Abstract
Subjective changes in concentration and memory are commonly reported by women during the second or third trimesters of pregnancy, but the nature of the problem is poorly understood. We hypothesized that these self-reports might reflect difficulties in working memory (WM). It was further hypothesized that antepartum depression (depression arising during pregnancy) may play an etiological role, either on its own or due to secondary changes in endocrine function or sleep. Using WM tasks that emphasized executive control processes mediated by the prefrontal cortex (PFC) we compared pregnant women tested at 34–36 weeks gestation (n = 28) with age- and education-matched non-pregnant controls (n = 26). All pregnant women were screened for depression. Evidence of a WM disturbance was found, and was evident only among pregnant women showing depressive symptoms. In contrast, pregnant women who were not depressed showed WM performance that equalled, or even significantly exceeded, non-pregnant controls. No significant differences were observed on control tests of other cognitive functions. Multiple regression revealed that serum estradiol concentrations, along with severity of depressive affect but not sleep disruption, significantly predicted variation in the WM scores. In agreement with studies of estradiol and WM in other contexts, higher estradiol was associated with better WM, while higher levels of depressive symptoms predicted poorer WM. We conclude that memory disturbance during gestation might not be as widespread as commonly believed, but can be seen among women experiencing antepartum depression. The high level of WM performance found in healthy, non-depressed, pregnant women is discussed from an adaptationist perspective.
Keywords: working memory, gestation, pregnancy, estradiol, estrogens, depression, antepartum, cortisol
Diminished concentration and memory is self-reported by a substantial proportion of women in the second and third trimesters of pregnancy (e.g., 64%, Parsons & Redman, 1991), a phenomenon termed gestational memory impairment (GMI; Brett & Baxendale, 2001). GMI is poorly understood in terms of confirming the existence, timing, nature and severity of symptoms through the use of standardized memory tests, or in terms of understanding its etiology. Failure of many studies using objective tests to verify impairment, despite the prevalent self-reports, has led some to conclude that GMI reflects only negative cultural expectations associated with pregnancy (Crawley et al., 2008), not a true loss of function. However, in a meta-analysis of 14 controlled studies done over a 17 year period, Henry and Rendell (2007) concluded that GMI is likely to be a domain-specific memory phenomenon, with some forms of memory being affected and others spared by pregnancy.
The target of most past studies has been explicit memory, the intentional recollection of previously experienced information or events. A few studies reported mild impairment during late gestation, primarily in verbal recall (Buckwalter et al., 1999; Sharp et al., 1993; Wilson et al., 2011). Keenan et al. (1998) for example, found a decline from the second to third trimester in the immediate and delayed conditions of a standard paragraph recall task in a sample of 10 women, which appeared to resolve postpartum. However, a majority of studies have found no evidence of impairment despite women’s self-perceptions of decreased memory function (e.g., Casey et al., 1999; Christensen et al., 2010; Crawley et al., 2003; Janes et al., 1999; Onyper et al., 2010). Based on their meta-analysis, Henry and Rendell (2007) argued that a deficit might exist in explicit memory tasks that require free recall but perhaps not if recognition is required, a finding re-iterated by Anderson and Rutherford in a recent re-review (2012). Recent studies employing larger samples have continued to produce inconsistent findings. Henry and Sherwin (2012) found poorer performance in pregnant women than controls on a composite measure of word list recall but not paragraph recall. A very large study by Glynn (2010) noted improvement in recall scores over four administrations throughout pregnancy of a paired-associates learning task, with no evidence of a differential rate of improvement in the pregnant group compared with non-pregnant controls. Neither study detected any change in memory postpartum, in contrast to the postpartum improvement reported by Keenan and colleagues (1998). In the largest study of its kind (n = 264 who became mothers out of 1058 women followed), Christensen et al. (2010) found no effects on the California Verbal Learning Test in primigravidae who, importantly, were tested prior to pregnancy and followed prospectively.
Explicit memory may not be the optimum focus to advance our understanding of GMI, given the nature of the symptoms self-described by pregnant women. Explicit memory is a form of long-term memory that is heavily dependent on medial temporal lobe structures including the hippocampus (Clark & Squire, 2013), and pertains to remembering facts and events. However, the content of women’s self-descriptions (e.g. distractibility, short-term memory lapses, difficulty concentrating, absent-mindedness; Parsons & Redman, 1991) suggests that the difficulties might arise instead from disturbed working memory (WM), a short-term memory system dependent on other brain regions, notably the prefrontal cortex (PFC). Consistent with this idea, Henry and Rendell (2007) predicted that disruption is most likely to be seen on memory tasks that place high demands on executive control processes. The passive storage component of WM involves the momentary passive retention of information, while the executive components of WM, which are mediated by the PFC, include the active holding of stored information in a state of sustained availability to guide behavior, as well as the on-line updating, monitoring, or manipulation of stored information (see Goldman-Rakic, 1996). Decades of research including electrophysiology studies, functional neuroimaging, and studies of monkeys or human patients with brain lesions, support the importance of the PFC for adequate WM function (for review see Goldman-Rakic, 1996; D’Esposito et al., 2000; Owen, 1997).
Most previous studies of GMI, if they assessed WM at all, have been limited to the passive storage elements of WM as assessed by forward digit span or similar tasks and failed to find impairment during pregnancy (Buckwalter et al., 1999; Casey, 2000). The backward digit span has been included in a few studies and does involve active re-ordering of information, but it too has largely failed to show significant changes during pregnancy (Cuttler et al., 2011; Henry & Sherwin, 2012; but see Janes et al., 1999; Christensen et al., 2010), perhaps because the task is relatively insensitive to individual differences within the normal range of performance. In total, WM tasks have been included in as many as 8 studies of GMI, but Anderson and Rutherford’s meta-analysis (2012) found a mean effect size of only r = −.07, which may reflect the choice of tasks employed. Two fairly recent studies found poorer performance in pregnant women than matched controls on tests of prospective memory, i.e., remembering to carry out a planned action at the appropriate future timepoint (Cuttler et al., 2011; Rendell & Henry, 2008). Like WM, prospective memory depends on the supervisory executive functions of the frontal lobe (Burgess et al., 2001) reinforcing the idea that tasks that evoke the executive processes of WM might be a fruitful avenue for understanding GMI.
The causal mechanisms responsible for GMI are not at all understood. Concentrations of estradiol, estriol, progesterone and several other hormones reach peak lifetime values during the third trimester. By altering activity in neurochemical pathways involved in cognitive processes, steroids have a potential to influence memory-related processes directly. Hormonal assays have seldom been included in studies of cognitive function, but several of the steroid hormones that rise dramatically during late gestation have regulatory effects on the neurochemistry of the PFC (Erickson et al., 2003; Kritzer & Kohama, 1999) and influence WM in other contexts. Estrogens in particular could be relevant. Behavioral (Duff & Hampson, 2000; Grigorova et al., 2006; Hampson & Moffat, 2004; Hampson & Morley, 2013; Krug et al., 2006), functional imaging (Joffe et al., 2006; Smith et al., 2006), and nonhuman primate studies (Rapp et al., 2003) increasingly suggest that in other contexts estrogens do influence the PFC-dependent WM system, but pregnancy has not been studied. Investigations of WM in postmenopausal women (e.g., Duff & Hampson, 2000; Keenan et al., 2001; Krug et al., 2006) or over the natural menstrual cycle (Hampson & Morley, 2013) would predict a positive effect of high estrogens on WM during gestation, not a negative one as implied by reports of GMI. However, some animal studies have found impaired WM at very high doses of estradiol, or other effects of estrogens on WM that are dose-dependent (e.g., Wide et al., 2004).
Other changes associated with advancing gestation could alternatively contribute to memory impairment, independently or as a consequence of the steroidal milieu. Sleep disruption and affective changes are obvious candidates, and are not mutually exclusive. Sleep loss is endemic among pregnant women, although previous studies have not found associations between quantitative indices of sleep disruption and memory performance (e.g., Casey et al., 1999). Recent work suggests depression is more prevalent during pregnancy than previously suspected. An estimated 12–25% of women in the USA and Canada experience significant depressive symptoms associated with pregnancy (Bennett et al., 2004; Yonkers et al., 2009) with similar rates in other developed countries (Lee et al., 2007). Antepartum depression is of concern in its own right and also because it is associated with poorer pregnancy outcomes, increased risk of preterm delivery, and an increased risk for depression in the postpartum period (Burt & Stein, 2002). Both psychosocial and biological factors are believed to contribute to the increased prevalence of depression seen during pregnancy including, potentially, increased hypothalamic-pituitary-adrenal (HPA) axis activity (O’Keane & Marsh, 2007). Subjective reports of GMI conceivably could be attributable to depression, either via increased self-reporting of negative symptoms or actual changes in memory function. Cognitive changes are well known to occur in major depressive disorder outside the pregnancy context (Veiel, 1997), and memory has been a prominent focus of investigation in patients who are clinically depressed (Landrø et al., 2001).
The purpose of the present study was to evaluate WM performance in pregnant women using tests that emphasize the active maintenance and manipulation of information within WM, which are thought to recruit components of the WM system subserved by the PFC (Goldman-Rakic, 1996). We also sought to examine the influence of incident depressive symptoms. Serum was collected to quantify six steroids that increase in the later stages of pregnancy when memory phenomena are reliably reported. Assessment included mood screening using well-established clinical instruments to assess depression and, where indicated, diagnosis as depressed or non-depressed by staff psychiatrists at a women’s mental health clinic. The overarching goal was to shed light on memory function during late gestation using objective psychometric measures of memory performance.
Method
Participants
Participants consisted of 28 pregnant women (Preg, n = 28) tested in the third trimester of pregnancy, and two groups of age- and education-matched controls (see Table 1). One control group consisted of non-pregnant nulliparous women (NonPreg, n = 26) and was used to establish whether any reduction in WM could be identified in the pregnant group. To further probe for any evidence of impairment, we recruited a second control group made up of age- and education-matched postpartum controls (PostPart, n = 10). This group was used to establish whether any WM impairment found during pregnancy resolved postpartum, as suggested by some authors (e.g., Keenan et al., 1998). Each group of women was tested twice, in a repeated-measures design. Late gestation was targeted at Visit 1. Therefore, Visit 1 data are the main focus of the present report.
