Abstract
Vav, a hematopoiesis-specific signaling protein, plays an important role in T-cell development and activation. Vav upregulates the expression of the interleukin-2 (IL-2) gene, primarily via activation of the distal NFAT site in the IL-2 gene promoter (NFAT–IL-2). However, since this site cooperatively binds NFAT and AP-1, the relative contribution of Vav to NFAT versus AP-1 activation has not been determined. Here, we studied the respective roles of the AP-1 and NFAT pathways in the T-cell receptor (TCR)-mediated, Vav-dependent activation of NFAT–IL-2. Although Vav stimulated the transcriptional activity of an NFAT–IL-2 reporter gene, it failed to stimulate the transcriptional or DNA-binding activities of an AP-1-independent NFAT site derived from the human gamma interferon gene promoter. Vav also did not stimulate detectable Ca2+ mobilization and nuclear translocation of NFATc or NFATp. On the other hand, Vav induced the activation of Rac1 or Cdc42 and c-Jun N-terminal kinase (JNK), enhanced the transcriptional and DNA-binding activities of AP-1, and induced increased phosphorylation of c-Jun. Dominant-negative Vav and/or Rac1 mutants blocked the TCR-mediated stimulation of these events, demonstrating the physiological relevance of these effects. Vav also associated with Rac1 or Cdc42 in T cells, and anti-CD3 antibody stimulation enhanced this association. These findings indicate that a Rac1-dependent JNK/c-Jun/AP-1 pathway, rather than the Ca2+/NFAT pathway, plays the predominant role in NFAT–IL-2 activation by Vav.
The proto-oncogene product Vav, which is expressed specifically in hematopoietic and trophoblast cells, plays crucial roles in the development and activation of T cells triggered through the antigen-specific T-cell receptor (TCR) (7, 48). Vav enhances basal and TCR-activated transcription of the interleukin-2 (IL-2) gene, and this enhancement is largely mediated by activation of the distal NFAT element in the IL-2 gene promoter (NFAT–IL-2) (12, 24, 63). As in the case of other NFAT-binding sites (44), this element represents a binding site for a cooperative complex of the transcription factors NFAT and AP-1 (25, 40). This fact confounds an accurate assessment of the relative importance of NFAT versus AP-1 in NFAT–IL-2 activation, as measured by standard reporter assays. Consistent with the ability of Vav to upregulate the activity of NFAT, several studies demonstrated reduced Ca2+ mobilization in T cells from Vav-deficient mice (9, 16, 17, 23, 60). However, this issue remains controversial in view of apparently contradictory findings that documented intact nuclear translocation and DNA-binding activities of NFAT (23) in Vav-deficient splenic T cells. Similarly, overexpressed Vav did not increase Ca2+ mobilization in transfected T cells (63).
Little is known regarding the potential role of Vav in AP-1 activation. The ability of Vav to activate c-Jun N-terminal kinase (JNK) in various cells (10, 42, 59) suggests that Vav may also enhance AP-1 activation, since JNK is one of the upstream kinases involved in AP-1 activation via the phosphorylation of c-Jun (13, 22, 55). Consistent with this notion, we recently found that transient overexpression of Vav greatly increases AP-1 activity in T cells (61), although another recent study reported that Vav does not play a role in AP-1 activation (15).
Here, we further analyzed the mechanism of Vav-mediated NFAT–IL-2 activation, with particular emphasis on the contribution of AP-1 and its potential importance as a Vav target in T cells. We also assessed the effects of Vav on the nuclear translocation and DNA-binding activities of NFAT proteins. Our findings indicate that Vav-induced activation of c-Jun/AP-1, which depends on an intact Rac or JNK pathway, plays a major role in NFAT–IL-2 activation and, furthermore, that Vav may have a relatively minor role in direct NFAT activation.
MATERIALS AND METHODS
Antibodies and reagents.
Mouse monoclonal antibodies (MAbs) against Vav or Rac1 and a rabbit anti-phospho-c-Jun (Ser-73) antibody were obtained from Upstate Biotechnology (Lake Placid, N.Y.). Polyclonal rabbit anti-c-Jun (H-79) or anti-c-Fos (K-25), goat anti-Cdc42 (P1), or anti-NFATx (C-20) antibodies as well as mouse anti-NFATc (7A6), anti-NFATp (4G6-G5), or anti-JNK1 (F-3) MAbs were obtained from Santa Cruz Biotechnology (Santa Cruz, Calif.). Anti-CD3 (OKT3) and anti-c-Myc (9E10) MAbs were purified from culture supernatants of the corresponding hybridomas by protein G-Sepharose chromatography. The antihemagglutinin (anti-HA; clone 12CA5) MAb was obtained from Boehringer Mannheim Biochemicals (Indianapolis, Ind.). Horseradish peroxidase-conjugated F(ab′)2 fragments of donkey anti-rabbit immunoglobulin G (IgG) or sheep anti-mouse IgG were obtained from Amersham (Piscataway, N.J.). All other reagents were obtained from Sigma (St. Louis, Mo.).
Plasmids.
The cDNA encoding c-Myc epitope-tagged Vav in the pEF mammalian expression vector has been described elsewhere (12). This cDNA was used as a template for oligonucleotide-based site-directed mutagenesis to generate the following mutants: (i) L213A, with a point mutation (Leu-213 to Ala) in the Dbl homology (DH) domain, and (ii) 6A-DH, containing the substitution of a six-amino-acid sequence, LLLQEL (residues 338 to 343), in the DH domain with alanine residues. HA-JNK1 and dominant-negative Rac1 (N17Rac1) were cloned in pcDNA3 and pEF, respectively. The NFAT–IL-2 luciferase reporter, obtained from G. Crabtree (Stanford University), has been described elsewhere (32). Three tandem repeats of the AP-1 site in the human metallothionein IIA gene (28, 31, 45) or two tandem repeats of the distal NFAT site in the human gamma interferon (IFN-γ) gene (NFAT-IFN) (57) were cloned in the pGL3-Basic vector (Promega, Madison, Wis.). As a control for transfection efficiencies, a β-galactosidase (β-Gal) expression plasmid in the pEF vector was used. The correct sequences of all constructs were verified by sequencing.
Cell cultures, transfection, and stimulation.
Simian virus 40 T antigen-transfected human leukemic Jurkat T (Jurkat-TAg) cells were grown in RPMI 1640 medium (Life Technologies, Inc., Gaithersburg, Md.) supplemented with 10% fetal bovine serum (Harlan, Indianapolis, Ind.), 2 mM l-glutamine, 1 mM sodium pyruvate, 10 mM HEPES, nonessential amino acid solution, and 100 U each of penicillin G and streptomycin per ml. Cells in logarithmic growth phase were transfected with various amounts of plasmid DNAs by electroporation as described previously (12, 32). In each experiment, cells in different groups were transfected with the same total amount of plasmid DNA by supplementing expression vector DNA with the proper amount of the corresponding empty vector. On average, ∼35% of the cells expressed the transfected plasmids, as determined by fluorescence-activated cell sorter (FACS) analysis of green fluorescent protein (GFP)-cotransfected cells (data not shown). After 24 h, cells were resuspended (2 × 107 to 4 × 107/ml) in RPMI 1640 medium, equilibrated at 37°C for 5 min, and either left unstimulated or stimulated with OKT3 (0.03 to 0.3 μg/ml), which had been cross-linked using a secondary anti-mouse IgG antibody (Organon Teknika Corp., Durham, N.C.). The cross-linking secondary antibody (1 μg/ml) was added 1 min after the addition of OKT3. The secondary antibody alone did not have any effect on the cells (data not shown).
Reporter assays.
After 8 h of stimulation, cells were harvested, washed twice with phosphate-buffered saline (pH 7.2), and lysed. Luciferase or β-Gal activities in cell extracts were determined as described previously (32, 61). Luciferase activity was determined in triplicate by luminometry, and β-Gal activity was measured by spectrophotometry (400 nm). Luciferase activity was expressed as arbitrary units normalized to β-Gal activity in the same cells. The standard deviation for triplicates was <10%, and each experiment was repeated at least four times.
Preparation of cell extracts.
