Abstract
Staphylococcus aureus is a major human pathogen causing serious implant–associated infections. Combination treatment with rifampin (10 to 15 mg/kg per day), which has dose-dependent activity, is recommended to treat S. aureus orthopedic implant–associated infections. Rifampin, however, has limited bone penetration. Here, dynamic 11C-rifampin positron emission tomography (PET) performed in prospectively enrolled patients with confirmed S. aureus bone infection (n = 3) or without orthopedic infection (n = 12) demonstrated bone/plasma area under the concentration-time curve ratio of 0.14 (interquartile range, 0.09 to 0.19), exposures lower than previously thought. PET-based pharmacokinetic modeling predicted rifampin concentration-time profiles in bone and facilitated studies in a mouse model of S. aureus orthopedic implant infection. Administration of high-dose rifampin (human equipotent to 35 mg/kg per day) substantially increased bone concentrations (2 mg/liter versus <0.2 mg/liter with standard dosing) in mice and achieved higher bacterial killing and biofilm disruption. Treatment for 4 weeks with high-dose rifampin and vancomycin was noninferior to the recommended 6-week treatment of standard-dose rifampin with vancomycin in mice (risk difference, −6.7% favoring high-dose rifampin regimen). High-dose rifampin treatment ameliorated antimicrobial resistance (0% versus 38%; P = 0.04) and mitigated adverse bone remodeling (P < 0.01). Last, whole-genome sequencing demonstrated that administration of high-dose rifampin in mice reduced selection of bacterial mutations conferring rifampin resistance (rpoB) and mutations in genes potentially linked to persistence. These data suggest that administration of high-dose rifampin is necessary to achieve optimal bone concentrations, which could shorten and improve treatments for S. aureus orthopedic implant infections.
INTRODUCTION
Staphylococcus aureus is a major human pathogen that causes various clinical syndromes, including osteoarticular and device-related infections (1, 2). It is one of the most common pathogens of biofilm-related infection on foreign implanted materials (3). Bacterial biofilm formation on the implant impedes penetration of immune cells and some antibiotics, creating chronic, persistent infections (4). S. aureus orthopedic implant–associated infections are exceedingly difficult to treat and may require surgery and prolonged systemic antibiotics. They are also associated with extended disability and rehabilitation, contributing to worse outcomes (5, 6). Although infection rates of orthopedic implant–associated infections have remained at 1 to 2% after primary and 3 to 6% after revision arthroplasty, inpatient costs average $25,000 to $107,000 per case, corresponding to an annual healthcare burden of $3 billion in the United States alone (7). The emergence of methicillin-resistant S. aureus (MRSA) strains poses yet another challenge for the treatment of these complicated infections.
Combination treatment with rifampin is recommended by the Infectious Diseases Society of America (IDSA) for the treatment of staphylococcal implant–associated infections (8–10). Moreover, some experts recommend the combination treatment with rifampin for MRSA infection even without hardware, particularly for osteomyelitis and central nervous system infection (11). This is because rifampin enhances microbiological clearance (12–14), and high clinical cure rates (>80%) are achieved only with the combinatory use of rifampin (14–20). The most beneficial effect of combinatory treatment with rifampin was noted in implant-associated, in particular orthopedic implant–associated, infections (19). The lipophilicity of rifampin, its activity within the acidic environment of biofilms, and its accumulation within neutrophils likely facilitate its activity against implant-associated infections (21). However, prolonged treatment duration (e.g., 6 to 12 weeks) is required to minimize treatment failure. In a recent randomized controlled study of patients with prosthetic joint infections, treatment failure was 18.1 and 9.4%, respectively, for 6 weeks versus 12 weeks of treatment, even with most patients receiving combinatory treatment with rifampin (70%) (22). Prolonged antibiotic duration is associated with an increased risk of adverse drug events. In one study, every additional 10 days of antibiotic treatment conferred a 3% increased risk of an antibiotic-associated adverse event (23). Prolonged use of antibiotics is also associated with emergence of multiresistant pathogens (24). Thus, development of shorter rifampin-containing antibiotic regimens for the treatment of orthopedic implant–associated infections is an urgent, unmet clinical need.
Rifampin has potent, dose-dependent sterilizing activity against Gram-positive bacteria including methicillin-susceptible S. aureus (MSSA) and MRSA (21). However, limited data are available regarding rifampin penetration into bone, and published data report rifampin bone concentrations at single time points using invasive methods (25–30), precluding the ability to measure area under the concentration-time curve (AUC), which is most predictive of bactericidal activity for rifampin (31–34). Furthermore, recent data showed that pathologically diverse lesions occur simultaneously at different sites within the same patient (35, 36). Thus, there is a need for holistic analysis of rifampin concentrations in various anatomic locations, which is generally not feasible with currently available methods that require invasive acquisition of tissue for drug measurement.
In this study, we used a bidirectional process to integrate findings from animal and human studies (fig. S1). 11C-Rifampin, a chemically identical radiolabeled analog of rifampin, was administered, and positron emission tomography (PET) with computed tomography (CT) (37–39) imaging was performed in prospectively enrolled patients with S. aureus bone infections or in patients without S. aureus infection (38) to noninvasively measure detailed intralesional rifampin bone concentration-time profiles at several anatomic sites. This provided a translational bridge to facilitate pharmacokinetic (PK) modeling and predict rifampin concentration-time profiles in bone tissues. 11C-Rifampin PET studies in a mouse model of S. aureus orthopedic implant infection (40) as well as direct rifampin measurements from postmortem bone tissues were performed and applied to an in vitro S. aureus biofilm system (41) to assess bacterial killing and biofilm disruption using live, time-lapse imaging. These data were used to design studies to evaluate high-dose rifampin–containing regimens in mice as compared to standard (IDSA-recommended) rifampin regimens, administered at human-equipotent doses achieving similar plasma AUC in mice with S. aureus orthopedic implant infection. Mice were assessed for relapse-free cure 6 weeks after completion of antibiotic treatment. High-resolution CT was used to measure bone remodeling (40), and antimicrobial susceptibility testing, as well as whole-genome bacterial sequencing, was performed for all bacterial isolates obtained from mice after treatment completion.
RESULTS
Dynamic 11C-rifampin PET/CT in human subjects
Dynamic 11C-rifampin PET/CT was performed in accordance with U.S. Food and Drug Administration (FDA) guidelines after an intravenous dose of 11C-rifampin [707.4 ± 43.7 megabecquerel (MBq)] in three prospectively enrolled patients with S. aureus bone infections: tibial MSSA, MRSA spine and midfoot osteomyelitis, and MSSA infection of the spine (tables S1 and S2 and fig. S2). The procedures were safe and well tolerated in all patients with 11C-rifampin biodistribution (Fig. 1A), consistent with previous studies (38, 39). Spatial distribution of 11C-rifampin AUC demonstrated lower exposures in the bone compared to the surrounding soft tissues (Fig. 1B). The 11C-rifampin PET signal in the bone was quantified using three-dimensional (3D) volumes of interest (VOIs) drawn around infected sites and the contralateral uninfected bone (fig. S3). These data were used to generate time-activity curves (TACs) (Fig. 1C) and calculate the bone-to-plasma AUC ratios (AUCbone/plasma). Median AUCbone/plasma ratios were 0.11 [interquartile range (IQR), 0.08 to 0.22] and 0.08 (IQR, 0.04 to 0.16) for infected and uninfected bones, respectively, and were not significantly different (P = 0.25) (Fig. 1D). 11C-Rifampin PET/CT performed after an intravenous dose of 11C-rifampin (337 ± 14 MBq) in another cohort of 12 prospectively enrolled patients with pulmonary tuberculosis (TB) (38) provided additional data on rifampin penetration in uninfected bone. Median AUCbone/plasma ratios were 0.13 (IQR, 0.10 to 0.16) and 0.22 (IQR, 0.18 to 0.25) for long bone (humerus) and spine, respectively (Fig. 1E). These data demonstrate low penetration of rifampin with an overall median AUCbone/plasma ratio of 0.14 (IQR, 0.09 to 0.19).
Fig. 1. Rifampin bone exposures.
