Abstract
Objective:
Inflammation plays an important role in the initiation of postoperative atrial fibrillation (PoAF) in individuals undergoing cardiac surgery, Thus, this study aimed to investigate the predictive value of the systemic immune inflammation index (SII) to develop PoAF in such patients.
Methods:
In total, 391 consecutive patients undergoing an isolated coronary artery bypass grafting (CABG) were retrospectively analyzed. PoAF was defined according to the current guideline. The SII is determined using the following equation: neutrophil (N) × platelet (P) ÷ lymphocyte (L).
Results:
The incidence of PoAF in the present study was 24% (n=97 cases). Multivariate logistic regression analysis revealed that the SII was an independent predictor of PoAF (Odds ratio: 1.002 95% confidence interval: (1.001-1.002), p<0.01). The optimal value of the SII in detecting PoAF was established by a receiver operating characteristic curve assessment, and it was >807.8 with 60.8% sensitivity and 80.9% specificity [area under the curve (AUC): 0.7107]. The AUC value of SII in detecting PoAF was much greater than the AUC values of both the neutrophil to lymphocyte ratio (NLR) and the platelet to lymphocyte ratio (PLR) (AUC: 0.6740 and AUC: 0.6426, respectively).
Conclusions:
This study revealed that SII was an independent predictor of PoAF in patients who were operated on for isolated CABG. Additionally, SII had a better discriminative ability for PoAF compared to either NLR or PLR among these cases.
Keywords: SII, postoperative atrial fibrillation, coronary artery bypass grafting, predictive
Abstract
Amaç:
Enflamasyon, kalp cerrahisi olan hastalarda postoperatif atriyal fibrilasyon (PoAF) başlangıcında önemli bir rol oynadığından, bu çalışma bu tür hastalarda PoAF gelişimi için sistemik immün enflamasyon indeksinin (SII) öngörücü değerini araştırmayı amaçladı.
Yöntemler:
Toplamda, izole bir koroner arter baypas greft (KABG) uygulanan 391 ardışık hasta geriye dönük olarak analiz edildi. PoAF mevcut kılavuza göre tanımlanmıştır. SII nötrofil (N) x trombosit (T) ÷ lenfosit (L) formülü kullanılarak belirlendi.
Bulgular:
Bu çalışmada PoAF insidansı %24’tür (n=97 olgu). Çok değişkenli lojistik regresyon analizi, SII’nin PoAF’nin bağımsız bir öngörücüsü olduğunu ortaya çıkarmıştır [olasılık oranı: %95 güven aralığı: 1,002 (1,001-1,002), p<0,01]. PoAF tespitinde SII’nin optimal değeri, alıcı işletim karakteristik eğri değerlendirmesi ile belirlendi ve SII’nin ideal değeri %60,8 duyarlılık ve %80,9 özgüllük [eğri altı alanı (EAA): 0,7107] ile 807,8’den büyük olarak belirlendi. PoAF’nin tespitinde SII’nin EAA, nötrofil/lenfosit oranının (NLO) veya trombosit/lenfosit oranının (TLO) (sırasıyla EAA: 0,6740 ve EAA: 0,6426) EAA’sından daha yüksekti.
Sonuçlar:
Bu araştırmada, izole KABG nedeniyle ameliyat edilen hastalarda SII’nin PoAF’nin bağımsız bir öngörücüsü olduğunu gösterdik. Ek olarak SII, bu olgular arasında NLO veya TLO’ya kıyasla PoAF için daha iyi ayırt etme yeteneğine sahipti.
