Abstract
There is no systematic histopathologic analysis of non-neoplastic polyps in the gallbladder. In this study, in addition to a computer search for cases designated as “polyp”, a systematic review of 2533 consecutive routinely sampled archival and 203 totally-submitted prospective cholecystectomies were analyzed for >2mm polyps(cut-off was based on radiologic sensitivity). 447 non-neoplastic polyps were identified. The frequency was 3% in archival cases, 5% in totally-submitted cases. Only 21(5%) were ≥1 cm. Average age was 52, F/M=3.1. Two distinct categories were delineated: 1. Injury-related polyps(n=273): (1a)Fibro(myo)glandular polyps(n=214) were small(mean=0.4 cm), broad-based, often multiple(45%), almost always(98%) gallstone-associated, composed of a mixture of (myo)fibroblastic tissue/lobular glandular units with chronic cholecystitis. Dysplasia seen in 9% seemed to be secondary involvement. (1b)Metaplastic pyloric glands forming polypoid collections(n=42). (1c)Inflammatory-type polyps associated with acute/subacute injury (11 granulation tissue, 3 xanthogranulomatous, 3 lymphoid). 2. Cholesterol polyps(n=174) occurred in un-injured gallbladders, revealing a very thin stalk, edematous cores devoid of glands but with cholesterol-laden macrophages in 85%, cholesterolosis in the uninvolved mucosa in 60%. Focal low-grade dysplasia was seen in 3%, always confined to the polyp, unaccompanied by carcinoma. In conclusion, non-neoplastic polyps are seen in 3% of cholecystectomies and are often small. Injury-related fibromyoglandular polyps are the most common. Cholesterol polyps have distinctive cauliflower architecture, often in a background of un-injured gallbladders with cholesterolosis and may lack the cholesterol-laden macrophages in the polyp itself. Although dysplastic changes can involve non-neoplastic polyps, they don’t seem to be the cause of invasive carcinoma by themselves.
INTRODUCTION
With the advent of imaging modalities, gallbladder polyps are increasingly being encountered in clinical practice. Most are not submitted to histological examination, but instead are generally followed with ultrasound, unless larger than 1 cm or showing rapid growth 1–4. In the meantime, a number of smaller polyps are removed incidentally during cholecystectomy performed for other conditions, especially for cholecystitis and often masked by the accompanying gallstones. These often lead to confusion in diagnosis and terminology because there has been no systematic pathology-based study examining the frequency, histopathology and clinicopathologic associations of non-neoplastic polyps. In fact, most of the studies and the lexicon used are based on the impression and opinion of experts rather than systematic data 5–8. Consequently, there is no formal evidence-based pathologic classification or terminology for gallbladder polyps. While cholesterol polyps have been well recognized, their clinicopathologic associations have not been elucidated. For other polypoid lesions occurring in this organ, a variety of terms and approaches have been applied, but their nature has not been clarified. In terms of neoplastic or malignant transformation in these polyps, there is virtually no data in the literature.
In this study, we performed a detailed histopathologic analysis of non-neoplastic polyps of gallbladder to better elucidate their pathologic characteristics, and to determine their relative frequency and clinicopathologic associations with the hope that this will lead to better understanding of these lesions towards their more refined management.
MATERIALS AND METHODS
Case identification
The study was accomplished in agreement with the respective institutions’ review board requirements.
The cases analyzed in this study originated in 3 different regions including South America (in Temuco, Chile, where the gallbladder cancer incidence is one of the highest in the world), North America (United States) and Turkey. The cases were identified through:
A targeted search of the archival pathology files of the respective institutions was performed to identify the cases reported as “polyp” in cholecystectomies. Separately, cases in the consultation files of one of the authors (VA) were also culled.
In order to determine the frequency of non-neoplastic polyps, a systematic re-review of 2533 consecutive cholecystectomies was performed by the authors.
203 consecutive cholecystectomies were prospectively submitted totally for microscopic examination in order to determine the frequency of subtle pathologic findings that may be missed by the macroscopic examination during daily practice.
For comparison with chronic cholecystitis, the findings in the same cohorts described above (2736 cases) were utilized. For gallbladder carcinomas, 636 cases identified in the same files were used.
Criteria for inclusion
For the purpose of evaluation, a polyp was defined as a mucosal elevation that formed a projection that stood out from the adjacent mucosa as a clearly recognizable polyp either on the gross bench or by examination of the whole mount of a glass slide, and that formed a lesion that was morphologically distinct from the adjacent mucosa as verified by microscopic examination. Instead of determining an arbitrary cut-off as is customary in other studies, we elected to review the radiology literature and also polled several radiologists in order to establish what is typically diagnosable as a “polyp” in the gallbladder clinically by imaging studies. This investigation resulted in the conclusion that polyps in the gallbladder are mostly discovered by ultrasound, and conversely ultrasound also appears to be the best modality for polyp detection 1,9. It is stated that if the patient is not overweight and if the polyp is not in the neck region, and especially if the gallbladder is not contracted, then polyps as small as 2 mm are commonly visible by ultrasound. Polyps that are as small as 2 mm are also detectable by MRI scan, but MRI is less commonly used to investigate gallbladder pathology. CT scan is not an ideal modality to visualize small gallbladder polyps. Overall, based on these observations, the 2 mm size cut-off was chosen for this study.
Exclusion criteria
Polypoid lesions measuring less than 2 mm in the largest diameter were excluded, because their radiological and gross recognition are both challenging and subjective.
Polyp types that are by nature neoplastic (intracholecystic papillary tubular neoplasms-ICPNs 10, including their <1 cm “incipient” versions and polypoid carcinomas) were excluded. Back to back monotonous collections of pyloric glands (clonal-appearing lesions, so called pyloric gland adenomas) were also excluded 10.
