Summary
New neurons are abnormal in the adult hippocampus of Alzheimer's disease (AD) mouse models. The effects of modulating adult neurogenesis on AD pathogenesis differ from study to study. We reported recently that ablation of adult neural stem cells (aNSCs) was associated with improved memory in AD models. Here, we found that long-term potentiation (LTP) was improved in the hippocampus of APP/PS1 mice after ablation of aNSCs. This effect was confirmed in hAPP-J20 mice, a second AD mouse model. On the other hand, we found that exposure to enriched environment (EE) dramatically increased the number of DCX+ neurons, promoted dendritic growth, and affected the location of newborn neurons in the dentate gyrus of APP/PS1 mice, and EE exposure significantly ameliorated memory deficits in APP/PS1 mice. Together, our data suggest that both inhibiting abnormal adult neurogenesis and enhancing healthy adult neurogenesis could be beneficial for AD, and they are not mutually exclusive.
Keywords: Alzheimer's disease, adult neurogenesis, synaptic plasticity, spatial memory, mouse
Highlights
-
•
Ablation of aNSCs improves hippocampal synaptic plasticity in AD mice
-
•
Ablation of aNSCs results in hippocampal remodeling in AD mice
-
•
EE accelerates development of new neurons and improves cognition in AD mice
-
•
Effects of inhibiting and enhancing AHN on AD are not mutually exclusive
In this study, Sun and colleagues show that ablation of adult neural stem cells (aNSCs) improves hippocampal synaptic plasticity in mouse models of Alzheimer's disease (AD). This effect is associated with the remodeling in the hippocampus. Furthermore, their data indicate that effects of inhibiting and enhancing adult hippocampal neurogenesis (AHN) on AD pathogenesis are not mutually exclusive.
Introduction
Accumulation of amyloid β (Aβ) in the brain is one of the hallmarks of Alzheimer's disease (AD) (Selkoe and Hardy, 2016). High level of Aβ induces impaired synaptic transmission, altered activity of neurons, and aberrant neural circuit activity, which may account for the impaired cognitive functions in AD (Busche et al., 2008, 2012; Mucke and Selkoe, 2012; Palop and Mucke, 2016; Shankar et al., 2008). Previous studies have reported that Aβ suppressed the reuptake of glutamate by astrocytes and then induced hyperactivation of hippocampal CA1 neurons (Zott et al., 2019). Aβ accumulation also impairs the function of GABAergic neurons and then leads to aberrant activity of neural circuits in the hippocampus and cortex (Palop and Mucke, 2016; Verret et al., 2012). In addition, Aβ interacts with different receptors that affect either inhibitory or excitatory synaptic transmission (Harris et al., 2020). However, the mechanisms underlying the Aβ-induced deficits remain to be further investigated.
Adult neurogenesis (AN) occurs in the hippocampus of mice, non-human primates, and probably humans as well (Abbott and Nigussie, 2020; Boldrini et al., 2018; Eriksson et al., 1998; Kuhn et al., 2018; Moreno-Jimenez et al., 2019; Spalding et al., 2013; Tobin et al., 2019). In contrast to the rapid maturation of neurons during the embryonic development, neurogenesis in the adult hippocampus is a much longer process, which takes at least 4 weeks for mice (Zhao et al., 2006). Therefore, different populations of neurons at different developmental stages with varieties of features and functions co-exist in the adult hippocampus. These new neurons integrate into different neural circuits and regulate neuronal activity, synaptic transmission/plasticity, and activity/functions of neural circuits (Tuncdemir et al., 2019). They are also involved in a variety of cognitive functions, and impaired AN is associated with several neurological disorders (Akers et al., 2014; Clelland et al., 2009; Sahay et al., 2011; Saxe et al., 2006; Snyder et al., 2011).
Aerobic exercising or exposure to an enriched environment (EE) enhances AN (Kempermann et al., 1997; van Praag et al., 1999). Meanwhile, toxic microenvironment impairs AN (Babcock et al., 2021; Toda et al., 2019). For instance, aberrant growth and migration of newborn neurons were observed in the hippocampus of epileptic mice (Overstreet-Wadiche et al., 2006). In the hippocampus of mice overexpressing hAPP/Aβ, the number of newborn neurons was either reduced or increased, but the morphology (length or branching of dendrites and spine density) and functional integration of new neurons were impaired (Fu et al., 2019; Jin et al., 2004; Krezymon et al., 2013; Pan et al., 2016; Richetin et al., 2015; Sun et al., 2009). The reduction of normal new neurons or the existence of abnormal neurons could disrupt the normal neural circuits in the hippocampus and then may result in deficits of cognitive functions. Therefore, modulating AN could be an approach to regulate the pathogenesis of AD or other neurological disorders. Actually, Richetin et al. (2015) found that delivering NeuroD1 into neural stem cells restored the morphological development and functional integration of newborn neurons, and rescued spatial memory in AD mice. There are reports showing that increased newborn neurons by lithium or allopregnanolone were associated with improved memory in AD mice (Fiorentini et al., 2010; Wang et al., 2010). However, Choi et al. (2018) found that activating AN accompanied with increased expression of BDNF but not increasing new neurons alone improved memory in AD mice. On the other hand, Hollands et al. (2017) reported that deleting neurogenesis starting from very young age (right after weaning) mildly exacerbated cognitive deficits in AD mice. But Choi et al. (2018) found that deleting neurogenesis alone was not sufficient and loss of mature granule neurons was minimally required to cause global hippocampal cognitive deficits in AD mice. Therefore, these reports indicate that the effects of modulating AN on AD pathogenesis differ from study to study, and further investigations are warranted.
In a recent study, we showed that spatial memory was improved in two AD models after deleting adult neural stem cells (aNSCs) by genetic or pharmacological approaches (Zhang et al., 2021). In this study, we further found that hippocampal LTP was also improved in AD mice after ablation of aNSCs. On the other hand, by exposing AD mice to an EE, our data revealed that enhanced AN was associated with the recovery of cognitive functions in AD mice. Our results suggest that the effects of inhibiting abnormal neurogenesis and enhancing healthy neurogenesis on AD pathogenesis are not mutually exclusive.
Results
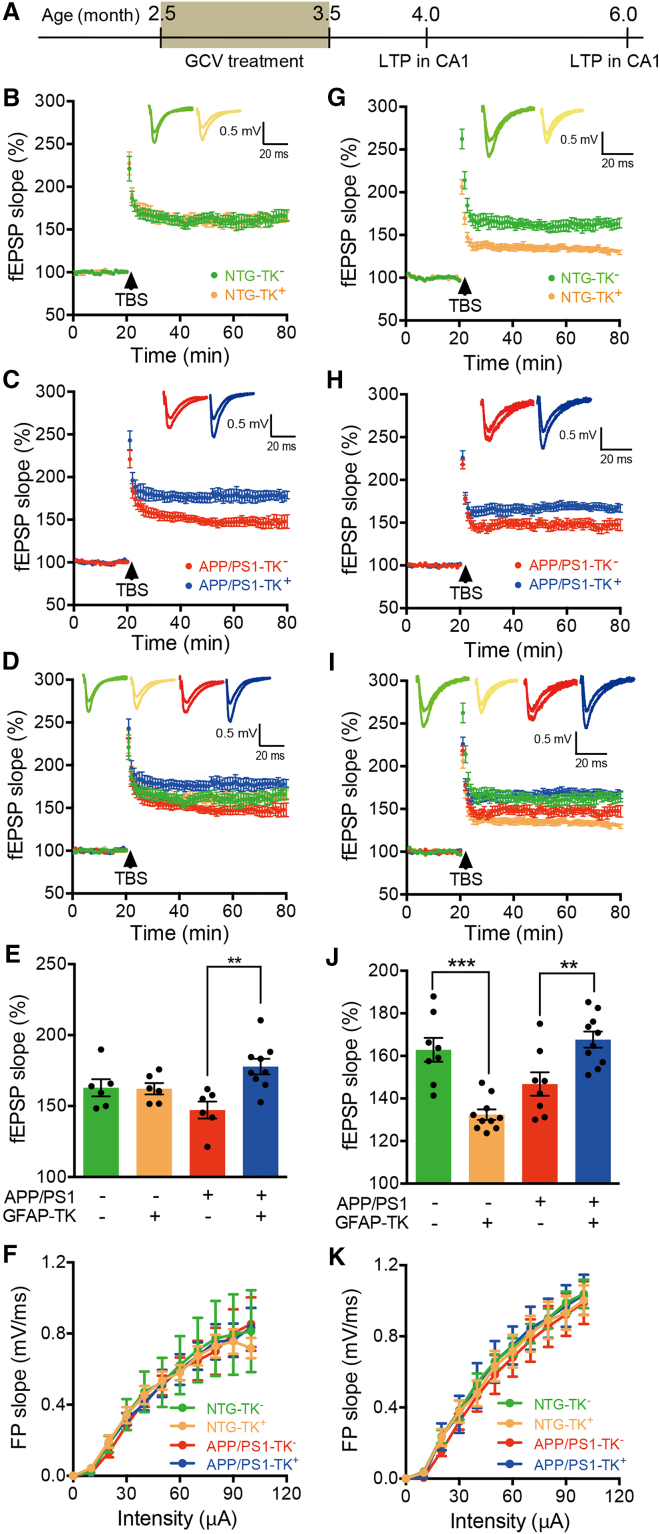
Genetic ablation of aNSCs improved LTP in the CA1 of APP/PS1 mice
In a recent study, we showed that ablating aNSCs improved spatial memory in APP/PS1 mice (Zhang et al., 2021). To determine whether aNSC ablation affects synaptic plasticity, which is associated with learning and memory, we used the same genetic approach as described (Zhang et al., 2021) to delete aNSCs in the hippocampus. In brief, APP/PS1 mice were bred with GFAP-TK mice (Snyder et al., 2011) and then were treated with GCV via subcutaneous mini-pumps for 4 weeks (starting from the age of 2.5–3 months). We have shown that this approach reduced AN and did not affect the number and morphology of astrocytes and microglia (Zhang et al., 2021). To further examine whether this approach affects inflammation in the brain, we performed western blots and ELISA to assess the expression of markers for astrocytes and microglia and inflammatory cytokines, respectively. Our data showed that there was no difference in the expression of the markers and cytokines between NTG-TK− and NTG-TK+ or APP/PS1-TK− and APP/PS1-TK+ mice after ganciclovir (GCV) treatment (Figure S1). We also did double staining (3D6 and glia markers) in the brain of older mice (8–9 months). Our results showed that there was no significant difference in the number of GFAP+ or Iba1+ cells between NTG-TK− and NTG-TK+ or APP/PS1-TK− and APP/PS1-TK+ mice after GCV treatment (Figures S2A, S2B, S3A, and S3B). These results further indicate that our approach to delete aNSCs did not affect the inflammation in the brain of both NTG and APP/PS1 mice. Previous studies reported that high dosage of GCV treatment might affect the inflammation in the intestine and body weight of mice (Bush et al., 1998). We measured the body weight of mice and did H&E staining to check the histology of intestines. We found that our approach did not affect the body weight of mice and did not induce inflammation in the intestine (Figure S4).