Crude nuclear and cytoplasmic extracts were prepared as described previously (51) with some modifications. Stimulated and unstimulated cells were washed in ice-cold phosphate-buffered saline and resuspended at 5 × 107 cells/ml in ice-cold lysis buffer A (10 mM HEPES-KOH [pH 7.9], 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol [DTT], 0.5 mM phenylmethylsulfonyl fluoride [PMSF]). After 15 min on ice, a 1/16 volume of 10% NP-40 was added, and the mixture was vigorously vortexed and then centrifuged (10,000 × g) for 30 s at 4°C. The supernatant (cytoplasmic fraction) was retained on ice, and the nuclear pellet was washed with the same buffer containing 0.625% NP-40. The pellet (nuclear fraction) was incubated with 3 volumes of ice-cold extraction buffer (10 mM HEPES-KOH [pH 7.9], 400 mM NaCl, 10 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM PMSF) at 5 × 106 nuclei/ml for 15 min and then centrifuged (16,500 × g) for 15 min at 4°C. The nuclear and cytoplasmic supernatants were kept frozen at −70°C. Protein concentrations in cytoplasmic and nuclear extracts were determined using a protein assay dye reagent (Bio-Rad, Hercules, Calif.) according to the manufacturer's instructions. In some experiments, the nuclear extracts were dialyzed against 500 volumes of 10 mM HEPES-KOH (pH 7.9)–50 mM NaCl–50% glycerol (vol/vol)–1 mM DTT–1 mM MgCl2 for 24 h at 4°C. Cross-contamination between nuclear and cytoplasmic extracts averaged <10%, as determined by immunoblot detection of nuclear (Oct-1) and cytoplasmic (β-Gal) proteins (data not shown).
EMSA.
The electrophoretic mobility shift assay (EMSA) was performed as described previously (39) using oligonucleotides corresponding to NFAT–IL-2 (5′-GGAGGAAAAACTGTTTCATACAGAAGGCGT-3′) (53), AP-1 (5′-GAGCCGCAAGTGACTCAGCGCGGG-3′) (28, 31, 45), or NFAT-IFN (5′-GGTACAAAAAAATTTCCAGTCCTTGAATG-3′) (57). A consensus AP-2 oligonucleotide (5′-GATCGAACTGACCGCCCGCGGCCCGT-3′) (Promega) was used as a specificity control for competition assays. Pairs of synthetic high-pressure liquid chromatography-purified oligonucleotides containing complementary sequences were annealed by boiling equimolar concentrations of each strand for 10 min and allowing the mixture to slowly cool in a water bath to room temperature. Then, 3.5 pmol of annealed oligonucleotide was incubated in a 10-μl reaction mixture containing 70 mM Tris-HCl (pH 7.6), 10 mM MgCl2, 5 mM DTT, 10 μCi of [γ-32P]ATP (3,000 Ci/mmol), and 10 U of T4 polynucleotide kinase for 30 min at 37°C. The reaction was stopped by the addition of 1 μl of 0.5 mM EDTA and 89 μl of TE buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA). Gel shift analysis was performed using a gel shift assay system (Promega) according to the manufacturer's protocol with slight modifications. 32P-end-labeled oligonucleotides (35 fmol) were incubated in a 10-μl reaction mixture containing 10 mM Tris-HCl (pH 7.5), 0.5 mM EDTA, 0.5 mM DTT, 4% glycerol, 50 mM NaCl, 1 mM MgCl2, 0.5 μg of poly(dI-dC) · poly(dI-dC), and 4 μg of nuclear extract for 30 min at room temperature. After incubation, bromophenol blue and xylene cyanol were added to 0.02%, and the resulting complexes were resolved on an 8% polyacrylamide gel (acrylamide-bisacrylamide, 37.5:1 [wt/wt]) by electrophoresis at 100 V in 0.5× TBE buffer (1× TBE buffer is 89 mM Tris-HCl [pH 8.0], 89 mM boric acid, and 2 mM EDTA) at room temperature. The gel was subsequently dried and exposed to RX film at −70°C.
Determination of the [Ca2+]i.
The intracellular free Ca2+ concentration ([Ca2+]i) was measured as described previously (19). Briefly, cells were incubated in assay buffer (Hanks' balanced salt solution containing 10 mM HEPES and 1 mM CaCl2) containing 5 μM indo-1-acetoxy-methyl ester (Tef Labs, Austin, Tex.) for 45 min at room temperature. The cells were washed twice, resuspended in assay buffer (3 × 106/ml), and placed in fluorometer cuvettes. Ca2+ mobilization was measured at 37°C by use of a luminescence spectrometer (SLM-AMINCO Bowman Series 2; Spectronic Instruments, Rochester, N.Y.) with stirring at an excitation wavelength of 350 nm and an emission wavelength of 405 nm. The standard deviation for triplicates was <10%. In each experiment, the percentage of cells expressing transfected plasmids was determined by FACS analysis of a parallel cell sample transfected with GFP. Since there was no detectable difference in the Ca2+ response between empty vector-transfected or pseudotransfected (electroporated only) cells on the one hand and nontransfected (nonelectroporated) cells on the other (data not shown), the elevation of [Ca2+]i in cells expressing the transfected Rac or Vav proteins was calculated by taking into account the transfection efficiency and the data obtained with empty vector-transfected cells.
Rac1 or Cdc42 activity.
After stimulation for various times, cell lysates were prepared by the addition of 2× NP-40 lysis buffer B (2% NP-40, 40 mM Tris-HCl [pH 7.5], 300 mM NaCl, 10 mM EDTA, 10 mM sodium or thophosphate, 4 mM Na3VO4, 20 μg each of aprotinin and leupeptin per ml). Cells were lysed for 10 min at 4°C, and insoluble material was removed by centrifugation at 15,000 rpm (4°C, 10 min). Lysates were mixed with 2 μg of a glutathione S-transferase (GST) fusion protein (GST-PBD) expressing the Rac1- or 8 Cdc42-binding domain of mouse p21-activated kinase 3 (Pak3) (2) for 1 h, followed by the addition of 40 μl of glutathione-coupled Sepharose 4B beads (Pharmacia Biotech Inc., Piscataway, N.J.) for an additional 1 h at 4°C. The beads were washed four times with NP-40 lysis buffer B, boiled in 20 μl of 2× Laemmli buffer, subjected to sodium dodecyl sulfate (SDS)–12% polyacrylamide gel electrophoresis (PAGE) analysis, and electrotransferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, Mass.). Membranes were immunoblotted with anti-Rac1 or anti-Cdc42 primary antibodies (1 μg/ml), followed by horseradish peroxidase-conjugated secondary antibodies. The membranes were washed and visualized with an enhanced chemiluminescence detection system (Amersham).
JNK kinase assay.
JNK1 was immunoprecipitated from cell lysates using an anti-HA MAb. Samples were washed twice with lysis buffer B, washed twice with JNK kinase buffer (25 mM HEPES [pH 7.5], 25 mM MgCl2, 25 mM β-glycerophosphate, 1 mM DTT, 0.1 mM Na3VO4), and resuspended in 20 μl of the same kinase buffer plus 10 μCi of [γ-32P]ATP, 20 μM cold ATP, and 2 μg of GST–c-Jun as a substrate. Samples were incubated for 20 min at 30°C with gentle shaking. The reactions were stopped by the addition of 5 μl of 5× Laemmli buffer, and the samples were separated by SDS-PAGE, transferred to PVDF membranes, and exposed to RX film. The membranes were immunoblotted with an anti-c-Jun antibody as a loading control.
Immunoprecipitation and immunoblotting.
Cells were lysed in NP-40 lysis buffer B for 10 min at 4°C, and insoluble material was removed by centrifugation at 16,500 × g (4°C, 10 min). Lysates were mixed with optimal concentrations of various antibodies for 2 h, followed by the addition of 40 μl of protein G-Sepharose beads for an additional 1 h at 4°C. The beads were washed four times with NP-40 lysis buffer B, boiled in 20 μl of 2× Laemmli buffer, subjected to SDS–10 to 12% PAGE, and electrotransferred to PVDF membranes. Membranes were immunoblotted with various primary antibodies (1 μg/ml) and processed as described above. When necessary, membranes were stripped by incubation in 62.5 mM Tris-HCl (pH 6.7)–100 mM 2-mercaptoethanol–2% SDS for 1 h at 70°C with constant agitation, washed, and then reprobed with other antibodies.
RESULTS
Vav activates NFAT–IL-2 and AP-1.
