Abstract
Dengue virus serotypes 1-4 (DENV1-4) and Zika virus (ZIKV) are mosquito-borne flaviviruses that induce both virus-specific and broadly-reactive antibodies. A first DENV infection is thought to induce antibodies that wane over two years to titers that can subsequently enhance severe dengue disease, causing large dengue epidemics. Secondary DENV infection with a different serotype is thought to induce stable, cross-serotype protective antibodies. Low dengue disease incidence following the recent Zika pandemic led to the hypothesis that ZIKV infection is also transiently cross-protective. We investigated antibody kinetics in 4189 children up to 11 years after one and multiple DENV and ZIKV infections in longitudinal cohorts in Nicaragua. We used a DENV inhibition enzyme linked immunosorbent assay (iELISA), which measures antibodies associated with protection against dengue and Zika disease and with enhancement of dengue disease severity. Surprisingly, we found that overall DENV iELISA titers stabilized by 8 months post-primary DENV infection to a half-life longer than a human life and waned, although gradually, after secondary DENV infection. Similarly, DENV iELISA titers were stable or rose after primary ZIKV infection but declined in individuals with histories of DENV and ZIKV infection. In contrast, kinetics of anti-ZIKV antibodies post-ZIKV infection were similar regardless of prior DENV immunity. We observed heterogeneity in DENV iELISA titer, suggesting that individual antibody titer setpoint, rather than waning, is important for future dengue disease risk. Together, these findings change our understanding of anti-flavivirus antibody kinetics and have implications for measuring vaccine efficacy and for predicting future dengue and Zika outbreaks.
One sentence summary:
Unexpectedly, cross-reactive antibodies are stable or rise after primary dengue and Zika virus infection and wane slowly post-secondary infection.
INTRODUCTION
Measurement of the quantity, repertoire, and durability of humoral immunity against emerging viral diseases is critical for developing vaccines, introducing appropriate interventions, and predicting outbreaks. Flaviviruses, including dengue virus serotypes 1 to 4 (DENV1-4) and Zika virus (ZIKV), have caused severe epidemics worldwide over the last fifty years (1). Prior infection with DENV induces antibodies that can be protective at high titers but at low to intermediate titers are capable of facilitating viral infection of target myeloid cells through the Fcγ receptor, a mechanism known as antibody-dependent enhancement (ADE). ADE increases the risk for Dengue Hemorrhagic Fever/Dengue Shock Syndrome (DHF/DSS) and other forms of severe dengue disease (2–8). ZIKV infection can also induce low to intermediate titers of anti-DENV antibodies that can enhance DENV2 infection in animal models and increase risk of symptomatic and severe DENV2 infections in humans (8–11). Although it is known that the titer of pre-existing antibodies against DENV and ZIKV is associated with disease outcome, the actual kinetics of anti-DENV and anti-ZIKV humoral immunity remain poorly characterized.
Primary DENV infection is thought to induce cross-serotype reactive antibodies that are initially neutralizing but wane to enhancing titers over time, with only antibodies to the infecting serotype remaining at protective titers (12–18). Early challenge studies describe a period of cross-serotype protection of at least 2 to 9 months (12). Epidemiological studies observed that the time between a first DENV infection and a subsequent symptomatic heterologous DENV infection was two years, after which individuals are at risk of severe disease (13, 14). Statistical models of dengue case data found that a two-year period of cross-serotype protection provided the best model fit (16). Spatiotemporal analyses have shown that dengue cases that occur within two years or within 1 kilometer of one another are more likely to be of the same serotype. In contrast, any two cases are more likely to be caused by different serotypes when they occur more than two years apart, an observation attributed to a two-year period of cross-protective immunity (17, 18). When the Americas experienced low dengue transmission for two years after the Zika pandemic followed by a continent-wide dengue resurgence in 2019 and 2020, it was proposed that ZIKV infection might also transiently cross-protect against dengue (19).
Unlike primary DENV infection, secondary DENV infection with a different serotype induces broadly protective, cross-serotype reactive anti-DENV antibodies that are associated with reduced risk of future dengue disease caused by any of the four serotypes (5, 6, 8, 20–22). It is currently believed that antibodies induced by secondary DENV infection are maintained at high titers over long periods of time. Interestingly, when the second infection is with ZIKV rather than a different DENV serotype it does not provide protection against future dengue disease or severity (8). However, to date, the long-term kinetics of cross-reactive antibodies following secondary DENV or sequential DENV and ZIKV infection have not been well described.
Here, we tested the hypothesis that cross-reactive antibody titers wane after primary DENV and ZIKV infection but are stable after sequential DENV infection or after DENV and ZIKV infection in two pediatric cohort studies in Managua, Nicaragua. Instead, we observed the opposite: cross-reactive antibody titers were stable or increased after one DENV or ZIKV infection but waned more and for longer periods of time in those with multiple prior DENV infections or in those with DENV and ZIKV infections. Our investigations of the longest running pediatric dengue cohort studies provide a detailed characterization of the kinetics and magnitude of antibodies after one and multiple flavivirus infections.
RESULTS
Study population.
We have previously reported that pre-infection DENV and ZIKV iELISA titers (measured the year before infection) are associated with dengue and Zika disease outcome in this population (5, 8). In the present study, we describe the long-term antibody kinetics after distinct DENV and ZIKV infection histories in these cohorts. We followed children after one or multiple DENV and ZIKV infections in a community-based cohort (n=3981) and a hospital-based study (n=208) in Managua, Nicaragua (Table 1). In the community-based cohort study, ongoing since 2004, children ages 2 to 17 years provided a healthy blood sample upon enrollment for measurement of baseline immunity and annually thereafter. Children were followed for many years, making this cohort ideal for evaluating long-lived immunity. Samples were provided in March, generally 4 to 8 months after the peak arbovirus transmission season. Participants who experienced febrile disease (and after 2016, rash) visited the health center and were tested for symptomatic dengue or Zika disease by molecular biological, virological, and serological methods in paired acute and convalescent samples. Each year, annual samples from all participants were tested by three assays side-by-side with the previous year’s samples to determine which children experienced DENV or ZIKV infections. Complete infection histories were based on confirmed dengue and Zika cases as well as seroconversion or greater than or equal to a four-fold rise in DENV or ZIKV antibody titers between paired annual samples (8). In total, 8399 participants at the time of analysis had contributed at least one sample to the study. We measured antibody kinetics in 3981 children for whom an infection history could be determined (fig. S1). This included all children who entered the cohort flavivirus-naïve and had one confirmed DENV or ZIKV infection as well as those who then experienced two sequential confirmed DENV infections (DENV-DENV) or DENV and ZIKV infections (DENV-ZIKV). We also followed children who experienced at least two confirmed DENV infections (2+DENV), including those with three or more confirmed DENV infections as well as those who entered DENV-immune and had one or more confirmed DENV infections. Some in the 2+DENV group experienced a subsequent ZIKV infection (2+DENV-ZIKV) and were analyzed separately.
Table 1.
Characteristics of participants in the community-based cohort and hospital-based studies with documented infection histories.
| Characteristics | Cohort study N (%) |
Hospital study N (%) |
|---|---|---|
| Total participants | 3981 | 208 |
| Sex | ||
| Female | 2018 (51%) | 96 (46%) |
| Male | 1950 (49%) | 112 (54%) |
| Age (years, first infection) | ||
| 2 to 5 years | 1233 (31%) | 35 (17%) |
| 6 to 9 years | 1542 (39%) | 69 (33%) |
| 10+ years | 1189 (30%) | 90 (43%) |
| Infection history1 | ||
| DENV/primary dengue | 1201 | 101 |
| DENV-DENV | 363 | |
| 2+DENV/secondary dengue | 1022 | 107 |
| ZIKV | 1530 | |
| DENV-ZIKV | 478 | |
| 2+DENV-ZIKV | 152 |
Some individuals had more than one infection and contribute to multiple infection history groups.
All annual and acute and convalescent samples were tested for anti-DENV1-4 antibodies using the DENV inhibition ELISA (iELISA), which we have previously shown measures cross-reactive antibodies to DENV1-4 and ZIKV such as those targeting the envelope (E) protein fusion loop and the premembrane (prM) protein (5, 8, 23). The databases of DENV iELISA titers have been extensively characterized and shown to have low assay variability and to be highly reproducible (5). Starting in 2016, samples were also tested for antibodies to the ZIKV E domain III (EDIII) using a ZIKV iELISA as well as antibodies to ZIKV non-structural protein 1 (NS1) using a blockade-of-binding (BOB) ELISA, assays that have also been evaluated previously (24–26). The ZIKV iELISA, like the DENV iELISA, is a competition ELISA that detects heterotypic antibody titers (8), whereas the ZIKV NS1 BOB is highly specific to ZIKV.
In the hospital-based study, ongoing since 2005, children ages 6 months to 14 years enrolled upon disease presentation (days 1 to 7 post symptom onset) and were followed during treatment and recovery. Blood samples were collected during illness, at convalescence (days 14 to 28), and at 3, 6, 12, and 18 months post-infection, making it ideal for investigating the early kinetics following infection. All samples were tested by the DENV iELISA. Primary versus secondary dengue disease was defined by convalescent DENV iELISA titer based on cut-offs developed for the gold-standard hemagglutination Inhibition assay (HAI) and evaluated previously (27–29).
Infection history is the strongest determinant of DENV iELISA titer magnitude and kinetics.
We first tested whether DENV iELISA titer magnitude and kinetics measured greater than 0.5 years post-infection differed by age, sex, disease during prior flavivirus infection, and infection history using linear mixed models. In the community-based cohort, the strongest predictor of DENV iELISA titer was infection history (Fig. 1, fig. S2, data file S1). Compared to primary DENV infection, secondary DENV and sequential DENV and ZIKV infections induced higher magnitude titers and more rapid decay (p<0.001), whereas primary ZIKV infection induced lower magnitude titers but greater titer increase (p<0.001) (Fig. 1A). Female sex (p<0.01), older age (p<0.01), and past symptomatic DENV infection (p<0.001) were associated with higher magnitude DENV iELISA titers (Fig. 1A). Older age (p<0.001) and past inapparent infection (p<0.01) were associated with more rapid decay (Fig. 1B). In 2012 and after, data on obesity and socioeconomic status were also available; however, neither variable was a determinant of antibody magnitude or kinetics (data file S2). Notably, even for the best-fit model, which explained 93% of the variance in DENV iELISA titers, these covariates explained only 53% of the heterogeneity in antibody magnitude and kinetics, suggesting many modifying factors remain unidentified.
Fig. 1. Anti-DENV antibody magnitude and kinetics are strongly determined by infection history.
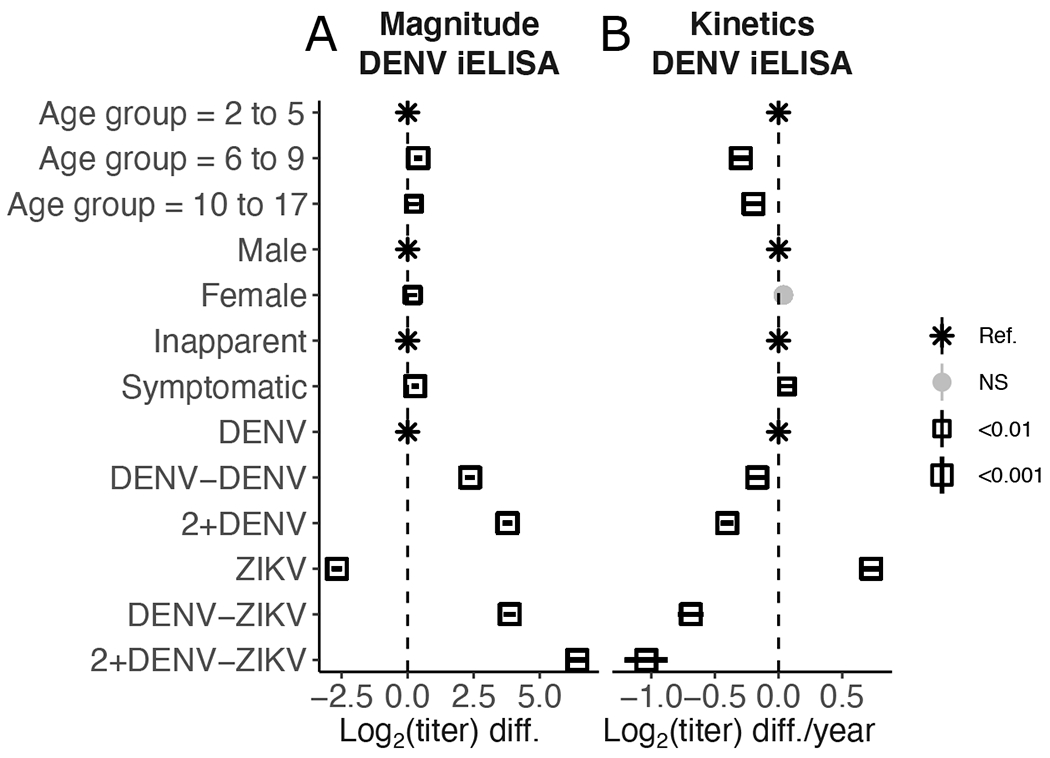
(A and B) Fixed effects of covariates on antibody titer magnitude (A) and kinetics (B) in the community-based cohort. Effects for age, sex, prior symptomatic infection, and infection history were estimated using linear mixed models fit to DENV iELISA titer data collected greater than 0.5 years post-infection. Point shape, size, and shade represent significance of estimated effects. Ref., reference; NS, not significant; diff., difference.
