Abstract
Background
Systemic atopic dermatitis treatments that have acceptable safety are needed.
Objective
To evaluate the safety of the oral Janus kinase inhibitor upadacitinib in combination with topical corticosteroids (TCSs) for the treatment of atopic dermatitis.
Methods
In this phase 3, double-blind study (Rising Up), Japanese patients (12-75 years) with moderate-to-severe atopic dermatitis were randomized in a 1:1:1 ratio to receive 15 mg of upadacitinib + TCS, 30 mg of upadacitinib + TCS, or a placebo + TCS (rerandomized in a 1:1 ratio to receive either 15 or 30 mg of upadacitinib + TCS at week 16). Adverse events and laboratory data were assessed for safety.
Results
In 272 treated patients, the serious adverse event rates were similar for 15- and 30-mg upadacitinib + TCS at week 24 (15 mg, 56%; 30 mg, 64%) but greater than those for placebo + TCS (42%). Acne (all mild or moderate; none leading to discontinuation) occurred more frequently with upadacitinib + TCS (15 mg, 13.2%; 30 mg, 19.8%) than with placebo + TCS (5.6%). Furthermore, herpes zoster infection (4.4% vs 0%), anemia (1.1% vs 0%), neutropenia (4.4% vs 1.1%), and creatine phosphokinase elevations (2.2% vs 1.1%) occurred more frequently with 30-mg upadacitinib + TCS than with 15-mg upadacitinib + TCS; none of these events were reported with placebo + TCS. No thromboembolic events, malignancies, gastrointestinal perforations, active tuberculosis, or deaths occurred.
Limitations
The limitations included a small sample size and short observation period as well as nongeneralizability of the results beyond Japanese populations.
Conclusions
The results were generally consistent with those of previous reports; no new safety risks were detected.
Key words: atopic dermatitis, clinical trial, eczema, Janus kinase inhibitors, safety, topical corticosteroids, upadacitinib
Abbreviations used: AD, atopic dermatitis; AE, adverse event; AESI, adverse event of special interest; CPK, creatine phosphokinase; EASI, Eczema Area and Severity Index; EASI 50, ≥50% improvement in eczema area and severity index; EASI 75, ≥75% improvement in Eczema Area and Severity Index; EASI 90, ≥90% improvement in Eczema Area and Severity Index; JAK, Janus kinase; SAE, serious adverse event; TCS, topical corticosteroid; TEAE, treatment-emergent adverse event; vIGA-AD, validated Investigator's Global Assessment for Atopic Dermatitis
Capsule Summary.
-
•
The Rising Up study's results are consistent with those of prior global phase 2b and phase 3 trial reports and upadacitinib's labeled safety profile, contributing to the totality of upadacitinib safety data for the treatment of atopic dermatitis.
-
•
Upadacitinib, in combination with topical corticosteroids, has an acceptable safety profile for treating patients with moderate-to-severe atopic dermatitis.
Introduction
Atopic dermatitis (AD) is an inflammatory skin condition characterized by intense pruritus that affects up to 20% of children and up to 16% of adults.1,2 AD is associated with excessive health costs and financial burden and can adversely affect the quality of life as well as social, academic, and occupational pursuits.3,4 Patients with moderate-to-severe AD in whom first-line topical treatments are insufficient may benefit from the addition of systemic agents.1
Upadacitinib—an oral Janus kinase (JAK) inhibitor with a greater inhibitory potency for JAK1 than for JAK2, JAK3, or tyrosine kinase 2,5,6 currently under development to treat AD and other inflammatory conditions—is safe and effective for patients with rheumatoid arthritis7, 8, 9, 10, 11, 12, 13, 14 and has been approved for the treatment of rheumatoid arthritis in several countries. Here, we analyzed the safety of upadacitinib in a prespecified 24-week interim analysis of a phase 3 trial of upadacitinib combined with topical corticosteroids (TCSs) in Japanese adolescents and adults with moderate-to-severe AD in whom topical treatment was inadequate.
Methods
Study design and participants
The Rising Up study is an ongoing phase 3, randomized, double-blind, multicenter study evaluating the safety of upadacitinib combined with TCS in adolescents and adults and is being conducted at clinical centers in Japan (Supplementary Fig 1, available via Mendeley at https://data.mendeley.com/datasets/h2krvxjtzv/1.)
There were no changes in the study design or study endpoints after the trial commenced. This report is based on the results of a prespecified 24-week interim safety analysis.
The eligible patients were adolescents (aged 12-17 years; weight, ≥40 kg) or adults (aged 18-75 years) who had moderate-to-severe AD for 3 years or more who met the Hanifin and Rajka criteria and had a documented history of an inadequate response to topical AD treatments or systemic AD treatment within 6 months. Moderate-to-severe AD was defined as an Eczema Area and Severity Index (EASI) score of ≥16, a validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) score of ≥3, an AD involvement of ≥10% of body surface area, and a weekly average of daily Worst Pruritus Numerical Rating Scale (NRS) score of ≥4.
