Abstract
Fms is the receptor for macrophage colony-stimulating factor (M-CSF) and contains intrinsic tyrosine kinase activity. Expression of exogenous Fms in a murine myeloid progenitor cell line, FDC-P1 (FD-Fms), results in M-CSF-dependent growth and macrophage differentiation. Previously, we described a 100-kDa protein that was tyrosine phosphorylated upon M-CSF stimulation of FD-Fms cells. In this report, we identify this 100-kDa protein as the recently cloned scaffolding protein Gab2, and we demonstrate that Gab2 associates with several molecules involved in M-CSF signaling, including Grb2, SHP2, the p85 subunit of phosphatidylinositol 3′-kinase, SHIP, and SHC. Tyrosine phosphorylation of Gab2 in response to M-CSF requires the kinase activity of Fms, but not that of Src. Overexpression of Gab2 in FD-Fms cells enhanced both mitogen-activated protein kinase (MAPK) activity and macrophage differentiation, but reduced proliferation, in response to M-CSF. In contrast, a mutant of Gab2 that is unable to bind SHP2 did not potentiate MAPK activity. Furthermore, overexpression of this mutant in FD-Fms cells inhibited macrophage differentiation and resulted in a concomitant increase in growth potential in response to M-CSF. These data indicate that Gab2 is involved in the activation of the MAPK pathway and that the interaction between Gab2 and SHP2 is essential for the differentiation signal triggered by M-CSF.
Macrophage colony-stimulating factor (M-CSF) is a growth factor that controls the growth, survival, and differentiation of cells belonging to the monocyte-macrophage lineage (reviewed in references 4, 12, and 36). The importance of M-CSF in mononuclear phagocyte development in vivo has been demonstrated in the naturally occurring osteopetrotic (op/op) mouse, which lacks functional M-CSF and shows severe defects in the production of certain macrophage populations (52, 53). The biological effects of M-CSF are mediated by its unique high-affinity receptor (Fms), which is encoded by the c-fms proto-oncogene (44). Fms is a member of the receptor tyrosine kinase family and is expressed primarily in cells of the monocytic lineage (39). Binding of M-CSF to the extracellular domain of Fms induces receptor homodimerization and autophosphorylation of several tyrosine residues in the cytoplasmic domain which serve as binding sites for src homology 2 (SH2) domain-containing signaling molecules (23, 34, 47). This, in turn, results in the activation of multiple intracellular signaling pathways, such as the Ras/Raf/mitogen-activated protein kinase (MAPK) pathway (reviewed in reference 4). It is believed that activation of these pathways plays an important role in regulating macrophage differentiation by controlling the activities of downstream transcription factors.
To investigate the mechanism by which these signals that are transduced from Fms lead to cell growth and differentiation, Fms receptors containing single or multiple tyrosine-to-phenylalanine mutations have been generated and expressed in various cell systems (33, 40, 41, 47, 48). In Rat2 cells, it was found that mutation of the binding sites for the adapter protein Grb2 and the 85-kDa subunit (p85) of phosphatidylinositol (PI) 3′-kinase completely abolished signal transduction by murine Fms (47). However, expression of Fms receptor mutants in the murine myeloid progenitor cell line FDC-P1 revealed that none of the three tyrosine residues in the kinase insertion domain of Fms, including the Grb2 and p85 binding sites, were required for M-CSF-induced growth and differentiation (3). These results imply the existence of additional signaling molecules in hematopoietic cells that can transduce growth and differentiation signals from the Fms receptor.
In an attempt to identify novel molecules that are involved in M-CSF signaling, Carlberg and Rohrschneider previously described a candidate protein of 100-kDa (p100) that undergoes rapid tyrosine phosphorylation in FD-Fms cells after M-CSF stimulation (5). This p100 protein was also found in immune complexes of the SHP2 tyrosine phosphatase and the p85 subunit of PI 3′-kinase. In this report, we demonstrate that p100 is in fact the previously identified p97/Gab2 adapter protein (11). Overexpression and mutant analysis revealed that p100, or Gab2 protein, plays a critical role in mediating the differentiation signal in hematopoietic cells.
MATERIALS AND METHODS
Mammalian expression constructs.
The open reading frame (ORF) of mouse Gab2 was obtained by reverse transcriptase (RT)-PCR based on the sequence information published previously (11). The sense primer contains the nucleotide sequence upstream of ATG (5′-GCG GCG GGC TCC AGT TTA GCC-3′), and the antisense primer contains the nucleotide sequence before the stop codon (5′-CAG CTT GGC ACC CTT GGA AGG TT-3′). The RT reaction was performed using mRNA from FD-Fms cells as template, and the Gab2 ORF was amplified using the above primers and Advantage HF-2 DNA polymerase mix (Clontech). The PCR fragment was then cloned into a pIND/V5-His TA vector (Invitrogen) in which the coding sequence of mouse Gab2 was fused in frame with a V5-His tag. A fragment that contains the Gab2 sequence with the V5-His tag was then generated by PCR using the ecdysone forward primer (Invitrogen) and a reverse primer (5′-AAG GAT CCG TTT AAA CTC AAT GGT GAT GGT G-3′), digested with BamHI, and subcloned into a pLXSH retroviral vector (28). The expression vector Gab2/PRD/V5 contains only the proline-rich domain of Gab2, which was amplified by the primers 5′-GAC AAT ATG GAT GTC CCA ACC ACT-3′ and 5′-GGA GTT AAA GGT GTG GCT GTT GAT-3′, fused with the V5-His tag, and subcloned into pLXSH vector by the same strategy as described above. The SHP2-binding mutant Gab2/ΔSHP2/V5 was derived from wild-type Gab2/V5 construct through two rounds of site-directed mutagenesis using a QuikChange mutagenesis kit (Stratagene) to introduce Y604F and Y633F mutations. The expression vector of c-Src was a kind gift from Jonathan Cooper (Fred Hutchinson Cancer Research Center), and it was subcloned by inserting a BamHI(blunt)-to-SalI fragment of chicken c-Src (7) into the HpaI-to-XhoI site of pLXSH vector (28). The expression vector of wild-type Fms pZen(Fms/wt), pZen(Fms/KD), and pZen(Fms/Y559F) were constructed as previously described (38). The expression vector pZen(Fms/ΔGrb2) and pZen(Fms/ΔGrb2/Δ85) contain Y697F and Y920F mutations or Y697F, Y721F, and Y920F mutations, respectively, and were constructed by introducing the desired mutations into the pZen(Fms/wt) plasmid using site-directed mutagenesis as described above. The mouse PIR-B expression vector used for antibody production was subcloned by inserting the full-length mouse PIR-B (21) into pLXSH vector using a NotI-XhoI site. All the constructs were verified by sequencing.
Cell culture, transfection, and retroviral infection.
FDC-P1 cells expressing Fms, Fms/KD, or Fms/Y559F were generated as previously described (6). FDC-P1 cells expressing Fms/ΔGrb2 or the Fms/ΔGrb2/Δ85 mutant were generated by transient transfection of the corresponding retroviral construct into Phoenix Ecotropic packaging cells (American Type Culture Collection) and subsequent infection of FDC-P1 cells by cocultivation as described before (3). Cells expressing the mutant Fms receptors on their surface were selected by fluorescence-activated cell sorting using a rabbit polyclonal antibody against the Fms extracellular domain. FD-Fms cells expressing Gab2/V5, Gab2/PRD/V5, or Gab2/ΔSHP2/V5 were generated by using a similar retroviral infection protocol but were selected by culturing the cells in hygromycin B (Boehringer) selection medium (800 μg/ml). All FDC-P1-derived cells were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum and 0.3% X63-IL-3 cell-conditioned medium as a source of interleukin-3 (IL-3) (19). To induce differentiation, cells were washed twice with serum-free DMEM to remove IL-3 and resuspended at a density of 5 × 104 cells/ml in DMEM containing 10% fetal bovine serum and 2,500 U of recombinant murine M-CSF/ml from a conditioned medium with Sf9 insect cells expressing M-CSF (49). The Src family kinase-deficient cell line SYF (20) was a kind gift from Philippe Soriano (Fred Hutchinson Cancer Research Center). SYF cells expressing both wild-type Fms and wild-type V5-tagged Gab2 were obtained by similar retroviral infections as described above. SYF, 293T, and Phoenix Ecotropic cells were maintained in DMEM with 10% fetal bovine serum, and transient transfection of these cells was performed using SuperFect transfection reagent (Qiagen).
Antibodies.