Among symptomatic dengue cases in the cohort study, secondary dengue was also the strongest determinant of DENV iELISA titer magnitude (p<0.001) and was also associated with more rapid antibody decay (p<0.05) (data file S3). Female sex (p<0.05) and older age (p<0.05) were associated with modestly higher titers. In the hospital study, older age (10-17 years, p<0.001) was associated with higher magnitude titers, whereas secondary dengue was associated with more rapid decay (p<0.05) (fig. S3, data file S4). Disease severity (DHF/DSS or Dengue with Warning Signs/Severe Dengue) was not associated with a detected difference DENV iELISA titers in either the cohort or hospital studies (data file S3 and S4). In the cohort study, dengue cases that were negative by reverse transcription polymerase chain reaction (RT-PCR) (p<0.001) or caused by DENV3 (p<0.01) had lower magnitude titers, whereas antibodies after DENV2 infection (p<0.01) decayed more rapidly (data file S3). We did not observe any differences by infecting serotype in the hospital-based study (data file S4).
Anti-DENV antibodies reach stable setpoints after primary DENV infection.
To test whether anti-DENV antibodies waned from protective to enhancing titers in the first two years after a single DENV infection, we followed children after their first DENV infection in both the hospital-based study (n=101; 505 titers) and community-based cohort study (n=1201 children; 5117 titers). For all analyses, we modeled the log2 antibody titers as a function of time post-infection using generalized additive mixed models (GAMM) (Fig. 2A and B). GAMMs are, by definition, flexible and do not incorporate specific assumptions about decay kinetics like exponential or power-law decay models; GAMMs were used to identify relevant inflection points in antibody kinetics (data file S5). We then modeled each ‘phase’ of decay using multi-phasic linear mixed models with bootstrap confidence intervals, which produce estimates of half-life that are easier to interpret and are more comparable across studies (Fig. 2C and D, accounting for various sources of error in time and titer estimates, fig. S4). A positive half-life indicates waning antibody titers, whereas a negative half-life indicates increasing antibody titers. Gradual antibody decay or growth correspond to half-lives around infinity, and confidence intervals that span infinity indicate no change in titer kinetics.
Fig. 2. Anti-DENV antibody titers after primary DENV infection reach stable setpoints.
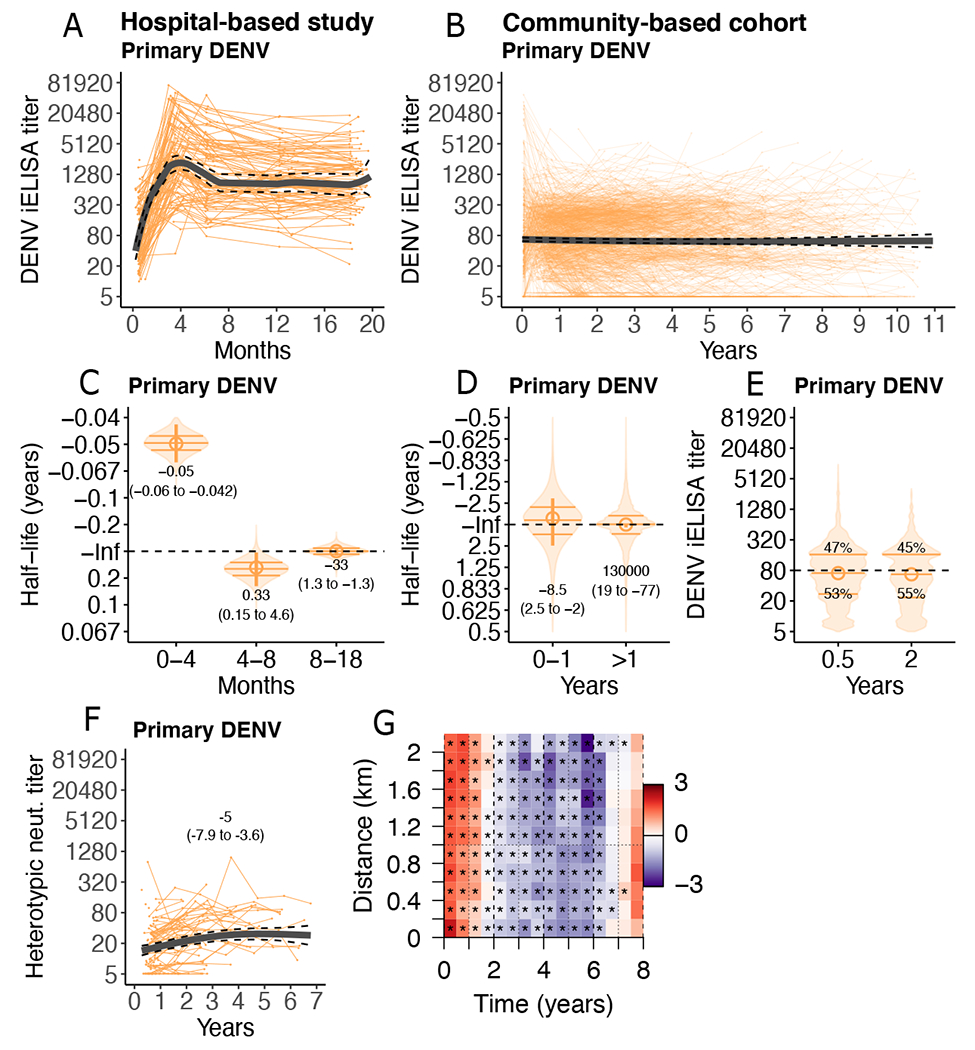
(A and B) Individual trajectories of DENV iELISA titers (reciprocal serum dilution) in children following primary DENV infection in the hospital-based study (n=101; 505 titers, A) and community-based cohort (n=1201; 5117 titers, B). Group GAMM models (thick lines) with 95% confidence intervals (CIs, dashed lines) are shown. (C and D) Bootstrap sampling of multi-phasic linear mixed models of individual half-lives (violin plots, with quartiles marked as horizontal bars) and group half-lives (circles, 95% CI marked as vertical lines, with printed estimates) after primary DENV infection in the hospital-based study (C) and community-based cohort (D) are shown. Inf indicates infinity (horizontal dashed line). (E) Bootstrap distributions from multi-phasic linear mixed models of individual DENV iELISA titer setpoints (violin plots, horizontal bars indicate quartiles) for the community-based cohort are shown. Printed estimates indicate percent of children below versus above the indicated threshold (1:80; horizontal dashed line). (F) Heterotypic DENV neutralizing (neut.) titers for a subset of primary DENV infections in the community-based cohort (n=108) are shown, with GAMM fit and 95% CI, and printed half-life estimates from a linear mixed model. (G) Log-ratio of the pairwise difference in date of symptom onset and home location between symptomatic dengue cases of the same versus different serotypes in the community cohort is shown. Red indicates more homologous cases, purple more heterologous cases. Stars mark ratios statistically significantly different from 1 (p-values <0.001).
In the hospital study, DENV iELISA titers increased between 14 days and 4 months post-symptom onset (half-life [t1/2]: −0.050 years, 95% confidence interval [CI] −0.06- to −0.042) then declined between 4 and 8 months (t1/2: 0.33 years, 0.15 to 4.6) (Fig. 2A and C). However, between 8 and 18 months, DENV iELISA titers were stable, with a half-life of −33 years (1.3 to −1.3) (Fig. 2C, magnitude in fig. S5). Strikingly, in the community-based cohort, DENV iELISA titers were stable from 1 to 11 years post-infection, with a half-life far exceeding that of a human life (estimate: 130,000 years, 95% CI: 19 to −77 years) (Fig. 2B and D, fig. S6). Only 11% of children experienced rapid antibody decay (t1/2 of less than 2 years). Younger individuals and those with past inapparent DENV infections were significantly more likely to be in the rapid decay group (defined as less than 8.6 years based on k-means clustering analysis and logistic regression adjusted for age and sex, p= 0.007). Among symptomatic dengue cases, greater decay was observed following DENV2 infection (p=0.03) compared to DENV1 infections, but not by disease severity (data file S3).
Across all DENV iELISA titer measurements in the hospital-study and community-based cohort, children settled to antibody titers or ‘setpoints’ that were stable over time but were heterogenous across individuals. An analysis of equivalent timepoints in both cohorts (0.4 to 0.7 years and 1.4 to 1.7 years) showed that past symptomatic and severe dengue (p<0.001, compared with inapparent infections, which make up 74% of infections in the cohort study) and older age (p<0.05 for age greater than 10 years, which is more common in the hospital study) were determinants of higher titer magnitude (data file S6). In the community-based cohort, DENV iELISA titers differed by individual, with 53% of children settling to titers below 1:80 (Fig. 2E, fig. S7). Individuals with past inapparent infections were significantly more likely to have titers less than 1:80 (p<0.001). We and others have previously shown that DENV iELISA titers between 1:21 and 1:80 (or HAI titers of 1:10 to 1:40) are associated with the highest risk of future severe dengue disease (5, 6, 8). Viral load may be a contributing factor: among symptomatic dengue cases in the cohort, as each log10 viral load was associated with a 1.2-fold difference in DENV iELISA titer (p=0.036, linear mixed model, accounting for day of symptom onset, age, sex, and disease severity, data file S7) (7). Past infecting serotype was also a predictor of antibody titer magnitude, with significantly lower magnitude titers for DENV3 (p=0.039) and RT-PCR negative cases (p<0.001) compared to DENV1 cases.
We further evaluated whether neutralizing antibody titers were stable for a subset of children in the cohort. Serum neutralizing antibodies were measured post-primary infection using DENV1-4 reporter virus particles in a flow cytometry-based neutralization assay using human Raji-DC-SIGNR cells (14, 30). In this group, cross-reactive neutralizing antibody titers (geometric mean of titers to the three non-infecting serotypes) also did not decline, and in fact significantly increased over time (P <0.001, Fig. 2F).
Primary DENV infection with a given serotype is thought to induce protective antibodies that transiently reduce individual risk of heterotypic disease as well as epidemics of other serotypes. These cross-reactive antibodies were thought to wane to enhancing titers by two years post-infection. We observed here that DENV iELISA titers were stable by about 8 months after a first DENV infection, suggesting that individual immunity may not explain why disease risk is elevated across the population only after two years post-infection (12–18). We hypothesized that perhaps lower probability of exposure to a different serotype due to temporal and spatial patterns of transmission, rather than individual antibody waning, may help explain the 2-year time-lag to dengue disease. We measured the spatial and temporal relationship between serotyped, RT-PCR-confirmed dengue cases in the community-based cohort. We observed that dengue cases in different individuals that occurred less 1.5 years apart were more likely to be caused by the same serotype, whereas cases that occurred greater than 2 years apart were more likely to be caused by different serotypes (Fig. 2G). When we adjusted for temporal variation in cases, we did not observe strong evidence for spatial clustering, although cases that occurred closest together in time and space (less than 6 months and less than 200m) were more likely to be homologous (fig. S8). Thus, a two-year gap between infections caused by different serotypes can be observed in a population with individual antibody waning only lasting 8 months.
