Abstract
Background
Cognitive dysfunction after surgery is a major issue in older adults. Here, we determined the effect of APOE4 on perioperative neurocognitive function in older patients.
Methods
We enrolled 140 English-speaking patients ≥60 yr old scheduled for noncardiac surgery under general anaesthesia in an observational cohort study, of whom 52 underwent neuroimaging. We measured cognition; Aβ, tau, p-tau levels in CSF; and resting-state intrinsic functional connectivity in six Alzheimer's disease-risk regions before and 6 weeks after surgery.
Results
There were no significant APOE4-related differences in cognition or CSF biomarkers, except APOE4 carriers had lower CSF Aβ levels than non-carriers (preoperative median CSF Aβ [median absolute deviation], APOE4 305 pg ml−1 [65] vs 378 pg ml−1 [38], respectively; P=0.001). Controlling for age, APOE4 carriers had significantly greater preoperative functional connectivity than non-carriers between several brain regions implicated in Alzheimer's disease, including between the left posterior cingulate cortex and left angular gyrus (β [95% confidence interval, CI], 0.218 [0.137–0.230]; PFWE=0.016). APOE4 carriers, but not non-carriers, experienced significant connectivity decreases from before to 6 weeks after surgery between several brain regions including between the left posterior cingulate cortex and left angular gyrus (β [95% CI], –0.196 [–0.256 to –0.136]; PFWE=0.001). Most preoperative and postoperative functional connectivity differences did not change after controlling for preoperative CSF Aβ levels.
Conclusions
Postoperative change trajectories for cognition and CSF Aβ, tau or p-tau levels did not differ between community dwelling older APOE4 carriers and non-carriers. APOE4 carriers showed greater preoperative functional connectivity and greater postoperative decreases in functional connectivity in key Alzheimer's disease-risk regions, which occur via Aβ-independent mechanisms.
Keywords: Alzheimer's disease, APOE4, cerebrospinal fluid, functional MRI, intrinsic functional connectivity, neuroimaging, perioperative neurocognitive disorders, surgery
Editor's key points.
-
•
Patients undergoing noncardiac surgery were enrolled in an observational cohort study of neuroimaging, APOE4 genotyping, cognition, CSF biomarkers, and resting-state functional connectivity before and 6 weeks after surgery.
-
•
There were no significant APOE4-related differences in cognition or CSF biomarkers, except APOE4 carriers had lower CSF Aβ levels than non-carriers.
-
•
APOE4 carriers showed greater preoperative functional connectivity and greater postoperative decreases in functional connectivity in key Alzheimer's disease-risk regions.
-
•
The disassociation between Alzheimer's disease biomarkers and cognitive change from functional brain connectivity change after surgery in APOE4 carriers underscores the value of multidisciplinary approaches to understanding the complex aetiology of perioperative cognitive trajectories.
Nearly seven decades of surgical outcome studies show that older patients are at increased risk for both acute and lingering cognitive deficits after anaesthesia and surgery.1 Postoperative cognitive dysfunction (POCD) is an objective cognitive decline of >1 or >2 standard deviations (sd) from preoperative baseline that occurs 1–12 months after surgery, also referred to as postoperative neurocognitive disorder when it is accompanied by subjective cognitive complaints.2 POCD incidence rates vary depending on surgery type (up to 50% 3 months after cardiac surgery3 vs 20% 3 months after noncardiac surgery3), age group (more than 10% in patients >60 yr vs 5% in middle-aged and younger adults4), and the threshold used to define cognitive decline.5
The exact relationship between POCD and Alzheimer's disease (AD) is unclear, although several lines of evidence suggest they are related. Patients who develop POCD within weeks to months after surgery have greater long-term cognitive decline 5 yr later,6,7 and patients with preclinical Alzheimer's disease and related dementias (ADRD) pathology (e.g. low CSF Aβ levels) are at increased risk of POCD.8 Thus, several studies have examined postoperative cognition in carriers of the apolipoprotein E4 allele (APOE4), the most common genetic risk factor for late-onset AD, although this literature is mixed. One retrospective study found no difference in 6–10 yr cognitive trajectories between middle-aged APOE4 carriers vs non-carriers.9 In older adults, however, retrospective and prospective studies have found greater long-term10,11 (i.e. over years) – but not short-term12 (i.e. ≤1 yr) – cognitive decline after surgery in older APOE4 carriers vs non-carriers. Prospective observational cohort studies have found that older APOE4 carriers have worse 5-yr postoperative cognitive trajectories after cardiac surgery than non-carriers.10 Furthermore, there is an interaction effect between APOE4 carrier status and surgery exposure for long-term cognitive decline.11 APOE4 carriers who underwent surgery had worse long-term cognitive decline than both APOE4 carriers who did not undergo surgery and non-carriers who had surgery.11
One potential mechanism for this interaction could be a postoperative acceleration of pre-clinical AD neuropathology and related brain connectivity changes in APOE4 carriers. To evaluate this hypothesis, we studied preoperative to postoperative changes in cognition, CSF Aβ, tau, and phospho-tau levels, and functional brain connectivity in community-dwelling older adult APOE4 carriers compared with non-carriers undergoing major noncardiac surgery.
Methods
Study registration, human subjects
Data reported here were obtained from patients prospectively enrolled in the observational cohort study Markers of Alzheimer's Disease and neuroCognitive Outcomes after Perioperative Care (MADCO-PC)13 at Duke University Medical Center (Durham, NC, USA). Patients signed written informed consent before participation in the MADCO-PC study, in accord with the Declaration of Helsinki. MADCO-PC was registered with clinicaltrials.gov (NCT01993836) and approved by Duke Medical Center Institutional Review Board (5/15/2013).
Patients were eligible to participate if they were English-speaking, age 60 yr or older, and scheduled for non-neurological, noncardiac surgery under general anaesthesia with a planned hospital stay of ≥1 night.13 Exclusion criteria included: imprisonment, anticoagulants precluding safe lumbar puncture, contraindication to receiving isoflurane or propofol, or systemic chemotherapy between preoperative and 6 week follow-up (to avoid confounding cognitive effects). There were no exclusions based on preoperative cognitive impairment.
Sample collection, neurological disease biomarkers, and APOE4 genotyping
CSF samples were collected before, 24 h and 6 weeks after surgery using a 25G pencil-point needle by our established protocol to reduce pain/complications.14 CSF Aβ42, tau, and phospho-tau181p were measured on an xMAP Luminex platform (Luminex Corp, Austin, TX, USA) using Innogenetics immunoassay reagents (INNO-BIA AlzBio3, Ghent, Belgium) per Alzheimer's Disease Neuroimaging Initiative protocols.15 All assays were performed in duplicate. Aβ42 levels were classified as normal or pathologic based on a cut-off of 250 pg ml−1 (5%) for indeterminate values.16 APOE4 genotyping was determined from whole blood samples as described (see Supplemental methods).17 Patients with an APOE2 allele were included in the APOE4 non-carrier group, except one patient with an APOE2/APOE4 compound heterozygous genotype, whom we excluded from analysis because of the counterbalancing effects of these two alleles on cognition and ADRD risk.18
In the case of missing cognitive or CSF data (see Supplementary materials), we utilised multiple imputation using SRCWare software (University of Michigan in Ann Arbor, MI, USA) based on all known patient and surgical factors in the full study cohort of 140 patients, and observed cognitive test scores and CSF biomarker concentrations in the full 140 patient data set.19 We created 10 imputation datasets and used standard methods to pool across imputed sets.20
We used Wilcoxon rank sum tests to compare CSF biomarker levels at baseline, 24 h, and 6 weeks between the APOE4 carrier groups because of non-normal data distribution(s). We corrected for multiple comparisons using a false discovery rate with q=0.05, and we report q-values rather than P-values. We used Friedman tests to examine CSF biomarker trajectories over time within each group (APOE4 carriers and non-carriers). As CSF biomarker data were non-normally distributed, data were summarised by medians and median absolute deviations.