Anti-Gab2 antibody was raised by immunizing rabbits with glutathione S-transferase (GST)-Gab2 fusion protein containing amino acids 285 to 676 of human Gab2 (see below for the details of this GST construct). The preparation of anti-PIRB rabbit polyclonal antibody (1423K) was obtained after multiple subcutaneous injections of New Zealand White rabbits with Rab-9 fibroblasts (American Type Culture Collection) expressing full-length mouse PIR-B on the cell surface. The rabbit polyclonal antiserum directed against the cytoplasmic domain of murine Fms (38), the extracellular domain (37), anti-SHC rabbit polyclonal antibody (23), or anti-SHIP (P1D7) mouse monoclonal antibody (24) were raised in our lab as described previously. Monoclonal antibody to phosphotyrosine (clone 4G10) was a kind gift from Brian Druker (Dana-Farber Cancer Institute). Anti-Grb2 and anti-SHP2 monoclonal antibodies were purchased from Transduction Laboratories. The anti-p85 polyclonal antibody was purchased from Upstate Biotechnology; anti-SHP2 rabbit polyclonal antibody was purchased from Santa Cruz. Antibodies against MAPK (rabbit polyclonal) and phospho-MAPK (mouse monoclonal) were purchased from New England Biolabs, and mouse monoclonal antibody against V5 epitope was purchased from Invitrogen.
GST constructs, GST fusion proteins, and GST pull-down assay.
The GST-fusion construct of Gab2 that was used for antibody production contains a cDNA fragment of human Gab2 encoding amino acids 285 to 676 (29). The construct was generated by PCR with 5′-ACG TGG ATC CCC TCC GAG ACA GAT AAT GAG GAT-3′ as the sense primer and 5′-ATA TAT ATA TAT CTC GAG CAG CTT GGC ACC CTT GGA AGG-3′ as the antisense primer. The amplified product was digested with BamHI and XhoI and subcloned in the BamH1 and XhoI sites of pGEX-5X-1 (Pharmacia). The bacterial expression vectors for GST-Grb2, GST-Grb2-SH2, GST-p85-SH2, GST-SHP2-SH2, GST-SHC-PTB, and GST-SHC-SH2 were constructed as described previously (5, 23). The GST-SHIP-SH2 domain was constructed by PCR from mouse SHIP cDNA (22) using sense primer 5′-GGG AAT GCG GCC GCA TGT CCC TGG GTG GAA CC-3′ and antisense primer 5′-CTT GAG CTC GAG GTC CTT GGC CTC GCT GGG CC-3′, and the amplified fragment was digested with NotI and XhoI and ligated in the same sites of pGSTag vector. All the GST fusion proteins were prepared from Escherichia coli BL21 lysates by absorption to glutathione-Sepharose as described before (5). For the GST pull-down assay, cell lysates were incubated with 20 μg of GST fusion protein coupled on glutathione-Sepharose beads for 2 h at 4°C. The precipitated proteins were washed, eluted, and then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting analysis as described previously (5).
Immunoprecipitation and immunoblotting.
The FDC-P1-derived cells (2 × 107 cells/ml) were starved in serum-free DMEM for 5 h, collected by centrifugation, and then stimulated with M-CSF at 50,000 U/ml, whereas SYF-derived cells were starved in serum-free DMEM for 5 h and then stimulated with M-CSF at 10,000 U/ml directly on the plate. Unless stated otherwise in the figure legends, all stimulations were performed at room temperature for 5 min. After stimulation, cells were immediately lysed in ice-cold lysis buffer (0.5% Igepal CA-630, 50 mM NaCl, 10 mM Tris base, 30 mM Na4PO7, 50 mM NaF, 2 mM C2H2IO2Na, 5 μM ZnCl2, 1 mM phenylmethylsulfonyl fluoride, 200 μM Na3VO4, 10 μM phenylarsine oxide, and 10 μg of aprotinin/ml), and cell lysates were cleared of cell debris by centrifugation at 10,000 × g for 10 min at 4°C. Immunoprecipitation and immunoblotting were then performed as previously described (5).
Cell proliferation analysis.
Cells were plated in triplicate at 5 × 104 cells/ml in M-CSF (2,500 U/ml)-containing medium in 12-well plates and incubated in a 5% CO2 incubator. Fresh medium was added during the culture to keep cells at the optimal density. Cells were counted periodically by Coulter particle count and size analyzers (Coulter Corporation). Each data point was assayed in triplicate and is presented as the average ± standard deviation using Microsoft Excel. Assays were repeated twice.
Flow cytometry analysis.
For selecting Fms-expressing cells, cells were washed with DMEM twice, incubated with anti-Fms (against the extracellular domain) antibody solution (1:200 dilution in DMEM) for 30 min on ice, washed, and then incubated with a fluorescein isothiocyanate (FITC)-conjugated anti-rabbit immunoglobulin G (Jackson Laboratories) secondary antibody solution (1:200 dilution in DMEM) for 30 min on ice. The cells were washed and resuspended in ice-cold DMEM for sorting using a fluorescence-activated cell sorter (Becton Dickinson), and positive cells were plated in regular growth medium. For cell morphology analysis (42) and PIR-B staining, samples were prepared through a similar procedure as described above, but with anti-PIRB rabbit polyclonal antibody instead of anti-Fms antibody. Cells were resuspended in DMEM containing 1 μg of propidium iodide (Sigma) per ml for staining and exclusion of dead cells. Samples were then analyzed using a FACScan apparatus to monitor cellular size (forward scatter), cellular granularity (side scatter), and the expression of PIR-B on the cell surface (FITC intensity). For V5 staining, cells were fixed with 3.7% formaldehyde in phosphate-buffered saline (PBS) for 10 min, permeabilized with 0.1% Triton X-100 for 5 min, washed with PBS, and blocked with 2% bovine serum albumin in PBS for 30 min. Cells were then incubated with anti-V5 antibody solution (1:500 dilution in blocking solution) for 30 min, washed with PBS, and incubated with FITC-conjugated anti-mouse secondary antibody (1:200 dilution in blocking solution; Jackson Laboratories) for another 30 min. Cells were washed, resuspended in PBS, and then analyzed by a FACScan apparatus. All procedures for V5 staining were performed at room temperature.
RESULTS
Determination of the identity of p100, a protein that is tyrosine phosphorylated in response to M-CSF stimulation.
In a search for novel proteins involved in M-CSF signaling, our laboratory has previously identified a 100-kDa protein (p100) that undergoes rapid tyrosine phosphorylation in murine FDC-P1 cells expressing exogenous Fms after M-CSF stimulation (5). It was also shown that p100 could be detected in immune complexes of the tyrosine phosphatase SHP2 and the p85 subunit of PI 3′-kinase in FD-Fms cells stimulated by M-CSF, suggesting that this protein interacts with both SHP2 and p85 and might be a substrate of SHP2.
Recently, several SHP2-binding proteins have been cloned, including the Drosophila Daughter of Sevenless (DOS) (13, 32) and the mammalian Gab1 (16) and Gab2 (11, 29) scaffolding proteins. Notably, Gab2 is a 95- to 100-kDa phosphoprotein that interacts with SHP2 and p85 in response to various hematopoietic stimuli (11, 29). Based on these observations, it seems very likely that our p100 is the Gab2 gene product.
To examine this possibility, we generated a cDNA encoding the ORF of mouse Gab2 by RT-PCR. The C terminus of the mouse Gab2-coding region was fused with a V5 epitope tag (Invitrogen), and the resultant cDNA was subcloned into a retroviral vector and expressed in FD-Fms cells where p100 was originally identified. As shown in Fig. 1A, exogenously expressed V5-tagged Gab2 (Gab2/V5) associated with both SHP2 and p85 in an M-CSF-dependent manner, which is consistent with the observations by Carlberg and Rohrschneider (5) for the endogenous p100 protein in FD-Fms.
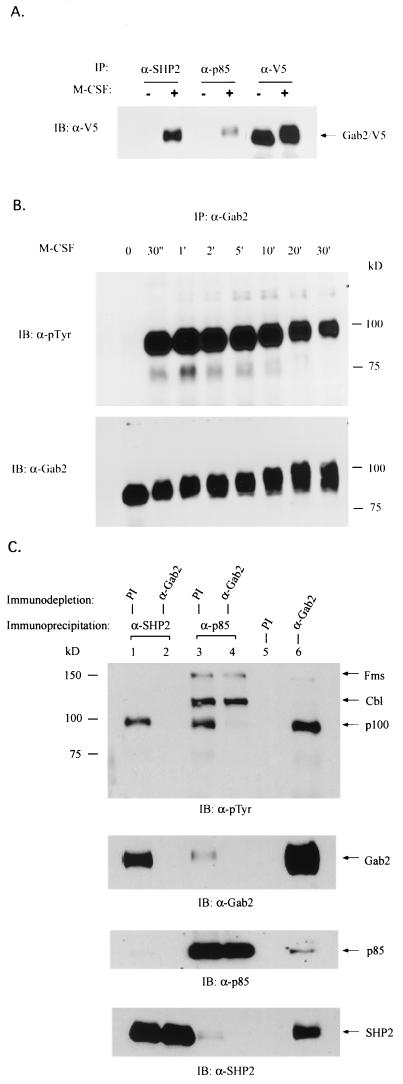
FIG. 1.
