Abstract
Background:
Phthalate exposure is ubiquitous and may affect biological pathways related to regulators of blood pressure. Given the profound changes in vasculature during pregnancy, pregnant women may be particularly susceptible to the potential effects of phthalates on blood pressure.
Objectives:
We examined associations of phthalate exposure during pregnancy with maternal blood pressure trajectories from mid-pregnancy through 72 months postpartum.
Methods:
Women with singleton pregnancies delivering a live birth in Mexico City were enrolled during the second trimester (). Spot urine samples from the second and third trimesters were analyzed for 15 phthalate metabolites. Blood pressure and covariate data were collected over nine visits through 72 months postpartum. We used linear, logistic, and linear mixed models; latent class growth models (LCGMs); and Bayesian kernel machine regression to estimate the relationship of urinary phthalate biomarkers with maternal blood pressure.
Results:
As a joint mixture, phthalate biomarker concentrations during pregnancy were associated with higher blood pressure rise during mid-to-late gestation. With respect to individual biomarkers, second trimester concentrations of monobenzyl phthalate (MBzP) and di(2-ethylhexyl) phthalate biomarkers () were associated with higher third trimester blood pressure. Two trajectory classes were identified by LCGM, characterized by increasing blood pressure through 72 months postpartum (“increase–increase”) or decreased blood pressure through 18 months postpartum with a gradual increase thereafter (“decrease–increase”). Increasing exposure to phthalate mixtures during pregnancy was associated with higher odds of being in the increase–increase class. Similar associations were observed for mono-2-ethyl-5-carboxypentyl terephthalate (MECPTP) and dibutyl phthalate () biomarkers. When specific time periods were examined, we observed specific temporal relationships were observed for , MECPTP, MBzP, and .
Discussion:
In our cohort of pregnant women from Mexico City, exposure to phthalates and phthalate biomarkers was associated with higher blood pressure during late pregnancy, as well as with long-term changes in blood pressure trajectories. https://doi.org/10.1289/EHP8562
Introduction
Women undergo major hemodynamic and cardiovascular changes during pregnancy, including changes in plasma volume, blood pressure, cardiac output, and vascular resistance (Ouzounian and Elkayam 2012). Arterial blood pressure decreases starting as early as 7 wk of gestation, and reaches its lowest point at , before gradually increasing until delivery (Costantine 2014; Ouzounian and Elkayam 2012). These major hemodynamic changes may make pregnancy a period of heightened susceptibility to environmental chemical exposures that may affect blood pressure.
Phthalates are a class of synthetic organic chemicals, commonly found in consumer products, and recognized as endocrine-disrupting compounds (Benjamin et al. 2017; Casals-Casas and Desvergne 2011). Phthalate exposure is ubiquitous in humans (Katsikantami et al. 2016). Recently, certain phthalates, such as di(2-ethylhexyl) phthalate (DEHP), which is well characterized for its endocrine-disrupting effects (Benjamin et al. 2017; Zoeller et al. 2012), are being replaced with alternative phthalates, such as di(2-ethylhexyl) terephthalate (DEHTP) (Eastman Chemical Company 2014), leading to changes in exposure profiles around the world (Lessmann et al. 2019; Silva et al. 2017; Wu et al. 2020).
Three studies have examined phthalate metabolites in relation to gestational blood pressure, with varying results. One study of a birth cohort in the state of Ohio, USA, reported that prenatal urinary concentrations of monobenzyl phthalate (MBzP) were associated with increased diastolic blood pressure and risk of pregnancy-induced hypertensive diseases (Werner et al. 2015). Two studies from Europe did not replicate this relationship (Philips et al. 2019; Warembourg et al. 2019a). One study reported nominal inverse associations between di-ethyl phthalate (DEP) and diisobutyl phthalate (DiBP) metabolite concentrations and blood pressure (Warembourg et al. 2019a), whereas the other study reported null associations (Philips et al. 2019). Importantly, these three studies of gestational blood pressure had methodological differences in modeling strategies and timing of exposure and blood pressure assessments, which likely contributed to the disparate findings. In studies of prenatal (Sol et al. 2020; Tran et al. 2017; Vafeiadi et al. 2018; Valvi et al. 2015) or early childhood (Amin et al. 2018; Jenkins et al. 2019; Trasande et al. 2013; Trasande and Attina 2015; Vafeiadi et al. 2018; Warembourg et al. 2019b; Wu et al. 2018; Yao et al. 2020) phthalate exposure, associations between DEHP (Amin et al. 2018; Jenkins et al. 2019; Sol et al. 2020; Tran et al. 2017; Trasande et al. 2013; Trasande and Attina 2015; Valvi et al. 2015), dibutyl phthalate (DBP) (Amin et al. 2018; Vafeiadi et al. 2018; Yao et al. 2020), benzyl butyl phthalate (BBzP) (Vafeiadi et al. 2018; Warembourg et al. 2019b), and DEP (Vafeiadi et al. 2018; Wu et al. 2018) metabolites and elevated blood pressure have been observed. In adults, a cross-sectional analysis found that the urinary metabolite concentrations of numerous phthalates, including DEHP, DBP, BBzP, and DEP, were associated with higher blood pressure (Shiue 2014b, 2014a; Shiue and Hristova 2014), but studies in Sweden (Olsén et al. 2012) and China have provided more mixed results (Dong et al. 2017; Han et al. 2020; Zhang et al. 2018). Given the totality of the evidence, it is plausible that phthalate exposure during pregnancy is associated with altered gestational blood pressure and, consequently, postpartum blood pressure trajectories.
We recently reported that a cohort of pregnant women in Mexico City had a relatively high burden of phthalate exposure, especially DBP, DiBP, and BBzP from 1997 to 2005 and 2007 to 2011, as well as an increase in exposure to DEHTP from 2007 to 2010 (Wu et al. 2020). Here, using the same cohort, we investigated associations of prenatal exposure to phthalates with blood pressure trajectories in mid-to-late pregnancy through 72 months postpartum. No previous study, to our knowledge, has examined prenatal phthalate exposure in relation to postpartum blood pressure trajectories.
Methods
Cohort Recruitment and Data Collection
The Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) study recruited 1,048 pregnant women in the second trimester. All women were receiving prenatal care from the Mexican Social Security System in Mexico City and were recruited from late 2007 to 2011. Women were eligible if they were of age, having a singleton pregnancy, at of gestation at the time of recruitment, planning on residing in Mexico City for the next 3 y, and free of heart or kidney disease and did not use steroids or anti-epilepsy drugs, were not daily consumers of alcohol, and had access to a telephone. Of the 948 recruited women who delivered a live birth, 892 had at least one follow-up visit and comprised the population for the present study. Written informed consent was obtained from all participants. The study protocols were approved by institutional review boards at the Harvard School of Public Health, the Icahn School of Medicine at Mount Sinai, and the Mexican National Institute of Public Health.
Demographic data were collected at baseline (second trimester) by trained personnel via questionnaires. Smoking habit data were collected at baseline via questionnaire, including current smoking and whether the participant lived with a smoker, which we defined as environmental smoke at home. Alcohol consumption data during pregnancy was collected at the third trimester. Anthropometric (weight and height) and blood pressure measurements were taken by trained personnel during all nine clinical visits: second trimester (baseline; 16–22 wk of gestation), third trimester (27–34 wk), and at 1, 6, 12, 18, 24, 48, and 72 months postpartum.
Outcome Measurement
At each visit, systolic and diastolic blood pressure were measured twice, on the right arm, using a sphygmomanometer (Tycos CE0050; Welch Allyn) and measures were averaged. In addition, triplicate oscillometer blood pressure readings were also collected using Spacelab automatic blood pressure monitors at the 48- and 72-month postpartum visits (Spacelabs Healthcare). Owing to the completeness of the data, we used sphygmomanometer data as the primary outcome and oscillometer data in sensitivity analyses.
