Abstract
Purpose of review
Present the value of a person-centered approach in diabetes management and review current evidence supporting its practice.
Recent findings
Early evidence from glycemic control trials in diabetes resulted in most practice guidelines adopting a glucose-centric intensive approach for management of the disease, consistently relying on HbA1c as a marker of metabolic control and success. This paradigm has been recently dispelled by new evidence that shows that intensive glycemic control does not provide a significant benefit regarding patient-important microvascular and macrovascular hard outcomes when compared to moderate glycemic targets.
Summary
The goals of diabetes therapy are to reduce the risks of acute and chronic complications and increase quality of life while incurring least burden of treatment and disruption to the patient’s life. A person-centered approach to diabetes management is achieved through shared decision making, integration of evidence-based care and patient’s needs, values and preferences, and minimally disruptive approaches to diabetes care and at the same time offer practical guidance to clinicians and patients on achieving this type of care.
Keywords: Patient-centered glycemic control, Minimally disruptive medicine, Shared-decision making, Diabetes
Introduction
Type 2 diabetes is one of the most important health threats in the USA and around the world. Over 34 million (one in eight) American adults and 422 million (one in eleven) adults worldwide are estimated to live with diabetes [1]. It is among the leading causes of morbidity, disability, impaired quality of life, high healthcare costs, and mortality; thus, reducing the burden of diabetes and its complications is a priority for patients, health systems, and governments around the world [2, 3]. The vast majority of people living with diabetes, between 90 and 95%, have type 2 diabetes, characterized by progressive insulin resistance and hyperglycemia.[4] Epidemiologic studies have consistently demonstrated a positive correlation between hyperglycemia—measured either by fasting blood glucose levels or hemoglobin A1c (HbA1c)—and increased incidence of microvascular and macrovascular complications [5,6, 7, 8, 9, 10, 11, 12, 13, 14]. The first major treat-to-target randomized controlled trial (RCT) of type 2 diabetes management, the UK Prospective Diabetes Study (UKPDS) 33, reinforced this glucose-centric paradigm of type 2 diabetes management by demonstrating reduced rates of microvascular complications with intensive (fasting glucose levels <15 mmol/l, corresponding to HbA1c <11%) as compared to standard (fasting glucose levels <6 mmol/l, corresponding to HbA1c <5.4%) control. This early evidence resulted in most clinical practice guidelines adopting HbA1c of 7% as the treatment goal for most non-pregnant adults with diabetes, with some individualized variation to higher or lower HbA1c levels depending on the patient’s clinical complexity, anticipated life expectancy, risk for hypoglycemia, and burden of treatment [15,16, 17, 18, 19].
This glucose-centric paradigm, however, has recently been challenged [20] by an body of evidence that among patients with type 2 diabetes there may not be a meaningful difference in patient-important microvascular and macrovascular health outcomes with pursuit of intensive (HbA1c <7.0%) as compared to moderate (HbA1c 7.0–8.5%) glycemic targets [21]. Moreover, hyperglycemia is often one of many medical and psychosocial health threats experienced by patients with type 2 diabetes. Patients also live with multiple other medical, social, and financial demands that shape their goals for care as well as their capacity for safe and effective self-management [22, 23]. These factors need to be considered when developing an individualized, patient-centered diabetes care plan that improves not only glucose levels but also quality of life. We therefore propose a patient centered, rather than glucose-centric, approach to diabetes management that can be achieved through shared decision making, integration of evidence-based care with the patient’s individual needs and preferences, and minimally-disruptive approaches to care [24].
The objective of this review is to summarize the evidence regarding glycemic control in the management of type 2 diabetes, present the value of a patient centered approach, and offer practical guidance to clinicians and patients on how to archive minimally-disruptive, individualized, and a patient centered diabetes care in routine clinical practice.
Case Vignette
GGS is a 56-year-old woman with a 10-year history of type 2 diabetes, as well as hypertension, dyslipidemia, obesity, osteoarthritis, mild depression, and history of ovarian cancer in remission after undergoing surgery 5 years ago. Her husband died from myocardial infarction 3 years ago and she lives with her daughter’s family. She works as a manager of a local convenience store and enjoys watching television and going on afternoon walks. She takes the maximum approved doses of three oral glucose-lowering medications (metformin, glyburide, and sitagliptin), losartan, hydrochlorothiazide, atorvastatin, ezetimibe, calcium, vitamin D, omega-3, clopidogrel, bezafibrate, alopurinol, gabapentin, tramadol, vitamin C, glucosamine, and a multivitamin. Despite apparent adherence to medical and nutritional therapy, her HbA1c was 8.2%. Following current guidelines for optimal diabetes care (which recommend HbA1c <7.0%) [15, 16, 17, 18, 19] and quality metrics set by the primary care clinician’s institution (which require HbA1c <8.0%), the patient’s clinician recommended treatment intensification and addition of basal insulin. At three-month follow up, the patient returned to the clinic with HbA1c 7.9%; she also reported occasional nocturnal hypoglycemia and insomnia.
What Is Glycemic Control?
Glycemic control refers to maintaining blood glucose levels as close as possible to the normal range in order to prevent the acute and chronic complications that would arise from living with glucose levels that are substantially higher or lower than the desired range [25]. There are several ways of ascertaining glycemic control in clinical practice. The most common, particularly for patients with type 2 diabetes, is the HbA1c– a measure of average glycemia over approximately three months. HbA1c is also used as the primary endpoint in clinical trials of glucose-lowering medications [26••, 27] and is the metric tracked as part of performance-based reimbursement to represent the quality of clinical diabetes care [15, 16, 17, 18, 19]. Fasting/pre-prandial glucose levels are another measure, used most frequently for medication (most notably insulin) dose adjustments. More recently, time-in-range (TIR) has emerged as a more holistic representation of glucose levels in insulin-treated patients using continuous glucose monitors, which correlates with both HbA1c levels and the risk of diabetes complications [15]. Therapeutic goals for HbA1c, pre-prandial glucose levels, and TIR have been established by professional societies based on evidence from numerous clinical trials [1, 15, 15, 17, 18, 18, 19, 19, 28, 29]. Glycemic control goals can be loosely categorized as intensive (i.e., targeting HbA1c ≤7.0%) or moderate (i.e., accepting HbA1c levels between 7.0 and 8.5%), as detailed in Table 1. Most clinical practice guidelines [31–32], with few exceptions [33], have advised intensive treatment targets for most non-pregnant adults living with type 2 diabetes.
Table 1.
Statements of major diabetes guidelines regarding glycemic control
| Organization | Year | Statements |
|---|---|---|
| VA/DoD [17] | 2017 |
|
| ADA/EASD [28] | 2018 |
|
| ACP [42] | 2018 |
|
| AACE/ACE [30] | 2019 |
|
| ADA [41] | 2021 |
|
| NICE [18] | 2019 |
|
| ACC/AHA [29] | 2019 |
|
AACE American Association of Clinical Endocrinologists; ACC American College of Cardiology; ACE American College of Endocrinology; ACP American College of Physicians; ADA American Diabetes Association; AHA American Heart Association; CV cardiovascular; CVD cardiovascular disease; EASD European Association for the Study of Diabetes; GLP-1 glucagon-like peptide-1; HbA1c glycated hemoglobin; NICE National Institute for Health and Care Excellence; SGLT-2 sodium-glucose transport protein 2; VA/DoD Veterans Affairs and Department of Defense
Glucose-Centric Paradigm of Glycemic Control
The glucose-centric paradigm considers hyperglycemia as the predominant factor in the development of diabetes and its complications and thus seeks to reduce glucose levels as close to as possible to euglycemia. This paradigm dates back to the 1960s when two epidemiologic studies linked intensive glycemic control with a reduction in the incidence of diabetes-related microvascular complications. This included reductions in >3 lines visual acuity (63% relative risk reduction), severe non-proliferative/proliferative retinopathy (47% relative risk reduction), laser treatment for diabetes eye disease (51% relative risk reduction), urinary albumin excretion (around 50% relative risk reduction), and clinical neuropathy (60% relative risk reduction) [5, 6, 34, 35].