Identification of p100 as Gab2. (A) SHP2 and p85 form complexes with exogenously expressed Gab2 in response to M-CSF. Cell lysates from quiescent (−) or M-CSF-stimulated (+) FD-Fms cells expressing exogenous V5-tagged Gab2 were immunoprecipitated (IP) using antibody against SHP2, p85, or V5-epitope and then immunoblotted (IB) with anti-V5 antibody. (B) Anti-Gab2 antibody recognizes endogenous p100 in FD-Fms cells. FD-Fms cells were stimulated with M-CSF for the indicated time at room temperature. Cell lysates from each time point were immunoprecipitated with anti-Gab2 antiserum and visualized by immunoblotting using either antiphosphotyrosine (α-pTyr) or anti-Gab2 antibody. (C) Endogenous p100 protein in the p85 or SHP2 complex can be removed by prior immunodepletion using anti-Gab2 antibody. Cell lysates from M-CSF-stimulated FD-Fms cells were subjected to first-round immunoprecipitations using either preimmune serum (PI) as a control or anti-Gab2 antiserum. Supernatants recovered from prior depletions were subjected to second-round immunoprecipitations using either anti-SHP2 (lanes 1 and 2) or anti-p85 (lanes 3 and 4) antibody. The immunoprecipitates from both first-round (lanes 5 and 6) and second-round (lanes 1, 2, 3, and 4) precipitations were then separated by SDS-PAGE and visualized by immunoblotting using antiphosphotyrosine (α-pTyr), anti-Gab2, anti-p85, or anti-SHP2 antibody. Note that while the p100 protein in the p85 complex was efficiently removed by the anti-Gab2 antibody, other proteins in the p85 complex (Fms and Cb1) were not affected (lanes 3 and 4).
Further confirmation that p100 was indeed Gab2 was provided by studies using antiserum raised against Gab2. As shown in Fig. 1B, a polyclonal anti-Gab2 antibody recognized an endogenous 90- to 100-kDa protein in FD-Fms cells that underwent rapid tyrosine phosphorylation and a decrease in mobility after M-CSF stimulation, which resembles the p100 protein described previously (5). More importantly, the endogenous p100 protein presented in the immune complexes of SHP2 and p85 could be efficiently removed by prior immunodepletion using anti-Gab2 antibody (Fig. 1C, lanes 2 and 4, α-pTyr blot), but not the preimmune serum (Fig. 1C, lanes 1 and 3, α-pTyr blot). The depletion of p100 by Gab2 antibody was very specific, as the Fms and Cb1 proteins presented in the p85 immune complex were not affected by prior removal of Gab2 (lanes 3 and 4, α-pTyr blot). Taken together, these observations verify that p100 is identical to Gab2. To be consistent with the nomenclature used previously by other researchers, we will hereafter refer to p100 as Gab2.
Association of Gab2 with other signaling molecules downstream of Fms receptor.
The sequence of Gab2 reveals the architecture of a scaffolding protein. It contains an N-terminal pleckstrin homology (PH) domain, a central proline-rich domain, and multiple tyrosines spanning the whole length of the protein (11, 29). Previously, it has been shown that antibodies specific for SHP2 and p85 can coimmunoprecipitate tyrosine-phosphorylated Gab2 in FD-Fms cells (5). Reciprocal experiments in which the Gab2 antiserum coimmunoprecipitated both SHP2 and p85 in M-CSF-stimulated FD-Fms cells further confirmed these interactions (Fig. 2A). In addition, SHIP and SHC were also found in the Gab2 immune complex in response to M-CSF, whereas the presence of Grb2 in the complex was independent of M-CSF stimulation (Fig. 2A).
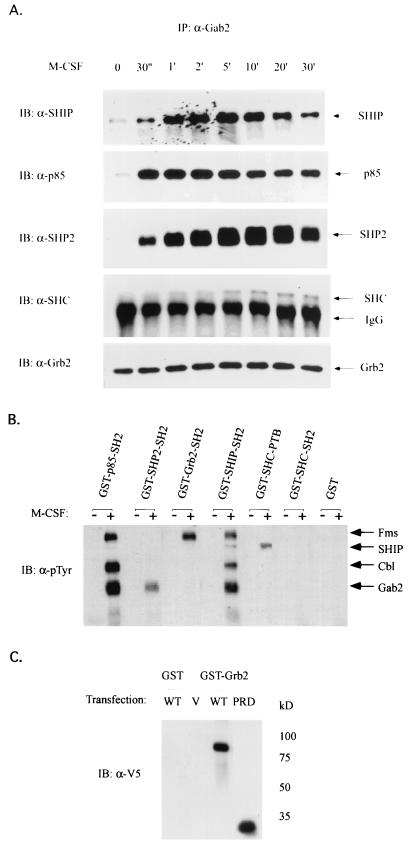
FIG. 2.
Association of Gab2 with signaling molecules in M-CSF pathway. (A) Endogenous Gab2 constitutively associates with Grb2 but interacts with SHP2, p85, SHC, and SHIP in an M-CSF-dependent manner. Gab2 immunoprecipitates shown in Fig. 1B were separated by 7.5% SDS-PAGE and subjected to immunoblotting (IB) using anti-SHIP, anti-p85, anti-SHP2, anti-SHC, or anti-Grb2 antibody, respectively. (B) SHP2, SHIP, and p85 associate with Gab2 via their SH2 domains. Cell lysates from quiescent (−) or M-CSF-stimulated (+) FD-Fms cells were incubated with the GST fusion protein (as indicated) coupled on glutathione-Sepharose beads. The coprecipitated proteins were then eluted, and run on an SDS–7.5% PAGE gel, and immunoblotted with antiphosphotyrosine (α-pTyr) antibody. (C) The proline-rich domain of Gab2 is sufficient to bind Grb2. 293T cells were transiently transfected with an expression vector containing V5-tagged wild-type Gab2 (WT), V5-tagged mutant Gab2 containing only the proline-rich domain (PRD), or the empty vector (V). Cell lysates from each sample were incubated with GST-Grb2 (full-length) fusion protein coupled on glutathione-Sepharose beads. The coprecipitated proteins were then eluted and run on a 7.5% SDS-PAGE gel and subjected to immunoblotting using anti-V5 antibody.
Although SHIP and SHC were present in the immune complex of Gab2, we were unable to detect Gab2 in the reciprocal experiments by using various anti-SHIP and anti-SHC antibodies (data not shown). Since both SHIP and SHC contain the tyrosine-binding domain(s), we then examined whether GST-fusion proteins containing the SHIP-SH2 domain, SHC-SH2 domain, or SHC-PTB domain would bind to phosphorylated Gab2 in lysates from M-CSF-stimulated FD-Fms cells. As shown in Fig. 2B, the SH2 domains of SHIP, as well as the SH2 domains of SHP2 and p85, pulled down Gab2 upon M-CSF stimulation, whereas neither the SHC-SH2 domain nor the SHC-PTB domain bound Gab2.
The constitutive association between Gab2 and Grb2 suggests that the interaction between these two molecules is not mediated by the SH2 domain of Grb2 and a phosphotyrosine on Gab2, although Gab2 does contain a potential binding site for the SH2 domain of Grb2 (11). We explored this further by using a GST fusion protein containing the SH2 domain of Grb2. Consistent with previous studies (23), the tyrosine-phosphorylated Fms receptor bound the SH2 domain of Grb2 upon M-CSF stimulation of FD-Fms cells. However, there was no detectable association between Gab2 and the Grb2 SH2 domain in these cells (Fig. 2B), suggesting that this interaction is not mediated by the SH2 domain of Grb2. Previously, Zhao et al. (55) reported that each of the two SH3 domains of Grb2 was sufficient to interact with Gab2. Considering that Gab2 contains a proline-rich domain with the potential to bind SH3 domain-containing proteins (8, 54), we examined the potential of the proline-rich domain of Gab2 to associate with Grb2 by generating an expression construct containing the proline-rich domain of Gab2 fused to the V5-epitope tag. This construct (Gab2/PRD/V5) and, as a control, the construct containing full-length Gab2/V5 were expressed in 293T cells, and the cell lysate from each sample was precipitated with a GST fusion protein containing full-length Grb2. As shown in Fig. 2C, Gab2/PRD/V5 bound to the GST-Grb2 fusion protein, indicating that the constitutive interaction between Gab2 and Grb2 is mediated by the proline-rich domain of Gab2.
The kinase activity of Fms, but not Src family, is required for tyrosine phosphorylation of Gab2.
