Abstract
Background
Sarcopenia has emerged as an important parameter to predict outcomes and treatment toxicity. However, limited data are available to assess sarcopenia prevalence in metastatic breast cancer and to evaluate its management.
Methods
The SCAN study was a cross-sectional multicenter French study that aimed to estimate sarcopenia prevalence in a real-life sample of metastatic cancer patients. Sarcopenia was identified by low muscle mass (estimated from the skeletal muscle index at the third lumbar, via computed tomography) and low muscle strength (defined by handgrip strength). Three populations were distinguished based on EWGSOP criteria: a sarcopenic group with low muscle mass AND strength, a pre-sarcopenic group with low muscle mass OR strength and a normal group with high muscle mass AND strength.
Results
Among 766 included patients, 139 patients with breast cancer and median age of 61.2 years (29.9–97.8 years) were evaluable; 29.5% were sarcopenic and 41.0% were pre-sarcopenic. Sarcopenic patients were older (P < 0.01), had a worse PS-score (P < 0.05), and a higher number of metastatic sites (P < 0.01), the majority being hepatic and bone. A moderate agreement between the oncologist's diagnosis and sarcopenia evaluation by muscle mass and strength was recognized (Cohen's kappa = 0.45). No associations were found between sarcopenia and adverse event occurrence in the 12 patients for whom these were reported. Sarcopenic patients were underdiagnosed and nutritional care and physical activity were less proposed.
Conclusion
It is necessary to evaluate sarcopenia due to its impact on patient prognosis, and its utility in guiding patient management in metastatic breast cancer.
Keywords: Sarcopenia, Breast cancer, Skeletal mass index, Nutritional status, Toxicity
Highlights
-
•
Sarcopenia prevalence was 29.5% in metastatic breast cancer patients.
-
•
Pre-sarcopenia was found in 41.0% of metastatic breast cancer patients.
-
•
Anthropometric parameters or oncologist assessments do not fully assess sarcopenia.
-
•
Sarcopenia is under-diagnosed in metastatic cancer.
-
•
Sarcopenic patients are under-referred for nutritional management.
1. Introduction
While being overweight and obese were shown to be strong risk factors for breast cancer [1], the relationship between body mass index (BMI) and metastatic breast cancer (MBC) survival is still in a debate [[2], [3], [4], [5]]. We recently showed in UNICANCER Epidemio-Strategy-Medical-Economical-MBC national cohort that overweight and obesity are not associated with poorer outcomes in MBC, while underweight appears as an independent adverse prognostic factor [6]. A way to improve the identification of patients at risk of death could be to analyze body composition instead of BMI. Prado et al. were the first to demonstrate the utility of body composition (BC) analysis on survival and treatment toxicities in cancers [7]. Since then, it has emerged as predictive factor of survival outcomes and toxicity in different type of cancers [8,9]. Computed tomography (CT) for the evaluation of BC has been validated, and a major advantage is that it could allow BC assessment simultaneously with tumor staging, monitoring and tumor response evaluation [10]. Sarcopenia has emerged as an interesting parameter for the assessment of BC features and the European Working Group on sarcopenia defined it as a progressive and generalized loss of skeletal muscle mass and strength, that may increase the risk of adverse outcomes [11].
Previous reports have highlighted that sarcopenia affects the efficacy and toxicity of chemotherapy in patients with non-metastatic [12,13] and metastatic breast cancers [14,15]. Sarcopenia was also associated with negative prognosis in adjuvant and metastatic breast cancers [[16], [17], [18]]. However, no data are available to assess the sarcopenia prevalence in MBC according to international consensus (including both low muscle mass with low muscle strength or performance) [19] and only one prospective study was conducted but with a small population of 55 [15].
The SCAN study is the first prospective investigation to measure sarcopenia (including both low muscle mass with low muscle strength) prevalence in a large group of real-life metastatic cancer patients, using CT-scan, biologic and anthropometric measurements. Other aims were to assess the impact and management of sarcopenia in real-life. Only results of breast cancer cohort are presented in this article.
2. Material and methods
2.1. Study design and patients
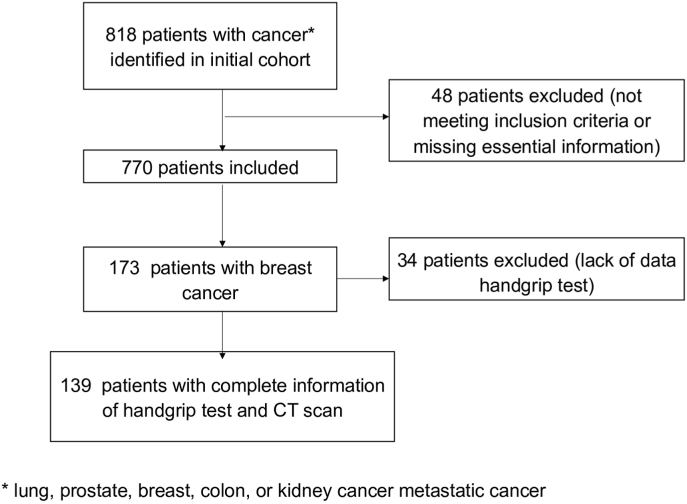
This cross-sectional study was performed in 29 private and public hospitals in France between September and October 2017. Patients were included if they were 18 years or older, had a confirmed diagnosis of metastatic lung, prostate, breast, colon, or kidney cancer, were undergoing chemotherapy, targeted therapy or hormonotherapy that was initiated since at least one cycle of treatment or for at least one month, irrespective of the line of ongoing treatment. Patients had a CT scan performed (for any reason) between 6 weeks before or 4 weeks after the inclusion study and included an L3 cross-section suitable for SMI evaluation of low muscle mass (Fig. 1). More details are available in the main publication [20]. In this present study, we present only the data from the breast cancer cohort.
