Summary
Background
Dyskeratosis congenita (DC) is a telomere biology disorder associated with high rates of bone marrow failure (BMF) and other medical complications. Oral androgens are successfully used to treat BMF in DC but often have significant side effects, including elevation of serum lipids. This study sought to determine the extent to which oral androgen therapy altered lipid and lipoprotein levels.
Methods
Nuclear magnetic resonance (NMR) was used to evaluate serum lipid profiles, and lipoprotein particle number and size in nine androgen-treated individuals with DC, 45 untreated individuals with DC, 72 unaffected relatives of DC patients, and 19 untreated individuals with a different inherited BMF syndrome, Fanconi anaemia (FA).
Findings
Androgen-treated individuals with DC had significantly decreased serum HDL cholesterol, HDL particle number and HDL particle size (p < 0·001, p < 0·001 and p < 0·001, respectively); significantly increased serum LDL cholesterol and LDL particle number (p < 0·001, p < 0·001, respectively), decreased apoA-I and increased apoB (p < 0⋅001, p < 0⋅05 respectively) when compared with untreated individuals with DC. There were no significant lipid profile differences between untreated DC and untreated FA participants; or between untreated DC participants and their unaffected relatives. Branched chain amino acids and lipoprotein insulin resistance were not significantly different with androgen treatment. GlycA, an inflammatory acute phase reactant, was significantly increased with androgen treatment (p < 0⋅001).
Interpretation
Androgen treatment in DC creates an atherogenic lipoprotein profile, raising concern for the potential of elevated cardiovascular disease risk. Clinical guidelines for individuals on androgens for DC-related BMF should include cardiovascular disease monitoring. These findings could be relevant in individuals treated with androgen for other indications.
Funding
Intramural research programs of the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and National Heart, Lung, and Blood Institute.
Keywords: Dyskeratosis congenita, Telomere, Bone marrow failure, Androgen, Lipoprotein particle, Lipid, Cholesterol, HDL, LDL, NMR, Lipoprotein
Research in context.
Evidence before this study
We searched PubMed for articles published from inception to 2021, using keywords “dyskeratosis congenita”, “lipoprotein particles”, “NMR”, “HDL”, “LDL” and “androgen”, with no language restrictions. Existing evidence at the time showed that oral androgens are successfully used to treat bone marrow failure (BMF) in dyskeratosis congenita (DC) but often have side effects, including elevated serum lipid levels.
Added value of this study
To the best of our knowledge, our study is the first to comprehensively evaluate serum lipid and lipoprotein particles in androgen-treated patients with DC and to show significant androgen-induced alterations in HDL and LDL particles. This is also the first time that branched chain amino acids (BCAAs), lipoprotein insulin resistance index (LP-IR), and GlycA have been investigated as potential biomarkers of disease and androgen effects in DC. Androgen treatment resulted in significantly decreased HDL cholesterol, HDL particle number and size, as well as increased serum LDL cholesterol and particle number when compared with untreated individuals with DC. Branched chain amino acids and LP-IR were not different between the groups, but GlycA was significantly increased with the use of oral androgens.
Implications of all the available evidence
Our study provides convincing evidence that androgen treatment for DC-associated BMF creates an atherogenic lipoprotein profile, raising the concern for cardiovascular disease risk. As management strategies and survival of patients with DC continues to improve, it is essential to carefully follow androgen-treated patients for cardiovascular disease related to abnormal lipid profiles. Additional studies are needed in individuals treated with androgens without DC to evaluate the full extent of androgen-related changes in lipid metabolism.
Alt-text: Unlabelled box
Introduction
Dyskeratosis congenita (DC) is a telomere biology disorder (TBD) and inherited bone marrow failure syndrome (IBMFS). In addition to the classic triad of nail dystrophy, reticular skin pigmentation, and oral leucoplakia, individuals with DC are at a high risk of a number of medical problems including bone marrow failure (BMF), pulmonary fibrosis, pulmonary and gastrointestinal vascular malformations, liver disease, haematological malignancies, solid malignancies, and other complications.1,2 Pathogenic variants in at least 15 different telomere biology genes with X-linked recessive, autosomal recessive and autosomal dominant inheritance, or de novo occurrence are reported to cause DC and the related TBDs (DC/TBD).1,3 Very short lymphocyte telomere length measured by flow cytometry with fluorescence in situ hybridization is diagnostic of DC.4
Hematopoietic cell transplantation (HCT) is the only curative therapeutic option for DC-related BMF but may not be available for those who lack a suitable donor or have other comorbidities. 5 Therefore, androgen therapy may be a preferred option instead of using HCT as a first therapeutic intervention for marrow failure. Oral androgens have been used for treatment of BMF in DC with up to 70% of patients showing blood count improvements leading to transfusion-independence.6, 7, 8 The side effects of androgens can be significant and include liver enzyme abnormalities, liver adenomas, pre-pubertal growth spurt, splenic peliosis, virilization, and serum lipid abnormalities. The long-term clinical consequences of these side effects in patients with DC are unknown.6,8
Serum lipid abnormalities due to anabolic steroid use have been described in variety of conditions such as endometriosis, hereditary angioedema, fibrocystic breast disease, and in weightlifters.9,10 Anabolic steroid use is associated with development of an atherogenic lipid profile, which includes a profound reduction in serum high-density lipoprotein C (HDL-C, also known as “good cholesterol” in layman terms) as well as by variable increases in serum low-density lipoprotein C (LDL-C, “bad cholesterol”) and total cholesterol (TC).9,10 The only study to date of serum lipids in DC showed significantly lower HDL-C, elevated LDL-C and TC in participants with DC on androgens.6
Serum lipid panels consisting of TC, HDL-C, and LDL-C are the standard initial screening tool used in cardiovascular disease (CVD) risk assessment,11,12 by measuring cholesterol content in circulating lipoprotein particles. HDL-C is well established to be inversely correlated with risk of cardiovascular disease (CVD) and LDL-C is directly correlated with CVD risk.13 The nuclear magnetic resonance (NMR) LipoProfile test measures both the number and size of serum lipoprotein particles in blood, along with standard lipid profile levels, branched chain amino acids (BCAA), GlycA and lipoprotein insulin resistance (LP-IR).14, 15, 16 The NMR-measured individual lipoprotein particle subclasses help derive the amount of cholesterol contained in the lipoproteins. HDL and LDL particle size and number (HDL-P and LDL-P) are emerging as better biomarkers of atherosclerosis compared with serum HDL-C and LDL-C and clinical indicators of CVD risk.17,18 The number of small LDL-P and small HDL-P are directly correlated with CVD and may be more sensitive in assessing CVD risk.14,17, 18, 19, 20 The essential BCAAs (valine, isoleucine, and leucine) are utilized as sources of muscle energy and, along with LP-IR, are biomarkers of insulin resistance, metabolic syndrome, and diabetes.21, 22, 23, 24 GlycA is a sensitive acute phase composite marker of systemic inflammation.25
In this study, we characterized HDL-P and LDL-P size, number, and subclasses by NMR spectroscopy to better understand androgen-induced lipid changes.
Methods
Study participants
Individuals with DC and their family members were enrolled in the National Cancer Institute's (NCI) IRB-approved IBMFS study (NCT00027274).4 Individuals with Fanconi anaemia (FA) enrolled in the same study and who had not received androgen treatment were included as a comparison group because they also have BMF and elevated cancer risk. Individuals were classified as having DC if they had a germline pathogenic variant in one of the known DC genes, or if they had at least two features of the diagnostic triad (oral leucoplakia, nail dystrophy, and/or abnormal skin pigmentations) and other clinical findings consistent with a DC diagnosis.1 Unaffected DC relatives were included in this analysis only if the causative gene and variant was known in their family and they did not carry the DC-associated pathogenic variant. Detailed questionnaires were completed, biospecimens were obtained, and medical records were reviewed. The medical management of the individuals was at the discretion of their primary haematologist, with input from the NCI IBMFS study physicians as expert consultants.
Ethics committee approval
This study was conducted in accordance with the Helsinki Declaration, as revised in 2008. It was approved by the institutional review board of the National Cancer Institute, National Institutes of Health, and all participants or their legal guardians signed informed written consent.
