Abstract
NMDA receptors are ligand-gated ion channels that are found throughout the brain and are required for both brain development and many higher order functions. A variety of human patients with diverse clinical phenotypes have been identified that carry autoantibodies directed against NMDA receptor subunits. Here we focus on two general classes of autoantibodies, anti-GluN1 antibodies associated with anti-NMDA receptor encephalitis and anti-GluN2 antibodies associated with systemic lupus erythematosus (SLE). These two general classes of anti-NMDA receptor autoantibodies display a wide range of pathophysiological mechanisms from altering synaptic composition to gating of NMDARs. While we have made progress in understanding how these autoantibodies work at the molecular and cellular level, many unanswered questions remain including their long-term actions on brain function, the significance of clonal variations, and their effects on different NMDA receptor-expressing cell types in local circuits. This information will be needed to define fully the transition from anti-NMDA receptor autoantibodies to a clinical phenotype.
Keywords: anti-NMDA receptor encephalitis, Systemic lupus erythematosus (SLE), neuropsychiatric lupus (NPLSE)
Introduction
NMDA receptors (NMDAR) are ion channels gated by the neurotransmitter glutamate, the major excitatory neurotransmitter in the brain. NMDAR signaling impacts nearly all forms of brain activity including those important to higher brain functions like learning and memory (Paoletti et al., 2013, Hansen et al., 2017, Herring and Nicoll, 2016). Dysfunctions in this signaling are associated with acute (e.g., stroke), chronic (e.g., Parkinson’s & Alzheimer’s Diseases), and neuropsychiatric (e.g., schizophrenia, depression) brain disorders (Coyle, 2017, Choi, 2020, Wang et al., 2020).
Highlighting the key role of NMDAR in brain function is the identification of numerous NMDAR channelopathies that are associated with psychiatric, neurological and neurodevelopmental disorders: these include missense and nonsense mutations in the genes encoding NMDAR subunits (Hu et al., 2016, Hardingham and Do, 2016, XiangWei et al., 2018, Garcia-Recio et al., 2020, Amin et al., 2021) and autoantibodies that target various NMDAR subunits (Diamond et al., 2009, Dalmau et al., 2017, Schwartz et al., 2019, Hunter et al., 2021). Here we will focus on anti-NMDAR autoantibodies, in particular those associated with anti-NMDAR encephalitis and with systemic lupus erythematosus (SLE) or lupus. We will focus on these two general classes since they are the best characterized examples yet highlight both the challenges of studying anti-NMDAR autoantibodies in disease and defining how these autoantibodies might lead to a clinical phenotype. Initially, we will describe general features of NMDAR signaling since this is what these autoantibodies presumably target to disrupt brain function. Subsequently, we will consider the challenges of relating anti-NMDAR autoantibodies to clinical phenotypes and then will discuss various evidence of how these autoantibodies affect NMDAR signaling and potentially lead to a clinical phenotype. Finally, we will discuss on-going and future efforts that are needed to move this critical field forward.
NMDA receptor-mediated signaling
The impact of NMDARs on brain function depends on three general considerations: (i) a charge transfer and Ca2+-mediated signaling that arises from the glutamate-induced opening of the associated ion channel (Figure 1)(Traynelis et al., 2010, Paoletti et al., 2013, Wollmuth, 2018); (ii) a metabotropic pathway that signals independently of ion channel opening (Nabavi et al., 2013, Valbuena and Lerma, 2016, Rajani et al., 2020); and (iii) NMDAR cell biology, which encompasses subunit composition and post-translational modifications as well as the number and distribution of NMDARs on the membrane (Paoletti et al., 2013, Lussier et al., 2015, Groc and Choquet, 2020). At present, there are no studies addressing any action of anti-NMDAR autoantibodies on NMDAR-mediated metabotropic signaling, and we will not discuss it further here. Still, this lack of information highlights a significant knowledge gap in understanding the pathophysiology of these autoantibodies.
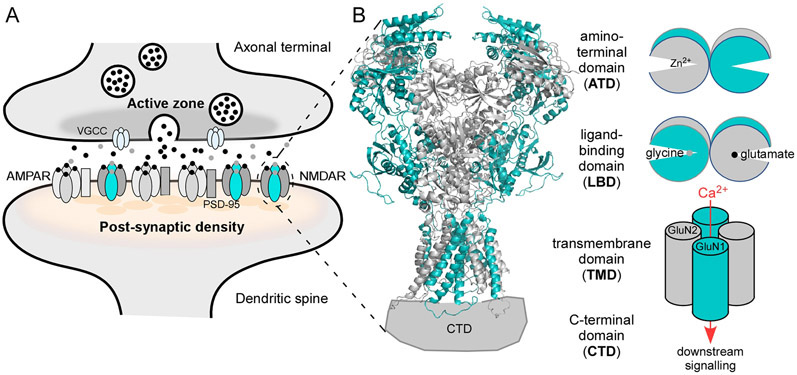
Figure 1. NMDA receptor (NMDAR) signaling.
(A) Features of glutamatergic synapses. Vesicular release of glutamate (black dots) is triggered by Ca2+ influx through voltage-gated calcium channels (VGCCs) at the active zone. Glutamate, along with glycine or D-serine (gray dots), activate AMPAR and NMDAR on the postsynaptic membrane. AMPAR are anchored at the postsynaptic density by PSD-95 via auxiliary subunits (gray rectangle). NMDARs are clustered at the PSD by a direct interaction with PSD-95 (Kornau et al., 1995).
(B) Left, NMDAR topology. GluN1 is teal; GluN2 is gray. The tetrameric complex is composed of four highly modular domains: the extracellularly located amino-terminal (ATD) and ligand-binding (LBD) domains; the membrane-spanning transmembrane domain (TMD) forming the ion channel; and the intracellular C-terminal domain (CTD), which is not resolved in any iGluR structures. Model structure of GluN1/GluN2B (4TLM) (Amin et al., 2017, Amin et al., 2018).
(B) Right, In addition to charge transfer, NMDAR mediate a Ca2+ component of excitatory neurotransmission. The clam-shell like LBD and ATD regulate ion channel activity. Glycine (GluN1) and glutamate (GluN2) binding to the LBD, which induces clam-shell closure, directly leads to ion channel opening (Armstrong and Gouaux, 2000, Kazi et al., 2014); clam-shell closure of the ATD by agents like Zn2+ act as a negative allosteric modulator (Romero-Hernandez et al., 2016).
The predominant postsynaptic glutamate-gated ion channels or ionotropic glutamate receptors (iGluRs) are AMPA (AMPAR) and NMDA (NMDAR) receptors (Figure 1A)(Bekkers and Stevens, 1989). AMPARs primarily mediate the depolarizing actions of synaptically-released glutamate (Traynelis et al., 2010, Huganir and Nicoll, 2013). On the other hand, NMDARs provide a more nuanced signaling capacity to neurons with opening of its ion channel affecting membrane depolarization as well as inducing Ca2+-dependent signaling (Figure 1B). This NMDAR-mediated charge injection and Ca2+ influx is fundamental to the physiology and pathophysiology of neurons affecting local events such synapse structure and strength of signaling (Herring and Nicoll, 2016) as well as distal events like membrane excitability (Stuart and Spruston, 2015) and gene expression (Tamminga and Zukin, 2015). NMDAR-mediated Ca2+ influx is also associated with excitotoxicity, a major form a cell death in the brain (Choi, 2020).
NMDARs are obligate heterotetramers composed of two GluN1 subunits, which are paired with varied combinations of GluN2(A-D) (Figure 1B) or GluN3(A,B) subunits. Alternative spicing of the GluN1 subunit and different association with GluN2 subunits impart distinct functional properties to the channel and foster different protein interactions through their intracellular domains (Paoletti et al., 2013, Hansen et al., 2018, Hardingham, 2019, Vieira et al., 2020). The most prominent GluN2 subunits in the brain are GluN2A and GluN2B.
NMDARs are distributed at synaptic sites, across from presynaptic active zones (Figure 1A), as well as at extrasynaptic sites (Parsons and Raymond, 2014, Papouin and Oliet, 2014, Zhou et al., 2015). In addition to subunit composition, key considerations in terms of signaling at synapses are the number of postsynaptic NMDARs and their distribution relative to release sites, which is controlled by both postsynaptic (e.g., PSD-95) as well as transsynaptic interactions (Sheng and Hoogenraad, 2007, Tang et al., 2016, Goncalves et al., 2020). There is a dynamic and regulated exchange of NMDARs between synaptic and extrasynaptic pools (Figure 2) (Tovar and Westbrook, 2002, Groc et al., 2004, Hiester et al., 2018, Groc and Choquet, 2020, McQuate and Barria, 2020). While extrasynaptic NMDARs can themselves play physiological roles in signaling, this function is distinct to that for synaptic receptors (Papouin et al., 2012), and both synaptic and extrasynaptic NMDARs are important mediators of excitotoxicity in neurons in response to acute injury (Wroge et al., 2012, Zhou et al., 2013). The different roles of synaptic and extrasynaptic NMDARs also depend on the subunit composition which greatly influence the receptor biophysics (Paoletti et al., 2013, Vieira et al., 2020), surface dynamics (Groc et al., 2006), and nanoscale organization in synapses (Kellermayer et al., 2018). The distinction between synaptic and extrasynaptic NMDAR to the pathology of anti-NMDAR autoantibodies is an important consideration given the functional and cell biological distinction between these pools as well as that the size of antibodies, which might in certain instances restrict access to receptors.
