Abstract
Background
Pumpkin seed oil is widely used to treat benign prostatic hyperplasia (BPH), a common disease in elder men. However, its active components and mechanism have remained to be elucidated.
Objective
The objective of the present study was to investigate the active components of pumpkin seed oil and its mechanism against BPH.
Design
Total phytosterol (TPS) was isolated from hull-less pumpkin (Cucurbita pepo L. var. Styriaca) seed oil and analyzed by gas chromatography/mass spectrometry (GC/MS). Three phytosterols were purified by preparative HPLC (high performance liquid chromatography) and confirmed by NMR (nuclear magnetic resonance). TPS (3.3 mg/kg body weight, 1 mL/day/rat) was administered intragastrically to the testosterone propionate-induced BPH rats for 4 weeks. The structure changes of prostate tissues were assessed by hematoxylin & eosin (H&E) staining. The expression of androgen receptor (AR) and steroid receptor coactivator 1 (SRC-1) was analyzed by immunohistochemistry, while that of 5α-reductase (5AR), apoptosis, or proliferation-related growth factors/proteins was detected by real-time quantitative polymerase chain reaction or western blotting.
Results
The ∆7-phytosterols in TPS reached up to 87.64%. Among them, 24β-ethylcholesta-7,22,25-trienol, 24β-ethylcholesta-7,25(27)-dien-3-ol, and ∆7-avenasterol were confirmed by NMR. TPS treatment significantly ameliorated the pathological prostate enlargement and restored histopathological alterations of prostate in BPH rats. It effectively suppressed the expressions of 5AR, AR, and coactivator SRC-1. TPS inhibited the expression of proliferation-related growth factor epidermal growth factor, whereas it increased the expressions of apoptosis-related growth factor/gene transforming growth factor-β1. The proliferation-inhibiting effect was achieved by decreasing the ERK (extracellular signal-regulated kinase) phosphorylation, while apoptosis was induced by Caspase 3 activation through JNK (c-Jun N-terminal kinase) and p38 phosphorylation.
Conclusion
TPS from hull-less pumpkin seed oil, with ∆7-phytosterols as its main ingredients, is a potential nutraceutical for BPH prevention.
Keywords: lower urinary tract symptoms, 5α-reductase inhibitor, MAPK pathway, apoptosis, proliferation, phytochemicals, Curcurbita pepo
Popular scientific summary
∆7-phytosterol was the main ingredients of total phytosterol (TPS), which was isolated from hull-less pumpkin (Cucurbita pepo L. var. Styriaca) seed oil, a well-known functional food.
TPS treatment significantly recovered the symptom of BPH in rats by lowering 5α-reductase expression and regulating the balance between cell proliferation and apoptosis.
As a functional food ingredient, phytosterol might be a promising candidate complementary and alternative medicine for the treatment of BPH in the future.
Benign prostatic hyperplasia (BPH) affects ~50% of men aged >50 years and ~90% in their 80s (1). Patients with BPH can exhibit various lower urinary tract symptoms (LUTSs), including hesitancy, incomplete voiding, post-void dribbling, or irritative symptoms (2). It is characterized by hyperplasia of the mesenchymal stromal and glandular epithelial cells in the prostate (3).
Although the pathophysiological mechanism of BPH still needs to be investigated, the predominant hypothesis is age-dependent alterations in hormone ratio and age-related tissue remodeling (4). Testosterone and dihydrotestosterone (DHT) are considered to be related to the development of BPH. By the action of 5α-reductase (5AR), testosterone is converted into DHT, a more potent androgen with higher affinity for the androgen receptor (AR). Serum concentration of testosterone decreases with age, whereas the activities of 5AR and AR are increased due to androgen balance. Recent studies suggest that the development of BPH involves the disruption of the DHT-supported homeostasis between cell proliferation and cell death, allowing proliferative processes to predominate (5). The mitogen-activated protein kinase (MAPK) signaling pathways, linking surface receptor-mediated signals to nuclear events, play key roles in the cellular proliferation, growth, and programmed cell apoptosis (6). Because of the different cell type and different stimulus, the activation of a MAPK signaling pathway might lead to opposite effect (7).
As one of the most frequently prescribed medications for BPH patients, the 5AR inhibitor can block the conversion of testosterone into DHT. Finasteride (Fi), a representative drug for BPH treatment, is one of 5AR inhibitors. It not only improves urinary flow rate but also reduces the risk of acute urinary retention and the requirement for surgical intervention. However, the sexual side effects, including decreased libido, reduced ejaculate volume, and erectile dysfunction, are significantly bothersome (8). Phytotherapeutic agents have recently gained interest worldwide due to their advantages of nature and harmlessness (9).
Pumpkin (Cucurbita spp.) has received considerable attention because of the nutritional and health-protective value of its seed oil. Pumpkin seed oil has been reported to be effective in improving BPH and other LUTS (10, 11). It contains a wide variety of bioactive compounds, including fatty acids, phytosterols, vitamins, etc. (12–14). However, the active ingredients and their mechanisms against BPH are unclear. Phytosterol, especially beta-sitosterol, is routinely suggested as the major active component against BPH (15–17). However, Tsai et al. considered that phytosterol produced a limited effect (18).
In this study, we isolated the total phytosterols (TPSs) of hull-less pumpkin seed oil and analyzed their composition. The hull-less seed pumpkin is a spontaneous mutant of standard pumpkin (Cucurbita pepo L. var. Styriaca), losing hard husk. Its seeds could be processed conveniently. To investigate the therapeutic potential of TPS against BPH, we observed the histopathological change of prostate in a male Sprague–Dawley (SD) rat model. Furthermore, to explore the molecular mechanism behind the effect, we evaluated the mRNA (messenger RNA) and/or protein expression of 5AR, AR, and coactivator SRC-1 (steroid receptor coactivator 1). Growth factors and related proteins in cell apoptosis and proliferation were also detected, including Caspase 3, JNK, ERK, p38, etc.
