Abstract
Since the 1960s, EEG has been used to assess the neurologic function of patients in the hours and days after cardiac arrest. Accurate and reliable prognostication after cardiac arrest is vital for tailoring aggressive patient care for those with a high likelihood of recovery and setting appropriate goals of care for those who have a high likelihood of a poor outcome. Attempts to define EEG’s role in this process has evolved over the years.
In this review, we provide important historical context about EEG’s use, it’s perceived unreliability in the post-cardiac arrest patient in the past and provide a detailed analysis of how this role has changed recently. A review of the 71 most recent and highest quality studies demonstrates that the introduction of a uniform classification and a timed approach to EEG analysis have positioned EEG as a complementary tool in the multimodal approach for prognostication.
The review was created and intended for medical staff in the intensive care units and emphasizes EEG patterns and timing which portend both favorable and poor prognoses. Also, the review addresses the overall quality of the existing studies and discusses future directions to address the knowledge gaps in this field.
Keywords: EEG, Prognosis, Post cardiac arrest, Electroencephalography, Review
Introduction
Determining if a patient may neurologically recover shortly after cardiac arrest (CA) resuscitation has long been challenging for medical staff. Published guidelines have advocated for clinicians to use a multi-modal approach to assess prognosis.1., 2., 3. To date, nearly all prognostication studies focus on poor outcomes, rather than good outcomes. Although targeted temperature management (TTM) has improved the overall outcome in CA, a large majority of patients have an indeterminate outcome initially.4., 5. The benefits of accurate prognostication in this indeterminate patient population will help prioritize deployment of resources.
In the 1960s, Hockaday et al devised a grading system (I-V) to categorize various EEG patterns and prognosis after cardiac or pulmonary arrest. The accuracy of prognosis was best for the grading extremes while indeterminate patterns gave an indeterminate prognosis. The authors recommended continued assessment with EEG over time for either rapid evolution of the EEG to normal or deterioration of EEG within a few days offering a “gloomy outlook”.6
In the absence of a uniformly accepted terminology7., 8. and the use of EEG for prognostication fell out of favor over the following decades. In 2006, the American Academy of Neurology (AAN) Quality Standards Subcommittee conducted an evidence-based review of the literature and published practice parameters about prediction of outcome in comatose survivors after cardiopulmonary resuscitation (CPR)9.and did not recommend EEG as a reliable outcome predictor. The review pointed out important confounders in the EEG literature including variable time intervals EEG recordings were conducted after CPR, and inconsistent classification systems.
Since the 2006 AAN practice parameter was published, major advances occurred within the field including increased use of continuous and serial EEG, broad adoption of standardized critical EEG terminology, and widespread adoption of TTM. Standardized critical care EEG terminology was published10 and recently updated11 which decreased inter-rater variability of EEG interpretation.12 TTM became the standard of care post-CA. Importantly though, hypothermia, sedatives, and neuromuscular blocking agents can mask the clinical exam, affect the EEG, and delay recovery of cerebral functioning for up to 5–6 days.13 In a worst-case scenario, this can lead to withdrawal of life sustaining therapy (WLST) inappropriately. In this situation, EEG provides an inexpensive and accurate method to monitor brain function in real time until normothermia and resolution of sedation. Hypothermia has a predicable effect on the EEG and at or above 34.2C, does not cause major changes on the EEG. However, EEG may be more unpredictable below that target temperature.14 While TTM has been revolutionary, the benefit is not conferred to all patients.15., 16.
More recently, the European Resuscitation Council (ERC) and the European Society of Intensive Care Medicine (ESICM) issued guidelines regarding prognostication in comatose survivors of cardiac arrest.1., 2., 3. With the recent advances in EEG literature, the guidelines promoted the use of EEG in the multimodal assessment of post-CA patients.
Further the 2015 ERC & ESICM guidelines noted that the overall quality of evidence for the review was low for almost all studies due to the risk of a self-fulfilling prophecy. For future studies, the 2015 ERC & ESICM guidelines called for blinding of treatment teams from results of the predictor under investigation.2 Subsequently, there was a substantial increase in neuro-prognostication studies per their recommendations. In 2021 the ERC-ESICM published updated guidelines after analyzing the most recent literature. The guidelines were informed in part by a systematic review recently conducted by Sandroni et al.17 The goal of this review is to discuss the most recent EEG prognostication literature and guidelines in detail.
Methods
Studies were located by searching PubMed using the words ‘adult’, ‘cardiac arrest’, ‘electroencephalogram’, ‘EEG’ and ‘prognostication’ for the years 2010–2020. This yielded 130 publications to review. The highest quality studies were selected based on the available data per the recent American Heart Association (AHA) Scientific Statement on the Standards of Studies in Neurologic Prognostication.18 The studies chosen were prospective, employed EEG early (within 24 hours after CA), used serial EEG or continuous EEG, and clearly stated a WLST algorithm. Trials after 2015 often attempted to make decisions about WLST independent of the result of the EEG thus allowing for reduced bias (although any knowledge off EEG results in the clinical context may introduce bias even on a subconscious level). Retrospective studies were included in the review if they addressed a knowledge gap. Ultimately, 71 studies were selected. Table 1 lists the studies and quality.
Table 1.
Studies reviewed in EEG Pattern section. An ‘asterisk’ ‘*’ denotes that a study provided and evaluation of not just one but multiple EEG patterns and was therefore mentioned in multiple sections.
