Abstract
The androgen receptor (AR) is a validated therapeutic target for prostate cancer and has been a focus for drug development for more than six decades. Currently approved therapies that inhibit AR signaling such as enzalutamide rely solely on targeting the AR ligand-binding domain and therefore have limited efficacy on prostate cancer cells that express truncated, constitutively active AR splice variants (AR-Vs). The LNCaP95 cell line is a human prostate cancer cell line that expresses both functional full-length AR and AR-V7. LNCaP95 is a heterogeneous cell population that is resistant to enzalutamide, with its proliferation dependent on transcriptionally active AR-V7. The purpose of this study was to identify a LNCaP95 clone that would be useful for evaluating therapies for their effectiveness against enzalutamide-resistant prostate cancer cells. Seven clones from the LNCaP95 cell line were isolated and characterized using morphology, in vitro growth rate, and response to ralaniten (AR N-terminal domain inhibitor) and enzalutamide (antiandrogen). In vivo growth of the clones as subcutaneous xenografts was evaluated in castrated immunodeficient mice. All of the clones maintained the expression of full-length AR and AR-V7. Cell proliferation of the clones was insensitive to androgen and enzalutamide but importantly was inhibited by ralaniten, which is consistent with AR-Vs driving the proliferation of parental LNCaP95 cells. In castrated immunodeficient animals, the growth of subcutaneous xenografts of the D3 clone was the most reproducible compared to the parental cell line and other clones. These data support that the enzalutamide-resistant LNCaP95-D3 subline may be suitable as a xenograft tumor model for preclinical drug development with improved reproducibility.
Keywords: prostate cancer, androgen receptor, AR-V7, LNCaP95, ralaniten, enzalutamide, animal models
INTRODUCTION
In the recent decade, several second-generation antiandrogens (enzalutamide, apalutamide, and darolutamide) have been developed for the treatment of castration-resistant prostate cancer (CRPC). These potent antagonists of the androgen receptor (AR) ligand-binding domain can extend patient survival but are not curative. All patients will eventually succumb to disease that is resistant to these therapies. One major mechanism of resistance to antiandrogens is the expression of constitutively active AR splice variants (AR-Vs) which lack the C-terminal ligand-binding domain [1, 2]. Most CRPC remains reliant on AR transcriptional activity to maintain tumor growth [3, 4]. Approximately one-third of patients with metastatic CRPC have detectable AR-Vs by liquid biopsy [5]. All current hormonal therapies inhibit the transcriptional activity of AR by targeting the ligand-binding domain, either directly (antiandrogens) or indirectly by androgen deprivation therapy (ADT) and inhibitors of steroidogenesis (abiraterone acetate). Since constitutively active AR-Vs do not encode a functional ligand-binding domain, these current hormonal therapies have limited to no effect on the proliferation of prostate cancer cells that are driven by transcriptional active AR-Vs [6–8].
Prostate cancer cell lines remain a vital component of preclinical research. Most human prostate cancer cell lines have characteristics that recapitulate some attributes of the clinical progression of prostate cancer [9]. LNCaP cells originated from lymph node metastasis of a prostate cancer patient treated with estrogens [10]. These cells express a functional full-length (fl)-AR that drives their proliferation in response to androgen. LNCaP cells express the clinical marker for prostate cancer, prostate-specific antigen (PSA), which is an androgen-regulated gene. The abilities of LNCaP cells to be grown in vivo as xenografts, progress to the castration-resistant stage, and secrete PSA mimics several important aspects of clinical disease. Unfortunately, parental LNCaP cells do not express AR-Vs and are sensitive to enzalutamide. Thus, there is a need for models of prostate cancer that express AR-Vs that are resistant to clinically used antiandrogens such as enzalutamide. In efforts to address this need, the long-term culture of LNCaP cells in hormone-depleted media resulted in creation of the LNCaP95 cell line, which is androgen-independent and resistant to enzalutamide in vitro and in vivo [11, 12]. However, since LNCaP95 cells were derived from parental LNCaP cells and were not generated from a single cell clone, they tend to exhibit a large degree of inherent variability when grown as subcutaneous xenograft tumors in vivo. In this study, we isolated and characterized several clones from the LNCaP95 cell line by serial dilution. This strategy led to the identification of cell clones that retained androgen-independent proliferation and the expression of AR-Vs but have improved reproducibility for in vivo studies.
MATERIALS AND METHODS
Reagents and compounds
Ralaniten (EPI-002) was provided by NAEJA (Edmonton, Alberta) and second-generation analog EPI-7170 was synthesized by Dr. Raymond Andersen (University of British Columbia, Canada). Enzalutamide was purchased from OmegaChem (Lévis, Québec). Synthetic androgen methyltrienolone (R1881) was purchased from Perkin-Elmer (Waltham, Massachusetts).
Cell culture
The LNCaP95 cell line was provided by Dr. Stephan Plymate from the University of Washington (Seattle, Washington). LNCaP95 (LN95) parental cells and clones were cultured in RPMI-1640 media with 10% charcoal-stripped serum (CSS).
Cell clonal selection
Cell clones of LNCaP95 cells were isolated by serial dilution in a 96-well plate with an initial inoculum of 4,000 cells. A sequential 1:2 dilution was performed down the first column from A1 to H1, followed by a second sequential 1:2 dilution across rows 1 to 12 using a multichannel pipette. Cell clones that were detectable by microscopy after three weeks were then further characterized. The colonies were subcultured from the 96-well plate to 48-well, 24-well, 6-well plate, and then to T25 and T75 flasks.