Neurocognitive assessment and delirium screening
Neurocognitive assessments were completed within 1 month before and 6 weeks after surgery using our established cognitive assessment battery (see Supplemental methods for individual test details).21 To minimise redundancy, a factor analysis with oblique rotation was performed on 10 scores from these individual tests, which resulted in four factors (i.e. cognitive domains) using factor weights from a comparable large cohort study.12 These four cognitive domain scores were averaged together to create a global cognitive index. Perioperative change in global cognition and within cognitive domains was defined as the interval change from before to 6 weeks after surgery, with 0 indicating no change, a negative score indicating cognitive decline, and vice versa.
Neuroimaging data acquisition, preprocessing, and analyses
By design, because of funding limitations, we planned that half of the study patients would undergo neuroimaging. In practice, this meant that 72 patients underwent functional MRI (fMRI) before surgery and 58 returned for postoperative neuroimaging. Neuroimaging data were collected within 1 month before surgery and 6 weeks after surgery.22 Structural and functional neuroimaging data were preprocessed and analysed using Statistical Parametric Mapping (SPM12, v. 7771; Wellcome Institute, London, UK) and functional connectivity CONN Toolbox (v. 19c; McGovern Institute for Brain Research, Massachusetts Institute of Technology, Cambridge, MA, USA) in MATLAB (v. R2017b 64-bit; The MathWorks, Inc., Natick, MA, USA).
Post-processed rsfMRI low-frequency blood oxygen level dependent (BOLD) MRI data were entered into seed-to-voxel analyses to test for preoperative differences and preoperative to 6 week postoperative change differences between APOE4 carriers and non-carriers controlling for age. Seed regions of interest (ROIs) were preselected from the SPM12 Neuromorphometrics Atlas23, 24, 25, 26 for six key AD-associated brain regions (i.e. left/right posterior cingulate cortices, left/right entorhinal cortices, and left/right hippocampal cortices). Each of the six AD-associated seed ROIs were interrogated for bivariate correlation with BOLD low-frequency signal throughout the rest of the brain using a canonical haemodynamic response function. Resulting participant seed-to-voxel first-level correlation maps were entered into random-effects analyses of covariance (ancova) with partitioned error variance modelling for each a priori ADRD-associated ROI seed. Statistical significance was set at a peak voxel threshold of P<0.001 and a family-wise error (FWE) corrected cluster extent threshold of PFWE<0.05.
The functional correlates of any brain regions identified by these seed-to-voxel analyses were determined through unbiased queries of a large open-source neuroimaging meta-analytic database, Neurosynth (http://neurosynth.org),27 using MNI atlas coordinates for the peak voxel within any statistically significant cluster (i.e. database studies reporting activation within 6 mm). Associated terms were retained if individual voxel posterior probability >0.75 and seed-based network meta-analytic coactivation r>0.10. See Supplementary materials for additional details of the neuroimaging methods.
Results
Patient characteristics by APOE4 carrier status
Figure 1 shows the study Consolidated Standards of Reporting Trials (CONSORT) diagram. We included all patients who returned for 6 week postoperative follow-up cognitive testing and had APOE genotyping in our primary analysis (n=109). Baseline patient characteristics based on APOE4 carrier status are shown in Table 1. None of the patients had a clinical diagnosis of AD or other related dementias; one patient had a diagnosis of mild cognitive impairment. There were 31 patients with the APOE3/APOE4 genotype, one patient had the APOE2/APOE4 genotype, and three patients were homozygous for APOE4. No statistically significant differences in baseline characteristics were noted by APOE4 genotype, except that baseline Aβ levels were lower in APOE4 carriers compared with non-carriers (median [median absolute deviation], APOE4 carriers 305 [65] pg ml−1 vs non-carriers 378 [38] pg ml−1; P=0.001). There was a trend towards a higher fraction of patients with low baseline CSF Aβ42 (i.e. <250 pg ml−1) among APOE4 carriers (9 of 35, 25.7%) vs non-carriers (9 of 74, 12.2%), but this difference did not reach statistical significance (P=0.075), perhaps because of insufficient sample size.
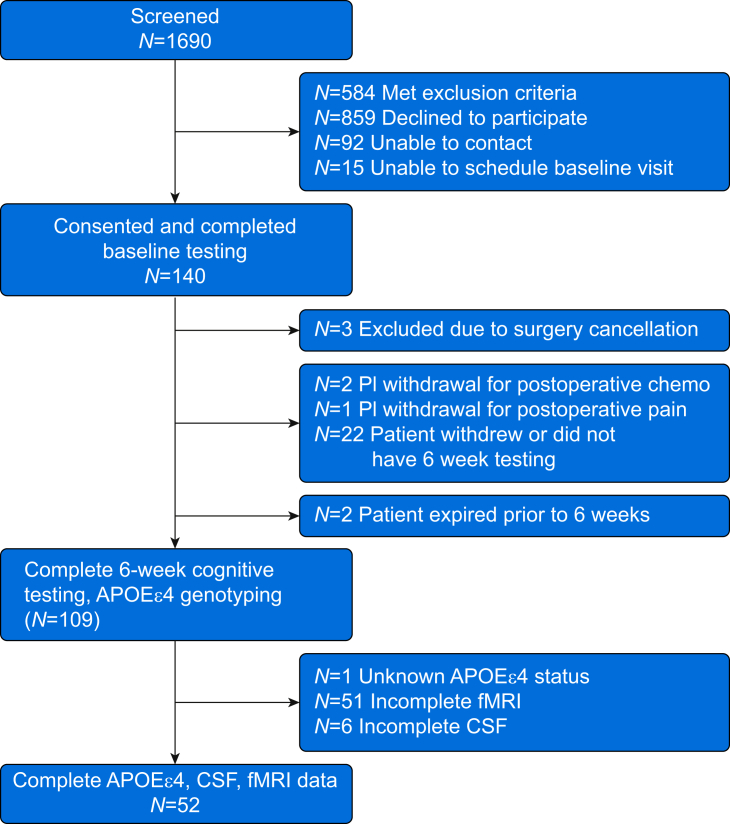
Fig 1.
MADCO-PC surgical patients consort diagram. Of the 109 patients who returned for 6 week cognitive testing and had APOEε4 genotyping, 52 patients had preoperative and 6 week postoperative neuroimaging and CSF samples obtained at all three time points. APOE4, apolipoprotein E4; fMRI, functional MRI; MADCO-PC, Markers of Alzheimer's Disease and neuroCognitive Outcomes after Perioperative Care.
Table 1.