Materials and methods
Preparation of TPS in hull-less pumpkin seed oil
The seeds of hull-less pumpkin (Cucurbita pepo L. var. Styriaca), which was identified by Prof. S.H. Dai (Hunan Agricultural University, Changsha, Hunan, China), were provided by Hunan provincial engineering research center for Cucurbitaceae. A voucher specimen (CPS-201710) was deposited at the Hunan provincial engineering research center for Cucurbitaceae. The pumpkin seed oil was extracted by supercritical carbon dioxide (CO2) fluid and then incubated in ethanolic potassium hydroxide. After extracted with an equal volume of n-hexane, the n-hexane solution was washed successively with 30% volume of ddH2O, 20% ethanol, ddH2O, 0.5 mol/L potassium hydroxide, ddH2O, until the n-hexane phase was clear. The light n-hexane was dehydrated by moderate anhydrous sodium sulfate. Afterward, the TPS was obtained by concentration, freeze-crystallization, aspirator filter, and drying.
Gas chromatography–mass spectrometry analysis, compounds isolation, and validation
The composition of TPS was analyzed using gas chromatography–mass spectrometry (GC-MS; Shimazu GCMS-QP 2020, Tokyo, Japan), equipped with an electrospray ionization (EI) source and a Rtx®-5MS Capillary column (30 m × 0.25 mm × 0.25 μm). TPS was silylated derived with BSTFA+TMCS (Bis(trimethylsilyl)trifluoroacetamide+Trimethylchlorosilane 99:1) at 80°C for 40 min. After cooled to room temperature, C2Cl2 was added for dissolving. The peaks were then tentatively identified from their retention characteristics and mass fragmentation patterns after initial pretreatment by using NIST.14 mass spectrum database. Three of the peaks were isolated by preparative high-performance liquid chromatography (HPLC) and were validated by NMR. 1H-NMR (400 MHz) and 13C-NMR (100 MHz) spectra were obtained at 25°C with CDCl3 as solvent on a Bruker AVANCE 500 M NMR instrument (Bruker, Switzerland).
Animals
The 12-week-old male SD rats (n = 40) weighing 160–200 g were purchased from Hunan Slake Jingda Experimental Animal Co. Ltd (No. 42004700048405). The experimental protocol was approved by the research Ethics Committee of the Third Xiangya Hospital, Central South University (SCXK [Xiang] 2016-0002). The rats were housed in a pathogen-free room maintained at a temperature of 23 ± 1°C and relative humidity of 70% with an alternating 12 h light/dark cycle. Water and standard laboratory diet were provided ad libitum. They were allowed for acclimation under climate-controlled conditions for 7 days before the experiments began.
Experimental design
All rats were randomly divided into four groups with 10 animals in each group, including 1) a normal control (NC) group, 2) BPH group, 3) Fi group: a positive control group, BPH + 1 mg/kg Fi, and 4) TPS group: BPH + 3.3 mg/kg TPS. TPS was dissolved in corn oil and orally administered to the mice (1 mL/day/rat), while the NC group were treated with the vehicle. To eliminate the influence of endogenous testosterone, rats in all groups except the NC group underwent bilateral orchiectomy 3 days prior to testosterone treatment. For the orchiectomy, the animals were anesthetized by intraperitoneal injections of sodium pentobarbital (25 mg/kg body weight, BW). The rats in BPH-induced groups were castrated and then induced by a pre-4-week treatment of daily subcutaneous injections of testosterone propionate (5 mg/kg) at the inguinal region (n = 30). TPS and Fi were intragastric administration once a day for 4 weeks. At the end of the experimental period, all rats were sacrificed by sodium pentobarbital anesthetization (25 mg/kg BW, i.p.) followed by cervical dislocation. The prostates were removed from all rats. Each prostate was weighed and divided into three parts. One was fixed in 10% formalin and embedded in paraffin for histomorphological analysis, and the others were stored at −80°C for further assays.
Hematoxylin & eosin staining and immunohistochemistry
The structure changes of prostate tissues, including the epithelial thickness and lumen area, were assessed by hematoxylin & eosin (H&E) staining. AR and SRC-1 were analyzed by immunohistochemistry (IHC), which was performed on formalin-fixed, paraffin-embedded tissue sections from archival blocks using standardized avidin-biotin techniques. Antibodies for AR and SRC-1 (dilution of 1:100) were purchased from Abcam, Inc. (Cambridge, United States) and Sigma-Aldrich Inc. (St. Louis, MO, USA), respectively.
Real-time quantitative polymerase chain reaction analysis and western blotting assay
The mRNA expression was analyzed by real-time quantitative polymerase chain reaction (RT-qPCR) and calculated by Livak 2-ΔΔCt method (19). Beta-actin was used as the house-keeping gene. All primers were designed by Beacon Designer 7.0 (PREMIER Biosoft International, San Francisco, USA) and synthesized by TsingKe Biological Technology Co. Ltd. (Changsha, China). Protein expressions were analyzed by western blotting. Total proteins extracted from liver tissue lysates were separated by 10% SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes. Then, PVDF membranes were blocked with skimmed milk (5% in PBS containing 0.2% Tween-20) at room temperature for 2 h and probed with primary antibodies overnight at 4°C. Antibodies for Caspase 3/Cleaved-Caspase 3, ERK/p-ERK, JNK/p-JNK, and p38/p-p38 were purchased from Abcam, Inc. (Cambridge, United States). Membranes were washed and labeled with secondary antibodies of goat anti-mouse IgG1 (Southern Biotech, USA) for 2 h at room temperature. The protein bands were visualized using ECL Prime Western Blotting Detection Reagent (Bio-Rad, USA). The chemiluminescent intensities of protein signals were quantified using Image J v1.8.0 software (National Institutes of Health, USA).
Statistical analysis
The results were expressed as mean ± standard deviation (SD). Comparison between groups was performed using one-way analysis of variance. Statistical significance was accepted at P < 0.05.