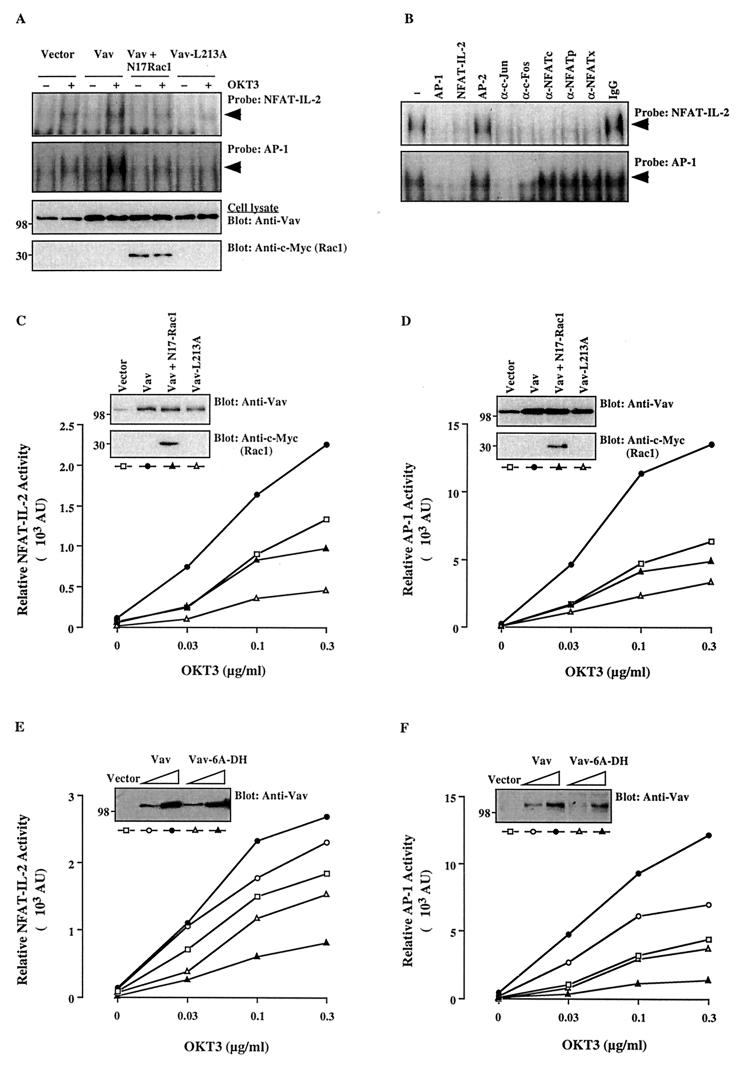
Earlier studies did not make it clear whether the upregulation of NFAT–IL-2 by Vav results from an increase in transcription factor complex binding to the cognate DNA sequence. Therefore, we first examined the effect of transient Vav overexpression on the DNA-binding and transcriptional activities of both NFAT–IL-2 and AP-1. As shown in Fig. 1A, EMSA analysis revealed that the stimulation of Jurkat T cells with an anti-CD3 antibody increased both NFAT–IL-2 and AP-1 DNA-binding activities in nuclear extracts of empty vector-transfected T cells. Both binding activities were further upregulated by transient overexpression of Vav. The transfected Vav protein was properly expressed in the cells (Fig. 1A).
FIG. 1.
The Vav/Rac pathway plays a role in NFAT–IL-2 and AP-1 activation. (A) Jurkat-TAg cells were transfected with empty pEF vector (10 μg) or wild-type Vav, Vav-L213A, or Vav plus N17Rac1 (5 μg each). After 24 h, cells (107/ml) either were left unstimulated or were stimulated with cross-linked OKT3 (0.3 μg/ml). Nuclear extracts were analyzed by an EMSA using NFAT–IL-2 and AP-1 oligonucleotide probes. The membranes were subjected to autoradiography (top two panels), and total cellular extracts from the same cells were immunoblotted with anti-Vav or anti-c-Myc antibodies (bottom two panels). The results shown are representative of four separate experiments. Arrowheads indicate specific NFAT and AP-1 complexes. (B) A similar EMSA was performed using a nuclear extract from OKT3-stimulated, Vav-transfected cells in the absence (−) or presence of the indicated unlabeled competing oligonucleotides, antibodies, or control IgG. A similar inhibition pattern was observed with an extract from unstimulated, Vav-transfected cells (data not shown). (C to F) Cells were cotransfected with NFAT–IL-2–Luc (C and E) or AP-1–Luc (D and F) reporter plasmids plus the indicated combinations of Vav, Vav-L213A, Vav-6A-DH, and/or N17Rac1. After 24 h, the cells either were left unstimulated or were stimulated with the indicated concentrations of cross-linked OKT3. Luciferase and β-Gal activities in cell extracts were determined 8 h later. AU, arbitrary units. The standard deviation for triplicates was <10%, and each experiment was repeated four times, with similar results. Samples of the same lysates were analyzed for the expression of Vav and N17Rac1 by immunoblotting with anti-Vav and anti-c-Myc antibodies, respectively (insets). The positions of molecular weight standards (in thousands) are shown.
To gain insight into the composition of the DNA-binding complex, an EMSA was performed in the presence of competing oligonucleotides or antibodies specific for NFAT or AP-1 proteins (Fig. 1B). NFAT–IL-2 binding was specifically reduced by cold NFAT–IL-2 or AP-1 oligonucleotides as well as by anti-c-Jun, anti-c-Fos, anti-NFATc, anti-NFATp, or anti-NFATx antibodies, indicating that the relevant binding complex consists of NFAT and AP-1 family proteins (Fig. 1B). On the other hand, AP-1 binding was reduced by cold AP-1 and NFAT–IL-2 oligonucleotides as well as by anti-c-Jun and anti-c-Fos antibodies but not by anti-NFAT antibodies, indicating that the AP-1-binding complex does not include any of the NFAT family proteins examined (Fig. 1B). A control IgG did not inhibit protein binding to either the NFAT–IL-2 or the AP-1 probe.
These results correlated with the transcriptional activities of NFAT–IL-2 and AP-1. Thus, luciferase reporter assays indicated that CD3 triggering stimulated in a dose-dependent manner the transcriptional activities of both NFAT–IL-2 (Fig. 1C) and AP-1 (Fig. 1D) in empty vector-transfected cells. Furthermore, these transcriptional activities were significantly upregulated by overexpression of wild-type Vav. The Vav-induced stimulation of AP-1 transcriptional and DNA-binding activities under both basal and stimulated conditions was confirmed by using additional AP-1 sites derived from the collagenase or mouse IL-4 gene promoters, as well as a consensus AP-1 oligonucleotide (data not shown).
Vav-mediated NFAT–IL-2 and AP-1 activation depends on guanine nucleotide exchange factor (GEF) activity and on Rac.
The DH domain of Vav mediates guanine nucleotide exchange activity for the Rho-Rac-Cdc42 family of GTPases, particularly for Rac (11, 20). To investigate the role of Rac as a potential intermediate in the pathway leading from Vav to NFAT–IL-2 or AP-1 activation, we examined the effect of a dominant-negative Rac1 mutant, N17Rac1. The Vav- and/or anti-CD3 antibody-mediated increase in the DNA-binding and transcriptional activities of both NFAT–IL-2 and AP-1 was blocked by coexpressed N17Rac1, even though Vav was properly overexpressed (Fig. 1A, C, and D). This result suggests that Vav activates NFAT–IL-2 and AP-1 through a Rac1-dependent pathway. Figure 1A shows that N17Rac1 was readily expressed in the transfected cells.
To further determine whether the GEF activity of Vav is important for transcriptional activation, we tested the effect of a Vav plasmid in which Leu-213 has been mutated to alanine (L213A). This mutation was found to abolish the GEF activity of Vav (11). In addition, this mutant behaves in a dominant-negative fashion by inhibiting the phosphatidylinositol 3′-kinase-mediated cytoskeletal reorganization (34) and the TCR-plus-CD28-induced activation of NFAT and JNK (M. Villalba, unpublished observations). In agreement with these findings, the Vav-L213A mutant failed to stimulate the DNA-binding and transcriptional activities of NFAT–IL-2 and AP-1, even though it was overexpressed at levels similar to wild-type Vav (Fig. 1A, C, and D). Another, DH domain Vav mutant, Vav-6A-DH, which is expected to lack GEF activity based on the effect of an analogous mutation in Dbl (21), also suppressed the anti-CD3 antibody-induced activation of NFAT–IL-2 and AP-1 reporters in a dose-dependent manner, even though it was properly overexpressed (Fig. 1E and F). These findings are consistent with the requirement for Rac and indicate that the GEF activity of Vav is essential for NFAT–IL-2 and AP-1 activation.
Vav does not induce detectable Ca2+ mobilization or NFAT nuclear translocation and DNA binding.
