Abstract
Pneumocystis jirovecii pneumonia (PCP) is a common opportunistic infection causing more than 400000 cases annually worldwide. Although antiretroviral therapy has reduced the burden of PCP in persons with human immunodeficiency virus (HIV), an increasing proportion of cases occur in other immunocompromised populations. In this review, we synthesize the available randomized controlled trial (RCT) evidence base for PCP treatment. We identified 14 RCTs that were conducted 25–35 years ago, principally in 40-year-old men with HIV. Trimethoprim-sulfamethoxazole, at a dose of 15–20 mg/kg per day, is the treatment of choice based on historical practice rather than on quality comparative, dose-finding studies. Treatment duration is similarly based on historical practice and is not evidence based. Corticosteroids have a demonstrated role in hypoxemic patients with HIV but have yet to be studied in RCTs as an adjunctive therapy in non-HIV populations. The echinocandins are potential synergistic treatments in need of further investigation.
Keywords: HIV, immunosuppressed, opportunistic infectious, Pneumocystis jirovecii pneumonia, TMP-SMX
Pneumocystis jirovecii is an opportunistic fungal infection, transmitted through the inhalation of airborne particles, which primarily affects immunocompromised patients by causing pneumocystis pneumonia (PCP) [1]. Humans are also thought to be a natural reservoir, and in times of immune suppression colonized people may develop the infectious syndrome [2]. Although the incidence of PCP in patients with human immunodeficiency virus (HIV) has diminished in many countries with the institution of antiretroviral therapy, major advances in biologic immunotherapies, chemotherapy, and transplantation have led to an increase among other immunocompromised populations [3]. Due to the expanding population of at-risk patients, the number of cases is increasing; in 2017, there were over 10000 PCP hospitalizations in the United States [4], and it continues to present a significant problem in patients with HIV, especially in Africa [5], Central, and South America [6]. Worldwide, there are an estimated 400000 cases per year [7]. Moreover, with newer diagnostic testing strategies boasting improved sensitivity, it is expected that the incidence of PCP will continue to increase, especially for patients without HIV [8].
More importantly, antimicrobial prophylaxis against PCP is highly effective, and it is routinely administered, in the context of certain immunocompromised conditions such as acquired immune deficiency syndrome (AIDS), early solid organ transplantation, or prolonged use of high-dose corticosteroids. However, cases may occur when prophylaxis is not routinely administered, as a result of breakthrough, due to resistance to second-line agents, or due to lapses in prophylaxis from toxicity or suboptimal adherence [9]. Furthermore, it has become apparent that PCP is associated with significantly more morbidity and mortality in non-HIV- immunocompromised populations. A growing number of retrospective cohort studies have reported fulminant infections with mortality rates ranging between 20% and 50% among non-HIV populations [10–13] and 10%–20% for patients with HIV [14–16]. Part of this increased mortality may be related to the comorbid illnesses and older age of patients without HIV who contract PCP. There remains a critical need for safe, effective, evidence-based treatments for PCP for people with HIV, as well as the growing population of immune suppressed individuals without HIV, who are at risk of more fulminant disease.
In this review, we (1) provide an overview of the body of randomized controlled trial (RCT) evidence that forms current guidelines for the treatment of PCP (including choice of agent, dose, and duration), (2) review identified ongoing clinical trials, and (3) highlight significant gaps in the evidence and areas in need of further research. For added perspective, we provide information on the history of some established practices, such as dosing of trimethoprim-sulfamethoxazole (TMP-SMX), duration of therapy, and the use of adjunctive corticosteroids.
THE EVIDENCE BEHIND CURRENT TREATMENT RECOMMENDATIONS: METHODS
To review the evidence behind current treatment recommendations we performed a search of PubMed database on April 15, 2021, with the search terms “(Pneumocystis OR Pneumocystosis OR P. jirovecii OR P. carinii OR PCP Infection OR PJP Infection OR PCP Pneumonia OR PJP Pneumonia) AND (‘Clinical Trial’[pt] OR ‘Randomized Controlled Trial’[pt])”. We looked for randomized controlled trials of PCP treatment. We excluded studies of primary or secondary prophylaxis and those that were noncomparative. We also searched clinicaltrials.gov for “Pneumocystis pneumonia” and cross-referenced them to those identified in the PubMed search. A more detailed version of the search strategy is available in the Supplement Material.
Patient Consent Statement
This study does not include factors necessitating patient consent.
RESULTS
Results of the search are summarized in Figure 1; 469 articles were screened by title and abstract leaving 25 for full-text review. Seven of these were corticosteroid trials, which have been previously described and meta-analyzed [17], 3 were noncomparative, and 1 was a study of prophylaxis. This left 14 RCTs of comparative treatments, which are summarized in Table 1 [18–31]. The search of the trials database identified 2 trials that never released any results and 3 ongoing trials that do not yet have results.
Figure 1.
PRISMA diagram.
Table 1.