Figure 2. Putative mechanisms of anti-NMDAR autoantibodies on NMDAR signaling.
AMPAR (gray) and NMDARs (teal/gray) are usually trafficked extrasynaptically and diffuse through the surface membrane to the synaptic space where they are anchored by synaptic anchoring proteins.
(1) Anti-GluN1 antibody disrupts interactions with synaptic anchoring proteins such as EphrinB2R, which may drive it out of the synaptic space by diffusion (3).
(2) Increased ion channel function by anti-GluN2 antibodies from SLE, presumably leading to excitotoxicity. Anti-GluN2 antibodies require glutamate to drive increases in NMDAR currents and hence may occur strongly only at synapses.
(4) Increased endocytosis and diffusion out of the synapse by anti-GluN1 antibodies for NMDARs. A similar internalization with anti-GluN2 antibodies from SLE also presumably occurs but this is unknown.
(5) Extrasynaptic signaling by anti-NMDAR autoantibodies for either liganded or unliganded receptors or for cross-linked receptors remains unknown.
Challenges of studying brain reactive autoantibodies
A variety of nervous system disorders are associated with antibodies that target self-antigens present in the nervous system including ion channels. The most well-known and perhaps best-defined example is myasthenia gravis where autoantibodies target the nicotinic acetylcholine receptor as well as associated proteins at the neuromuscular junction to disrupt muscle strength (Gilhus et al., 2019). However, there are many examples of autoimmune channelopathies, including for GABAA receptors (Pruss and Kirmse, 2018), voltage-gated K+ channels (van Sonderen et al., 2017), and aquaporin (Soltys et al., 2019).
Glutamate receptor autoantibodies are detectable in several neurological conditions, with strong evidence for contribution to disease pathology in a few cases (Levite, 2014, Dalmau et al., 2017, Pleasure, 2008, Tay et al., 2017). Autoantibodies against iGluRs, including AMPAR and NMDAR, have been discovered in patients with autoimmune encephalitis and paraneoplastic syndromes. The first identified anti-iGluR autoantibody was associated with Rasmussen’s encephalitis, where the antibody epitope was on the AMPAR GluA3 (historically, GluR3) (Rogers et al., 1994). Since then, numerous antibodies targeting iGluR have been discovered, along with putative mechanisms of action at synapses that presumably contribute to the disease phenotype (Table 1; Figure 2).
Table 1.
Anti-iGluR autoantibodies with pathophysiological mechanisms
| Disease | iGluR subunit epitope | Pathophysiological mechanisms |
|---|---|---|
| Anti-NMDAR encephalitis | GluN1 | Decreases in synaptic density of NMDARs through impaired surface diffusion and internalization (Hughes et al., 2010, Moscato et al., 2014, Planaguma et al., 2015, Kreye et al., 2016, Castillo-Gomez et al., 2017, Ladepeche et al., 2018). Displacement from EphrinB2R that normally stabilizes NMDARs in the synapse (Mikasova et al., 2012, Planaguma et al., 2015).No acute changes in NMDAR-mediated currents (Moscato et al., 2014) (but see (Castillo-Gomez et al., 2017)). Chronic decreases in NMDAR-mediated currents (Hughes et al., 2010, Moscato et al., 2014, Kreye et al., 2016). Decreases in synaptic plasticity (Mikasova et al., 2012, Planaguma et al., 2015). Behavior and memory deficits (Planaguma et al., 2015). |
| Acute psychosis/ schizophrenia | GluN1 | Disruption in EphrinB2R interactions Decrease in synaptic density of NMDARs through impaired surface diffusion Decrease in synaptic plasticity (Jezequel et al., 2017) |
| Systemic lupus erythematosus | GluN2A/N2B | NMDAR-dependent excitotoxicity (DeGiorgio et al., 2001, Kowal et al., 2004, Kowal et al., 2006, Faust et al., 2010, Gono et al., 2011, Kapadia et al., 2017). Acute changes in NMDAR-mediated currents (Faust et al., 2010, Gono et al., 2011, Kapadia et al., 2017). Behavior and memory deficits (Chang et al., 2015, Kapadia et al., 2017, Nestor et al., 2018). Recruitment of microglia (Nestor et al., 2018) Primarily acts via GluN2A (Chan et al., 2020) |
There are enormous challenges in relating the presence of autoantibodies to any disease progression. Autoantibodies can be found in healthy patients and may be a natural immune response and/or a progression of aging (Pan et al., 2019). This is particularly acute for NMDARs that show a wide distribution not only in neuronal tissue but also non-neuronal tissue including B and T cells (Ehrenreich, 2018, Leboyer et al., 2016). Additional complication include that isolated antibodies are often polyclonal and polyspecific, targeting multiple epitopes on ion channels as well as associated proteins. Furthermore, while autoantibodies can show correlations to a disease phenotype, it is often not clear whether the autoantibodies cause the disease or whether they only shape some symptom or some behavioral phenotype. Indeed, anti-NMDAR autoantibodies may be just one of several risk factors for clinical phenotypes and could clearly work across different diseases. Finally, the current methods that are used to detect autoantibodies have rather low sensitivity and high variability and are non-standardized, which constitute a major issue in comparing studies from different laboratories.
A variety of anti-GluN1 and anti-GluN2 autoantibodies have been reported in patients with diseases ranging from stroke to autism spectrum disorders, but with no known pathophysiological mechanisms (Hammer et al., 2014, Zerche et al., 2015, Steiner et al., 2014, Bokesch et al., 2006). Here, we will focus on those NMDAR-directed autoantibodies that have been well characterized. Perhaps the best characterized anti-iGluR autoantibodies are those targeting GluN1 found in anti-NMDA receptor encephalitis (Dalmau et al., 2007) and those targeting GluN2A and GluN2B in lupus (Diamond et al., 2009).
Anti-NMDA receptor encephalitis
Anti-NMDAR encephalitis is characterized by a prodromal flu-like malaise, followed by acute psychosis, paranoia, seizures, cognitive dysfunction, memory loss, and/or catatonia. The disease often affects women and may arise from ovarian teratomas that express NMDARs, exposing these receptors to the immune system in such a way as to induce formation of autoantibodies (Tuzun et al., 2009, Titulaer et al., 2013). Germline anti-GluN1 antibody-producing B cells and plasma cells that have escaped tolerance checkpoints may also be another cause (Irani et al., 2010, Kreye et al., 2016, Wenke et al., 2019). For some patients, anti-NMDAR encephalitis may also develop as a sequelae of herpes simplex virus (HSV) encephalitis or Toxoplasma gondii infection, but the mechanism is unclear (Pruss et al., 2012, Hacohen et al., 2014, Kannan et al., 2017).
Anti-GluN1 autoantibodies have at least one epitope near the hinge region of the bilobed ATD in GluN1, with critical residues at Asn368 and Gly369 (Kreye et al., 2016, Gleichman et al., 2012). The literature is ambiguous about direct effects of these autoantibodies on NMDAR ion channel function, with some showing no effect while others show a decrease or increase in channel function (Moscato et al., 2014, Castillo-Gomez et al., 2017, Gleichman et al., 2012, Mikasova et al., 2012). The primary disease mechanism is thought to involve impairment in trafficking and internalization of NMDAR (Figure 2). Anti-GluN1 antibody has been shown to decrease synaptic content of NMDARs, leading to a chronic decrease in EPSCs and LTP in the hippocampus after long periods of exposure to the antibody (Moscato et al., 2014, Mikasova et al., 2012, Planaguma et al., 2016). This decrease in synaptic NMDAR content is mediated by the antibody disrupting the interaction between NMDARs and transsynaptic anchoring proteins, such as the EphrinB2 receptor, altering NMDAR surface diffusion dynamics and mediating NMDAR internalization (Figure 2, 3)(Kreye et al., 2016, Hughes et al., 2010, Moscato et al., 2014, Ladepeche et al., 2018, Planaguma et al., 2015, Mikasova et al., 2012, Planaguma et al., 2016). Indeed, in basal condition, surface NMDAR diffuse along dendrite and get anchored within postsynaptic densities through protein-protein interaction. In presence of certain anti-GluN1 autoantibodies, NMDAR are not efficiently retained within postsynaptic areas and become cross-linked in the extrasynaptic compartment (Figure 3). Passive transfer of anti-GluN1 autoantibodies onto murine models have caused behavioral deficits and memory impairment (Planaguma et al., 2015), and in other instances epilepsy without memory deficits (Taraschenko et al., 2019), highlighting the variability of phenotypes. Anti-GluN1 antibodies do not appear to cause apoptosis, promote complement deposition, or increase brain lymphocytic infiltrates, suggesting that most of the pathophysiological effects observed stem from antibody-mediated NMDAR hypofunction (Planaguma et al., 2015).
Figure 3. Heterogeneity of the effects of anti-GluN1 antibodies’ effects on surface NMDAR.
(A) Schematic trace of a single membrane NMDAR diffusing at the surface of a hippocampal neuron. When NMDAR enter into a glutamatergic synapse it can be trapped in nanodomains through interactions with, for instance, intracellular PDZ and/or transsynaptic scaffolds. The receptor can be tracked using a single NP (green disk) coupled to the receptor by a linker.
(B) Schematic representation of the effect of different anti-GluN1 antibodies on the surface dynamics of NMDAR. In the presence of anti-GluN1 antibodies from AE/Psy+ patients, NMDAR are poorly stabilized within synapses and are cross-linked by the antibodies at extrasynaptic locations. These effects were not observed in presence of anti-GluN1 antibodies from healthy individuals or ASD+ patients. The effect of anti-GluN2 antibodies on membrane NMDAR trafficking remains unknown. Abbreviations: NP, nanoparticle (e.g. Quantum Dot); AE, autoimmune encephalitis; Psy+, seropositive patients diagnosed with psychosis; ASD+, seropositive patients diagnosed with Autism Spectrum Disorder (ASD).