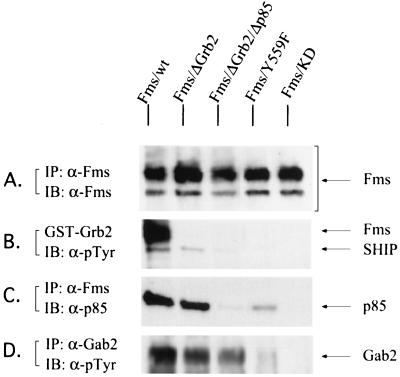
The cytoplasmic domain of Fms contains several tyrosine autophosphorylation sites, which serve to recruit substrates to the receptor via phosphotyrosine-dependent interactions. Two of these tyrosines have been identified as interacting with the Grb2 SH2 domain (25, 47), and another with either SH2 domain of p85 (34). Our observations that Gab2 associates with Grb2 and p85 (Fig. 2A) but not Fms (data not shown) suggest that Gab2 may be recruited to the receptor complex (and therefore tyrosine phosphorylated) via Fms-Grb2-Gab2 and/or Fms-p85-Gab2 interactions. To identify regions within Fms that are required for Gab2 tyrosine phosphorylation, we examined the tyrosine phosphorylation status of endogenous Gab2 in FDC-P1 cells overexpressing various Fms receptor mutants: Fms/KD, which is a kinase-dead mutant that bears a K614A mutation in the kinase domain; Fms/ΔGrb2, which contains Y-to-F mutations at two potential Grb2-binding sites, Y697 and Y920 (25, 47); Fms/ΔGrb2/Δp85, which contains an additional Y-to-F mutation at the p85-binding site Y721 (34); and Fms/Y559F, which contains a mutation at the potential Src-binding site (1). Figure 3A shows similar levels of wild-type and mutant Fms proteins in FDC-P1 cells. As expected, the Fms/ΔGrb2 mutant did not associate with Grb2, and neither Grb2 nor p85 associated with the Fms/ΔGrb2/Δp85 mutant (Fig. 3B and C). Cell lysates were also subjected to immunoprecipitation with Gab2 antiserum followed by immunoblotting with an antiphosphotyrosine antibody. In FDC-P1 cells overexpressing wild-type Fms, Gab2 was strongly tyrosine phosphorylated in response to M-CSF. However, no phosphorylation of Gab2 could be detected in FDC-P1 cells expressing the kinase-dead mutant of Fms (Fig. 3D). This indicates that the kinase activity of Fms is essential for the phosphorylation of Gab2 in response to M-CSF. On the other hand, M-CSF still induced tyrosine phosphorylation of Gab2, albeit at a lower level, in the presence of the Fms/ΔGrb2 and Fms/ΔGrb2/Δp85 receptor mutants (Fig. 3D). These data reveal that the binding of Grb2 and p85 to Fms is involved in, but not essential for, the tyrosine phosphorylation of Gab2, and the data suggest that additional mechanisms might exist to account for the M-CSF-induced phosphorylation of Gab2 in FD-Fms cells.
FIG. 3.
The kinase activity of Fms is essential for Gab2 phosphorylation in response to M-CSF. FDC-P1 cells expressing wild-type Fms or various mutant Fms proteins as indicated were stimulated with M-CSF for 5 min at room temperature, and cell lysates from each sample were subjected to immunoprecipitation (IP) or a GST pull- down assay. The precipitates were then separated on a 7.5% SDS-PAGE gel and immunoblotted (IB) with the antibodies indicated. (A) Expression levels of wild-type or mutant Fms in FDC-P1 cells. (B and C). The ability of wild-type or mutant Fms to associate with Grb2 (B) or p85 (C). (D) Tyrosine phosphorylation of Gab2 in FDC-P1 cells expressing wild-type or mutant Fms.
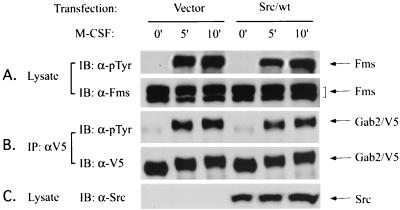
Although phosphorylation of Gab2 in response to M-CSF absolutely requires Fms kinase activity, it is still unclear whether Fms phosphorylates Gab2 directly or indirectly. A possible candidate for an indirect mechanism are the Src family kinases. It has been shown that Src family kinases can bind to the Y559 site on Fms (1), although this interaction has not been shown in the FD-Fms cells used in the present study. On the other hand, in FDC-P1 cells expressing the Fms/Y559F mutant, autophosphorylation of Fms receptor was found to be very low (data not shown), and the interactions between Fms and Grb2 or p85 were dramatically decreased (Fig. 3B and C). Therefore, although we found that Gab2 phosphorylation was substantially decreased in FDC-P1 cells expressing this mutant (Fig. 3D), it is unclear whether this was due to the loss of binding of Src family kinases or the low intrinsic kinase activity of this Fms mutant. To further examine the requirement of Src family kinases on the M-CSF-induced phosphorylation of Gab2, we utilized a Src-deficient cell line, SYF. SYF cells were derived from a Src/Yes/Fyn triple knockout mouse and do not express any other known Src family kinases (20). These cells do not express endogenous Fms or Gab2 either, and they have no response to M-CSF (data not shown). However, these cells became M-CSF responsive when Fms was introduced. We established a stable SYF cell line which ectopically expressed both wild-type Fms and V5-tagged wild-type Gab2, and we examined the tyrosine phosphorylation status of Gab2 in the absence or presence of reintroduced Src kinases. SYF/Fms/Gab2 cells were either transfected with vector or wild-type Src kinase (Src/wt), and 48 h after transfection cells were starved in serum-free medium for 5 h and then stimulated with M-CSF for the indicated times. As shown in Fig. 4, immunoblot analysis of total cell lysates revealed that Fms and Gab2 were equally expressed in these cells, and Src kinase was only expressed in the cells transfected by wild-type c-Src. Upon M-CSF stimulation, Fms underwent autophosphorylation, and this event was not affected by the absence or presence of Src kinase (Fig. 4A). Surprisingly, Gab2 was also efficiently phosphorylated in these Src-deficient cells after M-CSF stimulation, and cotransfection of Src kinase back into these cells (as shown in Fig. 4C) had no effect on Gab2 phosphorylation induced by M-CSF (Fig. 4B). Similar results were also obtained when we compared the tyrosine phosphorylation of Fms and Gab2 in SYF cells with that in SYF/Fyn-expressing cells (data not shown). These data indicate that Fms is necessary and sufficient for M-CSF-induced Gab2 phosphorylation, while Src family kinases are not required for this event, at least in SYF cells.
FIG. 4.
Src family kinase is not required for the phosphorylation of Gab2 in response to M-CSF. SYF cells expressing both Fms and V5-tagged Gab2 were transfected with either vector (Vector) or wild-type c-Src (Src/wt) and stimulated with M-CSF at room temperature at the indicated times. Cell lysates from the transfectants were then either subjected to direct Western blot or immunoprecipitation and Western analysis with the antibodies indicated. (A) Fms expression and tyrosine phosphorylation in the absence or presence of Src. (B) Gab2 expression and tyrosine phosphorylation in the absence or presence of Src. (C) Expression level of Src.
Gab2 is involved in the differentiation signaling pathway induced by M-CSF.
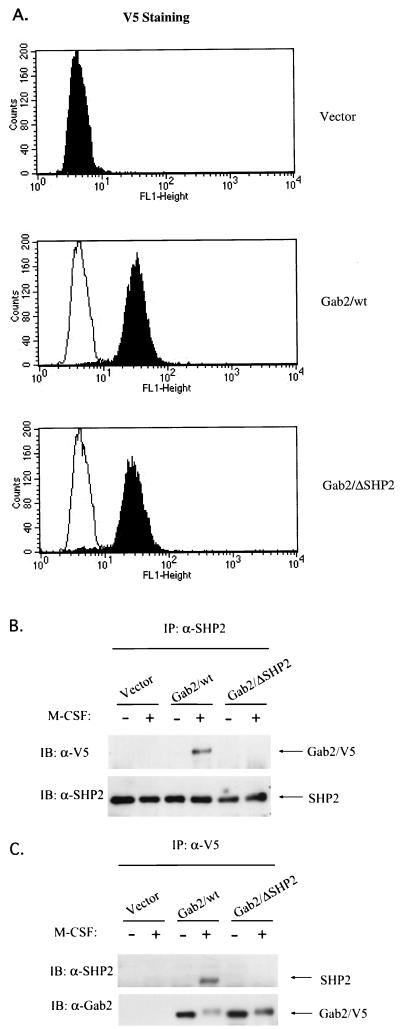
Gab2 was originally identified as a binding protein and potential substrate of SHP2 phosphatase (9). Mutant analysis of DOS, the Drosophila homolog of mammalian Gab proteins, revealed that the two tyrosines for SHP2 binding are necessary and sufficient to mediate Sevenless signaling (14). To determine the role of Gab2 and the functional significance of the Gab2-SHP2 interaction in M-CSF signaling, FD-Fms cells were infected with retroviruses carrying V5-tagged wild-type Gab2 (Gab2/wt) or a V5-tagged mutant of Gab2 (Gab2/ΔSHP2) containing phenylalanine substitutions at the two potential SHP2-binding sites (Y604/Y633) in Gab2. Figure 5A shows the successful transduction and similar expression levels of wild-type and mutant Gab2 in FD-Fms as determined by immunostaining using anti-V5 antibody and monitoring by flow cytometry analysis. Loss of interaction between Gab2/ΔSHP2 and SHP2 was confirmed by immunoprecipitation and Western analyses. Following stimulation with M-CSF, cell lysates were prepared and immunoprecipitated with an SHP2 antibody. Immunoblotting with the anti-V5 antibody revealed that the association of Gab2/ΔSHP2 with SHP2 was completely abolished (Fig. 5B, top panel), which was consistent with a previous report (11). Importantly, reblotting with the SHP2 antibody indicated that equal amounts of SHP2 were immunoprecipitated (Fig. 5B, bottom panel). The absence of an association between Gab2/ΔSHP2 and SHP2 was further confirmed by performing the reciprocal experiment (Fig. 5C, top panel). Reblotting with Gab2 antiserum confirmed that mutant and wild-type Gab2 proteins were efficiently immunoprecipitated (Fig. 5C, bottom panel).
