Abstract
Objective:
To evaluate outcomes of the first pregnancy after fertility-sparing surgery in patients with early-stage cervical cancer.
Methods:
We performed a population-based study of women 18–45 years old with a history of stage I cervical cancer reported to the 2000–2012 California Cancer Registry. Data were linked to the California Office of Statewide Health Planning and Development birth and discharge data sets. We included patients with cervical cancer who conceived ≥3 months after fertility-sparing surgery. The primary outcome was preterm birth. Secondary outcomes included growth restriction, neonatal morbidity, fetal demise, cesarean delivery, and severe maternal morbidity. We used propensity scores to match similar women from two groups in a 1:2 ratio of cases to controls: population controls without cancer and cervical cancer controls (women who delivered before their cervical cancer diagnosis). Wald statistics and logistic regressions were used to evaluate outcomes.
Results:
Of 4,087 patients with cervical cancer, 118 (2.9%) conceived following fertility-sparing surgery (conization/LEEP), and 107 met inclusion criteria and were matched to controls. Squamous cell carcinoma was the most common histology (63.2%) followed by adenocarcinoma (30.8%). Cases had higher odds of preterm birth before 37 weeks compared to both control groups (21.5% vs 9.3%, OR 2.66, 95% CI 1.38–5.10; 21.5% vs 12.7%, OR 1.88, 95% CI 1.01–3.57), but not preterm birth before 32 weeks. Neonatal morbidity was more common among the cases relative to cervical cancer controls (OR 15.9% vs 6.9%, 2.53, 95% CI 1.16–5.54). There were no differences in rates of growth restriction, fetal demise, cesarean delivery, and maternal morbidity.
Conclusion:
In a population-based cohort, patients who conceived after surgery for cervical cancer had higher odds of preterm delivery compared to controls.
Précis
Patients with cervical cancer who underwent fertility-sparing surgery had an increased risk of preterm birth and neonatal morbidity compared to matched controls.
Introduction
Most of the estimated 14,480 new cases of cervical cancer in 2021 will be diagnosed among reproductive-aged women.1 Given national trends toward delayed childbearing,2 the number of women with early-stage cervical cancer seeking fertility-sparing options, such as cervical conization, loop electrosurgical excision procedure (LEEP), and simple or radical trachelectomy, is growing.3,4 Though fertility-spearing surgery is accepted as oncologically safe in well-selected patients,4–6 the obstetric risks of this approach have been challenging to study because fewer than half of all patients attempt to conceive.6,7
Observational studies have demonstrated that more than a third of cervical cancer survivors who ultimately conceive will deliver prematurely.7–10 However, the available data are limited as they are derived from case series11–18 and systematic reviews of case series with few pregnancies.7,9,10 Population-based studies have included more pregnancies after cervical cancer surgery, but often lack a rigorous definition of pregnancy timing related to cancer diagnosis,8,19 match controls to cases on few demographic variables,8,19 or summarize outcomes from patients with different types of reproductive cancers.8,19 Moreover, estimates from studies that included patients diagnosed with cancer during pregnancy or postpartum20,21 are challenging to interpret as such patients are at higher risk of adverse obstetric outcomes.19,22
Therefore, we sought to characterize obstetric outcomes for the first pregnancy after fertility sparing surgery in patients with early-stage cervical cancer who delivered after cancer treatment compared to matched controls using a population-level database.
Methods
We linked the California Cancer Registry and California Office of Statewide Health Planning and Development (OSHPD) birth cohort databases to conduct a population-based analysis. With assistance from OSHPD, the California Cancer Registry data were linked to the California linked birth cohort, a database of maternal and neonatal hospital records from 9 months prior to delivery to 1 year postpartum (more details in Appendix 1). The linkage was conducted in a deterministic manner with a combination of maternal date of birth, social security number, and ZIP code to accurately link patients between the data sets. We used data collected from January 2000 through December 2012, the most recent years for which birth cohort data are available. We obtained approval for this study from The University of Texas MD Anderson Cancer Center Institutional Review Board, OSHPD, and the State of California Committee for the Protection of Human Subjects.
We identified women 18–45 years old who were diagnosed with American Joint Committee on Cancer eighth edition pathologic stage I cervical cancer from January 1, 2000 to December 31, 2012 in a manner similar to that described in our recent study.23 Of note, The International Federation of Gynecology and Obstetrics (FIGO) staging was not available in the cancer registry for some of the cohort. Patients who did not undergo surgery as part of primary treatment for cervical cancer were excluded. Women with cancer who met these eligibility criteria were screened for deliveries in the linked database, and those without deliveries were excluded. Also excluded were patients whose dates of birth in the California Cancer Registry and the linked OSHPD file did not match. In an effort to clearly define the case cohort, cases were defined as those who conceived at least 3 months after surgery. We excluded patients defined as having had pregnancy-associated cancer (conceived less than 1 year prior to diagnosis to within 3 months of surgery) as well as those whose births had no recorded date of delivery, had improbable combinations of living status and gestational age (<22 weeks or >46 weeks), or occurred before 20 weeks gestational age. For each included case, only the first post-diagnosis birth that occurred during the study period was considered.
We defined two control groups. The population-based control group consisted of women who were 18–45 years old, delivered in California during the study period, and did not link to a record in the California Cancer Registry (n=4,327,110). Because there are unmeasured covariates that may bias the exposure-outcome association in this study, such as cervical dysplasia and cervical procedures for dysplasia,24,25 we matched a second control group to better account for these variables: those who conceived more than 1 year prior to cervical cancer diagnosis (n=1,100). These patients did not have a diagnosis of cervical cancer at the time of pregnancy, but we assumed that they were more likely to have undergone procedures for dysplasia than population-based controls. We refer to this group as the cervical cancer control group. For both control groups, we used the same selection criteria as applied to the cancer patients, excluding patients with births with a missing delivery date, those with delivery before 20 weeks gestational age, and those with implausible combinations of live birth and gestational age (<22 weeks or >46 weeks).
