Abstract
Purpose
Women under 40 years old are at increased risk for developing human epidermal growth factor receptor 2 (HER2) positive or triple negative subtype and more advanced breast cancer, yet young age itself has also historically been an independent prognostic factor.
Methods
Using the Surveillance, Epidemiology, and End Results (SEER) Program, we examined data for 271,173 women with stage I-III breast cancer between 2010 and 2015. Using Fine and Gray regression models to account for competing risks, we examined the risk of breast cancer-specific death by age and clinical subtypes, considering grade, hormone receptor (HR) and HER2 status, adjusting for demographic, clinical and treatment variables.
Results
Of 271,173 women eligible for analysis, 14,109 were <40 years of age. Women under 40 years old were more likely to be non-white, uninsured, and to have higher stage, higher grade, HER2-positive and triple-negative subtype disease (all, p < 0.001). Compared to women ages 40–60, women ages <40 had higher breast cancer mortality (hazard ratio, 1.8; 95% confidence interval (CI) 1.6–1.9) in unadjusted analysis. In models controlling for demographic, clinical and treatment factors, young age was significantly associated with an increased risk of breast cancer mortality among women with HR-positive, lower grade disease (hazard ratio 1.7; 95% CI 1.4–2.1) but not for women with high grade/HR-positive, HER2-positive, or triple-negative disease. Women age >75 had increased breast cancer mortality in all subtypes.
Conclusion
With modern clinical subtyping, age under 40 remains independently associated with worse outcomes in 30 months follow-up only in HR-positive, lower grade disease.
Keywords: Breast cancer, Young women, Age, Outcomes, Subtype
Highlights
-
•
Young women present with more advanced and aggressive types of breast cancer.
-
•
Young age is not an independent prognostic factor in HER2+ breast cancer or TNBC.
-
•
Young age is independently associated with poor outcomes in HR+/lower grade disease.
1. Introduction
While 5-year breast cancer specific survival increased 74% from 1975 to 1979, and 88.5% from 2010 to 2015 in the United States using the Surveillance, Epidemiology, and End Results (SEER) database [1], a less favorable outcome has been demonstrated in younger women with breast cancer [2]. Adami et al. found that women under age 45 years had worse survival and higher annual hazard of recurrence compared with women diagnosed ages 45–49 [3]. Since that time, hormonal, cytotoxic and targeted therapies [4,5] have improved survival for women with breast cancer [1]. However, more recent data suggest patients aged <40 years continue to have significantly inferior overall and breast cancer-specific survival (BCSS) compared to middle-aged women [6].
Breast cancer is a heterogenous disease that can be divided into several intrinsic molecular subtypes with different clinical and prognostic characteristics. Sorlie and Perou classified breast cancer carcinomas based on variations in gene expression patterns in 2001 [7], allowing breast cancer to be classified into intrinsic subtypes including luminal A, luminal B, normal breast like, human epidermal growth factor receptor 2 (HER2) positive and basal like [8]. Clinical subtypes are defined by immunohistochemistry results of estrogen receptor (ER), progesterone receptor (PR) and HER2 status, with or without additional markers. These clinical subtypes have different targeted therapies and different risks of disease recurrence and survival [[9], [10], [11]].
Improved understanding of disparities in breast cancer outcomes are critical to their mitigation. Young women are more likely to present with advanced stage breast cancer, in part because of a lack of effective screening strategies for average risk young women [12]. Breast cancer arising in younger women is also more likely to have an aggressive phenotype such as hormone receptor (HR)-negative, HER2-positive and/or high grade disease [13]. In this analysis, we examined breast cancer-specific mortality by age and subtype, with a focus on the previously documented young age-related poor survival.
2. Materials and methods
2.1. Data source and patient population
We used SEER cancer registry data to identify a cohort of patients with a first diagnosis of stage I-III unilateral breast cancer. Stage IV disease is heterogeneous, and has different entities, and we purposely excluded them given that they are treated differently from diagnosis, due to their incurable status [14]. The 18 population-based SEER cancer registries cover areas that uniformly collect information on patient demographics, tumor characteristics, initial treatment utilization, and mortality for all incident cancers. Because this study used previously collected, de-identified data, it was deemed exempt for review by the Office for Human Research Studies at the Dana-Farber Cancer Institute. Data-Use Agreement for the SEER research file was completed.
We used SEER stat (version 8.3.5) to download data from the SEER 18 registries research database, which contains data from the SEER 13 registries (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, Utah, Los Angeles, San Jose-Monterey, rural Georgia, and the Alaska Native Tumor Registry) and the registries of greater California, Kentucky, Louisiana, New Jersey, and greater Georgia. Radiation and chemotherapy treatment variables were requested in an additional data agreement.