| Study | n | Study design | WLST algorithm stated? | Tx team Blinded to EEG | EEG evaluation blinded to outcome | Outcome measure |
|---|---|---|---|---|---|---|
| Voltage: Isoelectric EEG / Suppressed Background / Low voltage | ||||||
| *Sivaraju et al21 | 100 | Prospective cohort, single center | Not described | No | Yes | GOS at discharge: 4,5 good, 1–3 poor |
| *Tjepkema-Cloostermans et al22 | 142 | Prospective cohort, single center | 24-hour bilateral absent SSEP, lack of improvement, organ failure | No | Yes | 6 mos CPC: 1,2 good, 3–5 poor |
| *Ruijter et al23 | 850 | Prospective cohort, 5 center | 72hours after CA when normothermic and off sedation with 2 of following: Absent brainstem reflexes, Bilateral absent SSEP, Absent or extensor motor response | No | Yes | 6 mos CPC: 1–2 good, 3–5 poor |
| *Hofmeijer et al19 | 277 | Prospective cohort, single center | @ 72 hours, normothermic, off-sedation for bilateral absent SSEP, treatment resistant myoclonus, incomplete return of brainstem reflexes | No | Yes | 6 mos CPC: 1,2 good, 3–5 poor |
| *Sondag et al24 | 430 | Prospective, 2 centers | 72hours after CA when normothermic and off sedation with >=2 of following: Incomplete return of brainstem reflexes, Bilateral absent SSEP, Treatment resistant myoclonus, 48–72 hours for loss of SSEP | No | Yes | 6 mos CPC: 1–2 good, 3–5 poor |
| *Scarpino et al25 | 351 | Prospective, multicenter | WLST not performed on any patient during study | No | Yes | Discharge and 6 mos CPC: 1–3 good, 4,5 poor |
| *Westhall et al26 | 202 | Prospective cohort, single center | 72hours post-CA local neurologist rec’d WLST: GCS 1–2 + bilateral loss SSEP or tx refractory SE (at least 24 hours), or status myoclonus + bilateral loss of SSEP | No | Yes | 6 mos CPC: 1–2 good, 3–5 poor |
| Burst Suppression: Heterogeneous Bursts | ||||||
| *Sivaraju et al21 | 100 | Prospective cohort, single center | Not described | No | Yes | GOS at discharge: 4,5 good, 1–3 poor |
| *Scarpino et al25 | 351 | Prospective, multicenter | WLST not performed on any patient during study | No | Yes | Discharge and 6 mos CPC: 1–3 good, 4,5 poor |
| *Westhall et al26 | 202 | Prospective cohort, single center | 72 hours CA local neurologist rec’d WLST: GCS 1–2 + bilateral loss SSEP or treatment refractory SE (at least 24 hours), or Status myoclonus + bilateral loss of SSEP | No | Yes | 6 mos CPC: 1–2 good, 3–5 poor |
| *Barbella et al29 | 522 | Retrospective cohort, single center | 72 hours after CA when normothermic and off sedation with >=2 of following: Incomplete return brainstem reflexes, bilateral absent SSEP, Serum NSE > 75 ug/L. Unreactive EEG, Tx resistant myoclonus or SE, Massive DWI changes | No | No | 3 mos CPC: 1–2 good, 3–5 poor |
| *Ruijter et al23 | 850 | Prospective cohort, 5 center | 72 hours after CA when normothermic and off sedation with 2 of following: Absent brainstem reflexes, bilateral absent SSEP, Absent or extensor motor response | No | Yes | 6 mos CPC: 1–2 good, 3–5 poor |
| *Tjepkema-Cloostermans et al22 | 142 | Prospective cohort | 24 hour bilateral absent SSEP, lack of improvement, organ failure | No | Yes | 6 mos CPC: 1–2 good, 3–5 poor |
| Hofmeijer et al30 | 101 | Prospective cohort34 + retrospective cohort single center | 72 hour after CA when normothermic and off sedation with bilateral absent SSEP | No | Yes | 3 & 6 mos CPC: 1–2 good, 3–5 poor |
| Amorim et al31 | 120 | Retrospective single center | Not described | No | No | At discharge: CPC: 1–2 good, 3–4 poor |
| Burst Suppression: Identical Bursts (monomorphic) | ||||||
| *Hofmeijer et al19 | 277 | Prospective cohort, single center | @ 72 hours, normothermic, off-sedation for bilateral absent SSEP, treatment resistant myoclonus, incomplete return of brainstem reflexes | No | Yes | 6 mos CPC: 1,2 good, 3–5 poor |
| *Tjepkema-Cloostermans et al22 | 142 | Prospective cohort | 24 hour bilateral absent SSEP, lack of improvement, organ failure | No | Yes | 6 mos CPC: 1–2 good, 3–5 poor |
| *Ruijter et al23 | 850 | Prospective cohort, 5 center | 72 hours after CA when normothermic and off sedation with 2 of following: absent brainstem reflexes, bilateral absent SSEP, absent or extensor motor response | No | Yes | 6 mos CPC: 1–2 good, 3–5 poor |
| *Barbella et al29 | 522 | Retrospective cohort, single center | 72 hours after CA when normothermic and off sedation with >=2 of following: Incomplete return brainstem reflexes, bilateral absent SSEP, Serum NSE > 75 ug/L, Unreactive EEG, treatment resistant myoclonus or SE. massive DWI changes | No | No | 3 mos CPC: 1–2 good, 3–5 poor |
| Hofmeijer et al30 | 101 | Prospective cohort34 + retrospective cohort single center | 72 hour after CA when normothermic and off sedation with bilateral absent SSEP | No | Yes | 3 & 6 mos CPC: 1–2 good, 3–5 poor |
| van Putten et al58 | 11 | Prospective cohort, single center | @ 72 hours, normothermic, off-sedation for bilateral absent SSEP, treatment resistant myoclonus, incomplete return of brainstem reflexes | No | Yes | Postmortem analysis of brains of patients who expired in hospital during cohort study of post-CA patients |
| Epileptiform Activity (Status Epilepticus and Periodic Discharges) | ||||||
| *Sivaraju et al21 | 100 | Prospective cohort, single center | Not described | No | Yes | GOS at discharge: 4,5 good, 1–3 poor |
| *Tjepkema-Cloostermans et al22 | 142 | Prospective cohort, single center | >=24 hours bilateral absent SSEP, lack of improvement, organ failure | No | Yes | 6 mos CPC: 1,2 good, 3–5 poor |
| *Westhall et al26 | 202 | Prospective cohort, single center | 72 hours CA local neurologist rec’d WLST: GCS 1–2 + bilateral loss SSEP or treatment refractory SE (at least 24 hours), Status myoclonus + bilateral loss of SSEP | No | Yes | 6 mos CPC: 1–2 good, 3–5 poor |
| Rossetti et al35 | 61 | Prospective cohort, single cohort | Two of following at normothermia: Incomplete brainstem reflexes, Early myoclonus, unreactive EEG, bilaterally absent SSEP | No | Yes | 3 mos CPC: 1–2 good, 3–5 poor |
| Beretta et al36 | 166 | Prospective cohort, single center | Multimodal approach: Bilateral absent brainstem reflexes, NSE > 68 ng/ml@ 48 hours, 72 hours bilateral absent SSEP | No | No | 6 mos CPC: 1–2 good, 3–5 poor |
| Ruijter et al37 | 47 | Prospective cohort, 2 hospitals | Not described | No | Yes | 6 mos CPC: 1–2 good, 3–5 poor |
| Continuous Activity with frequencies greater than or less than 8 Hz | ||||||
| *Sivaraju et al21 | 100 | Prospective cohort, single center | Not described | No | Yes | GOS at discharge: 4,5 good, 1–3 poor |