Keywords: SII, postoperatif atriyal fibrilasyon, koroner arter baypas greft, öngörücü
INTRODUCTION
Postoperative atrial fibrillation (PoAF) is the most commonly observed supraventricular arrhythmia following cardiovascular surgery, with an estimated incidence of 20%-40%1. It typically occurs on postoperative 2-4 days and often spontaneously terminates without any interventions2. Prior studies showed that the development of PoAF after cardiac surgery was linked with longer hospitalization, higher risk of thromboembolic events, and a slightly increased risk of death during the index hospitalization3. Furthermore, the occurrence of PoAF has been demonstrated to be significantly related to an increased risk of stroke in the long-term follow-up4. The exact mechanisms of PoAF following cardiac surgery are complex and incompletely defined; however, inflammation, sympathetic activation, and cardiac ischemia are shown to induce and maintain PoAF5. Particularly, systemic inflammation had previously been shown to predict the onset and recurrence of PoAF in patients who had undergone cardiac surgery, including coronary artery bypass grafting (CABG)6,7,8.
The systemic immune inflammation index (SII), which is developed to assess patients’ inflammatory state, is determined using the following equation: neutrophil (N) × platelet (P) ÷ lymphocyte (L)9. Previously, cancer cases with higher SII were shown to have poor outcomes and increased mortality9,10. Additionally, the predictive value of this index has been examined in predicting deaths in cases with acute and chronic coronary syndromes (CCS)11,12. Nevertheless, the current literature reported insufficient data on the usefulness of SII in predicting the PoAF occurrence in patients after cardiac surgery, especially those undergoing an isolated CABG. Systemic inflammation plays an important role in the initiation of PoAF in patients following cardiac surgery, thus, this study mainly aimed to explore the predictive accuracy of this index for PoAF occurrence in such patients.
MATERIALS and METHODS
Study Population
Firstly, consecutive cases with symptomatic coronary artery disease that underwent an isolated CABG at our facility between January 2018 and January 2021 were screened. Then, those who had acute and chronic infections, chronic inflammatory or autoimmune dysfunction, liver problems, malignancy, and significant valvular heart diseases as well as those previously diagnosed with AF were excluded. Therefore, 391 patients undergoing an isolated CABG were analyzed in this study. All patients were operated on with isolated on-pump CABG. Clinical and laboratory data of all patients were obtained from the hospital electronic database. Additionally, data regarding the intraoperative procedures were collected, including the aortic cross-clamp time, the number of distal anastomoses, left internal mammary artery (LIMA) graft use, cardiopulmonary bypass (CPB) time, drainage amount, intubation time, the use of inotropic support, and the use of blood products. PoAF was defined according to the European Society of Cardiology AF guidelines, with a duration of at least 30 s. Per hospital protocol, patients with hemodynamically stable PoAF were firstly managed with medical cardioversion. Those who did not convert to sinus rhythm with medical cardioversion underwent electrical cardioversion. In the case of hemodynamic instability, direct electrical cardioversion was performed. Patients that were previously diagnosed with AF were excluded, thus no patients used any antiarrhythmic medication. Per hospital protocol, AF prophylaxis was left to the attending physician’s decision. The anatomical synergy between percutaneous coronary intervention with Taxus and cardiac surgery (SYNTAX) score was applied to assess the complexity of coronary artery disease using the web online calculator (http://syntaxscore.org). The University of Health Sciences Turkey, Hamidiye Scientific Research Ethics Committee has reviewed and approved our study protocol (decision number: 13/7, date: 09.04.2021).
SII and Transthoracic Echocardiography Examination
For each case, venous samples were collected from the antecubital vein before cardiac surgery. An auto-analyzer was used to assess all blood cell counts, including neutrophils, lymphocytes, and platelets. The SII was computed as P × N ÷ L ratio.
Transthoracic echocardiography was performed for each case included in the study. The left ventricle ejection fraction (LVEF) was determined based on the modified Simpson method. Additionally, the left anteroposterior atrial diameter (LAPd) was determined from the parasternal long-axis. The LA volume index (LAVI) was computed by dividing the volume of the LA by the surface body area.