Participant characteristics by APOE4 carrier status. Values listed as either mean (sd), or median [Q1, Q3], or MAD. ∗Based on pooling of imputed values for larger set of patients with complete follow-up and APOE4 status and the CSF error is MAD, for those with imaging the CSF data is complete, so no imputation and errors are (Q1, Q3). †Among those with available BIS data. ‡Among those receiving gas; P-value key: 1equal variance t-test;2χ2 test; 3Wilcoxon's test. ¶WMH burden = [White matter hyperintensity volume/(White matter volume + White matter hyperintensity volume)]. aaMAC, age-adjusted end-tidal minimum alveolar concentration of volatile anaesthetics; BIS, bispectral index; LOS, length of stay; MAD, median absolute deviation; MMSE, Mini-Mental State Examination; sd, standard deviation; Q1, first quartile; Q3, third quartile.
| Patients with complete follow-up and APOE4 status |
Patients with complete follow-up, APOE4 status, neuroimaging, and CSF samples |
|||||
|---|---|---|---|---|---|---|
| APOE4 negative (n=74) | APOE4 positive (n=35) | P value | APOE4 negative (n=33) | APOE4 positive (n=19) | P value | |
| Patient characteristics | ||||||
| Age (yr), mean (sd) | 69.6 (6.9) | 68.4 (5.6) | 0.3491 | 68.61 (5.73) | 68.74 (5.15) | 0.9351 |
| Race (%) | 0.3602 | 0.8662 | ||||
| Black or African American | 5 (6.8) | 5 (14.3) | 30 (90.9) | 17 (89.5) | ||
| Caucasian/White | 68 (91.9) | 30 (85.7) | 3 (9.1) | 2 (10.5) | ||
| Not reported/declined | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Sex (male), n (%) | 48 (64.9) | 21 (60.0) | 0.6232 | 22 (66.7) | 12 (63.2) | 0.7982 |
| Height (cm) (Q1, Q3) | 173 [165, 180] | 173 [163, 180] | 0.9533 | 173 [145, 193] | 175 [165, 183] | 0.5333 |
| Weight (kg), mean (sd) | 84.7 (18.0) | 86.7 (19.8) | 0.6101 | 84.95 (18.85) | 89.93 (16.22) | 0.3401 |
| BMI (kg m−2), median [Q1, Q3] | 28.0 [24.5, 31.4] | 29.5 [24.3, 33.1] | 0.5483 | 28.4 [25.1, 31.4] | 30.5 [24.8, 33.1] | 0.2843 |
| Years of education, median [Q1, Q3] | 14.0 [12.0, 17.0] | 16.0 [13.0, 18.0] | 0.1823 | 16 [13, 17] | 16 [12, 18] | 0.8783 |
| MMSE total score, median [Q1, Q3] | 29.0 [28.0, 29.0] | 29.0 [27.0, 29.0] | 0.0653 | 29 [28, 30] | 29 [27, 29] | 0.3993 |
| MMSE category (%) | 0.3422 | 0.3142 | ||||
| <20 | 0 (0.0) | 1 (2.9) | 0 (0.0) | 1 (5.3) | ||
| 20–24 | 4 (5.4) | 2 (5.7) | 1 (3.0) | 0 (0.0) | ||
| 25–30 | 70 (94.6) | 32 (91.4) | 32 (97.0) | 18 (94.7) | ||
| Baseline cognitive index, mean (sd)∗ | 0.09 (0.7) | 0.03 (0.8) | 0.6211 | 0.06 (0.7) | 0.05 (0.8) | 0.9641 |
| Baseline Aβ∗ | 378 (37.9)a | 305 (65.1)a | 0.0013 | 389 [292, 410]b | 298 [245, 376]b | 0.0123 |
| Baseline p-tau∗ | 28 (6.1)a | 25 (6.9)a | 0.2683 | 28 [22, 33]b | 25 [18, 34]b | 0.4193 |
| Baseline P-tau/Aβ∗ | 0.07 (0.02)a | 0.08 (0.02)a | 0.3753 | 0.07 [0.06, 0.09]b | 0.09 [0.06, 0.10]b | 0.4793 |
| Baseline tau∗ | 48 (7.57)a | 47 (9.5)a | 0.3143 | 48 [44, 59]b | 44 [35, 52]b | 0.1863 |
| Baseline tau/Aβ∗ | 0.13 (0.02)a | 0.15 (0.04)a | 0.1663 | 0.13 [0.11, 0.17]b | 0.15 [0.12, 0.19]b | 0.3503 |
| Surgical characteristics | ||||||
| ASA physical status (%) | 0.5802 | 0.7482 | ||||
| 1 | 1 (1.4) | 0 (0.0) | 1 (3.0) | 0 (0.0) | ||
| 2 | 14 (18.9) | 9 (25.7) | 6 (18.2) | 4 (21.1) | ||
| 3 | 57 (77.0) | 26 (74.3) | 25 (75.8) | 15 (78.9) | ||
| 4 | 2 (2.7) | 0 (0.0) | 1 (3.0) | 0 (0.0) | ||
| Surgical service (%) | 0.4732 | 0.3942 | ||||
| Thoracic | 8 (10.8) | 3 (8.6) | 4 (12.1) | 2 (10.5) | ||
| General surgery | 20 (27.0) | 11 (31.4) | 11 (33.3) | 6 (31.6) | ||
| Gynaecology | 1 (1.4) | 1 (2.9) | 1 (3.0) | 1 (5.3) | ||
| Orthopaedics | 14 (18.9) | 7 (20.0) | 7 (21.2) | 2 (10.5) | ||
| Otolaryngology head and neck | 0 (0.0) | 2 (5.7) | 0 (0.0) | 2 (10.5) | ||
| Plastic surgery | 2 (2.7) | 1 (2.9) | 0 (0.0) | 1 (5.3) | ||
| Urology | 29 (39.2) | 10 (28.6) | 10 (30.3) | 5 (26.3) | ||
| Block type (%) | 0.6912 | 0.1892 | ||||
| None | 62 (83.8) | 31 (88.6) | 27 (81.8) | 18 (94.7) | ||
| Regional | 11 (14.9) | 4 (11.4) | 6 (18.2) | 1 (5.3) | ||
| Epidural | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Surgery duration (min), median [Q1, Q3] | 141 [98, 194] | 143 [105, 228] | 0.6433 | 129 [98, 161] | 145 [84, 261] | 0.4783 |
| Case average BIS,† median [Q1, Q3] | 46.6 (42.0, 51.8) | 45.8 [40.2, 49.3] | 0.7643 | 44.5 [42.5, 51.5] | 46.1 [39.0, 50.6] | 0.9893 |
| Case average aaMAC,‡ median [Q1, Q3] | 0.7 [0.7, 0.8] | 0.8 [0.7, 0.8] | 0.4903 | 0.8 [0.7, 0.9] | 0.7 [0.7, 0.8] | 0.5583 |
| Intraoperative propofol dose (mg), median [Q1, Q3] | 305 [150, 1201] | 552 [170, 1335] | 0.4593 | 200 [140, 970] | 729 [150, 1665] | 0.1543 |
| Hospital LOS (days), median [Q1, Q3] | 2 [1, 3] | 2.0 [1, 3] | 0.6483 | 2 [1, 3] | 2 [1, 3] | 0.7873 |
| Global brain volume metrics | ||||||
| Mean cortical grey matter thickness | – | – | – | 2.52 (0.10) | 2.55 (0.12) | 0.3081 |
| sd cortical grey matter thickness | – | – | – | 0.75 (0.04) | 0.76 (0.06) | 0.5191 |
| Grey matter/total intracranial volume | – | – | – | 0.39 (0.03) | 0.39 (0.03) | 0.7751 |
| White matter/total intracranial volume | – | – | – | 0.33 (0.06) | 0.32 (0.02) | 0.6251 |
| CSF/total intracranial volume | – | – | – | 0.27 (0.05) | 0.28 (0.04) | 0.2501 |
| White matter hyperintensity burden¶ | – | – | – | 0.01 (0.01) | 0.01 (0.01) | 0.5751 |
Bolded values represent P < 0.05.