Western blot analysis
Approximately 250,000 cells per well were plated in 6-well plates in RPMI supplemented with 10% CSS. After three days, the cells were collected to prepare whole cell lysates. Protein concentrations were determined by bicinchoninic acid assay and 20 μg of cell lysate per sample was run on an SDS-PAGE gradient gel (4–15%), transferred to a nitrocellulose membrane, and then probed with antibodies raised against AR (N-20, Santa Cruz Biotechnology), AR-V7 (EPR15656, Abcam), or PSA (C-19, Santa Cruz Biotechnology). Membranes were re-probed with antibodies against β-actin (AC-15, Sigma-Aldrich) as a loading control. Western blot images were captured using the ChemiDoc MP Imaging System (Bio-Rad Laboratories, Hercules, California) and then quantified using ImageJ software.
BrdU cell proliferation assay
Approximately 6,000 cells per well were plated in 96-well plate in RPMI supplemented with 10% CSS. The media was changed to 1.5% CSS RPMI media forty-eight hours later, and cells were treated with vehicle (DMSO), R1881 (0.1 nM), enzalutamide (10 μM), or EPI-002 (25 μM) for 4 or 5 days. After incubating with the compounds, the cells were labeled with BrdU for two hours and BrdU incorporation was measured using the colorimetric BrdU ELISA kit (Roche Applied Science, Mannheim, Germany) with a VersaMax Microplate Reader (Molecular Devices, San Jose, California). Absorbance was measured at 370 nm with a reference wavelength of 492 nm.
Growth rate of the LNCaP95 cell clones
Approximately 40,000 cells per well were plated in a 24-well plate with RPMI media supplemented with 10% CSS. Cell counts were determined at 48, 72, 96, and 120 hours with a hemocytometer by trypan blue exclusion, and the doubling time was calculated by exponential regression using GraphPad Prism 8 software (San Diego, California).
Cell cycle analysis by flow cytometry
Asynchronous LNCaP95-D3 cells were seeded into 10 cm dishes and cultured in RPMI media supplemented with 10% CSS. After three days, the media was replaced with RPMI media containing 1.5% CSS and the specified inhibitors in the absence of androgen. After 48 hours of treatment, the cells were labeled with 10 μM of BrdU for two hours and then harvested with trypsin. After washing with phosphate-buffered saline (PBS), the samples were fixed with 70% EtOH/PBS and stored at −20°C until further analysis. For flow cytometric analyses, 5 ×105 fixed cells were labeled with fluorescein (FITC)-conjugated anti-BrdU antibody (B44; BD Biosciences, San Jose, California) and stained with 7-AAD (Sigma-Aldrich). The samples were analyzed by a FACSCalibur flow cytometer (BD Biosciences) using the FL1 and FL3 channels to detect FITC and 7-AAD respectively. For each sample, 10,000 events were recorded and analyzed by FlowJo software V10 (Ashland, Oregon). Pre-gating was performed for every sample by plotting FL3-W × FL3-H to exclude doublets from the analysis.
Xenograft models
All experiments involving animals conform to the relevant regulatory and ethical standards and were approved by the University of British Columbia Animal Care Committee (A18–0077). Six- to eight-week-old male NOD-scid were subcutaneously injected with LNCaP95 clones (5 million cells) in a 1:1 volume of matrigel (Corning Discovery Labware, Corning, New York). Animals were castrated four weeks after inoculating with cells, and they were monitored every two days until tumors became palpable. The tumors were measured twice a week and tumor volume was determined by the formula: length × width × height × 0.5236. Mice were euthanized when tumor volumes exceeded 1,000 mm3.
Statistical analyses
Statistical differences were determined by GraphPad Prism 8 by Analysis of Variance (ANOVA). An alpha level of .05 was used for all statistical tests, which is denoted in figures as *P<.05, **P<.01, ***P<.001.
RESULTS
Isolation and general characterization of LNCaP95 cell clones
The LNCaP95 cell line is an androgen-independent cell line generated from parental LNCaP cells that were cultured under long-term androgen-deprivation conditions in charcoal-stripped serum media [11]. Thus, LNCaP95 cells were not derived from a single cell clone and are considered a heterogeneous cell population. This likely accounts for substantial variability observed with the growth rate of LNCaP95 xenograft tumors in vivo. To address this heterogeneity, we isolated clones from the LNCaP95 cell line to develop sublines that would provide more reproducible data for in vivo experiments. Clones were isolated from the LNCaP95 cell line by serial dilution in a 96-well plate (Fig. 1a) and expanded from wells with 32 to 250 cells. Compared to parental LNCaP95 cells, which have an epithelial cell-like morphology and dendritic cell-like extensions, several of the clones had unique cell morphologies and appeared more flattened or cuboidal (Fig. 1b; clones D3, E2, and F2).
Fig. 1.