Three treat-to-target RCTs comparing intensive vs. conventional glycemic control among patients with type 1 or type 2 diabetes were published in the 1990s and bolstered the hypothesis that achieving euglycemia through an intensive glycemic control strategy would reduce the incidence of both micro- and macrovascular complications. The intensive treatment arm of the Diabetes Control and Complication Trial (DCCT) in type 1 diabetes, which targeted fasting and postprandial glucose levels of 70–120 mg/dL and <180 mg/dL [31], respectively; the UK Prospective Diabetes Study (UKPDS) in patients with newly diagnosed type 2 diabetes which compared targeting fasting glucose levels <106 mg/dL (6 mmol/L) vs. <270 mg/dL (15 mmol/L) [27]; and the Kumamoto Study in patients with non-insulin dependent Diabetes Mellitus, which compared targeting fasting glucose <140 mg/dL, post-prandial glucose <200 mg/dL, and HbA1c <7.0% vs. fasting glucose level <140 mg/dL in the conventional treatment group [33]. These trials revealed that intensive glycemic control was associated with a decrease in the risk of microvascular complications of the disease (retinopathy, nephropathy and neuropathy). UKPDS also demonstrated significant associations between the HbA1c level and the development of diabetes complications, paving the way for the evolution of glycemic ascertainment in both research and practice from glucose to HbA1c [16, 36, 37, 38].
Evidence from these early observational and treat-to-target RCTs, as well as popularization of HbA1c as a simple and efficient way of assessing glucose levels that also correlated with long-term risks of diabetes complications [5, 6, 17, 18, 27], resulted in a glucose-centric framework of diabetes management solidified within professional society guidelines, quality measurement and reporting, and research. Current clinical practice guidelines uniformly define optimal glycemic control by an HbA1c level below a specific threshold. For the major professional societies, the following HbA1c targets have been endorsed: 6.0–7.0% by the Veterans Affairs/Department of Defense (VA/DoD) [28], ≤6.5% by the American Association of Clinical Endocrinologists/American College of Endocrinology (AACE/ACE) [28], ≤7.0% by the American Diabetes Association (ADA) [33], 6.5–7.0% by the National Institute for Health and Care Excellence (NICE) [39], ≤7.0% by the ADA/European Association for the Study of Diabetes (EASD) [40], and 7.0–8.0% by the American College of Physicians (ACP) guidelines [40]. While all guidelines acknowledge the need to individualize therapy based on each patient’s clinical complexity, life expectancy, burden of glucose-lowering medications needed to achieve the desired targets, and risk for hypoglycemia, they fail to inform clinicians how such individualization can be implemented and ultimately most guidelines still focus on HbA1c as the central treatment target in non-pregnant adult patients with diabetes [41, 42].
As a further testament to the centrality of the HbA1c, trials of glucose-lowering medications used for regulatory approval as well as comparative effectiveness trials of optimal glucose-lowering therapy (e.g., Glycemia Reduction Approaches in Diabetes [GRADE] trial), all focus on HbA1c as the primary outcome. Hard outcomes are usually relegated to secondary/exploratory positions or are excluded entirely [43]. Similarly, quality measurement used for public reporting, internal quality initiatives, and performance-based reimbursement all focus on HbA1c, either singularly [44] or in combination with other surrogate metrics of risk factor control [45], as the sole indicators of optimal diabetes care.
The glucose-centric approach to diabetes management also distorts patients’ perceptions and preferences for their care. A recent systematic review of studies that evaluated patient preferences for different glucose-lowering medications found that 90% of studies considered HbA1c as one of the priority attributes, while cardiovascular risk reduction was considered in fewer than 50% of included studies and microvascular risk reduction was not considered at all [46]. In a survey of nearly 5,000 patients with diabetes in the USA, HbA1c was chosen as the most important outcome of diabetes by 24% of respondents, second only to the risk of death (chosen by nearly 30% of respondents). Hard outcomes such as myocardial infarction, stroke, end-stage renal disease, neuropathy, blindness etc. were ranked as the most important outcome less often than HbA1c [47•]. Thus, patients—just like clinicians, professional societies, and payers—appear to prioritize surrogate numbers (i.e., HbA1c) more than actual health.
Limitations of a Glucose- Centric Approach
a. Focus on Surrogate Outcomes
A surrogate (also called intermediate) outcome is a measure of effect for a specific treatment that is presumed to be directly correlated with a hard outcome that is ultimately important to the patient or another stake-holder (e.g., doubling of creatinine and albuminuria are surrogate outcomes for the hard outcome of end-stage renal disease) [48, 49, 50]. Surrogate outcomes generally are not felt by patients, do not by themselves impair quality of life, are present upstream of the hard outcome they intend to portent, and are often represented by laboratory values. Surrogate outcomes are commonly used in clinical trials to examine treatment effects because they occur earlier and more frequently than hard outcomes, allowing trials to be smaller, shorter, and less expensive [51].
As a result, while clinicians and patients make treatment decisions with the goal of improving the patients’ hard outcomes (e.g., preventing disability, improving quality of life, prolonging life), the vast majority of these decisions is grounded in evidence supported by surrogate, not hard, outcomes. A recent meta-epidemiological study of nearly 400 clinical trials published in top endocrinology journals (66.7% of these trials were diabetes-related) reported that 9 out of 10 RCTs had surrogate outcomes as their primary outcome and 70% did not include any hard or patient-important outcome. Such reliance on surrogate outcomes is problematic when the strength of evidence linking them to their corresponding hard outcomes is weak or inconsistent [52•].
Ultimately, the goals of diabetes care are to decrease the risks of acute (i.e., severe hypoglycemia and hyper-glycemia) and chronic micro- and macrovascular diabetes complications while increasing life expectancy and quality of life [50]. While early evidence suggested that the risks of these complications correlate directly with HbA1c [21, 27, 33], thereby justifying the reliance on HbA1c as the surrogate outcome for optimal diabetes care, research that emerged over the past 15 years has not supported this glucose-centric paradigm [34]. Instead, multiple RCTs and meta-analyses that examined the association between intensive glycemic control (defined as HbA1c <6.0%, <6.5%, <6.9% or <7.0% depending on the study) and moderate glycemic control (defined as HbA1c between 7.0 and 7.9% or <7.5%) with respect to hard outcomes and found no such association [53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63]. With the exception of a 10–15% relative risk reduction (RRR) of non-fatal myocardial infarction (MI), there was no benefit of intensive glycemic control with regard to patient-important microvascular outcomes (specifically: end-stage kidney disease, renal death, blindness, and clinical neuropathy), macrovascular outcomes (specifically: CV mortality, non-fatal stroke, and amputation), or all-cause mortality. At the same time, intensive glycemic control did result in a 2–3-fold increase in the risk of severe hypoglycemia, weight gain, increased burden of treatment, higher costs of care, and greater likelihood of polypharmacy [64].
