Abstract
Background
Cystic fibrosis is the most common life‐limiting autosomal recessive genetic disorder in white populations. Distal intestinal obstruction syndrome (DIOS) is an important morbidity in cystic fibrosis. It is the result of the accumulation of viscid faecal material within the bowel which combines with thick, sticky mucus produced in the intestines of people with cystic fibrosis. The intestine may be completely blocked (complete DIOS) or only partially blocked (incomplete DIOS). Once a diagnosis of DIOS has been made, the goal of therapy is to relieve the acute complete or incomplete faecal obstruction and ultimately prevent the need for surgical intervention.
Objectives
This review aimed to evaluate the effectiveness and safety of different treatment regimens for the treatment of DIOS (complete and incomplete) in children and adults with cystic fibrosis.
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register comprising references identified from comprehensive electronic database searches and handsearches of relevant journals and abstract books of conference proceedings. Date of search: 09 September 2021.
We also searched online trial registries. Date of last search: 12 October 2021.
Selection criteria
Randomised controlled trials, quasi‐randomised controlled trials (including cross‐over trials (to be judged on an individual basis)) comparing the use of laxative agents or surgery for treating DIOS in children, young people and adults with cystic fibrosis to each other, placebo or no intervention.
Data collection and analysis
Two authors independently screened papers, extracted trial details and assessed for risk of bias. The authors assessed the quality of evidence using GRADE.
Main results
There was one trial with 20 participants (16 females) included in the review. The mean age of participants was 13.1 years. The trial was a double‐blinded, randomised cross‐over trial which had a duration of 12 months in total and compared high‐dose and low‐dose pancreatic enzyme therapy. As only the abstract of the trial was available, the overall risk of bias was judged to be unclear. The trial did not address either of our primary outcomes (time until resolution of DIOS and treatment failure rate), but reported episodes of acute DIOS, presence of abdominal mass and abdominal pain. There were no numerical data available for these outcomes, but the authors stated that there was no difference between treatment with high‐dose or low‐dose pancreatic enzymes. The overall certainty of the evidence was found to be very low.
Authors' conclusions
There is a clear lack of evidence for the treatment of DIOS in people with cystic fibrosis. The included abstract did not address our primary outcome measures and did not provide numerical data for the two secondary outcomes it did address. Therefore, we cannot justify the use of high‐dose pancreatic enzymes for treating DIOS, nor can we comment on the efficacy and safety of other laxative agents. From our findings, it is clear that more randomised controlled trials need to be conducted in this area.
Plain language summary
Interventions for treating distal intestinal obstruction syndrome (DIOS) in cystic fibrosis
Review question
We reviewed the evidence about the effectiveness and safety of different treatments for distal intestinal obstruction syndrome (DIOS) in children and adults with cystic fibrosis.
Background
Cystic fibrosis is a common, life‐limiting, inherited disease. One of the main features of cystic fibrosis is the thick, sticky mucus produced by many organs including the lungs, pancreas and intestine. DIOS occurs when mucus in the intestine combines with faeces and builds up to produce a mass. This mass can partially or completely block the intestine and cause symptoms such as vomiting, severe abdominal pain and a swollen stomach (abdominal distension). Once a diagnosis of DIOS has been made, the goal of therapy is to relieve the complete or partial blockage and ultimately prevent the need for any surgical intervention.
Search date
The evidence is current to: 12 October 2021.
Trial characteristics
The review included one trial with 20 people with cystic fibrosis who were aged between 7.1 and 23.2 years of age (average 13.1 years old). The 12‐month trial compared a high dose of pancreatic enzymes with a low dose of pancreatic enzymes for treating chronic DIOS.
Key results
The trial did not report on our primary outcomes (time until DIOS successfully treated and treatment failure rate), but addressed two of our secondary outcomes; episodes of acute DIOS and the harmful effects that might occur in participants (the presence of an abdominal mass and abdominal pain). There were no numerical data available for these results, but the authors reported that there was no difference between high‐dose or low‐dose pancreatic enzymes.
Certainty of the evidence
We found the overall certainty of the evidence to be very low. The trial itself was only published as an abstract from a conference which did not include numerical data and it was not published as a full report. This meant that we do not know many details about the trial. We thought that the overall risk of bias was unclear, as the trial authors did not describe how participants were put into the treatment groups, whether any participants dropped out or whether the planned outcomes were the same as the reported outcomes. The trial also had a very small number of participants and a limited age range, making it difficult to draw conclusions about the relevance of the treatment for all people with cystic fibrosis.
Summary of findings
Summary of findings 1. Summary of findings ‐ high‐dose pancreatic enzymes compared with low‐dose pancreatic enzymes .
| High‐dose pancreatic enzymes compared with low‐dose pancreatic enzymes for treating DIOS | ||||||
|
Patient or population: children and adults with cystic fibrosis Settings: outpatient Intervention: high‐dose pancreatic enzymes Comparison: low‐dose pancreatic enzymes | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Low‐dose pancreatic enzymes | High‐dose pancreatic enzymes | |||||
| Time taken from start of treatment until the resolution of DIOS | Outcome not reported. |
N/A | ||||
| Treatment failure rate | Outcome not reported. | N/A | ||||
| Adherence | Outcome not reported. | N/A | ||||
|
Episodes of acute DIOS Follow‐up: at 12 months |
There was no difference between low‐dose and high‐dose pancreatic enzymes. | N/A | 20 (1 trial) |
⊕⊝⊝⊝
very lowa,b,c,d,e,f |
||
|
Adverse effects: abdominal mass Follow‐up: at 12 months |
There was no difference between low‐dose and high‐dose pancreatic enzymes. | N/A | 20 (1 trial) |
⊕⊝⊝⊝
very lowb,c,d,e,f |
||
|
Adverse effects: abdominal pain Follow‐up: at 12 months |
There was no difference between low‐dose and high‐dose pancreatic enzymes. | N/A | 20 (1 trial) |
⊕⊝⊝⊝
very lowb,c,d,e,f |
||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DIOS: distal intestinal obstruction syndrome; N/A: not applicable. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
a The method of measurement of episodes of acute DIOS (e.g. numbers, %, per person or total number of episodes) was not described in the trial.
b The small number of participants in the trial (20) decreases the precision of the results.
c The participants had a very limited age range (7.1 years to 23.2 years), making the evidence for these outcomes restricted to this group, and therefore increasing its indirectness.
d The majority of the risk of bias domains were ranked as unclear (selection bias, attrition bias and reporting bias) as there was little information provided in the trial.
eNo specific numerical data were provided for the results of these outcomes, therefore we cannot be sure of the significance or relevance of these results.
f There was no washout period described in this cross‐over trial, therefore we cannot be sure whether there was a carry‐over effect of the treatments.
Background
Please see the appendices for a glossary of terms used in the review (Appendix 1). For definitions of Cochrane statistical and methodological terms, please see the Cochrane Community Glossary.
Description of the condition
Cystic fibrosis (CF) is the most common life‐limiting autosomal recessive genetic disorder in white populations. An affected individual must possess two defective copies of the gene that encodes a protein called the cystic fibrosis transmembrane conductance regulator (CFTR). Approximately 1 in 25 of the UK white population carry a single defective copy of this gene, and 1 in 2500 newborns in the UK are born with CF (Tobias 2011). Worldwide, CF affects approximately 70,000 children and adults (Cystic Fibrosis Foundation Patient Registry 2012).