Patients with AD who had a lesional surface involvement of ≥30% and could not be safely treated with a medium- or higher-potency TCS were excluded, as were those with any prior exposure to JAK inhibitors or dupilumab. Patients were required to discontinue topical AD treatments other than moisturizers, systemic immunomodulating therapy for AD, phototherapy or related forms of light therapy, oral or parenteral traditional Chinese medicines, and biologic treatments for defined washout periods prior to the first dose of the study drug. Patients with skin conditions that could have interfered with AD assessments, an active systemic infection, or a history of disseminated herpes zoster or herpes simplex infection were excluded.
This study was conducted in accordance with the good clinical practice guideline, defined by the International Conference on Harmonisation and the Declaration of Helsinki. The study protocol was approved by institutional review boards (protocol M17-377 was reviewed and approved by the Nagasaki University Hospital Institutional Review Board on September 20, 2018, and the Fukuyama City Hospital Institutional Review Board on September 28, 2018), and all adult patients and parents or legal guardians of adolescent patients provided informed consent before the commencement of the study. This study is registered with ClinicalTrials.gov, number NCT03661138 (September 7, 2018; the first patient was enrolled on November 17, 2018).
Study treatments
Patients meeting the eligibility criteria were randomized in a 1:1:1 ratio to receive 15 mg of upadacitinib, 30 mg of upadacitinib, or a matching placebo (all administered once daily), in combination with a TCS. Randomization was stratified by age (<18, 18-40, >40 years) and baseline disease severity (moderate [vIGA-AD = 3] and severe [vIGA-AD = 4]). At the end of the double-blind period (week 16), placebo-treated patients were rerandomized in a 1:1 ratio to receive either 15 or 30 mg of upadacitinib (once daily), whereas patients originally assigned to active treatment continued blinded treatment as originally assigned. Rerandomization was stratified by EASI-50 response (≥50% improvement in EASI) and age (<18, 18-40, >40 years). Interactive response technology and a randomization schedule generated by the Statistics Department at AbbVie were used for randomization.
During the double-blind period, the patients were required to apply a medium-potency TCS once daily to areas with active lesions for ≤3 consecutive weeks. Once the lesions were “clear” or “almost clear” or after 3 consecutive weeks of the application of the medium-potency TCS, a low-potency TCS was to be applied once daily for 7 days. If the lesions returned or persisted, this step-down approach was to be repeated as long as there was no sign of local or systemic TCS toxicity. During the blinded extension period, topical medication for AD was allowed as per investigator discretion.
At minimum, patients were to apply moisturizers twice daily for ≥7 days before baseline through week 16. During all study periods, the use of topical calcineurin inhibitors was permitted in areas where TCS use was contraindicated. From week 4 through week 24, rescue treatment for AD was provided to patients without an EASI-50 response at 2 consecutive scheduled visits.
Safety assessment
Safety evaluations were performed at each study visit from the time of screening through week 24. At each study visit, adverse events (AEs), including treatment-emergent adverse events (TEAEs), serious adverse events (SAEs), AEs leading to discontinuation, and AEs of special interest (AESI) were recorded. The AESIs (specific AEs that warrant ongoing monitoring with this class of drugs) included serious or opportunistic infections, herpes zoster infection, active tuberculosis, malignancy, gastrointestinal perforations, adjudicated cardiovascular events (including major adverse cardiovascular events), anemia, neutropenia, lymphopenia, renal dysfunction, hepatic disorders, elevations in creatine phosphokinase (CPK) levels, and adjudicated venous thromboembolic events. Clinical laboratory parameters were also assessed.
Exploratory efficacy endpoints
The exploratory efficacy endpoints included proportions of patients achieving EASI 50, EASI 75, and/or EASI 90; a mean and percent change in EASI from the baseline; the proportion of patients achieving vIGA-AD of clear or almost-clear type with ≥2 grades of reduction; the proportion of patients achieving ≥4 points of reduction (improvement) in Worst Pruritus NRS scores; and a mean and percent change in Worst Pruritus NRS scores.
Statistical analysis
A sample size of 264 patients (88 patients per treatment group) was chosen to meet Japanese regulatory requirements for safety exposure.
The results were descriptively reported for all patients who received the study treatment. Hypothesis testing was not performed. Missing data for categorical exploratory efficacy endpoints were imputed using nonresponder imputation. Continuous variables were analyzed using the mixed-effect model repeated-measure method.
Results
Patient population
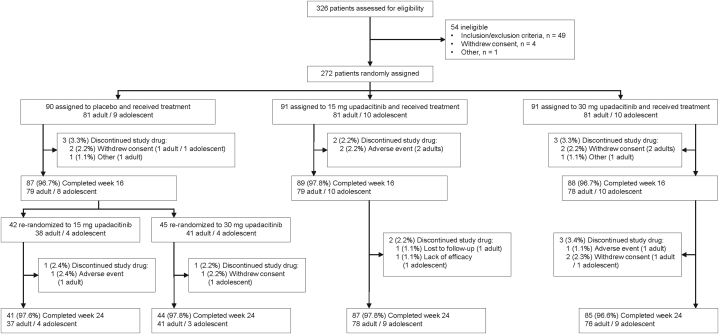
Of 326 patients screened for eligibility, 272 (243 adults and 29 adolescents) were randomized and treated beginning November 17, 2018 (Fig 1).
Fig 1.
Patient disposition.
