Abstract
Skeletal changes are a common complication in patients with chronic kidney disease and traditionally labelled as renal osteodystrophy. Uremic leontiasis ossea is a rare and severe form of renal osteodystrophy with characteristic overgrowth of the craniofacial bones. Imaging, in particular computed tomography, is valuable for the diagnosis and management of such rare condition. Uremic leontiasis ossea has distinctive imaging features with significant overgrowth of the jaw and characteristic internal serpiginous tunneling. The recognition of its radiological appearance and abrupt management are essential to avoid its devastating esthetic and functional impairments.
Keywords: ROD, renal osteodystrophy; SHPT, secondary hyperparathyroidism; CKD, chronic kidney disease; ULO, uremic leontiasis ossea; KDIGO, Kidney Disease-Improving Global Outcomes; CKD-MBD, chronic kidney disease–mineral and bone disorder; PTH, parathyroid hormone; ESRD, end-stage renal disease; CT, Computed tomography; 3D, three-dimensional; MRI, Magnetic resonance imaging; 4D, four-dimensional
Keywords: Renal osteodystrophy, Uremic, Leontiasis ossea, Craniofacial, Computed tomography
Introduction
The term leontiasis was classically used to describe the lion-like facial appearance in leprosy as a result of its cutaneous changes [1]. Later in 1869, the term ossea has been added to leontiasis by Virchow to describe inflammatory hyperostosis of the facial bones [2]. Indeed, leontiasis ossea is a general descriptive term rather than a disease in itself, which often applied to diffuse craniofacial overgrowth that resembles the lion face [3]. Such deformity was documented in varieties of medical conditions such as Paget's disease, fibrous dysplasia, and renal osteodystrophy (ROD) [4]. In 1953, Cohen et al. first described the facial manifestations in secondary hyperparathyroidism (SHPT) and chronic kidney disease (CKD) as uremic leontiasis ossea (ULO) [5].
Skeletal changes are a common complication in patients with CKD and traditionally labelled as ROD [6]. However, such patients’ group are also predisposed to other extra-skeletal manifestations for which the Kidney Disease-Improving Global Outcomes (KDIGO) Foundation recommended a broader term, chronic kidney disease–mineral and bone disorder (CKD-MBD) [7]. The KDIGO has also recommended that the term ROD be used exclusively to describe changes in bone morphology associated with CKD [7]. Hence, CKD-MBD is a complex systemic disorder consisted of biochemical alterations, bone changes, soft tissue and vascular calcifications [8]. The basic biochemical alterations in CKD-MBD are hyperphosphatemia, hypocalcemia, and decreased activation of vitamin D which eventually lead to SHPT with increased production of parathyroid hormone (PTH) [8].
ULO is a rare and severe form of ROD with characteristic overgrowth of the craniofacial bones [1,9]. Few cases of ULO are published in literature, which can be explained by the availability of dialysis, and the abrupt management of SHPT; as biochemical changes usually diagnosed earlier before development of skeletal changes [3,10]. Furthermore, there is a spectrum of skeletal changes caused by SHPT and labeled with different terminologies, for which the exact number of published cases can't be estimated [11]. The practitioners should be aware of the diagnosis and management of this rare condition, as it may results in devastating esthetic and functional impairments. This report aims to explore the role of imaging in ULO and its distinctive imaging features that allow differentiation of other clinical causes of leontiasis ossea. Additionally, the expected complications and treatment strategies will also be discussed.
Case report
A 28-year-old man presented with progressive painless facial swelling over the last seven months. Change in his voice was also documented. His past medical history included end-stage renal disease (ESRD) of undetermined etiology for the last nine years, currently on hemodialysis 3 times per week, severe uncontrolled SHPT, and hypertension. The patient denied any history of vision impairment, hearing loss, dyspnea, or dysphagia.
Upon arrival, the patient blood pressure was elevated (161/101 mmHg), and other vital signs were normal. On physical exam, the patient was noted to have hard, bony craniofacial protrusion, most pronounced along the maxilla. No tenderness was documented. Labs obtained included the following: 13.4 mmol/L urea (4.2-7.2), 920 umol/L creatinine (64-115), 6 mL/min/1.73m^2 e-GFR, 1.65 mmol/L phosphate (0.80-1.45), 1714.3 U/L alkaline phosphatase (50-116), 2.080 mmol/L calcium (2.100-2.550), and 3012 ng/L PTH (15-65).