Anti-GluN1 autoantibodies are also implicated in spontaneous acute psychosis/schizophrenia cases, which share some clinical features to those found in anti-NMDAR encephalitis (Jezequel et al., 2017, Lennox et al., 2017). The antibodies associated with the development of psychosis decrease synaptic NMDAR content, disrupt EphrinB2 and dopamine receptor interactions, and lead to decreases in hippocampal LTP. The anti-GluN1 autoantibodies associated with psychosis/schizophrenia do not compete with anti-NMDAR encephalitis antibodies, and they do not appear to bind to the same Asn368/Gly369 motif in the ATD (Castillo-Gomez et al., 2017, Jezequel et al., 2017). Interestingly, a few healthy controls in the psychosis studies also express anti-GluN1 autoantibodies that do not compete with patients’ autoantibodies and do not decrease synaptic NMDAR content (Jezequel et al., 2017, Jezequel et al., 2018). Furthermore, anti-GluN1 autoantibodies found in the circulation of patients with autism spectrum disorder, without psychosis, do not alter NMDAR surface dynamics (Grea et al., 2017). As all of the antibodies were isotype-controlled (i.e., all IgG), this would suggest that there may be intrinsic differences in the sample concentration, specific epitope, and/or avidity of the anti-GluN1 autoantibodies. It remains unclear whether anti-GluN1 autoantibodies isolated from patients with anti-NMDAR encephalitis, psychosis or healthy controls demonstrated similar capacity to internalize NMDARs and decrease NMDAR-mediated currents (Castillo-Gomez et al., 2017, Jezequel et al., 2017). Recent studies have suggested that clonal variations in anti-GluN1 autoantibodies could account for intrinsic differences in avidity for the NMDAR (Kreye et al., 2016, Ly et al., 2018). A similar titer of anti-GluN1 antibodies from one patient that elicits a clinical phenotype may not necessarily evoke a similar response in others, contributing to the variation in clinical presentation. Thus, the heterogeneity of anti-GluN1 antibodies found in patients with different neuropsychiatric conditions or healthy donors is likely to produce different molecular and cellular defects. (e.g. altered NMDAR surface dynamics; Figure 3).
Anti-NMDAR encephalitis patients benefit from immunoglobulin-depleting treatments including plasmapheresis and intravenous immunoglobulin (IVIG) (Titulaer et al., 2013). Second-line drugs that specifically target B cells also appear to eliminate symptoms for patients that are refractory to steroids and first-line immunosuppressants. Given that NMDAR hypofunction is implicated as a mechanistic feature of disease, the use of positive allosteric modulators (PAMs) has been explored in experimental anti-NMDAR encephalitis models with some recovery of synaptic function (Warikoo et al., 2018, Mannara et al., 2020). The development of new therapeutical strategies to directly control the receptor trafficking, and not the ionotropic function, will be of great interest as the removal of anti-GluN1 antibodies by immunotherapies is rather slow and as a low-titer of anti-GluN1 antibodies is suspected in patients following recovery or in purely psychiatric conditions. Thus, the investigation into anti-GluN1 autoantibody pathophysiology has guided exploration of treatment strategies with potential therapeutic benefit.
Anti-NMDA receptor autoantibodies in systemic lupus erythematosus
Systemic lupus erythematosus (SLE) or lupus is an autoimmune disease that disproportionally affects women and minorities (Reveille et al., 1998, Maningding et al., 2019). Lupus patients experience a highly diverse array of symptoms, including renal, cutaneous, neurologic and psychiatric dysfunctions. Nervous system-related manifestations can be classified as neuropsychiatric lupus (NPSLE) (Schwartz et al., 2019). Patients also experience subtle cognitive dysfunction such as spatial memory deficits that do not fall under the strict standardized ‘case definitions’ for NPSLE by the American College of Rheumatology (Rayes et al., 2018, Hanly et al., 2019, Kello et al., 2019).
Anti-NMDAR autoantibodies were first described in CSF samples from a SLE patient with declining cognitive function (DeGiorgio et al., 2001). These antibodies were originally identified as binding double-stranded DNA (dsDNA), with anti-dsDNA antibodies a hallmark of SLE (Tsokos, 2011), and recognized a short peptide epitope, the “DWEYS” motif. This pentapeptide consensus sequence is also found in the hinge region of the ATD in the GluN2A and GluN2B subunits, where it is DWDYS in GluN2A and EWDYG in GluN2B. (Tsokos, 2011)Given that these antibodies bound anti-dsDNA and NMDARs, they were later designated as “DNRAbs” (DNA and NMDAR-reactive antibodies) to distinguish them from other anti-GluN2 autoantibodies in SLE (Husebye et al., 2005, Chang et al., 2015, Nestor et al., 2018, Tay et al., 2017). DNRAbs promote cell death through enhancing NMDAR activity (DeGiorgio et al., 2001, Faust et al., 2010, Gono et al., 2011, Chan et al., 2020).
Anti-GluN2 antibodies associated with SLE were discovered before anti-NMDAR encephalitis antibodies (Omdal et al., 2005, Husebye et al., 2005, DeGiorgio et al., 2001). DNRAbs are expressed by 30-40% of lupus patients (Tay et al., 2017). Because nervous system assessments are not standardized in lupus, the prevalence of brain dysfunction varies between 20-90%, depending on the test employed (Hanly et al., 2010, Unterman et al., 2011, Borowoy et al., 2012, Schwartz et al., 2019). Still, anti-NMDAR autoantibodies have been significantly correlated with numerous brain dysfunctions, including spatial memory deficits (Chang et al., 2015, Mackay et al., 2019), acute confusion (Hirohata et al., 2014b), cognitive fatigue (Schwarting et al., 2019), and seizure disorders (Yang et al., 2017).
There have been discrepancies regarding serum samples from SLE patients, with serum samples often showing no significant correlation between anti-GluN2 antibodies and neuropsychiatric disease in SLE (Hanly et al., 2006, Harrison et al., 2006, Petri et al., 2010). However, CSF samples from SLE patients do demonstrate a correlation between anti-GluN2 antibodies and neuropsychiatric disease (Arinuma et al., 2008, Fragoso-Loyo et al., 2008, Hirohata et al., 2014a, Lauvsnes et al., 2014), suggesting that the status of the blood brain barrier (or blood-CSF barrier) may be important for determining whether the pathogenic anti-GluN2 antibodies can affect the brain. SLE patients with neuropsychiatric disease also benefit from immunoglobulin depletion and B-cell targeting drugs but the exact mechanism is unclear since SLE is a multifactorial and complex disease with many other autoantibodies and systemic inflammatory processes occurring beyond that of anti-GluN2 antibodies (Milstone et al., 2005, Lim et al., 2010).
Effect of DNRAbs in experimental models.
Because of the well-defined epitope in the GluN2 subunits (DWEYS), a variety of approaches have been employed to study the mechanism of anti-GluN2 autoantibodies in causing symptoms. The first study of DNRAbs employed passive transfer of human SLE antibodies from CSF into mice and onto primary neuronal cultures (DeGiorgio et al., 2001). These DNRAbs were isolated from patient CSF using affinity chromatography with a DWEYS-peptide conjugated column. DNRAbs caused neuronal apoptosis, but neurons were protected when antibodies were applied with the high-affinity NMDAR channel blocker MK-801. DNRAbs eluted from postmortem brains of SLE patients with cognitive impairment also caused neuronal apoptosis in the hippocampal CA1 region (Kowal et al., 2006).
Mouse models that endogenously generate DNRAbs were created by immunizing with the DWEYS peptide, and then administering lipopolysaccharide (LPS) to induce systemic inflammation and permeabilize the blood brain barrier (Figure 4)(Chang et al., 2015, Kowal et al., 2004). Without LPS, mice with circulating DNRAbs (DNRAb+) do not evidence hippocampal cell death. In contrast, following LPS treatment, DNRAb+ mice display reduced neuronal numbers in the hippocampal CA1 region along with increased apoptotic cells (Kowal et al., 2006). If epinephrine is used in place of LPS to induce BBB breakdown, the amygdala becomes the central target of DNRAbs; it is not clear why epinephrine and LPS differentially localize DNRAbs to different parts of the brain (Huerta et al., 2006). What is clear is that in SLE patients, there are microstructural defects in the hippocampus of patients with cognitive dysfunction and neuropsychiatric symptoms (Appenzeller et al., 2006, Lauvsnes et al., 2014, Mackay et al., 2019). Thus, DWEYS immunization followed by LPS treatment is a mouse model used to further study the role of DNRAbs in hippocampal and cognitive dysfunction in SLE. Interestingly, the B6.Nba2 SLE-prone mouse model develops autoantibodies that are reactive to DWEYS, which may be used to elucidate the role of anti-GluN2 antibodies in a SLE-prone milieu (Browne et al., 2021). The availability of monoclonal DNRAbs developed from SLE patients has circumvented the issue of limited patient antibody samples to significantly enable such mechanistic studies (Zhang et al., 2009). Indeed, the availability of monoclonal antibodies offers numerous advantages including being able to define concentrations and exclude the effects from other antibodies and other molecules commonly found in human bodily fluids.
Figure 4. Two phases of DNRAb-induced pathology in a mouse model.