Lipid NMR
Fasting serum samples from all individuals were frozen at -80⁰C until analysis on a 400-MHz proton Vantera Clinical Analyzer (LabCorp/LipoScience, Morristown, NC) in the Department of Laboratory Medicine, NIH Clinical Centre, Bethesda, MD. The NMR spectroscopy analysis used an advanced algorithm (LP4) to quantify lipoprotein subclasses.14,16 The LP4 algorithm is the most current NMR version which provides triglyceride-rich lipoprotein particles (TRL-Ps), high-density lipoprotein particles (HDL-Ps), and low-density lipoprotein particles (LDL-Ps). The algorithm also provides better detail of the TRL-P and HDL-P subclasses into 24 and 7 particle size subcategories, respectively. An important feature of the LP4 is the ability to locate and separate very large TRL-Ps.16
Lipoprotein classes (HDL-P, LDL-P, Triglyceride-rich lipoprotein (TRL-P) total particle size number and number of subclasses by size were measured.14,16 In addition to the lipoprotein particles, the NMR also analysed branch chain amino acids (BCAA), GlycA, LP-IR, apoA-I (major protein in HDL particles), and apoB (only protein on LDL).
Particle sizes HDL (7⋅4-12⋅0 nm), LDL (19-23 nm) and TRL (24-240 nm) were reported as nanometres (nm). HDL-P numbers (HDL-P total, large, medium, and small) were reported as micromoles per litre (µmol/L). HDL-P was further separated into seven HDL subspecies but reported as small (H1P, H2P), medium (H3P, H4P), and large (H5P, H6P, H7P) HDL-P. LDL-P numbers (LDL-P total, large, medium, and small) were reported as nanomoles per litre (nmol/L). LDL was reported as three LDL-P subclasses, small (SLDL-P), medium (MLDL-P), and large (LLDL-P).
TRL-P numbers (TRL-P total, very large, large, medium, small, very small) were reported as nmol/L. Intestinal chylomicrons were found in the very large TRL-P (VLTRLP-P). Traditional VLDL was separated into three main TRL-P subclasses designated small TRL-P (STRL-P), medium TRL-P (MTRL-P), and large TRL-P (LTRL-P). Each of these VLDL subclasses was further separated in three subspecies each designated small, medium, and large for a total of nine VLDL subspecies. For example, the LTRL-P was separated into three additional subspecies, small LTRL-P (VS-55), medium LTRL-P (VS-65), and large LTRL-P (VS-80). The number in each TRL-P parameter indicated the approximate diameter in nm. IDL, known as very small TRL-P (VSTRL-P), was separated into two additional subspecies, small VSTRL-P (VS-27) and large VSTRL-P (VS-28). The number in each subspecies parameter indicated the approximate diameter in nm.
Total cholesterol, HDL-C, LDL-C, TG, apoA-I and apoB were NMR-derived numbers reported as milligrams per decilitre (mg/dL).14
Statistical analysis
All statistical analyses were non-parametric. We used Mann-Whitney U test for pairwise analyses, P < 0·05 was considered significant. Multiple linear regression was performed to account for age at blood drawn and sex as confounders in this study. Analyses were performed with Microsoft Excel, SPSS (Armonk, New York) and Python 3 (version 3⋅8⋅3). Data were presented as median and ranges.
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of this report.
Results
Study subject characteristics
We analysed data from 53 individuals with DC (45 untreated and nine androgen-treated, one of whom had pre-and post-androgen data), 72 unaffected DC relatives, and 19 individuals with FA who were never treated with androgens. All participants were started on androgens for severe cytopenia(s). Tables 1 and 2 detail the demographics and type of androgen, respectively. The median age at sample collection for androgen-treated DC group was 17 years (range 9-60 years), 25 years (range 3-69 years) in the untreated group, 41 years (range 3-70 years) in the unaffected DC relatives, and 18 years (range 6-65 years) for untreated patients with FA (Table 1). As anticipated, the unaffected relatives were older than the untreated DC, treated DC and untreated FA cohorts. Forty-eight (90%) of the individuals with DC had a known germline pathogenic variant in one of the causative genes (five DKC1, five TINF2, nine TERC, nine TERT, two WRAP53, twelve RTEL1, four PARN, and two CTC1) (Table 1, Supplemental Table 2).
Table 1.
Characteristics of study subjects.
| Diagnosis | Androgen-Treated DC (n = 9) | Untreated DC (n = 45) | Unaffected DC Relative (n = 72) | FA (n = 19) |
|---|---|---|---|---|
| Male: Female | 6:3 | 3:2 | 34:38 | 8:11 |
| Median age, years (range) | 17 (9-60) |
25 (3-69) |
41 (3-70) |
18 (6-65) |
| Genetics | ||||
| DKC1 | 1 | 4 | ||
| TINF2 | 1 | 4 | ||
| TERC | 2 | 7 | ||
| TERT | 0 | 9 | ||
| WRAP53 | 1 | 1 | ||
| RTEL1* | 2 | 10 | ||
| PARN+ | 1 | 3 | ||
| CTC1 | 0 | 2 | ||
| Unknown | 1 | 5 | ||
Five RTEL1 patients were homozygotes/biallelic and 6 were heterozygotes
All 4 PARN patients were heterozygotes
One person had pre- and post- treatment data
Table 2.
Androgen dose and treatment duration in study subjects.
| Individual | Causative Gene | Androgen | Dose | Duration on Androgens |
|---|---|---|---|---|
| NCI-6 | TERC | Halotestin | 10mg/day | 15 years |
| NCI-74 | TINF2 | Anadrol | 150mg/day | 9 months |
| NCI-165 | PARN | Anadrol | 25-50mg/day | 5 years |
| NCI-180 | RTEL1 | Anadrol | 50mg/day | 20 months |
| NCI-202 | WRAP53 | Anadrol | 100mg/day for 2 weeks then 50mg/day | 22 months |
| NCI-297 | RTEL1 | Anadrol | 75mg/day | 1 year |
| NCI-350 | TERC | Danazol | 800mg/day | 5 months |
| NCI-397 | DKC1 | Danazol | 400mg/day | 2 years |
| NCI-474 | Unknown | Danazol | 200mg/day | 2 years |
Serum lipid profiles in untreated DC, unaffected DC relatives, and FA groups
Non-parametric analyses showed that HDL-C in untreated DC individuals was higher in the unaffected DC relatives (54 vs 48 mg/dL respectively, p < 0·001, Mann-Whitney U test). LDL-C was lower in the untreated DC group (93 vs 116 mg/dL respectively, p < 0·001, Mann-Whitney U test) (Table 3). TC (171 vs 189 mg/dL respectively, p < 0·05, Mann-Whitney U test) and triglyceride (TG) (58 vs 79 mg/dL respectively, p < 0·001, Mann-Whitney U test) levels were lower in untreated DC group compared with the unaffected relatives. Additionally, apoA-I was higher in the untreated DC group (137 vs 127 mg/dL respectively, p < 0·05, Mann-Whitney U test), while apoB was lower (69 vs 88 mg/dL respectively, p < 0.001, Mann-Whitney U test). Multiple linear regression accounting for age and sex as confounders showed that TC, LDL-C and TG were not significantly different between untreated DC and unaffected relatives, showing that the observed differences were likely due to differences in age and sex.
Table 3.