While HbA1c reduction itself did not appear to reduce microvascular and macrovascular complications risk in type 2 diabetes, subsequent trials revealed that use of specific classes of glucose-lowering medications did even in the absence of concurrent HbA1c reduction. Evidence from cardiovascular outcome trials (CVOT) of glucagon like peptide-1 receptor agonists and sodium glucose co-transporter-2 inhibitors demonstrated that micro- and macrovascular complications of type 2 diabetes could be observed without setting specific HbA1c thresholds or substantially lowering the HbA1c. HbA1c levels were approximately 8.0% in all the CVOTs, suggesting that (1) medications used to treat hyperglycemia matter with respect to improving health outcomes and (2) moderate glycemic control is adequate to see benefits in hard outcomes.
b. Conflation of Type 1 and Type 2 Diabetes
The strongest early evidence that supported intensive glycemic control and laid the foundation for the glucose-centric paradigm came from the DCCT [34, 35]. Conducted in patients with type 1 diabetes, DCCT demonstrated that intensive glycemic control (pre-prandial glucose 70–120 mg/dl, postprandial glucose <180 mg/dl, and HbA1c <6.0%) as compared to what was usual care at the time (no numeric glycemic targets, treatment intensification when HbA1c exceeded 13.5%) decreased the risk of >3 line visual acuity (63% relative risk reduction), severe non-proliferative/proliferative retinopathy (47% relative risk reduction), laser eye treatment (51% relative risk reduction), urinary albumin excretion (around 50% relative risk reduction), and clinical neuropathy (60% relative risk reduction) [34, 35]. Intensive treatment also tripled the risk of severe hypoglycemia. The Epidemiology of Diabetes Interventions and Complications (EDIC) Trial reporting on 17 years of observational follow-up after DCCT, further demonstrated that intensive glycemic control had a legacy effect and was associated with 42% relative risk reduction of any cardiovascular disease event and 57% relative risk reduction in the risk of nonfatal MI, stroke, or death from CV disease [65]. Many including guideline panelists, clinicians, and patients extrapolated these results into considering that intensive glycemic control is beneficial for all patients with diabetes. This overlooks two key factors. First, type 1 and type 2 diabetes, despite both presenting with high blood glucose levels and bearing the same name, vastly differ in their etiology, pathophysiology, treatment, and prognosis. Thus, reduction in hard outcomes with intensive glycemic control in type 1 diabetes does not translate to analogous inferences in type 2 diabetes [66]. Second, the DCCT compared intensive with substandard, by current definitions, glycemic control and thus cannot be extrapolated to suggest that moderate glycemic control is also inferior to intensive control. Thus, while HbA1c may be an adequate surrogate outcome for microvascular and macrovascular complications of type 1 diabetes, there is no similarly strong association between HbA1c and hard outcomes in type 2 diabetes.
c. The Glucose-Centric Approach Is Disease Specific and Context Blind
Clinical guidelines for the management of diabetes increasingly acknowledge the importance of a holistic, comprehensive, and patient-centered approach when caring for people living with diabetes [15, 28]. This is important as multi-morbidity is highly prevalent among patients with diabetes. Some comorbidities share their pathogenesis with diabetes and have aligned treatment goals; these diabetes-concordant comorbidities include metabolic disorders, cardiovascular disease, and kidney disease [67, 68]. Metabolic disorders such as hypertension, obesity, and dyslipidemia are present in at least three quarters of patients with diabetes [69, 70, 71, 67]. Approximately 30% of patients with diabetes have heart disease and 35% have some degree of kidney impairment. Other comorbidities are distinct from diabetes, yet also have significant effects on patient’s functional capacity and quality of life. These diabetes-discordant comorbidities include common conditions like hypothyroidism, osteoarthritis, depression, pulmonary disease, and cognitive impairment. Between 25 and 50% of patients with diabetes also experience depression [70, 71], and other mental health conditions like anxiety, obsessive compulsive disorder, and disordered eating are also common [72, 72]. In a recent survey, only 2.1% of Belgian patients with diabetes reported no comorbidities [73], and similar rates of multi-morbidity have been reported by other cross-sectional analyses [74, 75, 76]. All these health conditions impact the patient’s life. However, while their presence is acknowledged by the guidelines in the context of individualizing glycemic targets (reflective of the pervasive glucose-centric approach to the care for patients with diabetes), there is little acknowledgement or guidance on how to optimize the treatment of all the patient’s comorbidities to achieve the longest duration of high quality of life. This includes consideration of the burden of treatment – logistical and financial—as well as potentially contradictory treatment approaches of the different health conditions. A study estimated that in order to adhere to all guideline recommendations for the management of their chronic health conditions, patients must devote at least two hours every day to prescribed monitoring and treatment recommendations [77].
Thus, efforts to lower HbA1c levels to near normal—as recommended by the glucose-centric paradigm of contemporary clinical practice guidelines—may not achieve the desired hard outcomes, are likely to increase rates of iatrogenic hypoglycemia and weight gain, and can lead to high burden of treatment that impairs, not improves, their quality of life. The body of evidence on the management of type 2 diabetes therefore calls for a paradigm shift: a focus on treatment approaches that balance the totality of the patient’s medical and psychosocial needs, their capacity for self-management, and the goals of preventing the acute and chronic complications that truly matter to person living with diabetes [23].
A Patient-Centered Paradigm of Diabetes Management
High quality patient care needs to be effective (i.e., evidence-based), safe, and person-centered (i.e., responsive to the goals, preferences, and situation of the patient), while the means of delivering this care need to equitable, timely, and efficient. Pursuit of intensive glycemic control as part of a glucose-centric approach to type 2 diabetes care is neither effective (it does not translate to meaningful improvements in health outcomes in patients with type 2 diabetes), safe (it increases the risk of severe hypoglycemia and polypharmacy), nor person-centered (it is not individualized or responsive to the goals, preferences, and situation of the patient). We therefore propose an alternative, patient centered paradigm of type 2 diabetes care that seeks to prevent acute and chronic complications with least burden of treatment and risk for iatrogenic harm. This is a two-step process, whereby clinicians marry the best available evidence about treatment strategies that yield outcomes that are important to the patient with the patient’s goals, preferences, and capacity for adhering to that treatment regimen [78, 79, 80].
Effective type 2 diabetes management requires control not only of hyperglycemia, but also metabolic and other risk factors for microvascular and macrovascular complications associated with diabetes. For glycemic management, specifically, this is a combination of moderate glycemic control and preferential use of medications associated with greatest likelihood of benefit and least risk of harm [21]. Lowering blood glucose levels to a safe range is necessary to prevent symptoms of hyperglycemia (polyuria, polydipsia) and its immediate complications (poor wound healing, dehydration, hyperosmolar hyperglycemic syndrome, diabetic ketoacidosis, diabetic coma) without incurring hypoglycemia. Longer-term, moderate control of hyperglycemia—reflected by HbA1c levels between 7.0 and 8.0%—does correspond to lower rates of microvascular and macrovascular complications and is reasonable to pursue as long as real-time glucose levels remain within desired, safe parameters. Patients should also be treated with medications that are most likely to improve their health and are also affordable, tolerable, and do not cause undue burden of treatment.
In addition to managing hyperglycemia, clinicians will need to take the same approach to treating the patient’s other comorbidities, working alongside the patient to identify treatment goals and regimens that balance optimal outcomes with the patient’s goals, preferences, and capacity for self-care (Fig. 1). In the sections that follow, we offer guidance on how this can be achieved in real-world clinical practice.