Anti-DENV antibodies wane rapidly then gradually after secondary DENV infection.
We initially hypothesized that cross-reactive anti-DENV antibody titers are maintained at high titers after secondary DENV infection. To test this hypothesis, we measured DENV iELISA titers after secondary dengue in the hospital-based study (n=107; 535 titers) and after exactly two DENV infections (DENV-DENV) or two or more prior DENV infections (2+DENV) in the community-based cohort (n=1319; 5884 titers) (Fig. 3A to C). After hospitalized secondary dengue, DENV iELISA titers waned rapidly in the first 4 months (t1/2: 0.056 years, 0.048 to 0.064), followed by gradual waning up to 8 months (t1/2: 0.14, 0.085 to 0.35) (Fig. 3A and D). Thereafter, DENV iELISA titers stabilized (t1/2: 26 years, 1.3 to −1.6). In the community-based cohort, rapid waning was observed in the first year after exactly two DENV infections (t1/2: 0.30, 0.21 to 0.39) (Fig. 3B and E). Similar rates of waning were observed in those known to have experienced at least two prior DENV infections (t1/2: 0.23 years, 0.20 to 0.25) (Fig. 3C and F). Unexpectedly, between 1 and 8 years, DENV iELISA continued to gradually decline in both groups (t1/2: DENV-DENV, 6.8 years, 3.1 to 8.9; 2+DENV, 4.2 years, 3.2 to 4.4) (Fig. 3B and C, E and F). When the 2+DENV group was divided into smaller subgroups, those with three or more DENV infections had less long-term decay (t1/2: 17 years, 5.7 to −57), than those who entered immune and experienced one DENV infection (t1/2: 3.6 years, 2.7 to 4, fig. S9). Among symptomatic secondary dengue cases, we did not observe any differences by infecting serotype or viral load (data file S3 and S7); however, RT-PCR negative cases had significantly more rapid decay (p= 0.029, data file S3).
Fig. 3. Anti-DENV antibody titers after secondary DENV infection wane rapidly, then gradually decline.

(A to C) Antibody kinetics are shown for children in the hospital-based study (n=107; 535 titers, A), and with two prior DENV infections (n=363; 1330 titers, B) or two or more prior DENV infections (n=1022; 4554 titers, C) in the community-based cohort. Individual DENV iELISA titer trajectories (presented as reciprocal serum dilutions) and group GAMM fit (black line; 95% CI, dashed lines) are shown for children following secondary DENV infection. (D to F) Individual half-lives (violin plots) and group half-lives (vertical bars and circle, with printed estimates) are shown for children in the hospital-based study (D) and the community based study with two prior DENV infections (E) or with two or more prior DENV infections (F). Half-lives were estimated using bootstrap sampling and multi-phasic linear mixed model and are printed for each panel. Dashed horizontal lines infinity (no significant decay).
Others have previously described three periods of antibody kinetics, lasting from 0 to 1 year, 1 to 3 years, and greater than 3 years (31, 32). We re-analyzed the post-secondary DENV infection data to test for intermediate versus long-term waning (fig. S10). At greater than 3 years, we observed more gradual waning for the DENV-DENV group (t1/2: 18, 4.6 to 39) and 2+DENV group (t1/2: 8.8, 7.0 to 18) than at earlier time points. The relationships between individual estimates of titer magnitude and kinetics for the long-term waning period were visualized in a two-dimensional plot, with contour shapes indicating the regions with the highest density of points for each infection history (Fig. 4). Both DENV-DENV and 2+DENV infection histories are shown; primary DENV infection is also shown for comparison. Radial guidelines indicate time at which individuals with that titer magnitude and half-life are expected to have titers below a serum dilution of 1:80. This type of diagram is similar to plots presented in other studies of long-lived protective antibodies (33). The majority (51%) of children with secondary DENV infection histories were predicted to sustain antibodies above enhancing titers (with enhancement defined here as less than 1:80 based on previous observations (5, 6)) for a minimum of 10 years (Fig. 4). However, 21% were predicted to have titers drop into the enhancing range within 5 years (Fig. 4). For 5.8% of children per year (CI: 2.4 to 9.2%), raw DENV iELISA titers dropped to the titers observed immediately before their secondary DENV infection, although only 1% dropped more than 2-fold below pre-secondary titers.
Fig. 4. Contour plot summarizes long-term half-lives and antibody magnitude measured at greater than 3 years post-infection.
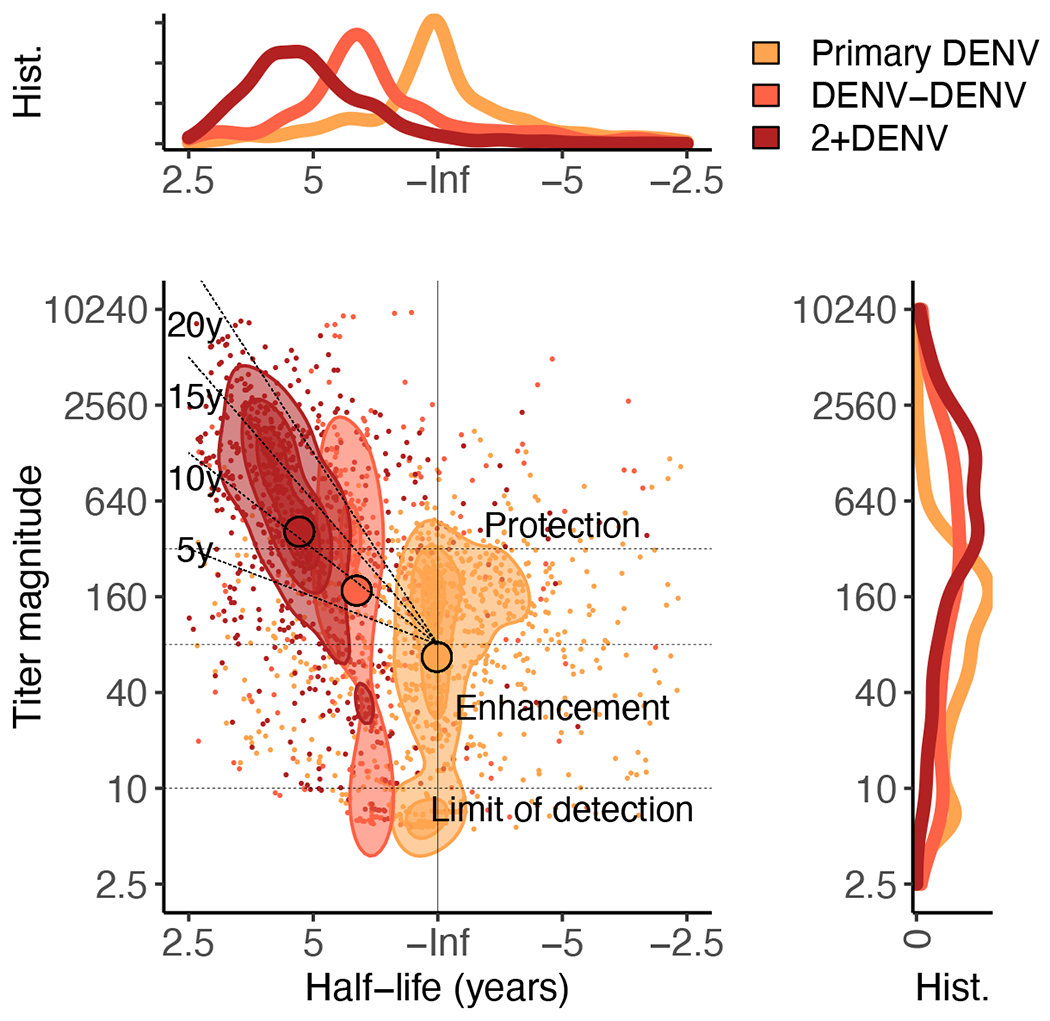
All individual estimates for primary and secondary DENV infections in the community-based cohort are shown. Contour shapes mark regions with the highest density of points for each infection history. Black horizontal guide lines show previously estimated titers of protection and enhancement (5, 8), radial lines indicate expected time (years post-infection) to DENV iELISA titers dropping to enhancing titers (less than 1:80). Histograms show the distributions of half-lives (top) or magnitudes (right) for each infection history.
Anti-DENV antibodies are stable or increase after primary ZIKV infection.
We have previously shown that ZIKV infection can induce antibodies detectable by the DENV iELISA and that these antibodies are associated with enhanced risk of future symptomatic and severe dengue (8). Here, we tested whether ZIKV infection initially induced high DENV iELISA titers that waned over time in 1530 children (4534 titers) in the three years following the Zika epidemic (2017 to 2019). Instead of waning, we found that DENV iELISA titers increased for multiple years after primary ZIKV infection (t1/2: −1.8 years, 95%CI: −2.2 to −1.7) (Fig. 5A). As a result, the fraction of children with DENV iELISA titers in the range associated with enhancement of DHF/DSS increased from 46% to 77% between the Zika epidemic and the subsequent dengue epidemic. Older age was associated with higher magnitude (p<0.01) and modest increases in titer (p<0.001), whereas symptomatic ZIKV infections was only associated with more gradual titer increases (p<0.001) (data file S1). It is possible that re-exposures contributed to this rise in antibodies; however, rates of DENV and ZIKV infection were very low during this period, with only 1.2% of flavivirus-naïve children seroconverting in 2017 and 0.3% seroconverting in 2018. Thus, we observed that anti-DENV iELISA titers increase after primary ZIKV infection, even in years with low DENV and ZIKV transmission.
Fig. 5. Anti-DENV and anti-ZIKV antibody titers display distinct kinetics after ZIKV infection.
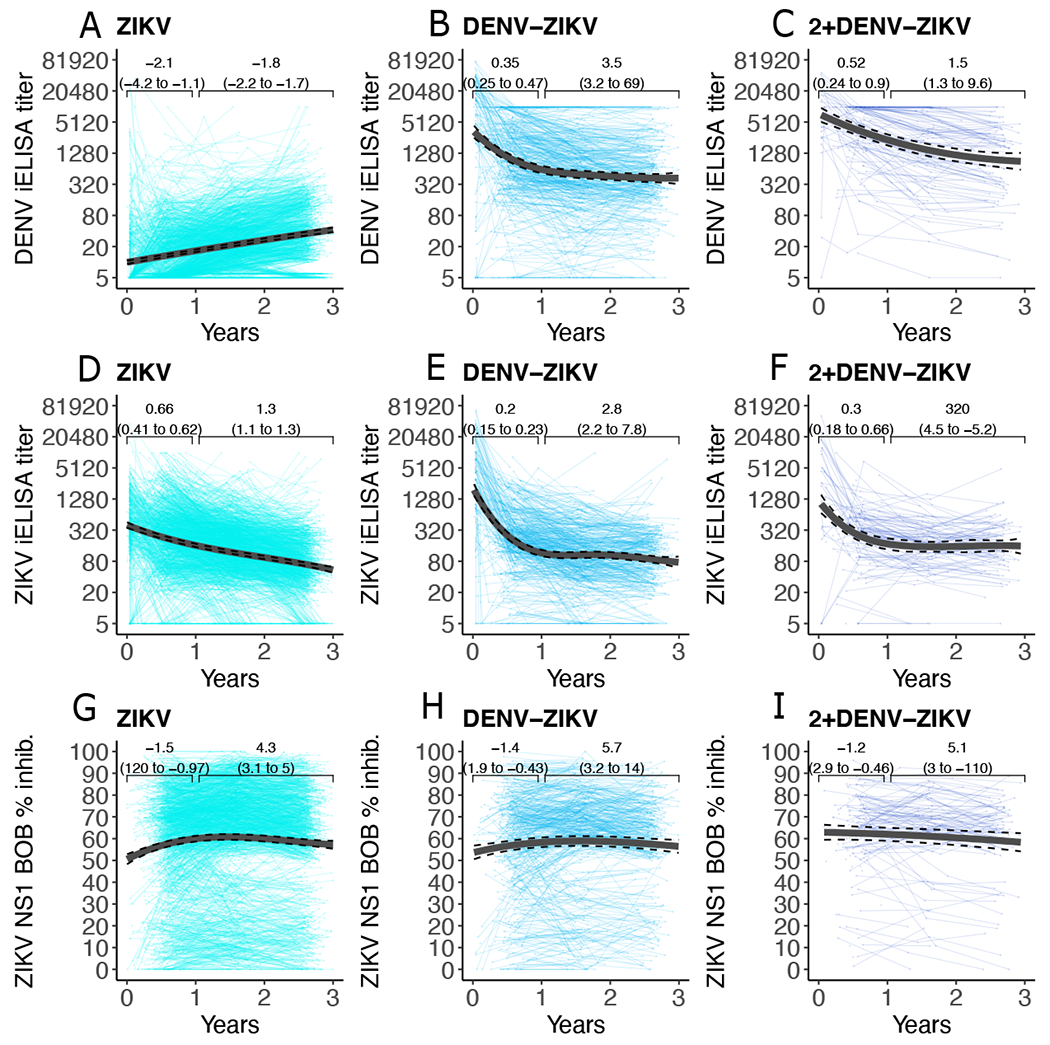
Antibody kinetics in the community-based cohort, as measured using the DENV iELISA (A to C), ZIKV iELISA (D to F), and ZIKV NS1 BOB assay (G to I) are shown. Data are shown for 0 to 3 years following primary ZIKV infection (A, D, G, n= 1530 children, 4534 titers), one DENV and one ZIKV infection (DENV-ZIKV, B, E, H, n=478 children, 1324 titers), and two or more DENV infections followed by ZIKV infection (2+DENV-ZIKV, C, F, I, n=152 children, 384 titers). Individual trajectories and group GAMM fits (black line; 95% CIs, dashed lines) are shown. Brackets indicate group linear mixed model half-lives and 95% CIs.