| *Sondag et al24 | 430 | Prospective, 2 centers | 72 hours after CA when normothermic and off sedation with >=2 of following Incomplete return brainstem reflexes, bilateral absent SSEP, Treatment resistant myoclonus, 48–72 hours for loss of SSEP | No | Yes | 6 mos CPC: 1–2 good, 3–5 poor |
| *Scarpino et al25 | 351 | Prospective, multicenter | WLST not performed on any patient during study | No | Yes | Discharge and 6 mos CPC: 1–3 good, 4,5 poor |
| Spalleti et al39 | 211 | Retrospective cohort, single center | Not described | No | No | 6 mos CPC: 1–2 good, 3–5 poor |
| Background Reactivity | ||||||
| *Sivaraju et al21 | 100 | Prospective cohort, single center | Not described | No | Yes | GOS at discharge: 4,5 good, 1–3 poor |
| *Barbella et al44 | 488 | Retrospective cohort, single center | 72 hours after CA when normothermic and off sedation with >=2 of following: Incomplete return brainstem reflexes, Bilateral absent SSEP, Serum NSE > 75 ug/L, Unreactive EEG, Tx resistant myoclonus or SE, Massive DWI changes | No | No | 3 mos CPC: 1–2 good, 3–5 poor |
| Admiraal et al40 | 149 | Prospective cohort, single center | 72 hours post CA normothermic and off sedation: neurologic exam, SSEP, and EEG patterns @ 72 hours | No | No | 6 mos CPC: 1–2 good, 3–5 poor |
| Rossetti et al42 | 357 | Prospective, 2 centers | 72 hours post CA, no improvement with incomplete brainstem reflexes and/or absent SSEP | No | No | 3 mos CPC: 1–2 good, 3–5 poor |
| Tsetou et al43 | 61 | Prospective cohort, single center | Post CA at normothermia and off sedation: 2 of following: unreactive eeg, treatment resistant myoclonus, bilateral absent SSEP, incomplete brainstem reflexes | No | No | 3 mos CPC: 1–2 good, 3–5 poor |
| Evolution of Background over time | ||||||
| *Hofmeijer et al19 | 277 | Prospective cohort, single center | @ 72 hours, normothermic, off-sedation for bilateral absent SSEP, treatment resistant myoclonus, incomplete return of brainstem reflexes | No | Yes | 6 mos CPC: 1,2 good, 3–5 poor |
| *Sivaraju et al21 | 100 | Prospective cohort, single center | Not described | No | Yes | GOS at discharge: 4,5 good, 1–3 poor |
| *Sondag et al24 | 430 | Prospective, 2 centers | 72 hours after CA when normothermic and off sedation with >=2 of following Incomplete return brainstem reflexes, bilateral absent SSEP, Treatment resistant myoclonus , 48–72 hours for loss of SSEP | No | Yes | 6 mos CPC: 1–2 good, 3–5 poor |
| *Ruijter et al22 | 850 | Prospective cohort, 5 center | 72 hours after CA when normothermic and off sedation with 2 of following: absent brainstem reflexes, bilateral absent SSEP, absent or extensor motor response | No | Yes | 6 mos CPC: 1–2 good, 3–5 poor |
| Rosetti et al49 | 364 | Prospective multicenter, randomized | Not described | No | No | 6 mos CPC: 1–2 good, 3–5 poor |
| Alvarez et al50 | Prospective cohort | After interdisciplinary discussion based on clinical and electrophysiological findings but not EEG | No | Yes | 3 mos CPC: 1–2 good, 3–5 poor | |
| Elmer et al51 | 759 | Observational cohort | Not described | No | No | Epileptiform EEG patterns associated with neurological outcome at discharge |
| Fatuzzo et al52 | 497 | Retrospective cohort | 72 hours post CA, no improvement with incomplete brainstem reflexes and/or absent SSEP | No | No | 3 mos CPC: 1–2 good, 3–5 poor |
| Myoclonus | ||||||
| *Sivaraju et al21 | 100 | Prospective cohort, single center | Not described | No | Yes | GOS at discharge: 4,5 good, 1–3 poor |
| Elmer et al55 | 401 | Prospective cohort, single center | Not described | No | No | Survival to hospital discharge |
| Beuchat et al56 | 78 | Retrospective cohort, 4 registries | Interdisciplinary consensus with involvement with family | No | No | 3 mos CPC: 1–2 good, 3–5 poor |
| Dhakar et al57 | 59 | Retrospective cohort, single center | Not described | No | Yes | Recovery of consciousness: CPC 1–3 or 4–5 |
EEG patterns that were 100% specific for a poor outcome with a 0% false positive rate (FPR) are highlighted. When possible, this review calls attention to EEG patterns associated with favorable outcomes and EEG patterns which should be intervened upon to improve clinical outcome.
The following sections are separated by EEG pattern defined by Hofmeijer el al.19 Background reactivity, evolution over time, and myoclonus (clinical and cortical) were added because these EEG features have reliable predictive value in post CA patients.
Eeg features:
-
1.Voltage
-
a.Isoelectric
-
b.Suppressed
-
c.Low voltage
-
a.
-
2.Burst suppression
-
a.Burst suppression with identical bursts
-
a.
-
3.
Epileptiform activity (status epilepticus and generalized periodic discharges)
-
4.
Continuous activity with frequencies < 8 Hz
-
5.
Continuous activity with frequencies > 8 Hz
-
6.
Background Reactivity
-
7.
Evolution / change of the EEG background over time
-
8.
Clinical and Cortical Myoclonus with EEG features
Voltage
Isoelectric EEG / suppressed background / low voltage
Ten to forty seconds after circulatory arrest, EEG recordings become isoelectric.20 After successful ROSC, in most cases electrical activity returns. However, a persistent isoelectric background (lack of any identifiable electrocortical activity at 3uV sensitivity) at 24 hours has been shown in multiple studies to be invariably associated with a poor outcome.19., 21., 22., 24.
A suppressed background (SB) is defined by all activity having voltages =< 10 uV. A low voltage background is defined by voltages =< 20 uV for most or all activity in longitudinal bipolar with standard 10–20 electrodes measured peak to trough.10 In general, low voltage activity is present in nearly all post-CA patients initially and is seen in otherwise healthy adults. A suppressed background and low voltage background are not interchangeable terms and have different clinical significance. Both backgrounds are included in this section because in some of the trials analyzed below, the two backgrounds were combined in results analysis.
In a recent prospective, multi-center study (n = 850), a suppressed background was 100% predictive of a poor outcome at >= 6 hours with a 0% FPR.23 This result was found in patients receiving TTM with sedation. It is important to note, though debatable and applied with caution, sedation rates ranging from 0.1-0.2 mg/kg/hr (midazolam) or 2–3 mg/kg/hr (propofol) have only a limited impact on the prognostic accuracy of EEG in some studies.14., 19., 21. These finding need to be confirmed in future studies.
