Abstract
On March 11, 2021, the AstraZeneca vaccine against COVID-19 was suspended in three Nordic countries and, on subsequent days, in other European countries. Using data on vaccine acceptance in eight Western countries obtained on a daily basis, we show that these decisions - and associated news - decreased public vaccine acceptance in several countries and part of this decrease happened in response to suspensions in other countries. The findings demonstrate the importance of international coordination between health authorities during a pandemic such that local authorities are able to put the decisions of foreign authorities into perspective.
Keywords: Vaccine acceptance, COVID-19, Side-effects, AstraZeneza suspension, International coordination
Within a week of March 2021, a number of countries officially announced that they were temporarily suspending the use of the AstraZeneca vaccine against COVID-19 after concerns about potential side effects in the form of blood clots. Austria was the first country to raise such concerns. On March 7, Austria announced that they would suspend a single batch of the vaccine. On March 11, Denmark, Norway and Iceland announced that they suspended the vaccine entirely until they had more evidence on its potential side effects. In the following days, a number of countries took the same decision. These suspensions created headlines worldwide [11].
It is well-known that concerns about side effects are significantly associated with vaccine skepticism also in the context of COVID-19 vaccines [2], [6], [9], [1], [8]. Accordingly, there has been raised debates over whether the decision to suspend the AstraZeneca vaccine in some countries has potential effects on levels of acceptance when it comes to COVID-19 vaccines more generally [3]. In this manuscript, we raise the specific question of whether the suspension decision of some countries impacted vaccine acceptance in other countries. During an international health crisis such as a pandemic, negative information about side-effects will travel quickly across borders, potentially affecting not just the immediate national audience but also international audiences.
We have continuously collected nationally representative surveys about acceptance of a COVID-19 vaccine across eight Western countries: France, Denmark, Germany, Hungary, Italy, Sweden, United Kingdom and United States (for further description, see [4]). In March, data was collected from March 10 to March 16. On this basis, we are able to assess the impact of the suspension decisions on March 11 and subsequent decisions on public COVID-19 vaccine acceptance by comparing levels of acceptance across different specific dates. This also allows us to examine if the decisions in some countries had spill-over effects on acceptance in other countries. In this article, we thus provide a real-world examination of what happens to vaccine acceptance when potential side-effects are massively discussed by authorities and news media.
1. Materials and methods
1.1. Data
The data we use for the present purpose was collected in online survey panels, collected over a week each month from December 2020 to April 2021 with approximately 500 participants per month and country (total N = 20.519). In each data round, survey respondents were quota sampled to match the population margins on age, gender, and geographic location for each of the eight countries. The data collection was carried out in accordance with Aarhus University's Code of Conduct as well as the Committee Act of the Danish National Committee of Health Research Ethics, which states that “Surveys using questionnaires and interviews that do not involve human biological material (section 14(2) of the Committee Act)” are exempted from approval (https://en.nvk.dk/how-to-notify/what-to-notify).
As our measure of vaccine acceptance, we use agreement with the statement: “If the health authorities advise people like me to get an approved vaccine against the coronavirus, I will follow their advice”. Individuals who answer “completely agree” and “somewhat agree” are considered “accepting” and coded as “1″. All other responses (”neither agree nor disagree“, ”somewhat disagree“, ”completely disagree“ and ”don't know“) are coded as ”0″.
1.2. Research design
To identify the causal impact of the suspension decisions and the associated news coverage, we compare levels of vaccine acceptance on March 10 with levels of vaccine acceptance on subsequent days. This strategy is limited by the fact that we are not able to assess the impact of events prior to March 10; specifically, Austria’s decision to suspend a single batch of the AstraZeneca vaccine on March 7. However, Fig. 1 shows that there is good reason to believe that public attention was not driven by this decision, but mainly generated from the decisions made on March 11. Fig. 1 provides an overview of all decisions (vertical dashed lines) by European societies in the focal days around the suspensions together with the development in popular attention about the AstraZeneca vaccine from March 6 to March 18.1 While the figure shows a marked increase in relative internet search volumes in most countries on March 11, there is no increase on March 6. Moreover, we see large country-specific increases in relative search volumes in Italy, Germany, and France on March 15 associated with their decisions to suspend the vaccine.