Urine Collection and Phthalate Metabolite Quantification
Details of urinary phthalate metabolites assessment are detailed elsewhere (Wu et al. 2020). In brief, maternal urine samples were collected during scheduled study visits in the second and third trimesters and stored at . The samples were analyzed by isotope dilution high-performance liquid chromatography coupled with tandem mass spectrometry (Silva et al. 2007) for 15 phthalate metabolites: mono- butyl phthalate (MBP), mono-isobutyl phthalate (MiBP), mono-hydroxybutyl phthalate (MHBP), mono-hydroxyisobutyl phthalate (MHiBP), mono-3-carboxypropyl phthalate (MCPP), monoethyl phthalate (MEP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), MBzP, mono(carboxy-isononyl) phthalate (MCNP) mono(carboxy-isooctyl) phthalate (MCOP), monooxononyl phthalate (MONP), and mono-2-ethyl-5-carboxypentyl terephthalate (MECPTP). Limits of detection (LOD) ranged from 0.2 to .
Values below the LOD for each metabolite were replaced by the lowest instrument-reported value, as in previous analysis (Wu et al. 2020). Although instrument values are not reportable for measuring the concentration of individual samples, they have been increasingly preferred to arbitrary or random values for imputing below-detection concentrations in population-based data analyses (Schisterman et al. 2006). Specific gravity (SG) was measured on a digital handheld refractometer (AR200; Reichert Technologies). Imputation of the median value (1.016) was used for 101 samples from the second trimester visit with missing specific gravity measures. The formula for urine dilution correction using specific gravity is , where Pc is the SG-corrected metabolite concentration (in nanograms per milliliter), P is the measured phthalate metabolite concentration, SGm is the median SG value of all samples, and SG is the specific gravity value for that individual urine sample.
We calculated molar sums of DEHP (), diisononyl phthalate [DiNP ()], DiBP (), and DBP () to reduce the number of correlated exposures and comparisons. For the analysis of gestational blood pressure, we restricted our analysis to only the second trimester phthalates data. For the analysis of postpartum blood pressure, to address the known variability in phthalate measurements, we computed the geometric mean across second and third trimester measurements for statistical modeling.
Outcome Definition and Statistical Analysis
Given the known fluctuations in blood pressure during pregnancy, we examined gestational and postpartum blood pressure separately. For blood pressure changes during gestation, we modeled second trimester urinary phthalate biomarker levels with third trimester blood pressure using linear regression, adjusting for baseline (second trimester) blood pressure so that model estimates represent the difference in third trimester blood pressure conditional on baseline levels, that is, the difference in the blood pressure change over this period of time. We fitted models with individual exposures (single biomarker in each model) and with coadjusted exposures (all biomarkers in a single linear regression model). We did not examine gestational hypertension because only one individual met the classification criteria (National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy 2000).
For postpartum blood pressure changes and trajectories, we first empirically identified temporal patterns via latent class growth models (LCGM) for systolic and diastolic blood pressure separately. To identify different trajectories, we centered all postpartum BP measurements to the individuals’ respective measurements at 1-month postpartum. We considered a range of possible parameters for LCGM models, including linear and spline link functions at different intervals, varying over two to five latent classes. To avoid local maxima, we set the log-likelihood convergence criterion to and used 20 sets of random starting values. The final LCGM model was decided based on the Bayesian information criterion (BIC), sample sizes in each trajectory class, and the mean posterior probability for each class. To account for the uncertainty in class assignment, we used a weighted approach (Bakk et al. 2013; Mooney et al. 2018; Vermunt 2010) by creating a pseudo population where each individual had records equal to the total number of classes and the posterior probability of assignment for that class was used as the weight for that record. We then analyzed the predicted classes as outcomes using general estimating equations with a logit link and an independent correlation structure to account for the weights and repeated observation nature of the data. Mean phthalate concentrations were modeled as individual (i.e., single biomarker) and multiple exposure (all biomarkers in the single model) models with and without adjusting for 1-month postpartum blood pressure. To complement the LCGM-defined log-binomial regression models, we also defined two periods in the postpartum: a) short-term, measured at 1, 6, 12, and 18 months postpartum, and b) long term, measured at 24, 48, and 72 months postpartum. These periods were based on our observations from LCGM models and were used to specifically examine distinct phases of the postpartum blood pressure trajectory with greater power. The short-term period corresponded to a phase of blood pressure decline following delivery, whereas the long-term period corresponded to a general rise in blood pressure. We used linear mixed models of geometric mean gestational phthalate biomarker concentrations with continuous blood pressure measurements. We specified an unstructured variance–covariance structure and random intercepts.
For each of the aforementioned statistical analyses, we also modeled all biomarkers jointly as a mixture using Bayesian kernel machine regression (BKMR) (Bobb et al. 2015, 2018). BKMR is adaptable to repeated measurements and can flexibly model multiple exposures simultaneously to estimate individual component importance and joint mixture effect. Prior to modeling, we scaled all continuous model inputs, including exposures, outcomes, and covariates via -standardization, and created dummy variables for categorical covariates. For the models, we specified Gaussian or binomial distributions, 50,000 iterations, and random intercepts per individual for models with multiple observations per person. For BKMR models, we used a single predicted class for each individual. To quantify the impact of the joint mixture, we used BKMR to estimate the change in blood pressure if all of the exposures were fixed at progressively higher quantiles.
For linear regressions and linear mixed models, we used -transformed biomarker concentrations, and the resulting model estimates can be interpreted as the change in blood pressure (in millimeters of mercury) per doubling of biomarker concentration. Similarly, logistic models of LCGM trajectory profiles are presented as odds ratios (ORs) for every doubling of biomarker concentration. For BKMR models with linear outcomes, the model-predicted effect estimates are expressed on the scale of standard deviations of the outcome. For BKMR models with binomial distributions, a Probit link was fitted and the resulting estimates were thus interpreted as the -score (or Probit index) change in outcome.
All postpartum models, including the LCGM classes, excluded women who were diagnosed with preeclampsia () or if they became pregnant again at any point during the follow-up (). We considered maternal age (in years), gestational age (in weeks), education (, high school, ), socioeconomic status (SES) (collapsed into three categories) was measured by The Mexican Association of Marketing Research and Public Opinion Agencies index (Carrasco 2002), primiparity (yes/no), height (in meters), alcohol use during pregnancy (yes/no), active smoking during pregnancy (yes/no), exposure to environmental smoking at home during pregnancy (yes/no), and seasonality of the visit (November–February, March–April, May–October; defined based on climate patterns in Mexico) as potential covariates. Maternal age, SES (collapsed from six to three categories), education, primiparity, baseline body mass index (BMI), baseline blood pressure, and height were included in all presented models. Because this data was collected at baseline, there were no missing data in these covariates. In addition, gestational age and seasonality were included in models of gestational blood pressure to control for the expected variability in maternal blood pressure. Alcohol use and environmental smoking at home were not included in the presented multivariable models because of a lack of association with phthalates in this population (Wu et al. 2020) and the presence of missing data. There were too few active smokers () to model. Exclusion of individuals diagnosed with preeclampsia in the gestational blood pressure models and the influence of concurrent gestational weight gain were separately examined in other sensitivity analyses.
To check the robustness of our BKMR models, we used quantile-based g-computation (qgcomp) to estimate the association of the phthalate biomarker exposure with gestational and postpartum blood pressure. We chose to present results from quintile and decile models without scaling any variables for ease of interpretation. Confidence intervals (CIs) and -values were calculated via 500 bootstraps, and we specified clusters based on participant identifier for models, with multiple observations per person.
All of our analyses were conducted using R (version 4.0.5; R Development Core Team). LCGM, generalized estimating equations, BKMR, linear mixed models, and qgcomp were modeled using lcmm (Proust-Lima et al. 2017), geepack (Hojsgaard 2005), bkmr (Bobb et al. 2018), and qgcomp (Keil et al. 2020) packages, respectively. BKMR fit was assessed using the bkmrhat package.