Table 1.
Demographic Data for the Pregnant Women and Matched Controls
| Pregnant (Preg, n = 28) |
Postpartum Controls (PostPart, n = 10) |
Non-Pregnant Controls (NonPreg, n = 26) |
|
|---|---|---|---|
| Age (yrs) | 29.96 (SD = 5.41) | 31.30 (3.92) | 27.35 (3.37) |
| Education (yrs) | 15.92 (1.53) | 16.60 (1.26) | 16.35 (1.41) |
| Ethnicity (% white) | 100% | 90% | 96% |
All women in the pregnant and postpartum groups had healthy singleton pregnancies and deliveries were uncomplicated and full-term. Participants were recruited from an obstetrics or perinatal health clinic at St. Joseph’s Healthcare in Hamilton, Canada, or from perinatal fitness or parenting classes and met the following criteria: 18 years of age or over, between 34–38 weeks gestation at enrollment into the study, able to communicate in English, no concurrent medical conditions or use of hormonal preparations. All participants gave informed consent and signed a form approved by the St. Joseph’s Healthcare Research Ethics Board.
All pregnant and postpartum women were screened for depressive symptoms using the Edinburgh Ante/Postnatal Depression Scale (EPDS, Cox et al., 1987) and Montgomery-Asberg Depression Rating Scale (MADRS, Montgomery & Asberg, 1979), and underwent the Mini International Neuropsychiatric Interview (MINI) (Sheehan et al., 1997) and an interview about psychiatric history. Routine clinical lab tests were performed to ensure that any symptoms of depression were not due to another medical condition (e.g., anemia, thyroid dysfunction).
Based on the depression screening, 28 women (18 pregnant, all 10 postpartum controls) showed no evidence of depression (EPDS < 7, Cox et al., 1987, or MADRS ≤ 10, Montgomery & Asberg, 1979). Ten of the pregnant women scored in the moderate to severe depression range (> 20) on the MADRS (M = 27.6, SD = 6.8) and/or ≥ 11 on the EPDS (M = 14.0, SD = 2.8) and met DSM-IV criteria for major depression with a peripartum onset. Thus the pregnant group varied considerably in depressive status, and consisted of two identifiable subgroups--pregnant women with clinical depression (Preg+) and pregnant women without depression (Preg−). The depressed women were not taking antidepressant medications. Importantly, though they differed in depression status, the Preg+ and Preg− women were closely matched on other demographic variables including age, education, ethnicity, parity, and gestational week at testing (see Table 2).
Table 2.
Demographic Matching of the Preg+ and Preg− Subgroups of Pregnant Women
| Pregnant (Preg, n = 28) | ||
|---|---|---|
| Preg− (n = 18) | Preg + (n = 10) | |
| Age (years) | 30.33 (SD = 4.65) | 29.30 (6.80) |
| Education (years) | 16.33 (1.53) | 15.20 (2.39) |
| Ethnicity (% white) | 100% | 100% |
| Parity (% primiparous) | 66 % | 60 % |
| Weeks prior to deliveryǂ | 2.9 (1.5) | 3.7 (2.6) |
| MADRS | 5.56 (3.51) | 27.60 (6.84)** |
| EPDS | 4.83 (2.66) | 14.00 (2.79)** |
Notes: Preg+ = pregnant women with clinical depression; Preg− = pregnant women without depression.
Refers to the gestational week when the cognitive and hormonal data were collected. MADRS = Montgomery-Asberg Depression Rating Scale; EPDS = Edinburgh Postnatal Depression Scale.
p < .001
Because our pregnant group was well-educated, a group of non-pregnant control women (NonPreg, n = 26) with natural menstrual cycles (not using oral contraceptives or other hormonal contraception) was recruited from the local affiliated university to form a healthy control group that matched the pregnant and postpartum groups on age, education, and ethnicity. The controls were recruited through poster advertisements on campus and compensated for their participation. Only healthy women with no concurrent medical conditions or use of medications were included. Depression was screened via questionnaire, and all controls were confirmed to be non-depressed.
General Procedure
Women underwent cognitive testing on two separate occasions at St. Joseph’s Healthcare or at the university. For the pregnant women (Preg), the first visit took place between 34 and 38 weeks gestation and the second took place postpartum, 3 to 4 months later (M = 14.2 weeks post-delivery). Timing was well-matched in the Preg− and Preg+ subgroups, both at the first visit (see Table 2) and at the second, postpartum, visit (M = 14.1 weeks and 14.5 weeks, respectively). For the postpartum control group (PostPart) the first visit took place 4 to 12 weeks after delivery (M = 9.0 weeks post-delivery) and the second visit 3 to 4 months later (M = 27 weeks postpartum). The MADRS, EPDS, and MINI were completed on the same day as the cognitive testing, and were repeated at the second visit. Length of time between visits was equated for all participants in order to control for practice effects on the cognitive tests.
The NonPreg controls were tested at the university. Recent evidence suggests that WM is influenced by circulating levels of estrogen in young women (Hampson & Morley, 2013), with greater numbers of WM errors seen at menses. Therefore the menstrual cycle is a relevant variable for studies of GMI, although it is rarely considered. In particular, the ovarian cycle has the potential to influence the level of performance seen in non-pregnant control groups used to evaluate the memory of pregnant women. In the present study, NonPreg controls were counterbalanced so that half were at menses by self-report on Visit 1 (the lowest estrogen phase of the cycle) and half were at higher-estrogen phases. Phase of cycle was reversed at Visit 2. Menses status was verified by a confidential health questionnaire given at each visit.
Neuropsychological Tests
Spatial Working Memory (SPWM; Duff & Hampson, 2000).
The SPWM is a WM task in which participants must try to find all 10 matching pairs of colors that are hidden from view beneath the doors of a 4 × 5 array. The objective was to find all 10 pairs in as few door openings as possible, without returning to already searched locations. When a door was opened, the color beneath was temporarily revealed, but was again hidden from view when the door was released. In order to keep track of where each color was located, a participant had to create and continually update throughout the task, a representation of the array in working memory. A WM error was committed whenever the participant chose a pair of locations that had already been searched but did not match, or revisited an already matched pair (for details see Duff & Hampson, 2000). A trial ended when all 10 matching pairs had been successfully found. Two consecutive trials were given. The dependent variable was the total number of WM errors, summed over the 2 trials. Alternate versions of the array were used on each visit, counterbalanced across participants.
The SPWM is modeled on a classic visual search task used in nonhuman primates to study WM (Passingham, 1985). Monkeys with lesions of the dorsolateral PFC were severely impaired, producing high numbers of WM errors.
Self-Ordered Pointing (SOP; Petrides and Milner, 1982).
The SOP was included as a second measure of WM. Like the SPWM, the SOP emphasizes the manipulation and updating functions of WM. Positron emission tomography confirms activation in the dorsolateral frontal cortex during performance of the SOP task (Petrides, 2000). In the present study, participants completed a practice set, followed by 8, 10, and 12-item sets of stimuli. Stimuli were unique at each sequence length and consisted of images arranged in a fixed two-column layout on each page. The image occupying each position varied unpredictably from page to page within each set. For each set, participants pointed to one item on each page, so that by the end of the set each image had been pointed to once and only once. Accordingly, participants had to keep track within memory of which images had already been pointed to and which had not, and continually update their mental record throughout the task. If the same image was pointed to more than once or if an image was missed within a set, then a WM error was recorded. The dependent variable was the total number of WM errors summed over the 8, 10, and 12-item sets.
Paragraph Recall (Wechsler Memory Scale-III, 1997, adapted).
Paragraph recall was used to measure explicit memory. Two short stories were read aloud and participants were asked to recall the details, immediately following presentation (immediate recall) and again after a 30 minute delay (delayed recall). In both the immediate and delayed conditions, the score was the number of verbatim story units correctly recalled summed over the 2 stories. Two parallel sets of stories were created and were counterbalanced across participants and study visits.
The Paragraph Recall task has been used in numerous studies of explicit memory during pregnancy (e.g., Henry & Sherwin, 2012; Keenan et al., 1998). In contrast to the 2 WM tasks, story recall is dependent on the hippocampus or surrounding cortex of the medial temporal lobe (Frisk & Milner, 1990).
Corsi Blocks (Milner, 1971).
The Corsi Blocks is a nonverbal analog of the digit span task. Instead of remembering digits, participants try to emulate a set of spatial locations tapped out by the examiner on an array of 10 identical cubes fixed to a small board. Sequence length is increased progressively. The score is the maximum number of blocks that can be tapped in correct sequential order. The Corsi task has minimal executive components, and requires only immediate attention without any retention of information over time.
Mooney-Harshman Closure (adapted from Mooney and Ferguson, 1951).
This non-memory task required participants to identify objects shown as fragmentary visual images. A total of 13 images were shown at each visit and participants tried to identify each object. The score was the number correct. Parallel versions of the task were given at each visit, in counterbalanced order. This visuoperceptual task requires object recognition processes dependent on posterior cortex (Wasserstein et al., 1984), but not WM. In contrast to the SPWM and SOP, no effect of gestation was predicted. It was intended to serve as a contrast to the WM measures, to help establish the selectivity of any pregnancy-related effects observed.
Mood and Sleep
The MADRS and EPDS were completed at each visit and provided a quantitative index of depression severity in the pregnant or postpartum women.
To probe sleep quantity and quality, women were asked to indicate: (a) the number of hours of sleep they had within the past 24 hrs, and (b) number of awakenings during the night before each visit.
Hormone Determinations
Serum was drawn at the outpatient laboratory at St. Joseph’s Healthcare on each visit for all pregnant and postpartum women. Six hormones (progesterone, estriol, estradiol, testosterone, cortisol, dehydroepiandrosterone) were quantified using 125I radioimmunoassays from Diagnostic Products Corporation (Los Angeles, CA) or MP Biomedicals (Orangeburg, NY). All intra-assay coefficients of variation were < 10%. Details of the assays have been described elsewhere (Hampson et al., 2013).