Statistical Analysis
All statistical calculations were performed using SAS University Edition (Copyright© 2015, SAS Institute Inc., Cary, NC, USA). The Kolmogorov-Smirnov test was used to assess the normality of data. Quantitative variables with a normal distribution were reported as mean (standard deviation), and those without a normal distribution were expressed as median (interquartile range). Categorical variables were presented as numbers and percentages. The independent Student’s t-test and Mann-Whitney U tests were used for inter-group comparisons of continuous variables. The chi-square test or Fisher’s Exact test was used to compare categorical variables, as appropriate. Univariate logistic regression analysis was used to determine the predictors of PoAF. A multivariable logistic regression analysis was performed with variables that exhibit statistical significance in univariate logistic regression (p<0.05). To avoid multicollinearity, neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), neutrophil, lymphocyte, and platelet were excluded from the multivariable logistic regression model. Receiver operating characteristic (ROC) curve analysis was performed to obtain the best SII cutoff value for predicting PoAF. The Bayesian information criterion (BIC), 2 Log likelihood (-2LL), Akaike information criterion (AIC), and area under the curve (AUC) were used to assess the comparisons of the abilities of variables to predict PoAF. Lower levels of AIC, BIC, and -2LL indicate a better model fit, and a high AUC value indicates effective discrimination ability for the diagnostic prediction. A 2-sided p-value of >0.05 was considered significant, and the confidence interval was set at 95%.
RESULTS
The research population was grouped into two categories as patients who developed PoAF and those who did not. The incidence of PoAF in the present study was 24% (n=97 cases). Baseline features and previous medications of all cases are displayed in Table 1. Both groups were similar in terms of comorbidities and previous medications. Additionally, LVEF, LAPd, and LAVI were not different in both groups.