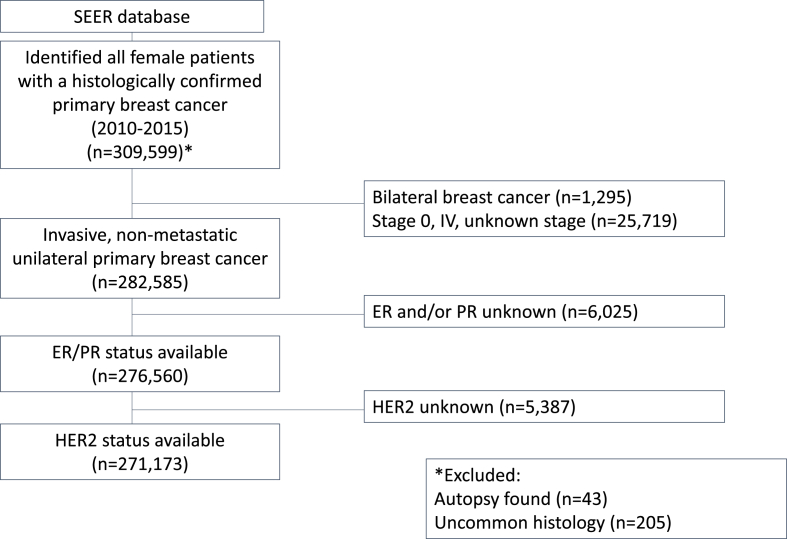
We identified 309,599 women who were diagnosed with their first stage I-III breast cancer between January 1, 2010 and December 31, 2015 who had cancer histology likely to be treated by standard guidelines and who were not diagnosed at autopsy or death. All breast cancers included were classified according to the American Joint Committee on Cancer (AJCC) 6th edition. Women with bilateral cancers (n = 1295), unknown ER and/or unknown PR (n = 6025) and unknown HER2 status (n = 5387) were excluded.
3. Outcome measures
The primary outcome was breast cancer specific death, with death due to other causes considered a competing event. Breast cancer specific survival was defined as the date of diagnosis until the date of death from any cause, or the date of censoring at the last follow-up date available of December 31, 2015. We ascertained deaths and causes of death from National Death Index data with the SEER file.
4. Independent variables
Our independent variables of interest included age, stage and clinical subtype. We first defined cohorts by age group (<40, 40–60, 61–75, >75 years) and then sub-cohorts of women by age and clinical subtypes. Clinical subtype was categorized using HR and HER2 status and grade (high grade; G3, lower grade; G2 or G1), with HR-positive defined ER- and/or PR-positive.
ER- or PR-positive included positive and borderline on immunohistochemical stain. ER- and PR-positive was defined as having any ER- and PR-positive staining. ER- and PR-negative was defined as those having no ER or PR staining. HER2 status was categorized as positive, negative, or unknown/borderline. Triple negative disease was defined as those having ER- and PR-negative and HER2-negative disease.
5. Control variables
Control variables included race/ethnicity (non-Hispanic White, non-Hispanic.
Black, Hispanic, other/unknown), Insurance (insured, Medicaid, unknown/uninsured), SEER region (Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, Atlanta/rural Georgia, California, Kentucky, Louisiana, New Jersey), tumor grade (low/intermediate, high, unknown/others), stage and treatment.
5.1. Statistical analyses
Characteristics of the cohort by age were compared by chi-square test. Inference regarding breast cancer-specific mortality was made using Fine and Gray regression models, allowing for a sub-distribution of hazards of death due to breast cancer when considering death due to other causes as a competing event. Univariate and multivariate competing regression models and wild type tests were conducted to identify independent prognostic factors and calculate the hazard ratio and 95% confidence interval (CI). All statistical analyses were conducted SAS version 9.4 (SAS Institute, Cary, NC).
6. Results
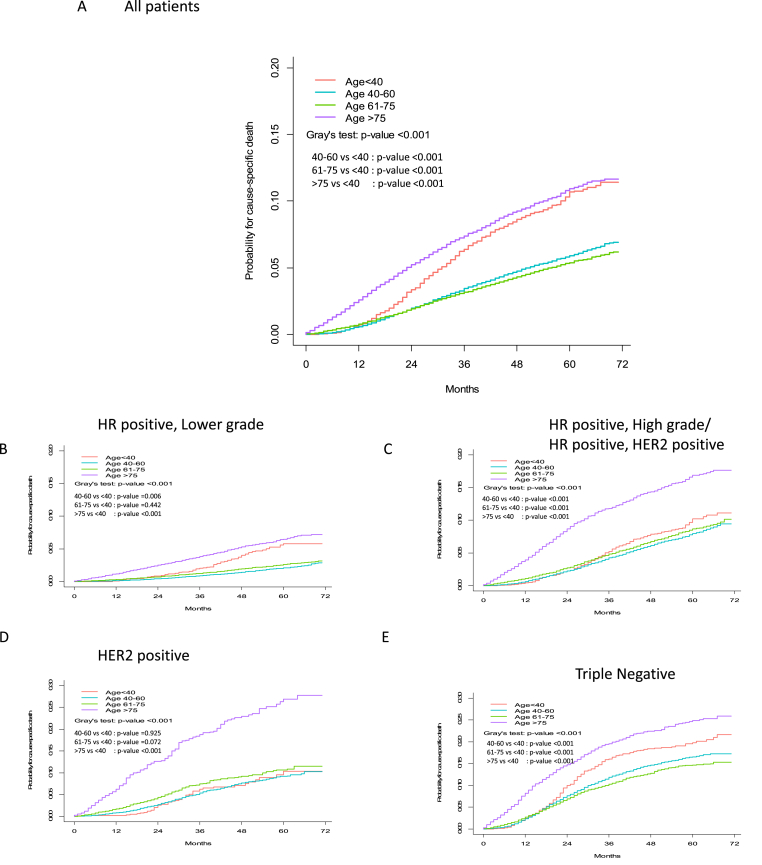
Among 271,173 women with breast cancer eligible for analysis, 14,109 were <40 years of age at diagnosis, with a mean age of 60 years old. Median follow-up time was 30 months overall. We present the cohort flow diagram in Fig. 1. Compared with older women, women age <40 years were more likely to be non-white, have Medicaid or be uninsured, with HR-negative and high-grade tumors, and to have received chemotherapy (p < 0.001) (Table 1). The risk of breast cancer-specific death was highest in women age >75 years and age <40 years (Fig. 2). In unadjusted analysis, compared with women age 40–60 years (reference), women <40 years (hazard ratio 1.8, 95% CI 1.6 to 1.9) and >75 years (hazard ratio 2.1, 95% CI 2.1 to 2.3) were more likely to die of breast cancer (Table 2). Stratifying by subtype, among women with HR-positive, lower grade disease, those <40 years of age were more than twice as likely to die of breast cancer compared with women ages 40–60 years (hazard ratio 2.5, 95% CI 2.0 to 3), unadjusted analysis. After controlling for sociodemographic, disease and treatment characteristics, the association was attenuated but women age <40 years were still more likely die of breast cancer than women age 40–60 years (Table 1, hazard ratio 1.7, 95% CI 1.4 to 2.1). In HER2-positive disease, young age was not significantly associated with mortality in unadjusted and adjusted analyses (p > 0.05, both). In triple-negative disease, age <40 years was associated with increased mortality in the unadjusted model but after controlling for tumor characteristics and treatment factors, the risk became non-significant (hazard ratio 1.1, 95% CI 1.0 to 1.2). Women age >75 years had the highest breast cancer mortality for all subtypes (Table 3).