We then examined the long-term potentiation (LTP) at the Schaffer collateral-CA1 pyramidal cell synapses of APP/PS1 mice at two time points: around 6 months of age when Aβ plaques have been visible and 3.5–4 months of age when Aβ plaques are absent in the hippocampus. For 6-month-old mice, deleting aNSCs by GFAP-TK with GCV treatment did not affect the LTP in CA1 area of NTG mice (Figures 1B, 1D, and 1E). LTP in CA1 area of APP/PS1 mice was depressed in comparison with control (NTG) mice. Interestingly, however, it was significantly improved after aNSC ablation (Figures 1C, 1D, and 1E). Deletion of aNSCs did not affect the strength of basal synaptic transmission reflected by input/output (I/O) relationships in CA1 of both NTG and APP/PS1 mice (Figure 1F).
Figure 1.
Genetic ablation of aNSCs improved LTP in the hippocampal CA1 of APP/PS1 mice
(A) Timeline for the experiments.
(B and G) Representative traces showing LTP in CA1 of 6-month-old (B) and 4-month-old (G) NTG-TK− and NTG-TK+ mice treated with GCV.
(C and H) Representative traces showing LTP in CA1 of 6-month-old (C) and 4-month-old (H) APP/PS1-TK− and APP/PS1-TK+ mice treated with GCV.
(D and I) Representative traces showing LTP in CA1 of 6-month-old (D) replotted from (B and C) and 4-month-old (I) replotted from (G and H) NTG-TK−, NTG-TK+, APP/PS1-TK−, and APP/PS1-TK+ mice treated with GCV.
(E) Quantification of the last 15 min of fEPSP recordings in 6-month-old mice (NTG-TK−, n = 6 slices from 3 mice; NTG-TK+, n = 6 slices from 5 mice; APP/PS1-TK−, n = 6 slices from 5 mice; APP/PS1-TK+, n = 9 slices from 7 mice). Two-way ANOVA: genotype (APP), F(1,23) < 0.0001, p = 0.9965; ablating aNSCs, F(1,23) = 6.949, p = 0.0148; interaction, F(1,23) = 7.599, p = 0.0112; ∗∗p < 0.01 with Bonferroni post hoc test. Data are represented as mean ± SEM.
(J) Quantification of the last 15 min of fEPSP recordings in 4-month-old mice treated with GCV (NTG-TK−, n = 8 slices from 4 mice; NTG-TK+, n = 10 slices from 3 mice; APP/PS1-TK−, n = 8 slices from 3 mice; APP/PS1-TK+, n = 10 slices from 3 mice). Two-way ANOVA: genotype (APP), F(1,32) = 4.975, p = 0.0329; ablating aNSCs, F(1,32) = 1.252, p = 0.2715; interaction, F(1,32) = 35.81, p < 0.0001; ∗∗p < 0.01, ∗∗∗p < 0.001 with Bonferroni post hoc test. Data are represented as mean ± SEM.
(F and K) The strength of basal synaptic transmission reflected by input/output (I/O) relationships at the Schaffer collateral-CA1 synapses of 6-month-old (F) and 4-month-old (K) NTG-TK−, NTG-TK+, APP/PS1-TK−, and APP/PS1-TK+ mice treated with GCV. Two-way ANOVA, data are represented as mean ± SEM. See also Figures S1–S4.
For the younger mice (3.5–4 months old), our results revealed that deleting aNSCs significantly decreased the LTP in CA1 area of NTG mice (Figures 1G, 1I, and 1J). Similar to that of 6-month-old mice, CA1 LTP was depressed in 3.5–4 months old APP/PS1 mice versus NTG mice, and again, CA1 LTP in the APP/PS1 mice without Aβ plaques was dramatically improved after ablation of aNSCs (Figures 1H, 1I, and 1J). The strength of basal synaptic transmission was not changed in both NTG and APP/PS1 mice after aNSC ablation (Figure 1K).
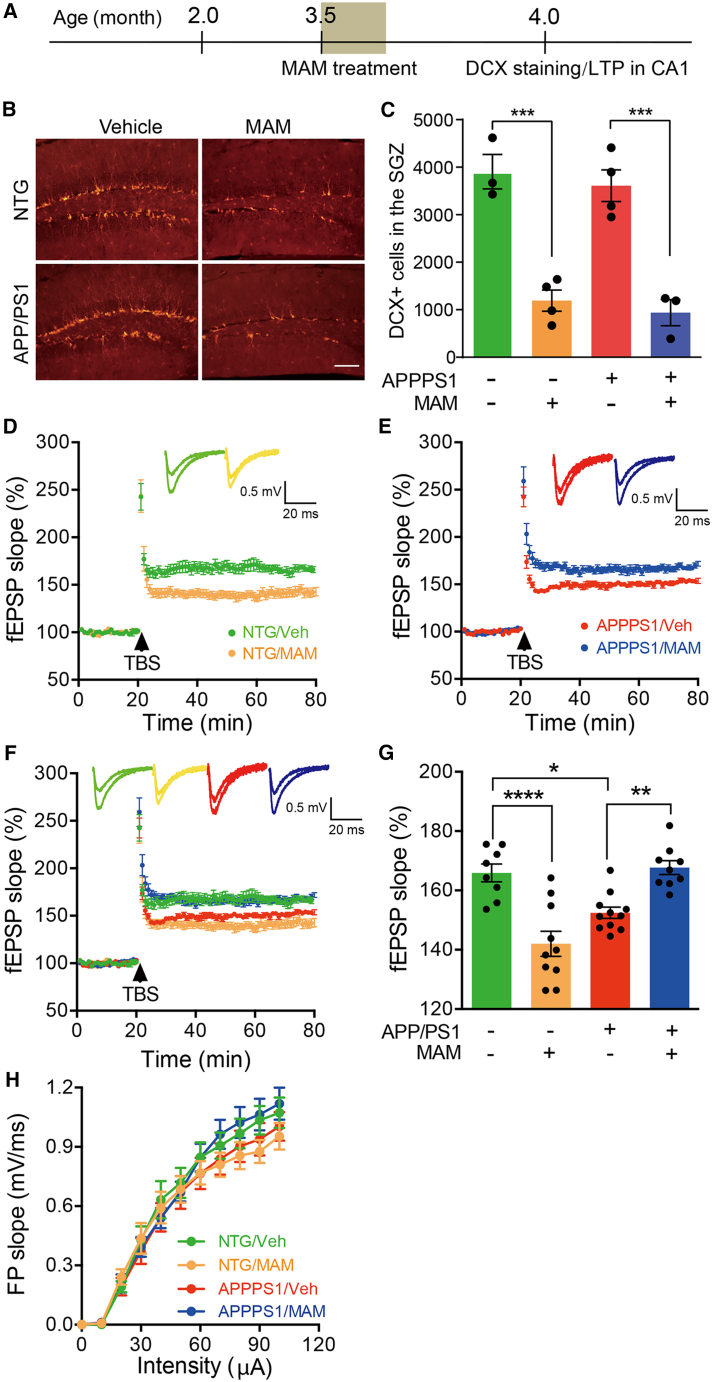
Drug-induced ablation of aNSCs improved LTP in the CA1 of APP/PS1 mice
To further evaluate the effect of aNSC ablation on LTP of APP/PS1 mice, an alternative approach was used to delete aNSCs in the hippocampus. Mice of around 3.5 months old were treated with methylazoxymethanol acetate (MAM), a DNA-methylating agent and a toxin for proliferating cells (Shors et al., 2001) once daily for 7 days. Staining with anti-DCX antibody revealed that aNSCs were effectively deleted in the hippocampus by MAM treatment for both NTG and APP/PS1 mice (Figures 2B and 2C). However, we found that the number of DCX+ cells was not decreased in the hippocampus of APP/PS1 mice versus NTG mice (Figures 2B and 2C), which is different from our previous results showing less DCX+ cells in the hippocampus of APP/PS1 mice versus NTG mice (Zhang et al., 2021). While we are not sure exactly what factors account for the discrepancy, one possibility is that the APP/PS1 mice used in our previous study (Zhang et al., 2021) were from our own animal facility but the APP/PS1 mice used in this study were purchased from Shanghai Model Organisms.
Figure 2.
Deleting aNSCs by MAM improved LTP in the CA1 area of APP/PS1 mice
(A) Timeline for the experiments.