Identified Randomized Controlled Trials
| Title | Author | Year | Total Patients | Mean Age | Male | Population | Intervention | Primary Outcome | Mortality | Change for Failure | Change for Toxicity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clindamycin with primaquine vs. trimethoprim-sulfamethoxazole therapy for mild and moderately severe Pneumocystis carinii pneumonia in patients with AIDS: a multicenter, double-blind, randomized trial (CTN 004). CTN-PCP Study Group | Toma | 1998 | 87 | <40 | 95%–100% | HIV-associated PJP with PaO2 ≥50 mmHg and weight >45 kg | Clindamycin-primaquine vs TMP-SMX (~20mg/kg) | Success (defined 2 or more points in “PCP Score” provided all items lower than baseline) AND no new mechanical ventilation AND no switch to alternative therapy AND no new steroids | 35 days | 21 days | 21 days |
| Comparison of three regimens for treatment of mild to moderate Pneumocystis carinii pneumonia in patients with AIDS. A double-blind, randomized, trial of oral trimethoprim-sulfamethoxazole, dapsone-trimethoprim, and clindamycin-primaquine. ACTG 108 Study Group | Safrin | 1996 | 97 | NA | 89% | HIV-positive patients with symptoms or signs of PJP and PaO2 ≥45 mmHg | Dapsone + TMP
clindamycin-primaquine TMP-SMX (~15–20mg/kg) |
Failure at day 7/21: Increase A-a gradient of 20 mmHg without improvement in symptoms; Change in therapy except for toxicity; Intubation; Death | 81 days | 21 days | 21 days |
| Pentamidine aerosol versus trimethoprim-sulfamethoxazole for Pneumocystis carinii in acquired immune deficiency syndrome | Montgomery | 1995 | 254 | 35 | 93% | All HIV patients with symptomatic PJP and resting A-a gradient of 55 mmHg or less | Inhaled pentamidine vs TMP-SMX (15mg/kg) | Survival (day 35). Failure was defined as change in treatment for slow or nonresponse | 35 days | 21 days | 21 days |
| Adjunctive folinic acid with trimethoprim-sulfamethoxazole for Pneumocystis carinii pneumonia in AIDS patients is associated with an increased risk of therapeutic failure and death | Safrin | 1994 | 92 | 37 | 98% | All patients with presumed PJP who were receiving TMP-SMX ≤15mg/kg per day | TMP-SMX (15mg/kg) vs TMP-SMX (15mg/kg) + folinic acid | Therapeutic failure: change to alternative agent due to lack of response or patient death | 1 month postdischarge | 21 days | 21 days |
| Oral atovaquone compared with intravenous pentamidine for Pneumocystis carinii pneumonia in patients with AIDS | Dohn | 1994 | 109 | 37 | 95% | All HIV patients with clinical presentations consistent with PJP | Atovaquone vs intravenous pentamidine | Success: sustained improvement 4 weeks after therapy had stopped with no alternative treatment during that time. Absence of response: new ventilation after 3 days, increase in A-a of 20 mmHg or more provided absolute is ≥30 mmHg; worsening radiographs or worsening symptoms without other reason. | 4 weeks posttherapy (and not ventilated) | 4 weeks | 4 weeks |
| Trimetrexate with leucovorin versus trimethoprim-sulfamethoxazole for moderate to severe episodes of Pneumocystis carinii pneumonia in patients with AIDS: a prospective, controlled multicenter investigation of the AIDS Clinical Trials Group Protocol 029/031 | Sattler | 1994 | 215 | 36 | 95% | All HIV-positive patients with suspected or confirmed PJP with A-a gradient >30mm Hg | Trimetrexate with leucovorin vs TMP-SMX (20mg/kg per day) | Survival day 21.
Therapeutic failure (day 10): RR >50/minute; A-a gradient increased by 20 mmHg; change in anti-PJP due clinician perceived failure; mechanical ventilation, death. Therapeutic Failure (day 21): Death, mechanical ventilation, increase in A-a gradient by 20 mmHg, change in PJP therapy, ongoing signs and symptoms of pulmonary infection |
4 weeks posttherapy | 21 days | 21 days |
| Clindamycin/primaquine versus trimethoprim-sulfamethoxazole as primary therapy for Pneumocystis carinii pneumonia in AIDS: a randomized, double-blind pilot trial | Toma | 1993 | 49 | 40 | 92% | All AIDS patients with first episode of possible PJP | TMP-SMX (15–20mg/kg) vs clindamycin-primaquine | Adverse reactions (21 days). Therapeutic outcome was classified as positive, failure, or discontinuation. “Failure” was no improvement after 7 days of treatment, or “deterioration” despite 5 days of therapy. | 2 months | 21 days | 21 days |
| Comparison of atovaquone (566C80) with trimethoprim-sulfamethoxazole to treat Pneumocystis carinii pneumonia in patients with AIDS | Hughes | 1993 | 322 | 35 | 96% | All patients with AIDS and untreated PJP (histology) with symptoms or radiographic evidence of disease | Atovaquone vs TMP-SMX (15–20mg/kg) | Therapeutic failure (21 days): one of the following: (1) deterioration after the first 3 days of therapy and a requirement for mechanical ventilation; (2) deterioration after 7 days of therapy (A-a increase by ≥20 mmHg, worsening x-ray, and worsening symptoms); (3) lack of improvement in A-a, x-ray, OR symptoms after day 10; (4) requirement of alternative therapy within 4 weeks of discontinuation | 4 weeks posttherapy (and not ventilated) | 21 days | 21 days |
| trimethoprim-sulfamethoxazole versus pentamidine for Pneumocystis carinii pneumonia in AIDS patients: results of a large prospective randomized treatment trial | Klein | 1992 | 160 | 36 | 80% | All patients with pneumonia clinically suggestive of PJP | Intravenous pentamidine vs TMP-SMX (20mg/kg). N.B. cross-over between groups at day 5 based on response | Therapeutic failure on day 5: persistent fever and worsening hypoxemia and/or progressive x-ray changes | Unclear (? 28 days) | 21 days | 21 days |