Results
Chemical characterization of TPS in hull-less pumpkin seed oil
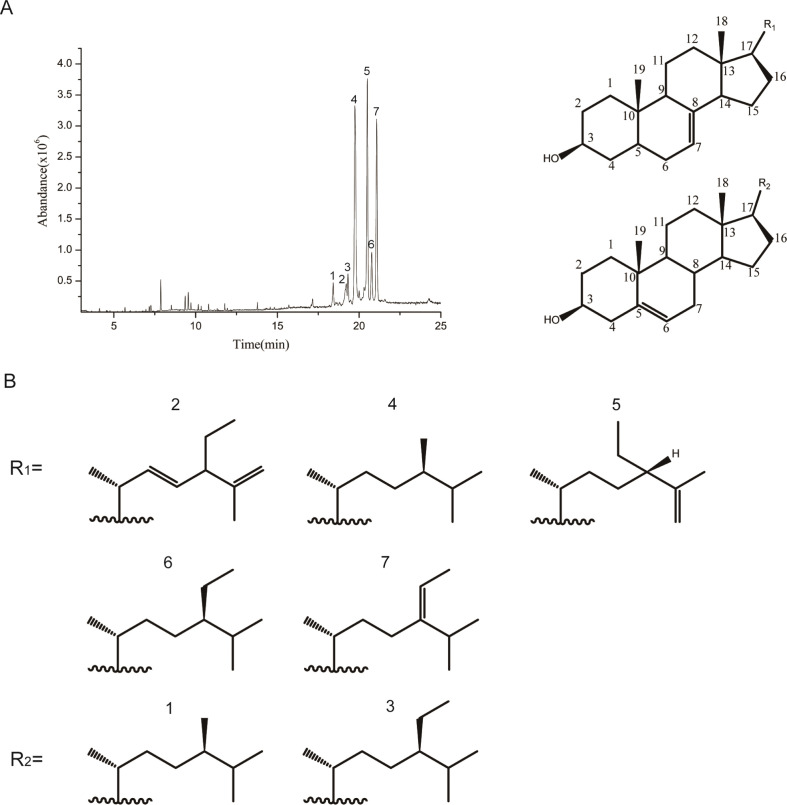
As shown in Fig. 1 and Table 1, the hull-less pumpkin seed oil was rich in phytosterols. The TPS compounds found in the oil included 1) campeterol, 2) 24β-ethylcholesta-7, 22, 25-trienol, 3) β-sitosterol, 4) ∆7-campeterol, 5) 24β-ethylcholesta-7, 25(27)-dien-3-ol, 6) ∆7-sitosterol, and 7) ∆7-avenasterol (Fig. 1 and Table 1). In contrast to the other vegetable oils, in which ∆5-phytosterols are the main ingredients, the TPS contained 87.64% of ∆7-phytosterols. We obtained compounds 2, 5, and 7 with a purity of 90, 94.6, and 91.3%, respectively, using preparative HPLC and validated by NMR. The physical, analytical, and spectral data of compounds 2, 5, and 7 were shown in the Supplementary material and identified as (2) 24β-ethylcholesta-7, 22, 25-trienol, (5) 24β-ethylcholesta-7, 25(27)-dien-3-ol, and (7) ∆7-avenasterol, according to the references (20, 21).
Fig. 1.
GC–MS analysis of TPS in hull-less pumpkin seed oil. (A) Total ion chromatogram of TPS by GC–MS. (B) Chemical structure of peaks (1–7) shown in (A). Peaks (1–7) were identified by comparing with NIST.14 standard library and defined in the text.
Table 1.
Compounds of TPS in hull-less pumpkin seed oil
| Peak | Compound | A% |
|---|---|---|
| 1 | Campeterol | 3.75 |
| 2 | 24β-Ethylcholesta-7,22,25-trienol | 2.41 |
| 3 | β-Sitosterol | 8.17 |
| 4 | ∆7-Campeterol | 23.56 |
| 5 | 24β-Ethylcholesta-7,25(27)-dien-3-ol | 30.75 |
| 6 | ∆7-Sitosterol | 12.59 |
| 7 | ∆7-Avenasterol | 18.33 |
Note: Peaks 1–7 are the peaks 1–7 in Fig. 1A.
Effect of TPS on histopathologic patterns
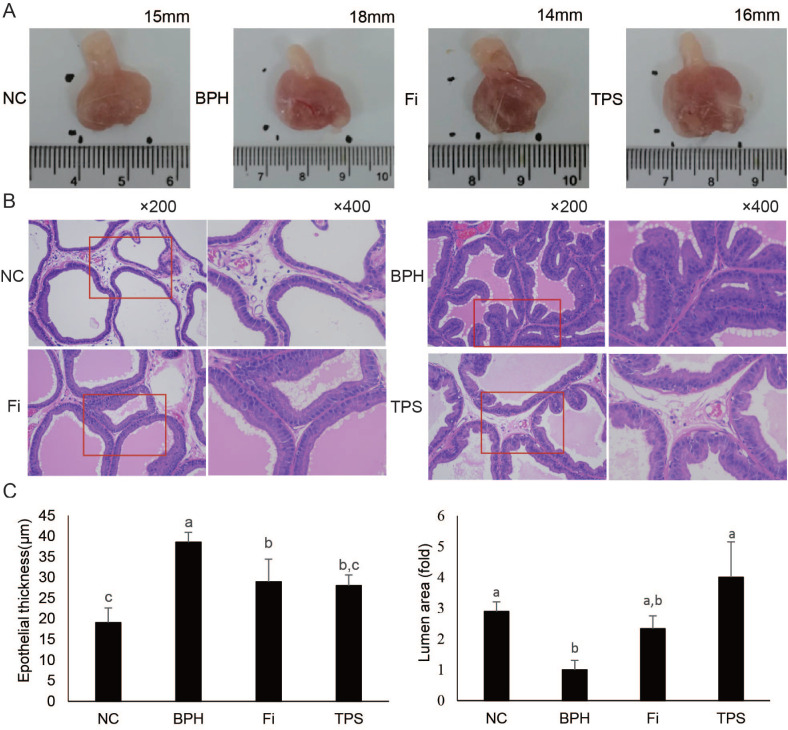
No significant difference was observed in the average BW of rats from all groups (Table 2). The average prostate size, prostate weight (PW), and prostate weight index (PWI) of the BPH group were significantly higher than those of the NC group (Fig. 2A and Table 2). The increment of PW of the TPS group was significantly lowered by 83.4% than those of the BPH group (Table 2). The effect was comparable as that of Fi’s. Similar results were observed in TPS group’s PWI, which was inhibited by 65.8% (Table 2).
Table 2.
Effect of TPS on prostatic parameters
| Group | Body weight (g) | PW (mg) | PWI (mg/100 g) | Inhibition of increase in PWI (%) |
|---|---|---|---|---|
| NC | 450.4 ± 47.7 | 835.6 ± 161.9b | 185.6 ± 29.5b | – |
| BPH | 415.8 ± 25.1 | 1072.9 ± 61.9a | 258.8 ± 21.0a | – |
| Fi | 439.2 ± 32.7 | 868.4 ± 188.3b | 198.1 ± 44.3b | 82.9 |
| TPS | 421.2 ± 48.9 | 875.1 ± 132.2b | 210.6 ± 41.1b | 65.8 |
Prostate weight index = the prostate weight (mg)/body weight of the rat (100 g).