Statistical Analysis
To test whether pregnancy is associated with diminished WM, one-way ANOVA (or ANCOVA) was used to compare the various groups on Visit 1, when the Preg women were in late gestation. Fisher’s protected least significant difference test was used to perform post-hoc tests, and p ≤ .05 was used as the criterion for significance. Age and education were potential covariates for all cognitive tests. Only age was significant and only for the SPWM. Therefore all SPWM data were analyzed using ANCOVAs that controlled for age.
A 3-step analysis was used to evaluate the WM hypothesis: (a) To simulate the method of analysis used in typical studies of GMI, we first compared pregnant women as a whole (Preg) with controls (NonPreg, PostPart). Dependent variables used were performance on the SPWM and SOP, our two WM tasks. (b) To evaluate the influence of depression during pregnancy, we next ran a one-way ANOVA (or ANCOVA) on the same WM tests, splitting the Preg group into the depressed and non-depressed subgroups (Preg+ and Preg−). By contrasting the performance of each subgroup with the controls (NonPreg, PostPart), we were able to dissociate the effects of depression during pregnancy (Preg+) from the effects of gestation itself (Preg−). In addition, a direct contrast of the Preg+ and Preg− subgroups addressed the incremental effects of depression on women’s performance during late gestation. (c) In the third step we checked whether the menstrual cycle status of the NonPreg control group influenced their WM performance and thus affected the level of WM to which the pregnant women were being compared. For this analysis, we separated the NonPreg controls tested at menses (Menses group) from those tested at higher-estrogen phases of the cycle (Other). An ANOVA was then carried out comparing the Preg+ and Preg− women with the NonPreg controls considered as separate groups (Menses or Other).
Differences in WM were, in fact, identified in the group comparisons. Accordingly, to investigate potential associations between the severity of depression, hormone levels, sleep, and memory, forced entry multiple regression was used to evaluate the relative contributions of the various predictors to WM functioning in the pregnant women.
Results
The total number of WM errors produced on the SPWM and the SOP was well-correlated (r = .58, p < .001), consistent with their shared working memory demands. ANOVAs revealed a parallel pattern of group differences on the 2 tasks, as described below.
Working Memory
Analysis 1: Pregnant Women vs. Controls.
ANOVA comparing pregnant women as a whole (Preg) with the 2 control groups (NonPreg, PostPart) revealed no significant difference in WM errors on the SOP, F(2, 61) = 0.18, p = .833. The same analysis for the SPWM (with age controlled) was likewise nonsignificant, F(2, 58) = 1.30, p = .281.
Thus, when analyzed using the standard method of statistical analysis typically used in most studies of GMI, there was no evidence of WM impairment during the third trimester for the pregnant group as a whole (n = 28).
Analysis 2: Impact of Antepartum Depression.
To evaluate the influence of antepartum depression on performance, the same analyses were repeated separating the pregnant women into the Preg− and Preg+ subgroups. The contrast between the Preg− and the non-pregnant (NonPreg) controls represents the effects of pregnancy on memory in ordinary healthy gestations. Contrasts between the Preg+ and controls represent the effects in gestations in which depression is present. Importantly, the Preg− and Preg+ groups were matched on demographic variables (see Table 2), including parity (66% primiparous vs 60% primiparous, χ2 (1) = 0.01, p = 0.96), and gestational week when the cognitive testing took place (2.9 weeks vs 3.7 weeks prior to parturition, t (26) = 1.11, p = .279), and differed significantly from one another only on depression status (see Table 2). Thus, the direct contrast of the Preg− vs Preg+ subgroups represents the effects of antepartum depression on WM in matched groups of pregnant women.
One-way ANOVA (or for the SPWM, ANCOVA) comparing the Preg+, Preg−, NonPreg, and PostPart groups revealed a significant effect of group on the WM tasks, SPWM: F(3, 57) = 3.09, p = .034; and SOP: F(3, 60) = 2.79, p = .048 (see Figure 1). Post-hoc tests revealed that on both tasks the Preg+ women committed elevated numbers of WM errors compared with Preg− women (p = .014, Cohen’s d = 0.99 for SPWM and p = .006, d = 1.24 for SOP), confirming the influence of antepartum depression on WM performance of pregnant women. The Preg+ group also tended to make more WM errors than the NonPreg controls (SOP: d = 0.67).
Figure 1.
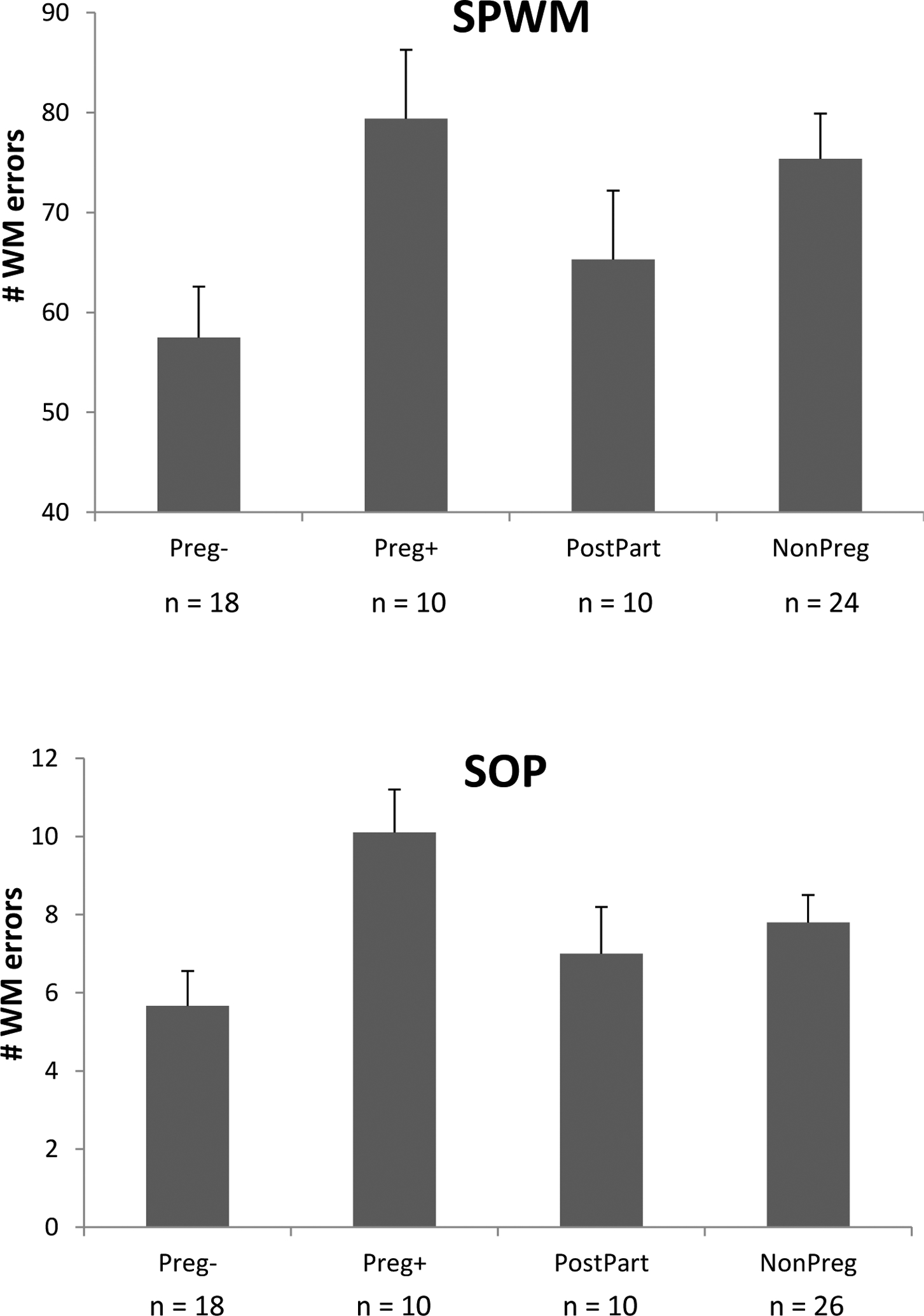
Mean number of WM errors produced on the SPWM task (top panel) and SOP task (bottom panel). Preg+ = pregnant women who were depressed; Preg− = pregnant women who were not depressed; PostPart = postpartum controls; NonPreg = non-pregnant controls. Bars represent the standard error of the mean. The two subgroups of pregnant women (Preg+, Preg−) differed markedly from one another in their patterns of WM performance. Preg− women showed excellent WM, making significantly fewer WM errors than NonPreg controls on the SPWM (p = .013) with the same trend seen on the SOP (p = .069). In contrast, Preg+ women, who were pregnant but exhibited antepartum depression, committed elevated numbers of WM errors and differed significantly (p = .014 and p = .006 on the SPWM and SOP respectively) from their non-depressed counterparts (Preg−) on both tasks. The Preg+ and Preg− groups were matched on gestational stage, parity, age, and education but differed in levels of depressive affect.
Interestingly, post-hoc contrasts for the Preg− group (i.e., pregnant women with ordinary gestations) revealed that the Preg− women displayed significantly better WM than the NonPreg controls (SPWM: p = .013 by post-hoc t-test; d = 0.81, see Figure 1). The same contrast for the SOP also approached significance (p = .069, d = 0.61). Thus in the absence of depression, Preg− women showed no impairment in WM. In fact, data from the SPWM suggested that WM was significantly superior during healthy gestations, relative to a group of healthy NonPreg controls. This effect was overridden by the presence of depression in the Preg+ group.
Analysis 3: Influence of the Menstrual Cycle on the WM Comparisons.