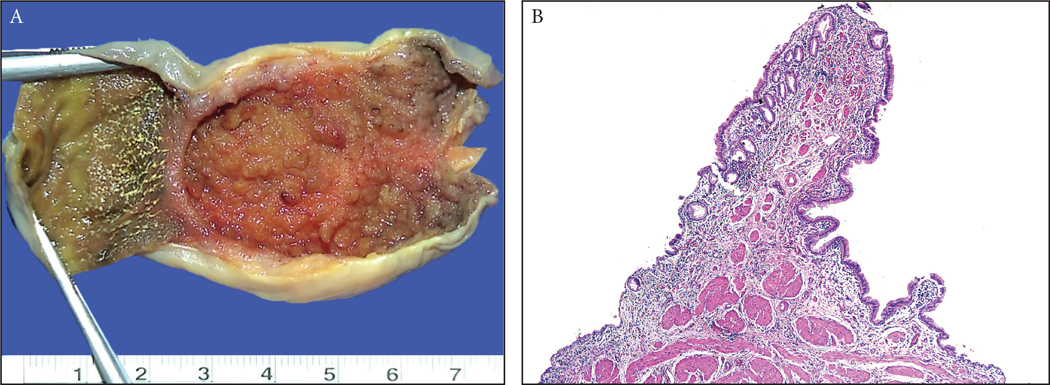
Polypoid anatomic folds, including spiral valve of Heister and Phrygian cap deformity were excluded: These plicae typically form a pencil-like projection that comes off the mucosa at an angle, have a well defined and relatively well organized muscle core at its center, and a normal mucosal covering, similar to the mucosa elsewhere (Figure 1).
Figure 1:
(A) There are anatomic polypoid folds such as Phrigian cap deformity as well as the valves at the neck/cystic-duct region and those occur due to impacted stones, all of which were excluded from the study. Typically, these form a pencil-like projection that come out of the surface with an angle. (B) Microscopically, these tend to have a well defined and organized muscle core at its center that tapers towards the tip, and a normal mucosal covering that is almost equidistant from the muscle similar to the mucosa elsewhere.
Mucosal neural hyperplasias (prominence of nerves forming minute polyps), a.k.a. mucosal “neuromas” or “(ganglio)neuromatosis”, were excluded.
Lesions that are fundamentally mural (submucosal) but can be misinterpreted radiologically to be polypoid such as adenomyomas or heterotopias were also excluded since it is often highly subjective whether such a process is truly polypoid or not, even on radiologic examination. And furthermore, such lesions do not fit to the strict definition of a polyp per se (defined in the dictionary as “a growth projecting from a mucous membrane”, https://www.merriam-webster.com/dictionary/polyp) because they typically push the mucosa from below rather than forming a true protrusion.
Evaluation of pathologic and clinical parameters
The polyp size measured during gross examination was verified microscopically to establish the true size of the lesion. The morphologic findings of the polyp was recorded. The findings in the remaining gallbladder (outside the non-neoplastic polyp) were also documented. For the recognition of the dysplasia, the criteria put forth for the bile ducts in the study by Zen et al (BilIN) and later refined for the gallbladder Santiago consensus meeting 11 was used, which is also covered in the most recent World Health Organization classification 12 and other textbooks 13.
The findings were correlated with the clinicopathologic parameters. The patients’ clinical information including gender and age was obtained through pathology databases and patients’ charts.
Statistical analysis
Categorical variables were presented as percentages and analyzed by Chi-square test. All the statistical analyses were performed by using Jamovi Project 2019 (Version 1.0) [Computer Software, retrieved from (https://www.jamovi.org)]. A p value of <0.05 was determined for the statistically significance.
RESULTS
Frequency of non-neoplastic polyps
In the 203 prospectively analyzed and totally submitted cholecystectomies, 11 cases (5%) had polyps of >2 mm in size. In the 2533 archival cholecystectomy specimens that had been processed by routine sampling, 76 revealed >2 mm non-neoplastic polyps, placing the frequency of >2 mm polyps at 3% in this cohort (p=0.059). Altogether, the number of polyps that are >2 mm in the combined cohorts was 447 (this number was 341 if 3 mm cut-off was applied, 221 for 4 mm cut-off and 143 for 5 mm cut-off).
General characteristics of cases with polyps
Overall, the targeted search together with the systematic review revealed 447 patients with polyps fulfilling the criteria outlined above.
Mean age for all patients with non-neoplastic polyps was 52 years (range: 21–93) [vs. 61 for ICPNs (range: 20–94), 64 (range: 26–95) for gallbladder carcinomas and 47 (range: 16–94) for chronic cholecystitis, NOS in the authors’ databases]. Female to male ratio was 3.1 (vs. 2.3 for ICPNs, 3.8 for gallbladder carcinomas, 2.4 for chronic cholecystitis, NOS and, 3.1 for all of the gallbladders in the authors’ databases). Mean polyp size was 0.43 cm (range, 0.2–5 cm; the lower end threshold of 2 mm was definitional). Only 21 cases (5% of all non-neoplastic polyps) were ≥1 cm in largest diameter. 45% (96/214) of fibromyoglandular polyps (range, 0.2 – 1.3 cm) and 33% (58/174) of cholesterol polyps (range: 0.2 – 5 cm) were multifocal. The frequency of polyps were similar in the Chilean, US and Turkish cohorts (data not shown).
Dysplasia was seen in 15 cases (3%) and was either as innocent bystander involvement of the polyp by dysplasia in gallbladder mucosa (as seen in the 9 cases of fibromyoglandular polyps, see below) or an innocuous type of a very low-grade dysplastic change that is polyp-specific (as seen in 5 cholesterol-type polyps) and clinically insignificant. There was no evidence of true malignant transformation (frank in-situ or invasive carcinoma) arising specifically within the polyp.