Characteristics of serum lipids, apolipoproteins, and nuclear magnetic resonance spectroscopy-derived lipoprotein particles.
| Parameter | Reference Range (5th–95th %ile)† | Untreated DC (n = 45) median (range) | Androgen-treated DC (n = 9) median (range) | Untreated FA (n = 19) median (range) | Unaffected relatives (n = 72) median (range) | p-value* | p-value⁎⁎ | p-value+ |
|---|---|---|---|---|---|---|---|---|
| Lipids and apolipoproteins (mg/dL) | ||||||||
| Total cholesterol | 140 - 256 | 171·6 (98·6 - 254·6) |
193·0 (130·0 - 316·8) |
166·1 (113·6 - 268·3) |
189·3 (83·1 - 280·3) |
0·10 | 0·48 | <0·05 |
| HDL-cholesterol | 41 - 88 | 54·1 (37·9 - 110·6) |
15·4 (8·1- 47·0) |
49·4 (35·6 - 97·7) |
48·4 (33·0 - 103·4) |
<0·001 | 0·11 | <0·001 |
| LDL-cholesterol | 63 - 163 | 93·0 (41·0 - 161·2) |
159·8 (69·0 - 256·3) |
95·1 (50·7 - 196·0) |
116 (40·0 - 191·1) |
<0·001 | 0·42 | <0·001 |
| Total triglyceride | 43 - 276 | 58·5 (19·0 - 427) |
58·3 (24·5 - 166·4) |
61·7 (27·2 - 196·4) |
79·1 (25·0 - 278·3) |
0·40 | 0·12 | <0·001 |
| TRL triglyceride | 25 - 238 | 41·6 (2·4 - 397·2) |
34·6 (6·7 - 126·8) |
47·2 (14·2 - 165·4) |
59·3 (10·0 - 248·4) |
0·48 | 0·13 | <0·001 |
| Apolipoprotein A-1 | 116 - 209 | 137·4 (98·0 - 241·2) |
43·1 (22·2 - 121·0) |
122·8 (88·2 - 208·2) |
127·9 (84·4 - 237·0) |
<0·001 | 0·14 | <0·05 |
| Apolipoprotein B | 53 - 127 | 69·6 (31·4 - 143·6) |
118·5 (52·0 - 197·3) |
76·5 (42·4 - 138·7) |
88·4 (33·1 - 136·2) |
<0·05 | 0·43 | <0·001 |
| Mean particle sizes (nm) | ||||||||
| HDL size | 8·3 - 9·8 | 9·0 (8·3 - 11·5) |
8·5 (7·8 - 9·1) |
9·0 (8·4 - 10·1) |
8·8 (8·1 - 9·7) |
<0·001 | 0·13 | <0·001 |
| LDL size | 20·1 - 21·7 | 20·9 (20·0 - 21·7) |
21·2 (20·0 - 21·9) |
21·1 (19·3 - 21·5) |
21·1 (19·7 - 22·1) |
0·08 | 0·15 | 0·08 |
| TRL size | 33·8 - 60·9 | 40·2 (32·7 - 71·2) |
35·7 (0 - 50·9) |
44·1 (32·5 - 58·4) |
42·5 (34·3 - 70·8) |
<0·05 | 0·06 | <0·05 |
| HDL particle number (μmol/L) | ||||||||
| Total HDL-P | 19·2 - 29·3 | 21·1 (13·3 - 32·9) |
8·2 (3·8 - 19·9) |
20·7 (14·7 - 31·7) |
21·4 (14·9 - 32·6) |
<0·001 | 0·46 | 0·30 |
| Small HDL-P | 8·1 - 19·6 | 12·3 (5·0 - 21·5) |
6·4 (2·4 - 13·0) |
12·0 (9·7 - 25·1) |
14·4 (7·0 - 22·8) |
<0·001 | 0·47 | <0·001 |
| Medium HDL-P | 3·7 - 12·6 | 5·9 (1·1 - 12·6) |
1·7 (0·4 - 10·9) |
6·5 (3·5 - 11·7) |
5·1 (1·0 - 12·5) |
<0·05 | 0·20 | 0·09 |
| Large HDL-P | 0·2 - 6·3 | 2·3 (0·2 - 11·3) |
0·0 (0 - 0·8) |
1·6 (0 - 8·3) |
1·4 (0 - 6·9) |
<0·001 | 0·05 | <0·05 |
| LDL particles (nmol/L) | ||||||||
| Total LDL-P | 891 - 2150 | 1260 (583 - 2504) |
2092 (893 - 3360) |
1265 (673 - 2449) |
1496 (578 - 2427) |
<0·001 | 0·49 | <0·001 |
| Small LDL-P | 13 - 1318 | 384 (0 - 2027) |
532 (0 - 2327) |
429 (0 - 1692) |
415 (0 - 2269) |
0·14 | 0·44 | 0·19 |
| Medium LDL-P | 0 - 1377 | 582 (0 - 1572) |
718 (0 - 1841) |
536 (0 - 1500) |
567 (0 - 1706) |
0·13 | 0·25 | 0·48 |
| Large LDL-P | 17 - 748 | 211 (0 - 962) |
474 (0 - 1173) |
214 (0 - 834) |
274 (0 - 1400) |
<0·05 | 0·35 | 0·07 |
| Triglyceride-rich particle number (TRL-P) (nmol/L) | ||||||||
| Total TRL-P | 42 - 239 | 75·5 (13·6 - 276·6) |
80·9 (35·6 - 264·9) |
87·7 (11·4 - 191·4) |
128·8 (5 - 291·4) |
0·08 | 0·35 | <0·001 |
| Very small TRL-P (IDL) |
0 - 142·3 | 21·6 (0 - 195·4) |
44·9 (3 - 145·7) |
13·9 (0 - 122·7) |
62·8 (0 - 216·0) |
0·08 | 0·25 | <0·001 |
| Small TRL-P | 7·3 - 124·4 | 36·5 (0 - 113·7) |
41·5 (0 - 224·1) |
48·2 (0·7 - 81·9) |
40·8 (0 - 124·4) |
0·28 | 0·16 | 0·15 |
| Medium TRL-P | 0·3 - 48·4 | 6·3 (0 - 43·2) |
2·5 (0 - 32·3) |
10·5 (0 - 33·3) |
12·1 (0 - 52·0) |
0·06 | 0·08 | <0·001 |
| Large TRL-P | 0 - 12·8 | 0·2 (0 - 21·5) |
0·1 (0 - 0·8) |
0·8 (0 - 11·2) |
0·8 (0 - 16·0) |
0·11 | <0·05 | <0·05 |
| Very large TRL-P (chylomicron) |
0 - 1·6 | 0·1 (0 - 1·0) |
0·1 (0 - 1·0) |
0·1 (0 - 1·4) |
0·1 (0 - 2·6) |
0·19 | 0·20 | 0·14 |
All p-values shown here are Mann-Whitney U analyses.
Analysis between the untreated DC group and androgen-treated DC group
Analysis between the untreated DC group and untreated FA group
Analysis between the untreated DC group and unaffected DC relatives
Reference range values are from a representative sampling (n = 698) of the general population, comprised of apparently healthy men (n = 284) and women (n = 414) aged 18 to 84 years (mean 39 years).
Abbreviations: DC: Dyskeratosis congenita, FA: Fanconi anaemia, HDL: high density lipoprotein, LDL: low density lipoprotein, NS: Not significant (p > 0·05)
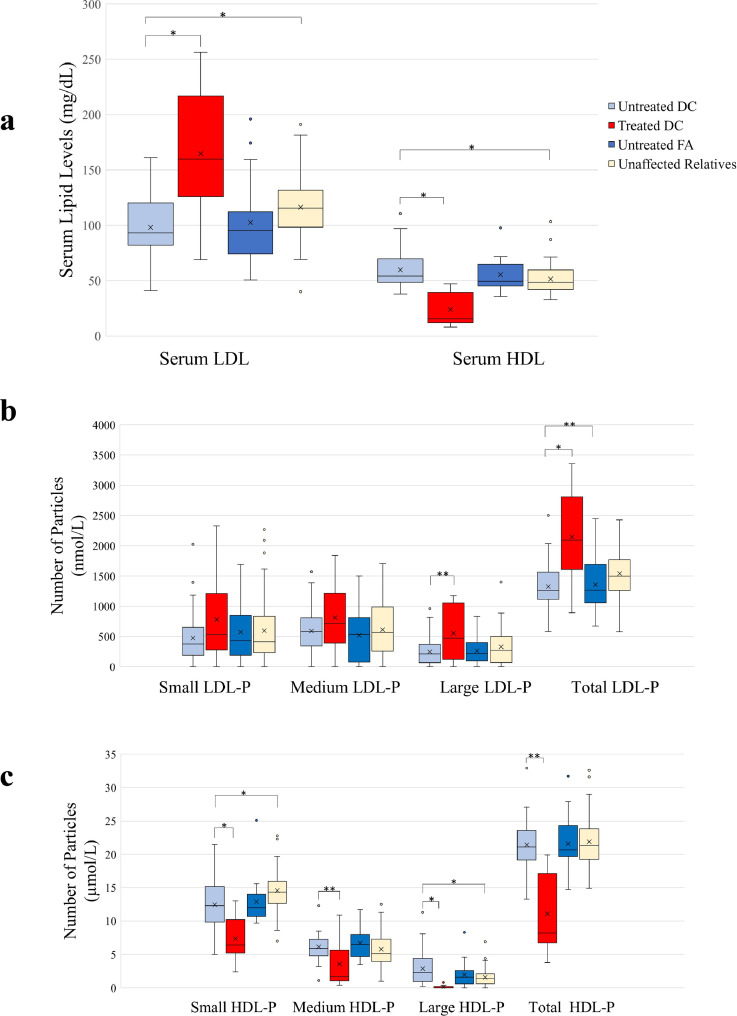
HDL-C, LDL-C, TC, TG, apoA-I and apoB levels were not statistically significantly different between the untreated DC and FA groups (p > 0·05, Mann-Whitney U test) (Fig. 1a, Table 3), indicating that abnormal lipid profiles are not a consequence of having DC. Multiple linear regression accounting for age and sex as confounders confirmed these results.