(B) Representative photomicrographs of DCX+ cells in the DG of NTG and APP/PS1 mice treated with vehicle or MAM. Scale bar, 100 μm.
(C) Quantification of DCX+ cells in the DG of 4-month-old NTG + Veh (n = 3), NTG + MAM (n = 4), APP/PS1 + Veh (n = 4), and APP/PS1 + MAM mice (n = 3). Two-way ANOVA: genotype × treatment, F(1,10) = 0.0052, p = 0.9437; genotype, F(1,10) = 77.83, p < 0.0001; treatment, F(1,10) = 0.8097, p = 0.3894; ∗∗∗p < 0.001 with Bonferroni post hoc test, data are represented as mean ± SEM.
(D) Representative traces showing LTP in CA1 of NTG mice treated with vehicle or MAM.
(E) Representative traces showing LTP in CA1 of APP/PS1 mice treated with vehicle or MAM.
(F) Representative traces showing LTP in CA1 of both NTG and APP/PS1 mice treated with vehicle or MAM, replotted from (D and E).
(G) Quantification of the last 15 min of fEPSP recordings (NTG + Veh, n = 8 slices from 3 mice; NTG + MAM, n = 10 slices from 3 mice; APP/PS1 + Veh, n = 11 slices from 3 mice; APP/PS1 + MAM, n = 9 slices from 4 mice). Two-way ANOVA: genotype × treatment, F(1,34) = 41.88, p < 0.0001; genotype, F(1,34) = 2.033, p = 0.1630; treatment, F(1,34) = 4.106, p = 0.0506; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001 with Bonferroni post hoc test, data are represented as mean ± SEM.
(H) The strength of basal synaptic transmission reflected by I/O relationships at the Schaffer collateral-CA1 synapses was not affected by deleting aNSCs. Two-way ANOVA, data are represented as mean ± SEM. See also Figures S2 and S3.
Right after the completion of the MAM treatment, CA1 LTP was measured in acute brain slices containing hippocampus. We found that MAM-induced ablation of aNSCs suppressed the LTP at Schaffer collateral-CA1 pyramidal cell synapses in NTG mice (Figures 2C, 2E, and 2F). While the LTP in CA1 area was impaired in APP/PS1 mice in comparison with NTG mice, this impairment was significantly ameliorated after aNSC ablation by MAM (Figures 2D, 2E, and 2F). Again, the basal synaptic transmission at the Schaffer collateral-CA1 synapses was not changed after MAM treatment (Figure 2G). These results are in line with those obtained in 3.5- to 4-month-old mice with genetic ablation of aNSCs (Figure 1).
MAM treatment affects not only proliferating aNSCs but also other proliferating cells, such as glia, in the brain. We stained the brain slices with GFAP/3D6 or Iba1/3D6 after LTP recording (half brain for LTP and the other half brain for staining). 3D6+ amyloid plaques were only occasionally found in the brain at this stage, and the number of GFAP+ or Iba1+ cells was comparable between NTG/saline and NTG/MAM or APP/PS1/saline and APP/PS1/MAM (Figures S2C, S2D, S3C, and S3D), suggesting that glial cells were not significantly affected by MAM in both NTG and APP/PS1 mice.
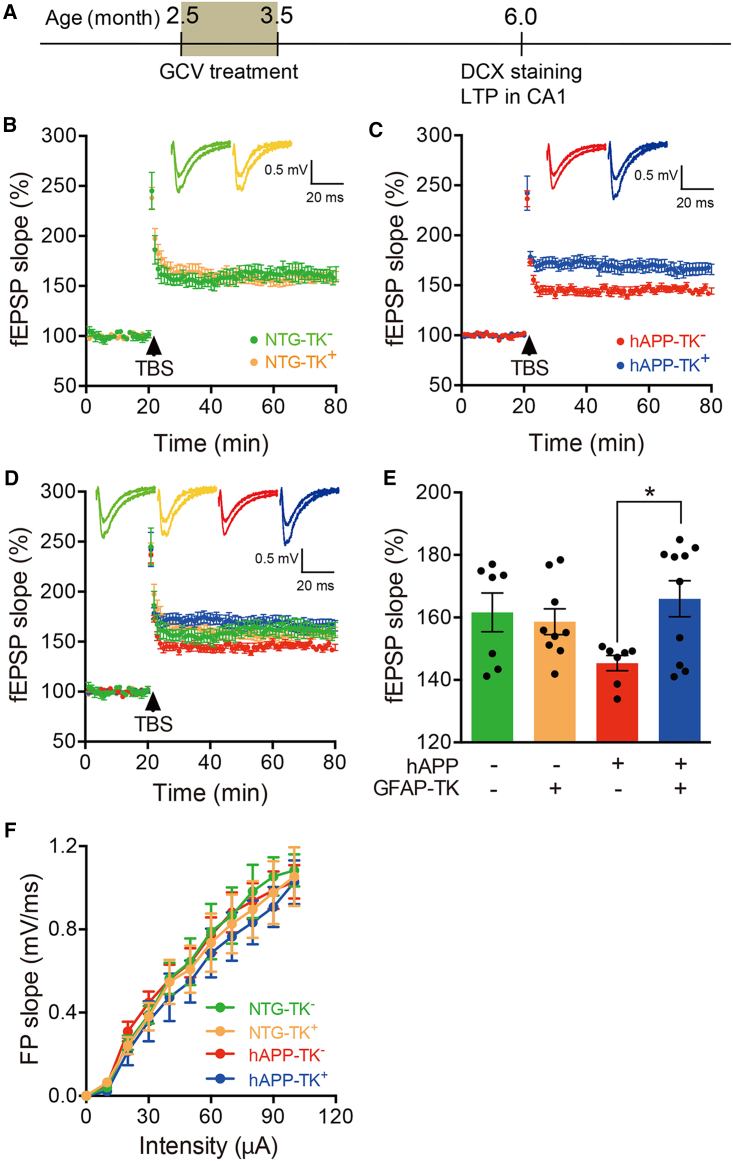
Ablation of aNSCs improved synaptic plasticity in the hippocampus of hAPP-J20 mice
To determine whether the effects of ablating aNSCs on synaptic plasticity could be validated in another line of AD models, we crossed GFAP-TK mice with hAPP-J20 mice (Mucke et al., 2000), a widely used mouse model of AD. The mice were then treated with GCV as in APP/PS1 mice, and LTP in CA1 was examined in acute brain slices from 5.5- to 6-month-old mice. Consistent with the data shown in Figure 1, deleting aNSCs did not affect the LTP in CA1 of NTG mice at around 6 months old but significantly improved the LTP in CA1 of hAPP-J20 mice (Figures 3A–3E), and the basal synaptic transmission was not affected by aNSC ablation (Figure 3F).
Figure 3.
Genetic ablation of aNSCs improved LTP in the hippocampal CA1 of hAPP-J20 mice
(A) Timeline for the experiments.
(B) Representative traces showing LTP in CA1 of 6-month-old NTG-TK− and NTG-TK+ mice treated with GCV.
(C) Representative traces showing LTP in CA1 of 6-month-old hAPP-TK− and hAPP-TK+ mice treated with GCV.
(D) Traces of LTP replotted from (B and C).
(E) Quantification of the last 15 min of fEPSP recordings (NTG-TK−, n = 7 slices from 4 mice; NTG-TK+, n = 9 slices from 4 mice; hAPP-TK−, n = 7 slices from 3 mice; hAPP-TK+, n = 10 slices from 4 mice) in CA1. Two-way ANOVA: genotype (hAPP), F(1,29) = 0.7573, p = 0.3913; ablating aNSCs, F(1,29) = 2.977, p = 0.0951; interaction, F(1,29) = 5.357, p = 0.0279; ∗p < 0.05 with Bonferroni post hoc test. Data are represented as mean ± SEM.
(F) The strength of basal synaptic transmission reflected by I/O relationships at the Schaffer collateral-CA1 synapses. Two-way ANOVA, data are represented as mean ± SEM. See also Figures S2 and S3.
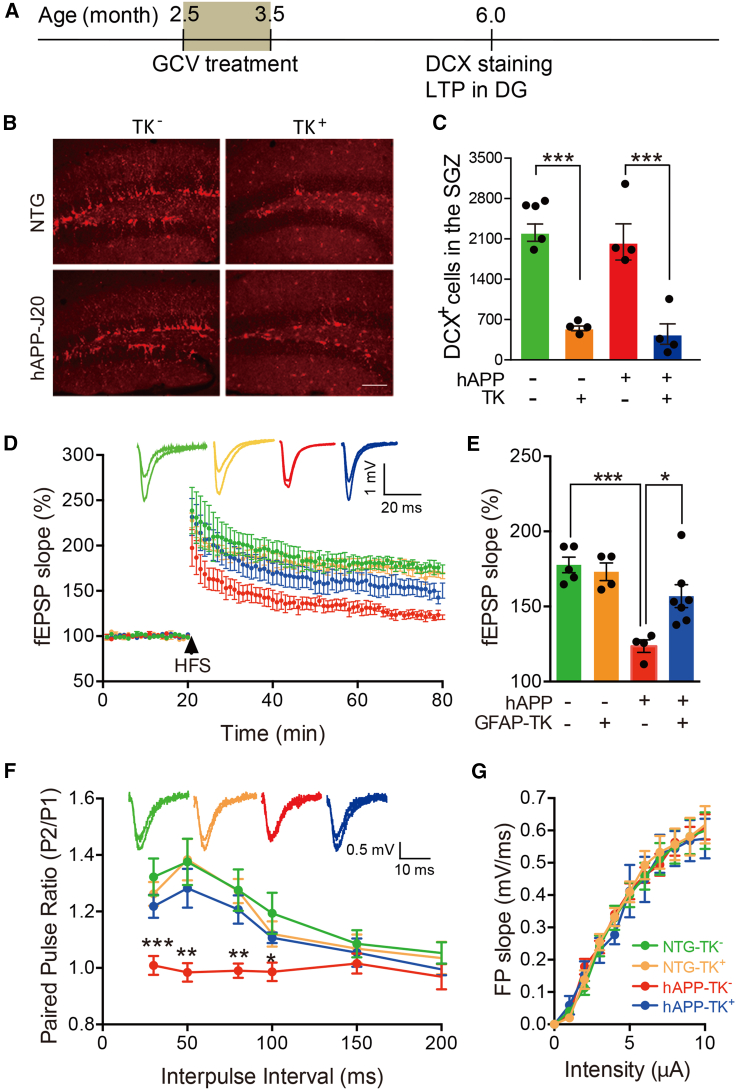
Considering that AN occurs in the subgranular zone (SGZ) and synaptic plasticity at the perforant path to the dentate gyrus (DG) granule cell synapses was impaired in hAPP-J20 mice (Palop et al., 2007; Sun et al., 2008), we then examined the effects of ablating aNSCs on LTP and paired-pulse facilitation (PPF) in this pathway. Consistent with the results of previous reports (Palop et al., 2007; Sun et al., 2008), we found that both LTP and PPF were impaired in the DG of hAPP-J20 mice versus NTG mice (Figures 4D–4F). Ablating aNSCs did not affect the LTP and PPF in the DG of NTG mice at this stage. However, both LTP and PPF were improved in hAPP-J20 mice after aNSC ablation (Figures 4D–4F), suggesting that ablation of aNSCs improved both long-term and short-term synaptic plasticity. The strength of basal synaptic transmission reflected by I/O relationships remained unchanged (Figure 4G).