Although respiratory symptoms are most prominent and often the focus of clinical care, CF also has important effects on the gastrointestinal and endocrine systems. The CFTR protein translates into an ion channel responsible for conducting negatively charged ions (notably chloride, bicarbonate and thiocyanate ions) across various cell membranes in the body, and thus indirectly influences water transport across these membranes. Absent or dysfunctional CFTR leads to thickened, dehydrated mucus. Affected individuals experience multi‐organ dysfunction, resulting in morbidity and reduced quality of life.
Distal intestinal obstruction syndrome (DIOS) is an important morbidity in CF. It occurs when the thick, faecal material combines with sticky mucus in the CF intestine, commonly in the terminal ileum and caecum, making it fixed in position and difficult to remove (Colombo 2011). This may cause complete blockage (complete DIOS) or partial blockage (incomplete DIOS).
DIOS affects between 10% to 22% of individuals with CF (Davidson 1987; Dray 2004); and is associated with meconium ileus, liver disease, diabetes mellitus and Pseudomonas aeruginosa infection (Munck 2016). It also occurs in individuals who have pancreatic enzyme deficiency and, anecdotally, in those who do not adhere to pancreatic enzyme replacement therapy (Hess 2015).
Distinguishing DIOS from other causes of bowel obstruction in CF
The CF gut is prone to obstruction from other causes due to its altered pathophysiology. A small but significant proportion of newborns with CF present either at birth or shortly afterwards with a type of bowel obstruction called meconium ileus. Meconium ileus occurs in 13% to 17% of all people with CF (Van der Doef 2011). Throughout life, children and adults with CF are prone to constipation, with almost half of all children studied (47%) having evidence of constipation (Van der Doef 2011).
However, it is possible to distinguish between constipation and DIOS clinically and radiologically. DIOS is an acute complete or incomplete faecal obstruction in the ileocecum, whereas constipation is defined as gradual faecal impaction of the total colon (Houwen 2010). A further important differential diagnosis that needs to be considered in individuals with suspected DIOS is obstruction caused by scar tissue (adhesions) from previous abdominal surgery.
Description of the intervention
Once a diagnosis of DIOS has been made, the goal of therapy is to relieve the acute complete or incomplete faecal obstruction and ultimately prevent the need for surgical intervention. A number of medical treatments are used for managing DIOS.
Osmotic laxatives
Osmotic laxatives are faecal softeners which work by increasing water in the large bowel, either by drawing fluid from body into bowel or by retaining fluid they were administered with.
Lactulose
Lactulose is an oral osmotic laxative which is widely used, but may cause flatulence or abdominal pain in high doses (Colombo 2011).
Macrogol 3350
Macrogol 3350 is recommended as first‐line treatment for constipation in children and adults (NICE 2015). Maintenance treatment with the oral powders (e.g. Movicol®) are given to children with chronic constipation. Intensive treatment courses may be necessary for cases of faecal impaction (BNF 2016; BNFc 2016). Macrogol 3350 can also be formulated as a bowel cleansing preparation (e.g. Klean‐Prep®). This solution is administered until clear fluid is passed per rectum. As large volumes are required, it is often necessary to administer via nasogastric tube or gastrostomy (Colombo 2011; NICE 2015).
Diatrizoate
Oral diatrizoate (known under the brand name Gastrografin®) is used by many centres to treat DIOS. It is given as a single dose, which can be repeated after 24 hours. Rectal diatrizoate can also be used in more severe cases (Colombo 2011). As diatrizoate is highly osmotic, the individual must be adequately hydrated prior to administration in order to avoid complications such as hypovolemia (a decrease of the volume of circulating blood) and perforation of the bowel (Tuladhar 1999).
Stimulant laxatives
Stimulant laxatives work by increasing intestinal motility and reducing gut transit time. They stimulate peristalsis by enhancing muscle contraction of the bowel wall. A common side effect includes abdominal cramp and prolonged use may cause diarrhoea and a loss of electrolytes (notably potassium ions) in the stools (BNF 2016).
Senna
Senna acts by stimulating peristalsis and increases emptying of the bowel. Senna is therefore useful when the individual has soft stools but find it difficult to pass them (NICE 2015).
Sodium docusate
Sodium docusate acts both as a stimulant and also as a stool softener. It can be administered orally, but if this does not relieve faecal impaction, the drug can also be given as an enema (NICE 2015).
Sodium picosulphate
Sodium picosulphate acts by stimulating the mucosa of the large bowel, increasing its motility. It is given as an oral solution (BNF 2016; BNFc 2016).
Mucolytics
Mucolytics work by breaking down the thick, viscid mucus produced in CF, so may be useful at disintegrating the mucofaeculant material that is adhered to the bowel wall in DIOS.
Oral N‐acetylcysteine
N‐acetylcysteine (known under the brand name Parvolex®) is indicated for abnormal or impaired mucus production. It can be given as a single oral dose for treatment of meconium ileus or DIOS. It is typically diluted in a sweeter drink such as orange juice or cola to mask the strong and bitter taste (BNFc 2016).
Other agents
In addition to laxative agents, other interventions may also be used to treat DIOS. These include prokinetic drugs, e.g. macrolide antibiotics, metoclopramide, cisapride that help to increase gastrointestinal motility (Longo 1993). Increasing the dose of pancreatic enzyme replacement therapy may also improve symptoms of DIOS, as optimum usage has been shown to prevent further episodes (Colombo 2011).
Surgery
Surgical decompression of DIOS is reserved for the most refractory cases not responding to medical management. This intervention is associated with high post‐operative morbidity and is therefore used as a last resort (Docherty 1992; Rescorla 1993). Other surgical techniques are described in the literature, e.g. caecostomy, right hemicolectomy and small bowel resection, but these are associated with bleeding, delayed healing of wounds and postoperative infection. In turn, these factors increase the risk of mortality in surgery (Hodson 1976; Lavie 2015).
How the intervention might work
The aim of DIOS treatment is to clear the luminal contents of the bowel and prevent complete obstruction. Different treatment regimens have different mechanisms of action. The simple laxatives can be broadly characterised as osmotic laxatives, stimulant laxatives and mucolytics. Some agents have more than one mechanism of action, e.g. macrogol 3350, which is both a stool softener and a stimulant. Diatriozate is a potent osmotic agent and works by drawing fluid into the bowel to soften the inspissated faecal material. N‐acetyl‐cysteine is a mucolytic agent, and is likely to work by breaking down the mucoid content of the intestinal mass.
Why it is important to do this review
For people with CF, DIOS is a common complication (Van der Doef 2011). If medical treatment fails and surgery is required, this is likely to increase the risks to the person with CF (Hodson 2007). Currently, there is variation in practice between centres and individual doctors and much of this variation is driven by anecdotal evidence and local experience. Identifying the best medical treatment strategy will enable clinicians to make better informed choices, sharing information about risks and benefits with individuals and their families.
Objectives
This review aimed to evaluate the effectiveness and safety of different treatment regimens for the treatment of DIOS (complete and incomplete) in children and adults with CF.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs. We planned to assess quasi‐RCTs on their merit using the Cochrane risk of bias tool and if satisfied that the groups were similar at baseline, we planned to include them. We also planned to assess cross‐over trials for possible inclusion on an individual basis. If we deemed the treatment to alter the condition to the extent that, on entry to subsequent phases, the participants differed from their initial state, we would exclude the trial unless we could use data from the first phase only (see Unit of analysis issues).