Fig. 1.
Flow scheme of the Study.
Table 1.
Descriptive characteristics of 271,173 patients with stage I to III breast cancer according to age at diagnosis from SEER data.
| Age at diagnosis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| <40 years N = 14,109 |
40–60 years N = 122,188 |
60–75 years N = 96,837 |
>75 years N = 38,039 |
||||||
| No. | % | No. | % | No. | % | No. | % | p value | |
| Race/Ethnicity | |||||||||
| White/non-Hispanic | 7304 | 51.8 | 77100 | 63.1 | 70433 | 72.7 | 29739 | 78.2 | <0.001 |
| Hispanic | 2798 | 32.8 | 16536 | 13.5 | 8572 | 8.9 | 2618 | 6.9 | |
| Black/non-Hispanic | 2086 | 24.5 | 14596 | 11.9 | 9449 | 9.8 | 3165 | 8.3 | |
| Asian, PI/non-Hispanic | 1726 | 20.2 | 12564 | 10.3 | 7363 | 7.6 | 2234 | 5.9 | |
| Others/unknown | 195 | 2.3 | 1392 | 1.1 | 1020 | 1.1 | 283 | 0.7 | |
| Insurance | |||||||||
| Insured | 11169 | 79.2 | 102651 | 84.0 | 87602 | 90.5 | 35084 | 92.2 | <0.001 |
| Medicaid | 2440 | 17.3 | 16723 | 13.7 | 8312 | 8.6 | 2831 | 7.4 | |
| Uninsured/unknown | 500 | 3.5 | 2814 | 2.3 | 923 | 1.0 | 124 | 0.3 | |
| SEER registry | |||||||||
| California | 5840 | 41.4 | 49705 | 40.7 | 39004 | 40.3 | 15141 | 39.8 | <0.001 |
| Connecticut | 643 | 4.6 | 6368 | 5.2 | 4659 | 4.8 | 2064 | 5.4 | |
| Detroit | 651 | 4.6 | 6346 | 5.2 | 4939 | 5.1 | 2094 | 5.5 | |
| Georgia | 1830 | 13.0 | 13933 | 11.4 | 10855 | 11.2 | 3765 | 9.9 | |
| Iowa | 458 | 3.2 | 4212 | 3.4 | 3752 | 3.9 | 1850 | 4.9 | |
| Kentucky | 668 | 4.7 | 6345 | 5.2 | 5534 | 5.7 | 2006 | 5.3 | |
| Louisiana | 717 | 5.1 | 6199 | 5.1 | 5295 | 5.5 | 2051 | 5.4 | |
| New Jersey | 1518 | 10.8 | 13921 | 11.4 | 10057 | 10.4 | 4346 | 11.4 | |
| New Mexico | 238 | 1.7 | 2265 | 1.9 | 2180 | 2.3 | 842 | 2.2 | |
| Others (Hawaii, Alaska) | 272 | 1.9 | 2426 | 2.0 | 1905 | 2.0 | 706 | 1.9 | |
| Seattle | 843 | 6.0 | 7665 | 6.3 | 6363 | 6.6 | 2213 | 5.8 | |
| Utah | 431 | 3.1 | 2803 | 2.3 | 2294 | 2.4 | 961 | 2.5 | |
| Stage | |||||||||
| I | 3701 | 26.2 | 56408 | 46.2 | 54925 | 56.7 | 19902 | 52.3 | <0.001 |
| II | 7384 | 52.3 | 49022 | 40.1 | 32492 | 33.6 | 13880 | 36.5 | |
| III | 3024 | 21.4 | 16758 | 13.7 | 9420 | 9.7 | 4257 | 11.2 | |
| Histologic grade | |||||||||
| High, G3 | 7743 | 54.9 | 42543 | 34.8 | 24718 | 25.5 | 9228 | 24.3 | <0.001 |
| Low,intermediate (G1, G2) | 5757 | 40.8 | 75099 | 61.5 | 68884 | 71.1 | 27305 | 71.8 | |
| Other (G4)/unknown | 609 | 4.3 | 4546 | 3.7 | 3235 | 3.3 | 1506 | 4.0 | |
| ER status | |||||||||
| Positive∗ | 4049 | 28.7 | 99373 | 81.3 | 83281 | 86.0 | 33071 | 86.9 | <0.001 |
| Negative | 10060 | 71.3 | 22815 | 18.7 | 13556 | 14.0 | 4968 | 13.1 | |
| PR status | |||||||||
| positive∗ | 5315 | 37.7 | 88155 | 72.1 | 72651 | 75.0 | 28689 | 75.4 | <0.001 |
| negative | 8794 | 62.3 | 34033 | 27.9 | 24186 | 25.0 | 9350 | 24.6 | |