Fig. 1.
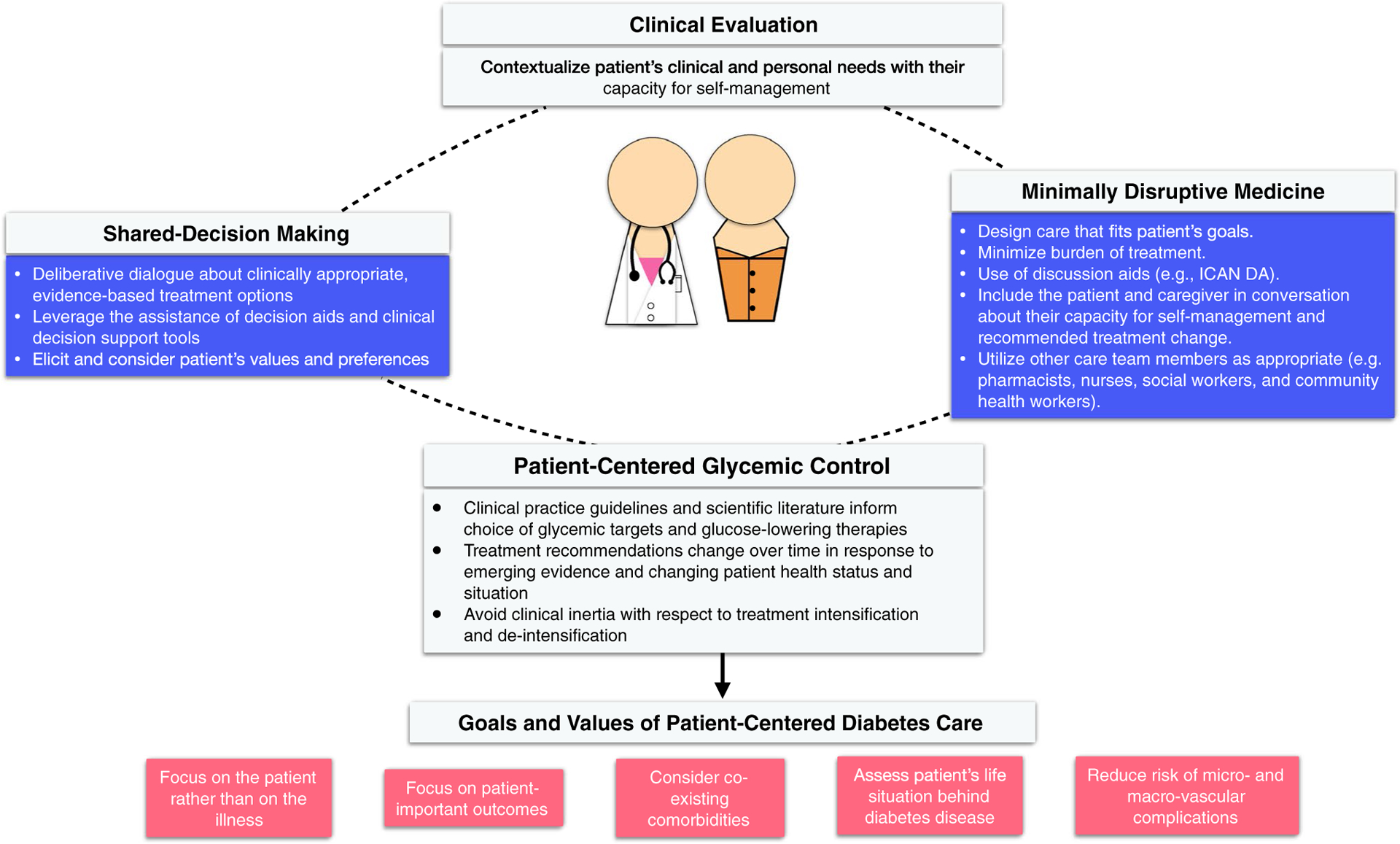
Patient’s goals, preferences, and capacity for self-care
In order to pursue person-centered diabetes care, clinicians therefore need to take the following steps:
Focus on the patient and not the illness.
Prioritize, and help the patient prioritize, as the ultimate goals of their therapy hard outcomes that translate to meaningful improvements in health (i.e., advanced kidney disease, visual impairment, clinical neuropathy) rather than surrogate outcomes that are mean to substitute for them (i.e., albuminuria, HbA1c).
Identify aspects of the patient’s life situation that may be hindered by diabetes and its treatment, as well as factors that influence the patient’s capacity for safe and effective self-management. These include a) symptomatic hyperglycemia, hypoglycemia, burden of treatment; b) challenges at home, work, community etc.; c) material support (financial, other resource); and d) social support (isolation, caregiver burden).
Identify and consider all of the patient’s co-existing comorbidities including how they need to be treated, how they impact capacity for self-management, and how their treatment interacts with diabetes treatment (and vice versa).
Use shared-decision making and minimally disruptive medicine tools to integrate scientific evidence with the patient’s goals, preferences, and situation.
In our clinical vignette, GGS’s HbA1c of 8.2% was above both the guideline-recommended target of 7% and the publicly reported quality threshold of 8%, and her clinician intensified therapy with the addition of basal insulin, also per the clinical guidelines [15, 16, 17, 18, 19]. Despite the addition of insulin, her HbA1c improved negligibly while nocturnal hypoglycemia and insomnia appeared with detriment to her quality of life. GGS ultimately saw a different clinician who—recognizing the medical (i.e., hypoglycemia) and personal (i.e., quality of life) impacts of treatment burden incurred by polypharmacy, multi-morbidity, and family stressors—stopped medications (i.e., insulin, omega-3, clopidogrel, bezafibrate, alopurinol, gabapentin, tramadol, vitamin C, glucosamine, ezetimibe, and multivitamin), prioritized depression management, and engaged a multidisciplinary team (i.e., endocrinologist, psychologist, psychiatrist, dietitian, diabetes educator) to support the patient in her self-management. This patient-centered, minimally disruptive approach was successful. Six months later, her medication list decreased from 20 to 9 and her HbA1c decreased to 7.4% without need for insulin. Her hypoglycemia resolved, sleep quality improved, depressive symptoms lessened, and she reported full adherence to her prescribed medications. Most importantly, she endorsed increased capacity and bandwidth to engage with her daily activities and enjoy her family.
Tools to Support Patient-Centered, Minimally Disruptive Care
Shared Decision-Making
Shared decision-making is an approach to medical decision-making in which patients and clinicians work together and engage in a deliberative dialogue about clinically appropriate treatment options [81, 82, 83, 84]. Shared decision-making recognizes that in situations where there are multiple reasonable therapeutic approaches from the clinician’s perspective of biomedical expertise, the patient’s perspective as the expert in their illness, life, and capacity needs to guide the ultimate choice of care to ensure that it is both right and feasible for them [85]. Shared decision-making supports patients’ autonomy and makes their care patient-centered [86, 87].