Types of participants
Children, young people and adults with CF (diagnosed with confirmed sweat test or mutation analysis, or both) who also have a confirmed diagnosis of complete or incomplete DIOS (diagnosed clinically or radiologically). We planned to include both pancreatic‐sufficient and pancreatic‐insufficient individuals.
Types of interventions
We planned to compare each type of pharmacological intervention (osmotic laxatives, stimulant laxatives, mucolytics and other laxative agents) or surgery used for the treatment of DIOS in children, young people and adults with CF to each other, to placebo or to no intervention.
Types of outcome measures
We assessed the following outcomes where possible.
Primary outcomes
Time taken from start of treatment until the resolution of DIOS (diagnosed clinically or radiologically)
Treatment failure rate (e.g. clinician‐determined need to change treatment regimen or need for surgical intervention)
Secondary outcomes
Recurrence rate of DIOS (diagnosed clinically or radiologically) after resolution of DIOS (see primary outcome)
-
Adverse effects
serious adverse effects of treatment regimens (including but not limited to rectal bleeding, intestinal perforation, mucosal erosions, anaphylactic reaction, vomiting with electrolyte disturbance)
other adverse effects of treatment (e.g. abdominal distension, soiling, loss of continence or pain)
Adherence to treatment (this will help to provide information about the tolerability of the treatment)
Search methods for identification of studies
We planned to search for all relevant published and unpublished trials without restrictions on language or publication status.
Electronic searches
The authors identified potentially relevant studies from the Group's Cystic Fibrosis Trials Register using the term: distal intestinal obstruction syndrome (DIOS). The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of The Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cochrane Cystic Fibrosis and Genetic Disorders Group's website.
Date of the most recent search: 09 September 2021.
We searched the following databases on 12 October 2021:
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library www.thecochranelibrary.com;
MEDLINE Ovid (1946 to October 2021)
EMBASE Ovid (1974 to October 2021)
We also searched the following trials registries and other resources. Date of last search 12 October 2021.
US National Institutes of Health Ongoing Trials Register Clinicaltrials.gov (www.clinicaltrials.gov);
International Standard Randomised Controlled Trial Number (ISRCTN) Registry (www.isrctn.com );
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch);
Open Grey (www.opengrey.eu/).
For details of our search strategies, please see the appendices (Appendix 2).
Searching other resources
We checked the bibliographies of included trials and any relevant systematic reviews identified for further references to relevant trials.
Data collection and analysis
Selection of studies
Once we had the complete list of identified references, one author (JG in 2018 and FG in 2021) checked for duplicates and removed them. Two authors (JG and FG in 2018 and FG and WC in 2021) reviewed all titles and abstracts and discarded references which clearly did not meet the inclusion criteria. We planned to resolve any disagreements by discussion, but if we could not reach a decision, the third author (WC in 2018 and JG in 2021) would have acted as an external arbiter to mediate until we could reach a final conclusion. Once we discarded trials on the basis of title and abstract, we planned to obtain full copies of the remaining references and screen these using a standardised screening form customised for this review.
We considered trials in any language and planned to translate them as necessary. We planned to include trials published as full texts; if there was only an abstract available, we would include it if it presented results. If there were no results presented within the abstract or on any trials registry sites, then we would classify the trial as 'Awaiting assessment' until more information was available. Similarly with ongoing trials, if a trial met our inclusion criteria and quality assessment then we would include it.
We have presented the results of the search using a standardised flow chart.
Data extraction and management
Two authors (JG and FG) independently performed data extraction for the included trial. Data extraction is a significant part of a Cochrane Review, as authors must collect important information from each of the included trials and record the data on a detailed form. We collected data using the data extraction form on Covidence, an online software program that provides detailed data extraction forms for Cochrane Reviews (Covidence 2017). We aimed to collect data on:
participant characteristics;
trial characteristics and trial design;
intervention and comparator;
outcome data ‐ we will report data for each outcome separately.
One author (JG) checked the two independently completed data extraction forms for discrepancies and if there had been any which we could not resolve by discussion, the third author (WC) would have arbitrated.
We would have entered the data extracted into Review Manager software for analysis, but none were available (RevMan 2014). We planned to report data at up to one week, up to two weeks, up to one month, up to three months, up to six months and up to one year. If data had been reported at other time points we would have considered reporting these too. We planned to initially carry out a comparison of any osmotic agents, stimulant laxatives or mucolytics versus placebo or usual treatment with further subgroup analyses planned as data allowed (see below).
Assessment of risk of bias in included studies
We used the risk of bias tool as described in the Cochrane Handbook for Systematic Reviews of Interventions to assess the risk of bias across six domains (sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and other potential sources of bias) (Higgins 2011).
If the trial described methods of randomisation in the allocation of participants to their intervention groups, we would assign a low risk of bias only if the described method of randomisation was adequate (e.g. computer‐generated random number lists). We also looked for methods of concealment of the allocation sequence from the researchers, and if we deemed these to be adequate, then we ranked the trial as having a low risk of bias for this domain. Examples of an adequate method of concealment may include the use of opaque sequentially numbered sealed envelopes. Where these methods were inadequate, we ranked the trial as being at a high risk and where it was unclear from the description given, then we ranked it as having an unclear risk of bias.
Similarly for blinding, the trial should state that participants and personnel were blinded, in order to have a low risk of bias for this domain. We also looked for the blinding of outcome assessors, which should be specifically mentioned for the rank of low risk of bias.
For the domain of incomplete outcome data, we planned to extract information on missing data and how the investigators recorded participant withdrawals and loss to follow‐up. We also planned to look at whether missing data were equally distributed between the intervention and control groups. If the review authors agree that missing data have been accounted for adequately, then we would judge the trial to be at a low risk of bias. We planned to record the trial as having a high risk of bias if the missing data had not been reported adequately and would have recorded it as having an unclear risk of bias if we were unable to see how the missing data had been reported. We planned to assess each included trial to determine whether the investigators used an intention‐to‐treat (ITT) analysis and again, once we had reached an agreement, we would rank the trials as being at a high, low or unclear risk of bias. If a trial had a high risk of bias for missing data but a low risk of bias for ITT analysis or vice versa, we would look into more detail at the data to make a final judgement. This would include looking at the proportion of randomised participants who have been analysed as ITT and how many individuals dropped out relative to the total number of participants in the trial. If these data were high, then the overall risk of bias for incomplete outcome data would be high.
If the trial investigators reported all outcomes in the paper, we planned to record a low risk of bias from selective reporting. If the paper stated that investigators measured outcomes, but they did not report the results of these, the review authors would rank the paper as being at high risk. If it was unclear to the us whether the trial reports all outcomes measured, then we planned to state this and rank it as unclear for this domain. We also planned to search for trial protocols to be able to assess outcome reporting. If we could not locate the protocol, we planned to assess outcome reporting based on a comparison between the methods section of the full published paper and the results section.
We planned to look for any other potential sources of bias in the included trials and record what we find. If we could not find any other source of bias, then we planned to rank the trial as having a low risk for this domain and high risk if the opposite is true.
We presented the results of the risk of bias assessment both individually and in a summary table.