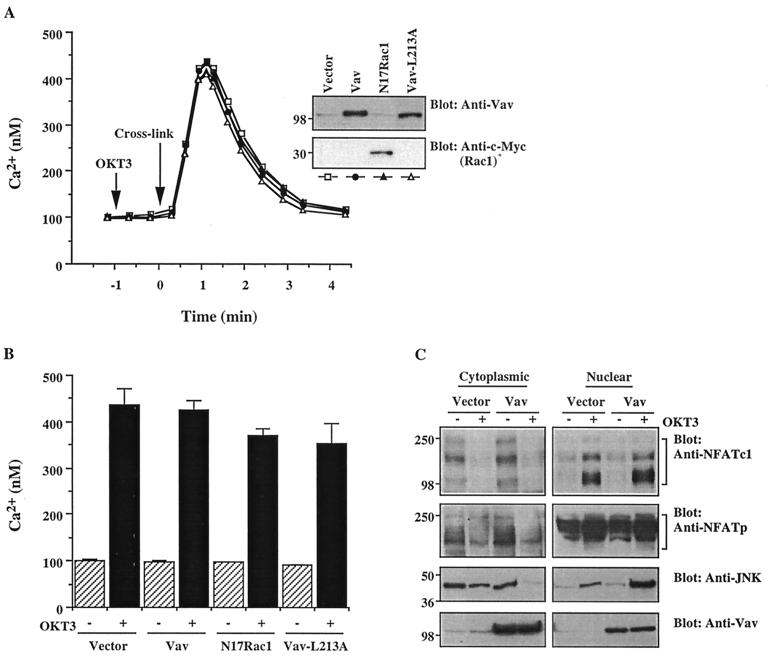
In addition to AP-1, the activation of NFAT–IL-2 also requires the binding of NFAT proteins themselves and is mediated by the nuclear translocation of these proteins (44). Nuclear NFAT translocation is regulated by the phosphatase calcineurin, which is activated by the elevation of [Ca2+]i (3, 6, 33, 44, 49, 54). Therefore, we evaluated the effect of Vav or Rac on Ca2+ mobilization in T cells. Transfection of a parallel cell sample with GFP in each experiment revealed a transfection efficiency of 35% ± 3.5% by FACS analysis (data not shown). The peak [Ca2+]i in the cell population expressing the transfected proteins was calculated based on this information (Fig. 2B). Stimulation with soluble anti-CD3 antibody did not result in detectable Ca2+ mobilization (Fig. 2A). However, the addition of a secondary cross-linking antibody dramatically elevated [Ca2+]i in empty vector-transfected cells. Overexpression of Vav did not have a detectable effect on TCR-stimulated Ca2+ mobilization, even though Vav was clearly overexpressed (Fig. 2A, inset) at a level similar to that observed in other experiments (e.g., Fig. 1). The Vav-L213A mutant, which inhibited the anti-CD3 antibody-induced activation of NFAT–IL-2 and AP-1, and the dominant-negative N17Rac1 mutant, which reversed the stimulatory effect of Vav on the same reporters, had minimal, if any, effects on Ca2+ mobilization (Fig. 2A and B).
FIG. 2.
Vav fails to induce detectable Ca2+ mobilization or NFAT nuclear translocation. (A) Cells were transfected with empty vector, Vav, Vav-L213A, or N17Rac1. After 24 h, cells either were left unstimulated or were stimulated with cross-linked OKT3. [Ca2+]i was determined by monitoring indo-1 fluorescence. The results are representative of three experiments. Samples of the same lysates were analyzed for the expression of Vav or Rac1 by immunoblotting (insets). The positions of molecular weight standards (in thousands) are shown. (B) In a parallel experiment, the percentage of cells expressing the transfected plasmids was determined by FACS analysis of GFP-transfected cells. Based on this information, the anti-CD3 antibody-stimulated peak [Ca2+]i in the transfected cell fraction was calculated. Error bars indicate standard deviations. (C) Cells were transfected with empty vector or with Vav plasmid DNA. After 24 h, the cells either were left unstimulated or were stimulated by cross-linked OKT3. Cytosolic and nuclear extracts were immunoblotted with anti-NFATc (top panel), anti-NFATp (second panel from top) or anti-JNK (third panel from top) antibodies, as indicated. Total cellular extracts from the same groups were immunoblotted with an anti-Vav antibody (bottom panel). The results shown are representative of four separate experiments. Brackets indicate NFATc and NFATp proteins. The positions of molecular weight standards (in thousands) are shown.
In order to determine whether Vav has a direct influence on the dynamics of NFAT, the nuclear translocation of NFAT proteins was analyzed in parallel by immunoblotting of cytoplasmic and nuclear extracts. In resting cells, the majority of NFATc existed in the cytoplasm (Fig. 2C). Upon TCR stimulation, the amount of NFATc in the cytoplasm was decreased and, conversely, that in the nucleus was increased. The levels of expression of both pools of NFATc in resting or TCR-stimulated cells were not affected by overexpression of Vav, even though Vav was properly overexpressed. A similar result was obtained for another NFAT family protein, NFATp, with the exception that a significant fraction of this protein was localized in the nucleus in resting cells (Fig. 2C). This result is consistent with the report that the DNA-binding activity of NFATp is constitutively expressed in the nucleus of unstimulated T cells (26, 35, 46), while the nuclear distribution of NFATc is very low in resting cells (3, 23). The functionality of the transfected Vav protein was confirmed by the finding that under the same conditions, Vav overexpression induced the nuclear translocation of JNK (Fig. 2C). Since phosphorylation and subsequent activation of JNK result in its nuclear translocation (27, 37), this result suggests that the Vav-mediated activation of JNK also induces its nuclear translocation. These results suggest that enhanced nuclear translocation of NFAT family proteins is not the primary mechanism underlying the activation of NFAT–IL-2 by Vav.
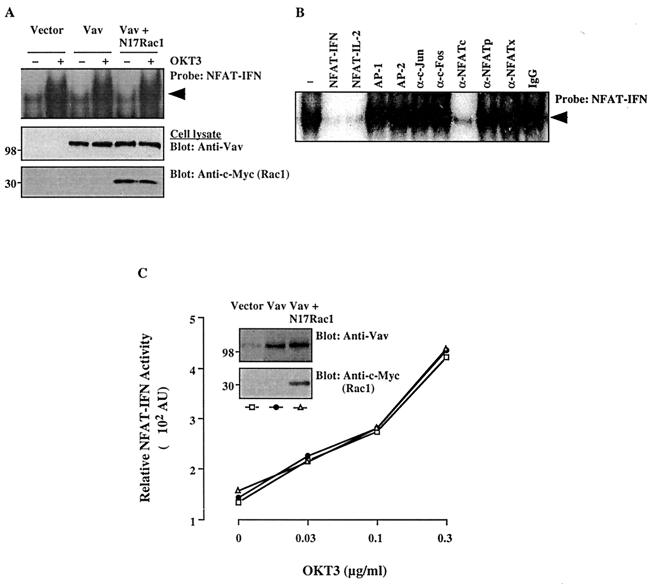
To directly evaluate the effect of Vav on NFAT binding to its cognate DNA sequence in the absence of a potential contribution by AP-1, an EMSA was performed using the distal NFAT site in the human IFN-γ gene promoter (NFAT-IFN), which does not include an AP-1-binding site (57). CD3 stimulation enhanced NFAT-IFN DNA binding in a dose-dependent manner (Fig. 3A). However, Vav, despite being properly overexpressed (Fig. 3A), did not further upregulate NFAT-IFN-binding activity in either the absence or the presence of coexpressed dominant-negative Rac1. Protein binding to the NFAT-IFN site was reduced by the addition of cold oligonucleotides specific for NFAT-IFN or NFAT–IL-2 and by an anti-NFATc antibody. In contrast, AP-1- or AP-2-specific oligonucleotides and anti-c-Fos, anti-c-Jun, anti-NFATp, or anti-NFATx antibodies had no effect (Fig. 3B). These results confirm the report (57) that the NFAT-IFN-binding complex does not include AP-1 and consists predominantly of NFATc. The effect of Vav on NFAT-IFN DNA binding correlated with the transcriptional activity of an NFAT-IFN reporter. Thus, the transcriptional activity of this reporter was enhanced in a dose-dependent manner by anti-CD3 antibody stimulation (Fig. 3C). However, Vav overexpression, with or without N17Rac1, did not affect the transcriptional activity of this reporter. These findings further support the notion that the activation of NFAT–IL-2 by Vav does not result from increased binding of NFAT family proteins.
FIG. 3.