Results
At baseline, the PROGRESS population had a mean age of 27.7 y [] and mean BMI of (). The majority of women were of low SES (74%), did not advance beyond high school (76%), and had at least one previous pregnancy (55%) (Table 1). Few women reported active smoking () or alcohol intake during pregnancy (, 3%), but 269 (30%) reported exposure to environmental smoke at home during pregnancy. All measured urinary phthalate metabolites were above the LOD in of all samples, with most of the metabolites being of all samples (Table S1) and correlated with each other (Figure S1). Summary statistics from 4,729 blood pressure measurements taken from 892 women with at least one follow-up visit after baseline are shown in Table S2. For our cohort, pregnancy-induced fluctuations in systolic and diastolic blood pressure peaked in the third trimester and at 1 month postpartum, respectively. Both systolic and diastolic blood pressure trajectories declined from the peak, reaching their respective postpartum lows at 24 months before rising again at 48 and 72 months postpartum.
Table 1.
Demographics of PROGRESS participants based on baseline information (2007–2011).
| Characteristic | Full cohort () | In this study () |
|---|---|---|
| Age (second trimester) [mean (SD)] | 27.7 (5.5) | 27.7 (5.5) |
| BMI (second trimester) [mean (SD)] | 26.9 (4.2) | 26.9 (4.2) |
| SES index [ (%)]a | ||
| 1 (lowest) | 86 (9) | 83 (9) |
| 2 | 400 (42) | 379 (42) |
| 3 | 218 (23) | 199 (22) |
| 4 | 138 (15) | 132 (15) |
| 5 | 88 (9) | 83 (9) |
| 6 (highest) | 18 (2) | 16 (2) |
| Education [ (%)] | ||
| 385 (41) | 360 (40) | |
| High school | 334 (35) | 320 (36) |
| 229 (24) | 212 (24) | |
| Parity [ (%)] | ||
| 0 | 428 (45) | 411 (46) |
| 520 (55) | 481 (54) | |
| Exposure to environmental smoke at home during pregnancy [ (%)] | ||
| No | 644 (68) | 616 (69) |
| Yes | 297 (31) | 269 (30) |
| Missing | 7 (1) | 7 (1) |
| Current smoking [ (%)] | ||
| Yes | 6 (1) | 5 (1) |
| No | 602 (64) | 559 (63) |
| Missing | 342 (36) | 328 (37) |
| Alcohol during pregnancy [ (%)] | ||
| Yes | 25 (3) | 25 (3) |
| No | 770 (81) | 770 (86) |
| Missing | 153 (16) | 97 (11) |
Note: AMAI, Mexican Association of Market Intelligence and Opinion Agencies; BMI, body mass index; PROGRESS, Programming Research in Obesity, Growth, Environment and Social Stress; SD, standard error; SES, socioeconomic status.
Calculated based on characteristics of the household according to AMAI criteria and collapsed into three categories for modeling, combining categories 1–2, 3–4, and 5–6.
Gestational Blood Pressure Changes
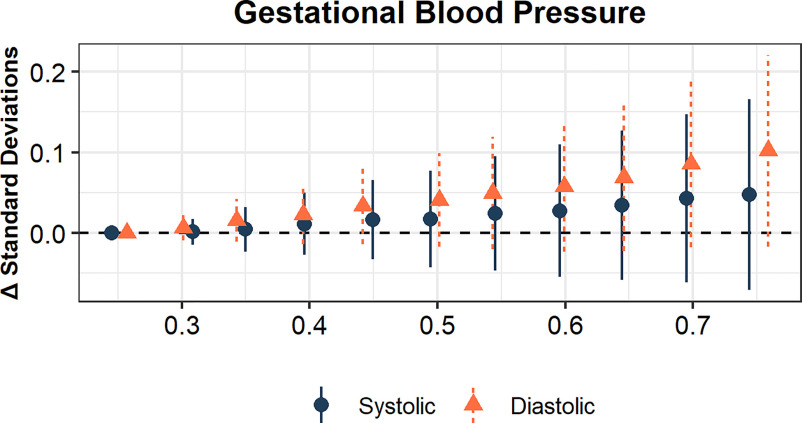
We modeled urinary phthalate biomarkers concentrations from second trimester urinary samples with third trimester blood pressure to assess whether exposure to phthalates was associated with changes in blood pressure during pregnancy. Adjusting for maternal age, SES, education, parity, second trimester BMI, second trimester height, second trimester blood pressure, seasonality, and gestation age, there was a positive trend between the second trimester phthalate mixture and third trimester diastolic blood pressure in BKMR models (Figure 1; Table S3).
Figure 1.
Bayesian kernel machine regression estimates of increasing phthalate metabolite mixture and blood pressure at third trimester. The model adjusted for maternal age, SES, education, parity, second trimester BMI, second trimester height, second trimester blood pressure, seasonality, and gestation age. The points and lines represent the point estimates (y-axis, in standard deviations for continuous blood pressure measures) and 95% credible intervals when all of the mixture components are at a given percentile (x-axis) compared with when all components are at the 25th percentile. All plots range from the 25th to the 75th percentile, in increments of 5%; the alignment are slightly jittered for presentation. Model estimates can be found in Table S3. Note: BMI, body mass index; SES, socioeconomic status.
In multivariable linear regression models with all nine biomarkers and the same covariates, we found that each doubling of second trimester urinary concentration of MBzP was associated with 0.5-mmHg higher third trimester diastolic blood pressure (95% CI: 0.1, 0.9) [Figure 2A; Table S4 (Model 2)]. There was also a general positive trend between MBzP and systolic blood pressure, although it was not statistically significant ( per doubling; 95% CI: , 0.8). BKMR models supported these findings; holding other biomarkers to the median, urinary concentrations of MBzP in the second trimester were positively associated with both systolic and diastolic blood pressure (Figure 2B). Associations were observed between and systolic blood pressure ( per doubling; 95% CI: 0, 1.5) and between and diastolic blood pressure ( per doubling; 95% CI: , 0.0), supported by both linear regression (Figure 2A) and BKMR (Figure 2B) models. All of the observed associations persisted after adjustment for concurrent gestational weight gain [Table S4 (Model 3)]. Exclusion of women with a preeclampsia diagnosis attenuated the inverse association between and diastolic blood pressure but otherwise did not substantially change the results [Table S4 (Model 4)]. Model estimates from single pollutant models can be found in Table S4 (Model 1).
Figure 2.

The associations of second trimester urinary phthalate metabolites and maternal blood pressure taken in the third trimester via (A) linear regression or (B) Bayesian kernel machine regression (BKMR). The linear models are interpreted as estimated change in blood pressure (in mmHg) per doubling of metabolite concentrations and the corresponding 95% confidence intervals. The BKMR models are presented as univariate dose–response curves with a scaled and centered exposure (x-axis) and outcome (y-axis) and can be interpreted as standard deviation changes in blood pressure compared with the median of exposure. The shaded areas represent the 95% credible intervals. Models included all nine metabolites in the same model and were adjusted for maternal age, SES, education, primiparity, height, second trimester BMI, second trimester blood pressure (systolic or diastolic), second trimester visit gestational age, and third trimester visit gestational age. Model estimates for (A) can be found in Table S4 (Model 3). Note: BMI, body mass index; DBP, dibutyl phthalate; DEHP, di(2-ethylhexyl) phthalate; DIBP, diisobutyl phthalate; DINP, diisononyl phthalate; MBzP, monobenzyl phthalate; MCNP, mono(carboxy-isononyl) phthalate; MCPP, mono-3-carboxypropyl phthalate; MECPTP, mono-2-ethyl-5-carboxypentyl terephthalate; MEP, monoethyl phthalate; SES, socioeconomic status.
Overall Postpartum Blood Pressure Trajectories
We applied LCGM to systolic and diastolic blood pressure separately and identified two latent trajectory classes describing postpartum blood pressure. As shown in Figure 3A,B, the shapes of the defined trajectories were similar for both systolic and diastolic blood pressure. All class assignments had moderately high posterior group assignment probabilities (; Table S5). The first class (“increase–increase”) was characterized by a small transient increase in blood pressure from 1 to 12 months postpartum followed by a long-term steady increase in blood pressure through 72 months postpartum. The second class (“decrease–increase”) was characterized by a sharp decrease in blood at pressure 1–18 months postpartum followed by the steady long-term increase in blood pressure through 72 months postpartum. Notably, mean second trimester blood pressure was nearly identical between the classes and distinct gestational blood pressure trends were observed for each class (Figure S2). Specifically, the increase–increase class demonstrated a milder increase in blood pressure slope in pregnancy and a monotonic increase in blood pressure through the duration of the follow-up. In contrast, the decrease–increase class had a steeper increase in pregnancy but fell below that of the increase–increase class starting at 12 months postpartum and then remained lower through the duration of the follow-up (Figure S2).