The baseline characteristics were generally well balanced among the treatment groups (Table I).
Table I.
Baseline demographics and characteristics
| Characteristic | Placebo (n = 90) | Upadacitinib 15 mg (n = 91) | Upadacitinib 30 mg (n = 91) |
|---|---|---|---|
| Female, n (%) | 16 (17.8) | 23 (25.3) | 22 (24.2) |
| Age (y), mean (SD) | 36.3 (12.6) | 35.9 (13.2) | 34.7 (12.7) |
| Age group (y), n (%) | |||
| Adolescents (<18) | 9 (10.0) | 10 (11.0) | 10 (11.0) |
| Adults (≥18) | 81 (90.0) | 81 (89.0) | 81 (89.0) |
| Weight (kg), mean (SD) | 67.6 (12.8) | 65.1 (14.2) | 66.2 (14.4) |
| BSA (%), mean (SD) | 62.0 (20.5) | 61.7 (23.7) | 66.7 (21.2) |
| Disease duration since diagnosis (y), mean (SD) | 24.7 (14.4) | 23.0 (14.3) | 20.7 (14.1) |
| hs-CRP (mg/L), mean (SD) | 3.1 (6.4) | 2.3 (3.8) | 3.9 (8.8) |
| vIGA-AD, n (%) | |||
| Moderate (score < 4) | 47 (52.2) | 47 (51.6) | 48 (52.7) |
| Severe (score = 4) | 43 (47.8) | 44 (48.4) | 43 (47.3) |
| EASI, mean (SD) | 34.4 (13.0) | 34.2 (14.1) | 36.1 (14.5) |
| Worst Pruritus NRS, mean (SD) | 6.8 (1.3) | 6.7 (1.4) | 7.0 (1.4) |
| Medical history, n (%) | |||
| Asthma | 34 (37.8) | 29 (31.9) | 28 (30.8) |
| Rhinitis allergic | 58 (64.4) | 45 (49.5) | 45 (19.5) |
| Conjunctivitis allergic | 11 (12.2) | 8 (8.8) | 12 (13.2) |
BSA, Body surface area; EASI, Eczema Area and Severity Index; hs-CRP, high-sensitivity C-reactive protein; NRS, numerical rating scale; vIGA-AD, validated Investigator's Global Assessment for Atopic Dermatitis.
Safety outcomes
During the double-blind period, TEAEs were reported for 56% and 64% of patients who received 15 and 30 mg of upadacitinib, respectively, compared with 42% of patients who received the placebo (Table II).
Table II.
Adverse event interim analysis summary
| Treatment-emergent AE | Double-blind period |
During the administration of upadacitinib∗ |
|||
|---|---|---|---|---|---|
| Patients, n (%) |
Event (E/100 PYs) |
||||
| Placebo (n = 90) | Upadacitinib 15 mg (n = 91) | Upadacitinib 30 mg (n = 91) | Upadacitinib 15 mg (n = 133) PYs = 82.8 | Upadacitinib 30 mg (n = 136) PYs = 81.5 | |
| Any TEAE | 38 (42.2) | 51 (56.0) | 58 (63.7) | 208 (251.3) | 245 (300.7) |
| Adults | 32 | 42 | 50 | 181 | 212 |
| Adolescents | 6 | 9 | 8 | 27 | 33 |
| Serious AE† | 1 (1.1) | 1 (1.1) | 1 (1.1) | 4 (4.8) | 3 (3.7) |
| Adults | 1 | 1 | 1 | 4 | 3 |
| Adolescents | 0 | 0 | 0 | 0 | 0 |
| AE leading to discontinuation of the study drug‡ | 1 (1.1) | 2 (2.2) | 1 (1.1) | 4 (4.8) | 1 (1.2) |
| Adults | 1 | 2 | 1 | 3 | 1 |
| Adolescents | 0 | 0 | 0 | 1 | 0 |
| Deaths | 0 | 0 | 0 | 0 | 0 |
| TEAE reported by ≥5% of patients in any group | |||||
| Acne | 5 (5.6) | 12 (13.2) | 18 (19.8) | 24 (29.0) | 39 (47.9) |
| Adults | 4 | 9 | 15 | 20 | 34 |
| Adolescents | 1 | 3 | 3 | 4 | 5 |
| Arthralgia | 0 | 0 | 5 (5.5) | 0 | 8 (9.8) |
| Adults | 0 | 0 | 5 | 0 | 8 |
| Adolescents | 0 | 0 | 0 | 0 | 0 |
| Herpes zoster | 0 | 0 | 4 (4.4) | 5 (6.0) | 11 (13.5) |
| Adults | 0 | 0 | 4 | 5 | 11 |
| Adolescents | 0 | 0 | 0 | 0 | 0 |
| Nasopharyngitis | 14 (15.6) | 12 (13.2) | 14 (15.4) | 30 (36.2) | 38 (46.6) |
| Adults | 11 | 11 | 13 | 27 | 32 |
| Adolescents | 3 | 1 | 1 | 3 | 6 |
Adults were aged ≥18 years, and adolescents were aged <18 years. The number of adults and adolescents was 81 and 9, respectively, for the placebo and 81 and 10 for 15 and 30 mg of upadacitinib each; the patient-years were 72.8 and 10.0, respectively, for 15 mg of upadacitinib and 72.8 and 8.6, respectively, for 30 mg of upadacitinib.