| Oral therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A controlled trial of trimethoprim-sulfamethoxazole versus trimethoprim-dapsone | Medina | 1990 | 60 | 35 | 98% | All patients with HIV and confirmed first episode of PJP | Dapsone + TMP vs TMP-SMX (20mg/kg) | Therapeutic failure: profound deterioration within 4 days of therapy (clinical, radiological, and/or laboratory) or lack of improvement after 1 week | 21 days | 21 days | 21 days |
| Intravenous or inhaled pentamidine for treating Pneumocystis carinii pneumonia in AIDS. A randomized trial | Conte | 1990 | 38 | Not specified | 98% | Confirmed PJP or probable (with specimen pending); infiltrate seen on x-ray; unlikely to deteriorate within 4 days even if untreated | Inhaled vs intravenous pentamidine | Therapeutic failure: profound deterioration within 4 days of therapy (clinical, radiological, and/or laboratory) or lack of improvement after 1 week; recurrence within 28 days of stopping | 3 months | 21 days | 21 days |
| Inhaled or intravenous pentamidine therapy for Pneumocystis carinii pneumonia in AIDS. A randomized trial | Soo Hoo | 1990 | 21 | 35–38 | 100% | All patients with AIDS or at risk of AIDS with suspected PJP | Inhaled vs intravenous pentamidine | Therapeutic failure: after at least 5 days of therapy PaO2 <67 mmHg with decrease of 20 mmHg and progressive infiltrates; mechanical ventilation. Days 7–14 fevers, rising LDH and progressive x-ray and oxygen requirements. | 3 months postcompletion | 21 days | 21 days |
| Pentamidine aerosol vs cotrimoxazole in the treatment of slight to moderate Pneumocystis carinii pneumonia | Arasteh | 1994 | 46 | 36–38 | 96% | All patients with confirmed HIV and PJP | Inhaled pentamidine vs TMP-SMX (20mg/kg) | Therapeutic failure: no increase in pO2 and FVC with unchanged x-ray infiltrates despite 1 week of treatment. N.B.: switched to other drug if failure or adverse drug reaction. | 4 weeks posttherapy | 21 days | 21 days |
| trimethoprim-sulfamethoxazole or pentamidine for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A prospective randomized trial | Wharton | 1986 | 40 | 36–37 | Not specified | All patients who met surveillance definition of AIDS and who had documented PJP | Intravenous pentamidine vs TMP-SMX (20mg/kg) | Therapeutic failure: death or clinical status deterioration after at least 7 days of therapy | 3 months | 21 days | 21 days |
Abbreviations: A-a, alveolar to arterial; AIDS, acquired immunodeficiency syndrome; FVC, forced vital capacity; HIV, human immunodeficiency virus; LDH, lactate dehydrogenase; N.B., Nota Bene; PJP, Pneumocystis jirovecii pneumonia; RR, risk ratio; TMP, trimethoprim; SMX, sulfamethoxazole.
Where possible, we conducted a random-effects meta-analysis of similar studies using Stata version 16 (StataCorp, College Station, TX) and the metan command [32]. Studies that were meta-analyzed were assessed for risk of bias using the Robbins-2 quality assessment tool and were all considered at an overall low risk of bias (Supplemental Material).
There are several findings worthy of special attention when summarizing the available data. Perhaps most significantly, all published, terminated, or completed trials occurred between 1986 and 1998, wherein TMP-SMX was always studied a dose of 15–20mg/kg per day, based on historical practice, as opposed to dose-finding studies. This is important for 2 reasons: (1) the patient population that is infected with PCP has changed significantly since this time with respect to age, sex, nature of immunosuppression, and other important comorbidities [33, 34]; and (2) all patients are currently treated with a dose and duration of TMP-SMX that may not represent the optimal balance between avoidance of toxicity and clinical cure.
Population Studied
First, all of the clinical trials we identified comprised predominantly of men (89%–100% men); second, the mean age of participants was less than 40 years old; third, all of the trials were completed in patients with HIV and none addressed the current predominant [34] at-risk population in North America (non-HIV immune suppression); fourth, few studies adequately represented participants with any degree of organ insufficiency (in particular a lack of inclusion of participants with chronic renal insufficiency or those taking medications that may interact with TMP-SMX, such as angiotensin-converting enzyme inhibitors, or angiotensin receptor blockers) [35]; fifth, most trials were small with an average number of subjects recruited ~100 (range 21 to 322) per study; finally, none of the trials were conducted in the era of modern antiretroviral therapy. Outcomes in these trials also do not reflect current management of acute respiratory failure, because they predate modern critical care management through low tidal volume ventilation for acute respiratory distress syndrome [36] and the availability of noninvasive ventilatory support [37, 38]. Hence, the external validity of these trial results is compromised in the management of almost all present-day populations of PCP, and application of the results has mainly been through inference.