Figure 3.

Trajectory classes for postpartum blood pressure identified by latent class growth models are shown for (A) systolic and (B) diastolic blood pressure. The associations of geometric mean urinary phthalate metabolites (second and third trimester) and the trajectory classes via (C) logistic regression or (D) Bayesian kernel machine regression (BKMR). In all cases, trajectory 1 (“increase–increase”) was the reference group and trajectory 2 (“decrease–increase”) was the comparison group. The logistic regression models are expressed as odds ratios per doubling of metabolite concentrations, along with the corresponding 95% confidence intervals. The BKMR models are presented as univariate dose–response curves with a scaled and centered exposure (x-axis) and outcome (y-axis). The y-axis can be interpreted as the change in -score (Probit index) of systolic/diastolic blood pressure compared with the median of exposure. Models included all nine metabolites in the same model and excluded women with preeclampsia or if they were pregnant again. Models were adjusted for maternal age, SES, education, primiparity, height, baseline BMI, and blood pressure at both baseline and 1 month postpartum. Model estimates for (C) can be found in Table S6. Note: BMI, body mass index; DBP, dibutyl phthalate; DEHP, di(2-ethylhexyl) phthalate; DIBP, diisobutyl phthalate; DINP, diisononyl phthalate; MBzP, monobenzyl phthalate; MCNP, mono(carboxy-isononyl) phthalate; MCPP, mono-3-carboxypropyl phthalate; MECPTP, mono-2-ethyl-5-carboxypentyl terephthalate; MEP, monoethyl phthalate; SES, socioeconomic status.
Adjusting for maternal age, SES, education, parity, second trimester BMI, second trimester height, second trimester blood pressure, and 1-month postpartum blood pressure, phthalate biomarker mixture was associated with a lower probability of being in the decrease–increase class for both systolic and diastolic blood pressure (Figure 4A; Table S3). In metabolic-specific analyses using weighted classes to account for class assignment uncertainty, geometric mean urinary concentrations of MECPTP ( per doubling; 95% CI: 0.79, 0.95), ( per doubling; 95% CI: 0.74, 0.1.02), and ( per doubling; 95% CI: 0.72, 1.01) were associated with lower odds of being in the decrease–increase class of systolic blood pressure (Figure 3C; Table S6). BKMR models also showed inverse relations between MECPTP, , and concentrations and the probability of being in the decrease–increase class (Figure 3D). In contrast, despite a notable mixture effect, no specific biomarker showed strong evidence of an association with diastolic blood pressure classes (Figure 3; Table S6).
Figure 4.
Bayesian kernel machine regression estimates of increasing phthalate metabolite mixture on probability of being in (A) trajectory class 2 (“decrease–increase”), (B) short-term changes in postpartum blood pressure, and (C) long-term changes in postpartum blood pressure. All models were adjusted for maternal age, SES, education, parity, second trimester BMI, second trimester height, and second trimester blood pressure. The model in (A) additionally was adjusted for 1 month postpartum blood pressure, whereas models in (B) and (C) were adjusted for visit (days since delivery). The points and lines represent the point estimates (y-axis, in standard deviations for continuous blood pressure measures and Probit index for the binary trajectory class models) and 95% credible intervals when all of the mixture components are at a given percentile (x-axis) compared with when all components are at the 25th percentile. All plots range from the 25th to the 75th percentile, in increments of 5%; the alignments are slightly jittered for presentation. Model estimates can be found in Table S3. Note: BMI, body mass index; LCGM, latent class growth model; SES, socioeconomic status.
LCGMs with more classes generally produced a higher BIC and small classes in addition to the same two classes observed in our two-class models (Table S7). Sensitivity analyses using the three-class LCGM model (Figure S3) showed results consistent with the two-class LCGM model (Table S8).
Short-Term vs. Long-Term Postpartum Blood Pressure
We independently examined the short-term phase (1–18 months) and long-term phase (24–72 months) of the postpartum period, mirroring the general temporal patterns observed both in the summary statistics and LCGM models, to better understand the associations of phthalate exposure on maternal blood pressure at distinct periods.
In the short-term phase, we analyzed outcomes at 6, 12, and 18 months postpartum and adjusted for maternal age, SES, education, primiparity, height, baseline blood pressure, 1-month postpartum blood pressure, baseline BMI, and visit. Consistent with the results from LCGM class-based BKMR models, increasing levels of the joint mixture were associated with higher systolic and diastolic blood pressure through 18 months postpartum (Figure 4B; Table S3). Both linear mixed models (Figure 5A; Table S9 Model 2) and BKMR models (Figure 5B) showed that MECPTP ( per doubling; 95% CI: , 1.0) and ( per doubling; 95% CI: 0.3, 2.1) were associated with higher systolic blood pressure. This is broadly consistent with our multivariable logistic regression models based on LCGM trajectory classes, where MECPTP and were associated with lower odds of the decrease–increase class. In addition, was associated with lower systolic blood pressure ( per doubling; 95% CI: , ) and MBzP was associated with lower systolic ( per doubling; 95% CI: , 0.1) and diastolic (; 95% CI: , ) blood pressure. The addition of visit-specific BMI, measured at the respective follow-up visits, to the model did not meaningfully change the results [Table S9 (Model 3)]. Model estimates from single pollutant models can be found in Table S9 (Model 1).
Figure 5.

The associations of mean urinary phthalate metabolites (second and third trimester) and short-term postpartum maternal blood pressure (6, 12, and 18 months) via (A) linear mixed models or (B) Bayesian kernel machine regression (BKMR). The linear mixed models are interpreted as estimated change in blood pressure (in mmHg) per doubling of metabolite concentrations and the corresponding 95% confidence intervals. The BKMR models are presented as univariate dose–response curves with a scaled and centered exposure (x-axis) and outcome (y-axis) and can be interpreted as standard deviation changes in blood pressure compared with the median of exposure. The shaded areas represent the 95% credible intervals. Models included all nine metabolites in the same model and excluded women with preeclampsia or if they were pregnant again. Models were adjusted for maternal age, SES, education, primiparity, height, baseline blood pressure, 1 month postpartum blood pressure, baseline BMI, and visit (1, 6, and 12 months postpartum). Model estimates for (B) can be found in Table S9 (Model 2). Note: BMI, body mass index; DBP, dibutyl phthalate; DEHP, di(2-ethylhexyl) phthalate; DIBP, diisobutyl phthalate; DINP, diisononyl phthalate; MBzP, monobenzyl phthalate; MCNP, mono(carboxy-isononyl) phthalate; MCPP, mono-3-carboxypropyl phthalate; MECPTP, mono-2-ethyl-5-carboxypentyl terephthalate; MEP, monoethyl phthalate; SES, socioeconomic status.
In the long-term phase, we analyzed blood pressure outcomes at 24, 48, and 72 months postpartum. Similar to the short-term phase, as a joint mixture, increasing levels of joint mean gestational phthalate biomarker exposure were associated with higher systolic and diastolic blood pressure (Figure 4C; Table S3). For individual biomarkers. was inversely associated with systolic blood pressure and MECPTP was positively associated with systolic blood pressure (Figure 6; Table S10 Model 2). The associations for MECPTP and were both attenuated by the addition of visit-specific BMI to the model [Table S10 (Model 3)]. We also analyzed oscillometer blood pressure data collected at 48 and 72 months postpartum because the oscillometric method avoids a rounding error and observer bias. In these models, we observed that the mixture was positively associated with oscillometer-measured systolic and diastolic blood pressure (Figure 7), similar to the models from sphygmomanometer data. However, the effect estimates from oscillometer data were stronger for systolic blood pressure and more precise (i.e., they had a more narrow credible interval) compared with the sphygmomanometer data. In biomarker specific models, was inversely associated with diastolic blood pressure, and MECPTP and DBP were positively associated with systolic and diastolic blood pressure (Table S11). Model estimates from single pollutant models can be found in Table S10 (Model 1).