Effects of Vav on DNA-binding and transcriptional activities of NFAT-IFN. (A) Cells were transfected with empty pEF vector, Vav, or Vav plus N17Rac1. After 24 h, cells either were left unstimulated or were stimulated with cross-linked OKT3. Nuclear extracts were prepared and analyzed by an EMSA using a 32P-labeled NFAT-IFN probe (top panel). Total cellular extracts from the same groups were immunoblotted with anti-Vav (middle panel) or anti–c-Myc (bottom panel) antibodies. The results are representative of three separate experiments. The arrowhead indicates the specific NFAT-IFN complex. (B) A similar EMSA was conducted using a nuclear extract from OKT3-stimulated, Vav-transfected cells in the absence (−) or presence of the indicated unlabeled competing oligonucleotides, antibodies, or control IgG. A similar inhibition pattern was observed with an extract from unstimulated, Vav-transfected cells (data not shown). (C) Cells were cotransfected with an NFAT-IFN–Luc reporter plasmid plus empty vector, Vav, or Vav plus N17Rac1. Stimulation and reporter activity determinations were done as described in the legend to Fig. 1. AU, arbitrary units. The data shown are representative of four experiments. Samples of the same lysates were analyzed for the expression of Vav or Rac1 by immunoblotting with anti-Vav or anti-c-Myc MAbs (insets). The positions of molecular weight standards (in thousands) are shown.
Vav activates and associates with Rac1 or Cdc42 in T cells.
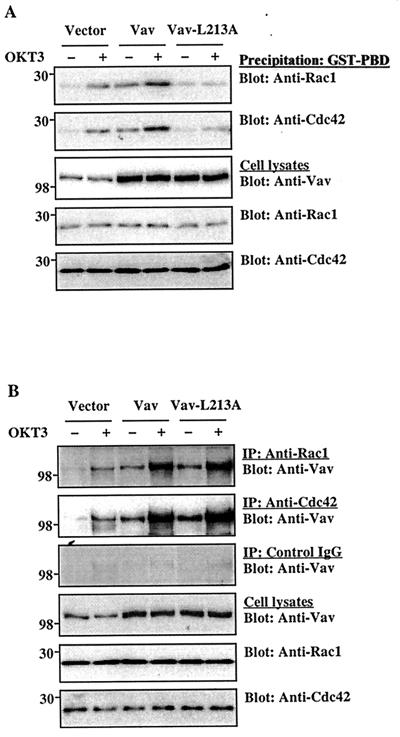
The next series of experiments was designed to elucidate the regulatory mechanisms through which Vav activates AP-1. Vav displays GEF activity for Rho family GTPases (11, 20). However, it is not clear whether Vav physiologically interacts with and activates Rho family GTPases in T cells or whether Vav couples TCR signals to Rac activation. Therefore, we determined whether Vav could modulate the activity of Rac1 or Cdc42 in T cells using a pull-down assay based on the selective binding of the activated (GTP-bound) forms of these GTPases to the Rac- or Cdc42-binding domain of mouse Pak3. In agreement with a recent report (50), TCR stimulation activated Rac1 and Cdc42 in empty vector-transfected control cells (Fig. 4A). Overexpression of Vav significantly increased the basal activity of Rac1 or Cdc42 in unstimulated cells and further enhanced the TCR-stimulated activity of these GTPases. The upregulation of Rac1 or Cdc42 activity was dose dependent in terms of the amount of Vav protein expressed (data not shown). The GEF activity-deficient Vav mutant Vav-L213A failed to stimulate Rac1 or Cdc42 activity, even though it was properly overexpressed (Fig. 4A).
FIG. 4.
Interaction between Vav and Rho family GTPases. Jurkat-TAg cells were transfected with the indicated plasmids (5 μg each). After 24 h, the cells either were left unstimulated or were stimulated with cross-linked OKT3. (A) The active, GTP-loaded forms of Rac1 or Cdc42 in cell lysates were captured by incubation with a GST-PBD fusion protein, followed by immobilization on glutathione-coupled Sepharose 4B beads. Washed precipitates were analyzed by immunoblotting with the indicated antibodies (top two panels). Total cellular extracts from the same groups were immunoblotted with anti-Vav, anti-Rac1, or anti-Cdc42 antibodies (bottom three panels). The results shown are representative of five separate experiments. (B) Cell lysates were immunoprecipitated (IP) with anti-Rac1 or anti-Cdc42 antibodies or with control IgG and immunoblotted with an anti-Vav MAb. Total cellular extracts were immunoblotted as described for panel A. The results shown are representative of three separate experiments. The positions of molecular weight standards (in thousands) are shown.
Since Vav can associate with recombinant Rho family GTPases in vitro (20), we assessed whether an association between Vav and Rac1 or Cdc42 can also be detected in intact T cells. Rac1 and Cdc42 were immunoprecipitated from resting or TCR-stimulated T cells, and the immunoprecipitates were probed with an anti-Vav antibody. Endogenous Vav was coimmunoprecipitated to a small extent with Rac1 and Cdc42 from unstimulated cells, but TCR stimulation enhanced these associations (Fig. 4B). As expected, these associations were further increased by transient Vav overexpression in the same cells. Leu-213, which is required for the GEF activity of Vav (11), was not essential for the association between Vav and Rho family GTPases. Thus, the mutated Vav-L213A protein associated with Rac1 or Cdc42 to a similar degree as the wild-type protein (Fig. 4B). Very little, if any, Vav was immunoprecipitated by a control IgG (Fig. 4B).
Vav promotes JNK activation and c-Jun phosphorylation.
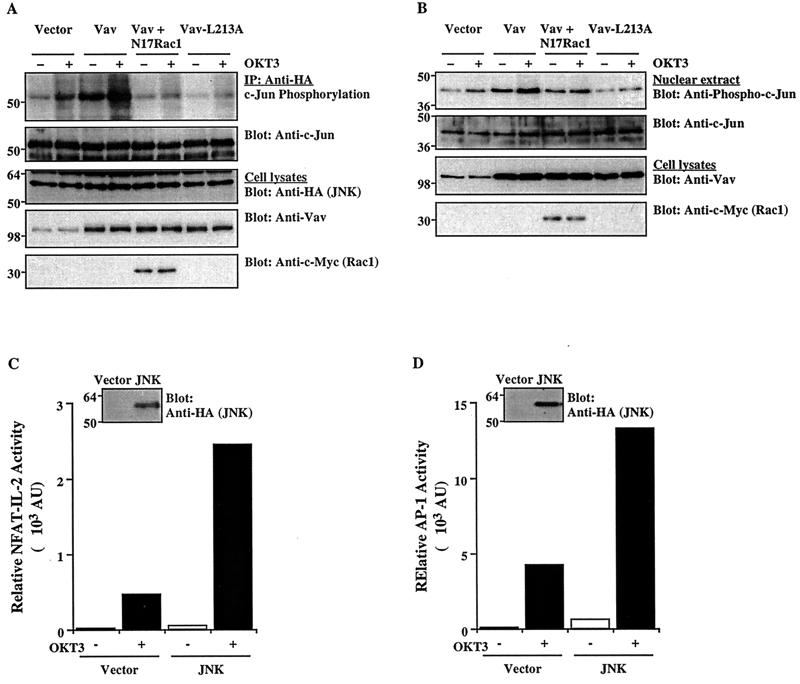
The GTP-bound forms of Rho family GTPases, Rac1 and Cdc42, bind to and activate members of the Pak family (1, 18), consequently leading to JNK activation (8, 38, 41). Activated JNK then phosphorylates c-Jun on two activating serine residues (Ser-63 and Ser-73) within its N-terminal activation domain, resulting in increased transcriptional activity of AP-1 (13, 22, 55). However, the effect of Vav on JNK activation and, in particular, on the resulting phosphorylation of c-Jun in T cells has not been studied in detail. One study indicated that JNK activation in Jurkat T cells required TCR plus CD28 costimulation, an effect that was mimicked by a combination of phorbol ester and Ca2+ ionophore (55). In the course of our studies, we found that, in contrast to stimulation with a soluble anti-CD3 antibody (reference 55 and data not shown), stimulation of Jurkat cells with a cross-linked anti-CD3 antibody was sufficient to induce JNK activation (Fig. 5A). This finding is consistent with a recent report that JNK activation in mouse immature (double-positive) thymocytes does not require CD28 costimulatory signals (47), as well as with our finding that CD3 cross-linking was also required for effective Ca2+ mobilization in Jurkat cells (Fig. 2). Overexpression of wild-type Vav induced significant JNK activation in resting or anti-CD3 antibody-stimulated cells, and these effects were reversed by the dominant-negative N17Rac1 mutant. In contrast, the GEF-deficient Vav-L213A mutant did not stimulate JNK activity and, moreover, it inhibited anti-CD3 antibody-stimulated JNK activity. The upregulation of JNK activity was proportional to the amount of Vav protein expressed (data not shown).
FIG. 5.