CSF biomarker trajectories by APOE4 carrier status
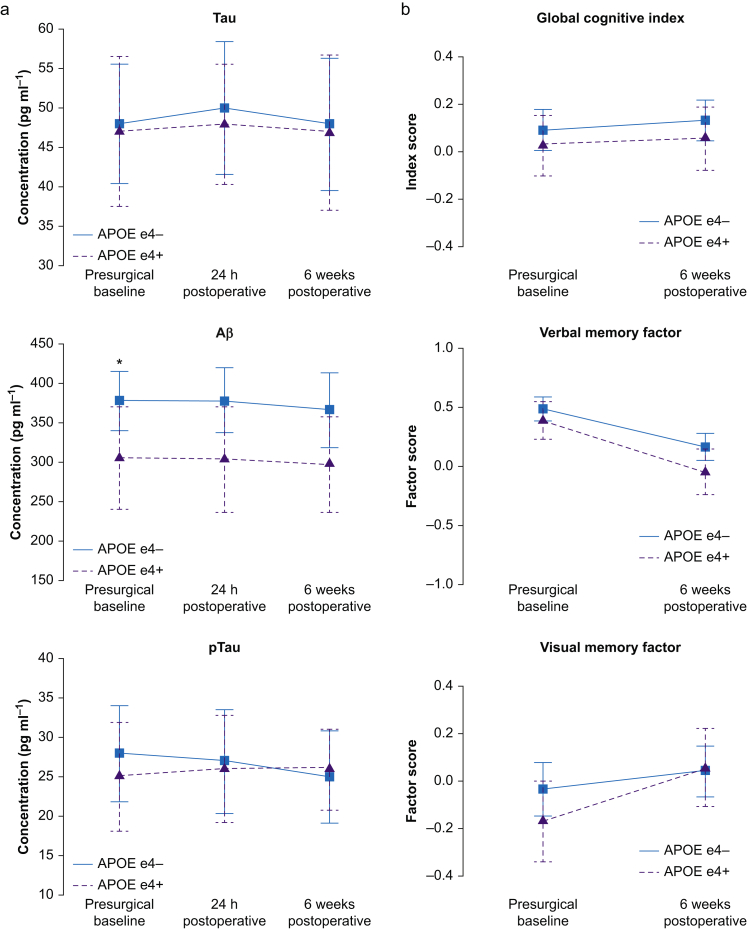
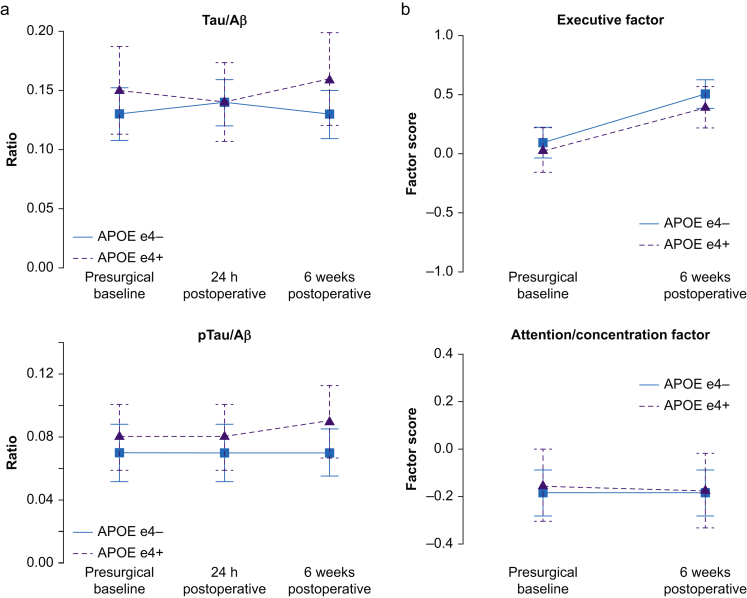
APOE4 carriers also had lower CSF Aβ levels compared with non-carriers at both 24 h after surgery (304 [68] pg ml−1 vs 378 [41] pg ml−1, respectively; q=0.020) and 6 weeks after surgery (297 [61] pg ml−1 vs 367 [47] pg ml−1, respectively; q=0.025; Fig. 2a). There were no differences in CSF tau or phospho-tau levels, or the phosphoTau/Aβ or Tau/Aβ ratios between APOE4 carriers and non-carriers at any time point (q>0.05 for all). Furthermore, there was no significant change over time in Aβ, phosphoTau, tau, phosphoTau/Aβ, or Tau/Aβ among either APOE4 carriers or non-carriers (all multiple corrected Friedman P-values >0.40).
Fig 2.
Perioperative neurocognitive trajectories by APOE4 genotype. (a) Perioperative CSF AD biomarker trajectories. ∗P<0.05. (b) Indicated cognitive trajectories. AD, Alzheimer's disease.