AE, Adverse event; E, event; MACE, major adverse cardiovascular events; PYs, patient-years; TEAE, treatment-emergent adverse event.
Included data up to the cutoff date for all patients with ≥1 dose of upadacitinib. The mean (SD) duration of exposure to study drug from the first dose to the analysis cutoff date in the 15-mg upadacitinib group and 30-mg upadacitinib group was 227.3 (82.8) and 218.8 (84.1) days, respectively.
The serious AE in the double-blind period was cholelithiasis (n = 1) for placebo, cerebellar hemorrhage (n = 1) for 15 mg of upadacitinib, and herpes simplex infection (n = 1) for 30 mg of upadacitinib; the serious AEs during the administration of upadacitinib were cellulitis (n = 1), herpes zoster infection (n = 1), Pneumocystis jirovecii pneumonia (n = 1), and cerebellar hemorrhage (n = 1) for 15 mg of upadacitinib and herpes simplex (n = 1) and herpes zoster infections (n = 2) for 30 mg of upadacitinib.
The AEs leading to the discontinuation of the study drug during the double-blind period were dermatitis atopic (n = 1) for placebo, Kaposi's varicelliform eruption (n = 1) and cerebellar hemorrhage (n = 1) for 15 mg of upadacitinib, and peripheral edema (n = 1) for 30 mg of upadacitinib; the AEs leading to the discontinuation of the study drug during the administration of upadacitinib were Kaposi varicelliform eruption (n = 1), P jirovecii pneumonia (n = 1), cerebellar hemorrhage (n = 1), and atopic dermatitis for 15 mg of upadacitinib and peripheral edema (n = 1) for 30 mg of upadacitinib.
The proportions of patients with SAEs and AEs leading to study drug discontinuation during the double-blind period were similar across the treatment groups, with no reports of these events in the adolescent groups (Table II). In the double-blind period, 1 SAE was reported in each treatment group: cerebellar hemorrhage (adjudicated as a major adverse cardiovascular event of hemorrhagic stroke) in the 15-mg upadacitinib group, herpes simplex infection in the 30-mg upadacitinib group, and cholelithiasis in the placebo group. Across the study periods up to the cutoff date, a slightly higher rate of TEAEs was observed for the 30-mg upadacitinib group than for the 15-mg upadacitinib group; however, the rates of SAEs and AEs leading to discontinuation were higher in the 15-mg upadacitinib group than in the 30-mg upadacitinib group (Table II). The most frequently reported TEAEs in any treatment group were acne, nasopharyngitis, and herpes zoster infection (Table II). The incidence of acne increased in a dose-dependent manner; all cases were mild or moderate in intensity, consisting primarily of papules and pustules on the face, and were more frequently reported in adolescents than in adults. No patients discontinued the study drug because of acne. Although 33.5% and 11.4% of the patients had a history of asthma and/or allergic conjunctivitis, respectively, at the baseline, TEAEs related to asthma and allergic conjunctivitis were infrequent (<2.5%) across the study periods up to the cutoff date.
During the double-blind period, AESIs were infrequently reported in the upadacitinib groups (most were reported for 0 or 1 patient), and no AESIs were reported in the placebo group (Table III).
Table III.
Adverse event of special interest interim analysis summary
| Event | Double-blind period |
During the administration of upadacitinib∗ |
|||
|---|---|---|---|---|---|
| Patients, n (%) |
Events (E/100 PYs) |
||||
| Placebo (n = 90) | Upadacitinib 15 mg (n = 91) | Upadacitinib 30 mg (n = 91) | Upadacitinib 15 mg (n = 133) PYs = 82.8 | Upadacitinib 30 mg (n = 136) PYs = 81.5 | |
| Adjudicated VTE | 0 | 0 | 0 | 0 | 0 |
| Adjudicated MACE | 0 | 1 (1.1) | 0 | 1 (1.2) | 0 |
| Adults | 0 | 1 | 0 | 1 | 0 |
| Adolescents | 0 | 0 | 0 | 0 | 0 |
| Serious infections | 0 | 0 | 1 (1.1) | 3 (3.6) | 3 (3.7) |
| Adults | 0 | 0 | 1 | 3 | 3 |
| Adolescents | 0 | 0 | 0 | 0 | 0 |
| Opportunistic infections (excluding tuberculosis and herpes zoster) | 0 | 3 (3.3) | 1 (1.1) | 5 (6.0) | 2 (2.5) |
| Kaposi's varicelliform eruption (eczema herpeticum) | 0 | 3 (3.3) | 1 (1.1) | 4 (4.8) | 2 (2.5) |
| Adults | 0 | 2 | 1 | 3 | 2 |
| Adolescents | 0 | 1 | 0 | 1 | 0 |
| Pneumocystis jirovecii pneumonia | 0 | 0 | 0 | 1 (1.2) | 0 |
| Adults | 0 | 0 | 0 | 1 | 0 |
| Adolescents | 0 | 0 | 0 | 0 | 0 |