Fig. 1.
Study Flow diagram in the SCAN-study and selection of patients. Among 818 case report forms (CRFs), 52 were excluded due to not meeting selection criteria or because they were missing essential information. 766 CRFs were ultimately included in the final study analysis. Among these patients, 173 patients had metastatic breast cancer, of whom 34 were excluded because of missing hand-grip test data. The final analysis population consisted of 139 patients.
2.2. Assessments and procedures
2.2.1. Clinical and biological characteristics
Oncologists, who were blinded to the CT-scan results, recorded information on clinical, demographic and pathological characteristics, treatments and toxicities, nutritional status and management, physical activity, food intake, biological data and anthropometric measures.
BMI was used to describe underweight, normal weight, overweight and obesity thresholds, as per the World Health Organization guidelines [21]. Weight prior to cancer diagnosis, at the study visit and at 1 and 6 months prior to the visit was also collected. Malnutrition was defined as weight loss >10% in 6 months, weight loss > 5% in 1 month, BMI ≤ 18.5 kg/m2 or < 21 kg/m2 if patients were 70 years old and older or serum albumin < 30 g/l or < 35 g/l if patients were 70 years old and older [22].
The mid-upper arm circumference (MUAC), thigh circumference and quadriceps atrophy was assessed by the oncologist. Muscle strength was assessed by handgrip strength of the dominant arm was measured using a JAMAR hydraulic hand dynamometer [20].
2.2.2. CT scan imaging
Radiologists were trained by a radiologist member of the SCAN study scientific committee in the measurement of the L3 total muscular surface area (TMS, cm2) using a standardized approach [23]. TMS was measured from the axial section of the third lumbar vertebra (L3). Skeletal muscle was identified and quantified by use of Hounsfield unit (HU) between thresholds (−29 to +150). Manual segmentation of residual structures that did not correspond to muscle was performed. The SMI at L3 (cm2/m2) was calculated as follows: SMI = TMS/height2.
2.2.3. Study end-points
The primary objective was to evaluate the prevalence of sarcopenia in MBC based on the EWGSOP criteria, defined by low muscle mass and strength.
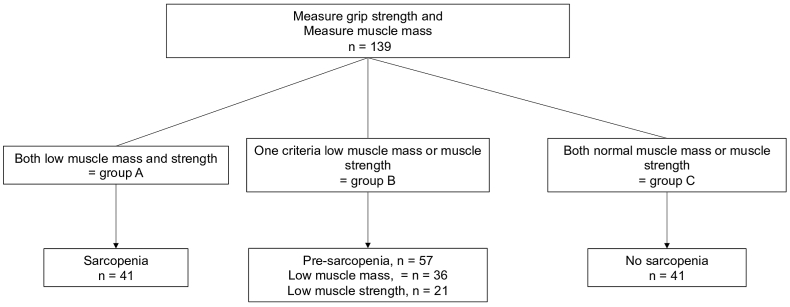
In this study, we defined three populations (Fig. 2) [11]: (1) population A or sarcopenic group with both low muscle mass (via the SMI at L3) and muscle strength (defined by handgrip strength) (2) population B or pre-sarcopenic group with low muscle mass or low muscle strength (3) population C or normal group with both high muscle mass and strength.
Fig. 2.
Criteria for the diagnosis of sarcopenia. Three populations were defined according to the conceptual stages of sarcopenia defined by the EWGSOP guidelines: (1) population A or sarcopenic group in case of low muscle mass (assessed by CT-scan) and low muscle strength (assessed by hand-grip strength test) (2) population B or pre-sarcopenic group in case of low muscle mass or low muscle strength (3) population C or normal group in case of absent of low muscle mass and low muscle strength.
The cut-off for low muscle mass was SMI < 55 cm2/m2 for men and < 39 cm2/m2 for women [24]. Low muscle strength was defined by handgrip strength < 30 kg for men and < 20 kg for women [11].
Secondary objectives were to evaluate the correlation between sarcopenia and the following variables: subjective assessment of sarcopenia by the oncologist (yes/no), MUAC (low if < 21.1 cm for men and < 19.9 cm for women) [25] and thigh circumference (low if < 38.8 for men and < 38.9 for women) [26], quadriceps atrophy assessed visually by the oncologist (yes/no). Objectives also included the evaluation of: the relationship between anthropometric characteristics and nutritional status, the impact of sarcopenia on cancer therapy tolerance, the relationship between sarcopenia and nutritional status, nutritional care, and physical activity for each patient.
2.2.4. Ethical statement
All patients were informed about the study and data collection. Clinical data were collected according to French bioethics laws regarding patient information and consent. The protocol was in line with the French data protection committee (CNIL, approval no. 2066086) regulations and was approved by the French ethical research committee, “Le Comité de Protection des Personnes” (CPP), on the July 6, 2017 (approval no. 2017-A01648-45).
2.2.5. Statistical analysis
Data management and statistical analyses were performed by Kantar Health (Paris, France). Statistical analyses were performed using DAISIE (version 2.4.25 & 2.4.45) and R i386 3.0.1. For continuous variables, descriptive analyses (means, SD, median and range) were provided, and the Z-test or t-test was used for comparisons between the sarcopenic and non-sarcopenic groups. Data are presented as percentages for categorical variables. The Cohen's kappa coefficient (κ) was used to assess the reliability of the oncologists' subjective assessment of sarcopenia diagnosis. All statistical tests had a level of significance established at P < 0.05.