FIG. 5.
Biochemical features of wild-type and mutant Gab2 in FD-Fms cells. (A) Wild-type and mutant Gab2 were expressed at equivalent levels in FD-Fms cells. FD-Fms cells expressing vector control (Vector), V5-tagged wild-type Gab2 (Gab2/wt), or the V5-tagged SHP2 binding mutant (Gab2/ΔSHP2) were fixed, immunostained with anti-V5 antibody and FITC-conjugated anti-mouse immunoglobulin G, and then subjected to flow cytometry analysis. The shaded peaks indicate the FITC intensity of each cell line. The unshaded peaks indicate the profile of the vector line that was overlaid on the profiles of the Gab2/wt or Gab2/ΔSHP2 line. (B and C) The Gab2/ΔSHP2 mutant lost the ability to interact with SHP2. Cell lysates from quies cent (−) or M-CSF-stimulated (+) FD-Fms cells expressing vector, Gab2/wt, or Gab2/ΔSHP2 were immunoprecipitated (IP) with either anti-SHP2 (A) or anti-V5 antibody (B), and the immunoprecipitates were separated on a 12% SDS-PAGE gel and immunoblotted (IB) with antibodies as indicated.
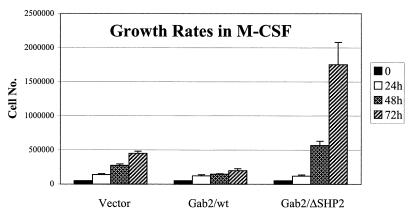
It has been previously shown that FD-Fms cells proliferate in response to IL-3 and maintain an immature blast-like phenotype, whereas in the presence of M-CSF their proliferative potential decreases and they differentiate into cells bearing the morphology of macrophages (37). The above-mentioned FD-Fms cell lines overexpressing wild-type Gab2 or the Gab2/ΔSHP2 mutant were routinely maintained in liquid culture in the presence of IL-3, and no significant differences in growth rate or morphology were observed. We then examined the proliferation and morphology of these cells in the presence of M-CSF. As shown in Fig. 6, the growth rate of FD-Fms cells expressing vector alone in the presence of M-CSF gradually declined over a 3-day period, consistent with a previous report for the parental FD-Fms cells (37). Interestingly, cells overexpressing wild-type Gab2 grew even slower than the control vector cells. On the other hand, overexpression of the Gab2/ΔSHP2 mutant appeared to enhance cell growth in the presence of M-CSF.
FIG. 6.
Growth rates of Gab2-expressing lines in M-CSF. FD-Fms cells expressing empty vector (Vector), V5-tagged wild-type Gab2 (Gab2/wt), or the V5-tagged SHP2-binding mutant of Gab2 (Gab2/ΔSHP2) were plated in triplicate at 5 × 104 cells/ml in M-CSF-containing medium and counted periodically by using a Coulter particle analyzer (Coulter Corporation). Each data point was assayed in triplicate and is presented as the average ± the standard deviation. Similar results were obtained from three independent experiments.
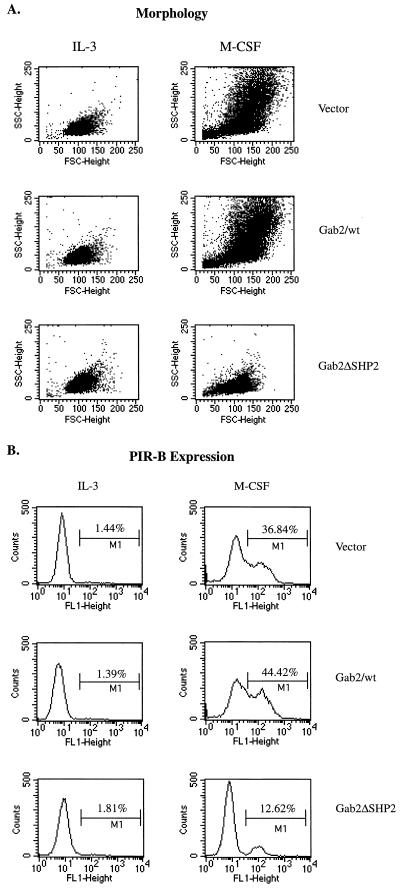
The decrease in proliferation rate of these Gab2 expression lines in the presence of M-CSF seems to correlate with their ability to differentiate. Control vector cells grown for 3 days in the presence of M-CSF displayed morphological changes consistent with those seen during macrophage differentiation, such as increased cell size (forward scatter) and granularity (side scatter) (Fig. 7A, Vector). Also, the expression of PIR-B, a mature macrophage surface protein that is normally expressed at low levels in the presence of IL-3, was upregulated in control vector cells grown in M-CSF (Fig. 7B, Vector). FD-Fms cells overexpressing wild-type Gab2 also differentiated in response to M-CSF. However, the macrophage phenotype, as determined by PIR-B expression and cell morphology, appeared even stronger than that seen for control vector cells in M-CSF (Fig. 7A and B, Gab2/wt). In contrast, we found that FD-Fms cells overexpressing the Gab2/ΔSHP2 mutant did not differentiate well in the presence of M-CSF (Fig. 7A and B, Gab2/ΔSHP2). The differences in phenotypes were also confirmed by the microscopic observations of the morphologies of these cells.
FIG. 7.
Morphology and PIR-B expression of Gab2-expressing lines in M-CSF. FD-Fms cells expressing empty vector (Vector), V5-tagged wild-type Gab2 (Gab2/wt), or the V5-tagged SHP2-binding mutant of Gab2 (Gab2/ΔSHP2) were either maintained in IL-3-containing medium (IL-3) or shifted to M-CSF-containing medium for 3 days (M-CSF). After incubation with rabbit polyclonal antibody against PIR-B and FITC-conjugated anti-rabbit secondary antibody, cells were subject to flow cytometry analysis. The forward and side scatter reflects cellular size and granularity, respectively (A), and the FITC intensity reflects the expression of PIR-B on the cell surface (B).
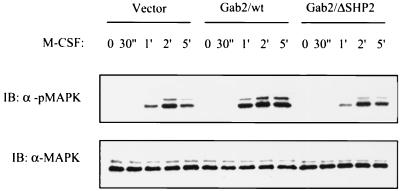
Numerous reports on SHP2 have established its positive role in MAP kinase activation (2, 27, 30, 45, 46). Overexpression of Gab2 has also been shown to increase MAPK activity in response to IL-3 and granulocyte CSF (11, 29). Considering that these studies link Gab2 and SHP2 to the MAPK pathway, we examined whether expression of wild-type Gab2 or the Gab2/ΔSHP2 mutant in FD-Fms cells affects the MAPK activity induced by M-CSF. FD-Fms cells expressing vector control, wild-type Gab2, or the Gab2/ΔSHP2 mutant were stimulated with M-CSF, and the cell lysates were analyzed by immunoblotting with a phospho-MAPK-specific antibody. As shown in Fig. 8, overexpression of wild-type Gab2 enhanced the MAPK activity following M-CSF stimulation, consistent with reports with other receptor systems (11, 29, 55). However, the level of M-CSF-induced MAPK activity in the cells overexpressing the Gab2/ΔSHP2 mutant was equivalent to that of the vector control, indicating that binding of SHP2 is essential for Gab2 to augment M-CSF-induced MAPK activity in FD-Fms cells. Intriguingly, SHP2 binding was found to be dispensable for Gab2-mediated enhancement of MAPK activity in BaF3 cells stimulated with IL-3 (11). It is possible that although both IL-3 and M-CSF stimulate the phosphorylation of Gab2, the actual sites of phosphorylation induced by IL-3 or M-CSF vary, and the subsequent stimulation of pathways leading to MAPK activation, therefore, is different too. Alternatively, different cell contexts may contribute to the functional differences of Gab2 in BaF3 versus FD-Fms cells.
