 31.Deputy NP. Prevalence and Changes in Preexisting Diabetes and Gestational Diabetes Among Women Who Had a Live Birth — United States, 2012–2016. MMWR Morb Mortal Wkly Rep 2018;67. https://doi.org/10.15585/mmwr.mm6743a2. [DOI] [PMC free article] [PubMed]
- 32.Padmanabhan S., Jiang S., Mclean M., Cheung N.W. Effect of pregnancy on insulin requirements differs between type 1 and type 2 diabetes: A cohort study of 222 pregnancies. Aust N Z J Obstet Gynaecol. 2016;56(4):352–357. doi: 10.1111/ajo.12446. [DOI] [PubMed] [Google Scholar]
- 33.Plows J.F., Stanley J.L., Baker P.N., Reynolds C.M., Vickers M.H. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342. doi: 10.3390/ijms19113342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonagra AD, Biradar SM, K. D, Murthy D.S. J. Normal Pregnancy- A State of Insulin Resistance. J Clin Diagn Res JCDR 2014;8:CC01–3. https://doi.org/10.7860/JCDR/2014/10068.5081. [DOI] [PMC free article] [PubMed]
- 35.Association AD. 14. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021;44:S200–10. https://doi.org/10.2337/dc21-S014. [DOI] [PubMed]
- 36.Hardin Dana S., Rice Julie, Cohen Richard C., Ellis K.J., Nick JA. The metabolic effects of pregnancy in cystic fibrosis. Obstet Gynecol. 2005;106(2):367–375. doi: 10.1097/01.AOG.0000172421.04007.74. [DOI] [PubMed] [Google Scholar]
- 37.HAPO Study Cooperative Research Group, Metzger B.E., Lowe L.P., Dyer A.R., Trimble E.R., Chaovarindr U., et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 38.Jensen Dorte M., Damm Peter, Sørensen Bente, Mølsted-Pedersen Lars, Westergaard Jes G., Klebe Joachim, et al. Clinical impact of mild carbohydrate intolerance in pregnancy: a study of 2904 nondiabetic Danish women with risk factors for gestational diabetes mellitus. Am J Obstet Gynecol. 2001;185(2):413–419. doi: 10.1067/mob.2001.115864. [DOI] [PubMed] [Google Scholar]
- 39.Yang X., Hsu-Hage B., Zhang H., Zhang C., Zhang Y., Zhang C. Women with impaired glucose tolerance during pregnancy have significantly poor pregnancy outcomes. Diabetes Care. 2002;25(9):1619–1624. doi: 10.2337/diacare.25.9.1619. [DOI] [PubMed] [Google Scholar]
- 40.Mackin Sharon T., Nelson Scott M., Wild Sarah H., Colhoun Helen M., Wood Rachael, Lindsay Robert S. Factors associated with stillbirth in women with diabetes. Diabetologia. 2019;62(10):1938–1947. doi: 10.1007/s00125-019-4943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Battarbee Ashley N., Venkatesh Kartik K., Aliaga Sofia, Boggess Kim A. The association of pregestational and gestational diabetes with severe neonatal morbidity and mortality. J Perinatol Off J Calif Perinat Assoc. 2020;40(2):232–239. doi: 10.1038/s41372-019-0516-5. [DOI] [PubMed] [Google Scholar]
- 42.Billionnet Cécile, Mitanchez Delphine, Weill Alain, Nizard Jacky, Alla François, Hartemann Agnès, et al. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia. 2017;60(4):636–644. doi: 10.1007/s00125-017-4206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020;43:S14–31. https://doi.org/10.2337/dc20-S002. [DOI] [PubMed]
- 44.Rosenstein M.G., Cheng Y.W., Snowden J.M., Nicholson J.M., Doss A.E., Caughey A.B. The risk of stillbirth and infant death stratified by gestational age in women with gestational diabetes. Am J Obstet Gynecol. 2012;206(309):e1–e7. doi: 10.1016/j.ajog.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gabbay-Benziv R., Reece E.A., Wang F., Yang P. Birth defects in pregestational diabetes: Defect range, glycemic threshold and pathogenesis. World J Diabetes. 2015;6:481–488. doi: 10.4239/wjd.v6.i3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hawthorne G., Robson S., Ryall E.A., Sen D., Roberts S.H., Platt M.P.W. Prospective population based survey of outcome of pregnancy in diabetic women: results of the Northern Diabetic Pregnancy Audit, 1994. BMJ. 1997;315(7103):279–281. doi: 10.1136/bmj.315.7103.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suhonen L., Hiilesmaa V., Teramo K. Glycaemic control during early pregnancy and fetal malformations in women with type I diabetes mellitus. Diabetologia. 2000;43(1):79–82. doi: 10.1007/s001250050010. [DOI] [PubMed] [Google Scholar]