Figure 6.

The associations of mean urinary phthalate metabolites (second and third trimester) and long-term postpartum maternal blood pressure (24, 48, 72 months) via (A) linear mixed models or (B) Bayesian kernel machine regression (BKMR) (B). The linear mixed models are interpreted as estimated change in blood pressure (in mmHg) per doubling of metabolite concentrations and the corresponding 95% confidence intervals. The BKMR models are presented as univariate dose–response curves with a scaled and centered exposure (x-axis) and outcome (y-axis) and can be interpreted as standard deviation changes in blood pressure compared with the median of exposure. The shaded areas represent the 95% credible intervals. Models included all nine metabolites in the same model and excluded women with preeclampsia or if they were pregnant again. Models were adjusted for maternal age, SES, education, primiparity, height, baseline BMI, baseline blood pressure, and visit (number of days postpartum), excluding those with preeclampsia (). Model estimates for (B) can be found in Table S10 (Model 2). Note: BMI, body mass index; DBP, dibutyl phthalate; DEHP, di(2-ethylhexyl) phthalate; DIBP, diisobutyl phthalate; DINP, diisononyl phthalate; MBzP, monobenzyl phthalate; MCNP, mono(carboxy-isononyl) phthalate; MCPP, mono-3-carboxypropyl phthalate; MECPTP, mono-2-ethyl-5-carboxypentyl terephthalate; MEP, monoethyl phthalate; SES, socioeconomic status.
Figure 7.

Bayesian kernel machine regression estimates of increasing phthalate metabolite mixture on long-term postpartum blood pressure measured at 48 and 72 months postpartum using sphygmomanometer and or SpaceLab oscillometers. Models were adjusted for maternal age, SES, education, primiparity, height, baseline BMI, baseline blood pressure, and visit (number of days postpartum). The estimates represent the estimated change and associated 95% credible interval (y-axis, in standard deviations) when all of the mixture components are at a given percentile (x-axis) compared with when all components are at the 25th percentile. All plots range from the 25th to the 75th percentile, in increments of 5%; the alignment are slightly jittered for presentation. Note: BMI, body mass index; SES, socioeconomic status.
Quantile g-Computation
The overall results from the quantile g-computation models are consistent with those from BKMR models (Table S12). Second trimester phthalate biomarker mixture was positively associated with third trimester diastolic blood pressure ( per decile; 95% CI: 0.01, 0.56). Postpartum systolic and diastolic blood pressure, during both the short- and long-term periods, showed positive associations with the mixture of mean urinary phthalate biomarkers. Using oscillometer data at 48 and 72 months, there was a robust association of the mean urinary phthalate biomarker mixture with long-term systolic ( per decile; 95% CI: 0.04, 0.64) and diastolic ( per decile; 95% CI: 0.05, 0.55) blood pressure.
Discussion
In this longitudinal analysis of 892 women from Mexico City, joint phthalate mixture levels were positively associated with a steeper systolic blood pressure rise during mid-to-late pregnancy, as well as with a higher postpartum blood pressure through 72 months postpartum. However, the associations and temporal patterns differed by phthalate biomarker. Considering the consistency in findings over different outcomes periods, as well as across the different types of statistical models, the evidence was strongest for , MECPTP, MBzP, and .
Higher urinary was associated with higher odds of a decrease–increase class of systolic blood pressure, which is characterized by a trajectory of greater systolic blood pressure rise during mid-to-late gestation and lower postpartum systolic blood pressure up to 72 months postpartum. Urinary concentrations of MECPTP, a metabolite of DEHTP, were associated with higher postpartum blood pressure. The associations between urinary MECPTP concentrations with long-term postpartum blood pressure were attenuated by the addition of visit-specific BMI to the model, suggesting that weight changes may underlie this relationship. In addition, using oscillometer measures of blood pressure, instead of the less precise sphygmomanometer measures, identified associations of and MECPTP with diastolic blood pressure, further suggesting that both are associated with long-term differences in diastolic blood pressure as well.
Associations of MBzP with both systolic and diastolic blood pressure revealed a different pattern that was somewhat similar to the decrease–increase latent class. Higher urinary MBzP concentrations were associated with a higher systolic and diastolic blood pressure rise during mid-to-late pregnancy and a greater decline of blood pressure through 18 months postpartum, similar to the relationship between and systolic blood pressure. However, there is no evidence of association past this point, suggesting that associations of MBzP and maternal blood pressure may be transient and that the exaggerated blood pressure increase in mid-to-late gestation is largely resolved by 18 months postpartum. It is possible that MBzP is associated with pregnancy-specific end points, such as gestational hypertension or preeclampsia, and future inquiry is required.
Last, urinary had sporadic associations with systolic and diastolic blood pressure in our analyses, suggesting that there is a possible relationship. For systolic blood pressure, urinary was associated with higher odds of being in the increase–increase trajectory class, which was also supported by our linear mixed models of short-term postpartum blood pressure. However, we did not find evidence that urinary was associated with gestational blood pressure fluctuations in mid-to-late gestation nor long-term postpartum blood pressure. For diastolic blood pressure, urinary was associated with a greater rise during the mid-to-late gestation, but no associations were observed with diastolic blood pressure in our postpartum models. The lack of associations with long-term postpartum blood pressure may be due to random measurement error. Using oscillometric measurements of blood pressure at 48 and 72 months postpartum resulted in more robust estimates that do suggest an association with long-term blood pressure. Overall, there is suggestive evidence that DBP may be associated with maternal blood pressure, but the exact nature and timing of the relationship is unclear.
In general, previous studies that assessed the relationship between prenatal phthalate exposure and gestational blood pressure reported mixed results (Philips et al. 2019; Warembourg et al. 2019a; Werner et al. 2015). It is difficult to directly compare findings across existing studies because the discrepancies may be rooted in differences in phthalate exposure patterns and study design differences, such as modeling strategies, the timing of phthalate exposure, and blood pressure assessments, among other possibilities. A study of the Health Outcomes and Measures of the Environment (HOME) cohort based in Ohio reported that urinary concentrations of MBzP and , but not , were positively associated with blood pressure measurements in early pregnancy ( of gestation) (Werner et al. 2015). Although these results generally support an association of MBzP and DBP on gestational blood pressure, this time period is reflective of the early declines in blood pressure during pregnancy and not the later rise in blood pressure captured in our study. The HOME Study also reported that none of the examined biomarkers, including , MBzP, and , were associated with peak blood pressure between 20 wk of gestation and delivery, but it is unclear whether a single measure of peak blood pressure is comparable to our analyses of blood pressure change over time. A study of the Generation R cohort from the Netherlands found no association between urinary concentrations of with overall blood pressure levels (Philips et al. 2019). In that study, blood pressure was measured during gestational weeks 12–14, 20–21, and 30–31, capturing the quadratic temporal relationship of blood pressure, but the authors examined overall blood pressure levels, not changes in blood pressure trajectory, as was done in the present study. Last, a study of the Human Early Life Exposome cohort based in Western Europe reported that urinary concentrations of , MBzP, and MBP (a metabolite of DBP) measured around gestational week 20 were not associated with blood pressure in the third trimester (Warembourg et al. 2019a). Although that study provides the best comparison with our gestational blood pressure models, it is possible that differences in populations and exposure levels contributed to the discordant findings. Overall, although the evidence for gestational blood pressure changes associated with phthalate exposure is somewhat variable, there is consistent support from previous studies of children and nonpregnant adults that DEHP (Amin et al. 2018; Jenkins et al. 2019; Sol et al. 2020; Tran et al. 2017; Trasande et al. 2013; Trasande and Attina 2015; Valvi et al. 2015), DBP (Amin et al. 2018; Vafeiadi et al. 2018; Yao et al. 2020), and MBzP (Vafeiadi et al. 2018; Warembourg et al. 2019b) are associated with blood pressure changes.