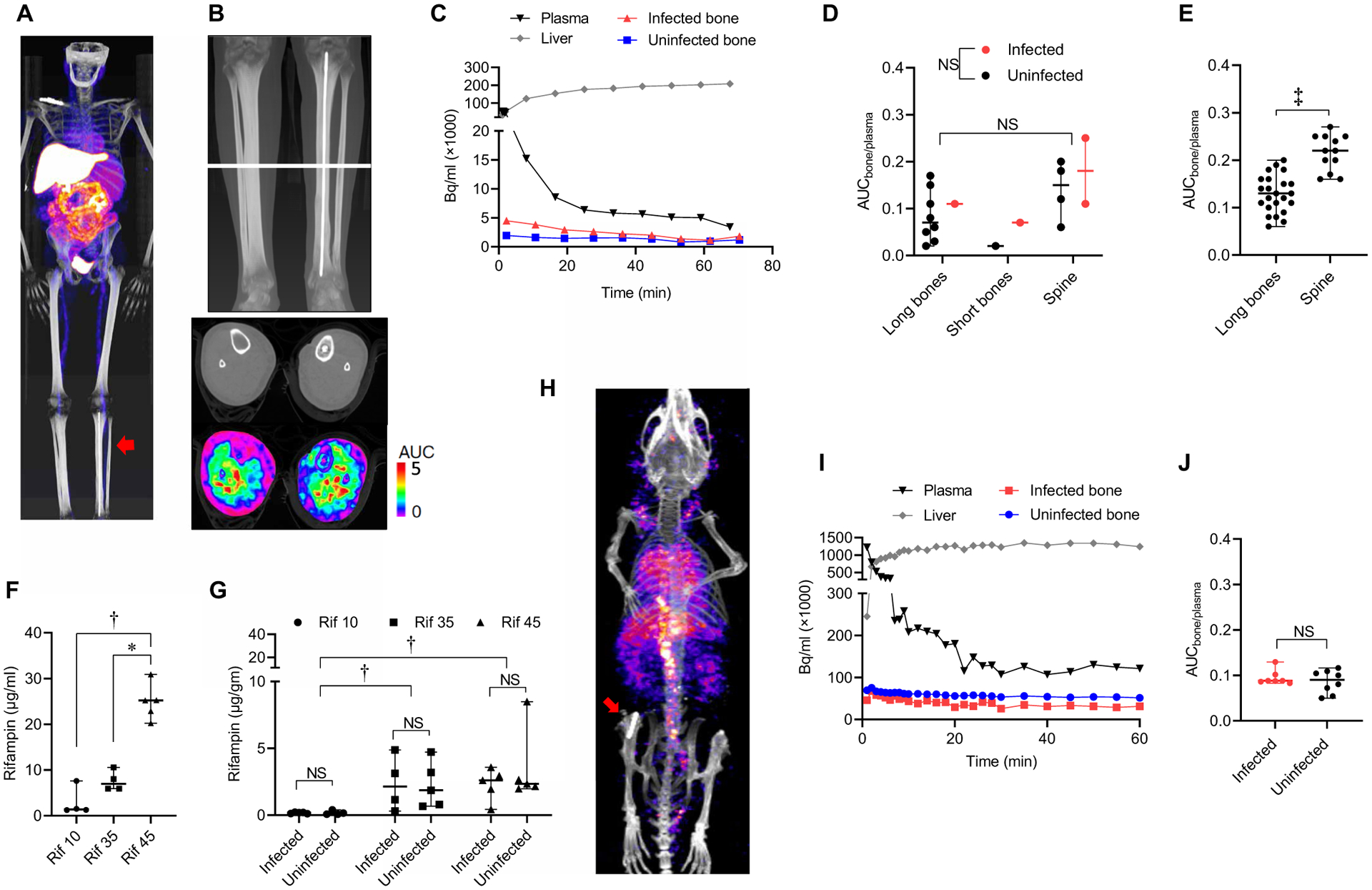
(A) Three prospectively enrolled patients with S. aureus bone infections underwent dynamic 11C-rifampin PET for 60 to 90 min, followed by a CT scan. Frontal view of maximum intensity projection (PET). Note the implant in the left tibia (arrow). (B) 11C-Rifampin PET area under the concentration-time curve (AUC) shown as a heat map overlay in the selected transverse section. (C) Representative 11C-rifampin PET time-activity curve (TAC) from a S. aureus–infected patient. (D) 11C-Rifampin exposure in long bones (tibia and humerus), short bones (tarsals), and spine of S. aureus–infected patients shown as the median (and range) bone-to-plasma AUC ratio (AUCbone/plasma). NS, not significant. (E) Dynamic 11C-rifampin PET in patients without an orthopedic infection (and with pulmonary tuberculosis; n = 12 patients) was used to get additional data in uninfected long bone (humerus) and spine. (F and G) Mice with a femoral orthopedic implant infected with methicillin-resistant S. aureus (SAP231) were administered rifampin (with vancomycin) corresponding to a human-equipotent rifampin dose of either 10, 35, or 45 mg/kg per day (n = 4 to 6 animals per treatment group in one experiment). Rifampin plasma (F) and bone (G) concentrations measured by mass spectrometry in postmortem samples. (H) Mice (after at least 2 weeks of antibiotic treatment) underwent dynamic 11C-rifampin PET for 60 min, followed by a CT scan (n = 8 animals total in two independent experiments). Frontal view of maximum intensity projection (PET). Note the implant in the right knee (arrow). (I) Representative TAC from an S. aureus–infected mouse. (J) 11C-Rifampin exposure in mouse shown as AUCbone/plasma. *P < 0.05, †P < 0.01, and ‡P < 0.001 as shown by the Kruskal-Wallis test (F and G) or by the Wilcoxon matched-pairs signed rank test (D and E), adjusted for multiple comparisons as indicated to preserve the desired false discovery rate or by the two-tailed Mann-Whitney test for comparison of infected to uninfected bone (G and J).
Rifampin bone exposures in a mouse model of S. aureus orthopedic implant infection
Given the poor penetration of rifampin into bone tissues demonstrated in the human studies, we evaluated whether higher oral doses of rifampin, at human-equipotent dosing, would result in higher bone concentration in mice. Consistent with previous reports (21, 42), administration of higher doses of rifampin led to a supralinear increase in plasma concentration (Fig. 1F). However, low bone rifampin concentrations (median, 0.16 μg/g; IQR, 0.10 to 0.21) were noted with standard dosing (10 mg/kg per day). Administration of rifampin (35 or 45 mg/kg per day) substantially increased rifampin bone concentration to 1.86 μg/g (IQR, 0.74 to 3.96) and 2.48 μg/g (IQR, 1.97 to 3.10), respectively (Fig. 1G). There was no significant difference between rifampin concentrations in infected versus uninfected bone (Fig. 1G), with bone-to-plasma ratios of 0.08 (IQR, 0.05 to 0.18) and 0.05 (IQR, 0.04 to 0.13) for infected and uninfected bone, respectively (P = 0.43).
Dynamic 11C-rifampin PET studies were also performed after an intravenous dose of 11C-rifampin in mice with S. aureus orthopedic implant infection (Fig. 1, H and I), with results similar to that noted in the human studies. Median AUCbone/plasma ratios were 0.09 (IQR, 0.09 to 0.10) and 0.09 (IQR, 0.06 to 0.11) for infected and uninfected bone, respectively (P = 0.61) (Fig. 1J).
High-dose rifampin enhances biofilm disruption and bacterial killing
Direct rifampin measurements from postmortem bone tissues from mice were applied to an in vitro S. aureus biofilm system to assess bacterial killing and biofilm disruption using live, time-lapse imaging. Bacterial killing within in vitro biofilms exposed to rifampin bone concentrations (0.2 mg/liter) achieved with standard-dosing (human-equipotent rifampin dose of 10 mg/kg per day) rifampin treatment resulted in killing of only up to 17% of bacteria at 4 hours with retained biofilm structure. Rifampin bone concentrations (2 mg/liter) achieved with a human-equipotent rifampin dose of 35 mg/kg per day (high-dose), however, disrupted the biofilm structure and achieved killing of 85% of bacteria (P < 0.05) (fig. S4).
PK modeling and simulations in human subjects
PK modeling was performed using the human 11C-rifampin PET-derived data to extrapolate rifampin bone exposures. A two-compartment PK model with an additional bone compartment was developed (fig. S5), which could predict plasma and bone rifampin concentrations in patients with and without S. aureus orthopedic infection (fig. S6). The model estimated a bone penetration of 12%, consistent with earlier analyses (table S3). Bone-related parameter estimates from the 11C-rifampin PET model were integrated into a previously published plasma PK rifampin model for oral rifampin (Fig. 2A and tables S3 and S4) (43). Because of the short radioactive half-life of 11C-rifampin, PET imaging was only performed for up to 90 min, which was sufficient to model rifampin microdosing, as 11C-rifampin PET plasma PK displayed distinct distribution and elimination phases within the hour.
Fig. 2. Pharmacokinetic model and simulations for human subjects.
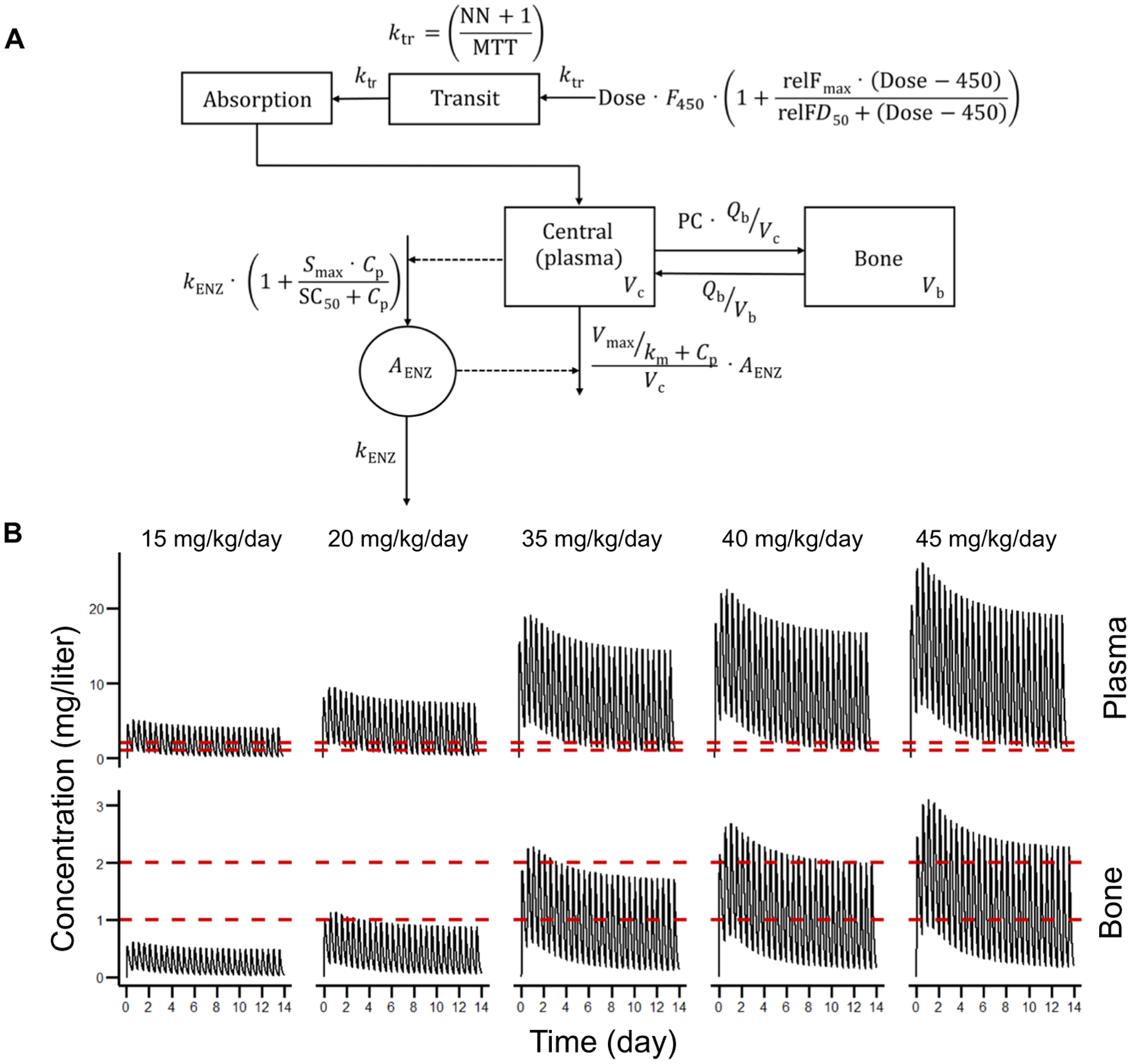
(A) One-compartment model with one bone compartment, relative bioavailability, transit absorption, autoinduction, and Michaelis-Menten elimination. Plasma volume (Vc), bone volume (Vb), blood flow between plasma and bone (Qb), partition coefficient (PC), maximal increase in bioavailability relative to 450-mg dose (relFmax), difference in dose from 450 mg at which half relFmax is achieved (relFD50), bioavailability for 450-mg dose (F450)—set to 1, number of transit compartments (NN), mean transit time (MTT), transit rate constant (ktr), first-order rate constant for enzyme degradation (kENZ), maximal increase in rate of enzyme production (Smax), plasma concentration (Cp), concentration at which half Smax is achieved (SC50), amount of enzyme (AENZ), maximal rate of elimination (Vmax), and concentration at which half Vmax is achieved (km). (B) Rifampin concentrations were simulated for 2 weeks with twice-daily dosing. Dashed red lines represent cutoffs of 1 and 2 mg/liter. The value of 1 mg/liter is the breakpoint suggested by the Clinical and Laboratory Standards Institute (CLSI).