Figure 4.
Deleting aNSCs improved both PPF and LTP in the medial perforant path to DG granule cell synapses of hAPP-J20 mice
(A) Timeline for the experiments.
(B) Representative photomicrographs of DCX+ cells in the DG of mice. Scale bar, 100 μm.
(C) Quantification of the numbers of DCX+ cells in the DG of 6-month-old NTG-TK− (n = 5), NTG-TK+ (n = 4), hAPP-TK− (n = 4), and hAPP-TK+ mice (n = 4) treated with GCV. Two-way ANOVA with Bonferroni post hoc test, data are represented as mean ± SEM. ∗∗∗p < 0.001.
(D) Representative traces showing LTP in the DG of NTG-TK−, NTG-TK+, hAPP-TK−, and hAPP-TK+ mice.
(E) Quantification of the last 15 min of fEPSP recordings (NTG-TK−, n = 5; NTG-TK+, n = 4; hAPP-TK−, n = 4; hAPP-TK+, n = 7). Two-way ANOVA: genotype (hAPP), F(1,16) = 26.14, p = 0.0001; ablating aNSCs, F(1,16) = 4.350, p = 0.0534; interaction, F(1,16) = 7.528, p = 0.0144; ∗p < 0.05, ∗∗∗p < 0.001 with Bonferroni post hoc test. Data are represented as mean ± SEM.
(F) Paired-pulse facilitation at the perforant path (MPP)-DG. Only hAPP mice (hAPP-TK−) had deficits of paired-pulse facilitation in comparison with NTG mice (NTG-TK−). Two-way ANOVA analysis revealed that 30 ms: genotype (hAPP), F(1,28) = 12.58, p = 0.0014; ablating aNSCs, F(1,28) = 2.194, p = 0.1479; interaction, F(1,28) = 7.194, p = 0.0121; 50 ms: genotype (hAPP), F(1,28) = 12.30, p = 0.0016; ablating aNSCs, F(1,28) = 4.747, p = 0.0379; interaction, F(1,28) = 4.269, p = 0.0482; 80 ms: genotype (hAPP), F(1,28) = 10.67, p = 0.0029; ablating aNSCs, F(1,28) = 3.929, p = 0.0573; interaction, F(1,28) = 4.188, p = 0.0502; 100 ms: genotype (hAPP), F(1,28) = 4.483, p = 0.0433; ablating aNSCs, F(1,28) = 0.2033, p = 0.6556; interaction, F(1,28) = 3.446, p = 0.0740; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 with Bonferroni post hoc test. Data are represented as mean ± SEM.
(G) The strength of basic synaptic transmission reflected by I/O relationships at the perforant path-DG was not affected by deleting aNSCs. Two-way ANOVA, data are represented as mean ± SEM. See also Figures S2 and S3.
Double staining of 3D6 and GFAP or 3D6 and Iba1 was done after LTP recordings. 3D6+ amyloid plaques were barely observed in the brain, and no significant difference in the number of GFAP+ or Iba1+ cells was found between TK− and TK+ mice for both NTG and hAPP-J20 (Figures S2E, S2F, S3E, and S3F).
Taken together, we demonstrated that genetic or pharmacological ablation of aNSCs improved synaptic plasticity in the hippocampus of AD mice.
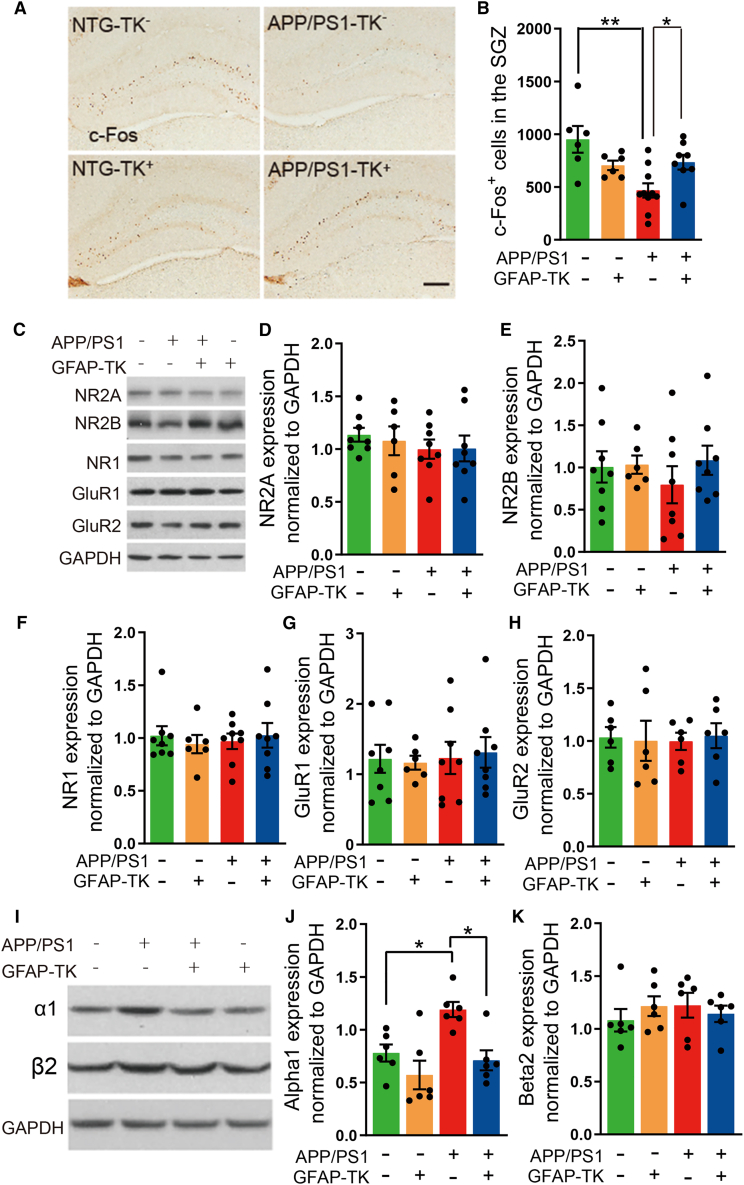
Deleting aNSCs increased the number of c-Fos+ cells in the DG of APP/PS1 mice
Consistent with previous reports (Palop et al., 2003; Sun et al., 2008), we found that the number of c-Fos+ cells in the granule layer of DG was significantly reduced in APP/PS1-TK− mice compared with that of NTG-TK− mice (Figures 5A and 5B), suggesting that the activity of granule cells was depressed in APP/PS1 mice. However, many more c-Fos+ cells were observed in the granule layer of DG of the APP/PS1-TK+ mice in comparison with APP/PS1-TK− mice after GCV treatment (Figures 5A and 5B), suggesting that inhibiting AN attenuated the depressive effect on the activity of granule cells, which may partially account for the improved LTP in the hippocampus of APP/PS1 mice.
Figure 5.
Remodeling in the hippocampus after ablation of aNSCs
(A) Photomicrographs of c-Fos immunostaining in the DG of NTG-TK−, APP/PS1-TK−, APP/PS1-TK+, and NTG-TK+ mice after GCV treatment. Scale bar, 200 μm.
(B) Quantification of the numbers of c-Fos+ cells in the DG granule layer (NTG-TK−, n = 6; APP/PS1-TK−, n = 10; APP/PS1-TK+, n = 8; NTG-TK+, n = 6). Two-way ANOVA: genotype × treatment, F(1,26) = 10.07, p = 0.0038; genotype, F(1,26) = 0.0158, p = 0.9010; treatment, F(1,26) = 7.857, p = 0.0094; ∗p < 0.05, ∗∗p < 0.01 with Bonferroni post hoc test, data are represented as mean ± SEM.
(C) Protein bands of NMDARs (NR2A, NR2B, and NR1) and AMPARs (GluR1 and GluR2) in the hippocampus; GAPDH severed as the loading control.
(D–H) Quantification of the levels of NR2A, NR2B, NR1, GluR1, and GluR2 in the hippocampus of NTG-TK−, APP/PS1-TK−, APP/PS1-TK+, and NTG-TK+ mice after GCV treatment (n = 6 mice per group). Two-way ANOVA with Bonferroni post hoc test, data are represented as mean ± SEM.