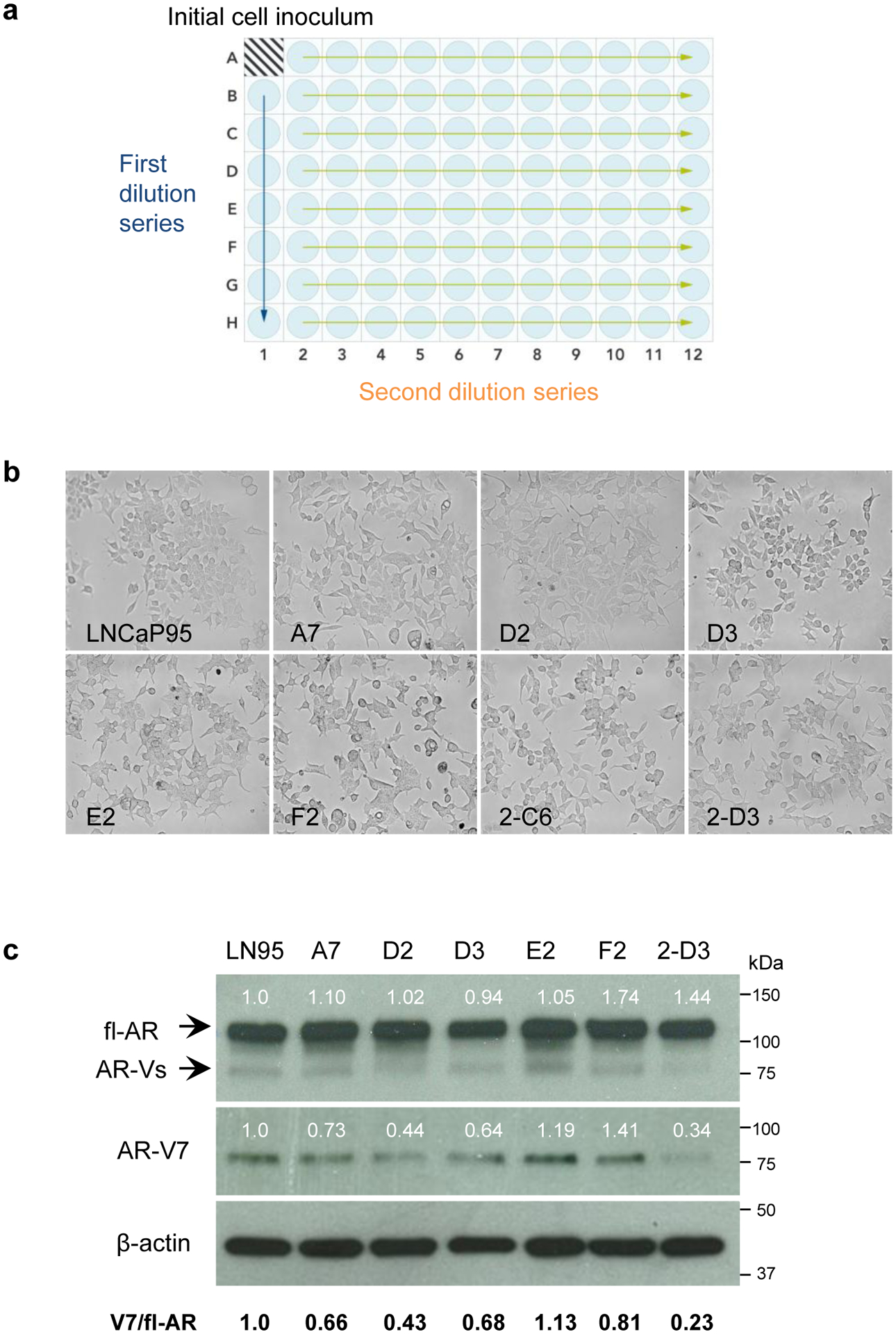
Generation of clones from the LNCaP95 parental cell line. a Schematic summarizing the serial dilution of LNCaP95 cells in a 96-well plate. Arrows represent the first and second dilution series. b Micrographs depicting the cell morphology of LNCaP95 parental cells and isolated clones. LNCaP95 cells have an epithelial cell morphology with pronounced dendritic-cell-like extensions, whereas several of the isolated clones had a unique flattened morphology. c Western blot analysis of fl-AR and AR-V7 expression in the isolated clones. Numbers above the protein bands indicate the relative fl-AR and AR-V7 expression after normalizing to β-actin. The ratio of AR-V7 expression relative to fl-AR is indicated below
We performed Western blot analyses to determine whether the isolated clones expressed fl-AR and AR-V7. An antibody recognizing the AR N-terminal domain (N-20) can detect fl-AR as well as truncated AR-Vs. The analysis revealed that all of the isolated clones expressed fl-AR at comparable levels to parental LNCaP95 cells (Fig. 1c). The expression of AR-V7 was confirmed using a V7-specific antibody which revealed a single band at approximately 75 kDa, consistent with the expected size of AR-V7 protein. AR-V7 was detected in all of the clones, but protein levels differed between separate clones. Comparing the levels of AR-V7 between clones, the E2 clone had a higher ratio of V7/fl-AR than the parental cell line, whereas the D2 and 2-D3 clones had relatively lower ratios. We then estimated the cell doubling time of the clones in vitro in a cell counting assay (Fig. 2a). The parental LNCaP95 cells had a doubling time of 52.2 hours, and the clones had equivalent or longer doubling times that ranged from 52.9 to 85.3 hours (Table 1). Overall, the doubling times of the clones did not correlate with the relative levels of fl-AR (r=0.56, P=.19) or the ratio of V7/fl-AR (r=0.12, P=.80) (Fig. 2b).
Fig. 2.