(A) At time 0, mice are inoculated either with the DWEYS decapeptide, which is a mimetope of dsDNA, multimerized on a polylysine backbone (MAP-DWEYS) or with the backbone alone (MAP). Immunization of wildtype mice with MAP-DWEYS induces production of DNRAbs (DNRAb+ mice) whereas MAP alone (control) does not (Putterman and Diamond, 1998).
(B) Two weeks later, mice are given lipopolysaccharide (LPS) to allow transient access of antibodies (Ab) to the hippocampus (Kowal et al., 2004, Nestor et al., 2018).
(C) One week after LPS, DNRAbs are still present in the hippocampus (acute phase) (Kowal et al., 2004, Chang et al., 2015) and CA1 pyramidal neurons show enhanced cell death (Kowal et al., 2004, Faust et al., 2010), due to GluN2A-mediated NMDAR excitotoxicity (Chan et al., 2020).
(D) Two weeks post-LPS, DNRAb levels are not detectable (chronic phase) (Chang et al., 2015). Eight weeks post LPS, mice show microglia activation, reduced CA1 dendritic complexity, reduced spatial memory and expanded place fields (Kowal et al., 2004, Chang et al., 2015, Nestor et al., 2018), with all effects GluN2A-dependent (Chan et al., 2020). Microglia activation is dependent on recruitment of C1q (Nestor et al., 2018).
The murine LPS/DNRAb+ models as well as DNRAb monoclonal antibodies have provided insights into the mechanisms and functional effects of DNRAbs. The CSF concentrations of DNRAbs in a cohort of SLE patients with neuropsychiatric dysfunction was approximately 30 – 180 μg/mL (median: ~70 μg/mL) (Faust et al., 2010). G11 is a monoclonal antibody derived from a lupus patient that specifically binds to GluN2A- or GluN2B-containing NMDARs. At clinically relevant concentrations, G11 acutely increased NMDAR field EPSPs and caused NMDAR-dependent cell death in the hippocampus, which could be prevented by NMDAR antagonists (Faust et al., 2010). Using the LPS/DNRAb+ model, chronic changes in the brain were observed including decreased hippocampal dendritic complexity, decreased object-place memory discrimination, and hippocampal place field expansion (Chang et al., 2015). DNRAbs require complement immune response (C1q deposition) to mediate these chronic changes, but not for inducing acute neuronal cell death (Nestor et al., 2018).
Notably, by testing G11 directly against NMDAR subunits expressed heterologously, G11 was found to act as a positive allosteric modulator (PAM) at NMDARs, enhancing the gating action of glutamate, and that this effect is nearly 100-fold more efficacious at GluN2A-containing receptors than at those only containing GluN2B subunit (Chan et al., 2020). This allostery occurs through the DWEYS motif, requires only a single GluN2A subunit to induce its full effect, and leads to NMDAR-mediated cell death since it is blocked by GluN2A-specific antagonists. Indeed, in the murine model, the deficits associated with DNRAbs were blocked in GluN2A, but not GluN2B −/− mice, indicating that most of the pathology in vivo is associated with the GluN2A subunit (Figure 4)(Chan et al., 2020). While the basis for this positive allostery remains unknown, it may act in part by counteracting the negative allostery induced by Zn2+ in GluN2A-containing subunits (Figure 5).
Figure 5. Possible mechanism of positive allostery of DNRAbs on GluN2A-containing NMDARs.
(A) Individual domains within a GluN2A subunit. DWEYS is a mimetope of dsDNA and is the major binding site for DNRAbs. Model structure of 4TLM (Amin et al., 2017).
(B) Zn2+ acts as a negative allosteric modulator of GluN2A-containing NMDARs by inducing clam-shell closure of the ATD. The DWEYS motif is at the hinge of the ATD clam-shell and DNRAb binding may potentiate currents by forcing open the clam-shell.
Synaptic currents recorded from a CA1 pyramidal neuron with Schaffer collateral stimulation. Currents recorded at −70 mV in a solution containing no added Mg2+ (LPW, unpublished data).
Future challenges
Despite considerable advances in terms of describing anti-iGluR autoantibodies and identifying potential disease pathways (Table 1; Figure 2), we still lack an understanding of how these classes of autoantibodies lead to their clinical phenotype. In addition, and as noted above, there remains uncertainty as our capacity to detect known, and obviously unknown, autoantibodies and how they contribute to disease progression. This is especially true when considering the diversity of clinical phenotypes associated within any one class. We discuss below several key issues for future considerations.
Clonal variations.
While it is easy to classify anti-NMDAR autoantibodies into simple categories, anti-NMDAR encephalitis or DNRAbs, this classification ignores the inherit diversity of antibodies arising from clonal variation, which are small variations in the complementarity determining regions.
In SLE patients, the clinical manifestation of brain dysfunction expressing DNRAbs is diverse (Tay et al., 2017, Schwartz et al., 2019). This diversity presumably has many origins – extent of break-down of the blood-brain barrier and production of brain reactive antibodies (BRA) in addition to DNRAbs (Kivity et al., 2015, Schwartz et al., 2019). Nevertheless, a key feature may be that diverse DNRAbs from different patients, while identified by their DWEYS binding (DeGiorgio et al., 2001, Kowal et al., 2006, Tay et al., 2017), show clonal variation – that is they have small variations in the complementarity determining regions of IgGH – which in turn lead to variations in the magnitude of their functional effects. Clonal variation is common (Dalmau et al., 2017), and DNRAbs from different patients show differential patterns of binding to kidney and brain antigens (Zhang et al., 2009) and differences in affinity for dsDNA and pathogenicity (Katz et al., 1994). Still, how diverse DNRAbs affect NMDAR-mediated signaling and hence brain dysfunction is completely unknown. The issue of clonal variations also occurs in anti-GluN1 autoantibodies and could account for intrinsic differences in avidity for the NMDAR (Kreye et al., 2016, Ly et al., 2018). Refining the view of clonal variation and how this diversity impacts synaptic function will provide a foundation for personalized medicine for patients with anti-iGluR autoantibodies. Notable in this regard is the development of monoclonal antibodies for different variants, which would allow more precise quantification of differences in action.
Circuit functions.
One of the great challenges is that anti-NMDAR autoantibodies are often studied in isolation typically on pyramidal neurons (Hunter et al., 2021). Yet, interneurons are likely to be involved into the disease mechanisms of anti-NMDAR autoantibodies action, both into the psychiatric presentation and seizures. Recent investigations have suggested that NMDAR hypofunction, specifically on fast-spiking interneuron populations, may be a key driver of psychosis phenotypes. In the presence of anti-GluN antibodies, one may speculate that antibody-induced receptor hypofunction on interneurons is a key mechanism for the generation of psychotic symptoms and seizures (Hunter et al., 2021). A key question will then be to precisely define how a given anti-GluN antibody target and act on NMDAR located at the surface of principal cells and interneurons (as well as non-neuronal cells). NMDAR subunit composition, the functional role of synaptic and extrasynaptic NMDARs, the accessibility of antibodies to the receptor (i.e., interneurons are surrounded by perineuronal net) is different between interneurons and principal cells and may thus constitute the basis for the differential impact of anti-GluN antibodies onto these cell populations. In addition, NMDARs are present on glial cells, including astrocytic processes and endothelial cells and these may also constitute an additional cellular target that will have profound network effects. Finally, the NMDAR is expressed throughout the whole nervous system with striking difference in its subunit composition, developmental expression and synaptic content (Paoletti et al., 2013). It is possible that anti-GluN antibodies differentially affect NMDAR functions in various brain areas across life. Decrypting, for instance, the mechanism through which certain anti-GluN antibodies mainly target limbic NMDAR will be of prime interest.
Transitions from acute to long-term effects.
Neurons are exposed to anti-NMDAR autoantibodies typically only transiently and have acute effects on NMDAR-mediated signaling, either hypofunction (anti-NMDAR encephalitis) or hyperfunction (DNRAbs), but mechanisms regulating the transition from acute to long-term outcomes remain poorly defined. This information is critical to devise treatments for patients at different pathological stages.
As an example of potential complexity let’s consider anti-GluN2 autoantibodies in SLE. Transient exposure of the hippocampus to DNRAbs in SLE leads to enhanced cell death but surviving neurons undergo microglia-dependent dendritic pruning and a presumed associated decrease in spatial memory (Figure 4). The complement factor C1q is required for DNRAb-mediated microglia recruitment to prune dendrites and reduce dendritic complexity (Nestor et al., 2018). DNRAb-mediated enhancement of NMDAR activity may release high mobility group box 1 (HMGB1), which is often released under conditions of cellular stress, and HMGB1 may recruit C1q to dendrites (Son et al., 2016, Nestor et al., 2018). However, HMGB1 preferentially interacts with GluN2B (Pedrazzi et al., 2012), yet GluN2B is not required for DNRAb-mediated microglia activation and dendritic pruning, which is solely GluN2A-dependent (Figure 4) (Chan et al., 2020). So how does C1q get recruited to dendrites? One possibility is that anti-GluN2 autoantibodies, in addition to acting as positive allosteric modulators, may cross-link NMDARs with the proximity of the Ab-antigen complexes enhancing C1q recruitment, as occurs for anti-aquaporin 4 autoantibodies in neuromyelitis optica (Soltys et al., 2019).