Fig. 1.
Suspension Events Related to the AstraZeneca Vaccine in Europe and Public Attention to the AstraZeneca Vaccine in the Same Period in Eight Western Democracies. Suspension events are divided into suspensions of single batches (“Batch”) and the entire vaccine (“Vaccine”). Dashed vertical lines and associated text indicate the suspension event, the country and the date. Public attention is operationalized as searches on Google for “astrazeneca”, measured via Google Trends.
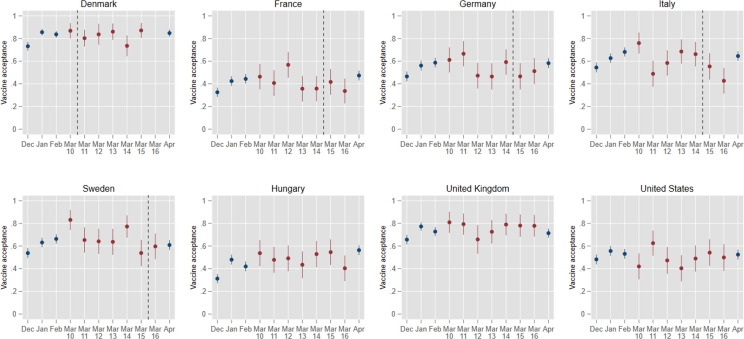
This identification strategy has the key strength—compared to alternatives—that it provides a comparison of observations immediately before and after the key suspension event. A previous study that compared rates of vaccine acceptance in Denmark in February (sampled between the 4th and 21st) and in March (sampled between the 15th and 25th) found no change in overall vaccine acceptance [10]. However, the study faces at least two challenges. First, by comparing post-suspension observations to a benchmark that was observed over a month prior to the suspension, estimates will be biased towards zero if vaccine acceptance is trending upwards. Fig. 2 displays our data setup. Red filled circles reflect the observations we use in the estimations below to estimate the effect of suspensions on vaccine acceptance, while the blue filled circles show vaccine acceptance in previous and later rounds of data. The figure clearly illustrates that vaccine acceptance, in fact, is trending upwards from December until March 10 across all countries in our sample, except in the United States. Potentially, this reflects that acceptance is increasing with a successful implementation of the vaccine program. In any case, it implies that comparing post-suspension vaccine acceptance to vaccine acceptance in early February will provide a biased counterfactual (i.e., what we would expect to have happened had there been no suspensions and no reports about potential side effects). Second, if the health communication strategies regarding the suspensions are successful, we might expect a recovery of vaccine acceptance rates after an initial downward drop. In other words, we might expect negative effects on vaccine acceptance to be only temporary. Fig. 2 also shows mean vaccine acceptance in April (about a month after the suspensions). Here, after an initial decrease in March, we observe some tendency towards recovery of acceptance in April. Consequently, it suggests that using post-suspension observations relatively far away from the suspension threshold may similarly create a bias in the estimates towards zero. Taken together, this suggests that the only way to obtain a credible counterfactual and reliably assess the suspensions' causal impact on vaccine acceptance is to compare vaccine acceptance just around the suspensions.
Fig. 2.
Vaccine Acceptance Across Eight Western Democracies in the Week the AstraZeneca Vaccine Was Suspended. Entries are monthly and daily national proportions (and associated 95 % confidence intervals) who answered “completely agree” or “somewhat agree” when asked whether they were willing to receive an approved COVID-19 vaccine. Red filled circles are the daily vaccine acceptance means from the March data collection that we use in the statistical estimations. Blue filled circles are means from data rounds in months before and after the suspensions, respectively. Vertical dashed lines indicate the day of suspension of the vaccine in each of the countries (Hungary, the UK, and the USA never suspended the AstraZeneca vaccine). There are approximately 500 respondents in each country per month (N = 20.519).
1.3. Statistical analysis
We compare the vaccine acceptance proportions before and after the AstraZeneca suspensions (see the red filled circles and vertical dashed lines in Fig. 2) using ordinary least squares regression with country-level fixed effects. Our identification strategy thus rests on the assumption that the samples are comparable on different days. Consistent with this, the sampling of the survey was intentionally designed to achieve an equal number of responses each day of the week. To adjust for remaining sampling imbalances, all analyses utilize post-stratification weights such that the samples on each day match the population margins on age, gender, education and geographic location.