Treatments Investigated
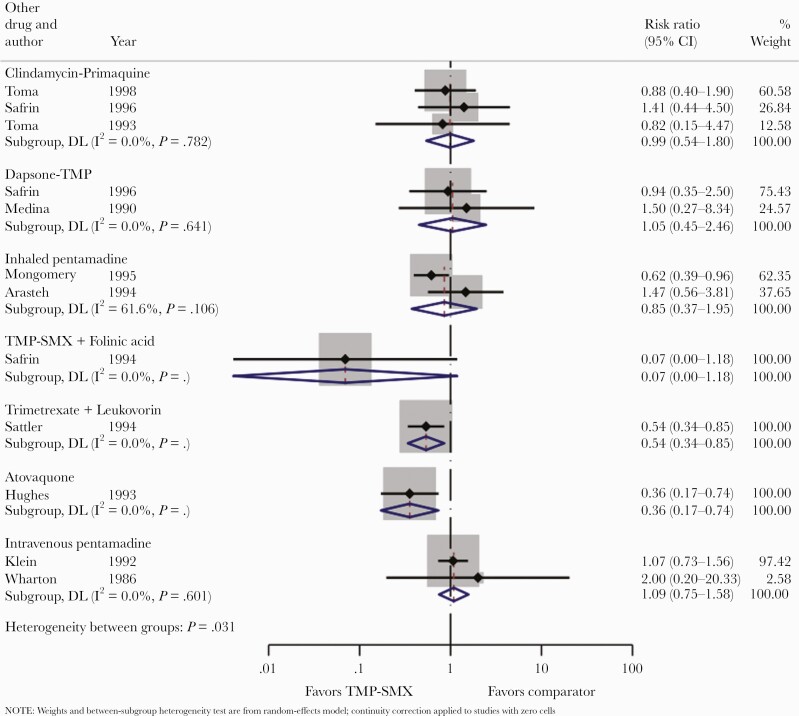
A total of 11 studies compared TMP-SMX to other treatments (clindamycin/primaquine, dapsone-TMP, inhaled or intravenous pentamidine, atovaquone, TMP/folinic acid, and trimetrexate/leucovorin) (Figures 2–4), 4 studies compared clindamycin/primaquine to other treatments (dapsone-TMP and TMP-SMX) (Supplemental Figures 1–3), and 5 studies compared intravenous pentamidine to other treatments (atovaquone, inhaled pentamidine, and TMP-SMX) (Supplemental Figures 4–6). For context, the North American guidelines for the management of PCP in adult patients with HIV [39] and in patients with solid organ transplant [40] are summarized in Supplemental Table 1.
Figure 2.
Trimethoprim sulfamethoxazole (TMP-SMX) vs comparators: treatment failure.
Figure 4.
Trimethoprim sulfamethoxazole (TMP-SMX) vs comparators: change of treatment due to toxicity.
All studies of TMP-SMX studied a dose of 15–20mg/kg of the trimethoprim component. The dose of 15–20mg/kg seems to be initially based on a single study of 20 children with leukemia. This underpowered study compared 20mg/kg with 4–7mg/kg and showed no statistical difference between the 2 regimens [41]. Nonetheless, the higher dose became standard of care for adult patients and is still used to this day in most countries. This is important given a 2020 systematic review, and meta-analysis of observational data suggested that a lower dose (10mg/kg) may be as effective and less toxic [42]. Some centers have attempted to highlight that a lower dose should be considered in some clinical scenarios pending results of a head-to-head comparative dosing trial [43]. Such a trial has started in a pilot phase and will be completed subject to funding (ClinicalTrials.gov Identifier NCT04851015).
Treatment Failure
Recommending TMP-SMX as first-line therapy is justified given that it is the most studied drug, and no agent has ever been shown to be superior in terms of treatment failure (Figure 2). There were only 2 comparisons in which one treatment was clearly superior to the other (TMP-SMX was superior to atovaquone [risk ratio {RR}, 0.36; 95% confidence interval {CI}, 0.17–0.74] and to trimetrexate/leucovorin [RR, 0.45; 95% CI, 0.34–0.85]) with respect to treatment failure. In no case was any treatment comparator demonstrated to be superior to TMP-SMX.
Mortality
With respect to overall mortality, 3 agents (dapsone-TMP and inhaled or intravenous pentamidine) had point estimates favoring them versus TMP-SMX, but the confidence intervals crossed 1 (Figure 3). Based on a single randomized controlled trial, TMP-SMX was superior to atovaquone with respect to mortality (RR, 0.09; 95% CI, 0.01–0.69).
Figure 3.
Trimethoprim sulfamethoxazole (TMP-SMX) vs comparators: overall mortality.
Treatment Toxicity
What clearly emerged from the review of the literature is that TMP-SMX more frequently leads to treatment discontinuation for reasons related to toxicity (Figure 4). Indeed, when toxicity is considered, all point estimates favor the comparator drugs with statistically significant differences in favor of less toxicity for dapsone-TMP (RR, 1.83; 95% CI, 1.26–2.65), inhaled pentamidine (RR, 4.40; 95% CI, 2.5–7.76), and atovaquone (RR, 2.87; 95% CI, 1.50–5.50), when compared to TMP-SMX. In the case of atovaquone, this reduced toxicity is offset by significantly increased mortality. In the prior systematic review and meta-analysis of observational data, lower doses of TMP-SMX had similar mortality to higher doses but markedly lower rates of treatment-associated adverse events leading to discontinuation [42]. Hence, lower doses of TMP-SMX may represent the ideal treatment.