Table 1. Clinical and demographic characteristics of the patients.
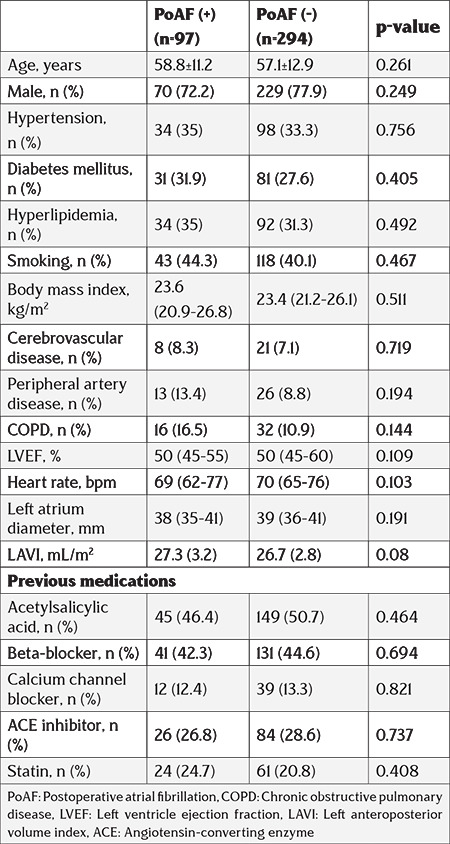
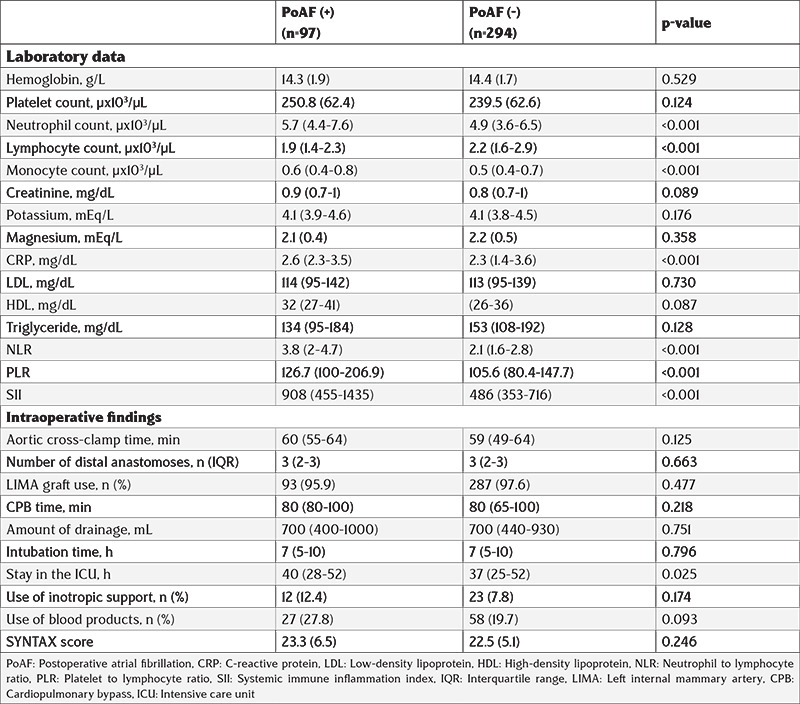
Table 2 presents the data of laboratory results and intraoperative data of each case that is included in the study. Patients who developed PoAF had higher neutrophil, monocyte count, C-reactive protein (CRP), NLR, PLR, and SII but lower lymphocyte counts. Other laboratory data were similar between the groups. Both groups had similar intraoperative data, including aortic cross-clamp time, number of distal anastomoses, LIMA graft use, CPB time, drainage amount, intubation time, the use of inotropic support, and the use of blood products. As expected, patients with PoAF had a longer stay in the intensive care unit (ICU). Additionally, the SYNTAX score was similar between the groups.
Table 2. Laboratory and intraoperative data of all cases.
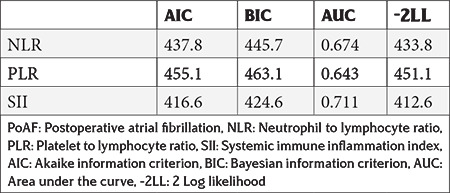
Both univariable and multivariable logistic regression analyses were applied to identify the determinants of PoAF. LVEF, neutrophil count, lymphocyte count, monocyte count, SII, CRP, and ICU stay were determinants of PoAF in univariable analysis. Multivariate logistic regression analysis revealed that monocyte count, SII (odds ratio: 95% confidence interval: 1.002 (1.001-1.002), p<0.01), CRP, and ICU stay were independent predictors for PoAF (Table 3). As shown in Table 4, SII had lower AIC, BIC, and -2LL, and higher AUC values.
Table 3. Univariable and multivariable logistic regression analysis for PoAF predictors.
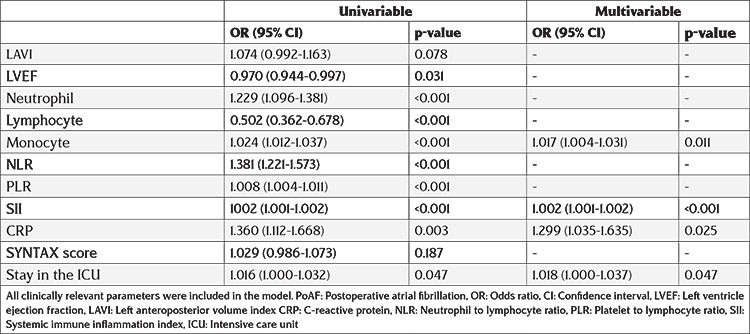
Table 4. Diagnostic performance comparison of PoAF predictors.
The ROC curve assessment revealed that the optimal value of the SII level in detecting PoAF was >807.8 with 60.8% sensitivity and 80.9% specificity (AUC: 0.7107) (Figure 1). Of note, the AUC value of SII in detecting PoAF was much higher than the AUC values of the NLR and PLR (AUC: 0.6740 vs. AUC: 0.6426, respectively). According to a box-plot assessment, individuals with PoAF had considerably greater SII than those without PoAF (Figure 2).