(I) Protein bands of α1 and β2 GABAA receptor subunits in the hippocampus, GAPDH severed as the loading control.
(J) Quantification of the levels of α1 GABAA receptor subunit in the hippocampus of 4-month-old NTG-TK−, APP/PS1-TK−, APP/PS1-TK+, and NTG-TK+ mice after GCV treatment (n = 6 mice per group). Two-way ANOVA: genotype × treatment, F(1,20) = 1.904, p = 0.1829; genotype, F(1,20) = 12.21, p = 0.0023; treatment, F(1,20) = 7.796, p = 0.0113; ∗p < 0.05 with Bonferroni post hoc test, data are represented as mean ± SEM.
(K) Quantification of the levels of β2 GABAA receptor subunit in the hippocampus of 4-month-old NTG-TK−, APP/PS1-TK−, APP/PS1-TK+, and NTG-TK+ mice after GCV treatment (n = 6 mice per group). Two-way ANOVA: genotype × treatment, F(1,20) = 1.144, p = 0.2976; genotype, F(1,20) = 0.0674, p = 0.7979; treatment, F(1,20) = 0.1242, p = 0.7282; with Bonferroni post hoc test, data are represented as mean ± SEM.
Deleting aNSCs affected the expression of α1 GABAA receptor in the hippocampus of APP/PS1 mice
We found previously that the amplitudes of both evoked and miniature post synaptic currents were changed in the DG granule cells of APP/PS1 mice (Zhang et al., 2021), suggesting that the responses of granule cells to synaptic activity were affected. To find out the possible underlying reasons, we did western blots to measure the expression of receptors for excitatory and inhibitory neurotransmissions. Our results revealed that there was no difference in the expression of NMDARs, AMPARs, and β2 GABAA receptor in the hippocampus of APP/PS1 mice compared with that of NTG mice (Figures 5C–5K). However, the expression of α1 GABAA receptor, a subunit of the GABAA receptor found throughout the dendritic areas of the hippocampus proper including the strata oriens and radiatum of CA1 and the molecular layer and hilus of the DG (Hortnagl et al., 2013), was increased (Figures 5I and 5J). Similarly, the expression of α1 GABAA receptor was also increased in the hippocampus of AD patients versus controls, especially in the DG, and CA3 (Kwakowsky et al., 2018). We found that inhibiting AN prevented this increase in the hippocampus of APP/PS1 mice (Figures 5I and 5J), indicating that the change of the α1 GABAA receptor expression may account for the improved LTP after ablation of aNSCs, at least partially.
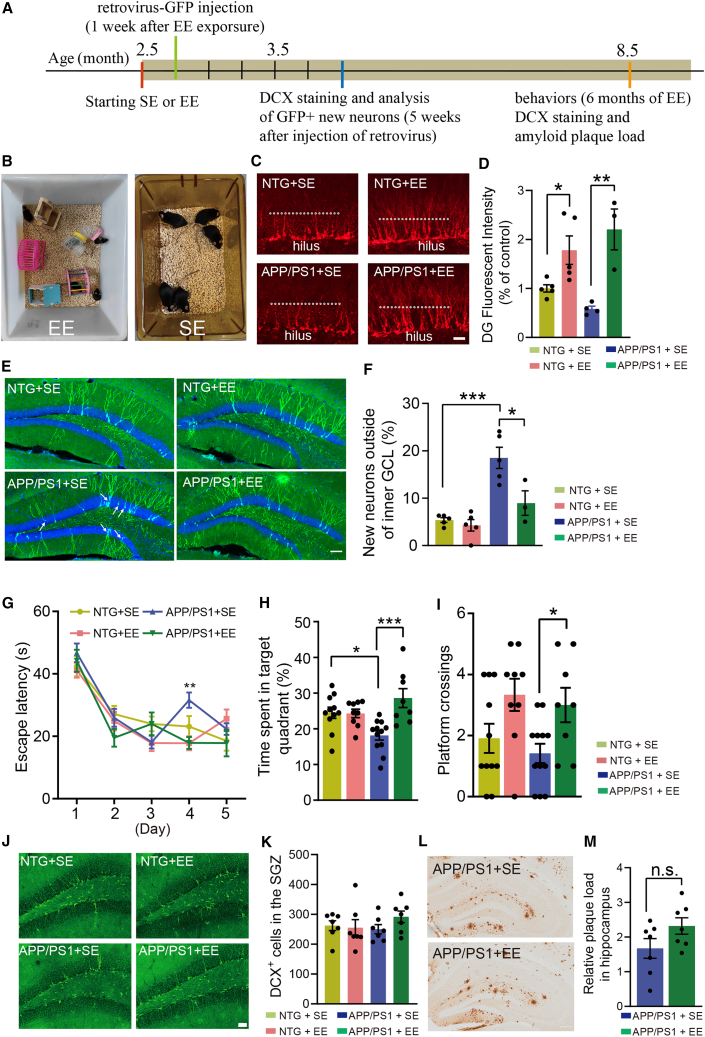
Enhancing AN in an early stage of EE was associated with improved spatial memory in APP/PS1 mice
We proposed previously that the effects of inhibiting abnormal AN and enhancing healthy AN on AD pathogenesis were not mutually exclusive (Zhang et al., 2021). In that study, our data showed that ablation of aNSCs was associated with improved memory in AD mice (Zhang et al., 2021). In this study, we found that the synaptic plasticity reflected by LTP in AD mice was also improved after deleting aNSCs. To determine whether enhancing AN is associated with changes of memory in AD mice, we subjected 2.5-month-old male NTG and APP/PS1 mice to an EE or home cages (standard environment [SE]) (Figures 6A and 6B). To assess the effects of EE on AN, we injected retrovirus-expressing GFP into the DG of mice (1 week after EE) and mice were perfused 5 weeks after the injection of the retrovirus (Figure 6A). Staining with anti-DCX antibody showed that the number of DCX+ cells was dramatically increased in the DG of both NTG and APP/PS1 mice in EE cages versus SE cages (Figures 6C and 6D). On the other hand, while the dendrites of DCX+ neurons in the DG of mice in SE cages are generally confined to the granule layer or inner molecular layer of DG (Figure 6C), in line with the fact that DCX+ neurons are 2- to 3-week-old immature neurons (Duan et al., 2008), dendrites of DCX+ neurons in the DG of mice in EE cages extend extensively into the middle or even the outer molecular layer of the DG (Figure 6C), suggesting that EE not only induces increased number but also promotes the dendritic development of immature newborn neurons. Analysis of the GFP-labeled mature newborn neurons (5 weeks after the injection of retrovirus-GFP) in the DG of NTG mice revealed that the majority of their somas were located on the border between hilus and granule layer or the inner granule layer (Figures 6E and 6F), consistent with the previous literature reporting that newborn neurons were located in the SGZ plus inner third GCL of mice (Kempermann et al., 2003). In APP/PS1 mice, however, a higher percentage of the new neuron's somas were located out of the inner granule layer (18.5% in APP/PS1 versus 5.38% in NTG) (Figures 6E and 6F). No significant difference in the soma's location in the DG of NTG mice was observed between SE and EE (5.38% versus 4.28%), but EE significantly reduced the percentage of new neurons located outside of the inner granule layer in the DG of APP/PS1 mice (18.5% for SE versus 8.97% for EE) (Figures 6E and 6F). We found that the dendritic architecture and spine density of newborn neurons labeled with CAG-GFP were impaired in APP/PS1 mice versus NTG mice (Figures S5A, S5B, and S5E). However, EE exposure did not significantly affect the dendritic architecture and spine density of newborn neurons in both NTG and APP/PS1 mice (Figures S5A–S5D and S5F–S5H). Collectively, these results demonstrated that EE increased the number, promoted the dendritic development, and affected the location of newborn neurons in the DG of APP/PS1 mice.
Figure 6.
Enriched environment induced memory recovery and enhanced adult neurogenesis in APP/PS1 mice
(A) Timeline for the experiments.
(B) Setup of the enriched environment (EE) and standard environment (SE).
(C) Representative photomicrographs of DCX+ cells in the DG of 4-month-old mice (6 weeks after exposure to EE). White dotted lines indicate the border between granule layer and molecular layer in the DG. Scale bar, 50 μm.
(D) Quantification of DCX expression in the DG of mice as in (C), n = 5 (NTG +SE or EE), 4 (APP/PS1 + SE), and 3 (APP/PS1 + EE). Two-way ANOVA: genotype × treatment, F(1,13) = 3.236, p = 0.0953; genotype, F(1,13) = 26.34, p = 0.0002; treatment, F(1,13) = 4.884e-005, p = 0.9945; ∗p < 0.05, ∗∗p < 0.01 with Bonferroni post hoc test, data are represented as mean ± SEM.
(E) Representative photomicrographs of newborn neurons labeled with retrovirus-GFP (5 weeks after the virus injection). White arrows indicate the newborn neurons located outside of the inner granule layer in the DG. Scale bar, 100 μm.
(F) Quantification of newborn neurons located in the middle or outer granule layer, n = 5 (NTG + SE or EE), 5 (APP/PS1 + SE), and 3 (APP/PS1 + EE). Two-way ANOVA: genotype × treatment, F(1,14) = 6.085, p = 0.0272; genotype, F(1,14) = 9.702, p = 0.0076; treatment, F(1,14) = 27.16, p = 0.0001; ∗p < 0.05, ∗∗∗p < 0.001 with Bonferroni post hoc test, data are represented as mean ± SEM.