| HER2 status | |||||||||
| positive | 3540 | 25.1 | 21072 | 17.2 | 11580 | 12.0 | 3852 | 10.1 | <0.001 |
| borderlinea | 269 | 1.9 | 2594 | 2.1 | 2069 | 2.1 | 956 | 2.5 | |
| negative | 10300 | 73.0 | 98522 | 80.6 | 83188 | 85.9 | 33231 | 87.4 | |
| Molecular subtype | |||||||||
| HR+, lower grade (HR+, HER2- and G1/2) | 4483 | 31.8 | 65949 | 54.0 | 62554 | 64.6 | 25267 | 66.4 | <0.001 |
| HR+, high grade/HR + HER2+ | 5724 | 40.6 | 33323 | 27.3 | 20195 | 20.9 | 7488 | 19.7 | |
| HER2 (HR- and HER2+) | 976 | 6.9 | 6427 | 5.3 | 3437 | 3.5 | 1134 | 3.0 | |
| TN (HR- and HER2-) | 2757 | 19.5 | 14599 | 11.9 | 9105 | 9.4 | 3432 | 9.0 | |
| Unknown | 169 | 1.2 | 1890 | 1.5 | 1546 | 1.6 | 718 | 1.9 | |
| Chemotherapy | |||||||||
| Yes | 11252 | 79.8 | 65411 | 53.5 | 31884 | 32.9 | 3717 | 9.8 | <0.001 |
| No | 2857 | 20.2 | 56777 | 46.5 | 64953 | 67.1 | 34322 | 90.2 | |
| Radiotherapy | |||||||||
| Yes | 6553 | 46.4 | 65333 | 53.5 | 55515 | 57.3 | 14036 | 36.9 | <0.001 |
| No | 7556 | 53.6 | 56855 | 46.5 | 41322 | 42.7 | 24003 | 63.1 | |
| Surgery | |||||||||
| Yes | 13326 | 94.5 | 117738 | 96.4 | 93804 | 96.9 | 34689 | 91.2 | <0.001 |
| No | 783 | 5.5 | 4450 | 3.6 | 3033 | 3.1 | 3350 | 8.8 | |
Abbreviation: HER2, human epidermal growth factor receptor 2.
HR+, low grade: ER positive and/or PR positive, HER2 negative/low grade HR+, high grade/HR+, HER2+: ER positive and/or PR positive, HER2 negative/high grade, or ER positive and/or PR positive, HER2 positive.
HER2: ER negative, PR negative, and HER2 positive.
∗TN(Triple negative): ER negative, PR negative, and HER2 negative.
∗Borderline was included as positive.
The result of IHC, FISH, SISH etc.
Fig. 2.
Cumulative incidence functions for cancer specific death according to age group in: A) all patients, B) HR positive, lower grade, C) HR positive, high grade/HR positive, HER2 positive, D) HER2 positive, E) Triple negative from SEER data.
Table 2.
Age and breast cancer mortality using SEER registry database.
| Age | No. of breast cancers | HR (95% CI)a | HR (95% CI)b | HR (95% CI)c | HR (95% CI)d |
|---|---|---|---|---|---|
| <40 years | 14109 | 1.8 (1.6–1.9) | 1.7 (1.53–1.79) | 1.1 (1.04–1.22) | 1.1 (1.04–1.22) |
| 40–60 years | 122188 | 1.0 (REF) | 1.0 (REF) | 1.0 (REF) | 1.0 (REF) |
| 60–75 years | 96837 | 0.9 (0.9–1.0) | 1 (1.0–1.1) | 1.3 (1.2–1.4) | 1.3 (1.2–1.3) |
| >75 years | 38039 | 2.2 (2.1–2.3) | 2.4 (2.3–2.5) | 3.1 (3.0–3.3) | 2.5 (2.4–2.7) |
Abbreviations: CI, confidence interval; HR, hazard ratio; REF, reference.
unadjusted.
Adjusted for race/ethnicity, insurance, SEER registry.
Adjusted for race/ethnicity, insurance, SEER registry, Stage at diagnosis, subtype.
Adjusted for race/ethnicity, insurance, SEER registry, Stage at diagnosis, subtype, treatment.
Table 3.