Inhibition of increase in PWI (%) = (PWI of BPH group – PWI of TPS group)/(PWI of BPH group – PWI of NC group).
Values are mean ± SD (n = 10 for each group).
Different lowercase letters (a, b) show a significant difference between groups (P < 0.05).
Fig. 2.
The effect of TPS on histopathologic patterns of prostate tissue. (A) Size of prostate tissue; (B) representative photomicrograph of H&E-stained prostate tissues (left panel magnification ×200 and right panel magnification ×400); (C) epithelial thickness and lumen area level. Data are means ± SD (n = 6 for each group). Different lowercase letters (a–c) show a significant difference between groups (P < 0.05).
The tubular glands of the BPH group demonstrated typical features of glandular hypertrophy, including thickening of the prostatic epithelial layer, papillary fronds protruding into the glandular cavities, and decreased luminal volume (Fig. 2B). Administration of TPS decreased the prostatic epithelial thickness and increased the glandular luminal area (P < 0.05, Fig. 2B and C).
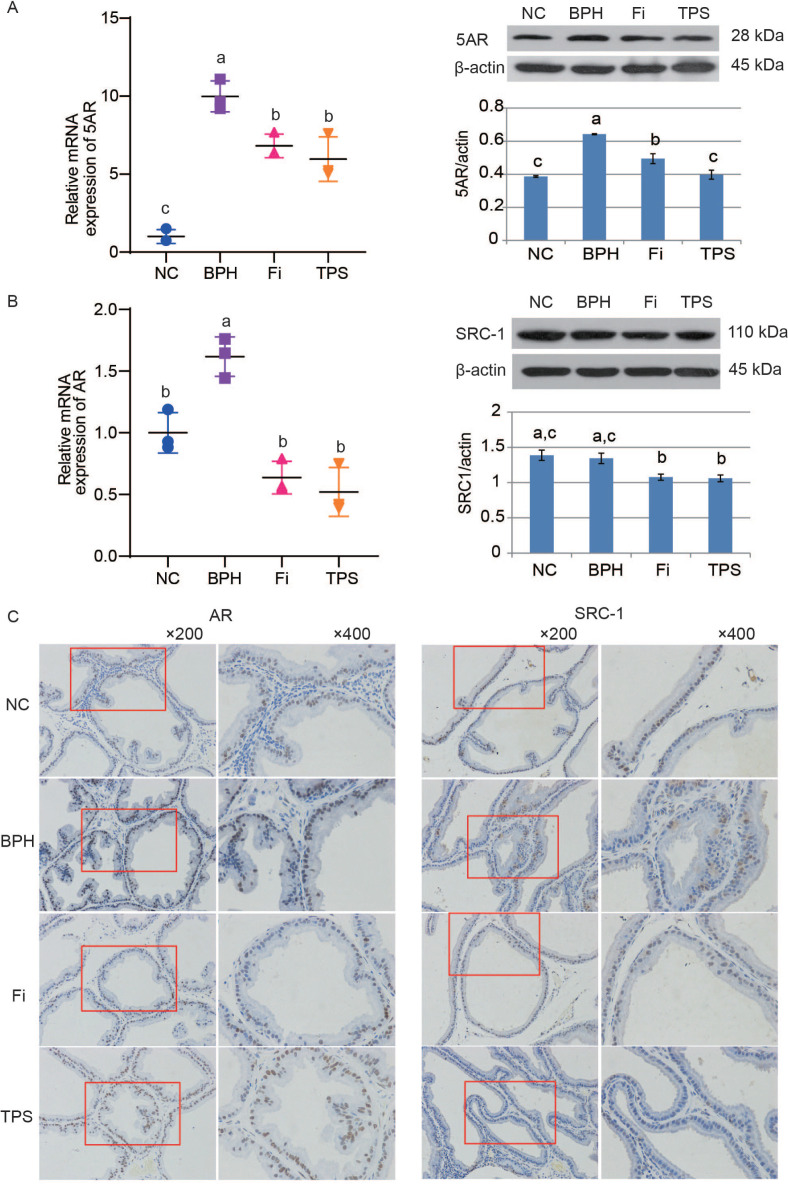
Effect of TPS on 5AR, AR and SRC-1 expression
Testosterone propionate enhanced the 5AR expression in both mRNA and protein levels in the BPH group (Fig. 3A). Treatment with TPS was demonstrated to reduce the testosterone propionate-induced 5AR expression alteration (Fig. 3A). RT-qPCR results showed that the mRNA expression of AR was upregulated in the BPH group by comparing with those of the NC group. TPS reduced the elevated AR mRNA expression in rats (Fig. 3B). The immunofluorescence staining of AR protein confirmed the RT-qPCR results (Fig. 3B and C). Although the protein expression of SRC-1, a typical type of AR adjuvant, showed no significant difference in the BPH group by western blotting, it showed a reduction by TPS treatment, both in the western blotting and IHC assay (Fig. 3B and C).
Fig. 3.
The effect of TPS on 5AR, AR, and its coactivator SRC-1 expression of the prostate tissues in testosterone propionate-induced BPH rats. (A) Relative mRNA expression and protein expression of 5AR in prostate tissues; (B) relative mRNA expression of AR and protein expression of SRC-1; (C) representative photomicrograph of IHC-stained prostate tissues with anti-AR and anti-SRC-1 antibodies (left panel magnification ×200 and right panel magnification ×400). Data are mean ± SD from three independent experiments. Different lowercase letters (a–c) show a significant difference between groups (P < 0.05).