| Malignancy | 0 | 0 | 0 | 0 | 0 |
| Lymphoma | 0 | 0 | 0 | 0 | 1 (1.2)† |
| Adults | 0 | 0 | 0 | 0 | 0 |
| Adolescents | 0 | 0 | 0 | 0 | 1 |
| Hepatic disorder | 0 | 1 (1.1) | 1 (1.1) | 7 (8.5) | 6 (7.4) |
| Adults | 0 | 1 | 1 | 7 | 6 |
| Adolescents | 0 | 0 | 0 | 0 | 0 |
| Gastrointestinal perforations | 0 | 0 | 0 | 0 | 0 |
| Anemia | 0 | 0 | 1 (1.1) | 2 (2.4) | 5 (6.1) |
| Adults | 0 | 0 | 1 | 2 | 5 |
| Adolescents | 0 | 0 | 0 | 0 | 0 |
| Neutropenia | 0 | 1 (1.1) | 4 (4.4) | 1 (1.2) | 6 (7.4) |
| Adults | 0 | 1 | 4 | 1 | 6 |
| Adolescents | 0 | 0 | 0 | 0 | 0 |
| Lymphopenia | 0 | 0 | 0 | 0 | 0 |
| Herpes zoster | 0 | 0 | 4 (4.4) | 6 (7.2) | 12 (14.7) |
| Adults | 0 | 0 | 4 | 6 | 12 |
| Adolescents | 0 | 0 | 0 | 0 | 0 |
| CPK elevation | 0 | 1 (1.1)‡ | 2 (2.2)§ | 2 (2.4) | 4 (4.9) |
| Adults | 0 | 0 | 2 | 1 | 4 |
| Adolescents | 0 | 1 | 0 | 1 | 0 |
| Renal dysfunction | 0 | 0 | 0 | 0 | 0 |
| Active tuberculosis | 0 | 0 | 0 | 0 | 0 |
Adults were aged ≥18 years, and adolescents were aged <18 years. The number of adults and adolescents was 81 and 9, respectively, for the placebo and 81 and 10 for 15 and 30 mg of upadacitinib each; the patient-years were 72.8 and 10.0, respectively, for 15 mg of upadacitinib and 72.8 and 8.6, respectively, for 30 mg of upadacitinib.
CPK, Creatine phosphokinase; E, event; MACE, major adverse cardiovascular events; PYs, patient-years; VTE, venous thromboembolic event.
Included data up to the cutoff date for all patients with ≥1 dose of upadacitinib. The mean (SD) duration of exposure to study drug from the first dose to the analysis cutoff date in the 15-mg upadacitinib group and 30-mg upadacitinib group was 227.3 (82.8) and 218.8 (84.1) days, respectively.
Event of atypical lymphocytes seen in peripheral blood that was not a malignancy.
Maximum CPK elevation = 2052 U/L.
Maximum CPK elevation = 1660 U/L.
Till the cutoff date, there were no deaths, malignancies, venous thromboembolic events, gastrointestinal perforations, or active tuberculosis events.
Across the study periods up to the cutoff date, the most frequently reported AESIs were serious infection, opportunistic infections (excluding tuberculosis and herpes zoster infection), herpes zoster infection, hepatic disorder, anemia, neutropenia, and increased CPK levels (Table III). The rates of serious infection and hepatic disorders were similar between 15 mg (events/100 patient-years, 3.6 vs 3.7, respectively) and 30 mg (8.5 vs 7.4, respectively) of upadacitinib (Table III). The serious infections included cellulitis (n = 1 and 0 for 15 and 30 mg of upadacitinib, respectively), herpes simplex infection (n = 0 and 1), herpes zoster infection (n = 1 and 2), and Pneumocystis jirovecii pneumonia (n = 1 and 0); only P jirovecii pneumonia led to treatment discontinuation. Most hepatic disorders were nonserious, caused by asymptomatic transaminase enzyme level elevations.
The rate of opportunistic infections (excluding tuberculosis and herpes zoster infection) was higher in the 15-mg upadacitinib group than in the 30-mg upadacitinib group (Table III).
The event rates of herpes zoster infection, anemia, neutropenia, and CPK elevations were higher in the 30-mg upadacitinib group than in the 15-mg upadacitinib group (Table III). Most events caused by herpes zoster were mild or moderate and involved a single dermatome; no events of herpes zoster infection involving the central nervous system were reported. Of 7 patients with anemia, all patients had mild anemia, and none discontinued the treatment. Of 7 patients with neutropenia, 6 patients had moderate- or lower-intensity neutropenia and 1 had severe neutropenia; none led to treatment discontinuation. All elevations occurring in upadacitinib-treated patients were asymptomatic, and none required discontinuation of the study drug.
In adolescents, CPK elevations and Kaposi varicelliform eruptions were the only AESIs reported, and each was reported in 1 patient. One event associated with an abnormal lymphocyte morphology was detected using a blood test in an adolescent who was taking 30 mg of upadacitinib (Table III). However, this event was included in the lymphoma category, the abnormality was a transient finding, no confirmed malignancy was reported, and the abnormal lymphocyte morphology was resolved in subsequent testing.