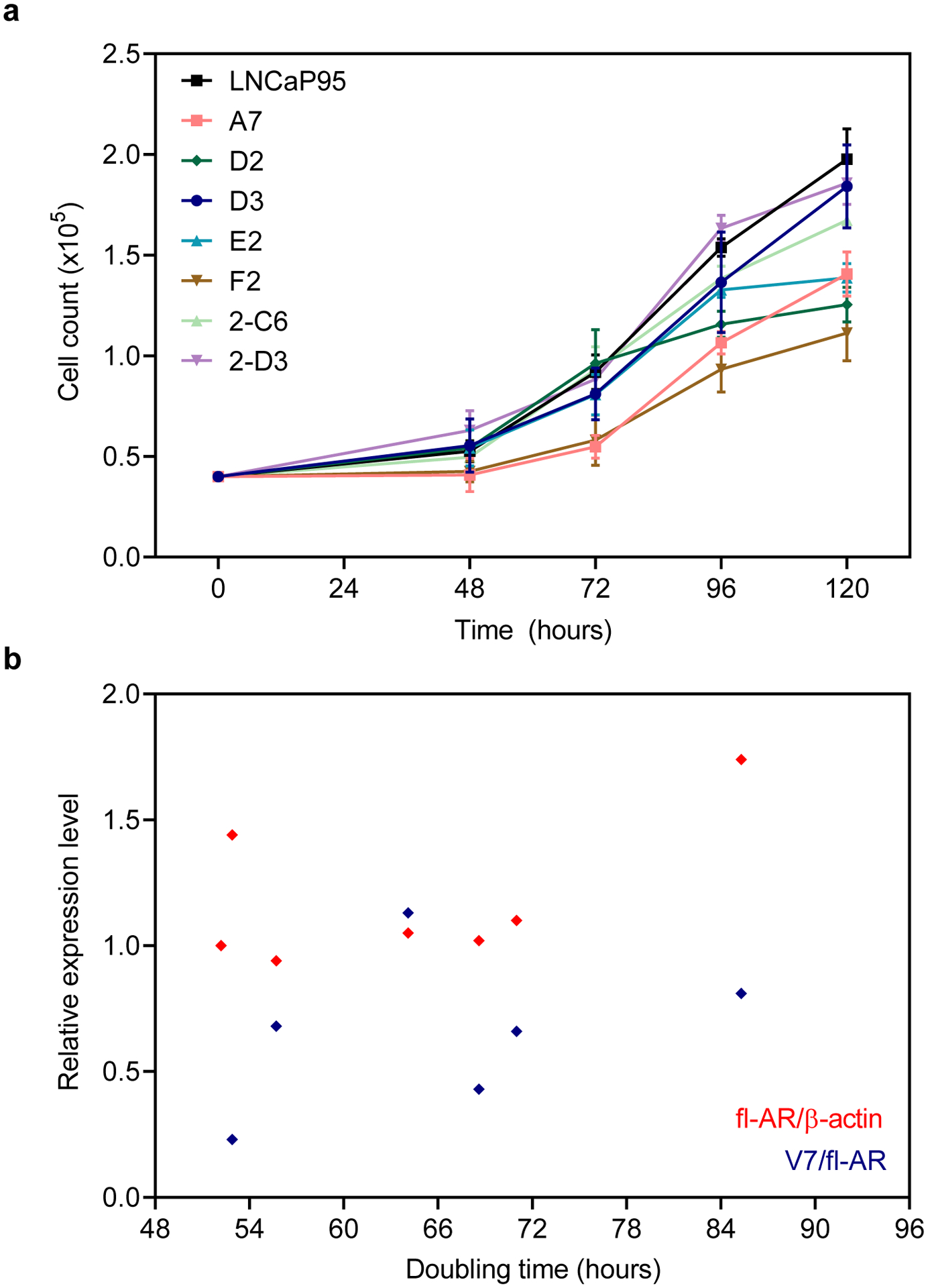
Cell growth of the LNCaP95 clones. a Cell counts for the isolated clones were determined after 48, 72, 96, and 120 hours from an initial population of 4,000 cells. Data shown represent the mean ± SE, n=3. b Pearson correlation analysis showing no association between expression of fl-AR or AR-V and cell doubling times determined by exponential regression
Table 1.
Doubling time of parental LNCaP95 cells and clones
| Clone | Doubling time (h) | R 2 |
|---|---|---|
| LNCaP95 (parental) | 52.2 | .92 |
| A7 | 71.0 | .78 |
| D2 | 68.6 | .80 |
| D3 | 55.7 | .80 |
| E2 | 64.1 | .87 |
| F2 | 85.3 | .70 |
| 2-C6 | 57.6 | .91 |
| 2-D3 | 52.9 | .91 |
Androgen-independent LNCaP95 clones express PSA and are resistant to enzalutamide but sensitive to ralaniten
LNCaP95 clones were evaluated in cell proliferation assays for their responsiveness to androgen (R1881), an AR ligand-binding domain inhibitor (enzalutamide), and an AR N-terminal domain inhibitor (ralaniten). In response to R1881 (0.1 nM), the parental LNCaP95 cells and most of the clones showed a decrease in proliferation compared to the vehicle control (Fig. 3a,b). Ralaniten (EPI-002, 25 μM), which targets both fl-AR and AR-V7, reduced the proliferation of parental LNCaP95 cells as previously reported [12] and all of the clones to a certain extent after four or five days of treatment (Fig. 3a,b). Except for D2, none of the clones showed a substantial decrease in proliferation in response to enzalutamide (10 μM) compared to ralaniten (25 μM). Interestingly, the inhibitory effect of ralaniten and enzalutamide was not durable in D2 clones and by 5 days no significant difference was observed compared to controls (Fig. 3b). Parental LNCaP95 cells and the D3 and 2-C6 clones were the most sensitive to ralaniten and were inhibited by 66–79% after five days. Clones A7, E2, F2, and 2-D3 were moderately inhibited by ralaniten by 54–56%. Collectively these data suggest that the LNCaP95 clones maintain characteristics of the parental LNCaP95 cell line such that their proliferation is resistant to enzalutamide and sensitive to ralaniten, suggestive of reliance on constitutively active AR-Vs.
Fig. 3.