In a prospective cohort study (n = 100) in which EEG results were taken into consideration for WLST, the best predictor for poor outcome was an isoelectric background, suppressed background, and low voltage activity at 24 hours post-ROSC.21 In three additional prospective studies (n = 277,142, 430, respectively) in which EEG results were not taken into account for WLST, 100% of patients with an isoelectric background, or low voltages at 24 hours post-ROSC did poorly.19., 21., 24. Importantly, other studies showed that a suppressed pattern25 or low voltage pattern21., 26. at 12 hours was not invariably associated with a poor outcome. Taken together, these studies show that despite a patient receiving standard TTM treatment and sedation, medical staff may be able to predict outcome at 24 hours post-ROSC. Importantly, the prospective study (ProNeCA Multicenter Study) did not allow WLST and showed isoelectric backgrounds or burst suppression at 24 hours was always associated with a poor outcome.25 Notably, a recent systemic review by Sandroni et al concluded that EEG suppression, especially after 24 hours after ROSC almost invariably predict poor outcome.17
These finding could help teams better decipher which patients may do poorly early in the post-CA course. Low voltage backgrounds do not universally confer a bad outcome and may improve with time. This data provides justification for WLST at 24 hours in patients with an isoelectric background or suppressed background. however, decisions regarding WLST should be made in the context of a robust multimodal assessment.
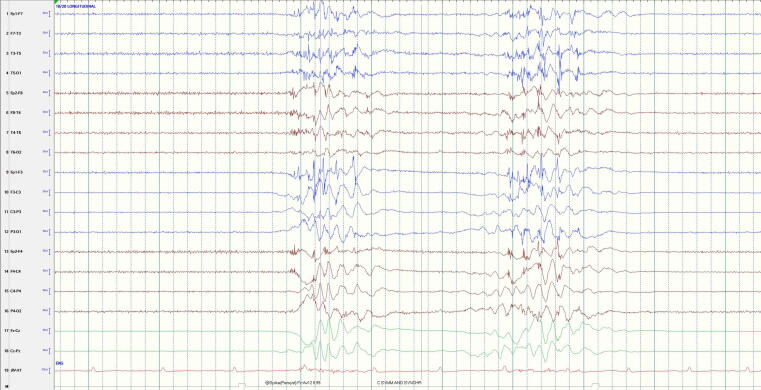
Burst suppression: Heterogeneous bursts (Fig. 1)
Fig. 1.
Burst Suppression with heterogenous bursts: In this example the ∼1 second bursts (consisting of sharply contoured delta-theta) are notably different in morphology and frequency composition from one another and are separated by ∼1.5 seconds of voltage suppression (<10 uV). (Note that the space between each dotted vertical line denotes 1 second).
Burst suppression is defined as bursts (>500 ms AND > 3 phases) of generalized activity on a suppressed background.10 Burst suppression can occur for physiologic reasons (e.g., in early development), in the context of cooling, due to the administration of an anesthetic, or from pathologic causes. The prognostic value of burst-suppression patterns after CA are conflicting.23 In a prospective study (n = 100), burst suppression at any time point (including 6 hours after ROSC) indicated a poor prognosis with 100% specificity and 0% FPR.21 In two studies, patients with a burst suppression pattern at 24 hours and 72 hours after ROSC had a poor outcome with a probability of 100%.25., 26. However, several other studies have shown that burst suppression is not solely associated with a poor outcome regardless of timing.23., 27., 28. and in fact can be a time-dependent finding early in the course or a function of treatment. In a systematic review, Sandroni et al concluded spontaneous burst suppression almost always predicts poor outcome, especially after 24 hours.17
Based on these conflicting results and the possibility of sedation and cooling confounders, the prognostic significance of a burst suppression with heterogenous bursts is still unclear regardless of timing. Therefore, the patient and EEG should be assessed after rewarming and off sedation.
Burst suppression: Identical bursts (monomorphic) (Fig. 2)
Fig. 2.
Burst suppression with identical bursts: The very brief spike bursts are of identical in morphology with periods of suppression in between.
In contrast to burst suppression with heterogeneous bursts, burst suppression with identical bursts appears to be exclusively indicative of a poor outcome. Indeed, burst suppression with identical bursts has been shown to be highly correlated with severe post-anoxic brain injury, high neuron specific enolase (NSE) levels29 and is 100% specific for poor outcome regardless of timing in relation to ROSC based on multiple studies.19., 22., 23., 29., 30. Thus, it is important for physicians to evaluate whether bursts are monomorphic (identical) versus heterogeneous. Bursts are defined as identical if the first 500 ms are identical, irrespective of amplitude or subsequent duration of bursts or inter-burst intervals.35 The authors in one study quantified independent bursts by sampling the first 500 ms of 128 bursts to better define their correlation.21 This EEG pattern can emerge early, around 12 hours post-CA, and evolve into less predictive patterns around 32 hours.29 It is important to note this is a pathological EEG pattern that can occur after diffuse cerebral ischemia and is invariably associated with a poor outcome.30
Epileptiform activity (status epilepticus (SE) and periodic discharges) (Figs. 3a, 3b, and 4)
Fig. 3.
Generalized Periodic Discharges (GPDs): a. GPDs on a continuous background. b. GPDS on a suppressed background a. GPDs on continuous background: The background is continuous (meaning not suppressed) with sharply contoured generalized periodic discharges occurring at ∼1 HZ (1 per second). b. GPDs on a suppressed background: The background is suppressed (<10 uV) between the generalized periodic discharges (GPDs) occurring at ∼1 Hz. The discharges are sharp waves with phase reversal at F3 and F4 (frontocentral head region).
Fig. 4.
Status Epilepticus: The EEG demonstrated bilateral low to medium voltage 4–5 Hz spike and slow wave discharges (meeting ACNS criteria for seizure activity, spike and slow wave discharges greater than or equal to 3/s). It is important to note that this ten second EEG epoch cannot encapsulate the evolution and devolution present in this (or any typical) seizure. (ACNS = American Clinical Neurophysiology Society).
Electrographic status epilepticus in post-CA patients occurs in 10–35% of cases. A significant portion of epileptic seizures occur during the first 24 hours post-arrest while patients are actively undergoing TTM and receiving sedating medication.31., 32. American Clinical Neurophysiology Society (ACNS) criteria define unequivocal electrographic seizures as either generalized spike-wave discharges 3 Hz or faster or as clearly evolving discharges of any type that reach a frequency of >4 Hz, whether focal or generalized.10 Nonconvulsive status epilepticus (NCSE) on the other hand, has been more difficult to define. The Salzburg criteria describe NCSE by two distinct EEG patterns with epileptiform discharges greater than 2.5 Hz, or less than or equal 2.5 HZ with EEG and clinical improvement after IV AED, or subtle clinical signs, or typical spatiotemporal evolution.33., 34.
