Significance
Significant calcium absorption across renal and intestinal epithelia occurs via the paracellular pathway. However, the identity of the paracellular pore involved is unknown. Claudin-2 and claudin-12 contribute paracellular calcium permeability in cell models, but single knockout animals don’t have altered serum calcium or bone mineralization. To investigate this, Cldn2/12 double knockout mice were generated. They display decreased intestinal calcium absorption and renal calcium wasting, resulting in hypocalcemia and markedly reduced bone mineralization. Claudin-2 and claudin-12 don’t physically interact in vitro, and coexpression has an additive effect on calcium permeability. Our work identifies claudin-2 and claudin-12 as important constituents of the paracellular Ca2+ pathway in intestine and kidney enabling calcium transport and highlights their important complementary roles in maintaining calcium homeostasis.
Keywords: claudins, calcium, paracellular
Abstract
Calcium (Ca2+) homeostasis is maintained through coordination between intestinal absorption, renal reabsorption, and bone remodeling. Intestinal and renal (re)absorption occurs via transcellular and paracellular pathways. The latter contributes the bulk of (re)absorption under conditions of adequate intake. Epithelial paracellular permeability is conferred by tight-junction proteins called claudins. However, the molecular identity of the paracellular Ca2+ pore remains to be delineated. Claudins (Cldn)-2 and -12 confer Ca2+ permeability, but deletion of either claudin does not result in a negative Ca2+ balance or increased calciotropic hormone levels, suggesting the existence of additional transport pathways or parallel roles for the two claudins. To test this, we generated a Cldn2/12 double knockout mouse (DKO). These animals have reduced intestinal Ca2+ absorption. Colonic Ca2+ permeability is also reduced in DKO mice and significantly lower than single-null animals, while small intestine Ca2+ permeability is unaltered. The DKO mice display significantly greater urinary Ca2+ wasting than Cldn2 null animals. These perturbations lead to hypocalcemia and reduced bone mineral density, which was not observed in single-KO animals. Both claudins were localized to colonic epithelial crypts and renal proximal tubule cells, but they do not physically interact in vitro. Overexpression of either claudin increased Ca2+ permeability in cell models with endogenous expression of the other claudin. We find claudin-2 and claudin-12 form partially redundant, independent Ca2+ permeable pores in renal and colonic epithelia that enable paracellular Ca2+ (re)absorption in these segments, with either one sufficient to maintain Ca2+ balance.
Calcium (Ca2+) is an essential mineral for physiological processes, including cell signaling, muscle contraction, and bone mineralization. Serum Ca2+ is tightly regulated within a narrow range, and homeostasis maintained through coordinated regulation between the intestines, kidneys, and bones (1). Failure to optimally deposit Ca2+ into bone results in an increased risk of osteoporosis (2). This disease is responsible for a fracture every 3 s worldwide, presenting a significant economic and healthcare burden globally (3). Moreover, a failure to reabsorb Ca2+ along the renal tubule results in increased urinary Ca2+ excretion, the greatest risk for kidney stone formation (4). Therefore, understanding the mechanisms of Ca2+ balance will aid in understanding and treating these diseases.
Intestinal Ca2+ absorption occurs via transcellular or paracellular pathways. Paracellular diffusion is a bidirectional process whereby Ca2+ moves down an electrochemical gradient. It predominates when Ca2+ intake is adequate (5–7). Ca2+ absorption across the small intestine is thought to occur via the paracellular pathway, with the role of the colon still incompletely elucidated (5, 8). However, several reports suggest the colon is essential to Ca2+ homeostasis (8–11).
Paracellular permeation of ions across epithelia requires the formation of a pore. Claudins are membrane proteins with two extracellular loops that interact at the tight junction between cells to form pores for, or barriers to, paracellular movement of solutes by altering the charge and size selectivity characteristics (12). In this manner, claudins confer permeability properties to epithelia. Claudins-2, -12, -16, and -19 contribute to the formation of Ca2+-permeable paracellular pores. Both claudin-2 and claudin-12 are expressed throughout intestinal epithelia and contribute to Ca2+ permeability in cell culture (13). Claudin-2 (Cldn2) knockout (KO) mice have decreased colonic Ca2+ permeability but unaltered small intestine permeability. They display decreased fecal Ca2+ excretion (i.e., increased intestinal Ca2+ absorption) and have unaltered bone mineral content and serum Ca2+, relative to wild-type (WT) mice (8, 14). The intestinal phenotype of claudin-12 (Cldn12) KO mice has not been fully described, although fecal Ca2+ and serum Ca2+ levels are unaltered relative to WT mice (15). To date, no other claudins have been implicated in forming Ca2+ permeable pores in the intestine.
In the kidney, paracellular Ca2+ reabsorption occurs across the renal proximal tubule and thick ascending limb. Claudin-2 and claudin-12 are expressed in the proximal tubule, in which two-thirds of filtered Ca2+ is reabsorbed (14, 15). Paracellular Ca2+ reabsorption is better characterized in the thick ascending limb, in which claudin-16 and claudin-19 form Ca2+ permeable pores and mutations in these genes cause the syndrome familial hypomagnesemia with hypercalciuria and nephrocalcinosis associated with severe renal Ca2+ wasting (16, 17). In contrast, activation of the Ca2+-sensing receptor by increased plasma Ca2+ increases the expression of the pore blocking claudin, claudin-14, which prevents Ca2+ reabsorption from this segment (18–20).