Anti-DENV antibodies wane in individuals with prior DENV and ZIKV infection.
We previously showed that ZIKV infection does not act like a secondary DENV infection; in those with monotypic DENV immunity, a subsequent ZIKV infection fails to reduce future risk of severe dengue disease (8). Here, we tested whether antibody kinetics after sequential DENV and ZIKV infection differed compared to kinetics observed after secondary DENV infection. Our analyses included those with one DENV followed by one ZIKV infection (DENV-ZIKV, 478 children, 1324 titers) as well as two or more DENV infections followed by one ZIKV infection (2+DENV-ZIKV, n=152 children, 384 titers). Interestingly, antibody kinetics in the sequential DENV and ZIKV infection groups were similar to those seen after secondary DENV infection, with initially rapid decay (DENV-ZIKV, t1/2: 0.35 years, 0.25 to 0.47; 2+DENV-ZIKV, t1/2: 0.52,0.24 to 0.90) followed by more gradual long-term decay (DENV-ZIKV, t1/2: 3.5 years, 3.2 to 69; 2+DENV-ZIKV, t1/2 1.5, 1.3 to 9.6) (Fig. 5B and C).
We previously found that in the year before the 2019 dengue epidemic, those with a history of DENV-ZIKV infection had higher DENV iELISA titers than those with DENV-DENV infection. A similar difference was observed between the 2+DENV-ZIKV group and the 2+DENV group (8). This observation was unexpected because the high DENV iELISA titers induced by DENV-ZIKV infection did not translate into protection against future dengue disease. We revisited this observation and compared the DENV-ZIKV to DENV-DENV group using linear mixed effects models. Even after adjustment for age, sex, and past disease, the DENV-ZIKV group on average had DENV iELISA titers 4.0-fold higher than the DENV-DENV infection group (p<0.001); similarly, the 2+DENV-ZIKV group had 11.3-fold higher titers than the 2+DENV group (p<0.001) (data file S1).
Prior DENV infection does not affect the kinetics of ZIKV-specific antibodies following ZIKV infection.
Others have shown that ZIKV infection induces neutralizing antibodies that target ZIKV EDIII and ZIKV NS1, and both antigens are being used as Zika vaccine candidates and to measure ZIKV seroprevalence (24, 26, 34–36). Here, we measured the kinetics of antibodies that compete with an anti-ZIKV EDIII monoclonal antibody for binding to ZIKV virions using the ZIKV iELISA (25, 26) and antibodies that compete with an anti-ZIKV NS1 monoclonal antibody for binding to ZIKV NS1 using the ZIKV NS1 BOB assay (24, 26). After both primary ZIKV and sequential DENV and ZIKV infection, ZIKV iELISA titers declined rapidly out to one year, then decreased gradually thereafter (Fig. 5D to F). ZIKV NS1 BOB inhibition increased modestly out to one year, then decreased very gradually after one year, again with similar kinetics across infection histories (Fig. 5G to I). The kinetics of ZIKV-specific antibodies were similar between those with and without prior DENV infections: infection history was not an important predictor of ZIKV iELISA titers or ZIKV NS1 BOB inhibition (fig. S11; data file S8). Female sex (p<0.05), younger age (p<0.001), and past inapparent ZIKV infection (p<0.001) were associated with slightly higher magnitude ZIKV iELISA, whereas older age (p<0.001) was associated with more gradual decay (fig. S11). Female sex (p<0.001), older age (p<0.05), and being overweight or obese (p<0.01) were associated with slightly higher ZIKV NS1 BOB percent inhibition, consistent with previous observations (fig. S11, data file S2 and S8) (37).
DISCUSSION
It was previously thought that after primary DENV infection or primary ZIKV infection, anti-DENV antibodies wane to enhancing titers that lead to subsequent dengue disease and large epidemics. Here, we evaluated the kinetics of cross-reactive anti-DENV antibodies up to 11 years after one or multiple DENV and ZIKV infections in thousands of children. Our findings suggest that, instead of waning over many years, each individual reaches a long-term antibody setpoint by about 8 months after primary DENV or ZIKV infection. In contrast, anti-DENV antibodies induced after secondary DENV or sequential DENV and ZIKV infection wane rapidly for 8 months and then gradually decay over longer periods. We also evaluated kinetics of ZIKV-specific antibodies and found that they undergo different kinetics compared to anti-DENV antibodies, suggesting that antibody kinetics to flaviviruses are specific to each infecting virus and viral antigen. Our findings also reveal extensive heterogeneity in individual titers, suggesting that individual setpoint, rather than time since prior infection, may determine future dengue and Zika disease risk (5, 8) Together, these findings challenge existing theories about the relationship between the kinetics of anti-DENV and anti-ZIKV antibodies, disease outcomes, and DENV and ZIKV transmission dynamics.
Many viruses and viral vaccines stimulate long-lived plasma cells that continuously secrete antibodies and result in serum antibody titers with half-lives that are close to life-long (32, 38). These long-lived plasma cells neither divide nor require replenishment from the memory B cell population, suggesting that their fate is determined or ‘imprinted’ at the time of infection (39). The specific factors governing the germinal center reaction, affinity maturation, and the induction of circulating memory B cells and long-lived plasma cells is complex and remains an active area of research (40). However, highly repetitive antigens, such as whole virions, effectively cross-link B cells and induce strong cellular signaling associated with very long-lived plasma cells and serum antibody titers (32, 41). The magnitude or setpoint of serum antibodies is thought to be a function of the initial viral or antigenic threshold (39). It is difficult to directly interrogate the long-lived plasma cells response, as it requires bone marrow samples; thus, here we examine serum antibodies, as done in other studies. We found that on average, anti-DENV antibodies measured using the DENV iELISA stabilize to long-term antibody titers, or setpoints, by 8 months after primary DENV infection. Although this observation is counter to the existing theory of the kinetics of anti-DENV antibodies, it is consistent with smaller, earlier studies showing that both anti-DENV binding and neutralizing antibody titers are stable and even increase after a first DENV infection (6, 30, 42, 43). Antibody ‘boosting’, or re-infections with either the same or different serotypes that stimulate immunity but fail to reach the cut-off to be defined as a new inapparent infection, may help maintain these titers (30, 43, 44). However, our observation that antibody titers are sustained across thousands of individuals in two distinct studies suggests that antibody stability may be a general feature of post-primary cross-reactive flavivirus immunity.
Unexpectedly, we also observe that anti-DENV antibodies measured using the DENV iELISA but induced by primary ZIKV infection increased up to a year and more after infection. A similar pattern was observed for cross-neutralizing antibodies induced by primary DENV infection. We have previously found that DENV iELISA titers (which are thought to correspond to cross-reactive antibodies) in the hospital-based study peaked later, at the three-month timepoint, than serotype-specific neutralizing antibodies, which peaked at convalescence (42, 45–47). Together, these findings suggest that antibody cross-reactivity and breadth are stable or even increase in the early period after primary flavivirus infection. These observations may be consistent with recent findings that the process of affinity maturation is longer than a few months. A recent study found yellow fever virus-specific memory B cells increase in binding affinity up to at least 9 months post-vaccination (48), whereas we and others have shown that memory B cell breadth also increases up to at least a year following primary DENV and ZIKV infections (49–51).
Although we observed that individual antibody waning after primary DENV infection lasted less than two years, we still found a two-year lag across the population in disease caused by heterologous serotypes. This may be in part related to local epidemiology and the dynamics of introduction of new DENV serotypes. Mechanistic transmission models fit to dengue epidemic data have generally considered immune interactions between DENV serotypes and include parameters that modulate enhancement or cross-protection (16, 52–57). Although unexpected, DENV and ZIKV transmission models with individual cross-protection lasting less than one year can produce epidemics two or more years apart (58). Further, models incorporating spatial heterogeneities alone can also convincingly reproduce natural DENV1-4 epidemic dynamics, without requiring parameters for antibody waning or enhancement (59). Alternatively, perhaps individual antibody kinetics following secondary DENV infection are an underappreciated factor modulating dengue epidemic periodicity and severity. In support of this hypothesis, we also observed that anti-DENV antibodies rise in the years after primary ZIKV infection but wane after sequential DENV and ZIKV infection. These trends may have contributed to a greater proportion of individuals with enhancing quantities of anti-DENV antibodies two to three years after the Zika pandemic than immediately afterwards. Whether these changes in anti-DENV antibodies in this population help explain the later resurgence of dengue requires further modeling studies. Overall, our observations show that individual antibody kinetics following primary DENV infection do not directly predict population epidemic dynamics, an important consideration for vaccine evaluation and epidemic preparedness.
Secondary DENV infection induces broadly neutralizing antibodies that confer long-term protection against future dengue disease, although symptomatic and severe dengue can occur (14, 20, 22). After secondary DENV and sequential DENV-ZIKV infection, we observed that anti-DENV antibody titers were highest at convalescence, consistent with an early and rapid plasmablast response (60). Antibody titers waned rapidly out to 8 months, and then gradually waned thereafter. When we measured three periods of waning – 01 year, 1-3 years, and 3 years onward, we observed that titers waned even more slowly beyond three years, consistent with previous observations of long-lived antibody kinetics following vaccination (32). The observation that anti-DENV antibodies may wane for longer periods of time after secondary infection, in some individuals to titers seen before secondary infection, may mean that re-exposure to DENV is important for maintaining protective antibody titers in those whose ‘plateau’ or setpoint is below a protective titer (61). Alternatively, differences in antibody repertoire between primary and secondary DENV immunity, such as the presence of broadly neutralizing antibodies targeting the E-dimer epitope (EDE), may mean that post-secondary immunity is protective even if DENV iELISA titers are not higher than after primary DENV infection (20, 21). Consistent with this hypothesis, one DENV infection followed by ZIKV infection induced high titers of cross-reactive anti-DENV antibodies as measured by the DENV iELISA, similar to after sequential DENV infections, but without providing protection, suggesting differences in antibody quality (8). This observation is consistent with studies showing that antibodies cross-reactive to DENV and ZIKV strongly bind but only weakly neutralize DENV and ZIKV and that they primarily target flavivirus-conserved epitopes associated with enhancement, such as the fusion loop (34, 62). Another possible explanation is that sequential DENV and ZIKV infection may fail to stimulate as strong of a T cell response as secondary DENV infection, leaving individuals unable to mount a rapid and effective cellular immune response against future DENV infection (63).
