Abstract
Background
NVX-CoV2373 is an adjuvanted, recombinant spike protein nanoparticle vaccine that was shown to have clinical efficacy for the prevention of coronavirus disease 2019 (Covid-19) in phase 2b–3 trials in the United Kingdom and South Africa, but its efficacy had not yet been tested in North America.
Methods
We conducted a phase 3, randomized, observer-blinded, placebo-controlled trial in the United States and Mexico during the first half of 2021 to evaluate the efficacy and safety of NVX-CoV2373 in adults (≥18 years of age) who had not had severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Participants were randomly assigned in a 2:1 ratio to receive two doses of NVX-CoV2373 or placebo 21 days apart. The primary objective was to determine vaccine efficacy against reverse-transcriptase–polymerase-chain-reaction–confirmed Covid-19 occurring at least 7 days after the second dose. Vaccine efficacy against moderate-to-severe disease and against different variants was also assessed.
Results
Of the 29,949 participants who underwent randomization between December 27, 2020, and February 18, 2021, a total of 29,582 (median age, 47 years; 12.6% ≥65 years of age) received at least one dose: 19,714 received vaccine and 9868 placebo. Over a period of 3 months, 77 cases of Covid-19 were noted — 14 among vaccine recipients and 63 among placebo recipients (vaccine efficacy, 90.4%; 95% confidence interval [CI], 82.9 to 94.6; P<0.001). Ten moderate and 4 severe cases occurred, all in placebo recipients, yielding vaccine efficacy against moderate-to-severe disease of 100% (95% CI, 87.0 to 100). Most sequenced viral genomes (48 of 61, 79%) were variants of concern or interest — largely B.1.1.7 (alpha) (31 of the 35 genomes for variants of concern, 89%). Vaccine efficacy against any variant of concern or interest was 92.6% (95% CI, 83.6 to 96.7). Reactogenicity was mostly mild to moderate and transient but was more frequent among NVX-CoV2373 recipients than among placebo recipients and was more frequent after the second dose than after the first dose.
Conclusions
NVX-CoV2373 was safe and effective for the prevention of Covid-19. Most breakthrough cases were caused by contemporary variant strains. (Funded by Novavax and others; PREVENT-19 ClinicalTrials.gov number, NCT04611802.)
The global pandemic of coronavirus disease 2019 (Covid-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been met with an unprecedented response in vaccine development.1 Several Covid-19 vaccines based on different technologies are available, all of which use the SARS-CoV-2 spike protein, usually based on the ancestral strain, as an antigen.2-5 Although these vaccines have been shown to provide a remarkable reduction in disease burden in clinical trials, development efforts must continue to address global supply shortages and the emergence of variants that affect virulence or susceptibility to vaccine-induced immunity (variants of concern or variants of interest).6-8
NVX-CoV2373 (Novavax), a SARS-CoV-2 vaccine made up of full-length, stabilized, prefusion, recombinant spike protein trimers produced from the Wuhan-Hu-1 sequence, is assembled into nanoparticles coformulated with a saponin-based adjuvant (Matrix-M). The vaccine, which is stable at refrigerated temperatures (2 to 8°C), may help to address the continually evolving Covid-19 pandemic and the concurrent global vaccine shortage.9
NVX-CoV2373 was found to be safe and immunogenic in adults10,11 and provided high vaccine efficacy against severe disease caused by the B.1.351 (beta) variant of concern in a phase 2b trial in South Africa12 and against Covid-19 of any severity caused by the B.1.1.7 (alpha) variant of concern in a phase 3 trial in the United Kingdom.13 We describe the results of PREVENT-19 (Prefusion Protein Subunit Vaccine Efficacy Novavax Trial–COVID-19), a phase 3 trial of NVX-CoV2373 involving adults 18 years of age or older in the United States and Mexico conducted during a period in which the circulating variants were predominantly alpha, beta, P.1 (gamma), B.1.427 and B.1.429 (epsilon), and B.1.526 (iota).14
Methods
Trial Design, Participants, Procedures, and Oversight
We conducted this phase 3, randomized, observer-blinded, placebo-controlled trial at 113 clinical sites in the United States and 6 in Mexico to evaluate the efficacy and safety of NVX-CoV2373. Adults 18 years of age or older who were healthy or who had stable chronic medical conditions, including chronic pulmonary, renal, or cardiovascular disease, diabetes mellitus type 2, or well-controlled human immunodeficiency virus (HIV) infection, were eligible for participation. Key exclusion criteria included known previous laboratory-confirmed SARS-CoV-2 infection or known immunosuppression. In accordance with the Food and Drug Administration (FDA) and funding agency guidance,1,15 enrollment targets were specified for the inclusion of racial and ethnic minorities, and site selection prioritized accessibility to participants at high risk for acquisition or complications of Covid-19. Participants received initial injections between December 27, 2020, and February 18, 2021, and were followed through April 19, 2021. During this period, the predominant viral variants detected in the United States and Mexico were alpha, beta, gamma, epsilon, and iota.14 Additional details regarding trial design, conduct, oversight, and analyses are provided in the Supplementary Appendix and in the protocol and statistical analysis plan, available with the full text of this article at NEJM.org.