- 48.Temple R., Aldridge V., Greenwood R., Heyburn P., Sampson M., Stanley K. Association between outcome of pregnancy and glycaemic control in early pregnancy in type 1 diabetes: population based study. BMJ. 2002;325:1275–1276. doi: 10.1136/bmj.325.7375.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dunne F., Brydon P., Smith K., Gee H. Pregnancy in women with Type 2 diabetes: 12 years outcome data 1990–2002. Diabet Med J Br Diabet Assoc. 2003;20:734–738. doi: 10.1046/j.1464-5491.2003.01017.x. [DOI] [PubMed] [Google Scholar]
- 50.Martin Robert B., Duryea Elaine L., Ambia Anne, Ragsdale Alexandra, Mcintire Donald, Wells Chet Edward, et al. Congenital Malformation Risk According to Hemoglobin A1c Values in a Contemporary Cohort with Pregestational Diabetes. Am J Perinatol. 2021;38(12):1217–1222. doi: 10.1055/s-0041-1730435. [DOI] [PubMed] [Google Scholar]
- 51.Jensen D.M., Korsholm L., Ovesen P., Beck-Nielsen H., Moelsted-Pedersen L., Westergaard J.G., et al. Peri-conceptional A1C and risk of serious adverse pregnancy outcome in 933 women with type 1 diabetes. Diabetes Care. 2009;32(6):1046–1048. doi: 10.2337/dc08-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cundy T., Gamble G., Townend K., Henley P.G., MacPherson P., Roberts A.B. Perinatal mortality in Type 2 diabetes mellitus. Diabet Med J Br Diabet Assoc. 2000;17(1):33–39. doi: 10.1046/j.1464-5491.2000.00215.x. [DOI] [PubMed] [Google Scholar]
- 53.Reynaud Quitterie, Poupon-Bourdy Stéphanie, Rabilloud Muriel, Al Mufti Lina, Rousset Jablonski Christine, Lemonnier Lydie, et al. Pregnancy outcome in women with cystic fibrosis-related diabetes. Acta Obstet Gynecol Scand. 2017;96(10):1223–1227. doi: 10.1111/aogs.13185. [DOI] [PubMed] [Google Scholar]
- 54.Giacobbe Lauren E., Nguyen Ruby H.N., Aguilera Marijo N., Mikhaelian Marina, Jacobs Katherine, Ramin Kirk D., et al. Effect of maternal cystic fibrosis genotype on diabetes in pregnancy. Obstet Gynecol. 2012;120(6):1394–1399. doi: 10.1097/AOG.0b013e31826d7eca. [DOI] [PubMed] [Google Scholar]
- 55.Bryson C.L., Ioannou G.N., Rulyak S.J., Critchlow C. Association between Gestational Diabetes and Pregnancy-induced Hypertension. Am J Epidemiol. 2003;158:1148–1153. doi: 10.1093/aje/kwg273. [DOI] [PubMed] [Google Scholar]
- 56.Weissgerber T.L., Mudd L.M. Preeclampsia and Diabetes. Curr Diab Rep. 2015;15:579. doi: 10.1007/s11892-015-0579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Östlund Ingrid, Haglund Bengt, Hanson Ulf. Gestational diabetes and preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2004;113(1):12–16. doi: 10.1016/j.ejogrb.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Negrato C.A., Mattar R., Gomes M.B. Adverse pregnancy outcomes in women with diabetes. Diabetol Metab Syndr. 2012;4:41. doi: 10.1186/1758-5996-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bellamy Leanne, Casas Juan-Pablo, Hingorani Aroon D, Williams David. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet Lond Engl. 2009;373(9677):1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 60.Bao Wei, Yeung Edwina, Tobias Deirdre K., Hu Frank B., Vaag Allan A., Chavarro Jorge E., et al. Long-term risk of type 2 diabetes mellitus in relation to BMI and weight change among women with a history of gestational diabetes mellitus: a prospective cohort study. Diabetologia. 2015;58(6):1212–1219. doi: 10.1007/s00125-015-3537-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peters R.K., Xiang A., Kjos S.L., Buchanan T.A. Long-term diabetogenic effect of single pregnancy in women with previous gestational diabetes mellitus. Lancet Lond Engl. 1996;347(8996):227–230. doi: 10.1016/S0140-6736(96)90405-5. [DOI] [PubMed] [Google Scholar]