Each control group was matched to cases in a 2:1 ratio (when possible) to balance cervical cancer patients and controls on observed covariates. Demographic characteristics included age at delivery (continuous and grouped into 18–29, 30–34, 35–37, 38–40, 41–44, or ≥45 years), duration of education (<12 years or ≥12 years), insurance type (public, private, or uninsured), race (White, Black, Hispanic, Asian/Pacific Islander, or other [pre-specified formal category in the database]), and annual income quartile. Obstetric covariates included parity (nulliparous vs multiparous), pregnancy type (singleton or multiples), timing of entry to prenatal care, and presence of maternal co-morbidities including hypertension (none, chronic, gestational, preeclampsia, and severe preeclampsia/eclampsia), diabetes (none, pregestational, or gestational), and abnormal placentation (placenta previa, vasa previa, and placenta accreta). Importantly, we found that only one case reported smoking during pregnancy, and therefore smoking was not included in the matching algorithm.
The primary outcome was preterm birth, defined as birth at <37 weeks of gestation. Secondary outcomes included preterm birth at <32 weeks, fetal growth restriction (determined by national standards for U.S. births adjusted for gestational age and sex, published by Aris and colleagues26 and defined as <10th percentile and <5th percentile), fetal demise, cesarean delivery, severe maternal morbidity as defined by the Centers for Disease Control and Prevention algorithm,27 and neonatal morbidity adapted from Grobman and colleagues.28 Neonatal morbidity was thus defined as the need for respiratory support within 72 hours after birth or the occurrence of hypoxic-ischemic encephalopathy, seizure, infection (pneumonia or sepsis within 28 days of birth), meconium aspiration syndrome, birth trauma, or intracranial or subgaleal hemorrhage.
For clinical and demographic data, we used the Pearson chi-square test or Fisher exact test, when appropriate, to analyze categorical variables and the Student t test or Wilcoxon rank-sum test to analyze continuous variables. To match controls to cases, we used greedy nearest neighbor propensity-score matching without replacement in a 2:1 ratio of controls to cases with a caliper width set to 0.2 times the standard deviation of the propensity score. To optimally adjust the analyses for the factors most likely to influence the outcomes, we preferentially matched on parity and singleton vs multiple gestations. Multiple logistic regressions were used to evaluate the associations between cervical cancer and the obstetric outcomes of interest compared to each control group. Wald statistics were used to calculate 95% CIs. We stratified the cervical cancer cases by months from surgery to conception (3–6, >6–12, or >12 months) to assess whether outcome distribution differed by subgroup. STROBE guidelines for observational research were followed. All statistical tests were two-sided. A P-value of <0.05 and a 95% CI not inclusive of the null (1.0) were considered statistically significant. These analyses were implemented in SAS Enterprise Guide version 7.11 (SAS Institute)
We undertook quantitative bias analyses to evaluate the robustness of the derived estimates to unmeasured confounding. The E-value29 is unlike other sensitivity analyses in that it does not use subjective investigator parameters but instead uses estimates derived from the study to quantify how strong an unmeasured confounder would have to be to fully explain an observed treatment–outcome relationship. We used the E-value to calculate the required magnitude of the association an unmeasured confounder with both the exposure (cervical cancer) and the various obstetric outcomes, to fully explain away the derived estimate (Appendix 2). We also addressed statistical uncertainty by calculating the E-value required to explain away the lower confidence limit in our estimates. These analyses were implemented in Stata/MP version 16.0 (StataCorp).
Results
From January 2000 to December 2012 there were 4,087 patients with AJCC stage I cervical cancer, of whom 118 met inclusion criteria. A flow chart of the cohort selection process is given in Figure 1, and a comparison between patients who met and did not meet inclusion criteria is provided in Appendix 3. The majority of included patients were non-Hispanic White women (n=60, 51.3%), aged 26–35 years (n=90, 76.9%), and had no additional comorbidities (n=113, 96.6% with Charlson comorbidity index=0). The predominant histology was squamous cell carcinoma (n=74, 63.2%) followed by adenocarcinoma (n=36, 30.8%). Of those with known type of primary surgery (n=113, 95.8%), 103 (91.2%) underwent cervical conization/LEEP and 10 (8.8%) underwent a trachelectomy prior to pregnancy. Given the higher risk of preterm birth associated with trachelectomies compared to conization/LEEP and the low number of trachelectomies in this cohort, these 10 patients were excluded from the main analysis. The final cohort before matching was 108 patients. None of the included patients received neoadjuvant chemotherapy, adjuvant chemotherapy, or radiation.