Simulations were performed with rifampin doses administered every 12 hours for 2 weeks to achieve steady-state concentrations in human subjects (Fig. 2B and Table 1). Consistent with previous literature (44), rifampin exposures for plasma and bone increased with higher than dose-proportional increases in AUC. Rifampin dose of at least 35 mg/kg per day was required to achieve bone concentrations above the breakpoint (1 mg/liter) suggested by the Clinical and Laboratory Standards Institute (CLSI) (45), and 40 mg/kg per day would achieve a bone rifampin concentration of 2 mg/liter through steady state.
Table 1. Simulated human rifampin exposures.
AUC, area under the curve; SS, steady state.
| Dose (mg/kg per day) | Plasma AUCss,24hr (mg*hour/liter) | Bone AUCss,24hr (mg*hour/liter) |
|---|---|---|
| 15 | 37.8 | 4.54 |
| 20 | 70.6 | 8.46 |
| 35 | 146 | 17.5 |
| 40 | 173 | 20.8 |
| 45 | 202 | 24.2 |
High-dose rifampin enhances treatment outcomes in mice
Using mice with S. aureus (MRSA bioluminescent strain) orthopedic implant infection, we administered 1- or 3-week-long antibiotic treatments of vancomycin in combination with standard-dose (10 mg/kg per day) or high-dose (45 mg/kg per day) rifampin. The bacterial burden was serially monitored noninvasively using bioluminescence imaging (BLI) (40), and we observed a decrease in the BLI signal (bacterial burden) with treatment, which reappeared in most animals after cessation of treatments (fig. S7, A and B).
Treatment failure or recurrence may occur weeks after cessation of treatment (46, 47). Therefore, mice were followed for an additional 6 weeks after discontinuing antibiotics to determine relapse-free cure. Overall, compared with the 1-week regimens, treatment regimens lasting 3 weeks led to a greater reduction in bacterial burden in tissues (fig. S7C) and in implants (fig. S7D). The reappearance of BLI signal was also significantly less frequent in mice receiving 3 weeks versus 1 week of treatment (P < 0.01) (fig. S7E). To confirm the rate of relapse, homogenized tissue specimens and sonicated implants were cultured in broth medium for 48 hours followed by overnight culture on plates, and the presence or absence of bacterial growth was determined. The 3-week high-dose rifampin regimen was better at reducing relapse versus the corresponding standard-dose regimen (10% versus 20%) (fig. S7F).
Rifampin is also used in combination with other antibiotics. Specifically, MRSA infection can be treated by an all-oral regimen of linezolid and rifampin (40). Therefore, similar experiments were performed with linezolid in combination with standard- or high-dose rifampin (fig. S8). Similar to the vancomycin regimens, the 3-week high-dose rifampin regimen, in combination with linezolid, was better at reducing relapse versus the corresponding standard-dose regimen (47% versus 80%) (fig. S8F).
Treatment shortening studies
Because high-dose rifampin was more effective in achieving relapse-free cure, we hypothesized that a shorter treatment course using the high dose will result in cure rates similar to the recommended standard 6-week treatment regimen. To test this, vancomycin with standard-dose [human-equipotent rifampin dose (10 mg/kg per day)] rifampin for 6 weeks (standard regimen recommended by the IDSA) was compared to vancomycin with high-dose [human-equipotent rifampin dose (35 mg/kg per day)] rifampin for 4 weeks using a noninferiority study design in mice with prosthetic joint infection (Fig. 3A). Unlike monotherapy with vancomycin, both rifampin-containing regimens sustained a BLI signal below the limit of detection (Fig. 3, B and C). Ex vivo tissue colony-forming unit (CFU) enumeration was performed to determine the bacterial burden 6 weeks after cessation of antibiotic treatments. This resulted in significantly reduced bacterial burden in the two rifampin groups (Fig. 3D) with no bacteria recovered from the implants. Tissue broth cultures were used to determine whether the antibiotics had eradicated the infection (cure). The 4 weeks of high-dose rifampin and the 6 weeks of standard-dose rifampin regimen resulted in a relapse rate of 11.1% (5 of 45) and 17.8% (8 of 45), respectively (Fig. 3E). This risk difference of −6.7% (90% confidence interval, −20 to 6.9%), favoring the high-dose rifampin regimen, was below the preset limit for noninferiority (Fig. 3F).
Fig. 3. Treatment shortening studies in mice with S. aureus implant infection.
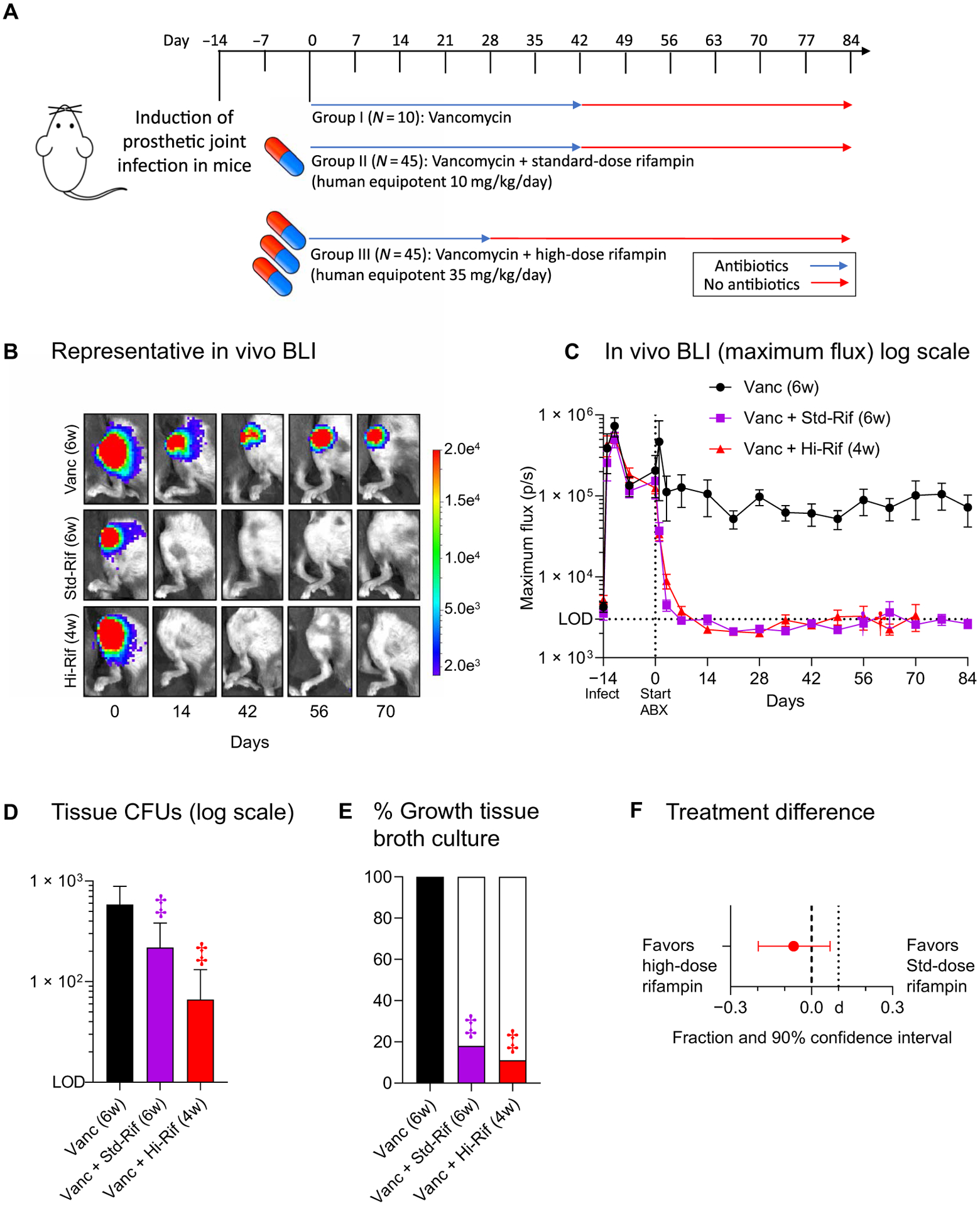
(A) Intramuscular vancomycin (equipotent to 2 g/day intravenously in human) and oral rifampin were initiated 2 weeks after infection (designated as day 0) and continued for 6 weeks (IDSA regimen, standard-dose rifampin; n = 45 animals) or 4 weeks (high-dose rifampin; n = 45 animals). Standard- and high-dose rifampin doses were (human equipotent) 10 and 35 mg/kg per day, respectively. Monotherapy with vancomycin (equipotent to 2 g/day intravenously in humans) over 6 weeks was also performed (n = 10 animals). Data from one experiment are presented. Stable cure was determined at day 84 by assessing sterilization of the tissues and the implant. (B) Representative in vivo bioluminescence images from mice in each group. (C) Mean maximum flux (photons s−1 cm−2 sr−1) (and SEM). LOD, level of detection (3 × 103 photons s−1 cm−2 sr−1). (D) Six weeks after completion of antibiotic treatment, peri-implant joint and bone tissue were homogenized, implants were sonicated, and colony-forming units (CFUs) were enumerated ex vivo. Data are presented as the mean number of CFUs (and SEM) isolated from the peri-implant bone and joint tissue. (E) To evaluate whether the antibiotic treatment eradicated infection, tissue homogenates and implants were cultured for an additional 48 hours in broth followed by overnight plate culture, and the presence or absence of bacterial growth was determined. Data are presented as the percentage of tissue samples with any bacterial growth. (F) Statistical analysis showing treatment difference in favor of high-dose rifampin and 90% confidence interval within the preset limit for noninferiority (d = 10%). *P < 0.05, †P < 0.01, and ‡P < 0.001 as shown by the Kruskal-Wallis test adjusted for multiple comparisons to preserve the desired false discovery rate.