We also observed that the kinetics of ZIKV-specific antibodies were similar following primary ZIKV and sequential DENV-ZIKV infection. This finding is consistent with previous studies showing that in both DENV-naïve and DENV-experienced individuals, ZIKV-specific memory B cells, and in all likelihood, serum antibodies, arise from naïve B cells (34, 51, 62). We also found that type-specific antibody kinetics depend on the viral antigen, as antibodies to the ZIKV EDIII waned whereas antibodies to the secreted NS1 protein increased over time. Recent studies suggest that antibodies targeting epitopes present only on the ZIKV virion and that are virus-maturation state-insensitive, rather than antibodies targeting EDIII, are likely key determinants of protection (64, 65). These distinct antibody kinetics indicate differences in the quality of antibodies each antigen induces and highlight the importance of measuring the relevant antibody populations in studies of immune correlates.
Even after accounting for similar kinetics by infection history, virus, and antigen specificity, we observe extensive heterogeneity in the magnitude of antibody setpoints, which can be associated with future dengue disease risk. These differences are only partially explained by other measured covariates such as age, sex, and the severity of the previous infection. Most individuals reached their long-term antibody setpoints by 8 months post-infection, which coincides with the timing of the loss of broad cross-serotype protection after the final vaccine dose in Phase 3 studies of the licensed dengue vaccine (66). The 8-month time-point may thus be useful for measuring immunity in vaccine studies; however, participants should still be followed for 4 to 5 years post-vaccination to detect changes in vaccine efficacy and safety (66).
Our study has several limitations. Although we modeled anti-DENV antibody titers in the longest-running cohort study of dengue available to date, our data still only followed individuals for a maximum of 11 years, which is far less than an average human life. Further, because we only followed a pediatric cohort, antibody kinetics may differ in an adult population or in a non-endemic population. Further studies are needed to measure the long-term antibody half-lives for DENV over decades to measure the true longevity of antibodies derived from long-lived plasma cells (38, 67). Another limitation of the cohort study was that we were only able to sample children once per year: more frequent sampling may reveal finer-scale kinetics, although frequent sampling often leads to greater loss to follow-up. Further, we only measured antibody kinetics with a limited set of assays: additional studies tracking the specificities of neutralizing antibodies or antibodies targeting specific protective epitopes are underway and will likely provide further information on long-lived protective immunity.
Here, we identified four key features of long-term antiviral humoral immunity, including differences in antibody kinetics by primary versus secondary infection, infecting virus (DENV versus ZIKV), antigen specificity, and individual, using the largest and longest running cohort study of individual DENV and ZIKV immunity. Collectively, this work is of broad relevance to ongoing efforts to evaluate DENV and ZIKV vaccines and predict the risk of future dengue and Zika epidemics, as well as to define the kinetics of antibodies to other emerging viral diseases.
MATERIALS AND METHODS
Study design.
The objective of this study was to quantify the magnitude and kinetics of anti-DENV and anti-ZIKV antibodies following sequential DENV and ZIKV infection. We conducted a retrospective analysis of serological data collected as part of two longitudinal observational cohort studies in Nicaragua, following the guidelines in the Strengthening the reporting of observational studies in epidemiology (STROBE) checklist. Participants were not randomized nor was blinding conducted as part of the study. In total, we measured anti-DENV antibodies in 58,600 samples. Only individuals for whom DENV and ZIKV infection histories could be classified (see infection history criteria below) were included in our analysis. In total, we studied 17,714 independent samples from 4,135 individuals. Each sample is titered at least twice for both anti-DENV and anti-ZIKV antibodies: once in the first year it is collected, paired with the previous year’s sample, and again the following year, paired with the next year’s sample. All samples are tested two or more times in independent experiments (5). Sample size for the initial cohort and inclusion criteria have been described previously (68). All data available at the time the manuscript was being prepared were included. The primary endpoint was individual and group antibody magnitude and kinetics following each infection history as measured using each assay used in the cohort (DENV iELSIA, ZIKV iELISA, ZIKV NS1 BOB assays).
Ethics statement.
The Pediatric Dengue Cohort Study was reviewed and approved by the institutional review boards of the University of California, Berkeley (protocol: 2010-09-2245), the University of Michigan (study ID: HUM00091606), and the Nicaraguan Ministry of Health (protocol NIC-MINSA/CNDR CIRE-09/03/07-008). The Pediatric Dengue Hospital-based Study was reviewed and approved by the institutional review boards of the University of California, Berkeley (protocol 2010-06-1649) and the Nicaraguan Ministry of Health (protocol NIC-MINSA/CNDR CIRE-01/10/06-13). Parents or legal guardians of all participants provide written informed consent, and participants greater than 6 years old provide assent (68).
Community-based dengue cohort.
The Pediatric Dengue Cohort Study has followed an active cohort of about 3,800 individuals, aged 2 to 14 years, since 2004 and up to age 17 by 2020 in Managua, Nicaragua. The average length of participation was 6.8 years (Standard deviation: 3.5 years). Study participants provide a healthy blood sample each year. Each year, paired annual samples are tested for a rise in serum antibodies using the DENV inhibition ELISA (iELISA) to detect DENV infections. Since 2016, annual samples have also been tested by the ZIKV iELISA and the ZIKV NS1 blockade-of-binding (BOB) assay. During the year, children who present to the study health center meeting the clinical definitions for arboviral disease are laboratory-confirmed by: 1) RT-PCR/viral isolation in acute serum (and urine for Zika); 2) seroconversion by ZIKV, CHIKV, or DENV IgM capture (MAC)-ELISA or 3) ≥4-fold increase in DENV, ZIKV, and CHIKV iELISA in paired acute and convalescent sera (25, 69–72). Ninety percent of acute samples are collected between days 1 to 4 post-symptom onset and convalescent samples between days 14 to 28 post-symptom onset. For symptomatic DENV infections, time since infection is defined as the exact time since symptom onset; post-infection convalescent and annual samples were included in all analyses. For inapparent infections, the exact time of infection is unknown. We randomly sampled infection dates from the distribution of symptomatic infection dates, assigned infection dates to inapparent infections, and measured time relative to the assigned infection date. To account for sources of error in modeling cohort antibody titers, we considered random error in assay measurements. Resulting antibody kinetics remained similar (fig. S4).
Hospital-based dengue study.
We measured the kinetics of DENV iELISA titers following hospitalized primary and secondary dengue for 208 children participating in our hospital-based dengue study, ongoing since 2005 (29, 73). Children were sampled at 14 to 28 days, and 3, 6, 12, and 18 months post-infection; exact time of sampling since infection is known for each study participant. Primary versus secondary DENV infection was defined based on convalescent DENV iELISA titer greater than 1:2560, a cut-off that has been previously established in this study and corresponds to cut-offs in the hemagglutination inhibition assay, the gold-standard assay used for this purpose (27–29).
Serological assays.
The methods for the DENV iELISA, ZIKV iELISA and ZIKV NS1 BOB have been described in detail elsewhere (5, 24, 25). Briefly, in the DENV iELISA, plates are coated with equivalent titers of DENV1-4 antigen of DENV prototype strains (KM204119, KM204118, KU050695, KR011349) (5, 28, 74, 75). Until first seroconversion, annual samples are tested at a 1:10 dilution. Thereafter, all samples are tested in 10-fold serial dilutions from 1:10 to 1:10,000. Sample antibodies are competed for binding with a peroxidase-labeled (EZ-Link Plus Activated Peroxidase Kit, Thermo Scientific, Waltham, MA) polyclonal mix of high-titer DENV-specific antibodies (in house preparation). Antibody titers are estimated using the Reed-Muench method (76). The ZIKV iELISA is based on the same principle as the DENV ELISA, except that sample antibodies compete for binding to ZIKV antigen (strain MR766 (28)) with conjugated monoclonal antibody (mAb) ZKA64 (Humabs Biomed SA) (26), a potent ZIKV-specific neutralizing antibody that targets ZIKV EDIII (25). In the ZIKV NS1 BOB assay, serum antibodies compete with mAb ZKA35 (HuMabs), a horseradish peroxidase-labeled or biotinylated anti-NS1 mAb, for binding to recombinant ZIKV NS1 protein (MR766 strain, Native Antigen Co.) (24, 26). Unlike the DENV and ZIKV iELISA, the ZIKV NS1 BOB is always run at a 1:10 dilution, and results are reported as negative (<50% inhibition) or positive (≥50% inhibition) relative to wells without sample.
Infection histories.
Children were grouped by infection history based on documented symptomatic dengue and Zika cases or observed DENV and ZIKV infections between annual samples, as described previously (8). Inapparent DENV infection is defined as seroconversion or a ≥4-fold rise in DENV iELISA titer between annual samples without seroconversion or ≥4-fold rise by either ZIKV serological assay (NS1 BOB or iELISA) or an observed dengue, Zika, or flavivirus case in that year. Inapparent ZIKV infection is defined as 1) seroconversion by ZIKV NS1 BOB, or 2) seroconversion or ≥4-fold rise in the ZIKV iELISA between paired annual samples and without an observed dengue, Zika, or flavivirus case in that year. We used these documented DENV and ZIKV infections to define infection histories. Primary DENV infection includes children who entered the study flavivirus-naïve and were observed to have exactly one DENV infection. Secondary DENV infection is split into two separate groups. Children who entered the cohort flavivirus-naïve and experienced exactly two distinct DENV infections are called DENV-DENV. Children who entered the study flavivirus-naïve and had three or more observed DENV infections or who entered the study flavivirus-immune and had one or more observed DENV infections are called two or more prior DENV infections (2+DENV). Primary ZIKV infection (ZIKV) was defined as children who: 1) entered the study flavivirus-naïve and had one observed ZIKV infection (n= 1465), or 2) children who entered the study ZIKV NS1 BOB-positive (≥50%) or ZIKV iELISA-positive (≥1:10) but with low DENV iELISA titers (≤ 1:160) (n=65). Sequential DENV and ZIKV infections was also split into two groups. Children with one DENV infection followed by one ZIKV infection and children who entered the cohort ZIKV NS1 BOB-positive or ZIKV iELISA-positive who also had high DENV iELISA titers (> 1:160), were grouped as DENV-ZIKV. Children with multiple documented prior DENV infections followed by ZIKV infection were labeled as 2+DENV-ZIKV.
Individual determinants.
For the Pediatric Dengue Cohort Study, all surveys were administered once a year orally during the annual sample, and answers were recorded on custom-built software through table computers or smartphones (68). Household and individual participant surveys were administered. The participant questionnaires included demographic information such as sex, age, and education. Questions on assets of the home were covered in the household survey, which served to determine a household’s asset-based socioeconomic status (SES) every year. The wealth index was based on: household construction material, dirt floor, number of fans, ownership of cars, and a refrigerator. We used principal components analysis (PCA) to define the weights for an index of the household asset variables based on the first principal component (PC1) score (77). The PC1 index was split into terciles, and households were categorized as Non-poor, Poor, and Poorest according to their terciles. These categories were used to reflect the general SES of the study area. We assigned the individual SES status to their corresponding household SES status and year of study. Each participant’s height and weight were also measured during each annual sampling in the Cohort Study (78). In the Hospital Study, demographic information and participant’s height and weight were collected during admission to the study. Body mass index (BMI)-for-age z-scores were calculated based on height and weight, accounting for age and sex and comparing them to the WHO reference populations (79, 80). BMI categories were defined using the WHO Child Growth Standards (81, 82). Overweight and Obese categories were merged so that children less than 5 years old whose BMI-for-age z-scores were greater than 2 standard deviations, and children 5 to 19 years old whose z-scores were greater than 1 standard deviation were categorized as Overweight/Obese. Otherwise, they were categorized as Non-Overweight/Obese.
Spatiotemporal clustering of dengue cases.
The distance between dengue cases of the same versus different serotypes as determined by serotyped RT-PCR in the community-based cohort was measured as the amount of time (years) and space (kilometers) between each pair of cases. Home Global Positioning System (GPS) coordinates were used to define location in space for the case; the day the child presented with symptoms to the clinic was defined as the time of the case.