Figure 1:
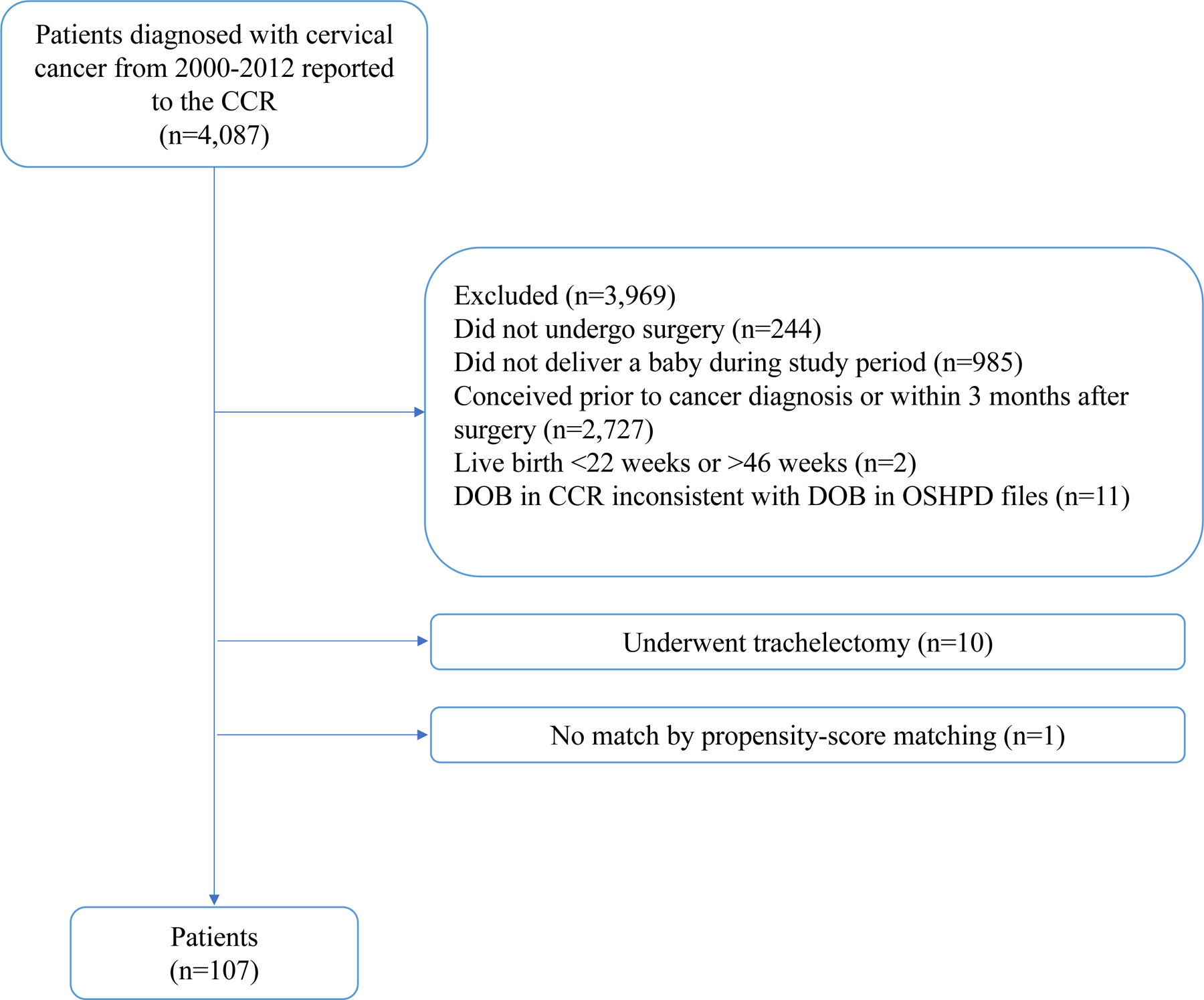
Cohort selection process
Abbreviations: CCR, California Cancer Registry; DOB, date of birth; OSHPD, California Office of Statewide Health Planning and Development.
Before propensity-score matching, the groups demonstrated significantly different covariate distributions (Appendix 4). Matching yielded 107 cervical cancer cases (1 case had no match), 214 population controls, and 173 cervical cancer controls. Table 1 shows that, after matching, the clinical and demographic covariates were well balanced between groups. There were no missing data in the propensity-score matched population. Among the cases, the median time from cervical cancer surgery to conception was 14.1 months (IQR 8.4–29.0, range 3.2–100.8 months).
Table 1.
Characteristics of Women with Stage I Cervical Cancer Who Underwent Fertility-Sparing Surgery and Controls After Propensity-Score Matching
| Propensity Score-Matched Cohort* |
||||
|---|---|---|---|---|
| Characteristic† | Cervical Cancer Patients (n=107) | Cervical Cancer Controls (n=173) | Population Controls (n=214) | P-Value§ |
| Age, y, median (IQR) | 33 (29–36) | 33 (29–36) | 33 (29–36) | .4 |
| Education | .9 | |||
| <12 y | 33 (30.8%) | 52 (30.1%) | 69 (32.2%) | |
| ≥12 y | 70 (65.4%) | 117 (67.6%) | 137 (64.0%) | |
| Insurance type | .7 | |||
| Private | 84 (78.5%) | 141 (81.5%) | 169 (79.0%) | |
| Public | 23 (21.5%) | 32 (18.5%) | 45 (21.0%) | |
| Race/ethnicity | 1.0 | |||
| White | 59 (55.1%) | 95 (54.9%) | 120 (56.1%) | |
| Black | 1 (0.9%) | 1 (0.6%) | 2 (0.9%) | |
| Hispanic | 32 (29.9%) | 50 (28.9%) | 62 (29.0%) | |
| Asian/Pacific Islander | 3 (2.8%) | 10 (5.8%) | 7 (3.3%) | |
| Other‡ | 12 (11.2%) | 17 (9.8%) | 23 (10.7%) | |
| Annual income, quartiles | .8 | |||
| Lowest | 40 (37.4%) | 67 (38.7%) | 83 (38.8%) | |
| Low-middle | 15 (14.0%) | 23 (13.3%) | 27 (12.6%) | |
| High-middle | 30 (28.0%) | 56 (32.4%) | 59 (27.6%) | |