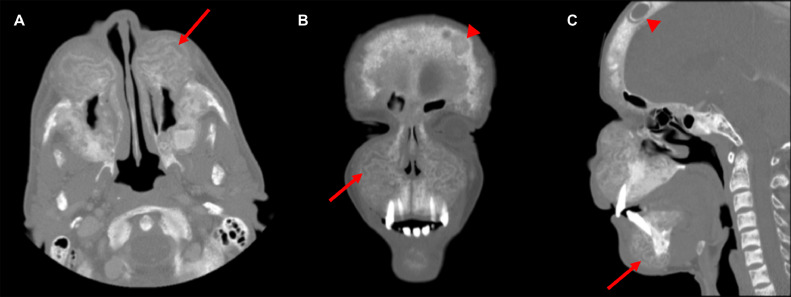
Computed tomography (CT) of the facial bones was then requested to rule out bone tumors. The scout film demonstrates overgrowth of the jaw, in particular the maxilla, with multiple tiny well-defined lucencies in the calvaria (Fig. 1). The axial, coronal, and sagittal CT images show significant overgrowth of the jaw with internal serpiginous tunneling or channeling; found as alternative rings of hypo- and hyperattenuation associated with loss of corticomedullary differentiation (Fig. 2). The sagittal CT image shows sclerosis of the vertebral endplates (Fig. 2).
Fig. 1.
The scout film demonstrates overgrowth of the jaw, in particular the maxilla, with classical salt and pepper sign (arrow); found as multiple tiny well-defined calvarial lucencies in background of sclerotic ground-glass appearance associated with indistinct inner and outer tables
Fig. 2.
Axial (A), coronal (B), and sagittal (C) CT images of the facial bones show significant overgrowth of the jaw with internal serpiginous tunneling or channeling (arrows); found as alternative rings of hypo- and hyperattenuation associated with loss of corticomedullary differentiation. The coronal (B) and sagittal (C) CT images show multifocal lytic lesions (arrowheads) as a result of Brown tumors. The sagittal (C) CT image shows the classical rugger jersey spine sign with sclerosis of the vertebral endplates
The history of ESRD, biochemical results of SHPT, and the distinctive imaging features in the craniofacial bones are diagnostic of ULO. The patient currently enrolled in active transplant waiting list. The SHPT failed to be controlled by medical therapy for which endocrine surgery consultation was requested for possible parathyroidectomy.
Discussion
Generally, the skeletal manifestations in ROD fall into three main patterns: high bone turnover in SHPT, low bone turnover in rickets/osteomalacia, or mixed [12]. Although poorly controlled SHPT can be labelled as a key feature of ULO, the exact pathogenesis is still unknown [13]. Furthermore, the predilection of ULO to involve the craniofacial bones is still unclear [14]. Massicotte-Azarniouch et al. describe the morphological changes in ULO not limited to osseous changes but include soft tissue changes which eventually gives the lion-like facial appearance [15]. In 2004, Sagliker et al. found 25 patients with CKD, SHPT, severe craniofacial deformities, tumoral tissues in the mouth, fingertips changes, and psychological problems; and they labeled it as Sagliker syndrome [16].
Although ULO is clinically diagnosed, however, imaging allows differentiation from other diseases with similar craniofacial overgrowth. Imaging is also valuable prior to the reconstructive surgeries for patients with cosmetic or functional impairments [13,17]. Furthermore, imaging can be used as prognostic tool in ULO to confirm improvement in craniofacial deformities following successful management [1]. Cross-sectional imaging studies, primarily CT, with or without three-dimensional (3D) reconstruction is the imaging modality of choice. Magnetic resonance imaging (MRI) can be requested when CT findings are inconclusive [13]. Panoramic radiograph is useful for assessment of lamina dura which tends to be affected in hyperparathyroidism. As a part of management of ULO, imaging plays a vital role in preoperative location of parathyroid adenoma. Ultrasound and 99mTc-sestamibi scintigraphy are the imaging techniques of choice for such scenario [18]. Contrast enhanced CT and MRI can be reserved for failed parathyroidectomy or discordant ultrasound and scintigraphy findings [18]. Four-dimensional (4D) CT is valuable technique which demonstrates high diagnostic accuracy for parathyroid adenoma localization in particular for indeterminate findings of ultrasound or scintigraphy [19].