3. Results
3.1. Clinical characteristics of the patients
Among 766 included patients, 139 patients (2 men and 137 women) with breast cancer having available data for SMI and hand-grip strength were analyzed. Patient demographics and cancer characteristics are presented in Table 1. The median age was 61.2 years (29.9–97.8 years). Most patients (83.5%) had a PS of 0 or 1. The median time between primary diagnosis and discovery of the first metastasis was 40.3 months (0.0–329.0 months); 23.7% were metastatic from the start. The median time between the onset of primary diagnosis and current chemotherapy was 62.0 months (0.0–285.0 months).
Table 1.
Breast cancer patient and cancer characteristics with and without sarcopenia.
| Total (n = 139) |
Population A (n = 41) | Population B (n = 57) | Population C (n = 41) | Pop A vs. B p-value | Pop A vs. C p-value | Pop B vs. C p-value | |
|---|---|---|---|---|---|---|---|
| Female, n (%) | 137 (98.6) | 40 (97.6) | 56 (98.2) | 41 (100.0) | NS | NS | NS |
|
Age (yrs)a Mean ± SD |
(n = 137) 60.4 ± 13.4 |
(n = 40) 67.3 ± 13.3 |
(n = 56) 57.6 ± 12.7 |
(n = 41) 57.5 ± 11.8 |
P < 0.01 | P < 0.01 | NS |
| ≥ 70 years, n (%) | 34 (24.5) | 17 (41.5%) | 10 (17.5) | 7 (17.1) | P < 0.01 | P = 0.03 | NS |
| Performance status, n (%)a | |||||||
| 0 | 59 (42.4) | 10 (24.4) | 29 (50.9) | 20 (48.8) | P < 0.01 | P = 0.04 | NS |
| 1 | 57 (41.0) | 20 (48.8) | 22 (38.6) | 15 (36.6) | NS | NS | NS |
| 2 | 17 (12.2) | 10 (24.4) | 5 (8.8) | 2 (4.9) | P = 0.05 | P = 0.03 | NS |
| 3 | 3 (2.2) | 1 (2.4) | 1 (1.8) | 1 (2.4) | NS | NS | NS |
|
Number of metastatic sites Mean ± SD median (range) |
2.2 ± 1.1 1.6 (1.0–7.0) |
2.5 ± 1.2 1.8 (1.0–7.0) |
2.3 ± 1.2 1.7 (1.0–6.0) |
1.9 ± 0.9 1.3 (1.0–4.0) |
NS | P < 0.01 | NS |
| Main metastatic sites, n (%) | |||||||
| Bones | 102 (73.4) | 32 (78.0) | 40 (70.2) | 30 (73.2) | NS | NS | NS |
| Liver | 64 (46.0) | 24 (58.5) | 24 (42.1) | 16 (39.0) | NS | NS | NS |
| Lymph nodes | 54 (38.8) | 16 (39.0) | 25 (43.9) | 13 (31.7) | NS | NS | NS |
| Lung | 50 (36.0) | 17 (41.5) | 24 (42.1) | 9 (22.0) | NS | NS | P = 0.05 |
| Brain | 11 (7.9) | 3 (7.3) | 7 (12.3) | 1 (2.4) | NS | NS | NS |
| Current metastatic sites, n (%) | |||||||
| Bone-only metastasis | 21 (15.1) | 3 (7.3) | 6 (10.5) | 12 (29.3) | NS | P = 0.02 | P = 0.04 |
| Visceral metastases (excluding brain metastases) | 91 (65.5) | 31 (75.6) | 36 (63.2) | 24 (58.5) | NS | NS | NS |
| Non-visceral metastases (skin, lymph nodes and ovaries) | 54 (38.8) | 17 (41.5) | 23 (40.4) | 14 (34.1) | NS | NS | NS |
| Brain metastases | 11 (7.9) | 3 (7.3) | 7 (12.3) | 1 (2.4) | NS | NS | NS |
| Visceral metastasis (excluding brain)b | |||||||
| Yes | 91 (65.5) | 31 (75.6) | 36 (63.2) | 24 (58.5) | NS | NS | NS |
| No | 48 (34.5) | 10 (24.4) | 21 (36.8) | 17 (41.5) | NS | NS | NS |
| Current therapies, n (%) | |||||||
| Chemotherapy alone | 63 (45.3) | 23 (56.1) | 21 (36.8) | 19 (46.3) | NS | NS | NS |
| Targeted therapy alone | 24 (17.3) | 3 (7.3) | 15 (26.3) | 6 (14.6) | P = 0.03 | NS | NS |
| Hormonotherapy alone | 13 (9.4) | 3 (7.3) | 5 (8.8) | 5 (12.2) | NS | NS | NS |
| Chemotherapy and Targeted therapy | 24 (17.3) | 9 (22.0) | 11 (19.3) | 4 (9.8) | NS | NS | NS |
| Targeted therapy and hormonotherapy | 14 (10.1) | 3 (7.3) | 4 (7.0) | 7 (17.1) | NS | NS | NS |
| Chemotherapy and hormonotherapy | 1 (0.7) | 0 (0.0) | 1 (1.8) | 0 (0.0) | NS | NS | NS |
|
Number of treatment lines Mean ± SD median (range) |
2.5 ± 2.0 1.2 (1–10) |
2.9 ± 2.5 1.0 (1–10) |
2.4 ± 1.8 1.3 (1–8) |
2.4 ± 1.7 1.4 (1–7) |
NS | NS | NS |
| Current line of treatment, n (%) | |||||||
| 1st line, | 63 (45.3) | 20 (48.8) | 25 (43.9) | 18 (43.9) | NS | NS | NS |
| 2nd line | 20 (14.4) | 2 (4.9) | 12 (21.1) | 6 (14.6) | P = 0.05 | NS | NS |
| 3rd line and more | 52 (37.4) | 16 (39.0) | 19 (33.3) | 17 (41.5) | NS | NS | NS |
Results are presented by N (%) or mean ± SD. NS: not significant.
Unknown responses and non-responses are not reported in the Table.