Classification and their clinicopathologic associations:
Based on morphologic characteristics and mucosal background changes, non-neoplastic polyps were classified as follows:
I. Injury-related polyps
A. Fibromyoglandular polyp (214 cases; 48%)
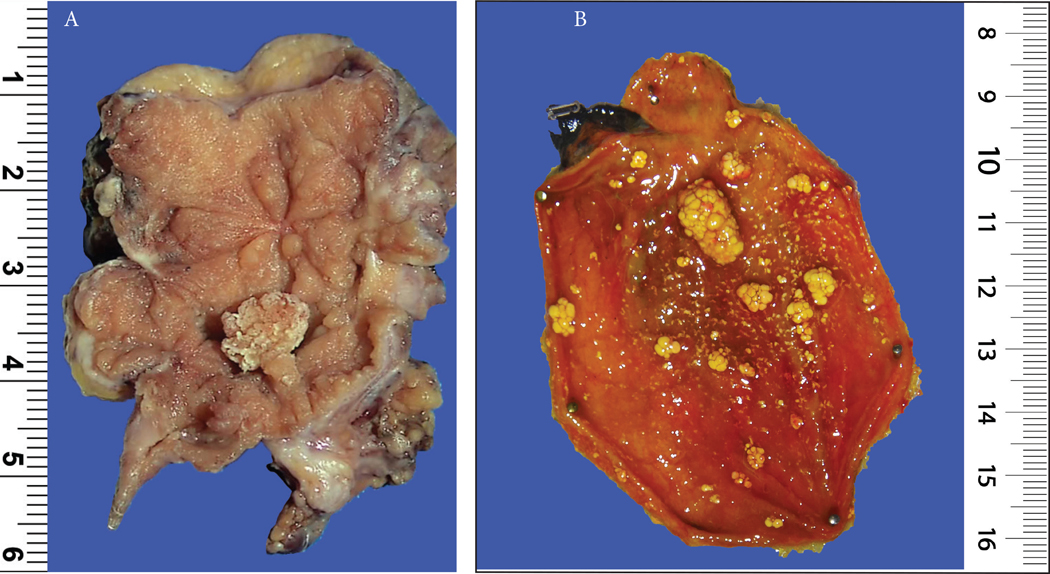
These were broad-based polyps, composed of lobular units of small glands, often of pyloric type, separated by fibroblastic stroma with variable amounts of smooth muscle. Most were incidental and small (mean=0.43 cm; largest 1.3 cm), commonly multifocal (96 cases; 45%) with only 9 (4%) measuring ≥1 cm. These were almost always associated with stones (149/151 cases with available information had gallstones). Inflammation was predominantly mucosal, with minimal changes in the deeper layers (burned-out chronic cholecystitis) and occurred in background of wall thickening, fibrosis and inflammation (n=148, 69%) (Figure 2 and 3).
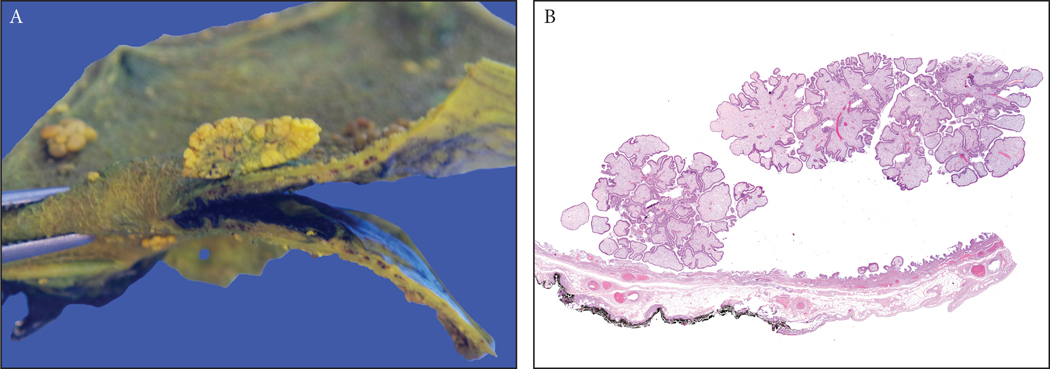
Figure 2:
(A) A 0.7 cm fibromyoglandular polyp is seen in the background of a polypoid/nodular gallbladder mucosa with prominent wall thickening and gallstones (See Figure 3 for microscopy).
(B) A case of multiple cholesterol polyps, which were typically seen in gallbladders devoid of any significant chronic changes (notice the non-polypoid mucosa). Because of the presence of cholesterol-laden macrophages, which were often in abundance, they had the distinctive yellow color in macroscopic examination. They were characterized by a striking cauliflower pattern which was highly consistent in virtually all examples.
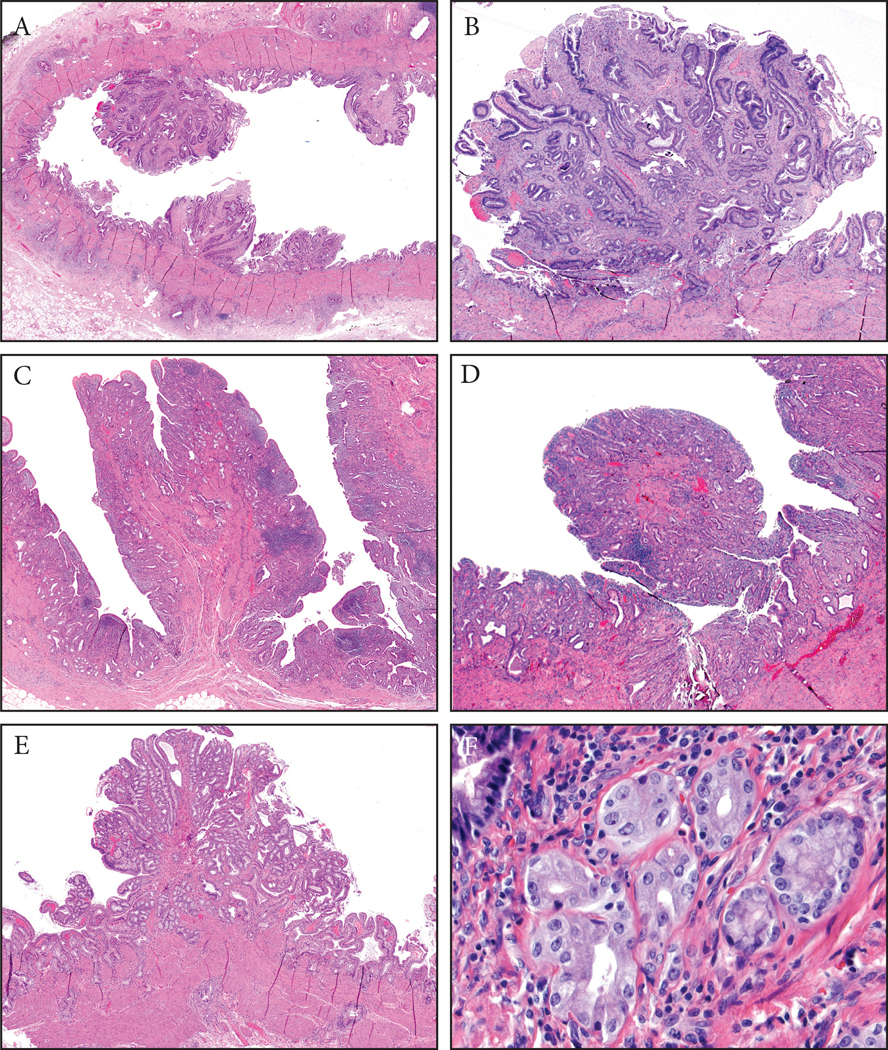
Figure 3:
Fibromyoglandular polyps. (A) These occurred in the background of mucosal injury and were commonly multifocal. They had a spectrum of glandular and stromal components with variable muscle participation. The glands were also distributed variably. (B) Some had more fibrotic stroma and relatively evenly distriuted glands. (C) Others had substantial amount of muscular stroma with mucosally-oriented glandular components. (D) Inflammation can be prominent in some. (E) Lobulated collections of glands could be seen and (F) these were often of pyloric gland type.