The diagnosis of ULO pose challenges radiologically and pathologically in particular with lack of history of ESRD and biochemical results of SHPT. Radiologically, the features of ULO are complex as in ROD which can overlap in between rickets/osteomalacia and SHPT [20]. Even pathologically, ULO can be interpreted as other causes of craniofacial overgrowth such as fibrous dysplasia [10]. Generally, the findings of osteomalacia on imaging are non-specific and include reduced bone density, loss of cortical definition, and coarsening of the trabecular pattern in addition to Looser zones or pseudofractures; a type of insufficiency fractures [20,21]. On the other hand, hyperparathyroidism presents with bone resorption as most common radiological manifestations [22]. The bone resorption in the skull best described as “salt and pepper” appearance caused by multiple lucencies in background of sclerotic ground-glass appearance associated with indistinct inner and outer tables [21,22]. Such ground-glass pattern can be mistaken with fibrous dysplasia, however, in ULO appears to be diffuse with loss of corticomedullary differentiation [10]. Subperiosteal resorption can also be observed in the lamina dura, which is the bone surrounding the tooth sockets [20]. Osteitis fibrosa cystica is another manifestation of hyperparathyroidism as a result of activation of the osteoclasts and replacement of the bone marrow by vascular and fibrous tissues [21,22]. Brown tumor is an extreme form of osteitis fibrosa cystica which presents as solitary or multifocal lytic lesions [20,21]. Osteosclerosis can be also seen in particular during the healing phase with characteristic increased bone density of the vertebral end-plates (rugger-jersey spine) [22]. Significant overgrowth of the jaw with internal serpiginous tunneling or channeling and loss of corticomedullary differentiation are specific appearance of ULO [4]. The cause of such appearance still unknown without histopathological explanation [10]. However, Wang et al. reported the serpiginous tunneling or channeling as a zonal pattern with alternative rings of hypo- and hyperattenuation as a result of osteoclastic and osteoblastic activity [23]. Lee et al. reported such zonal pattern to be related to concentric layers of repetitive cells and trabeculae in addition to blood products surrounding involuted dense brown tumors [13].
The main differential diagnoses of ULO are Paget's disease and fibrous dysplasia based on clinical findings of craniofacial overgrowth. However, the overgrowth in such conditions tends to be asymmetrical and multifocal as opposite to diffuse symmetrical involvement in ULO [4,11]. Histopathological confirmation is not necessary as ULO, fibrous dysplasia, and Paget's disease can have similar findings [10,11]. Hence, the diagnosis of ULO in patients with craniofacial changes depends on clinical findings of ESRD, biochemical results of SHPT, and classical imaging findings. ULO presents clinically with progressive painless massive overgrowth of the craniofacial bones in particular the jaw, widening of the nares, flattening of the nasal bridge, and increased interdental spacing [15,24]. Beside its cosmetic effects, the involvement of the craniofacial bones in ULO causes progressive narrowing of the skull base foramina which may results in compressive cranial neuropathy [3,25]. Sundaram et al. documented bilateral compressive optic neuropathy caused by ULO with spontaneous improvement of the visual symptoms following parathyroidectomy [9]. Hearing loss was also previously reported [26]. Furthermore, ULO may results in other functional impairments such as life-threatening upper airway obstruction, nasal breathing, dysarthria, and dysphagia [3,10,13,25].
There is a limited published data on the treatment strategies of ULO which can medical and surgical. The majority of cases are resistant to medical therapy alone [1]. The surgical strategies aim to treat the primary cause of SHPT in addition to attempt to regain the form and function. Lee et al. documented mild improvement or at least stabilization of the facial deformities following parathyroidectomy in their series of 5 patients with ULO [13]. Yang et al. confirmed the prior published results when they retrospectively assessed 6 patients with relieve SHPT and improve quality of life after total parathyroidectomy and autotransplantation [27]. Duan et al. documented notable reversible skeletal changes on imaging following parathyroidectomy and autotransplantation in addition to medical therapy [1]. However, ULO may have high biochemical recurrence rate (60%) within 1 year following the surgery [27]. Hence, they concluded, ULO is not a simple disease with craniofacial malformations but is a severe systemic disease. Furthermore, abnormal levels of PTH after parathyroidectomy can be explained by residual parathyroid tissue which can be ectopic in location and necessitates post-operative 99mTc-sestamibi scintigraphy to detect the location as confirm by prior reported case [24]. Successful resection of such residual promises to normalize the PTH levels [24]. Based on rarity of such case, no clear strategies for maxillofacial reconstruction in order to improve the patient's quality of life. Such strategies have to be timely planned to ensure higher percentage of mature bone within the lesion [24].