Bacterial evolution during antibiotic treatments
Exposure to low antibiotic concentrations [equal or lower than the minimal inhibitory concentration (MIC)] may result in development of bacterial resistance (48–50). Therefore, at the end of the study (and 6 weeks after cessation of antibiotics), rifampin MICs were obtained for 21 bacterial isolates cultured from tissue homogenates. As anticipated, none of the isolates (0 of 8) from mice receiving vancomycin alone had developed rifampin resistance. However, 38% (3 of 8) and 0% (0 of 5) of isolates recovered from mice receiving the standard- or high-dose rifampin regimen, respectively, had developed rifampin resistance (P = 0.04) (Fig. 4A). Antibiotic treatments may also lead to emergence of genetic mutations that help in bacterial adaptation and tolerance or persistence (51, 52). We therefore performed whole-genome bacterial sequencing for these 21 isolates, each from a unique animal, and compared each to the parent strain. Eighteen genetic variants including 16 single-nucleotide polymorphisms (SNPs) with 11 unique SNPs and 2 insertions were found in 9 isolates (table S5). Most genetic variants were in isolates from the standard-dose treatment group: 12 genetic variants in 4 different isolates, compared to 3 variants each in 3 or 2 isolates, for the vancomycin alone and vancomycin with high-dose rifampin groups, respectively (table S5). A missense mutation (Ser464Pro) in RNA polymerase β subunit (rpoB), conferring rifampin resistance (53), was found in two of the three resistant strains in the standard-dose rifampin group (Fig. 4B). In these two bacterial isolates, an additional missense mutation (Asn51Ser) was found in succinate dehydrogenase iron-sulfur subunit (sdhB) (Fig. 4B). An SNP (A➔G) found 25 base pairs upstream to lysM was found in four other isolates (two each in the vancomycin alone and the standard-dose rifampin groups). Two of three genetic variants in the high-dose rifampin group were in a single strain with two synonymous variants in clumping factor B (clfB). Together, these results indicate that administration of standard-dose rifampin-containing regimens resulted in higher resistance and in selection for more bacterial genetic changes in genes related to persistence. Both were ameliorated by high-dose rifampin.
Fig. 4. Bacterial characteristics and bone remodeling.
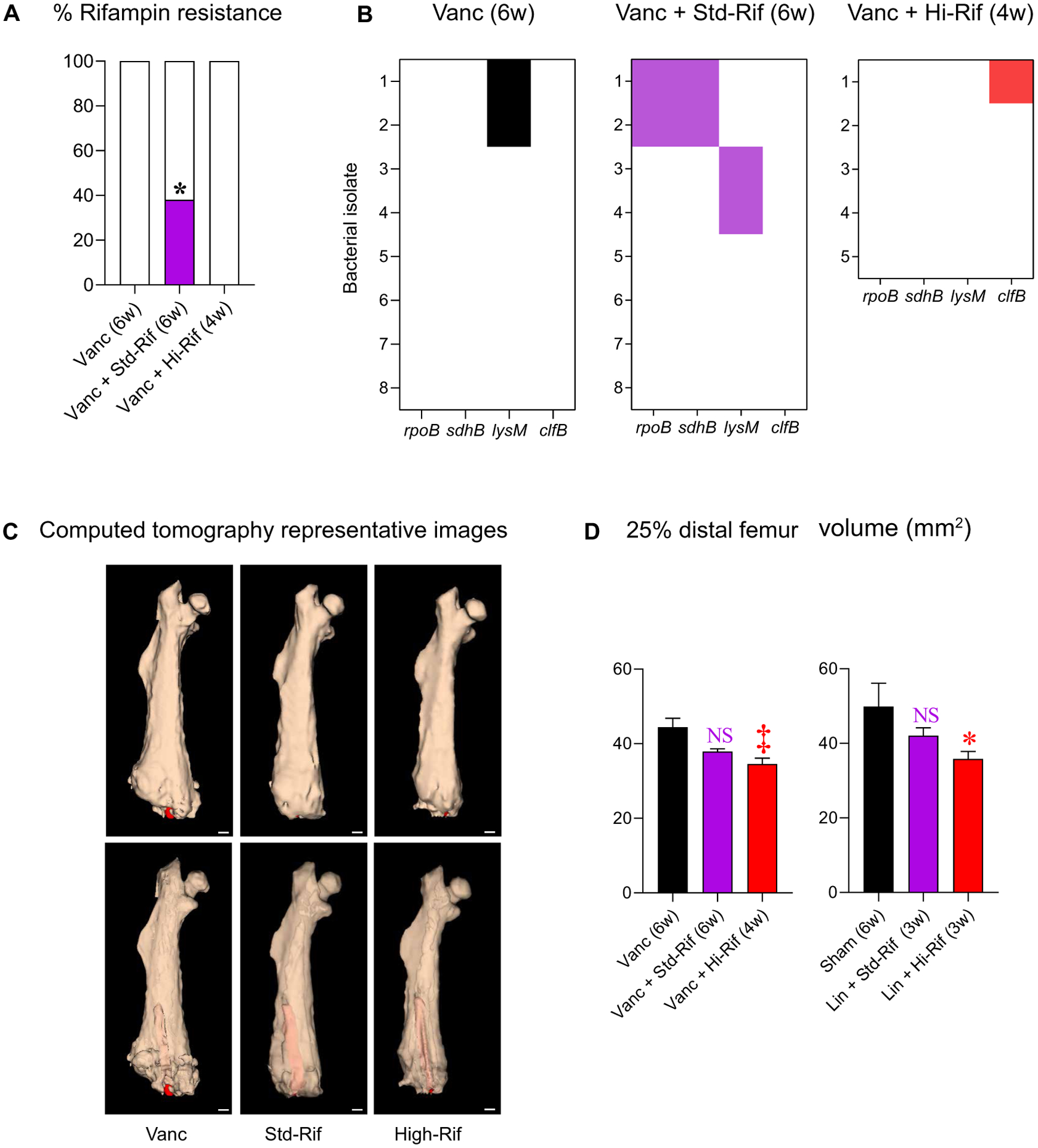
Twenty-one isolates, each from a unique mouse from the noninferiority study, were subjected to (A) phenotypic rifampin resistance testing (BD Phoenix), with results presented as the percentage of resistant isolates for each treatment group and (B) whole-genome sequencing (Illumina; MiGS) and compared to the parent strain SAP231. Heat maps display isolates with variants in genes related to rifampin resistance (rpoB) or to bacterial persistence (lysM, sdhB, and clfB). (C) Representative 3D reconstructed μCT images [opaque (top) and translucent (bottom) mouse femora with implants in red; scale bars, 1 mm]. (D) Mean (and SEM) volume of the distal 25% of the infected femurs (excluding the implant) (n = 5 to 15 per group in one experiment). *P < 0.05, †P < 0.01, and ‡P < 0.001 as shown by the χ2 test (A) or by the Kruskal-Wallis test adjusted for multiple comparisons to preserve the desired false discovery rate (D).
Bone remodeling and antibiotic treatment
Orthopedic implant–associated infections can cause reactive bone changes, including periprosthetic osteolysis and cortical expansion, which can be detrimental to clinical outcomes and necessitate revision arthroplasty (40). We therefore assessed reactive bone changes by high-resolution CT in mice (Fig. 4C). While standard-dose rifampin regimens were not able to mitigate adverse bony changes compared to monotherapy with vancomycin (Fig. 4D), high-dose rifampin regimens mitigated these adverse changes. This was evident even when the high-dose rifampin regimen was administered for a shorter duration (4 weeks) compared with the standard-dose rifampin regimen (6 weeks), both in combination with vancomycin (Fig. 4D).
DISCUSSION
Studies have demonstrated that current rifampin dosing (10 to 15 mg/kg per day), which was chosen on the basis of economic necessity in the 1970s (54), achieves concentrations that are on the steep part of the dose-response curve (31, 55). Higher doses of rifampin (up to 35 mg/kg per day) are safe in adults with TB (56, 57). Moreover, a recent study suggested that 40 mg/kg per day is also safe and well tolerated (58). These regimens may shorten treatments (31, 55) as well as improve patient outcome (59). This is facilitated by the high-dose rifampin–containing regimens achieving much higher tissue rifampin concentrations at infection sites, where the pathogen resides (38).