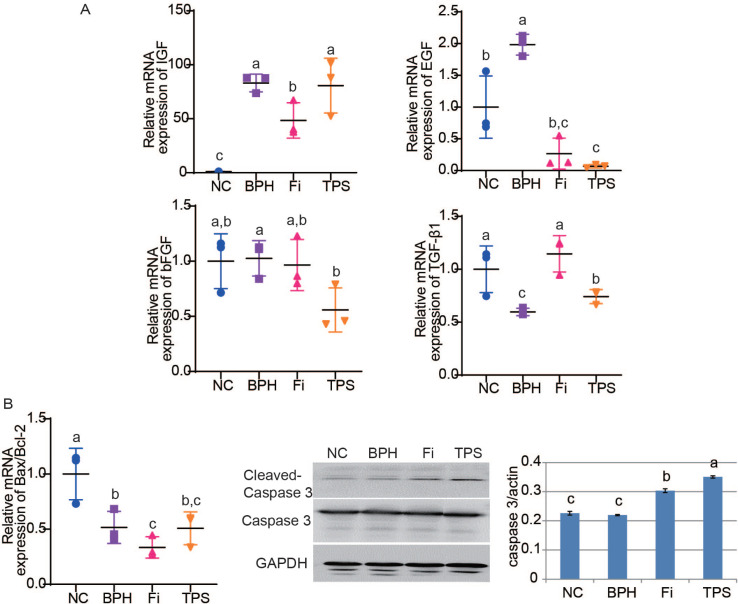
Effect of TPS on cell proliferation and apoptosis
After treated with testosterone propionate, the mRNA relative expressions of growth factors insulin-like growth factor (IGF) and epidermal growth factor (EGF) were markedly increased (Fig. 4A). With the administration of TPS, the mRNA relative expressions of EGF significantly decreased, whereas those of IGF showed no significant difference (Fig. 4A). The basic fibroblast growth factor (bFGF) expression showed no significant difference in the BPH group, while it was decreased in the TPS group (Fig. 4A). For the proapoptotic gene transforming growth factor-β1 (TGF-β1), it was downregulated in the BPH group and upregulated after treating with TPS (Fig. 4A). The expression of the apoptosis-effector, cleaved-Caspase 3, was elevated after treated with TPS in the western blotting; however, the Bax/Bcl-2 mRNA expression showed no significant difference (Fig. 4B).
Fig. 4.
The effect of TPS on cell proliferation and apoptosis in testosterone propionate-induced BPH rats. (A) Relative mRNA expression of proliferation-related growth factors IGF, EGF, bFGF, and TGF-β1; (B) relative Bax/Bcl-2 mRNA expression and Cleaved-Caspase 3 protein expression. Data are means ± SD from three independent experiments. Different lowercase letters (a–c) show a significant difference between groups (P < 0.05).
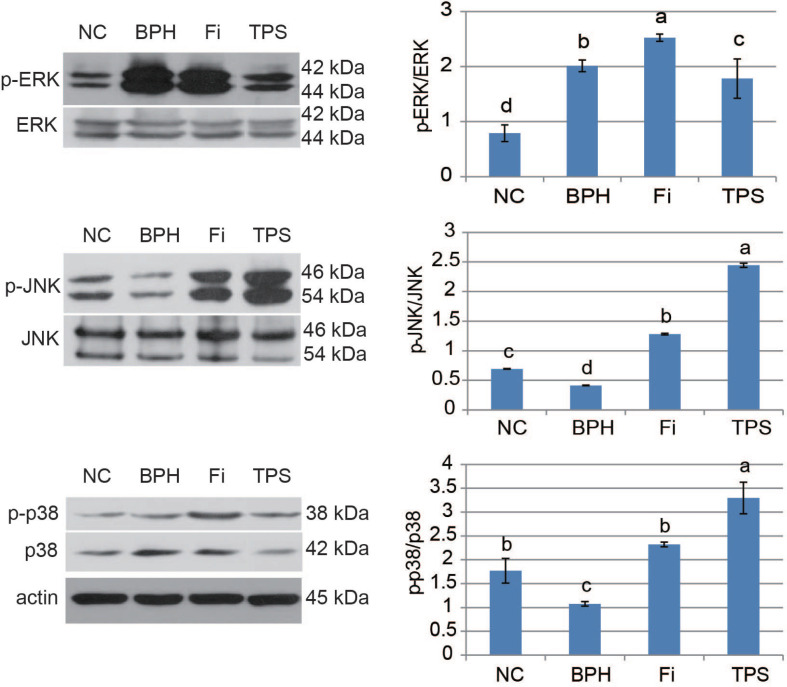
Effect of TPS on MAPK signal expression
Induced by testosterone propionate, the expression levels of p-ERK were increased obviously (Fig. 5). After the management of TPS, the expression level of p-ERK in the TPS group was significantly reduced (Fig. 5). For p-JNK and p-p38, it showed reduction in the BPH group and increase in the Fi and TPS groups (Fig. 5).
Fig. 5.
The effect of TPS on MAPK signal expressions in prostate tissues of testosterone propionate-induced BPH rat. Phosphorylation levels of ERK, JNK, and p38 were analyzed by western blotting. Data are means ± SD from three independent experiments. Different lowercase letters (a–d) show a significant difference between groups (P < 0.05).
Discussion
In this study, TPS, composed of ∆7-phytosterols and ∆5-phytosterols, was isolated from hull-less pumpkin seed oil. It showed admirably inhibited effects on BPH. Beta-sitosterol, one of ∆5-phytosterols, has been reported to improve the symptoms and urinary flow parameters of BPH (22). Beta-sitosterol reduced the conversion of testosterone to DHT and inhibited proliferation of human prostate cancer cells (23, 24). In contrast, Tsai et al. fed rats with pumpkin seed oil combined with ∆5-phytosterol and found that ∆5-phytosterol (β-sitosterol, campesterol, stigmasterol, and brassicasterol) produced a limited additive effect of pumpkin seed oil on the BPH (18). In this study, β-sitosterol only accounts for 8.17% of TPS, and ∆7-phytosterols reached up to 87.64%. Thus, we supposed that ∆7-phytosterols might be the active ingredients for BPH treatment.
DHT overproduction could lead to the development and exacerbation of BPH. Numerous researchers have aimed to reduce DHT levels by inhibiting 5AR (25). The 5AR is responsible for the synthesis of DHT, which has substantially greater affinity for AR than testosterone does. DHT could induce the overexpression of AR, which promotes BPH development via enhancing the recruitment of infiltrating macrophages that resulted in increased stromal cell proliferation (26). DHT binds to an AR and SRC1 and forms a complex, which shows high affinity to specific androgen response elements. It causes the production of prostate-specific antigen and regulatory proteins that modulate cell proliferation and death (5). As reported, the positive agent Fi showed a significant inhibition of 5AR, AR, and coactivator SRC-1 expression, resulting in reduction of prostate size and improvement of LUTS (27, 28). In this study, TPS showed similar therapeutic potential against BPH as Fi did.