Figure 1.
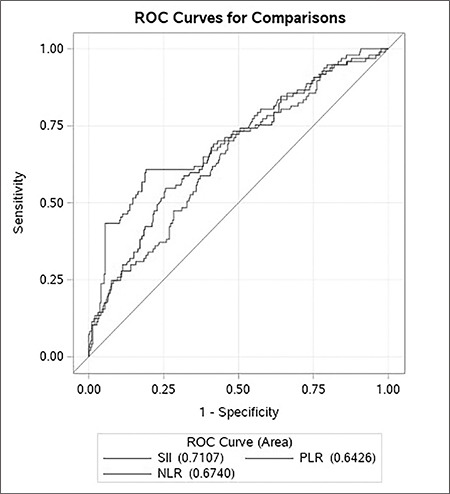
Receiver operating characteristics curve comparisons of systemic immune inflammation index, neutrophil to lymphocyte ratio, and platelet to lymphocyte ratio.
SII: Systemic immune inflammation index, NLR: Neutrophil to lymphocyte ratio, PLR: Platelet to lymphocyte ratio, ROC: Receiver operating characteristic
Figure 2.
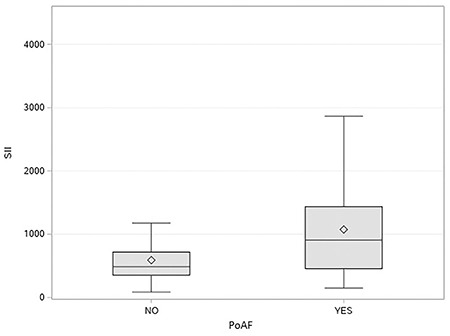
A box-plot analysis of systemic immune inflammation index in patients with and without postoperative atrial fibrillation.
PoAF: Postoperative atrial fibrillation, SII: Systemic immune inflammation index
DISCUSSION
This study revealed that SII was a significant and independent predictor of PoAF in patients who were operated on for isolated CABG. Additionally, elevated preoperative SII had a better effective discriminative ability for PoAF compared to either NLR or PLR among these cases.
PoAF is the most commonly observed arrhythmia complication that develops after cardiovascular surgery. The incidence of such complication is slightly higher after valve-replacement surgery; however, it occurs in approximately 30% of cases undergoing CABG. Allying with the current literature, the incidence of PoAF in our study was 24%13. Current data indicates that PoAF does slightly prolong the hospital stay duration; however, it is less likely to have an impact on the survival of cases compared with chronic AF14. Similarly, in the present investigation, patients who developed PoAF were observed to have longer ICU stays than those who did not. In the current knowledge, growing evidence shows that inflammation plays a crucial role both in the onset and maintenance of PoAF after cardiovascular surgery6,7. Previously, several well-known markers of inflammation, such as CRP and interleukin-6, were linked with an increased risk of PoAF following cardiac surgery15,16. Similar to these previous studies, an independent relationship was observed between the CRP and PoAF in our study. Hence, it can be concluded that inflammatory markers can be used to determine the risk of PoAF in patients undergoing CABG. A previous study demonstrated that a high SYNTAX score was linked with more frequent PoAF in patients undergoing isolated on-pump CABG17. However, the SYNTAX score was not an independent predictor of PoAF in our study since our study population included only patients undergoing isolated on-pump CABG, in whom the SYNTAX score was high. Our study revealed that both LA diameters and LAVI were comparable between the groups. Patients with significant valvular heart disease and those previously diagnosed with AF were eliminated, thus these findings might be expected.