We used a bidirectional process to integrate findings from animal and human studies. Dynamic 11C-rifampin PET/CT, a clinically translatable, noninvasive imaging technology, was performed in patients with and without S. aureus orthopedic infection, demonstrating rifampin exposures considerably lower (plasma-to-bone AUC ratio of 0.14) than previously thought, based on single time-point invasive measurements (plasma-to-bone ratio of 0.4) (25–30). This is relevant as AUC is most predictive of bactericidal activity for rifampin (31–34). Similar 11C-rifampin PET findings were noted in a mouse model of S. aureus orthopedic implant infection, where direct measurements of plasma rifampin were in agreement with previous reports (55, 60) and direct measurements of bone concentrations validated the imaging data. These concentrations led to higher bacterial killing and biofilm disruption in an in vitro S. aureus biofilm system. PET data facilitated an integrated PK model to predict rifampin bone biodistribution in humans, demonstrating that high-dose rifampin results in supralinear increase in rifampin bone exposure. PK simulations suggested that treatment regimens with rifampin (35 mg/kg per day; high dose) may be necessary to achieve sufficiently high bone concentrations. A series of studies in the mouse model of S. aureus orthopedic implant–associated infection demonstrated that high-dose rifampin regimens (combined with vancomycin or linezolid) were superior to the corresponding standard-dose regimens in achieving cure. Last, 4 weeks of high-dose rifampin with vancomycin was noninferior to the recommended 6 weeks of standard-dose rifampin with vancomycin in mice. Collectively, these data suggest that administration of high-dose rifampin is needed to achieve optimal bone concentrations, and high-dose rifampin–containing regimens could shorten the treatment for S. aureus orthopedic implant infections.
If administration of standard-dose rifampin only achieves sub-therapeutic concentrations in bone, it may cultivate resistance. Thirty-eight percent of bacterial isolates were rifampin-resistant after treatment with standard-dose rifampin and vancomycin in mice, and the high-dose rifampin regimen completely ameliorated development of bacterial resistance. Moreover, whole-genome sequencing revealed selection of genetic variations in genes associated with biofilm formation and persistence, which was more common under standard-dose rifampin treatment. These included mutations in sdhB, a gene associated with biofilm formation and persistence (61, 62), in lysM, which is a peptidoglycan binding protein related to bacterial cell wall structure and dormancy (63, 64), and in clfB, a well-studied gene associated with biofilm formation (65). However, further studies are required to establish a direct link between the specific SNPs identified in the current study (other than those in rpoB) and the associated bacterial phenotype. Last, high-dose rifampin regimens mitigated adverse bone remodeling better than standard-dose rifampin regimens when combined with either vancomycin or linezolid. This is clinically relevant and would help in reducing pathological fractures and implant subsidence and overall decrease the morbidity associated with these infections.
This study has some limitations. First, the efficacy of high-dose rifampin was tested against a single community-acquired MRSA (CA-MRSA) strain, and it is not yet known whether these results could be expanded to other staphylococcal strains as well as to other staphylococcal species, particularly coagulase-negative staphylococci, which are often involved in orthopedic implant–associated infections (66). Second, rifampin combination treatment with either vancomycin or linezolid was evaluated; however, other antibiotic combinations are commonly used. Standard-dose rifampin was previously shown to increase treatment success in implant-associated infection when combined with β-lactam antibiotics (12, 13, 16, 17), fluoroquinolones (19), trimethoprim/sulfamethoxazole (14, 15), and other antibiotics. These antibiotics should also be evaluated in combination with high-dose rifampin. In addition, although we demonstrated that combination treatment with high-dose rifampin suppressed the development of rifampin resistance, our sample size was limited, and therefore, further studies are needed to validate this finding. Third, antibiotics were started 14 days after infection to ensure biofilm formation (67), which we thought should be a prerequisite for testing the efficacy of the treatment regimens. Fourth, human study participants with orthopedic infection had significant comorbidities; therefore, derived PK data may not be generalizable; however, many adult patients with S. aureus orthopedic infection have significant comorbidities (e.g., diabetes). In addition, our findings related to rifampin bone penetration were consistent with the 11C-rifampin PET data from TB patients without comorbidities. Fifth, whereas rifampin is generally administered orally, 11C-rifampin was administered intravenously in this study to assess the bone-to-plasma AUC ratios. Because data on oral bioavailability of rifampin are readily available, the bone-to-plasma AUC ratios combined with plasma concentrations achieved after oral dosing were used to predict bone concentrations of rifampin. Sixth, rifampin bone penetration may vary on the basis of the anatomical location (e.g., spine versus long bones), which warrants further investigation. Last, although high-dose rifampin (up to 40 mg/kg per day) has been demonstrated to be safe in patients with TB (56, 57), further studies are needed to evaluate the safety of high-dose rifampin in other patient populations, particularly in those with comorbidities and orthopedic infections. These results should be the basis of future clinical studies to evaluate the efficacy of high-dose rifampin–containing regimens for S. aureus orthopedic implant–associated infections.
MATERIALS AND METHODS
Study design
The overall goal of this study was to measure rifampin concentration-time profiles within infected human bone (using noninvasive imaging tools) and develop optimized rifampin-containing regimens for S. aureus orthopedic implant infections. All protocols were approved by the Johns Hopkins University Biosafety, Radiation Safety, Animal Care and Use (MO19M382, MO15M421, and SP17M176) and Institutional Review Board Committees (IRB00067243 and IRB00179210). We used a bidirectional process to integrate findings from animal and human studies. Dynamic 11C-rifampin PET/CT was used in patients with S. aureus bone infections (n = 3) as well as patients with pulmonary TB but without orthopedic infection (n = 12) (38) to measure concentration-time profiles in bone and plasma to determine bone-to-plasma AUC ratios (AUCbone/plasma) at several anatomic sites. 11C-Rifampin PET in a mouse model of S. aureus orthopedic implant infection as well as direct rifampin measurements from postmortem bone tissues were performed and applied to an in vitro S. aureus biofilm system to assess bacterial killing and biofilm disruption using live, time-lapse imaging. These data provided a translational bridge to facilitate PK modeling and predict rifampin concentration-time profiles in bone tissues and were used to design efficacy studies to assess relapse-free cure in mice with S. aureus orthopedic implant infection. High-resolution CT was used to measure bone remodeling, and antimicrobial susceptibility testing as well as whole-genome bacterial sequencing were performed to assess the development of bacterial mutations. Sample size, selection, and replicates are provided in the figure legends. Although the study was not blinded, a unique identification number was provided to each subject, and VOIs and mass spectrometry were performed without knowledge of group assignment.
Human studies
11C-Rifampin was synthesized as a sterile, pyrogen-free solution of high specific activity (595.87 ± 158.15 GBq/μmol) and high radio-chemical purity at the Johns Hopkins PET Radiotracer Center using Current Good Manufacturing Practices and used per the U.S. FDA Radioactive Drug Research Committee program guidelines (41). Three patients with microbiologically confirmed S. aureus bone infections were prospectively recruited between February and December 2020. Written informed consent was obtained from all patients, and deidentified images are presented. The eligibility criteria are outlined in table S1. The subject received an intravenous bolus of 707.4 ± 43.7 MBq of 11C-rifampin followed immediately by a dynamic PET/CT for 60 to 90 min (Biograph mCT, Siemens) using a multi-bed dynamic protocol. The study team had no role in the diagnosis or clinical management of the patients. There was no external data and safety monitoring board.
Dynamic PET/CT (Biograph mCT, Siemens) data from 12 patients with confirmed pulmonary TB from a previous study (38) were used to measure bone penetration at various anatomic sites. All patients had a physical exam before imaging by a trained physician, and no findings related to the musculoskeletal system were noted.
Bacterial strains
The USA300 bioluminescent S. aureus strain SAP231 derived from the NRS384 CA-MRSA isolated from an outbreak in the Mississippi prison system was used for all experiments (68). SAP231 has a stably integrated modified luxABCDE operon from the bacterial insect pathogen Photorhabdus luminescens and was used previously to study orthopedic implant infections (40, 67, 69). The MICs for SAP231 were ≤0.5 μg/ml for rifampin, 1 μg/ml for vancomycin, and ≤1 μg/ml for linezolid, as determined by the microdilution assay (n = 3 replicates per antibiotic).
SAP231 was streaked onto tryptic soy agar (TSA) plates [tryptic soy broth (TSB; TSA plus 1.5% Bacto Agar)] (BD Biosciences) and grown overnight at 37°C in a bacterial incubator. Two to three colonies were picked and cultured in TSB at 37°C in a shaking incubator (MaxQ HP 420, Thermo Fisher) (240 rpm) overnight (16 hours), followed by a 1:50 subculture at 37°C for 2 hours to obtain mid-logarithmic phase bacteria. The bacteria were pelleted, washed three times, and resuspended in sterile phosphate-buffered saline (PBS). The absorbance (A600) was measured to estimate the number of CFU, which was verified after overnight culture on TSA plates.
Animal infection and antibiotic treatments
An orthopedic-grade titanium Kirschner wire (0.5 × 9 mm; Modern Grinding, Custom Wire technologies) was surgically placed into the distal femoral intramedullary canal with 1 mm protruding into the knee joint of 10- to 12-week-old male C57BL/6 mice (The Jackson Laboratory) (40, 67, 69). Bacteria (1 × 103 CFU SAP231) were inoculated onto the implant in the knee joint before closure. Antibiotic treatments were initiated 2 weeks after the infection to allow biofilm formation on the Kirschner wire (67).
Rifampin, vancomycin, and linezolid doses approximated human exposure based on matching AUC of human and mice. Please see table 1 in (40), which compares mice and human AUCs for the relevant antibiotics based on previous literature (60, 70–72). Mice with S. aureus implant infection received vancomycin (110 mg/kg per day intramuscularly divided twice a day; equipotent to 1000 mg twice a day intravenously in humans) in combination with rifampin at 15, 45, and 60 mg/kg per day (orally divided twice a day) and equipotent to rifampin (10, 35, and 45 mg/kg per day) in humans. Studies using linezolid (160 mg/kg per day orally divided twice a day; equipotent to 600 mg twice a day orally in humans) in combination with rifampin were also performed in some cohorts of mice. Body weight and mouse behavior, posture, and activity level were used to assess for drug intolerance or toxicity. There were no significant differences in initial body weight among the groups. CFUs were determined with serial dilutions on plates after overnight cultures of homogenized tissue (Pro200 Series homogenizer; Pro Scientific).