(G) Learning curve in the Morris water maze test, n = 11 (NTG +SE), 9 (NTG + EE), 12 (APP/PS1 + SE), and 8 (APP/PS1 + EE). Day 4: two-way ANOVA, genotype × treatment, F(1,36) = 1.760, p = 0.1930; genotype, F(1,36) = 10.53, p = 0.0025; treatment, F(1,36) = 1.826, p = 0.1851; ∗∗p < 0.01 with Bonferroni post hoc test, data are represented as mean ± SEM.
(H) Time spent in the target quadrant during the probe trial of the Morris water maze test, n = 11 (NTG +SE), 9 (NTG + EE), 12 (APP/PS1 + SE), and 8 (APP/PS1 + EE). Two-way ANOVA: genotype × treatment, F(1,36) = 10.24, p = 0.0029; genotype, F(1,36) = 9.574, p = 0.0038; treatment, F(1,36) = 0.3785, p = 0.5423; ∗p < 0.05, ∗∗∗p < 0.001 with Bonferroni post hoc test, data are represented as mean ± SEM.
(I) The number of platform crossings during the probe trial of the Morris water maze test, n = 11 (NTG +SE), 9 (NTG + EE), 12 (APP/PS1 + SE), and 8 (APP/PS1 + EE). Two-way ANOVA: genotype × treatment, F(1,36) = 0.0293, p = 0.8651; genotype, F(1,36) = 10.47, p = 0.0026; treatment, F(1,36) = 0.7889, p = 0.3803; ∗p < 0.05 with Bonferroni post hoc test, data are represented as mean ± SEM.
(J) Representative photomicrographs of DCX+ cells in the DG of 9-month-old mice (6.5 months after exposure to EE). Scale bar, 50 μm.
(K) Quantification of DCX+ cells in the DG of mice as in (K), n = 7. Two-way ANOVA: genotype × treatment, F(1,24) = 1.436, p = 0.2426; genotype, F(1,24) = 0.7201, p = 0.4045; treatment, F(1,24) = 0.3715, p = 0.5479; with Bonferroni post hoc test, data are represented as mean ± SEM.
(L) Representative photomicrographs of amyloid deposition (staining with 3D6) in the hippocampus of 9-month-old APP/PS1 mice (6.5 months after exposure to EE or SE). Scale bar, 100 μm.
(M) Quantification of the amyloid plaque load in the hippocampus of APP/PS1 mice as shown in (L). Unpaired t test, data are represented as mean ± SEM. See also Figures S5–S7.
Mice were tested for behaviors at around 8.5 months old (6 months in EE cages). In the open-field test, NTG mice in EE cages moved a slightly longer distance and spent more time in the center area in comparison with mice in SE cages (Figures S6A–S6C), suggesting increased locomotion activity and reduced anxiety after EE. Interestingly, the effects of EE on locomotion and anxiety were more prominent for APP/PS1 mice than NTG mice (Figures S6A–S6C). In the Morris water maze test, we observed a significant difference in the latency to the platform between APP/PS1 + SE and the other three groups at the 4th day of training (Figure 6G). In the probe trials, APP/PS1 mice in SE cages spent shorter time in the target quadrant in comparison with NTG mice in SE cages (Figure 6H), suggesting impaired spatial memory of APP/PS1 mice. Interestingly, in comparison with APP/PS1 mice in SE cages, APP/PS1 mice in EE cages spent significantly longer time in the target quadrant and crossed the platform more times during the probe trials, suggesting improved spatial memory of APP/PS1 mice after EE exposure (Figures 6H and 6I).
We analyzed the correlation between increased locomotion/reduced anxiety in open-field test and memory performance in Morris water maze test of individual mouse for APP/PS1 mice exposed to SE or EE. We found that there was a correlation between locomotion and memory but no correlation between anxiety and memory in APP/PS1 mice exposed to SE (Figures S7A and s7B). In APP/PS1 mice exposed to EE, however, no correlation between the increased locomotion and memory or reduced anxiety and the memory performance in the Morris water maze test was observed (Figures S7C and S7D), suggesting that the improved spatial memory of APP/PS1 mice after EE exposure was not due to the increased locomotion or reduced anxiety.
After behavioral tests, we perfused the mice and did the immunostaining to check Aβ plaques and DCX+ neurons. Staining with 3D6 revealed that there was no significant difference in the Aβ plaque load in both cortex and hippocampus of 9-month-old APP/PS1 mice between SE and EE, although there was a trend of increase of plaque load in the hippocampus after EE (Figures 6L and 6M). DCX+ neurons were barely observed in the hippocampus of all tested mice, and no significant differences in the number of DCX+ neurons were found between either NTG and APP/PS1 or SE and EE (Figures 6J and 6K).
Taken together, our data showed that EE improved the spatial memory and attenuated anxiety in APP/PS1 mice, and these effects were not due to reducing the Aβ pathology but were associated with the enhanced AN in an early stage of EE.
Discussion
In this study, our data indicate that ablation of aNSCs attenuates the impairment of LTP in AD mice, which is in line with our recent report that deleting aNSCs improved memory in AD models (Zhang et al., 2021). We found that not only the LTP in DG but also the CA1 LTP were improved in AD mice after aNSC ablation. Although a previous study showed that ablating AN did not affect LTP in CA1 of normal mice (Saxe et al., 2006), a recent study found that reduced AN was associated with LTP suppression in CA1 (Zimmermann et al., 2018). Similarly, our results showed that both genetic and pharmacological ablation of aNSCs reduced the CA1 LTP in NTG mice, suggesting that the effect of deleting aNSCs on synaptic plasticity of NTG or AD mice was beyond the DG area where AN occurs.
It seems that there is a long-lasting effect of aNSC ablation on LTP in AD mice, because the LTP of APP/PS1-TK+ or hAPP-J20-TK+ mice was still improved 2 months after the completion of GCV treatment. Similarly, Cho et al. (2015) reported previously that ablating AN by nestin-TK mice treated with GCV for 4 weeks led to long-term (more than 40 weeks) suppression of spontaneous recurrent seizures. For NTG mice, however, the effect of aNSC ablation on LTP was only observed shortly after the GCV treatment and it returned to normal level 2 months afterward, although the number of DCX+ neurons was still significantly less than that of mice without ablation of aNSCs. This result is similar to a previous report showing that the LTP suppressed by inhibiting AN (nestin-TK mice treated with GCV) in control mice recovered 42 days after the completion of GCV treatment (Singer et al., 2011).
The mechanisms underlying the improved LTP in the hippocampus of AD mice after aNSC ablation are not clear. However, a number of factors associated with the remodeling of the hippocampus may provide some explanations: (1) expression of calbindin was decreased in the DG of AD mice (Palop et al., 2003), which was associated with enhanced expression of ΔFosB (You et al., 2017). Recovery of calbindin has shown to be related with the improvement of LTP in the hippocampus of AD mice (Sanchez et al., 2012; Sun et al., 2008). We found previously that ablation of aNSCs reduced the expression of ΔFosB and attenuated the reduction of calbindin in the DG of AD mice (Zhang et al., 2021). (2) Newborn neurons activate the GABAergic interneurons and therefore may affect the activity and synaptic transmission in principal neurons in the hippocampus (Drew et al., 2016; Lacefield et al., 2012). We found previously that ablation of aNSCs restored the synaptic transmission in the DG granule cells of AD mice (Zhang et al., 2021). In this study, our data showed that the number of c-Fos+ cells was increased in the DG granule layer of AD mice after aNSC ablation, indicating that the activity of DG granule cells was increased by deleting aNSCs. (3) The expression of GABAA α1 receptor, a subunit of the GABAA receptor found throughout the dendritic areas of the hippocampus proper including the strata oriens and radiatum of CA1, and the molecular layer and hilus of the DG (Hortnagl et al., 2013), was increased in the hippocampus of AD mice (Figures 5I and 5J) and AD patients (Kwakowsky et al., 2018). Ablation of aNSCs attenuated the expression of GABAA α1 receptor in the hippocampus of AD mice. The altered expression of these factors, which are important in remodeling the hippocampus circuits, may account for, at least partially, the improved LTP in the hippocampus of AD mice after deleting aNSCs.
Although deleting aNSCs improved spatial memory and LTP in AD mice, our data did not exclude the possibility that enhancing healthy AN could be beneficial for AD as well. Actually, several studies have shown that enhanced AN was associated with improved LTP or cognitive functions in AD mice (Fiorentini et al., 2010; Hu et al., 2010; Wang et al., 2010). In this study, we found that exposure to EE significantly increased the number, promoted the dendritic development, and affected the location of newborn neurons in the DG of APP/PS1 mice. The Morris water maze test revealed that the deficits of spatial memory were significantly attenuated in APP/PS1 mice after exposure to EE. Although some studies reported that exposure to EE reduced the deposition of Aβ in the brain of AD mice (Lazarov et al., 2005), our data showed that exposure to EE for around 6 months did not reduce the Aβ plaque load in the cortex or hippocampus of APP/PS1 mice. Actually, we found a trend of increase of the Aβ deposition in the hippocampus of APP/PS1 mice after EE versus SE, which is similar to the results of the report of Jankowsky et al. (2005). Therefore, we believe that the improved spatial memory in APP/PS1 mice after exposure to EE was not due to reducing Aβ deposition but was associated with early enhanced AN.
Our data indicate that abnormal AN may contribute to the synaptic and cognitive deficits in AD. However, a previous study reported that rare individuals who exhibited full AD pathological symptoms but had intact cognitive functions had more NSCs in the DG in comparison with AD patients (Briley et al., 2016), suggesting a potential correlation between increased neural stem cells (under pathological conditions) and preserved cognitive functions. Although it is not clear whether this increased number of NSCs will result in increased abnormal neurogenesis in the DG of those individuals, more studies are definitely needed to further investigate the involvement of AN in AD pathogenesis.