Figure 1.
Serum lipid profiles of study participants. a) Boxplot showing serum lipid levels in in untreated DC, treated DC, untreated FA, and unaffected relatives. The solid line indicates the median and the ‘x’ indicated the mean. The whiskers show the 25th and 75th percentile. b) Boxplot showing low density lipoprotein particle number (LDL-P) in untreated DC, treated DC, untreated FA, and unaffected DC relatives. The solid line indicates the median and the ‘x’ indicated the mean. The whiskers show the 25th and 75th percentile. c) Boxplot showing high density lipoprotein particle (HDL-P) in untreated DC, treated DC, untreated FA, and unaffected DC relatives. The solid line indicates the median and the ‘x’ indicates the mean. The whiskers show the 25th and 75th percentile.
Abbreviations: DC: Dyskeratosis congenita, FA: Fanconi anaemia, LDL: low density lipoprotein, HDL: high density lipoprotein
*p < 0·001; ⁎⁎p < 0·05.
(n = 144)
Androgen treatment causes significant alterations in serum lipid profiles in DC
HDL-C in the androgen-treated DC group was significantly lower than the untreated DC group (15 vs 54 mg/dL respectively, p < 0·001, Mann-Whitney U test) (Fig. 1a, Table 3). LDL-C was significantly higher in the androgen-treated group with DC compared with the untreated group (160 vs 93 mg/dL respectively, p < 0·001), consistent with our prior findings. (6) TC was minimally higher in the androgen-treated group (193 vs 172 mg/dL, p = 0·10, Mann-Whitney U test), while triglycerides were not significantly different between groups (p = 0.40, Mann-Whitney U test) (Table 3). ApoA-1 was significantly lower in the androgen-treated DC group (43 vs 137 mg/dL, p < 0·001, Mann-Whitney U test) and apoB was significantly higher with androgen treatment (119 vs 70 mg/dL, p < 0·05, Mann-Whitney U test). Multiple linear regression analyses accounting for age and sex confirmed these results, except showing that TC was significantly higher in the androgen-treated DC group.
Lipoprotein particle characteristics in untreated DC, unaffected DC relatives, and FA
Non-parametric analyses showed that the overall size of the HDL-P was larger in the untreated DC group compared with the unaffected DC relatives (9·0 vs 8·8 nm respectively, p < 0·001, Mann-Whitney U test) (Table 3). Compared with DC relatives, the small HDL-P number was decreased and the large HDL-P number was increased (12·3 vs 14·4 µmol/L, p < 0·001; and 2·3 vs 1·4 µmol/L, p < 0·05, respectively, Mann-Whitney U test). The total LDL-P number was decreased (1260 vs 1496 nmol/L, p < 0·001, Mann-Whitney U test), this was shown to be not statistically significant with multiple linear regression accounting for age and sex. There were no specific changes in the individual LDL-P subclasses in the untreated DC group (Table 3, Fig. 1). The TRL-P particles were smaller in size and total TRL-P number was decreased in the untreated DC group compared with the unaffected DC relatives (40·2 vs 42·5 nm, p < 0⋅05; and 75·5 vs 128·8 nmol/L, respectively, p < 0⋅001, Mann-Whitney U test) (Table 3). Of the three subclasses of the VLDL particles, medium TRL-P (MTRL-P) was decreased (6·3 vs 12·1 nmol/L, p < 0⋅001, Mann-Whitney U test) and the very small TRL-P, which represents a more atherogenic remnant IDL, was lower in the untreated DC group compared with the unaffected DC relatives (21·6 vs 62·8 nmol/L respectively, p < 0·001, Mann-Whitney U test) (Table 3). All these findings, except change in MTRL-P were shown to be non-significant by multiple linear regression adjusting for age and sex.
The overall lipoprotein size and number of HDL-P, LDL-P, and TRL-P and their subclasses were not different between the untreated FA group and the untreated DC group (p > 0⋅05, Mann-Whitney U test) (Tables 3 and 4, Fig. 1). This was confirmed by multiple linear regression analyses accounting for age and sex as confounding factors.
Table 4.
Characteristics of nuclear magnetic spectroscopy-derived lipoprotein subclasses, branched chain amino acid, lipoprotein insulin resistance and GlycA.
| Parameter | Reference Range (5th–95th %ile)† | Untreated DC (n =45) median (range) | Androgen-treated DC (n = 9) median (range) | Untreated FA (n = 19) median (range) | Unaffected relatives (n = 72) median (range) | p-value* | p-value⁎⁎ | p-value+ |
|---|---|---|---|---|---|---|---|---|
| HDL Subspecies (µmol/L) | ||||||||
| H1P | 0 - 6·0 | 3·0 (0 - 8·0) |
1·7 (0 - 3·0) |
4·1 (1·2 - 7·7) |
4·7 (0 - 10·0) |
<0·05 | <0·05 | <0·001 |
| H2P | 6·8 - 16·0 | 9·3 (3 - 17·5) |
5·7 (2 - 13) |
8·4 (4·2 - 23·8) |
9·7 (4·5 - 17·1) |
<0·05 |
0·08 | 0·19 |
| H3P | 1·58 - 9·23 | 3·5 (0 - 7·2) |
1·1 (0 - 7·3) |
2·4 (0 - 8·3) |
2·2 (0 - 9·0) |
<0·05 | 0·21 | 0·08 |
| H4P | 0·70 - 4·59 | 3·1 (0·6 - 8·8) |
0·6 (0·1 - 3·7) |
3·4 (2·1 - 8·1) |
2·8 (0 - 9·4) |
<0·05 |
0·06 | 0·44 |
| H5P | 0 - 3·02 | 0·9 (0 - 3·5) |
0·0 (0 - 0·2) |
1·0 (0 - 3·2) |
0·6 (0 - 4·5) |
<0·001 |
0·46 | 0·24 |
| H6P | 0 - 3·64 | 0·7 (0 - 7·2) |
0·0 (0 - 0·3) |
0·3 (0 - 4·4) |
0·3 (0 - 3·0) |
<0·001 | 0·11 | <0·001 |
| H7P | 0 - 1·09 | 0·2 (0 - 8·0) |
0·0 (0 - 0·4) |
0·1 (0 - 1·5) |
0·1 (0 - 1·6) |
<0·05 | 0·06 | <0·05 |
| TRL-P Subspecies (nmol/L) | ||||||||
| Very small TRL-Ps (IDL) | 0 - 142·3 | 21·6 (0 - 195·4) |
44·9 (3 - 146·0) |
13·9 (0 - 123) |
62·8 (0 - 216) |
0·08 | 0·25 | <0·001 |
| VVS-27 | 3·2 (0 - 195·4) |
25·9 (0 - 119·0) |
0 (0 - 81·1) |
24·5 (0 - 203·3) |
0·05 | 0·16 | <0·05 | |
| VVS-28 | 0·5 (0 - 181·9) |
1·1 (0 - 70·0) |
10·0 (0 - 61) |
15·8 (0 - 140·4) |
0·32 | 0·12 | <0·05 | |
| Small TRL-Ps | 7·3 - 124·4 | 36·5 (0 - 113·7) |
41·5 (0 - 224·1) |
48·2 (0·7 - 82) |
40·8 (0 - 124·4) |
0·28 | 0·16 | 0·15 |
| VS-30 | 0 (0 - 40·8) |
0 (0 - 195) |
3·3 (0 - 65) |
0·6 (0 - 124·4) |
0·45 | <0·05 | <0·05 | |
| VS-33 | 25·4 (0 - 70·4) |
30·7 (0 - 56·2) |
19·2 (0 - 68) |
23·7 (0 -104) |
0·36 | 0·12 | 0·15 | |
| VS-35 | 0 (0 - 42·7) |
0 (0 - 0) |
0 (0 - 23) |
0 (0 - 49) |
<0·05 | 0·17 | 0·10 | |
| Medium TRL-Ps | 0·3 - 48·4 | 6·3 (0 - 43·2) |
2·5 (0 - 32·3) |
10·5 (0 - 33·3) |
12·1 (0 - 52) |
0·06 | 0·08 | <0·001 |
| VM-38 | 0 (0 - 7·6) |
0 (0 - 0) |
0 (0 - 11) |
0 (0 - 12) |
0·10 | <0·05 | 0·17 | |
| VM-42 | 0·1 (0 - 29·1) |