Participants provided written informed consent before enrollment, were stratified according to age (18 to 64 years or ≥65 years), and were randomly assigned with the use of a Web-based interactive response system in a 2:1 ratio to receive two 0.5-ml intramuscular injections of either NVX-CoV2373 (5 μg of SARS-CoV-2 recombinant spike protein adjuvanted with 50 μg of Matrix-M) or saline placebo 21 days apart. Site personnel who were aware of the randomization assignments managed the logistics of the injections and the preparation of the vaccine and placebo but had no other role in trial conduct. Novavax was the trial sponsor and was responsible for the trial design and for the development and manufacture of the NVX-CoV2373 clinical trial material. The trial was part of the U.S. Government–funded harmonized trial program. Site selection, monitoring of clinical data, data collection and analysis, and preparation of the manuscript (the first draft of which was written by authors who are employees of Novavax) were conducted in collaboration with the Biomedical Advanced Research and Development Authority, the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health, the Covid-19 Prevention Network, and the trial cochairs.16 Trial data were available to all the authors, who vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. The trial is continuing after the time of this analysis, and investigators and Novavax clinical team remain unaware of the participant-level randomization assignments. The protocol, amendments, and overall oversight were provided by institutional review boards and ethics committees according to local regulations. Safety oversight was provided by an NIAID-sponsored protocol safety review team, and safety, efficacy, and potential vaccine-induced harm were monitored through regular review of unblinded data by the NIAID-sponsored data and safety monitoring board.17
Efficacy Assessments
The aim of the trial was to estimate, as the primary end point, the efficacy of NVX-CoV2373 for the prevention of a first occurrence of reverse-transcriptase–polymerase-chain-reaction (RT-PCR)–confirmed symptomatic mild, moderate, or severe Covid-19 (defined with the use of FDA criteria15) (Table S3 in the Supplementary Appendix) with onset at least 7 days after the second dose. The primary end point was assessed in the per-protocol efficacy analysis population, which included participants who underwent randomization and received both doses as assigned, were seronegative for anti–SARS-CoV-2 nucleoprotein and had a SARS-CoV-2 RNA RT-PCR–negative nasal swab at baseline, and did not have a censoring event at any time before 7 days after the second injection. Symptoms of suspected Covid-19 (Table S2) were reported daily by participants using an electronic diary. When specified symptoms were reported for 2 or more consecutive days, participants were instructed to obtain three nasal swab specimens themselves daily and to undergo in-clinic medical evaluation, including collection of a nasal swab specimen for SARS-CoV-2 testing at a central laboratory. Covid-19 cases for end-point assessment were considered to be confirmed when at least one of four nasal swab specimens tested positive for SARS-CoV-2 RNA by RT-PCR at the central laboratory. Whole-genome sequencing and clade and lineage assignment were performed on RT-PCR–positive samples with sufficient viral RNA loads (see the Supplementary Appendix).
The key secondary objective was to determine the vaccine efficacy of NVX-CoV2373 for the prevention of RT-PCR–confirmed Covid-19 cases caused by SARS-CoV-2 that was neither a variant of concern nor a variant of interest (i.e., virus that did not have mutations associated with variants of concern or interest), which could be considered to most closely resemble the ancestral Wuhan-Hu-1 strain. Conversely, vaccine efficacy against RT-PCR–confirmed Covid-19 caused by variants of concern or variants of interest, as defined by the Centers for Disease Control and Prevention (CDC) at the time of end-point case accrual,6 was estimated as an exploratory end point.
We also estimated vaccine efficacy against moderate and severe Covid-19 as secondary end points, with moderate Covid-19 defined as high fever and objective evidence of lower respiratory tract infection and severe Covid-19 defined as the presence of clinically significant tachypnea, tachycardia, or hypoxia; receipt of intensive respiratory support; major dysfunction of one or more organ systems; admission to an intensive care unit; or death. Additional analyses of vaccine efficacy, although they were not statistically powered, were performed in relevant subgroups (based on race, ethnic group, age, and coexisting conditions associated with an increased risk of severe Covid-19 complications according to CDC criteria,18 as well as on the risk of exposure related to occupation or living conditions).
Covid-19 severity as an end point was assessed by investigators and trial physicians; severe cases were confirmed by an external independent end-point review committee. All evaluations were blinded to randomization assignments.
Safety Assessments
Data on solicited local and systemic adverse events were collected with the use of a participant-recorded electronic diary for 7 days after each injection. Data on unsolicited adverse events were collected from the first dose through 28 days after the second dose; data on serious adverse events, adverse events of special interest, and medically attended adverse events were collected from the first dose through data cutoff (secondary end points).
Data on solicited local and systemic adverse events were assessed for severity (according to FDA criteria19) and duration after each injection. Unsolicited adverse events were coded according to preferred term and system organ class with the use of the Medical Dictionary for Regulatory Activities, version 23.1, and were summarized according to severity and relationship to vaccine or placebo.
Statistical Analysis
Vaccine efficacy was assessed in both the full analysis population (i.e., all participants who underwent randomization and received at least one dose of vaccine or placebo) and the per-protocol efficacy analysis population (defined above in “Efficacy Assessments”). In analyses involving the full analysis population, two observation periods were used — one starting at dose 1 and the other starting 7 days after dose 2. In analyses involving the per-protocol efficacy population, only the observation period that started 7 days after dose 2 was used.
The primary efficacy analysis was based on the per-protocol efficacy analysis population. Vaccine efficacy was defined as (1−RR)×100, where RR is the relative risk of the end point of interest based on the incidence in the NVX-CoV2373 group as compared with that in the placebo group. The estimated relative risk and two-sided 95% confidence interval were derived with the use of a Poisson regression with robust error variance. The prespecified criteria for success in the primary efficacy analysis were a lower bound of the two-sided 95% confidence interval for vaccine efficacy of greater than 30% and a point estimate for vaccine efficacy of 50% or greater.15
The trial was designed to evaluate efficacy on the basis of the number of events expected to be required in order to achieve significance with respect to the primary end point, originally estimated to be 144 cases meeting the definition of the primary end point. However, to maintain the viability of the trial and retain participants in the face of nationwide availability of vaccines under emergency use authorization, a blinded crossover (i.e., participants who had originally been randomly assigned to receive placebo were administered NVX-CoV2373 and vice versa) was implemented. This coincided with the accumulation of a median of 2 months of safety follow-up and ensured that all participants received active vaccine at the earliest possible time without compromising FDA-required placebo-controlled safety follow-up. The final analysis of the placebo-controlled portion of the trial, which was approved by the data and safety monitoring board before general access to unblinded data and is reported here, includes the number of confirmed cases of Covid-19 fulfilling the definition of the primary end point that occurred before the crossover. As a result of the blinded crossover, the two initially planned formal interim analyses of efficacy and futility were replaced with a single analysis of efficacy in which the full two-sided type I error of 5% was used for the primary end point.