Patients with at least one site of visceral metastases (liver, lung, pleural effusion peritoneum, adrenal gland, pancreas, retinal, mediastinum, breast, epididymis).
The main metastatic sites were bone (73.4%), liver (46.0%), lymph nodes (38.8%), lung (36.0%) or brain (7.9%). Patients presented a median of 1.6 metastatic sites (1.0–7.0). At diagnosis of metastatic disease, 21 patients had bone-only metastases (15.1%); 91 (65.5%) had visceral metastases (excluding brain metastases) while 11 had brain metastases at diagnosis (7.9%).
Most patients (45.3%) were on their first line of treatment, 14.4% received 2 treatment lines and 37.4% were undergoing a third or higher line of treatment. The main treatment was chemotherapy (45.3%) followed by targeted therapy (17.3%) and hormonotherapy (9.4%). In 28.1% of cases, patients had a combination treatment.
Generally, sarcopenic patients were older (P < 0.01 vs the normal group), had a worse PS-score (P = 0.03 vs the normal group), more bone metastases (P = 0.02 vs the normal group) and a higher number of metastatic sites (P < 0.01 vs the normal group) (Table 1).
3.2. Sarcopenia prevalence and anthropometric characteristics
According to the EWGSOP criteria, 29.5% of patients (n = 41) were sarcopenic and 41.0% of patients (n = 57) were pre-sarcopenic, among whom 63.2% (n = 36) had low SMI and 36.8% (n = 21) had low muscle strength (Fig. 2).
In the sarcopenia group, sarcopenia was correctly assessed by physicians in 61.0% of cases, whereas patients in the normal group were correctly assessed as non-sarcopenic in 78.0% of cases by oncologists. A Cohen's kappa index of 0.45 indicated a moderate level of agreement between oncologist assessment and sarcopenic evaluation as per EWGSOP criteria.
In the sarcopenia group, atrophy of the quadriceps was noted in 46.3% of patients, contrary to pre-sarcopenic or normal group (19.3% and 7.3% respectively, P < 0.01). In the sarcopenia group, 29.3% had a low thigh circumference versus 1.8% in the pre-sarcopenic group and 0.0% in the normal group (P < 0.01). There was no difference in the mean brachial circumference of the dominant arm between the sarcopenia and normal groups. Pearson's correlation coefficients (r) indicated a moderate correlation between sarcopenic status and low thigh circumference (r = 0.55) and low MUAC (r = 0.52).
3.3. Sarcopenia, nutritional status and care
Table 2 shows the nutritional status and characteristics of patients. Mean BMI at inclusion was 24.3 ± 4.2 kg/m2, of which 30.2% were overweight and 9.4% were obese. A weight loss of > 5% in the previous month was found in 5.0% of patients. In sarcopenic group, BMI was lower at initial cancer diagnosis (P = 0.01 vs the normal group) and at study inclusion (P < 0.01). Sarcopenic patients tended to be malnourished or underweight with low serum albumin. A poor correlation was found between sarcopenia status and dietary assessment (r = 0.13).
Table 2.
Anthropometric characteristics and nutritional status in breast cancer patients with and without sarcopenia.
|
Total (n = 139) |
Population A (n = 41) | Population B (n = 57) | Population C (n = 41) | Pop A vs. B p-value | Pop A vs. C p-value |
Pop B vs. C p-value | |
|---|---|---|---|---|---|---|---|
| Weight prior to cancer diagnosis (kg) | 66.9 ± 13.1 | 62.6 ± 11.6 | 68.3 ± 12.9 | 69.3 ± 13.6 | P = 0.01 | P = 0.01 | NS |
| BMI prior to cancer diagnosis (kg/m2)a | 25.4 ± 5.0 | 24.1 ± 4.4 | 25.4 ± 4.8 | 26.7 ± 5.5 | NS | P = 0.01 | NS |
| Underweight (≥16.0 < 18.5) | 3 (2.2) | 1 (2.4) | 2 (3.5) | 0 (0.0) | NS | NS | NS |
| Normal (≥18.5 < 25.0) | 58 (41.7) | 22 (53.7) | 22 (38.6) | 14 (34.1) | NS | NS | NS |
| Overweight (≥25.0 < 30.0) | 41 (29.5) | 10 (24.4) | 18 (31.6) | 13 (31.7) | NS | NS | NS |
| Obese (≥30.0) | 20 (14.4) | 4 (9.8) | 7 (12.3) | 9 (22.0) | NS | NS | NS |
| Current data | |||||||
| Current weight (kg) | 63,8 ± 11.0 | 58.0 ± 9.7 | 65.1 ± 10.3 | 67.9 ± 10.7 | P < 0.01 | P < 0.01 | NS |
| Current BMI (kg/m2)a | 24.3 ± 4.2 | 22.3 ± 3.3 | 24.4 ± 3.8 | 26.1 ± 4.6 | P < 0.01 | P < 0.01 | NS |
| Malnourished (<16.0) | 1 (0.7) | 1 (2.4) | 0 (0.0) | 0 (0.0) | NS | NS | NS |
| Underweight (≥16.0 < 18.5) | 5 (3.6) | 4 (9.8) | 1 (1.8) | 0 (0.0) | NS | NS | NS |
| Normal (≥18.5 < 25.0) | 78 (56.1) | 25 (61.0) | 33 (57.9) | 20 (48.8) | NS | NS | NS |
| Overweight (≥25.0 < 30.0) | 42 (30.2) | 11 (26.8) | 18 (31.6) | 13 (31.7) | NS | NS | NS |
| Obese (≥30.0) | 13 (9.4) | 0 (0.0) | 5 (8.8) | 8 (19.5) | NS | NS | NS |
| Weight loss > 5% (in preceding 1 month) | 7 (5.0) | 2 (4.9) | 4 (7.0) | 1 (2.4) | NS | NS | NS |
| Weight loss > 10% (in preceding 6 months) | 11 (7.9) | 3 (7.3) | 4 (7.0) | 4 (9.8) | NS | NS | NS |
| Serum albumin (g/l) | 37.0 ± 6.2 | 35.6 ± 6.2 | 38.1 ± 5.1 | 36.7 ± 7.5 | NS | NS | NS |
| Normal | 83 (59.7) | 20 (48.8) | 39 (68.4) | 24 (58.5) | NS | NS | NS |
| Malnutrition | 6 (4.3) | 4 (9.8) | 2 (3.5) | 0 (0.0) | NS | NS | NS |
| Severe malnutrition | 7 (5.0) | 4 (9.8) | 1 (1.8) | 2 (4.9) | NS | NS | NS |
Results are presented by n (%) or mean ± SD. NS: not significant.