Treatment Duration
The recommended treatment duration for PJP is not well validated. The guideline-recommended treatment course of 21 days for patients with HIV [44] refers to a single retrospective observation study from 1984 that compared 49 episodes of PCP in patients with HIV to 39 episodes in patients with other immunosuppressive diseases and found that people with HIV had a longer median duration of symptoms (28 days vs 5 days). Patients in this study received variable treatment durations (10 to 14 days, or longer), and 40% of those initiated on TMP-SMX had their treatment course altered with the addition of or change to pentamidine primarily due to suspected treatment failure or due to toxicity. Of note, duration of symptoms had no correlation with survival, and the study did not conclude with any recommendation addressing treatment duration; it merely stated that the clinical course of PCP was noted to be more subacute in patients with HIV. All RCTs of PCP treatment were exclusively for a 21-day course, regardless of the choice of agent, presumably based on this observed duration of more prolonged symptoms.
The guideline-recommended duration of treatment in non-HIV cases of immune suppression is at least 14 days with longer durations often recommended [40]. For patients with other forms of immunosuppression, decision on duration of therapy is guided by observational evidence, with a 14-day course having been used successfully since early reports [41]. Some observational studies suggest that a 14-day course could also be considered in HIV [45]. This discrepancy between HIV and non-HIV recommendations highlights another major gray area of PCP treatment management in need of RCT evidence. Patients with PCP may be receiving a longer than necessary treatment course.
Treatment in Pregnancy and Breastfeeding
Pregnant or breastfeeding people have not been included in any large, randomized trial of PCP treatment. The preferred initial therapy for pregnant people with PCP is TMP-SMX [44]. Although there may be a small (<1%) increased risk of congenital malformation (based on case-control studies of TMP-SMX), increased mortality from PCP has also been observed in this population, and so the benefits are believed to outweigh the risks. The alternatives in pregnancy include the following: atovaquone, clindamycin-primaquine, dapsone-TMP, and intravenous pentamidine. Both dapsone and primaquine can rarely cause hemolytic anemia in a newborn if they have concurrent glucose-6-phosphate dehydrogenase deficiency, and both have been demonstrated to cross the placenta [46, 47]. Regulatory agencies should consider including pregnant people in future treatment trials of PCP, given that they currently receive TMP-SMX without any supporting data [48].
The Role of Adjunctive Therapies: Corticosteroids
We found 7 RCTs that examined the role of adjective corticosteroids that have been previously published in a systematic review [17]. The number and size of trials included in the review (they included 6 RCTs) were small, but they demonstrated that adjunctive corticosteroids for HIV-infected people with PCP were beneficial for patients with hypoxemia and likely decreased mortality. Each of these RCTs is subject to the same limitations as those comparing treatments: namely, they primarily involve young men with HIV in an era before modern critical care and antiretroviral therapy. There has never been an RCT of adjunctive corticosteroids in patients with PCP and solid organ transplant, hematological malignancy, or other populations, and so use in these settings is based entirely on extrapolation from the HIV data.
A systemic review of adjunctive corticosteroids in non-HIV populations summarized the available observational data (16 retrospective studies) and found that in patients with respiratory failure, they were possibly beneficial, and in an unselected population, they might be harmful and associated with increased mortality [49]. We identified 1 ongoing randomized controlled trial (ClinicalTrials.gov Identifier NCT02603575) comparing a 21-day tapered dose of methylprednisolone against placebo in patients with PCP without HIV and being treated with sulfanilamide. If steroids are beneficial for non-HIV populations, the questions of which steroid, and at what dose and duration, are also candidates for study.
The Role of Adjunctive Therapies: Echinocandins
On the basis of bioplausibility [50] and observational reports [51–53], there has been interest in the use of the echinocandins as adjunctive agents for the treatment of PCP. There are currently 2 randomized controlled trials in critically ill patients without HIV examining the use of adjunctive caspofungin listed as actively recruiting (ClinicalTrials.gov Identifier NCT03978559 and NCT02603575); however, the use of echinocandins in patients with HIV and/or before critical illness will need to be inferred from those results unless additional trials are conducted. Rezafungin is a long-acting echinocandin that is currently being studied in phase III trials for the prevention of invasive fungal diseases in stem cell transplant patients, and it has in vivo data demonstrating its ability to prevent PCP in a neutropenic mouse model [54]. Rezafungin as a single dose could be an interesting single-dose adjunctive treatment to evaluate in PCP.
Special Populations: Consideration of Low-Dose Trimethoprim-Sulfamethoxazole Treatment Pending Randomized Controlled Trial Evidence
Guidelines allude to the potential efficacy and reduced toxicity of lower dose TMP-SMX without elaborating further [39]. Some populations are at higher risk of toxicity (primarily hyperkalemia, renal failure, and sudden death) and pending RCT evidence, a lower dose of TMP-SMX could be considered. Based on the informed opinion of the authors, these populations include the following: (1) older adults (we suggest 66 years and older) [35], especially those who are concurrently treated with spironolactone and/or renin-angiotensin-aldosterone inhibitors (where these agents cannot be held) [35]; (2) baseline chronic kidney disease and/or baseline potassium levels at the upper limit of normal before the initiation of TMP-SMX treatment (risk of acute kidney injury and/or progression to severe hyperkalemia); and (3) patients with a low-intermediate pretest probability of disease, who are clinically stable, pending the results of definitive testing (low-stakes scenarios whereby the risk of toxicity outweighs real or perceived risk of undertreatment). In these scenarios, a dose of 10mg/kg per day can be considered. We have provided a link to an online dose calculator (http://tmpdose.idtrials.com).