An imbalance between cell proliferation and cell death leads to the abnormal growth of prostate cells, the major pathological feature of BPH (29, 30). Three MAPK cascades were implicated in these pathological alterations by modulating the local prostatic environment to favor the progression of BPH. It is known that MAPK family members participate in regulating the cell cycle in various ways (31). ERK mainly is involved in regulating the progression of G0/G1 to the S phase, and p38 primarily regulates the G2 checkpoint (32, 33). ERK pathway is reported to be essential for the proliferation of human prostate (28, 34). Its activation might result in mitogenesis and induces motility (35). JNK-dependent apoptosis was suppressed by the activation of ERK MAPK (36). JNK not only induce the Bax phosphorylation and mitochondrial translocation to promote apoptosis but also phosphorylate and inactivate Bcl-2 to suppress its antiapoptotic function. JNK and p38 always work in tune, and the activation and role of p38 seem to be similar to the JNK pathway (7). In this study, there is a dominance of ERK cascade and suppression of JNK and p38 cascades in BPH, which is agreed with the notion of Athanasios et al. (6). TPS could inhibit the ERK cascade and activate the JNK and p38 cascades, leading to the repression of proliferation and activation of apoptosis. However, in the apoptosis-promoting process, TPS could not increase the expression of downregulated Bax/Bcl-2. The down-effector of JNK and p38 after TPS administration needs further study.
Growth factors EGF, bFGF, IGF, and TGF-β are activators of MAPK signaling (37–39). The production and secretion of EGF and IGF may be stimulated by DHT (5). In prostate, the activation of EGF receptor and IGF receptor leads to ERK/MAPK activation (35). In benign and malignant prostate, the overexpression of EGF, bFGF, and IGF protein has been observed (37, 40, 41). EGF is a mitogen for prostatic epithelial cells and has been proven to promote the growth of prostate (42). Additionally, EGF can greatly enhance the expression of VEGF in BPH (43). The strong EGF-induced responses of BPH could contribute to the transformation/malignant potential (44). The activity of TGF-β may be affected by DHT (5). TGF-β has an inhibitory role in BPH, as well as in the normal prostate, inhibiting proliferation and inducing apoptosis in epithelial cells (45, 46). Although the activation mechanisms and its biological consequences of TGF-β poorly characterized, it is known that it can activate the ERK, JNK, and p38 MAPK kinase pathways (47). In this study, TPS inhibited BPH development by decreased the mRNA expression of EGF and increased the expression of TGF-β.
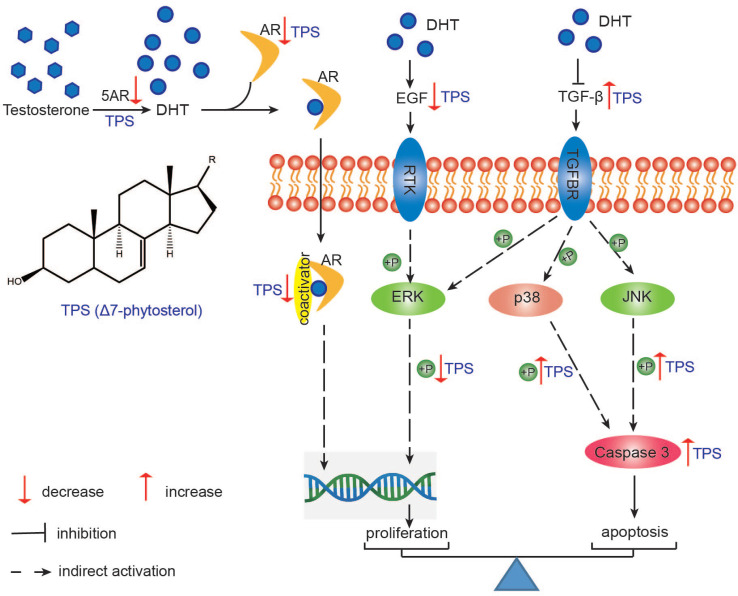
In conclusion, TPS from hull-less pumpkin seed oil, with ∆7-phytosterols as the main ingredients, exhibited anti-BPH effect via lowering the 5AR expression, regulating the balance between proliferation and apoptosis. The proliferation-inhibited action was conducted by lowering the AR and SRC-1 expressions and decreasing the phosphorylation of ERK. The apoptosis was promoted by Caspase 3 activation through JNK and p38 phosphorylation (Fig. 6). This study indicated that phytosterols of hull-less pumpkin seed oil could be a potential nutraceutical for the prevention and treatment of BPH.
Fig. 6.
The proposed mechanism of TPS ameliorating BPH in testosterone propionate-induced BPH rat. This graph is a summary of the mechanism speculated from this study. TPS: total phytosterols; 5AR: 5α-reductase; AR: androgen receptor; TGFBR: TGF-β receptor; RTK: receptor tyrosine kinase.
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (81773850), Natural Science Foundation of Hunan Province (2020JJ5266), the Youth Science Fund Project of Hunan Agriculture University (19QN26), Science and Technology Major Project of Hunan Province (2017SK1020), and Changsha Science and Technology Project (kh1801123).