Univariate and Multivariate analysis of age and breast cancer mortality according to breast cancer clinical subtype from SEER data.
| Breast cancer clinical subtype and age | No. of Breast Cancers | sHR (95% CI)a | sHR (95% CI)b | sHR (95% CI)c | sHR (95% CI)d |
|---|---|---|---|---|---|
| HR+, lower grade (HR+, HER2- and G1/2) | 2.45 (1.99–3.01) | 2.29 (1.86–2.82) | 1.71 (1.39–2.1) | 1.73 (1.4–2.13) | |
| <40 years | 4483 | 1.0 (REF) | 1.0 (REF) | 1.0 (REF) | 1.0 (REF) |
| 40–60 years | 65949 | 1.34 (1.21–1.49) | 1.4 (1.26–1.55) | 1.68 (1.51–1.86) | 1.67 (1.5–1.85) |
| 60–75 years | 45802 | 3.79 (3.42–4.19) | 4.04 (3.65–4.47) | 4.67 (4.22–5.17) | 3.79 (3.37–4.26) |
| >75 years | 42019 | ||||
| HR+, high grade/HR + HER2+ | 1.2 (1.05–1.37) | 1.16 (1.02–1.32) | 1.02 (0.89–1.16) | 1.03 (0.91–1.18) | |
| <40 years | 5724 | 1.0 (REF) | 1.0 (REF) | 1.0 (REF) | 1.0 (REF) |
| 40–60 years | 33323 | 1.14 (1.04–1.24) | 1.2 (1.1–1.31) | 1.33 (1.22–1.45) | 1.26 (1.16–1.38) |
| 60–75 years | 20195 | 2.73 (2.5–2.99) | 2.97 (2.71–3.26) | 3.11 (2.84–3.42) | 2.3 (2.06–2.56) |
| >75 years | 7488 | ||||
| HER2 (HR- and HER2+) | 0.98 (0.72–1.34) | 0.97 (0.71–1.33) | 0.8 (0.58–1.1) | 0.84 (0.61–1.16) | |
| <40 years | 976 | 1.0 (REF) | 1.0 (REF) | 1.0 (REF) | 1.0 (REF) |
| 40–60 years | 6427 | 1.32 (1.1–1.58) | 1.4 (1.17–1.68) | 1.52 (1.27–1.82) | 1.43 (1.19–1.71) |
| 60–75 years | 3437 | 3.75 (3.11–4.52) | 4.06 (3.35–4.91) | 4.15 (3.42–5.04) | 2.89 (2.35–3.55) |
| >75 years | 1134 | ||||
| TN (HR- and HER2-) | 1.27 (1.13–1.43) | 1.24 (1.1–1.4) | 1.1 (0.98–1.24) | 1.09 (0.96–1.23) | |
| <40 years | 2757 | 1.0 (REF) | 1.0 (REF) | 1.0 (REF) | 1.0 (REF) |
| 40–60 years | 14599 | 0.9 (0.82–0.98) | 0.95 (0.87–1.04) | 1.06 (0.97–1.16) | 1.03 (0.94–1.13) |
| 60–75 years | 9105 | 1.81 (1.64–2) | 2.01 (1.82–2.22) | 2.08 (1.88–2.3) | 1.81 (1.61–2.03) |
| >75 years | 3432 | 2.45 (1.99–3.01) | 2.29 (1.86–2.82) | 1.71 (1.39–2.1) | 1.73 (1.4–2.13) |
Abbreviations: HER2, human epidermal growth factor receptor 2; HR, hormone receptor; REF, reference; sHR, subdistribution hazard ratio; TN, triple-negative; +, positive; -, negative.
Unadjusted.
Adjusted for race/ethnicity, insurance, SEER registry.
Adjusted for race/ethnicity, insurance, SEER registry, Stage at diagnosis, subtype.
Adjusted for race/ethnicity, insurance, SEER registry, Stage at diagnosis, subtype, treatment.
7. Discussion
In this modern dataset representative of the U.S. population, young women with breast cancer presented with more advanced and aggressive types of breast cancer and had higher breast cancer-specific mortality compared with women ages 40–60 years, particularly in HR-positive, low grade breast cancer. Importantly, young age was not independently associated with lower survival in other tumor subtypes.
This analysis also provides additional evidence that among women with triple-negative disease or HER2-positive disease, regardless of HR status, there is no increased risk of mortality among women <40 years of age, which confirms and expands on prior research in this area [2,10]. Prior analysis in the setting of HER2-positive disease demonstrated that young age was neither prognostic nor predictive among women treated with chemotherapy, whether followed by trastuzumab or not [2]. Partridge et al. [10] also published similar data in 2016 using the National Comprehensive Cancer Network (NCCN) database, concluding that age does not seem to be an independent predictor of outcome in HER2-positive breast cancer. Modern systemic therapy with targeted treatment in patients with HER2 disease improves survival in young women, and treatment principles should be similar, regardless of age, in women with HER2-positive disease.
In the setting of triple-negative disease, the risk of recurrence also seems to be worse in younger women compared with other age groups, but when controlling for tumor factors and treatment factors, breast cancer mortality seems to be similar with middle-aged women. In this report, we confirmed that in women with HER2 or triple-negative breast cancer, there was no clear increased risk of breast cancer mortality among women <40 years of age compared with middle-aged women. The study using a Korean national database showed that even women in their 20s with breast cancer have worse survival in luminal subtype, but no survival differences were observed in HER2 and triple negative subtype compared with women in their 30s and 40s [15].