Despite the recent attempts to introduce uniform terminology and criteria for epileptic seizures and NCSE, studies have varied widely in their use of diagnostic criteria for epileptiform activity.19 As an example, discharges that may be perceived as epileptiform activity initially often become more monomorphic and decrease in frequency over time. If there is a transition to a discontinuous background, what was once considered epileptiform activity may later be more aptly described as monomorphic burst suppression pattern. This highlights the construct that the EEG in the early post-CA period is dynamic, and therefore timing always needs to be considered when interpreting EEG patterns. Adding to this confusion, it is unclear whether treating epileptiform patterns improves outcomes or if they are just a reflection of irreversible neuronal damage.
In multiple prior studies, patients with NCSE and/or epileptiform activity post-CA exclusively had poor outcomes.21., 22., 26., 35. However, there were some confounding aspects to these results. First, studies inconsistently used the validated EEG ACNS criteria to describe epileptiform activity. Second, antiepileptic treatment approaches were varied, generally of moderate intensity, and only given for a predefined period (e.g., 24 hours of anesthetics). Third, EEG timing and attention to evolution of the EEG background varied amongst the studies. Finally, refractory SE (RSE) was considered a reason for WLST in one study rendering its prognostic significance unclear.27
Recent research has challenged the notion that epileptiform activity and SE heralds a poor prognosis. In a recent prospective cohort study (n = 166) a portion of patients were defined as having SE and/or RSE.36 The ACNS and Salzburg NCSE criteria were employed in this study. RSE was defined as epileptiform discharges (sharp waves and spikes) > 2.5 Hz for 30 minutes or longer and Generalized Periodic Discharges (GPDs) were defined as sharply contoured, sharp, or spiky discharges at <2.5 Hz for 30 minutes or longer. Interestingly, patterns less than 2.5 Hz were not considered NCSE despite the Salzburg criteria making possible allowances for this as mentioned above. Nonetheless, this frequency distinction was generally not made in prior studies. In patients identified with SE and favorable multimodal prognostic indicators, aggressive standardized antiepileptic therapy without a predefined duration was given. Clinical motor seizures were more prevalent in patients with GPDs (61.5%) compared to RSE (44.4%). A good argument could be made whether the patients with GPDs and clinical motor seizures should have been placed in the SE group. Even so, in patients with RSE, 44.4% had a good neurologic outcome at 6 mos. Only 15.4% of patients with GPDs had a good outcome. Of note, the study assigned seven patients who initially had RSE and later developed GPDs to the GPD group. This emphasizes the need to continuously evaluate the EEG in these patients over time rather than once. This study provided surprising insights into the prognosis of post CA patients with RSE. A key difference compared to prior research was the aggressive treatment approach without predefined duration. Currently, Ruijter et al are conducting the Treatment of ELectrographic STatus epilepticus After cardiopulmonary Resuscitation (TELSTAR) which hopefully provides additional guidance regarding post CA SE.
Another interesting paper by Ruijter et al noted that post CA patients with SE had a better chance for recovery if SE started later in the course (45 hr vs 29 hr post ROSC), evolved from a continuous background, had a higher discharge frequency, lower relative discharge power, and lower discharge periodicity. They noted that patients with post-anoxic SE without a preceding continuous background always had a poor outcome.37 Sandroni et al reviewed the recent literature concluding, along with the ERC-ESICM 2021 Guidelines3, that true early seizure activity (first 72 hours post ROSC) is associated with a poor outcome.17
Overall, the data regarding epileptiform activity is mixed. However, EEG should be started promptly, epileptiform activity should be described according to the ACNS EEG criteria, and care should be taken to utilize the Salzburg and ACNS criteria for NCSE. Background continuity should be assessed because epileptiform activity arising from a continuous background can be effectively treated with antiseizure drugs (ASD)38 and a suppressed background appears to be an ominous sign.37 Clinicians should consider aggressive and prolonged treatment for patients with true SE if a multimodal approach otherwise suggests a favorable prognosis.36 Future studies should attempt to decipher epileptiform pattern timing and evolution, treatment effects, and outcomes.
Continuous activity with frequencies greater than or less than 8 Hz
All studies looking at EEG prognostication early after CA have shown that a continuous, normal amplitude background at 12 hours post ROSC was associated with a good outcome.21., 24., 25., 39. Also, normal voltage backgrounds without epileptiform discharges, even if discontinuous, were largely associated with good outcomes.21 However, the absence of such finding does not necessarily indicate poor neurological outcome based on current evidence.
Background reactivity
Background reactivity is generally assessed in patients in a stepwise fashion with auditory, visual, and tactile stimulation. Reactivity emerges as brief voltage attenuation or a change in amplitude of frequency in electrical activity.10 Importantly, muscle artifact should not be classified as reactivity.40 The absence of EEG reactivity has been shown in some studies to predict a poor outcome with a low or no FPR during TTM when assessed early and repeatedly during the post-CA course.41., 42., 43. Other studies did not show findings that were as robust. In one study, a nonreactive EEG predicted poor outcome in 70% of patients, however, the authors conceded this pattern had high interrater variability.12 In another study, a lack of background activity was more common in the poor outcome group (48/61) than the good outcome group (4/28).21 Importantly, the ERC-ESICM 2021 Guidelines3 and 2019 AHA Scientific Statement18 state that absence of background reactivity is an indicator of poor prognosis.
Recently, a prospective cohort study (n = 160) investigated specifically for EEG reactivity as a predictor of outcome. A standardized assessment was performed twice a day on patients post-CA and analyzed by three blinded EEG readers. Absence of reactivity as a poor outcome predictor had a specificity of 82% and sensitivity 73%. The authors concluded that EEG reactivity is not sufficient as a sole outcome predictor in patients with CA. They further stated that the presence of reactivity rather than absence of reactivity adds value to a multi-modal assessment.40 Indeed, a reactive EEG under TTM has been shown to be highly sensitive for a good outcome.43
Intriguingly, a retrospective paper hypothesized that stimulus-induced rhythmic periodic discharges (SIRPIDs) may be a form a reactivity, in an abnormally functioning brain, when occurring later in the recovery period (36–72 hours post ROSC) and were associated with a good outcome.44 This is a finding which challenged prior study results which associated SIRPIDs with a poor outcome.21., 45.
In general, assessment of reactivity is reader-dependent, lacks standardization in application and assessment, and is non-quantitative when evaluated on the raw EEG. While being assessed for the presence of absence of reactivity, patients should not be on sedation.46 Recent prospective studies have attempted to standardize reactivity approaches and assessment.4., 47. Algorithms for automated determination of reactivity based on quantitative EEG parameters and machine learning are currently being developed and have shown promise.48 Until reactivity assessments on raw and/or quantitative EEG have been sufficiently standardized and validated, reactivity should only be used as one parameter within a multi-model prognosis assessment of post-CA patients.