A related issue is the significance of anti-NMDAR-induced hypofunction or hyperfunction on gene expression. NMDARs signaling regulates gene expression (Chen et al., 2007, Tamminga and Zukin, 2015) including their own expression (Snyder and Gao, 2020) with the specific action often depending on whether the receptors are synaptic or extrasynaptic (Vanhoutte and Bading, 2003). The altered signaling induced by anti-NMDAR autoantibodies, whether occurring at synaptic or at extrasynaptic sites (Figure 2), presumably would have long-term consequences on gene expression, which might change the whole profile of the affected cell. Nevertheless, the long-term consequences of transient anti-NMDAR autoantibody exposure on neurons and glia remain unknown and unexplored.
Conclusion
Overall, it emerges that anti-GluN autoantibodies impact the NMDAR signaling through different ways: pushing them away from synapse and cross-linking them in the extrasynaptic compartment and modulating their ionotropic transmission. Yet, our understanding of the mechanism of action of these antibodies is still in its infancy. Defining the molecular, cellular, and network effects of the antibodies will certainly shed new and unprecedented lights on the basis of neuropsychiatric and neurological disorders. Translational efforts, combining in-depth multiscale investigations and clinical characterization, should thus be strongly encouraged.
Funding.
This work was supported by National Institute of Health grants NRSA F30MH115618 (KC) and R01 NS088479 (LPW) and by the Centre National de la Recherche Scientifique, Agence Nationale de la Recherche, EraNet Neuron Mental Disorders Program, and Fondation pour la Recherche Médicale. (LG).
References
- Amin JB, Leng X, Gochman A, Zhou HX & Wollmuth LP 2018. A conserved glycine harboring disease-associated mutations permits NMDA receptor slow deactivation and high Ca(2+) permeability. Nat Commun, 9, 3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin JB, Moody GR & Wollmuth LP 2021. From bedside-to-bench: What disease-associated variants are teaching us about the NMDA receptor. J Physiol, 599, 397–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin JB, Salussolia CL, Chan K, Regan MC, Dai J, Zhou HX, Furukawa H, Bowen ME & Wollmuth LP 2017. Divergent roles of a peripheral transmembrane segment in AMPA and NMDA receptors. J Gen Physiol, 149, 661–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenzeller S, Carnevalle AD, Li LM, Costallat LT & Cendes F 2006. Hippocampal atrophy in systemic lupus erythematosus. Ann Rheum Dis, 65, 1585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arinuma Y, Yanagida T & Hirohata S 2008. Association of cerebrospinal fluid anti-NR2 glutamate receptor antibodies with diffuse neuropsychiatric systemic lupus erythematosus. Arthritis Rheum, 58, 1130–5. [DOI] [PubMed] [Google Scholar]
- Armstrong N & Gouaux E 2000. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron, 28, 165–81. [DOI] [PubMed] [Google Scholar]
- Bekkers JM & Stevens CF 1989. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature, 341, 230–3. [DOI] [PubMed] [Google Scholar]
- Bokesch PM, Izykenova GA, Justice JB, Easley KA & Dambinova SA 2006. NMDA receptor antibodies predict adverse neurological outcome after cardiac surgery in high-risk patients. Stroke, 37, 1432–6. [DOI] [PubMed] [Google Scholar]
- Borowoy AM, Pope JE, Silverman E, Fortin PR, Pineau C, Smith CD, Arbillaga H, Gladman D, Urowitz M, Zummer M, Hudson M, Tucker L & Peschken C 2012. Neuropsychiatric lupus: the prevalence and autoantibody associations depend on the definition: results from the 1000 faces of lupus cohort. Semin Arthritis Rheum, 42, 179–85. [DOI] [PubMed] [Google Scholar]
- Browne K, Zhang E, Sullivan JK, Evonuk KS, Desilva TM & Jorgensen TN 2021. Lupus-prone B6.Nba2 male and female mice display anti-DWEYS reactivity and a neuropsychiatric phenotype. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Gomez E, Oliveira B, Tapken D, Bertrand S, Klein-Schmidt C, Pan H, Zafeiriou P, Steiner J, Jurek B, Trippe R, Pruss H, Zimmermann WH, Bertrand D, Ehrenreich H & Hollmann M 2017. All naturally occurring autoantibodies against the NMDA receptor subunit NR1 have pathogenic potential irrespective of epitope and immunoglobulin class. Mol Psychiatry, 22, 1776–1784. [DOI] [PubMed] [Google Scholar]
- Chan K, Nestor J, Huerta TS, Certain N, Moody G, Kowal C, Huerta PT, Volpe BT, Diamond B & Wollmuth LP 2020. Lupus autoantibodies act as positive allosteric modulators at GluN2A-containing NMDA receptors and impair spatial memory. Nat Commun, 11, 1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EH, Volpe BT, Mackay M, Aranow C, Watson P, Kowal C, Storbeck J, Mattis P, Berlin R, Chen H, Mader S, Huerta TS, Huerta PT & Diamond B 2015. Selective Impairment of Spatial Cognition Caused by Autoantibodies to the N-Methyl-D-Aspartate Receptor. EBioMedicine, 2, 755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, He S, Hu XL, Yu J, Zhou Y, Zheng J, Zhang S, Zhang C, Duan WH & Xiong ZQ 2007. Differential roles of NR2A- and NR2B-containing NMDA receptors in activity-dependent brain-derived neurotrophic factor gene regulation and limbic epileptogenesis. J Neurosci, 27, 542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW 2020. Excitotoxicity: Still Hammering the Ischemic Brain in 2020. Front Neurosci, 14, 579953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT 2017. Schizophrenia: Basic and Clinical. Adv Neurobiol, 15, 255–280. [DOI] [PubMed] [Google Scholar]
- Dalmau J, Geis C & Graus F 2017. Autoantibodies to Synaptic Receptors and Neuronal Cell Surface Proteins in Autoimmune Diseases of the Central Nervous System. Physiol Rev, 97, 839–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmau J, Tuzun E, Wu HY, Masjuan J, Rossi JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, Mason W, Sansing LH, Dichter MA, Rosenfeld MR & Lynch DR 2007. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol, 61, 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT & Diamond B 2001. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med, 7, 1189–93. [DOI] [PubMed] [Google Scholar]
- Diamond B, Huerta PT, Mina-Osorio P, Kowal C & Volpe BT 2009. Losing your nerves? Maybe it's the antibodies. Nat Rev Immunol, 9, 449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H 2018. Autoantibodies against N-methyl-d-aspartate receptor 1 in health and disease. Curr Opin Neurol, 31, 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust TW, Chang EH, Kowal C, Berlin R, Gazaryan IG, Bertini E, Zhang J, Sanchez-Guerrero J, Fragoso-Loyo HE, Volpe BT, Diamond B & Huerta PT 2010. Neurotoxic lupus autoantibodies alter brain function through two distinct mechanisms. Proc Natl Acad Sci U S A, 107, 18569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoso-Loyo H, Cabiedes J, Orozco-Narvaez A, Davila-Maldonado L, Atisha-Fregoso Y, Diamond B, Llorente L & Sanchez-Guerrero J 2008. Serum and cerebrospinal fluid autoantibodies in patients with neuropsychiatric lupus erythematosus. Implications for diagnosis and pathogenesis. PLoS One, 3, e3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Recio A, Santos-Gomez A, Soto D, Julia-Palacios N, Garcia-Cazorla A, Altafaj X & Olivella M 2020. GRIN database: A unified and manually curated repertoire of GRIN variants. Hum Mutat. [DOI] [PubMed] [Google Scholar]
- Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM & Verschuuren J 2019. Myasthenia gravis. Nat Rev Dis Primers, 5, 30. [DOI] [PubMed] [Google Scholar]
- Gleichman AJ, Spruce LA, Dalmau J, Seeholzer SH & Lynch DR 2012. Anti-NMDA receptor encephalitis antibody binding is dependent on amino acid identity of a small region within the GluN1 amino terminal domain. J Neurosci, 32, 11082–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves J, Bartol TM, Camus C, Levet F, Menegolla AP, Sejnowski TJ, Sibarita JB, Vivaudou M, Choquet D & Hosy E 2020. Nanoscale co-organization and coactivation of AMPAR, NMDAR, and mGluR at excitatory synapses. Proc Natl Acad Sci U S A, 117, 14503–14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gono T, Takarada T, Fukumori R, Kawaguchi Y, Kaneko H, Hanaoka M, Katsumata Y, Yoneda Y & Yamanaka H 2011. NR2-reactive antibody decreases cell viability through augmentation of Ca(2+) influx in systemic lupus erythematosus. Arthritis Rheum, 63, 3952–9. [DOI] [PubMed] [Google Scholar]
- Grea H, Scheid I, Gaman A, Rogemond V, Gillet S, Honnorat J, Bolognani F, Czech C, Bouquet C, Toledano E, Bouvard M, Delorme R, Groc L & Leboyer M 2017. Clinical and autoimmune features of a patient with autism spectrum disorder seropositive for anti-NMDA-receptor autoantibody. Dialogues Clin Neurosci, 19, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L & Choquet D 2020. Linking glutamate receptor movements and synapse function. Science, 368. [DOI] [PubMed] [Google Scholar]