Unknown responses and non-responses are not reported in the Table.
Personalized nutritional counselling was proposed only in 27.3% of cases, regardless of the patient's nutritional status (Table 3). More sarcopenic patients with counselling were consulting dieticians (19.5%) and 17.0% used oral nutritional supplements or enteral nutrition. More sarcopenic patients were undertaking physiotherapy than those in the normal group (P = 0.04). Conversely, sarcopenic patients spent more time in bed each day, including sleep time (P < 0.01 vs the normal group) (see Table 3).
Table 3.
Nutritional management and physical activity interventions in cancer patients.
| Total (n = 139) | Population A (n = 41) | Population B (n = 57) | Population C (n = 41) | Pop A vs. B p-value | Pop A vs. C p-value | Pop B vs. C p-value | |
|---|---|---|---|---|---|---|---|
| Patients benefiting from personalized nutrition follow-upa(Yes) | 38 (27.3) | 11 (26.8) | 16 (28.1) | 11 (26.8) | NS | NS | NS |
| From a nutritionist | 4 (2.9) | 0 (0.0) | 2 (3.5) | 2 (4.9) | NS | NS | NS |
| From a dietician | 27 (19.4) | 8 (19.5) | 11 (19.3) | 8 (19.5) | NS | NS | NS |
| From an oncologist | 4 (2.9) | 1 (2.4) | 2 (3.5) | 1 (2.4) | NS | NS | NS |
| Special nutritional managementa(Yes) | 16 (11.5) | 7 (17.0) | 5 (8.8) | 4 (9.8) | NS | NS | NS |
| Oral nutritional supplements | 14 (10.1) | 6 (14.6) | 4 (7.0) | 4 (9.8) | NS | NS | NS |
| Enteral nutrition | 2 (1.4) | 1 (2.4) | 1 (1.8) | 0 (0.0) | NS | NS | NS |
| Patients undertaking physiotherapy | 31 (22.3) | 14 (34.1) | 12 (21.1) | 5 (12.2) | NS | P = 0.04 | NS |
| Patients consulting a sports coach | 9 (6.5) | 1 (2.4) | 3 (5.3) | 5 (12.2) | NS | NS | NS |
|
Frequency of physical activity per week Mean ± SD |
3.0 ± 4.1 | 2.6 ± 2.9 | 2.3 ± 2.6 | 4.3 ± 6.1 | NS | NS | NS |
|
Number of hours spent in bed per dayb(hrs/day) Mean ± SD |
9.7 ± 3.1 | 10.4 ± 2.9 | 9.8 ± 3.3 | 8.7 ± 2.6 | NS | P < 0.01 | NS |
Results are presented by N (%) or mean ± SD. NS not significant.
Unknown responses and non-responses are not reported in the Table.
Including sleeping time.
3.4. Impact of sarcopenia on cancer therapy tolerance
Adverse events (AEs) were reported in 12 patients. Since the initiation of treatment and specifically in the last month of its administration, AE of grade 3 or higher included hematological abnormalities, gastrointestinal side effects and hand and foot syndrome. No impact of sarcopenia status on anti-cancer treatment related toxicities and treatment management was noticed in this small population (Table 4).
Table 4.
Impact of sarcopenia on anti-cancer treatment related toxicities and treatment management.
| Total (n = 139) | Population A (n = 41) | Population B (n = 57) | Population C (n = 41) | Pop A vs. B p-value | Pop A vs. C p-value | Pop B vs. C p-value | |
|---|---|---|---|---|---|---|---|
|
Dose reduction due to toxicitiesa | |||||||
| Yes | 15 (10.8) | 5 (12.2) | 5 (8.8) | 5 (12.2) | NS | NS | NS |
| No |
121 (87.1) |
36 (87.8) |
51 (89.5) |
34 (82.9) |
NS |
NS |
NS |
|
Treatment interruptions due to toxicities | |||||||
| Yes | 13 (9.4) | 4 (9.8) | 4 (7.0) | 5 (12.2) | NS | NS | NS |
| No |
123 (88.5) |
37 (90.2) |
52 (91.2) |
34 (82.9) |
NS |
NS |
NS |
|
Treatment delay due to toxicities during the previous month | |||||||
| Yes | 17 (12.2) | 7 (17.1) | 5 (8.8) | 5 (12.2) | NS | NS | NS |
| No |
119 (85.6) |
34 (82.9) |
51 (89.5) |
34 (82.9) |
NS |
NS |
NS |
|
Adverse events (AE), ≥ grade 3 during the previous month | |||||||
| Yes | 12 (8.6) | 3 (7.3) | 4 (7.0) | 5 (12.2) | NS | NS | NS |
| No | 122 (87.8) | 38 (92.7) | 50 (87.7) | 34 (82.9) | NS | NS | NS |
Results are presented by N (%). NS not significant.
Unknown responses and non-responses are not reported in the Table.