In summary, in concord with our recent report that ablation of aNSCs improved memory in AD mice (Zhang et al., 2021), we showed in this study that LTP in the hippocampus of AD mice was also improved after deleting aNSCs. Furthermore, our data indicate that enhanced AN was associated with attenuated memory deficits in AD mice as well. Therefore, the effects of inhibiting abnormal AN and enhancing healthy AN on AD are not mutually exclusive. In terms of improving synaptic and cognitive functions in AD, it will be important to develop better approaches to specifically inhibit abnormal AN or enhance healthy AN.
Experimental procedures
Animals
APP/PS1 (JAX, 34832) and hAPP-J20 mice (JAX MMRRC, 034836) were used as AD models. GFAP-TK mice were provided by Dr. Tianming Gao (Southern Medical University, China) with permission from Dr. Heather Cameron (NIH). Male mice (NTG and APP/PS1) purchased from Shanghai Model Organisms were used in the EE exposure and the subsequent behavioral tests. Both male and female mice were used for other experiments. All mice were housed under standard conditions at 22°C and a 12-h light:dark cycle with free access to food and water. All experiments were approved by the Institutional Animal Care and Use Committee of the Zhejiang University.
Drug treatments
Mice were treated with GCV or MAM as described previously (Zhang et al., 2021). See supplemental experimental procedures for details.
Preparation and stereotaxic injection of retrovirus
The retroviral vector (CAG–EGFP) (Zhao et al., 2006) was used to prepare the retrovirus as described (Tashiro et al., 2006). See supplemental experimental procedures for details.
Analysis of the location and morphology of newborn neurons in the DG
The ratio of new neurons located outside of the inner granular layer was calculated as number of new neurons outside of the inner GCL/number of total new neurons × %100. Sholl analysis of the dendritic architecture and spine density of newborn neurons were performed as described previously (Sun et al., 2009; Zhang et al., 2021). See supplemental experimental procedures for details.
Immunostaining and quantification
Immunostaining and quantification were performed according to the procedures as described previously (Zhang et al., 2021). See supplemental experimental procedures for details.
Western blot
Western blot analyses were performed as described previously (Zhang et al., 2017, 2021). See supplemental experimental procedures for details.
ELISA
The levels of interleukin-6 and tumor necrosis factor alpha in the brain were determined by using the quantification kit (RayBiotech, USA). See supplemental experimental procedures for details.
Enriched environment
APP/PS1 and NTG mice (2.5 months) were housed in SE or EE for 6 months. See supplemental experimental procedures for details.
Behavioral tests
NTG and APP/PS1 mice at around 8.5 months old (exposure to SE or EE for 6 months) were used for behavioral tests. See supplemental experimental procedures for details of the open-field test and the Morris water maze test.
Electrophysiology
Brain slices for electrophysiology were prepared and LTP in CA1 and DG were recorded as described previously (Zhang et al., 2017, 2021). See supplemental experimental procedures for details.
Statistical analyses
Statistical analyses were performed with GraphPad Prism 5 (San Diego, CA). All data were presented as mean ± SEM. Differences among multiple means were evaluated by two-way ANOVA with Bonferroni post-tests. Differences between two means were assessed with unpaired two-tailed t test. Linear regression was performed in GraphPad Prism 5 to assess the correlation between locomotion/anxiety and memory performance in the Morris water maze of individual mouse for all APP/PS1 mice exposed to SE or EE. Only values with p < 0.05 were accepted as significant.
Data and code availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
X.Q.Z. did the electrophysiology, ELISA, and western blot analysis. X.J.W. did the EE exposure, behavioral tests, immunostaining, and retrovirus injection, with assistance from Y.F.M., D.P.W., J.W., and Y.P.Z. Y.G. provided assistance in preparation of the retrovirus. X.Q.Z., X.J.W., X.K.L., G.P.P., and B.S. analyzed the data. B.S., X.Q.Z., and X.J.W. wrote the manuscript with inputs from other authors. B.S. supervised the project.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
We thank Drs Heather Cameron and Tianming Gao for the GFAP-TK mice, Dr. Fred Gage for retroviral vector-expressing eGFP, and Janssen Research & Development, L.L.C., for 3D6. We also thank the Core Facilities of Zhejiang University School of Medicine for technical support. This work was supported by grants from National Key Research and Development Program of China (2019YFA0110103), the Natural Science Foundation of Zhejiang Province (LZ19C090001 and LQ20C090002), the National Natural Science Foundation of China (31871025, 32071031, 91132713, and 82071182), and the National Key Basic Research Program of China (2017YFE0196600).
Published: December 2, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2021.11.003.
Supplemental information
References
- Abbott L.C., Nigussie F. Adult neurogenesis in the mammalian dentate gyrus. Anat. Histol. Embryol. 2020;49:3–16. doi: 10.1111/ahe.12496. [DOI] [PubMed] [Google Scholar]
- Akers K.G., Martinez-Canabal A., Restivo L., Yiu A.P., De Cristofaro A., Hsiang H.L., Wheeler A.L., Guskjolen A., Niibori Y., Shoji H., et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344:598–602. doi: 10.1126/science.1248903. [DOI] [PubMed] [Google Scholar]
- Babcock K.R., Page J.S., Fallon J.R., Webb A.E. Adult hippocampal neurogenesis in aging and Alzheimer's disease. Stem Cell Reports. 2021;16:681–693. doi: 10.1016/j.stemcr.2021.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M., Fulmore C.A., Tartt A.N., Simeon L.R., Pavlova I., Poposka V., Rosoklija G.B., Stankov A., Arango V., Dwork A.J., et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22:589–599.e5. doi: 10.1016/j.stem.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briley D., Ghirardi V., Woltjer R., Renck A., Zolochevska O., Taglialatela G., Micci M.A. Preserved neurogenesis in non-demented individuals with AD neuropathology. Sci. Rep. 2016;6:27812. doi: 10.1038/srep27812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busche M.A., Chen X.W., Henning H.A., Reichwald J., Staufenbiel M., Sakmann B., Konnerth A. Critical role of soluble amyloid-beta for early hippocampal hyperactivity in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. U S A. 2012;109:8740–8745. doi: 10.1073/pnas.1206171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busche M.A., Eichhoff G., Adelsberger H., Abramowski D., Wiederhold K.H., Haass C., Staufenbiel M., Konnerth A., Garaschuk O. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science. 2008;321:1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- Bush T.G., Savidge T.C., Freeman T.C., Cox H.J., Campbell E.A., Mucke L., Johnson M.H., Sofroniew M.V. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- Cho K.O., Lybrand Z.R., Ito N., Brulet R., Tafacory F., Zhang L., Good L., Ure K., Kernie S.G., Birnbaum S.G., et al. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat. Commun. 2015;6:6606. doi: 10.1038/ncomms7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.H., Bylykbashi E., Chatila Z.K., Lee S.W., Pulli B., Clemenson G.D., Kim E., Rompala A., Oram M.K., Asselin C., et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer's mouse model. Science. 2018;361:eaan8821. doi: 10.1126/science.aan8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland C.D., Choi M., Romberg C., Clemenson G.D., Fragniere A., Tyers P., Jessberger S., Saksida L.M., Barker R.A., Gage F.H., et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew L.J., Kheirbek M.A., Luna V.M., Denny C.A., Cloidt M.A., Wu M.V., Jain S., Scharfman H.E., Hen R. Activation of local inhibitory circuits in the dentate gyrus by adult-born neurons. Hippocampus. 2016;26:763–778. doi: 10.1002/hipo.22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X., Kang E., Liu C.Y., Ming G.L., Song H. Development of neural stem cell in the adult brain. Curr. Opin. Neurobiol. 2008;18:108–115. doi: 10.1016/j.conb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P.S., Perfilieva E., Bjork-Eriksson T., Alborn A.M., Nordborg C., Peterson D.A., Gage F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fiorentini A., Rosi M.C., Grossi C., Luccarini I., Casamenti F. Lithium improves hippocampal neurogenesis, neuropathology and cognitive functions in APP mutant mice. PLoS One. 2010;5:e14382. doi: 10.1371/journal.pone.0014382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C.H., Iascone D.M., Petrof I., Hazra A., Zhang X.H., Pyfer M.S., Tosi U., Corbett B.F., Cai J.L., Lee J., et al. Early seizure activity accelerates depletion of hippocampal neural stem cells and impairs spatial discrimination in an Alzheimer's disease model. Cell. Rep. 2019;27:3741–3751. doi: 10.1016/j.celrep.2019.05.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S.S., Wolf F., De Strooper B., Busche M.A. Tipping the scales: peptide-dependent dysregulation of neural circuit dynamics in Alzheimer's disease. Neuron. 2020;107:417–435. doi: 10.1016/j.neuron.2020.06.005. [DOI] [PubMed] [Google Scholar]