Cldn2 KO but not Cldn12 KO mice have hypercalciuria, although direct measurement of perfused proximal tubules from Cldn12 KO mice confirm reduced Ca2+ permeability (8, 15). Interestingly, neither Cldn2 KO nor Cldn12 KO animals have increased parathyroid hormone (PTH) or calcitriol [1,25(OH)2-vitamin D] levels (8, 15). Given that paracellular reabsorption in the proximal tubule is a major contributor to renal Ca2+ reabsorption, it is unclear why these phenotypes are not more severe. These data suggest that other yet-to-be-identified pathways exist or that claudin-2 and claudin-12 compensate for each other.
We hypothesized that claudin-2 and claudin-12 form independent cation-permeable pores across intestinal and renal epithelia, contributing independently to paracellular Ca2+ transport and maintenance of Ca2+ homeostasis. To test this hypothesis, we generated Cldn2 and Cldn12 double KO (DKO) mice. Unlike previous models, DKO mice were unable to maintain normal serum Ca2+. Decreased Ca2+ permeability across the colon but not small intestine resulted in reduced intestinal Ca2+ absorption in DKO mice, which also had severe hypercalciuria and markedly decreased bone mineral density. We found that while claudin-2 and -12 were expressed in the same cells, they did not physically interact and coexpression of each claudin had an additive effect on Ca2+ permeability in vitro. These results suggest that claudin-2 and -12 mediate paracellular Ca2+ absorption and reabsorption independently and that only one of these claudins is sufficient to maintain a normal Ca2+ balance.
Results
Claudin-2 and Claudin-12 Contribute Paracellular Ca2+ Permeability to the Proximal Colon.
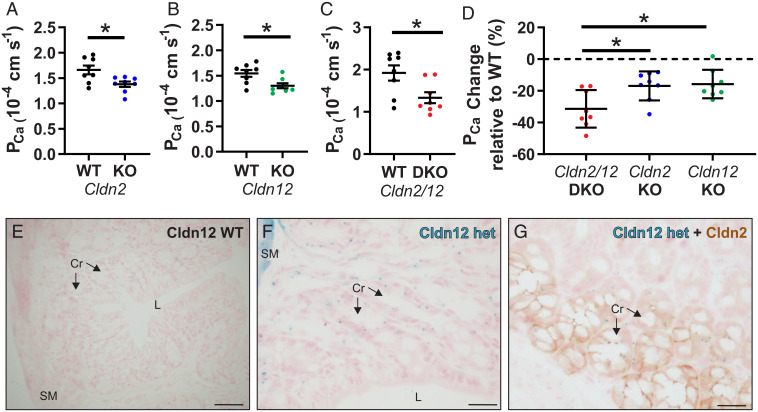
We generated a global Cldn2 and Cldn12 DKO mouse by crossing mice from Cldn2 KO and Cldn12 KO colonies. DKO mice were born to typically sized litters, and the only observed difference to WT mice was an 8% lower body weight in DKO males (SI Appendix, Table S1). We previously reported decreased colonic Ca2+ permeability in Cldn2 KO mice (8). We confirmed this and also found decreased colonic Ca2+ permeability in Cldn12 KO mice (Fig. 1 A and B and SI Appendix, Tables S2 and S3). These results are consistent with both claudin-2 and claudin-12 conferring Ca2+ permeability to the proximal colon. We repeated the experiment and found decreased Ca2+ permeability across the colon of DKO mice (Fig. 1C and SI Appendix, Table S4). To determine whether the loss of both Cldn2 and Cldn12 in the DKO mice results in an additive loss of Ca2+ permeability, we analyzed the results of each KO or DKO relative to their respective WT mice. Colonic Ca2+ permeability was decreased by 16.9% (±9.1%) in Cldn2 KO animals, 16.7% (±9.0%) in Cldn12 KO animals, and 31.4% (±11.8%) in DKO mice (Fig. 1D). The additive results are consistent with claudin-2 and claudin-12 contributing independently to Ca2+ permeability across the colon.
Fig. 1.
Cldn2 and Cldn12 confer independent Ca2+ permeability to the proximal colon. PCa measured ex vivo in Ussing’s chambers across the proximal colon compared to WT of Cldn2 KO (A) (n = 8 per group and P = 0.016); Cldn12 KO (B) (n = 8 per group and P = 0.012); and Cldn2/12 DKO (C) (n = 8 per group and P = 0.019). Full results of bionic dilution potential experiments on small intestine and colon of DKO mice are in SI Appendix, Fig. S2 and Tables S1–S4. Means were compared by Student’s t test. (D) Data from A–C expressed as the percentage change in PCa relative to WT for each genotype. Data are presented as mean ± SD. One-way ANOVA with Dunnett correction for multiple comparisons to compare mean from DKO mice to Cldn2 KO (P = 0.017) and Cldn12 KO (P = 0.010) was performed. The Cldn12 coding exon was replaced with a LacZ cassette. (E and F) We used this to localize Cldn12 expression by X-gal staining of colon from Cldn12 WT (E) and Cldn12 heterozygous mice (F). Staining is present in colonic crypt epithelial cells in Cldn12 heterozygous mouse (cyan). (Scale bars, 100 µm [E] and 25 µm [F]). (G) X-gal staining of colon for Cldn12 (cyan) and immunohistochemical staining for claudin-2 (brown) from a Cldn12 heterozygous mouse. Cr = crypts, SM = smooth muscle, and L = colonic lumen. (Scale bar, 25 µm.) *P < 0.05.