One effective tool to support shared decision-making is a decision aid. Decision aids are evidence-based tools designed to communicate the best available evidence about a therapeutic approach and to facilitate informed conversation between the patient and the clinician [88]. Decision aids can be videos, worksheets, interactive cards, or web applications. Several systematic reviews demonstrated that interactions that utilize decision aids result in patients feeling more engaged decision-making and confident in their knowledge of what is important to them, better understanding of the balance between the harms and benefits of treatment alternatives, and reduced decisional conflict [89, 90]. Decision aids have been developed and validated for multiple conditions, including diabetes, depression, asthma, atrial fibrillation, and statin use [91, 92, 93, 94, 95, 96, 97, 98]. The Diabetes Medication Choice decision aid, in particular, is freely available and has been formally tested and that helps clinicians and patients identify preferred glucose-lowering medication(s) based on their efficacy, side effect, administration, and cost profiles [94] (Table 2). Unfortunately, currently there is no decision aid for HbA1c targets. However, a quality indicator for the appropriateness of type 2 diabetes management was proposed recently [108]. This quality indicator is based on HbA1c but is placed in the context of the patient complexity and the treatment intensity. This quality indicator proposes classifying the patients into treated appropriately, over treated, or undertreated based on a matrix of clinical complexity, HbA1c level, and medications used [108].
Table 2.
Decision aids for shared decision making
| Decision aid | Institution | Location | Link |
|---|---|---|---|
| Diabetes Medication Choice[91] | Knowledge and Evaluation Research Unit, Mayo Clinic. | Rochester, MN, USA | https://carethatfits.org/diabetes-medication-choice/ |
| Type 2 diabetes in adults: controlling your blood glucose by taking a second medicine – what are your options?[99] | National Institute for Health and Care Excellence | London, UK | https://www.nice.org.uk/guidance/ng28/resources/patient-decision-aid-2187281197 |
| Diabetes, Type 2: Should I take insulin?[100] | Healthwise, Incorporated. | Idaho, US | https://decisionaid.ohri.ca/AZlist.html |
| Diabetes: Should I Get an Insulin Pump?[101] | Healthwise, Incorporated. | Idaho, US | https://decisionaid.ohri.ca/AZlist.html |
| Diabetes: Should I Get Pregnant?[102] | Healthwise, Incorporated. | Idaho, US | https://decisionaid.ohri.ca/AZlist.html |
| Type 2 diabetes in adults: management[103] | National Institute for Health and Care Excellence | London, UK | https://www.nice.org.uk/guidance/ng28/resources/patient-decision-aid-1687717 |
| PANDAs decision aid[95] | Academic Unit of Primary Medical Care, Northern General Hospital, University of Sheffield | Sheffield, UK | https://bmjopen.bmj.com/content/2/6/e001469 |
| OPTIMAL decision aid[104] | Julius Centre for Health Sciences and Primary Care, University Medical Centre Utrecht | Utrecht, The Netherlands | https://bmcfampract.biomedcentral.com/articles/10.1186/s12875-015-0230-0 |
| PORTDA-diab(105) | Department of Clinical Pharmacology, University of Groningen, University Medical Center Groningen | Groningen, The Netherlands | https://trialsjournal.biomedcentral.com/articles/10.1186/1745-6215-13-219 |
| The Interactiva Diabetes Medication Aid for Type 2 diabetes [106] | EPI-Q Incorporated, Shared Decision-making Resources, and Janssen Scientific Affairs LLC. | Oak Brook, IL; George-town, ME; and Raritan, NJ, US. | https://www.dovepress.com/development-of-a-patient-decision-aid-for-type-2-diabetes-mellitus-for-peer-reviewed-fulltext-article-PPA |
| iDecide [107] | Center of Health Communications Research, University of Michigan | Michigan, US. | https://muse.jhu.edU/article/510563#info_wrap |
Minimally Disruptive Medicine
Diabetes is a complex and multifaceted disease that requires the simultaneous management of hyperglycemia and other cardiovascular risk factors (e.g., hypertension, hyperlipidemia, chronic kidney disease), is often just one of many comorbidities the patient is living with, and necessitates frequent monitoring and clinical touch points. This high disease burden translates to high burden of treatment, diminished bandwidth for non-clinical activities, and disruption of life’s routines. Minimally disruptive medicine (MDM) is a patient-centered approach to care that strives to impose the least possible treatment burden [24]. It is a collaborative effort between the patient and the clinician to design care that fits the patient’s goals and priorities and does not exceed their capacity for self-care [24]. In essence, shared decision making seeks to balance evidence-based medicine with the patient’s goals, preferences, and situation to identify a MDM approach to care. One tool that have been formally tested to implement MDM is the ICAN Discussion Aid (minimallydisruptivemedicine.org/ican/) [109]. This conversation tool is designed for clinicians, nurses, care coordinators, social workers, community health workers, and health coaches to engage in a conversation with their patients and help understand their capacity and treatment burden. ICAN is intended to focus patient care on the patient instead of the illness [109].
Implementing patient-centered, minimally disruptive diabetes care in routine clinical practice may represent a challenge in terms of time, effort, and education for both patients and clinicians. The emergence of shared-decision making and MDM aids can make it easier for the healthcare system to adopt this approach, but will require a paradigm shift in how the quality and value of diabetes care are measured, communicated, and reimbursed. While the preponderance of evidence supports moderate, rather than intensive, glycemic targets, contemporary professional society guidelines, institutional initiatives, public health campaigns, and performance-based reimbursement all focus on intensive control of surrogate metrics rather than long-term reduction of complications, goal-concordant care, and improved quality of life. Similarly, while there is robust evidence for the use of glucose-lowering medications that improve hard outcomes that patients care about, these medications are often not accessible to patients because of insurance restrictions on their use (e.g., step therapy, prior authorization requirements) and high cost sharing responsibility making them unaffordable even if technically covered by the patient’s health plan.
Conclusion
The goals of diabetes therapy are to reduce the risks of acute and chronic complications and increase quality of life while incurring least burden of treatment and disruption to the patient’s life. This paradigm of minimally disruptive medicine can be achieved through shared decision making, whereby evidence-based medicine is contextualized in the patient’s goals, preferences, and situation through informed conversation. Importantly, contemporary evidence supports moderate—not intensive—glycemic control targets and preferential use of medications that result in outcomes that patients and society ultimately care about, i.e., the prevention of complications. Achieving such high-quality care will require a paradigm shift away from the simplicity of surrogate metrics and a one-size-fits-all approach to care. Patient-centered care can be supported in clinical practice by validated SDM decision aids and MDM conversational tools. However, professional societies, health systems, regulatory bodies, and insurance companies will also need to play an active role in supporting patient-centered care if we are to see meaningful improvements in the health and wellbeing of all people living with diabetes.
Funding
RGM is funded by the National Institute of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant number K23DK114497. Study contents are the sole responsibility of the authors and do not necessarily represent the official views of NIH. The study sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Competing Interests
In the past 36 months, Dr. McCoy also received research support from NIDDK (P30DK111024; R03DK127010), AARP (AARP® Quality Measure Innovation Grant through a collaboration with OptumLabs® and the NQF Measure Incubator™), and Mayo Clinic. All other authors declare no competing interests.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Organization WH. Factsheet/Diabetes World Health Organization2021 [cited 2021 21/05/2021]. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes.
- 2.Organization WH. Diabetes Infographic 2016 [cited 2021 05/14/2021]. Available from: https://www.who.int/diabetes/global-report/WHD2016_Diabetes_Infographic_v2.pdf?ua=1.
- 3.Prevention CfDCa. Type 2 Diabetes 2019 [cited 2021 05/14/2021]. Available from: https://www.cdc.gov/diabetes/basics/type2.html.