[Microscopic photographs of Figure 2a]
Fibromyoglandular polyps were more common in high cancer-risk regions (67% in South American cohort vs. 33% of North American). Mean age was almost a decade older than in chronic cholecystitis, NOS (55 vs. 47 years, respectively). F/M ratio was 4.6.
The surface epithelium ranged from regenerative/hyperplastic (less common) to denuded and atrophic with stromal sclerosis, creating a fibroblastic polyp-type picture. However, the overall pattern and accompanying findings were otherwise characteristic of the other fibromyoglandular polyps. Conversely, in some examples, glandular component was prominent, and if more pyloric-type, transitioning to polypoid metaplasias presented below.
Dysplastic changes were seen in 9% and occurred in the non-polypoid mucosa as a continuum (mucosal pagetoid spread) of the dysplasia elsewhere. 7 small and incidental (mean=0.3 cm) fibromyoglandular polyps were detected adjacent to gallbladder carcinoma.
B. Polypoid metaplasias in injury (42 cases, 9%, all <1 cm).
In some cases, regenerative post-injury mucosa showed polypoid aggregates of pyloric gland metaplasia. These have formerly been termed mucinous type “pyloric gland adenoma”, or “incipient” form of pyloric mucinous type ICPN. However, unlike pyloric gland adenoma and ICPN, the pyloric gland collections in fibromyoglandular polyps did not form back-to-back clonal pyloric-type glands, and were also often seen in the background of healing/healed mucosal injury.
C. Inflammatory polyps (17 cases, 4%, all <1 cm)
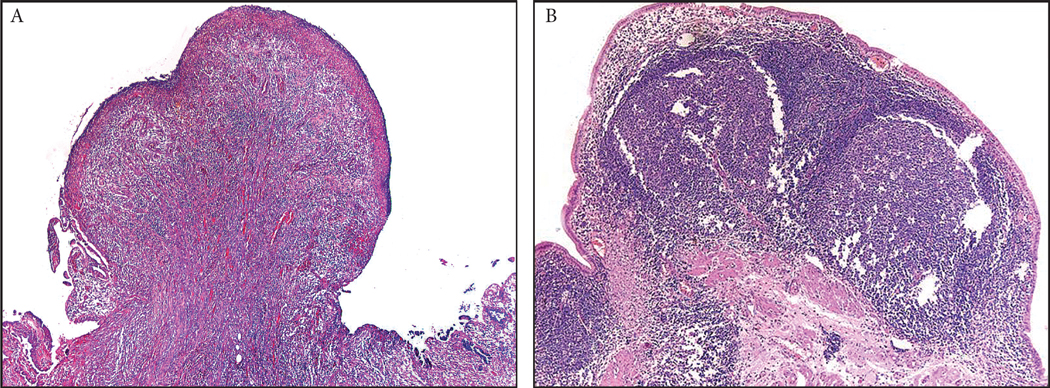
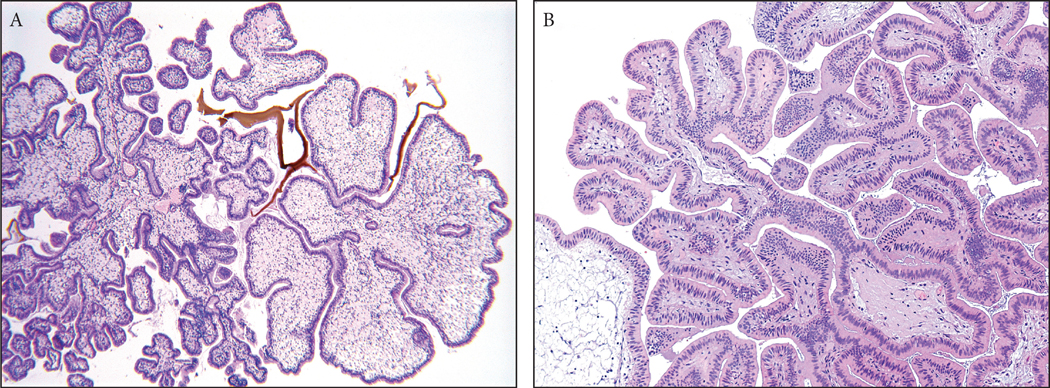
Small polyps composed entirely of prominent lymphoid aggregates (lymphoid polyp; n=3), granulation tissue (granulation tissue polyp; n=11) and xanthogranulomas (xanthogranulomatous polyps; n=3) were in this group (Figure 4).