The Cldn12 KO mouse was created by replacing the Cldn12 coding exon with β-galactosidase (15). Given the lack of sufficient antibodies against claudin-12 (15, 21), we performed X-gal staining on fixed tissue sections to determine the expression of claudin-12 in the mouse colon. X-gal staining (cyan in the section) was present in the crypts of Cldn12 heterozygous mice but not WT mice (Fig. 1 E and F). Costaining for claudin-2 (brown) and X-gal (cyan) revealed that both proteins are present but restricted to crypt epithelium (Fig. 1G and SI Appendix, Fig. S1).
The paracellular pathway is proposed to contribute significant Ca2+ absorption from the small intestine. However, prior work found no difference in Ca2+ permeability between Cldn2 KO and WT mice across the small intestine (8). Similarly, in DKO mice, we failed to detect a difference in Ca2+ permeability from any small intestinal segment (SI Appendix, Fig. S2).
Claudin-2 and Claudin-12 DKO Mice Exhibit Hypocalcemia, Hypercalciuria, and Decreased Ca2+ Balance.
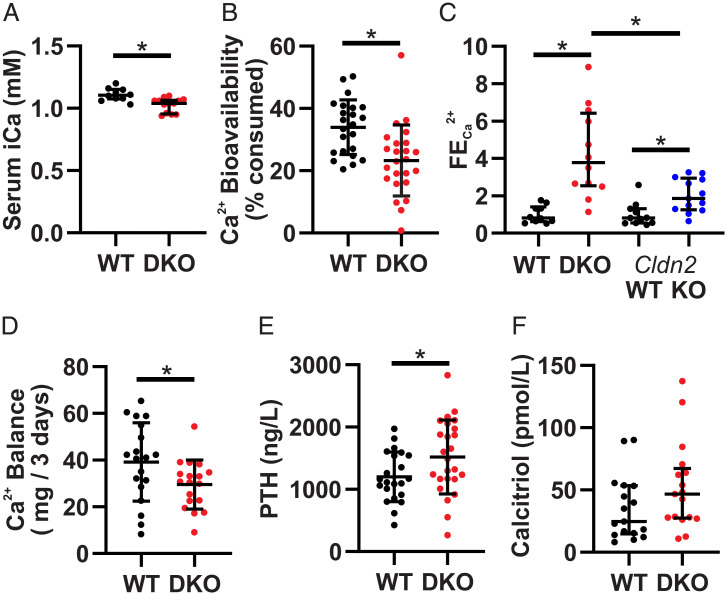
Cldn2 KO mice exhibit hypercalciuria and increased net intestinal Ca2+ absorption, while Cldn12 KO mice do not have an overt Ca2+ phenotype. To ascertain whether DKO mice have a phenotype with altered Ca2+ homeostasis, we performed metabolic cage studies. Water and chow consumption, urine volume, and fecal mass were not different between genotypes (SI Appendix, Table S1). Blood analysis revealed significantly decreased ionized calcium (iCa) in DKO animals (Fig. 2A). No differences in other chemistry, hematology, or blood gas parameters were noted, except that the DKO mice had slightly increased blood sodium, which is unlikely to be physiologically relevant. (SI Appendix, Table S5). These results suggest that the DKO mice have a phenotype with altered Ca2+ balance.
Fig. 2.
Cldn2/12 DKO mice have hypocalcemia, hypercalciuria, and decreased intestinal Ca2+ absorption. (A) Serum-ionized Ca2+ (median ± interquartile range [IQR], n = 10 WT, 13 DKO, Mann–Whitney U Test, and P = 0.002). (B) Ca2+ bioavailability as a percent of Ca2+ consumed (mean ± SD, n = 23 WT, 24 DKO, Student’s t test, and P = 0.001). (C) Fractional excretion of urinary Ca2+ normalized to WT for each genotype (median ± IQR, n = 10 WT, 12 DKO, 11 Cldn2 WT, 13 Cldn2 KO, Mann–Whitney U test, P < 0.0001 WT versus DKO, P = 0.003 Cldn2 WT versus KO, and P = 0.011 DKO versus Cldn2 KO). (D) Net 3 d Ca2+ balance (mean ± SD, n = 19 WT, 18 DKO, Student’s t test, and P = 0.043). (E) Serum PTH levels (mean ± SD, n = 22 WT, 25 DKO, Student’s t test, and P = 0.039). (F) Serum calcitriol (median ± IQR, n = 17 WT, 17 DKO, Mann–Whitney U Test, and P = 0.099). *P < 0.05.
We measured fecal Ca2+ excretion to assess intestinal absorption as a percentage of Ca2+ consumed (i.e., bioavailability). DKO mice had decreased Ca2+ bioavailability (Fig. 2B). This suggests decreased paracellular absorption from the colon, the only intestinal segment with altered Ca2+ permeability (Fig. 1 and SI Appendix, Fig. S2). Claudin-2 and claudin-12 are also expressed in the renal proximal tubule (SI Appendix, Fig. S3). No differences were observed in urinary Na+, K+, PO43−, Cl−, or Mg2+ excretion. However, we found an almost fourfold increase in Ca2+ excretion in the DKO compared to WT mice (SI Appendix, Table S5). To account for glomerular filtration and decreased plasma Ca2+, we calculated fractional excretion of Ca2+ (FECa). Relative to WT mice, the FECa in DKO mice was 4.3-fold greater (Fig. 2C). Cldn2 KO but not Cldn12 KO mice display hypercalciuria (14, 15). Of note, when normalized to their respective WT mice, FECa of DKO mice was significantly greater than single-Cldn2 KO animals (Fig. 2C). Together, these parameters contribute to decreased overall Ca2+ balance in the DKO mice (Fig. 2D).