0 (0 -15·3) |
0 (0 - 18·1) |
1·3 (0 - 52) |
0·07 | 0·21 | <0·05 | |
| VM-48 | 2·9 (0 - 43·2) |
2·2 (0 - 17) |
7·2 (0 - 26) |
7·1 (0 - 44) |
0·16 | 0·16 | <0·05 | |
| Large TRL-Ps | 0 - 12·8 | 0·2 (0 - 21·5) |
0·1 (0 - 0·8) |
0·8 (0 - 11·2) |
0·8 (0 - 16) |
0·11 | <0·05 | <0·05 |
| VL-55 | 0 (0 - 1·1) |
0 (0 - 0) |
0 (0 - 2·0) |
0 (0 - 3·2) |
0·35 | 0·26 | 0·43 | |
| VL-65 | 0·2 (0 - 8·1) |
0·1 (0 - 0·8) |
0·7 (0 - 11·2) |
0·7 (0 - 16) |
0·17 | <0·05 | <0·05 | |
| VL-80 | 0 (0 - 20·7) |
0 (0 - 0·04) |
0 (0 - 0·3) |
0 (0 - 7·0) |
0·27 | 0·28 | <0·05 | |
| (The additional TRL-P parameters are distinguished by size. The number in each TRL-P parameter indicates the approximate diameter in nm.) | ||||||||
| Inflammatory Marker (µmol/L) | ||||||||
| GlycA | 307 - 524 | 364 (248 - 711) |
572 (363 - 774) |
378 (307 - 574) |
399 (268 - 608) |
<0·001 | 0·11 | <0·05 |
| Lipoprotein Insulin Resistance (LP-IR) | ||||||||
| LP-IR score 0-100 |
3 - 83 | 23 (0 -100) |
38 (19 – 48) |
32 (4 - 78) |
38 (4 - 96) |
0·05 | 0·09 | <0·05 |
| Total Branched Chain Amino Acid (BCAA) (µmol/L) | ||||||||
| BCAA | 309 - 658 | 465 (296 - 736) |
469 (358 - 624) |
423 (330 - 697) |
488 (323 - 891·1) |
0·50 | 0·08 | <0·05 |
| Valine | 166 - 332 | 226 (156 - 342) |
234 (170 - 276) |
242 (182 - 429) |
253 (166 - 424) |
0·29 | 0·10 | <0·001 |
| Isoleucine | 19 - 96 | 47 (3·7 - 91) |
53 (33 – 69) |
53 (31 - 119) |
59 (21 - 129) |
0·18 | <0·05 | <0·001 |
| Leucine | 110 - 239 | 163 (95 - 260) |
143 (103 - 220) |
189 (129 - 343) |
195 (120·3 - 267) |
0·28 | <0·05 | <0·001 |
All p-values shown here are Mann-Whitney U analyses.
Analysis between the untreated and treated DC group
Analysis between untreated FA group and untreated DC group
Analysis between unaffected relatives and untreated DC group
Reference range values are from a representative sampling (n = 698) of the general population, comprised of apparently healthy men (n = 284) and women (n = 414) aged 18 to 84 years (mean 39 years).
Abbreviations: DC: Dyskeratosis congenita, FA: Fanconi anaemia, HDL: high density lipoprotein, LDL: low density lipoprotein, TRL: triglyceride-rich lipoprotein, NS: Not significant (p > 0·05)
Androgen-induced changes in lipoprotein particles in DC
HDL-P size was smaller and HDL-P number was significantly decreased in androgen-treated DC individuals compared with the untreated (8·5 vs 9·0 nm, p < 0·001; and 8·2 vs 21·1 µmol/L, p < 0·001, respectively, Mann-Whitney U test) (Table 3, Fig. 1c). The numbers of all HDL-P size subclasses were significantly decreased in the androgen-treated DC group compared with untreated DC (p < 0·05, Mann-Whitney U test) (Fig. 1c). There was a reduced number of all seven HDL-P size subclasses, most notably seen in H5P and H6P (0·9 vs 0 µmol/L, p < 0·001; and 0·7 vs 0 µmol/L, p < 0·001, respectively, Mann-Whitney U test) subspecies of large HDL-P that essentially disappeared with androgen-treatment (Table 4). Multiple linear regression accounting for age and sex as confounders confirmed these results, except showing the change in H7P and H3P to be not statistically significant, while all other subclasses showed significant change.
Non-parametric LDL-P analysis showed that while the overall LDL-P size was not significantly different between androgen-treated and untreated group (21·2 vs 21 nm respectively, p = 0·08, Mann-Whitney U test), there was a significant increase in total LDL-P number in the androgen-treated group (2092 vs 1260 nmol/L, p < 0·001, Mann-Whitney U test) (Table 3, Fig. 1b). When stratifying by the three LDL-P subclasses, small LDL-P and medium LDL-P were increased (532 vs 384 nmol/L, p = 0·14; and 718 vs 582 nmol/L, p = 0·13, respectively, Mann-Whitney U test) and large LDL-P number more than doubled in the androgen-treated group (474 vs 211 nmol/L, p < 0·05) (Table 3, Fig. 1b). TRL-P size was smaller in the androgen-treated DC group compared with the untreated DC individuals (35·7 vs 40·2 nm respectively, p < 0·05). Total TRL-P number and subclasses by size were not significantly different between the groups (p > 0·05, Mann-Whitney U test). Multiple linear regression accounting for age and sex as confounding factors confirmed these results.
BCAA, LP-IR and GlycA
Non-parametric analyses showed that BCAA concentrations were unchanged with androgen treatment (469 vs 465 µmol/L in the untreated, p = 0·50, Mann-Whitney U test) (Table 4). Total BCAA and valine concentrations were minimally increased in the unaffected DC relatives compared to the untreated DC group (488 vs 465 µmol/L, p < 0·05; and 253 vs 226 µmol/L, p < 0·001, respectively, Mann-Whitney U test) (Table 4). Isoleucine and leucine concentrations were increased in the unaffected DC relatives and untreated FA group compared to the untreated DC group (p < 0·05, Mann-Whitney U test). Multiple linear regression accounting for age and sex showed that all the total and individual BCAA changes were not statistically significant between untreated and androgen-treated DC, all BCAA changes were significantly different between unaffected relatives and untreated DC as well as between untreated DC and untreated FA (except total BCAA that was not significant).
The LP-IR score, a combination of six lipoprotein parameters associated with insulin resistance, was not significantly different between androgen-treated and untreated DC (38 vs 23 respectively, p = 0·05, Mann-Whitney U test) (Table 4), confirmed by multiple linear regression analyses accounting for age and sex
GlycA was significantly elevated at 572 µmol/L in the androgen-treated DC group compared to 364 µmol/L in the untreated DC participants (p < 0·001) (Table 4). The GlycA scores were not different between the untreated DC or FA participants (364 vs 378 µmol/L respectively, p = 0·11, Mann-Whitney U test). The GlycA scores were minimally increased in the unaffected relatives compared to the untreated DC participants (399 vs 364 µmol/L respectively, p < 0·05, Mann-Whitney U test). Multiple linear regression showed that while accounting for age and sex, the changes in GlycA were not significantly different except when comparing androgen-treated to untreated DC groups.