Figure 4:
Inflammatory polyps constituted about 4% of all non-neoplastic polyps of the gallbladder. They were composed of various types of inflammatory process, including (A) granulation tissue, (B) lymphoid aggregates or xantogranulomas.
II. Cholesterol polyps (174 cases; 39%)
Cholesterol polyps were pedunculated, arborizing polypoid lesions, lined by single-layered normal gallbladder epithelium with widened edematous cores that were mostly devoid of glands. These polyps had a distinctive cauliflower architecture, a very consistent and distinguishing feature present in every case, and not seen in other polyps. The stalk was so thin that the polyps often detached during manipulation of the unfixed gallbladder. In some cases cholesterol-laden macrophages were identifiable only after careful inspection or additional sections. In 15% of the cases the cholesterol-laden macrophages were not documentable in the polyp itself having left behind an edemeatous stroma in polyp otherwise showing all the characteristics of cholesterol polyps (Figures 2, 5 and 6).
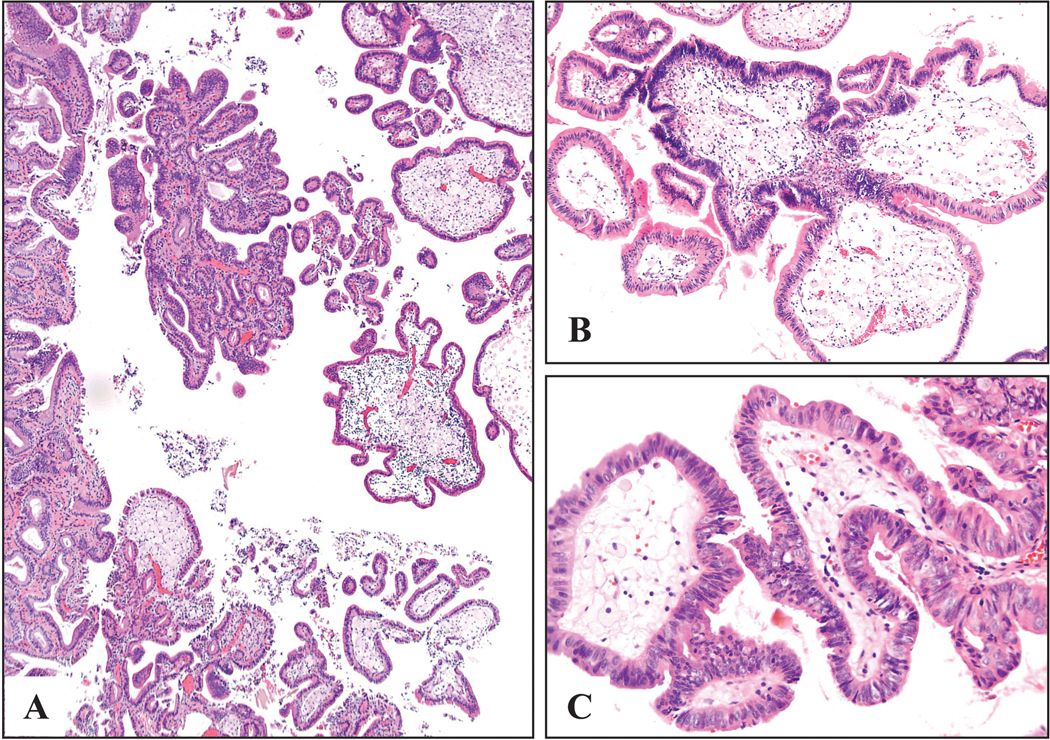
Figure 5:
Cholesterol polyps were typically found in gallbladders that were devoid of any significant chronic changes. (A) They could be multifocal as seen in this example, though not as frequently as the fibromyoglandular polpys. (B) They had very thin stalks and as a result they commonly were detached from the surface. The main characteristic of cholesterol polyps, even more so than the cholesterol macrophages (lacking in 15% and barely visible in many others), was their distinctive cauliflower architecture. Another consistent finding was the conspicuous lack of epithelial elements in the cores of the polyp.
Figure 6:
Cholesterol polyps were characterized by the cauliflower architecture, (A) even when the polyp acquired more complex architecture and (B) was devoid of lipid-laden macrophages.
103 of 174 cholesterol polyps (59%) were associated with cholesterolosis in the uninvolved gallbladder. Mean size for all cases was 0.45 cm (range: 0.2–5 cm) (mean size was 0.39 cm for female and 0.55 cm for male patients); mean age 46 years; 33% (58/174) multiple and 55% (96/174) was associated with gallstones. A peculiar and significant feature of this group was the inconspicious absence of significant injury, fibrosis or inflammation in the gallbladder. Also, cholesterol polyps were relatively common in males, compared to other gallbladder pathologies, especially in western cohort (F/M ratio was 1.5 in Western cohort and 3 in Chilean cohort, in contrast with the majority of gallbladder pathologies for which F/M ratio was 3 or higher). From the perspective of polyps occurring in males, 77% and 37% of the polyps occurring in Western and Chilean males, respectively.
There were 3 cases in which the papilla were even more complex, creating a picture that mimicking the so-called biliary-type ICPN (Figure 6B).
5 cases (3%) showed a subtle cellular atypia amounting to “low-grade dysplastic changes” composed of uniform pencillate cells with pseudo-stratified, hyperchromatic nuclei. These cases were classified as low-grade dysplastic solely on the basis of pseudo-stratification of relatively elongated nuclei, a diversion from the normal mucosal changes in the absence of injury. Epithelial maturation was always maintained and features of high-grade dysplasia (pleomorphism, mitotic activity, chromatin clumping, tufting or architectural complexity) were not seen (Figure 7).
Figure 7:
Dysplastic type changes in a cholesterol polyp. This polyp shows the distinctive cauliflower pattern of a cholesterol polyp. The epithelium shows atypia that is substantially different than the normal epithelium that typically covers a cholesterol polyp. No substantial injury changes are noted to attribute the atypical changes to. This was classified as dysplasia in the original report, and was also classified as low-grade dysplasia also in this study although the precise nature of this process remains to be determined. No high-grade dysplasia or carcinoma is seen (and it was not seen in any of the other cases either).