Evolution of the EEG background over time
Clues as to which patient may have a good versus poor outcomes can now be determined within the first 24 hours of ROSC despite TTM and sedation,24., 25. An EEG pattern like burst suppression with identical bursts or a persistent isoelectric study by 24 hours are strongly correlated with a poor outcome regardless of timing. However, prognostic value is time-dependent for most other EEG patterns. For example, in a prospective multicenter trial (n = 850) a discontinuous pattern at 6 hours was associated with an 80% chance of a good outcome but a 0% chance of a good outcome at 120 hours.22 Similarly, while most EEGs are usually of low voltage just after ROSC, it is not until 24 hours that a low voltage EEG has been correlated with a poor outcome.19., 21., 24.
It should be noted that serial and continuous EEG monitoring gives the treatment team the best opportunity to recognize treatable conditions like electrographic seizures early and to recognize patterns associated with poor outcomes in a timely fashion. However, multiple recent studies have demonstrated that continuous EEG does not confer an advantage over serial routine EEGs with respect to outcome.49., 50., 51., 52. These studies allowed WLST which may have biased results. Nonetheless, this is potentially good news for health systems with limited resources. Serial routine EEGs should be performed at regular time points (e.g., 24 hr, 48 hr, and 72 hr) for at least 2–3 days.
Myoclonus
Myoclonus is a clinical phenomenon which consists of sudden, brief, involuntary jerks caused by muscular contractions or inhibitions. It can range from massive clinical myoclonic jerking to a more subtle Lance-Adam syndrome. To date, there is no consensus-based definition for post-anoxic myoclonus (PAM). This has led to confusion within the field because the term has been used but described differently in various studies.53 Despite the confusion over PAM, there is a clear definition of status myoclonus (SM) which has been described as patients in a coma with continuous and generalized (clinical) myoclonic jerks for 30 minutes or longer within 5 days after cardiac arrest. Status myoclonus has often been classified to be an indicator of a poor outcome.2., 9.
EEG can help distinguish between cortical myoclonus and subcortical myoclonus which typically require different treatment approaches.54 Cortical myoclonus is described as clinical myoclonus with concurrent generalized polyspikes, spikes, or sharp waves that are time-locked with the jerks. Subcortical myoclonus is clinical myoclonus with no EEG correlate. In addition to clinical semiology, post-anoxic (cortical) myoclonus has been further described in a recent study as two distinct EEG patterns which are differentiated by the presence or absence of a continuous background. All patients with a discontinuous background had a poor outcome, while 50% of patients with a continuous background survived to have Lance Adams Syndrome.55 Patients with a discontinuous background had a predictable pattern evolution over a few days from ROSC. Initially, the EEG showed high voltage polyspike burst suppression, followed by a softening of the bursts and dissipation of myoclonus. Next, the bursts lengthened which almost gave the appearance of continuity before the EEG rapidly lost complexity and the background became suppressed.
Based on these findings, patients with early myoclonus should not be considered to have a universally poor outcome. While we acknowledge the importance of PAM in post cardiac arrest syndrome, we also highlight this critical knowledge gap. We believe that myoclonus and its related issues deserve a dedicated and separate review and continued analysis.
Conclusion
Since the 1960s, the role of EEG in post-CA evaluation has evolved. Over the years with rigorous research and a more uniform classification system, EEG has become a valuable tool for neurological prognostication. Availability of EEG (routine and continuous) has increased over the years and new EEG methodologies including quantitative analysis, machine learning, and deep learning of EEG patterns look promising.56 Unfortunately, WLST bias continues to plague prognostication studies, though some steps have been taken to reduce it. More randomized trials evaluating different treatment modalities, like the upcoming TELSTAR trial, should be pursued. Efforts to classify and stratify post-CA patients with electro-clinical phenotyping have shown promise. Finally, studies should continue to determine who may do poorly post-CA but perhaps more importantly, who may do well.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Dr. Geocadin is supported in part by grants on cardiac arrest: NIH UG3 HL145269-01A1, 2R01HL071568-15, 1 RO1 NS119825-01, R01 HL155760, and an unrestricted grant from the Wenzel Family Foundation.
Contributor Information
Jay Bronder, Email: Jbronde1@jhmi.edu.
Sung-Min Cho, Email: csungmi1@jhmi.edu.
Romergryko G. Geocadin, Email: rgeocad1@jhmi.edu.
Eva Katharina Ritzl, Email: Eritzl1@jhmi.edu.
References
- 1.Nolan J.P., Soar J., Cariou A., Cronberg T., Moulaert V.R., Deakin C. European Resuscitation Council and European Society of Intensive Care Medicine 2015 guidelines for post-resuscitation care. Intensive Care Med. 2015;41:2039–2056. doi: 10.1007/s00134-015-4051-3. [DOI] [PubMed] [Google Scholar]
- 2.Sandroni C., Cariou A., Cavallaro F., Cronberg T., Friberg H., Hoedemaekers C. Prognostication in comatose survivors of cardiac arrest: an advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Resuscitation. 2014;85:1779–1789. doi: 10.1016/j.resuscitation.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Nolan J.P., Sandroni C., Böttiger B.W., et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: post-resuscitation care. Intensive Care Med. 2021;47:369–421. doi: 10.1007/s00134-021-06368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holzer M. Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med. 2010;363:1256–1264. doi: 10.1056/NEJMct1002402. [DOI] [PubMed] [Google Scholar]
- 5.Kamps M.J., Horn J., Oddo M., et al. Prognostication of neurologic outcome in cardiac arrest patients after mild therapeutic hypothermia: A meta-analysis of the current literature. Intensive Care Med. 2013;39:1671–1682. doi: 10.1007/s00134-013-3004-y. [DOI] [PubMed] [Google Scholar]
- 6.Hockaday J.M., Potts F., Epstein E., Bonazzi A., Schwab R.S. Electroencephalographic changes in acute cerebral anoxia from cardiac or respiratory arrest. Electroencephalogr Clin Neurophysiol. 1965;18:575–586. doi: 10.1016/0013-4694(65)90075-1. [DOI] [PubMed] [Google Scholar]