Reduced serum ionized Ca2+ in DKO mice should increase serum PTH and, consequently, calcitriol levels. As expected, DKO had increased serum PTH. Despite this, DKO mice did not have a statistically significant increase in serum calcitriol levels (Fig. 2 E and F). This suggests that DKO mice are unable to appropriately compensate for a decreased Ca2+ balance.
Renal and Intestinal Gene Expression Changes in DKO Mice.
We examined expression of renal genes involved in Ca2+ reabsorption to assess whether compensation for marked hypercalciuria occurs. Expression of Cldn14 and Cldn16, which respectively blocks and permits Ca2+ reabsorption in the thick ascending limb, were slightly increased (SI Appendix, Fig. S4 A–C) (18, 22). Expression of Trpv5, Calb1, and Slc8a1, which contribute to transcellular reabsorption from the distal nephron, was also increased in DKO mice; there was also an increased abundance of intracellular binding protein calbindin-D28k (encoded by Calb1; SI Appendix, Fig. S4 D–I). Cyp27b1 and Cyp24a1, genes encoding enzymes that activate and deactivate calcitriol, respectively, were not significantly increased in DKO mice (SI Appendix, Fig. S4 J and K).
In the colon, although the expression of the apical Ca2+ channels Trpv6 and Cacna1d were not changed, the expression of the intracellular Ca2+-binding protein S100g and basolateral extrusion pump Atp2b1 were increased by fivefold and 0.5-fold, respectively. We observed no change in Cldn3 or Cldn4 expression but found a slight decrease in the expression of Cldn15, a gene that encodes a cation-permeable pore (23). No changes were observed in the duodenum (SI Appendix, Fig. S5). These results altogether suggest that the DKO mice have increased transcellular Ca2+ absorption from the colon, which is not stimulated by calcitriol. The intestinal expression of genes encoding Ca2+ transporters have not been reported for the Cldn12 KO mouse. We therefore measured them and found that the only difference was a twofold increase in S100g expression in the colon (SI Appendix, Fig. S6). Taken together, the results indicate that the DKO mice have impaired intestinal Ca2+ absorption and renal reabsorption with insufficient compensation to maintain serum iCa.
Claudin-2 and Claudin-12 DKO Mice Have Reduced Bone Mineralization.
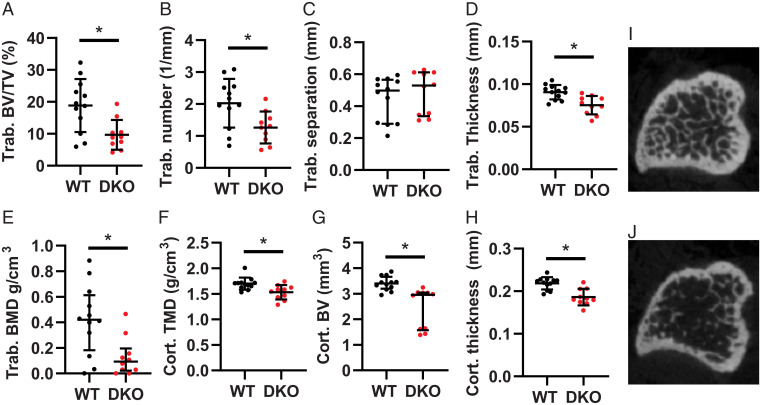
Bone mineralization and microarchitecture is not altered in Cldn2 KO mice but has not been examined in Cldn12 KO mice (8). We therefore performed microcomputed tomography (µCT) studies on Cldn12 KO mice at 3 and 6 mo of age and observed no differences to WT (SI Appendix, Table S6 and S7). We next examined bone microarchitecture and mineral density in the DKO mice and found a more-than-fourfold decrease in trabecular bone mineral density and both decreased trabecular thickness and number in DKO mice. Similarly, we found decreased bone volume, thickness, and tissue mineral density in cortical bone of DKO mice (Fig. 3). These results are consistent with reduced bone mineralization or accelerated demineralization in DKO mice and support the notion that claudin-2 and -12 compensate for one another when one is lost.
Fig. 3.
Cldn2/12 DKO mice have altered bone morphometry at 3 mo. Microarchitecture of trabecular (trab) (A–E) and cortical (cort) (F–H) bone from tibia of WT and Cldn2/12 DKO mice analyzed by micro-CT. (A) Trabecular bone volume/tissue volume (P = 0.006). (B) Trabecular number (P = 0.014). (C) Trabecular separation (P = 0.228). (D) Trabecular thickness (P = 0.001). (E) Trabecular bone mineral density (P = 0.014). (F) Cortical tissue mineral density (P = 0.006). (G) Cortical bone volume (P = 0.0001). (H) Cortical thickness (P = 0.0003). Representative micro-CT images of the tibial metaphyses shown at 40 slices from growth plate from WT (I) and DKO (J) mice. Data are presented as mean ± SD compared by unpaired t test (A, B, D, G, and H) or as median (IQR) compared by Mann–Whitney U test (C, E, and F). *P < 0.05.
Claudin-2 and -12 Do Not Physically Interact In Vitro yet Confer Increased Ca2+ Permeability.