Cognitive outcomes by APOE4 carrier status
Figure 2b shows global cognitive index and cognitive factor domain scores before and 6 weeks after surgery. There were no significant differences in global cognitive change from before to 6 weeks after surgery between APOE4 carriers and non-carriers (mean change [sd], 0.03 [0.05] vs 0.04 [0.04], respectively; P=0.830). There was a decrease in verbal memory and increases in spatial memory and executive function performance from before to 6 weeks after surgery in both APOE4 carriers and non-carriers (P<0.05 for each). However, there was no difference in baseline performance or postoperative change within any individual cognitive domain between APOE4 carriers and non-carriers (q>0.20 for all). There was also no difference in the percentage of APOE4 carriers and non-carriers with a ≥1 sd decrease in ≥1 cognitive domain from before to 6 weeks after surgery (28.6% vs 27.0%, respectively), which is a cognitive change threshold used in numerous prior studies.21,28, 29, 30
Neuroimaging differences by APOE4 carrier status
Preoperative/baseline intrinsic functional connectivity
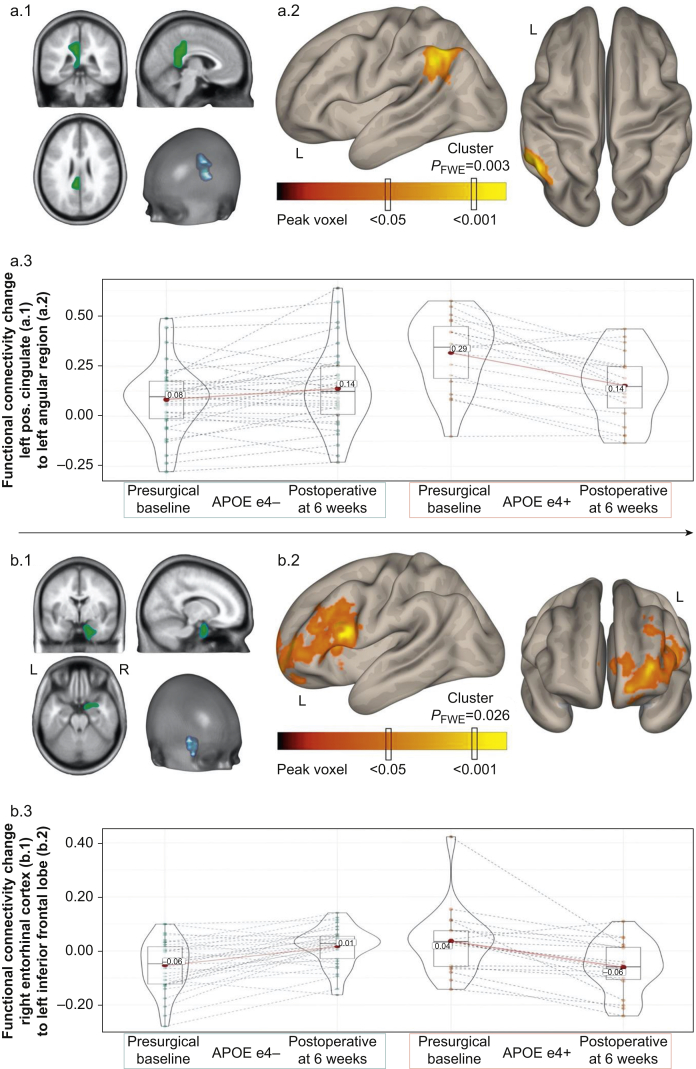
Seed-to-voxel analyses controlling for age identified greater intrinsic functional connectivity in APOE4 carriers compared with non-carriers between the left hippocampus seed and left angular/supramarginal gyrus (β [95% confidence interval, CI], 0.163 [0.108–0.219]; PFWE=0.005; Table 2) and between the left hippocampus seed and right angular/supramarginal gyrus (β [95% CI], 0.157 [0.099–0.216]; PFWE=0.031). Similarly, APOE4 carriers had greater intrinsic functional connectivity from the right hippocampus seed to the angular/supramarginal gyrus region (β [95% CI], 0.159 [0.112–0.206]; PFWE<0.001; Table 2). APOE4 carriers also had greater preoperative baseline functional connectivity than non-carriers from the left posterior cingulate to left angular gyrus region (β [95% CI], 0.218 [0.137–0.230]; PFWE=0.016) and right posterior cingulate to the ventral anterior cingulate gyrus (β [95% CI], 0.163 [0.111–0.215]; PFWE=0.003; Table 2).
Table 2.
Statistically significant preoperative baseline and postoperative change intrinsic functional connectivity differences in APOE4 carrier groups for Alzheimer's disease risk-associated regions of interest (ROIs). ∗Anatomical and Brodmann area labels based upon 7 mm3 search range of the Talairach Daemon Database using MNI-to-Talairach non-linear transform coordinates.31†Effect size of the difference in mean standardised regression coefficients (β) within brain region clusters demonstrating statistically significant group-wise effects. ‡Montreal Neurological Space (MNI) ICBM152 non-linear sixth-generation brain atlas coordinates. Seed-to-voxel, random-effects, between-group (APOE4+ vs APOE4–) comparison of preoperative baseline functional connectivity maps, adjusted for age¶ (or age and preoperative baseline Aβ)§, or comparison of 6 weeks postoperative minus preoperative baseline functional connectivity difference maps, adjusting for age|| (or age and preoperative baseline Aβ)#, using a priori ROI seeds from known Alzheimer's disease risk regions (i.e. Neuromorphometrics ROI Atlas left/right entorhinal cortices, left/right hippocampi, left/right posterior cingulate cortices) by hemisphere [APOE4+ (n=19), APOE4− (n=33); peak voxel threshold P<0.001, cluster spatial extent (kE) threshold PFWE <0.05].
| Seed/ROI | Associated regions (Brodmann areas)∗ | Cluster extent (kE) | Cluster (PFWE) | SPM(T) | Hedges' g (effect r)† | Cluster maxima‡ |
||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Preoperative baseline (controlling for age)¶ | ||||||||
| Left hippocampus | Left angular gyrus (BA 39)/supramarginal gyrus (BA 40) | 364 | 0.005 | 5.40 | 1.41 (0.57) | –56 | –50 | 48 |
| Right angular gyrus (BA 39)/supramarginal gyrus (BA 40) | 244 | 0.031 | 4.88 | 1.27 (0.53) | 58 | –48 | 34 | |
| Right hippocampus | Left angular gyrus (BA 39)/supramarginal gyrus (BA 40) | 1027 | <0.001 | 5.77 | 1.62 (0.62) | –52 | –50 | 50 |
| Left posterior cingulate region | Left angular gyrus (BA 39)/supramarginal gyrus (BA 40) | 260 | 0.016 | 4.57 | 1.27 (0.53) | –54 | –56 | 34 |
| Right posterior cingulate region | Posterior aspect of ventral anterior cingulate gyrus (BA 24)/juxtapositional lobule (BA 6) | 374 | 0.003 | 4.88 | 1.49 (0.59) | 10 | –20 | 50 |
| Preoperative baseline (controlling for age and preoperative CSF Aβ levels)§ | ||||||||
| Left hippocampus | Left angular gyrus (BA 39)/supramarginal gyrus (BA 40) | 460 | 0.023 | 4.26 | 1.40 (0.57) | –56 | –52 | 48 |
| Right hippocampus | Left angular gyrus (BA 39)/supramarginal gyrus (BA 40) | 845 | <0.001 | 5.63 | 1.61 (0.62) | –54 | –48 | 50 |
| Right posterior cingulate region | Posterior aspect of ventral anterior cingulate gyrus (BA 24)/juxtapositional lobule (BA 6) | 317 | 0.006 | 4.36 | 1.48 (0.59) | 12 | –22 | 48 |
| Postoperative change (controlling for age)|| | ||||||||
| Right entorhinal | Left inferior frontal gyrus (pars opercularis; BA 44) | 363 | 0.001 | 5.26 | 1.73 (0.65) | –48 | 12 | 20 |
| Left posterior cingulate region | Left angular gyrus (BA 39)/supramarginal gyrus (BA 40) | 369 | 0.001 | 5.50 | 1.55 (0.60) | –58 | –54 | 44 |
| Postoperative change (controlling for age and preoperative CSF Aβ levels)# | ||||||||
| Right entorhinal | Left inferior frontal gyrus (pars opercularis; BA 44) | 327 | 0.002 | 5.39 | 1.74 (0.65) | –50 | 12 | 18 |
| Left posterior cingulate region | Left angular gyrus (BA 39)/supramarginal gyrus (BA 40) | 204 | 0.026 | 5.04 | 1.58 (0.61) | –58 | –54 | 44 |
Meta-analytic Neurosynth27 associations for the angular/supramarginal gyrus regions were ‘dorsal attention (network)’ and ‘memory retrieval’. Neurosynth associations for the posterior aspect of ventral anterior cingulate gyrus were ‘motor imagery’, ‘supplementary motor’, and ‘sensorimotor’ (posterior probability >0.75 and meta-analytic coactivation r >0.10 for all).25
Postoperative resting-state functional connectivity change
Next, we examined differences in preoperative to 6 week postoperative functional connectivity change between APOE4 carriers and non-carriers. There were APOE4-related differences in connectivity change between the left posterior cingulate and left angular/supramarginal gyrus (β [95% CI], –0.196 [–0.256 to –0.136]; PFWE=0.001) and between the right entorhinal cortex and left inferior frontal gyrus (β [95% CI], –0.172 [–0.218 to –0.125]; PFWE=0.001; Table 2). In both cases, the significant group by time interactions were driven largely by significant postoperative reductions in increased baseline functional connectivity in APOE4 carriers.