FIG. 8.
Effects of overexpression of wild-type or mutant Gab2 on MAPK activity induced by M-CSF. FD-Fms cells expressing empty vector (Vector), wild-type Gab2 (Gab2/wt), or the SHP2-binding mutant of Gab2 (Gab2/ΔSHP2) were stimulated with M-CSF at 37°C for the times indicated. Cell lysates from each time point were run on a 12% SDS-PAGE gel and then subjected to immunoblotting (IB) using either the antibody against MAPK (α-MAPK) or the antibody only recognizing the activated form of MAPK (α-pMAPK).
DISCUSSION
In exploring the molecular basis of the M-CSF-mediated signal transduction pathway, we previously identified a 100-kDa phosphoprotein downstream of the Fms receptor tyrosine kinase (5). In the present study, we found that this 100-kDa protein is identical to the recently cloned Gab2 gene product (11, 29). Furthermore, we demonstrated that Gab2 plays an important role in regulating growth and differentiation of myeloid cells in response to M-CSF.
Gab2 belongs to a family of scaffolding proteins which includes the mammalian Gab1 and Drosophila DOS protein (reviewed in reference 17). These proteins are structurally similar, each containing an N-terminal PH domain, a central proline-rich domain, and multiple tyrosines that serve as docking sites for SH2 domain-containing proteins. As is the case for Gab1, Gab2 is tyrosine phosphorylated and interacts with SHP2 and the p85 subunit of PI 3′-kinase in response to various stimuli (5, 11, 29, 51, 55). The specificities of these two family members are likely determined by their distinct expression pattern (29, 51), although functional differences in mediating Elk activation have also been reported (55). Gene targeting of Gab1 in mice resulted in an early embryonic lethal phenotype (18), indicating that Gab2 is not able to substitute for the function of Gab1 during mouse development. On the other hand, although Gab1 and Gab2 have overlapping expression in many different tissues, only Gab2 was found in the myeloid progenitor cell lines Baf3 (29) and DA3ER (51). In FDC-P1 cells, only Gab2, but not Gab1, was detected at the protein level (data not shown). These data suggest that Gab2 might be the major player of this family in mediating the signaling in myeloid cells.
Upon M-CSF stimulation, a variety of cellular proteins, including Fms, Cb1, Gab2, SHC, and SHIP, undergo rapid tyrosine phosphorylation in FD-Fms cells (5, 23). It has been shown that tyrosine-phosphorylated Fms binds directly to the SH2 domains of Grb2 and p85, whereas phosphorylated SHIP binds to the PTB domain of SHC and phosphorylated SHC binds to the SH2 domain of Grb2 (5, 22, 23). By coimmunoprecipitation using Gab2 antibody, we observed constitutive association between Gab2 and Grb2, as well as M-CSF-dependent associations between Gab2 and SHP2, p85, SHIP, or SHC (Fig. 2A). Interaction between Gab2 and Grb2 appears to occur through the proline-rich domain of Gab2 and the SH3 domain of Grb2 (Fig. 2C and reference 55). On the other hand, associations between Gab2 and SHP2 or p85 are mediated by the phosphotyrosines on Gab2 and the SH2 domains of SHP2 or p85 (Fig. 2B and references 5, 10, and 11). The interactions of Gab2-SHP2 and Gab2-p85 seem to be solely dependent on the tyrosine phosphorylation status of Gab2, but not that of SHP2 or p85, since Gab2-associated SHP2 or p85 was barely phosphorylated in M-CSF-stimulated FD-Fms cells (Fig. 1C). SHIP and SHC are another two molecules that are found in the immune complex of Gab2. The SHIP-SH2 domain was shown to bind phosphorylated Gab2 in the GST pull-down assay (Fig. 2B). However, no Gab2 could be detected in the coimmunoprecipitation experiments with a variety of SHIP antibodies (data not shown). Therefore, it remains unclear whether these two molecules associate directly in vivo. Regarding the interaction between Gab2 and SHC, evidence from both the reciprocal coimmunoprecipitation experiment using anti-SHC antibody and the GST pull-down assays using GST-SHC-PTB and GST-SHC-SH2 fusion proteins supports the conclusion that this interaction is indirect. Certainly, more work is required to further elucidate the nature of these interactions and their functional significance in M-CSF signaling.
Considering that Grb2 and p85 directly bind to phosphotyrosines on Fms, we speculated that the association of Gab2 with Grb2 and p85 would provide a mechanism for receptor recruitment and subsequent phosphorylation of Gab2. However, while the kinase activity of Fms is essential for Gab2 phosphorylation, M-CSF stimulation of cells expressing an Fms mutant that is unable to bind either p85 or Grb2 still induced Gab2 phosphorylation (Fig. 3). This is consistent with previous observations that Grb2- and p85-binding sites on Fms are essential for M-CSF signaling in Rat2 cells, which do not contain endogenous Gab2 (5), but are not required in FDC-P1 cells, which express endogenous Gab2 (3, 47).
Although Gab2 may be recruited to the receptor via Fms-Grb2 and/or Fms-p85-Gab2 interactions, there are clearly additional mechanisms by which Gab2 can be recruited to the receptor and phosphorylated upon M-CSF stimulation of FD-Fms cells. One possible mechanism could involve an unidentified Fms-binding docking protein(s) which recruits Gab2 to the receptor complexes through a direct or indirect association with Gab2. It has been shown that Gab2 can be recruited to the IL-2 and IL-3 receptors via a SHC-Grb2-Gab2 pathway (10). However, this is not very likely to be the case in M-CSF signaling, since SHC does not appear to bind Fms or Gab2 directly (Fig. 2B). Another mechanism for Gab2 recruitment could involve the PH domain of Gab2 itself, based on the observations made for Gab1 (26, 35). It is possible that the PH domain is sufficient to localize Gab2 to the vicinity of Fms and enable it to be phosphorylated by the kinase domain of Fms in response to M-CSF. However, it is conceivable that the PH domain is not necessary for this event, since Grb2 and p85 could also provide redundant mechanisms for recruiting Gab2 to the receptor complex. Future experiments using various Gab2 mutants in combination with Fms mutants should give us a clearer picture of how Gab2 is recruited and phosphorylated in response to M-CSF.
Unlike Gab1 and DOS, whose functions have been well studied both in vitro and in vivo (13, 14, 18, 26, 32, 50), the role of Gab2 in development and differentiation in response to various stimuli remains largely unknown. To characterize the function of Gab2 in M-CSF signaling, we expressed either wild-type Gab2 or the Gab2/ΔSHP2 mutant in FD-Fms cells. Interestingly, we found that overexpression of wild-type Gab2 promoted both M-CSF-induced MAPK activity and macrophage differentiation of FD-Fms cells. In contrast, overexpression of a Gab2/ΔSHP2 mutant in FD-Fms cells did not promote M-CSF-induced MAPK activity. Furthermore, overexpression of this mutant in FD-Fms cells inhibited macrophage differentiation and resulted in a concomitant increase in growth in response to M-CSF. These results establish an essential role for Gab2 in M-CSF signaling and highlight the importance of the interaction between Gab2 and SHP2 during macrophage differentiation of FD-Fms cells.
Intriguingly, the MAPK activities induced by M-CSF in FD-Fms cells expressing wild-type or mutant Gab2 proteins do not correlate with the growth rates. It has been shown that overexpression of v-Ha-RAS in FDC-P1 cells caused a differentiated phenotype as well as tumorigenicity of the cells (15), indicating that the RAS-MAPK pathway contributes to both proliferation and differentiation signals. However, the enhanced MAPK activity of the cells expressing wild-type Gab2 coincided with a reduced growth potential instead. Furthermore, the Gab2/ΔSHP2 mutant showed loss of function for activation of MAPK but dominant-negative function in inhibiting differentiation. If the enhanced differentiation phenotype of cells expressing wild-type Gab2 was entirely due to increased MAPK activity, then the Gab2/ΔSHP2 and the vector control cells would be expected to have similar levels of differentiation, since both were stimulated to equivalent levels of MAPK activity after M-CSF treatment (Fig. 8). However, cells expressing Gab2/ΔSHP2 actually showed a decreased differentiation phenotype compared with vector control, suggesting that this mutant functioned in a dominant-negative manner to inhibit the differentiation signals delivered by endogenous Gab2 without affecting the MAPK activity. We believe that these data, taken together, indicate that there is an additional pathway(s) through Gab2 which leads to growth inhibition and differentiation in addition to the MAPK pathway.