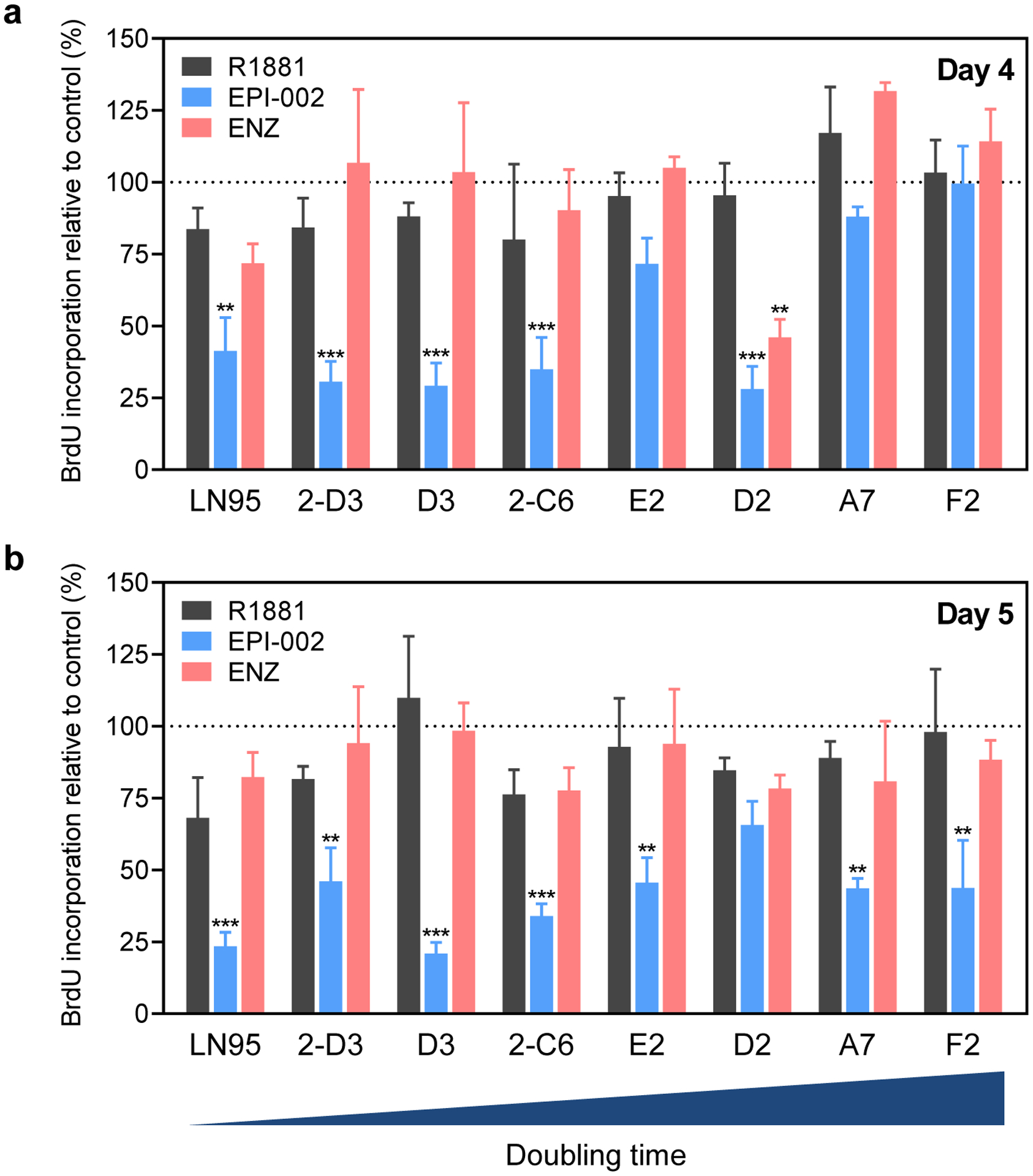
Proliferation of the LNCaP95 clones in response to androgen, ralaniten, and enzalutamide. Parental LNCaP95 cells and clones were treated with R1881 (0.1 nM), EPI-002 (25 μM), enzalutamide (ENZ, 10 μM), or vehicle for a 4, or b 5 days, and then labeled with BrdU for 2 hrs. Data shown represents BrdU incorporation determined by ELISA after normalizing to the vehicle control and represent the mean ± SE, n=3. Statistical significance was determined by two-way ANOVA using the Dunnett multiple comparisons test
We evaluated the D3 clone for the expression of PSA, a well-characterized and validated marker of the clinical progression of prostate cancer. Western blot analysis of cell lysates prepared from the D3 clone revealed expression of PSA protein, which was induced by treatment with androgen by approximately 8-fold, consistent with the parental cell line (Fig. 4a). Moreover, treatment of androgen reduced the levels of AR-V7 in the D3 clone, which was also observed for parental cells and is consistent with the notion that AR-V7 expression is attenuated when fl-AR transcriptional activity is predominant [13].
Fig. 4.
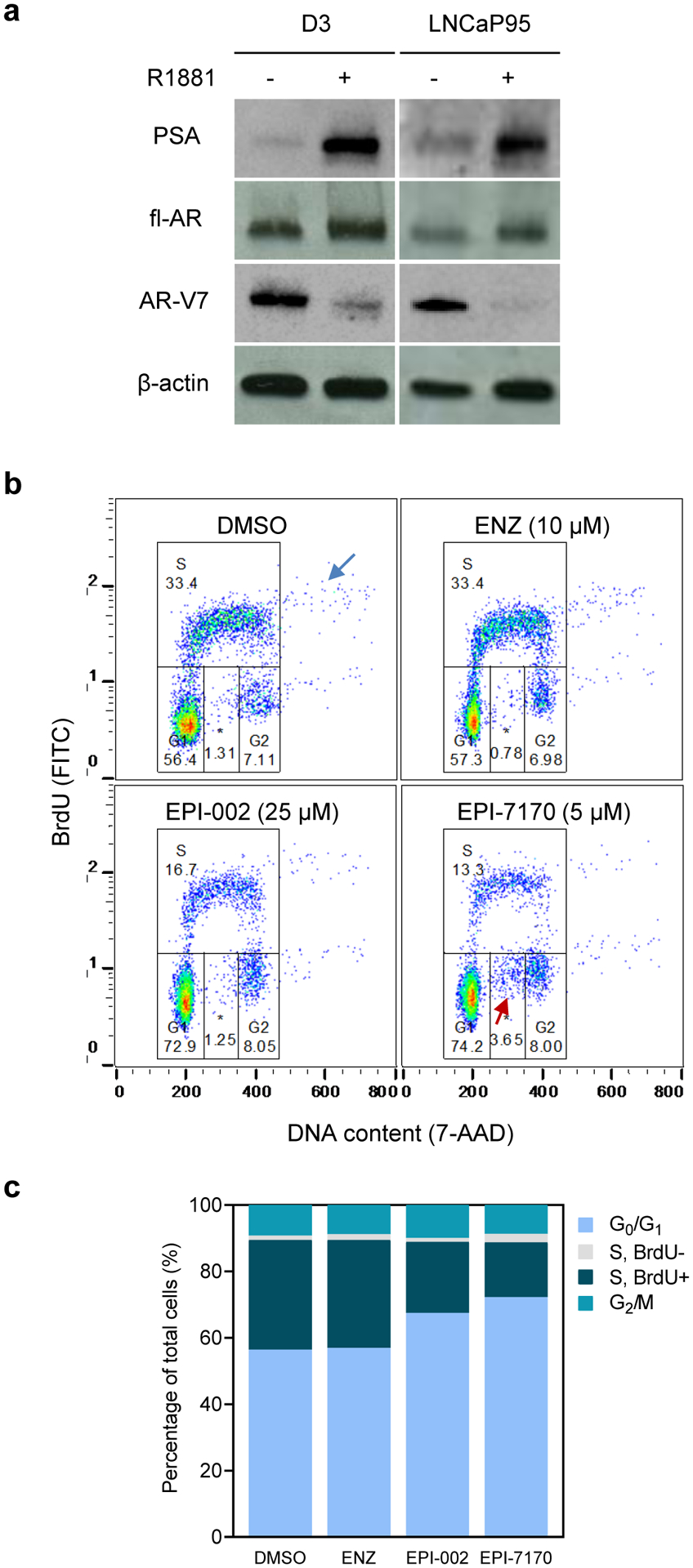
PSA expression and cell cycle analysis of the D3 clone in response to ralaniten and enzalutamide. a Western blot analysis of cell lysates prepared from D3 or parental LNCaP95 cells incubated with androgen (R1881, 1 nM) for 48 hours, n=2. b Cell cycle analysis of D3 cells incubated in the absence of androgen with vehicle (DMSO), enzalutamide (ENZ), EPI-002, EPI-7170 for 48 hours before labeling with BrdU for two hours. The blue arrow indicates a proportion of cells with DNA content >4n and the red arrow shows BrdU-negative cells induced by EPI-7170. *BrdU-negative subpopulation in S phase. c Stacked bar graph summarizes the average cell cycle distribution from n=3 independent experiments