The SII is an innovative inflammatory biomarker that combines neutrophil, lymphocyte, and platelet counts to reflect the overall inflammatory status of the body. The predictive value of this index has been examined for poor outcomes in individuals who suffer from a variety of cancers10,18. Additionally, a prior investigation by Seo et al.19 revealed the predictive accuracy of SII for mortality in chronic heart failure cases. Moreover, some previous studies compared the prognostic significance of this index to its components, including NLR or PLR. For example, in a recent investigation by Erdoğan et al.20, SII levels have been shown to hemodynamically predict severe coronary obstruction better than either NLR or PLR in patients with the CCS. Another study by Yang et al.21 revealed that SII predicted major cardiovascular events better than traditional risk factors in CCS cases after the intervention. However, the current knowledge reported no data about the use of SII in predicting PoAF in patients who underwent an isolated CABG. To our knowledge, our study is the first to evaluate SII in predicting PoAF in patients after CABG and revealed that SII was independently associated with PoAF in patients undergoing isolated CABG. Remarkably, SII was also observed to have a higher AUC value for PoAF compared to the AUC value of either NLR or PLR.
Our study results are considered valuable in daily clinical practice. As a simple and readily available prognostic biomarker, SII can be easily obtained from routine complete blood tests without additional costs. Preoperative SII level is believed to be used in assisting the clinicians in identifying high-risk patients and making better medical decisions in clinical practice.
Study Limitations
This study has some major limitations. First, this study had a retrospective design. Second, the study included a limited number of cases despite the adequate sample size in the power analysis. Third, multivariable analysis was conducted to determine independent predictors; however, some unmeasured confounders might be present, which might affect the study results. Fourth, spot laboratory data was used to determine the relationship between PoAF and SII. Unfortunately, data regarding the post-op SII was not collected. Fifth, data regarding the in-hospital mortality and the choice of arrhythmic medications were not collected. Finally, more prospective studies are warranted to validate the link between PoAF and SII.
CONCLUSIONS
The present study shows that preoperative SII can be used in predicting PoAF in patients undergoing an isolated CABG.
Footnotes
Ethics
Ethics Committee Approval: The University of Health Sciences Turkey, Hamidiye Scientific Research Ethics Committee has reviewed and approved our study protocol (decision number: 13/7, date: 09.04.2021).
Informed Consent: Retrospective study.
Peer-review: Externally and internally peer-reviewed.
Author Contributions
Concept: M.S., T.C., F.S., S.D., I.S., A.L.O., Design: M.S., T.C., F.S., S.D., I.S., A.L.O., Data Collection and/or Processing: M.S., T.C., F.S., S.D., I.S., A.L.O., Analysis and/or Interpretation: M.S., T.C., F.S., Critical Revision: M.S., T.C., F.S., S.D., A.L.O., Writing: M.S., T.C., F.S., I.S.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Filardo G, Damiano RJ Jr, Ailawadi G, et al. Epidemiology of new-onset atrial fibrillation following coronary artery bypass graft surgery. Heart. 2018;104:985–92. doi: 10.1136/heartjnl-2017-312150. [DOI] [PubMed] [Google Scholar]
- 2.Peretto G, Durante A, Limite LR, Cianflone D. Postoperative arrhythmias after cardiac surgery: incidence, risk factors, and therapeutic management. Cardiol Res Pract. 2014;2014:615987. doi: 10.1155/2014/615987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowres N, Mulcahy G, Jin K, Gallagher R, Neubeck L, Freedman B. Incidence of postoperative atrial fibrillation recurrence in patients discharged in sinus rhythm after cardiac surgery: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2018;26:504–11. doi: 10.1093/icvts/ivx348. [DOI] [PubMed] [Google Scholar]