Previous work has suggested that WM performance on the SPWM is influenced by levels of ovarian hormones present over the menstrual cycle (Hampson & Morley, 2013). Although NonPreg controls in the present study were not depressed (see Participants), an effect of the menstrual cycle conceivably could alter the numbers of WM errors seen in the NonPreg women against which the Preg− and Preg+ were compared, and thus alter whether or not a WM decrement appeared to be evident in the Preg women. To test whether the menstrual cycle was an important consideration, we ran a third WM analysis which separated the NonPreg women tested at menses (Menses) from those tested at other phases of the menstrual cycle (Other).
The main effect of group was significant for the SOP, F(4, 59) = 3.40, p = .014 (Figure 2) and approached significance for the SPWM, F(4, 57) = 2.21, p = .0791 (data not shown). Post-hoc tests were run for the SOP task only, and showed that women tested at menses made more WM errors than women tested at other phases of the cycle (p = .034), consistent with Hampson and Morley (2013). As already reported in Analysis 2, the pregnant women showing depression (Preg+) made elevated numbers of WM errors and performed significantly more poorly than Preg− women (p = .005, d = 1.24). Post-hoc tests comparing Preg+ with the controls (Menses, Other) confirmed that the Preg+ also performed significantly more poorly than controls tested at phases of the cycle other than the menstrual phase (Other, p = .020, d = 1.19). In contrast, the healthy non-depressed pregnant women (Preg−) made the lowest absolute numbers of WM errors of all the groups (Figure 2). They significantly outperformed NonPreg controls tested at Menses (p = .009, d = 1.13), though their performance was not significantly superior to those tested at higher-estrogen phases of the cycle (Other).
Figure 2.
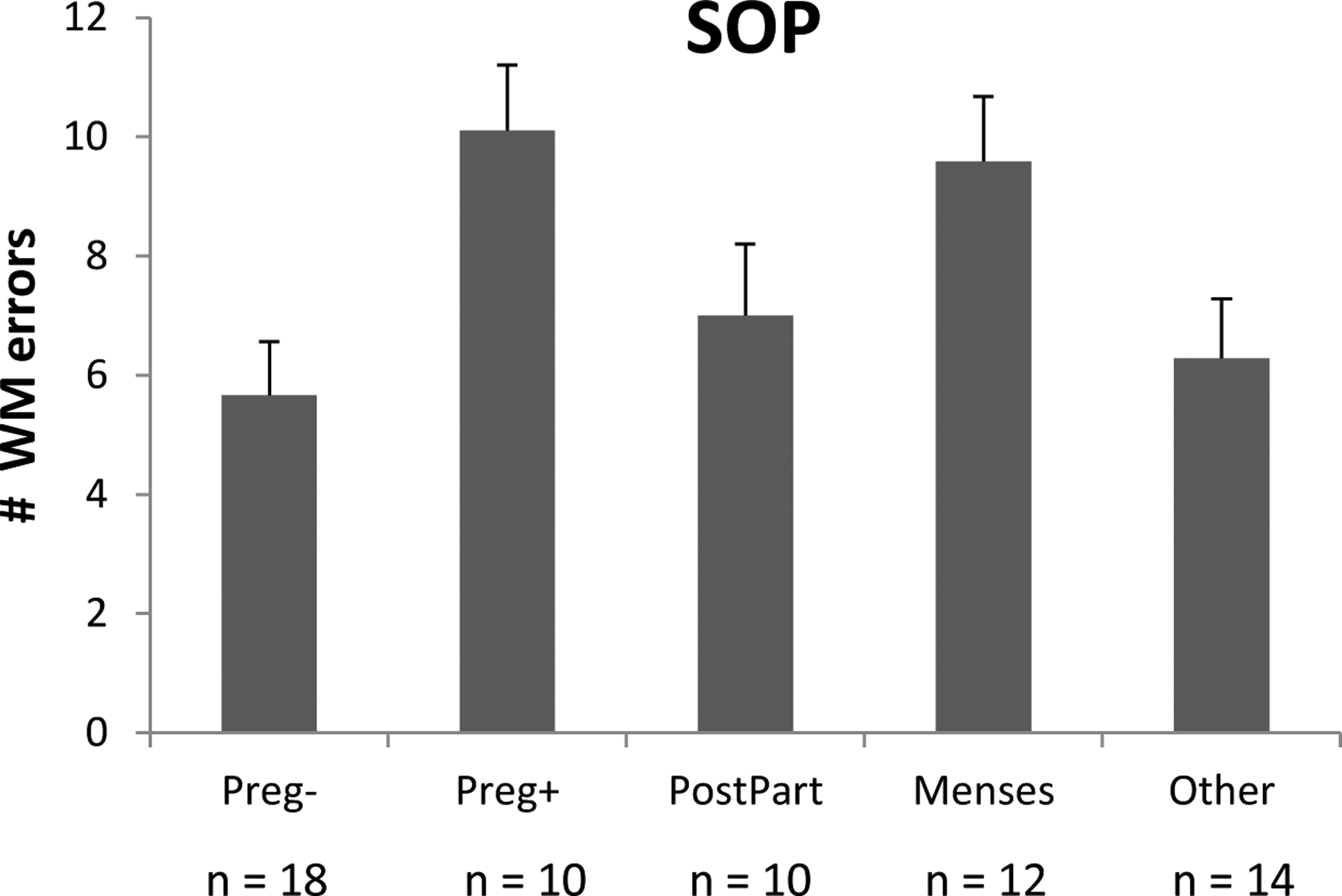
Mean number of WM errors produced on the SOP by the Preg+ and Preg− groups, and by NonPreg controls who were tested at menses (Menses) or at other phases of the ovarian cycle (Other). Preg+ = pregnant women who were depressed; Preg− = pregnant women who were not depressed; PostPart = postpartum controls; Menses = NonPreg controls tested at menses; Other = NonPreg controls tested at other phases of the cycle. Bars represent the standard error of the mean. Higher numbers of WM errors were produced at menses than at other phases of the cycle (p = .034). The Preg+ women showed elevated numbers of WM errors compared with the Preg− women (p = .005), or compared with NonPreg controls tested at Other phases of the cycle (p = .020).
Thus the Preg+ showed evidence of a WM impairment on the SOP task relative to Preg− women matched for stage of gestation, and relative to NonPreg women (except for those at the menstrual phase, characterized by the lowest estrogen levels). In contrast, Preg− women showed no evidence of a WM impairment.
Explicit Memory and Control Measures
There were no significant differences on the other cognitive tasks, including Corsi Blocks and Paragraph Recall. Importantly, no differences were seen as a function of depression status among the pregnant women. On the measure of explicit memory, Paragraph Recall, there was no evidence of a difference in either immediate recall or delayed recall, or in the percentage recalled after the delay (all p’s > .60), and the scores were average to above average, compatible with the education level of the present sample. In particular, the Preg+ and Preg− women did not differ, achieving a percent recall of M = 81.4% ± 5.6 (SEM) and M = 80.9% ± 3.6 respectively, after a delay. This contrasts with the large and significant difference in WM performance between the same two groups.
Changes in WM Performance in the Postpartum
Visit 1 data were the primary focus of the present report. However, because diminished WM was, in fact, identified in the Preg+ subgroup, an exploratory analysis was run to compare pre- to postpartum changes in the same women. At time of writing, Visit 2 data were incomplete but a mixed ANOVA was run to investigate whether there was a significant change in women’s WM performance or mood from Visit 1 to Visit 2. The Preg− and Preg+ groups, who were both pregnant at Visit 1, were tested in the postpartum period at Visit 2, and were well-matched on the timing of their postpartum cognitive testing (M = 14.1 weeks and 14.5 weeks post-delivery, respectively).
ANOVA with visit (Visit 1, Visit 2) as a within-subjects factor and group (Preg+, Preg−, PostPart) as a between-subjects factor revealed a significant improvement in depressive mood in the postpartum period that was limited to the Preg+ group (MADRS: F(2, 30) = 44.32, p < .001; EPDS: F(2, 30) = 14.58, p < .001; see Figure 3 top). The depression seen on Visit 1 resolved in most of the Preg+ women by Visit 2 (p < .001) with no significant change in the other 2 groups. Coincident with the mood change, a parallel ANOVA carried out for the WM tasks showed an improvement in WM that varied as a function of group (Visit × Group interaction: F(4, 44) = 5.70, p = .001 (SOP); F(4, 45) = 2.51, p = .055 (SPWM). Decomposition of the significant interaction for the SOP revealed that the formerly depressed group (Preg+) showed a substantial decrease in WM errors from Visit 1 to Visit 2 (M = 10.71 to 6.28 errors at the postpartum testing, p < .01; see Figure 3), while the Preg− group (and PostPart) showed no evidence of change. As a result, at Visit 2 there were no significant differences in WM performance among the 3 groups of women (Preg+, Preg−, PostPart), all of whom were postpartum at the second visit (Visit 2).
Figure 3.