The safety analysis population included all participants who received at least one dose of vaccine or placebo. Data on safety were summarized descriptively.
Results
Participants
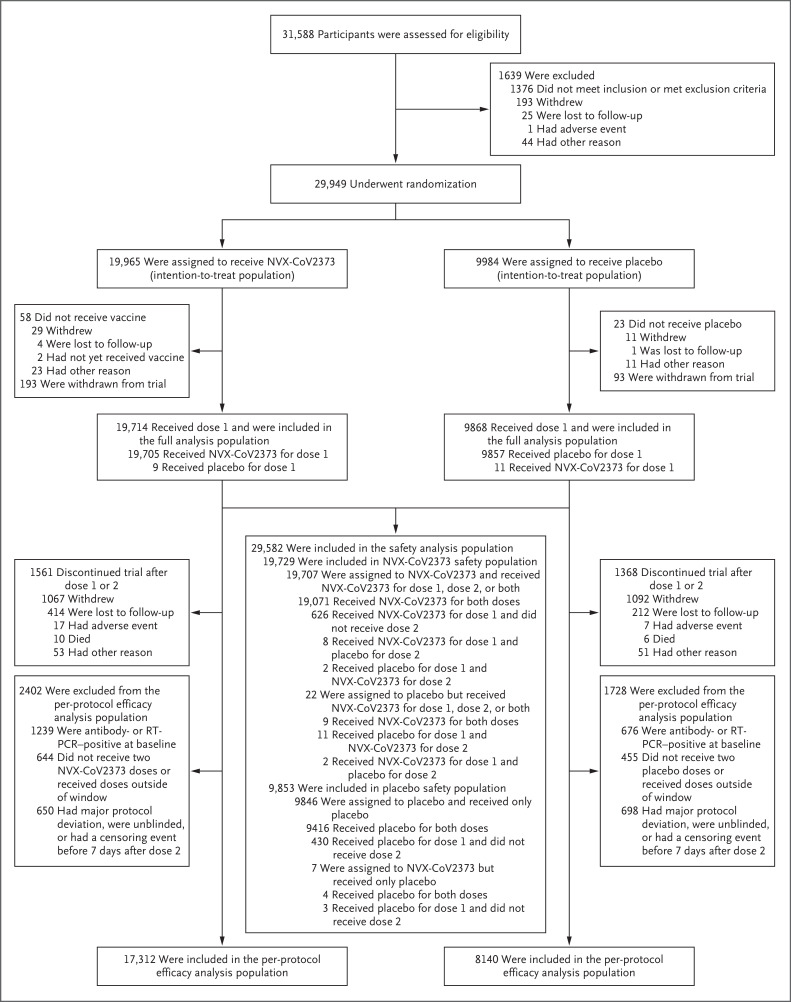
Of the 31,588 participants who were screened, 29,949 underwent randomization between December 27, 2020, and February 18, 2021 (Figure 1). Among the 29,582 participants who received at least one dose of NVX-CoV2373 (19,714 participants) or placebo (9868 participants) and were included in the full analysis population (median age, 47 years; 12.6% ≥65 years of age), 25,452 participants (17,312 NVX-CoV2373 recipients and 8140 placebo recipients) were included in the per-protocol efficacy analysis population. The main reasons for exclusion from the per-protocol efficacy analysis population included baseline positivity for anti–SARS-CoV-2 nucleoprotein or SARS-CoV-2 RNA on RT-PCR testing, failure to receive both doses as prescribed, unblinding, or other censoring event (Table S18). At baseline, 6.3% of the NVX-CoV2373 recipients and 6.9% of the placebo recipients in the safety analysis population were seropositive or RT-PCR–positive for SARS-CoV-2 (Table S4).
Figure 1. Screening, Randomization, and Follow-up.
The full analysis population included all participants who underwent randomization and received at least one dose of vaccine or placebo, regardless of protocol violations or missing data; participants in the full analysis population are included in the analysis according to the group to which they were randomly assigned. The safety analysis population included all participants who received at least one dose of vaccine or placebo. The per-protocol efficacy analysis population included all participants who underwent randomization and received both doses as assigned, were seronegative for anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleoprotein and had a SARS-CoV-2 RNA reverse-transcriptase–polymerase-chain-reaction (RT-PCR)–negative nasal swab at baseline, and did not have a censoring event at any time before 7 days after the second injection. Data from participants at two sites (193 assigned to receive NVX-CoV2373 and 93 assigned to receive placebo) were excluded from analyses because of Good Clinical Practice quality concerns. The window for the first dose of vaccine or placebo was December 20, 2020, to February 18, 2021.
The demographic characteristics of the participants in the per-protocol efficacy analysis population were well balanced between the treatment groups; 48.2% identified as female, 75.9% as White, 11.0% as Black or African American, 6.2% as Native American or Alaska Native (including American Indians and Native Mexicans), and 21.5% as Hispanic or Latino. One or more coexisting conditions18 were reported by 47.3% of the participants. The median age was 47 years, and 11.8% of the participants were 65 years of age or older (Table 1).