We estimated the proportion of all dengue case pairs caused by the same serotype that occurred within a given distance in a given time and space ‘bin’ (homologous cases). We separately estimated the proportion of all dengue case pairs caused by different serotype that occurred within a given distance in a given time and space ‘bin’ (heterologous cases). To isolate spatial clustering independent of annual variation in epidemic magnitude, we normalized homologous and heterologous space-time bins by the total cases in the corresponding time bin (as opposed to all bins). The two-way histogram plots (Fig. 1G, fig. S8) show the log-ratio of homologous to heterologous dengue cases, estimated by dividing the normalized space-time bins from homologous pairs by the corresponding space-time bin for heterologous pairs. Stars mark bins with ratios statistically different from 1 (|Z scores| >3.1, corresponding to p-values <0.001).
Statistical analyses.
We estimated the magnitude and half-life of log2 antibody titers in the years after primary and secondary DENV and sequential DENV and ZIKV infection. Magnitude is a measure of antibody titer at a given time point; magnitude is interchangeable with the term ‘setpoint’ when no significant antibody decay is observed. Half-life is estimated over a specific range of time and corresponds to: −1/(slope of the log2 antibody titers). Positive half-lives indicate antibody decay and negative half-lives are used to indicate antibody increase, or ‘doubling times’. For slope values near 0, the half-life approaches infinity; thus, slope estimates that span 0 (half-lives = infinity) indicate titer stability.
All statistical analyses were conducted using R 3.6.3 (R Foundation for Statistical Computing) (83). For each infection history, antibody titers were modeled as a potentially non-linear function of time using generalized additive mixed models (GAMM, gamm4 in the mgcv package in R (84)). We used mixed models so as to incorporate random effects accounting for clustering at the individual, enabling estimation of individual differences in titer magnitude/setpoints and half-lives, as is commonly performed for longitudinal analysis of antibody kinetics (33). We chose to start by modeling kinetics using generalized additive models, as opposed to generalized linear models, because they are non-parametric and thus do not impose model assumptions on estimates of antibody kinetics (85). Numerous papers have shown that antibodies likely have multiple phases of antibody decay resulting from distinct underlying processes, including 1) early phase decay resulting from loss of plasmablasts and physical decay of antibodies, 2) moderate-term decay resulting from loss of short to medium-lived plasma cells, and 3) long-term decay resulting from loss of long-lived plasma cells (31, 32). Thus, unsurprisingly, our preliminary analyses suggested that assuming exponential decay, power-law decay, or log-linear decay did not provide an accurate description of these kinetics. Smooth terms for the GAMM of each dataset were determined based on model Akaike’s Information Criterion (AIC), overall best fit (conditional R2), best performance in gam.check, and consistency of the resulting dynamics across a range of values (data file S5). The best-fit GAMM, with 95% confidence intervals, are shown for each dataset, overlayed on the plot of individual antibody trajectories (Figs. 2, 3, and 5, fig. S4). For each dataset, we tested the significance of group antibody decay by estimating the instantaneous slope of the GAMM at each time point. We sought to use the estimation of slope to identify ‘cut-points’ or changes in the phase of antibody decay, similar to approaches used previously (86). The time at which the instantaneous half-life plateaued to a non-significant half-live is shown in data file S5. Due to less frequent sampling in the community-based cohort, we are not able to observe high-frequency antibody dynamics, and the resulting times when titers plateau were later than in the hospital cohort. We used the cut-points estimated from these GAMMs to define periods of antibody change in multi-phasic linear mixed models of each dataset (lmer, with random intercepts and slopes from the lme4 package) (33, 86, 87). We used bootstrap sampling (n=1000) to construct 95% confidence intervals for individual and group titer magnitude and half-lives from the multi-phasic linear mixed models for each period (vertical bars and points, with printed numeric estimates, fig. S5 to S7). Individual magnitude and half-lives are shown either separately as violin plots or combined as contour plots (Fig. 2 to 4, fig. S5 to S7). To test whether heterotypic anti-DENV neutralizing titers have distinct kinetics after primary and secondary DENV infection, we fit GAMMs to neutralizing antibody titer data previously collected on a subset of cohort participants (Fig. 1F) (14, 30).
We also built linear mixed models that incorporated covariates hypothesized to be related to individual antibody magnitude and kinetics, including: sex, age, overweight/obesity, socioeconomic status, and past symptomatic infection, disease severity, past infecting serotype, and viral load (tables S1 to S4 and S6 to S8). In these models, we only used annual sample data so as not to bias the magnitude estimates between symptomatic and inapparent groups. Each variable was included as a fixed-effect with an interaction term with years since infection, enabling estimation of how each variable modified both titer magnitude and half-life. We built a combined model for each antibody measure in which we adjusted for infection history (Fig. 1, fig. S11), as well as models of each infection history separately (fig. S2). The same method was used to determine variables associated with antibody magnitude and kinetics using DENV iELISA titer data from the hospital-based study (fig. S3, data file S2 and S4). We included sex, age, infection history, overweight/obesity and dengue severity as covariates. P-values were estimated using lmerTest (88). To determine the percent of explained heterogeneity in both antibody magnitude and kinetics by the observed covariates and the individuality, we calculated conditional and marginal R2 for the combined models and the models for each infection history (89).
We also conducted additional analyses of determinants of antibody decay to address specific hypotheses. We tested for factors to explain the difference in antibody titer magnitude among primary infections in the cohort and hospital. Given the difference in the timepoints of blood collection between the hospital and cohort studies, we built two separate models using the samples that were collected at equivalent times in the cohort and hospital study: 0.4 to 0.9 years and 1.4 to 1.9 years. We built linear models of DENV iELISA titer as a function of disease severity (Inapparent, Dengue Fever, Dengue Hemorrhagic Fever/Dengue Shock Syndrome, OR Inapparent, Dengue without Warning Signs, Dengue with Warning Signs/Severe Dengue), age, sex, and time of sampling.
Supplementary Material
Data file S1 to S8.
Fig. S1 to S11.
Acknowledgments:
We thank the Pediatric Dengue Cohort Study and Pediatric Dengue Hospital-based Study participants and their families. We are grateful to past and present members of the study team based at the Centro de Salud Sócrates Flores Vivas, Hospital Infantil Manuel de Jesús Rivera, the Laboratorio Nacional de Virología in the Centro Nacional de Diagnóstico y Referencia, and the Sustainable Sciences Institute in Nicaragua for their dedication and high-quality work.
Funding:
This research was funded by National Institutes of Health (NIH) grant P01AI106695 (to E.H.). The Pediatric Dengue Cohort Study was supported by NIH grants P01AI106695 (to E.H.), U19AI118610 (to E.H.), and R01AI099631 (to A.B.), as well as the Pediatric Dengue Vaccine Initiative grant VE-1 (to E.H.) and FIRST grant (to E.H. and J.C.), both from the Bill and Melinda Gates Foundation. L.C.K. was supported in part by the Global Health Equity Scholar Fogarty International Center/National Institutes of Health training grant D43TW010540 and the Intramural Research Program of the National Institute of Allergy and Infectious Diseases.
Footnotes
Competing interests: E.H.’s laboratory received research funds from Takeda Vaccines, Inc. to analyze samples from vaccine recipients. E.H. served on one-time Advisory Boards for Merck and Takeda. A.G. serves on a RSV vaccine scientific advisory board for Janssen.
Data and materials availability:
All data associated with this study are in the paper or supplementary materials. Sequence data are available on GenBank (accession numbers KM204119, KM204118, KU050695, KR011349, and KX421193) and code is available on Zenodo (10.5281/zenodo.4817916). Individual data for reproducing figures may be shared with outside investigators following UC Berkeley IRB approval. Please contact Eva Harris (eharris@berkeley.edu) to arrange for data access. The materials and data used in this study are covered by standard data and material transfer agreements.
REFERENCES
- 1.Morens DM, Fauci AS, Emerging Infectious Diseases: Threats to Human Health and Global Stability, PLoS Pathog. 9, 7–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halstead SB, In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody., J. Infect. Dis 140, 527–533 (1979). [DOI] [PubMed] [Google Scholar]
- 3.Kliks SC, Nisalak A, Brandt WE, Wahl L, Burke DS, Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever., Am. J. Trop. Med. Hyg 40, 444–451 (1989). [DOI] [PubMed] [Google Scholar]
- 4.Sangkawibha N, Rojanasuphot S, Ahandrik S, Viriyapongse S, Jatanasen S, Salitul V, Phanthumachinda B, Halstead SB, Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak., Am. J. Epidemiol 120, 653–69 (1984). [DOI] [PubMed] [Google Scholar]
- 5.Katzelnick LC, Gresh L, Halloran ME, Mercado JC, Kuan G, Gordon A, Balmaseda A, Harris E, Antibody-dependent enhancement of severe dengue disease in humans., Science 358, 929–932 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salje H, Cummings DAT, Rodriguez-Barraquer I, Katzelnick LC, Lessler J, Klungthong C, Thaisomboonsuk B, Nisalak A, Weg A, Ellison D, Macareo L, Yoon I-K, Jarman R, Thomas S, Rothman AL, Endy T, Cauchemez S, Reconstruction of antibody dynamics and infection histories to evaluate dengue risk., Nature 557, 719–723 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waggoner JJ, Katzelnick LC, Burger-Calderon R, Gallini J, Moore RH, Kuan G, Balmaseda A, Pinsky BA, Harris E, Pinksy B, Harris E, Antibody-Dependent Enhancement of Severe Disease Is Mediated by Serum Viral Load in Pediatric Dengue Virus Infections, J. Infect. Dis 221, 1846–1854 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katzelnick LC, Narvaez C, Arguello S, Lopez Mercado B, Collado D, Ampie O, Elizondo D, Miranda T, Bustos Carillo F, Mercado JC, Latta K, Schiller A, Segovia-Chumbez B, Ojeda S, Sanchez N, Plazaola M, Coloma J, Halloran ME, Premkumar L, Gordon A, Narvaez F, de Silva AM, Kuan G, Balmaseda A, Harris E, Zika virus infection enhances future risk of severe dengue disease., Science 369, 1123–1128 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breitbach ME, Newman CM, Dudley DM, Stewart LM, Aliota MT, Koenig MR, Shepherd PM, Yamamoto K, Crooks CM, Young G, Semler MR, Weiler AM, Barry GL, Heimsath H, Mohr EL, Eichkoff J, Newton W, Peterson E, Schultz-Darken N, Permar SR, Dean H, Capuano S, Osorio JE, Friedrich TC, O’Connor DH, Kuhn RJ, Ed. Primary infection with dengue or Zika virus does not affect the severity of heterologous secondary infection in macaques, PLOS Pathog. 15, e1007766 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George J, Valiant WG, Mattapallil MJ, Walker M, Huang YJS, Vanlandingham DL, Misamore J, Greenhouse J, Weiss DE, Verthelyi D, Higgs S, Andersen H, Lewis MG, Mattapallil JJ, Prior Exposure to Zika Virus Significantly Enhances Peak Dengue-2 Viremia in Rhesus Macaques, Sci. Rep 7, 1–10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pérez-Guzmán EX, Pantoja P, Serrano-Collazo C, Hassert MA, Ortiz-Rosa A, Rodríguez IV, Giavedoni L, Hodara V, Parodi L, Cruz L, Arana T, White LJ, Martinez MI, Weiskopf D, Brien JD, de Silva A, Pinto AK, Sariol CA, Time elapsed between Zika and dengue virus infections affects antibody and T cell responses, Nat. Commun 10, 4316 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabin AB, Research on dengue during World War II., Am. J. Trop. Med. Hyg 1, 30–50 (1952). [DOI] [PubMed] [Google Scholar]