2. Results
Table 1 reports the results of our regression analyses. Model I gives the results from regressing vaccine acceptance on a dummy that indicates the March 11 suspension across all countries. Pooling across all countries, we find that vaccine acceptance fell with an average of 8 percentage points (b = -0.08, p = .002) after March 10. In Model II and III, we split the sample into the countries in the sample that at some point during the analyses period suspended the AstraZeneca vaccine (Denmark, Sweden, Germany, France, and Italy) and the countries that did not suspend the vaccine (Hungary, the United Kingdom, and the United States). Taken together, Models II and III show that the pooled suspension effect estimate was driven by the countries that decided to suspend the AstraZeneca vaccine within the analyses period. More specifically, pooling across the countries that decided to suspend the AstraZeneca-vaccine, Model II shows lower acceptance after March 10 (b = -0.11, p < .0001). At the same time, Model III shows that there was no effect in the countries that did not suspend the vaccine (b = -0.01, p = .845). The difference (modeled as an interaction term) between the two groups of countries that did and did not suspend the AstraZeneca vaccine, respectively, is statistically significant (p = .041). In Model IV, we estimate whether the local suspensions in Germany, France, Italy, and Sweden (see Fig. 1 for details) resulted in additional decreases. Here, we regress vaccine acceptance on a variable with three levels that indicates (1) the period before March 11, (2) the period between March 11 and the local suspensions (reference category), and (3) the period after the local suspension. The results show an additional decrease of 7 percentage points (b = -0.07, p = .044) after the local national suspension.
Table 1.
Estimated suspension effect on vaccine acceptance. Entries are unstandardized regression coefficients. Robust standard errors in parentheses. Model I includes all countries. Model II includes Denmark, Sweden, France, Germany, and Italy. Model III includes Hungary, the UK, and the USA. Model IV includes Sweden, France, Germany, and Italy.
| Model I |
Model II |
Model III |
Model IV |
|||||
|---|---|---|---|---|---|---|---|---|
| Pooled effect | Sig (p) | Suspending countries | Sig (p) | No suspension countries | Sig (p) | Own suspensions | Sig (p) | |
| March 11 suspension | −0.08 (0.03) | 0.002 | −0.11 (0.03) | <0.0001 | −0.01 (0.04) | 0.845 | – | |
| Prior to March 11 | – | – | – | 0.11 (0.04) | 0.002 | |||
| March 11 suspensions | – | – | – | Ref. | ||||
| Local suspensions | – | – | – | −0.07 (0.03) | 0.044 | |||
| Country fixed-effects | √ | √ | √ | √ | ||||
| N (countries) | 8 | 5 | 3 | 4 | ||||
| N (individuals) | 4,089 | 2,599 | 1,490 | 2,047 | ||||
3. Discussion and conclusion
These findings suggest that news about the side-effects of the AstraZeneca vaccine and the associated decisions to suspend the vaccine on March 11 had negative, cross-national effects on acceptance of a vaccine against COVID-19 and co-occurred with the ending of a period of increased vaccine acceptance across several countries. The findings thus provide a real-world demonstration of how concerns about side-effects in some countries can depress vaccine acceptance in other countries in the context of the COVID-19 pandemic, creating international ripple effects of national decisions.
Importantly, the identified adverse ripple effects were limited in the sense that they were specific for individuals within Western European countries who are accustomed to relying on shared health information (e.g., from the European Medical Agency). Furthermore, the ripple effects may also be limited in another sense. Data from another study on vaccine acceptance among Danish respondents also obtained daily data but used a simple “Yes vs. No”-response format for vaccine acceptance. In these data, there is no decrease in vaccine acceptance in the days following the suspension among Danes [5]. This suggests that the suspensions and associated news may not depress acceptance as such, but rather induce psychological uncertainty about the individual's acceptance; something which is more likely to be captured by the more fine-grained measure used here.