| Highest | 22 (20.6%) | 27 (15.6%) | 45 (21.0%) | |
| Parity | 1.0 | |||
| Nulliparous | 47 (43.9%) | 77 (44.5%) | 94 (43.9%) | |
| Multiparous | 60 (56.1%) | 96 (55.5%) | 120 (56.1%) | |
| Entry to prenatal care | .6 | |||
| 1st trimester | 98 (91.6%) | 161 (93.1%) | 199 (93.0%) | |
| 2nd trimester | 8 (7.5%) | 10 (5.8%) | 15 (7.0%) | |
| 3rd trimester | 1 (0.9%) | 2 (1.2%) | 0 (0.0%) | |
| Pregnancy type | .7 | |||
| Singleton | 103 (96.3%) | 169 (97.7%) | 206 (96.3%) | |
| Twins | 4 (3.7%) | 4 (2.3%) | 8 (3.7%) | |
| Chronic hypertension | .8 | |||
| No | 104 (97.2%) | 167 (96.5%) | 209 (97.7%) | |
| Yes | 3 (2.8%) | 6 (3.5%) | 5 (2.3%) | |
| Pregestational diabetes | .7 | |||
| No | 104 (97.2%) | 169 (97.7%) | 206 (96.3%) | |
| Yes | 3 (2.8%) | 4 (2.3%) | 8 (3.7%) | |
| Gestational diabetes | .5 | |||
| No | 95 (88.8%) | 159 (91.9%) | 189 (88.3%) | |
| Yes | 12 (11.2%) | 14 (8.1%) | 25 (11.7%) | |
| Pregnancy-associated hypertension | .6 | |||
| No | 99 (92.5%) | 166 (96.0%) | 200 (93.5%) | |
| Gestational hypertension | 3 (2.8%) | 3 (1.7%) | 5 (2.3%) | |
| Preeclampsia | 3 (2.8%) | 2 (1.2%) | 8 (3.7%) | |
| Severe preeclampsia/eclampsia | 2 (1.9%) | 2 (1.2%) | 1 (0.5%) | |
| Renal disease | .5 | |||
| No | 106 (99.1%) | 173 (100.0%) | 213 (99.5%) | |
| Yes | 1 (0.9%) | 0 (0.0%) | 1 (0.5%) | |
| Abnormal placentation | .8 | |||
| No | 105 (98.1%) | 171 (98.8%) | 212 (99.1%) | |
| Placenta previa | 2 (1.9%) | 2 (1.2%) | 2 (0.9%) | |
| Delivery year | .05 | |||
| 2002 | 10 (9.3%) | 19 (11.0%) | 15 (7.0%) | |
| 2003 | 11 (10.3%) | 24 (13.9%) | 23 (10.7%) | |
| 2004 | 6 (5.6%) | 16 (9.2%) | 11 (5.1%) | |
| 2005 | 9 (8.4%) | 20 (11.6%) | 17 (7.9%) | |
| 2006 | 6 (5.6%) | 8 (4.6%) | 13 (6.1%) | |
| 2007 | 13 (12.1%) | 25 (14.5%) | 25 (11.7%) | |
| 2008 | 11 (10.3%) | 23 (13.3%) | 24 (11.2%) | |
| 2009 | 10 (9.3%) | 17 (9.8%) | 17 (7.9%) | |
| 2010 | 9 (8.4%) | 10 (5.8%) | 20 (9.3%) | |
| 2011 | 8 (7.5%) | 9 (5.2%) | 20 (9.3%) | |
| 2012 | 14 (13.1%) | 2 (1.2%) | 29 (13.6%) | |