Rifampin assays
To obtain steady-state kinetics, mice were treated for at least 1 week before euthanasia. Four hours after the administration of the last rifampin dose, mice were euthanized and blood (collected in BD Microtainer tubes, Fisher Scientific Co. LLC) as well as the infected and contralateral uninfected femora were carefully dissected. Blood was separated into plasma, and the distal 25% of the femora were homogenized. Rifampin concentrations were determined using validated ultraperformance liquid chromatography–tandem mass spectrometry assays at the University of Florida. The standard curves for each compound ranged from 50.00 to 0.05 μg/ml. The assay measured total (free and protein bound) concentrations.
Bioluminescence imaging
In vivo bioluminescence imaging was performed using the IVIS Lumina III Imaging System (PerkinElmer) in live mice at several time points after starting antibiotic treatments to noninvasively monitor bacterial burden (40). Bioluminescent signals were localized on a grayscale image of the mice and quantified within an oval 0.5 × 0.75–cm region of interest using maximum flux (photons per second per square centimeter per steradian) (level of detection = 2.5 × 103 photons s−1 cm−2 sr−1).
PET/CT imaging
Live mice with S. aureus implant infection were imaged after an intravenous dose of 5.03 ± 0.02 MBq of 11C-rifampin via the tail vein, and dynamic PET was performed over 60 min using a nanoScan PET/CT (Mediso) small-animal imager (37). High-resolution CT was also performed using the nanoScan PET/CT to assess bone remodeling.
Image analysis
11C-Rifampin PET/CT were visualized using Mirada XD 3.6 (Mirada Medical) or VivoQuant 2020 (Invicro) for human and mice, respectively. 3D spherical VOIs were manually drawn using CT as a guide and applied to the dynamic PET data (38, 39). VOIs are shown in fig. S3. PET signal in blood (left ventricle; corrected to plasma using the individual hematocrit values from each patient or using a 50% hematocrit value in mice), infected bone (visualized on magnetic resonance imaging or CT in human subjects or 25% distal femur in mice), and contralateral uninfected region (e.g., contralateral tibia or foot in humans and contralateral 25% of distal femur in mice) as well as other uninfected bones (humerus head, humerus shaft, and cervical and lumbar spine) was measured. PMOD 4.1 (PMOD Technologies) and VivoQuant 2020, for human and mice, respectively, were used to generate TACs. Tissue density [Hounsfield unit (HU) obtained by CT] was used to convert the PET data to per mass of tissue. The implants (identified on the basis of radiodensity >5000 HUs) were excluded from the image analysis in all studies. Heat map overlays were implemented using R software (R Foundation for Statistical Computing).
In vitro S. aureus biofilm system
Biofilms were cultivated as previously described (41). Briefly, bacteria (SAP231) were cultured overnight in TSB and then diluted 1:100 into fresh medium, and 400 μl was pipetted onto circular glass coverslips in individual wells of a 24-well plate. Biofilm was incubated 48 hours at 37°C, and then the biofilm-covered coverslips were removed and inverted into wells containing fresh medium containing 3 μM propidium iodide (PI; BD Pharmingen), 5 μM Syto9 (Invitrogen), and the indicated dose of rifampin (Sigma-Aldrich). Plates were subsequently incubated at 37°C using a temperature-controlled environmental chamber associated with a Zeiss Axio Observer.Z1 (Zeiss) fluorescent microscope with Colibri.2 light-emitting diode light source and an ORCA-R2 digital charge-coupled device camera (Hamamatsu). Time-lapse images were taken with ×200 total magnification every hour for 4 hours. Images were analyzed using ImageJ (73). The ratio between the area positive for PI (dead cells) and the area positive for Syto9 (dead and live cells) was quantified.
PK modeling
Nonlinear mixed effects modeling was performed with Pumas 2.0 (Pumas-AI) (74). To describe the intravenous 11C-rifampin PET data, a two-compartment model was developed from 15 patients with an additional bone compartment and a bone partition coefficient (PC) to model rifampin bone penetration (fig. S5). As bone PK was not consistently different between infected and uninfected bones, infected and uninfected bone PK were combined for modeling. Patients with and without orthopedic infection were also combined.
As rifampin exhibits dose- and time-dependent PK, a previously published PK model was used to simulate the PK for standard- and high-dose rifampin (Fig. 2A) (43). The model incorporates relative increases in bioavailability and saturable clearance to model higher than dose proportional increases in exposure. Autoinduction was included to model the time-dependent decrease in exposure. Bone-related parameters for blood flow (Qb), volume (Vb), and bone PC from the 11C-rifampin PET model were incorporated into the therapeutic dosing model (tables S3 and S4). Doses below daily 15 mg/kg per day were unable to be simulated because of bioavailability being set relative to 450 mg.
Treatment shortening studies
Mice were euthanized 6 weeks after completion of the antibiotic course to determine relapse-free cure in the mouse model of S. aureus orthopedic implant infection. Whole tissues (bone and surrounding joint and soft tissues) were homogenized in 1 ml of PBS. Bacteria adherent to the Kirschner wire implants were isolated by sonication in 1 ml of 0.3% Tween solution (Sigma-Aldrich) for 10 min followed by vortexing for 2 min. The CFUs were counted after overnight culture with serial dilutions on plates of 100 μl of homogenate or sonicate. Homogenates and sonicates (100 μl) were also cultured for an additional 48 hours in TSB, and the presence or absence of bacterial growth was determined after overnight culture on plates. Detection of any viable bacteria from an animal was considered as a relapse event.
Bacterial isolates (21 of 23 isolates) cultured from tissue homogenates and implant sonicates were analyzed by the Johns Hopkins Hospital microbiological laboratory to determine rifampin MICs (BD Phoenix) using CLSI guidelines (two isolates from the mice treated with vancomycin alone were excluded for technical reasons). In addition, whole-genome bacterial sequencing using NextSeq 550 platform (Illumina; MiGS) was also performed on 21 of 23 isolates and compared to the original stock of SAP231 (USA300-NRS384). Mouse tissues were cultured on TSA plates and TSB broth. Positive broth cultures were subcultured onto a TSA plate, and single colonies were picked and subcultured for genomic DNA isolation. Whole-genome bacterial sequences were deposited to the National Center for Biotechnology Information (NCBI) database, and the associated BioProject accession number is PRJNA763376.
Statistical analysis
Data were analyzed using (GraphPad Software Inc.). Bacterial burden (CFU) is presented as means ± SD on a logarithmic scale (base 10). PET, BLI, and mass spectrometry data are presented as median ± IQR. Continuous variables were compared between two groups using the two-tailed Mann-Whitney U test (mass spectroscopy and mouse PET for infected versus noninfected bone), one-tailed Mann-Whitney U test when appropriate (in vitro biofilm assay for anticipation that higher-dose rifampin would be more efficient), two-tailed Wilcoxon matched-pairs signed rank test when paired comparisons were appropriate (PET data for infected versus noninfected bone and for TB patients), and Kruskal-Wallis for comparisons of multiple groups (CFU, radiographic findings, and mass spectroscopy). BLI was compared using two-way analysis of variance (ANOVA). Analyses were adjusted for multiple comparisons when indicated to preserve the false discovery rate. χ2 test or a two-tailed Fisher exact test was used to compare proportions (bacterial growth in broth and rifampin resistance). Infection-free survival was compared by the log-rank (Mantel-Cox) test. P < 0.05 was considered statistically significant.
Sample size for the noninferiority studies was calculated assuming 85 and 95% cure rates for standard- and high-dose rifampin, respectively. Noninferiority limit (d) was set to 10%, power (1 – β) was set to 90%, and significance (α) was set to 5% (Sealed Envelope Ltd. 2012; www.sealedenvelope.com/power/binary-noninferior/). Two-sided 90% confidence intervals were calculated using the Wilson method (GraphPad Software Inc.). Individual subject-level data are reported in data file S1.
Supplementary Material
Acknowledgments:
We thank the generosity of the study participants. We also thank R. Abdallah, C. Voicu, L. Shinehouse, E. W. Tucker, and M. K. Brosnan (Johns Hopkins Hospitals) for coordinating the human imaging studies.
Funding:
This work was funded by the NIH (Director’s Transformative Research Award R01-EB020539, R01-HL131829, R01-EB025985, and R01-AI153349 as well as T32-AI052071) to S.K.J. NIH grants (R01-AR073665 and R01-AR069502) to L.S.M. and N.K.A. provided salary support for L.S.M. and N.K.A.
Competing interests:
J.V.S.G. is a co-founder of Pumas-AI Inc., which commercializes Pumas software. L.S.M. is currently a full-time employee of Janssen Research & Development; has received grant support from AstraZeneca, Pfizer, Boehringer Ingelheim, Regeneron Pharmaceuticals, and Moderna Therapeutics; holds stock from Johnson & Johnson and Noveome Biotherapeutics; and was a paid consultant for AstraZeneca, Almirall, and Janssen Research and Development, which are all developing therapeutics against S. aureus and other pathogens.
Footnotes
SUPPLEMENTARY MATERIALS
www.science.org/doi/10.1126/scitranslmed.abl6851
Materials Design Analysis Reporting (MDAR) Checklist for Authors
Data and materials availability:
All data associated with this study are present in the paper or the Supplementary Materials. Whole-genome sequences were submitted to the NCBI, and the associated BioProject accession number is PRJNA763376.
