Abstract
Large granular lymphocytic leukemia (LGLL) represents a clonal/oligoclonal lymphoproliferation of cytotoxic T and natural killer cells often associated with STAT3 mutations. When symptomatic, due to mostly anemia and neutropenia, therapy choices are often empirically-based, because only few clinical trials and systematic studies have been performed. Incorporating new molecular and flow cytometry parameters, we identified 204 patients fulfilling uniform criteria for LGLL diagnoses and analyzed clinical course with median follow-up of 36 months, including responses to treatments. While selection of initial treatment was dictated by clinical features, the initial responses, as well as overall responses to methotrexate (MTX), cyclosporine (CsA), and cyclophosphamide (CTX), were similar at 40–50% across drugs. Sequential use of these drugs resulted in responses in most cases: only 10–20% required salvage therapies such as ATG, Campath, tofacitinib, splenectomy or abatacept. MTX yielded the most durable responses. STAT3-mutated patients required therapy more frequently and had better overall survival.
Keywords: T-LGLL, NK-LGLL, STAT3 mutation
Introduction
Large granular lymphocytic leukemia (LGLL) is characterized by lymphoproliferation of cytotoxic T cells (CTL) or natural killer (NK) cells with some features consistent with a chronic lymphocytic leukemia/low-grade lymphoma and it, is in some cases, indistinguishable from a chronic reactive immune process [1–3]. Commonly diagnosed in the elderly, LGLL often follows an indolent clinical course. Unlike typical low-grade lymphomas/lymphocytic leukemias, T-cell proliferation in LGLL generally appears to be under control of homeostatic T-cell regulation and thus high lymphocyte counts are found only in a minority of patients. The main manifestations of LGLL prompting therapy are single lineage cytopenias, including neutropenia or reticulocytopenic anemia [3,4]. The pathophysiology of LGLL-associated cytopenia is incompletely understood, but it is thought to involve CTLs through either their secretion of inhibitory effector cytokines, their direct cytotoxicity, or as a consequence of hypersplenism [5,6].
Clonal expansion of CTL or NK cells in LGLL often resembles a chronic reactive immune process driven by putative viral, autoimmune, or even tumor antigens [7]. Supporting this hypothesis, LGLL is frequently associated with chronic B-cell leukemias and lymphomas, various cancers, autoimmune diseases, or alloresponses in transplant recipients [8,9]. Distinguishing LGLL from a skewed reactive process may be difficult. Confounding this important distinction is the fact that LGLL can emerge as a clonal outgrowth from an originally polyclonal reactive lymphoproliferation. STAT3 mutations present in a substantial proportion of patients is compatible with clonal evolution in the context of initially physiologic immune responses [10–12].
Because LGLL is relatively rare, few clinical trials have been conducted in this disease [13,14], and thus, current standard of care is derived from empiric experiences, retrospective analysis of responses to rationally applied drugs, or inferences from treatment results of related conditions [4,15–18]. Here, we analyze the clinical features, associated conditions, therapy responses, and outcomes, in the largest single-center cohort to date that includes STAT3 mutational status.
Materials and methods
Patients and diagnosis
This research was approved by the institutional review board for retrospective chart review. Informed consent was obtained from all study patients for blood and marrow aspirate collection. For the diagnosis of T- and NK-LGLL, modified criteria were applied [9]. Briefly, at least three out of four of the following had to be fulfilled for the diagnosis of T-LGL leukemia: (i) presence of large granular lymphocytes (>500/μL) in blood for more than 6 months; (ii) abnormal cytotoxic T or NK lymphocytes expressing CD2, CD56 and CD57 and lacking CD28; (iii) preferential usage of a TCR Vb family by flow cytometry; and (iv) TCR gene rearrangement by PCR [19–21]. Bone marrow biopsy and aspiration was performed in most cases to exclude other conditions and confirm the diagnosis. In occasional asymptomatic yet classical cases meeting diagnostic criteria bone marrow examination was omitted. Supplementary Table S1 includes the distribution of patients across diagnostic criteria.
Clinical parameters and responses
Clinical data collected included patient age, sex, presence of splenomegaly, associated autoimmune conditions, and underlying cancers or hematologic neoplasms, including chronic B-cell leukemias and lymphomas. Cytopenias were defined as follows: neutropenia = absolute neutrophil count (ANC) < 1.5 × 109/L; anemia = hemoglobin <11 g/dL; and thrombocytopenia = platelet count <150 × 109/L. Table 1 summarize the clinical characteristics of the patients and associated conditions.