Table 1. Demographic and Clinical Characteristics of the Participants at Baseline (Per-Protocol Efficacy Analysis Population).*.
| Characteristic | NVX-CoV2373 (N=17,312) |
Placebo (N=8140) |
Total (N=25,452) |
|---|---|---|---|
| Median age (range) — yr | 47.0 (18–95) | 47.0 (18–90) | 47.0 (18–95) |
| Age group — no. (%) | |||
| 18 to 64 yr | 15,264 (88.2) | 7,194 (88.4) | 22,458 (88.2) |
| ≥65 yr | 2,048 (11.8) | 946 (11.6) | 2,994 (11.8) |
| Sex — no. (%) | |||
| Male | 9,050 (52.3) | 4,131 (50.7) | 13,181 (51.8) |
| Female | 8,262 (47.7) | 4,009 (49.3) | 12,271 (48.2) |
| Race or ethnic group — no. (%)† | |||
| White | 13,140 (75.9) | 6,184 (76.0) | 19,324 (75.9) |
| Black or African American | 1,893 (10.9) | 900 (11.1) | 2,798 (11.0) |
| American Indian or Alaska Native, including Mexican Natives | 1,074 (6.2) | 498 (6.1) | 1,572 (6.2) |
| Asian | 761 (4.4) | 366 (4.5) | 1,127 (4.4) |
| Multiple | 293 (1.7) | 132 (1.6) | 425 (1.7) |
| Native Hawaiian or other Pacific Islander | 47 (0.3) | 10 (0.1) | 57 (0.2) |
| Not reported | 104 (0.6) | 45 (0.6) | 149 (0.6) |
| Hispanic or Latino | |||
| No | 13,538 (78.2) | 6,379 (78.4) | 19,917 (78.3) |
| Yes | 3,733 (21.6) | 1,751 (21.5) | 5,484 (21.5) |
| Not reported | 22 (0.1) | 9 (0.1) | 31 (0.1) |
| Unknown | 19 (0.1) | 1 (<0.1) | 20 (0.1) |
| Overall high risk of Covid-19 — no. (%)‡ | |||
| Yes | 16,493 (95.3) | 7,737 (95.0) | 24,230 (95.2) |
| No | 819 (4.7) | 403 (5.0) | 1,222 (4.8) |
| High risk of severe Covid-19 — no. (%)§ | |||
| Yes | 9,046 (52.3) | 4,294 (52.8) | 13,340 (52.4) |
| No | 8,266 (47.7) | 3,846 (47.2) | 12,112 (47.6) |
| Coexisting conditions — no. (%) | |||
| Any | 8,117 (46.9) | 3,910 (48.0) | 12,027 (47.3) |
| Obesity | 6,400 (37.0) | 3,070 (37.7) | 9,470 (37.2) |
| Chronic lung disease | 2,442 (14.1) | 1,218 (15.0) | 3,660 (14.4) |
| Diabetes mellitus type 2 | 1,303 (7.5) | 677 (8.3) | 1,980 (7.8) |
| Cardiovascular disease | 191 (1.1) | 91 (1.1) | 282 (1.1) |
| Chronic kidney disease | 109 (0.6) | 50 (0.6) | 159 (0.6) |
| HIV infection — no. (%) | 128 (0.7) | 38 (0.5) | 166 (0.7) |
| Country — no. (%) | |||
| United States | 16,294 (94.1) | 7,638 (93.8) | 23,932 (94.0) |
| Mexico | 1,018 (5.9) | 502 (6.2) | 1,520 (6.0) |
The per-protocol efficacy analysis population included all participants who underwent randomization and received both doses as assigned, were seronegative for anti–SARS-CoV-2 nucleoprotein and had a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA RT-PCR–negative nasal swab at baseline, and did not have a censoring event at any time before 7 days after the second injection. HIV denotes human immunodeficiency virus.
Race and ethnic group were reported by the participants.
Participants at overall high risk included those 65 years of age or older and those of any age with chronic health conditions or an increased risk for Covid-19 because of work or living conditions.
Participants were classified as having a high risk of severe Covid-19 if they had one or more of the following coexisting conditions: obesity (defined as a body-mass index [the weight in kilograms divided by the square of the height in meters] of ≥30.0), chronic lung disease, diabetes mellitus type 2, cardiovascular disease, or chronic kidney disease.
Efficacy
In the full analysis population, the incidence of Covid-19 was 21.2 cases per 1000 person-years (95% confidence interval [CI], 16.2 to 27.7) in the NVX-CoV2373 group and 51.9 cases per 1000 person-years (95% CI, 40.9 to 66.0) in the placebo group when the observation period started after dose 1. The cumulative incidence curves separated between days 14 and 21 (Figure 2A).
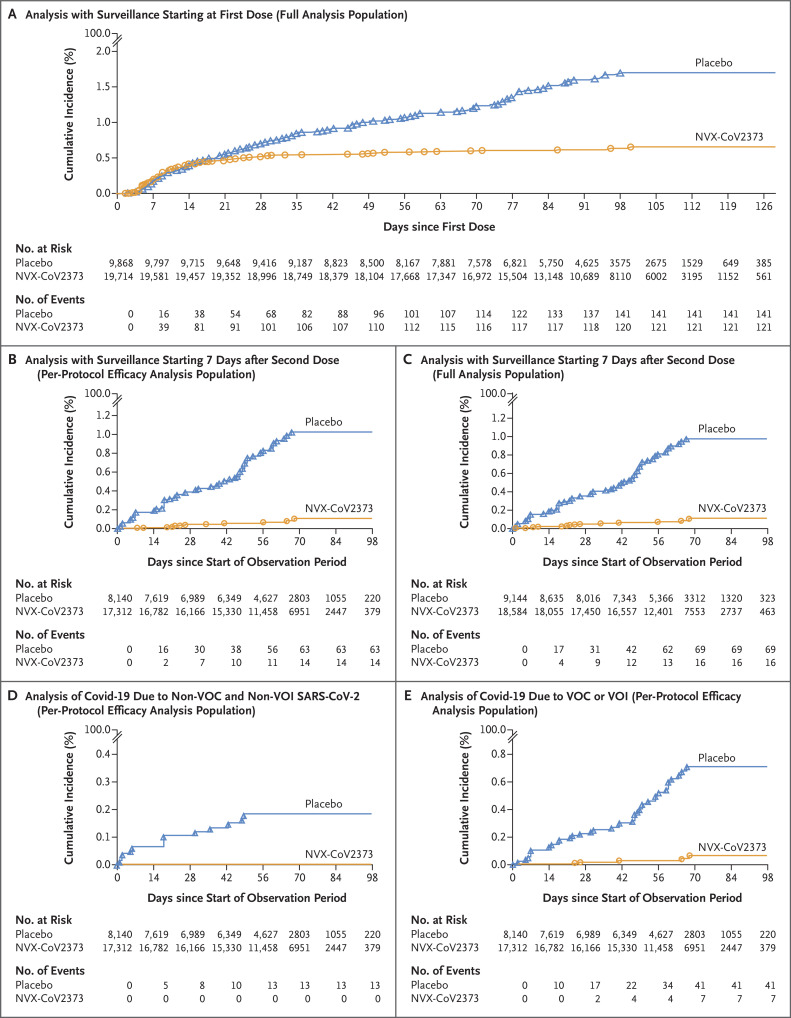
Figure 2. Overall Efficacy of NVX-CoV2373 against Covid-19.