It is important to emphasize that findings should not be taken to suggest that authorities should not transparently disclose negative information about vaccines. A recent experimental study find that while such transparency may hinder short-term vaccine acceptance, it can uphold trust in the health authorities [8], which is the biggest predictor of overall acceptance of COVID-19 vaccines [4], [5]. Instead, the contribution from the present analyses is that they provide the first evidence that decisions from one national health authority during a pandemic can reach and impact the populations of other countries. These findings thereby highlight the importance of rapid information sharing and collaboration between national health authorities such that each national authority immediately can qualify the decisions of foreign health authorities and put them into an appropriate national perspective.
Funding statement
The study was funded by The Carlsberg Foundation with grant CF20-0044 to Michael Bang Petersen. The funders had no role in the conduct of this research.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Alexander Bor for help with the design and collection of the data. We thank Magnus Storm Rasmussen for help with data management. The data was collected by the survey company, Epinion, on behalf of the researchers. The data and code files for reproducing the analyses are publicly available at Open Science Framework: https://osf.io/knq8e/.
Footnotes
As a metric of popular attention, we use Google searches for "astrazeneca", accessed via Google Trends. Google Trends provide a relative search volume, which is the query share of a particular term for a given location and time period, normalized by the highest query share of that term over the time-series (see [7]).
References
- 1.Jørgensen, F. J., Bor, A., & Petersen, M. (2021, June 28). How the Development, Features and Roll-Out of a SARS-COV-2 Vaccine Shape Public Acceptance: A Conjoint Experiment in a Large Representative Sample of Danes. https://doi.org/10.31234/osf.io/4y8ap
- 2.Kreps S., Prasad S., Brownstein J.S., Hswen Y., Garibaldi B.T., Zhang B., et al. Factors associated with US adults’ likelihood of accepting COVID-19 vaccination. JAMA network open. 2020;3(10):e2025594. doi: 10.1001/jamanetworkopen.2020.25594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larson H.J., Broniatowski D.A. Volatility of vaccine confidence. Science. 2021;317(6536):1289. doi: 10.1126/science.abi6488. [DOI] [PubMed] [Google Scholar]
- 4.Lindholt, M. F., Jørgensen, F. J., Bor, A., & Petersen, M. B. (2021a). Public Acceptance of COVID-19 Vaccines: Cross-National Evidence on Levels and Individual-Level Predictors Using Observational Data. BMJ Open, in press. [DOI] [PMC free article] [PubMed]
- 5.Lindholt, M. F., Jørgensen, F. J., Bor, A., & Petersen, M. B. (2021b). Danskernes Smitteforebyggende Adfærd og Opfattelser. Report from the HOPE-project, March 15, 2021: https://raw.githubusercontent.com/mariefly/HOPE/master/Danskernes_Smitteforebyggende_Adfærd_Og_Opfattelser_0316.pdf.
- 6.Motta M. Can a COVID-19 vaccine live up to Americans’ expectations? A conjoint analysis of how vaccine characteristics influence vaccination intentions. Soc Sci Med. 2021;272:113642. doi: 10.1016/j.socscimed.2020.113642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nuti S.V., Wayda B., Ranasinghe I., Wang S., Dreyer R.P., Chen S.I., et al. The use of google trends in health care research: a systematic review. PLoS ONE. 2014;9(10):e109583. doi: 10.1371/journal.pone.0109583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen M.B., Bor A., Jørgensen F., Lindholt M.F. Transparent communication about negative features of COVID-19 vaccines decreases acceptance but increases trust. Proc Natl Acad Sci USA. 2021;118(29) doi: 10.1073/pnas.2024597118. e2024597118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwarzinger M., Watson V., Arwidson P., Alla F., Luchini S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. The Lancet Public Health. 2021;6(4):e210–e221. doi: 10.1016/S2468-2667(21)00012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sønderskov K.M., Dinesen P.T., Østergaard S.D. Sustained COVID-19 vaccine willingness after safety concerns over the Oxford-AstraZeneca vaccine. Dan Med J. 2021;68:A03210292. [PubMed] [Google Scholar]
- 11.Vogel G., Kupferschmidt K. New problems erode confidence in AstraZeneca's vaccine. Science. 2021;371(6536):1294–1295. doi: 10.1126/science.371.6536.1294. [DOI] [PubMed] [Google Scholar]