Data are no. (%) unless otherwise specified
Propensity-score matching based on all tabulated characteristics
Due to missing data, sums may not add up to total number of patients per column
P-values derived by Pearson chi-square test or Wilcoxon rank-sum test
Pre-specified formal category in the database
Abbreviations: IQR, interquartile range.
Cervical cancer cases had higher odds of preterm birth <37 weeks compared to population controls (21.5% vs 9.3%, odds ratio [OR] 2.66, 95% CI 1.38–5.10) and cervical cancer controls (21.5% vs 12.7%, OR 1.88, 95% CI 1.01–3.57). Cervical cancer cases had higher odds of neonatal morbidity compared to cervical cancer controls (15.9% vs 6.9%, OR 2.53, 95% CI 1.16–5.54) but not population controls (15.9% vs 9.8%, OR 1.74, 95% CI 0.87–3.45). There were no statistically significant differences between cases and both control groups in any of the other studied outcomes (Figure 2). Of note, there was one fetal demise in the case group, but we could not estimate odds ratios as there were no cases in either control group. Subgroup analysis stratified by time after diagnosis demonstrated no significant differences in studied outcomes (Table 2).
Figure 2:
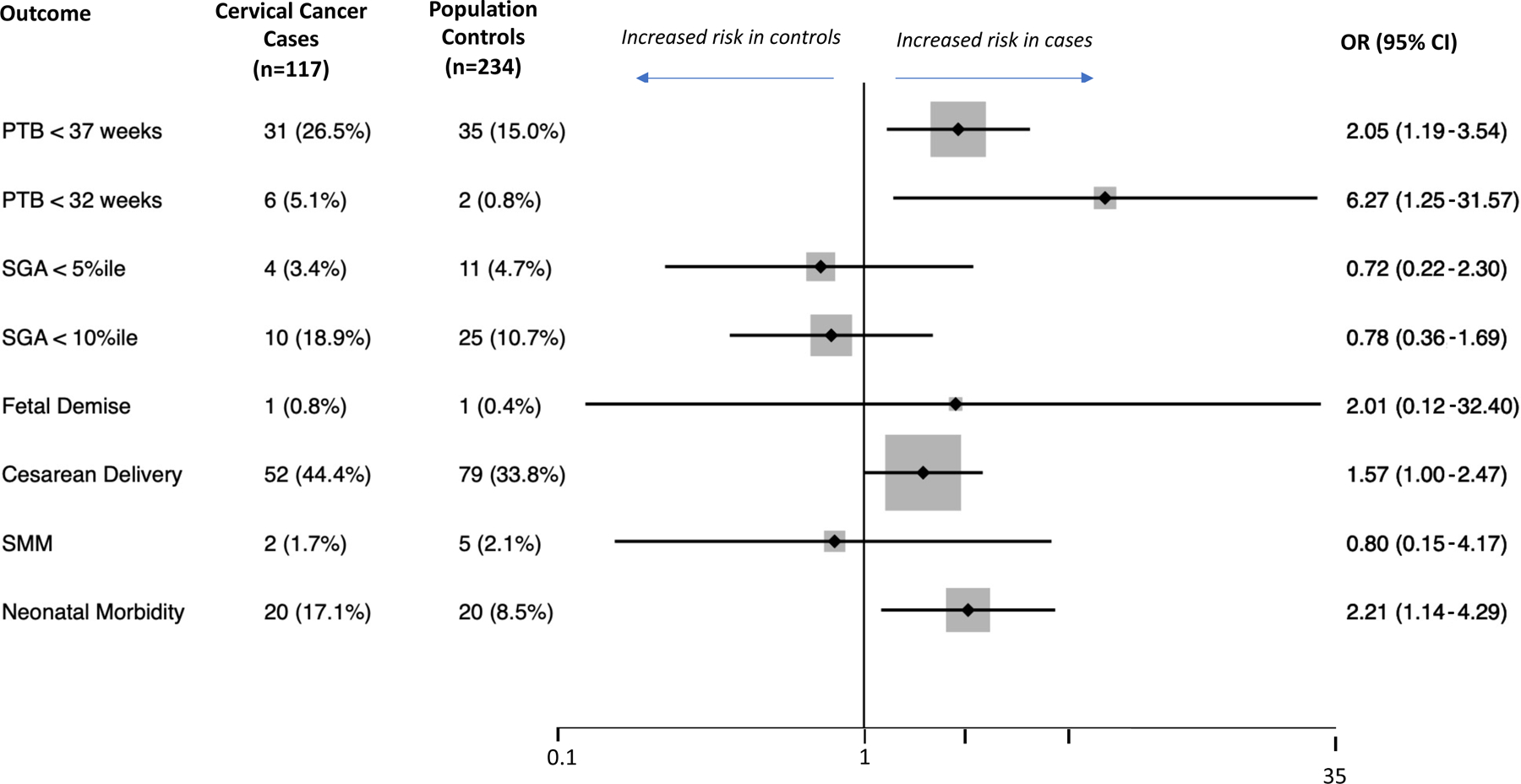
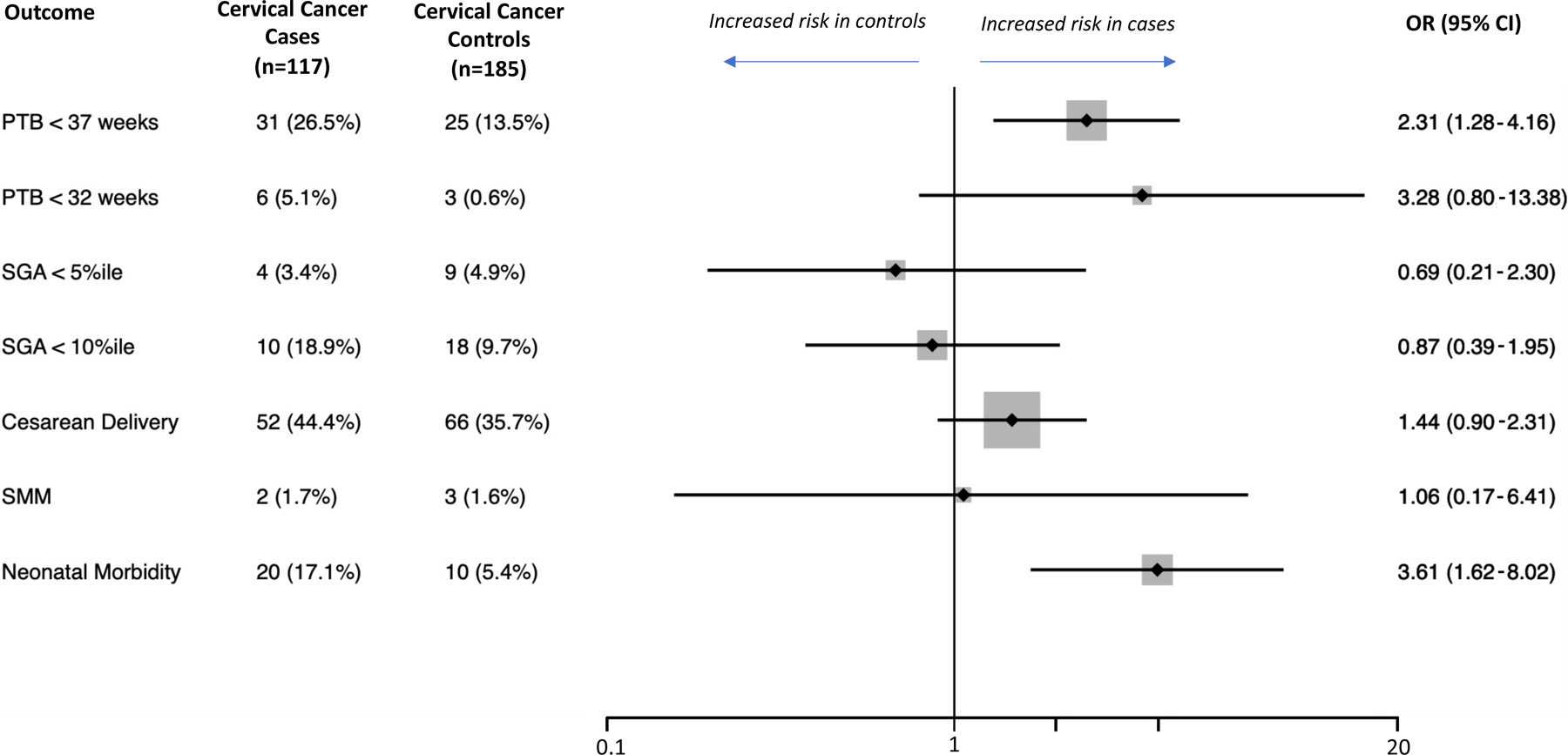
Outcomes of interest in cervical cancer patients and propensity score–matched controls as well as associated odds ratios (OR) of the adverse outcome in the cervical cancer patients compared to the controls. ORs greater than 1 indicate a higher risk in the cervical cancer cases. The diamonds and lines represent point estimates and 95% confidence intervals (CIs) for the estimates, respectively. The vertical line is centered at the null (1.0). We were unable to estimate the odds of fetal demise as there were no cases in the control groups. (A) Cervical cancer cases compared to population-based controls. (B) Cervical cancer cases compared to cervical cancer controls.