Effect of Vav on JNK activation and c-Jun phosphorylation. (A) Cells were cotransfected with an HA-tagged JNK1 plasmid plus the indicated combinations of Vav, Vav-L213A, and/or N17Rac1 (5 μg each). After 24 h, the cells either were left unstimulated or were stimulated with cross-linked OKT3. JNK1 was immunoprecipitated (IP) from cell lysates with an anti-HA MAb and subjected to an in vitro kinase assay using GST–c-Jun as a substrate. The SDS-PAGE-separated kinase reaction was visualized by autoradiography (top panel), followed by immunoblotting with an anti-c-Jun antibody (second panel from top). Total cellular extracts were immunoblotted with anti-HA, anti-Vav, or anti-c-Myc antibodies (bottom three panels). The results shown are representative of four separate experiments. (B) Cells were transfected and stimulated as described for panel A. Nuclear extracts were prepared, resolved by SDS-PAGE, and immunoblotted with anti-phospho-c-Jun or anti-c-Jun antibodies (top two panels). Total cellular extracts from the same groups were immunoblotted with anti-Vav or anti-c-Myc antibodies (bottom two panels). (C and D) Cells were cotransfected with NFAT–IL-2–Luc or AP-1–Luc reporter plasmids plus empty pcDNA3 or an HA-tagged JNK1 plasmid. Reporter activity in unstimulated or OKT3-stimulated cells was determined as described in the legend to Fig. 1. AU, arbitrary units. Samples of the same lysates were immunoblotted with an anti-HA MAb to detect JNK expression (insets). The results are representative of three separate experiments. The positions of molecular weight standards (in thousands) are shown.
Next, we determined the effect of Vav on the phosphorylation of c-Jun in intact T cells. Anti-CD3 antibody stimulation increased the level of phosphorylated c-Jun in empty vector-transfected cells, and transient overexpression of wild-type Vav further enhanced this phosphorylation as well as the level of phosphorylated c-Jun in unstimulated cells (Fig. 5B). Vav-L213A suppressed TCR-induced c-Jun phosphorylation, and N17Rac1 inhibited the effect of Vav, indicating that Vav stimulates c-Jun phosphorylation via a Rac1-dependent pathway. This activation of the JNK/c-Jun cascade correlated with the transcriptional activation of NFAT–IL-2 and AP-1. Luciferase reporter assays indicated that, under similar conditions, JNK1 overexpression upregulated the activities of these two transcription factors in both unstimulated and anti-CD3 antibody-activated cells (Fig. 5C and D).
DISCUSSION
The essential role of Vav in T-cell development, activation, and IL-2 production is well established through the analysis of T cells from Vav-deficient mice (9, 16, 17, 23, 58, 60, 64). The majority of studies that have analyzed the role of Vav in transcriptional activation of the IL-2 gene have focused on the distal NFAT site in the corresponding gene promoter as a potential target (12, 16, 23, 24, 63). However, since activation of this site requires cooperative binding of a complex of the transcription factors NFAT and AP-1 (25, 40, 44), it is difficult, if not impossible, to analyze the relative contribution of NFAT versus AP-1 to the Vav-mediated activation of this site. Two earlier studies reported apparently conflicting results, i.e., that Vav can activate AP-1 in Jurkat T cells (61) or that Vav has no apparent role in AP-1 activation (15).
Here, we analyzed the role of Vav in AP-1 versus NFAT activation in more detail by relying not only on reporter gene activation assays but also on assessment of DNA binding, nuclear NFAT translocation, and c-Jun phosphorylation. In addition, we analyzed the effects of Vav on an NFAT site that does not bind AP-1, i.e., the distal NFAT site in the human IFN-γ gene promoter (57). Our findings demonstrate that AP-1 is an important target of Vav in the TCR signaling pathway leading to IL-2 gene induction and that AP-1 activation is mediated by a Rac1/JNK pathway leading to c-Jun phosphorylation. Furthermore, direct NFAT activation by Vav may play a relatively minor role in IL-2 gene induction. The data supporting these conclusions can be summarized as follows. First, transient Vav overexpression enhanced the transcriptional and DNA-binding activities of several AP-1 sites and also induced the increased phosphorylation of c-Jun. TCR-mediated stimulation of all these events was blocked by dominant-negative Vav and/or Rac1 mutants, demonstrating the physiological relevance of these events. Second, although Vav, in agreement with earlier studies (12, 24, 63), stimulated the transcriptional activity of an NFAT–IL-2 reporter gene, it failed to stimulate the transcriptional or DNA-binding activities of an AP-1-independent NFAT site derived from the human IFN-γ gene promoter (57). In accordance with this finding and an earlier report (23), Vav also did not stimulate detectable nuclear translocation of NFATc or NFATp.
It is not clear why Vav failed to stimulate AP-1 activity in an earlier study (15). We and Fang and Koretzky (15) both used an AP-1 site derived from the metallothionein-IIA gene (28, 31, 45). However, whereas our reporter plasmid contained three tandem repeats of this site derived from the human gene, the one used by Fang and Koretzky (15) contained five tandem repeats based on the corresponding sheep promoter sequence. Furthermore, in the latter case, the tandem AP-1 repeats were placed upstream of a minimal IL-2 promoter (15). These differences in the reporter gene used, as well as a potentially low level of expression of Vav, which was not documented in the earlier study (15), could explain the apparent conflict in the findings.
The role of Vav in regulating TCR-driven Ca2+ and NFAT responses is not well understood and has been controversial. Our findings are consistent with other reports showing that T cells from Vav-deficient mice display intact nuclear NFAT translocation (23) and, furthermore, that transient Vav overexpression does not stimulate an increase in [Ca2+]i in transfected Jurkat cells (63). Nevertheless, other studies demonstrated defective Ca2+ mobilization (9, 16, 17, 23, 60) and NFATc upregulation (16) in the same T cells. However, this defect was not absolute, and TCR stimulation could still induce substantial Ca2+ mobilization in the cells which apparently was sufficient for NFAT activation (23). In addition, vaccinia virus-driven Vav overexpression was recently found to increase the TCR-stimulated Ca2+ response (4). Overall, these findings suggest that, although Vav has the potential to regulate Ca2+ pathways in T cells, it is not absolutely essential, and that other TCR-associated Ca2+ signaling pathways, which are independent of Vav, exist in T cells.
Our findings implicate a role for Rac1 and JNK in the TCR-induced pathway leading from Vav to AP-1 activation and, furthermore, suggest that Vav- or Rac1-dependent phosphorylation of c-Jun, most likely mediated by JNK (13, 22, 55), accounts, at least in part, for this activation. However, the physiological role of a Vav/Rac pathway in JNK–AP-1 activation and the function of activated JNK in the induction of the IL-2 gene are not completely understood. First, T cells of Vav-deficient mice displayed intact JNK and AP-1 activation in the presence of defective proliferation (16, 23). T cells from Vav-deficient mice were also found by other groups to display diminished IL-2 production (9, 58). These reports are in apparent contradiction to our findings that overexpression of dominant-negative Vav mutants suppresses JNK and AP-1 activation induced by a cross-linked anti-CD3 antibody (Fig. 5) or by a combination of soluble anti-CD3 and anti-CD28 antibodies (61). These discrepancies suggest that a compensatory mechanism(s) leading to JNK/AP-1 activation (e.g., Vav2 upregulation) might be upregulated in the chronic absence of Vav, consistent with the results of a very recent study (29). Under those conditions, inhibition of NFAT induction (16) but not its nuclear translocation might contribute to the disruption of T-cell activation and IL-2 production. Second, JNK activation is not required for IL-2 production by TCR- or CD28-stimulated primary T cells (14), suggesting that JNK is not a major target of Vav in such cells. Nevertheless, Vav could play a physiological role in JNK activation in memory or effector T cells. Last, JNK could, under certain conditions, even play a negative regulatory role in T-cell activation, as indicated by the finding that JNK phosphorylates and inhibits the nuclear translocation of NFATc and causes the nuclear exclusion of NFAT4 (NFATx) (5, 43).
Two different mutations that abolish the GEF activity of Vav ablated its biological activity in the functional assays that we conducted, i.e., reporter gene stimulation, JNK activation, and c-Jun phosphorylation. Furthermore, the mutants inhibited anti-CD3 antibody-stimulated activation events in a dose-dependent manner, consistent with their dominant-negative phenotype. These findings indicate that the catalytic activity of Vav is required for these functions and are in general agreement with the results of other studies in which similar mutations also abolished the cellular activity of Vav (34). However, in contrast to our present findings, a recent study concluded that the GEF activity of Vav was not required for NFAT activation (30). The reason for this apparent discrepancy is not clear but may involve different experimental conditions. Indeed, the GEF-deficient Vav mutant used by Kuhne et al. was toxic to Jurkat cells and, as a result, NFAT activation could be measured in unstimulated cells only at an early time following transfection (30).