- 4.Eikelboom R, Sanjanwala R, Le M, Yamashita MH, Arora RC. Postoperative atrial fibrillation after cardiac surgery: a systematic review and meta-analysis. Ann Thorac Surg. 2021;111:544–54. doi: 10.1016/j.athoracsur.2020.05.104. [DOI] [PubMed] [Google Scholar]
- 5.Maesen B, Nijs J, Maessen J, Allessie M, Schotten U. Post-operative atrial fibrillation: a maze of mechanisms. Europace. 2012;14:159–74. doi: 10.1093/europace/eur208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12:230–43. doi: 10.1038/nrcardio.2015.2. [DOI] [PubMed] [Google Scholar]
- 7.Jacob KA, Nathoe HM, Dieleman JM, van Osch D, Kluin J, van Dijk D. Inflammation in new-onset atrial fibrillation after cardiac surgery: a systematic review. Eur J Clin Invest. 2014;44:402–28. doi: 10.1111/eci.12237. [DOI] [PubMed] [Google Scholar]
- 8.Gibson PH, Cuthbertson BH, Croal BL, et al. Usefulness of neutrophil/lymphocyte ratio as predictor of new-onset atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 2010;105:186–91. doi: 10.1016/j.amjcard.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Yang R, Chang Q, Meng X, Gao N, Wang W. Prognostic value of Systemic immune-inflammation index in cancer: a meta-analysis. J Cancer. 2018;9:3295–302. doi: 10.7150/jca.25691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong JH, Huang DH, Chen ZY. Prognostic role of systemic immune-inflammation index in solid tumors: a systematic review and meta-analysis. Oncotarget. 2017;8:75381–8. doi: 10.18632/oncotarget.18856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Candemir M, Kiziltunç E, Nurkoç S, Şahinarslan A. Relationship between Systemic Immune-Inflammation Index (SII) and the severity of stable coronary artery disease. Angiology. 2021;72:575–81. doi: 10.1177/0003319720987743. [DOI] [PubMed] [Google Scholar]
- 12.Huang J, Zhang Q, Wang R, et al. Systemic immune-inflammatory index predicts clinical outcomes for elderly patients with acute myocardial infarction receiving percutaneous coronary intervention. Med Sci Monit. 2019;25:9690–701. doi: 10.12659/MSM.919802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omae T, Kanmura Y. Management of postoperative atrial fibrillation. J Anesth. 2012;26:429–37. doi: 10.1007/s00540-012-1330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almassi GH, Schowalter T, Nicolosi AC, et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997;226:501–11. doi: 10.1097/00000658-199710000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karabacak K, Kubat E, Akyol FB, et al. The C-reactive protein/albumin ratio as a new predictor for postoperative atrial fibrillation after coronary artery bypass graft surgery. J Card Surg. 2020;35:2747–53. doi: 10.1111/jocs.14898. [DOI] [PubMed] [Google Scholar]
- 16.Jacob KA, Buijsrogge MP, Frencken JF, et al. White blood cell count and new-onset atrial fibrillation after cardiac surgery. Int J Cardiol. 2017;228:971–6. doi: 10.1016/j.ijcard.2016.11.038. [DOI] [PubMed] [Google Scholar]
- 17.Geçmen Ç, Babür Güler G, Erdoğan E, et al. SYNTAX score predicts postoperative atrial fibrillation in patients undergoing on-pump isolated coronary artery bypass grafting surgery. Anatol J Cardiol. 2016;16:655–61. doi: 10.5152/AnatolJCardiol.2015.6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 19.Seo M, Yamada T, Morita T, et al. Prognostic value of systemic immune-inflammation index in patients with chronic heart failure. Eur Heart J. 2018;39 (suppl 1):P589. [Google Scholar]
- 20.Erdoğan M, Erdöl MA, Öztürk S, Durmaz T. Systemic immune-inflammation index is a novel marker to predict functionally significant coronary artery stenosis. Biomark Med. 2020;14:1553–61. doi: 10.2217/bmm-2020-0274. [DOI] [PubMed] [Google Scholar]
- 21.Yang YL, Wu CH, Hsu PF, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patievnts with coronary artery disease. Eur J Clin Invest. 2020;50:e13230. doi: 10.1111/eci.13230. [DOI] [PubMed] [Google Scholar]