4. Discussion
In the present study, we evaluated for the first time the prevalence of sarcopenia and its impact in real-life MBC patients. Sarcopenia prevalence was 29.5%, taking into account muscle mass and muscle strength measurements as recommended [11]. Our work is the first large-scale study in metastatic situation which established sarcopenia according muscle mass and muscle strength criteria [19] and by using cut-off defined by the international consensus [24].
Until now, data on the prevalence of sarcopenia are limited in breast cancer compared to others cancers [9,27], with most reports being retrospective. Three studies report the prevalence of low muscle mass in MBC in patients with a similar median age to our cohort [19]. The median age of our cohort is also similar to the ESME program which represents a real-life cohort on MBC, involving more than 22,000 patients screened between 2008 and 2016 [28].
The prevalence of low muscle mass varied from 25% (cut-off: 38.5 cm2/m2) [15] to 66.9% (cut-off: 41 cm2/m2) in metastatic setting [29]. In adjuvant breast cancer, this rate was fairly similar (34%–41.8%) [19]. Only one study in early breast cancer used the criteria defined by the EWGSOP and detected a sarcopenia prevalence 22.4% in adjuvant breast cancer patients [30].
To correctly assess sarcopenia, the nature of screening is very important. We showed that an incorrect evaluation by the oncologist occulted nearly 39.0% of sarcopenia diagnoses. While, each parameter (quadriceps’ atrophy, MUAC, thigh circumference) was found to be related to sarcopenia status, a single method on its own was insufficient to conclusively determine sarcopenia status. The most effective way to identify sarcopenia would thus be to combine screening tools, including CT-scan. The relationship between hand-grip strength and muscle mass that was previously explored in early adjuvant breast cancer confirms the above comment [30]. We also showed that the rate of sarcopenia was independent of the treatment line. There is little data on this in breast cancer but this is consistent with another publication in pancreatic cancer [31].
Although previous studies showed a link between sarcopenia and treatment intolerance in breast cancer [[12], [13], [14], [15]], we could not confirm this because a small number of AEs had occurred in the sample. In our study, AEs has occurred in less than 10% of patients, compared to 33% of patients in a study by Shachar et al. [13] or to 50% of patients described by Prado et al. [15]. The differing percentage of toxicities could be related to the design of our study, which had a cross-sectional design, in contrast to these two studies that were especially designed to evaluate the relation between toxicities and sarcopenia. Another explanation is the use of treatments different from chemotherapy or a less toxic chemotherapy than in previous studies.
Finally, we showed that nutritional care and physical activity were under-proposed to sarcopenic patients. It is well known that sarcopenia is linked to poor prognosis [[16], [17], [18],[32], [33], [34]] and that using simple tools to screen early for sarcopenia and to propose a nutritional management plan could help to counter sarcopenia worsening. One explanation is the lack of knowledge about a patient's sarcopenic status through inadequate assessment, but even correctly assessed sarcopenic patients did not receive adequate nutritional support and physical exercise management. A French study previously highlighted the difficulty of assessing nutrition status and its management in elderly patients with cancer [35].
Proper nutrition and exercise have been shown to have a synergistic effect in the prevention and improvement of sarcopenia symptoms, and it is known that combined resistance and aerobic exercise intervention could improve BC in cancer and specifically in breast cancer survivors [36]. Exercise intervention for breast cancer survivors also showed beneficial effects on BC, fatigue and quality of life [37,38]. Similarly, a recent review showed that physical exercise had a positive effect on muscle mass in cancer patients [39]. Exercise improves lean body mass and muscular strength in BC patients. These improvements have been associated with improved of quality of life (QoL). One randomized trial highlighted that resistance exercise training during adjuvant chemotherapy in patients with breast cancer induced reversal of sarcopenia which was also associated with clinically meaningful improvements in QoL [40]. More recently, Delrieu et al. showed the evolution of sarcopenia in patients with metastatic breast cancer during a six-month physical activity intervention and showed that physical activity maintained muscle mass [41].
This cross-sectional study evaluated patient's sarcopenia status at a time point. One of the limitations of our study is that patients are more representative of a patient population on a certain day, rather than during consultations because of the way patients are recruited. Moreover, histological and immunohistochemical characteristics of breast cancer were not collected that could limit the precision in population characterization. It should also be noted that concomitant treatment may have an impact on the impact of extended therapy with corticosteroids on muscle wasting. This data was unfortunately not collected but should be considered in a future prospective study.
In conclusion, CT imaging to assess low muscle mass, and hand-grip test for muscle strength are feasible, rapid and non-invasive methods for sarcopenia assessment. Both examinations together allow for a better assessment of sarcopenia but individually, they can also identify patients at risk of sarcopenia. We also showed that sarcopenia or pre-sarcopenic situation in real-life were underestimated and it resulted in inappropriate management of nutritional care and physical activity, despite the knowledge of sarcopenia's negative impact on patient prognosis. Therefore, we need to better integrate sarcopenia evaluation and nutritional management in patients with cancer by increasing the awareness of oncologists in clinical practice.
Funding
Fresenius Kabi France sponsored this study.
Availability of data and material
N/A.
Code availability
N/A.
Authors’ contributions
B. Raynard, F. Pigneur, M. Di Palma, E. Deluche and F. Goldwasser equally contributed to the conception and design of the research; B. Raynard, F. Pigneur, M. Di Palma, E. Deluche and F. Goldwasser equally contributed to the acquisition and analysis of the data; E. Deluche contributed to the interpretation of the data; and E. Deluche drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Ethics approval
This study, conducted in accordance with the Declaration of Helsinki, was approved by the French ethical research committee, “Le Comité de Protection des Personnes” (CPP), on the July 6, 2017 (approval no. 2017-A01648-45). National data protection regulations were also met (CNIL, approval no. 2066086).