Clinicopathologic features of cholesterol polyps and fibromyoglandular polyps are summarized in Table 1.
Table 1.
Clinicopathologic characteristics of the two main polyp types in the gallbladder
| Fibromyoglandular polyp (n=214) | Cholesterol polyp (n=174) | |
|---|---|---|
| Mean age of patients (years) | 55 (range: 27–88) | 46 (range: 21–83) |
| Female:Male | 4.6 | 2.2 |
| Mean size (cm) | 0.43 (range: 0.2–1.3) | 0.45 (range: 0.2–5) |
| Percentage of cases >1 cm | 4% | 6% |
| Histology | - Broad based polyps - Lobular units of small glands - Fibroblastic and/or muscular stroma |
- Distinctive cauliflower-like architecture - Single-layered normal gallbladder epithelium with widened edematous cores devoid of glands - In 15% of cases, cholesterol-laden macrophages were not identified in the polyp itself |
| Gallstone (%) | 98% | 55% |
| Presence of significant inflammation and fibrosis(%) | 69% | None |
| Multiplicity (%) | 44% | 33% |
Characteristics of non-neoplastic polyps >1 cm (n=21):
Since 1 cm cut-off is widely used as clinical indication for cholecystectomy, the cases with polyps larger than 1 cm were evaluated separately as well.
Only 5% (n=21) of the non-neoplastic polyps were >1 cm in size. The vast majority of these were cholesterol polyps [6% (10 cases) of all cholesterol-type polyps] followed by fibromyoglandular polyps [4% (9 cases) of all fibromyoglandular polyps]. The F/M ratio was 1.3.
DISCUSSION
This systematic analysis reveals that clinically detectable non-neoplastic polyps are encountered in approximately 3% of cholecystectomies, and of similar frequency in regions with high and low-risk for gallbladder cancer. Most are incidental and small with a mean size of 0.4 cm; only 5% are ≥1 cm and fall into “clinically significant” category.
When all clinically significant (≥1 cm) polyps are considered separately, non-neoplastic polyps constitute only 5% of this group in the authors’ gallbladder pathology database (data not shown) and showed no evidence of malignant transformation other than the observation that complex/pyloric/non-mucinous ICPNs, known high-grade dysplasic lesions, may in fact evolve from cholesterol-type polyps (see below); the details of this group is the subject of another study. Otherwise, although focal dysplasia was identified in 3% of otherwise classical non-neoplastic polyps in this study, this was either as an innocent bystander, or a subtle (and presumably clinically insignificant) form of low-grade dysplasia, none of these showed malignant degeneration of the type that is seen in adenomas of gastrointestinal tract. Therefore, we conclude that the non-neoplastic polyps analyzed and discussed here do not have any notable malignant transformation potential. We have also not observed in our daily practice any invasive carcinoma with an adjacent residual non-neoplastic polyp. Having said that, it is well known that, once developed, an invasive carcinoma can destroy a pre-existing lesion and therefore it may be difficult to exclude such an association entirely. It should also be noted here that there are a whole host of mass-forming pathologic conditions of the gallbladder, including submucosal, mural and otherwise, that can be interpreted as “polypoid” clinically but were excluded from this analysis.
Non-neoplastic polyps of the gallbladder have been reported in the literature, from many different perspectives, including radiology, surgery and pathology, mostly in the company of neoplastic polyps. The main terms that have been applied and mostly encountered histologic types of non-neoplastic polyps of the gallbladder in these reports were cholesterol polyps and “inflammatory polyps”, followed by “hyperplastic polyps”, as well as adenomyoma and mesenchymatous tumors 14–20.
In virtually none of these studies a detailed pathologic documentation of these different polyps was performed. Other uncommon types of non-neoplastic polyps, including inflammatory myofibroblastic tumor, heterotopic polyps and leiomyomas are also reported 17,21. While some of the polyps described in this study were recognized by the authors and mentioned in reference texts 7,8,13, this was partly criticized due to lack of evidence-based analysis. For example, this is the first study to officially analyze the associations of the polyp type that we propose to refer to as fibromyoglandular polyp (see below) and virtually none of the clinicopathologic characteristics of this group have thus far been documented.
This study brings new perspectives to the classification and terminology of non-neoplastic polyps of the gallbladder. A large subset of non-neoplastic polyps are a product of mucosal injury and regeneration. The main polyp type in this category is one we propose to classify as fibromyoglandular polyps 7,8. Fibromyoglandular polyps are characterized by broad based and stubby architecture and are composed of small glandular collections (often with pyloric-type features), separated by variable amounts of fibroblastic and myoid stroma. These are often multifocal and small (mean: 0.4 cm), and they invariably occur in older patients showing marked mucosal (but not as much mural) injury indicating that they are a long term sequale of chronic mucosal-focused injury due to gallstones and inflammation, all supporting their regenerative nature. Although they can have collection of pyloric glands (polypoid metaplasias), they do not have back-to-back glandular proliferation and clonal cytologic findings characteristic of the so-called “pyloric gland adenoma” type ICPNs. In this study, fibromyoglandular polyps were higher in South American population, both in incidence as well as proportion; however, this is probably at least partly due to the fact that chronic inflammatory injurious changes (and gallstones) were more common and more abundant in this cohort. This would also explain the noticable co-existence of fibromyoglandular polyps with carcinomas and dysplasia, both of which are relatively common in the Chilean cohort in this study. In all the fibromyoglandular polyps showing dysplastic changes, there were also dysplastic changes (and/or carcinoma) in the remainder of the gallbladder, and if present, these did not appear to be arising specifically from the polyp. In fact, it is possible that the polyps may be secondary to the effect of the carcinoma or is a manifestation of the underlying chronic injury that may be responsible for both the carcinoma and the independent polyp formation.