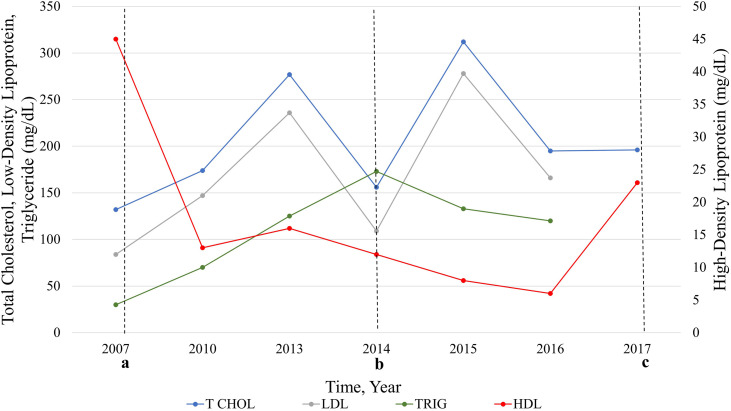
Case study: Nine year follow-up
A male with the Hoyeraal-Hreidarsson syndrome subtype of DC due to biallelic pathogenic RTEL1 variants developed progressive BMF requiring treatment by age nine years. 26 HCT was not an option at the time, and he was started on oral oxymetholone. After three years on androgens, he was noted to have abnormal serum lipids, with normal levels prior to commencement of androgens (Fig. 2). A carotid ultrasound conducted at that time that showed no evidence of hemodynamically significant stenosis. Further evaluations during androgen treatment showed progressively worsening serum lipids, and carotid plaque development was seen on doppler ultrasound. He was started on a low dose of simvastatin for management at 15 years of age until he underwent a successful HCT at age 18 years at which time his lipids normalized, and simvastatin was stopped (Supplemental Table 1, Fig. 2). On retrospective analysis with NMR pre-and post-androgen treatment, we observed a decrease in his HDL-P number (across all size subclasses with disappearance of all subsets of large HDL-P) and increase in his LDL-P number (mainly due to increase in small and medium LDL-P) with androgen treatment (additional details in Supplemental Table 1).
Figure 2.
Serum lipids in a paediatric participant with dyskeratosis congenita before and during oral androgen treatment. Start of oral androgen treatment with oxymetholone indicated at time point a. Time point b was the start of statin treatment for abnormal lipids. c denotes the end of oxymetholone treatment and receipt of hematopoietic stem cell transplant.
(n = 1)
Discussion
DC and related TBDs are complex disorders with very high rates of BMF that may require androgen therapy, with approximately 80% of individuals with DC developing clinically significant cytopenia by age 30 years. 1 This study is the first to comprehensively evaluate serum lipid and lipoprotein particles in DC as well as the first to show significant androgen-induced changes in HDL and LDL particles in DC.
Androgen treatment in individuals with DC significantly decreased HDL-C by 72% from 54 to 15 mg/dL, while apoA-I levels decreased by 70%. Since HDL is heterogeneous in size and cholesterol composition and carries variable amounts of apoA-I, it is a suboptimal biomarker for CVD risk. HDL-P has been well established as a superior predictor of CVD risk compared with HDL-C.13,27 HDL-P size is known to be inversely related to CVD risk.13 HDL-P and small HDL-P number have been reported to inversely correlate with cardiovascular mortality.27 Androgens decreased the overall average size of the HDL-P by approximately 50%, generating smaller particles that are established to be more atherogenic.13 In particular, the large H5P and H6P subspecies, considered the most atheroprotective subspecies of HDL-P,13,18 essentially disappeared with androgen treatment. Further studies will be needed to elucidate the effects of specific androgens (e.g., danazol, oxymetholone) on HDL-P number and size.
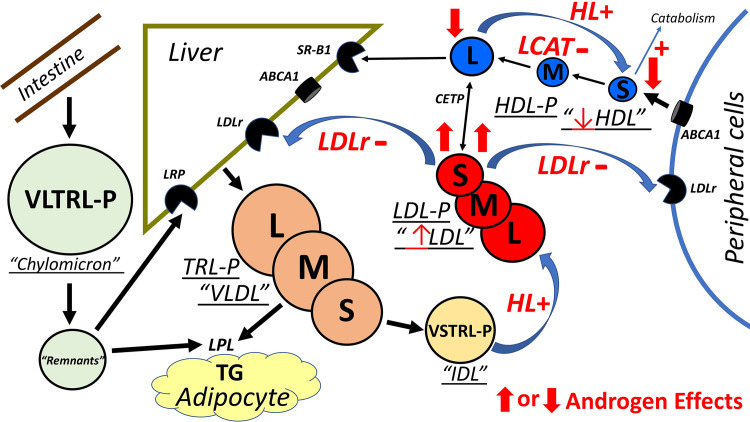
The exact mechanisms by which androgens decrease HDL are unknown but there are two postulated mechanisms which are supported by our data. Androgens stimulate hepatic lipase (HL) promoting the conversion or recycling of large-sized HDL to small-sized HDL particles.28, 29, 30 Our data demonstrate that androgens significantly reduced or eliminated the number of large HDL-P supporting the accelerated conversion to small HDL-P, which are known to be more rapidly cleared, further contributing to the significantly decreased HDL-C.31,32 The second effect of androgens on HDL is to inhibit lecithin cholesterol acyltransferase (LCAT) activity also resulting in reduced large HDL-P.30, 31, 32 LCAT activity plays a major role in removing cholesterol from the blood and tissues leading to HDL maturation, converting small-sized HDL into large-sized HDL that is then delivered to the liver. Consequently, inhibiting LCAT further reduces large HDL-P numbers. The androgen-induced elimination of large HDL-P in DC individuals may be hypothesized to be due to reduced LCAT activity. The combination of increased HL and decreased LCAT activity in the androgen-treated DC group resulted in the complete disappearance of large HDL-P, the product of LCAT activity (Fig. 3).31, 32, 33
Figure 3.
Schematic of the effects of androgens on lipid metabolism. The liver secretes large triglyceride-rich (TG-rich) VLDL (LTRL-P) that is lipolyzed to TG by LPL converting LTRL-P to STRL-P (VLDL). STRL-P are then further converted to VSTRL-P (IDL). VSTRL-P are delipidated by hepatic lipase to LDL. Large LDL (LLDL-P) is hydrolysed to smaller LDL (SLDL–P) and cleared by LDLr in the liver and in peripheral cells including macrophages. Free Cholesterol (FC) is exported by hepatic (not shown) and peripheral cells via ABCA1 to form small HDL (SHDL-P). LCAT esterifies FC to CE by the action of LCAT converting small HDL (SHDL-P) to large HDL (LHDL-P). HDL CE can be transferred to LDL and other apoB TRL-Ps by CETP or directly to the liver by SR-B1. Hepatic lipase hydrolyses LHDL-P to SHDL-P converting HDL back to smaller HDL lipoproteins. Dietary cholesterol and TG are absorbed through the intestine by chylomicrons (VLTRL-P). The TG is hydrolysed by LPL on adipocytes and the remnant are cleared by LRP on the liver. The postulated mechanism of action of androgens on HDL is to 1) stimulate hepatic lipase (HL) hydrolysis of TG leading to accelerated conversion of large HDL (LHDL-P) to small HDL (SHDL-P) and 2) suppress LCAT activity reducing the formation of large HDL (LHDL-P) that leads to smaller HDL particles which are then catabolized more rapidly resulting in the significantly decreased HDL levels. The postulated mechanism of action of androgens on LDL is to 1) stimulate hepatic lipase that promotes the conversion of IDL (VSTRL-P) to LDL (LLDL-P) and 2) inhibit LDL receptor activity in hepatic and peripheral cells which results in increased LDL levels containing smaller LDL particles (SLDL-P). Curved arrows are effects of androgens on major pathways. + or – is effect on enzymes or receptor. Thick arrows are androgen change in individual NMR isolated lipoprotein subparticles. Thin arrow is androgen effect on standard lipoprotein level (HDL, LDL). Abbreviations: HDL; high-density lipoprotein, IDL; intermediate-density lipoprotein, LDL; low-density lipoprotein, VLDL; very low-density lipoprotein, LCAT; lecithin: cholesterol acyltransferase, CETP; cholesteryl ester transfer protein, LPL; lipoprotein lipase, LDLr; LDL receptor, LRP; LDL receptor related protein, SR-B1; scavenger receptor Class B type1, TG; triglyceride, VLTRL-P; very large TRL-P (chylomicron), VSTRL-P; very small TRL-P (IDL), L; large, M; medium, S; small.