CONCLUSIONS
Present day treatment of PCP relies on data from trials that were conducted 25–35 years ago. Not only do these trials fail to capture many populations at risk today, the choice of dose and duration of treatment are based almost entirely on anecdote. Based on the available data, the choice of agent for the standard of care remains TMP-SMX, which seems appropriate. However, there is a need for clinical trials conducted in the modern era of critical care and HIV management that have better representation of women, patients with hematologic malignancies and solid organ transplants, and those with renal insufficiency. Patients with and without HIV equally stand to benefit from shorter course and/or lower dose treatment regimens in terms of reduced toxicity. It will be important to examine TMP-SMX dosing and treatment duration to maximize safety and efficacy, to elucidate whether adjunctive corticosteroids for non-HIV populations are safe and effective (as well as which class, duration, and dose), and to answer questions about the role of adjuvant echinocandin therapy. In the meantime, consideration should be given to downgrading the strength of recommendations in current guidelines. This review should serve as an overview for clinicians who need to understand both the history and substantial limitations that underlie all current treatment recommendations for this common but neglected opportunistic infection.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Author contributions. T. C. L., E. G. M., and M. P. C. contributed to conceptual ideas; T. C. L. contributed to methodology; T. C. L., O. D. C., J. M. H., A. L., J. S., and Z. N. S. contributed to validation; T. C. L. and G. B.-L. contributed to formal analysis; T. C. L., O. D. C., J. M. H., A. L., J. S., and Z. N. S. contributed to investigation; T. C. L. contributed to resources; T. C. L. contributed to data curation; E. G. M., M. P. C., and T. C. L. contributed to writing the original draft; all authors contributed to writing and review and editing; T. C. L. contributed to visualization, supervision, and project administration.
Disclaimer. The findings and conclusions in this study are those of the authors and do not necessarily represent the official position of the National Institutes of Health.
Financial support. This research was funded in part by the Intramural Research Program of the National Institutes of Health, Clinical Center. T. C. L. and E. G. M. receive research salary support from the Fonds de Recherche du Québec – Santé. G. B. L. is supported by a scholarship from the Fonds de Recherche Québec - Santé and the Ministère de la Santé et des Services sociaux.
Potential conflicts of interest. The authors have received funding from the McGill University Health Centre Association of Physicians to conduct a study of lower dose trimethoprim-sulfamethoxazole in the treatment of Pneumocystis jirovecii pneumonia and have applied to the Canadian Institutes of Health Research for operating funds for a national trial. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Sokulska M, Kicia M, Wesołowska M, Hendrich AB.. Pneumocystis jirovecii–from a commensal to pathogen: clinical and diagnostic review. Parasitol Res 2015; 114:3577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ponce CA, Gallo M, Bustamante R, Vargas SL.. Pneumocystis colonization is highly prevalent in the autopsied lungs of the general population. Clin Infect Dis 2010; 50:347–53. [DOI] [PubMed] [Google Scholar]
- 3. Pereira-Díaz E, Moreno-Verdejo F, de la Horra C, et al. Changing trends in the epidemiology and risk factors of pneumocystis pneumonia in Spain. Front Public Health 2019; 7:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benedict K, Jackson BR, Chiller T, Beer KD.. Estimation of direct healthcare costs of fungal diseases in the United States. Clin Infect Dis 2019; 68:1791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wills NK, Lawrence DS, Botsile E, et al. The prevalence of laboratory-confirmed Pneumocystis jirovecii in HIV-infected adults in Africa: a systematic review and meta-analysis. Med Mycol 2021; 59:802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Armas Rodríguez Y, Wissmann G, Müller AL, et al. Pneumocystis jirovecii pneumonia in developing countries. Parasite 2011; 18:219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown GD, Denning DW, Gow NA, et al. Hidden killers: human fungal infections. Sci Transl Med 2012; 4:165rv13. [DOI] [PubMed] [Google Scholar]
- 8. Fauchier T, Hasseine L, Gari-Toussaint M, et al. Detection of Pneumocystis jirovecii by quantitative PCR to differentiate colonization and pneumonia in immunocompromised HIV-positive and HIV-negative patients. J Clin Microbiol 2016; 54:1487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lundberg BE, Davidson AJ, Burman WJ.. Epidemiology of Pneumocystis carinii pneumonia in an era of effective prophylaxis: the relative contribution of non-adherence and drug failure. AIDS 2000; 14:2559–66. [DOI] [PubMed] [Google Scholar]
- 10. Enomoto T, Azuma A, Kohno A, et al. Differences in the clinical characteristics of Pneumocystis jirovecii pneumonia in immunocompromized patients with and without HIV infection. Respirology 2010; 15:126–31. [DOI] [PubMed] [Google Scholar]
- 11. Roblot F, Le Moal G, Godet C, et al. Pneumocystis carinii pneumonia in patients with hematologic malignancies: a descriptive study. J Infect 2003; 47:19–27. [DOI] [PubMed] [Google Scholar]