Conflict of interest and funding
The authors declare no potential conflicts of interest. The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- 1.Egan KB. The epidemiology of benign prostatic hyperplasia associated with lower urinary tract symptoms: prevalence and incident rates. Urol Clin North Am 2016; 43(3): 289–97. doi: 10.1016/j.ucl.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 2.Marberger M. Drug insight: 5alpha-reductase inhibitors for the treatment of benign prostatic hyperplasia. Nat Clin Pract Urol 2006; 3(9): 495–503. doi: 10.1038/ncpuro0577 [DOI] [PubMed] [Google Scholar]
- 3.Foster CS. Pathology of benign prostatic hyperplasia. Prostate Suppl 2000; 9: 4–14. doi: [DOI] [PubMed] [Google Scholar]
- 4.Untergasser G, Madersbacher S, Berger P. Benign prostatic hyperplasia: age-related tissue-remodeling. Exp Gerontol 2005; 40(3): 121–8. doi: 10.1016/j.exger.2004.12.008 [DOI] [PubMed] [Google Scholar]
- 5.Carson C, 3rd, Rittmaster R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology 2003; 61(4 Suppl 1): 2–7. doi: 10.1016/s0090-4295(03)00045-1 [DOI] [PubMed] [Google Scholar]
- 6.Papatsoris AG, Papavassiliou AG. Molecular ‘palpation’ of BPH: a tale of MAPK signalling? Trends Mol Med 2001; 7(7): 288–92. doi: 10.1016/s1471-4914(01)02015-9 [DOI] [PubMed] [Google Scholar]
- 7.Yue J, Lopez JM. Understanding MAPK signaling pathways in apoptosis. Int J Mol Sci 2020; 21(7): 1–22. doi: 10.3390/ijms21072346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim EH, Larson JA, Andriole GL. Management of benign prostatic hyperplasia. Annu Rev Med 2016; 67: 137–51. doi: 10.1146/annurev-med-063014-123902 [DOI] [PubMed] [Google Scholar]
- 9.Allkanjari O, Vitalone A. What do we know about phytotherapy of benign prostatic hyperplasia? Life Sci 2015; 126: 42–56. doi: 10.1016/j.lfs.2015.01.023 [DOI] [PubMed] [Google Scholar]
- 10.Hong H, Kim CS, Maeng S. Effects of pumpkin seed oil and saw palmetto oil in Korean men with symptomatic benign prostatic hyperplasia. Nutr Res Pract 2009; 3(4): 323–7. doi: 10.4162/nrp.2009.3.4.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishimura M, Ohkawara T, Sato H, Takeda H, Nishihira J. Pumpkin seed oil extracted from Cucurbita maxima improves urinary disorder in human overactive bladder. J Tradit Complement Med 2014; 4(1): 72–4. doi: 10.4103/2225-4110.124355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morakul B, Teeranachaideekul V, Buraphacheep Junyaprasert V. Niosomal delivery of pumpkin seed oil: development, characterisation, and physical stability. J Microencapsul 2019; 36(2): 120–9. doi: 10.1080/02652048.2019.1607597 [DOI] [PubMed] [Google Scholar]
- 13.Stevenson DG, Eller FJ, Wang L, Jane JL, Wang T, Inglett GE. Oil and tocopherol content and composition of pumpkin seed oil in 12 cultivars. J Agric Food Chem 2007; 55(10): 4005–13. doi: 10.1021/jf0706979 [DOI] [PubMed] [Google Scholar]
- 14.Montesano D, Blasi F, Simonetti MS, Santini A, Cossignani L. Chemical and nutritional characterization of seed oil from Cucurbita maxima L. (var. Berrettina) pumpkin. Foods 2018; 7(3): 1–14. doi: 10.3390/foods7030030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klippel KF, Hiltl DM, Schipp B. A multicentric, placebo-controlled, double-blind clinical trial of beta-sitosterol (phytosterol) for the treatment of benign prostatic hyperplasia. German BPH-Phyto Study group. Br J Urol 1997; 80(3): 427–32. doi: 10.1046/j.1464-410x.1997.t01-1-00362 [DOI] [PubMed] [Google Scholar]
- 16.Wilt TJ, MacDonald R, Ishani A. beta-sitosterol for the treatment of benign prostatic hyperplasia: a systematic review. BJU Int 1999; 83(9): 976–83. doi: 10.1046/j.1464-410x.1999.00026.x [DOI] [PubMed] [Google Scholar]
- 17.Wang R, Kobayashi Y, Lin Y, Rauwald HW, Fang L, Qiao H, et al. A phytosterol enriched refined extract of Brassica campestris L. pollen significantly improves benign prostatic hyperplasia (BPH) in a rat model as compared to the classical TCM pollen preparation Qianlie Kang Pule’an Tablets. Phytomedicine 2015; 22(1): 145–52. doi: 10.1016/j.phymed.2014.10.001 [DOI] [PubMed] [Google Scholar]
- 18.Tsai YS, Tong YC, Cheng JT, Lee CH, Yang FS, Lee HY. Pumpkin seed oil and phytosterol-F can block testosterone/prazosin-induced prostate growth in rats. Urol Int 2006; 77(3): 269–74. doi: 10.1159/000094821 [DOI] [PubMed] [Google Scholar]
- 19.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008; 3(6): 1101–8. doi: 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- 20.Vipin K. Garg WRN. Occurrence of Δ5-sterols in plants producing predominantly Δ7-sterols: studies on the sterol compositions of six cucurbitaceae seeds. Phytochemistry 1986; 25(11): 2591–7. doi: 10.1016/s0031-9422(00)84516-1 [DOI] [Google Scholar]
- 21.Zhang X, Cambrai A, Miesch M, Roussi S, Raul F, Aoude-Werner D, et al. Separation of Delta5- and Delta7-phytosterols by adsorption chromatography and semipreparative reversed phase high-performance liquid chromatography for quantitative analysis of phytosterols in foods. J Agric Food Chem 2006; 54(4): 1196–202. doi: 10.1021/jf052761x [DOI] [PubMed] [Google Scholar]
- 22.Berges RR, Windeler J, Trampisch HJ, Senge T. Randomised, placebo-controlled, double-blind clinical trial of beta-sitosterol in patients with benign prostatic hyperplasia. Beta-sitosterol Study Group. Lancet 1995; 345(8964): 1529–32. doi: 10.1016/s0140-6736(95)91085-9 [DOI] [PubMed] [Google Scholar]
- 23.Awad AB, Fink CS, Williams H, Kim U. . In vitro and in vivo (SCID mice) effects of phytosterols on the growth and dissemination of human prostate cancer PC-3 cells. Eur J Cancer Prev 2001; 10(6): 507–13. doi: 10.1097/00008469-200112000-00005 [DOI] [PubMed] [Google Scholar]
- 24.Cabeza M, Bratoeff E, Heuze I, Ramirez E, Sanchez M, Flores E. Effect of beta-sitosterol as inhibitor of 5 alpha-reductase in hamster prostate. Proc West Pharmacol Soc 2003; 46: 153–5. PMID: . [PubMed] [Google Scholar]