We further assessed the D3 clone for its response to ralaniten and enzalutamide in the absence of androgen in flow cytometric cell cycle analyses. To do this, D3 cells were incubated with vehicle (DMSO), ralaniten (25 μM), and a more potent ralaniten analog EPI-7170 (5 μM) [14], or enzalutamide (10 μM) for 48 hours. After treatment with inhibitors, D3 cells were labeled with BrdU to identify the S phase population. Ralaniten compounds effectively reduced the percentage of BrdU-labeled cells in S phase whereas enzalutamide had no effect compared to the vehicle (Fig. 4b), which is consistent with previous in vitro data [14, 15]. Interestingly, polyploid cells with DNA content >4n were detected consistently in our analyses (Fig. 4b, blue arrow). These polyploid cells showed a separate S phase (BrdU-positive) subpopulation, which was decreased by ralaniten compounds, implying that they are actively cycling cells and sensitive to ralaniten and EPI-7170 (Fig. 4b). Notably, a separate BrdU-negative subpopulation with 2n to 4n DNA content was induced by EPI-7170 by 3-fold relative to the control (Fig. 4b,c; red arrow). Together, these results suggest that ralaniten compounds inhibit cell cycle progression of D3 cells, which is consistent with previous in vitro data with the parental LNCaP95 cell line [14, 15].
Growth rate of the LNCaP95 clones in xenograft tumor models
We characterized the in vivo growth of the LNCaP95 clones as subcutaneous xenograft tumors in immunodeficient mice. Each LNCaP95 clone was injected subcutaneously into the flank of NOD-scid mice and the mice were castrated four weeks later. The time for tumors to become established varied between the clones where most tumors were palpable within 13 to 36 days after castration (Fig. 5a). There was substantial variability in tumor growth rates amongst the different clones, but the D3 and F2 clones showed the most consistent growth in terms of when tumors became palpable and their doubling time (Table 2).
Fig. 5.