Meta-analytic Neurosynth27 associations for the angular/supramarginal gyri were ‘recollection’ processes, ‘memory retrieval’, and ‘reappraisal’. Similarly, Neurosynth associations for the left inferior frontal gyrus were ‘lexical decision’, ‘language network’, and ‘rehearsal’ (posterior probability >0.75 and meta-analytic coactivation r >0.10 for all).
Relationship of APOE4-related connectivity changes and CSF Aβ levels
APOE4 carriers in this and prior studies had lower CSF Aβ levels (Fig. 2a).32,33 Lower CSF Aβ levels (an indicator of increased brain Aβ plaque deposition) have been associated with altered resting state functional brain connectivity patterns.34 Thus, we examined whether the altered intrinsic functional connectivity patterns seen in APOE4 carriers were attributable to CNS Aβ pathology (as indicated by lower CSF Aβ levels). We observed only a few differences in preoperative baseline intrinsic functional results when CSF Aβ levels were included as a covariate with age in the statistical models (Table 2). Namely, the left hippocampus seed connectivity differences with the right angular gyrus and left posterior cingulate seed connectivity difference with the left angular gyrus decreased below statistical thresholds (Table 2; Supplementary Fig. S1). The postoperative intrinsic functional connectivity changes observed between APOE4 carriers and non-carriers remained unchanged (Table 2; Fig. 3). For both preoperative and postoperative analyses, altered intrinsic functional connectivity to the left angular gyrus region in APOE4 carriers remained significant after controlling for CSF Aβ levels (Table 2, Fig. 3; Supplementary Fig. S1). Thus, the differential intrinsic functional connectivity patterns seen in APOE4 carriers compared with non-carriers in these regions were not mediated by lower CSF Aβ levels in APOE4 carriers.
Fig 3.
Postoperative resting-state functional connectivity changes in APOE4 carriers and non-carriers for Alzheimer's disease-associated brain regions. (a.1) A priori left posterior cingulate cortex Alzheimer's disease-risk seed region-of-interest (SPM12 Neuromorphometrics ROI Atlas). (a.2) Left angular/supramarginal gyrus region demonstrating statistically significant (peak P<0.001; cluster PFWE<0.05) resting-state functional connectivity change from preoperative baseline to 6 weeks postoperatively between APOE e4 carrier and non-carrier groups for a.1 seed ROI. (a.3) Distribution of resting-state functional connectivity contrast values (i.e. β, standardised regression coefficient) for APOE groups between a.1 and a.2 regions at preoperative baseline and postoperatively at 6 weeks. (See Table 2, Postoperative Change section.). (b.1) A priori right entorhinal cortex Alzheimer's disease-risk seed region-of-interest (SPM12 Neuromorphometrics ROI Atlas). (b.2) Left dorsolateral prefrontal cortex region demonstrating statistically significant (peak P<0.001; cluster PFWE<0.05) resting-state functional connectivity change from preoperative baseline to 6 weeks postoperatively between APOE e4 carrier and non-carrier groups for b.1 seed ROI. (b.3) Distribution of resting-state functional connectivity contrast values (i.e. β, standardised regression coefficient) for APOE groups between b.1 and b.2 regions at preoperative baseline and postoperatively at 6 weeks. (See Table 2, Postoperative Change section.). PFWE, family-wise error probability.
Discussion
We found that older APOE4 carriers had lower CSF Aβ levels before and after surgery compared with non-carriers, although there were no statistically significant APOE4-related differences in postoperative changes in CSF Aβ, tau, or phospho-tau levels or their ratios. We also did not detect APOE4-related differences in global or domain-specific postoperative cognitive change. APOE4 carriers showed significantly increased resting-state fMRI (rsfMRI) intrinsic functional connectivity between specific AD-risk brain regions before surgery and a greater postoperative decrease in connectivity in some of the same brain regions. Furthermore, the altered functional connectivity patterns seen before and after surgery in APOE4 carriers were largely unchanged after additionally controlling for CSF Aβ levels. This suggests that altered functional brain connectivity in community dwelling older APOE4 carriers occurs independently of Aβ pathology; these changes may be related to other CNS proteomic changes that occur in APOE4 carriers independent of Aβ alterations.35
Our findings corroborate the prior finding of no difference in 6 week postoperative cognitive change between older APOE4 carriers and non-carriers.12 These findings are also consistent with prior studies demonstrating that older adult APOE4 carriers have lower CSF Aβ levels,32,33 and increased connectivity patterns in AD-associated brain functional connectivity networks.36,37 Indeed, a recent large cohort study of APOE4 carriers and non-carriers found that APOE4 carriers had greater intrinsic functional brain network connectivity across a wide age range (18–88 yr).38 Furthermore, prior studies have also shown that functional connectivity changes in APOE4 carriers may occur independently of amyloid beta plaque pathology.39
The functional relevance of the greater connectivity within specific brain regions and networks observed in APOE4 carriers here before surgery (and in prior studies34) and the reduction of this greater connectivity after surgery in APOE4 carriers remains unclear. One hypothesis is that increased functional brain connectivity helps compensate to maintain normal cognition despite the greater AD pathology typically present in APOE4 carriers than in non-carriers. Indeed, several regions displaying greater intrinsic connectivity in APOE4 carriers from this study were associated with memory retrieval and recollection processes. Thus, a loss of increased connectivity among these memory-associated regions could lead to later episodic memory deficits, which are among the first observable cognitive deficits in aging APOE4 carriers.
Alternatively, the increased connectivity phenotype seen in APOE4 carriers may reflect neuronal over-excitation or excitotoxity, which could accelerate AD-associated neuropathologic processes and contribute to cognitive decline.40 The present findings do not firmly support one of these hypotheses over the other, although a loss of a compensatory process would be generally consistent with the interaction effect previously observed between APOE4 and surgery exposure for worsened long-term cognitive decline.11 Studying longer-term postoperative cognitive outcomes in this and other ADRD risk cohorts could help differentiate between these two hypotheses. If increased connectivity is a compensatory process that helps maintain normal cognition in APOE4 carriers, then a postoperative loss of this increased connectivity should lead to worsened long-term cognitive decline than normally seen in APOE4 carriers. Conversely, if this preoperative increased connectivity pattern is detrimental, then a postoperative loss of this increased connectivity pattern would be expected to lead to reduced long-term cognitive decline in APOE4 carriers.