- 25.Roehrborn CG. Pathology of benign prostatic hyperplasia. Int J Impot Res 2008; 20 Suppl 3: S11–8. doi: 10.1038/ijir.2008.55 [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Lin WJ, Izumi K, Jiang Q, Lai KP, Xu D, et al. Increased infiltrated macrophages in benign prostatic hyperplasia (BPH): role of stromal androgen receptor in macrophage-induced prostate stromal cell proliferation. J Biol Chem 2012; 287(22): 18376–85. doi: 10.1074/jbc.M112.355164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan SA, Roehrborn CG, McConnell JD, Meehan AG, Surynawanshi S, Lee JY, et al. Long-term treatment with finasteride results in a clinically significant reduction in total prostate volume compared to placebo over the full range of baseline prostate sizes in men enrolled in the MTOPS trial. J Urol 2008; 180(3): 1030–2; discussion 2–3. doi: 10.1016/j.juro.2008.05.004 [DOI] [PubMed] [Google Scholar]
- 28.Youn DH, Park J, Kim HL, Jung Y, Kang J, Lim S, et al. Corrigendum: berberine improves benign prostatic hyperplasia via suppression of 5 alpha reductase and extracellular signal-regulated kinase in vivo and in vitro. Front Pharmacol 2019; 10: 541. doi: 10.3389/fphar.2019.00541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minutoli L, Rinaldi M, Marini H, Irrera N, Crea G, Lorenzini C, et al. Apoptotic pathways linked to endocrine system as potential therapeutic targets for benign prostatic hyperplasia. Int J Mol Sci 2016; 17(8): 1–15. doi: 10.3390/ijms17081311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kyprianou N, Tu H, Jacobs SC. Apoptotic versus proliferative activities in human benign prostatic hyperplasia. Hum Pathol 1996; 27(7): 668–75. doi: 10.1016/s0046-8177(96)90396-2 [DOI] [PubMed] [Google Scholar]
- 31.Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene 2007; 26(22): 3227–39. doi: 10.1038/sj.onc.1210414 [DOI] [PubMed] [Google Scholar]
- 32.MacCorkle RA, Tan TH. Mitogen-activated protein kinases in cell-cycle control. Cell Biochem Biophys 2005; 43(3): 451–61. doi: 10.1385/CBB:43:3:451 [DOI] [PubMed] [Google Scholar]
- 33.Tsai SC, Huang WW, Huang WC, Lu CC, Chiang JH, Peng SF, et al. ERK-modulated intrinsic signaling and G(2)/M phase arrest contribute to the induction of apoptotic death by allyl isothiocyanate in MDA-MB-468 human breast adenocarcinoma cells. Int J Oncol 2012; 41(6): 2065–72. doi: 10.3892/ijo.2012.1640 [DOI] [PubMed] [Google Scholar]
- 34.Youn DH, Park J, Kim HL, Jung Y, Kang J, Jeong MY, et al. Chrysophanic acid reduces testosterone-induced benign prostatic hyperplasia in rats by suppressing 5alpha-reductase and extracellular signal-regulated kinase. Oncotarget 2017; 8(6): 9500–12. doi: 10.18632/oncotarget.13430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wells A, Gupta K, Chang P, Swindle S, Glading A, Shiraha H. Epidermal growth factor receptor-mediated motility in fibroblasts. Microsc Res Tech 1998; 43(5): 395–411. doi: [DOI] [PubMed] [Google Scholar]
- 36.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell 2000; 103(2): 239–52. doi: 10.1016/s0092-8674(00)00116-1 [DOI] [PubMed] [Google Scholar]
- 37.Ropiquet F, Giri D, Lamb DJ, Ittmann M. FGF7 and FGF2 are increased in benign prostatic hyperplasia and are associated with increased proliferation. J Urol 1999; 162(2): 595–9. doi: 10.1016/S0022-5347(05)68632-6 [DOI] [PubMed] [Google Scholar]
- 38.Lucia MS, Lambert JR. Growth factors in benign prostatic hyperplasia: basic science implications. Curr Urol Rep 2008; 9(4): 272–8. doi: 10.1007/s11934-008-0048-6 [DOI] [PubMed] [Google Scholar]
- 39.Poncet N, Guillaume J, Mouchiroud G. Epidermal growth factor receptor transactivation is implicated in IL-6-induced proliferation and ERK1/2 activation in non-transformed prostate epithelial cells. Cell Signal 2011; 23(3): 572–8. doi: 10.1016/j.cellsig.2010.11.009 [DOI] [PubMed] [Google Scholar]
- 40.Sreenivasulu K, Nandeesha H, Dorairajan LN, Rajappa M, Vinayagam V, Cherupanakkal C. Gene expression of insulin receptor, insulin-like growth factor increases and insulin-like growth factor-binding protein-3 reduces with increase in prostate size in benign prostatic hyperplasia. Aging Male 2018; 21(2): 138–44. doi: 10.1080/13685538.2017.1401994 [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, Chisholm GD, Habib FK. Epidermal growth factor and transforming growth factor alpha concentrations in BPH and cancer of the prostate: their relationships with tissue androgen levels. Br J Cancer 1993; 67(1): 152–5. doi: 10.1038/bjc.1993.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hennenberg M, Schreiber A, Ciotkowska A, Rutz B, Waidelich R, Strittmatter F, et al. Cooperative effects of EGF, FGF, and TGF-beta1 in prostate stromal cells are different from responses to single growth factors. Life Sci 2015; 123: 18–24. doi: 10.1016/j.lfs.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 43.Wei H, Wu G, Shi D, Song S, Zhang X, Lei Y, et al. Total flavan glycoside from Abacopteris penangiana rhizomes and its acid hydrolysate: characterisation and anti-benign prostatic hyperplasia potential. Food Chem 2012; 134(4): 1959–66. doi: 10.1016/j.foodchem.2012.03.128 [DOI] [PubMed] [Google Scholar]
- 44.Hayward SW, Wang Y, Cao M, Hom YK, Zhang B, Grossfeld GD, et al. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res 2001; 61(22): 8135–42. PMID: . [PubMed] [Google Scholar]
- 45.Ilio KY, Sensibar JA, Lee C. Effect of TGF-beta 1, TGF-alpha, and EGF on cell proliferation and cell death in rat ventral prostatic epithelial cells in culture. J Androl 1995; 16(6): 482–90. doi: 10.1002/j.1939-4640.1995.tb00568.x [DOI] [PubMed] [Google Scholar]
- 46.Martikainen P, Kyprianou N, Isaacs JT. Effect of transforming growth factor-beta 1 on proliferation and death of rat prostatic cells. Endocrinology 1990; 127(6): 2963–8. doi: 10.1210/endo-127-6-2963 [DOI] [PubMed] [Google Scholar]
- 47.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003; 425(6958): 577–84. doi: 10.1038/nature02006 [DOI] [PubMed] [Google Scholar]