- 4.Classification and Diagnosis of Diabetes 2021. Standards of Medical Care in Diabetes—2021 Diabetes care. 44 Supplement 1 S15. [DOI] [PubMed] [Google Scholar]
- 5.Hardin RC, Jackson RL, Johnston TL, Kelly HG. The development of diabetic retinopathy; effects of duration and control of diabetes. Diabetes. 1956;5(5):397–405. [DOI] [PubMed] [Google Scholar]
- 6.Keiding NR, Root HF, Marble A. Importance of control of diabetes in prevention of vascular complications. J Am Med Assoc. 1952;150(10):964–9. [DOI] [PubMed] [Google Scholar]
- 7.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ (Clinical research ed). 2000;321(7258):405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003;63(1):225–32. [DOI] [PubMed] [Google Scholar]
- 9.Frank RN. Diabetic retinopathy. New Engl J Med. 2004;350(1):48–58. [DOI] [PubMed] [Google Scholar]
- 10.Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, Pach JM, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993;43(4):817–24. [DOI] [PubMed] [Google Scholar]
- 11.Morris AD, McAlpine R, Steinke D, Boyle DI, Ebrahim AR, Vasudev N, et al. Diabetes and lower-limb amputations in the community. A retrospective cohort study. DARTS/MEMO Collaboration. Diabetes Audit and Research in Tayside Scotland/Medicines Monitoring Unit. Diabetes Care. 1998;21(5):738–43. [DOI] [PubMed] [Google Scholar]
- 12.Chen R, Ovbiagele B, Feng W. Diabetes and Stroke: Epidemiology, Pathophysiology, Pharmaceuticals and Outcomes. AmJ Med Sci. 2016;351(4):380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pazin-Filho A, Kottgen A, Bertoni AG, Russell SD, Selvin E, Rosamond WD, et al. HbA 1c as a risk factor for heart failure in persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia. 2008;51(12):2197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Int Med. 2004;141(6):421–31. [DOI] [PubMed] [Google Scholar]
- 15.Glycemic Targets 2019. Standards of Medical Care in Diabetes—2019 Diabetes care. 42 Supplement 1 S61. [DOI] [PubMed] [Google Scholar]
- 16.The American Association of Clinical Endocrinologists Medical Guidelines for the Management of Diabetes Mellitus: the AACE system of intensive diabetes self-management--2000 update. Endocrine Pract. 2000;6(1):43–84. [PubMed] [Google Scholar]
- 17.US Veterans Affairs DoD. VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care 2017. [cited 2021 3/3/2021]. Available from: https://www.healthquality.va.gov/guidelines/cd/diabetes/.
- 18.Excellence NIfHaC. Type 2 diabetes in adults: management NICE 2015. [cited 2021 3/3/2021]. Available from: https://www.nice.org.uk/guidance/ng28.
- 19.Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA. Hemoglobin A1c Targets for Glycemic Control With Pharmacologic Therapy for Nonpregnant Adults With Type 2 Diabetes Mellitus: A Guidance Statement Update From the American College of Physicians. Ann Int Med. 2018;168(8):569–76. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Gutierrez R, Lipska KJ, McCoy RG. Intensive Glycemic Control in Type 2 Diabetes Mellitus – A Balancing Act of Latent Benefit and Avoidable Harm: A Teachable Moment. JAMA Int Med. 2016;176(3):300–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Gutierrez R, Gonzalez-Gonzalez JG, Zuñiga-Hernandez JA, McCoy RG. Benefits and harms of intensive glycemic control in patients with type 2 diabetes. BMJ (Clinical research ed). 2019;367. [DOI] [PubMed] [Google Scholar]
- 22.Espinoza P, Varela CA, Vargas IE, Ortega G, Silva PA, Boehmer KB, et al. The burden of treatment in people living with type 2 diabetes: A qualitative study of patients and their primary care clinicians. PloS one. 2020;15(10):e0241485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serrano Larrea V, Spencer G, Boehmer K, Montori V. Minimally Disruptive Medicine for Patients with Diabetes. 2017. [DOI] [PubMed]
- 24.Leppin AL, Montori VM, Gionfriddo MR. Minimally Disruptive Medicine: A Pragmatically Comprehensive Model for Delivering Care to Patients with Multiple Chronic Conditions. Healthcare (Basel, Switzerland). 2015;3(1):50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Diabetes A. Postprandial Blood Glucose Diabetes Care. 2001;24(4):775. [DOI] [PubMed] [Google Scholar]
- 26.••.Rodríguez-Gutiérrez R, Montori VM. Glycemic control for patients with type 2 diabetes mellitus: our evolving faith in the face of evidence. Circulation. 2016;9(5):504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]; Meta-analysis that reported no significant impact of tight glycemic control on the risk of dialysis/transplantation/renal death, blindness, or neuropathy even when most published statements from medical associations endorsed benefit.
- 27.Group UPDS. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–53. [PubMed] [Google Scholar]
- 28.Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garber AJ, Handelsman Y, Grunberger G, Einhorn D, Abraham-son MJ, Barzilay JI, et al. Consensus statement by the american association of clinical endocrinologists and american college of endocrinology on the comprehensive type 2 diabetes management algorithm - 2020 executive summary. Endoc Pract. 2020;26(1):107–39. [DOI] [PubMed] [Google Scholar]
- 31.The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. New Engl J Med. 1993;329(14):977–86. [DOI] [PubMed] [Google Scholar]
- 32.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-Year Follow-up of Intensive Glucose Control in Type 2 Diabetes. New Engl J Med. 2008;359(15):1577–89. [DOI] [PubMed] [Google Scholar]
- 33.Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28(2):103–17. [DOI] [PubMed] [Google Scholar]
- 34.Diabetes Control and Complications Trial (DCCT): results of feasibility study. The DCCT Research Group. Diabetes Care. 1987;10(1):1–19. [DOI] [PubMed] [Google Scholar]
- 35.Nathan DM, Group DER. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Association AD. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2002;25(1):213–29. [DOI] [PubMed] [Google Scholar]
- 37.Song SH, Gray TA. Management of type 2 diabetes and lipids: a critique of the NICE guidelines 2008. Brit J Diabetes Vasc Dis. 2009;9(2):69–74. [Google Scholar]
- 38.Force ICGT. Global Guideline for Type 2 Diabetes: recommendations for standard, comprehensive, and minimal care. Diabetic Med. 2006;23(6):579–93. [DOI] [PubMed] [Google Scholar]
- 39.Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New Engl J Med. 1993;329(14):977–86. [DOI] [PubMed] [Google Scholar]
- 40.Handelsman Y, Bloomgarden ZT, Grunberger G, Umpierrez G, Zimmerman RS, Bailey TS, et al. American Association of Clinical Endocrinologists and American College of Endocrinology–clinical practice guidelines for developing a diabetes mellitus comprehensive care plan–2015—executive summary. Endoc Pract 2015;21(4):413–37. [PubMed] [Google Scholar]
- 41.Association AD. 6. Glycemic Targets: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(Supplement 1):S73–S84. [DOI] [PubMed] [Google Scholar]
- 42.Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA. Hemoglobin A1c Targets for Glycemic Control With Pharmacologic Therapy for Nonpregnant Adults With Type 2 Diabetes Mellitus: A Guidance Statement Update From the American College of Physicians. Ann Int Med 2018;168(8):569–76. [DOI] [PubMed] [Google Scholar]
- 43.Nathan DM, Buse JB, Kahn SE, Krause-Steinrauf H, Larkin ME, Staten M, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36(8):2254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LaVecchia CM, Montori VM, Shah ND, McCoy RG. Values informing the development of an indicator of appropriate diabetes therapy: qualitative study. BMJ Open. 2020;10(12):e044395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MNCM. Minnesota community measurement data collection guide: optimal diabetes care specifications, 2019 report year (01/01/2018 to 12/31/2018 dates of service). 2018.