Our study has several limitations. First, this report includes data on 109 patients who completed 6 week postoperative cognitive testing and had APOE4 genotype data, which is smaller than some,12 but not other,33 studies on this topic. Second, some of the phenotypes related to APOE4 genotype have been related to other genomic regions in linkage disequilibrium with the APOE4 allelic variant, rather than the C-158-R amino acid mutation in the APOE4-encoded protein. Third, owing to the lack of non-surgical controls, our comparison of cognitive change between APOE4 carriers and non-carriers do not account for test–retest or potential practice effects, although they do describe relative differences between the two genotype groups (APOE4 carriers and non-carriers) over time.41 Lastly, only 140 of the 1014 eligible patients whom we contacted agreed to participate in this study; the majority of patients were unwilling to participate (often because of concern about lumbar punctures). Thus, future studies will be necessary to study the extent to which the findings reported here generalise to the larger population of older surgical patients.
Our study also has several strengths. First, 52 patients in this study underwent preoperative and postoperative rsfMRI scans, cognitive testing, and CSF sampling, making this one of the largest studies performed to date using these combined modalities in older surgical patients.22,28 This combination of techniques (CSF biomarker assays, neuroimaging, cognitive assessment) provides multifaceted insights into APOE4-related neurocognitive differences before and after surgery in older adults. Second, to our knowledge, these findings represent the first evidence for a human genetic variant associated with altered intrinsic brain functional connectivity patterns weeks after surgery and general anaesthesia, providing initial support for the general idea that there may be human genetic modifiers of brain functional connectivity patterns after general anaesthesia.
Conclusions
Older APOE4 carriers exhibited greater intrinsic functional brain connectivity within specific AD-associated brain regions before surgery but displayed a larger reduction in connectivity than non-carriers after surgery and anaesthesia, within many of these regions from before to 6 weeks after major noncardiac surgery. Future studies with longer-term follow-up should determine if this postoperative decrease in functional hyperconnectivity between the identified brain regions in APOE4 carriers is associated with worsened long-term cognitive trajectories. More broadly, the disassociation of neuropathologic biomarker (CSF Aβ) and cognitive change from functional brain connectivity change after surgery observed here in APOE4 carriers underscores the value of multi-disciplinary approaches to understand the complex aetiology of perioperative cognitive trajectories.
Authors' contributions
Study conception and design, data acquisition, statistical analysis, manuscript drafting: JB.
Statistical analysis, manuscript drafting, data interpretation: MC.
Manuscript drafting, data interpretation: RY, AS, JP, AH.
Study conception and design, data acquisition, data interpretation: KM.
Data acquisition, data interpretation: MD, EM.
Study conception and design, data interpretation: HW, HC, JM.
Study conception and design, data acquisition and interpretation, manuscript drafting: MB.
All authors contributed to data interpretation, manuscript revisions, and gave their approval to publish the current version. All authors also agree to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgements
We acknowledge the use of MRI scan data from the OASIS-3 project. The labeled data from this project was provided by Neuromorphometrics, Inc.26 under academic subscription.
Handling editor: Hugh C Hemmings Jr
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2021.08.012.
Contributor Information
Jeffrey N. Browndyke, Email: j.browndyke@duke.edu.
Miles Berger, Email: miles.berger@duke.edu.
The MADCO-PC Investigators:
S. Bengali, E. Bennett, R. Brassard, B. Brigman, M. Bullock, J. Carter, J. Chapman, B. Colin, T. D'Amico, J. DeOrio, R. Esclamado, M. Ferrandino, J. Gadsden, J. Gardner, G. Garrigues, C. Giattino, S. Grant, J. Guercio, D. Gupta, A. Habib, D. Harpole, M. Hartwig, J. Hu, E. Iboaya, B. Inman, A. Khan, S. Lagoo-Deenadayalan, D. Laskowitz, P. Lee, W. Lee, J. Lemm, H. Levinson, C. Mantyh, D. McDonagh, J. Migaly, S. Mithani, J. Moul, M. Newman, B. Ohlendorf, A. Perez, A. Peterson, G. Preminger, Q. Quinones, A. Ray, K. Roberts, C. Robertson, S. Roman, S. Runyon, A. Sandler, F. Sbahi, R. Scheri, K. Smith, L. Talbot, J. Thacker, J. Thomas, B. Tong, Y. Toulgoat-Dubois, A. Tu, S. Vaslef, M. Woldorff, N. Waldron, X. Wang, and C. Young
Declarations of interest
MB has received material support (i.e. EEG monitors) for a postoperative recovery study in older adults from Masimo, unrelated to this manuscript. MB has also received legal consulting fees related to postoperative cognition in older adults. JB acknowledges funding from Claret Medical, Inc. The other authors have no relevant conflicts to disclose.
Funding
US National Institutes of Health (R01-HL130443 to JM and JB; U01-HL088942 to JM and JB; U01-AG050618 to JB), Duke Anaesthesiology departmental funds, and a mentored research grant from the International Anesthesia Research Society. MB acknowledges additional support from the Alzheimer’s Drug Discovery Foundation (NIH grants T32 GM08600, R03AG050918, 1K76AG057022 to MB), Duke Claude D. Pepper Older American Independence Centre (P30AG028716), and William L. Young neuroscience research award from the Society for Neuroscience in Anaesthesiology and Critical Care (SNACC).