- 7.Sutter R., Kaplan P.W. Electroencephalographic criteria for nonconvulsive status epilepticus: synopsis and comprehensive survey. Epilepsia. 2012;53(Suppl 3):1–51. doi: 10.1111/j.1528-1167.2012.03593.x. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch L.J., Brenner R.P., Drislane F.W., et al. The ACNS Subcommittee on Research Terminology for Continuous EEG Monitoring: proposed standardized terminology for rhythmic and periodic EEG patterns encountered in critically ill patients. J Clin Neurophysiol. 2005;22:128–135. doi: 10.1097/01.wnp.0000158701.89576.4c. [DOI] [PubMed] [Google Scholar]
- 9.Wijdicks E.F.M., Hijdra A., Young G.B., Bassetti C.L., Wiebe S. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67:203–210. doi: 10.1212/01.wnl.0000227183.21314.cd. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch L.J., LaRoche S.M., Gaspard N., et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol. 2013;30:1–27. doi: 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch L.J., Fong M.W.K., Leitinger M., et al. American Clinical Neurophysiology Society's Standardized Critical Care EEG Terminology: 2021 Version. J Clin Neurophysiol. 2021;38:1–29. doi: 10.1097/WNP.0000000000000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westhall E., Rosen I., Rossetti A.O., et al. Interrrater variability of EEG interpretation in comatose cardiac arrest patients. Clin Neurophysiol. 2015;126:2397–2404. doi: 10.1016/j.clinph.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Samaniego E.A., Mlynash M., Caulfield A.F., Eyngorn I., Wijman C.A.C. Sedation confounds outcome prediction in cardiac arrest survivors treated with hypothermia. Neurocrit Care. 2011;15:113–119. doi: 10.1007/s12028-010-9412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stecker M.M., Cheung A.T., Pochettino A., et al. Deep hypothermic circulatory arrest: I. Effects of cooling on electroencephalogram and evoked potentials. Ann Thorac Surg. 2001;71:14–21. doi: 10.1016/s0003-4975(00)01592-7. [DOI] [PubMed] [Google Scholar]
- 15.The Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen N., Wetterslev J., Cronberg T., et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369:2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 17.Sandroni C., D’Arrigo S., Cacciola S., et al. Prediction of poor neurological outcome in comatose survivors of cardiac arrest: a systematic review. Intensive Care Med. 2020;46:1803–1851. doi: 10.1007/s00134-020-06198-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geocadin R.G., Callaway C.W., Fink E.L., et al. Standards for studies of neurological prognostication in comatose survivors of cardiac arrest: a scientific statement from the American Heart Association. Circulation. 2019;140:e517–e542. doi: 10.1161/CIR.0000000000000702. [DOI] [PubMed] [Google Scholar]
- 19.Hofmeijer J., Beernink T.M.J., Bosch F.H., Beishuizen A., Tjepkema-Cloostermans M.C., van Putten M.J.A.M. Early EEG contributes to multimodal outcome prediction of postanoxic coma. Neurology. 2015;85:137–143. doi: 10.1212/WNL.0000000000001742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Dijk J.G., Thijs R.D., van Zwet E., et al. The semiology of tilt-induced reflex syncope in relation to electroencephalographic changes. Brain. 2014;137:576–585. doi: 10.1093/brain/awt332. [DOI] [PubMed] [Google Scholar]
- 21.Sivaraju A., Gilmore E.J., Wira C.R., et al. Prognostication of post-cardiac arrest coma: early clinical and electroencephalographic predictors of outcome. Intensive Care Med. 2015;41:1264–1272. doi: 10.1007/s00134-015-3834-x. [DOI] [PubMed] [Google Scholar]
- 22.Tjepkema-Cloostermans M.C., Hofmeijer J., Trof R.J., Blans M.J., Beishuizen A., van Putten M.J.A.M. Electroencephalogram predicts outcome in patients with postanoxic coma during mild therapeutic hypothermia. Crit Care Med. 2015;43:159–167. doi: 10.1097/CCM.0000000000000626. [DOI] [PubMed] [Google Scholar]
- 23.Ruijter B.J., Tjepkema-Cloostermans M.C., Tromp S.C., et al. Early electroencephalography for outcome prediction of postanoxic coma: a prospective cohort study. Ann Neurol. 2019;86:203–214. doi: 10.1002/ana.25518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sondag L., Ruijter B.J., Tjepkema-Cloostermans M.C., et al. Early EEG for outcome prediction of postanoxic coma: prospective cohort study with cost-minimization analysis. Critical Care. 2017;21:111. doi: 10.1186/s13054-017-1693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scarpino M., Carrai R., Lolli F., et al. Neurophysiology for predicting good and poor neurological outcome at 12 and 72 hr after cardiac arrest: The ProNeCA multicenter prospective study. Resuscitation. 2020;147:95–103. doi: 10.1016/j.resuscitation.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Westhall E., Rossetti A., van Rootselaar A.F., et al. Standardized EEG interpretation accurately predicts prognosis after cardiac arrest. Neurology. 2016;16:1482–1490. doi: 10.1212/WNL.0000000000002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cloostermans M.C., van Meulen F.B., Eertman C.J., Hom H.W., van Putten M.J. Continuous electroencephalography monitoring for early prediction of neurological outcome in postanoxic patients after cardiac arrest: A prospective cohort study. Crit Care Med. 2012;40:2867–2875. doi: 10.1097/CCM.0b013e31825b94f0. [DOI] [PubMed] [Google Scholar]
- 28.Amorim E., Rittenberger J.C., Baldwin M.E., Callaway C.W., Popescu A. Post Cardiac Arrest Service. Malignant EEG patterns in cardiac arrest patients treated with targeted temperature management who survive to hospital discharge. Resuscitation. 2015;90:127–132. doi: 10.1016/j.resuscitation.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbella G., Novy J., Marques-Vidal P., Oddo M., Rossetti A.O. Prognostic role of EEG identical bursts in patients after cardiac arrest: Multimodal correlation. Resuscitation. 2020;148:140–144. doi: 10.1016/j.resuscitation.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 30.Hofmeijer J., Tjepkema-Cloostermans M.C., van Putten M.J.A.M. Burst-suppression with identical bursts: A distinct EEG pattern with poor outcome in postanoxic coma. Clin Neurophysiol. 2014;125:947–954. doi: 10.1016/j.clinph.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Geocadin R.G., Ritzl E.K. Seizures and status epilepticus in post cardiac arrest syndrome: therapeutic opportunities to improve outcome or basis to withhold life sustaining therapies. Resuscitation. 2012;83:791–792. doi: 10.1016/j.resuscitation.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Mani R., Schmitt S.E., Mazer M., Putt M.E., Gaieski D.F. The frequency and timing of epileptiform activity on continuous electroencephalogram in comatose post-cardiac arrest syndrome patients treated with therapeutic hypothermia. Resuscitation. 2012;83:840–847. doi: 10.1016/j.resuscitation.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beniczky S., Hirsch L.J., Kaplan P.W., et al. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia. 2013;54(suppl 6):28–29. doi: 10.1111/epi.12270. [DOI] [PubMed] [Google Scholar]