Measures of treatment effect
For dichotomous data (adverse effects, treatment failure, recurrence and adherence), we planned to calculate a pooled estimate of the treatment effects for each outcome across trials using risk ratio (RR) and 95% confidence intervals (CIs) where appropriate.
For continuous data, we planned to record the mean change and standard deviation (SD) from baseline for each group. We intended to calculate a pooled estimate of treatment effect using the mean difference (MD) and 95% CIs. Where trials use different units of measurement or measurement scales for reporting the same outcome, we planned to use the standardised MD (SMD) to report the results. Where trials only report only a pre‐intervention mean (SD) and post‐intervention mean (SD) then we intended to calculate the mean change but not the SD of the change. We planned to report these results narratively.
For time‐to‐event data (time to resolution of DIOS) we intended to express the intervention effect as a hazard ratio (HR) with 95% CIs using the generic inverse variance method. It may be that time taken to resolution of DIOS is reported as continuous data (rather than time‐to‐event data) in some trials. If this is the case, we planned to seek advice from Cochrane, but would analyse the results according to how the majority of included trials present the data, so that we could obtain an accurate estimate of treatment effect.
Where end‐points are semantically different but report to similar outcomes then we planned to group outcomes. Thus, synonymous terms were considered jointly. We considered:
abdominal distension to be synonymous with bloating, swelling, gaseous distension;
pain to be synonymous with discomfort or ache;
vomiting to be synonymous with emesis; and
constipation to be synonymous with straining at stool or dyschezia.
Unit of analysis issues
We assessed any trials using a cross‐over design to establish how much data we could include in the analysis. We included the trial if the authors had taken account of the cross‐over design in the analysis, any carry‐over effect (i.e. included a washout period for the intervention) and within‐person differences. Where the original authors had not analysed the data appropriately, we planned to include data from the first phase of the cross‐over trial as if it were a parallel design; although the advantage of the cross‐over design (using participants as their own controls) would be lost (Elbourne 2002).
If we found trials which were multi‐arm they would possibly fall into more than one comparison. In such cases, where the two active treatment arms are different types of laxative regimen, e.g. macrogol 3350 versus lactulose and senna versus placebo, we planned to analyse each treatment arm separately against placebo and where appropriate included in a meta‐analysis. If the two active treatment arms were of the same type of laxative (e.g. softening agents), but employ a different laxative or dose, we intended to combine them against the placebo arm to look at the effect of the type of laxative rather than an individual drug. When analysing multi‐arm studies, it would also be sensible to split the placebo group in order to avoid active treatments being compared to the same individuals. If there was heterogeneity between trials looking at different types of laxative regimen, we planned to carry out a subgroup analysis to look at the effect of individual drugs (see Subgroup analysis and investigation of heterogeneity).
Dealing with missing data
We planned to request additional data from the trial author(s) if there were insufficient data in the published paper or uncertainty about data we are able to extract from the included trials. We planned to undertake an intention‐to‐treat (ITT) analyses wherever possible throughout the review.
We also planned to assess the extent to which trial authors had employed an ITT analysis and we planned to report the numbers of participants who dropped out of each arm of the trial, where possible.
Where data are incomplete but partially available, we intended to use the last available measurement.
Assessment of heterogeneity
Where there are trials reporting the same outcomes which we were able to include in a meta‐analysis, we planned to assess the level of heterogeneity using the I² statistic. We also planned to look at the overlap of the CIs on the forest plots to gauge the significance of the I² value.
We planned to base our definitions of different levels of heterogeneity on the levels described in the Cochrane Handbook for Systematic Reviews of Interventions:
low (might not be important) = 0% to 40%;
moderate = 30% to 60%;
substantial = 50% to 90%; and
considerable = 75% to 100%.
The Cochrane Handbook for Systematic Reviews of Interventions states that this is a rough guide because the importance of inconsistency depends on several factors (Deeks 2011).
Assessment of reporting biases
Where we were able to include at least 10 trials, we planned to generate a funnel plot to attempt to identify any publication bias in the included trials (Sterne 2011). We would also attempt to identify any selective reporting in the included publications, by comparing the trial protocols with the final papers and by careful examination of the trial publications and consideration of reporting of both positive and negative effects of the intervention. Where trial protocols were not available, we planned to compare the outcomes reported in the results section against the methods section of the paper. We planned to extract information on the sponsors, sources of funding and competing interests of the authors to determine the role of external bias being introduced. To minimise publication bias, we planned to contact pharmaceutical companies for unpublished data; we searched trial registries (as detailed above).
Data synthesis
Where we were able to combine trials in a meta‐analysis, we planned to use the data from the selected trials to generate forest plots using the Review Manager software (RevMan 2014). We planned to carry out separate meta‐analyses for different groups of laxative agents (e.g. osmotic laxatives, stimulant laxatives and mucolytics and those with a combined mechanism of action) versus placebo, usual treatment or each other. We intended to examine the level of heterogeneity to determine which type of analysis model to use. If there was low heterogeneity (less than 40%) then we planned to use a fixed‐effect model and if the I² statistic was greater than 40% then we would use a random‐effects model to summarize the data. However, it is important to note that as the random‐effects model allows for heterogeneity, the CI for the pooled estimate will be wider and therefore, less precise. If heterogeneity was considerable (I² over 75%), we planned to report results narratively as it would not be appropriate in these cases to combine results in a meta‐analysis.
Subgroup analysis and investigation of heterogeneity
If there was greater than 40% heterogeneity in the included trials, we planned to undertake the following subgroup analyses:
comparison of individual treatment agents (e.g. lactulose versus senna) or combinations or agents (e.g. lactulose plus senna versus diatrizoate);
children (under 18 years of age) versus adults;
route of administration (e.g. oral, via nasogastric tube, via gastrostomy or rectally).
Sensitivity analysis
If we had performed a meta‐analysis, we planned to carry out sensitivity analyses to look at the effect of the risk of bias findings. We intended to look at the effect of adding in and taking out trials with a high risk of bias. For example, we would include the high risk of bias trials in the main meta‐analysis, but would look at the effect of excluding those trials whose eligibility criteria was questionable (e.g. quasi‐RCTs with unclear baseline characteristics) or those with a high risk of bias (e.g. high degree of missing data not accounted for) in the sensitivity analysis in order to examine their overall effects on the review. We also planned to attempt to examine the effect of cross‐over trials on the results by carrying out a sensitivity analysis to include and exclude them.
Summary of findings and assessment of the certainty of the evidence
We reported summary of findings information; we planned to present a separate table for each treatment comparison, where there was at least one trial assessing our chosen outcomes comparing laxative agents versus control, placebo or alternate regimens for the outcomes: time to resolution of DIOS; treatment failure; recurrence of DIOS; adverse effects; and adherence. Thus we currently present a single table.
For each outcome we planned to report the illustrative risk with and without the intervention, magnitude of effect (RR or MD), numbers of trials and participants addressing each outcome and a grade of the overall certainty of the body of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) with comments (Schünemann 2011).
Results
Description of studies
Please see tables for additonal information.
Results of the search
There were 3054 references retrieved after electronic searches. Of these, nine references (eight trials) were considered eligible after screening. After full text screening, one trial was included in the review. Details of the included trial and reasons for excluding the remaining seven trials can be found below. The flow diagram can be found in Figure 1.
1.