Abbreviations: PTB, preterm birth; SGA, small for gestational age based on curves by Aris and colleagues26; SMM, severe maternal morbidity based on the CDC algorithm.27
Table 2:
Obstetric Outcomes in Cervical Cancer Cases Stratified by Time to Conception
| Time from Surgery to Conception, mo |
||||
|---|---|---|---|---|
| Outcome | 3–6 (n=18) | 6–12 (n=28) | >12 (n=61) | P-Value* |
| PTB < 37 w | 22% (3–41) | 21% (6–37) | 21% (11–32) | 1 |
| PTB < 32 w | 6% (0–16) | 4% (0–10) | 2% (0–5) | .4 |
| SGA < 5th percentile | 0% | 0% | 7% (0–13) | .4 |
| SGA < 10th percentile | 11% (0–26) | 11% (0–22) | 8% (1–15) | .8 |
| Fetal demise | 0% | 0% | 2% (0–5) | 1 |
| Cesarean delivery | 44% (21–67) | 39% (21–57) | 38% (25–50) | .9 |
| SMM | 6% (0–16) | 4% (0–10) | 0% | .2 |
| Neonatal morbidity | 17% (0–34) | 18% (4–32) | 15% (6–24) | .9 |
Quantitative bias analysis showed that an unmeasured confounder would need to be strongly associated with cervical cancer and each outcome to explain the observed associations: OR of at least 3.17 for preterm birth <37 weeks, and OR of at least 4.5 for neonatal morbidity (Appendix 5).
Discussion
In this population-based study, women who conceived at least three months after fertility-sparing surgery for cervical cancer had higher odds of preterm birth and neonatal morbidity, likely reflective of their earlier gestational age at birth, compared to matched controls. However, pregnancies after fertility-sparing surgery for cervical cancer did not have an increased risk of preterm birth<32 weeks, fetal demise, cesarean delivery, growth restriction, or severe maternal morbidity.
Prematurity occurs in one in 10 births nationwide and is a leading cause of neonatal morbidity and mortality.2 In our study, the odds of preterm birth <37 weeks were approximately double among patients with cervical cancer compared to controls consistent with prior studies. 6,10,30–32 Our study design allows for more reliable estimates compared to case series with few pregnancies and limited obstetric outcomes11–18, systematic reviews that pool these same uncontrolled studies,7,9,10 and population-based studies that used ICD codes to identify cancer diagnoses.20 Our findings are consistent with those of a database study by Hartnett and colleagues8 that demonstrated a 28% rate of preterm birth <37 weeks, which translated to more than double the risk of preterm birth compared to controls. Our analysis, however, is strengthened by our ability to match on more characteristics that are likely to influence the risk of preterm birth, such as parity and maternal comorbidities, and our clear definition of the “pregnancy after cancer” exposure.
Our analysis is also unique in its effort to control for cervical dysplasia and procedures for pre-invasive disease, which are important unmeasured confounders.25 Though we were unable to adjust for these directly, we matched a second control group of women who delivered prior to their diagnosis of cervical cancer. Patients in this group did not have a diagnosis of cervical cancer at the time of pregnancy; however, given the latency of cervical cancer, they were more likely to have had cervical dysplasia and to have undergone procedures for dysplasia than population-based controls. We found that even compared to women with an a priori higher risk of preterm birth relative to controls, patients who conceived after fertility-sparing surgery for cervical cancer were at higher risk. It is important to note that the type of fertility-sparing surgery determines the magnitude of risk of preterm birth, which has been demonstrated to increase with radicality of resection.7,10,30 The analysis does not include patients with trachelectomies and these results do not apply to women who underwent a trachelectomy and consequentially are at a higher risk of preterm birth.
The reliability of the databases and variables used in this study have been previously demonstrated. Preterm birth in the linked data set, which includes vital statistics and discharge data from California hospitals, has been shown to have good validity (positive predictive value [PPV] 95–96%).33 Likewise, validity has been demonstrated for the variables used for propensity-score matching such as race and ethnicity (PPV 96–97%), insurance status (PPV 84–97%), and birth order (PPV 93%) as well as other outcomes such as mode of delivery (PPV 99%). 33–36
There are several limitations to our study. We were unable to control for some covariates that may contribute to differences in preterm birth rate and neonatal morbidity, such as history of preterm birth, maternal BMI, and smoking. Our quantitative bias analysis, however, demonstrated that the estimates are moderately robust to unmeasured confounding. We also cannot exclude the possibility that our study was underpowered to detect more subtle differences between the groups. In addition, we assume that patients in the cervical cancer control group had higher rates of dysplasia and excisional procedures compared to population-based controls; however, we are unable to assess the validity of this assumption with this dataset. Furthermore, our data are subject to inherent error in the reporting and coding process used to generate the databases. Given that the database includes the 9 months before and 1 year after delivery, we were unable to address miscarriages or deliveries prior to 20 weeks, which were likely to be unreported. If a miscarriage occurred close to the time of a new conception, it may have been captured by discharge data, but in most cases, it would not have been captured in our dataset. Finally, given the high proportion of cases missing FIGO staging data, we were unable to provide estimates of adverse outcomes based on the standard staging system for cervical cancer.