- 34.Leitinger M., Trinka E., Gardella E., et al. Diagnostic accuracy of the Salzburg EEG criteria for non-convulsive status epilepticus: a retrospective study. Lancet Neurol. 2016;15:1054–1062. doi: 10.1016/S1474-4422(16)30137-5. [DOI] [PubMed] [Google Scholar]
- 35.Rossetti A.O., Carrera E., Oddo M. Early EEG correlates of neuronal injury after brain anoxia. Neurology. 2012;78:796–802. doi: 10.1212/WNL.0b013e318249f6bb. [DOI] [PubMed] [Google Scholar]
- 36.Beretta S., Coppo A., Bianchi E., et al. Neurologic outcome of postanoxic refractory status epilepticus after aggressive treatment. Neurology. 2018;91:e2153–e2162. doi: 10.1212/WNL.0000000000006615. [DOI] [PubMed] [Google Scholar]
- 37.Ruijter B.J., van Putten M.J.A.M., Hofmeijer J. Generalized epileptiform discharges in postanoxic encephalopathy: quantitative characterization in relation to outcome. Epilepsia. 2015;56:1845–1854. doi: 10.1111/epi.13202. [DOI] [PubMed] [Google Scholar]
- 38.Solanki P., Coppler P.J., Kvaløy J.T., Baldwin M.A., Callaway C.W., Elmer J. Pittsburgh Post-Cardiac Arrest Service. Association of antiepileptic drugs with resolution of epileptiform activity after cardiac arrest. Resuscitation. 2019;142:82–90. doi: 10.1016/j.resuscitation.2019.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spalletti M., Carrai R., Scarpino M., et al. Single electroencephalographic patterns as specific and time-dependent indicators of good and poor outcome after cardiac arrest. Clin Neurophysiol. 2016;127:2610–2617. doi: 10.1016/j.clinph.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Admiraal M.M., van Rootselaar A.F., Hofmeijer J., et al. Electroencephalographic reactivity as predictor of neurological outcome in postanoxic coma: a multicenter prospective cohort study. Ann Neurol. 2019;86:17–27. doi: 10.1002/ana.25507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossetti A.O., Urbano L.A., Delodder F., Kaplan P.W., Oddo M. Prognostic value of continuous EEG monitoring during therapeutic hypothermia after cardiac arrest. Crit Care. 2010;14:R173. doi: 10.1186/cc9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossetti A.O., Tovar Quiroga D.F., Juan E., et al. Electroencephalography predicts poor and good outcomes after cardiac arrest: a two-center study. Crit Care Med. 2017;45:e674–e682. doi: 10.1097/CCM.0000000000002337. [DOI] [PubMed] [Google Scholar]
- 43.Tsetsou S., Novy J., Pfeiffer C., Oddo M., Rossetti A.O. Multimodal Outcome Prognostication After Cardiac Arrest and Targeted Temperature Management: Analysis at 36 °C. Neurocrit Care. 2018 Feb;28:104–109. doi: 10.1007/s12028-017-0393-8. [DOI] [PubMed] [Google Scholar]
- 44.Barbella G., Lee J.W., Alvarez V., et al. Prediction of regaining consciousness despite an early epileptiform EEG after cardiac arrest. Neurology. 2020;94:e1675–e1683. doi: 10.1212/WNL.0000000000009283. [DOI] [PubMed] [Google Scholar]
- 45.Alvarez V., Oddo M., Rossetti A.O. Stimulus-induced rhythmic, periodic or ictal discharges (SIRPIDs) in comatose survivors of cardiac arrest: characteristics and prognostic value. Clin Neurophysiol. 2013;124:204–208. doi: 10.1016/j.clinph.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 46.Benghanem S., Paul M., Charpentier J., et al. Value of EEG reactivity for prediction of neurologic outcome after cardiac arrest: Insights from the Parisian registry. Resuscitation. 2019;142:168–174. doi: 10.1016/j.resuscitation.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 47.Tsetsou S., Novy J., Oddo M., Rossetti A. EEG reactivity to Pain in Comatose Patients: Importance of the Stimulus Type. Resuscitation. 2015 Dec;97:34–37. doi: 10.1016/j.resuscitation.2015.09.380. [DOI] [PubMed] [Google Scholar]
- 48.Amorim E., van der Stoel M., Nagaraj S.B., et al. Quantitative EEG reactivity and machine learning for prognostication in hypoxic-ischemic brain injury. Clin Neurophysiol. 2019;130:1908–1916. doi: 10.1016/j.clinph.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rossetti A.O., Schindler K., Sutter R., Rüegg S., et al. Continuous vs Routine Electroencephalogram in Critically Ill Adults With Altered Consciousness and No Recent Seizure: A Multicenter Randomized Clinical Trial. JAMA Neurol. 2020;77:1–8. doi: 10.1001/jamaneurol.2020.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alvarez V., Sierra-Marcos A., Oddo M., Rossetti A.O. Yield of intermittent versus continuous EEG in comatose survivors of cardiac arrest treated with hypothermia. Crit Care. 2013;17:R190. doi: 10.1186/cc12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elmer J., Coppler P.J., Solanki P., et al. Sensitivity of Continuous Electroencephalography to Detect Ictal Activity After Cardiac Arrest. JAMA Net Open. 2020;3:e2037. doi: 10.1001/jamanetworkopen.2020.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fatuzzo D., Beuchat I., Alvarez V., Novy J., Oddo M., Rossetti A.O. Does continuous EEG influence prognosis in patients after cardiac arrest? Resuscitation. 2018;132:29–32. doi: 10.1016/j.resuscitation.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 53.Mikhaeil-Demo Y., Gavvala J.R., Bellinski I.I., et al. Clinical classification of post anoxic myoclonic status. Resuscitation. 2017;119:76–80. doi: 10.1016/j.resuscitation.2017.07.035. [DOI] [PubMed] [Google Scholar]
- 54.Caviness J.N. Treatment of myoclonus. Neurotherapeutics. 2014 Jan;11:188–200. doi: 10.1007/s13311-013-0216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elmer J., Rittenberger J.C., Faro J., et al. Pittsburgh Post-Cardiac Arrest Service. Clinically distinct electrographic phenotypes of early myoclonus after Cardiac Arrest. Ann Neurol. 2016;80:175–184. doi: 10.1002/ana.24697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jonas S., Rossetti A.O., Oddo M., Jenni S., Favaro P., Zubler F. EEG-based outcome prediction after cardiac arrest with convolutional neural networks: Performance and visualization of discriminative features. Hum Brain Mapp. 2019;40:4606–4617. doi: 10.1002/hbm.24724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dhakar M.B., Sivaraju A., Maciel C.B., et al. Electro-clinical characteristics and prognostic significance of post anoxic myoclonus. Resuscitation. 2018;131:114–120. doi: 10.1016/j.resuscitation.2018.06.030. [DOI] [PubMed] [Google Scholar]
- 58.Fananeanu T.A., Tolchin B., Alvarez V. Effect of Stimulus Type and Temperature on EEG reactivity in Cardiac Arrest. Clin Neurophysiol. 2016;127:3412–3417. doi: 10.1016/j.clinph.2016.09.002. [DOI] [PubMed] [Google Scholar]