Table 1.
Patient characteristics (LGLL cases seen at CCF 2001–2016).
| Parameter | N |
|---|---|
|
| |
| Number of patients | 204 |
| Sex (M/F) | 110/94 |
| Median age (IQR) | 63 (54–72) |
| Median follow-up in months (IQR) | 36 (13–84) |
| T-LGL (%) | 183 (90) |
| NK-LGL (%) | 21 (10) |
| Patients with STAT3 mutation (%) | 66/183 (36) |
| Percentage of patients treated (%) | 118 (58) |
| Median LGL count, × 109/L (IQR) | 1.74 (0.8–3.3) |
| Median ANC, × 109/L (IQR) | 1.56 (0.8–2.6) |
| Median ALC, × 109/L (IQR) | 3.23(1.7–4.8) |
| Lymphocyte, % (IQR) | 55 (42–72) |
| Hemoglobin, g/dL (IQR) | 11.7 (9.8–13) |
| Median platelet count, × 109/L (IQR) | 192 (138–260) |
| Hematologic manifestation (%) | |
| Neutropenia (<1.5 × 109/L) (%) | 93 (46) |
| Severe neutropenia (<0.5 × 109/L) (%) | 36 (17) |
| Anemia (<11 g/dL) (%) | 79 (40) |
| RBC transfusions at presentation (%) | 45 (22) |
| Thrombocytopenia (<150 × 106/L) (%) | 59 (30) |
| Splenomegaly (%) | 49 (24) |
| Splenectomy (%) | 19 (9) |
| MGUS (%) | 41 (20) |
| Rheumatoid arthritis (%) | 31 (15) |
| Other autoimmune conditions (%) | 20 (10) |
| Carcinomas (%) | 67 (33) |
| Hematologic (%) | 39 (19) |
| Non-hematologic (%) | 35 (17) |
| >1 malignancy (%) | 7 (3.5) |
IQR: inter quartile range; T-LGLL: T-cell large granular lymphocytic leukemia; NK-LGLL: natural killer cell large granular lymphocytic leukemia; ANC: absolute neutrophil count; ALC: absolute lymphocyte count; MGUS: monoclonal gammopathy of undetermined significance. NK-LGLL: 18 chronic, 3 aggressive. For type of STAT3 mutations: Supplementary Table S2. For associated conditions: Supplementary Table S3.
We analyzed differences associated with the use of methotrexate (MTX), cyclosporine (CsA), and cyclophosphamide (CTX). MTX was given at doses of 10–15 mg PO every week, CsA doses of dose 50–100 mg PO BID, and CTX doses of 50–100 mg PO daily. Treatment responses were assessed after a minimum of 12 weeks of therapy. Complete responses (according to the definition) were not asserted in most of the cases because in lieu of hematologic responses, marrow sampling had no actionable clinical consequences. Drug efficacy was therefore determined using hematologic response criteria. We defined a complete hematological response as blood lineages normalizing (ANC >1.5 × 109/L; hemoglobin >11 g/dL; platelet count >150 × 109/L). In the absence of CR, partial response (PR) was defined as ANC >50% of baseline, hemoglobin increased by >1 g/dL, or a decrease in transfusion requirements by >50% for at least 4 months [14].
Next-generation sequencing
To detect mutations and determine the STAT3 clonal burden, targeted multiamplicon deep DNA sequencing was performed on fresh blood using the Illumina Miseq platform [10].
Results
Clinical features of LGLL
In our longitudinal cohort of 204 patients, 90% had T-LGLL and 10% had NK-LGLL (Table 1). Of T-LGLL cases, 13% showed expression or co-expression of CD4. The median follow-up was 36 months with an interquartile range (IQR) of 13–84 months. Among LGLL patients, median observations were LGL count = 1.7k/μL (IQR 0.8–3), ANC = 1.56k/μL (IQR 0.8–2.6), hemoglobin = 11.7 g/dL (IQR 10–13), and platelet count = 192k/μL (IQR 138–260). Of our 204 patients, 93 (46%) had neutropenia, 79 (40%) had anemia, and 49 (24%) had splenomegaly. Compared to rare CD4+ and classical T-LGLL, those with aberrant expression of CD4 were less cytopenic (data not shown). Solid tumors were present prior to LGL in 30 (15%) cases, CLL, Hodgkin lymphoma (HL) and non-HL (NHL) preceded LGL in 28 (14%) cases, and myeloid neoplasms preceded LGL in 10 (5%). Monoclonal gammopathy of undetermined significance (MGUS) was identified in 40 (20%) LGL diagnostic work ups. Autoimmune conditions were found in 50 (25%) patients, of which rheumatoid arthritis (RA) was the most common at 21 (15%). Nine patients had prior transplants: six solid organs and three hematopoietic stem cells (Supplementary Table 2).