At the time of this interim analysis, there were few instances of laboratory values that met the definition of grade 3 or 4 laboratory abnormalities based on the Common Terminology Criteria for Adverse Events, version 4.03 (Supplementary Table I, available via Mendeley at https://data.mendeley.com/datasets/h2krvxjtzv/1.)15
Exploratory endpoints
Because the main objective of this study was to evaluate safety, exploratory measures were assessed only for reference. At the time of this interim analysis, the response rates were numerically greater for both the upadacitinib groups than for the placebo group for all the exploratory endpoints at week 16 (Table IV).
Table IV.
Exploratory endpoints at week 16, interim analysis (intent-to-treat population)∗
| Endpoint | Overall |
Adolescents |
||||
|---|---|---|---|---|---|---|
| Placebo (n = 90) | Upadacitinib 15 mg (n = 91) | Upadacitinib 30 mg (n = 91) | Placebo (n = 9) | Upadacitinib 15 mg (n = 10) | Upadacitinib 30 mg (n = 10) | |
| vIGA-AD 0/1 with at least 2 grades of reduction from baseline (NRI), n (%) | 6 (6.7) | 37 (40.7) | 43 (47.3) | 1 (11.1) | 7 (70.0) | 5 (50.0) |
| EASI 90 (NRI), n (%) | 6 (6.7) | 38 (41.8) | 44 (48.4) | 1 (11.1) | 4 (40.0) | 6 (60.0) |
| EASI 75 (NRI), n (%) | 17 (18.9) | 59 (64.8) | 68 (74.7) | 2 (22.2) | 7 (70.0) | 9 (90.0) |
| EASI 50 (NRI), n (%) | 26 (28.9) | 77 (84.6) | 79 (86.8) | 3 (33.3) | 9 (90.0) | 10 (100) |
| Percent reduction in EASI from baseline (MMRM), LSM | −36.9 | −75.4 | −82.3 | −23.9 | −77.3 | −90.9 |
| Worst Pruritus NRS improvement ≥4 from baseline (NRI), n (%)† | 11 (12.2) | 37 (41.1)‡ | 43 (47.3) | 0 | 1 (10.0) | 3 (30.0) |
| Percent reduction in Worst Pruritus NRS from baseline (MMRM), LSM | −28.3 | −47.1 | −53.7 | −34.8 | −46.4 | −47.1 |
EASI 90/75/50, ≥90%/75%/50% reduction in Eczema Area and Severity Index; LSM, least squares mean; MMRM, mixed-effect model repeated measures; NRI, nonresponder imputation; NRS, numeric rating scale; vIGA-AD, validated Investigator Global Assessment for Atopic Dermatitis.
Adults were aged ≥18 years, and adolescents were aged <18 years.
Among patients with a baseline Worst Pruritus NRS score of ≥4.
N = 90.
The response rates for 30 mg of upadacitinib were consistently numerically greater than those for 15 mg of upadacitinib, and the responses among patients in the upadacitinib groups were consistently numerically greater than the responses among patients in the placebo group (Supplementary Figs 2 to 5, available via Mendeley at https://data.mendeley.com/datasets/h2krvxjtzv/1.)
Discussion
This study contributes to the totality of upadacitinib safety data for AD. The results were consistent with those reported in the prior global phase 2b16 and phase 317,18 trials and the labeled safety profile of upadacitinib.19 Similar to the phase 2b16 and phase 317,18 studies, the incidence of acne was reported to increase in a dose-dependent fashion in this study, but this did not result in study drug discontinuation. The overall TEAE rates were low and similar between the upadacitinib dose groups in adolescents and adults, and few SAEs were observed. The rate of serious infections was not higher than that expected and was similar between the upadacitinib groups. All opportunistic infections included Kaposi varicelliform eruption (synonymous with eczema herpeticum), except for 1 case of P jirovecii pneumonia. All 4 events of Kaposi varicelliform eruption were nonserious and moderate in intensity; only 1 event of Kaposi varicelliform eruption led to study drug discontinuation. The single event of P jirovecii pneumonia was reported in a 51-year-old man in the 15-mg upadacitinib group. The patient was diagnosed with a common cold 5 days prior to the event and admitted to the hospital for a fever, cough, and dyspnea. The diagnosis of P jirovecii pneumonia was made based on an elevated β-d-glucan value, considered to be consistent with the clinical presentation. The patient recovered with antibiotic treatment and discontinued the study. Kaposi varicelliform eruption (eczema herpeticum), which is one of the most common viral infections in patients with AD caused by skin barrier defects and other skin alterations associated with AD,20 was reported at a higher rate in the 15-mg upadacitinib group than in the 30-mg upadacitinib group. The characteristics (ie, severity, seriousness, and involvement) of herpes zoster infections observed in this study were similar to those reported previously for JAK inhibitors, including upadacitinib.7, 8, 9, 10, 11 The risk of herpes zoster infection observed in this Japanese population is generally consistent with previous findings for JAK inhibitors as a class in Asian populations; however, the reasons for these effects are not clear and may be due to intrinsic and/or extrinsic factors (eg, genetics, regional differences in reporting, or medical care).21 One major adverse cardiovascular event of hemorrhagic stroke was reported in a 22-year-old man in the 15-mg upadacitinib group. The patient was hospitalized because of falls, headache, and vomiting; the presence of a cerebellar hemorrhage was confirmed using magnetic resonance imaging. The event resolved after surgical removal of the hematoma. No deaths, malignancies, venous thromboembolic events, gastrointestinal perforations, or active tuberculosis events were reported.