- Hollands C., Tobin M.K., Hsu M., Musaraca K., Yu T.S., Mishra R., Kernie S.G., Lazarov O. Depletion of adult neurogenesis exacerbates cognitive deficits in Alzheimer's disease by compromising hippocampal inhibition. Mol. Neurodegener. 2017;12:64. doi: 10.1186/s13024-017-0207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortnagl H., Tasan R.O., Wieselthaler A., Kirchmair E., Sieghart W., Sperk G. Patterns of mRNA and protein expression for 12 GABA(A) RECEPTOR subunits in the mouse brain. Neuroscience. 2013;236:345–372. doi: 10.1016/j.neuroscience.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.S., Xu P., Pigino G., Brady S.T., Larson J., Lazarov O. Complex environment experience rescues impaired neurogenesis, enhances synaptic plasticity, and attenuates neuropathology in familial Alzheimer's disease-linked APPswe/PS1 Delta E9 mice. FASEB J. 2010;24:1667–1681. doi: 10.1096/fj.09-136945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowsky J.L., Melnikova T., Fadale D.J., Xu G.M., Slunt H.H., Gonzales V., Younkin L.H., Younkin S.G., Borchelt D.R., Savonenko A.V. Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer's disease. J. Neurosci. 2005;25:5217–5224. doi: 10.1523/JNEUROSCI.5080-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K.L., Galvan V., Xie L., Mao X.O., Gorostiza O.F., Bredesen D.E., Greenberg D.A. Enhanced neurogenesis in Alzheimer's disease transgenic (PDGF-APP(sw,lnd))mice. Proc. Natl. Acad. Sci. U S A. 2004;101:13363–13367. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G., Gast D., Kronenberg G., Yamaguchi M., Gage F.H. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kempermann G., Kuhn H.G., Gage F.H. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Krezymon A., Richetin K., Halley H., Roybon L., Lassalle J.M., Frances B., Verret L., Rampon C. Modifications of hippocampal circuits and early disruption of adult neurogenesis in the Tg2576 mouse model of Alzheimer's disease. PLoS One. 2013;8:e76497. doi: 10.1371/journal.pone.0076497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn H.G., Toda T., Gage F.H. Adult hippocampal neurogenesis: a coming-of-age story. J. Neurosci. 2018;38:10401–10410. doi: 10.1523/JNEUROSCI.2144-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakowsky A., Guzman B.C.F., Pandya M., Turner C., Waldvogel H.J., Faull R.L. GABA(A) receptor subunit expression changes in the human Alzheimer's disease hippocampus, subiculum, entorhinal cortex and superior temporal gyrus. J. Neurochem. 2018;145:374–392. doi: 10.1111/jnc.14325. [DOI] [PubMed] [Google Scholar]
- Lacefield C.O., Itskov V., Reardon T., Hen R., Gordon J.A. Effects of adult-generated granule cells on coordinated network activity in the dentate gyrus. Hippocampus. 2012;22:106–116. doi: 10.1002/hipo.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O., Robinson J., Tang Y.P., Hairston I.S., Korade-Mirnics Z., Lee V.M.Y., Hersh L.B., Sapolsky R.M., Mirnics K., Sisodia S.S. Environmental enrichment reduces A beta levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Moreno-Jimenez E.P., Flor-Garcia M., Terreros-Roncal J., Rabano A., Cafini F., Pallas-Bazarra N., Avila J., Llorens-Martin M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease. Nat. Med. 2019;25:554–560. doi: 10.1038/s41591-019-0375-9. [DOI] [PubMed] [Google Scholar]
- Mucke L., Masliah E., Yu G.Q., Mallory M., Rockenstein E.M., Tatsuno G., Hu K., Kholodenko D., Johnson-Wood K., McConlogue L. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke L., Selkoe D.J. Neurotoxicity of amyloid beta-protein: synaptic and network dysfunction. Cold Spring Harb. Perspect. Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet-Wadiche L.S., Bromberg D.A., Bensen A.L., Westbrook G.L. Seizures accelerate functional integration of adult-generated granule cells. J. Neurosci. 2006;26:4095–4103. doi: 10.1523/JNEUROSCI.5508-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop J.J., Chin J., Roberson E.D., Wang J., Thwin M.T., Bien-Ly N., Yoo J., Ho K.O., Yu G.Q., Kreitzer A., et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop J.J., Jones B., Kekonius L., Chin J., Yu G.Q., Raber J., Masliah E., Mucke L. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer's disease-related cognitive deficits. Proc. Natl. Acad. Sci. U S A. 2003;100:9572–9577. doi: 10.1073/pnas.1133381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop J.J., Mucke L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2016;17:777–792. doi: 10.1038/nrn.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H.Y., Wang D.P., Zhang X.Q., Zhou D.M., Zhang H., Qian Q., He X., Liu Z.L., Liu Y.J., Zheng T.T., et al. Amyloid beta is not the major factor accounting for impaired adult hippocampal neurogenesis in mice overexpressing amyloid precursor protein. Stem Cell. Reports. 2016;7:707–718. doi: 10.1016/j.stemcr.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richetin K., Leclerc C., Toni N., Gallopin T., Pech S., Roybon L., Rampon C. Genetic manipulation of adult-born hippocampal neurons rescues memory in a mouse model of Alzheimer's disease. Brain. 2015;138:440–455. doi: 10.1093/brain/awu354. [DOI] [PubMed] [Google Scholar]
- Sahay A., Scobie K.N., Hill A.S., O'Carroll C.M., Kheirbek M.A., Burghardt N.S., Fenton A.A., Dranovsky A., Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez P.E., Zhu L., Verret L., Vossel K.A., Orr A.G., Cirrito J.R., Devidze N., Ho K., Yu G.Q., Palop J.J., et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer's disease model. Proc. Natl. Acad. Sci. U S A. 2012;109:E2895–E2903. doi: 10.1073/pnas.1121081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe M.D., Battaglia F., Wang J.W., Malleret G., David D.J., Monckton J.E., Garcia A.D.R., Sofroniew M.V., Kandel E.R., Santarelli L., et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol. Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar G.M., Li S.M., Mehta T.H., Garcia-Munoz A., Shepardson N.E., Smith I., Brett F.M., Farrell M.A., Rowan M.J., Lemere C.A., et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat. Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors T.J., Miesegaes G., Beylin A., Zhao M.R., Rydel T., Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Singer B.H., Gamelli A.E., Fuller C.L., Temme S.J., Parent J.M., Murphy G.G. Compensatory network changes in the dentate gyrus restore long-term potentiation following ablation of neurogenesis in young-adult mice. Proc. Natl. Acad. Sci. U S A. 2011;108:5437–5442. doi: 10.1073/pnas.1015425108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder J.S., Soumier A., Brewer M., Pickel J., Cameron H.A. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding K.L., Bergmann O., Alkass K., Bernard S., Salehpour M., Huttner H.B., Bostrom E., Westerlund I., Vial C., Buchholz B.A., et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B.G., Halabisky B., Zhou Y.G., Palop J.J., Yu G.Q., Mucke L., Gan L. Imbalance between GABAergic and glutamatergic transmission impairs adult neurogenesis in an animal model of Alzheimer's disease. Cell Stem Cell. 2009;5:624–633. doi: 10.1016/j.stem.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B.G., Zhou Y.G., Halabisky B., Lo I., Cho S.H., Mueller-Steiner S., Devidze N., Wang X., Grubb A., Gan L. Cystatin C-cathepsin B axis regulates amyloid beta levels and associated neuronal deficits in an animal model of Alzheimer's disease. Neuron. 2008;60:247–257. doi: 10.1016/j.neuron.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A., Zhao C., Gage F.H. Retrovirus-mediated single-cell gene knockout technique in adult newborn neurons in vivo. Nat. Protoc. 2006;1:3049–3055. doi: 10.1038/nprot.2006.473. [DOI] [PubMed] [Google Scholar]
- Tobin M.K., Musaraca K., Disouky A., Shetti A., Bheri A., Honer W.G., Kim N., Dawe R.J., Bennett D.A., Arfanakis K., et al. Human hippocampal neurogenesis persists in aged adults and Alzheimer's disease patients. Cell Stem Cell. 2019;24:974–982.e3. doi: 10.1016/j.stem.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Parylak S.L., Linker S.B., Gage F.H. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry. 2019;24:67–87. doi: 10.1038/s41380-018-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuncdemir S.N., Lacefield C.O., Hen R. Contributions of adult neurogenesis to dentate gyrus network activity and computations. Behav. Brain Res. 2019;374:112112. doi: 10.1016/j.bbr.2019.112112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H., Kempermann G., Gage F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Verret L., Mann E.O., Hang G.B., Barth A.M., Cobos I., Ho K., Devidze N., Masliah E., Kreitzer A.C., Mody I., et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149:708–721. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.M., Singh C., Liu L.F., Irwin R.W., Chen S.H., Chung E.J., Thompson R.F., Brinton R.D. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. U S A. 2010;107:6498–6503. doi: 10.1073/pnas.1001422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J.C., Muralidharan K., Park J.W., Petrof I., Pyfer M.S., Corbett B.F., LaFrancois J.J., Zheng Y., Zhang X.H., Mohila C.A., et al. Epigenetic suppression of hippocampal calbindin-D28k by Delta FosB drives seizure-related cognitive deficits. Nat. Med. 2017;23:1377–1383. doi: 10.1038/nm.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhang L., Zhou D.M., He X., Wang D.P., Pan H.Y., Zhang X.Q., Mei Y.F., Qian Q., Zheng T.T., et al. Ablating ErbB4 in PV neurons attenuates synaptic and cognitive deficits in an animal model of Alzheimer's disease. Neurobiol. Dis. 2017;106:171–180. doi: 10.1016/j.nbd.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Zhang X.Q., Mei Y.F., He Y., Wang D.P., Wang J., Wei X.J., Yang E.L., Zhou D.M., Shen H.W., Peng G.P., et al. Ablating adult neural stem cells improves synaptic and cognitive functions in Alzheimer models. Stem Cell. Rep. 2021;16:89–105. doi: 10.1016/j.stemcr.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C.M., Teng E.M., Summers R.G., Ming G.L., Gage F.H. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J. Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann T., Maroso M., Beer A., Baddenhausen S., Ludewig S., Fan W.Q., Vennin C., Loch S., Berninger B., Hofmann C., et al. Neural stem cell lineage-specific cannabinoid type-1 receptor regulates neurogenesis and plasticity in the adult mouse hippocampus. Cereb. Cortex. 2018;28:4454–4471. doi: 10.1093/cercor/bhy258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zott B., Simon M.M., Hong W., Unger F., Chen-Engerer H.J., Frosch M.P., Sakmann B., Walsh D.M., Konnerth A. A vicious cycle of beta amyloid-dependent neuronal hyperactivation. Science. 2019;365:559–565. doi: 10.1126/science.aay0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.