Out of 183 patients, STAT3 mutations were found in 66 (36%), with 9 (5%) having more than one mutation. The most frequent mutation was Y640F, found in 32 (43%) cases, followed by D661Y in 22 (32%), D661V in 10 (6%), and N647I in 5 (3%) (Supplementary Table S3). There were differences in clinical characteristics based on mutational status: patients with STAT3 mutation were more likely to have neutropenia 39/66 (62%) than those with STAT3 wild type 41/117 (37%; p= . 001). Anemia was also more prevalent in STAT3mutated patients, at 31/66 (49%) versus 38/117 (34%) (p= .005). RA also more common at 17 (26%) versus 11 (9%; p= .005), MGUS was more common among wild type 24 versus 14% (p= .08). Similarly, all hematological malignancies combined, including lymphomas, were more common among wild type STAT3 cases, at 29 (25%) versus 7 (11%; p= .02) (Table 2).
Table 2.
Clinical characteristics based on mutational status.
| Parameter | STAT3MU (%) | STAT3WT (%) | p |
|---|---|---|---|
|
| |||
| Number of patients | 66 | 117 | |
| Sex (M/F) | 36/30 | 60/57 | |
| Median age (IQR) | 63 (54–72) | 63 (56–71) | |
| Median follow-up in months (IQR) | 56 (11–61) | 31 (28–130) | .0002 |
| T-LGL (%) | 62 (94) | 105 (90) | |
| NK-LGL (%) | 4 (6) | 12 (10) | |
| Percentage of patients treated (%) | 47 (71) | 63 (54) | .019 |
| Median LGL count, × 109/L (IQR) | 1.8 (0.8–3.7) | 1.46 (0.75–3) | |
| Median ANC, × 109/L (IQR) | 1(0.6–2) | 1.8 (1–3) | .001 |
| Median ALC, × 109/L (IQR) | 3 (1.8–5) | 3.4 (1.6–4.6) | |
| Lymphocyte, % (IQR) | 64 (45–75) | 51 (41–63) | .006 |
| Hemoglobin, g/dL (IQR) | 11 (8.9–13) | 12 (10–13.6) | .037 |
| Median platelet count, × 109/L (IQR) | 203 (161–274) | 184 (131–249) | |
| Hematologic manifestation (%) | |||
| Neutropenia (<1.5 × 109/L) (%) | 39 (62) | 41 (37) | .001 |
| Severe neutropenia (<0.5 × 109/L) (%) | 13 (20.6) | 17 (15) | |
| Anemia (<11 g/dL) (%) | 31 (49) | 38 (34) | .005 |
| RBC transfusions at presentation (%) | 16 (27) | 24 (23) | |
| Thrombocytopenia (<150 × 106/L) (%) | 14 (22) | 38 (34) | |
| Splenomegaly (%) | 15 (23) | 28 (25) | |
| Splenectomy (%) | 8 (13) | 11 (10) | |
| MGUS (%) | 9 (14) | 26 (24) | .08 |
| Rheumatoid arthritis (%) | 17 (26) | 11 (9) | .005 |
| Other autoimmune conditions (%) | 4 (6) | 14 (12) | |
| Other malignancies (%) | 18 (27) | 41 (35) | |
| Hematologic (%) | 7 (11) | 29(25) | .02 |
| Non-hematologic (%) | 12(18) | 18(15) | |
| >1 malignancy (%) | 1 (1.5) | 6 (5) | |
Clinical management and outcomes
Indications for treatment included neutropenia (46%), anemia (40%), or mixed cytopenia (8%). Of anemic patients, 22% were transfusion dependent (≥2 U of blood per 3 months).