Grade 3 or 4 laboratory values were infrequent in the current study, and reports of these grades were consistent with those described in previous studies of rheumatoid arthritis,11,12 AD,16 and other JAK inhibitors.22, 23, 24, 25 These studies reported that patients experienced changes in laboratory values, consisting of elevations in serum transaminases, lipids, and CPK levels and reductions in hemoglobin and white blood cells.11,12 These effects are not unexpected, given that JAK signaling pathways play a key role in maintaining hemopoietic homeostasis.7 In contrast to decreases in platelet counts observed with another JAK1 inhibitor,23, 24, 25 no grade 3 or 4 thrombocytopenia was observed with upadacitinib use up to the data cutoff date.
In this study, patients with baseline AD disease severity comparatively higher than that of patients previously enrolled in the global 2b trial of upadacitinib in patients with AD were enrolled.16 The exploratory endpoints indicated numerically higher response rates in the upadacitinib groups than in the placebo group, with a trend for higher response rates with 30 mg of upadacitinib, although no testing for statistical significance was performed.17,18
Limitations
The limitations of this interim safety analysis include the relatively small sample size and the short 24-week observation period. In addition, the findings in this study's population may not be generalizable to all patients with moderate-to-severe AD, although the safety findings are consistent with those reported in the previous phase 2b and phase 3 studies, which included broader study populations.16, 17, 18
Conclusions
In conclusion, these interim safety results demonstrate acceptable safety profiles for 15 and 30 mg of upadacitinib in patients with moderate-to-severe AD. Taken together with the safety data collected from the global phase 2b16 and phase 3 studies,17,18 the results of this interim analysis support the acceptable safety profile of upadacitinib to treat patients with moderate-to-severe AD.
Conflicts of interest
Dr Katoh has received honoraria as a speaker or consultant for AbbVie, Eli Lilly Japan, Janssen, LEO Pharma, Maruho, Mitsubishi Tanabe Pharma, Sanofi, and Taiho Pharmaceutical and has received grants as an investigator from AbbVie, Eli Lilly Japan, Kyowa Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe Pharma, and Sanofi. Dr Ohya has received funding or grant support from Maruho and Yakult and honorarium as a speaker or consultant from AbbVie, Bayer, Chugai, Lilly, Torii Pharmaceutical, Towa Pharmaceutical, Maruho, Mylan, and Sanofi. Dr Murota has received funding or grant support from Maruho, Mitsubishi Tanabe Pharma, and Taiho Pharmaceutical and honorarium as a consultant from Japan Tobacco, Kaken Pharmaceutical, Maruho, Mitsubishi Tanabe Pharma, Sanofi, Shiseido Japan, and Taiho Pharmaceutical. Dr Ikeda has received a scholarship donation from the Central Research Institute of Pias Co, Ltd. Drs Chu, Hu, Ikeda, Liu, and Teixeira and Author Sasaki are full-time employees of AbbVie Inc and may own AbbVie stock or stock options. Dr Saeki has received funding or grant support from Eisai, Maruho, Mitsubishi Tanabe Pharma, and Torii Pharmaceutical and honorarium as a consultant from AbbVie, LEO Pharma, and Sanofi.
Acknowledgments
AbbVie and the authors thank all study investigators for their contributions and the patients who participated in this study. Medical writing support, funded by AbbVie, was provided by Michelle R. Roberts, PhD, Kersten Reich, MPH, CMPP, and Lamara D. Shrode, PhD, CMPP, of JB Ashtin, who developed the first draft of the manuscript based on an author-approved outline and assisted in implementing author revisions throughout the editorial process. JB Ashtin adheres to the good publication practice (GPP3) guidelines and the International Committee of Medical Journal Editors recommendations.
Footnotes
Funding sources: AbbVie funded the research for this study and provided writing support for this manuscript.
IRB approval status: The protocol (M17-377) was reviewed and approved by the Nagasaki University Hospital Institutional Review Board (September 20, 2018; Sakamoto, Nagasaki, Japan) and the Fukuyama City Hospital Institutional Review Board (September 28, 2018; Fukuyama-shi, Hiroshima, Japan).