- 62.Noctor E., Dunne F.P. Type 2 diabetes after gestational diabetes: The influence of changing diagnostic criteria. World J Diabetes. 2015;6:234–244. doi: 10.4239/wjd.v6.i2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carr D.B., Utzschneider K.M., Hull R.L., Tong J., Wallace T.M., Kodama K., et al. Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care. 2006;29(9):2078–2083. doi: 10.2337/dc05-2482. [DOI] [PubMed] [Google Scholar]
- 64.Shah B.R., Retnakaran R., Booth G.L. Increased Risk of Cardiovascular Disease in Young Women Following Gestational Diabetes Mellitus. Diabetes Care. 2008;31(8):1668–1669. doi: 10.2337/dc08-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shostrom D.C.V., Sun Y., Oleson J.J., Snetselaar L.G., Bao W. History of Gestational Diabetes Mellitus in Relation to Cardiovascular Disease and Cardiovascular Risk Factors in US Women. Front Endocrinol. 2017;8:144. doi: 10.3389/fendo.2017.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gunderson EP, Chiang V, Pletcher MJ, Jacobs DR, Quesenberry CP, Sidney S, et al. History of Gestational Diabetes Mellitus and Future Risk of Atherosclerosis in Mid‐life: The Coronary Artery Risk Development in Young Adults Study. J Am Heart Assoc n.d.;3:e000490. https://doi.org/10.1161/JAHA.113.000490. [DOI] [PMC free article] [PubMed]
- 67.Vargas R., Repke J.T., Ural S.H. Type 1 Diabetes Mellitus and Pregnancy. Rev Obstet Gynecol. 2010;3:92–100. [PMC free article] [PubMed] [Google Scholar]
- 68.Evers Inge M, de Valk Harold W, Visser Gerard H A. Risk of complications of pregnancy in women with type 1 diabetes: nationwide prospective study in the Netherlands. BMJ. 2004;328(7445):915. doi: 10.1136/bmj.38043.583160.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Owens L.A., Sedar J., Carmody L., Dunne F. Comparing type 1 and type 2 diabetes in pregnancy- similar conditions or is a separate approach required? BMC Pregnancy Childbirth. 2015;15:69. doi: 10.1186/s12884-015-0499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jensen D.M., Damm P., Moelsted-Pedersen L., Ovesen P., Westergaard J.G., Moeller M., et al. Outcomes in Type 1 Diabetic Pregnancies: A nationwide, population-based study. Diabetes Care. 2004;27(12):2819–2823. doi: 10.2337/diacare.27.12.2819. [DOI] [PubMed] [Google Scholar]
- 71.Rasmussen K.L., Laugesen C.S., Ringholm L., Vestgaard M., Damm P., Mathiesen E.R. Progression of diabetic retinopathy during pregnancy in women with type 2 diabetes. Diabetologia. 2010;53(6):1076–1083. doi: 10.1007/s00125-010-1697-9. [DOI] [PubMed] [Google Scholar]
- 72.Egan Aoife M., McVicker Lyle, Heerey Adrienne, Carmody Louise, Harney Fiona, Dunne Fidelma P. Diabetic retinopathy in pregnancy: a population-based study of women with pregestational diabetes. J Diabetes Res. 2015;2015:1–7. doi: 10.1155/2015/310239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Amoaku Winfried M., Ghanchi Faruque, Bailey Clare, Banerjee Sanjiv, Banerjee Somnath, Downey Louise, et al. Diabetic retinopathy and diabetic macular oedema pathways and management: UK Consensus Working Group. Eye Lond Engl. 2020;34(S1):1–51. doi: 10.1038/s41433-020-0961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klein B.E.K., Moss S.E., Klein R. Effect of pregnancy on progression of diabetic retinopathy. Diabetes Care. 1990;13(1):34–40. doi: 10.2337/diacare.13.1.34. [DOI] [PubMed] [Google Scholar]
- 75.Spotti Donatella. Pregnancy in women with diabetic nephropathy. J Nephrol. 2019;32(3):379–388. doi: 10.1007/s40620-018-0553-8. [DOI] [PubMed] [Google Scholar]
- 76.Davidson A.J.F., Park A.L., Berger H., Aoyama K., Harel Z., Cook J.L., et al. Risk of severe maternal morbidity or death in relation to elevated hemoglobin A1c preconception, and in early pregnancy: A population-based cohort study. PLOS Med. 2020;17 doi: 10.1371/journal.pmed.1003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bornstein E, Eliner Y, Chervenak FA, Grünebaum A. Concerning trends in maternal risk factors in the United States: 1989–2018. EClinicalMedicine 2020;29. https://doi.org/10.1016/j.eclinm.2020.100657. [DOI] [PMC free article] [PubMed]