As initial treatment, 44/118 (37%) LGLL patients received CSA, 34 cases (29%) received MTX and 22 (19%) cases received CTX. Fifteen (13%) cases received other old chemotherapeutic regimens as outlined in Table 3(A). Initial response rates (RR) were as follows: CsA 20/44 (45%); CTX 10/22 (47%); and MTX 15/34 (44%). The mean response duration as initial agent was 43 months for MTX, 21 months for CSA, and 17 months for CTX (p= .01). Overall response durations, respectively, were 36, 21, and 14 months (p= .0005). Due to lack of initial response and/or toxicity, many patients received multiple therapies. The combined overall response rate (ORR) was 48% for CsA, 53% for CTX, and 43% for MTX (p= .55). In total, 24 patients received low-dose alemtuzumab (10–20 mg subcutaneous/week) as a salvage therapy with an overall response rate (ORR) of 46%. ORR in patients who underwent splenectomy was 11/19 (58%) with median response duration of 30 months (1.8–115 months). Several salvage therapies were effective in a proportion of patients, with RR of 6/8 (75%) for abatacept, 6/9 (67%) for tofacitinib, and 2/6 (33%) for ATG. (Table 3(B)).
Table 3.
Summary of therapeutic responses.
| Drug | Initial response rate | Overall response rate |
|---|---|---|
|
| ||
| (A) Common therapies | ||
| MTX | 15/34 (44) | 26/61 (43) |
| CSA | 20/44 (45) | 36/74 (48) |
| CTX | 10/22 (47) | 28/53 (53) |
| Old regimens | 7/11 (63) | 7/11(63) |
| p | .9 | .55 |
| Treatment | Median number of previous regimens | No. responded (%) |
|---|---|---|
|
| ||
| (B) Salvage therapies | ||
| MTX | 11/27 (41) | |
| CSA | 16/30 (53) | |
| CTX | 18/31 (58) | |
| Splenectomy | 2 | 11/19 (58) |
| Alemtuzumab | 2 | 11/24 (46) |
| Tacrolimus | 2 | 6/15 (40) |
| Abatacept | 4 | 6/8 (75) |
| Tofacitinib | 4 | 6/9 (67) |
| ATG | 5 | 2/6 (33) |
Old regimens include CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone), CVP (cyclophosphamide, vincristine, and prednisone), HE-CEPP (cyclophosphamide, etoposide and prednisone) Velcade, Cholambucil, Thalidomide and HyperCVAD.
MTX: methotrexate; CsA: cyclosporine; CTX: cyclophosphamide; ATG: antithymocyte globulin.
We also analyzed RR based on mutational status and indication for treatment: for anemia, RR for STAT3 mutated versus wild-type with MTX, CsA, and CTX were 5/10 (50%) versus 2/6 (33%), 3/12 (25%) versus 4/10 (40%), and 4/4 (100%) versus 3/8 (38%), respectively (p= .2); for neutropenia, RR for STAT3 mutated versus wild-type with MTX, CsA, and CTX were 6/13 (46%) versus 4/9 (44%), 9/17 (53%) versus 5/12 (42%), and 1/1 (100%) versus 0/6 (0%), respectively (p= .89). Initial response for STAT3 mutated versus wild-type with MTX, CsA, and CTX were 9/18 (50%) versus 6/13 (46%), 10/21 (48%) versus 9/22(41%), and 4/5 (80%) versus 5/15 (33%), respectively (p= .6). Overall response for STAT3 mutated versus wild-type with MTX, CsA, and CTX were 16/30 (53%) versus 11/29 (38%), 17/35 (49%) versus 16/35 (49%), and 13/22 (59%) versus 14/30(47%), respectively.
Various laboratory and immunogenetic parameters may be predictors of clinical outcomes. LGL count correlated with major Vbb clone size as assessed by flow cytometry (p= .0002; Supplementary Figure S1). HLADQB1 03 was more frequent in responders (40 versus 24%, p=09) while HLA-DRB1 03 was less common (11 versus 25%; p= .06). Response to MTX was better in STAT3-mutated patients with a mean duration of response of 46 versus 29 months (p= .1). For CsA, response duration was 25 versus 20 months in STAT3 mutated and wild-type patients (p= .4); duration of responses did not differ for those treated with CTX (p= .3; Supplementary Figure S2).