Shown is the cumulative incidence of symptomatic (mild, moderate, or severe) coronavirus disease 2019 (Covid-19). Panel A shows all participants in the full analysis population during the period of surveillance that started at the first dose of NVX-CoV2373 or placebo. Panel B shows all participants in the per-protocol efficacy analysis population during the period of surveillance starting 7 days after the second dose (i.e., up to 28 days after the first dose) through approximately 3 months of follow-up, until unblinding, or until receipt of a vaccine under emergency use authorization. Panel C shows all participants in the full analysis population during the period of surveillance that started 7 days after the second dose. Panel D shows all participants in the per-protocol efficacy analysis population with cases of Covid-19 due to variants that are not considered variants of concern (VOC) or variants of interest (VOI). Panel E shows all participants in the per-protocol efficacy analysis population with cases of Covid-19 due to VOC or VOI.
Among the 25,452 participants in the per-protocol efficacy analysis population who were followed through April 19, 2021 (median follow-up for efficacy, approximately 3 months), 77 central laboratory–confirmed Covid-19 cases occurred: 14 cases in NVX-CoV2373 recipients and 63 in placebo recipients (incidence, 3.3 cases per 1000 person-years [95% CI, 1.6 to 6.9] and 34.0 cases per 1000 person-years [95% CI, 20.7 to 55.9], respectively). These results yielded a vaccine efficacy of 90.4% (95% CI, 82.9 to 94.6; P<0.001) (Figure 2B and Table S5). The rate of new Covid-19 cases among NVX-CoV2373 recipients was higher during the first 42 days of follow-up than during the remainder of the accrual period covered in this report. Subsequently, the incidence of new cases declined among vaccine recipients and increased among placebo recipients (Figure 2B).
Similar results for vaccine efficacy were obtained in the full analysis population when the observation period started 7 days after the second dose: 85 Covid-19 cases occurred (16 in NVX-CoV2373 recipients and 69 in placebo recipients; incidence, 3.7 cases per 1000 person-years [95% CI, 1.8 to 7.4] and 34.6 cases per 1000 person-years [95% CI, 22.3 to 53.6], respectively). These results yielded a vaccine efficacy of 89.3% (95% CI, 81.6 to 93.8) (Figure 2C and Table S17).
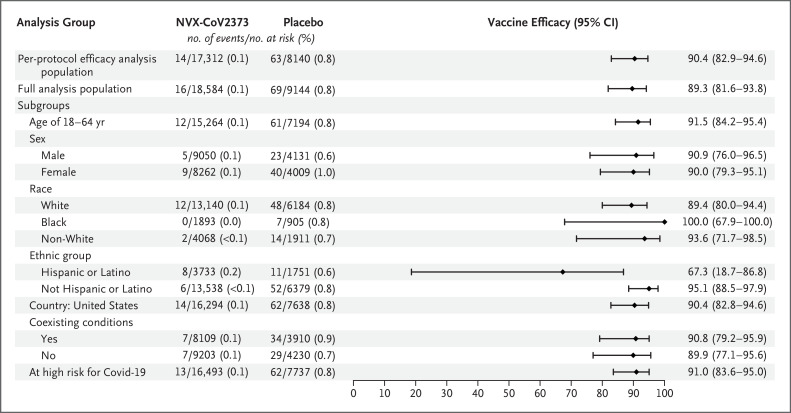
All the cases reported in NVX-CoV2373 recipients were mild in severity, whereas 14 moderate-to-severe cases (10 moderate and 4 severe) occurred in the placebo group, yielding a vaccine efficacy against moderate-to-severe Covid-19 of 100% (95% CI, 87.0 to 100) (Table S7). Vaccine efficacy against severe Covid-19 alone (4 cases) was 100% (95% CI, 34.6 to 100 in a post hoc analysis). Vaccine efficacy against confirmed Covid-19 meeting the definition of the primary end point among participants who were at high risk for acquisition or complications of Covid-19 was 91.0% (95% CI, 83.6 to 95.0) (Figure 3). Vaccine efficacy in several prespecified demographic subgroups was in most cases found to be similarly high. Among Black participants, vaccine efficacy was 100% (95% CI, 67.9 to 100) (Figure 3). Hispanic or Latino participants were the only demographic group in which vaccine efficacy was lower than in other subgroups (67.3%; 95% CI, 18.7 to 86.8) (Figure 3).
Figure 3. Vaccine Efficacy of NVX-CoV2373 in Specific Subgroups (Per-Protocol Efficacy Analysis Population).
Vaccine efficacy was defined as 1 minus the relative risk (NVX-CoV2373 minus placebo). Non-White race included end-point cases in participants from all other races to ensure that these subgroups would be large enough for meaningful analyses. The assessment of coexisting conditions is based on the Centers for Disease Control and Prevention definitions18 of persons at risk for complications of Covid-19. Participants at overall high risk for Covid-19 included those 65 years of age or older and those of any age with chronic health conditions or an increased risk for Covid-19 because of work or living conditions.