- Groc L, Heine M, Cognet L, Brickley K, Stephenson FA, Lounis B & Choquet D 2004. Differential activity-dependent regulation of the lateral mobilities of AMPA and NMDA receptors. Nat Neurosci, 7, 695–6. [DOI] [PubMed] [Google Scholar]
- Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L & Choquet D 2006. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci U S A, 103, 18769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacohen Y, Deiva K, Pettingill P, Waters P, Siddiqui A, Chretien P, Menson E, Lin JP, Tardieu M, Vincent A & Lim MJ 2014. N-methyl-D-aspartate receptor antibodies in post-herpes simplex virus encephalitis neurological relapse. Mov Disord, 29, 90–6. [DOI] [PubMed] [Google Scholar]
- Hammer C, Stepniak B, Schneider A, Papiol S, Tantra M, Begemann M, Siren AL, Pardo LA, Sperling S, Mohd Jofrry S, Gurvich A, Jensen N, Ostmeier K, Luhder F, Probst C, Martens H, Gillis M, Saher G, Assogna F, Spalletta G, Stocker W, Schulz TF, Nave KA & Ehrenreich H 2014. Neuropsychiatric disease relevance of circulating anti-NMDA receptor autoantibodies depends on blood-brain barrier integrity. Mol Psychiatry, 19, 1143–9. [DOI] [PubMed] [Google Scholar]
- Hanly JG, Kozora E, Beyea SD & Birnbaum J 2019. Review: Nervous System Disease in Systemic Lupus Erythematosus: Current Status and Future Directions. Arthritis Rheumatol, 71, 33–42. [DOI] [PubMed] [Google Scholar]
- Hanly JG, Robichaud J & Fisk JD 2006. Anti-NR2 glutamate receptor antibodies and cognitive function in systemic lupus erythematosus. J Rheumatol, 33, 1553–8. [PubMed] [Google Scholar]
- Hanly JG, Urowitz MB, Su L, Bae SC, Gordon C, Wallace DJ, Clarke A, Bernatsky S, Isenberg D, Rahman A, Alarcon GS, Gladman DD, Fortin PR, Sanchez-Guerrero J, Romero-Diaz J, Merrill JT, Ginzler E, Bruce IN, Steinsson K, Khamashta M, Petri M, Manzi S, Dooley MA, Ramsey-Goldman R, Van Vollenhoven R, Nived O, Sturfelt G, Aranow C, Kalunian K, Ramos-Casals M, Zoma A, Douglas J, Thompson K, Farewell V & Systemic Lupus International Collaborating, C. 2010. Prospective analysis of neuropsychiatric events in an international disease inception cohort of patients with systemic lupus erythematosus. Ann Rheum Dis, 69, 529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Yi F, Perszyk RE, Furukawa H, Wollmuth LP, Gibb AJ & Traynelis SF 2018. Structure, function, and allosteric modulation of NMDA receptors. J Gen Physiol, 150, 1081–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Yi F, Perszyk RE, Menniti FS & Traynelis SF 2017. NMDA Receptors in the Central Nervous System. Methods Mol Biol, 1677, 1–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham G 2019. NMDA receptor C-terminal signaling in development, plasticity, and disease. F1000Res, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE & Do KQ 2016. Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat Rev Neurosci, 17, 125–34. [DOI] [PubMed] [Google Scholar]
- Harrison MJ, Ravdin LD & Lockshin MD 2006. Relationship between serum NR2a antibodies and cognitive dysfunction in systemic lupus erythematosus. Arthritis Rheum, 54, 2515–22. [DOI] [PubMed] [Google Scholar]
- Herring BE & Nicoll RA 2016. Long-Term Potentiation: From CaMKII to AMPA Receptor Trafficking. Annu Rev Physiol, 78, 351–65. [DOI] [PubMed] [Google Scholar]
- Hiester BG, Becker MI, Bowen AB, Schwartz SL & Kennedy MJ 2018. Mechanisms and Role of Dendritic Membrane Trafficking for Long-Term Potentiation. Front Cell Neurosci, 12, 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirohata S, Arinuma Y, Yanagida T & Yoshio T 2014a. Blood-brain barrier damages and intrathecal synthesis of anti-N-methyl-D-aspartate receptor NR2 antibodies in diffuse psychiatric/neuropsychological syndromes in systemic lupus erythematosus. Arthritis Res Ther, 16, R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirohata S, Sakuma Y, Yanagida T & Yoshio T 2014b. Association of cerebrospinal fluid anti-Sm antibodies with acute confusional state in systemic lupus erythematosus. Arthritis Res Ther, 16, 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Chen W, Myers SJ, Yuan H & Traynelis SF 2016. Human GRIN2B variants in neurodevelopmental disorders. J Pharmacol Sci, 132, 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta PT, Kowal C, Degiorgio LA, Volpe BT & Diamond B 2006. Immunity and behavior: antibodies alter emotion. Proc Natl Acad Sci U S A, 103, 678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir RL & Nicoll RA 2013. AMPARs and synaptic plasticity: the last 25 years. Neuron, 80, 704–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Peng X, Gleichman AJ, Lai M, Zhou L, Tsou R, Parsons TD, Lynch DR, Dalmau J & Balice-Gordon RJ 2010. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci, 30, 5866–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D, Jamet Z & Groc L 2021. Autoimmunity and NMDA receptor in brain disorders: Where do we stand? Neurobiol Dis, 147, 105161. [DOI] [PubMed] [Google Scholar]
- Husebye ES, Sthoeger ZM, Dayan M, Zinger H, Elbirt D, Levite M & Mozes E 2005. Autoantibodies to a NR2A peptide of the glutamate/NMDA receptor in sera of patients with systemic lupus erythematosus. Ann Rheum Dis, 64, 1210–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani SR, Bera K, Waters P, Zuliani L, Maxwell S, Zandi MS, Friese MA, Galea I, Kullmann DM, Beeson D, Lang B, Bien CG & Vincent A 2010. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain, 133, 1655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezequel J, Johansson EM, Dupuis JP, Rogemond V, Grea H, Kellermayer B, Hamdani N, Le Guen E, Rabu C, Lepleux M, Spatola M, Mathias E, Bouchet D, Ramsey AJ, Yolken RH, Tamouza R, Dalmau J, Honnorat J, Leboyer M & Groc L 2017. Dynamic disorganization of synaptic NMDA receptors triggered by autoantibodies from psychotic patients. Nat Commun, 8, 1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezequel J, Lepleux M, Kahn RS, Honnorat J, Leboyer M & Groc L 2018. Molecular Pathogenicity of Anti-NMDA Receptor Autoantibody From Patients With First-Episode Psychosis. Am J Psychiatry, 175, 382–383. [DOI] [PubMed] [Google Scholar]
- Kannan G, Gressitt KL, Yang S, Stallings CR, Katsafanas E, Schweinfurth LA, Savage CLG, Adamos MB, Sweeney KM, Origoni AE, Khushalani S, Bahn S, Leweke FM, Dickerson FB, Yolken RH, Pletnikov MV & Severance EG 2017. Pathogen-mediated NMDA receptor autoimmunity and cellular barrier dysfunction in schizophrenia. Transl Psychiatry, 7, e1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia M, Bijelic D, Zhao H, Ma D, Stojanovich L, Milosevic M, Andjus P & Sakic B 2017. Effects of sustained i.c.v. infusion of lupus CSF and autoantibodies on behavioral phenotype and neuronal calcium signaling. Acta Neuropathol Commun, 5, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JB, Limpanasithikul W & Diamond B 1994. Mutational analysis of an autoantibody: differential binding and pathogenicity. J Exp Med, 180, 925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazi R, Dai J, Sweeney C, Zhou HX & Wollmuth LP 2014. Mechanical coupling maintains the fidelity of NMDA receptor-mediated currents. Nat Neurosci, 17, 914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermayer B, Ferreira JS, Dupuis J, Levet F, Grillo-Bosch D, Bard L, Linares-Loyez J, Bouchet D, Choquet D, Rusakov DA, Bon P, Sibarita JB, Cognet L, Sainlos M, Carvalho AL & Groc L 2018. Differential Nanoscale Topography and Functional Role of GluN2-NMDA Receptor Subtypes at Glutamatergic Synapses. Neuron, 100, 106–119 e7. [DOI] [PubMed] [Google Scholar]
- Kello N, Anderson E & Diamond B 2019. Cognitive Dysfunction in Systemic Lupus Erythematosus: A Case for Initiating Trials. Arthritis Rheumatol, 71, 1413–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivity S, Agmon-Levin N, Zandman-Goddard G, Chapman J & Shoenfeld Y 2015. Neuropsychiatric lupus: a mosaic of clinical presentations. BMC Med, 13, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB & Seeburg PH 1995. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science, 269, 1737–40. [DOI] [PubMed] [Google Scholar]
- Kowal C, Degiorgio LA, Lee JY, Edgar MA, Huerta PT, Volpe BT & Diamond B 2006. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc Natl Acad Sci U S A, 103, 19854–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal C, Degiorgio LA, Nakaoka T, Hetherington H, Huerta PT, Diamond B & Volpe BT 2004. Cognition and immunity; antibody impairs memory. Immunity, 21, 179–88. [DOI] [PubMed] [Google Scholar]
- Kreye J, Wenke NK, Chayka M, Leubner J, Murugan R, Maier N, Jurek B, Ly LT, Brandl D, Rost BR, Stumpf A, Schulz P, Radbruch H, Hauser AE, Pache F, Meisel A, Harms L, Paul F, Dirnagl U, Garner C, Schmitz D, Wardemann H & Pruss H 2016. Human cerebrospinal fluid monoclonal N-methyl-D-aspartate receptor autoantibodies are sufficient for encephalitis pathogenesis. Brain, 139, 2641–2652. [DOI] [PubMed] [Google Scholar]