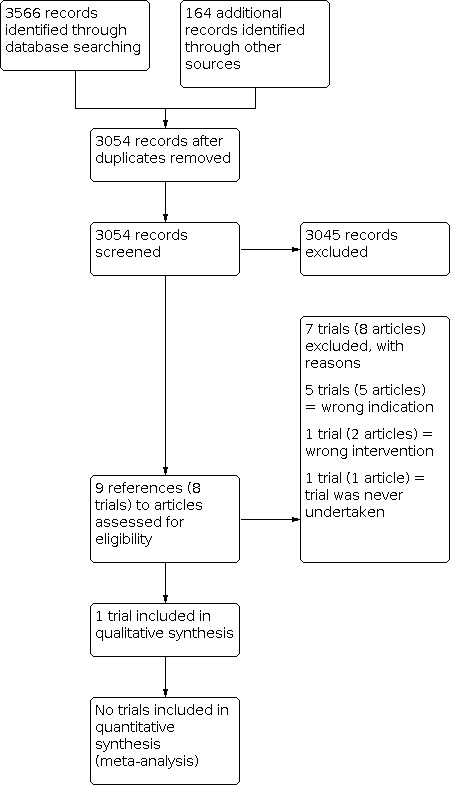
Study flow diagram
Included studies
There was one trial included in the review which was only available as an abstract (Dalzell 1993). We attempted to contact the authors, who informed us that there was no full text version of the trial available. It was a single‐centre, double‐blind, randomised, cross‐over trial that took place at the Royal Liverpool Children's Hospital, Alder Hey in the UK. The trial included 20 participants (16 female) with an age range of 7.1 years to 23.2 years, giving a mean age of 13.1 years. Participants were required to have a diagnosis of chronic DIOS to be included in the trial.
Participants were randomly given either high‐dose or low‐dose pancreatic enzymes for six months each. Trial investigators measured the difference in acute episodes of DIOS, presence of an abdominal mass and abdominal pain. Other outcomes measured in the trial included the coefficient for fat absorption and weight gain.
More details of the included trial can be found in the Characteristics of included studies table.
Excluded studies
Please see the Characteristics of excluded studies table for more information.
Seven trials were excluded in total. Five of these were excluded because the indication was wrong, i.e. N‐acetylcysteine was not used to treat DIOS. In four trials N‐acetylcysteine was used as an intervention for CF lung disease (Baran 1980; Dietzsch 1980; Gotz 1980; Howatt 1966). In one trial N‐acetylcysteine was used to improve malabsorption in CF (Mitchell 1981). One trial was excluded because the intervention was used for preventing DIOS rather than treating DIOS (Koletzko 1990) and the final trial was excluded as it was a trial proposal and the trial was never undertaken (as confirmed by the lead author) (Rotolo 201923).
Risk of bias in included studies
The general risk of bias of the included trial was unclear, as there was not enough information to judge most of the domains. For more information on the risk of bias, please see the Characteristics of included studies table and the risk of bias summary (Figure 2).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The trial was described as randomised, but the method of how this was done was unclear. There was no information in the trial about whether the allocation to high‐dose or low‐dose pancreatic enzymes was concealed; this was therefore also judged as having an unclear risk of bias.
Blinding
The trial, which was only published as a conference abstract, was described as double‐blinded, so we judged this to have a low risk of bias. We accepted this description because it is unlikely that the authors would have gone into detail on exactly who was blinded and how it was done (e.g. making the two doses of PERT look, smell and taste identical) in the abstract. However, we also recognise that these details should be discussed fully in the text of the trial. Detection bias was low risk for two outcomes as these were objective measures:the episodes of acute DIOS and presence of an abdominal mass. However, there was not information about the blinding of outcome assessors for the outcome of abdominal pain. We judged this as having an unclear risk of bias because pain is a subjective outcome.
Incomplete outcome data
The trial did not describe any withdrawal of participants, nor whether there was an intention‐to‐treat analysis. There was insufficient information to judge the risk of bias in this domain, therefore, we ranked it as unclear.
Selective reporting
As the trial was only available as an abstract (and there was no protocol available), we could not compare the outcomes in the methods with those in the results section. There was insufficient information to judge whether there was selective reporting, so we ranked it as having an unclear risk of bias for this domain.
Other potential sources of bias
There was insufficient information in the abstract to judge whether there was a washout period between the first and second phase of the trial. No other potential sources of bias were identified.
Effects of interventions
See: Table 1
Please see the summary of findings table (Table 1).
As there was only one trial in this review, we were unable to perform meta‐analysis of the data. Furthermore, as the included trial was only available as an abstract, no data could be presented in the results, although three outcomes were relevant to our review. We tried to contact the authors for a full‐text copy of the review, but we were unsuccessful.
Primary outcomes
1. Time taken from start of treatment until the resolution of DIOS
The included trial did not report this outcome measure.
2. Treatment failure rate
The included trial did not report this outcome measure.
Secondary outcomes
1. Recurrence rate of DIOS after resolution of DIOS
The included trial reported the number of episodes of acute DIOS during the 12‐month trial period. It stated that there was no difference in episodes between high‐dose and low‐dose pancreatic enzymes.
2. Adverse effects
a. serious adverse effects of treatment regimens
The included trial did not report this outcome measure.
b. other adverse effects of treatment
The included trial reported the adverse effects abdominal mass and abdominal pain. It stated that there was no difference in either event between high‐dose or low‐dose pancreatic enzymes. Numerical data were not available for these outcomes.
3. Adherence to treatment
The included trial did not report this outcome measure.
Discussion
Summary of main results
As there was one included trial in this review, we could not perform a meta‐analysis of data. The trial was only available as an abstract and did not provide numerical data for any of our outcomes. The trial did not address our primary outcomes (time until the resolution of DIOS and treatment failure rate) but addressed two of our secondary outcomes (episodes of acute DIOS and adverse effects (abdominal mass and abdominal pain)). The authors stated that there were no differences in the episodes of acute DIOS or in the adverse effects between the two treatment arms, but did not provide numerical data to support this.
A summary of the evidence from the single comparison in this review (high‐dose pancreatic enzymes compared with low‐dose pancreatic enzymes) is presented in the tables (Table 1).
Overall completeness and applicability of evidence
In this review, there was an absence of assessment regarding the efficacy and safety of various laxative agents for treating DIOS. The only included trial failed to address our review objective or our primary outcomes, therefore, the overall completeness and applicability of this evidence to the wider CF population is extremely limited.
Quality of the evidence
The lack of included trials does not allow a robust conclusion regarding the objective of this review. The only included trial was small (comprising 20 participants) adding to the imprecision of the results. The participants also had a limited age range, between 7.1 and 23.2 years. This restricts the main review objective to a small group of participants and thus contributes to the indirectness of the evidence in the review. Furthermore, although the trial was a randomised, double‐blind and cross‐over in design, it failed to address our primary outcomes and two of our secondary outcomes.
The included trial also only compared low‐dose to high‐dose pancreatic enzyme therapy, but specific dose levels were not described. There were also no other types of laxatives (osmotic, stimulant or bowel cleansing agents) assessed for the treatment of DIOS, which contributes to the indirectness of the evidence. Only the abstract of this trial was available, so we are unable to comment on the consistency or inconsistency of the results, or whether there was any selective reporting. Other areas of potential bias such as allocation or incomplete outcome data were also unclear.