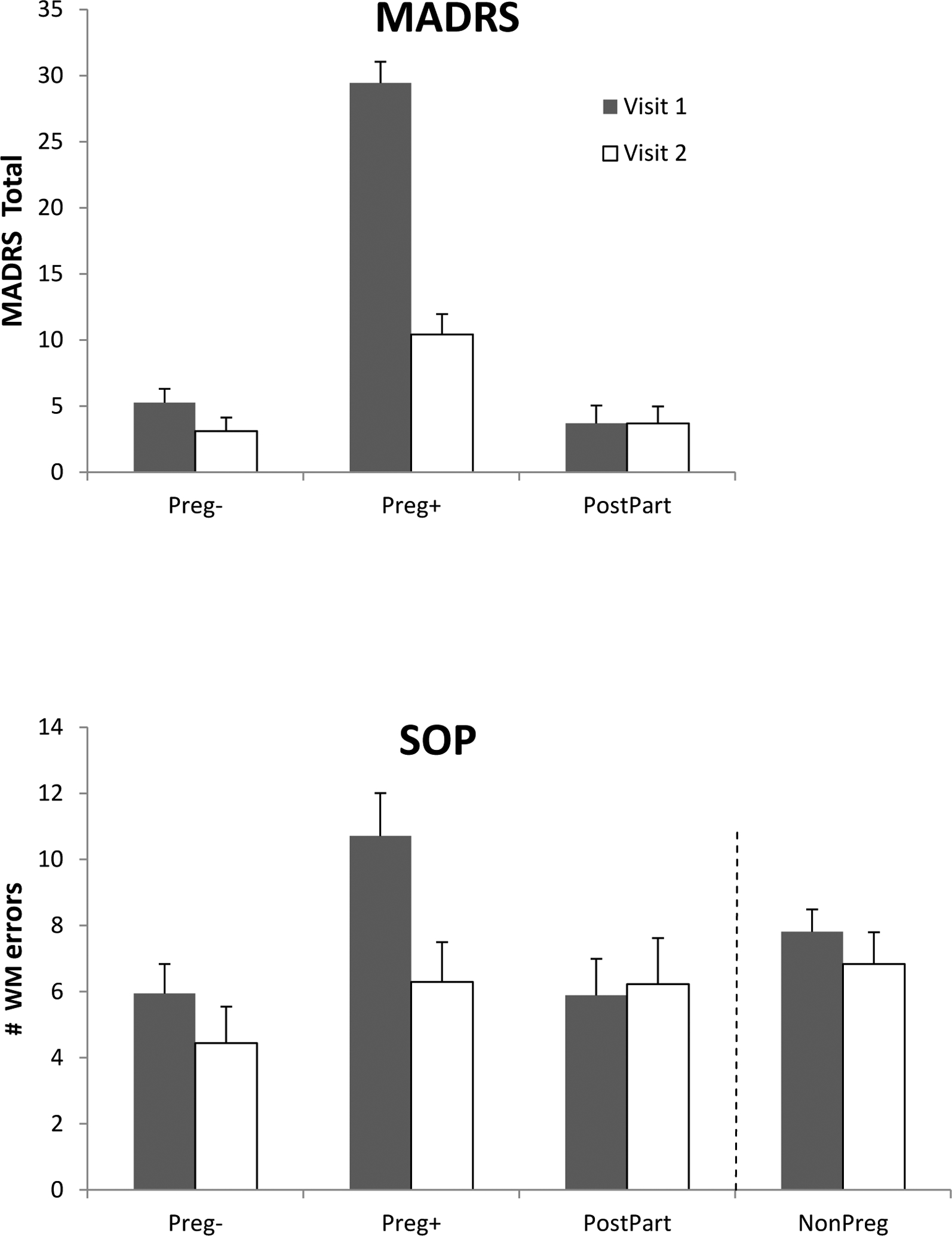
Changes from Visit 1 to Visit 2 in MADRS depression scores (top panel) and in the numbers of WM errors produced on the SOP task (bottom panel). The Preg− and Preg+ women were pregnant on Visit 1 but on Visit 2 they were tested in the postpartum, approximately 14.0 to 14.5 weeks post-delivery. On Visit 2, the Preg+ women, who had been clinically depressed at Visit 1, showed a significant reduction in depressive affect (top panel) and a significant reduction in WM errors on the SOP, p < .01 (bottom panel). Other groups showed no significant change between Visits. The NonPreg controls are shown for reference in the bottom panel based on all available data at each timepoint (Visit 1, Visit 2), and irrespective of phase of cycle when tested. Bars represent the standard error of the mean.
The postpartum results were unchanged if we controlled for individual differences in the timing of women’s postpartum assessment via the use of a covariate. The effect of the covariate (number of weeks post-delivery) was non-significant, F(1, 28) = 0.04, p = .844.
Depression was not the only variable that affected WM performance. Inclusion of the NonPreg controls in the ANOVA showed that the Menses and Other subgroups each showed a reversal in their SOP scores on Visit 2 relative to Visit 1 (data not shown), coincident with the reversal in their phase of cycle at the second testing (see General Procedure).
Predictors of WM Performance During Pregnancy
Major depression is often associated with disturbances in sleep and endocrine function as well as affective changes such as those sampled by the MADRS or EPDS. Although tending to covary, there is individual variation across persons who are depressed. To illuminate the factors most closely associated with WM impairment (or improvement) seen in the antepartum, multiple regression was used to explore the degree to which WM scores could be predicted by the level of depression, maternal hormone levels, or sleep disruption in the pregnant women (n = 28).
To limit the number of hormones considered, we checked for any differences between the Preg+ and Preg− groups in the 6 steroids that had been measured (see Hormone Determinations). For increased power, an alpha level of .10 was used. The Preg+ and Preg− differed by less than a week in the gestational timing of their Visit 1 assessments (M = 3.7 vs 2.9 wks prior to delivery, t(26) = 1.11, p = .279), and on that basis would be expected to have similar hormone levels. As revealed in Table 3, however, the women who were depressed (Preg+) had significantly higher serum cortisol than the non-depressed women (Preg−), and lower estradiol levels. Progesterone, estriol, DHEA, and testosterone did not differ between the 2 groups (all p’s > .480). Therefore, only cortisol and estradiol were considered hormones of potential interest when performing the multiple regressions predicting the WM scores. The sleep variables used consisted of the 2 items described under Mood and Sleep (see Method).
Table 3.
Serum Concentrations (M ± SD) of Six Steroids in the Preg+ and Preg− Women
| Preg− (n = 18) | Preg+ (n = 10) | Cohen’s d | |
|---|---|---|---|
| Progesterone (nmol/L) | 844.78 (373.32) | 790.59 (334.75) | d = 0.15 |
| Estradiol (nmol/L) | 63.64 (21.51) | 48.45 (22.09)* | d = 0.70 |
| Free Estriol (nmol/L) | 70.50 (30.95) | 62.36 (24.03) | d = 0.28 |
| Cortisol (nmol/L) | 892.94 (159.53) | 1001.12 (164.31)* | d = −0.67 |
| DHEA (nmol/L) | 16.71 (3.00) | 16.80 (4.16) | d = −0.02 |
| Free Testosterone (pmol/L) | 5.64 (2.60) | 5.89 (1.86) | d = −0.11 |
p ≤ .10
Simple correlations are given in Table 4. Data shown are partial correlations, controlling for minor individual differences across participants in the number of days prior to parturition when the cognitive and hormonal testing took place. Consistent with reports from non-pregnant women (Krug et al., 2006; Hampson & Morley, 2013), high estradiol levels were inversely correlated with the number of WM errors produced--i.e. higher estradiol predicted fewer WM errors. Conversely, greater depression severity (higher depression score on the MADRS) was correlated with higher numbers of WM errors. High EPDS or MADRS scores predicted greater night-time wakefulness (r = .55, p = .003 and r = .65, p < .001 respectively) and reduced sleep over the past 24 hr (r = −.40), but sleep per se did not predict WM performance. Cortisol levels were positively correlated with depression severity (EPDS) in the depressed subset of women (Preg+, r = .64; not shown separately in Table).
Table 4.
Correlations Between WM Performance, Sleep Variables, Depression Severity, and Maternal Hormone Levels in the Pregnant Women (n = 28)
| Estradiol | Cortisol | EPDS | MADRS | #Hours Sleep | # Awakenings | |
|---|---|---|---|---|---|---|
| SOP errors | −.45* | −.19 | .34‡ | .48* | −.10 | .26 |
| SPWM errors | −.33‡ | −.23 | .34‡ | .36‡ | .09 | .01 |
| EPDS | −.12 | .36‡ | −.40* | .55* | ||
| MADRS | −.25 | .28 | −.37‡ | .65* |
p < .05,
p < .10;
all p-values are two-tailed. Partial correlations are shown, controlling for individual variation in the number of days prior to parturition when testing took place. EPDS = Edinburgh Depression Scale; MADRS = Montgomery-Asberg Depression Scale; # Hours Sleep = number of hours slept in past 24 hrs; # Awakenings = number of nighttime awakenings; SOP = Self-Ordered Pointing; SPWM = Spatial Working Memory Test. Not shown in table: the correlation between serum cortisol and EPDS score in the Preg+ subgroup considered on its own was r = .64.
Multiple regression to evaluate the relative importance of the various predictors showed that only estradiol and depression severity (but not cortisol or sleep) were significant predictors of WM. The regressions were run with and without the number of days prior to parturition as a control variable, to adjust for differences in the timing of women’s assessments. Results showed that estradiol levels and EPDS scores together predicted the number of WM errors observed on the SOP, F(3, 24) = 3.22, p = .041, and jointly explained 20% of the variance in the SOP scores. Higher depression (standardized β = .29, p = .110) and lower estradiol concentrations (β = −.44, p = .025) were associated with greater numbers of WM errors. The same result was seen if the MADRS instead of EPDS was used as a predictor, F(3, 24) = 4.32, p = .014, explaining 27% of the variance. The beta coefficient for the MADRS was β = .40, p = .029 and for estradiol was β = −.37, p = .050. Findings for the SPWM were similar but the F-test did not achieve significance.
If estradiol and cortisol were forced into the model simultaneously, omitting depression severity, then significance was lost, F(2, 24) = 2.06, p = .132. The beta coefficient for estradiol (β = −.48, p = .035) remained significant but cortisol was not (β = .01, p = .966), confirming that depression severity captured unique variance not shared by cortisol.
Discussion
In keeping with the possibility that WM is disrupted during the second or third trimesters of pregnancy, we found a medium to large effect (Cohen’s d) on tests of WM but not on a test of explicit memory in pregnant women. The effect was seen only in women exhibiting antepartum depression. Specifically, significantly diminished WM was evident in the Preg+ group relative to pregnant women tested at a matched point in gestation who were not depressed (Preg−) and relative to non-pregnant controls tested at mixed phases of the menstrual cycle (NonPreg, Other). The fact that WM returned to normal postpartum, when mood returned to normal, confirms the association between the WM decrement and depressive affect, and underscores the reversibility of the pregnancy deficit as reported in some previous studies of GMI (e.g., Keenan et al., 1998).