- 13.Anderson KB, Gibbons RV, Cummings DAT, Nisalak A, Green S, Libraty DH, Jarman RG, Srikiatkhachorn A, Mammen MP, Darunee B, Yoon I-K, Endy TP, A shorter time interval between first and second dengue infections is associated with protection from clinical illness in a school-based cohort in Thailand., J. Infect. Dis 209, 360–368 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montoya M, Gresh L, Mercado JC, Williams KL, Jose M, Gutierrez G, Kuan G, Gordon A, Balmaseda A, Harris E, Vargas MJ, Gutierrez G, Kuan G, Gordon A, Balmaseda A, Harris E, Symptomatic versus inapparent outcome in repeat dengue virus infections is influenced by the time interval between infections and study year., PLoS Negl. Trop. Dis 7, e2357 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsang TK, Ghebremariam SL, Gresh L, Gordon A, Halloran ME, Katzelnick LC, Rojas DP, Kuan G, Balmaseda A, Sugimoto J, Harris E, Longini IM, Yang Y, Effects of infection history on dengue virus infection and pathogenicity, Nat. Commun 10, 1246 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reich NG, Shrestha S, King AA, Rohani P, Lessler J, Kalayanarooj S, Yoon I, Gibbons RV, Burke DS, Cummings DAT, Interactions between serotypes of dengue highlight epidemiological impact of cross-immunity, J. R. Soc. Interface 10, 20130414 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salje H, Lessler J, Endy TP, Curriero FC, Gibbons RV, Nisalak A, Nimmannitya S, Kalayanarooj S, Jarman RG, Thomas SJ, Burke DS, Cummings DAT, Revealing the microscale spatial signature of dengue transmission and immunity in an urban population., Proc. Natl. Acad. Sci 109, 9535–9538 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salje H, Lessler J, Maljkovic Berry I, Melendrez MC, Endy T, Kalayanarooj S, A-Nuegoonpipat A, Chanama S, Sangkijporn S, Klungthong C, Thaisomboonsuk B, Nisalak A, Gibbons RV, Iamsirithaworn S, Macareo LR, Yoon I-K, Sangarsang A, Jarman RG, Cummings DAT, Berry IM, Melendrez MC, Kalayanarooj S, A-Nuegoonpipat A, Chanama S, Klungthong C, Thaisomboonsuk B, Nisalak A, Gibbons V, Iamsirithaworn S, Macareo LR, Yoon I-K, Sangarsang A, Jarman RG, Cummings DAT, Dengue diversity across spatial and temporal scales: Local structure and the effect of host population size., Science 355, 1302–1306 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borchering RK, Huang AT, Mier-y-Teran-Romero L, Rojas DP, Rodriguez-Barraquer I, Katzelnick LC, Martinez SD, King GD, Cinkovich SC, Lessler J, Cummings DAT, Impacts of Zika emergence in Latin America on endemic dengue transmission, Nat. Commun 10, 5730 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dejnirattisai W, Wongwiwat W, Supasa S, Zhang X, Dai X, Rouvinski A, Jumnainsong A, Edwards C, Quyen NTH, Duangchinda T, Grimes JM, Tsai W-Y, Lai C-Y, Wang W-K, Malasit P, Farrar J, Simmons CP, Zhou ZH, Rey FA, Mongkolsapaya J, Screaton GR, A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus, Nat. Immunol 16, 170–177 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rouvinski A, Guardado-Calvo P, Barba-Spaeth G, Duquerroy S, Vaney M-C, Kikuti CM, Navarro Sanchez ME, Dejnirattisai W, Wongwiwat W, Haouz A, Girard-Blanc C, Petres S, Shepard WE, Desprès P, Arenzana-Seisdedos F, Dussart P, Mongkolsapaya J, Screaton GR, Rey FA, Recognition determinants of broadly neutralizing human antibodies against dengue viruses., Nature 520, 109–113 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Patel B, Longo P, Miley MJ, Montoya M, Harris E, de Silva AM, Morrison AC, Ed. Dissecting the human serum antibody response to secondary dengue virus infections, PLoS Negl. Trop. Dis 11, e0005554–e0005554 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Alwis R, Williams KL, Schmid MA, Lai C-Y, Patel B, Smith SA, Crowe JE, Wang W, Harris E, de Silva AM, De Alwis R, Williams KL, Schmid MA, Lai C-Y, Patel B, Smith SA, Crowe JE, Wang W, Harris E, De Silva AM, Dengue viruses are enhanced by distinct populations of serotype cross-reactive antibodies in human immune sera., PLoS Pathog. 10, e1004386 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balmaseda A, Stettler K, Medialdea-Carrera R, Collado D, Jin X, Zambrana JV, Jaconi S, Cameroni E, Saborio S, Rovida F, Percivalle E, Ijaz S, Dicks S, Ushiro-Lumb I, Barzon L, Siqueira P, Brown DWG, Baldanti F, Tedder R, Zambon M, de Filippis AMB, Harris E, Corti D, Antibody-based assay discriminates Zika virus infection from other flaviviruses., Proc. Natl. Acad. Sci 114, 8384–8389 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balmaseda A, Zambrana JV, Collado D, García N, Saborío S, Elizondo D, Mercado JC, Gonzalez K, Cerpas C, Nuñez A, Corti D, Waggoner JJ, Kuan G, Burger-Calderon R, Harris E, Tang Y-W, Ed. Comparison of Four Serological Methods and Two Reverse Transcription-PCR Assays for Diagnosis and Surveillance of Zika Virus Infection., J. Clin. Microbiol 56, e01785–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, Foglierini M, Pedotti M, Simonelli L, Dowall S, Atkinson B, Percivalle E, Simmons CP, Varani L, Blum J, Baldanti F, Cameroni E, Hewson R, Harris E, Lanzavecchia A, Sallusto F, Corti D, Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection., Science 353, 823–6 (2016). [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization, Dengue: Guidelines for diagnosis, treatment, prevention and control, (2009) (available at http://www.who.int/mediacentre/factsheets/fs117/en). [PubMed]
- 28.Clarke DH, Casals J, Techniques for Hemagglutination and Hemagglutination-Inhibition with Arthropod-Borne Viruses, Am. J. Trop. Med. Hyg 7, 561–573 (1958). [DOI] [PubMed] [Google Scholar]
- 29.Narvaez F, Gutierrez G, Pérez MA, Elizondo D, Nuñez A, Balmaseda A, Harris E, Hirayama K, Ed. Evaluation of the traditional and revised WHO classifications of dengue disease severity, PLoS Negl. Trop. Dis 5, 1–8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katzelnick LC, Montoya M, Gresh L, Balmaseda A, Harris E, Neutralizing antibody titers against dengue virus correlate with protection from symptomatic infection in a longitudinal cohort, Proc. Natl. Acad. Sci 113, 728–733 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andraud M, Lejeune O, Musoro JZ, Ogunjimi B, Beutels P, Hens N, Living on three time scales: The dynamics of plasma cell and antibody populations illustrated for hepatitis a virus, PLoS Comput. Biol 8, 1–8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slifka MK, Amanna IJ, Role of multivalency and antigenic threshold in generating protective antibody responses, Front. Immunol 10, 1–16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antia A, Ahmed H, Handel A, Carlson NE, Amanna IJ, Antia R, Slifka M, Heterogeneity and longevity of antibody memory to viruses and vaccines, PLoS Biol. 16, 1–15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogers TF, Goodwin EC, Briney B, Sok D, Beutler N, Strubel A, Nedellec R, Le K, Brown ME, Burton DR, Walker LM, Zika virus activates de novo and cross-reactive memory B cell responses in dengue-experienced donors, Sci. Immunol 2, eaan6809 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robbiani DF, Bozzacco L, Keeffe JR, Khouri R, Olsen PC, Gazumyan A, Schaefer-Babajew D, Avila-Rios S, Nogueira L, Patel R, Azzopardi SA, Uhl LFK, Saeed M, Sevilla-Reyes EE, Agudelo M, Yao K-H, Golijanin J, Gristick HB, Lee YE, Hurley A, Caskey M, Pai J, Oliveira T, Wunder EA, Sacramento G, Nery N, Orge C, Costa F, Reis MG, Thomas NM, Eisenreich T, Weinberger DM, de Almeida ARP, West AP, Rice CM, Bjorkman PJ, Reyes-Teran G, Ko AI, MacDonald MR, Nussenzweig MC, Recurrent Potent Human Neutralizing Antibodies to Zika Virus in Brazil and Mexico., Cell 169, 597–609.e11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brault AC, Domi A, McDonald EM, Talmi-Frank D, McCurley N, Basu R, Robinson HL, Hellerstein M, Duggal NK, Bowen RA, Guirakhoo F, A Zika Vaccine Targeting NS1 Protein Protects Immunocompetent Adult Mice in a Lethal Challenge Model, Sci. Rep 7, 1–11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zambrana JV, Bustos Carrillo F, Burger-Calderon R, Collado D, Sanchez N, Ojeda S, Carey Monterrey J, Plazaola M, Lopez B, Arguello S, Elizondo D, Aviles W, Coloma J, Kuan G, Balmaseda A, Gordon A, Harris E, Seroprevalence, risk factor, and spatial analyses of Zika virus infection after the 2016 epidemic in Managua, Nicaragua, Proc. Natl. Acad. Sci 115, 9294–9299 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amanna IJ, Carlson NE, Slifka MK, Duration of Humoral Immunity to Common Viral and Vaccine Antigens, N. Engl. J. Med 357, 1903–1915 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Hammarlund E, Thomas A, Amanna IJ, Holden LA, Slayden OD, Park B, Gao L, Slifka MK, Plasma cell survival in the absence of B cell memory, Nat. Commun 8, 1781 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akkaya M, Kwak K, Pierce SK, B cell memory: building two walls of protection against pathogens, Nat. Rev. Immunol 20, 229–238 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slifka MK, Amanna I, How advances in immunology provide insight into improving vaccine efficacy, Vaccine 32, 2948–2957 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai CY, Williams KL, Wu YC, Knight S, Balmaseda A, Harris E, Wang WK, Analysis of Cross-Reactive Antibodies Recognizing the Fusion Loop of Envelope Protein and Correlation with Neutralizing Antibody Titers in Nicaraguan Dengue Cases, PLoS Negl. Trop. Dis 7, 1–11 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clapham HE, Rodriguez-Barraquer I, Azman AS, Althouse BM, Salje H, Gibbons RV, Rothman AL, Jarman RG, Nisalak A, Thaisomboonsuk B, Kalayanarooj S, Nimmannitya S, Vaughn DW, Green S, Yoon I-KK, Cummings DATT, Dengue virus (DENV) neutralizing antibody kinetics in children after symptomatic primary and postprimary DENV infection, J. Infect. Dis 213, 1428–1435 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alexander LW, Ben-Shachar R, Katzelnick LC, Kuan G, Balmaseda A, Harris E, Boots M, Boosting can explain patterns of fluctuations of ratios of inapparent to symptomatic dengue virus infections, Proc. Natl. Acad. Sci 118, e2013941118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puschnik A, Lau L, Cromwell EA, Balmaseda A, Zompi S, Harris E, Correlation between dengue-specific neutralizing antibodies and serum avidity in primary and secondary dengue virus 3 natural infections in humans., PLoS Negl. Trop. Dis 7, e2274 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montoya M, Collins M, Dejnirattisai W, Katzelnick LC, Puerta-Guardo H, Jadi R, Schildhauer S, Supasa P, Vasanawathana S, Malasit P, Mongkolsapaya J, de Silva AD, Tissera H, Balmaseda A, Screaton G, de Silva AM, Harris E, Longitudinal Analysis of Antibody Cross-neutralization Following Zika Virus and Dengue Virus Infection in Asia and the Americas., J. Infect. Dis 218, 536–545 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clapham HE, Rodriguez-Barraquer I, Azman AS, Althouse BM, Salje H, Gibbons RV, Rothman AL, Jarman RG, Nisalak A, Thaisomboonsuk B, Kalayanarooj S, Nimmannitya S, Vaughn DW, Green S, Yoon IK, Cummings DAT, Dengue Virus (DENV) Neutralizing Antibody Kinetics in Children after Symptomatic Primary and Postprimary DENV Infection, J. Infect. Dis 213, 1428–1435 