Despite these limitations, we used a novel linkage between population-level databases to rigorously identify cervical cancer cases and compare their obstetric outcomes to controls. We found greater incidence of preterm delivery and neonatal morbidity after fertility-sparing surgery in cervical cancer patients compared to matched controls, but we found no differences in live birth rates and maternal morbidity. The magnitude of this effect did not change with increasing time from surgery. This study provides evidence to foster shared decision-making discussions regarding obstetric outcomes after fertility-sparing treatment for early cervical cancer. With greater knowledge of these risks and close antenatal surveillance, there may be room for intervention to prolong gestation and achieve more favorable neonatal outcomes.
Supplementary Material
Acknowledgments:
This work was supported by grants from The National Institutes of Health National Cancer Institute (JARH: K08 CA234333; RN, SG, SF, and JARH: P30 CA016672; RN 5T32 CA101642) and National Center for Advancing Translational Sciences (AM: KL2TR001874) The funding sources were not involved in the development of the research hypothesis, study design, data analysis, or manuscript writing. Editorial support was provided by Bryan Tutt, ELS, of the Research Medical Library at The University of Texas MD Anderson Cancer Center.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin 2021;71(1):7–33. 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: final data for 2018. Natl Vital Stat Rep 2019;68(13):1–47. [PubMed] [Google Scholar]
- 3.Machida H, Mandelbaum RS, Mikami M, Enomoto T, Sonoda Y, Grubbs BH, et al. Characteristics and outcomes of reproductive-aged women with early-stage cervical cancer: trachelectomy vs hysterectomy. Am J Obstet Gynecol 2018;219(5):461.e1–461.e18. 10.1016/j.ajog.2018.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui RR, Chen L, Tergas AI, Hou JY, St Clair CM, Neugut AI, et al. Trends in use and survival associated with fertility-sparing trachelectomy for young women with early-stage cervical cancer. Obstet Gynecol 2018;131(6):1085–1094. 10.1097/AOG.0000000000002613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nitecki R, Woodard T, Rauh-Hain JA. Fertility-sparing treatment for early-stage cervical, ovarian, and endometrial malignancies. Obstet Gynecol 2020;136(6):1157–1169. 10.1097/AOG.0000000000004163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuurman T, Zilver S, Samuels S, Schats W, Amant F, van Trommel N, et al. Fertility-sparing surgery in gynecologic cancer: A systematic review. Cancers (Basel) 2021;13(5):1008. 10.3390/cancers13051008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nezhat C, Roman RA, Rambhatla A, Nezhat F. Reproductive and oncologic outcomes after fertility-sparing surgery for early stage cervical cancer: a systematic review. Fertil Steril 2020;113(4):685–703. 10.1016/j.fertnstert.2020.02.003 [DOI] [PubMed] [Google Scholar]
- 8.Hartnett KP, Ward KC, Kramer MR, Lash TL, Mertens AC, Spencer JB, et al. The risk of preterm birth and growth restriction in pregnancy after cancer. Int J Cancer 2017;141(11):2187–2196. 10.1002/ijc.30914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mogos MF, Rahman S, Salihu HM, Salinas-Miranda AA, Sultan DH. Association between reproductive cancer and fetal outcomes: A systematic review. Int J Gynecol Cancer 2013;23(7):1171–1177. 10.1097/IGC.0b013e31829e9fe2 [DOI] [PubMed] [Google Scholar]
- 10.Bentivegna E, Maulard A, Pautier P, Chargari C, Gouy S, Morice P. Fertility results and pregnancy outcomes after conservative treatment of cervical cancer: a systematic review of the literature. Fertil Steril 2016;106(5):1195–1211. 10.1016/j.fertnstert.2016.06.032 [DOI] [PubMed] [Google Scholar]
- 11.Okugawa K, Kobayashi H, Sonoda K, Kaneki E, Kawano Y, Hidaka N, et al. Oncologic and obstetric outcomes and complications during pregnancy after fertility-sparing abdominal trachelectomy for cervical cancer: a retrospective review. Int J Clin Oncol 2017;22(2):340–346. 10.1007/s10147-016-1059-9 [DOI] [PubMed] [Google Scholar]
- 12.Ditto A, Martinelli F, Bogani G, Fischetti M, Di Donato V, Lorusso D, et al. Fertility-sparing surgery in early-stage cervical cancer patients. Int J Gynecol Cancer 2015;25(3):493–497. 10.1097/IGC.0000000000000371 [DOI] [PubMed] [Google Scholar]
- 13.Tamauchi S, Kajiyama H, Sakata J, Sekiya R, Suzuki S, Mizuno M et al. Oncologic and obstetric outcomes of early stage cervical cancer with abdominal radical trachelectomy: Single-institution experience. J Obstet Gynaecol Res 2016;42(12):1796–1801. 10.1111/jog.13100 [DOI] [PubMed] [Google Scholar]
- 14.Li X, Xia L, Li J, Chen X, Ju X, Wu X. Reproductive and obstetric outcomes after abdominal radical trachelectomy (ART) for patients with early-stage cervical cancers in Fudan, China. Gynecol Oncol 2020;157(2):418–422. 10.1016/j.ygyno.2020.02.016 [DOI] [PubMed] [Google Scholar]
- 15.Martinelli F, Ditto A, Filippi F, Vinti D, Bogani G, Maggiore UL, et al. Conization and lymph node evaluation as a fertility-sparing treatment for early stage cervical cancer. Int J Gynecol Cancer 2021;31(3):457–461. 10.1136/ijgc-2020-001740 [DOI] [PubMed] [Google Scholar]
- 16.Fanfani F, Anchora LP, Di Martino G, Bizzarri N, Di Meo ML, Carbone V, et al. Oncologic and obstetric outcomes after simple conization for fertility-sparing surgery in FIGO 2018 stage IB1 cervical cancer. Int J Gynecol Cancer 2021;31(3):452–456. 10.1136/ijgc-2020-001750 [DOI] [PubMed] [Google Scholar]