Nasal swabs from 61 of 77 participants (79%) with RT-PCR–confirmed Covid-19 yielded sequences by whole-genome sequencing. Sequences from 48 of these 61 specimens were identified as variants of concern or interest, and 13 were identified as other variants (Fig. S1); vaccine efficacy against the latter variants was 100% (95% CI, 85.8 to 100) (Figure 2D). The alpha variant accounted for most specimens with sequences that were identified as variants of concern (31 of 35, 89%); vaccine efficacy against the alpha variant was 93.6% (95% CI, 81.7 to 97.8 in a post hoc analysis), and vaccine efficacy against any variant of concern or interest was 92.6% (95% CI, 83.6 to 96.7) (Figure 2E). An assessment of vaccine efficacy for cases that yielded no sequence information (16 of 77, 21%) was not conducted.
Safety
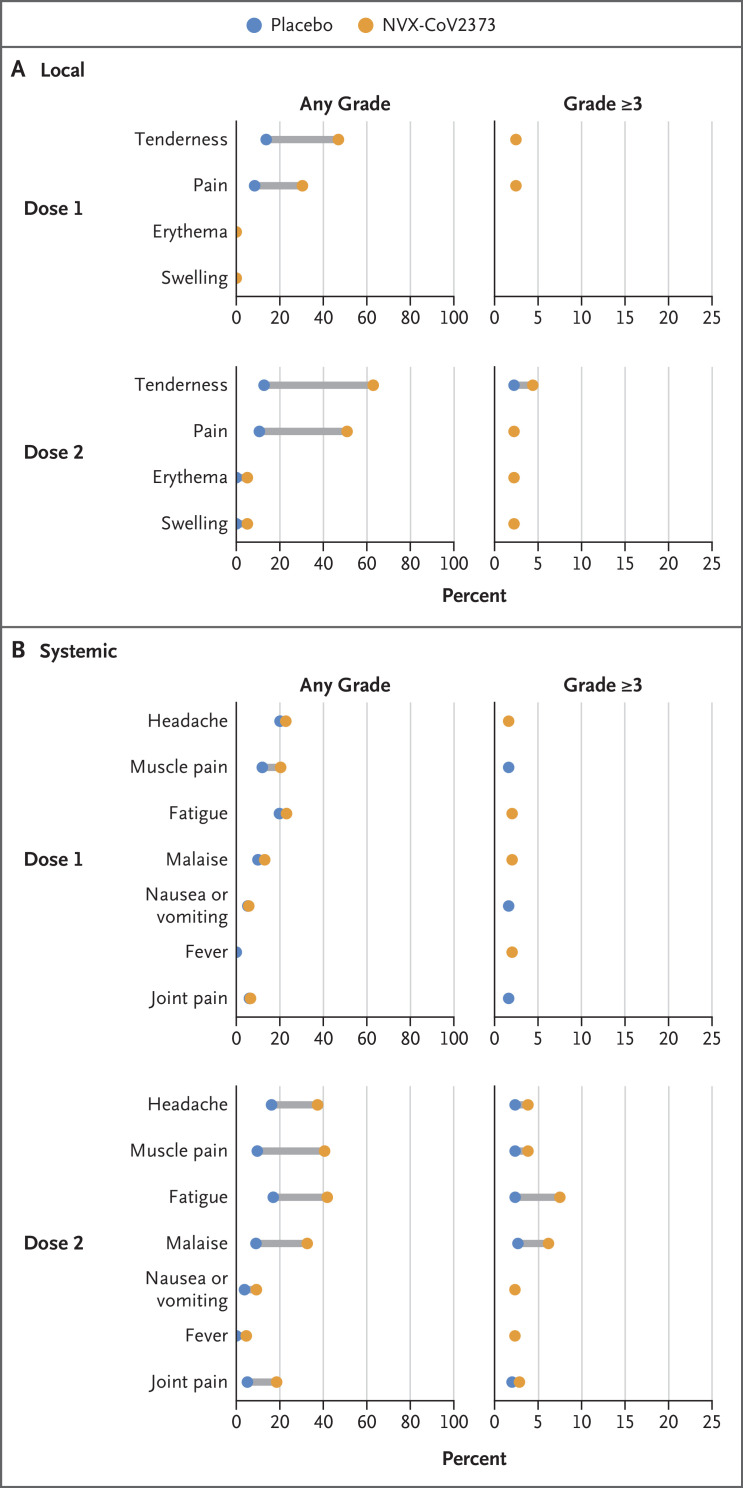
Solicited local and systemic adverse events were predominantly mild to moderate and transient. These events occurred more frequently among NVX-CoV2373 recipients than among placebo recipients (any local adverse event, 58.0% and 21.1%, respectively, after dose 1 and 78.9% and 21.7% after dose 2; any systemic adverse event, 47.7% and 40.0%, respectively, after dose 1 and 69.5% and 35.9% after dose 2).
After each dose, the most frequently reported solicited local adverse events were tenderness and injection-site pain. The median duration of these events was 2 days or less (Table S11). Severe (grade ≥3) local reactions were infrequent overall but were more common among NVX-CoV2373 recipients than among placebo recipients, particularly after dose 2 (1.1% of NVX-CoV2373 recipients and <1% of placebo recipients after dose 1 and 6.7% and <1%, respectively, after dose 2) (Figure 4 and Table S10).
Figure 4. Solicited Local and Systemic Adverse Events (Safety Analysis Population).
The percentage of participants in each group with solicited local and systemic adverse events during the 7 days after each dose is plotted according to Food and Drug Administration toxicity grade, either as any grade (mild, moderate, severe, or potentially life-threatening) or as grade 3 or higher (severe or potentially life-threatening).19 An expanded x axis is used for events of grade 3 or higher to highlight differences between small percentages.