- 46.Toroski M, Kebriaeezadeh A, Esteghamati A, Karyani AK, Abbasian H, Nikfar S. Patient and physician preferences for type 2 diabetes medications: a systematic review. J Diabetes Metab Disord. 2019;18(2):643–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.•.Murad MH, Shah ND, Van Houten HK, Ziegenfuss JY, Deming JR, Beebe TJ, et al. Individuals with diabetes preferred that future trials use patient-important outcomes and provide pragmatic inferences. J Clin Epidemiol. 2011;64(7):743–8. [DOI] [PubMed] [Google Scholar]; Survey on 2,036 patients with diabetes that reported a strong preference for trials using patient-important outcomes as primary outcomes rather than HbA1c.
- 48.Montori VM, Gandhi GY, Guyatt GH. Patient-important outcomes in diabetes–time for consensus. Lancet (London, England). 2007;370(9593):1104–6. [DOI] [PubMed] [Google Scholar]
- 49.Yudkin JS, Lipska KJ, Montori VM. The idolatry of the surrogate. BMJ (Clinical research ed). 2011;343:d7995. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez-Gutierrez R, McCoy RG. Measuring What Matters in Diabetes. JAMA. 2019;321(19):1865–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fleming TR. Surrogate endpoints in clinical trials. Drug Inf J. 1996;30(2):545–51. [Google Scholar]
- 52.•.Dorsey-Treviño EG, Alvarez-Villalobos N, González-González JG, González-Colmenero AD, Barrera-Flores FJ, McCoy RG, et al. Outcomes that patients perceive and value are systematically unassessed in randomized clinical trials of endocrine-related illnesses: a systematic review. J Clin Epidemiol. 2019;106:140–3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Meta-epidemiological study that demonstrated a scarcity of assessment of patient-important outcomes in clinical trials of endocrine-related illnesses.
- 53.Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassaï B, et al. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ (Clinical research ed). 2011;343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coca SG, Ismail-Beigi F, Haq N, Krumholz HM, Parikh CR. Role of intensive glucose control in development of renal end points in type 2 diabetes mellitus: systematic review and meta-analysis. Arch Int Med. 2012;172(10):761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buehler AM, Cavalcanti AB, Berwanger O, Figueiro M, Laranjeira LN, Zazula AD, et al. Effect of tight blood glucose control versus conventional control in patients with type 2 diabetes mellitus: a systematic review with meta-analysis of randomized controlled trials. Cardiovas Therap. 2013;31(3):147–60. [DOI] [PubMed] [Google Scholar]
- 56.Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal T, Hemmingsen C, et al. Intensive glycaemic control for patients with type 2 diabetes: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. BMJ (Clinical research ed). 2011;343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herrera-Gómez F, Asensio-González M, González-López A, Álvarez FJ. Effects of Intensive Control of Glycemia on Clinical Kidney Outcomes in Type 2 Diabetes Patients Compared with Standard Control: A Meta-Analysis. Front Pharmacol. 2017;8:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Int Med 2009;151(6):394–403. [DOI] [PubMed] [Google Scholar]
- 59.Montori VM, Fernández-Balsells M. Glycemic control in type 2 diabetes: time for an evidence-based about-face? Ann Int Med 2009;150(11):803–8. [DOI] [PubMed] [Google Scholar]
- 60.Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nether-cott S, Preiss D, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet (London, England). 2009;373(9677):1765–72. [DOI] [PubMed] [Google Scholar]
- 61.Sardar P, Udell JA, Chatterjee S, Bansilal S, Mukherjee D, Farkouh ME. Effect of Intensive Versus Standard Blood Glucose Control in Patients With Type 2 Diabetes Mellitus in Different Regions of the World: Systematic Review and Meta-analysis of Randomized Controlled Trials. J Am Heart Assoc. 2015;4(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tkác I Effect of intensive glycemic control on cardiovascular outcomes and all-cause mortality in type 2 diabetes: Overview and metaanalysis of five trials. Diabetes Res Clin Pract. 2009;86(Suppl 1):S57–62. [DOI] [PubMed] [Google Scholar]
- 63.Zhang X, Zhao J, Zhao T, Liu H. Effects of intensive glycemic control in ocular complications in patients with type 2 diabetes: a meta-analysis of randomized clinical trials. Endocrine. 2015;49(1):78–89. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez-Gutierrez R, Lipska KJ, McCoy RG, Ospina NS, Ting HH, Montori, Victor M. Hypoglycemia as an indicator of good diabetes care. BMJ (Clinical research ed). 2016;352:i1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22(1):99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skyler JS, Bakris GL, Bonifacio E. Differentiation of Diabetes by Pathophysiology. Nat Hist Progn. 2017;66(2):241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29(3):725–31. [DOI] [PubMed] [Google Scholar]
- 68.McCoy RG, Lipska KJ, Van Houten HK, Shah ND. Paradox of glycemic management: multimorbidity, glycemic control, and high-risk medication use among adults with diabetes. BMJ Open Diabetes Res Care. 2020;8(1):e001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nowakowska M, Zghebi SS, Ashcroft DM, Buchan I, Chew-Graham C, Holt T, et al. The comorbidity burden of type 2 diabetes mellitus: patterns, clusters and predictions from a large English primary care cohort. BMC Med. 2019;17(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hermans MP, Dath N. Prevalence and co-prevalence of comorbidities in Belgian patients with type 2 diabetes mellitus: a transversal, descriptive study. Acta Clin Belgica. 2018;73(1):68–74. [DOI] [PubMed] [Google Scholar]
- 71.Jelinek HF, Osman WM, Khandoker AH, Khalaf K, Lee S, Almahmeed W, et al. Clinical profiles, comorbidities and complications of type 2 diabetes mellitus in patients from United Arab Emirates. BMJ Open Diabetes Res Care. 2017;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin P-J, Pope E, Zhou FL. Comorbidity type and health care costs in type 2 diabetes: a retrospective claims database analysis. Diabetes Therapy. 2018;9(5):1907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pashaki MS, Mezel JA, Mokhtari Z, Gheshlagh RG, Hesabi PS, Nematifard T, et al. The prevalence of comorbid depression in patients with diabetes: A meta-analysis of observational studies. Diabetes Metab Syndrome. 2019;13(6):3113–9. [DOI] [PubMed] [Google Scholar]
- 74.Khaledi M, Haghighatdoost F, Feizi A, Aminorroaya A. The prevalence of comorbid depression in patients with type 2 diabetes: an updated systematic review and meta-analysis on huge number of observational studies. Acta Diabetol. 2019;56(6):631–50. [DOI] [PubMed] [Google Scholar]
- 75.Winston AP. Eating Disorders and Diabetes. Curr Diabetes Rep. 2020;20(8):32. [DOI] [PubMed] [Google Scholar]
- 76.Buchberger B, Huppertz H, Krabbe L, Lux B, Mattivi JT, Siafarikas A. Symptoms of depression and anxiety in youth with type 1 diabetes: A systematic review and meta-analysis. Psycho-neuroendocrinology. 2016;70:70–84. [DOI] [PubMed] [Google Scholar]
- 77.Russell LB, Suh DC, Safford MA. Time requirements for diabetes self-management: too much for many? J Family Pract. 2005;54(1):52–6. [PubMed] [Google Scholar]
- 78.Evidence-based medicine. A new approach to teaching the practice of medicine. JAMA. 1992;268(17):2420–5. [DOI] [PubMed] [Google Scholar]
- 79.Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ (Clinical research ed). 1996;312(7023):71–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Straus SE, Sackett DL. Applying evidence to the individual patient. Ann Oncol. 1999;10(1):29–32. [DOI] [PubMed] [Google Scholar]
- 81.Stiggelbout AM, Van der Weijden T, De Wit MP, Frosch D, Légaré F, Montori VM, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ (Clinical research ed). 2012;344:e256. [DOI] [PubMed] [Google Scholar]
- 82.Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med (1982). 1999;49(5):651–61. [DOI] [PubMed] [Google Scholar]
- 83.Elwyn G, Lloyd A, May C, van der Weijden T, Stiggelbout A, Edwards A, et al. Collaborative deliberation: a model for patient care. Patient Educ Counsel. 2014;97(2):158–64. [DOI] [PubMed] [Google Scholar]
- 84.Rodriguez-Gutierrez R, Gionfriddo MR, Ospina NS, Maraka S, Tamhane S, Montori VM, et al. Shared decision making in endocrinology: present and future directions. Lancet Diabetes Endocrinol. 2016;4(8):706–16. [DOI] [PubMed] [Google Scholar]
- 85.Guyatt GMM, Rennie D, Cook DJ. Users’ guides to the medical literature: a manual for evidence-based clinical practice. 3rd ed. New York: McGraw-Hill Medical; 2015. [Google Scholar]
- 86.Institute of Medicine Committee on Quality of Health Care in A. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington (DC): National Academies Press (US) [Google Scholar]
- 87.Copyright 2001 by the National Academy of Sciences. All rights reserved.; 2001.