REFERENCES AND NOTES
- 1.Rasigade JP, Vandenesch F, Staphylococcus aureus: A pathogen with still unresolved issues. Infect. Genet. Evol 21, 510–514 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr., Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev 28, 603–661 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richter K, Van den Driessche F, Coenye T, Innovative approaches to treat Staphylococcus aureus biofilm-related infections. Essays Biochem. 61, 61–70 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Costerton JW, Stewart PS, Greenberg EP, Bacterial biofilms: A common cause of persistent infections. Science 284, 1318–1322 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Del Pozo JL, Patel R, Infection associated with prosthetic joints. N. Engl. J. Med 361, 787–794 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf BR, Lu X, Li Y, Callaghan JJ, Cram P, Adverse outcomes in hip arthroplasty: Long-term trends. J. Bone Joint Surg. Am 94, e103 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J, Economic burden of periprosthetic joint infection in the United States. J. Arthroplasty 27, 61–65.e1 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr., Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O’Gara P, Taubert KA; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council, Infective endocarditis in adults: Diagnosis, antimicrobial therapy, and management of complications: A scientific statement for healthcare professionals from the American Heart Association. Circulation 132, 1435–1486 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR; Infectious Diseases Society of America, Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis 56, e1–e25 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Tunkel AR, Hasbun R, Bhimraj A, Byers K, Kaplan SL, Scheld WM, van de Beek D, Bleck TP, Garton HJL, Zunt JR, 2017 infectious diseases society of America’s clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin. Infect. Dis 64, e34–e65 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF, Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: Executive summary. Clin. Infect. Dis 52, 285–292 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Van der Auwera P, Meunier-Carpentier F, Klastersky J, Clinical study of combination therapy with oxacillin and rifampin for staphylococcal infections. Rev. Infect. Dis 5 (suppl. 3), S515–S521 (1983). [DOI] [PubMed] [Google Scholar]
- 13.Van der Auwera P, Klastersky J, Thys JP, Meunier-Carpentier F, Legrand JC, Double-blind, placebo-controlled study of oxacillin combined with rifampin in the treatment of staphylococcal infections. Antimicrob. Agents Chemother 28, 467–472 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Euba G, Murillo O, Fernandez-Sabe N, Mascaro J, Cabo J, Perez A, Tubau F, Verdaguer R, Gudiol F, Ariza J, Long-term follow-up trial of oral rifampin-cotrimoxazole combination versus intravenous cloxacillin in treatment of chronic staphylococcal osteomyelitis. Antimicrob. Agents Chemother 53, 2672–2676 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen S, Pasquet A, Legout L, Beltrand E, Dubreuil L, Migaud H, Yazdanpanah Y, Senneville E, Efficacy and tolerance of rifampicin-linezolid compared with rifampicin-cotrimoxazole combinations in prolonged oral therapy for bone and joint infections. Clin. Microbiol. Infect 15, 1163–1169 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Norden CW, Bryant R, Palmer D, Montgomerie JZ, Wheat J, Chronic osteomyelitis caused by Staphylococcus aureus: Controlled clinical trial of nafcillin therapy and nafcillin-rifampin therapy. South. Med. J 79, 947–951 (1986). [DOI] [PubMed] [Google Scholar]
- 17.Norden CW, Fierer J, Bryant RE, Chronic staphylococcal osteomyelitis: Treatment with regimens containing rifampin. Rev. Infect. Dis 5 (suppl. 3), S495–S501 (1983). [DOI] [PubMed] [Google Scholar]
- 18.Li HK, Rombach I, Zambellas R, Walker AS, McNally MA, Atkins BL, Lipsky BA, Hughes HC, Bose D, Kumin M, Scarborough C, Matthews PC, Brent AJ, Lomas J, Gundle R, Rogers M, Taylor A, Angus B, Byren I, Berendt AR, Warren S, Fitzgerald FE, Mack DJF, Hopkins S, Folb J, Reynolds HE, Moore E, Marshall J, Jenkins N, Moran CE, Woodhouse AF, Stafford S, Seaton RA, Vallance C, Hemsley CJ, Bisnauthsing K, Sandoe JAT, Aggarwal I, Ellis SC, Bunn DJ, Sutherland RK, Barlow G, Cooper C, Geue C, McMeekin N, Briggs AH, Sendi P, Khatamzas E, Wangrangsimakul T, Wong THN, Barrett LK, Alvand A, Old CF, Bostock J, Paul J, Cooke G, Thwaites GE, Bejon P, Scarborough M; OVIVA Trial Collaborators, Oral versus intravenous antibiotics for bone and joint infection. N. Engl. J. Med 380, 425–436 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE, Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: A randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA 279, 1537–1541 (1998). [DOI] [PubMed] [Google Scholar]
- 20.Karlsen OE, Borgen P, Bragnes B, Figved W, Grogaard B, Rydinge J, Sandberg L, Snorrason F, Wangen H, Witsoe E, Westberg M, Rifampin combination therapy in staphylococcal prosthetic joint infections: A randomized controlled trial. J. Orthop. Surg. Res 15, 365 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandell GL, Bennett JE, Dolin R, Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases (Churchill Livingstone/Elsevier, ed. 7, 2010). [Google Scholar]
- 22.Bernard L, Arvieux C, Brunschweiler B, Touchais S, Ansart S, Bru JP, Oziol E, Boeri C, Gras G, Druon J, Rosset P, Senneville E, Bentayeb H, Bouhour D, Le Moal G, Michon J, Aumaitre H, Forestier E, Laffosse JM, Begue T, Chirouze C, Dauchy FA, Devaud E, Martha B, Burgot D, Boutoille D, Stindel E, Dinh A, Bemer P, Giraudeau B, Issartel B, Caille A, Antibiotic therapy for 6 or 12 weeks for prosthetic joint infection. N. Engl. J. Med 384, 1991–2001 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE, Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern. Med 177, 1308–1315 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chastre J, Wolff M, Fagon JY, Chevret S, Thomas F, Wermert D, Clementi E, Gonzalez J, Jusserand D, Asfar P, Perrin D, Fieux F, Aubas S, Pneum ATG, Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: A randomized trial. JAMA 290, 2588–2598 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Cluzel RA, Lopitaux R, Sirot J, Rampon S, Rifampicin in the treatment of osteoarticular infections due to staphylococci. J. Antimicrob. Chemother 13 (suppl. C), 23–29 (1984). [DOI] [PubMed] [Google Scholar]
- 26.Roth B, Penetration of parenterally administered rifampicin into bone tissue. Chemotherapy 30, 358–365 (1984). [DOI] [PubMed] [Google Scholar]
- 27.Sirot J, Lopitaux R, Cluzel R, Delisle JJ, Sauvezie B, Rifampicin diffusion in non-infected human bone (author’s transl). Ann. Microbiol 128, 229–236 (1977). [PubMed] [Google Scholar]
- 28.Sirot J, Prive L, Lopitaux R, Glanddier Y, Diffusion of rifampicin into spongy and compact bone tissue during total hip prosthesis operation. Pathol. Biol 31, 438–441 (1983). [PubMed] [Google Scholar]
- 29.Thabit AK, Fatani DF, Bamakhrama MS, Barnawi OA, Basudan LO, Alhejaili SF, Antibiotic penetration into bone and joints: An updated review. Int. J. Infect. Dis 81, 128–136 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Iversen P, Nielsen OS, Jensen KM, Madsen PO, Comparison of concentrations of rifampin and a new rifamycin derivative, DL 473, in canine bone. Antimicrob. Agents Chemother 23, 338–340 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diacon AH, Patientia RF, Venter A, van Helden PD, Smith PJ, McIlleron H, Maritz JS, Donald PR, Early bactericidal activity of high-dose rifampin in patients with pulmonary tuberculosis evidenced by positive sputum smears. Antimicrob. Agents Chemother 51, 2994–2996 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chigutsa E, Pasipanodya JG, Visser ME, van Helden PD, Smith PJ, Sirgel FA, Gumbo T, McIlleron H, Impact of nonlinear interactions of pharmacokinetics and MICs on sputum bacillary kill rates as a marker of sterilizing effect in tuberculosis. Antimicrob. Agents Chemother 59, 38–45 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T, Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J. Infect. Dis 208, 1464–1473 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swaminathan S, Pasipanodya JG, Ramachandran G, Hemanth Kumar AK, Srivastava S, Deshpande D, Nuermberger E, Gumbo T, Drug concentration thresholds predictive of therapy failure and death in children with tuberculosis: Bread crumb trails in random forests. Clin. Infect. Dis 63, S63–S74 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunter RL, The pathogenesis of tuberculosis: The early infiltrate of post-primary (adult pulmonary) tuberculosis: A distinct disease entity. Front. Immunol 9, 2108 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cassat JE, Moore JL, Wilson KJ, Stark Z, Prentice BM, Van de Plas R, Perry WJ, Zhang Y, Virostko J, Colvin DC, Rose KL, Judd AM, Reyzer ML, Spraggins JM, Grunenwald CM, Gore JC, Caprioli RM, Skaar EP, Integrated molecular imaging reveals tissue heterogeneity driving host-pathogen interactions. Sci. Transl. Med 10, eaan6361 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeMarco VP, Ordonez AA, Klunk M, Prideaux B, Wang H, Zhuo Z, Tonge PJ, Dannals RF, Holt DP, Lee CK, Weinstein EA, Dartois V, Dooley KE, Jain SK, Determination of [11C]rifampin pharmacokinetics within Mycobacterium tuberculosis-infected mice by using dynamic positron emission tomography bioimaging. Antimicrob. Agents Chemother 59, 5768–5774 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ordonez AA, Wang H, Magombedze G, Ruiz-Bedoya CA, Srivastava S, Chen A, Tucker EW, Urbanowski ME, Pieterse L, Fabian Cardozo E, Lodge MA, Shah MR, Holt DP, Mathews WB, Dannals RF, Gobburu JVS, Peloquin CA, Rowe SP, Gumbo T, Ivaturi VD, Jain SK, Dynamic imaging in patients with tuberculosis reveals heterogeneous drug exposures in pulmonary lesions. Nat. Med 26, 529–534 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tucker EW, Guglieri-Lopez B, Ordonez AA, Ritchie B, Klunk MH, Sharma R, Chang YS, Sanchez-Bautista J, Frey S, Lodge MA, Rowe SP, Holt DP, Gobburu JVS, Peloquin CA, Mathews WB, Dannals RF, Pardo CA, Kannan S, Ivaturi VD, Jain SK, Noninvasive 11C-rifampin positron emission tomography reveals drug biodistribution in tuberculous meningitis. Sci. Transl. Med 10, eaau0965 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson JM, Saini V, Ashbaugh AG, Miller RJ, Ordonez AA, Ortines RV, Wang Y, Sterling RS, Jain SK, Miller LS, Oral-only linezolid-rifampin is highly effective compared with other antibiotics for periprosthetic joint infection: Study of a mouse model. J. Bone Joint Surg. Am 99, 656–665 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merritt JH, Kadouri DE, O’Toole GA, Growing and analyzing static biofilms. Curr. Protoc. Microbiol Chapter 1, Unit 1B 1 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Acocella G, Pharmacokinetics and metabolism of rifampin in humans. Rev. Infect. Dis 5 (suppl. 3), S428–S432 (1983). [DOI] [PubMed] [Google Scholar]