The most common solicited systemic adverse events were headache, myalgia, fatigue, and malaise, which were detected more frequently among NVX-CoV2373 recipients and after the second injection and lasted for a median duration of 1 day or less (Table S13). Fever of any severity was rare (<1%) and was similarly distributed in the vaccine and placebo groups after each dose. Severe systemic reactions were more common among NVX-CoV2373 recipients, particularly after dose 2 (2.4% of NVX-CoV2373 recipients and 2.1% of placebo recipients after dose 1 and 12.1% and 2.1%, respectively, after dose 2) (Figure 4 and Table S12), but they were less frequent than has been reported for other Covid-19 vaccines.2
In this preliminary safety “snapshot,” unsolicited adverse events were slightly more frequent among NVX-CoV2373 recipients than among placebo recipients (16.3% and 14.8%, respectively), although the imbalance appeared to include duplicate reporting by investigators of reactogenicity that had also been derived from participant-reported outcomes. The frequencies of medically attended adverse events, serious adverse events, severe adverse events, adverse events of special interest related to Covid-19, and potential immune-mediated medical conditions were balanced between the two groups (Table S9). No episodes of anaphylaxis, no evidence of vaccine-associated enhanced Covid-19, and no events that triggered prespecified pause rules were observed. No episodes of the Guillain–Barré syndrome20 and no imbalance in myocarditis or pericarditis21 or in vaccine-induced immune thrombosis with thrombocytopenia22 were observed during the relatively short safety follow-up period reported here (Tables S14 through S16). All-cause mortality was balanced: nine deaths occurred among NVX-CoV2373 recipients (0.5%), and five occurred among placebo recipients (0.5%).
Discussion
In this randomized, controlled trial assessing the efficacy and safety of an adjuvanted SARS-CoV-2 recombinant spike protein vaccine in approximately 30,000 participants, we continue to assess, in a blinded fashion, the duration of protection after blinded crossover, as well as the overall safety profile in the trial participants. The trial includes a demographically diverse population in the United States and Mexico and provides strong evidence of high short-term vaccine efficacy of NVX-CoV2373 for the prevention of Covid-19 (>90%) and for the prevention of moderate-to-severe disease (100%).
NVX-CoV2373 was safe during a median safety follow-up of 2 months and showed mild-to-moderate, transient reactogenicity. No safety concerns related to vaccination were seen, as indicated by the similar percentages of participants reporting unsolicited adverse events in the NVX-CoV2373 group and the placebo group. None of the safety signals under observation with other Covid-19 vaccines20-22 were reported in this trial in the limited safety follow-up period reported here. Long-term safety monitoring is planned to continue through 24 months after initial vaccination.
Our trial expands the understanding of NVX-CoV2373 efficacy in the evolving pandemic. SARS-CoV-2 has undergone extensive genetic evolution since it was first reported in December 2019, resulting in the emergence of multiple variants that may cause evasion of spike protein–based vaccine-induced immunity.6-8 NVX-CoV2373 showed vaccine efficacy of greater than 90% against variants circulating during case accrual in the United States and Mexico, largely represented by the alpha variant. Similar protection against this variant was observed in the earlier phase 3 trial conducted in the United Kingdom13 (vaccine efficacy, 93.6% in the current trial and 86.3% in the U.K. trial). Moreover, efficacy against the alpha variant in both trials was similar to that against the 13 variants that were not variants of concern or interest and that may more closely resemble the ancestral Wuhan-Hu-1 spike sequence from which the vaccine antigen was derived; this suggests that the vaccine induces protective immunity to a broad spectrum of variants beyond the prototype strain.12
Some additional strengths of the current trial include the demographic diversity of the trial population, which permitted an assessment of vaccine efficacy among racial and ethnic minorities reflecting U.S. and Mexican populations that are particularly affected by the pandemic, including Black persons, among whom vaccine efficacy was 100% (95% CI, 67.9 to 100). Vaccine efficacy among Hispanic or Latino persons in the United States was lower than that in other demographic groups (albeit still higher than the prespecified success criterion for vaccine efficacy); this could be related to chance alone or to unidentified viral or host factors, although the former appears to be more plausible biologically. All end-point cases in Hispanic or Latino NVX-CoV2373 recipients were mild in severity. A similar trend was reported for another Covid-19 vaccine, but these findings were not explicitly addressed.23
Several limitations of this trial are noteworthy. Covid-19 vaccines became available under emergency use authorization concurrently with the initiation of this trial, reducing the enrollment of older adult participants, which then resulted in only four end-point cases in participants 65 years of age or older and prevented a useful estimation of vaccine efficacy in older adults. However, the U.K. phase 3 trial of NVX-CoV2373 enrolled enough older adults to establish a vaccine efficacy of 88.9%, similar to that among younger adults.13
Another limitation is the imbalance in the number of unblinding requests by trial participants at early stages of the trial. This coincided with the introduction of vaccines under emergency use authorization in the United States. Participants who apparently surmised, on the basis of an absence of reactogenicity symptoms, that they had received placebo requested unblinding more often than did those who had received NVX-CoV2373. This led to the exclusion of a higher number of placebo recipients from the per-protocol population for the assessment of efficacy. The limited efficacy follow-up period (approximately 3 months) was another limitation of this analysis; however, this period is consistent with the limited vaccine efficacy follow-up requested for emergency use authorization15 and with data that supported all other authorized Covid-19 vaccines.
In this trial, we implemented a blinded crossover approximately 3 to 4 months after the first vaccination series to allow all trial participants to receive NVX-CoV2373, after vaccine efficacy and required safety had been established and reviewed by the data and safety monitoring board. To address the durability of vaccine efficacy and long-term safety after blinded crossover, hazard models have been proposed for subsequent analyses24; this will allow comparisons between groups of participants who were vaccinated early and those vaccinated later, although the estimate of the durability of vaccine efficacy after the crossover may be less reliable than it would have been if continued placebo-controlled follow-up had been feasible.
Finally, as with all major Covid-19 vaccine trials, vaccine efficacy was assessed over a relatively short period during a rapidly evolving pandemic. Initial trials of messenger RNA vaccines in the United States2,3 tested vaccine efficacy largely against viral variants that were not substantially different from the source vaccine antigen and showed high levels of vaccine efficacy against Covid-19 of any severity. The vaccine efficacy results from our trial not only recapitulate the overall high vaccine efficacy observed in earlier trials but also indicate that vaccine efficacy was high regardless of the then-circulating variant strains: 100% (95% CI, 85.8 to 100) against those that are not variants of concern or interest and 92.6% (95% CI, 83.6 to 96.7) against any circulating variant of concern or interest. Because case accrual for this analysis occurred during the first half of 2021, when the delta variant of concern had not been widely established throughout the United States or Mexico,14 no Covid-19 efficacy end-point cases due to this variant were accrued, and thus the vaccine efficacy against delta and other newer variants could not be established.