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Berger M., Terrando N., Smith S.K., Browndyke J.N., Newman M.F., Mathew J.P. Neurocognitive function after cardiac surgery: from phenotypes to mechanisms. Anesthesiology. 2018;129:829–851. doi: 10.1097/ALN.0000000000002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evered L., Silbert B., Knopman D.S., et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery—2018. Br J Anaesth. 2018;121:1005–1012. doi: 10.1016/j.bja.2017.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evered L., Scott D.A., Silbert B., Maruff P. Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth Analg. 2011;112:1179–1185. doi: 10.1213/ANE.0b013e318215217e. [DOI] [PubMed] [Google Scholar]
- 4.Monk T.G., Weldon B.C., Garvan C.W., et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 5.Daiello L.A., Racine A.M., Yun Gou R., et al. Postoperative delirium and postoperative cognitive dysfunction: overlap and divergence. Anesthesiology. 2019;131:477–491. doi: 10.1097/ALN.0000000000002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newman M.F., Kirchner J.L., Phillips-Bute B., et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 7.Evered L.A., Silbert B.S., Scott D.A., Maruff P., Ames D. Prevalence of dementia 7.5 years after coronary artery bypass graft surgery. Anesthesiology. 2016;125:62–71. doi: 10.1097/ALN.0000000000001143. [DOI] [PubMed] [Google Scholar]
- 8.Evered L., Silbert B., Scott D.A., Ames D., Maruff P., Blennow K. Cerebrospinal fluid biomarker for Alzheimer disease predicts postoperative cognitive dysfunction. Anesthesiology. 2016;124:353–361. doi: 10.1097/ALN.0000000000000953. [DOI] [PubMed] [Google Scholar]
- 9.Dokkedal U., Wod M., Thinggaard M., et al. Apolipoprotein E epsilon4 and cognitive function after surgery in middle-aged and elderly Danish twins. Eur J Anaesthesiol. 2020;37:984–991. doi: 10.1097/EJA.0000000000001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartels K., Li Y.J., Li Y.W., et al. Apolipoprotein epsilon 4 genotype is associated with less improvement in cognitive function five years after cardiac surgery: a retrospective cohort study. Can J Anaesth. 2015;62:618–626. doi: 10.1007/s12630-015-0337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schenning K.J., Murchison C.F., Mattek N.C., Silbert L.C., Kaye J.A., Quinn J.F. Surgery is associated with ventricular enlargement as well as cognitive and functional decline. Alzheimers Dement. 2016;12:590–597. doi: 10.1016/j.jalz.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonagh D.L., Mathew J.P., White W.D., et al. Cognitive function after major noncardiac surgery, apolipoprotein E4 genotype, and biomarkers of brain injury. Anesthesiology. 2010;112:852–859. doi: 10.1097/ALN.0b013e3181d31fd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giattino C.M., Gardner J.E., Sbahi F.M., et al. Intraoperative frontal alpha-band power correlates with preoperative neurocognitive function in older adults. Front Syst Neurosci. 2017;11:24. doi: 10.3389/fnsys.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nobuhara C.K., Bullock W.M., Bunning T., et al. A protocol to reduce self-reported pain scores and adverse events following lumbar punctures in older adults. J Neurol. 2020;267:2002–2006. doi: 10.1007/s00415-020-09797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trojanowski J.Q., Vandeerstichele H., Korecka M., et al. Update on the biomarker core of the Alzheimer’s disease neuroimaging initiative subjects. Alzheimers Dement. 2010;6:230–238. doi: 10.1016/j.jalz.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw L.M., Waligorska T., Fields L., et al. Derivation of cutoffs for the Elecsys® amyloid beta (1–42) assay in Alzheimer’s disease. Alzheimers Dement (Amst) 2018;10:698–705. doi: 10.1016/j.dadm.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berger M., Ponnusamy V., Greene N., et al. The effect of propofol vs. isoflurane anesthesia on postoperative changes in cerebrospinal fluid cytokine levels: results from a randomized trial. Front Immunol. 2017;8:1528. doi: 10.3389/fimmu.2017.01528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z., Shue F., Zhao N., Shinohara M., Bu G. APOE2: protective mechanism and therapeutic implications for Alzheimer's disease. Mol Neurodegener. 2020;15:63. doi: 10.1186/s13024-020-00413-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raghunathan T.E., Lepkowski J.M., Hoewyk J.V., Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001;27:85–95. [Google Scholar]
- 20.Rubin D. Wiley; New York: 1987. Multiple imputation for nonresponse in surveys. [Google Scholar]
- 21.Klinger R.Y., Cooter M., Bisanar T., et al. Intravenous lidocaine does not improve neurologic outcomes after cardiac surgery: a randomized controlled trial. Anesthesiology. 2019;130:958–970. doi: 10.1097/ALN.0000000000002668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Browndyke J.N., Berger M., Smith P.J., et al. Task-related changes in degree centrality and local coherence of the posterior cingulate cortex after major cardiac surgery in older adults. Hum Brain Mapp. 2018;39:985–1003. doi: 10.1002/hbm.23898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landman B.A., Warfield S.K. CreateSpace Independent Publishing Platform; 2012. MICCAI 2012 workshop on multi-atlas labeling. [Google Scholar]
- 24.LaMontagne P.J., Benzinger T.L., Morris J.C., et al. OASIS-3: longitudinal neuroimaging, clinical, and cognitive dataset for normal aging and Alzheimer disease. medRxiv. 2019 doi: 10.1101/2019.12.13.19014902. [DOI] [Google Scholar]
- 25.Bakker R., Tiesinga P., Kotter R. The scalable brain atlas: instant web-based access to public brain atlases and related content. Neuroinformatics. 2015;13:353–366. doi: 10.1007/s12021-014-9258-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neuromorphometrics, Inc. SPM12 software 2020 http://Neuromorphometrics.com/ Available from: [Accessed 1/13/12020]
- 27.Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berger M., Oyeyemi D., Olurinde M.O., et al. The INTUIT study: investigating neuroinflammation underlying postoperative cognitive dysfunction. J Am Geriatr Soc. 2019;67:794–798. doi: 10.1111/jgs.15770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith P.J., Browndyke J.N., Monge Z.A., et al. Longitudinal changes in regional cerebral perfusion and cognition after cardiac operation. Ann Thorac Surg. 2019;107:112–118. doi: 10.1016/j.athoracsur.2018.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klinger R.Y., James O.G., Borges-Neto S., et al. 18F-florbetapir positron emission tomography-determined cerebral beta-amyloid deposition and neurocognitive performance after cardiac surgery. Anesthesiology. 2018;128:728–744. doi: 10.1097/ALN.0000000000002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lancaster J.L., Woldorff M.G., Parsons L.M., Liotti M., Freitas C.S., Rainey L., Kochunov P.V., Nickerson D., Mikiten S.A., Fox P.T. Automated Talairach Atlas labels for functional brain mapping. Human Brain Map. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jack C.R., Jr., Knopman D.S., Jagust W.J., et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaw L.M., Vanderstichele H., Knapik-Czajka M., et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo X., Qiu T., Jia Y., et al. Intrinsic functional connectivity alterations in cognitively intact elderly APOE epsilon4 carriers measured by eigenvector centrality mapping are related to cognition and CSF biomarkers: a preliminary study. Brain Imaging Behav. 2017;11:1290–1301. doi: 10.1007/s11682-016-9600-z. [DOI] [PubMed] [Google Scholar]
- 35.Berger M., Cooter M., Roesler A.S., et al. APOE4 copy number-dependent proteomic changes in the cerebrospinal fluid. J Alzheimers Dis. 2021;79:511–530. doi: 10.3233/JAD-200747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weissberger G.H., Nation D.A., Nguyen C.P., Bondi M.W., Han S.D. Meta-analysis of cognitive ability differences by apolipoprotein e genotype in young humans. Neurosci Biobehav Rev. 2018;94:49–58. doi: 10.1016/j.neubiorev.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koelewijn L., Lancaster T.M., Linden D., et al. Oscillatory hyperactivity and hyperconnectivity in young APOE-varepsilon4 carriers and hypoconnectivity in Alzheimer's disease. eLife. 2019;8 doi: 10.7554/eLife.36011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henson R.N., Suri S., Knights E., et al. Effect of apolipoprotein E polymorphism on cognition and brain in the Cambridge Centre for ageing and neuroscience cohort. Brain Neurosci Adv. 2020;4 doi: 10.1177/2398212820961704. 2398212820961704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheline Y.I., Morris J.C., Snyder A.Z., et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Abeta42. J Neurosci. 2010;30:17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones D.T., Knopman D.S., Gunter J.L., et al. Cascading network failure across the Alzheimer’s disease spectrum. Brain. 2016;139:547–562. doi: 10.1093/brain/awv338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Racine A.M., Gou Y., Fong T.G., et al. Correction for retest effects across repeated measures of cognitive functioning: a longitudinal cohort study of postoperative delirium. BMC Med Res Methodol. 2018;18:69. doi: 10.1186/s12874-018-0530-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.