Potential biases in the review process
We conducted comprehensive electronic searches on medical databases and registries in order to identify suitable trials for this review, therefore reducing bias. However, since the only RCT on this topic took place in 1992 and to our knowledge, there have been none since then that matched our inclusion criteria, it may suggest a degree of publication bias. Furthermore, we were unable to obtain a full‐text version of the included trial, even after contacting one of the authors, meaning that we were unable to access the relevant data in the trial. This may also indicate that there was publication bias.
Within the review process, two authors independently screened and assessed trials for eligibility, as well as assessing for risk of bias. A third author acted as external arbiter in order to solve any disagreements. This process reduced the risk of bias.
Agreements and disagreements with other studies or reviews
As far as we are aware, there have not been any other systematic reviews or RCTs (other than the included trial in this review) comparing interventions for the treatment of DIOS. It is generally accepted that good adherence to PERT can reduce constipation and gastrointestinal symptoms in CF, but there is no high‐quality evidence that assesses the use of PERT for the treatment of DIOS.
Authors' conclusions
Implications for practice.
Despite the use of various laxative agents for the treatment of distal intestinal obstruction syndrome (DIOS) in clinical practice, this review concludes that there is no consensus or evidence‐base regarding the efficacy and safety of current interventions used to treat DIOS. There is no high‐quality evidence that compares the use of pancreatic enzyme replacement therapy (PERT) or any other laxative regimen for treating DIOS.
Implications for research.
This review has highlighted that there is a severe lack of evidence for the treatment of DIOS in children and adults with cystic fibrosis (CF). Therefore, there is a need for further randomised controlled trials with much larger numbers of participants to be carried out, comparing the current laxatives used in clinical practice (e.g. osmotic laxatives, stimulants, mucolytics and bowel cleansing agents) at any dose, with placebo, no treatment or with other laxatives. However, one must consider the ethical implications of using a placebo to treat DIOS, as it is a serious and potentially dangerous condition, especially if the individual experiences complete obstruction.
Future trials should include a range of participants including children and adults, as DIOS occurs in both of these groups. Future research should also address important outcomes for the treatment of DIOS, as highlighted in this review. These outcomes may include the time taken from the start of the treatment until the resolution of DIOS, recurrence of DIOS after successful treatment and adverse effects from treatments. In addition, future trials should include a follow‐up period in order to identify participants who may experience a recurrence of DIOS. They should also be detailed in their description of the methodology used, to ensure that an accurate assessment of the risk of bias can be carried out.
What's new
| Date | Event | Description |
|---|---|---|
| 16 December 2021 | New citation required but conclusions have not changed | Since no new data have been added at this update, our conclusions remain the same. |
| 16 December 2021 | New search has been performed | A search of the Cochrane Cystic Fibrosis and Genetic Disorders Review Group's Cystic Fibrosis Trials Register identified a single reference which was potentially eligible for inclusion in the review, however this trial proposal was never accepted and the trial was never undertaken. We have listed this as an excluded study (Rotolo 2019). On 12 October 2021, the authors repeated the full search strategy using available databases including EMBASE and MEDLINE. A further 510 articles were identified, of which 88 were duplicates and so excluded from further review. FG and WC reviewed titles and abstracts, and found no additional studies for inclusion. The PRISMA diagram (Figure 1) has been amended to reflect the larger number of studies identified. |
History
Protocol first published: Issue 9, 2017 Review first published: Issue 8, 2018
Acknowledgements
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Glossary of terms
| Term | Explanation |
| anaphylactic reaction | a life‐threatening allergic reaction that may result in severe respiratory and/or cardiovascular distress and skin reactions; it is a medical emergency |
| autosomal recessive | a form of genetic inheritance in which two copies of a gene are required for a characteristic or condition to be carried to the offspring |
| caecum | this is the beginning of the large intestine and is connected to the end of the small intestine (known as the terminal ileum); it is located in the right lower quadrant of the abdomen |
| dyschezia | painful defecation |
| emesis | vomiting |
| intestinal perforation | a hole that forms in the intestine causing its contents to leak into the abdomen; this is a surgical emergency |
| mucosal erosions | the wearing away or abrasion of a surface or lining, e.g. gastric erosions relates to abrasion of the stomach lining |
| synonymous | terms or words that have the same meaning e.g. small is synonymous with petite |
| terminal ileum | the end of the small intestine, it is connected to the caecum (see above) |
| vomiting with electrolyte disturbance | severe vomiting that leads to important electrolytes e.g. sodium, potassium, calcium being lost from the body |
For definitions of statistical and methodological Cochrane terms (e.g. cross‐over trial, funnel plot, forest plot, heterogeneity, quasi‐randomised controlled trial) please see the Cochrane Community Glossary.
Appendix 2. Search strategies
| Database/Resource | Strategy |
| Cochrane Central Register of Controlled Trials (CENTRAL) | #1 Cystic Fibrosis [MeSH descriptor] #2 cystic fibrosis:ti,ab #3 fibrocystic near/10 disease near/10 pancreas #4 mucoviscidos*:ti,ab #5 cystic* near/10 fibros*:ti,ab #6 #1 or #2 or #3 or #4 or #5 #7 distal intestinal obstruction syndrome*:ti,ab #8 dios or mie:ti,ab #9 Intestinal Obstruction [MeSH descriptor] #10 meconium ileus equivalent:ti,ab #11 faecal near/3 (obstruction or impact*):ti,ab #12 Constipation [MeSH descriptor] #13 constipat*:ti,ab #14 laxative*:ti,ab #15 Laxatives [MeSH descriptor] #16 lactulose:ti,ab #17 Lactulose [MeSH descriptor] #18 (macrogol or polyethylene glycol*):ti,ab #19 Polyethylene Glycols [MeSH descriptor] #20 movicol:ti,ab #21 klean*:ti,ab #22 diatriozate:ti,ab #23 gastrografin:ti,ab #24 sennati:ti,ab #25 docusate:ti,ab #26 bicosulfate:ti,ab #27 acetylcysteine or fibrol:ti,ab #28 parvolex:ti,ab #29 fibre:ti,ab #30 picosulphate:ti,ab #31 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 #30 #32 #6 and #31 |