- 78.Heller S., Damm P., Mersebach H., Skjøth T.V., Kaaja R., Hod M., et al. Hypoglycemia in Type 1 Diabetic Pregnancy. Diabetes Care. 2010;33:473–477. doi: 10.2337/dc09-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nielsen L.R., Pedersen-Bjergaard U., Thorsteinsson B., Johansen M., Damm P., Mathiesen E.R. Hypoglycemia in Pregnant Women With Type 1 Diabetes: Predictors and role of metabolic control. Diabetes Care. 2008;31(1):9–14. doi: 10.2337/dc07-1066. [DOI] [PubMed] [Google Scholar]
- 80.Milla C.E., Billings J., Moran A. Diabetes is associated with dramatically decreased survival in female but not male subjects with cystic fibrosis. Diabetes Care. 2005;28(9):2141–2144. doi: 10.2337/diacare.28.9.2141. [DOI] [PubMed] [Google Scholar]
- 81.Landon Mark B., Spong Catherine Y., Thom Elizabeth, Carpenter Marshall W., Ramin Susan M., Casey Brian, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339–1348. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Crowther C.A., Hiller J.E., Moss J.R., McPhee A.J., Jeffries W.S., Robinson J.S., et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 83.Zheng Xueying, Yang Daizhi, Luo Sihui, Yan Jinhua, Guo Xiaohui, Yang Huixia, et al. Association of Implementation of a Comprehensive Preconception-to-Pregnancy Management Plan With Pregnancy Outcomes Among Chinese Pregnant Women With Type 1 Diabetes: The CARNATION Study. Diabetes Care. 2021;44(4):883–892. doi: 10.2337/dc20-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Owens Lisa A., Egan Aoife M., Carmody Louise, Dunne Fidelma. Ten Years of Optimizing Outcomes for Women With Type 1 and Type 2 Diabetes in Pregnancy-The Atlantic DIP Experience. J Clin Endocrinol Metab. 2016;101(4):1598–1605. doi: 10.1210/jc.2015-3817. [DOI] [PubMed] [Google Scholar]
- 85.Moran A., Brunzell C., Cohen R.C., Katz M., Marshall B.C., Onady G., et al. Clinical Care Guidelines for Cystic Fibrosis-Related Diabetes: A position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33(12):2697–2708. doi: 10.2337/dc10-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Edenborough F.P., Borgo G., Knoop C., Lannefors L., Mackenzie W.E., Madge S., et al. Guidelines for the management of pregnancy in women with cystic fibrosis. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2008;7:S2–S32. doi: 10.1016/j.jcf.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 87.Moran Antoinette, Pillay Kubendran, Becker Dorothy, Granados Andrea, Hameed Shihab, Acerini Carlo L. ISPAD Clinical Practice Consensus Guidelines 2018: Management of cystic fibrosis-related diabetes in children and adolescents. Pediatr Diabetes. 2018;19:64–74. doi: 10.1111/pedi.12732. [DOI] [PubMed] [Google Scholar]
- 88.Hughan K.S., Daley T., Rayas M.S., Kelly A., Roe A. Female reproductive health in cystic fibrosis. J Cyst Fibros. 2019;18:S95–S104. doi: 10.1016/j.jcf.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 89.Kaminski B.A., Goldsweig B.K., Sidhaye A., Blackman S.M., Schindler T., Moran A. Cystic fibrosis related diabetes: Nutrition and growth considerations. J Cyst Fibros. 2019;18:S32–S37. doi: 10.1016/j.jcf.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 90.Nutritional management of cystic fibrosis – an update for the 21st century. Paediatr Respir Rev 2018;26:4–6. https://doi.org/10.1016/j.prrv.2017.03.006. [DOI] [PubMed]
- 91.Chan Christine L., Hope Emma, Thurston Jessica, Vigers Timothy, Pyle Laura, Zeitler Philip S., et al. Hemoglobin A1c Accurately Predicts Continuous Glucose Monitoring-Derived Average Glucose in Youth and Young Adults With Cystic Fibrosis. Diabetes Care. 2018;41(7):1406–1413. doi: 10.2337/dc17-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.ACOG Practice Bulletin No. 201: Pregestational Diabetes... : Obstetrics & Gynecology. LWW n.d. https://doi.org/10.1097/AOG.0000000000002960.