Survival
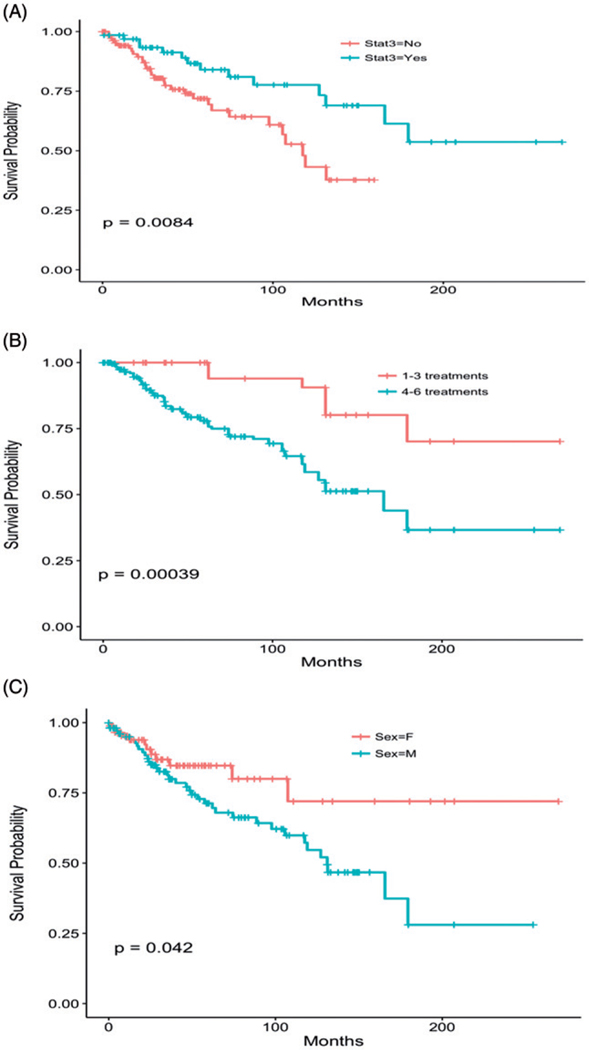
The median overall survival among all LGLL patients was 166 months. There were no major differences in survival between NK-LGL, CD4+ and CD8+ T-LGLL patients (Supplementary Figure S3(A,B)). When patients were subdivided according to the presence of a STAT3 mutation, median overall survival was significantly different, as patients with a STAT3 mutation tended to live longer than those without (p= .0052; Figure 1(A)). However, LGL-related mortality was not significantly different between STAT3-mutated patients and patients without mutation. Patients that required more than three lines of treatment had poor survival compared to patients that required less than three lines of therapy (Figure 1(B); p= .00039). In our cohort of patients, females had better overall survival than males (p= .04; Figure 1(C)). In multivariate analyses, presence of STAT3 mutation, female sex, and younger age, associated with favorable survival (Supplementary Table S4). When we explored causes of death, out of 37 patients with an identifiable cause, complications of LGL were responsible for 11 (30%); six of these were NK LGL patients. Other causes of deaths include five patients with MDS/AML, three with lymphomas, two lung and one breast cancers and nine with cardiac or respiratory failure.
Figure 1.
Survival. (A) Based on STAT3 mutation. Median survival was 118 months in patients without a STAT3 mutation and median survival was not reached in patients with a STAT3 mutation. Hazard ratio of 0.31 (0.18–0.52, p= .006). (B) Survival based on rounds of treatment. Patients who required more than three lines of therapy had a hazard ratio of 3.44 (1.58–7.5, p= .019). (C) Survival based on sex. Male sex has slightly increased hazard ratio of 1.88 (1.08–3.25, p= .025).
Discussion
Selection of most suitable therapies in orphan diseases like LGLL are often based on empiric experiences and retrospective analyses due to the lack of experimental evidence from prospective clinical trials. In our study, we assembled the largest to date cohort of well-annotated LGLL patients seen at a single center, who received a standardized diagnostic work-up and uniform therapeutic management. Previous studies were often smaller and involved only selected drugs rather than comparison between currently available modalities, or relied on multi-institutional registries with inherent variation in clinical assessments (Table 4) [4,14–19]. To date, evidence-based treatment guidelines have not been established because of the lack of objective clinical evidence derived from formal phase 3 trials. Thus, choices of initial and subsequent therapies in LGLL have been empiric. Our retrospective results may help to support rational therapy choices and their timing.
Table 4.