- 43.Svensson RJ, Aarnoutse RE, Diacon AH, Dawson R, Gillespie SH, Boeree MJ, Simonsson USH, A population pharmacokinetic model incorporating saturable pharmacokinetics and autoinduction for high rifampicin doses. Clin. Pharmacol. Ther 103, 674–683 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stott KE, Pertinez H, Sturkenboom MGG, Boeree MJ, Aarnoutse R, Ramachandran G, Requena-Mendez A, Peloquin C, Koegelenberg CFN, Alffenaar JWC, Ruslami R, Tostmann A, Swaminathan S, McIlleron H, Davies G, Pharmacokinetics of rifampicin in adult TB patients and healthy volunteers: A systematic review and meta-analysis. J. Antimicrob. Chemother 73, 2305–2313 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinstein MP, Performance Standards for Antimicrobial Susceptibility Testing, 31st Edition (Clinical and Laboratory Standards Institute, 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mansour A, Nabos JF, Nabos RF, Psoas abscess: Thirty-four years after pyogenic osteomyelitis of the spine. Orthopedics 2, 262–264 (1979). [DOI] [PubMed] [Google Scholar]
- 47.Zimmerli W, Trampuz A, Ochsner PE, Prosthetic-joint infections. N. Engl. J. Med 351, 1645–1654 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Bhattacharya G, Dey D, Das S, Banerjee A, Exposure to sub-inhibitory concentrations of gentamicin, ciprofloxacin and cefotaxime induces multidrug resistance and reactive oxygen species generation in meticillin-sensitive Staphylococcus aureus. J. Med. Microbiol 66, 762–769 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Dheda K, Lenders L, Magombedze G, Srivastava S, Raj P, Arning E, Ashcraft P, Bottiglieri T, Wainwright H, Pennel T, Linegar A, Moodley L, Pooran A, Pasipanodya JG, Sirgel FA, van Helden PD, Wakeland E, Warren RM, Gumbo T, Drug-penetration gradients associated with acquired drug resistance in patients with tuberculosis. Am. J. Respir. Crit. Care Med 198, 1208–1219 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roch M, Clair P, Renzoni A, Reverdy ME, Dauwalder O, Bes M, Martra A, Freydiere AM, Laurent F, Reix P, Dumitrescu O, Vandenesch F, Exposure of Staphylococcus aureus to subinhibitory concentrations of β-lactam antibiotics induces heterogeneous vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother 58, 5306–5314 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brauner A, Fridman O, Gefen O, Balaban NQ, Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol 14, 320–330 (2016). [DOI] [PubMed] [Google Scholar]
- 52.Liu J, Gefen O, Ronin I, Bar-Meir M, Balaban NQ, Effect of tolerance on the evolution of antibiotic resistance under drug combinations. Science 367, 200–204 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Wichelhaus T, Schafer V, Brade V, Böddinghaus B, Differential effect of rpoB mutations on antibacterial activities of rifampicin and KRM-1648 against Staphylococcus aureus. J. Antimicrob. Chemother 47, 153–156 (2001). [DOI] [PubMed] [Google Scholar]
- 54.Grobbelaar M, Louw GE, Sampson SL, van Helden PD, Donald PR, Warren RM, Evolution of rifampicin treatment for tuberculosis. Infect. Genet. Evol 74, 103937 (2019). [DOI] [PubMed] [Google Scholar]
- 55.Jayaram R, Gaonkar S, Kaur P, Suresh BL, Mahesh BN, Jayashree R, Nandi V, Bharat S, Shandil RK, Kantharaj E, Balasubramanian V, Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob. Agents Chemother 47, 2118–2124 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boeree MJ, Diacon AH, Dawson R, Narunsky K, du Bois J, Venter A, Phillips PP, Gillespie SH, McHugh TD, Hoelscher M, Heinrich N, Rehal S, van Soolingen D, van Ingen J, Magis-Escurra C, Burger D, Plemper van Balen G, Aarnoutse RE; PanACEA Consortium A dose-ranging trial to optimize the dose of rifampin in the treatment of tuberculosis. Am. J. Respir. Crit. Care Med 191, 1058–1065 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Boeree MJ, Heinrich N, Aarnoutse R, Diacon AH, Dawson R, Rehal S, Kibiki GS, Churchyard G, Sanne I, Ntinginya NE, Minja LT, Hunt RD, Charalambous S, Hanekom M, Semvua HH, Mpagama SG, Manyama C, Mtafya B, Reither K, Wallis RS, Venter A, Narunsky K, Mekota A, Henne S, Colbers A, van Balen GP, Gillespie SH, Phillips PPJ, Hoelscher M; PanACEA Consortium, High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: A multi-arm, multi-stage randomised controlled trial. Lancet Infect. Dis 17, 39–49 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Te Brake LHM, de Jager V, Narunsky K, Vanker N, Svensson EM, Phillips PPJ, Gillespie SH, Heinrich N, Hoelscher M, Dawson R, Diacon AH, Aarnoutse RE, Boeree MJ, Pan AC, Increased bactericidal activity but dose-limiting intolerability at 50 mg.kg−1 rifampicin. Eur. Respir. J 58, 2000955 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pasipanodya JG, Smythe W, Merle CS, Olliaro PL, Deshpande D, Magombedze G, McIlleron H, Gumbo T, Artificial intelligence-derived 3-way concentration-dependent antagonism of gatifloxacin, pyrazinamide, and rifampicin during treatment of pulmonary tuberculosis. Clin. Infect. Dis 67, S284–S292 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Steenwinkel JEM, Aarnoutse RE, de Knegt GJ, ten Kate MT, Teulen M, Verbrugh HA, Boeree MJ, van Soolingen D, Bakker-Woudenberg IA, Optimization of the rifampin dosage to improve the therapeutic efficacy in tuberculosis treatment using a murine model. Am. J. Respir. Crit. Care Med 187, 1127–1134 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Wang W, Chen J, Chen G, Du X, Cui P, Wu J, Zhao J, Wu N, Zhang W, Li M, Zhang Y, Transposon mutagenesis identifies novel genes associated with Staphylococcus aureus persister formation. Front. Microbiol 6, 1437 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gaupp R, Schlag S, Liebeke M, Lalk M, Gotz F, Advantage of upregulation of succinate dehydrogenase in Staphylococcus aureus biofilms. J. Bacteriol 192, 2385–2394 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bateman A, Bycroft M, The structure of a LysM domain from E. coli membrane-bound lytic murein transglycosylase D (MltD). J. Mol. Biol 299, 1113–1119 (2000). [DOI] [PubMed] [Google Scholar]
- 64.Pascoe B, Dams L, Wilkinson TS, Harris LG, Bodger O, Mack D, Davies AP, Dormant cells of Staphylococcus aureus are resuscitated by spent culture supernatant. PLOS ONE 9, e85998 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Speziale P, Pietrocola G, Foster TJ, Geoghegan JA, Protein-based biofilm matrices in Staphylococci. Front. Cell. Infect. Microbiol 4, 171 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Geno Tai DB, Patel R, Abdel MP, Berbari EF, Tande AJ, Microbiology of hip and knee periprosthetic joint infections: A database study. Clin. Microbiol. Infect S1198–743X(21)00323–2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bernthal NM, Stavrakis AI, Billi F, Cho JS, Kremen TJ, Simon SI, Cheung AL, Finerman GA, Lieberman JR, Adams JS, Miller LS, A mouse model of post-arthroplasty Staphylococcus aureus joint infection to evaluate in vivo the efficacy of antimicrobial implant coatings. PLOS ONE 5, e12580 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Plaut RD, Mocca CP, Prabhakara R, Merkel TJ, Stibitz S, Stably luminescent Staphylococcus aureus clinical strains for use in bioluminescent imaging. PLOS ONE 8, e59232 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pribaz JR, Bernthal NM, Billi F, Cho JS, Ramos RI, Guo Y, Cheung AL, Francis KP, Miller LS, Mouse model of chronic post-arthroplasty infection: Noninvasive in vivo bioluminescence imaging to monitor bacterial burden for long-term study. J. Orthop. Res 30, 335–340 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crandon JL, Kuti JL, Nicolau DP, Comparative efficacies of human simulated exposures of telavancin and vancomycin against methicillin-resistant Staphylococcus aureus with a range of vancomycin MICs in a murine pneumonia model. Antimicrob. Agents Chemother 54, 5115–5119 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Healy DP, Polk RE, Garson ML, Rock DT, Comstock TJ, Comparison of steady-state pharmacokinetics of two dosage regimens of vancomycin in normal volunteers. Antimicrob. Agents Chemother 31, 393–397 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ruslami R, Nijland HM, Alisjahbana B, Parwati I, van Crevel R, Aarnoutse RE, Pharmacokinetics and tolerability of a higher rifampin dose versus the standard dose in pulmonary tuberculosis patients. Antimicrob. Agents Chemother 51, 2546–2551 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schneider CA, Rasband WS, Eliceiri KW, NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rackauckas ANC, Dixit V, Ma Y, Mogensen PK, Maddhashiya S, Byrne S, Calderón JBS, Nyberg J, Gobburu JVS, Ivaturi V, Accelerated predictive healthcare analytics with pumas, a high performance pharmaceutical modeling and simulation platform. bioRxiv, 2020.11.28.402297 (2020). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are present in the paper or the Supplementary Materials. Whole-genome sequences were submitted to the NCBI, and the associated BioProject accession number is PRJNA763376.