The NF-κB cascade may represent a major target of the Vav or Rac pathway, at least in primary T cells. This notion is supported by the findings that both Vav-deficient (9) and PKCθ-deficient (56) T cells display a defect in NF-κB activation and that PKCθ mediates the transcriptional effects of Vav (61). In this regard, combined Vav- or Rac1-mediated activation of AP-1 and NF-κB may be important for activation of the CD28 response element in the IL-2 gene promoter, which is known to bind a complex of these two transcription factors (36, 52). However, many details of this functional connection between Vav or Rac and NF-κB activation remain to be analyzed.
We conclude that the principal mechanism through which Vav activates NFAT–IL-2 is the upregulation of AP-1 DNA-binding and transcriptional activities via the activation of a Rac1/JNK/c-Jun cascade. This mechanism is consistent with our recent finding that Vav acts in synergy with Ca2+ ionophore or with constitutively active calcineurin to activate NFAT–IL-2 in human T cells (62). This synergy is most likely due to the combined activation of AP-1 and NFAT by Vav and calcineurin-mediated signals, respectively. Further studies are required in order to elucidate additional details of the pathway used by Vav to regulate AP-1 activity.
ACKNOWLEDGMENTS
We thank L. Qiu, H. Tang, and J. Hernandez for expert technical assistance; M. Villalba, N. Coudronniere, and Y. Saso for helpful comments and discussions; and N. Weaver for manuscript preparation.
This work was supported by National Institutes of Health grant GM50819.
Footnotes
Publication 377 from the La Jolla Institute for Allergy and Immunology.
REFERENCES
- 1.Bagrodia S, Derijard B, Davis R J, Cerione R A. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J Biol Chem. 1995;270:27995–27998. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- 2.Bagrodia S, Taylor S J, Creasy C L, Chernoff J, Cerione R A. Identification of a mouse p21Cdc42/Rac activated kinase. J Biol Chem. 1995;270:22731–22737. doi: 10.1074/jbc.270.39.22731. [DOI] [PubMed] [Google Scholar]
- 3.Beals C R, Clipstone N A, Ho S N, Crabtree G R. Nuclear localization of NF-ATc by a calcineurin-dependent, cyclosporin-sensitive intramolecular interaction. Genes Dev. 1997;11:824–834. doi: 10.1101/gad.11.7.824. [DOI] [PubMed] [Google Scholar]
- 4.Billadeau D D, Mackie S M, Schoon R A, Leibson P J. Specific subdomains of Vav differentially affect T cell and NK cell activation. J Immunol. 2000;164:3971–3981. doi: 10.4049/jimmunol.164.8.3971. [DOI] [PubMed] [Google Scholar]
- 5.Chow C-W, Rincon M, Cavanagh J, Dickens M, Davis R J. Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science. 1997;278:1638–1641. doi: 10.1126/science.278.5343.1638. [DOI] [PubMed] [Google Scholar]
- 6.Clipstone N A, Crabtree G R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- 7.Collins T, Deckert M, Altman A. Views on Vav. Immunol Today. 1997;18:221–225. doi: 10.1016/s0167-5699(97)01037-2. [DOI] [PubMed] [Google Scholar]
- 8.Coso O A, Chiariello M, Yu J C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 9.Costello P S, Walters A E, Mee P J, Turner M, Reynolds L F, Prisco A, Sarner N, Zamoyska R, Tybulewicz V L J. The Rho-family GTP exchange factor Vav is a critical transducer of T cell receptor signals to the calcium, ERK, and NF-κB pathways. Proc Natl Acad Sci USA. 1999;96:3035–3040. doi: 10.1073/pnas.96.6.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crespo P, Bustelo X R, Aaronson D S, Coso O A, Lopez B M, Barbacid M, Gutkind J S. Rac-1 dependent stimulation of the JNK/SAPK signaling pathway by Vav. Oncogene. 1996;13:455–460. [PubMed] [Google Scholar]
- 11.Crespo P, Schuebel K E, Ostrom A A, Gutkind J S, Bustelo X R. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- 12.Deckert M, Tartare Deckert S, Couture C, Mustelin T, Altman A. Functional and physical interactions of Syk family kinases with the Vav proto-oncogene product. Immunity. 1996;5:591–604. doi: 10.1016/s1074-7613(00)80273-3. [DOI] [PubMed] [Google Scholar]
- 13.Derijard B, Hibi M, Wu I H, Barrett T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 14.Dong C, Yang D D, Tournier C, Whitmarsh A J, Xu J, Davis R J, Flavell R A. JNK is required for effector T-cell function but not for T-cell activation. Nature. 2000;405:91–94. doi: 10.1038/35011091. [DOI] [PubMed] [Google Scholar]
- 15.Fang N, Koretzky G A. SLP-76 and Vav function in separate, but overlapping pathways to augment interleukin-2 promoter activity. J Biol Chem. 1999;274:16206–16212. doi: 10.1074/jbc.274.23.16206. [DOI] [PubMed] [Google Scholar]
- 16.Fischer K D, Kong Y Y, Nishina H, Tedford K, Marengère L E, Kozieradzki I, Sasaki T, Starr M, Chan G, Gardener S, Nghiem M P, Bouchard D, Barbacid M, Bernstein A, Penninger J M. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr Biol. 1998;8:554–562. doi: 10.1016/s0960-9822(98)70224-6. [DOI] [PubMed] [Google Scholar]
- 17.Fischer K D, Zmuidzinas A, Gardner S, Barbacid M, Bernstein A, Guidos C. Defective T-cell receptor signalling and positive selection of Vav-deficient CD4+ CD8+ thymocytes. Nature. 1995;374:474–477. doi: 10.1038/374474a0. [DOI] [PubMed] [Google Scholar]
- 18.Frost J A, Xu S, Hutchison M R, Marcus S, Cobb M H. Actions of Rho family small G proteins and p21-activated protein kinases on mitogen-activated protein kinase family members. Mol Cell Biol. 1996;16:3707–3713. doi: 10.1128/mcb.16.7.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldsmith M A, Weiss A. Isolation and characterization of a T-lymphocyte somatic mutant with altered signal transduction by the antigen receptor. Proc Natl Acad Sci USA. 1987;84:6879–6883. doi: 10.1073/pnas.84.19.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han J, Das B, Wei W, Van Aelst L, Mosteller R D, Khosravi-Far R, Westwick J K, Der C J, Broek D. Lck regulates Vav activation of members of the Rho family of GTPases. Mol Cell Biol. 1997;17:1346–1353. doi: 10.1128/mcb.17.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart M J, Eva A, Zangrilli D, Aaronson S A, Evans T, Cerione R A, Zheng Y. Cellular transformation and guanine nucleotide exchange activity are catalyzed by a common domain on the dbl oncogene product. J Biol Chem. 1994;269:62–65. [PubMed] [Google Scholar]
- 22.Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 23.Holsinger L J, Graef I A, Swat W, Chi T, Bautista D M, Davidson L, Lewis R S, Alt F W, Crabtree G R. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr Biol. 1998;8:563–572. doi: 10.1016/s0960-9822(98)70225-8. [DOI] [PubMed] [Google Scholar]
- 24.Holsinger L J, Spencer D M, Austin D J, Schreiber S L, Crabtree G R. Signal transduction in T lymphocytes using a conditional allele of Sos. Proc Natl Acad Sci USA. 1995;92:9810–9814. doi: 10.1073/pnas.92.21.9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain J, McCaffrey P G, Valge-Archer V E, Rao A. Nuclear factor of activated T cells contains Fos and Jun. Nature. 1992;356:801–804. doi: 10.1038/356801a0. [DOI] [PubMed] [Google Scholar]
- 26.Jain J, Miner Z, Rao A. Analysis of the preexisting and nuclear forms of nuclear factor of activated T cells. J Immunol. 1993;151:837–848. [PubMed] [Google Scholar]
- 27.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 28.Karin M, Richards R I. Human metallothionein genes—primary structure of the metallothionein-II gene and a related processed gene. Nature. 1982;299:797–802. doi: 10.1038/299797a0. [DOI] [PubMed] [Google Scholar]
- 29.Krawczyk C, Bachmaier K, Sasaki T, Jones R G, Snapper S B, Bouchard D, Kozieradzki I, Ohashi P S, Alt F W, Penninger J M. Cbl-b is a negative regulator of receptor clustering and raft aggregation in T cells. Immunity. 2000;13:463–473. doi: 10.1016/s1074-7613(00)00046-7. [DOI] [PubMed] [Google Scholar]