The mechanism by which Gab2 exerts its function through interaction with SHP2 remains unknown, although there are several possibilities. Firstly, Gab2 may function as an activator of SHP2 phosphatase. Deletion of the N-terminal SH2 domain of SHP2 renders it catalytically inactive, and mice bearing such a mutation are embryonic lethal (31, 43), implying that the catalytic and therefore biological activity of SHP2 requires the binding of phosphotyrosine(s) to its SH2 domain. Such an interaction could be provided by the SHP2-binding phosphotyrosine on Gab2. This hypothesis is further supported by the observation that mutant DOS bearing only the two tyrosines for SHP2 binding is sufficient to mediate Sevenless signaling and to rescue the developmental lethality of the DOS deficiency in Drosophila (14). Secondly, Gab2 may function as a substrate and downstream effector of SHP2. Gab2 was originally recognized as a binding protein and potential substrate of SHP2, since it was hyperphosphorylated in cells expressing a catalytically inactive mutant of SHP2 (9). Furthermore, Gab2 is dephosphorylated by SHP2 in vitro (9, 29). These data support that Gab2 is a substrate of SHP2. Thirdly, Gab2 may function as an adapter to recruit substrates for SHP2. This is supported by the discovery of a p90 protein in the Gab1 complex which is hyperphosphorylated in cells expressing an N-terminal SH2 domain deletion mutant of SHP2 (45). Importantly, these three mechanisms need not be mutually exclusive, and future investigation is required to clarify these possibilities.
In summary, we found Gab2 is a critical component in the signal transduction pathway of M-CSF, and the interaction between Gab2 and SHP2 is essential for macrophage differentiation of FD-Fms cells. The fact that Gab2 is phosphorylated in response to various growth factors and cytokines (5, 11, 29, 51, 55) suggests that this protein might play important roles in other signaling pathways as well, and alteration to either the expression or structure of Gab2 might lead to aberrant signaling and subsequent pathological disorders.
ACKNOWLEDGMENTS
We thank all the members of L. R. Rohrschneider's lab for their suggestions and technical help. Special thanks go to Kristen Carlberg, whose work provided the background for this work, Tamara Anderson for expert technical assistance, Leslie Cary and Rich Klinghoffer for the help on SYF cells, David Nochimson for excellent secretarial help, and Lisa Connell-Crowley, Michael Harkey, and Weiguo Zhang for critical readings of the manuscript.
This work was supported by U.S. Public Health Service grants CA6608 and CA6648 to L.R.R. from the National Institutes of Health.
REFERENCES
- 1.Alonso G, Koegl M, Mazurenko N, Courtneidge S A. Sequence requirements for binding of Src family tyrosine kinases to activated growth factor receptors. J Biol Chem. 1995;270:9840–9848. doi: 10.1074/jbc.270.17.9840. [DOI] [PubMed] [Google Scholar]
- 2.Bennett A M, Hausdorff S F, O'Reilly A M, Freeman R M, Neel B G. Multiple requirements for SHPTP2 in epidermal growth factor-mediated cell cycle progression. Mol Cell Biol. 1996;16:1189–1202. doi: 10.1128/mcb.16.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourette R P, Myles G M, Carlberg K, Rohrschneider L R. Uncoupling of the proliferation and differentiation signals mediated by the murine macrophage colony-stimulating factor receptor expressed in myeloid FDC-P1 cells. Cell Growth Differ. 1995;6:631–645. [PubMed] [Google Scholar]
- 4.Bourette R P, Rohrschneider L R. Early events in M-CSF receptor signaling. Growth Factors. 2000;17:155–166. doi: 10.3109/08977190009001065. [DOI] [PubMed] [Google Scholar]
- 5.Carlberg K, Rohrschneider L R. Characterization of a novel tyrosine phosphorylated 100-kDa protein that binds to SHP-2 and phosphatidylinositol 3′-kinase in myeloid cells. J Biol Chem. 1997;272:15943–15950. doi: 10.1074/jbc.272.25.15943. [DOI] [PubMed] [Google Scholar]
- 6.Carlberg K, Rohrschneider L R. The effect of activating mutations on dimerization, tyrosine phosphorylation, and internalization of the macrophage colony stimulating factor receptor. Mol Biol Cell. 1994;5:81–95. doi: 10.1091/mbc.5.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper J A, MacAuley A. Potential positive and negative autoregulation of p60c-src by intermolecular autophosphorylation. Proc Natl Acad Sci USA. 1988;85:4232–4236. doi: 10.1073/pnas.85.12.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng S, Chen J K, Yu H, Simon J A, Schreiber S L. Two binding orientations for peptides to the Src SH3 domain: development of a general model for SH3-ligand interactions. Science. 1994;266:1241–1247. doi: 10.1126/science.7526465. [DOI] [PubMed] [Google Scholar]
- 9.Gu H, Griffin J D, Neel B G. Characteriztion of two SHP-2-associated binding proteins and potential substrates in hematopoietic cells. J Biol Chem. 1997;272:16421–16430. doi: 10.1074/jbc.272.26.16421. [DOI] [PubMed] [Google Scholar]
- 10.Gu H, Maeda H, Moon J J, Lord J D, Yoakim M, Nelson B H, Neel B G. New role for SHC in activation of the phosphatidylinositol 3-kinase/Akt pathway. Mol Cell Biol. 2000;20:7109–7120. doi: 10.1128/mcb.20.19.7109-7120.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu H, Pratt J C, Burakoff S J, Neel B G. Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation. Mol Cell. 1998;2:729–740. doi: 10.1016/s1097-2765(00)80288-9. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton J A. CSF-1 signal transduction. J Leukoc Biol. 1997;62:145–155. doi: 10.1002/jlb.62.2.145. [DOI] [PubMed] [Google Scholar]
- 13.Herbst R, Carroll P M, Allard J D, Schilling J, Raabe T, Simon M A. Daughter of sevenless is a substrate of the phosphotyrosine phosphatase corkscrew and functions during sevenless signaling. Cell. 1996;85:899–909. doi: 10.1016/s0092-8674(00)81273-8. [DOI] [PubMed] [Google Scholar]
- 14.Herbst R, Zhang X, Qin J, Simon M A. Recruitment of the protein tyrosine phosphatase CSW by DOS is an essential step during signaling by the Sevenless receptor tyrosine kinase. EMBO J. 1999;18:6950–6961. doi: 10.1093/emboj/18.24.6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hibi S, Löhler J, Friel J, Stocking C, Ostertag W. Induction of monocytic differentiation and tumorigenicity by v-Ha-ras in differentiation-arrested hematopoietic cells. Blood. 1993;81:1841–1848. [PubMed] [Google Scholar]
- 16.Holgado-Madruga M, Emlet D R, Moscatello D K, Godwin A K, Wong A J. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature. 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- 17.Huyer G, Alexander D R. Immune signalling: SHP-2 docks at multiple ports. Curr Biol. 1999;9:R129–R132. doi: 10.1016/s0960-9822(99)80080-3. [DOI] [PubMed] [Google Scholar]
- 18.Itoh M, Yoshida Y, Nishida K, Narimatsu M, Hibi M, Hirano T. Role of Gab1 in heart, placenta, and skin development and growth factor- and cytokine-induced extracellular signal-regulated kinase mitogen-activated protein kinase activation. Mol Cell Biol. 2000;20:3695–3704. doi: 10.1128/mcb.20.10.3695-3704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karasuyama H, Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4, or 5, using modified cDNA expression vectors. Eur J Immunol. 1988;18:97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- 20.Klinghoffer R A, Sachsenmaier C, Cooper J A, Soriano P. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 1999;18:2459–2471. doi: 10.1093/emboj/18.9.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubagawa H, Burrows P, Cooper M D. A novel pair of immunoglobulin-like receptors expressed by B cells and myeloid cells. Proc Natl Acad Sci USA. 1997;94:5261–5266. doi: 10.1073/pnas.94.10.5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lioubin M N, Algate P A, Tsai S, Carlberg K, Aebersold R, Rohrschneider L R. p150Ship, a signal transduction molecule with inositol polyphosphate-5-phosphatase activity. Genes Dev. 1996;10:1084–1095. doi: 10.1101/gad.10.9.1084. [DOI] [PubMed] [Google Scholar]
- 23.Lioubin M N, Myles G M, Carlberg K, Bowtell D, Rohrschneider L R. Shc, Grb2, Sos1, and a 150-kilodalton tyrosine-phosphorylated protein form complexes with Fms in hematopoietic cells. Mol Cell Biol. 1994;14:5682–5691. doi: 10.1128/mcb.14.9.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucas D M, Rohrschneider L R. A novel spliced form of SH2-containing inositol phosphatase SHIP is expressed during myeloid development. Blood. 1999;93:1922–1933. [PubMed] [Google Scholar]