Different tumor or host biology [[16], [17], [18]], lower hormonal therapy effectiveness [19], early restoration of ovarian function after chemotherapy [20,21], and decreased adherence to hormonal therapy [22], likely contribute to this disparity and further research to address differences is warranted. Even in patients untreated with adjuvant therapy [16] or treated with more aggressive adjuvant therapy [23], survival in young women was worse only in patients with luminal subtypes and not in those with other subtypes. Breast cancer arising at a young age seems to be biologically distinct beyond subtype distribution [24]. Azim and colleagues showed that independent of subtype, grade and stage, younger patients have higher expression of RANK-ligand, c-kit, mammary stem cell and luminal progenitors and BRCA 1 mutation signatures [24]. In addition, several recent studies have reported somatic mutations in breast cancer using next generation sequencing, including point mutations in TP53 and PIK3CA genes [[25], [26], [27]]. Accumulating evidence also suggests differences in breast stroma in younger patients, and changes that occur with pregnancy and breastfeeding likely contribute to the different biology of tumors arising thereafter. However, data are still insufficient to conclude that such effects play a fundamental role in carcinogenesis and tumor biology [16]. Somatic gene alteration of young tumors versus older may be different, and especially somatic mutation of TP53 [28] and GATA3 [29] have been associated with an early age at presentation of breast cancer. GATA3 directly upregulates proto-oncogenes and ER, suggesting that it may promote tumorigenesis in luminal subtypes of cancer [30]. Mutations in GATA3 affect ER binding to DNA and modulate the response of tumor cells to estrogen signaling, which might be associated with endocrine resistance and tumor growth [31,32]. These results may have clinical relevance, since the adverse prognosis associated with younger age at diagnosis has been observed mainly in patients with ER-positive breast cancer [10,33].
Neugut et al. found that patients with breast cancer who were younger than 45 years of age had an odds ratio of 2.0 of non-adherence to oral endocrine therapy compared with women 55–64 years of age in a large medical and pharmacy insurance claims database [34]. Several observational studies reported that younger age is associated with lower rates of treatment compliance with endocrine therapy, possibly suggesting the level of toxicity, especially sexual toxicity, is less acceptable to women younger than 35 years of age [[35], [36], [37], [38]]. Rosenberg and colleagues reported that the experience of side effects, feeling less informed, and negative emotions about endocrine therapy are the main reasons for non-adherence [39], and attention to symptom management on endocrine therapy may reduce symptom burden and improve quality of life, potentially improving endocrine therapy adherence [40]. Hopefully, increased use of ovarian function suppression among young women with higher risk disease will further improve survival in HR-positive disease. Continued research efforts are focused on making anti-hormonal treatment more effective and tolerable, although the present analysis is limited by a lack of information regarding the use of this strategy in the population [41,42].
Interestingly, while women <40 and those >75 years of age received less radiotherapy, there was no information regarding types of surgery. Gu et al. reported that young and old age were associated with increased likelihood of mastectomy [43]. Fear of recurrence and the increasing rate of contralateral prophylactic mastectomy seems to support more mastectomy in young age groups [44,45]. Women of older age may choose mastectomy for a more expedient treatment, avoiding radiation and placing less value on cosmetic outcomes as age increases [43].
This study should be considered in the context of its limitations. First, 30-month follow up is a relatively short period to access survival differences, particularly HR-positive disease, and longer term survival outcomes are worth observing. Second, we grouped HER2+, ER + disease with high grade HER2-, ER + disease as luminal B-like, as has been done in previous studies and in light of the limitation of not having the ability to assess the use of anti-HER2 therapy in this dataset. Third, we classified grades 1 and 2 as lower grade disease. Sotiriou et al. showed that grade 2 is a heterogeneous group according to gene expression, therefore, HR-positive, lower grade disease may include genetic high risk patients [46]. Fourth, for the analysis of survival, cause-of-death information in the SEER database was used, which has limitations with regard to reliability and completeness. And finally, adherence with adjuvant therapy could not be adjusted for in the analysis and may impact outcomes.
8. Conclusions
Nevertheless, the strengths of this study include the population-based, national sample of women with breast cancer with modern clinical subtyping, including tumor HER2 status. This study supports and expands upon the growing evidence from North America [10], Europe [33], and Asia [19,47] that young age remains an independent prognostic factor in HR-positive/lower grade subtype breast cancer, and further research to understand and improve the outcomes of this vulnerable population is imperative.
Prior presentations
This study was presented in part during the AACR outstanding investigator award lecture at the 2018 San Antonio Breast Cancer Symposium.
Data availability statement
All data are available publicly through SEER.
Funding
The work was supported by Susan G. Komen (AHP) and Breast Cancer Research Foundation (AHP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
Conceptualization: HJK SK, RAF, AHP. Formal analysis: SK. Writing – original draft: HJK, SK, RAF, AHP. Writing – review & editing: HJK SK, RAF, AHP.
Declaration of competing interest
None.
Acknowledgments
We greatly appreciate the support of Kate Bifolck, BA (editor, full-time employee of Dana-Farber Cancer Institute) in formatting and submitting this manuscript.