- 30.Kuhne M R, Ku G, Weiss A. A guanine nucleotide exchange factor-independent function of Vav1 in transcriptional activation. J Biol Chem. 2000;275:2185–2190. doi: 10.1074/jbc.275.3.2185. [DOI] [PubMed] [Google Scholar]
- 31.Lee W, Mitchell P, Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987;49:741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y-C, Elly C, Langdon W Y, Altman A. Ras-dependent, Ca2+-stimulated activation of NFAT by a constitutively active Cbl mutant in T cells. J Biol Chem. 1997;272:168–173. doi: 10.1074/jbc.272.1.168. [DOI] [PubMed] [Google Scholar]
- 33.Loh C, Carew J A, Kim J, Hogan P G, Rao A. T-cell receptor stimulation elicits an early phase of activation and a later phase of deactivation of the transcription factor NFAT1. Mol Cell Biol. 1996;16:3945–3954. doi: 10.1128/mcb.16.7.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma A D, Metjian A, Bagrodia S, Taylor S, Abrams C S. Cytoskeletal reorganization by G protein-coupled receptors is dependent on phosphoinositide 3-kinase γ, a Rac guanosine exchange factor, and Rac. Mol Cell Biol. 1998;18:4744–4751. doi: 10.1128/mcb.18.8.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCaffrey P G, Perrino B A, Soderling T R, Rao A. NF-ATp, a T lymphocyte DNA-binding protein that is a target for calcineurin and immunosuppressive drugs. J Biol Chem. 1993;268:3747–3752. [PubMed] [Google Scholar]
- 36.McGuire K L, Iacobelli M. Involvement of Rel, Fos, and Jun proteins in binding activity to the IL-2 promoter CD28 response element/AP-1 sequence in human T cells. J Immunol. 1997;159:1319–1327. [PubMed] [Google Scholar]
- 37.Minden A, Karin M. Regulation and function of the JNK subgroup of MAP kinases. Biochim Biophys Acta. 1997;1333:F85–F104. doi: 10.1016/s0304-419x(97)00018-8. [DOI] [PubMed] [Google Scholar]
- 38.Minden A, Lin A, Claret F X, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 39.Mori A, Suko M, Kaminuma O, Inoue S, Ohmura T, Hoshino A, Asakura Y, Terada E, Miyazawa K, Nosaka C, Okumura Y, Ito K, Okudaira H. IL-2-induced IL-5 synthesis, but not proliferation, of human CD4+ T cells is suppressed by FK506. J Immunol. 1997;158:3659–3665. [PubMed] [Google Scholar]
- 40.Northrop J P, Ullman K S, Crabtree G R. Characterization of the nuclear and cytoplasmic components of the lymphoid-specific nuclear factor of activated T cells (NF-AT) complex. J Biol Chem. 1993;268:2917–2923. [PubMed] [Google Scholar]
- 41.Olson M F, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 42.Olson M F, Pasteris N G, Gorski J L, Hall A. Fasciogenital dysplasia protein (FGD1) and Vav, two related proteins required for normal embryonic development, are upstream regulators of Rho GTPases. Curr Biol. 1996;6:1628–1633. doi: 10.1016/s0960-9822(02)70786-0. [DOI] [PubMed] [Google Scholar]
- 43.Porter C M, Havens M A, Clipstone N A. Identification of amino acid residues and protein kinases involved in the regulation of NFATc subcellular localization. J Biol Chem. 2000;275:3543–3551. doi: 10.1074/jbc.275.5.3543. [DOI] [PubMed] [Google Scholar]
- 44.Rao A, Luo C, Hogan P G. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 45.Rauscher F J, Sambucetti L C, Curran T, Distel R J, Spiegelman B M. Common DNA binding site for Fos protein complexes and transcription factor AP-1. Cell. 1988;52:471–480. doi: 10.1016/s0092-8674(88)80039-4. [DOI] [PubMed] [Google Scholar]
- 46.Rincon M, Flavell R A. Transcription mediated by NFAT is highly inducible in effector CD4+ T helper 2 (Th2) cells but not in Th1 cells. Mol Cell Biol. 1997;17:1522–1534. doi: 10.1128/mcb.17.3.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rincón M, Whitmarsh A, Yang D D, Weiss L, Dérijard B, Jayaraj P, Davis R J, Flavell R A. The JNK pathway regulates the in vivo deletion of immature CD4+CD8+ thymocytes. J Exp Med. 1998;188:1817–1830. doi: 10.1084/jem.188.10.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romero F, Fischer S. Structure and function of Vav. Cell Signal. 1996;8:545–553. doi: 10.1016/s0898-6568(96)00118-0. [DOI] [PubMed] [Google Scholar]
- 49.Ruff V A, Leach K L. Direct demonstration of NFATp dephosphorylation and nuclear localization in activated HT-2 cells using a specific NFATp polyclonal antibody. J Biol Chem. 1995;270:22602–22607. doi: 10.1074/jbc.270.38.22602. [DOI] [PubMed] [Google Scholar]
- 50.Salojin K, Zhang J, Delovitch T. TCR and CD28 are coupled via ZAP-70 to the activation of the Vav/Rac-1/PAK-1/p38 MAPK signaling pathway. J Immunol. 1999;163:844–853. [PubMed] [Google Scholar]
- 51.Schreiber E, Matthias P, Muller M M, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts,’ prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shapiro V S, Truitt K E, Imboden J B, Weiss A. CD28 mediates transcriptional upregulation of the interleukin-2 (IL-2) promoter through a composite element containing the CD28RE and NF–IL-2B AP-1 sites. Mol Cell Biol. 1997;17:4051–4058. doi: 10.1128/mcb.17.7.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shaw J-P, Utz P J, Durand D B, Toole J J, Emmel E A, Crabtree G R. Identification of putative regulator of early T cell activation genes. Science. 1988;241:202–205. [PubMed] [Google Scholar]
- 54.Shibasaki F, Price E R, Milan D, McKeon F. Role of kinases and the phosphatase calcineurin in the nuclear shuttling of transcription factor NF-AT4. Nature. 1996;382:370–373. doi: 10.1038/382370a0. [DOI] [PubMed] [Google Scholar]
- 55.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben Neriah Y. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994;77:727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 56.Sun Z, Arendt C W, Ellmeier W, Schaeffer E M, Sunshine M J, Gandhi L, Annes J, Petrzilka D, Kupfer A, Schwartzberg P L, Littman D R. PKC-θ is required for TCR-induced NF-κB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 57.Sweetser M T, Hoey T, Sun Y-L, Weaver W M, Price G A, Wilson C B. The roles of nuclear factor of activated T cells and Ying-Yang 1 in activation-induced expression of the interferon-γ promoter in T cells. J Biol Chem. 1998;273:34775–34783. doi: 10.1074/jbc.273.52.34775. [DOI] [PubMed] [Google Scholar]
- 58.Tarakhovsky A, Turner M, Schaal S, Mee P J, Duddy L P, Rajewsky K, Tybulewicz V L J. Defective antigen receptor-mediated proliferation of B and T cells in the absence of Vav. Nature. 1995;374:467–470. doi: 10.1038/374467a0. [DOI] [PubMed] [Google Scholar]
- 59.Teramoto H, Salem P, Robbins K C, Bustelo X R, Gutkind J S. Tyrosine phosphorylation of the vav proto-oncogene product links FcɛRI to the Rac1-JNK pathway. J Biol Chem. 1997;272:10751–10755. doi: 10.1074/jbc.272.16.10751. [DOI] [PubMed] [Google Scholar]
- 60.Turner M, Mee P J, Walters A E, Quinn M E, Mellor A L, Zamoyska R, Tybulewicz V L J. A requirement for the Rho-family GTP exchange factor Vav in positive and negative selection of thymocytes. Immunity. 1997;7:451–460. doi: 10.1016/s1074-7613(00)80367-2. [DOI] [PubMed] [Google Scholar]
- 61.Villalba M, Coudronniere N, Deckert M, Teixeiro E, Mas P, Altman A. Functional interactions between Vav and PKCθ are required for TCR-induced T cell activation. Immunity. 2000;12:151–160. doi: 10.1016/s1074-7613(00)80168-5. [DOI] [PubMed] [Google Scholar]
- 62.Villalba M, Hernandez J, Deckert M, Altman A. Vav modulation of the Ras/MEK/ERK signaling pathway plays a role in NFAT activation and CD69 upregulation. Eur J Immunol. 2000;30:1587–1596. doi: 10.1002/1521-4141(200006)30:6<1587::AID-IMMU1587>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 63.Wu J, Katzav S, Weiss A. A functional T-cell receptor signaling pathway is required for p95vav activity. Mol Cell Biol. 1995;15:4337–4346. doi: 10.1128/mcb.15.8.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang R, Alt F W, Davidson L, Orkin S H, Swat W. Defective signalling through the T- and B-cell antigen receptors in lymphoid cells lacking the vav proto-oncogene. Nature. 1995;374:470–473. doi: 10.1038/374470a0. [DOI] [PubMed] [Google Scholar]