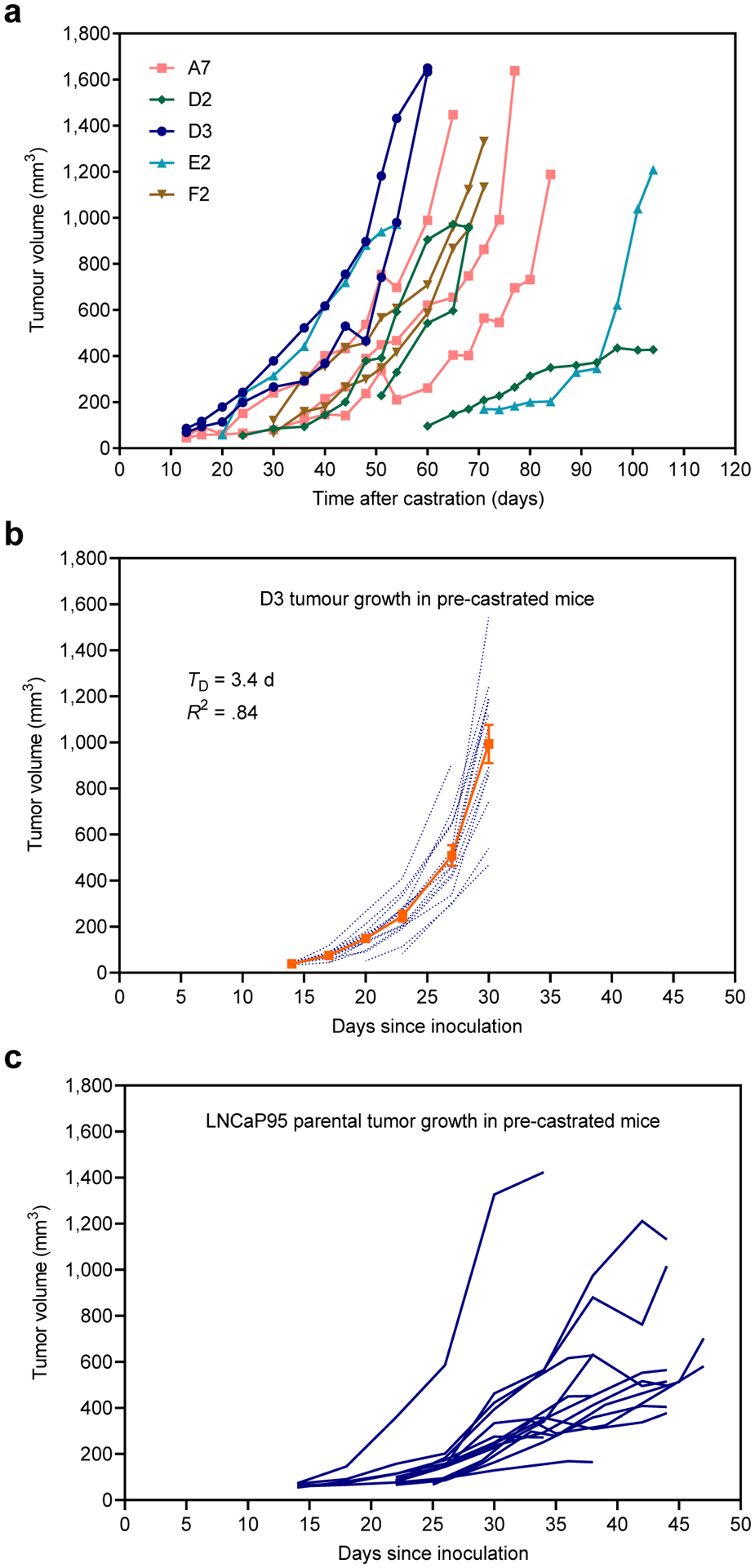
In vivo xenograft growth of LNCaP95 clones. a Tumor growth of xenografts established from the clones in NOD-scid mice, where hosts were castrated four weeks after inoculating the cells. Data shown are the individual tumors for each clone starting from a group of 3 mice. b Tumor growth of D3 xenografts in pre-castrated male NSG (NOD-scid IL2Rγnull) mice. The dotted blue lines represent individual tumors and the solid orange line shows the average tumor volume ± SE, n=14. TD, doubling time. c Tumor growth of the parental LNCaP95 cells in pre-castrated male NSG mice, where the growth curves of individual tumors are shown
Table 2.
Growth rate of the LNCaP95 clones in NOD/SCID animals
| Clone | Tumors | Initiation time (d) | SD | Doubling time (h) | R 2 |
|---|---|---|---|---|---|
| A7 | 3/3 | 20.7 | 13.3 | 31.8 | 0.36 |
| D2 | 3/3 | 29.0 | 20.0 | 23.0 | 0.40 |
| D3 | 2/3 | 13.0 | - | 11.6 | 0.92 |
| E2 | 2/3 | 45.5 | 36.1 | 11.0 | 0.92 |
| F2 | 2/3 | 30.0 | - | 13.7 | 0.94 |
Note. Animals were inoculated with the LNCaP95 clones and castrated four weeks later, from Figure 5A.
Average days for tumors to become palpable after castration
The D3 clone was selected for further characterization in immunodeficient pre-castrated animals. In this study, subcutaneous D3 tumors were palpable in pre-castrated NSG (NOD-scid IL2Rγnull) mice within 13 to 17 days after inoculation, with a tumor take rate of 75% (Fig. 5b; 12/16). The growth rate of the D3 xenograft tumors was fairly consistent between animals and the doubling time for tumor volume was approximately 3.4 days (R2=0.84, P=.010). These D3 tumors grew quicker in comparison to those that were grown in animals that were castrated after tumors had established, where the doubling time was 13 days (Table 2). The D3 xenografts were also more consistent and uniform in their growth compared to parental LNCaP95 tumors in pre-castrated animals (Fig. 5c). Based on these in vivo studies, the D3 clone produced subcutaneous tumors with the most consistent growth rate in vivo and maintained characteristics to the parental LNCaP95 cell line in terms of its response to androgen, ralaniten, and enzalutamide and expression of PSA in vitro. These data support that the LNCaP95-D3 subline is suitable as a xenograft tumor model with improved reproducibility for in vivo experiments.
DISCUSSION
The LNCaP xenograft model has historically provided a reliable preclinical model that was predictive of clinical responses to antiandrogens that target the C-terminal ligand-binding domain of the AR [16, 17]. Unfortunately, this model does not represent the current major clinical challenge of enzalutamide-resistant CRPC that is mediated by expression of constitutively active AR-Vs. To address this need, here we have characterized several new clones derived from the LNCaP95 cell line. These LNCaP95 cell clones were isolated by serial dilution and maintained in media with charcoal-stripped serum. All of the clones isolated retained expression of AR-V7 and were not dependent on androgen for proliferation. These findings are consistent with the role of AR-Vs in facilitating androgen-independent proliferation of CRPC cells. Accordingly, proliferation of most of the clones (6/7) in vitro was resistant to enzalutamide but sensitive to inhibition by ralaniten. Only the D2 clone responded to enzalutamide treatment, but its response to both enzalutamide and ralaniten diminished with a longer 5 days incubation. Ralaniten is metabolized primarily by oxidation and glucuronidation [18], whereas enzalutamide is predominantly oxidized by cytochrome P450 isoenzymes CYP2C8 and to a lesser extent by a CYP3A4 [19]. Thus, loss of inhibition to both ralaniten and enzalutamide by 5 days may indicate more rapid metabolism in D2 cells, or alternatively a greater reliance on other pathways that contribute to their proliferation. The lack of correlation of doubling times with the levels of expression of fl-AR or AR-Vs may also be suggestive of additional pathways influencing the proliferation of these clones.