Conclusion
ULO has distinctive imaging features with significant overgrowth of the jaw and characteristic internal serpiginous tunneling. The recognition of its radiological appearance and abrupt management are essential to avoid its devastating esthetic and functional impairments. Long-term follow-up is required for possible recurrence following successful management. The biochemical screening during hemodialysis is vital as abnormal laboratory results can proceed the skeletal changes for which can be used as preventive tools.
Patient consent
No consent obtained for this case report as this is a retrospective study with no patient identifiers. “Formal consents are not required for the use of entirely anonymised images from which the individual cannot be identified- for example, xrays, ultrasound images, pathology slides or laparoscopic images, provided that these do not contain any identifying marks and are not accompanied by text that might identify the individual concerned”.
Footnotes
Conflict of Interest: The author declares that there are no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Duan S.Y., Xing C.Y., Yang G., Wang N.N., Zhang B. Dramatic alteration of the skull in a uremic patient with leontiasis ossea. Intern Med. 2014;53(17):1971–1976. doi: 10.2169/internalmedicine.53.2217. [DOI] [PubMed] [Google Scholar]
- 2.Virchow R., Arrhonsson P. Pathologie des tumours, vol 2. Paris: Bailliere; 1869. p. 22–3.
- 3.Dimkovic N., Piscevic V., Jankovic A., Djuric P. Fatal uremic leontiasis ossea in long-lasting uncontrolled hyperparathyroidism: a case report. Hippokratia. 2015;19(3):266–267. [PMC free article] [PubMed] [Google Scholar]
- 4.Haroyan H., Bos A., Ginat D.T. Uremic leontiasis ossea. Am J Otolaryngol. 2015;36(1):74–76. doi: 10.1016/j.amjoto.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Cohen J., Diamond I. Leontiasis ossea, slipped epiphyses, and granulosa cell tumor of testis with renal disease; report of a case with autopsy findings. AMA Arch Pathol. 1953;56(5):488–500. [PubMed] [Google Scholar]
- 6.Martin K.J., González E.A. Pathophysiology of renal osteodystrophy. Clin Rev Bone Miner Metab [Internet] 2007;5(1):11–19. doi: 10.1007/BF02736667. [DOI] [Google Scholar]
- 7.Moe S., Drüeke T., Cunningham J. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: improving Global Outcomes (KDIGO) Kidney Int. 2006;69(11):1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 8.Lim C.Y., Ong K.O. Various musculoskeletal manifestations of chronic renal insufficiency. Clin Radiol. 2013;68(7):e397–e411. doi: 10.1016/j.crad.2013.01.025. doi: 10.1016/j.crad.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 9.Sundaram A.N.E., Abhayambika A., Kumar S. Bilateral compressive optic neuropathy from renal osteodystrophy caused by Branchio-oto-renal syndrome stabilised after parathyroidectomy. Neuroophthalmology. 2017;41(6):321–325. doi: 10.1080/01658107.2017.1315145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang J.I., Som P.M., Lawson W. Unique imaging findings in the facial bones of renal osteodystrophy. AJNR Am J Neuroradiol. 2007;28(4):608–609. [PMC free article] [PubMed] [Google Scholar]
- 11.Collum J., Jones R.H., Lynham A., Hirst J. Leontiasis ossea: a presentation of hyperparathyroidism in an indigenous Australian man secondary to chronic renal failure. J Oral Maxillofac Surg. 2013;71(1):56–61. doi: 10.1016/j.joms.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Slatopolsky E., Gonzalez E., Martin K. Pathogenesis and treatment of renal osteodystrophy. Blood Purif. 2003;21(4-5):318–326. doi: 10.1159/000072552. [DOI] [PubMed] [Google Scholar]