LDL is the main lipoprotein carrier of cholesterol in the circulation and contains one apoB per particle regardless of LDL size. We found that LDL-C was significantly increased by 72% from 93 to 160 mg/dL and apoB levels increased 70% with androgen treatment (Table 3). High levels of LDL-C and apoB are strongly correlated with CVD risk13 and the observed changes are concerning for potential future CVD risk in androgen-treated individuals with DC.
LDL particle size has a strong inverse association with CVD risk. LDL-P number is an established strong predictor of CVD risk compared with LDL-C and is currently in clinical use to guide cholesterol management.12, 13, 14,17,22 In contrast to HDL, the overall average size of LDL particles was unchanged in the androgen-treated DC group, suggesting the significant increase in LDL-C was not due to changes in LDL size but in total LDL-P number. LDL-P number greater than 1,600 nmol/L place patients at a very high risk of CVD.14 While untreated DC individuals did not exhibit concerning LDL-P number, the significant increase to median of >2000 nmol/L with androgen treatment may portend significance for future CVD risk and atherogenicity possibly at a younger age given the age at which androgen treatment is typically started and young age at which these findings were observed in this study.
Similar to the HDL-P changes, the mechanisms by which androgens induce atherogenic LDL changes are unclear. The two postulated mechanisms of action of androgens on LDL seemed consistent with our results. Androgens stimulate hepatic lipase activity which promote lipolysis through the conversion of IDL (VSTRL-P) to LDL (LLDL-P) resulting in increased LDL-C (Fig. 3).31,32 Also, androgens affect LDL catabolism by inhibiting LDL receptor activity in hepatic cells leading to a delay in LDL clearance further increasing LDL-C containing smaller LDL particles (SLDL-P). The significant androgen-induced increases in LDL-C and LDL-P number in individuals with DC are consistent with both increased LDL production and delayed catabolism, which are both atherogenic processes.14,22, 23, 24, 25,31,32
Differences seen between the unaffected DC relatives, such as higher very small TRL-P (an atherogenic IDL), may be attributed to the older age of the ‘control’ relatives compared with individuals with DC. The results of multiple linear regression analyses showed that total cholesterol, LDL, and triglycerides were not statistically significantly different between untreated DC and unaffected relatives. Along with these findings, the essentially similar serum lipid and lipoprotein particle profiles between the untreated FA and the untreated DC cohorts indicate that the underlying disease processes do not influence lipid metabolism, and further highlight the effect of androgens on lipid profile changes.
This study is the first time BCAAs, LP-IR and GlycA were explored as potential novel biomarkers of disease and androgen effects in DC. BCAAs play an important role in glucose haemostasis and protein synthesis. High concentrations of BCAAs are associated with prediabetes, metabolic syndrome, obesity and diabetes.21, 22, 23, 24 LP-IR combines six TRL-P, LDL-P, and HDL-P subclass and size parameters and high levels are strongly associated with insulin resistance or predispose to prediabetes or diabetes.24 The results of both normal BCAA and LP-IR suggest that the androgen-induced lipid changes in DC are not associated with adverse changes in insulin resistance or glucose homeostasis and may not predispose to the development of future risk of diabetes.21, 22, 23, 24 Future prospective studies of clinical outcomes will be necessary to validate these results.
GlycA provides a composite measurement of acute phase reactants and systemic inflammation, with levels >400 µmol/L associated with high risk of systemic inflammation; and increased GlycA associated with future CVD mortality.25,34 The significant increase of GlycA in androgen-treated DC may indicate androgen-induced alterations in systemic inflammation, or a hereto unknown components of inflammation with the underlying BMF for which they are treated.
There are several strengths of this study including the detailed clinical data available phenotypic manifestations, androgen treatment and long-term follow-up in all the participant groups analysed. Additionally, we performed analyses accounting for age and sex as confounding factors, that overall confirmed the results of the analyses between androgen-treated and untreated DC: and showed that the dyslipidaemia observed in the unaffected relatives was due to differences in age demographics. We acknowledge that the sample size in this study is limited and that larger, prospective cohort studies of both NMR-based lipoprotein particle characteristics with associated biomarkers and clinical outcomes could help validate these findings and further characterize the potential atherogenic effects of androgen therapy in DC. While our analyses accounted for potential confounder, there may be other underlying biological mechanisms that are yet to be understood.
In summary, androgen treatment is a mainstay for BMF management in DC, especially when curative HCT is not an option. The combined atherogenic effects of androgens on HDL and LDL lipoprotein particles due to androgens observed in this study provide additional concern that androgen-treated individuals with DC may potentially be at a higher risk for CVD that is yet to be clinically characterized. As management strategies and survival of patients with DC improve, it is imperative to prospectively follow the androgen-treated individuals with DC to study clinical outcomes such as CVD and inflammation, as well as the effects of specific androgens on lipoprotein particles size and number. However, the significant degree of androgen-related change seen in this study raise caution and suggest that longitudinal evaluation of serum and NMR-based lipid profiles are warranted to guide management of androgen-treated DC. Until further prospective studies can be performed in dyskeratosis congenita patients, current cholesterol guidelines, including measurement of LDL and HDL particle number and size, should be used for the treatment of androgen-induced dyslipidaemia. Additional studies in individuals without DC who are treated with androgens for BMF, or other indications are required to understand the full extent of androgen-related alterations in lipid metabolism.
Contributors
- Conceptualization: SAS, PPK- Data curation: MBT, DM, NG, PPK- Formal analysis: MBT, DM, KCA, PPK- Funding acquisition: SAS, BPA- Investigation: RDS- Writing-original draft: MBT, SAS, PPK, RDS- Writing-review and editing: all authors
Data sharing
Upon publication of the manuscript, participant data including germline gene and variant data, age, sex, androgen treatment, lipid and lipoprotein profile data will be available, after appropriate de-identification. These data dictionaries will be shared after data sharing agreements are established between the NCI and requesting institution, for scientists with relevant expertise and hypotheses to test using these data, as long as the request is consistent with study consent forms.
Declaration of interests
The authors have no conflicts of interest to disclose.
Acknowledgements
We thank the patients, their families, and the referring clinicians for their valuable contributions to this study. Lisa Leathwood, RN, and Maureen Risch, RN of Westat, Inc. provided valuable study management under contract HHSN261201700004C with the NCI. This work was supported by the intramural research program of the Division of Cancer Epidemiology and Genetics, NCI.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103760.