- Ladepeche L, Planaguma J, Thakur S, Suarez I, Hara M, Borbely JS, Sandoval A, Laparra-Cuervo L, Dalmau J & Lakadamyali M 2018. NMDA Receptor Autoantibodies in Autoimmune Encephalitis Cause a Subunit-Specific Nanoscale Redistribution of NMDA Receptors. Cell Rep, 23, 3759–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauvsnes MB, Beyer MK, Kvaloy JT, Greve OJ, Appenzeller S, Kvivik I, Harboe E, Tjensvoll AB, Goransson LG & Omdal R 2014. Association of hippocampal atrophy with cerebrospinal fluid antibodies against the NR2 subtype of the N-methyl-D-aspartate receptor in patients with systemic lupus erythematosus and patients with primary Sjogren's syndrome. Arthritis Rheumatol, 66, 3387–94. [DOI] [PubMed] [Google Scholar]
- Leboyer M, Berk M, Yolken RH, Tamouza R, Kupfer D & Groc L 2016. Immuno-psychiatry: an agenda for clinical practice and innovative research. BMC Med, 14, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennox BR, Palmer-Cooper EC, Pollak T, Hainsworth J, Marks J, Jacobson L, Lang B, Fox H, Ferry B, Scoriels L, Crowley H, Jones PB, Harrison PJ, Vincent A & Team PPS 2017. Prevalence and clinical characteristics of serum neuronal cell surface antibodies in first-episode psychosis: a case-control study. Lancet Psychiatry, 4, 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levite M 2014. Glutamate receptor antibodies in neurological diseases: anti-AMPA-GluR3 antibodies, anti-NMDA-NR1 antibodies, anti-NMDA-NR2A/B antibodies, anti-mGluR1 antibodies or anti-mGluR5 antibodies are present in subpopulations of patients with either: epilepsy, encephalitis, cerebellar ataxia, systemic lupus erythematosus (SLE) and neuropsychiatric SLE, Sjogren's syndrome, schizophrenia, mania or stroke. These autoimmune anti-glutamate receptor antibodies can bind neurons in few brain regions, activate glutamate receptors, decrease glutamate receptor's expression, impair glutamate-induced signaling and function, activate blood brain barrier endothelial cells, kill neurons, damage the brain, induce behavioral/psychiatric/cognitive abnormalities and ataxia in animal models, and can be removed or silenced in some patients by immunotherapy. J Neural Transm (Vienna), 121, 1029–75. [DOI] [PubMed] [Google Scholar]
- Lim KS, Cheong KL & Tan CT 2010. Periodic lateralized epileptiform discharges in neuropsychiatric lupus: association with cerebritis in magnetic resonance imaging and resolution after intravenous immunoglobulin. Lupus, 19, 748–52. [DOI] [PubMed] [Google Scholar]
- Lussier MP, Sanz-Clemente A & Roche KW 2015. Dynamic Regulation of N-Methyl-d-aspartate (NMDA) and alpha-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) Receptors by Posttranslational Modifications. J Biol Chem, 290, 28596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly LT, Kreye J, Jurek B, Leubner J, Scheibe F, Lemcke J, Wenke NK, Reincke SM & Pruss H 2018. Affinities of human NMDA receptor autoantibodies: implications for disease mechanisms and clinical diagnostics. J Neurol, 265, 2625–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay M, Vo A, Tang CC, Small M, Anderson EW, Ploran EJ, Storbeck J, Bascetta B, Kang S, Aranow C, Sartori C, Watson P, Volpe BT, Diamond B & Eidelberg D 2019. Metabolic and microstructural alterations in the SLE brain correlate with cognitive impairment. JCI Insight, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maningding E, Dall'era M, Trupin L, Murphy LB & Yazdany J 2019. Racial/Ethnic Differences in Prevalence of and Time to Onset of SLE Manifestations: The California Lupus Surveillance Project (CLSP). Arthritis Care Res (Hoboken). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannara F, Radosevic M, Planaguma J, Soto D, Aguilar E, Garcia-Serra A, Maudes E, Pedreno M, Paul S, Doherty J, Quirk M, Dai J, Gasull X, Lewis M & Dalmau J 2020. Allosteric modulation of NMDA receptors prevents the antibody effects of patients with anti-NMDAR encephalitis. Brain. [DOI] [PubMed] [Google Scholar]
- Mcquate A & Barria A 2020. Rapid exchange of synaptic and extrasynaptic NMDA receptors in hippocampal CA1 neurons. J Neurophysiol, 123, 1004–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikasova L, De Rossi P, Bouchet D, Georges F, Rogemond V, Didelot A, Meissirel C, Honnorat J & Groc L 2012. Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain, 135, 1606–21. [DOI] [PubMed] [Google Scholar]
- Milstone AM, Meyers K & Elia J 2005. Treatment of acute neuropsychiatric lupus with intravenous immunoglobulin (IVIG): a case report and review of the literature. Clin Rheumatol, 24, 394–7. [DOI] [PubMed] [Google Scholar]
- Moscato EH, Peng X, Jain A, Parsons TD, Dalmau J & Balice-Gordon RJ 2014. Acute mechanisms underlying antibody effects in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol, 76, 108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S, Kessels HW, Alfonso S, Aow J, Fox R & Malinow R 2013. Metabotropic NMDA receptor function is required for NMDA receptor-dependent long-term depression. Proc Natl Acad Sci U S A, 110, 4027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor J, Arinuma Y, Huerta TS, Kowal C, Nasiri E, Kello N, Fujieda Y, Bialas A, Hammond T, Sriram U, Stevens B, Huerta PT, Volpe BT & Diamond B 2018. Lupus antibodies induce behavioral changes mediated by microglia and blocked by ACE inhibitors. J Exp Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omdal R, Brokstad K, Waterloo K, Koldingsnes W, Jonsson R & Mellgren SI 2005. Neuropsychiatric disturbances in SLE are associated with antibodies against NMDA receptors. Eur J Neurol, 12, 392–8. [DOI] [PubMed] [Google Scholar]
- Pan H, Oliveira B, Saher G, Dere E, Tapken D, Mitjans M, Seidel J, Wesolowski J, Wakhloo D, Klein-Schmidt C, Ronnenberg A, Schwabe K, Trippe R, Matz-Rensing K, Berghoff S, Al-Krinawe Y, Martens H, Begemann M, Stocker W, Kaup FJ, Mischke R, Boretius S, Nave KA, Krauss JK, Hollmann M, Luhder F & Ehrenreich H 2019. Uncoupling the widespread occurrence of anti-NMDAR1 autoantibodies from neuropsychiatric disease in a novel autoimmune model. Mol Psychiatry, 24, 1489–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Bellone C & Zhou Q 2013. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci, 14, 383–400. [DOI] [PubMed] [Google Scholar]
- Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M, Groc L, Pollegioni L, Mothet JP & Oliet SH 2012. Synaptic and Extrasynaptic NMDA Receptors Are Gated by Different Endogenous Coagonists. Cell, 150, 633–46. [DOI] [PubMed] [Google Scholar]
- Papouin T & Oliet SH 2014. Organization, control and function of extrasynaptic NMDA receptors. Philos Trans R Soc Lond B Biol Sci, 369, 20130601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MP & Raymond LA 2014. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron, 82, 279–93. [DOI] [PubMed] [Google Scholar]
- Pedrazzi M, Averna M, Sparatore B, Patrone M, Salamino F, Marcoli M, Maura G, Cervetto C, Frattaroli D, Pontremoli S & Melloni E 2012. Potentiation of NMDA receptor-dependent cell responses by extracellular high mobility group box 1 protein. PLoS One, 7, e44518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri M, Naqibuddin M, Carson KA, Wallace DJ, Weisman MH, Holliday SL, Sampedro M, Padilla PA & Brey RL 2010. Depression and cognitive impairment in newly diagnosed systemic lupus erythematosus. J Rheumatol, 37, 2032–8. [DOI] [PubMed] [Google Scholar]
- Planaguma J, Haselmann H, Mannara F, Petit-Pedrol M, Grunewald B, Aguilar E, Ropke L, Martin-Garcia E, Titulaer MJ, Jercog P, Graus F, Maldonado R, Geis C & Dalmau J 2016. Ephrin-B2 prevents N-methyl-D-aspartate receptor antibody effects on memory and neuroplasticity. Ann Neurol, 80, 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planaguma J, Leypoldt F, Mannara F, Gutierrez-Cuesta J, Martin-Garcia E, Aguilar E, Titulaer MJ, Petit-Pedrol M, Jain A, Balice-Gordon R, Lakadamyali M, Graus F, Maldonado R & Dalmau J 2015. Human N-methyl D-aspartate receptor antibodies alter memory and behaviour in mice. Brain, 138, 94–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasure D 2008. Diagnostic and pathogenic significance of glutamate receptor autoantibodies. Arch Neurol, 65, 589–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss H, Finke C, Holtje M, Hofmann J, Klingbeil C, Probst C, Borowski K, Ahnert-Hilger G, Harms L, Schwab JM, Ploner CJ, Komorowski L, Stoecker W, Dalmau J & Wandinger KP 2012. N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol, 72, 902–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss H & Kirmse K 2018. Pathogenic role of autoantibodies against inhibitory synapses. Brain Res, 1701, 146–152. [DOI] [PubMed] [Google Scholar]