In contrast, pregnant women without depression (Preg−) represented healthy gestations uncomplicated by depression and were of considerable interest in their own right. Preg− women showed a high level of accuracy on our WM tasks, achieving scores equal or even superior to the NonPreg controls (Figure 1). Our findings show that while a WM decrement can occur during pregnancy, it is not characteristic of all women. In particular, it might not be typical of healthy gestations. The high level of WM competency identified in the Preg− subgroup is of theoretical interest in light of observations from other species implying that pregnancy is a time of active neurocognitive re-organization (Macbeth & Luine, 2010; Anderson & Rutherford, 2012), a point we will return to below.
WM and Depression
WM was found to be disrupted during pregnancy in the present study, but only in women showing signs of clinical depression. Depression was carefully screened using the EPDS, a tool that has high predictive validity in pregnant women (Murray & Cox, 1990), and was verified by professional clinician interview. Although our sample size was small and our findings will need to be replicated in a larger cohort, our data suggest that depression associated with pregnancy is one possible basis for gestational memory impairment (GMI). WM dysfunction is a prominent feature of major depression appearing in other contexts, and a relatively specific impairment of WM/central executive function is said to underlie the pattern of cognitive dysfunction observed (Landrø et al., 2001; Rose & Ebmeier, 2006). The dorsolateral PFC is particularly important in the control of WM, and functional imaging reveals underactivity in this region during depressive episodes (Murrough et al., 2011), as well as more effortful processing in depressed patients than controls during WM tasks (Matsuo et al., 2007). The diminished WM seen in our Preg+ women is thus consistent with a broader picture of decreased WM function in people with depression.
Depression occurring during gestation (‘antepartum’ depression) is often overlooked by physicians, but the past decade has revealed that its prevalence rivals or exceeds that of post-partum depression, afflicting up to 20% of pregnancies or up to 25–30% of pregnancies in low socioeconomic status women (Bennett et al., 2004; Marcus et al., 2003; Parcells, 2010; Yonkers et al., 2009). If depressive symptoms are linked with poorer memory, and if depression during pregnancy is more prevalent than formerly realized, then it could potentially explain the widely held societal beliefs of defective memory in pregnant women (see also Crawley et al., 2008).
Although we are not the first to study WM during gestation, the present study is the first to find a significant WM effect using tasks with a strong executive component which are derived from cognitive neuroscience, and is the first to show that the effect is specific to only a subset of pregnant women. This is different from the prevailing view that memory disruption is a part of normal pregnancy. Most past studies assessing WM in pregnant women reported nonsignificant findings (Henry & Sherwin, 2012; Cuttler et al., 2011; Casey et al., 1999; 2000; Onyper et al., 2010; Christiansen et al., 2010; but for an exception see Janes et al., 1999). However, a meta-analysis of existing studies suggested that a WM effect might still be present (Henry & Rendell, 2007) and proposed that it is specifically memory tasks that place heavy demands on executive control processes (e.g. WM, prospective memory, self-initiated retrieval) that are likely to show a decrement during pregnancy. This notion meshes well with our finding that depression may be a basis for GMI, in that the same processes are also affected by depression. While in agreement on the types of memory processes affected, our data (especially the data from the Preg− women) offer an important qualification by implying that disturbed WM may not be a feature of ordinary pregnancy, but a departure from it.
Our data do not reveal which factor associated with depression is the basis for the WM impairment we observed. Although depression is often associated with disturbed sleep and with elevated cortisol, both of which were seen in the present sample, neither was found to be a significant predictor of WM errors in our regression analyses. Even though higher MADRS or EPDS depression scores were correlated with greater sleeplessness, the regressions showed that sleep disruption did not predict WM performance. This agrees with other work suggesting sleep loss does not mediate memory disruption during pregnancy (Casey et al., 1999; Cuttler et al., 2011; Henry & Sherwin, 2012; Janes et al., 1999; Keenan et al., 1998; Rendell & Henry, 2008).
Nor were we able to demonstrate any significant association between WM and cortisol. Elevated cortisol is associated with depression in many contexts (Strickland et al., 2002; Dinan, 1994) and it is a plausible candidate to mediate a disruption in WM because randomized trials show that glucocorticoids administered at depression-like levels disrupt WM in young adults (Young et al., 1999). Cortisol levels are normally elevated in the third trimester of pregnancy, stimulated by rising estradiol (Wilson et al., 1979). However, even during pregnancy higher basal cortisol may be seen in women who are experiencing depression with high levels of self-perceived life stress (Diego et al., 2006; Parcells, 2010, but see Evans et al., 2008). Consistent with this, we found that cortisol levels were significantly higher in the depressed (Preg+) than non-depressed women (Preg−) matched on gestational age, and were correlated with depression severity on the EPDS. But our regressions were unable to demonstrate any association between WM errors and cortisol.
Two recent studies have observed associations between cortisol levels during pregnancy and word list learning tasks (Glynn, 2010; Henry & Sherwin, 2012), and suggest a quadratic relationship by which verbal recall is better at moderately elevated cortisol concentrations but is impaired at the highest levels. It is possible that a similar relationship could exist for WM, and might potentially be shown in future studies with larger sample sizes that can adequately test for nonlinear associations.
Estrogen, WM, and the Maternal Brain
A disturbance in WM in women suffering from antepartum depression is of clinical and theoretical interest to help us understand the etiology of GMI. In the present data, however, the lack of any impairment in our non-depressed group of pregnant women is of equal interest. This is especially true in the context of recent suggestions that pregnancy might represent a time of active cognitive re-organization (Macbeth & Luine, 2010; Anderson & Rutherford, 2012), when the brain adapts in ecologically relevant ways by facilitating cognitive functions relevant to the current reproductive phase and the emerging demands of child-rearing. Working memory, the ability to keep track of and juggle competing short-term demands, would certainly qualify as one such function. Therefore it may be relevant that the Preg− women showed significantly better WM performance than NonPreg controls (see Figure 1). This was particularly true if they were compared with NonPreg controls tested in the lowest-estrogen state of menses (Analysis 3). In contrast to this picture, WM errors were significantly increased not decreased during pregnancy among women who were depressed (Preg+), underscoring the maladaptive impact of depression on the maternal brain. Although the present study was not designed to follow WM performance into the extended postpartum, an adaptationist perspective would predict persistence of the WM changes, in order to facilitate early child-rearing. Although constrained by the available sample size, Figure 3 is consistent with this possibility, in that the formerly pregnant women (Preg−) maintained their WM advantage at the postpartum visit (i.e., at Visit 2) compared with the WM performance of NonPreg controls2.
Estradiol is a possible basis for the high level of WM competence seen during gestation in the Preg− group. A growing body of evidence suggests that high estrogen states are associated with improvements in WM. This may also be true during pregnancy (when not impaired by an overlay of depression). The present study is the first to discover an association between estradiol and WM during pregnancy, but previous work has revealed that WM is better in postmenopausal women or nonhuman primates treated with estrogens compared with untreated controls (Duff & Hampson, 2000; Keenan et al., 2001; Krug et al., 2006; Rapp et al., 2003), and improves at high estradiol phases of the menstrual cycle compared with low (Hampson & Moffat, 2004; Hampson & Morley, 2013), while WM is diminished by leuprolide acetate, a medication which suppresses ovarian function (Grigovora et al., 2006). Functional imaging studies using randomized placebo-controlled designs show that estradiol administration alters regional activation levels seen in the PFC during the performance of WM tasks (Smith et al., 2006). Collectively, such observations suggest that estrogens may regulate the functioning of the female WM system, and may do so by modulating neuronal activity in the PFC. Menstrual cycle work has further shown that women’s accuracy on the SPWM resembles males’ at the menstrual phase, characterized by low estradiol, but a significant female superiority is found at high estradiol phases of the cycle (Hampson & Morley, 2013). Though it will need to be confirmed in future studies, the present findings imply the sex difference could potentially be further enhanced in Preg− women, who made the fewest WM errors of all (e.g. Figure 1). This fits with the adaptationist concept of preparation of the maternal CNS for child rearing, and with the possibility that hormonal adaptations attributable to estradiol may participate in this preparation.
In favor of an estradiol effect on WM in the present data, multiple regression revealed a significant association between the numbers of WM errors produced and serum estradiol levels measured in maternal serum taken on the same day as the cognitive testing. Importantly, these regressions were limited to the pregnant women only, and controlled for both gestational stage (number of days prior to parturition) and controlled for the independent influence of depression on WM through simultaneous forced entry. Higher circulating estradiol levels were found to be associated with lower numbers of WM errors (as revealed by the standardized coefficients). In contrast, depression severity had an independent, opposite, influence on WM function. Thus, in the absence of a countervailing effect of depression, higher estradiol was associated with greater WM accuracy. Our regression data support the view that high estradiol levels found during late gestation may play a facilitative role in the WM of Preg− women3, reinforcing effects of estradiol reported using other sorts of paradigms.
If estradiol is important, then why did we not see a larger difference in WM between the Preg− women and high-estradiol phases of the menstrual cycle (Other), given that serum estradiol levels are higher in late gestation? Mean level of performance was slightly superior in the Preg− women, but the groups did not differ significantly. It might only reflect the already high level of accuracy seen over the menstrual cycle, as the scope for further improvement on our WM tasks is not very large (see Figure 2). Our WM tasks were chosen in anticipation of finding impaired not facilitated WM during pregnancy, and future studies using different WM tests might reveal a group difference. Alternatively, physiological adaptations do occur during gestation to diminish the effects on the mother of the dramatic increases in hormone production by the fetoplacental unit. For instance, total serum estradiol differs 50- to 100-fold between the menstrual cycle and the final weeks of pregnancy, but the increase in the concentration that is biologically available is much smaller because of protection afforded by SHBG which also rises during gestation. There also may be reductions in tissue sensitivity in response to chronically raised estrogen exposure, and this blunting may explain why we did not see a more prominent WM enhancement. These same arguments have long been offered to explain the lack of Cushingoid features near the end of gestation in the face of greatly increased serum cortisol at that time (e.g., Nolten et al., 1980; Scott et al., 1990), or to explain the absence of maternal virilization under the increased levels of circulating testosterone.