- 12. Pagano L, Fianchi L, Mele L, et al. Pneumocystis carinii pneumonia in patients with malignant haematological diseases: 10 years’ experience of infection in GIMEMA centres. Br J Haematol 2002; 117:379–86. [DOI] [PubMed] [Google Scholar]
- 13. Liu Y, Su L, Jiang SJ, Qu H.. Risk factors for mortality from Pneumocystis carinii pneumonia (PCP) in non-HIV patients: a meta-analysis. Oncotarget 2017; 8:59729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kelley CF, Checkley W, Mannino DM, et al. Trends in hospitalizations for AIDS-associated Pneumocystis jirovecii pneumonia in the United States (1986 to 2005). Chest 2009; 136:190–7. [DOI] [PubMed] [Google Scholar]
- 15. Mansharamani NG, Garland R, Delaney D, Koziel H.. Management and outcome patterns for adult Pneumocystis carinii pneumonia, 1985 to 1995: comparison of HIV-associated cases to other immunocompromised states. Chest 2000; 118:704–11. [DOI] [PubMed] [Google Scholar]
- 16. Walzer PD, Evans HE, Copas AJ, et al. Early predictors of mortality from Pneumocystis jirovecii pneumonia in HIV-infected patients: 1985-2006. Clin Infect Dis 2008; 46:625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ewald H, Raatz H, Boscacci R, et al. Adjunctive corticosteroids for Pneumocystis jiroveci pneumonia in patients with HIV infection. Cochrane Database Syst Rev 2015; 2015:Cd006150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Toma E, Thorne A, Singer J, et al. Clindamycin with primaquine vs. trimethoprim-sulfamethoxazole therapy for mild and moderately severe Pneumocystis carinii pneumonia in patients with AIDS: a multicenter, double-blind, randomized trial (CTN 004). CTN-PCP Study Group. Clin Infect Dis 1998; 27:524–30. [DOI] [PubMed] [Google Scholar]
- 19. Safrin S, Finkelstein DM, Feinberg J, et al. Comparison of three regimens for treatment of mild to moderate Pneumocystis carinii pneumonia in patients with AIDS. A double-blind, randomized, trial of oral trimethoprim-sulfamethoxazole, dapsone-trimethoprim, and clindamycin-primaquine. ACTG 108 Study Group. Ann Intern Med 1996; 124:792–802. [DOI] [PubMed] [Google Scholar]
- 20. Montgomery AB, Feigal DW Jr, Sattler F, et al. Pentamidine aerosol versus trimethoprim-sulfamethoxazole for Pneumocystis carinii in acquired immune deficiency syndrome. Am J Respir Crit Care Med 1995; 151:1068–74. [DOI] [PubMed] [Google Scholar]
- 21. Safrin S, Lee BL, Sande MA.. Adjunctive folinic acid with trimethoprim-sulfamethoxazole for Pneumocystis carinii pneumonia in AIDS patients is associated with an increased risk of therapeutic failure and death. J Infect Dis 1994; 170:912–7. [DOI] [PubMed] [Google Scholar]
- 22. Dohn MN, Weinberg WG, Torres RA, et al. Oral atovaquone compared with intravenous pentamidine for Pneumocystis carinii pneumonia in patients with AIDS. Atovaquone Study Group. Ann Intern Med 1994; 121:174–80. [DOI] [PubMed] [Google Scholar]
- 23. Sattler FR, Frame P, Davis R, et al. Trimetrexate with leucovorin versus trimethoprim-sulfamethoxazole for moderate to severe episodes of Pneumocystis carinii pneumonia in patients with AIDS: a prospective, controlled multicenter investigation of the AIDS Clinical Trials Group Protocol 029/031. J Infect Dis 1994; 170:165–72. [DOI] [PubMed] [Google Scholar]
- 24. Toma E, Fournier S, Dumont M, et al. Clindamycin/primaquine versus trimethoprim-sulfamethoxazole as primary therapy for Pneumocystis carinii pneumonia in AIDS: a randomized, double-blind pilot trial. Clin Infect Dis 1993; 17:178–84. [DOI] [PubMed] [Google Scholar]
- 25. Hughes W, Leoung G, Kramer F, et al. Comparison of atovaquone (566C80) with trimethoprim-sulfamethoxazole to treat Pneumocystis carinii pneumonia in patients with AIDS. N Engl J Med 1993; 328:1521–7. [DOI] [PubMed] [Google Scholar]
- 26. Klein NC, Duncanson FP, Lenox TH, et al. Trimethoprim-sulfamethoxazole versus pentamidine for Pneumocystis carinii pneumonia in AIDS patients: results of a large prospective randomized treatment trial. AIDS 1992; 6:301–5. [DOI] [PubMed] [Google Scholar]
- 27. Medina I, Mills J, Leoung G, et al. Oral therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A controlled trial of trimethoprim-sulfamethoxazole versus trimethoprim-dapsone. N Engl J Med 1990; 323:776–82. [DOI] [PubMed] [Google Scholar]
- 28. Conte JE Jr, Chernoff D, Feigal DW Jr, et al. Intravenous or inhaled pentamidine for treating Pneumocystis carinii pneumonia in AIDS. A randomized trial. Ann Intern Med 1990; 113:203–9. [DOI] [PubMed] [Google Scholar]
- 29. Soo Hoo GW, Mohsenifar Z, Meyer RD.. Inhaled or intravenous pentamidine therapy for Pneumocystis carinii pneumonia in AIDS. A randomized trial. Ann Intern Med 1990; 113:195–202. [DOI] [PubMed] [Google Scholar]
- 30. Arasteh K, Heise W, L’age M.. Treatment of mild to moderately severe Pneumocystis carinii pneumonia with cotrimoxazole versus pentamidine aerosol. Preliminary results of a prospective randomized therapy study. Med Klin (Munich) 1990; 85(Suppl 2):260–3. [PubMed] [Google Scholar]
- 31. Wharton JM, Coleman DL, Wofsy CB, et al. Trimethoprim-sulfamethoxazole or pentamidine for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A prospective randomized trial. Ann Intern Med 1986; 105:37–44. [DOI] [PubMed] [Google Scholar]
- 32. Harris RJ. Metan: fixed- and random-effects meta-analysis. Available at: https://www.stata-journal.com/article.html?article=sbe24_2. Accessed 4 August 2021.