Summary of literature review of treatment results.
| Study | N | MTX (N) | OR (%) | CsA (N) | OR (%) | CTX (N) | OR (%) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Our studya | 118 | 61 | 26 (43) | 74 | 36 (48) | 53 | 28 (53) |
| French Registryb | 100 | 62 | 34 (55) | 24 | 5 (21) | 32 | 21 (66) |
| Loughran et al. 1994c | 10 | 10 | 6 (60) | ||||
| Battiwalla et al.a | 25 | 25 | 14 (56) | ||||
| Moignet et al.b | 45 | 45 | 32 (71) | ||||
| Osuji et al.b | 29 | 8 | 6 (85) | 23 | 18 (78) | 4 | 1 (25) |
| Fujishima et al.b | 14 | 4 | 1 (25) | 8 | 6 (75) | ||
| Dhodapkara | 47 | 2 | 2 (100) | 16 | 11 (69) | ||
| Loughran et al. 2015c | 59 | 55 | 21 (38) | 14 | 9 (64) | ||
N: number of patients received treatment; MTX: methotrexate; CsA: cyclosporine; CTX: cyclophosphamide.
Retrospective analysis in single right.
Retrospective analysis from multiple rights.
Multi-right phase 2 trial.
LGLL is derived from slow-cycling aberrant memory cells serving as progenitors for the bulk of mature effector CTL, and various lines of evidence including associations with autoimmune diseases suggest that various autoantigens incite or maintain CTL proliferation and activation [7,22]. Thus chronic ‘constant exposure’ to lower intensity treatments rather than high-dose pulse modalities produce more effective and durable responses.
Our large cohort of uniformly diagnosed LGLL patients afforded an opportunity to define common and less common clinical features of this disease. Normal blood counts were found in 6% of patients with no associated autoimmune conditions, chronic Bcell leukemias or lymphomas, other cancers or transplant history. These patients remained asymptomatic, and we classified them as T-cell clonopathy of undermined significance (TCUS) [16]. Most of our presumptive TCUS patients (83%) were most likely early/ subclinical TLGL. Median lymphocyte percentage of these mostly female patients (67%) was 48% with median absolute lymphocyte count of 4.3k/μL, their median LGL count was 2.7k/μL. Of note is that burden by Vb flow cytometry and LGL counts were comparable between TCUS and to non-TCUS patients. None of TCUS patients died after a median follow-up of 27 months indicating benign clinical course.
Demographic features of our LGLL patients were similar to those previously reported [4,14,19]. Anemia was present in 40% similar to previously reported [14,16,19]. However, the French registry reported a lower incidence of anemia of 25% with only 6% transfusion being dependent [4] compared to 22% in our cohort. Neutropenia was 46% in our cohort, consistent with other studies [14], but French registry reported higher neutropenia at 60% [4], albeit with only 44% requiring treatment compared to 58% in our cohort. Splenomegaly in 24% of our patients is comparable to other studies [4,16]. STAT3 mutations were found in 36% of patients comparable to 35% in 45 LGL patients [19]. However, Loughran et al. [14] reported, in a cohort of 59 patients, a slightly higher mutational frequency of 48%. In our study, RA was found in 15% of cases similar to other studies, [4,14] whereas Dhodapkar et al. [16] reported a slightly higher rate of 26%. We found MGUS in 20%, compared to only 7–10% reported by previuos studies [4,16]. We tested all patients for monoclonal protein at that time of diagnosis whereas systematic testing was not undertaken in other previous studies, explaining lower detection rates. We found other cancers in 33% of patients, a higher rate than 13% reported by the French group [4]. It is possible that a higher frequency of our cohort may be due to longer and closer and systematic follow up at our center. Interstingly, LGLL associated with hematologic malignacies was less commonly associated with STAT3 mutations and thus resembled a reactive process.