References
- 1.Guo F., Kuo Y.F., Shih Y.C.T., Giordano S.H., Berenson A.B. Trends in breast cancer mortality by stage at diagnosis among young women in the United States. Cancer. Sep 1 2018;124(17):3500–3509. doi: 10.1002/cncr.31638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Partridge A.H., Gelber S., Piccart-Gebhart M.J., et al. Effect of age on breast cancer outcomes in women with human epidermal growth factor receptor 2-positive breast cancer: results from a herceptin adjuvant trial. J Clin Oncol: official journal of the American Society of Clinical Oncology. Jul 20 2013;31(21):2692–2698. doi: 10.1200/jco.2012.44.1956. [DOI] [PubMed] [Google Scholar]
- 3.Adami H.O., Malker B., Holmberg L., Persson I., Stone B. The relation between survival and age at diagnosis in breast cancer. N Engl J Med. Aug 28 1986;315(9):559–563. doi: 10.1056/nejm198608283150906. [DOI] [PubMed] [Google Scholar]
- 4.Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. N Engl J Med. Dec 29 1988;319(26):1681–1692. doi: 10.1056/nejm198812293192601. [DOI] [PubMed] [Google Scholar]
- 5.Sainsbury R. The development of endocrine therapy for women with breast cancer. Cancer Treat Rev. Aug 2013;39(5):507–517. doi: 10.1016/j.ctrv.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Chen H.L., Zhou M.Q., Tian W., Meng K.X., He H.F. Effect of age on breast cancer patient prognoses: a population-based study using the SEER 18 database. PLoS One. 2016;11(10) doi: 10.1371/journal.pone.0165409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorlie T., Perou C.M., Tibshirani R., et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. Sep 11 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldhirsch A., Winer E.P., Coates A.S., et al. Personalizing the treatment of women with early breast cancer: highlights of the st gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol. Sep 2013;24(9):2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arvold N.D., Taghian A.G., Niemierko A., et al. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol: official journal of the American Society of Clinical Oncology. Oct 10 2011;29(29):3885–3891. doi: 10.1200/jco.2011.36.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Partridge A.H., Hughes M.E., Warner E.T., et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol: official journal of the American Society of Clinical Oncology. Sep 20 2016;34(27):3308–3314. doi: 10.1200/jco.2015.65.8013. [DOI] [PubMed] [Google Scholar]
- 11.Engstrom M.J., Opdahl S., Hagen A.I., et al. Molecular subtypes, histopathological grade and survival in a historic cohort of breast cancer patients. Breast cancer research and treatment. Aug 2013;140(3):463–473. doi: 10.1007/s10549-013-2647-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruddy K.J., Gelber S., Tamimi R.M., et al. Breast cancer presentation and diagnostic delays in young women. Cancer. Jan 1 2014;120(1):20–25. doi: 10.1002/cncr.28287. [DOI] [PubMed] [Google Scholar]
- 13.Keegan T.H., DeRouen M.C., Press D.J., Kurian A.W., Clarke C.A. Occurrence of breast cancer subtypes in adolescent and young adult women. Breast Cancer Res. Mar 27 2012;14(2):R55. doi: 10.1186/bcr3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen M.T., Sun H.F., Zhao Y., Fu W.Y., Yang L.P., Gao S.P., Li L.D., Jiang H.L., Jin W. Comparison of patterns and prognosis among distant metastatic breast cancer patients by age groups: a SEER population-based analysis. Sci Rep. 2017 Aug 23;7(1):9254. doi: 10.1038/s41598-017-10166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryu J.M., Yu J., Kim S.I., et al. Different prognosis of young breast cancer patients in their 20s and 30s depending on subtype: a nationwide study from the Korean Breast Cancer Society. Dec 2017;166(3):833–842. doi: 10.1007/s10549-017-4472-5. [DOI] [PubMed] [Google Scholar]
- 16.Azim H.A., Jr., Brohee S., Peccatori F.A., et al. Biology of breast cancer during pregnancy using genomic profiling. Endocr Relat Cancer. Aug 2014;21(4):545–554. doi: 10.1530/erc-14-0111. [DOI] [PubMed] [Google Scholar]
- 17.Paluch-Shimon S., Pagani O., Partridge A.H., et al. ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3) Breast. Oct 2017;35:203–217. doi: 10.1016/j.breast.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Gómez-Flores-Ramos L., Castro-Sánchez A., Peña-Curiel O., Mohar-Betancourt A. Molecular biology in young women with breast cancer: from tumor gene expression to DNA mutations. Revista de investigacion clinica; organo del Hospital de Enfermedades de la Nutricion. Jul-Aug 2017;69(4):181–192. doi: 10.24875/ric.17002225. [DOI] [PubMed] [Google Scholar]
- 19.Ahn S.H., Son B.H., Kim S.W., et al. Poor outcome of hormone receptor–positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in korea—a report from the Korean breast cancer society. J Clin Oncol. 2007;25(17):2360–2368. doi: 10.1200/jco.2006.10.3754. [DOI] [PubMed] [Google Scholar]
- 20.Walshe J.M., Denduluri N., Swain S.M. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol: official journal of the American Society of Clinical Oncology. Dec 20 2006;24(36):5769–5779. doi: 10.1200/jco.2006.07.2793. [DOI] [PubMed] [Google Scholar]
- 21.Swain S.M., Jeong J.H., Geyer C.E., Jr., et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. Jun 3 2010;362(22):2053–2065. doi: 10.1056/NEJMoa0909638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagani O., Partridge A., Korde L., et al. Pregnancy after breast cancer: if you wish, ma'am. Breast Cancer Res Treat. Sep 2011;129(2):309–317. doi: 10.1007/s10549-011-1643-7. [DOI] [PubMed] [Google Scholar]