- 33. Grønseth S, Rogne T, Hannula R, et al. Epidemiological and clinical characteristics of immunocompromised patients infected with Pneumocystis jirovecii in a twelve-year retrospective study from Norway. BMC Infect Dis 2021; 21:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kanj A, Samhouri B, Abdallah N, et al. Host factors and outcomes in hospitalizations for Pneumocystis jirovecii pneumonia in the United States. Mayo Clin Proc 2021; 96:400–7. [DOI] [PubMed] [Google Scholar]
- 35. Fralick M, Macdonald EM, Gomes T, et al. ; Canadian Drug Safety and Effectiveness Research Network. Co-trimoxazole and sudden death in patients receiving inhibitors of renin-angiotensin system: population based study. BMJ 2014; 349:g6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brower RG, Matthay MA, Morris A, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342:1301–8. [DOI] [PubMed] [Google Scholar]
- 37. Zayed Y, Banifadel M, Barbarawi M, et al. Noninvasive oxygenation strategies in immunocompromised patients with acute hypoxemic respiratory failure: a pairwise and network meta-analysis of randomized controlled trials. J Intensive Care Med 2020; 35:1216–25. [DOI] [PubMed] [Google Scholar]
- 38. Azoulay E, Lemiale V, Mokart D, et al. Effect of high-flow nasal oxygen vs standard oxygen on 28-day mortality in immunocompromised patients with acute respiratory failure: the HIGH randomized clinical trial. JAMA 2018; 320:2099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Names of contributors are listed in Appendix C. Available at: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/Adult_OI.pdf. Accessed 4 August 2021.
- 40. Fishman JA, Gans H; AST Infectious Diseases Community of Practice. Pneumocystis jiroveci in solid organ transplantation: Guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant 2019; 33:e13587. [DOI] [PubMed] [Google Scholar]
- 41. Hughes WT, Feldman S, Sanyal SK.. Treatment of Pneumocystis carinii pneumonitis with trimethoprim-sulfamethoxazole. Can Med Assoc J 1975; 112:47–50. [PMC free article] [PubMed] [Google Scholar]
- 42. Butler-Laporte G, Cheng MP, Thirion DJG, et al. Clinical trials increase off-study drug use: a segmented time-series analysis. Open Forum Infect Dis 2020; 7:ofaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thomas M, Rupali P, Woodhouse A, Ellis-Pegler R.. Good outcome with trimethoprim 10mg/kg/day-sulfamethoxazole 50mg/kg/day for Pneumocystis jirovecii pneumonia in HIV infected patients. Scand J Infect Dis 2009; 41:862–8. [DOI] [PubMed] [Google Scholar]
- 44. Benson C, Brooks JT, Holmes K, Masur H, Pau A.. Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Panel members are listed in Appendix B. Available at: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-opportunistic-infection/pneumocystis-pneumonia. Accessed 5 August 2021.
- 45. Creemers-Schild D, Kroon FP, Kuijper EJ, de Boer MG.. Treatment of Pneumocystis pneumonia with intermediate-dose and step-down to low-dose trimethoprim-sulfamethoxazole: lessons from an observational cohort study. Infection 2016; 44:291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thornton YS, Bowe ET.. Neonatal hyperbilirubinemia after treatment of maternal leprosy. South Med J 1989; 82:668. [DOI] [PubMed] [Google Scholar]
- 47. Nosten F, McGready R, d’Alessandro U, et al. Antimalarial drugs in pregnancy: a review. Curr Drug Saf 2006; 1:1–15. [DOI] [PubMed] [Google Scholar]
- 48. Malhamé I, D’Souza R, Cheng MP.. The moral imperative to include pregnant women in clinical trials of interventions for COVID-19. Ann Intern Med 2020; 173:836–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ding L, Huang H, Wang H, He H.. Adjunctive corticosteroids may be associated with better outcome for non-HIV Pneumocystis pneumonia with respiratory failure: a systemic review and meta-analysis of observational studies. Ann Intensive Care 2020; 10:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Luraschi A, Richard S, Hauser PM.. Site-directed mutagenesis of the 1,3-β-glucan synthase catalytic subunit of Pneumocystis jirovecii and susceptibility assays suggest its sensitivity to caspofungin. Antimicrob Agents Chemother 2018; 62:e01159-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tian Q, Si J, Jiang F, et al. Caspofungin combined with TMP/SMZ as a first-line therapy for moderate-to-severe PCP in patients with human immunodeficiency virus infection. HIV Med 2021; 22:307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang G, Chen M, Zhang S, et al. Efficacy of caspofungin combined with trimethoprim/sulfamethoxazole as first-line therapy to treat non-HIV patients with severe pneumocystis pneumonia. Exp Ther Med 2018; 15:1594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang M, Lang G, Chen Y, et al. A pilot study of echinocandin combination with trimethoprim/sulfamethoxazole and clindamycin for the treatment of AIDS patients with pneumocystis pneumonia. J Immunol Res 2019; 2019:8105075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Miesel L, Cushion MT, Ashbaugh A, et al. Efficacy of rezafungin in prophylactic mouse models of invasive candidiasis, aspergillosis, and pneumocystis pneumonia. Antimicrob Agents Chemother 2021; 65:e01992-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.