Uniform diagnosis and treatment parameters of a single institution may allow for a better comparison of treatment results obtained with different regimens. We compared therapy responses between most commonly used medications based on indication and STAT3 mutational status. Previous studies with fewer patients (≤10 patients) reported highly variable results for MTX (primary RR 66–100%) [13–17]. In our cohort, the primary hematologic response rate for MTX was 44% (ORR 45%) and comparable with the recently completed phase II study (38%) and retrospective French Registry analysis (44% and ORR of 55%) [4]. For CsA, our primary hematologic response rate was 45% (N= 44), whereas Battiwalla et al. [18] reported a response rate of 56% (N= 14) linked to HLA-DR4 expression [18], an association we did not find. Osuji et al. [15] described a higher CsA RR (78%), whereas the French Registry showed a lower primary response rate in a handful of patients 1/6 (17%) and ORR of 21%, in contrast to a 49% ORR in our study [4]. For CTX, our initial RR (45%) was lower than that reported by others (64–75% RR) [4,14,16,19,20]; a higher RR of 75% was seen in LGLL patients with PRCA [20]. In the interest of properly interpreting this study, it should be mentioned that our treatment algorithms avoided CTX as a first line therapy in LGLL with neutropenia and thus our results may be influenced by this bias. Our ORR to CTX (53%; N= 42) was comparable to the French Registry (ORR 66%; N= 32) [4]. We found no differences in response to MTX, CSA and CTX based on presence of MGUS or RA. Of relevance to future treatment selection decisions, MTX resulted in more durable responses (36 months) than CSA (21 months) or CTX (14 months), but due to its carcinogenic potential, we made every effort to minimize CTX exposure. By comparison, the previously reported mean duration of response for MTX was shorter, with 12/18 relapsing during the MTX treatment [4], while CTX responses were more durable [4].
A significant minority of patients requiring treatment did not achieve response to any of the standard therapies, and such patients may constitute a difficult clinical problem. For such cases, the ORR of splenectomy was 32% in the French registry, whereas in our study it was 58% with a median duration of 30 months (1.8–115 months). We only selected patients for splenectomy if they had adequate reticulocyte counts, splenomegaly, or hemolytic anemia. Alemtuzumab as an LGLL salvage agent yielded 6/21 responses versus 14/25 in a clinical trial [23]. However, in this trial, a dose-intense regimen was utilized (10 mg i.v. for 10 days compared to our regimen of 10 mg s.c. weekly) and thus while our responses seem comparable, our regimen with proper prophylaxis was associated with a minimal toxicity and was well tolerated. Treatment results with other salvage agents were similar e.g. for tacrolimus (6/8 responses), abatacept (6/8 responses), tofacitinib (6/9 responses) and ATG (2/6 responses). Patients requiring more than three lines of treatment had poor survival with a hazard ratio of 3.4 compared to those who were less refractory (p= .00039).
The choice of first-line agent in LGLL should take potential toxicities into consideration. For example, the presence of renal failure precludes the use of CsA, whereas CTX should be avoided as a chronic therapy in younger patients due to the risk of secondary malignancies, or in neutropenic patients in which myelotoxicity may preclude detection of response. Consequently, CTX was not commonly used as primary treatment in patients with severe neutropenia. Similarly, MTX was not used as a primary treatment in patients with severe anemia. Similar considerations determine the choice of salvage therapy (e.g. presence of RA for abatacept or tofacitininb, alemtuzamab in renal failure, or ATG for younger patients in good physical health). Experience has to be collected in systematic fashion to make firm recommendations. New rheumatologic agents targeting the IL-6 signaling axis may find their utility in LGLL, particularly in cases without intrinsic mutations in the STAT3 pathway. In our cohort, STAT3 mutated patients were more likely to have neutropenia or anemia and, as such, were more likely to be treated. Both STAT3 mutant and WT patients had similar initial and overall response rates for MTX, CsA, CTX, the three most commonly used first line agents. However, patients with STAT3 mutation had better overall survival than patients without a STAT3 mutation, suggesting that there may be a clinical distinction between patients with and without a STAT3 mutation. This finding certainly requires further investigation and has implications for future LGLL studies of novel STAT3 inhibitors.
Supplementary Material
Acknowledgements
This work was supported by NIH U54 RR019397 (J.P.M.), K24 HL077522 (J.P.M.), R01 CA113972 (J.P.M.) and LLS 624–13 (J.P.M.).
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article online at https://doi.org/10.1080/10428194.2017.1339880.