Consent to participate
All persons provided their informed consent prior to enrollment.
Consent for publication
All persons were informed that this study would be submitted for publication.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
All authors were remunerated for their participation as investigators in the SCAN study.
Acknowledgements
We thank all the other investigators of the SCAN Study Group for their contribution to this research.
We thank Kantar Health France for undertaking data management and statistical analyses, and Saannya Sequeira of ClinSearch, France, for medical writing assistance.
References
- 1.Neuhouser M.L., Aragaki A.K., Prentice R.L., Manson J.E., Chlebowski R., Carty C.L., et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the women's Health initiative randomized clinical trials. JAMA Oncol. 2015;1:611–621. doi: 10.1001/jamaoncol.2015.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trestini I., Carbognin L., Monteverdi S., Zanelli S., De Toma A., Bonaiuto C., et al. Clinical implication of changes in body composition and weight in patients with early-stage and metastatic breast cancer. Crit Rev Oncol Hematol. 2018;129:54–66. doi: 10.1016/j.critrevonc.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Martel S., Poletto E., Ferreira A.R., Lambertini M., Sottotetti F., Bertolini I., et al. Impact of body mass index on the clinical outcomes of patients with HER2-positive metastatic breast cancer. Breast. 2018;37:142–147. doi: 10.1016/j.breast.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Pizzuti L., Sergi D., Sperduti I., Lauro L.D., Mazzotta M., Botti C., et al. Body mass index in HER2-negative metastatic breast cancer treated with first-line paclitaxel and bevacizumab. Cancer Biol Ther. 2018;19:328–334. doi: 10.1080/15384047.2017.1416938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gennari A., Nanni O., Puntoni M., DeCensi A., Scarpi E., Conte P., et al. Body mass index and prognosis of metastatic breast cancer patients receiving first-line chemotherapy. Cancer Epidemiol Prev Biomark. 2013;22:1862–1867. doi: 10.1158/1055-9965.EPI-13-0595. [DOI] [PubMed] [Google Scholar]
- 6.Saleh K., Carton M., Dieras V., Heudel P.-E., Brain E., D'Hondt V., et al. Impact of body mass index on overall survival in patients with metastatic breast cancer. Breast. 2021;55:16–24. doi: 10.1016/j.breast.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prado C.M.M., Lieffers J.R., McCargar L.J., Reiman T., Sawyer M.B., Martin L., et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 8.Vrieling A., Kampman E., Knijnenburg N.C., Mulders P.F., Sedelaar J.P.M., Baracos V.E., et al. Body composition in relation to clinical outcomes in renal cell cancer: a systematic review and meta-analysis. Eur Urol Focus. 2016 doi: 10.1016/j.euf.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Shachar S.S., Williams G.R., Muss H.B., Nishijima T.F. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67. doi: 10.1016/j.ejca.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 10.Cooper C., Fielding R., Visser M., van Loon L.J., Rolland Y., Orwoll E., et al. Tools in the assessment of sarcopenia. Calcif Tissue Int. 2013;93:201–210. doi: 10.1007/s00223-013-9757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F., et al. Sarcopenia: European consensus on definition and diagnosis report of the European working group on sarcopenia in older people. Age Ageing. 2010 doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prado C.M.M., Lima I.S.F., Baracos V.E., Bies R.R., McCargar L.J., Reiman T., et al. An exploratory study of body composition as a determinant of epirubicin pharmacokinetics and toxicity. Cancer Chemother Pharmacol. 2011;67:93–101. doi: 10.1007/s00280-010-1288-y. [DOI] [PubMed] [Google Scholar]
- 13.Shachar S.S., Deal A.M., Weinberg M., Williams G.R., Nyrop K.A., Popuri K., et al. Body composition as a predictor of toxicity in patients receiving anthracycline and taxane-based chemotherapy for early-stage breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2017;23:3537–3543. doi: 10.1158/1078-0432.CCR-16-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shachar S.S., Deal A.M., Weinberg M., Nyrop K.A., Williams G.R., Nishijima T.F., et al. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane based chemotherapy. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-0940. 2016:clincanres.0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prado C.M., Baracos V.E., McCargar L.J., Reiman T., Mourtzakis M., Tonkin K., et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15:2920–2926. doi: 10.1158/1078-0432.CCR-08-2242. [DOI] [PubMed] [Google Scholar]
- 16.Caan B.J., Cespedes Feliciano E.M., Prado C.M., Alexeeff S., Kroenke C.H., Bradshaw P., et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. 2018;4:798–804. doi: 10.1001/jamaoncol.2018.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deluche E., Leobon S., Desport J.C., Venat-Bouvet L., Usseglio J., Tubiana-Mathieu N. Impact of body composition on outcome in patients with early breast cancer. Support Care Cancer Off J Multinatl Assoc Support Care Cancer. 2018;26:861–868. doi: 10.1007/s00520-017-3902-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rier H.N., Jager A., Sleijfer S., van Rosmalen J., Kock M.C.J.M., Levin M.-D. Low muscle attenuation is a prognostic factor for survival in metastatic breast cancer patients treated with first line palliative chemotherapy. The Breast. 2017;31:9–15. doi: 10.1016/j.breast.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Rossi F., Valdora F., Bignotti B., Torri L., Succio G., Tagliafico A.S. Evaluation of body Computed Tomography-determined sarcopenia in breast cancer patients and clinical outcomes: a systematic review. Cancer Treat Res Commun. 2019;21:100154. doi: 10.1016/j.ctarc.2019.100154. [DOI] [PubMed] [Google Scholar]