- 13.Lee V.S., Webb M.S., Jr, Martinez S., McKay C.P., Leight G.S., Jr. Uremic leontiasis ossea: "bighead" disease in humans? Radiologic, clinical, and pathologic features. Radiology. 1996;199(1):233–240. doi: 10.1148/radiology.199.1.8633151. [DOI] [PubMed] [Google Scholar]
- 14.Shim J., Yu R. Uremic Leontiasis Ossea. Sultan Qaboos Univ Med J. 2018;18(2) doi: 10.18295/squmj.2018.18.02.023. e243-e244. doi: 10.18295/squmj.2018.18.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massicotte-Azarniouch D., McLean L., Brown P.A. Uremic leontiasis ossea due to secondary hyperparathyroidism complicated by vitamin C deficiency in a non-adherent chronic hemodialysis patient: A case report. Clin Nephrol Case Stud. 2019;7:54–59. doi: 10.5414/CNCS109788. Sep 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sagliker Y., Balal M., Sagliker Ozkaynak P., et al. Sagliker syndrome: uglifying human face appearance in late and severe secondary hyperparathyroidism in chronic renal failure. Semin Nephrol. 2004;24(5):449–455. doi: 10.1016/j.semnephrol.2004.06.021. doi: 10.1016/j.semnephrol.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Aggunlu L., Akpek S., Coskun B. Leontiasis ossea in a patient with hyperparathyroidism secondary to chronic renal failure. Pediatr Radiol. 2004;34(8):630–632. doi: 10.1007/s00247-004-1188-6. [DOI] [PubMed] [Google Scholar]
- 18.Johnson N.A., Tublin M.E., Ogilvie J.B. Parathyroid imaging: technique and role in the preoperative evaluation of primary hyperparathyroidism. AJR Am J Roentgenol. 2007;188(6):1706–1715. doi: 10.2214/AJR.06.0938. [DOI] [PubMed] [Google Scholar]
- 19.Chazen J.L., Gupta A., Dunning A., Phillips C.D. Diagnostic accuracy of 4D-CT for parathyroid adenomas and hyperplasia. AJNR Am J Neuroradiol. 2012;33(3):429–433. doi: 10.3174/ajnr.A2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang C.Y., Rosenthal D.I., Mitchell D.M., Handa A., Kattapuram S.V., Huang A.J. Imaging findings of metabolic bone disease. Radiographics. 2016;36(6):1871–1887. doi: 10.1148/rg.2016160004. doi: 10.1148/rg.2016160004. PMID: 27726750. [DOI] [PubMed] [Google Scholar]
- 21.Andreu-Arasa V.C., Chapman M.N., Kuno H., Fujita A., Sakai O. Craniofacial Manifestations of Systemic Disorders: CT and MR imaging findings and imaging approach. Radiographics. 2018;38(3):890–911. doi: 10.1148/rg.2018170145. [DOI] [PubMed] [Google Scholar]
- 22.Adams J.E. Renal bone disease: radiological investigation. Kidney Int Suppl. 1999;73 doi: 10.1046/j.1523-1755.1999.07302.x. S38-41. doi: 10.1046/j.1523-1755.1999.07302.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang J., Zhao X., Shi H., Zhu L., Tao X. Radiological diagnostic features of uremic leontiasis ossea: a case report. Dentomaxillofac Radiol. 2020;49(1) doi: 10.1259/dmfr.20190253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donoso-Hofer F., Gunther-Wood M., Romero-Romano P., Pezoa-Opazo N., Fernández-Toro M.A., Ortega-Pinto A.V. Uremic leontiasis ossea, a rare presentation of severe renal osteodystrophy secondary to hyperparathyroidism. J Stomatol Oral Maxillofac Surg. 2018;119(1):56–60. doi: 10.1016/j.jormas.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S., Thuraisingham R., Yaqoob M. Big-head disease: uremic leontiasis ossea. Kidney Int. 2006;69(10) doi: 10.1038/sj.ki.5001526. 1709. [DOI] [PubMed] [Google Scholar]
- 26.Erkan A.N., Sagliker Y., Yildiz I., Ozluoglu L. Audiological findings in chronic kidney disease patients with Sagliker syndrome. J Ren Nutr. 2010;20 doi: 10.1053/j.jrn.2010.06.002. (5 Suppl):S56-8. [DOI] [PubMed] [Google Scholar]
- 27.Yang G., Zhang B., Zha X.M., Wang N.N., Xing C.Y. Total parathyroidectomy with autotransplantation for a rare disease derived from uremic secondary hyperparathyroidism, the uremic leontiasis ossea. Osteoporos Int. 2014;25(3):1115–1121. doi: 10.1007/s00198-013-2488-1. [DOI] [PubMed] [Google Scholar]