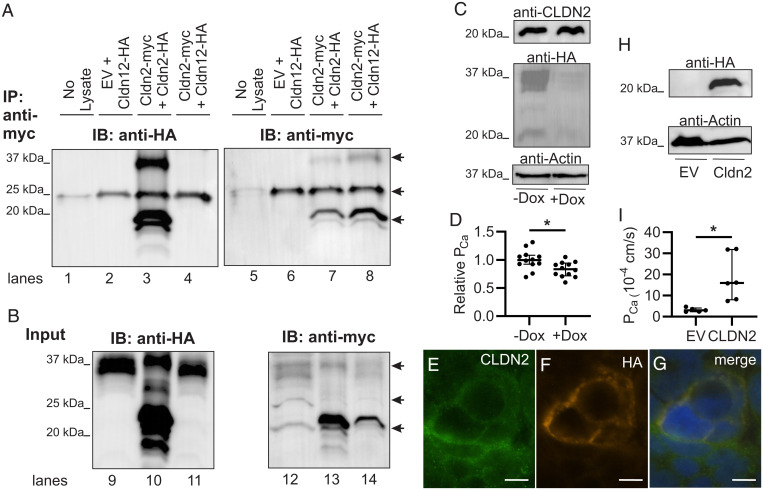
Given that the loss of claudin-2 and -12 have an additive effect on Ca2+ permeability and urinary Ca2+ excretion, we hypothesized that these proteins form independent pores. To assess this, we performed coimmunoprecipitation studies using human embryonic kidney (HEK293) cells expressing epitope-tagged claudin-2 and claudin-12. As a positive control, we found that myc-tagged claudin-2 was able to immunoprecipitate hemagglutinin (HA)-tagged claudin-2. However, myc-tagged claudin-2 was unable to immunoprecipitate HA-tagged claudin-12 (Fig. 4 A and B). Together, these results are consistent with claudin-2 and claudin-12 forming separate Ca2+ permeable pores.
Fig. 4.
Claudin-2 and claudin-12 form separate Ca2+ permeable pores. (A) HEK293 cells transfected as indicated were lysed or the buffer without cell lysate as control (no lysate lane) and proteins immunoprecipitated (IP) with antibodies to myc before sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were electrotransferred and blotted with anti-HA antibody (lanes 1 through 4) and then stripped and reprobed with an anti-myc antibody (lanes 5 through 8). IB, immunoblot. (B) Protein from HEK293 cells above directly resolved on SDS-PAGE (Input) and blotted with anti-HA (lanes 9 through 11) and then anti-myc (lanes 12 through 14). Claudin dimers (37 kDa) and monomers (20 kDa) are highlighted by arrows. The arrow at 25 kDa illustrates IgG light chain in the IP samples as seen in the lane with no cell lysate added. EV, empty vector. Representative blot of three repeats is shown. (C) Caco-2 cells endogenously express CLDN2 and express HA-tagged CLDN12 via a tet-off system. (D) Caco-2 cells expressing CLDN2 and CLDN12 (−Dox) have greater Ca2+ permeability than cells expressing only CLDN2 (+Dox) (P = 0.014 and unpaired t test). (E–G) Immunofluorescence of Caco-2 cells for endogenous CLDN2 (green) and HA-tagged CLDN12 (orange) demonstrating colocalization of CLDN2 and CLDN12 in the same cell membrane. (H) Opossum kidney (OK) cells endogenously expressing CLDN12 and expressing either EV or CLDN2. (I) OK cells expressing CLDN2 and CLDN12 (“CLDN2” data) have greater Ca2+ permeability than cells expressing only CLDN12 (“EV” data) (P = 0.018, Welch’s t test). Dox, doxycycline. TER, pNa, pCl, and relative permeabilities are presented in SI Appendix, Tables S8 and S9. *P < 0.05.
If claudin-2 and claudin-12 form separate Ca2+ permeable pores in renal and intestinal epithelia, Ca2+ permeability should be greater with both proteins present than with only one. We therefore established a colonic epithelial cell line, Caco-2, that stably expressed HA-tagged CLDN12 under a tet-off system. CLDN2 is endogenously present in these cells (Fig. 4C). After doxycycline treatment, CLDN12 was only very faintly detected (Fig. 4C). Colonic epithelial cells expressing both CLDN2 and CLDN12 had higher Ca2+ permeability than cells only expressing CLDN2 (Fig. 4D and SI Appendix, Table S8). Immunofluorescence of this cell line demonstrates colocalization of CLDN2 and CLDN12 at the plasma membrane (Fig. 4 E–G). Similarly, we employed a renal proximal tubule cell line (opossum kidney cells) that endogenously expresses CLDN12 but not CLDN2 and expressed either the empty vector or a HA-tagged CLDN2 (Fig. 4H) (24). Proximal tubule cells expressing both CLDN2 and CLDN12 had significantly greater Ca2+ permeability than cells only expressing CLDN12 (Fig. 4I and SI Appendix, Table S9). These results are consistent with claudin-2 and claudin-12 forming separate Ca2+-permeable pores.
Discussion
Employing murine models, detailed balance studies, and ex vivo and in vitro techniques, we provide evidence that either claudin-2 or claudin-12 is necessary to maintain Ca2+ homeostasis and optimal bone mineralization. This conclusion is supported by Cldn2/Cldn12 DKO mice having decreased Ca2+ permeability across the colon, contributing decreased net intestinal Ca2+ absorption. Furthermore, DKO mice have even greater hypercalciuria than the single-Cldn2 KO animals. Intestinal and renal compensatory mechanisms in the DKO mice are inadequate to maintain serum ionized Ca2+ levels. Ultimately, the loss of both proteins results in decreased bone mineral density. Moreover, we did not find that claudin-2 and -12 physically interact, despite being expressed in the same epithelia. Our results are consistent with additive effects of their deletion and their formation of independent Ca2+ pores in the proximal colon and renal proximal tubule.