Appendix. Supplementary materials
References
- 1.Niewisch MR, Savage SA. An update on the biology and management of dyskeratosis congenita and related telomere biology disorders. Expert Rev Hematol. 2019;12(12):1037–1052. doi: 10.1080/17474086.2019.1662720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Himes RW, Chiou EH, Queliza K, Shouval DS, Somech R, Agarwal S, et al. Gastrointestinal Hemorrhage: A Manifestation of the Telomere Biology Disorders. J Pediatr. 2020 doi: 10.1016/j.jpeds.2020.09.038. [DOI] [PubMed] [Google Scholar]
- 3.Bertuch AA. The molecular genetics of the telomere biology disorders. RNA Biol. 2016;13(8):696–706. doi: 10.1080/15476286.2015.1094596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in the National Cancer Institute inherited bone marrow failure syndrome cohort after fifteen years of follow-up. Haematologica. 2018;103(1):30–39. doi: 10.3324/haematol.2017.178111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calado RT, Cle DV. Treatment of inherited bone marrow failure syndromes beyond transplantation. Hematology Am Soc Hematol Educ Program. 2017;2017(1):96–101. doi: 10.1182/asheducation-2017.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khincha PP, Wentzensen IM, Giri N, Alter BP, Savage SA. Response to androgen therapy in patients with dyskeratosis congenita. Br J Haematol. 2014;165(3):349–357. doi: 10.1111/bjh.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Townsley DM, Dumitriu B, Young NS. Danazol treatment for telomere diseases. N Engl J Med. 2016;375(11):1095–1096. doi: 10.1056/NEJMc1607752. [DOI] [PubMed] [Google Scholar]
- 8.Khincha PP, Bertuch AA, Gadalla SM, Giri N, Alter BP, Savage SA. Similar telomere attrition rates in androgen-treated and untreated patients with dyskeratosis congenita. Blood Adv. 2018;2(11):1243–1249. doi: 10.1182/bloodadvances.2018016964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glazer G. Atherogenic effects of anabolic steroids on serum lipid levels. A literature review. Arch Intern Med. 1991;151(10):1925–1933. [PubMed] [Google Scholar]
- 10.Shahidi NT. A review of the chemistry, biological action, and clinical applications of anabolic-androgenic steroids. Clin Ther. 2001;23(9):1355–1390. doi: 10.1016/s0149-2918(01)80114-4. [DOI] [PubMed] [Google Scholar]
- 11.Ballantyne FC, Clark RS, Simpson HS. Ballantyne D. High density and low density lipoprotein subfractions in survivors of myocardial infarction and in control subjects. Metabolism. 1982;31(5):433–437. doi: 10.1016/0026-0495(82)90230-x. [DOI] [PubMed] [Google Scholar]
- 12.Cromwell WC, Otvos JD. Low-density lipoprotein particle number and risk for cardiovascular disease. Curr Atheroscler Rep. 2004;6(5):381–387. doi: 10.1007/s11883-004-0050-5. [DOI] [PubMed] [Google Scholar]
- 13.Kontush A. HDL particle number and size as predictors of cardiovascular disease. Front Pharmacol. 2015;6:218. doi: 10.3389/fphar.2015.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26(4):847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Rankin NJ, Preiss D, Welsh P, Burgess KE, Nelson SM, Lawlor DA, et al. The emergence of proton nuclear magnetic resonance metabolomics in the cardiovascular arena as viewed from a clinical perspective. Atherosclerosis. 2014;237(1):287–300. doi: 10.1016/j.atherosclerosis.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinzer AB, Shamburek RD, Lightbourne M, Muniyappa R, Brown RJ. Advanced lipoprotein analysis shows atherogenic lipid profile that improves after metreleptin in patients with lipodystrophy. J Endocr Soc. 2019;3(8):1503–1517. doi: 10.1210/js.2019-00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matyus SP, Braun PJ, Wolak-Dinsmore J, Jeyarajah EJ, Shalaurova I, Xu Y, et al. NMR measurement of LDL particle number using the Vantera Clinical Analyzer. Clin Biochem. 2014;47(16-17):203–210. doi: 10.1016/j.clinbiochem.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Matyus SP, Braun PJ, Wolak-Dinsmore J, Saenger AK, Jeyarajah EJ, Shalaurova I, et al. HDL particle number measured on the Vantera(R), the first clinical NMR analyzer. Clin Biochem. 2015;48(3):148–155. doi: 10.1016/j.clinbiochem.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996;276(11):875–881. [PubMed] [Google Scholar]
- 20.Freedman DS, Otvos JD, Jeyarajah EJ, Barboriak JJ, Anderson AJ, Walker JA. Relation of lipoprotein subclasses as measured by proton nuclear magnetic resonance spectroscopy to coronary artery disease. Arterioscler Thromb Vasc Biol. 1998;18(7):1046–1053. doi: 10.1161/01.atv.18.7.1046. [DOI] [PubMed] [Google Scholar]
- 21.Connelly MA, Wolak-Dinsmore J, Dullaart RPF. Branched chain amino acids are associated with insulin resistance independent of leptin and adiponectin in subjects with varying degrees of glucose tolerance. Metab Syndr Relat Disord. 2017;15(4):183–186. doi: 10.1089/met.2016.0145. [DOI] [PubMed] [Google Scholar]
- 22.Wolak-Dinsmore J, Gruppen EG, Shalaurova I, Matyus SP, Grant RP, Gegen R, et al. A novel NMR-based assay to measure circulating concentrations of branched-chain amino acids: Elevation in subjects with type 2 diabetes mellitus and association with carotid intima media thickness. Clin Biochem. 2018;54:92–99. doi: 10.1016/j.clinbiochem.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Oste MCJ, Flores-Guerrero JL, Gruppen EG, Kieneker LM, Connelly MA, Otvos JD, et al. High Plasma Branched-Chain Amino Acids Are Associated with Higher Risk of Post-Transplant Diabetes Mellitus in Renal Transplant Recipients. J Clin Med. 2020;9(2) doi: 10.3390/jcm9020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shalaurova I, Connelly MA, Garvey WT, Otvos JD. Lipoprotein insulin resistance index: a lipoprotein particle-derived measure of insulin resistance. Metab Syndr Relat Disord. 2014;12(8):422–429. doi: 10.1089/met.2014.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otvos JD, Shalaurova I, Wolak-Dinsmore J, Connelly MA, Mackey RH, Stein JH, et al. GlycA: a composite nuclear magnetic resonance biomarker of systemic inflammation. Clin Chem. 2015;61(5):714–723. doi: 10.1373/clinchem.2014.232918. [DOI] [PubMed] [Google Scholar]
- 26.Ballew BJ, Yeager M, Jacobs K, Giri N, Boland J, Burdett L, et al. Germline mutations of regulator of telomere elongation helicase 1, RTEL1, in Dyskeratosis congenita. Hum Genet. 2013;132(4):473–480. doi: 10.1007/s00439-013-1265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duparc T, Ruidavets JB, Genoux A, Ingueneau C, Najib S, Ferrieres J, et al. Serum level of HDL particles are independently associated with long-term prognosis in patients with coronary artery disease: The GENES study. Sci Rep. 2020;10(1):8138. doi: 10.1038/s41598-020-65100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haffner SM, Kushwaha RS, Foster DM, Applebaum-Bowden D, Hazzard WR. Studies on the metabolic mechanism of reduced high density lipoproteins during anabolic steroid therapy. Metabolism. 1983;32(4):413–420. doi: 10.1016/0026-0495(83)90052-5. [DOI] [PubMed] [Google Scholar]
- 29.Applebaum-Bowden D, Haffner SM, Hazzard WR. The dyslipoproteinemia of anabolic steroid therapy: increase in hepatic triglyceride lipase precedes the decrease in high density lipoprotein2 cholesterol. Metabolism. 1987;36(10):949–952. doi: 10.1016/0026-0495(87)90130-2. [DOI] [PubMed] [Google Scholar]
- 30.Kantor MA, Bianchini A, Bernier D, Sady SP, Thompson PD. Androgens reduce HDL2-cholesterol and increase hepatic triglyceride lipase activity. Med Sci Sports Exerc. 1985;17(4):462–465. doi: 10.1249/00005768-198508000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Packard CJ, Shepherd J. Action of danazol on plasma lipids and lipoprotein metabolism. Acta Obstet Gynecol Scand Suppl. 1994;159:35–40. [PubMed] [Google Scholar]
- 32.Shepherd J. Danazol and plasma lipoprotein metabolism. Int J Gynaecol Obstet. 1995;50 Suppl 1:S23–S26. doi: 10.1016/0020-7292(95)02511-a. [DOI] [PubMed] [Google Scholar]
- 33.Fahraeus L, Larsson-Cohn U, Ljungberg S, Wallentin L. Profound alterations of the lipoprotein metabolism during danazol treatment in premenopausal women. Fertil Steril. 1984;42(1):52–57. doi: 10.1016/s0015-0282(16)47957-4. [DOI] [PubMed] [Google Scholar]
- 34.Lawler PR, Akinkuolie AO, Chandler PD, Moorthy MV, Vandenburgh MJ, Schaumberg DA, et al. Circulating N-Linked Glycoprotein Acetyls and Longitudinal Mortality Risk. Circ Res. 2016;118(7):1106–1115. doi: 10.1161/CIRCRESAHA.115.308078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.