In conclusion, there is currently a lack of preclinical models of enzalutamide-resistant CRPC that are reliant on AR-Vs. Here the D3 clone of LNCaP95 cells was characterized and shown to express fl-AR, PSA, and AR-V7. In vitro, the proliferation of the D3 clone was androgen-independent and resistant to enzalutamide but importantly sensitive to ralaniten which targets the N-terminal domain of AR required for transcriptional activity of both fl-AR and AR-Vs. In vivo, the D3 clone had a growth rate that was more consistent than the parental cell line as well as the other clones tested. Further investigation may involve the analysis of growth factor receptors, signaling transduction pathways, and metastatic potential of the D3 clone. In summary, the LNCaP95 clones we report are a valuable addition to the limited number of prostate cancer cell lines reliant on AR-Vs for proliferation and are useful preclinical models to evaluate drug candidates for inhibiting AR-Vs.
ACKNOWLEDGMENTS
This work is supported by a grant from the National Cancer Institute of the National Institutes of Health (R01CA105304).
Footnotes
CONFLICTS OF INTERESTS
The authors declare the following competing interests: J.K.L, T.T., J.W., and M.D.S. are inventors of technology pertaining to AR N-terminal domain inhibitors, which was licensed by BC Cancer to ESSA Pharma Inc. M.D.S. owns equity in and is a Scientific Advisor for ESSA Pharma Inc. Their interests are reviewed and managed by BC Cancer in accordance with its research conflict of interest policy.
REFERENCES
- 1.Ware KE, Garcia-Blanco MA, Armstrong AJ, Dehm SM. Biologic and clinical significance of androgen receptor variants in castration resistant prostate cancer. Endocr Relat Cancer. 2014;21(4):T87–T103. doi: 10.1530/ERC-13-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu C, Brown LC, Antonarakis ES, Armstrong AJ, Luo J. Androgen receptor variant-driven prostate cancer II: advances in laboratory investigations. Prostate Cancer Prostatic Dis. 2020. doi: 10.1038/s41391-020-0217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandrasekar T, Yang JC, Gao AC, Evans CP. Targeting molecular resistance in castration-resistant prostate cancer. BMC Med. 2015;13(1):206. doi: 10.1186/s12916-015-0457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 5.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371(11):1028–38. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joseph JD, Lu N, Qian J, Sensintaffar J, Shao G, Brigham D et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 2013;3(9):1020–9. doi: 10.1158/2159-8290.CD-13-0226. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73(2):483–9. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Nakazawa M et al. Androgen Receptor Splice Variant 7 and Efficacy of Taxane Chemotherapy in Patients With Metastatic Castration-Resistant Prostate Cancer. Jama Oncol. 2015;1(5):582–91. doi: 10.1001/jamaoncol.2015.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pienta KJ, Abate-Shen C, Agus DB, Attar RM, Chung LW, Greenberg NM et al. The current state of preclinical prostate cancer animal models. Prostate. 2008;68(6):629–39. doi: 10.1002/pros.20726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L et al. The LNCaP cell line--a new model for studies on human prostatic carcinoma. Prog Clin Biol Res. 1980;37:115–32. [PubMed] [Google Scholar]
- 11.Hu R, Lu C, Mostaghel EA, Yegnasubramanian S, Gurel M, Tannahill C et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012;72(14):3457–62. doi: 10.1158/0008-5472.CAN-11-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang YC, Banuelos CA, Mawji NR, Wang J, Kato M, Haile S et al. Targeting Androgen Receptor Activation Function-1 with EPI to Overcome Resistance Mechanisms in Castration-Resistant Prostate Cancer. Clin Cancer Res. 2016;22(17):4466–77. doi: 10.1158/1078-0432.CCR-15-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu LL, Xie N, Sun S, Plymate S, Mostaghel E, Dong X. Mechanisms of the androgen receptor splicing in prostate cancer cells. Oncogene. 2014;33(24):3140–50. doi: 10.1038/onc.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banuelos CA, Ito Y, Obst JK, Mawji NR, Wang J, Hirayama Y et al. Ralaniten Sensitizes Enzalutamide-Resistant Prostate Cancer to Ionizing Radiation in Prostate Cancer Cells that Express Androgen Receptor Splice Variants. Cancers (Basel). 2020;12(7). doi: 10.3390/cancers12071991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imamura Y, Tien AH, Pan J, Leung JK, Banuelos CA, Jian K et al. An imaging agent to detect androgen receptor and its active splice variants in prostate cancer. Jci Insight. 2016;1(11). doi: 10.1172/jci.insight.87850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu X, Gong S, Roy-Burman P, Lee P, Culig Z. Current mouse and cell models in prostate cancer research. Endocr Relat Cancer. 2013;20(4):R155–70. doi: 10.1530/ERC-12-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunningham D, You Z. In vitro and in vivo model systems used in prostate cancer research. Journal of biological methods. 2015;2(1). doi: 10.14440/jbm.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obst JK, Wang J, Jian K, Williams DE, Tien AH, Mawji N et al. Revealing Metabolic Liabilities of Ralaniten To Enhance Novel Androgen Receptor Targeted Therapies. ACS Pharmacol Transl Sci. 2019;2(6):453–67. doi: 10.1021/acsptsci.9b00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibbons JA, de Vries M, Krauwinkel W, Ohtsu Y, Noukens J, van der Walt JS et al. Pharmacokinetic Drug Interaction Studies with Enzalutamide. Clin Pharmacokinet. 2015;54(10):1057–69. doi: 10.1007/s40262-015-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