- 93.ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet Gynecol 2018;131:e49–64. https://doi.org/10.1097/AOG.0000000000002501. [DOI] [PubMed]
- 94.Feig Denice S, Donovan Lois E, Corcoy Rosa, Murphy Kellie E, Amiel Stephanie A, Hunt Katharine F, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet Lond Engl. 2017;390(10110):2347–2359. doi: 10.1016/S0140-6736(17)32400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Murphy HR, Rayman G, Lewis K, Kelly S, Johal B, Duffield K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. BMJ 2008;337:a1680. https://doi.org/10.1136/bmj.a1680. [DOI] [PMC free article] [PubMed]
- 96.Kristensen Karl, Ögge Linda E., Sengpiel Verena, Kjölhede Karin, Dotevall Annika, Elfvin Anders, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes: an observational cohort study of 186 pregnancies. Diabetologia. 2019;62(7):1143–1153. doi: 10.1007/s00125-019-4850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Battelino Tadej, Danne Thomas, Bergenstal Richard M., Amiel Stephanie A., Beck Roy, Biester Torben, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.O'Riordan S.M.P., Hindmarsh P., Hill N.R., Matthews D.R., George S., Greally P., et al. Validation of continuous glucose monitoring in children and adolescents with cystic fibrosis: a prospective cohort study. Diabetes Care. 2009;32(6):1020–1022. doi: 10.2337/dc08-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moheet A., Chan C.L., Granados A., Ode K.L., Moran A., Battezzati A. Hypoglycemia in cystic fibrosis: Prevalence, impact and treatment. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2019;18(Suppl 2):S19–S24. doi: 10.1016/j.jcf.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 100.Ode K.L., Chan C.L., Granados A., Moheet A., Moran A., Brennan A.L. Cystic fibrosis related diabetes: Medical management. J Cyst Fibros. 2019;18:S10–S18. doi: 10.1016/j.jcf.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 101.Alexopoulos A.-S., Blair R., Peters A.L. Management of Preexisting Diabetes in Pregnancy: A Review. JAMA. 2019;321:1811–1819. doi: 10.1001/jama.2019.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blum Alyson K. Insulin Use in Pregnancy: An Update. Diabetes Spectr. 2016;29(2):92–97. doi: 10.2337/diaspect.29.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hardin Dana S., Rice Julie, Rice Mark, Rosenblatt Randall. Use of the insulin pump in treat cystic fibrosis related diabetes. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2009;8(3):174–178. doi: 10.1016/j.jcf.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 104.Scheuing Nicole, Badenhoop Klaus, Borkenstein Martin, Konrad Katja, Lilienthal Eggert, Laubner Katharina, et al. Why is insulin pump treatment rarely used in adolescents and young adults with cystic fibrosis-related diabetes? Pediatr Diabetes. 2015;16(1):10–15. doi: 10.1111/pedi.12158. [DOI] [PubMed] [Google Scholar]
- 105.Marks BE, Kilberg MJ, Aliaj E, Fredkin K, Hudson J, Riva D, et al. Perceptions of Diabetes Technology Use in Cystic Fibrosis-Related Diabetes Management. Diabetes Technol Ther 2021. https://doi.org/10.1089/dia.2021.0201. [DOI] [PubMed]
- 106.Kallas-Koeman Melissa M., Kong Jason M., Klinke Jennifer A., Butalia Sonia, Lodha Abhay K., Lim Ken I., et al. Insulin pump use in pregnancy is associated with lower HbA1c without increasing the rate of severe hypoglycaemia or diabetic ketoacidosis in women with type 1 diabetes. Diabetologia. 2014;57(4):681–689. doi: 10.1007/s00125-014-3163-6. [DOI] [PubMed] [Google Scholar]