NVX-CoV2373 is a new adjuvanted recombinant protein vaccine that can be added to the portfolio of vaccines that are safe and highly protective against contemporary SARS-CoV-2 strains and that have an acceptable side-effect profile. The extended stability and easy storage requirements (up to 6 months at refrigerator temperatures) make it well suited for global deployment. The efficacy of NVX-CoV2373 in preventing moderate-to-severe Covid-19 as well as any symptomatic Covid-19 in people at high risk for acquisition and complications of Covid-19 will make this vaccine a valuable tool in controlling the pandemic and its most serious health and economic consequences.
Acknowledgments
We thank all the participants who volunteered for the trial and who have contributed their clinical experience to the assessment of the safety and efficacy of NVX-CoV-2373; the members of the NIAID data and safety monitoring board and the protocol safety review team, whose monitoring of all trial data contributed to ensuring the safety and well-being of the trial participants; our NIAID and National Institutes of Health colleagues, who provided valuable infrastructure support for the NIAID-affiliated investigative sites; community leadership groups throughout the country who assisted with community engagement and recruitment; unnamed colleagues at each of the trial sites who generously contributed to the trial in many ways; and all our unnamed colleagues at Novavax who worked tirelessly and gave unlimited efforts to the development, testing, and support for this trial. Editorial assistance with the preparation of an earlier version of this manuscript was provided by Phase Five Communications, supported by Novavax.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This article was published on December 15, 2021, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by Novavax; the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority (contract Operation Warp Speed: Novavax Project Agreement number 1 under Medical CBRN [Chemical, Biological, Radiological, and Nuclear] Defense Consortium base agreement no. 2020-530; Department of Defense no. W911QY20C0077); and the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health. The NIAID provides grant funding to the HIV Vaccine Trials Network (HVTN) Leadership and Operations Center (UM1 AI68614), the HVTN Statistics and Data Management Center (UM1 AI68635), the HVTN Laboratory Center (UM1 AI68618), the HIV Prevention Trials Network Leadership and Operations Center (UM1 AI68619), the AIDS Clinical Trials Group Leadership and Operations Center (UM1 AI68636), and the Infectious Diseases Clinical Research Consortium leadership group (UM1 AI148684).
Disclosure forms provided by the authors are available with the full text of the article at NEJM.org.
References
- 1.Slaoui M, Hepburn M. Developing safe and effective Covid vaccines — Operation Warp Speed’s strategy and approach. N Engl J Med 2020;383:1701-1703. [DOI] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021;397:671-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.SARS-CoV-2 variant classifications and definitions. Atlanta: Centers for Disease Control and Prevention, 2021. (https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html). [Google Scholar]
- 7.Harvey WT, Carabelli AM, Jackson B, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol 2021;19:409-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krause PR, Fleming TR, Longini IM, et al. SARS-CoV-2 variants and vaccines. N Engl J Med 2021;385:179-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holder J. Tracking coronavirus vaccinations around the world. New York Times. 2021. (https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html).
- 10.Keech C, Albert G, Cho I, et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med 2020;383:2320-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Formica N, Mallory R, Albert G, et al. Different dose regimens of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373) in younger and older adults: a phase 2 randomized placebo-controlled trial. PLoS Med 2021;18(10):e1003769-e1003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shinde V, Bhikha S, Hoosain Z, et al. Efficacy of NVX-CoV2373 Covid-19 vaccine against the B.1.351 variant. N Engl J Med 2021;384:1899-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heath PT, Galiza EP, Baxter DN, et al. Safety and efficacy of the NVX-CoV2373 Covid-19 vaccine. N Engl J Med 2021;385:1172-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nexstrain. Genomic epidemiology of novel coronavirus — global subsampling. 2021. (https://nextstrain.org/ncov/gisaid/global).
- 15.Guidance for industry: development and licensure of vaccines to prevent COVID-19. Silver Spring, MD: Food and Drug Administration, June 2020. (https://www.fda.gov/media/139638/download). [Google Scholar]
- 16.Corey L, Mascola JR, Fauci AS, Collins FS. A strategic approach to COVID-19 vaccine R&D. Science 2020;368:948-950. [DOI] [PubMed] [Google Scholar]
- 17.Joffe S, Babiker A, Ellenberg SS, et al. Data and safety monitoring of COVID-19 vaccine clinical trials. J Infect Dis 2021. May 19 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.People with certain medical conditions. Atlanta: Centers for Disease Control and Prevention, 2021. (https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html). [Google Scholar]
- 19.Center for Biologics Evaluation and Research. Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. Silver Spring, MD: Food and Drug Administration, September 2007. (https://www.fda.gov/media/73679/download). [Google Scholar]
- 20.Coronavirus (COVID-19) update. Silver Spring, MD: Food and Drug Administration, July 13, 2021. (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-july-13-2021). [Google Scholar]
- 21.Myocarditis and pericarditis after mRNA COVID-19 vaccination. Atlanta: Centers for Disease Control and Prevention, 2021. (https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html). [Google Scholar]
- 22.Hunter PR. Thrombosis after covid-19 vaccination. BMJ 2021;373:n958-n958. [DOI] [PubMed] [Google Scholar]
- 23.Falsey AR, Sobieszczyk ME, Hirsch I, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 vaccine. N Engl J Med 2021;385:2348-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Follmann D, Fintzi J, Fay MP, et al. A deferred-vaccination design to assess durability of COVID-19 vaccine effect after the placebo group is vaccinated. Ann Intern Med 2021;174:1118-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.