- 20.Raynard B., Pigneur F., Di Palma M., Deluche E., Goldwasser F. 2021. CT-defined sarcopenia prevalence in patients with metastatic cancer: a cross-sectional multicenter French study (the scan study) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization (WHO) 2018. Body mass index - BMI.http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi [Google Scholar]
- 22.Senesse P., Bachmann P., Bensadoun R.J., Besnard I., Bourdel-Marchasson I., Bouteloup C., et al. Nutrition chez le patient adulte atteint de cancer: introduction. Nutr Clin Metab. 2012 [Google Scholar]
- 23.Shen W., Punyanitya M., Wang Z., Gallagher D., St-Onge M.-P., Albu J., et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol Bethesda Md 1985. 2004;97:2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 24.Fearon K., Strasser F., Anker S.D., Bosaeus I., Bruera E., Fainsinger R.L., et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 25.Landi F., Russo A., Liperoti R., Pahor M., Tosato M., Capoluongo E., et al. Midarm muscle circumference, physical performance and mortality: results from the aging and longevity study in the Sirente geographic area (ilSIRENTE study) Clin Nutr Edinb Scotl. 2010;29:441–447. doi: 10.1016/j.clnu.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Ishii S., Tanaka T., Shibasaki K., Ouchi Y., Kikutani T., Higashiguchi T., et al. Development of a simple screening test for sarcopenia in older adults. Geriatr Gerontol Int. 2014;14:93–101. doi: 10.1111/ggi.12197. [DOI] [PubMed] [Google Scholar]
- 27.Pamoukdjian F., Bouillet T., Lévy V., Soussan M., Zelek L., Paillaud E. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: a systematic review. Clin Nutr. 2018;37:1101–1113. doi: 10.1016/j.clnu.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Deluche E., Antoine A., Bachelot T., Lardy-Cleaud A., Dieras V., Brain E., et al. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008-2016. Eur J Cancer. 2020;129:60–70. doi: 10.1016/j.ejca.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Rier H.N., Jager A., Sleijfer S., van Rosmalen J., Kock M.C.J.M., Levin M.-D. Changes in body composition and muscle attenuation during taxane-based chemotherapy in patients with metastatic breast cancer. Breast Cancer Res Treat. 2018;168:95–105. doi: 10.1007/s10549-017-4574-0. [DOI] [PubMed] [Google Scholar]
- 30.Benavides-Rodríguez L., García-Hermoso A., Rodrigues-Bezerra D., Izquierdo M., Correa-Bautista J.E., Ramírez-Vélez R. Relationship between handgrip strength and muscle mass in female survivors of breast cancer: a mediation analysis. Nutrients. 2017;9 doi: 10.3390/nu9070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurita Y., Kobayashi N., Tokuhisa M., Goto A., Kubota K., Endo I., et al. Sarcopenia is a reliable prognostic factor in patients with advanced pancreatic cancer receiving FOLFIRINOX chemotherapy. Pancreatology. 2019;19:127–135. doi: 10.1016/j.pan.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Fabbro E.D., Parsons H., Warneke C.L., Pulivarthi K., Litton J.K., Dev R., et al. The relationship between body composition and response to neoadjuvant chemotherapy in women with operable breast cancer. Oncol. 2012;17:1240–1245. doi: 10.1634/theoncologist.2012-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villaseñor A., Ballard-Barbash R., Baumgartner K., Baumgartner R., Bernstein L., McTiernan A., et al. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Surviv. 2012;6:398–406. doi: 10.1007/s11764-012-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinberg M.S., Shachar S.S., Muss H.B., Deal A.M., Popuri K., Yu H., et al. Beyond sarcopenia: characterization and integration of skeletal muscle quantity and radiodensity in a curable breast cancer population. Breast J. 2018;24:278–284. doi: 10.1111/tbj.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lacau St Guily J., É Bouvard, Raynard B., Goldwasser F., Maget B., Prevost A., et al. NutriCancer: a French observational multicentre cross-sectional study of malnutrition in elderly patients with cancer. J Geriatr Oncol. 2018;9:74–80. doi: 10.1016/j.jgo.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Cornette T., Vincent F., Mandigout S., Antonini M.T., Leobon S., Labrunie A., et al. Effects of home-based exercise training on VO2 in breast cancer patients under adjuvant or neoadjuvant chemotherapy (SAPA): a randomized controlled trial. Eur J Phys Rehabil Med. 2016;52:223–232. [PubMed] [Google Scholar]
- 37.Jones L.W., Liang Y., Pituskin E.N., Battaglini C.L., Scott J.M., Hornsby W.E., et al. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. Oncol. 2011;16:112–120. doi: 10.1634/theoncologist.2010-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ballinger T.J., Reddy A., Althouse S.K., Nelson E.M., Miller K.D., Sledge J.S. Breast Cancer Res Treat; 2018. Impact of primary breast cancer therapy on energetic capacity and body composition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stene G.B., Helbostad J.L., Balstad T.R., Riphagen, Kaasa S., Oldervoll L.M. Effect of physical exercise on muscle mass and strength in cancer patients during treatment--a systematic review. Crit Rev Oncol Hematol. 2013;88:573–593. doi: 10.1016/j.critrevonc.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Adams S.C., Segal R.J., McKenzie D.C., Vallerand J.R., Morielli A.R., Mackey J.R., et al. Impact of resistance and aerobic exercise on sarcopenia and dynapenia in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. Breast Cancer Res Treat. 2016;158:497–507. doi: 10.1007/s10549-016-3900-2. [DOI] [PubMed] [Google Scholar]
- 41.Delrieu L., Martin A., Touillaud M., Pérol O., Morelle M., Febvey-Combes O., et al. Sarcopenia and serum biomarkers of oxidative stress after a 6-month physical activity intervention in women with metastatic breast cancer: results from the ABLE feasibility trial. Breast Cancer Res Treat. 2021;188:601–613. doi: 10.1007/s10549-021-06238-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N/A.