Blood pressure is regulated by a range of biological mechanisms involving the cardiac, vascular, renal, endocrine, and neural systems (Raven and Chapleau 2014; Shahoud et al. 2020). The potential biological mechanisms that underlie the observed associations of phthalate biomarkers with maternal blood pressure are unclear, particularly given that it is unknown whether there are any pregnancy-specific mechanisms involved. In addition, because the association between gestational phthalate concentrations and blood pressure appeared to vary by biomarker, it is possible, if not likely, that multiple mechanisms are involved. Phthalate metabolites are agonists of peroxisome proliferator-activated receptor gamma (), which is known to inhibit the rennin–angiotensin–aldosterone system, a key regulator of blood pressure (Corrales et al. 2018; Sugawara et al. 2012). Alternatively, numerous phthalate metabolites have been associated with oxidative stress during pregnancy in human (Ferguson et al. 2014, 2015, 2017; van T Erve et al. 2019; Wu et al. 2017) and animal (Rahmani et al. 2016) studies. Oxidative stress has been associated with pregnancy-induced hypertension (Draganovic et al. 2016), arterial stiffness (Mannaerts et al. 2018), and impaired endothelial function (Mannaerts et al. 2018) in some studies, but results have been inconsistent (Ouyang et al. 2018). Oxidative stress has also been hypothesized to contribute to preeclampsia (Rana et al. 2019), another potential cause of blood pressure changes during pregnancy. Rodent studies have also reported that DEHP can act on blood pressure via a variety of mechanisms, including changes to cardiac nitric oxide synthetase (Lee et al. 2016; Rahmani et al. 2016), calcium channels (Mariana et al. 2018), angiotensin type I (Deng et al. 2019b; Lee et al. 2016) and II (Martinez-Arguelles et al. 2013) receptors, angiotensin converting enzyme (Deng et al. 2019a, 2019b), and endothelial nitric oxide synthase (Deng et al. 2019a, 2019b).
To our knowledge, this was the first study to examine associations of gestational phthalates exposure and long-term, longitudinal maternal blood pressure, spanning pregnancy through 72 months postpartum. We were able to assess the impact of phthalate exposure on blood pressure trajectories over y of follow-up in a relatively healthy population, demonstrating that phthalates exposure at earlier life stages can affect blood pressure trajectories over years and likely influence disease risk later in life. The longitudinal repeated measures design of the study, including blood pressure measurements over nine visits, allowed us to capture differences in blood pressure, as well as different postpartum trajectories and the timing of the examined relationships. We were able to capture the expected natural rise in blood pressure during mid-to-late pregnancy with minimal misclassification from the decline in blood pressure that occurs in early pregnancy because our baseline visit (at 16–22 wk) closely aligned with the expected nadir in blood pressure (at of gestation). In addition, we were able to incorporate BKMR, a flexible mixture model that can handle correlated exposures, repeated measurements, and, potentially, opposite directions of effect. Last, one of the major strengths of the study was our ability to study MECPTP, an emerging exposure of interest. Exposure to replacement phthalates, such as DEHTP (the parent compound of MECPTP) and other alternatives, are increasing globally and it is vital for us to explore and understand their impact on human health. To that end, it is of great public health concern that this replacement phthalate appears to be associated with long-term changes in systolic blood pressure.
Our study has some notable limitations. First, we were limited by the lack of data on diet and personal care product use habits, two major sources of phthalates exposure (Schettler 2006). Diet, particularly foods packaged in plastic, may act as a potential confounder to the phthalate–blood pressure relationship. Although we adjusted for BMI and weight gain, which may act as proxies for diet, residual confounding is possible and future studies are warranted to address this question. Second, we did not have blood pressure data during preconception, the first trimester of pregnancy, or delivery and were not able to more finely capture blood pressure changes prior to, within the first month, and immediately following pregnancy. It is possible that some women experienced more rapid changes in blood pressure in the transition from pregnancy to the postpartum period. Third, there was likely nondifferential misclassification of exposure and outcome. Sphygmomanometer data are subject to rounding error and the use of oscillometer data resulted in stronger effect estimates for several biomarkers, possibly as a consequence of reduced measurement error. Misclassification of exposure is also likely, given the short biological half-lives and episodic nature of phthalate exposures. In addition, we do not have exposure data after delivery and thus were not able to distinguish between potential effects of gestational vs. postpartum exposures. Fourth, selection bias due to loss to follow-up is possible; however, because 86% of women (814 of 948) provided data for at least one postpartum visit, our statistical analyses were able to take advantage of all available data without requiring complete cases. Furthermore, distributions of demographic factors and exposures did not meaningfully differ between the baseline population and the sample with data available at any follow-up visit. Last, although mixture models can remove confounding by coexposures, they may suffer from coexposure amplification bias (Weisskopf et al. 2018) and reversal paradox (Hernán et al. 2011). Our results do show some differences between the individual exposure models and mixture models (Tables S4, S10, and S11), which may be indicative of coexposure amplification bias under specific scenarios of uncontrolled confounders correlated with some, but not all, mixture components. However, it is notable that if the uncontrolled confounder acts as a common source for the affected components, then mixture modeling is overall beneficial because it partially removes the bias (Weisskopf et al. 2018). Ultimately, these biases do not affect the estimates for the joint mixture, which can be interpreted with greater confidence, but our biomarker-specific results should be interpreted with caution.
Conclusion
In this longitudinal analysis of gestational urinary phthalate biomarker concentrations and maternal blood pressure changes during and after pregnancy, we found that urinary concentrations of phthalate biomarkers, as a mixture, were associated with higher gestational blood pressure during mid-to-late pregnancy through 72 months postpartum. This suggests that exposure to phthalates at earlier life stages may have lifelong consequences on the blood pressure trajectory, potentially elevating the risk for chronic illnesses later in life, such as hypertension. The trajectory associated with each phthalate biomarker differed slightly and further follow-up and future studies are necessary to study potential changes to blood pressure trajectories in early pregnancy as well as other long-term cardiometabolic health consequences.
Supplementary Material
Acknowledgments
We gratefully acknowledge all members of the Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) team for their tireless efforts in maintaining the cohort. In addition, we thank the American British Cowdray Hospital for providing research facilities for the PROGRESS study, and we thank the study participants, without whom this work would not be possible.
This work was supported by grants from the National Institutes of Health/National Institute of Environmental Health Sciences (R00 ES023474 to A.L.D.; R00 ES027508 to A.P.S.; R01 ES021357 and P30 ES009089 to A.A.B.; R01 ES024381 to J.M.B.; and P30 ES023515, R01 ES013744, R01ES014930, and R24 ES028522 to R.O.W.). The funding source did not have any role in the interpretation of the study results, writing of the manuscript, or decision to submit for publication.
The data used for this study are available upon request from the authors.
References
- Amin MM, Ebrahimpour K, Parastar S, Shoshtari-Yeganeh B, Hashemi M, Mansourian M, et al. 2018. Association of urinary concentrations of phthalate metabolites with cardiometabolic risk factors and obesity in children and adolescents. Chemosphere 211:547–556, PMID: , 10.1016/j.chemosphere.2018.07.172. [DOI] [PubMed] [Google Scholar]
- Bakk Z, Tekle FB, Vermunt JK. 2013. Estimating the association between latent class membership and external variables using bias-adjusted three-step approaches. Sociol Methodol 43(1):272–311, 10.1177/0081175012470644. [DOI] [Google Scholar]
- Benjamin S, Masai E, Kamimura N, Takahashi K, Anderson RC, Faisal PA. 2017. Phthalates impact human health: epidemiological evidences and plausible mechanism of action. J Hazard Mater 340:360–383, PMID: , 10.1016/j.jhazmat.2017.06.036. [DOI] [PubMed] [Google Scholar]
- Bobb JF, Claus Henn B, Valeri L, Coull BA. 2018. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health 17(1):67, PMID: , 10.1186/s12940-018-0413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, et al. 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16(3):493–508, PMID: , 10.1093/biostatistics/kxu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco AV. 2002. The AMAI system of classifying households by socio-economic level: the experience of Mexico and its comparison with Brazil and Argentina. In: Proceedings of the Latin American Conference, May 2002. Sao Paulo, Brazil: ESOMAR, https://ana.esomar.org/authors/adrian-villegas-carrasco.