- 88.Drake RE, Deegan PE. Shared decision making is an ethical imperative. Psychiatric services (Washington, DC: ). 2009;60(8):1007. [DOI] [PubMed] [Google Scholar]
- 89.Agoritsas T, Heen AF, Brandt L, Alonso-Coello P, Kristiansen A, Akl EA, et al. Decision aids that really promote shared decision making: the pace quickens. BMJ (Clinical research ed). 2015;350:g7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stacey D, Légaré F, Col NF, Bennett CL, Barry MJ, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane database Syst Rev. 2014(1):Cd001431. [DOI] [PubMed] [Google Scholar]
- 91.Durand MA, Carpenter L, Dolan H, Bravo P, Mann M, Bunn F, et al. Do interventions designed to support shared decision-making reduce health inequalities? A systematic review and meta-analysis. PloS one. 2014;9(4):e94670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mann DM, Ponieman D, Montori VM, Arciniega J, McGinn T. The Statin Choice decision aid in primary care: a randomized trial. Patient Educ Counsel. 2010;80(1):138–40. [DOI] [PubMed] [Google Scholar]
- 93.Fraenkel L, Street RL Jr, Towle V, O’Leary JR, Iannone L, Van Ness PH, et al. A pilot randomized controlled trial of a decision support tool to improve the quality of communication and decision-making in individuals with atrial fibrillation. J Am Geriatr Society. 2012;60(8):1434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thomson RG, Eccles MP, Steen IN, Greenaway J, Stobbart L, Murtagh MJ, et al. A patient decision aid to support shared decision-making on anti-thrombotic treatment of patients with atrial fibrillation: randomised controlled trial. Qual Saf Health Care. 2007;16(3):216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mullan RJ, Montori VM, Shah ND, Christianson TJ, Bryant SC, Guyatt GH, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med. 2009;169(17):1560–8. [DOI] [PubMed] [Google Scholar]
- 96.Mathers N, Ng CJ, Campbell MJ, Colwell B, Brown I, Bradley A. Clinical effectiveness of a patient decision aid to improve decision quality and glycaemic control in people with diabetes making treatment choices: a cluster randomised controlled trial (PANDAs) in general practice. BMJ Open. 2012;2(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Man-Son-Hing M, Laupacis A, O’Connor AM, Biggs J, Drake E, Yetisir E, et al. A patient decision aid regarding antithrombotic therapy for stroke prevention in atrial fibrillation: a randomized controlled trial. JAMA. 1999;282(8):737–43. [DOI] [PubMed] [Google Scholar]
- 98.Loh A, Simon D, Wills CE, Kriston L, Niebling W, Härter M. The effects of a shared decision-making intervention in primary care of depression: a cluster-randomized controlled trial. Patient Educ Counsel. 2007;67(3):324–32. [DOI] [PubMed] [Google Scholar]
- 99.Boehmer KR, Dobler CC, Thota A, Branda M, Giblon R, Behnken E, et al. Changing conversations in primary care for patients living with chronic conditions: pilot and feasibility study of the ICAN Discussion Aid. BMJ Open. 2019;9(9):e029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Excellence NIfHaC. Type 2 diabetes in adults: controlling your blood glucose by taking a second medicine – what are your options? 2015. [05/21/2021]. Available from: https://www.nice.org.uk/guidance/ng28/resources/patient-decision-aid-2187281197.
- 101.Healthwise. Diabetes, Type 2: Should I take insulin? : Healthwise; 2020. [05/21/2021]. Available from: https://www.healthwise.org.
- 102.Healthwise. Diabetes: Should I Get an Insulin Pump? : Healthwise; 2020. [05/21/2021]. Available from: https://www.healthwise.org.
- 103.Healthwise. Diabetes: Should I Get Pregnant? : Healthwise; 2020. [05/21/2021]. Available from: https://www.healthwise.org.
- 104.Excellence NIfHaC. Type 2 diabetes in adults: management: National Institute for Health and Care Excellence; 2015. [05/21/2021]. Available from: https://www.nice.org.uk/guidance/ng28/ifp/chapter/About-this-information. [PubMed]
- 105.den Ouden H, Vos RC, Reidsma C, Rutten GE. Shared decision making in type 2 diabetes with a support decision tool that takes into account clinical factors, the intensity of treatment and patient preferences: design of a cluster randomised (OPTIMAL) trial. BMC Family Pract. 2015;16:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Denig P, Dun M, Schuling J, Haaijer-Ruskamp FM, Voorham J. The effect of a patient-oriented treatment decision aid for risk factor management in patients with diabetes (PORTDA-diab): study protocol for a randomised controlled trial. Trials. 2012;13:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shillington AC, Col N, Bailey RA, Jewell MA. Development of a patient decision aid for type 2 diabetes mellitus for patients not achieving glycemic control on metformin alone. Patient Prefer Adherence. 2015;9:609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gagné ME, Légaré F, Moisan J, Boulet LP. Development of a patient decision aid on inhaled corticosteroids use for adults with asthma. J Asthma. 2016;53(9):964–74. [DOI] [PubMed] [Google Scholar]
- 109.McCoy RG, Lipska KJ, Van Houten HK, Shah ND. Development and evaluation of a patient-centered quality indicator for the appropriateness of type 2 diabetes management. BMJ Open Diabetes Res Care. 2020;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Henderson VA, Barr KL, An LC, Guajardo C, Newhouse W, Mase R, et al. Community-based participatory research and user-centered design in a diabetes medication information and decision tool. Prog Community Health Partnersh. 2013;7(2):171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]