Exactly how estradiol governs WM is not presently understood. Several neurotransmitter systems that figure prominently in the control of WM (in particular, dopaminergic, serotonergic, and cholinergic pathways, Chudasama & Robbins, 2006) have been shown in laboratory animals to be influenced by circulating levels of estradiol, and could potentially mediate facilitation in WM under the high estradiol conditions of pregnancy. ERα may provide an important molecular entranceway for estradiol to exert its effects, because data from other primates suggest that ERα is present in the dorsolateral PFC (Wang et al., 2010; see also Perlman et al., 2005), a region of particular significance to human WM (Owen, 1997). The expression of ERα in the dorsolateral cortex broadly supports the idea that WM may be modulated by estradiol during pregnancy, but because our data are only behavioral they do not speak directly to questions of mechanism.
Strengths and Limitations
The current study has strengths and limitations. Limitations include the fact that our data were collected only as pilot level data, and will need to be re-confirmed in a larger cohort of pregnant women. Although it would have allowed for a balanced experimental design, we were unable to recruit a comparison group of women who were moderately to severely depressed in the postpartum period. Women with postpartum depression are in need of medical management and are not usually able or willing to take part in memory research. Strengths of the present study include: its focus on WM instead of the more often studied explicit memory as a basis for GMI; identification of depression as a possible basis for memory impairment; the relatively large effect sizes observed on our WM tasks; convergence of these findings derived from pregnancy with other current research on the role of estrogen in WM; the use of not one but several control groups (whereas many past studies of GMI have lacked control groups altogether); the use of nulliparous controls; attention to the stage of the menstrual cycle amongst our controls; a within-subjects design; limitation of repeat testing to only 2 time points to minimize practice effects on cognition; and the inclusion of hormonal measures. Only about half a dozen past studies of GMI have included hormonal assessments, usually of a single hormone only.
Conclusions
Our data suggest that memory disturbance during gestation (GMI) does occur in certain women, but is not a feature of all pregnancies. We identify depression in the antepartum period as a potential risk factor for GMI. We found an effect in the domain of WM, but whether or not there is any adverse effect of pregnancy on explicit memory, and under what conditions, remains an unresolved question. Not all women showed reduced memory. To the contrary, a high level of WM proficiency was detected in normal pregnancies, consistent with certain adaptationist perspectives (Macbeth & Luine, 2010; Hampson, 2008; Anderson & Rutherford, 2012). While human research on pregnancy and cognition has tended to focus almost exclusively on memory deficits, from a biologist’s perspective a more interesting outcome is perhaps the positive (and potentially enduring) adaptations of the brain that may occur to promote maternal care and infant fitness. While an influential meta-analysis by Henry and Rendell (2007) concluded that effects of pregnancy on memory functioning in humans are comparable irrespective of gravidae status, enduring effects of maternal parity have been reported in other species (Macbeth & Luine, 2010). Thus future studies should examine the possibility of persisting adaptations that reflect maternal programming.
Acknowledgements
This work was supported by a pilot grant from the Society for Women’s Health Research through its Isis Fund Network (EH, WS, MS) and by a grant to the first author from the Natural Sciences and Engineering Research Council of Canada and to author WS from the National Institutes of Health (U01 GM092655). EH held a Chair in Women’s Health from the Canadian Institutes of Health Research and Ontario Women’s Health Council. We thank M. Coote for her assistance with the radioimmunoassays. SJ Duff-Canning is now at Toronto Western Hospital and author SD Phillips is now at Mount Sinai Hospital in Toronto. KL Evans is a postdoctoral fellow at the University of Toronto.
Footnotes
The weaker finding for the SPWM reflects the presence of one influential datapoint.
The PostPart group, too, showed a high level of WM accuracy when evaluated at a similar postpartum timepoint (Visit 1, for the PostPart group), but all contrasts for the PostPart controls were non-significant in the present study.
Though not presented in the current report, we have also found in the same sample of women reported here that a genetic variant in ESR1, the gene that codes ERα, was a significant predictor of the # WM errors committed on the SPWM in genetic association analyses based on whole blood DNA. A single nucleotide polymorphism in the 3’UTR region of ESR1 (rs3798577) was significant in a genotype test (for the SPWM: p = .001; for the SOP: p = .063). The minor allele has a frequency of 48% and was associated with higher numbers of WM errors. This too suggests a role for estradiol in WM, because a woman’s ESR1 genotype would be expected to assort randomly with the number of WM errors she committed unless ligand binding at ERα is an important consideration for WM performance.
References
- Anderson MV, & Rutherford MD (2012). Cognitive reorganization during pregnancy and the postpartum period: An evolutionary perspective. Evolutionary Psychology, 10, 659–687. [PubMed] [Google Scholar]
- Bennett HA, Einarson A, Taddio A, Koren G, & Einarson TR (2004). Prevalence of depression during pregnancy: Systematic review. Obstetrics and Gynecology, 103, 698–709. [DOI] [PubMed] [Google Scholar]
- Brett M, & Baxendale S (2001). Motherhood and memory: A review. Psychoneuroendocrinology, 26, 339–362. [DOI] [PubMed] [Google Scholar]
- Buckwalter JG, Stanczyk FZ, McCleary CA, Bluestein BW, Buckwalter DK, Rankin KP, Chang L, & Goodwin TM (1999). Pregnancy, the postpartum, and steroid hormones: Effects on cognition and mood. Psychoneuroendocrinology, 24, 69–84. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Quayle A, & Frith CD (2001). Brain region involved in prospective memory as determined by positron emission tomography. Neuropsychologia, 39, 545–555. [DOI] [PubMed] [Google Scholar]
- Burt VK, & Stein K (2002). Epidemiology of depression throughout the female life cycle. Journal of Clinical Psychiatry, 63, 9–15. [PubMed] [Google Scholar]
- Casey P (2000). A longitudinal study of cognitive performance during pregnancy and new motherhood. Archives of Women’s Mental Health, 3, 65–76. [Google Scholar]
- Casey P, Huntsdale C, Angus G, & Janes C (1999). Memory in pregnancy. II: Implicit, incidental, explicit, semantic, short-term, working and prospective memory in primigravid, multigravid and postpartum women. Journal of Psychosomatic Obstetrics and Gynecology, 20, 158–164. [DOI] [PubMed] [Google Scholar]
- Christensen H, Leach LS, & Mackinnon A (2010). Cognition in pregnancy and motherhood: Prospective cohort study. British Journal of Psychiatry, 196, 126–132. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, & Robbins TW (2006). Functions of frontostriatal systems in cognition: Comparative neuropsychopharmacological studies in rats, monkeys, and humans. Biological Psychology, 73, 19–38. [DOI] [PubMed] [Google Scholar]
- Clark RE, & Squire LR (2013). Similarity in form and function of the hippocampus in rodents, monkeys, and humans. Proceedings of the National Academy of Sciences, 110, 10365–10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JL, Holden JM, & Sagovsky R (1987). Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry,150, 782–786. [DOI] [PubMed] [Google Scholar]
- Crawley RA, Dennison K, & Carter C (2003). Cognition in pregnancy and the first year post-partum. Psychology and Psychotherapy: Theory, Research and Practice, 76, 69–84. [DOI] [PubMed] [Google Scholar]
- Crawley R, Grant S, & Hinshaw K (2008). Cognitive changes in pregnancy: Mild decline or societal stereotype? Applied Cognitive Psychology, 22, 1142–1162. [Google Scholar]
- Cuttler C, Graf P, Pawluski JL, & Galea LAM (2011). Everyday life memory deficits in pregnant women. Canadian Journal of Experimental Psychology, 65, 27–37. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, & Rypma B (2000). Prefrontal cortical contributions to working memory: Evidence from event-related fMRI studies. Experimental Brain Research, 133, 3–11. [DOI] [PubMed] [Google Scholar]
- Diego MA, Jones NA, Field T, Hernandez-Reif M, Schanberg S, Kuhn C, & Gonzalez-Garcia A (2006). Maternal psychological distress, prenatal cortisol, and fetal weight. Psychosomatic Medicine, 68, 747–753. [DOI] [PubMed] [Google Scholar]
- Dinan TG (1994). Glucocorticoids and the genesis of depressive illness: A psychobiological model. British Journal of Psychiatry, 164, 365–371. [DOI] [PubMed] [Google Scholar]
- Duff SJ, & Hampson E (2000). A beneficial effect of estrogen on working memory in postmenopausal women taking hormone replacement therapy. Hormones and Behavior, 38, 262–276. [DOI] [PubMed] [Google Scholar]
- Erickson K, Drevets W, & Schulkin J (2003). Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neuroscience and Biobehavioral Reviews, 27, 233–246. [DOI] [PubMed] [Google Scholar]
- Evans J, Heron J, Francomb H, Oke S, & Golding J (2001). Cohort study of depressed mood during pregnancy and after childbirth. British Medical Journal, 323, 257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans LM, Myers MM, & Monk C (2008). Pregnant women’s cortisol is elevated with anxiety and depression – but only when comorbid. Archives of Women’s Mental Health, 11, 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisk V, & Milner B (1990). The role of the left hippocampal region in the acquisition and retention of story content. Neuropsychologia, 28, 349–359. [DOI] [PubMed] [Google Scholar]