- Casals-Casas C, Desvergne B. 2011. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol 73:135–162, PMID: , 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- Corrales P, Izquierdo-Lahuerta A, Medina-Gómez G. 2018. Maintenance of kidney metabolic homeostasis by PPAR gamma. Int J Mol Sci 19(7):2063, PMID: , 10.3390/ijms19072063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantine MM. 2014. Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol 5:65, PMID: , 10.3389/fphar.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng T, Xie X, Duan J, Chen M. 2019a. Di-(2-ethylhexyl) phthalate induced an increase in blood pressure via activation of ACE and inhibition of the bradykinin-NO pathway. Environ Pollut 247:927–934, PMID: , 10.1016/j.envpol.2019.01.099. [DOI] [PubMed] [Google Scholar]
- Deng T, Xie X, Duan J, Chen M. 2019b. Exposure to diisononyl phthalate induced an increase in blood pressure through activation of the ACE/AT1R axis and inhibition of NO production. Toxicol Lett 309:42–50, PMID: , 10.1016/j.toxlet.2019.03.011. [DOI] [PubMed] [Google Scholar]
- Dong R, Zhao S, Zhang H, Chen J, Zhang M, Wang M, et al. 2017. Sex differences in the association of urinary concentrations of phthalates metabolites with self-reported diabetes and cardiovascular diseases in Shanghai adults. Int J Environ Res Public Health 14(6):598, PMID: , 10.3390/ijerph14060598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganovic D, Lucic N, Jojic D. 2016. Oxidative stress marker and pregnancy induced hypertension. Med Arch 70(6):437–440, PMID: , 10.5455/medarh.2016.70.437-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman Chemical Company. 2014. Why Eastman 168 is a non-phthalate plasticizer: a regulatory memo. https://www.eastman.com/Literature_Center/Internal/L243.pdf [accessed 25 November 2021].
- Ferguson KK, Cantonwine DE, Rivera-González LO, Loch-Caruso R, Mukherjee B, Anzalota Del Toro LV, et al. 2014. Urinary phthalate metabolite associations with biomarkers of inflammation and oxidative stress across pregnancy in Puerto Rico. Environ Sci Technol 48(12):7018–7025, PMID: , 10.1021/es502076j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Chen YH, VanderWeele TJ, McElrath TF, Meeker JD, Mukherjee B. 2017. Mediation of the relationship between maternal phthalate exposure and preterm birth by oxidative stress with repeated measurements across pregnancy. Environ Health Perspect 125(3):488–494, PMID: , 10.1289/EHP282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Chen YH, Mukherjee B, Meeker JD. 2015. Urinary phthalate metabolites and biomarkers of oxidative stress in pregnant women: a repeated measures analysis. Environ Health Perspect 123(3):210–216, PMID: , 10.1289/ehp.1307996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Li J, Wang Y, Xu S, Li Y, Liu H, et al. 2020. Association between phthalate exposure and blood pressure during pregnancy. Ecotoxicol Environ Saf 189:109944, PMID: , 10.1016/j.ecoenv.2019.109944. [DOI] [PubMed] [Google Scholar]
- Hernán MA, Clayton D, Keiding N. 2011. The Simpson’s paradox unraveled. Int J Epidemiol 40(3):780–785, PMID: , 10.1093/ije/dyr041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojsgaard S, Halekoh U, Yan J. 2005. The R Package geepack for Generalized Estimating Equations. J Stat Softw 15(2):1–11, 10.18637/jss.v015.i02. [DOI] [Google Scholar]
- Jenkins R, Tackitt S, Gievers L, Iragorri S, Sage K, Cornwall T, et al. 2019. Phthalate-associated hypertension in premature infants: a prospective mechanistic cohort study. Pediatr Nephrol 34(8):1413–1424, PMID: , 10.1007/s00467-019-04244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsikantami I, Sifakis S, Tzatzarakis MN, Vakonaki E, Kalantzi OI, Tsatsakis AM, et al. 2016. A global assessment of phthalates burden and related links to health effects. Environ Int 97:212–236, PMID: , 10.1016/j.envint.2016.09.013. [DOI] [PubMed] [Google Scholar]
- Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. 2020. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ Health Perspect 128(4):47004, PMID: , 10.1289/EHP5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KI, Chiang CW, Lin HC, Zhao JF, Li CT, Shyue SK, et al. 2016. Maternal exposure to di-(2-ethylhexyl) phthalate exposure deregulates blood pressure, adiposity, cholesterol metabolism and social interaction in mouse offspring. Arch Toxicol 90(5):1211–1224, PMID: , 10.1007/s00204-015-1539-0. [DOI] [PubMed] [Google Scholar]
- Lessmann F, Kolossa-Gehring M, Apel P, Rüther M, Pälmke C, Harth V, et al. 2019. German Environmental Specimen Bank: 24-hour urine samples from 1999 to 2017 reveal rapid increase in exposure to the para-phthalate plasticizer di(2-ethylhexyl) terephthalate (DEHTP). Environ Int 132:105102, PMID: , 10.1016/j.envint.2019.105102. [DOI] [PubMed] [Google Scholar]
- Mannaerts D, Faes E, Cos P, Briedé JJ, Gyselaers W, Cornette J, et al. 2018. Oxidative stress in healthy pregnancy and preeclampsia is linked to chronic inflammation, iron status and vascular function. PLoS One 13(9):e0202919, PMID: , 10.1371/journal.pone.0202919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariana M, Feiteiro J, Cairrao E. 2018. Cardiovascular response of rat aorta to di-(2-ethylhexyl) phthalate (DEHP) exposure. Cardiovasc Toxicol 18(4):356–364, PMID: , 10.1007/s12012-017-9439-6. [DOI] [PubMed] [Google Scholar]
- Martinez-Arguelles DB, McIntosh M, Rohlicek CV, Culty M, Zirkin BR, Papadopoulos V. 2013. Maternal in utero exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate affects the blood pressure of adult male offspring. Toxicol Appl Pharmacol 266(1):95–100, PMID: , 10.1016/j.taap.2012.10.027. [DOI] [PubMed] [Google Scholar]
- Mooney SJ, Joshi S, Cerdá M, Kennedy GJ, Beard JR, Rundle AG. 2018. Longitudinal patterns of physical activity among older adults: a latent transition analysis. Am J Epidemiol 187(7):1549–1558, PMID: , 10.1093/aje/kwy027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. 2000. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 183(1):S1–S22, PMID: , 10.1067/mob.2000.107928. [DOI] [PubMed] [Google Scholar]
- Olsén L, Lind L, Lind PM. 2012. Associations between circulating levels of bisphenol A and phthalate metabolites and coronary risk in the elderly. Ecotoxicol Environ Saf 80:179–183, PMID: , 10.1016/j.ecoenv.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Ouyang Z, Ren C, Liu F, An G, Bo X, Shu W. 2018. The landscape of the A-to-I RNA editome from 462 human genomes. Sci Rep 8(1):12069, PMID: , 10.1038/s41598-018-30583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouzounian JG, Elkayam U. 2012. Physiologic changes during normal pregnancy and delivery. Cardiol Clin 30(3):317–329, PMID: , 10.1016/j.ccl.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Philips EM, Trasande L, Kahn LG, Gaillard R, Steegers EAP, Jaddoe VWV. 2019. Early pregnancy bisphenol and phthalate metabolite levels, maternal hemodynamics and gestational hypertensive disorders. Hum Reprod 34(2):365–373, PMID: , 10.1093/humrep/dey364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proust-Lima C, Philipps V, Liquet B. 2017. Estimation of extended mixed models using latent classes and latent processes: the R package lcmm. J Stat Softw 78(2):1–56, 10.18637/jss.v078.i02. [DOI] [Google Scholar]
- Rahmani A, Soleimannejad K, Hafezi Ahmadi MR, Asadollahi K, Khalighi Z. 2016. Prenatal exposure to phthalic acid induces increased blood pressure, oxidative stress, and markers of endothelial dysfunction in rat offspring. Cardiovasc Toxicol 16(4):307–315, PMID: , 10.1007/s12012-015-9337-8. [DOI] [PubMed] [Google Scholar]