Paracellular intestinal Ca2+ absorption is proposed as the predominant pathway when dietary Ca2+ is adequate (5). However, this has never been directly tested. Claudin-2 and -12 have been implicated in mediating paracellular Ca2+ diffusion between epithelial cells (13, 15, 25). Herein, we describe a genetic model in which these two tight junction proteins are deleted. The DKO mice display decreased intestinal Ca2+ absorption when fed an adequate Ca2+-containing diet, likely due to reduced colonic Ca2+ permeability. Previous work clearly demonstrates Ca2+ absorption via the paracellular pathway from the large intestine under normal and high-dietary Ca2+ conditions (6, 7, 26). However, this paracellular pathway can mediate bidirectional diffusion of Ca2+, and thus, Ca2+ secretion has also been observed (26, 27). Therefore, depending on the electrochemical properties across the tissue, net secretion or absorption may occur. Previous work found that the loss of Cldn2 leads to increased net Ca2+ absorption, likely due to reduced colonic Ca2+ secretion (8). Despite reduced colonic Ca2+ permeability, Cldn12 KO mice do not have altered net intestinal Ca2+ absorption (15). In contrast to the single-KO animals, we observe decreased net Ca2+ absorption in DKO mice. Moreover, again in contrast to the single-KO mice, the DKO animals demonstrate reduced plasma Ca2+ levels and reduced bone mineral density. In Cldn2 KO mice, we only identified changes in Ca2+ permeability across the colon. However, in DKO and Cldn12 KO mice, transepithelial resistance was increased, and absolute Na+ and Cl− permeabilities as well as relative cation permeability were decreased. These alterations may contribute to an altered electrochemical driving force across the colon and explain the differences observed between Cldn2 KO and DKO mice. Regardless, our work provides evidence supporting a significant role for paracellular colonic Ca2+ absorption in the maintenance of Ca2+ homeostasis.
The DKO mice display marked hypercalciuria. Both claudin-2 and claudin-12 contribute Ca2+ permeability to the proximal tubule (15, 28). Perfused proximal tubules from Cldn12 KO mice have decreased Na+ and Ca2+ permeability but unaffected Cl− permeability (15), whereas both Cldn2 KO and Cldn12 KO proximal tubules are anion selective in contrast to proximal tubules from WT mice, which are cation selective (14, 15). However, Cldn12 KO mice do not have increased urinary Ca2+ excretion, perhaps due to compensation (15). Conversely, Cldn2 KO mice have a threefold increase in fractional Ca2+ excretion compared to WT (14, 18). The DKO mice have even greater renal Ca2+ wasting. The loss of claudin-2 and claudin-12 likely results in markedly inadequate Ca2+ reabsorption from the proximal tubule that overwhelms the compensatory capacity of the more distal segments.
A physical interaction between claudin-16 and -19 is required for the formation of a Mg2+- and Ca2+-permeable pore in the renal thick ascending limb (22, 29, 30). This physical coupling is highlighted by the fact that the loss of either claudin-16 or claudin-19 results in the same disease, familial hypomagnesemia with hypercalciuria and nephrocalcinosis (16, 17). In contrast, the loss of claudin-2 or another cation-permeable claudin, claudin-15, both result in decreased sodium permeability across the small intestine, and loss of both together appears to have an additive effect (23, 31). Thus, claudin-2 and -15 appear to form independent, cation-permeable pores across intestinal epithelia. Similarly, we have now provided evidence that claudin-2 and claudin-12 form independent, cation-permeable pores across renal and intestinal epithelia.
Previous in vitro work identified two other claudins, claudin-10a and claudin-3, predominantly expressed in the renal proximal tubule. Claudin-2 was found to weakly interact with claudin-10a but not with claudin-3 (28), and the interaction between claudin-2 and -10a is not within the same pore but through their formation of independent, parallel pores in the tight junction (28). Our findings regarding claudin-2 and -12 are consistent with the two claudins analogously being present in the same cell but forming independent pores.
The altered bone microarchitecture and decreased bone mineral density observed in the DKO mice is likely due to both inadequate intestinal absorption and renal reabsorption of Ca2+ as the effects of genetic alterations disrupting either intestinal absorption or renal reabsorption can be compensated by the other organ. Delineating the relative contribution of altered intestinal Ca2+ absorption or renal reabsorption in the DKO mice is an important future direction. However, this requires the generation of floxed models to allow for Cre-dependent, tissue-specific intestinal or renal DKO mice to tease apart these organ-specific effects. Cldn2 and Cldn12 are expressed in osteoblasts, although their function in bone is yet to be elucidated (32, 33). However, if our observed phenotype was due primarily to altered bone homeostasis, it is likely we would have observed greater intestinal Ca2+ absorption or renal reabsorption due to compensation rather than the decreased levels we observed. Regardless of the relative contributions of altered renal or intestinal paracellular Ca2+ absorption to the observed phenotype, our results support a significant role for the paracellular pathway in intestinal Ca2+ absorption under adequate dietary intake.
In summary, we present evidence that the loss of paracellular Ca2+ permeability across the colon and proximal tubule leads to a decreased net Ca2+ balance, decreased bone mineralization, and inability to maintain serum Ca2+ levels. Our work supports two independent but partially redundant, paracellular pathways mediated by claudin-2 and claudin-12. Overall, we highlight the critical role of claudins-2 and -12 paracellular Ca2+ pores in the colon and proximal tubule in maintaining Ca2+ balance.