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wec AZ, Haslwanter D, Abdiche YN, Shehata L, Pedreño-Lopez N, Moyer CL, Bornholdt ZA, Lilov A, Nett JH, Jangra RK, Brown M, Watkins DI, Ahlm C, Forsell MN, Rey FA, Barba-Spaeth G, Chandran K, Walker LM, Longitudinal dynamics of the human B cell response to the yellow fever 17D vaccine, Proc. Natl. Acad. Sci. U. S. A 117, 6675–6685 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathew A, West K, Kalayanarooj S, Gibbons RV, Srikiatkhachorn A, Green S, Libraty D, Jaiswal S, Rothman AL, B-cell responses during primary and secondary dengue virus infections in humans., J. Infect. Dis 204, 1514–1522 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andrade P, Narvekar P, Montoya M, Michlmayr D, Balmaseda A, Coloma J, Harris E, Primary and Secondary Dengue Virus Infections Elicit Similar Memory B-Cell Responses, but Breadth to Other Serotypes and Cross-Reactivity to Zika Virus Is Higher in Secondary Dengue, J. Infect. Dis 222, 590–600 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andrade P, Gimblet-Ochieng C, Modirian F, Collins M, Cárdenas M, Katzelnick LC, Montoya M, Michlmayr D, Kuan G, Balmaseda A, Coloma J, de Silva AM, Harris E, Impact of pre-existing dengue immunity on human antibody and memory B cell responses to Zika., Nat. Commun 10, 938 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferguson NM, a Donnelly C, Anderson RM, Transmission dynamics and epidemiology of dengue: insights from age-stratified sero-prevalence surveys., Philos. Trans. R. Soc. Lond. B. Biol. Sci 354, 757–68 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cummings DAT, Schwartz IB, Billings L, Shaw LB, Burke DS, Dynamic effects of antibody-dependent enhancement on the fitness of viruses., Proc. Natl. Acad. Sci 102, 15259–15264 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adams B, Holmes EC, Zhang C, Mammen MP, Nimmannitya S, Kalayanarooj S, Boots M, Cross-protective immunity can account for the alternating epidemic pattern of dengue virus serotypes circulating in Bangkok, Proc. Natl. Acad. Sci 103, 14234–14239 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wearing HJ, Rohani P, Ecological and immunological determinants of dengue epidemics., Proc. Natl. Acad. Sci 103, 11802–7 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perkins TA, Reiner RC, Rodriguez-Barraquer I, Smith DL, Scott TW, Cummings DAT, in Dengue and dengue hemorrhagic fever, 2nd edition, Gubler DJ, Ooi EE, Vasudevan SG, Farrar J, Eds. (CAB International, Wallingford, 2014), pp. 99–114. [Google Scholar]
- 57.Lourenco J, Tennant W, Faria NR, Walker A, Gupta S, Recker M, Challenges in dengue research: A computational perspective, Evol. Appl 11, 516–533 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borchering RK, Huang AT, Mier-y-Teran-Romero L, Rojas DP, Rodriguez-Barraquer I, Katzelnick LC, Martinez SD, King GD, Cinkovich SC, Lessler J, Cummings DAT, Impacts of Zika emergence in Latin America on endemic dengue transmission, Nat. Commun 10, 5730 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lourenco J, Recker M, Pascual M, Ed. Natural, Persistent Oscillations in a Spatial Multi-Strain Disease System with Application to Dengue, PLoS Comput. Biol 9, e1003308 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wrammert J, Onlamoon N, Akondy RS, Perng GC, Polsrila K, Chandele A, Kwissa M, Pulendran B, Wilson PC, Wittawatmongkol O, Yoksan S, Angkasekwinai N, Pattanapanyasat K, Chokephaibulkit K, Ahmed R, Rapid and massive virus-specific plasmablast responses during acute dengue virus infection in humans., J. Virol 86, 2911–2918 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagao Y, Koelle K, Decreases in dengue transmission may act to increase the incidence of dengue hemorrhagic fever, Proc. Natl. Acad. Sci 105, 2238–2243 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhaumik S, Priyamvada L, Kauffman R, Lai L, Natrajan M, Cho A, Rouphael N, Suthar M, Mulligan M, Wrammert J, Pre-Existing Dengue Immunity Drives a DENV-Biased Plasmablast Response in ZIKV-Infected Patient, Viruses 11, 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grifoni A, Pham J, Sidney J, O’Rourke PH, Paul S, Peters B, Martini SR, de Silva AD, Ricciardi MJ, Magnani DM, Silveira CGT, Maestri A, Costa PR, de-Oliveira-Pinto LM, de Azeredo EL, Damasco PV, Phillips E, Mallal S, de Silva AM, Collins M, Durbin A, Diehl SA, Cerpas C, Balmaseda A, Kuan G, Coloma J, Harris E, Crowe JE, Stone M, Norris PJ, Busch M, Vivanco-Cid H, Cox J, Graham BS, Ledgerwood JE, Turtle L, Solomon T, Kallas EG, Watkins DI, Weiskopf D, Sette A, Prior Dengue Virus Exposure Shapes T Cell Immunity to Zika Virus in Humans, J. Virol 91, 1–19 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gallichotte EN, Young EF, Baric TJ, Yount BL, Metz SW, Begley MC, de Silva AM, Baric RS, Role of Zika Virus Envelope Protein Domain III as a Target of Human Neutralizing Antibodies., MBio 10, 1–6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maciejewski S, Ruckwardt TJ, Morabito KM, Foreman BM, Burgomaster KE, Gordon DN, Pelc RS, DeMaso CR, Ko S, Fisher BE, Yang ES, Nair D, Foulds KE, Todd JP, Kong W, Roy V, Aleshnick M, Speer SD, Bourne N, Barrett AD, Nason MC, Roederer M, Gaudinski MR, Chen GL, Dowd KA, Ledgerwood JE, Alter G, Mascola JR, Graham BS, Pierson TC, Distinct neutralizing antibody correlates of protection among related Zika virus vaccines identify a role for antibody quality, Sci. Transl. Med 12, eaaw9066 (2020). [DOI] [PubMed] [Google Scholar]
- 66.Sridhar S, Luedtke A, Langevin E, Zhu M, Bonaparte M, Machabert T, Savarino S, Zambrano B, Moureau A, Khromava A, Moodie Z, Westling T, Mascareñas C, Frago C, Cortés M, Chansinghakul D, Noriega F, Bouckenooghe A, Chen J, Ng S-P, Gilbert PB, Gurunathan S, DiazGranados CA, Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy, N. Engl. J. Med 379, 327–340 (2018). [DOI] [PubMed] [Google Scholar]
- 67.Kareko BW, Booty BL, Nix CD, Lyski ZL, Slifka MK, Slifka MK, Amanna IJ, Messer WB, Messer WB, Messer WB, Persistence of Neutralizing Antibody Responses among Yellow Fever Virus 17D Vaccinees Living in a Nonendemic Setting, J. Infect. Dis 221, 2018–2025 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuan G, Gordon A, Aviles W, Ortega O, Hammond SN, Elizondo D, Nunez A, Coloma J, Balmaseda A, Harris E, Avilés W, Ortega O, Hammond SN, Elizondo D, Nuñez A, Coloma J, Balmaseda A, Harris E, Kuan G, Gordon A, Avile W, Coloma J, Balmaseda A, Harris E, Elizondo D, Nun A, The nicaraguan pediatric dengue cohort study: Study design, methods, use of information technology, and extension to other infectious diseases, Am. J. Epidemiol 170, 120–129 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Waggoner JJ, Ballesteros G, Gresh L, Mohamed-Hadley A, Tellez Y, Sahoo MK, Abeynayake J, Balmaseda A, Harris E, Pinsky BA, Clinical evaluation of a single-reaction real-time RT-PCR for pan-dengue and chikungunya virus detection, J Clin Virol 78, 57–61 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gordon A, Gresh L, Ojeda S, Katzelnick LC, Sanchez N, Mercado JC, Chowell G, Lopez B, Elizondo D, Coloma J, Burger-Calderon R, Kuan G, Balmaseda A, Harris E, von Seidlein L, Ed. Prior dengue virus infection and risk of Zika: A pediatric cohort in Nicaragua, PLOS Med. 16, e1002726 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harris E, Roberts TG, Smith L, Selle J, Kramer LD, Valle S, Sandoval E, Balmaseda A, Typing of dengue viruses in clinical specimens and mosquitoes by single-tube multiplex reverse transcriptase PCR., J. Clin. Microbiol 36, 2634–9 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Balmaseda A, Sandoval E, Pérez L, Gutiérrez CM, Harris E, Application of molecular typing techniques in the 1998 dengue epidemic in Nicaragua., Am. J. Trop. Med. Hyg 61, 893–7 (1999). [DOI] [PubMed] [Google Scholar]
- 73.Gutiérrez G, Gresh L, Pérez MÁ, Elizondo D, Avilés W, Kuan G, Balmaseda Á, Harris E, Reithinger R, Ed. Evaluation of the Diagnostic Utility of the Traditional and Revised WHO Dengue Case Definitions, PLoS Negl. Trop. Dis 7, e2385 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fernandez RJ, Vazquez S, Serological diagnosis of dengue by an ELISA inhibition method (EIM), Mem. Inst. Oswaldo Cruz 85, 347–351 (1990). [DOI] [PubMed] [Google Scholar]
- 75.Balmaseda A, Hammond SN, Tellez Y, Imhoff L, Rodriguez Y, Saborío SI, Mercado JC, Perez L, Videa E, Almanza E, Kuan G, Reyes M, Saenz L, Amador JJ, Harris E, High seroprevalence of antibodies against dengue virus in a prospective study of schoolchildren in Managua, Nicaragua., Trop. Med. Int. Health 11, 935–42 (2006). [DOI] [PubMed] [Google Scholar]
- 76.Reed LJ, Muench H, A simple method of estimating fifty per cent endpoints, Am. J. Epidemiol 27, 493–497 (1938). [Google Scholar]
- 77.Vyas S, Kumaranayake L, Constructing socio-economic status indices: how to use principal components analysis, Health Policy Plan. 21, 459–468 (2006). [DOI] [PubMed] [Google Scholar]
- 78.Gordon A, Kuan G, Aviles W, Sanchez N, Ojeda S, Lopez B, Gresh L, Balmaseda A, Harris E, The Nicaraguan pediatric influenza cohort study: design, methods, use of technology, and compliance, BMC Infect. Dis 15, 504 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J, Development of a WHO growth reference for school-aged children and adolescents., Bull. World Health Organ 85, 660–667 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Onis M, Garza C, Onyango AW, Rolland-Cachera M-F, Les standards de croissance de l’Organisation mondiale de la santé pour les nourrissons et les jeunes enfants, Arch. Pédiatrie 16, 47–53 (2009). [DOI] [PubMed] [Google Scholar]
- 81.World Health Organization, Training Course on Child Growth Assessment - WHO Child Growth Standards - Interpreting Growth Indicators (2008; https://www.who.int/childgrowth/training/module_c_interpreting_indicators.pdf).
- 82.World Health Organization, Growth reference data for 5-19 years - Interpretation of cut-offs, (2007) (available at https://www.who.int/tools/growth-reference-data-for-5to19-years/indicators/bmi-for-age).
- 83.R Core Team, R: A language and environment for statistical computing (Vienna, Austria, 2016). [Google Scholar]
- 84.Pedersen EJ, Miller DL, Simpson GL, Ross N, Hierarchical generalized additive models: an introduction with mgcv, PeerJ (2018), doi: 10.7287/peerj.preprints.27320v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hastie T, Tibshirani R, Generalized Additive Models (Chapman & Hall, New York, 1990). [Google Scholar]
- 86.Desai K, Coudeville L, Bailleux F, Modelling the long-term persistence of neutralizing antibody in adults after one dose of live attenuated Japanese encephalitis chimeric virus vaccine, Vaccine 30, 2510–2515 (2012). [DOI] [PubMed] [Google Scholar]
- 87.Bates D, Mächler M, Bolker B, Walker S, Fitting Linear Mixed-Effects Models Using lme4, J. Stat. Softw 67, 1–48 (2015). [Google Scholar]
- 88.Kuznetsova A, Brockhoff PB, Christensen RHB, lmerTest Package: Tests in Linear Mixed Effects Models, J. Stat. Softw 82 (2017), doi: 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- 89.Nakagawa S, Schielzeth H, O’Hara RB, Ed. A general and simple method for obtaining R 2 from generalized linear mixed-effects models, Methods Ecol. Evol 4, 133–142 (2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data file S1 to S8.
Fig. S1 to S11.
Data Availability Statement
All data associated with this study are in the paper or supplementary materials. Sequence data are available on GenBank (accession numbers KM204119, KM204118, KU050695, KR011349, and KX421193) and code is available on Zenodo (10.5281/zenodo.4817916). Individual data for reproducing figures may be shared with outside investigators following UC Berkeley IRB approval. Please contact Eva Harris (eharris@berkeley.edu) to arrange for data access. The materials and data used in this study are covered by standard data and material transfer agreements.