References
- [1].Loughran TP Jr, Kadin ME, Starkebaum G, et al. Leukemia of large granular lymphocytes: association with clonal chromosomal abnormalities and autoimmune neutropenia, thrombocytopenia and hemolytic anemia. Ann Intern Med. 1985;102:169–175. [DOI] [PubMed] [Google Scholar]
- [2].Loughran TP Jr. Clonal diseases of large granular lymphocytes. Blood. 1993;82:1–14. [PubMed] [Google Scholar]
- [3].Lamy T, Loughran TP Jr. How I treat LGL leukemia. Blood. 2011;117:2764–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bareau B, Rey J, Hamidou M, et al. Analysis of a French cohort of patients with large granular lymphocyte leukemia: a report on 229 cases. Haematologica. 2010;95:1534–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Epling-Burnette PK, Loughran TP Jr. Survival signals in leukemic large granular lymphocytes. Semin Hematol. 2003;40:213–220. [DOI] [PubMed] [Google Scholar]
- [6].Lamy T, Liu JH, Landowski TH, et al. Dysregulation of CD95/CD95 ligand-apoptotic pathway in CD3(+) large granular lymphocyte leukemia. Blood. 1998;92: 4771–4777. [PubMed] [Google Scholar]
- [7].Clemente MJ, Wlodarski MW, Makishima H, et al. Clonal drift demonstrates unexpected dynamics of the T-cell repertoire in T-large granular lymphocyte leukemia. Blood. 2011;118:4384–4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Viny AD, Lichtin A, Pohlman B, et al. Chronic B-cell dyscrasias are an important clinical feature of T-LGL leukemia. Leukemia & Lymphoma. 2008;49:9382–9387. [DOI] [PubMed] [Google Scholar]
- [9].Viny AD, Maciejewski J. High rate of both hematopoietic and solid tumors associated with large granular lymphocyte leukemia. Leukemia & Lymphoma. 2015;56:503–504. [DOI] [PubMed] [Google Scholar]
- [10].Jerez A, Clemente MJ, Makishima H, et al. STAT3 mutations unify the pathogenesis of chronic lymphoproliferative disorders of NK cells and T-cell large granular lymphocyte leukemia. Blood. 2012;120: 3048–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Koskela HL, Eldfors S, Ellonen P, et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. N Engl J Med. 2012;366:1905–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fasan A, Kern W, Grossmann V, et al. STAT3 mutations are highly specific for large granular lymphocytic leukemia. Leukemia. 2013;27:1598–1600. [DOI] [PubMed] [Google Scholar]
- [13].Loughran TP Jr, Kidd PG, Starkebaum G. Treatment of large granular lymphocyte leukemia with oral lowdose methotrexate. Blood. 1994;84:2164–2170. [PubMed] [Google Scholar]
- [14].Loughran TP Jr, Zickl L, Olson TL, et al. Immunosuppressive therapy of LGL leukemia: prospective multicenter phase II study by the Eastern Cooperative Oncology Group (E5998). Leukemia. 2015;29:886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Osuji N, Matutes E, Tjonnfjord G, et al. T-cell large granular lymphocyte leukemia: a report on the treatment of 29 patients and a review of the literature. Cancer. 2006;107:570–578. [DOI] [PubMed] [Google Scholar]
- [16].Dhodapkar MV, Li CY, Lust JA, et al. Clinical spectrum of clonal proliferations of T-large granular lymphocytes: a T-cell clonopathy of undetermined significance? Blood. 1994;84:1620–1627. [PubMed] [Google Scholar]
- [17].Mohan SR, Clemente MJ, Afable M, et al. Therapeutic implications of variable expression of CD52 on clonal cytotoxic T cells in CD8+ large granular lymphocyte leukemia. Haematologica. 2009;94:1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Battiwalla M, Melenhorst J, Saunthararajah Y, et al. HLA-DR4 predicts haematological response to cyclosporine in T-large granular lymphocyte lymphoproliferative disorders. Br J Haematol. 2003;123:449–453. [DOI] [PubMed] [Google Scholar]
- [19].Moignet A, Hasanali Z, Zambello R, et al. Cyclophosphamide as a first-line therapy in LGL leukemia. Leukemia. 2014;28:1134–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fujishima N, Sawada K, Hirokawa M, et al. Long-term responses and outcomes following immunosuppressive therapy in large granular lymphocyte leukemia-associated pure red cell aplasia: a Nationwide Cohort Study in Japan for the PRCA Collaborative Study Group. Haematologica. 2009;93:1555–1559. [DOI] [PubMed] [Google Scholar]
- [21].Semenzato G, Zambello R, Starkebaum G, et al. The lymphoproliferative disease of granular lymphocytes: updated criteria for diagnosis. Blood. 1997;89: 256–260. [PubMed] [Google Scholar]
- [22].Sokol L, Loughran TP Jr. Large granular lymphocyte leukemia. Oncologist. 2006;11:263–273. [DOI] [PubMed] [Google Scholar]
- [23].Dumitriu B, Ito S, Feng X, et al. Alemtuzumab in T-cell large granular lymphocytic leukaemia: interim results from a single-arm, open-label, phase 2 study. Lancet Haematol. 2016;3:e22–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.