The second main category of polyp in the gallbladder is the cholesterol polyp, which has almost opposite characteristics of fibromyoglandular polyps. The patients are relatively younger (mean age: 46 years in this study) and cholesterol polyps constitute a higher percentage of male patients with polyps in the Western population. Although cholesterol polyps can also be multiple on occasion, unlike fibromyoglandular polyps, they are not associated with chronic and inflammatory changes. Gallstones are also relatively less common companions of cholesterol polyps, but instead mucosal cholesterolosis is seen in close to two-thirds of the cases. The fact that there are not much injury-related pathology in the gallbladder in patients with cholesterol polyps supports the impression put forth by experts in the high-risk regions that cholesterolosis (and cholesterol polyps) may be representative of a milieu resistant (or at least, not as prone) to both inflammation as well as to the carcinomatous changes. It also signifies that, unlike fibromyoglandular polyps, cholesterol polyps are not a result of injury-regeneration cascade, but rather, a result of chemical milieu change, an issue that warrants further investigation. Cholesterol polyps also have a very different morphology than the fibromyoglandular polyps. In contrast with the broad-based sessile architecture of fibromyoglandular polyps, cholesterol polyps have a very thin stalk that is often invisible in histologic sections, leading to detached appearance and easy fragility in the gross room. The defining architecture of cholesterol polyp is its cauliflower-like architecture with its underlying thick cholestrol-laden or edematous cores devoid of glandular elements. This distinctive polyp type can be devoid of cholesterol-laden macrophages in 15% of the cases, with the cores showing only edema (Figure 5). This kind of lobulated branching complexity is not a feature of normal or injured mucosa even in misoriented sections. This growth pattern is also in stark contradistinction with the pattern of the other polyp types which have a less complex appearance, and display more broad-based sessile appearance absence of significant injury, fibrosis or inflammation in the gallbladder are also characteristic of these macrophage-deficient cholesterol polyps as their more classical counterparts. The cholesterol polyps that lack the macrophages, in addition to the pathognomonic cauliflower architecture, the cores at the tips of the projections often show a peculiar edema giving the impression that the resorption of the macrophages had left behind edematous acellular stroma.
When cholesterol polyps become more complex and arborizing and if they lose the cholesterol-laden macrophages, they may closely mimic what has been referred as “biliary-type adenoma”. Careful inspection of such cases reveals that the epithelium is not dysplastic/neoplastic, and there are in fact cholesterol-laden macrophages, along with the distinctive cauliflower architecture. On the other hand, a distinctive and not uncommon subset of ICPNs, characterized by complex-pyloric/tubular architecture and non-mucinous cells (technically similar to intraductal tubulo-papillary neoplasms of pancreatobiliary tract), have the overall cauliflower-like architecture of cholesterol polyps, often has cholesterol-laden macrophages, as well as background cholesterolosis in the absence of inflammatory changes, leading to the impression that they may be in fact arising from cholesterol polyps 22. However, no transitional-type cases were observed between these two entities in this study, and therefore it is possible that the association occurs very early in their pathogenesis and is not clearly evident after their full evolution.
This study also illustrates that non-neoplastic polyps are often multifocal; 45% of fibromyoglandular polyps and 33% of cholesterol polyps revealed multiplicity. However, rather than qualifying as a “polyposis” this seems to be multifocal manifestations of the underlying injury (chronic erosion and exaggerated healing, in the case of fibromyoglandular polyp) or chemical milieu (fatty acid chemistry, in the case of cholesterol polyps), rather than an inherent (congenital/germline) tendency of the epithelium itself to generate polyps. Additionally, no other associated polyposis disorders in any other organs were noted in any of the cases, although the clinical information was limited in most. Further studies are needed to establish the precise mechanism that lead to the development of these polyps.
In conclusion, non-neoplastic polyps are encountered in about 3% of cholecystectomies. A significant proportion of these are the result of reparation of mucosal injury and develop as a sequale of cholecystitis. The prototype of this phenomenon is what we propose to call fibromyoglandular polyp, which is often focal and may be involved by dysplasia as a bystander in a gallbladder inflicted with gallstone-related mucosal damage. The second major group of polyps, cholesterol polyps, occur in slightly younger patients and are unrelated to gallbladder injury, but rather are a byproduct of cholesterolosis (possibly a metabolic phenomenon), although some cases may be devoid of cholesterol-laden macrophages at the time of diagnosis. A subtle form of low-grade and clinically inconsequential dysplasia can be seen in 3% of cholesterol polyps. As radiologic methods become much more versatile in identifying fat-rich cells (“fat suppresion” technique) their frequency will rise, making their distinction from clinically relevant polyps even more critical. Recognition of these polyps and their clinicopathologic associations will hopefully allow more accurate diagnosis and analysis in order to determine their true pathogenesis. Now that the clinicopathologic features of these polyps are well documented and hopefully a more unified terminology is available, the next steps will be to conduct other analyses in an attempt to 1) Determine the radiologic correlates of these pathologically characterized entities, and their relative findings as opposed to pseudo-polyps 2) Understand the significance of seemingly-innocuous dysplastic type changes occurring in some of these polyps, and 3) Establish more reliable size cut-offs to distinguish these non-neoplastic polyps from neoplastic ones by a comprehensive comparative pathologic analysis that also includes a large number of well characterized carcinomas.
Acknowledgments
- Funding: The authors received no specific funding for this work.
Footnotes
Compliance with Ethical Standards:
- Conflict of Interest: Authors declare that they have no conflict of interest to disclose.