References
- 1.Tamagawa-Mineoka R., Katoh N. Atopic dermatitis: identification and management of complicating factors. Int J Mol Sci. 2020;21(8):2671. doi: 10.3390/ijms21082671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamamoto-Hanada K., Yang L., Ishitsuka K., et al. Allergic profiles of mothers and fathers in the Japan Environment and Children’s Study (JECS): a nationwide birth cohort study. World Allergy Organ J. 2017;10(1):24. doi: 10.1186/s40413-017-0157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drucker A.M., Wang A.R., Li W.Q., Sevetson E., Block J.K., Qureshi A.A. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137(1):26–30. doi: 10.1016/j.jid.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Murota H., Inoue S., Yoshida K., Ishimoto A. Cost of illness study for adult atopic dermatitis in Japan: a cross-sectional web-based survey. J Dermatol. 2020;47(7):689–698. doi: 10.1111/1346-8138.15366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nader A., Stodtmann S., Friedel A., Mohamed M.F., Othman A.A. Pharmacokinetics of upadacitinib in healthy subjects and subjects with rheumatoid arthritis, Crohn's disease, ulcerative colitis, or atopic dermatitis: population analyses of phase 1 and 2 clinical trials. J Clin Pharmacol. 2020;60(4):528–539. doi: 10.1002/jcph.1550. [DOI] [PubMed] [Google Scholar]
- 6.Parmentier J.M., Voss J., Graff C., et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494) BMC Rheumatol. 2018;2(1):1–11. doi: 10.1186/s41927-018-0031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burmester G.R., Kremer J.M., Van den Bosch F., et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet (London, England) 2018;391(10139):2503–2512. doi: 10.1016/S0140-6736(18)31115-2. [DOI] [PubMed] [Google Scholar]
- 8.Genovese M.C., Fleischmann R., Combe B., et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet (London, England) 2018;391(10139):2513–2524. doi: 10.1016/S0140-6736(18)31116-4. [DOI] [PubMed] [Google Scholar]
- 9.Genovese M.C., Smolen J.S., Weinblatt M.E., et al. Efficacy and safety of ABT-494, a selective JAK-1 inhibitor, in a phase IIb study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol. 2016;68(12):2857–2866. doi: 10.1002/art.39808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kremer J.M., Emery P., Camp H.S., et al. A phase IIb study of ABT-494, a selective JAK-1 inhibitor, in patients with rheumatoid arthritis and an inadequate response to anti-tumor necrosis factor therapy. Arthritis Rheumatol. 2016;68(12):2867–2877. doi: 10.1002/art.39801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smolen J.S., Pangan A.L., Emery P., et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet (London, England) 2019;393(10188):2303–2311. doi: 10.1016/S0140-6736(19)30419-2. [DOI] [PubMed] [Google Scholar]
- 12.Fleischmann R.M., Genovese M.C., Enejosa J.V., et al. Safety and effectiveness of upadacitinib or adalimumab plus methotrexate in patients with rheumatoid arthritis over 48 weeks with switch to alternate therapy in patients with insufficient response. Ann Rheum Dis. 2019;78(11):1454–1462. doi: 10.1136/annrheumdis-2019-215764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleischmann R., Pangan A.L., Song I.H., et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol. 2019;71(11):1788–1800. doi: 10.1002/art.41032. [DOI] [PubMed] [Google Scholar]
- 14.van Vollenhoven R., Takeuchi T., Pangan A.L., et al. A phase 3, randomized, double-blind study comparing upadacitinib monotherapy to MTX monotherapy in MTX-naïve patients with active rheumatoid arthritis. Arthritis Rheumatol. 2018;70(suppl 9):891. doi: 10.1002/art.41384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Common terminology criteria for adverse events (CTCAE) v4.03. National Cancer Institute. Accessed August 30, 2021. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- 16.Guttman-Yassky E., Thaçi D., Pangan A.L., et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145(3):877–884. doi: 10.1016/j.jaci.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 17.Guttman-Yassky E., Teixeira H.D., Simpson E.L., et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis: results from 2 pivotal, phase 3, randomised, double-blind, monotherapy, placebo-controlled studies (Measure Up 1 and Measure Up 2) Lancet (London, England) 2021;397(10290):2151–2168. doi: 10.1016/S0140-6736(21)00588-2. [DOI] [PubMed] [Google Scholar]
- 18.Reich K., Teixeira H.D., de Bruin-Weller M., et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis: results from a pivotal phase 3, randomised, double-blind, placebo-controlled study (AD Up) Lancet (London, England) 2021;397(10290):2169–2181. doi: 10.1016/S0140-6736(21)00589-4. [DOI] [PubMed] [Google Scholar]
- 19.Rinvoq. (updacitinib) extended-release tablets, for oral use. AbbVie Inc. Accessed August 30, 2021. https://www.rxabbvie.com/pdf/rinvoq_pi.pdf
- 20.Damour A., Garcia M., Seneschal J., Lévêque N., Bodet C. Eczema herpeticum: clinical and pathophysiological aspects. Clin Rev Allergy Immunol. 2020;59(1):1–18. doi: 10.1007/s12016-019-08768-3. [DOI] [PubMed] [Google Scholar]
- 21.Yamaoka K., Tanaka Y., Kameda H., et al. The safety profile of upadacitinib in patients with rheumatoid arthritis in Japan. Drug Saf. 2021;44(6):711–722. doi: 10.1007/s40264-021-01067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guttman-Yassky E., Silverberg J.I., Nemoto O., et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J Am Acad Dermatol. 2019;80(4):913–921. doi: 10.1016/j.jaad.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 23.Gooderham M.J., Forman S.B., Bissonnette R., et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155(12):1371–1379. doi: 10.1001/jamadermatol.2019.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silverberg J.I., Simpson E.L., Thyssen J.P., et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(8):863–873. doi: 10.1001/jamadermatol.2020.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson E.L., Sinclair R., Forman S., et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet (London, England) 2020;396(10246):255–266. doi: 10.1016/S0140-6736(20)30732-7. [DOI] [PubMed] [Google Scholar]