- 17.Iwata T, Machida H, Matsuo K, Okugawa K, Saito T, Tanaka K, et al. The validity of the subsequent pregnancy index score for fertility-sparing trachelectomy in early-stage cervical cancer. Fertil Steril February 2021. 10.1016/j.fertnstert.2020.09.162 [DOI] [PubMed]
- 18.Lee CY, Chen YL, Chiang YC, Cheng CY, Lai YL, Tai YJ, et al. Outcome and subsequent pregnancy after fertility-sparing surgery of early-stage cervical cancers. Int J Environ Res Public Health 2020;17(19):7103. 10.3390/ijerph17197103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson C, Engel SM, Mersereau JE, Black KZ, Wood WA, Anders CK et al. Birth outcomes among adolescent and young adult cancer survivors. JAMA Oncol 2017;3(8):1078. 10.1001/jamaoncol.2017.0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mogos MF, Salihu HM, Aliyu MH, Whiteman VE, Sultan DH. Association between reproductive cancer and fetal outcomes: a population-based study. Int J Gynecol Cancer 2013;23(2):218–226. 10.1097/IGC.0b013e31827b877b [DOI] [PubMed] [Google Scholar]
- 21.van der Kooi AL, Brewster DH, Wood R, Nowell S, Fischbacher C, van den Heuvel-Eibrink MM, et al. Perinatal risks in female cancer survivors: A population-based analysis. PLoS One 2018;13(8):e0202805. 10.1371/journal.pone.0202805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puzzi-Fernandes C, Surita FG, Schettini CS, Parpinelli MA, Guida JP, Costa ML. Awareness towards an increasing concern during pregnancy: maternal and perinatal outcomes of women with cancer. Am J Obstet Gynecol MFM 2020;2(3):100168. 10.1016/j.ajogmf.2020.100168 [DOI] [PubMed] [Google Scholar]
- 23.Pfaendler KS, Chang J, Ziogas A, Bristow RE, Penner KR. Disparities in adherence to national comprehensive cancer network treatment guidelines and survival for stage IB–IIA cervical cancer in California. Obstet Gynecol 2018;131(5):899–908. 10.1097/AOG.0000000000002591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jar‐Allah T, Kärrberg C, Wiik J, Sengpiel V, Strander B, Holmberg E, et al. Abnormal cervical cytology is associated with preterm delivery: a population based study. Acta Obstet Gynecol Scand 2019;98(6):777–786. 10.1111/aogs.13543 [DOI] [PubMed] [Google Scholar]
- 25.Kyrgiou M, Athanasiou A, Kalliala IEJ, Paraskevaidi M, Mitra A, Martin‐Hirsch PP, et al. Obstetric outcomes after conservative treatment for cervical intraepithelial lesions and early invasive disease. Cochrane Database of Systematic Reviews 2017(11). 10.1002/14651858.CD012847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aris IM, Kleinman KP, Belfort MB, Kaimal A, Oken E. A 2017 US reference for singleton birth weight percentiles using obstetric estimates of gestation. Pediatrics 2019;144(1):e20190076. 10.1542/peds.2019-0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Severe maternal morbidity indicators and corresponding ICD codes during delivery hospitalizations. 2019 Accessed February 1, 2021. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/smm/severe-morbidity-ICD.htm
- 28.Grobman WA, Rice MM, Reddy UM, Tita AT, Silver RM, Mallett G, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med 2018;379(6):513–523. 10.1056/NEJMoa1800566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-Value. Ann Intern Med 2017;167(4):268–274. 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 30.Zhang Q, Li W, Kanis MJ, Qi G, Li M, Yang X Oncologic and obstetrical outcomes with fertility-sparing treatment of cervical cancer: a systematic review and meta-analysis. Oncotarget 2017;8(28):46580–46592. 10.18632/oncotarget.16233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jolley J, Battista L, Wing D. Management of pregnancy after radical trachelectomy: case reports and systematic review of the literature. Am J Perinatol 2007;24(9):531–539. 10.1055/s-2007-986680 [DOI] [PubMed] [Google Scholar]
- 32.Plante M, Gregoire J, Renaud MC, Roy M. The vaginal radical trachelectomy: an update of a series of 125 cases and 106 pregnancies. Gynecol Oncol 2011;121(2):290–297. 10.1016/j.ygyno.2010.12.345 [DOI] [PubMed] [Google Scholar]
- 33.Alvarez R, Biliatis I, Rockall A, Papadakou E, Sohaib SA, deSouza NM, et al. MRI measurement of residual cervical length after radical trachelectomy for cervical cancer and the risk of adverse pregnancy outcomes: a blinded imaging analysis. BJOG An Int J Obstet Gynaecol 2018;125(13):1726–1733. 10.1111/1471-0528.15429 [DOI] [PubMed] [Google Scholar]
- 34.Yasmeen S, Romano PS, Schembri ME, Keyzer JM, Gilbert WM. Accuracy of obstetric diagnoses and procedures in hospital discharge data. Am J Obstet Gynecol 2006;194(4):992–1001. 10.1016/j.ajog.2005.08.058 [DOI] [PubMed] [Google Scholar]
- 35.Baumeister L, Marchi K, Pearl M, Williams R, Braveman P. The validity of information on “race” and “Hispanic ethnicity” in California birth certificate data. Health Serv Res 2000;35(4):869–883. [PMC free article] [PubMed] [Google Scholar]
- 36.Braveman P, Pearl M, Egerter S, Marchi K, Williams R. Validity of insurance information on California birth certificates. Am J Public Health 1998;88(5):813–816. 10.2105/AJPH.88.5.813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romano P, Rainwater J, Schembri M, Yasmeen S, Gilbert WM, Boe N, et al. OSHPD Postpartum Maternal Outcomes Validation Study UC Davis: Center for Healthcare Policy and Research. Accessed February 1, 2020. https://escholarship.org/uc/item/20h9h32c [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