- 25.Mancini A, Niedenthal R, Joos H, Koch A, Trouliaris S, Niemann H, Tamura T. Identification of a second Grb2 binding site in the v-Fms tyrosine kinase. Oncogene. 1997;15:1565–1572. doi: 10.1038/sj.onc.1201518. [DOI] [PubMed] [Google Scholar]
- 26.Maroun C R, Holgado-Madruga M, Royal I, Naujokas M A, Fournier T M, Wong A J, Park M. The Gab1 PH domain is required for localization of Gab1 at sites of cell-cell contact and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol Cell Biol. 1999;19:1784–1799. doi: 10.1128/mcb.19.3.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milarski K L, Saltiel A R. Expression of catalytically inactive syp phosphatase in 3T3 cells blocks stimulation of mitogen-activated protein kinase by insulin. J Biol Chem. 1994;269:21239–21243. [PubMed] [Google Scholar]
- 28.Miller A D, Miller D G, Garcia J V, Lynch C M. Use of retroviral vectors for gene transfer and expression. Methods Enzymol. 1993;217:581–599. doi: 10.1016/0076-6879(93)17090-r. [DOI] [PubMed] [Google Scholar]
- 29.Nishida K, Yoshida Y, Itoh M, Fukada T, Ohtani T, Shirogane T, Atsumi T, Takahashi-Tezuka M, Ishihara K, Hibi M, Hirano T. Gab-family adapter proteins act downstream of cytokine and growth factor receptors and T- and B-cell antigen receptors. Blood. 1999;93:1809–1816. [PubMed] [Google Scholar]
- 30.Noguchi T, Matozaki T, Horita K, Fujioka Y, Kasuga M. Role of SH-PTP2, a protein-tyrosine phosphatase with Src homology 2 domains, in insulin-stimulated Ras activation. Mol Cell Biol. 1994;14:6674–6682. doi: 10.1128/mcb.14.10.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qu C K, Shi Z Q, Shen R, Tsai F Y, Orkin S H, Feng G S. A deletion mutation in the SH2-N domain of SHP-2 severely suppresses hematopoietic cell development. Mol Cell Biol. 1997;17:5499–5507. doi: 10.1128/mcb.17.9.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raabe T, Riesgo-Escovar J, Liu X, Bausenwein B S, Deak P, Maröy P, Hafen E. DOS, a novel pleckstrin homology domain-containing protein required for signal transduction between sevenless and Ras1 in Drosophila. Cell. 1996;85:911–920. doi: 10.1016/s0092-8674(00)81274-x. [DOI] [PubMed] [Google Scholar]
- 33.Reedijk M, Liu X, Pawson T. Interactions of phosphatidylinositol kinase, GTPase-activating protein (GAP), and GAP-associated proteins with the colony-stimulating factor 1 receptor. Mol Cell Biol. 1990;10:5601–5608. doi: 10.1128/mcb.10.11.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reedijk M, Liu X, van der Geer P, Letwin K, Waterfield M D, Hunter T, Pawson T. Tyr721 regulates specific binding of the CSF-1 receptor kinase insert to PI 3′-kinase SH2 domains: a model for SH2-mediated receptor-target interactions. EMBO J. 1992;11:1365–1372. doi: 10.1002/j.1460-2075.1992.tb05181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodrigues G A, Falasca M, Zhang Z, Ong S H, Schlessinger J. A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol Cell Biol. 2000;20:1448–1459. doi: 10.1128/mcb.20.4.1448-1459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rohrschneider L R, Bourette R P, Lioubin M N, Algate P A, Myles G M, Carlberg K. Growth and differentiation signals regulated by the M-CSF receptor. Mol Reprod Dev. 1997;46:96–103. doi: 10.1002/(SICI)1098-2795(199701)46:1<96::AID-MRD15>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 37.Rohrschneider L R, Metcalf D. Induction of macrophage colony-stimulating factor-dependent growth and differentiation after introduction of the murine c-fms gene into FDC-P1 cells. Mol Cell Biol. 1989;9:5081–5092. doi: 10.1128/mcb.9.11.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rohrschneider L R, Rothwell V M, Nicola N A. Transformation of murine fibroblasts by a retrovirus encoding the murine c-fms proto-oncogene. Oncogene. 1989;4:1015–1022. [PubMed] [Google Scholar]
- 39.Rohrschneider L R, Woolford J F. Structural and functional comparison of viral and cellular fms. Semin Virol. 1992;2:385–395. [Google Scholar]
- 40.Roussel M F, Cleveland J L, Shurtleff S A, Sherr C J. Myc rescue of a mutant CSF-1 receptor impaired in mitogenic signalling. Nature. 1991;353:361–363. doi: 10.1038/353361a0. [DOI] [PubMed] [Google Scholar]
- 41.Roussel M F, Shurtleff S A, Downing J R, Sherr C J. A point mutation at tyrosine 809 in the human colony-stimulating factor 1 receptor impairs mitogenesis without abrogating tyrosine kinase activity, association with phosphatidylinositol 3-kinase, or induction of fos and junB genes. Proc Natl Acad Sci USA. 1990;87:6738–6742. doi: 10.1073/pnas.87.17.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salzman G C, Crowell J M, Martin J C, Trujillo T T, Romero A, Mullaney P F, LaBauve P M. Cell classification by laserlight scattering: identification and separation of unstained leukocytes. Acta Cytol. 1975;19:374–377. [PubMed] [Google Scholar]
- 43.Saxton T M, Henkemeyer M, Gasca S, Shen R, Rossi D J, Shalaby F, Feng G S, Pawson T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 1997;16:2352–2364. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sherr C J, Rettenmier C W, Sacca R, Roussel M F, Look A T, Stanley E R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985;41:665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- 45.Shi Z-Q, Yu D-H, Park M, Marshall M, Feng G-S. Molecular mechanism for the Shp-2 tyrosine phosphatase function in promoting growth factor stimulation of Erk activity. Mol Cell Biol. 2000;20:1526–1536. doi: 10.1128/mcb.20.5.1526-1536.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang T L, Freeman R M J, O'Reilly A M, Neel B, Sokol S Y. The SH2-containing protein-tyrosine phosphatase SH-PTP2 is required upstream of MAP kinase for early xenopus development. Cell. 1995;80:473–483. doi: 10.1016/0092-8674(95)90498-0. [DOI] [PubMed] [Google Scholar]
- 47.van der Geer P, Hunter T. Mutation of Tyr697, a GRB2-binding site, and Tyr721, a PI 3-kinase binding site, abrogates signal transduction by the murine CSF-1 receptor expressed in Rat-2 fibroblasts. EMBO J. 1993;12:5161–5172. doi: 10.1002/j.1460-2075.1993.tb06211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Geer P, Hunter T. Tyrosine 706 and 807 phosphorylation site mutants in the murine colony-stimulating factor-1 receptor are unaffected in their ability to bind or phosphorylate phosphatidylinositol-3 kinase but show differential defects in their ability to induce early response gene transcription. Mol Cell Biol. 1991;11:4698–4709. doi: 10.1128/mcb.11.9.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z, Myles G, Brandt C S, Lioubin M N, Rohrschneider L R. Identification of the ligand-binding regions in the macrophage colony-stimulating factor receptor extracellular domain. Mol Cell Biol. 1993;13:5348–5359. doi: 10.1128/mcb.13.9.5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weidner K M, Di Cesare S, Sachs M, Brinkmann V, Behrens J, Birchmeier W. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996;384:173–176. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- 51.Wickrema A, Uddin S, Sharma A, Chen F, Alsayed Y, Ahmad S, Sawyer S T, Krystal G, Yi T, Nishada K, Hibi M, Hirano T, Platanias L C. Engagement of Gab1 and Gab2 in erythropoietin signaling. J Biol Chem. 1999;274:24469–24474. doi: 10.1074/jbc.274.35.24469. [DOI] [PubMed] [Google Scholar]
- 52.Wiktor-Jedrzejczak W, Bartocci A, Ferrante A W, Jr, Ahmed-Ansari A, Sell K W, Pollard J W, Stanley E R. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci USA. 1990;87:4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz L D, Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 54.Yu H, Chen J K, Feng S, Dalgarno D C, Brauer A W, Schreiber S L. Structural basis for the binding of proline-rich peptides to SH3 domains. Cell. 1994;76:933–945. doi: 10.1016/0092-8674(94)90367-0. [DOI] [PubMed] [Google Scholar]
- 55.Zhao C, Yu D-H, Shen R, Feng G-S. Gab2, a new pleckstrin homology domain-containing adapter protein, acts to uncouple signaling from ERK kinase to Elk-1. J Biol Chem. 1999;274:19649–19654. doi: 10.1074/jbc.274.28.19649. [DOI] [PubMed] [Google Scholar]