- 107.Bruttomesso D., Bonomo M., Costa S., Dal Pos M., Di Cianni G., Pellicano F., et al. Type 1 diabetes control and pregnancy outcomes in women treated with continuous subcutaneous insulin infusion (CSII) or with insulin glargine and multiple daily injections of rapid-acting insulin analogues (glargine-MDI) Diabetes Metab. 2011;37(5):426–431. doi: 10.1016/j.diabet.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 108.Giménez M., Conget I., Nicolau J., Pericot A., Levy I. Outcome of pregnancy in women with type 1 diabetes intensively treated with continuous subcutaneous insulin infusion or conventional therapy. A case-control study. Acta Diabetol. 2007;44(1):34–37. doi: 10.1007/s00592-007-0239-5. [DOI] [PubMed] [Google Scholar]
- 109.Scott Eleanor M., Feig Denice S., Murphy Helen R., Law Graham R. CONCEPTT Collaborative Group. Continuous Glucose Monitoring in Pregnancy: Importance of Analyzing Temporal Profiles to Understand Clinical Outcomes. Diabetes Care. 2020;43(6):1178–1184. doi: 10.2337/dc19-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Farrar D, Tuffnell DJ, West J, West HM. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes. Cochrane Database Syst Rev 2016:CD005542. https://doi.org/10.1002/14651858.CD005542.pub3. [DOI] [PMC free article] [PubMed]
- 111.Pozzilli Paolo, Battelino Tadej, Danne Thomas, Hovorka Roman, Jarosz‐Chobot Przemyslawa, Renard Eric. Continuous subcutaneous insulin infusion in diabetes: patient populations, safety, efficacy, and pharmacoeconomics. Diabetes Metab Res Rev. 2016;32(1):21–39. doi: 10.1002/dmrr.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Feig Denice S., Corcoy Rosa, Donovan Lois E., Murphy Kellie E., Barrett Jon F.R., Sanchez J. Johanna, et al. Pumps or Multiple Daily Injections in Pregnancy Involving Type 1 Diabetes: A Prespecified Analysis of the CONCEPTT Randomized Trial. Diabetes Care. 2018;41(12):2471–2479. doi: 10.2337/dc18-1437. [DOI] [PubMed] [Google Scholar]
- 113.Rys P.M., Ludwig-Slomczynska A.H., Cyganek K., Malecki M.T. Continuous subcutaneous insulin infusion vs multiple daily injections in pregnant women with type 1 diabetes mellitus: a systematic review and meta-analysis of randomised controlled trials and observational studies. Eur J Endocrinol. 2018;178:545–563. doi: 10.1530/EJE-17-0804. [DOI] [PubMed] [Google Scholar]
- 114.Berget Cari, Messer Laurel H., Forlenza Gregory P. A Clinical Overview of Insulin Pump Therapy for the Management of Diabetes: Past, Present, and Future of Intensive Therapy. Diabetes Spectr. 2019;32(3):194–204. doi: 10.2337/ds18-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kayani K., Mohammed R., Mohiaddin H. Cystic Fibrosis-Related Diabetes. Front Endocrinol. 2018;9:20. doi: 10.3389/fendo.2018.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Henen Sara, Denton Christine, Teckman Jeff, Borowitz Drucy, Patel Dhiren. Review of gastrointestinal motility in cystic fibrosis. J Cyst Fibros. 2021;20(4):578–585. doi: 10.1016/j.jcf.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 117.Dorsey J., Gonska T. Bacterial overgrowth, dysbiosis, inflammation, and dysmotility in the Cystic Fibrosis intestine. J Cyst Fibros. 2017;16:S14–S23. doi: 10.1016/j.jcf.2017.07.014. [DOI] [PubMed] [Google Scholar]