- Glynn LM (2010). Giving birth to a new brain: Hormone exposures of pregnancy influence human memory. Psychoneuroendocrinology, 35, 1148–1155. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS (1996). Regional and cellular fractionation of working memory. Proceedings of the National Academy of Sciences USA, 93, 13473–13480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorova M, Sherwin BB, & Tulandi T (2006). Effects of treatment with leuprolide acetate depot on working memory and executive functions in young premenopausal women. Psychoneuroendocrinology, 31, 935–947. [DOI] [PubMed] [Google Scholar]
- Hampson E (2008). Endocrine contributions to sex differences in visuospatial perception and cognition. In: Becker JB, Berkley KJ, Geary N, Hampson E, Herman JP, & Young EA (Eds.), Sex Differences in the Brain: From Genes to Behavior (pp. 311–325). New York: Oxford University Press. [Google Scholar]
- Hampson E, & Moffat SD (2004). The psychobiology of gender: Cognitive effects of reproductive hormones in the adult nervous system. In: Eagly AH, Beall AE, & Sternberg RJ (Eds.), The Psychology of Gender (pp. 38–64). New York: Guilford Press. [Google Scholar]
- Hampson E, & Morley EE (2013). Estradiol concentrations and working memory performance in women of reproductive age. Psychoneuroendocrinology, 38, 2897–2904. [DOI] [PubMed] [Google Scholar]
- Hampson E, Phillips SD, Soares CN, & Steiner M (2013). Steroid concentrations in antepartum and postpartum saliva: Normative values in women and correlations with serum. Biology of Sex Differences, 4:7. http://www.bsd-journal.com/content/4/1/7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JD, & Rendell PG (2007). A review of the impact of pregnancy on memory function. Journal of Clinical and Experimental Neuropsychology, 29, 793–803. [DOI] [PubMed] [Google Scholar]
- Henry JF, & Sherwin BB (2012). Hormones and cognitive functioning during late pregnancy and postpartum: A longitudinal study. Behavioral Neuroscience, 126, 73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes C, Casey P, Huntsdale C, & Angus G (1999). Memory in pregnancy. I: Subjective experiences and objective assessment of implicit, explicit and working memory in primigravid and primiparous women. Journal of Psychosomatic Obstetrics and Gynecology, 20, 80–87. [DOI] [PubMed] [Google Scholar]
- Joffe H, Hall JE, Gruber S, Sarmiento IA, Cohen LS, Yurgelun-Todd D, & Martin KA (2006). Estrogen therapy selectively enhances prefrontal cognitive processes: A randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause, 13, 411–422. [DOI] [PubMed] [Google Scholar]
- Keenan PA, Ezzat WH, Ginsburg K, & Moore GJ (2001). Prefrontal cortex as the site of estrogen’s effect on cognition. Psychoneuroendocrinology, 26, 577–590. [DOI] [PubMed] [Google Scholar]
- Keenan PA, Yaldoo DT, Stress ME, Fuerst DR, & Ginsburg KA (1998). Explicit memory in pregnant women. American Journal of Obstetrics and Gynecology, 179, 731–737. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, & Kohama SG (1999). Ovarian hormones differentially influence immunoreactivity for dopamine ß-hydroxylase, choline acetyltransferase, and serotonin in the dorsolateral prefrontal cortex of adult rhesus monkeys. Journal of Comparative Neurology, 409, 438–451. [DOI] [PubMed] [Google Scholar]
- Krug R, Born J, & Rasch B (2006). A 3-day estrogen treatment improves prefrontal cortex-dependent cognitive function in postmenopausal women. Psychoneuroendocrinology, 31, 965–975. [DOI] [PubMed] [Google Scholar]
- Landrø NI, Stiles TC, & Sletvold H (2001). Neuropsychological function in nonpsychotic unipolar major depression. Neuropsychiatry, Neuropsychology, and Behavioral Neurology, 14, 233–240. [PubMed] [Google Scholar]
- Lee AM, Lam SK, Sze Mun Lau SM, Chong CS, Chui HW, & Fong DY (2007). Prevalence, course and risk factors for antenatal anxiety and depression. Obstetrics and Gynecology, 110, 1102–1112. [DOI] [PubMed] [Google Scholar]
- Marcus SM, Flynn HA, Blow FC, & Barry KL (2003). Depressive symptoms among pregnant women screened in obstetrics settings. Journal of Women’s Health, 12, 373–380. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Glahn DC, Peluso MAM, Hatch JP, Monkul ES, Najt P, Sanches M, Zamarripa F, Li J, Lancaster JL, Fox PT, Gao J-H, & Soares JC (2007). Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Molecular Psychiatry, 12, 158–166. [DOI] [PubMed] [Google Scholar]
- Macbeth AH, & Luine VN (2010). Changes in anxiety and cognition due to reproductive experience: A review of data from rodent and human mothers. Neuroscience and Biobehavioral Reviews, 34, 452–467. [DOI] [PubMed] [Google Scholar]
- Milner B (1971). Interhemispheric differences in the localization of psychological processes in man. British Medical Journal, 27, 272–277. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, & Asberg M (1979). A new depression scale designed to be sensitive to change. British Journal of Psychiatry, 134, 382–389. [DOI] [PubMed] [Google Scholar]
- Mooney CM, & Ferguson GA (1951). A new closure test. Canadian Journal of Psychology, 5, 129–133. [DOI] [PubMed] [Google Scholar]
- Murray D, & Cox JL (1990). Screening for depression during pregnancy with the Edinburgh Depression Scale. Journal of Reproductive and Infant Psychology, 8, 99–107. [Google Scholar]
- Murrough JW, Iacoviello B, Neumeister A, Charney DS, Iosifescu DV (2011). Cognitive dysfunction in depression: Neurocircuitry and new therapeutic strategies. Neurobiology of Learning and Memory, 96, 553–563. [DOI] [PubMed] [Google Scholar]
- Nolten WE, Lindheiner MD, Ruechert PA, Poaril S & Ehrlich EN (1980). Diurnal patterns and regulation of cortisol secretion in pregnancy. Journal of Clinical Endocrinology and Metabolism, 51, 466–472. [DOI] [PubMed] [Google Scholar]
- O’Keane V, & Marsh MS (2007). Depression during pregnancy. British Medical Journal, 334, 1003–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyper SV, Searleman A, Thacher PV, Maine EE, & Johnson AG (2010). Executive functioning and general cognitive ability in pregnant women and matched controls. Journal of Clinical and Experimental Neuropsychology, 32, 986–99. [DOI] [PubMed] [Google Scholar]
- Owen AM (1997). The functional organization of working memory processes within human lateral frontal cortex: The contribution of functional neuroimaging. European Journal of Neuroscience, 9, 1329–1339. [DOI] [PubMed] [Google Scholar]
- Parcells DA (2010). Women’s mental health nursing: Depression, anxiety and stress during pregnancy. Journal of Psychiatric and Mental Health Nursing, 17, 813–820. [DOI] [PubMed] [Google Scholar]
- Parsons C, & Redman S (1991). Self-reported cognitive change during pregnancy. The Australian Journal of Advanced Nursing, 9, 20–29. [PubMed] [Google Scholar]
- Passingham RE (1985). Memory of monkeys (Macaca mulatta) with lesions in prefrontal cortex. Behavioral Neuroscience, 99, 3–21. [DOI] [PubMed] [Google Scholar]
- Petrides M (2000). Frontal lobes and memory. In: Boller F and Grafman J (Eds.), Handbook of Neuropsychology Vol. 2 (pp. 67–84). Amsterdam: Elsevier Science BV. [Google Scholar]
- Petrides M, & Milner B (1982). Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia, 20, 249–262. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, & Roberts JA (2003). Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. Journal of Neuroscience, 23, 5708–5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell PG, & Henry JD (2008). Prospective memory functioning is affected during pregnancy and postpartum. Journal of Clinical and Experimental Neuropsychology, 30, 913–919. [DOI] [PubMed] [Google Scholar]
- Rose EJ, & Ebmeier KP (2006). Pattern of impaired working memory during major depression. Journal of Affective Disorders, 90, 149–161. [DOI] [PubMed] [Google Scholar]
- Scott EM, McGarrigle HHG, & Lachelin GCL (1990). The increase in plasma and saliva cortisol levels in pregnancy is not due to the increase in corticosteroid-binding globulin levels. Journal of Clinical Endocrinology and Metabolism, 71, 639–644 [DOI] [PubMed] [Google Scholar]
- Sharp K, Brindle PM, Brown MW, & Turner GM (1993). Memory loss during pregnancy. British Journal of Obstetrics and Gynaecology, 100, 209–215. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Janavs J, Weiller E, Keskiner A, Schinka J, Knapp E, Sheehan MF, & Dunbar GC (1997). The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. European Psychiatry, 12, 232–241. [Google Scholar]
- Smith YR, Love T, Persad CC, Tkaczyk A, Nichols TE, & Zubieta JK (2006). Impact of combined estradiol and norethindrone therapy on visuospatial working memory assessed by functional magnetic resonance imaging. Journal of Clinical Endocrinology and Metabolism, 91, 4476–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland PL, Deakin JFW, Percival C, Dixon J, Gater RA, & Goldberg DP (2002). Bio-social origins of depression in the community. British Journal of Psychiatry, 180, 168–173. [DOI] [PubMed] [Google Scholar]
- Veiel HOF (1997). A preliminary profile of neuropsychological deficits associated with major depression. Journal of Clinical and Experimental Neuropsychology, 19, 587–603. [DOI] [PubMed] [Google Scholar]
- Wang ACJ, Hara Y, Janssen WGM, Rapp PR, & Morrison JH (2010). Synaptic estrogen receptor-α levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance. Journal of Neuroscience, 30, 12770–12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wide JK, Hanratty K, Ting J, & Galea LAM (2004). High level estradiol impairs and low level estradiol facilitates non-spatial working memory. Behavioural Brain Research, 155, 45–53. [DOI] [PubMed] [Google Scholar]
- Wilson DL, Barnes M, Ellett L, Permezel M, Jackson M, & Crowe SF (2011). Compromised verbal episodic memory with intact visual and procedural memory during pregnancy. Journal of Clinical and Experimental Neuropsychology, 33, 680–691. [DOI] [PubMed] [Google Scholar]
- Wilson EA, Finn AE, Rayburn W, & Jawad MJ (1979). Corticosteroid-binding globulin and estrogens in maternal and cord blood. American Journal of Obstetrics and Gynecology, 135, 215–218. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, Ramin S, Chaudron L, & Lockwood C (2009). The management of depression during pregnancy: A report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstetrics & Gynecology, 114, 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AH, Sahakian BJ, Robbins TW, & Cowen PJ (1999). The effects of chronic administration of hydrocortisone on cognitive function in normal male volunteers. Psychopharmacology, 145, 260–266. [DOI] [PubMed] [Google Scholar]