- 23.Fredholm H., Eaker S., Frisell J., Holmberg L., Fredriksson I., Lindman H. Breast cancer in young women: poor survival despite intensive treatment. PLoS One. Nov 11 2009;4(11) doi: 10.1371/journal.pone.0007695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azim H.A., Jr., Partridge A.H. Biology of breast cancer in young women. Breast Cancer Res. Aug 27 2014;16(4):427. doi: 10.1186/s13058-014-0427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephens P.J., Tarpey P.S., Davies H., et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. May 16 2012;486(7403):400–404. doi: 10.1038/nature11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah S.P., Roth A., Goya R., et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. Apr 4 2012;486(7403):395–399. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nik-Zainal S., Alexandrov L.B., Wedge D.C., et al. Mutational processes molding the genomes of 21 breast cancers. Cell. May 25 2012;149(5):979–993. doi: 10.1016/j.cell.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Encinas G., Maistro S., Pasini F.S., et al. Somatic mutations in breast and serous ovarian cancer young patients: a systematic review and meta-analysis. Rev Assoc Med Bras. Sep-Oct 2015;61(5):474–483. doi: 10.1590/1806-9282.61.05.474. 1992. [DOI] [PubMed] [Google Scholar]
- 29.Azim H.A., Jr., Nguyen B., Brohee S., Zoppoli G., Sotiriou C. Genomic aberrations in young and elderly breast cancer patients. BMC Med. Oct 15 2015;13:266. doi: 10.1186/s12916-015-0504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen H., Ben-Hamo R., Gidoni M., et al. Shift in GATA3 functions, and GATA3 mutations, control progression and clinical presentation in breast cancer. Breast Cancer Res. Nov 20 2014;16(6):464. doi: 10.1186/s13058-014-0464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adomas A.B., Grimm S.A., Malone C., Takaku M., Sims J.K., Wade P.A. Breast tumor specific mutation in GATA3 affects physiological mechanisms regulating transcription factor turnover. BMC Cancer. Apr 22 2014;14:278. doi: 10.1186/1471-2407-14-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chou J., Provot S., Werb Z. GATA3 in development and cancer differentiation: cells GATA have it. J Cell Physiol. Jan 2010;222(1):42–49. doi: 10.1002/jcp.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johansson A.L.V., Trewin C.B., Hjerkind K.V., Ellingjord-Dale M., Johannesen T.B., Ursin G. Breast cancer-specific survival by clinical subtype after 7 years follow-up of young and elderly women in a nationwide cohort. Int J Cancer. Oct 26 2018 doi: 10.1002/ijc.31950. [DOI] [PubMed] [Google Scholar]
- 34.Neugut A.I., Zhong X., Wright J.D., Accordino M., Yang J., Hershman D.L. Nonadherence to medications for chronic conditions and nonadherence to adjuvant hormonal therapy in women with breast cancer. JAMA Oncol. 2016;2(10):1326–1332. doi: 10.1001/jamaoncol.2016.1291. [DOI] [PubMed] [Google Scholar]
- 35.Hershman D.L., Kushi L.H., Shao T., et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. Sep 20 2010;28(27):4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hershman D.L., Shao T., Kushi L.H., et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. Apr 2011;126(2):529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Partridge A.H., Wang P.S., Winer E.P., Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol: official journal of the American Society of Clinical Oncology. Feb 15 2003;21(4):602–606. doi: 10.1200/jco.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 38.Huiart L., Ferdynus C., Giorgi R. A meta-regression analysis of the available data on adherence to adjuvant hormonal therapy in breast cancer: summarizing the data for clinicians. Breast Cancer Res Treat. Feb 2013;138(1):325–328. doi: 10.1007/s10549-013-2422-4. [DOI] [PubMed] [Google Scholar]
- 39.Walker H.E., Rosenberg S.M., Stanton A.L., Petrie K.J., Partridge A.H. Perceptions, attributions, and emotions toward endocrine therapy in young women with breast cancer. J Adolesc Young Adult Oncol. Mar 2016;5(1):16–23. doi: 10.1089/jayao.2015.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenberg S.M., Stanton A.L., Petrie K.J., Partridge A.H. Symptoms and symptom attribution among women on endocrine therapy for breast cancer. Oncol. Jun 2015;20(6):598–604. doi: 10.1634/theoncologist.2015-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoon T.I., Hwang U.K., Kim E.T., et al. Survival improvement in hormone-responsive young breast cancer patients with endocrine therapy. Breast Cancer Res Treat. Sep 2017;165(2):311–320. doi: 10.1007/s10549-017-4331-4. [DOI] [PubMed] [Google Scholar]
- 42.Francis P.A., Pagani O., Fleming G.F., et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. Jul 12 2018;379(2):122–137. doi: 10.1056/NEJMoa1803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gu J., Groot G., Boden C., Busch A., Holtslander L., Lim H. Review of factors influencing women's choice of mastectomy versus breast conserving therapy in early stage breast cancer: a systematic review. Clin Breast Cancer. Aug 2018;18(4):e539–e554. doi: 10.1016/j.clbc.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 44.Tuttle T.M., Abbott A., Arrington A., Rueth N. The increasing use of prophylactic mastectomy in the prevention of breast cancer. Curr Oncol Rep. 2010;12(1):16–21. doi: 10.1007/s11912-009-0070-y. 2010/01/01. [DOI] [PubMed] [Google Scholar]
- 45.Tuttle T.M., Habermann E.B., Grund E.H., Morris T.J., Virnig B.A. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol: official journal of the American Society of Clinical Oncology. Nov 20 2007;25(33):5203–5209. doi: 10.1200/jco.2007.12.3141. [DOI] [PubMed] [Google Scholar]
- 46.Sotiriou C., Wirapati P., Loi S., et al. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst: J Natl Cancer Inst. 2006;98(4):262–272. doi: 10.1093/jnci/djj052. [DOI] [PubMed] [Google Scholar]
- 47.Tang L.C., Yin W.J., Di G.H., Shen Z.Z., Shao Z.M. Unfavourable clinicopathologic features and low response rate to systemic adjuvant therapy: results with regard to poor survival in young Chinese breast cancer patients. Breast Cancer Res Treat. Jul 2010;122(1):95–104. doi: 10.1007/s10549-009-0537-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available publicly through SEER.