| MEDLINE Ovid (1946 onwards) | 1. Cystic Fibrosis/ 2. cystic fibrosis.tw. 3. (fibrocystic adj10 disease adj10 pancreas).tw. 4. mucoviscidos$.tw. 5. (cystic$ adj10 fibros$).tw. 6. 1 or 2 or 3 or 4 or 5 7. "distal intestinal obstruction syndrome*".tw. 8. (dios or mie).tw. 9. Intestinal Obstruction/ 10. meconium ileus equivalent.tw. 11. (faecal adj3 (obstruction or impact*)).tw. 12. Constipation/ 13. "constipat*".tw. 14. "laxative*".tw. 15. Laxatives/ 16. lactulose.tw. or Lactulose/ 17. (macrogol or polyethylene glycol*).tw. or Polyethylene Glycols/ 18. movicol.tw. 19. klean*.tw. 20. diatriozate.tw. 21. gastrografin.tw. 22. senna.tw. 23. docusate.tw. 24. bicosulfate.tw. 25. acetylcysteine or fibrol.tw. 26. parvolex.tw. 27. fibre.tw. 28. picosulphate.tw. 29. 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 30. 6 and 29 |
| Embase Ovid (1974 onwards) | 1. CYSTIC FIBROSIS/ 2. cystic fibrosis.tw. 3. (fibrocystic adj10 disease adj10 pancreas).tw. 4. mucoviscidos$.tw. 5. (cystic$ adj10 fibros$).tw. 6. 1 or 2 or 3 or 4 or 5 7. "distal intestinal obstruction syndrome*".tw. 8. (dios or mie).tw. 9. INTESTINE OBSTRUCTION/ 10. meconium ileus equivalent.tw. 11. (faecal adj3 (obstruction or impact*)).tw. 12. CONSTIPATION/ 13. "constipat*".tw. 14. "laxative*".tw. 15. LAXATIVE/ 16. lactulose.tw. or LACTULOSE/ 17. (macrogol or polyethylene glycol*).mp,hw. 18. movicol.tw. 19. klean*.tw. 20. diatriozate.tw. 21. gastrografin.tw. 22. senna.tw. 23. docusate.tw. 24. bicosulfate.tw. 25. acetylcysteine or fibrol.tw. 26. parvolex.tw. 27. fibre.tw. 28. picosulphate.tw. 29. 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 30. 6 and 29 |
| Clinicaltrials.gov | ADVANCED SEARCH Search 1 Search terms: laxative OR laxatives OR lactulose OR macrogol OR polyethylene OR movicol OR klean OR diatriozate OR gastrografin OR senna OR docusate OR bicosulfate OR acetylcysteine OR fibrol OR parvolex OR picosulphate OR fibre Study type: Interventional Studies Conditions: cystic fibrosis Search 2 Search terms: intestinal OR DIOS OR constipation OR constipated OR faecal OR meconium Study type: Interventional Studies Conditions: cystic fibrosis |
| ISRCTN Registry | ADVANCED SEARCH Condition: cystic fibrosis |
| WHO ICTRP | BASIC SEARCHES Search 1: cystic fibrosis AND intestinal Search 2: cystic fibrosis AND constipation Search 3: cystic fibrosis AND faecal Search 4: cystic fibrosis AND meconium Search 5: mucoviscidose ADVANCED SEARCH Condition: cystic fibrosis Intervention: laxative OR laxatives OR lactulose OR macrogol OR polyethylene OR movicol OR klean OR diatriozate OR gastrografin OR senna OR docusate OR bicosulfate OR acetylcysteine OR fibrol OR parvolex OR picosulphate OR fibre Recruitment Status: All |
| Open Grey | (cystic fibrosis OR cf OR mucoviscidos*) AND (intestin* OR constipat* OR faecal OR meconium OR laxative* OR lactulose OR macrogol OR polyethylene OR movicol OR klean* OR diatriozate OR gastrografin OR senna OR docusate OR bicosulfate OR acetylcysteine OR fibrol OR parvolex OR picosulphate OR fibre) |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dalzell 1993.
| Study characteristics | ||
| Methods | Randomised double‐blind trial. Cross‐over design. Single centre (UK). |
|
| Participants | 20 participants (16 female) with mean age of 13.1 years (range 7.1 to 23.2) with CF and a diagnosis of chronic DIOS. | |
| Interventions | High‐dose pancreatic enzymes compared to low‐dose pancreatic enzymes for the treatment of chronic DIOS. | |
| Outcomes |
|
|
| Notes | There was no information available regarding the source of funding for the trial. No declarations of the interest from the primary researchers were stated in the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough information to make a judgement on whether there was true random sequence generation, although the trial stated that the participants were randomised. |
| Allocation concealment (selection bias) | Unclear risk | Not enough information to make a judgement on whether there was allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated that this was a "double‐blind" trial. |
| Blinding of outcome assessment (detection bias) Episodes of acute DIOS | Low risk | This is an objective measurement, so the blinding of outcome assessors is not important. |
| Blinding of outcome assessment (detection bias) Abdominal mass | Low risk | This is an objective measurement, so the blinding of outcome assessors is not important. |
| Blinding of outcome assessment (detection bias) Abdominal pain | Unclear risk | This is a subjective measurement, so the blinding of outcome assessors is important. There is not enough information to make a judgement on whether there was blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not enough information to make a judgement on whether there was incomplete outcome data. No intention‐to‐treat analysis was described, nor was there any information on withdrawal of participants. |
| Selective reporting (reporting bias) | Unclear risk | Not enough information to make a judgement on whether there was selective reporting. There was no protocol available and the trial was only available as an abstract. |
| Other bias | Unclear risk | There was insufficient information to judge whether there was a washout period between the first and second phase of the trial. There was not enough information to make a judgement on whether there were other forms of bias. |
CF: cystic fibrosis DIOS: distal intestinal obstruction syndrome
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baran 1980 | Wrong indication: N‐acetylcysteine was used to treat respiratory complications of CF rather than DIOS. |
| Dietzsch 1980 | Wrong indication: N‐acetylcysteine was used to treat respiratory complications of CF rather than DIOS. |
| Gotz 1980 | Wrong indication: N‐acetylcysteine was used to treat respiratory complications of CF rather than DIOS. |
| Howatt 1966 | Wrong indication: N‐acetylcysteine was used to treat respiratory complications of CF rather than DIOS. |
| Koletzko 1990 | Wrong intervention: trial to assess interventions for preventing DIOS rather than treating it. |
| Mitchell 1981 | Wrong indication: N‐acetylcysteine was used to improve malabsorption in CF rather than for treating DIOS. |
| Rotolo 2019 | This is a trial proposal regarding a treatment intervention, but the lead author confirmed that the study was never undertaken due to a lack of approval |
CF: cystic fibrosis DIOS: distal intestinal obstruction syndrome
Differences between protocol and review
There were no differences between the protocol and the review.
Contributions of authors
| Roles and responsibilities | |
| TASK | WHO WILL UNDERTAKE THE TASK? |
| Protocol stage: draft the protocol | JG & FG |
| Review stage: select which trials to include (2 + 1 arbiter) | JG, FG & WC |
| Review stage: extract data from trials (2 people) | JG & FG |
| Review stage: enter data into RevMan | JG |
| Review stage: carry out the analysis | JG, FG & WC |
| Review stage: interpret the analysis | JG, FG & WC |
| Review stage: draft the final review | JG & FG |
| Update stage: update the review | FG |
Sources of support
Internal sources
No sources of support provided
External sources
-
National Institute for Health Research, UK
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
Dr Will Carroll declares no known potential conflict of interest.
Dr Fran E Gilchrist declares no known potential conflict of interest.