Materials and Methods
The collection of serum, urine, and feces and measurement of electrolytes and calciotropic hormones has been described previously (15). Real-time qPCR, Ca2+ permeability of intestinal tissue, microcomputed tomography, immunofluorescence staining, and immunoblotting were performed as previously (8, 15, 18, 34–40). Refer to SI Appendix, Supplementary Methods for detailed descriptions.
Ethics Approval.
Experiments were approved by the University of Alberta Research Ethics Board animal ethics committee, Health Sciences Section (AUP00000213).
Animals and Husbandry.
We generated Cldn2 and Cldn12 global DKO mice (Cldn2/12 DKO) by cross-breeding Cldn2 (MMRRC, University of California, Davis) with Cldn12 KO (15) animals which had been backcrossed onto an FVB/N WT background (Taconic Biosciences, Rensselaer). DKO genotyping was confirmed by real-time qPCR as described in SI Appendix, Supplementary Methods using kidney and intestinal tissue. Cldn2 and Cldn12 gene expression was detected in WT but not DKO animals, whereas β-galactosidase was detected in tissue of DKO animals only. N.B. Cldn12 KO animals were generated by homologous recombination of exon 4 of the Cldn12 gene with the β-galactosidase coding sequence from Escherichia coli, and therefore, this gene will only be detected in mice carrying the mutant form (15). Metabolic cage studies were performed as previously described (18, 34).
Coimmunoprecipitation.
HEK293 cells (ATCC) were transiently transfected with pTRE2hyg vector expressing HA-tagged claudin-12, pTRE2hyg vector expressing myc-tagged claudin-2, pcDNA 3.1 vector expressing HA-tagged claudin-2, and/or empty pTRE2hyg vector as described in the Fig. 4 legend. Cells were lysed in a EDTA and nonidet P-40 containing Tris buffer, and lysate was incubated with mouse anti-myc antibody (Thermo Fisher Scientific, catalog No. MAI-21316) and precipitated with Dynabeads with protein G (magnetic beads, Invitrogen) overnight at 4 °C. Protein was eluted with Laemmli buffer and detected by immunoblot as above with rat anti-HA (Roche, catalog No. 11867423001) or rabbit anti-myc (Covance catalog No. PRB-150P) antibodies.
Immunohistochemistry and X-gal Staining.
Tissue was subjected to X-gal staining and, subsequently, immunohistochemistry, as described in detail previously (19). Briefly, sections of colon and kidney tissue were fixed in 4% PFA, cryoprotected in sucrose, and frozen before being sectioned and stained for X-gal. Some of the X-gal–stained sections were subsequently stained for CLDN2 using rabbit anti-CLDN2 antibodies (#51–6100, Invitrogen).
Statistics.
Data were analyzed using GraphPad Prism 9.0. The Shapiro–Wilk test was used to evaluate for normal distribution and F test to compare variances. Data were analyzed and presented as indicated in figure and table legends. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Inger Nissen at the University of Southern Denmark for expert technical assistance and Life Sciences editors for assistance. This work was funded by grants from the Women and Children’s Health Research Institute, which is supported by the Stollery Children’s Hospital Foundation, and the National Sciences and Engineering Research Council to R.T.A., who is the Canada Research Chair in Renal Epithelial Transport Physiology and Independent Research Fund Denmark (to H.D.).
Footnotes
Author contributions: M.R.B., A.P., J.R., M.R.D., H.D., and R.T.A. designed research; M.R.B., K.Y., W.P., D.D.O., M.S., A.P., J.R., H.D., and R.T.A. performed research; M.R.B., K.Y., W.P., D.D.O., M.S., A.P., J.R., M.R.D., E.C., H.D., and R.T.A. analyzed data; K.Y., W.P., D.D.O., M.S., A.P., J.R., M.R.D., and E.C. edited the paper; and M.R.B., H.D., and R.T.A. wrote the paper.
The authors declare no competing interest.
This article is a PNAS Direct Submission.