- Rana S, Lemoine E, Granger JP, Karumanchi SA. 2019. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res 124(7):1094–1112, PMID: , 10.1161/CIRCRESAHA.118.313276. [DOI] [PubMed] [Google Scholar]
- Raven PB, Chapleau MW. 2014. Blood pressure regulation XI: overview and future research directions. Eur J Appl Physiol 114(3):579–586, PMID: , 10.1007/s00421-014-2823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schettler T. 2006. Human exposure to phthalates via consumer products. Int J Androl 29(1):134–139, PMID: , 10.1111/j.1365-2605.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Vexler A, Whitcomb BW, Liu A. 2006. The limitations due to exposure detection limits for regression models. Am J Epidemiol 163(4):374–383, PMID: , 10.1093/aje/kwj039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahoud JS, Sanvictores T, Aeddula NR. 2020. Physiology, arterial pressure regulation. In: StatPearls. Treasure Island, FL: StatPearls Publishing. [PubMed] [Google Scholar]
- Shiue I. 2014a. Higher urinary heavy metal, arsenic, and phthalate concentrations in people with high blood pressure: US NHANES, 2009–2010. Blood Press 23(6):363–369, PMID: , 10.3109/08037051.2014.925228. [DOI] [PubMed] [Google Scholar]
- Shiue I. 2014b. Higher urinary heavy metal, phthalate, and arsenic but not parabens concentrations in people with high blood pressure, U.S. NHANES, 2011–2012. Int J Environ Res Public Health 11(6):5989–5999, PMID: , 10.3390/ijerph110605989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiue I, Hristova K. 2014. Higher urinary heavy metal, phthalate and arsenic concentrations accounted for 3–19% of the population attributable risk for high blood pressure: US NHANES, 2009–2012. Hypertens Res 37(12):1075–1081, PMID: , 10.1038/hr.2014.121. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL Jr, Reidy JA, Needham LL, Calafat AM. 2007. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci 860(1):106–112, PMID: , 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Wong LY, Samandar E, Preau JL, Calafat AM, Ye X. 2017. Exposure to di-2-ethylhexyl terephthalate in a convenience sample of U.S. adults from 2000 to 2016. Arch Toxicol 91(10):3287–3291, PMID: , 10.1007/s00204-017-1956-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sol CM, Santos S, Asimakopoulos AG, Martinez-Moral MP, Duijts L, Kannan K, et al. 2020. Associations of maternal phthalate and bisphenol urine concentrations during pregnancy with childhood blood pressure in a population-based prospective cohort study. Environ Int 138:105677, PMID: , 10.1016/j.envint.2020.105677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara A, Uruno A, Matsuda K, Saito-Ito T, Funato T, Saito-Hakoda A, et al. 2012. Effects of PPARγ agonists against vascular and renal dysfunction. Curr Mol Pharmacol 5(2):248–254, PMID: , 10.2174/1874467211205020248. [DOI] [PubMed] [Google Scholar]
- Tran V, Tindula G, Huen K, Bradman A, Harley K, Kogut K, et al. 2017. Prenatal phthalate exposure and 8-isoprostane among Mexican-American children with high prevalence of obesity. J Dev Orig Health Dis 8(2):196–205, PMID: , 10.1017/S2040174416000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Attina TM. 2015. Association of exposure to di-2-ethylhexylphthalate replacements with increased blood pressure in children and adolescents. Hypertension 66(2):301–308, PMID: , 10.1161/HYPERTENSIONAHA.115.05603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Sathyanarayana S, Spanier AJ, Trachtman H, Attina TM, Urbina EM. 2013. Urinary phthalates are associated with higher blood pressure in childhood. J Pediatr 163(3):747–753.e1, PMID: , 10.1016/j.jpeds.2013.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafeiadi M, Myridakis A, Roumeliotaki T, Margetaki K, Chalkiadaki G, Dermitzaki E, et al. 2018. Association of early life exposure to phthalates with obesity and cardiometabolic traits in childhood: sex specific associations. Front Public Health 6:327, PMID: , 10.3389/fpubh.2018.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvi D, Casas M, Romaguera D, Monfort N, Ventura R, Martinez D, et al. 2015. Prenatal phthalate exposure and childhood growth and blood pressure: evidence from the Spanish INMA-Sabadell birth cohort study. Environ Health Perspect 123(10):1022–1029, PMID: , 10.1289/ehp.1408887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van T Erve TJ, Rosen EM, Barrett ES, Nguyen RHN, Sathyanarayana S, Milne GL, et al. 2019. Phthalates and phthalate alternatives have diverse associations with oxidative stress and inflammation in pregnant women. Environ Sci Technol 53(6):3258–3267, PMID: , 10.1021/acs.est.8b05729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermunt JK. 2010. Latent class modeling with covariates: two improved three-step approaches. Polit Anal 18(4):450–469, 10.1093/pan/mpq025. [DOI] [Google Scholar]
- Warembourg C, Basagaña X, Seminati C, de Bont J, Granum B, Lyon-Caen S, et al. 2019a. Exposure to phthalate metabolites, phenols and organophosphate pesticide metabolites and blood pressure during pregnancy. Int J Hyg Environ Health 222(3):446–454, PMID: , 10.1016/j.ijheh.2018.12.011. [DOI] [PubMed] [Google Scholar]
- Warembourg C, Maitre L, Tamayo-Uria I, Fossati S, Roumeliotaki T, Aasvang GM, et al. 2019b. Early-Life environmental exposures and blood pressure in children. J Am Coll Cardiol 74(10):1317–1328, PMID: , 10.1016/j.jacc.2019.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Seals RM, Webster TF. 2018. Bias amplification in epidemiologic analysis of exposure to mixtures. Environ Health Perspect 126(4):047003, PMID: , 10.1289/EHP2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner EF, Braun JM, Yolton K, Khoury JC, Lanphear BP. 2015. The association between maternal urinary phthalate concentrations and blood pressure in pregnancy: the HOME Study. Environ Health 14(1):75, PMID: , 10.1186/s12940-015-0062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Kupsco AJ, Deierlein AL, Just AC, Calafat AM, Oken E, et al. 2020. Trends and patterns of phthalates and phthalate alternatives exposure in pregnant women from Mexico City during 2007–2010. Environ Sci Technol 54(3):1740–1749, PMID: , 10.1021/acs.est.9b05836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Olmsted A, Cantonwine DE, Shahsavari S, Rahil T, Sites C, et al. 2017. Urinary phthalate and phthalate alternative metabolites and isoprostane among couples undergoing fertility treatment. Environ Res 153:1–7, PMID: , 10.1016/j.envres.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Wu P, Yang F, Sun DL, Zhang DX, Zhou YK. 2018. Association of phthalate exposure with anthropometric indices and blood pressure in first-grade children. Environ Sci Pollut Res Int 25(23):23125–23134, PMID: , 10.1007/s11356-018-2447-7. [DOI] [PubMed] [Google Scholar]
- Yao Y, Chen DY, Yin JW, Zhou L, Cheng JQ, Lu SY, et al. 2020. Phthalate exposure linked to high blood pressure in Chinese children. Environ Int 143:105958, PMID: , 10.1016/j.envint.2020.105958. [DOI] [PubMed] [Google Scholar]
- Zhang SH, Shen YX, Li L, Fan TT, Wang Y, Wei N. 2018. Phthalate exposure and high blood pressure in adults: a cross-sectional study in China. Environ Sci Pollut Res Int 25(16):15934–15942, PMID: , 10.1007/s11356-018-1845-1. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, et al. 2012. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology 153(9):4097–4110, PMID: , 10.1210/en.2012-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.