REFERENCES
- 1.Wiles R, Thoeni RF, Barbu ST, et al. Management and follow-up of gallbladder polyps : Joint guidelines between the European Society of Gastrointestinal and Abdominal Radiology (ESGAR), European Association for Endoscopic Surgery and other Interventional Techniques (EAES), International Society of Digestive Surgery - European Federation (EFISDS) and European Society of Gastrointestinal Endoscopy (ESGE). Eur. Radiol. 2017;27:3856–3866. doi: 10.1007/s00330-017-4742-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCain RS, Diamond A, Jones C, et al. Current practices and future prospects for the management of gallbladder polyps: A topical review. World J. Gastroenterol. 2018;24:2844–2852. doi: 10.3748/wjg.v24.i26.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babu BI, Dennison AR, Garcea G. Management and diagnosis of gallbladder polyps: a systematic review. Langenbeck’s Arch. Surg. 2015;400:455–62. doi: 10.1007/s00423-015-1302-2. [DOI] [PubMed] [Google Scholar]
- 4.Elmasry M, Lindop D, Dunne DFJ, et al. The risk of malignancy in ultrasound detected gallbladder polyps: A systematic review. Int. J. Surg. 2016;33 Pt A:28–35. doi: 10.1016/j.ijsu.2016.07.061. [DOI] [PubMed] [Google Scholar]
- 5.Albores-Saavedra J, Henson DE. Tumors of the Gallbladder and Extrahepatic Bile Ducts. In: Atlas of Tumor Pathology. 2nd series. Washington, DC: Armed Forces Institute of Pathology; 1986. [Google Scholar]
- 6.Albores-Saavedra J, Henson DE, Klimstra DS. Tumors of the gallbladder, extrahepatic bile ducts and ampulla of Vater. In: Atlas of Tumor Pathology. 3rd series. Washington, DC: Armed Forces Institute of Pathology; 2000. [Google Scholar]
- 7.Adsay V, Klimstra D. Benign and malignant tumors of the gallbladder and extrahepatic biliary tract. In: Odze R, Goldblum J, eds. Surgical Pathology of the GI Tract, Liver, Biliary Tract, and Pancreas. Philadelphia: Elsevier; 2009:845–875. [Google Scholar]
- 8.Adsay V. Gallbladder, Extrahepatic Biliary Tree, and Ampulla. In: Mills SE, ed. Sternberg’s Diagnostic Surgical Pathology. Philadelphia, PA: Lippincott Williams and Wilkins; 2010:1600–1638. [Google Scholar]
- 9.Pedersen MRV, Dam C, Rafaelsen SR. Ultrasound follow-up for gallbladder polyps less than 6 mm may not be necessary. Dan. Med. J. 2012;59:A4503. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23158888. Accessed October 8, 2019. [PubMed] [Google Scholar]
- 10.Adsay V, Jang K-T, Roa JC, et al. Intracholecystic papillary-tubular neoplasms (ICPN) of the gallbladder (neoplastic polyps, adenomas, and papillary neoplasms that are ≥1.0 cm): clinicopathologic and immunohistochemical analysis of 123 cases. Am. J. Surg. Pathol. 2012;36:1279–301. doi: 10.1097/PAS.0b013e318262787c. [DOI] [PubMed] [Google Scholar]
- 11.Roa J, Basturk O, Torres J, et al. Marked Geographic Differences in the Pathologic Diagnosis of Non-Invasive (Tis) vs Minimally Invasive (T1) Gallbladder Cancer: Santiago Consensus Conference Highlights the Need for the Unifying Category “Early Gallbladder Cancer” (EGBC) (Abstract). Mod. Pathol. 2016;29:447A. [Google Scholar]
- 12.Klimstra D, Lam A, Paradis V, et al. , eds. Tumours of the gallbladder and extrahepatic bile ducts. In: WHO Classification of Tumors: Digestive System Tumours. 5th ed. Lyon (France): International Agency for Research on Cancer; 2019:265–294. [Google Scholar]
- 13.Quigley BC, Adsay NV. 10. Diseases of the Gallbladder. In: Burt AD, Ferrell LD, Hübscher SG, eds. Macsween’s Pathology of the Liver. Seventh Ed. Elsevier; 2018:594–635. doi: 10.1016/B978-0-7020-6697-9.00010-8. [DOI] [Google Scholar]
- 14.Wennmacker SZ, van Dijk AH, Raessens JHJ, et al. Polyp size of 1 cm is insufficient to discriminate neoplastic and non-neoplastic gallbladder polyps. Surg. Endosc. 2019;33:1564–1571. doi: 10.1007/s00464-018-6444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Limaiem F, Sassi A, Talbi G, et al. Routine histopathological study of cholecystectomy specimens. Useful? A retrospective study of 1960 cases. Acta Gastroenterol. Belg. 80:365–370. [PubMed] [Google Scholar]
- 16.Xu A, Zhang Y, Hu H, et al. Gallbladder Polypoid-Lesions: What Are They and How Should They be Treated? A Single-Center Experience Based on 1446 Cholecystectomy Patients. J. Gastrointest. Surg. 2017;21:1804–1812. doi: 10.1007/s11605-017-3476-0. [DOI] [PubMed] [Google Scholar]
- 17.Zemour J, Marty M, Lapuyade B, et al. Gallbladder tumor and pseudotumor: Diagnosis and management. J. Visc. Surg. 2014;151:289–300. doi: 10.1016/j.jviscsurg.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Andrén-Sandberg A. Diagnosis and management of gallbladder polyps. N. Am. J. Med. Sci. 2012;4:203–11. doi: 10.4103/1947-2714.95897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mellnick VM, Menias CO, Sandrasegaran K, et al. Polypoid lesions of the gallbladder: disease spectrum with pathologic correlation. Radiographics 2015;35:387–99. doi: 10.1148/rg.352140095. [DOI] [PubMed] [Google Scholar]
- 20.Mazlum M, Dilek FH, Yener AN, et al. Profile of gallbladder diseases diagnosed at Afyon Kocatepe University: a retrospective study. Turk Patoloji Derg. 2011;27:23–30. [PubMed] [Google Scholar]
- 21.Yamada T, Hisa T, Shiozawa S, et al. Inflammatory myofibroblastic tumor of the gallbladder: a case report and literature review. J. Med. Ultrason. (2001) 2018;45:175–180. doi: 10.1007/s10396-017-0798-1. [DOI] [PubMed] [Google Scholar]
- 22.Balci S, Bagci P, Dursun N, et al. Complex Tubular Type Intracholecystic Papillary Tubular Neoplasms (ICPN): Further Clinicopathologic and Molecular Characterization (Abstract). Mod. Pathol. 2014;27:446A. [Google Scholar]