Jessica Green declares no known potential conflict of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Dalzell 1993 {published data only}
- Dalzell AM, Heaf DP. High dose pancreatic enzymes in distal intestinal obstruction syndrome. In: Pediatric Research Community. Vol. 7. 1993:149. [CFGD REGISTER: GN170]
References to studies excluded from this review
Baran 1980 {published data only}
- Baran D. Mucolytic treatment in cystic fibrosis. Double-blind clinical trial with oral acetylcysteine and placebo. European Journal of Respiratory Diseases 1980;61:134. [Google Scholar]
Dietzsch 1980 {published data only}
- Dietzsch HJ, Berger G, Gottschalk B. Results of oral acetylcysteine therapy in children with cystic fibrosis. European Journal of Respiratory Diseases 1980;61:135. [Google Scholar]
Gotz 1980 {published data only}
- Gotz M, Kraemer R, Kerrebijn KF, Popow C. Oral acetylcysteine in cystic fibrosis. A co-operative study. European Journal of Respiratory Diseases - Supplement 1980;111:122-6. [PubMed] [Google Scholar]
Howatt 1966 {published data only}
- Howatt WF, DeMuth GR. A double-blind study of the use of acetylcysteine inpatients with cystic fibrosis. University of Michigan Medical Centre Journal 1966;32(2):82-5. [PubMed] [Google Scholar]
Koletzko 1990 {published data only}
- Koletzko S, Corey M, Ellis L, Spino M, Durie P, Hospital for Sick Children TC. Effects of cisapride in patients with cystic fibrosis (CF) and chronic distal intestinal obstruction syndrome (DIOS). In: Proceedings of the 3rd North American Cystic Fibrosis conference; 1989 Oct 11-14; Florida. Vol. Suppl 4. 1989:138. [CFGD REGISTER: GN15a]
- Koletzko S, Corey M, Ellis L, Spino M, Stringer DA, Durie PR. Effects of cisapride in patients with cystic fibrosis and distal intestinal obstruction syndrome. Journal of Pediatrics 1990;117(5):815-22. [CFGD REGISTER: GN15b] [DOI] [PubMed] [Google Scholar]
Mitchell 1981 {published data only}
- Mitchell EA, Elliott RB. Failure of oral N-acetylcysteine to improve the malabsorption of cystic fibrosis. Australian Paediatric Journal 1981;17(3):207-208. [DOI] [PubMed] [Google Scholar]
Rotolo 2019 {published data only}
- Rotolo N, Papale M, Parisi GF, Franzonello C, Bongiovanni A, Tardino L, et al. Treatment of distal intestinal obstruction syndrome (DIOS) in cystic fibrosis: proposal of a multicenter protocol. Italian Journal of Pediatrics 2019;45(Supplement 1):Abstract no. A22. [CFGD REGISTER: GN298] [Google Scholar]
Additional references
BNF 2016
- Joint Formulary Committee. British National Formulary. 72nd edition. London: BMJ Group and Pharmaceutical Press, 2016. [Google Scholar]
BNFc 2016
- Paediatric Formulary Committee. British National Formulary for Children. London: BMJ Group and Pharmaceutical Press, 2016-7. [Google Scholar]
Colombo 2011
- Colombo C, Ellemunter H, Houwen R, Munck A, Taylor C, Wilschanski M. Guidelines for the diagnosis and management of distal intestinal obstruction syndrome in cystic fibrosis patients. Journal of Cystic Fibrosis 2011;10(2):24-8. [DOI] [PubMed] [Google Scholar]
Covidence 2017 [Computer program]
- Veritas Health Innovation Covidence. Version accessed 13 September 2017. Melbourne, Australia: Veritas Health Innovation, 2017. Available at www.covidence.org.
Cystic Fibrosis Foundation Patient Registry 2012
- Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry. 2012 Annual Data Report.
Davidson 1987
- Davidson AC, Harrison K, Steinfort CL, Geddes DM. Distal intestinal obstruction syndrome in cystic fibrosis treated by oral intestinal lavage, and a cause of recurrent obstruction despite normal pancreatic function. Thorax 1987;42:538-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG, editor(s) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analysis. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Docherty 1992
- Docherty JG, Zaki A, Coutts JA, Evans TJ, Carachi R. Meconium ileus: a review 1972–1990. British Journal of Surgery 1992;79(6):571-3. [DOI] [PubMed] [Google Scholar]
Dray 2004
- Dray X, Bienvenu T, Desmazes-Dufeu N, Dusser D, Marteau P, Hubert D. Distal intestinal obstruction syndrome in adults with cystic fibrosis. Clinical Gastroenterology and Hepatology 2004;2(6):498-503. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [DOI] [PubMed] [Google Scholar]
Hess 2015
- Hess DR, MacIntyre NR, Galvin WF, Mishoe SC, Volsko TA, O’Malley C, et al. Chapter 37: Cystic Fibrosis. In: Respiratory Care: Principles and Practice. 3rd edition. Sudbury (MA): Jones and Bartlett Publishers Inc, 2015:896. [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, editor(s) on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hodson 1976
- Hodson ME, Mearns MB, Batten JC. Meconium ileus equivalent in adults with cystic fibrosis of pancreas: a report of six cases. BMJ 1976;2(6039):790-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hodson 2007
- Hodson M, Bush A, Geddes D. Gastrointestinal disease in cystic fibrosis. In: Cystic Fibrosis. 3rd edition. Boca Raton (FA): CRC Press, 2007:216-7. [Google Scholar]
Houwen 2010
- Houwen RH, Doef HP, Sermet I, Munck A, Hauser B, Walkowiak J, et al. Defining DIOS and constipation in cystic fibrosis with a multicentre study on the incidence, characteristics, and treatment of DIO. Journal of Paediatric Gastroenterology and Nutrition 2010;50(1):38-42. [DOI] [PubMed] [Google Scholar]
Lavie 2015
- Lavie M, Manovitz T, Vilozni D, Levy-Mendelovich S, Sarouk I, Weintraubv I, et al. Long-term follow-up of distal intestinal obstruction syndrome in cystic fibrosis. World Journal of Gastroenterology 2015;21(1):318-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Longo 1993
- Longo WE, Vernava AM. Prokinetic agents for lower gastrointestinal motility disorders. Diseases of the Colon and Rectum 1993;36(7):696-708. [DOI] [PubMed] [Google Scholar]
Munck 2016
- Munck A, Alberti C, Colombo C, Kashirskaya N, Ellemunter H, Fotoulaki M, et al. International prospective study of distal intestinal obstruction syndrome in cystic fibrosis: Associated factors and outcome. Journal of Cystic Fibrosis 2016;15(4):531-9. [DOI] [PubMed] [Google Scholar]
NICE 2015
- National Institute for Healthcare and Excellence (NICE). Clinical knowledge summaries. Constipation in children (last revised June 2015). cks.nice.org.uk/constipation-in-children#!scenario (accessed 03 October 2016).
Rescorla 1993
- Rescorla FJ, Grosfeld JL. Contemporary management of meconium ileus. World Journal of Surgery 1993;17(3):318-25. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH on behalf of the Cochrane Applicability and Recommendations Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Presenting results and ‘Summary of findings’ tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org..
Sterne 2011
- Sterne JA, Egger M, Moher D, editor(s) on behalf of the Cochrane Bias Methods Group. Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Tobias 2011
- Tobias ES, Connor M, Ferguson-Smith M. Strong family history – typical Mendelian disease. In: Essential Medical Genetics. 6th edition. Hoboken, NJ: Wiley-Blackwell, 2011:210-2. [Google Scholar]
Tuladhar 1999
- Tuladhar R, Daftary A, Patole SK, Whitehall JS. Oral gastrografin in neonates: a note of caution. International Journal of Clinical Practice 1999;53(7):565. [PubMed] [Google Scholar]
Van der Doef 2011
- Van der Doef HPJ, Kokke FTM, Ent CK, Houwen RHJ. Intestinal obstruction syndromes in cystic fibrosis: meconium ileus, distal intestinal obstruction syndrome, and constipation. Current Gastroenterology Reports 2011;13(3):265-70. [DOI] [PMC free article] [PubMed] [Google Scholar]