1Present address: Cambridge Academy of Therapeutic Sciences, University of Cambridge, Cambridge, CB2 1RX, UK.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2111247118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Hoenderop J. G. J., Nilius B., Bindels R. J. M., Calcium absorption across epithelia. Physiol. Rev. 85, 373–422 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Klibanski A., et al. , NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, Osteoporosis prevention, diagnosis, and therapy. JAMA 285, 785–795 (2001).11176917 [Google Scholar]
- 3.Johnell O., Kanis J. A., An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 17, 1726–1733 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Plain A., Alexander R. T., Claudins and nephrolithiasis. Curr. Opin. Nephrol. Hypertens. 27, 268–276 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Christakos S., et al. , Vitamin D and the intestine: Review and update. J. Steroid Biochem. Mol. Biol. 196, 105501 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duflos C., Bellaton C., Pansu D., Bronner F., Calcium solubility, intestinal sojourn time and paracellular permeability codetermine passive calcium absorption in rats. J. Nutr. 125, 2348–2355 (1995). [DOI] [PubMed] [Google Scholar]
- 7.Mineo H., Hara H., Tomita F., Short-chain fatty acids enhance diffusional ca transport in the epithelium of the rat cecum and colon. Life Sci. 69, 517–526 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Curry J. N., et al. , Claudin-2 deficiency associates with hypercalciuria in mice and human kidney stone disease. J. Clin. Invest. 130, 1948–1960 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hylander E., Ladefoged K., Jarnum S., The importance of the colon in calcium absorption following small-intestinal resection. Scand. J. Gastroenterol. 15, 55–60 (1980). [DOI] [PubMed] [Google Scholar]
- 10.Hylander E., Ladefoged K., Jarnum S., Calcium absorption after intestinal resection. The importance of a preserved colon. Scand. J. Gastroenterol. 25, 705–710 (1990). [DOI] [PubMed] [Google Scholar]
- 11.Jiang H., et al. , Targeting 1,25(OH)2D-mediated calcium absorption machinery in proximal colon with calcitriol glycosides and glucuronides. J. Steroid Biochem. Mol. Biol. 198, 105574 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Alexander R. T., Rievaj J., Dimke H., Paracellular calcium transport across renal and intestinal epithelia. Biochem. Cell Biol. 92, 467–480 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Fujita H., et al. , Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol. Biol. Cell 19, 1912–1921 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muto S., et al. , Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc. Natl. Acad. Sci. U.S.A. 107, 8011–8016 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plain A., et al. , Claudin-12 knockout mice demonstrate reduced proximal tubule calcium permeability. Int. J. Mol. Sci. 21, 2074 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon D. B., et al. , Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285, 103–106 (1999). [DOI] [PubMed] [Google Scholar]
- 17.Konrad M., et al. , Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am. J. Hum. Genet. 79, 949–957 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dimke H., et al. , Activation of the Ca2+-sensing receptor increases renal claudin-14 expression and urinary Ca excretion. Am. J. Physiol. Renal Physiol. 304, F761–F769 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frische S., et al. , Localization and regulation of claudin-14 in experimental models of hypercalcemia. Am. J. Physiol. Renal Physiol. 320, F74–F86 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Gong Y., et al. , Claudin-14 regulates renal Ca++ transport in response to CaSR signalling via a novel microRNA pathway. EMBO J. 31, 1999–2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castro Dias M., et al. , German Mouse Clinic Consortium, Claudin-12 is not required for blood-brain barrier tight junction function. Fluids Barriers CNS 16, 30 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou J., et al. , Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc. Natl. Acad. Sci. U.S.A. 106, 15350–15355 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamura A., et al. , Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterology 140, 913–923 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Borovac J., et al. , Claudin-4 forms a paracellular barrier, revealing the interdependence of claudin expression in the loose epithelial cell culture model opossum kidney cells. Am. J. Physiol. Cell Physiol. 303, C1278–C1291 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu A. S., et al. , Molecular basis for cation selectivity in claudin-2-based paracellular pores: Identification of an electrostatic interaction site. J. Gen. Physiol. 133, 111–127 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karbach U., Rummel W., Calcium transport across the colon ascendens and the influence of 1,25-dihydroxyvitamin D3 and dexamethasone. Eur. J. Clin. Invest. 17, 368–374 (1987). [DOI] [PubMed] [Google Scholar]
- 27.Karbach U., Bridges R. J., Rummel W., The role of the paracellular pathway in the net transport of calcium across the colonic mucosa. Naunyn Schmiedebergs Arch. Pharmacol. 334, 525–530 (1986). [DOI] [PubMed] [Google Scholar]
- 28.Curry J. N., Tokuda S., McAnulty P., Yu A. S. L., Combinatorial expression of claudins in the proximal renal tubule and its functional consequences. Am. J. Physiol. Renal Physiol. 318, F1138–F1146 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou J., et al. , Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J. Clin. Invest. 118, 619–628 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong Y., et al. , Biochemical and biophysical analyses of tight junction permeability made of claudin-16 and claudin-19 dimerization. Mol. Biol. Cell 26, 4333–4346 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wada M., Tamura A., Takahashi N., Tsukita S., Loss of claudins 2 and 15 from mice causes defects in paracellular Na+ flow and nutrient transport in gut and leads to death from malnutrition. Gastroenterology 144, 369–380 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Alshbool F. Z., Mohan S., Emerging multifunctional roles of Claudin tight junction proteins in bone. Endocrinology 155, 2363–2376 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wongdee K., et al. , Osteoblasts express claudins and tight junction-associated proteins. Histochem. Cell Biol. 130, 79–90 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Pan W., et al. , The epithelial sodium/proton exchanger, NHE3, is necessary for renal and intestinal calcium (re)absorption. Am. J. Physiol. Renal Physiol. 302, F943–F956 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beggs M. R., et al. , Expression of transcellular and paracellular calcium and magnesium transport proteins in renal and intestinal epithelia during lactation. Am. J. Physiol. Renal Physiol. 313, F629–F640 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Beggs M. R., et al. , TRPV6 and Cav1.3 mediate distal small intestine calcium absorption before weaning. cell. mol. gastroenterol. hepatol. 8, 625–642 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexander R. T., et al. , Ultrastructural and immunohistochemical localization of plasma membrane Ca2+-ATPase 4 in Ca2+-transporting epithelia. Am. J. Physiol. Renal Physiol. 309, F604–F616 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Plain A., et al. , Corticomedullary difference in the effects of dietary Ca2+ on tight junction properties in thick ascending limbs of Henle’s loop. Pflugers Arch. 468, 293–303 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Günzel D., et al. , Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function. J. Cell Sci. 122, 1507–1517 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Rievaj J., Pan W., Cordat E., Alexander R. T., The Na+/H+ exchanger isoform 3 is required for active paracellular and transcellular Ca2+ transport across murine cecum. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G303–G313 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.