- Putterman C & Diamond B 1998. Immunization with a peptide surrogate for double-stranded DNA (dsDNA) induces autoantibody production and renal immunoglobulin deposition. J Exp Med, 188, 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajani V, Sengar AS & Salter MW 2020. Tripartite signalling by NMDA receptors. Mol Brain, 13, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayes HA, Tani C, Kwan A, Marzouk S, Colosimo K, Medina-Rosas J, Mustafa A, Su J, Lambiris P, Mosca M & Touma Z 2018. What is the prevalence of cognitive impairment in lupus and which instruments are used to measure it? A systematic review and meta-analysis. Semin Arthritis Rheum, 48, 240–255. [DOI] [PubMed] [Google Scholar]
- Reveille JD, Moulds JM, Ahn C, Friedman AW, Baethge B, Roseman J, Straaton KV & Alarcon GS 1998. Systemic lupus erythematosus in three ethnic groups: I. The effects of HLA class II, C4, and CR1 alleles, socioeconomic factors, and ethnicity at disease onset. LUMINA Study Group. Lupus in minority populations, nature versus nurture. Arthritis Rheum, 41, 1161–72. [DOI] [PubMed] [Google Scholar]
- Rogers SW, Andrews PI, Gahring LC, Whisenand T, Cauley K, Crain B, Hughes TE, Heinemann SF & Mcnamara JO 1994. Autoantibodies to glutamate receptor GluR3 in Rasmussen's encephalitis. Science, 265, 648–51. [DOI] [PubMed] [Google Scholar]
- Romero-Hernandez A, Simorowski N, Karakas E & Furukawa H 2016. Molecular Basis for Subtype Specificity and High-Affinity Zinc Inhibition in the GluN1-GluN2A NMDA Receptor Amino-Terminal Domain. Neuron, 92, 1324–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarting A, Mockel T, Lutgendorf F, Triantafyllias K, Grella S, Boedecker S, Weinmann A, Meineck M, Sommer C, Schermuly I, Fellgiebel A, Luessi F & Weinmann-Menke J 2019. Fatigue in SLE: diagnostic and pathogenic impact of anti-N-methyl-D-aspartate receptor (NMDAR) autoantibodies. Ann Rheum Dis, 78, 1226–1234. [DOI] [PubMed] [Google Scholar]
- Schwartz N, Stock AD & Putterman C 2019. Neuropsychiatric lupus: new mechanistic insights and future treatment directions. Nat Rev Rheumatol, 15, 137–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M & Hoogenraad CC 2007. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem, 76, 823–47. [DOI] [PubMed] [Google Scholar]
- Snyder MA & Gao WJ 2020. NMDA receptor hypofunction for schizophrenia revisited: Perspectives from epigenetic mechanisms. Schizophr Res, 217, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltys J, Liu Y, Ritchie A, Wemlinger S, Schaller K, Schumann H, Owens GP & Bennett JL 2019. Membrane assembly of aquaporin-4 autoantibodies regulates classical complement activation in neuromyelitis optica. J Clin Invest, 129, 2000–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son M, Porat A, He M, Suurmond J, Santiago-Schwarz F, Andersson U, Coleman TR, Volpe BT, Tracey KJ, Al-Abed Y & Diamond B 2016. C1q and HMGB1 reciprocally regulate human macrophage polarization. Blood, 128, 2218–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Teegen B, Schiltz K, Bernstein HG, Stoecker W & Bogerts B 2014. Prevalence of N-methyl-D-aspartate receptor autoantibodies in the peripheral blood: healthy control samples revisited. JAMA Psychiatry, 71, 838–9. [DOI] [PubMed] [Google Scholar]
- Stuart GJ & Spruston N 2015. Dendritic integration: 60 years of progress. Nat Neurosci, 18, 1713–21. [DOI] [PubMed] [Google Scholar]
- Tamminga CA & Zukin RS 2015. Schizophrenia: Evidence implicating hippocampal GluN2B protein and REST epigenetics in psychosis pathophysiology. Neuroscience, 309, 233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AH, Chen H, Li TP, Metzbower SR, Macgillavry HD & Blanpied TA 2016. A transsynaptic nanocolumn aligns neurotransmitter release to receptors. Nature, 536, 210–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraschenko O, Fox HS, Pittock SJ, Zekeridou A, Gafurova M, Eldridge E, Liu J, Dravid SM & Dingledine R 2019. A mouse model of seizures in anti-N-methyl-d-aspartate receptor encephalitis. Epilepsia, 60, 452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay SH, Fairhurst AM & Mak A 2017. Clinical utility of circulating anti-N-methyl-d-aspartate receptor subunits NR2A/B antibody for the diagnosis of neuropsychiatric syndromes in systemic lupus erythematosus and Sjogren's syndrome: An updated meta-analysis. Autoimmun Rev, 16, 114–122. [DOI] [PubMed] [Google Scholar]
- Titulaer MJ, Mccracken L, Gabilondo I, Armangue T, Glaser C, Iizuka T, Honig LS, Benseler SM, Kawachi I, Martinez-Hernandez E, Aguilar E, Gresa-Arribas N, Ryan-Florance N, Torrents A, Saiz A, Rosenfeld MR, Balice-Gordon R, Graus F & Dalmau J 2013. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol, 12, 157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR & Westbrook GL 2002. Mobile NMDA receptors at hippocampal synapses. Neuron, 34, 255–64. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, Mcbain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ & Dingledine R 2010. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev, 62, 405–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsokos GC 2011. Systemic lupus erythematosus. N Engl J Med, 365, 2110–21. [DOI] [PubMed] [Google Scholar]
- Tuzun E, Zhou L, Baehring JM, Bannykh S, Rosenfeld MR & Dalmau J 2009. Evidence for antibody-mediated pathogenesis in anti-NMDAR encephalitis associated with ovarian teratoma. Acta Neuropathol, 118, 737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterman A, Nolte JE, Boaz M, Abady M, Shoenfeld Y & Zandman-Goddard G 2011. Neuropsychiatric syndromes in systemic lupus erythematosus: a meta-analysis. Semin Arthritis Rheum, 41, 1–11. [DOI] [PubMed] [Google Scholar]
- Valbuena S & Lerma J 2016. Non-canonical Signaling, the Hidden Life of Ligand-Gated Ion Channels. Neuron, 92, 316–329. [DOI] [PubMed] [Google Scholar]
- Van Sonderen A, Petit-Pedrol M, Dalmau J & Titulaer MJ 2017. The value of LGI1, Caspr2 and voltage-gated potassium channel antibodies in encephalitis. Nat Rev Neurol, 13, 290–301. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P & Bading H 2003. Opposing roles of synaptic and extrasynaptic NMDA receptors in neuronal calcium signalling and BDNF gene regulation. Curr Opin Neurobiol, 13, 366–71. [DOI] [PubMed] [Google Scholar]
- Vieira M, Yong XLH, Roche KW & Anggono V 2020. Regulation of NMDA glutamate receptor functions by the GluN2 subunits. J Neurochem, 154, 121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang F, Mai D & Qu S 2020. Molecular Mechanisms of Glutamate Toxicity in Parkinson's Disease. Front Neurosci, 14, 585584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warikoo N, Brunwasser SJ, Benz A, Shu HJ, Paul SM, Lewis M, Doherty J, Quirk M, Piccio L, Zorumski CF, Day GS & Mennerick S 2018. Positive Allosteric Modulation as a Potential Therapeutic Strategy in Anti-NMDA Receptor Encephalitis. J Neurosci, 38, 3218–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenke NK, Kreye J, Andrzejak E, Van Casteren A, Leubner J, Murgueitio MS, Reincke SM, Secker C, Schmidl L, Geis C, Ackermann F, Nikolaus M, Garner CC, Wardemann H, Wolber G & Pruss H 2019. N-methyl-D-aspartate receptor dysfunction by unmutated human antibodies against the NR1 subunit. Ann Neurol, 85, 771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmuth LP 2018. Ion permeation in ionotropic glutamate receptors: Still dynamic after all these years. Curr Opin Physiol, 2, 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wroge CM, Hogins J, Eisenman L & Mennerick S 2012. Synaptic NMDA receptors mediate hypoxic excitotoxic death. J Neurosci, 32, 6732–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiangwei W, Jiang Y & Yuan H 2018. De Novo Mutations and Rare Variants Occurring in NMDA Receptors. Curr Opin Physiol, 2, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Lin J, Peng X, Zhang Q, Zhang Y, Guo N, Zhou S & Li Q 2017. Effects of picrotoxin on zebrafish larvae behaviors: A comparison study with PTZ. Epilepsy Behav, 70, 224–231. [DOI] [PubMed] [Google Scholar]
- Zerche M, Weissenborn K, Ott C, Dere E, Asif AR, Worthmann H, Hassouna I, Rentzsch K, Tryc AB, Dahm L, Steiner J, Binder L, Wiltfang J, Siren AL, Stocker W & Ehrenreich H 2015. Preexisting Serum Autoantibodies Against the NMDAR Subunit NR1 Modulate Evolution of Lesion Size in Acute Ischemic Stroke. Stroke, 46, 1180–6. [DOI] [PubMed] [Google Scholar]
- Zhang J, Jacobi AM, Wang T, Berlin R, Volpe BT & Diamond B 2009. Polyreactive autoantibodies in systemic lupus erythematosus have pathogenic potential. J Autoimmun, 33, 270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Chen Z, Yun W, Ren J, Li C & Wang H 2015. Extrasynaptic NMDA Receptor in Excitotoxicity: Function Revisited. Neuroscientist, 21, 337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Hollern D, Liao J, Andrechek E & Wang H 2013. NMDA receptor-mediated excitotoxicity depends on the coactivation of synaptic and extrasynaptic receptors. Cell Death Dis, 4, e560. [DOI] [PMC free article] [PubMed] [Google Scholar]